Abstract
SATB2 (Special AT-rich sequence-binding protein 2) has recently been shown to be a specific biomarker of colorectal cancer (CRC). The aim of this study was to investigate the diagnostic potential of SATB2 as a means of detecting a CRC origin for liver metastases. SATB2 expression was examined in a resection cohort of 101 CRC and 273 non-CRC adenocarcinoma samples using immunohistochemistry (IHC). The diagnostic accuracy of CRC origins of liver metastases based on SATB2 and a three marker panel of SATB2, CK20 and CDX2 was evaluated using an independent cohort of 192 liver biopsies. IHC showed 97 of the 101 (96.0%) primary CRC samples were SATB2 positive, compared to only 6 of the 273 (2.1%) samples of other cancer types. The sensitivity, specificity and AUC values of SATB2 expression in resection samples were 97%, 97.1% and 0.977, respectively. Meanwhile, for the liver biopsy samples, the sensitivity, specificity and AUC values of a CRC liver metastases was 92.2%, 97.8% and 0.948 for SATB2, 95.1%, 91.0% and 0.959 for CK20, and 100%, 85.4% and 0.976 for CDX2, respectively. Further analysis demonstrated that all three-marker positivity was detected in 92/103 (89.3%) CRC and 2/89 (2.2%) non-CRC liver metastases sampled by biopsy. Our findings suggest that SATB2, as measured by IHC, could serve as a promising diagnostic biomarker of CRC metastases. Combining evaluation of SATB2 with CK20 and CDX2 to form a three marker panel further improved the detection of metastatic CRCs in liver biopsy tissues.
Keywords: Diagnostic biomarker, Metastatic colorectal cancer, Liver biopsy, SATB2
Highlights
-
•
SATB2 protein could serve as a promising diagnostic biomarker of CRC.
-
•
SATB2 expression can distinguish liver metastases with CRC origins from other forms of adenocarcinoma.
-
•
A three marker panel of SATB2, CK20 and CDX2 used on liver biopsy samples is valuable when assessing g a CRC origin.
Liver metastasis is the leading cause of cancer-related morbidity and mortality in colorectal cancer (CRC) patients. Since histological diagnosis from liver needle biopsy is based solely on tiny fragments of tissue, it can sometimes be quite difficult to distinguish CRC from non-CRCs. Thus, there is a current need for searching and identifying of specific biomarkers that can improve the accuracy of diagnosis. Our results indicate that SATB2 could serve as a promising diagnostic biomarker of CRC metastases; and that a three marker panel of SATB2, CK20 and CDX2 used on liver biopsy samples is valuable.
1. Introduction
Colorectal cancer (CRC) liver metastasis is one of the major causes of tumor-related death worldwide (Disibio and French, 2008). Recent advances in the detection and treatment of CRC have resulted in a significant increase in the 5-year overall survival rate and reduced the recurrence rate of CRC patients with liver metastasis (Chan et al., 2014, Pinson et al., 1996, Sharma et al., 2008). The benefit of complete hepatic resection is well recognized in CRC patients with liver metastasis, with 5-year overall survival rate up to 60% (Abdalla et al., 2004). Therefore, improving methods of identifying if the liver neoplasm originated from a CRC could increase the proportion of patients who are candidates for complete surgical treatment or preoperative chemotherapy, and, thus, aid in patient survival. Preoperative examinations, which include assessing signs and symptoms and imaging and laboratory studies, can be very informative, but are not perfect means of detection (Berger et al., 2000, Soyer et al., 1994, Tan et al., 2009, Zhou et al., 2006). Liver needle biopsies are generally recommended as follow-up studies to confirm assessments of hepatic nodules not satisfactorily addressed by imaging (Bruix et al., 2005). Troublingly, liver metastases with CRC origins often mimic the histological patterns of other cancers with other primary origins, as well as cholangiocarcinomas. For example, CRC liver metastases are usually tubular, but may also be mucinous, signet-ring, papillary, cystic or undifferentiated. Therefore, distinguishing between liver metastases of CRC origin and other forms of adenocarcinomas can be difficult, and diagnostic panels of immunohistochemical staining are needed. Importantly, histological analysis of liver needle biopsies is based solely on the analysis of tiny fragments of tissue, thus making it very difficult to make a histological distinction between metastatic CRC and other forms of adenocarcinoma, resulting in diagnostic delays.
Specific immunohistochemical markers play a very important role when making a histological distinction between metastatic CRC and other forms of adenocarcinoma in the liver. It is well known that CRCs typically express cytokeratin 20 (CK20) and caudal-type homeobox 2 (CDX2). However, although CK20 is a highly sensitive marker, it is also expressed in several other types of adenocarcinomas (Tot, 1999). Meanwhile, while CDX2 is helpful when diagnosing metastatic CRCs, it is non-specific and can be expressed in cholangiocarcinomas, gastric carcinomas, and ovarian mucinous tumors (Dennis et al., 2005, Werling et al., 2003). Overall, the availability of better and more specific immunohistochemical markers by which to identify metastatic CRCs would be advantageous when making diagnoses.
The special AT-rich sequence-binding protein 2 (SATB2) was initially introduced as a novel marker of osteoblastic differentiation (Sheehan-Rooney et al., 2010). Recent studies have shown that SATB2 could serve as a clinically useful diagnostic marker for CRCs (Dragomir et al., 2014, Kim et al., 2016, Lin et al., 2014, Magnusson et al., 2011, Ordonez, 2014) as 71–97% of primary and metastatic CRCs have been reported to stain positively for SATB2 expression (Dragomir et al., 2014, Eberhard et al., 2012, Lin et al., 2014, Moh et al., 2016). Moreover, when the SATB2 antibody was tested for specificity, it was rare to find positive SATB2 expression within a wide range of other non-CRC tissues, including gastric, cholangiocellular, lung, breast and ovarian cancers (Kim et al., 2016, Magnusson et al., 2011, Perez Montiel et al., 2015). These studies support this work verifying the role of SATB2 as a means by which to distinguish between liver metastases with a CRC origin and other types of metastatic adenocarcinoma.
The current study was designed to assess the specificity of the SATB2 protein for metastatic CRCs in livery biopsy specimens. First, SATB2 protein levels were measured in primary CRC and non-CRC adenocarcinoma tissues by immunohistochemistry (IHC). Previously, two other biomarkers, CK20 and CDX2, have been used for the differential diagnosis of CRC in the clinic. Therefore, the diagnostic value of SATB2 was examined further by IHC both alone and in combination with CK20 and CDX2 in an additional cohort of liver needle biopsy samples that contained liver metastases of CRCs and non-CRC adenocarcinomas. Overall, SATB2 protein was found to be a useful diagnostic biomarker, especially when used in combination with CK20 and CDX2, that improved identification of a CRC origin for liver metastases in liver needle biopsy tissues.
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
The study was approved by the Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center and the Six Affiliated Hospital of Sun Yat-sen University. The experiments were undertaken with the understanding and written consent of each subject. All clinical investigations were conducted according to the principles expressed in the World Medical Association Declaration of Helsinki.
2.2. Patient Cohorts
Specimens from 374 patients with adenocarcinomas, including CRC, gastric cancer (GC), pancreatic cancer (PC), duodenal cancer (DC), intrahepatic cholangiocellular cancer (ICC), pulmonary cancer (PUC), and mammary cancer (MC), were collected, formalin-fixed, paraffin-embedded and archived. All of these patients had undergone surgical resection between March 2003 and August 2006 at the Sun Yat-sen University Cancer Center (Guangzhou, China).
To validate the results, liver biopsies taken with 18-gauge needles from an independent cohort of 192 patients with adenocarcinoma were selected and classified as having either: (Abdalla et al., 2004) a primary liver origin (cholangiocarcinoma) or (Baba et al., 2015) an extrahepatic origin (including metastases from colorectal, gastric, pancreatic, lung and breast carcinomas). All 192 cases were selected from our institute and the Six Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China). The tumor grades were defined according to the World Health Organization (WHO) Classification of Tumours of the Digestive System (2010), Lung (2015) and Breast (2014), respectively. Tumor staging was determined based on the tumor node metastasis (TNM, 8th Edition) staging system of the International Union against Cancer (UICC). The baseline clinicopathologic characteristics for this study are summarized in Table 1.
Table 1.
Clinicopathological features of 7 types of human adenocarcinomas.
| Colorectal adenocarcinoma | n = 101 | Cholangiocellular adenocarcinoma | n = 56 |
|---|---|---|---|
| Gender | Gender | ||
| Male | 61 | Male | 30 |
| Female | 50 | Female | 26 |
| Average age of patient (year) | 58 (35–85) | Average age of patient (year) | 55 (23–85) |
| Average size of tumor (cm) | 4.6 (1.5–10.5) | Average size of tumor (cm) | 5.3 (0.7–11) |
| Tumor grade | Tumor grade | ||
| 1 | 10 | 1 | 0 |
| 2 | 66 | 2 | 46 |
| 3 | 25 | 3 | 10 |
| TNM stage | TNM stage | ||
| 1 | 23 | 1 | 15 |
| 2 | 39 | 2 | 23 |
| 3 | 29 | 3 | 18 |
| 4 | 10 | 4 | 0 |
| Gastric adenocarcinoma | n = 97 | Lung adenocarcinoma | n = 32 |
| Gender | Gender | ||
| Male | 65 | Male | 12 |
| Female | 32 | Female | 20 |
| Average age of patient (year) | 58 (24–89) | Average age of patient (year) | 59 (44–77) |
| Average size of tumor (cm) | 4.5 (0.3–13) | Average size of tumor (cm) | 2.9 (1–7) |
| Tumor grade | Tumor grade | ||
| 1 | 0 | 1 | 5 |
| 2 | 56 | 2 | 24 |
| 3 | 41 | 3 | 3 |
| TNM stage | TNM stage | ||
| 1 | 14 | 1 | 17 |
| 2 | 24 | 2 | 6 |
| 3 | 46 | 3 | 8 |
| 4 | 13 | 4 | 1 |
| Pancreatic adenocarcinoma | n = 30 | Breast adenocarcinoma | n = 31 |
| Gender | Gender | ||
| Male | 17 | Male | 0 |
| Female | 13 | Female | 31 |
| Average age of patient (year) | 57 (35–79) | Average age of patient (year) | 52 (23–80) |
| Average size of tumor (cm) | 3.5 (0.5–6) | Average size of tumor (cm) | 3.1 (0.8–11) |
| Tumor grade | Tumor grade | ||
| 1 | 4 | 1 | 15 |
| 2 | 18 | 2 | 11 |
| 3 | 8 | 3 | 5 |
| TNM stage | TNM stage | ||
| 1 | 10 | 1 | 14 |
| 2 | 15 | 2 | 11 |
| 3 | 4 | 3 | 6 |
| 4 | 1 | 4 | 0 |
| Duodenal adenocarcinoma | n = 27 | ||
| Gender | |||
| Male | 13 | ||
| Female | 14 | ||
| Average age of patient (year) | 55 (44–77) | ||
| Average size of tumor (cm) | 3.4 (0.3–12) | ||
| Tumor grade | |||
| 1 | 2 | ||
| 2 | 19 | ||
| 3 | 6 | ||
| TNM stage | |||
| 1 | 12 | ||
| 2 | 11 | ||
| 3 | 4 | ||
| 4 | 0 | ||
2.3. Immunohistochemistry
Assays were performed as described previously (Cai et al., 2011). Immunohistochemical staining for SATB2 (Abcam, Cambridge, MA, 1:100 dilution) was performed on all specimens. Additional immunohistochemical staining was performed on liver biopsy specimens with antibodies against CDX2 (Biogenex, Fremont, CA, 1:100 dilution), CK20 (Dako, Glostrup, Denmark, 1:50 dilution) and CK7 (ZSGB-BIO, Beijing, China, 1:100). Positive staining for SATB2 and CDX2 was considered to be localized in the nucleus and CK20 in the cytoplasm. The intensity of SATB2, CK20 and CDX2 staining was scored as follows: 0 (negative staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). Percentage scores were assigned as follows: 1 (1%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (76%–100%). A positive control was obtained by replacing cancer tissues with the colorectal mucosal tissues. The intensity and percentage scores assigned to each tumor sample were multiplied to give a final score of 0 to 12. Expression of these markers was assessed by two independent pathologists (M-YC and Y-JZ), who were blind to the clinicopathological data. The pathologists were in agreement for approximately 85% of the cases, indicating this scoring method is highly reproducible. If the two assessments were consistent with one another, that value was selected. In cases where different results occurred, the two pathologists discussed their assessments and agreed on the appropriate score.
2.4. Statistical Analysis
Statistical analyses were performed using SPSS software (SPSS standard version 16.0). Receiver operating characteristic (ROC) curve analysis was applied to determine the cut-point for marker positivity by the 0,1-criterion, and sensitivity, specificity and the areas under the ROC curves (AUC) were calculated. To combine the three markers (SATB2, CK20 and CDX2), the linear coefficient that maximized the AUC for all three in combination was determined. A difference was considered significant if the p value from a two-tailed test was < 0.05.
3. Results
3.1. Characterization of SATB2 Expression by IHC in CRC and Other Adenocarcinomas
Immunohistochemical analysis showed distinct nuclear expression of SATB2 of high intensity in a large fraction of positive tumor cells in the majority of analyzed CRCs (77/101) (Fig. 1A-1F). And there are still some of CRC cases showing focal positive (22/101) or negative (2/101) expression (Fig. 1G-1J). Further correlation analysis showed that there is no significant association between the high intensity nuclear expression of SATB2 and the clinicopathological features (including age, gender, tumor size, localization, differentiation and TNM stage, Supplemental Table 1). To identify a single, optimal cut-off to indicate positive SATB2 expression, ROC curve analysis was applied to the cohort of surgical resection tissues. The score closest to the point that maximized both sensitivity and specificity on the curve (0.0, 1.0) was selected as the cut-off score. According to ROC curve analysis, tumors designated positive for SATB2 were those with scores above 1. Using these criteria, 97 of 101 (96.0%) of the primary CRCs evaluated expressed SATB2. Meanwhile, only 6 of the 273 (2.1%) of the non-CRC adenocarcinoma tissues, which had gastric (n = 97), pancreatic (n = 30), duodenal (n = 27), intrahepatic cholangiocellular (n = 54), pulmonary (n = 32), and mammary (n = 31) origins, were positive for SATB2 upon immunostaining (Fig. 2). The duodenal and mammary adenocarcinomas were all negative for SATB2, while 1 of the 97 (1%) of the analyzed gastric adenocarcinomas was positive for SATB2 staining. Of the 54 cholangiocellular adenocarcinomas, 3 (5.7%) cases were positive for SATB2 expression. Only 1 of the 30 (3.3%) pancreatic cancer and 1 of the 32 (3.1%) lung cancer samples stained positive for SATB2, all of which displayed a notably weak intensity. The immunohistochemical findings for the above-described 7 types of adenocarcinoma are summarized in Table 2.
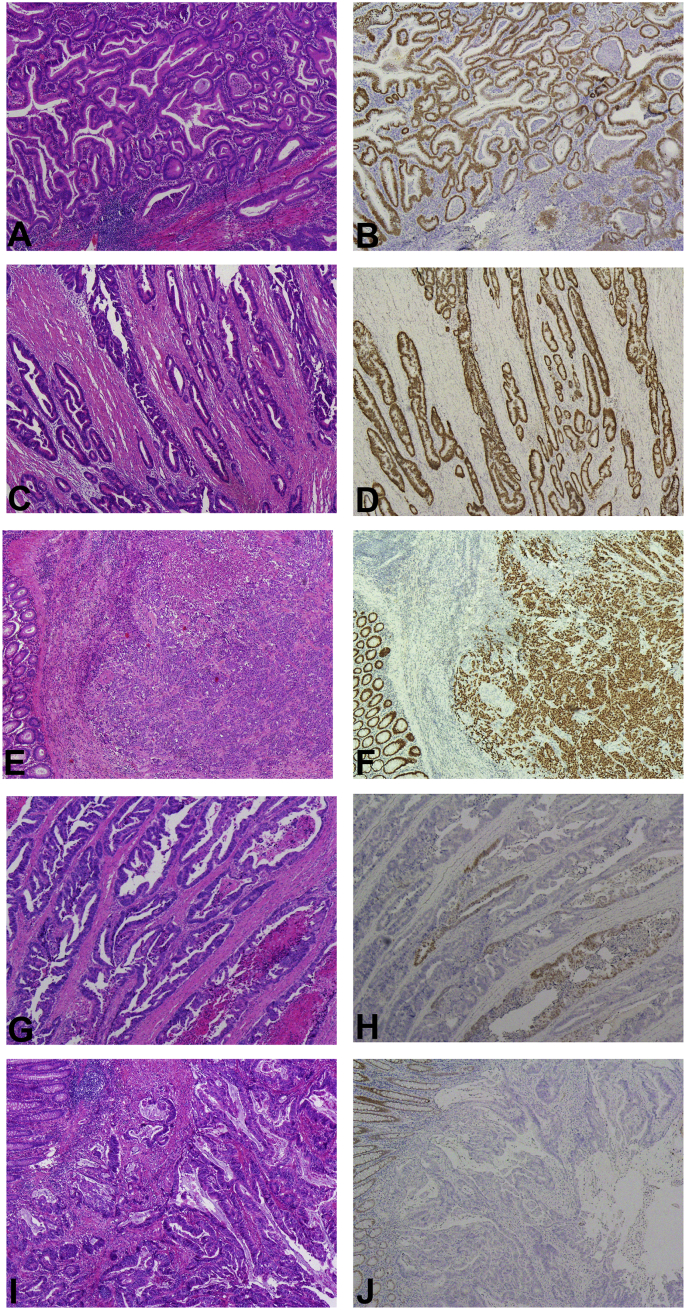
Fig. 1.
The expression pattern of SATB2 in colorectal cancer. SATB2 protein expression is distinctive with high intensity nuclear staining occurring in CRCs, regardless of high (A, HE and B, IHC), moderate (C, HE and D, IHC) or poor (E, HE and F, IHC) differentiation. There are still some of cases showing focal positive SATB2 expression (G, HE and H, IHC). There are a few cases showing negative SATB2 expression (I, HE and J, IHC).
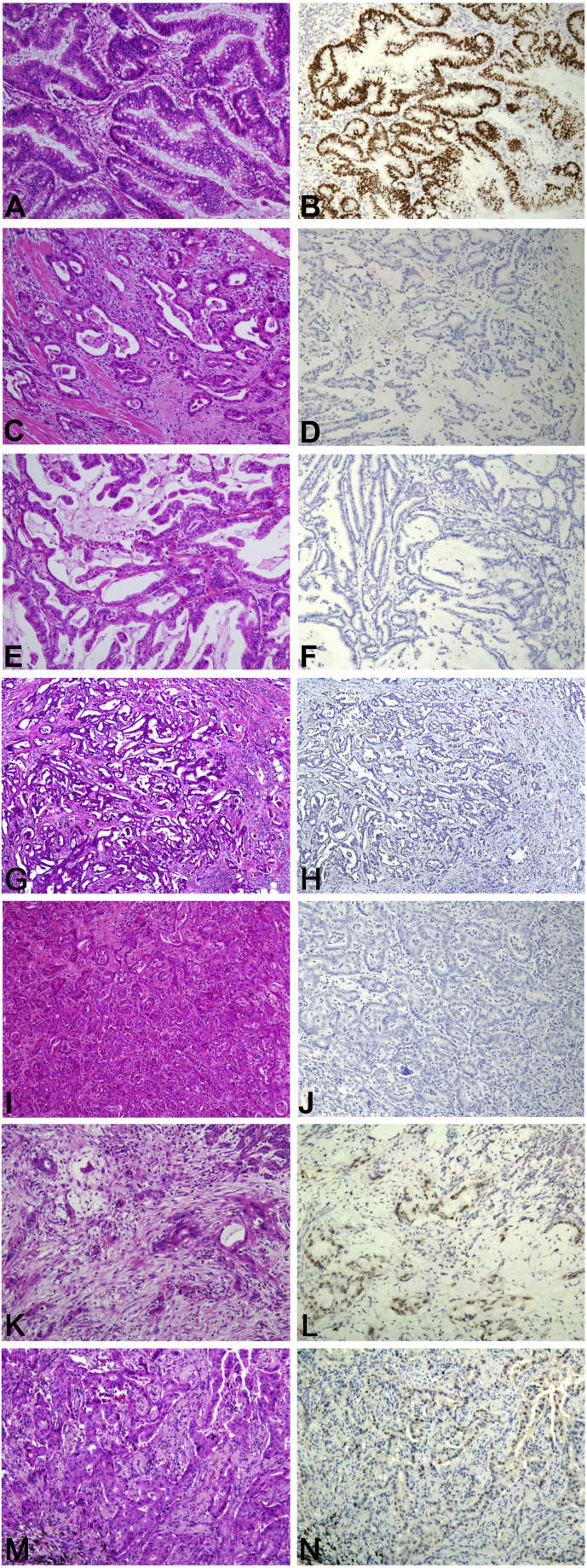
Fig. 2.
The expression of SATB2 in different histological types of human adenocarcinomas. Immunohistochemical analysis found high intensity nuclear expression of SATB2 in a large fraction of SATB2 positive tumor cells in the majority of analyzed CRCs (A, HE and B, IHC), while the 6 other types of adenocarcinoma show negative expression and focal staining of SATB2, including negative staining of gastric (C, HE and D, IHC), duodenal (E, HE and F, IHC), breast (G, HE and H, IHC), intrahepatic cholangiocellular cancer (I, HE and J, IHC). And focal staining of SATB2 is shown in pancreatic (K, HE and L, IHC) and lung cancer (M, HE and N, IHC) (× 100).
Table 2.
SATB2 expression in 7 types of human adenocarcinoma.
| No. of cases (%) |
||
|---|---|---|
| Positive | Negative | |
| Primary colorectal adenocarcinoma | 97/101 (96%) | 4/101 (4%) |
| Primary gastric adenocarcinoma | 1/97 (1%) | 96/97 (99%) |
| Primary pancreatic adenocarcinoma | 1/30 (3.3%) | 29/30 (96.7%) |
| Primary duodenal adenocarcinoma | 0/27 | 27/27 (100%) |
| Primary cholangiocellular adenocarcinoma | 3/54 (5.7%) | 51/54 (94.3%) |
| Primary lung adenocarcinoma | 1/32 (3.1%) | 31/32 (96.9%) |
| Primary breast adenocarcinoma | 0/31 | 31/31 (100%) |
3.2. SATB2 Expression in Liver Needle Biopsy Samples can Distinguish Liver Metastases with CRC Origins from Other Forms of Adenocarcinoma
In the clinic, a needle biopsy is normally recommended to characterize malignancies when hepatic nodules are detected. Sometimes, it can be difficult to distinguish between CRC and other sites as the origin of adenocarcinoma liver metastases. To aid in determining the origin of an adenocarcinoma from a liver needle biopsy sample, the expression of SATB2 was evaluated by IHC in a second cohort (i.e. “validation cohort”) that consisted of 103 CRCs, 28 cholangiocellular carcinomas, 14 gastric cancers, 12 pancreatic cancers, 28 breast cancers and 7 lung adenocarcinomas. Because CK20 and CDX2 are routinely used during differential diagnoses for CRC clinically, the diagnostic value of assessing SATB2 expression along with that of CK20 and CDX2 was evaluated for CRCs. In liver metastatic CRC with CK20 and/or CDX2 negative expression, SATB2 still shows the specificity of CRC origin in liver needle biopsy (Fig. 3).
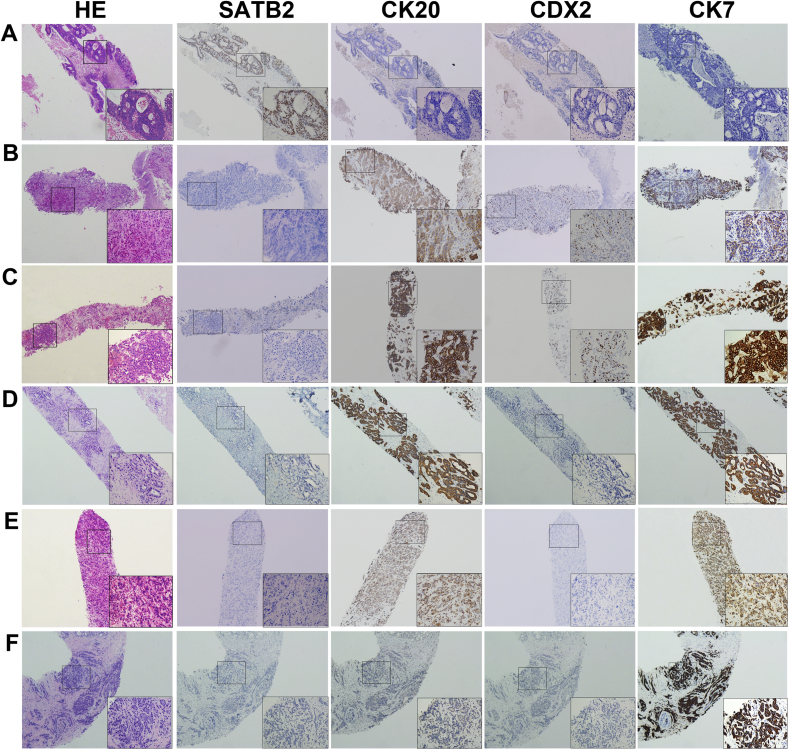
Fig. 3.
The expression of SATB2, CK20 and CDX2 in liver needle biopsy tissues. (A) Liver biopsies of metastatic CRCs positively expressed SATB2, but not CK20 and CDX2. Biopsies of liver metastases of (B) gastric and (C) pancreatic adenocarcinomas did not express SATB2, but stained positively for CK20 and CDX2. (D) Intrahepatic cholangiocellular carcinoma and (E) liver metastases of lung adenocarcinoma were negative for SATB2 and CDX2 expression, but positive for CK20 expression. (F) Expression of SATB2, CK20 and CDX2 was negative for liver metastases of a breast carcinoma. Representative liver needle biopsy samples at low (× 40) and high (× 200) magnification are shown.
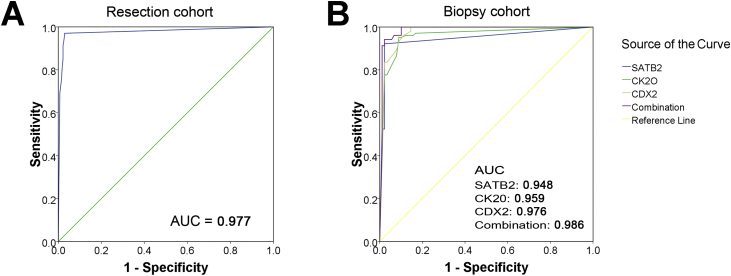
In order to determine the diagnostic value of SATB2 for CRCs, ROC curve analysis was performed on the resection and biopsy cohorts. In the resection cohort, the sensitivity, specificity and AUC values of SATB2 expression for detection of a CRC origin versus other origins were 97%, 97.1% and 0.977 (p < 0.0001, Fig. 4A), while in the biopsy cohort, they were 92.2%, 97.8% and 0.948, respectively (p < 0.0001, Fig. 4B). In the biopsy cohort, the sensitivity, specificity and AUC values for an accurate diagnosis of CRC were 95.1%, 91.0% and 0.959 when only CK20 was concerned, and 100%, 85.4% and 0.976 when only CDX2 was concerned, respectively. As shown in the ROC curves, although SATB2 is not so sensitive as CK20 and CDX2, it is the most specific of the 3 markers. These data suggested that SATB2 is a potential biomarker for identifying liver metastasis with CRC origin. Next, it was determined whether a combination of these three potential markers improved upon identification of a CRC origin. Interestingly, the AUC values increased to 0.986 when SATB2 expression was measured alongside CK20 and CDX2 (p < 0.0001, Fig. 4B). These values indicate that SATB2 expression is significantly different between liver metastases originating from CRC and other origins in liver needle biopsy samples. Moreover, SATB2 expression is more specific for assessing a CRC origin than either CK20 or CDX2. Therefore, combining these three markers could greatly improve the accuracy of diagnosing a hepatic CRC metastasis from a needle biopsy.
Fig. 4.
ROC curve analysis was performed to evaluate the contribution of SATB2, CK20 and CDX2 expression when diagnosing the origin as colorectal cancer. (A) ROC curve of SATB2 expression [area under curve (AUC) = 0.977, p < 0.0001] in CRCs compared to non-CRCs in the resection cohort. (B) ROC curves of SATB2 (AUC = 0.948, p < 0.0001), CK20 (AUC = 0.959, p < 0.0001), and CDX2 (AUC = 0.976, p < 0.0001) expression, as well as a combination of all three markers (AUC = 0.986, p < 0.0001), in liver needle biopsies from patients with liver metastases with CRC origins versus other origins. ROC, receiver operating characteristics.
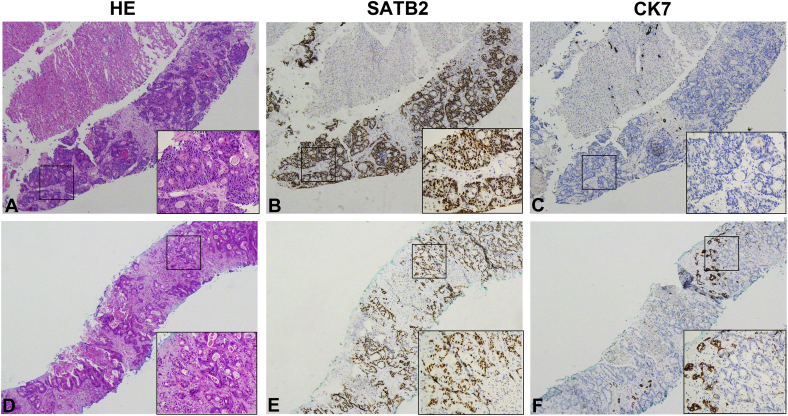
In order to evaluate the correlation between SATB2 and CK7 expression in a subset of liver ADCs, we further examined the IHC expression of CK7 in the biopsy cohort (192 cases). Our results showed that SATB2 was positive in only one of the liver ADC of pulmonary origin that was CK7+ CK20− CDX2− and SATB2 was expressed in 94.7% (89/94) of liver metastatic CRC with CK7− CK20+ CDX2+ (Fig. 5). Additionally, SATB2 staining was observed in 66.7% (6/9) that coexpressed CK20/CDX2 along with CK7 (Table 3).
Fig. 5.
The paired SATB2 and CK7 expression in metastatic adenocarcinomas originated from colorectal cancer (CRC) in liver needle biopsy. The representative expression patterns of SATB2+ and CK7− in majority of metastatic CRC (A–C). Minority of metastatic CRCs show diffuse positive staining of SATB2 and focal staining of CK7.
Table 3.
The expression patterns of SATB2, CK7, CK20 and CDX2 in the different adenocarcinoma in liver needle biopsies.
| CK7 | CK20 and/or CDX2 | Total Cases | SATB2 Positive |
|---|---|---|---|
| Colorectal Cancer | |||
| Positive | Negative | 0 | – |
| Positive | Positive | 9 | 6/9 |
| Negative | Positive | 94 | 89/94 |
| Gastric Cancer | |||
| Positive | Negative | 4 | 0/4 |
| Positive | Positive | 10 | 2/10 |
| Negative | Positive | 3 | 0/3 |
| Pancreatic Cancer | |||
| Positive | Negative | 10 | 0/10 |
| Positive | Positive | 2 | 0/2 |
| Negative | Positive | 0 | – |
| Intrahepatic Cholangiocellular cancer | |||
| Positive | Negative | 24 | 0/24 |
| Positive | Positive | 4 | 0/4 |
| Negative | Positive | 0 | – |
| Lung Cancer | |||
| Positive | Negative | 5 | 1/5 |
| Positive | Positive | 2 | 0/2 |
| Negative | Positive | 0 | – |
| Breast Cancer | |||
| Positive | Negative | 28 | 0/28 |
| Positive | Positive | 0 | – |
| Negative | Positive | 0 | – |
3.3. Potential Utility of SATB2, CK20 and CDX2 Expression in Liver Needle Biopsies during Diagnosis of a Colorectal Origin for Liver Metastases
According to the ROC curve analysis described above, liver needle biopsies of tumors designated positive for SATB2, CK20 and CDX2 expression were all those with scores above 1. Using these criteria on our biopsy cohort, all 28 cases of breast cancer liver metastases were negative for each of the three markers, whereas the number of immuno-positive cases where at least one marker was expressed was 10/14 GCs, 1/12 PCs, 4/28 ICCs, 3/7 LCs, and 103/103 (100%) CRCs. Positive expression of at least two markers occurred in 1/12 PCs, 8/14 GCs and 103/103 (100%) CRCs. Expression of all three markers was observed in 92/103 (89.3%) CRC liver metastases, but of the adenocarcinomas with a non-CRC origin, only 2 (2.2%) liver metastatic GC expressed all three markers (Table 4).
Table 4.
Immunohistochemical analysis of the different adenocarcinoma in liver needle biopsies.
| CRC |
GC |
PC |
LC |
BC |
ICC |
|
|---|---|---|---|---|---|---|
| n = 103 | n = 14 | n = 12 | n = 7 | n = 28 | n = 28 | |
| All three positive | 92 | 2 | 0 | 0 | 0 | 0 |
| At least two positive | 103 | 8 | 2 | 0 | 0 | 0 |
| At least one positive | 103 | 10 | 2 | 3 | 0 | 4 |
| SATB2 +/CK20 + | 92 | 2 | 0 | 0 | 0 | 0 |
| SATB2 +/CDX2 + | 95 | 2 | 0 | 0 | 0 | 0 |
| CK20 +/CDX2 + | 100 | 8 | 2 | 0 | 0 | 0 |
| SATB2 + | 95 | 2 | 0 | 1 | 0 | 0 |
| CK20 + | 100 | 8 | 2 | 2 | 0 | 3 |
| CDX2 + | 103 | 10 | 2 | 0 | 0 | 1 |
CRC, colorectal cancer; GC, gastric cancer; PC, pancreatic cancer; ICC, intrahepatic cholangiocellular cancer; LC, lung cancer; BC, breast cancer.
Further statistical analysis showed that when the expression of at least one marker (SATB2, CK20 or CDX2) was considered, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and Youden index for differentiating CRCs from non-CRCs was 100%,78.6%, 84.4%, 100%, 90.1% and 0.786, while for at least two positive markers, they were 100%, 88.8%, 91.2%, 100%, 94.8% and 0.888, respectively. Finally, when all three markers were used, the sensitivity, specificity, PPV, NPV, accuracy and Youden index were 97.8%, 89.3%, 97.8%, 88.7%, 92.5% and 0.871, respectively (Table 5).
Table 5.
Degree of diagnostic accuracy in CRCs and Non-CRCs.
| CRC n = 103 | Non-CRC n = 89 | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | Youden index | |
|---|---|---|---|---|---|---|---|---|
| All three positive | 92 | 2 | 97.8 | 89.3 | 97.8 | 88.7 | 92.5 | 0.871 |
| At least two positive | 103 | 10 | 100 | 88.8 | 91.2 | 100 | 94.8 | 0.888 |
| At least one positive | 103 | 19 | 100 | 78.6 | 84.4 | 100 | 90.1 | 0.786 |
| SATB2 +/CK20 + | 92 | 1 | 89.3 | 97.8 | 97.9 | 88.8 | 93.2 | 0.871 |
| SATB2 +/CDX2 + | 95 | 1 | 92.2 | 97.8 | 97.9 | 91.6 | 94.8 | 0.900 |
| CK20 +/CDX2 + | 103 | 9 | 97.1 | 88.7 | 90.9 | 96.3 | 93.2 | 0.858 |
| SATB2 + | 95 | 1 | 92.2 | 96.7 | 96.9 | 91.5 | 94.3 | 0.889 |
| CK20 + | 100 | 13 | 97.1 | 83.1 | 86.9 | 96.1 | 90.6 | 0.802 |
| CDX2 + | 103 | 12 | 100.0 | 85.4 | 60.4 | 88.8 | 93.2 | 0.854 |
CRC, colorectal cancer.
4. Discussion
SATB2 is a nuclear matrix-associated transcription factor and epigenetic regulator (Dobreva et al., 2006, Dobreva et al., 2003). The effects of SATB2 expression on prognosis and metastasis are different in CRC than any other cancer type. Low SATB2 expression was found to be correlated with tumor progression and an independent prognostic marker in colorectal cancer (Wang et al., 2009). Eberhard et al. indicated that overexpression of SATB2 in CRC enhanced the patient's benefit from adjuvant therapy (Eberhard et al., 2012). Positive staining with an antibody directed against this protein serves as a fairly novel biomarker that is chiefly utilized during clinical diagnosis of lower gastrointestinal tract adenocarcinomas (Magnusson et al., 2011). SATB2 has been reported to be expressed in the majority, 97% of CRCs, but only rarely in pancreatic and upper GI tract adenocarcinomas (Lin et al., 2014, Wang et al., 2009). Therefore, we hypothesized that SATB2 might aid in considerably improving differential diagnosis of liver adenocarcinomas.
In the present study, IHC staining was used to examine SATB2 expression in two large independent cohorts containing samples of both CRCs and non-CRCs. It was found that SATB2 protein expression was distinctive with high intensity nuclear staining occurring in CRCs, regardless of differentiation grade and region. This finding is inconsistent with the previous studies (Wang et al., 2009), which imply an association between SATB2 expression and tumor differentiation in CRC. Selection of SATB2 cutoff and patient heterogeneity may contribute to these discrepancy. However, expression of SATB2 is notably lower in the other 6 types of adenocarcinoma evaluated in our study. Our findings are consistent with previous reports of a high incidence (96% in our study) of SATB2 expression in primary CRCs, and a low incidence in gastric, duodenal, pancreatic, cholangiocellular, lung and breast adenocarcinomas (Kim et al., 2016, Magnusson et al., 2011). These observations strongly suggest that the expression levels of SATB2 can be used to distinguish CRCs from non-CRCs with high sensitivity, specificity and AUC.
Liver metastasis is the leading cause of cancer-related morbidity and mortality in colorectal cancer patients. The only potentially curative treatment for patients with CRC with liver metastasis is liver resection, but only 15–20% of these patients are suitable for surgical resection (Baba et al., 2015). The development of efficient molecular targeted drugs, such as cetuximab or bevacizumab, has opened new avenues of treating respectable and unrespectable liver metastases of CRC (Baba et al., 2015, Hasegawa et al., 2014). Therefore, it is very important to develop tools to accurately diagnose the origin of liver tumors. In the clinic, liver needle biopsies are recommended to confirm diagnoses of single hepatic nodules that are not satisfactorily addressed by imaging. However, since histological diagnosis from liver needle biopsy is based solely on tiny fragments of tissue, it can sometimes be quite difficult to distinguish between CRCs and other adenocarcinomas, such as gastric, duodenal, pancreatic, cholangiocellular, lung and breast adenocarcinomas. However, several biomarkers have been suggested to aid in distinguishing between CRCs and non-CRCs in liver needle biopsies. The diagnostic yield of a panel of putative CRC markers, such as CK20, CDX2 and Villin has been examined (Shin et al., 2010, Suh et al., 2005). In the present study, the diagnostic value of SATB2 was evaluated using resection adenocarcinoma tissues and then validated on liver needle biopsy samples. As expected, SATB2 alone showed a high sensitivity, specificity and AUC for detection of CRC.
In order to compare the application of SATB2 with traditional markers (CK20 and CDX2) when characterizing liver adenocarcinomas, we next evaluated a combination of three markers (SATB2, CK20 and CDX2) on a cohort of liver needle biopsy samples. We found that of these samples, breast adenocarcinomas were all negative for all three markers, while expression of at least one marker increased from 19/89 (28.1%) in non-CRCs to 103/103 (100%) in CRCs. Using this three-marker panel, only 2 of the non-CRCs positively expressed all three markers (2/89), compared to frequent expression (92/103) of all three in CRCs. Further statistical analysis demonstrated that the sensitivity was 100% when at least one marker was used, but decreased to 97.8% when all three markers were used. Specificity increased from 78.6% when at least one marker was used to 89.3% when all three markers were used, while the PPV increased from 84.4% to 97.8%. The accuracy and Youden index remained nearly identical. These findings were intriguing as PPV is highly useful in both marker situations as well as for NPV. Collectively, our findings not only confirm the value of SATB2 for the detection of CRC, but also demonstrate that the use of a three marker panel of SATB2, CK20 and CDX2 improves the accuracy of CRC detection in liver biopsy tissues.
Our findings are consistent with a previous report, where SATB2 expression in CRCs displayed a similar sensitivity (Magnusson et al., 2011). In our study, SAB2 shows to be less sensitive for the detection of CRC than CK20. However, there was a higher degree of specificity when compared to CK20 expression, suggesting that the use of both SATB2 and CK20 improves the accuracy of CRC detection. We also observed that SATB2 expression was rarely positive in non-CRCs, such as in liver metastatic adenocarcinoma (e.g. gastric cancer). In the few cases of non-CRCs expressing SATB2, the expression was weak and scattered. By contrast, for liver metastases of CRCs, the majority of cases demonstrated strong and diffuse nuclear expression of the SATB2 protein. These findings strongly suggest that if a liver metastatic adenocarcinoma displays strong and distinct nuclear staining of SATB2, it is highly indicative of CRC origin.
In summary, we verified the expression levels of SATB2 in CRC and non-CRCs tissues by IHC, and showed that SATB2 expression is different in liver needle biopsy samples between liver metastases of CRC origin and other origins. This study demonstrates, for the first time, that a three marker panel of SATB2, CK20 and CDX2 used on liver biopsy samples is valuable when identifying a CRC origin for adenocarcinomas. We believe that the use of SATB2 protein, as examined by IHC, as a diagnostic biomarker of CRCs could improve differential diagnosis based on liver needle biopsies.
The following is the supplementary data related to this article.
The correlation between the high intensity nuclear expression of SATB2 and the clinical characteristics of 101 colorectal cancer (CRC) patients.
Acknowledgements/Funding
This work was supported by Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2015A030306001) and the Nature Science Foundation of China (No. 81672407). The funding sources had no role in writing, data collection, analysis, or interpretation, or any aspect pertinent to the study.
Conflict of Interest
All authors declare no commercial nor associative interest that represents a conflict of interest in connection with the work submitted.
Author Contributions
MYC, DX, JPY and YJZ designed the concept and wrote the protocol. YJZ, JWC and SXH created the original cohort and undertook data collection. JHW, HZZ, PL, YHL and WHD performed the statistical analyses. All authors revised the manuscript, contributed to iterations and approved the final submission. MYC take responsibility for the integrity of the work as a whole, from inception to published article.
References
- Abdalla E.K. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. (discussion 825-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K. Successful treatment of conversion chemotherapy for initially unresectable synchronous colorectal liver metastasis. World J. Gastroenterol. 2015;21:1982–1988. doi: 10.3748/wjg.v21.i6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K.L. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am. J. Roentgenol. 2000;174:1005–1008. doi: 10.2214/ajr.174.4.1741005. [DOI] [PubMed] [Google Scholar]
- Bruix J. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- Cai M.Y. EZH2 protein: a promising immunomarker for the detection of hepatocellular carcinomas in liver needle biopsies. Gut. 2011;60:967–976. doi: 10.1136/gut.2010.231993. [DOI] [PubMed] [Google Scholar]
- Chan K.M. Prognostic significance of the number of tumors and aggressive surgical approach in colorectal cancer hepatic metastasis. World J Surg Oncol. 2014;12:155. doi: 10.1186/1477-7819-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J.L. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin. Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- Disibio G., French S.W. Metastatic patterns of cancers: results from a large autopsy study. Arch. Pathol. Lab. Med. 2008;132:931–939. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- Dobreva G. SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin mu gene expression. Genes Dev. 2003;17:3048–3061. doi: 10.1101/gad.1153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobreva G. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125:971–986. doi: 10.1016/j.cell.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Dragomir A. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: results of a pathology-based clinical prospective study. Am. J. Clin. Pathol. 2014;141:630–638. doi: 10.1309/AJCPWW2URZ9JKQJU. [DOI] [PubMed] [Google Scholar]
- Eberhard J. A cohort study of the prognostic and treatment predictive value of SATB2 expression in colorectal cancer. Br. J. Cancer. 2012;106:931–938. doi: 10.1038/bjc.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K. Cetuximab for resectable colorectal liver metastasis: new EPOC trial. Lancet Oncol. 2014;15:e305–6. doi: 10.1016/S1470-2045(14)70216-5. [DOI] [PubMed] [Google Scholar]
- Kim C.J. Value of SATB2 immunostaining in the distinction between small intestinal and colorectal adenocarcinomas. J. Clin. Pathol. 2016;69:1046–1050. doi: 10.1136/jclinpath-2015-203588. [DOI] [PubMed] [Google Scholar]
- Lin F. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Arch. Pathol. Lab. Med. 2014;138:1015–1026. doi: 10.5858/arpa.2013-0452-OA. [DOI] [PubMed] [Google Scholar]
- Magnusson K. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am. J. Surg. Pathol. 2011;35:937–948. doi: 10.1097/PAS.0b013e31821c3dae. [DOI] [PubMed] [Google Scholar]
- Moh M. SATB2 expression distinguishes ovarian metastases of colorectal and Appendiceal origin from primary ovarian tumors of mucinous or Endometrioid type. Am. J. Surg. Pathol. 2016;40:419–432. doi: 10.1097/PAS.0000000000000553. [DOI] [PubMed] [Google Scholar]
- Ordonez N.G. SATB2 is a novel marker of osteoblastic differentiation and colorectal adenocarcinoma. Adv. Anat. Pathol. 2014;21:63–67. doi: 10.1097/PAP.0000000000000001. [DOI] [PubMed] [Google Scholar]
- Perez Montiel D. The value of SATB2 in the differential diagnosis of intestinal-type mucinous tumors of the ovary: primary vs metastatic. Ann. Diagn. Pathol. 2015;19:249–252. doi: 10.1016/j.anndiagpath.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Pinson C.W. Repeat hepatic surgery for colorectal cancer metastasis to the liver. Ann. Surg. 1996;223:765–773. doi: 10.1097/00000658-199606000-00015. (discussion 773-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. Management of hepatic metastasis from colorectal cancers: an update. J. Hepato-Biliary-Pancreat. Surg. 2008;15:570–580. doi: 10.1007/s00534-008-1350-x. [DOI] [PubMed] [Google Scholar]
- Sheehan-Rooney K. A cross-species analysis of Satb2 expression suggests deep conservation across vertebrate lineages. Dev. Dyn. 2010;239:3481–3491. doi: 10.1002/dvdy.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.H. CK7, CK20, CDX2 and MUC2 Immunohistochemical staining used to distinguish metastatic colorectal carcinoma involving ovary from primary ovarian mucinous adenocarcinoma. Jpn. J. Clin. Oncol. 2010;40:208–213. doi: 10.1093/jjco/hyp150. [DOI] [PubMed] [Google Scholar]
- Soyer P. Hepatic metastases from colorectal cancer: detection and false-positive findings with helical CT during arterial portography. Radiology. 1994;193:71–74. doi: 10.1148/radiology.193.1.8090923. [DOI] [PubMed] [Google Scholar]
- Suh N. Value of CDX2, villin, and alpha-methylacyl coenzyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod. Pathol. 2005;18:1217–1222. doi: 10.1038/modpathol.3800407. [DOI] [PubMed] [Google Scholar]
- Tan E. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg. Oncol. 2009;18:15–24. doi: 10.1016/j.suronc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Tot T. Adenocarcinomas metastatic to the liver: the value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer. 1999;85:171–177. doi: 10.1002/(SICI)1097-0142(19990101)85:1<171::AID-CNCR24>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wang S. Down-regulated expression of SATB2 is associated with metastasis and poor prognosis in colorectal cancer. J. Pathol. 2009;219:114–122. doi: 10.1002/path.2575. [DOI] [PubMed] [Google Scholar]
- Werling R.W. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am. J. Surg. Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Zhou L. Serum tumor markers for detection of hepatocellular carcinoma. World J. Gastroenterol. 2006;12:1175–1181. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The correlation between the high intensity nuclear expression of SATB2 and the clinical characteristics of 101 colorectal cancer (CRC) patients.