Abstract
Twiddler's syndrome is an uncommon hardware complication involving the lead and pulse generators in cardiac pacemakers and defibrillators, deep brain stimulators, and vagal nerve stimulators. However, until very recently, it had not been reported in spinal cord stimulation (SCS). Considering the incidence of hardware complications of spinal cord stimulation, there may be an underreporting of Twiddler's syndrome due to lack of awareness. Two cases of Twiddler's syndrome as a hardware complication of SCS were identified between 2005 and 2015. One patient with hardware failure due to Twiddler's syndrome refused to have a revision surgery. The other patient who had a lead migration associated with coiling of the lead and twisting of pulse generator needed a revision surgery. Twiddler's syndrome in patients treated with SCS is an uncommon but important adverse event. Awareness of characteristic presentation and radiologic finding is essential in the identification of Twiddler's syndrome in SCS.
Keywords: Failed back surgery syndrome, hardware complication, pain, spinal cord stimulation, Twiddler's syndrome
Introduction
Twiddler's syndrome is a rare hardware complication of implantable pulse generators (IPGs) and is most commonly described as lead retraction with implanted cardiac devices, such as pacemakers and defibrillators.[1] It was first described in 1968 by Bayliss et al.[2] who reported a single case of a cardiac pacemaker flipping on its long axis resulting in retraction of the leads and coiling around the pacemaker boot. Since then, Twiddler's syndrome has been mostly described with implanted cardiac devices, such as pacemakers and defibrillators,[1] and has also been reported with deep brain and vagal nerve stimulators.[3,4,5,6,7,8,9,10]
Twiddler's syndrome in spinal cord stimulation (SCS) was first reported by Al-Mahfoudh et al.[11] in 2016. The reason why Twiddler's syndrome in SCS has not been reported is unclear. However, considering the reported incidence of about 0.39% by Al-Mahfoudh et al.,[11] there is a possibility that it has not been recognized and has been underreported. In the authors' personal series of 187 cases of SCS between 2005 and 2015, we experienced two cases (1.07%) of Twiddler's syndrome.
Case Report
Case 1
A 74-year-old female patient presented with a 2-year history of chronic back and buttock pain radiating to the bilateral legs. Her pain developed after a long-level decompression and posterior fixation at T12–L4 for osteoporotic L1 bursting fracture and did not respond to conservative care. Considering the chronic nature of pain and medical intractability, SCS trial with a Tripole 16™ paddle lead (St. Jude Medical, Plano, Texas, USA) at T8–T9 was performed. Pain relief exceeding 60% was obtained during trial stimulation. An Eon™ (IPG; St. Jude Medical) was implanted in her left lower abdominal wall, and chronic stimulation was given in May 2010 [Figure 1]. At the point of IPG implantation, she weighed 85 kg, with a height of 166 cm representing a body mass index (BMI) of 30.8 kg/m2.
Figure 1.
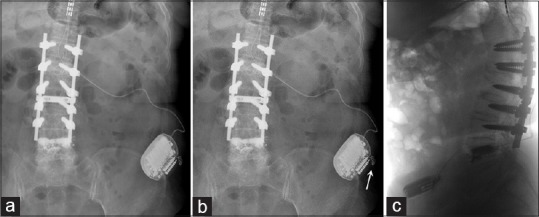
A radiographic findings of Twiddler's syndrome in spinal cord stimulation in patient #1. (a) Kidneys, ureters, and bladder radiograph taken immediately following spinal cord stimulator placement. (b) Kidneys, ureters, and bladder radiograph obtained 18 months after implantation. The pulse generator has been flipped and coiling of the lead is noted. (c) An inverted image of abdominal lateral X-ray film showing a flipping of the implanted pulse generator
In November 2011, she complained of aggravated back and leg pain. Loss of telemetry of the IPG was found. A plain X-ray showed that the IPG was turned over along its long axis and the extension wires were twisted [Figure 1]. She denied excessive manipulation of IPG except when adjustment of stimulation intensity was needed. She refused to have a revision surgery, and the SCS hardware was left untouched.
Case 2
A 63-year-old female patient with failed back surgery syndrome underwent a paddle lead SCS at T8–T9 for chronic neuropathic pain of the right buttock and right leg pain along the L5, S1 dermatomes in August 2011. She underwent low back surgery five times including vertebroplasties, decompression and posterior lumbar instrumentation, and fusion at L3–L5. Although more than 50% pain relief could be achieved with initial SCS using a Tripole 8™ 3-column paddle lead (St. Jude Medical), the patient requested stronger paresthesia in her right sole. An additional Quattrode™ cylindrical lead (St. Jude Medical) was placed at the T12–L1 level, and the distal end of the lead was connected to the remaining slot of the implanted Eon Mini™ IPG (St. Jude Medical) in the left lower abdomen in October 2011 [Figure 2]. At the point of IPG implantation, she weighed 54 kg, with a height of 162 cm, representing a BMI of 20.58 kg/m2.
Figure 2.
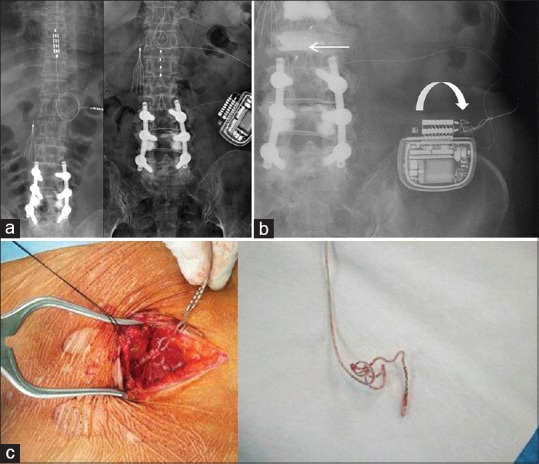
Radiographic findings of Twiddler's syndrome in spinal cord stimulation in patient #2. (a) X-ray obtained 2 weeks after implantation of the implanted pulse generator. Note the location of paddle lead at T8–T9 (left) and a four-contact cylindrical lead at T12–L1 (right). (b) X-ray finding showing a rotation of the implanted pulse generator along its short axis with lead twisting (curved arrow) and distal migration of the cylindrical lead (arrow). (c) An intraoperative photograph showing the twisted and coiled leads
Six months later, stimulation-induced paresthesia was lost abruptly in her right sole, and no telemetry with the cylindrical lead was found. X-ray revealed distal migration of cylindrical lead and twisting of the distal lead with some rotation of the IPG [Figure 2]. A revision surgery implanting a new cylindrical lead with untwisting of the distal leads was performed in the original site of the IPG implantation. Adequate stimulation that relieved in the buttock and leg pain was achieved.
Discussion
Twiddler's syndrome
Since the original description by Bayliss et al.,[2] there have been multiple cases of Twiddler's syndrome reported in the literature.[3,4,5,6,7,8,9,10,11,12,13,14] Two types of “twiddling” have been reported according to the direction of flipping axis of the IPG (long and short axis) and the status of pacemaker lead (twisting and potential fracturing vs. coiling and retraction).[12,13,14] Flipping of the IPG along its long axis commonly results in lead twisting and potential fracturing of the lead while rotation along the short axis of the device results in coiling and retraction of the leads.[1,12,13,14] Short-axis rotation with coiling of the lead around the device has been termed “Reel syndrome.”[13]
Although the exact pathogenesis of Twiddler's syndrome is still vague, widely accepted explanations are deliberate or subconscious manipulation of the device, spontaneous rotation of the device in the pocket, and the effects of muscle contraction on the implanted device.[1,12] The ability to “twiddle” an implanted device is believed to relate to an overly large or compliant soft tissue pocket.[12,15] As such, obesity, old age, and female gender are believed to be increased risk factors of Twiddler's syndrome due to laxity of the subcutaneous tissue.[12,15] Other predisposing factors mentioned include: psychiatric disorders, developmental delay, weight loss, looping of the lead outside the pocket, abdominal wall implantation, and replacement of an implant in the previous larger pacemaker pocket.[8,12,15,16] In addition, children might also represent a group at increased risk of “twiddling” due to thinner subcutaneous soft tissues, which make the devices more accessible.[1,12]
Twiddler's syndrome in SCS
Although Twiddler's syndrome may be well known to cardiologists, its occurrence following implantation of neurostimulators, such as deep brain stimulator and vagal nerve stimulator, is rare. A single-cohort study including 226 patients with deep brain stimulation (DBS) for movement disorders[7] reported a patient incidence of 1.3% (3 of 226 patients) and in 1.4% of leads (5 of 362 leads). Although SCS with similar IPG technology has been widely performed for more than 30 years, the occurrence of Twiddler's syndrome in SCS was first presented early in 2016.[11] Examination of the cohort from 2007 to 2013 revealed only 3 of 550 patients (0.54%) with the radiological and clinical evidence of Twiddler's syndrome. Considering this incidence, we speculate that there may be a possibility that the development of Twiddler's syndrome might have been underreported in SCS [Table 1].
Table 1.
Reported cases of Twiddler's syndrome in spinal cord stimulation
Lead migration is by far the most common complication of SCS malfunction and vertical (craniocaudal) migration is generally known to be more common than lateral (horizontal) migration.[17,18] The majority of the instances of lead migration require minor reoperation to relocate the lead to its original position, and most will incur the cost of a new lead.[17] Indeed, we did not figure out the occurrence of Twiddler's syndrome in patient #2 and we thought it a simple, distal migration, and breakage of the cylindrical lead. Cognizant of the report of Al-Mahfoudh et al.,[11] we reexamined the kidneys, ureters, and bladder and realized that it should be classified as Twiddler's syndrome, a rare complication of the IPGs. We admit that we did not pay attention to the position of the IPG and only focused on the distal migration of the lead. Although it is difficult to draw a general conclusion about the relationship between distal migration of the lead and Twiddler's syndrome, we speculate that there is a possibility that this kind of lead migration associated with lead malfunction could have been simply classified as lead migration.
Unlike parameter adjustment in deep brain stimulation performed by medical professionals, there are many instances for the patients in which SCS patients manipulate their IPGs to adjust stimulation intensity and to recharge the IPGs. In addition, unlike the infraclavicular chest wall location of the IPGs for DBS, there is no rigid supporting structure for IPGs in the abdominal wall.
Prevention and management of Twiddler's syndrome in SCS
Since Twiddler's syndrome has been mostly reported in cardiac pacemakers and DBS IPGs, it is hard to suggest specific measures to prevent in SCS. Reported measures to reduce the incidence of IPG displacement and Twiddler's syndrome include suture sleeves, use of prolene mesh or nonabsorbable sutures, fascial anchoring and submuscular placement of the IPG, and limiting the pocket size.[4,7,11,12,19] All three previously reported cases of Twiddler's syndrome in SCS[11] featured initial IPGs in the gluteal region over the iliac fascia, which were relocated to the lumbar region above the iliac crest anchored to the lumbar fascia. The authors recommended a revision of the IPG with lumbar fascial anchoring because it is less accessible and visible to the patient. However, the experience of Twiddler's syndrome in SCS is limited. Further studies involving more patients are warranted to provide adequate management of Twiddler's syndrome in SCS. Due to the potential adverse effects of “twiddling” any device, and because early recognition of “twiddling” might prevent damage, it is important to recognize the radiologic findings and understand the sequelae of “twiddling.”[12]
Conclusions
Although Twiddler's syndrome is a rare complication of implanted devices, its occurrence in SCS might have been underrecognized due to lack of awareness by virtue of its rarity. Cases may have been classified as a simple lead migration or lead breakage. Early recognition of “twiddling” might prevent damage. It is important to recognize the radiologic findings and understand the sequel of this phenomenon.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Abrams S, Peart I. Twiddler's syndrome in children: An unusual cause of pacemaker failure. Br Heart J. 1995;73:190–2. doi: 10.1136/hrt.73.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss CE, Beanlands DS, Baird RJ. The pacemaker-twiddler's syndrome: A new complication of implantable transvenous pacemakers. Can Med Assoc J. 1968;99:371–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Silva PA, Chamadoira C, Costa H, Linhares P, Rosas MJ, Vaz R. Twiddler (or not) syndrome: Questioning etiology for an uncommon form of hardware malfunction in deep brain stimulation. Surg Neurol Int. 2014;5(Suppl 8):S410–2. doi: 10.4103/2152-7806.140201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menghetti C, Zekaj E, Saleh C, Porta M, Servello D. How to avoid Twiddler's syndrome in deep brain stimulation for dystonia? Neuromodulation. 2014;17:198–9. doi: 10.1111/ner.12067. [DOI] [PubMed] [Google Scholar]
- 5.Penn DL, Wu C, Skidmore C, Sperling MR, Sharan AD. Twiddler's syndrome in a patient with epilepsy treated with deep brain stimulation. Epilepsia. 2012;53:e119–21. doi: 10.1111/j.1528-1167.2012.03489.x. [DOI] [PubMed] [Google Scholar]
- 6.Astradsson A, Schweder PM, Joint C, Green AL, Aziz TZ. Twiddler's syndrome in a patient with a deep brain stimulation device for generalized dystonia. J Clin Neurosci. 2011;18:970–2. doi: 10.1016/j.jocn.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Burdick AP, Okun MS, Haq IU, Ward HE, Bova F, Jacobson CE, et al. Prevalence of Twiddler's syndrome as a cause of deep brain stimulation hardware failure. Stereotact Funct Neurosurg. 2010;88:353–9. doi: 10.1159/000319039. [DOI] [PubMed] [Google Scholar]
- 8.Gelabert-Gonzalez M, Relova-Quinteiro JL, Castro-García A. “Twiddler syndrome” in two patients with deep brain stimulation. Acta Neurochir (Wien) 2010;152:489–91. doi: 10.1007/s00701-009-0366-6. [DOI] [PubMed] [Google Scholar]
- 9.Israel Z, Spivak A. A tremulous twiddler. Stereotact Funct Neurosurg. 2008;86:297–9. doi: 10.1159/000155231. [DOI] [PubMed] [Google Scholar]
- 10.Geissinger G, Neal JH. Spontaneous twiddler's syndrome in a patient with a deep brain stimulator. Surg Neurol. 2007;68:454–6. doi: 10.1016/j.surneu.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 11.Al-Mahfoudh R, Chan Y, Chong HP, Farah JO. Twiddler's syndrome in spinal cord stimulation. Acta Neurochir (Wien) 2016;158:147–54. doi: 10.1007/s00701-015-2627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trout AT, Larson DB, Mangano FT, Gonsalves CH. Twiddler syndrome with a twist: A cause of vagal nerve stimulator lead fracture. Pediatr Radiol. 2013;43:1647–51. doi: 10.1007/s00247-013-2736-8. [DOI] [PubMed] [Google Scholar]
- 13.Carnero-Varo A, Pérez-Paredes M, Ruiz-Ros JA, Giménez-Cervantes D, Martínez-Corbalán FR, Cubero-López T, et al. “Reel syndrome”: A new form of Twiddler's syndrome. Circulation. 1999;100:e45–6. doi: 10.1161/01.cir.100.8.e45. [DOI] [PubMed] [Google Scholar]
- 14.Von Bergen NH, Atkins DL, Gingerich JC, Law IH. “Ratchet” syndrome, another etiology for pacemaker lead dislodgement: A case report. Heart Rhythm. 2007;4:788–9. doi: 10.1016/j.hrthm.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Dursun I, Yesildag O, Soylu K, Yilmaz O, Yasar E, Meric M. Late pacemaker twiddler syndrome. Clin Res Cardiol. 2006;95:547–9. doi: 10.1007/s00392-006-0417-4. [DOI] [PubMed] [Google Scholar]
- 16.Pereira PL, Trübenbach J, Farnsworth CT, Huppert PE, Claussen CD. Pacemaker and defibrillator Twiddler's syndrome. Eur J Radiol. 1999;30:67–9. doi: 10.1016/s0720-048x(98)00003-5. [DOI] [PubMed] [Google Scholar]
- 17.Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Thomson S, et al. The appropriate use of neurostimulation: Avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:571–97. doi: 10.1111/ner.12206. [DOI] [PubMed] [Google Scholar]
- 18.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: 20-year literature review. J Neurosurg. 2004;100(3 Suppl):254–67. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 19.Mehta D, Lipsius M, Suri RS, Krol RB, Saksena S. Twiddler's syndrome with the implantable cardioverter-defibrillator. Am Heart J. 1992;123(4 Pt 1):1079–82. doi: 10.1016/0002-8703(92)90728-e. [DOI] [PubMed] [Google Scholar]


