Abstract
Background
The authors reviewed the evidence regarding the existence of age-related declines in central auditory processes and the consequences of any such declines for everyday communication.
Purpose
This report summarizes the review process and presents its findings.
Data Collection and Analysis
The authors reviewed 165 articles germane to central presbycusis. Of the 165 articles, 132 articles with a focus on human behavioral measures for either speech or nonspeech stimuli were selected for further analysis.
Results
For 76 smaller-scale studies of speech understanding in older adults reviewed, the following findings emerged: (1) the three most commonly studied behavioral measures were speech in competition, temporally distorted speech, and binaural speech perception (especially dichotic listening); (2) for speech in competition and temporally degraded speech, hearing loss proved to have a significant negative effect on performance in most of the laboratory studies; (3) significant negative effects of age, unconfounded by hearing loss, were observed in most of the studies of speech in competing speech, time-compressed speech, and binaural speech perception; and (4) the influence of cognitive processing on speech understanding has been examined much less frequently, but when included, significant positive associations with speech understanding were observed.
For 36 smaller-scale studies of the perception of nonspeech stimuli by older adults reviewed, the following findings emerged: (1) the three most frequently studied behavioral measures were gap detection, temporal discrimination, and temporal-order discrimination or identification; (2) hearing loss was seldom a significant factor; and (3) negative effects of age were almost always observed.
For 18 studies reviewed that made use of test batteries and medium-to-large sample sizes, the following findings emerged: (1) all studies included speech-based measures of auditory processing; (2) 4 of the 18 studies included nonspeech stimuli; (3) for the speech-based measures, monaural speech in a competing-speech background, dichotic speech, and monaural time-compressed speech were investigated most frequently; (4) the most frequently used tests were the Synthetic Sentence Identification (SSI) test with Ipsilateral Competing Message (ICM), the Dichotic Sentence Identification (DSI) test, and time-compressed speech; (5) many of these studies using speech-based measures reported significant effects of age, but most of these studies were confounded by declines in hearing, cognition, or both; (6) for nonspeech auditory-processing measures, the focus was on measures of temporal processing in all four studies; (7) effects of cognition on nonspeech measures of auditory processing have been studied less frequently, with mixed results, whereas the effects of hearing loss on performance were minimal due to judicious selection of stimuli; and (8) there is a paucity of observational studies using test batteries and longitudinal designs.
Conclusions
Based on this review of the scientific literature, there is insufficient evidence to confirm the existence of central presbycusis as an isolated entity. On the other hand, recent evidence has been accumulating in support of the existence of central presbycusis as a multifactorial condition that involves age- and/or disease-related changes in the auditory system and in the brain. Moreover, there is a clear need for additional research in this area.
Keywords: Aging, central hearing loss, presbycusis
Over a two-year period, 2009–2011, the America Academy of Audiology Task Force on Central Presbycusis reviewed and discussed the evidence regarding age-related changes in auditory portions of the central nervous system and the impact of such changes on everyday communication and function. This proved to be a challenging task! Many older adults, for example, have high-frequency sensorineural hearing loss, and this loss alone can have a negative impact on tests of central auditory function, as well as everyday speech communication and function. Further, there is evidence in laboratory animals that long-standing sensorineural hearing loss can induce secondary changes in some auditory structures in the central nervous system. To complicate things even more, many older adults may also experience age-related declines in cognitive function. This is not referring to clinical declines in cognition, such as mild cognitive impairment (MCI) or dementia of various types, including Alzheimer’s disease, but to the typical age-related decline in cognition that occurs in many older adults as a part of “healthy aging.” Such cognitive declines can also impact some measures of central auditory function, as well as everyday speech communication and function. In the end, the task force came to the conclusion that there was insufficient evidence to establish that a “pure” or “isolated” form of age-related central auditory decline existed in humans. Rather, central auditory declines in aging were most often intertwined with age-related declines in peripheral hearing, cognition, or both. This is not to say that pure, age-related declines in central auditory function do not exist or cannot occur but just that the evidence to date does not support this in humans. More research is needed to resolve this important issue. In the meantime, clinicians need to be fully aware that an older adult in the clinic may have various combinations of peripheral and higher-level processing deficits—cognitive, central auditory, or a combination—and that a higher-level-processing deficit may be an important contributing factor to the difficulties experienced by older adults in everyday speech communication and function, as well as to the attempts to reduce those difficulties through various forms of intervention. More clinical research is needed to develop reliable and valid measures of higher-level processing for use with older patients in the clinic. Some promising behavioral measures of higher-level processing, based on several small-scale laboratory studies in humans, were identified by the task force.
INTRODUCTION AND GENERAL BACKGROUND
This report summarizes the processes and findings of the American Academy of Audiology (Academy) Task Force on Central Presbycusis. Before proceeding further, central presbycusis should be defined. This was one of the earliest tasks pursued by the task force. The group’s deliberations resulted in the following definition:
Central presbycusis refers to age-related change in the auditory portions of the central nervous system negatively impacting auditory perception, speech-communication performance, or both. Attributing auditory-perception or speech-communication difficulties of older adults to central presbycusis is challenging, however, because many older adults have concomitant peripheral (sensorineural) hearing loss, age-related cognitive changes, or both. Also, central presbycusis precludes those older adults with frank presentation of lesions, such as tumors or vascular insults, impacting auditory portions of the central nervous system, as well as older adults with a diagnosis of significant cognitive decline, such as dementia of the Alzheimer’s type.
This definition was used to guide the task force’s selection of literature to review and was used as a framework for interpreting findings. Clearly, this definition requires that central presbycusis negatively impacts auditory perception or speech communication of older adults and that the negative impacts can be attributable primarily to alterations in the structure and function of the auditory portions of the central nervous system from the cochlear nucleus to primary auditory cortex. This is explicitly a historical or traditional, narrow structural form of central presbycusis. In contrast, a broad view of “central presbycusis” encompasses not only modality-specific central auditory forms but also amodal cognitive declines that might impact speech communication or the processing of auditory information. Given that speech processing in the brain uses cognitive resources, such as short-term memory, attention, and inhibition (Craik, 2007), a theoretical case can be made that, in some instances, declines in certain cognitive processes (the so-called executive functions) may contribute to the observed changes in performance.
With regard to speech communication, it is well known that many older adults, over the age of 60, have difficulties understanding speech (e.g., Plomp, 1978; Committee on Hearing, Bioacoustics, and Biomechanics [CHABA], 1988). In 1988, a working group of the National Research Council published an extensive summary and critique of the research literature on the speech-understanding problems of older adults (CHABA, 1988). In that report, it was noted that there had been little debate as to whether many older adults have difficulties understanding speech. Rather, the debates had been centered more on identifying the conditions under which older adults experienced such difficulties and the factors underlying those difficulties. In the more than two decades that have passed since the CHABA working group’s report, those debates have continued.
Basically, as noted by Humes (1996), the CHABA report offered three primary hypotheses regarding the mechanisms underlying the speech-understanding difficulties of older adults: (1) the peripheral hypothesis, (2) the central auditory hypothesis, and (3) the cognitive hypothesis. Of course, as noted then and in subsequent reviews by Humes (1996) and Humes and Dubno (2010), combinations of these three hypotheses were also viable options. CHABA (1988) also identified two versions of the peripheral hypothesis: (1) a simple version, which was basically the loss of audibility associated with age-related hearing loss, and (2) a more complex version, one that conjectured additional deficits in suprathreshold processing, such as frequency resolution, associated with the underlying inner-ear pathology (Humes, 1996).
Not only can multiple hypotheses apply to a given research study or clinical patient, interactions, including causal interactions, between hypothesized mechanisms can occur. For example, there is evidence in laboratory animals that some auditory structures in the central nervous system, such as the inferior colliculus, demonstrate age-related anatomical or physiological deficits without concomitant peripheral deficits (e.g., Walton et al, 1998, 2002). This would be evidence in support of a “direct” or “pure” form of the central auditory hypothesis applied to aging. Willott (1996) referred to this type of effect as a “central effect of biological aging,” or “CEBA.” Presumably, the individual, in the absence of peripheral pathology, would have normal or near-normal hearing thresholds for pure tones as central lesions typically show no effects on pure-tone thresholds. However, there is also evidence from other similar studies that central auditory changes can be induced, from the cochlear nucleus through the auditory portions of the cortex, by the presence of a peripheral hearing loss (see Willott [1996] and recent reviews by Canlon et al [2010] and Ison et al [2010]). This would be evidence of an “indirect” form of the central auditory hypothesis. Willott (1996) referred to this as a “central effect of peripheral pathology,” or “CEPP.” In either case, the presence of the central auditory deficit could be problematic for speech communication by older adults. In the direct case (CEBA), however, only the central auditory deficit would be present to impact performance. In contrast, in the indirect case (CEPP), the central auditory deficit only exists in combination with a concomitant peripheral hearing loss, and this peripheral loss itself may further exert a negative impact on speech communication due to reduced audibility, deficits in suprathreshold processing, or both. The foregoing is not meant to imply that the only time one might expect to see both peripheral and central auditory deficits in older adults would be through such causal interactions. There is no reason to believe, for instance, that older adults with peripheral impairments would be protected from experiencing a truly age-related direct and independent decline in a central auditory structure. For instance, let us assume that pure central effects of biologic aging are known to exist in the inferior colliculus. Further, assume that central effects from peripheral pathology are common in the cochlear nucleus. As a result, it is conceivable that an older adult with peripheral pathology may experience a central effect from this pathology in the cochlear nucleus and also have a central effect from biologic aging in the inferior colliculus. Thus, noncausal combinations or interactions among the mechanisms hypothesized in the CHABA (1988) report are also feasible.
It should also be noted that causal and noncausal interactions are not confined to combinations of the mechanisms underlying the peripheral and central auditory hypotheses. There is considerable evidence, for example, for the same types of interactions between peripheral hearing loss and various measures of cognitive function (see review by Akeroyd, 2008; Peelle, Troiani, Grossman and Wingfield, 2011). Many studies have demonstrated that degrading the peripheral auditory input can lead to poorer performance on cognitive measures (e.g., Rabbitt, 1968, 1990; Pichora-Fuller et al, 1995; Schneider and Pichora-Fuller, 2000; Wingfield et al, 2005; Surprenant, 2007), as well as clinical assessments of expressive language (Skenes et al, 1989) and dementia (Weinstein and Amsel, 1986) used frequently with older adults. Beyond the influence of degraded perceptual information on cognitive performance, it has been hypothesized that long-term deprivation of sensory input can lead to diminished cognition and that there may also be common causal mechanisms underlying a mutual coincident decline in sensory and cognitive function (e.g., Lindenberger and Baltes, 1994; Baltes and Lindenberger, 1997; Schneider and Pichora-Fuller, 2000).
Interactions among the various hypotheses outlined originally by the CHABA working group add to the complexity of the problem. Such interactions, however, can also challenge the very validity of one or more of the hypotheses or of the test measures used to confirm a given hypothesis. Consider, for example, the construct validity of measures for central auditory processing, the primary focus of this task force report. As will be demonstrated in the review to follow, behavioral measures using broadband speech stimuli have been used most commonly in the assessment of central auditory function in humans. As a consequence, performance on speech-based measures of central auditory function will likely be impacted negatively by concomitant peripheral hearing loss in many older adults. Likewise, there are often cognitive components to many commonly used measures of central auditory processing. Consider, for example, the multitude of tests involving dichotic presentation of speech stimuli. Whereas there are certainly auditory and linguistic factors contributing to performance on such tasks (e.g., Kimura, 1967; Berlin et al, 1973), cognitive abilities, such as executive function and attention, may also underlie individual differences in performance on dichotic measures (e.g., Cherry, 1953; Broadbent, 1954, 1971; Jerger et al, 1991; Jerger et al, 1994; Hallgren et al, 2001; Humes, 2005; Humes et al, 2006). Similarly, one might ask whether another popular measure of presumed central auditory processing, time-compressed speech, is tapping modality-specific auditory temporal processing, cognitive speed of processing, or both (e.g., Wingfield et al, 1985;Wingfield et al, 1999;Gordon-Salant and Fitzgibbons, 1993, 1997, 2001; Gordon-Salant et al, 2007; Humes et al, 2007). Finally, when competing stimuli have been employed in clinical measures of central auditory processing, more frequently than not, the competition is competing speech, rather than noise. This tends to also increase the cognitive demands of the task via increased distraction and need for sustained attention, or via age-related deficits in inhibition in older adults (e.g., Sommers, 1997; Tun et al, 2002). As an illustration of the likely overlap between cognitive function and central auditory function, as assessed with speech-understanding measures and primarily competing speech, Jerger et al (1989), in a study of 130 older adults, identified half (65) of the participants as having central auditory processing deficits, but 54%(35) of these individuals were identified as also having abnormal cognitive status. Thus, interactions between cognitive and central auditory processing can be expected to be quite common among older adults. To the extent that cognitive elements, such as executive function (e.g., short-term memory, attention, inhibition, arousal), play a role in speech understanding in competing stimuli by older adults, the distinction between auditory, central auditory, and cognitive factors is further blurred (Rönnberg et al, 2011).
Why have such challenging tests, such as tests comprised of speech in competing speech, dichotic speech presentation, and time-compressed speech, been used in the assessment of central auditory processing if the validity of assessment with such materials is questionable? Behavioral testing in the area of central auditory processing historically has made use of tests that have been “sensitized” to detect a lesion or dysfunction in the auditory portions of the central nervous system. This notion is built on the foundation established by Bocca and Calearo (1963), early pioneers of central auditory testing, which advanced the notions of “extrinsic redundancy” of the speech stimulus and “intrinsic redundancy” of the auditory central nervous system. In the presence of a known lesion in the central auditory structures, many patients have excellent scores on measures of speech perception under optimal conditions (moderate presentation level in quiet). This is because of the high extrinsic redundancy of the speech stimulus and the availability of multiple pathways from the auditory periphery to the cortex (intrinsic redundancy). If the extrinsic redundancy can be decreased, as through speech-in-noise or speech-in-speech masking, filtering of the speech signal, or various forms of temporal distortion, including time compression, then performance will be more sensitive to diminished intrinsic redundancy due to, for example, the presence of a lesion in the auditory portions of the central nervous system. Although this is a reasonable rationale for the development and use of such speech-based tests of central auditory processing, as noted, the degradation of the speech stimuli in the name of “sensitizing” the tests to central auditory deficits often also opened the door to potential cognitive interpretations for diminished performance, especially for older adults with no central auditory lesions that could be documented otherwise (e.g., via radiological techniques).
The coexistence of peripheral hearing loss and declines in auditory/cognitive processing with measures of central auditory processing complicates the interpretation of research studies directed toward attaining a better understanding of central presbycusis. This is the case, in part, because both peripheral hearing loss and cognitive dysfunction are prevalent deficits among older adults. For example, epidemiological studies of hearing loss among older adults reveal a prevalence of significant hearing loss in 40–60% for those over age 60 (e.g., Cruickshanks, 2010; Lin, Thorpe, et al, 2011). Similarly, the prevalence of MCI in a nondemented population of older adults (70–89 yr) is 16% (Petersen et al, 2010), although estimates range from 3–18%, increasing with age (Lopez et al, 2003; Portet et al, 2006). Even in healthy populations not diagnosed with either dementia or MCI, many cognitive functions decline with age over the adult life span (e.g., Schaie, 1983; Salthouse, 1985, 1991, 2010), some of which may influence the processing of speech or performance on tests designated as “central auditory” tests. Those assessing central auditory function in older adults in the laboratory or in the clinic must be cognizant of the likelihood that the older adults being tested may have concomitant peripheral deficits, cognitive declines, or both, and that each of these other deficits may negatively impact performance on presumed measures of central auditory processing. In addition, several longitudinal studies have shown increased risk of dementia in people with peripheral hearing loss or very poor speech recognition in noise (as measured by the Synthetic Sentence Identification (SSI) test with Ipsilateral Competing Message (ICM), and the Dichotic Sentence Identification [DSI] test) compared to people with better hearing (Gates et al 2002, 2011; Lin, Metter, et al, 2011). These findings suggest that auditory and cognitive function may be linked and underscore the need for neuropsychological testing in studies of age-related audition, as well as the pressing need for imaging and electrophysiological assessment of participants in studies of central presbycusis.
With regard to peripheral auditory impairment, there are strategies that researchers and clinicians can use to minimize the influence of such impairment on central auditory measures. Recall that the CHABA working group identified two forms of the peripheral hypothesis: a simple audibility-based version and a more complex version including suprathreshold processing deficits. The type of hearing loss most prevalent among older adults is sensorineural in nature, typically attributed, in large part, to underlying age-related changes in cochlear structures or mechanisms (e.g., Schuknecht, 1974; Schuknecht and Gacek, 1993; Schmiedt, 2010), and the cochlear pathology underlying the hearing loss is permanent. The same can be said for pathology of the first-order afferent nerves innervating the cochlea, which may also contribute to the measured peripheral sensorineural hearing loss. Although the underlying inner-ear pathology is permanent and cannot be minimized, the effects of reduction in audibility accompanying the inner-ear pathology often can be minimized through the judicious selection of stimulus parameters (e.g., Humes, 2007). As noted previously, the broadband nature of the speech signal used in many measures of central auditory processing poses a problem for use with older adults because of the likelihood of concomitant peripheral hearing loss. The typical age-related hearing loss is a sloping configuration impacting the high frequencies more than the lower frequencies, an observation documented for over a century (Schacht and Hawkins, 2005) and so well established as to be described in an international standard (ISO-7029; International Standards Organization, 2000). In contrast, broadband speech stimuli have most of their energy in the lower frequencies and midfrequencies (e.g., Fletcher, 1953), frequency regions of relatively normal hearing in older adults. As a result, conventional rules for the presentation of speech-based tests at suprathreshold levels, which are based on midfrequency pure-tone average (500, 1000, and 2000 Hz) or speech-recognition threshold, do not ensure audibility across the full bandwidth of speech even at relatively high sensation levels (e.g., Humes, 2009; Humes and Dubno, 2010). Further, use of high presentation levels can result in additional difficulties in and of itself that may lead to a reduction in speech-understanding performance even in young normal-hearing listeners (e.g., Fletcher and Galt, 1950; Pollack and Pickett, 1958; Studebaker et al, 1999; Dubno et al, 2005a, 2005b, 2006).
For research studies, there are various options available to control for the reduction in audibility, including judicious selection of the range of hearing loss and the speech presentation level to ensure sufficient audibility through at least 4000 Hz; spectrally shaping the speech signal to provide gain in the high frequencies to compensate fully for the loss of audibility; designing the study to include appropriate comparison groups, such as younger and older adults with both normal and equally impaired hearing (minimum of four groups required) or groups with hearing loss simulated via noise masking or other types of distortion; evaluating performance relative to that predicted by established standards, such as the American National Standards Institute (ANSI) standard for the Speech Intelligibility Index (SII; ANSI, 1997); statistically partialling out the effects of hearing loss in data analyses (e.g., Dubno et al, 1984; Dubno and Dirks, 1993; Gordon-Salant and Fitzgibbons, 1993, 1997, 2001; Gordon-Salant et al, 2007; Humes and Roberts, 1990; Humes, 2002; Humes and Dubno, 2010); selecting samples of older adults for whom age and hearing loss are not strongly correlated (e.g., Humes, 2002; Souza et al, 2007); or measuring performance on central auditory tasks longitudinally, controlling statistically for variations in other variables that may accompany changes in hearing. Most of these approaches have been pursued to varying degrees in much of the research reviewed by the task force. Each approach alone is not without shortcomings. However, when research involving multiple studies and approaches converges on the same outcome, there is greater confidence in the outcome that has emerged. This principle was a key component of the approach to the review of the available literature by the task force. To the extent that such research studies reviewed below demonstrate an influence of peripheral hearing loss on speech-understanding performance, the validity of using such broadband speech-based measures of central auditory processing is compromised.
There are alternatives, however, to the use of broadband speech stimuli in the assessment of central auditory processing. One could, for example, use low-pass filtered speech and reasonably high presentation levels to minimize the impact of the reduction in audibility expected in older adults (e.g., Fogerty et al, 2010; Humes et al, 2010). This strategy, however, rarely has been employed in the assessment of central auditory processing in older adults, although it has been used in other contexts to minimize the impact of reduced high-frequency audibility on speech-recognition performance (e.g., Horwitz et al, 2002).
A much more common alternative has been to make use of nonspeech stimuli, such as tones, to assess central auditory function behaviorally. In this case, one can specify the stimulus frequencies and levels to ensure sufficient audibility of the stimuli for older listeners and compare performance to young adults tested under acoustically identical stimulus conditions. Because the most appropriate comparison condition for the young adults is not always obvious, it is important to obtain normative data from young adults for both equivalent sensation levels and equivalent sound pressure levels, or to evaluate presentation levels using young adults with hearing loss, or young adults who have a hearing loss simulated by the addition of background noise, matched to the hearing loss of the older adults. These comparison conditions are important, even for narrow-band nonspeech stimuli positioned in the region of normal or near-normal hearing, because performance on some tasks may be mediated by the upward spread of cochlear stimulation to off-frequency high-frequency regions in young adults with a broad region of normal hearing, a frequency region unavailable to older listeners with high-frequency sensorineural hearing loss (e.g., Humes, 1982; Bacon and Viemeister, 1985; Dubno and Dirks, 1993). Use of such comparisons, however, is not without problems. Comparing the performance of young and older adults with comparably impaired hearing, for example, most likely will not involve similar etiologies underlying the observed hearing loss. Likewise, simulation of the presbycusic hearing loss via noise may capture some perceptual effects associated with reduced audibility and dynamic range but cannot simulate any lasting long-term effects on central structures or functions induced by such loss (i.e., CEPP).
Although the use of nonspeech stimuli makes it possible to minimize the contributions of inaudibility to performance, this approach is by no means problem free. For instance, if one wishes to assess potential central auditory deficits that are indirect or secondary to the development of a peripheral hearing loss, employing nonspeech measures in the normal-hearing frequency region likely will not enable one to assess such deficits. This is because the principle of tonotopic organization begins in the cochlea and is evident throughout the auditory portions of the central nervous system. As a result, the peripherally induced changes to central auditory structures will likely be frequency-specific, mirroring the cochlear lesion (Willott, 1991, 1996). Thus, use of low-frequency or midfrequency narrow-band nonspeech stimuli, while avoiding problems of inaudibility, will likely miss the identification of central auditory deficits induced by the high-frequency hearing loss (i.e., CEPP). In addition, various large-scale studies of individual differences for the perception of nonspeech and speech stimuli in young (e.g., Surprenant and Watson, 2001; Kidd et al, 2007) and older adults (Humes et al, 1994, 2010) have often failed to observe a strong association between performance for speech and nonspeech stimuli. This may prove problematic if the ultimate objective of documenting the presence of central auditory deficits is to better understand the reasons underlying the speech-understanding difficulties of older adults. Finally, although the potentially confounding influences of peripheral hearing loss may be minimized to a greater extent with narrow-band nonspeech stimuli than with broadband speech stimuli, tasks making use of nonspeech stimuli may still be impacted by cognitive processing (e.g., Humes et al, 1994; Humes, 1996, 2005, 2009; George et al, 2007). Thus, whether the measure of central auditory processing is comprised of speech or nonspeech stimuli, the validity of such tests as measures of central auditory processing is not easy to establish.
With regard to potential cognitive confounds, another form of confounding is that some older subjects, with typical or above-average cognitive function, may be able to successfully compensate for reduced or distorted input arriving from lower level peripheral or central auditory structures by exerting increased cognitive control and attention or by tapping more abundant lexical resources (Wingfield et al, 1991; Schneider and Pichora-Fuller, 2000; Bertoli et al, 2002; Alain, McDonald, Ostroff and Schneider, 2004; Wingfield et al, 2005; Pichora-Fuller and Singh, 2006; Pichora-Fuller, 2008; Peelle et al, 2011). Probably the area of speech-understanding performance in older adults for which this has been noted most frequently has been with regard to the use of semantic contextual information by older adults (e.g., Pichora-Fuller et al, 1995; Wingfield et al, 1995; Dubno et al, 2000; Humes et al, 2007). In general, unlike many other measures of cognitive function, vocabulary-related verbal measures are very resistant to age-related declines (e.g., Salthouse, 2010), perhaps even showing increases throughout much of the adult life span. If speech understanding is assessed with highly contextual speech materials, older adults may be able to compensate for lower-level peripheral or central auditory deficits to perform like young normal-hearing adults. Whereas, overall, this compensation may be beneficial for the individual involved, it may also serve to mask the true extent of auditory involvement, including any underlying central auditory deficits.
It has been argued that one way to possibly disentangle cognitive and central auditory processing is through the principle of modality specificity (Humes et al, 1992; McFarland and Cacace, 1995; Cacace and McFarland, 1998, 2005; George et al, 2007; Humes et al, 2007; Humes, 2009). That is, does the older individual only manifest a processing problem when presented with sound, rather than other forms of sensory stimulation, such as optical stimulation of the visual system? Although this is still an emerging and active area of research interest, at this point, some evidence supporting modality specificity of some measures of auditory temporal processing has been obtained (Humes et al, 2007, 2010). However, complicating this argument, recent anatomical and physiological studies in laboratory animals (Bizley and King, 2009; Budinger and Scheich, 2009; Cappe et al, 2009) and humans (Kayser et al, 2009) suggest that many cortical areas previously assumed to be exclusively auditory centers now appear to be responsive to stimulation from other senses as well. This is an active and complex area of investigation, however, with definitive implications for behavioral central auditory testing and central presbycusis yet to be established (e.g., Lemus et al, 2010; Meyer et al, 2011).
An emerging hypothesis regarding the coexistence of central auditory dysfunction (in particular, difficulty understanding speech in noise) and age-related cognitive declines (in particular, declines in executive function) views speech processing in the auditory association areas as a cognitive process (Craik, 2007) and suggests that a part of the conceptual blurring (“auditory” vs. “cognitive”) may be reconciled by considering that speech processing is tightly linked to executive function. Certainly, the association of tests of executive functioning and dichotic speech identification (Gates et al, 2010) in older people who passed cognitive screening tests and had comparable magnitude of hearing loss supports this notion. Further investigation, both functional and structural, is needed to delineate the extent and boundaries of this emerging hypothesis. Difficulties in examining the evidence for or against this hypothesis include, among others, the absence of data on executive function in earlier studies, the general custom of not differentiating among cognitive functions, and the unclear role played by individual differences in hearing loss on both measures of speech perception and executive function.
Most studies of central presbycusis rely on cross-sectional comparisons in highly selected subjects. It is important to recognize that, in spite of efforts described above to select appropriate comparison groups or control analytically for confounding effects, these studies are not, by themselves, able to provide sufficient evidence of central declines in aging. Many other exposures and behaviors may differ between groups and act as additional confounders, and with known generational differences in hearing loss (Zhan et al, 2010), comparisons across generations may be problematic. Participants in these limited studies may not reflect the typical experience of aging populations. In addition, longitudinal data are necessary to confirm that the observed auditory performance is, indeed, a change with time, rather than reflecting long-standing poorer performance. The longitudinal data gathered, however, should be sufficiently broad to control for other factors that might impact changes in performance over time, including varied interventions introduced (e.g., hearing aids, cognitive training) during the course of the longitudinal study as well as practice or learning effects from repeated assessment (e.g., Salthouse, 2010).
Finally, with regard to the potential cognitive “confound” noted above, one could make use of such a “confound” to develop an auditory-based measure of cognitive function. That is, a test initially designed to assess central auditory function in older adults, but found to have significant associations with cognitive function, may prove useful as a simpler measure of cognitive function (Gates et al, 2008, 2010).
In addition to the numerous threats to the construct validity of central auditory testing in older adults noted above, the reliability of these measures is equally important. Concerns regarding the reliability of several commonly used measures of central auditory processing have been reviewed recently by Humes (2009). In addition to theoretical concerns stemming from the number of items comprising tests commonly used, often 10 to 25 items per score, some central auditory measures, such as the SSI-ICM and DSI, have unacceptable reliability when assessed in older adults (e.g., Dubno and Dirks, 1983; Cokely and Humes, 1992; Humes et al, 1996; Pugh et al, 1998; Feeney and Hallowell, 2000). In contrast, other measures of auditory processing appear to have acceptable reliability, reflected in a lack of significant test-retest differences and at least moderately high test-retest correlations (r > 0.8), when used with older adults. In particular, the reliability of several tests from the Test of Basic Auditory Capabilities (Watson, 1987) and the Veterans Administration compact disc for auditory perceptual assessment (Noffsinger et al, 1994) has been established for older adults (Christopherson and Humes, 1992; Humes et al, 1996).
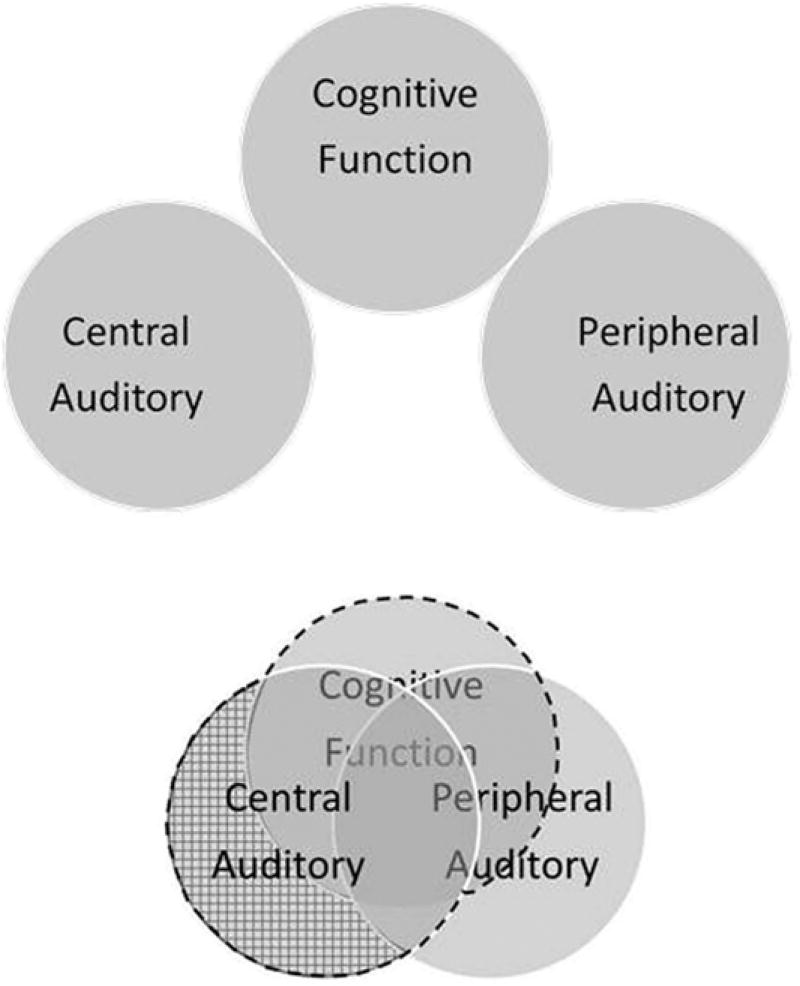
In summary, when viewed in the context of a general anatomical or structural framework that attempts to relegate the auditory-perception and speech understanding difficulties of older adults to peripheral, central auditory, or cognitive factors, singly or in combination, there are many threats to the validity and reliability of existing measures of central auditory processing. This structural approach is summarized by the two Venn diagrams in Figure 1. In the top diagram, each of the three contributing factors, peripheral auditory, central auditory, and cognitive, is assumed to be independent of the other factors, as in the structural form of central presbycusis. Based on the results of the review included in the task force report, the lower Venn diagram is likely a more appropriate depiction of the associations among these three factors affecting auditory perception and speech understanding in older adults. In the functional form of central presbycusis, the entire area encompassed by central auditory and/or cognitive factors (the larger area outlined by the dashed line) is relevant as these areas involve processing beyond the auditory periphery that might impact auditory perception and speech understanding. In the structural form of central presbycusis, which considers central auditory effects independent of the other factors, only the portion of central auditory factors not overlapping with peripheral-auditory or cognitive factors are relevant. This is illustrated by the smaller cross-hatched area to the left in the lower Venn diagram. Although the lower Venn diagram in Figure 1, reflecting interactions among the three contributing factors, is likely a more appropriate representation than the independence of factors assumed in the top Venn diagram of Figure 1, the precise overlap or interactions among the contributing factors, and the distinctions between “auditory” and “cognitive” functions, are largely unknown. Extreme and symmetrical overlap illustrated in the lower Venn diagram of Figure 1 may or may not be an accurate depiction. More research with older adults is needed to address these important questions, by supplementing behavioral measures with nonbehavioral measures based on newer technologies such as EEG, MEG, eye-tracking, and structural, spectroscopic, and functional neuroimaging to identify neurobiological markers of auditory and cognitive aging. As noted previously and articulated in the task force’s definition of “central presbycusis,” the focus of the task force was the important first step of evaluating the evidence base with regard to the traditional, structural form of central presbycusis. In the context of a clinical scope of practice, assessment of peripheral auditory function and central auditory function are clearly within the domain of audiology, whereas full cognitive assessments are not. As a result, understanding the interdependence of peripheral-auditory, central auditory, and cognitive factors underlying central presbycusis has practical implications for clinical assessment.
Figure 1.
Venn diagrams illustrating contributions of peripheral auditory, central auditory, and cognitive factors to auditory perception and speech communication in older adults. In the top diagram, each factor is assumed to make independent contributions. In the bottom diagram, a more realistic scenario is depicted in which each factor interacts with the others. The cross-hatched area and the area bounded by the heavy dashed line in the lower diagram contrast the structural and functional forms of central presbycusis, respectively.
One could argue that establishing the anatomical locus of the impairment is not critical. Rather, consistent with World Health Organization (WHO) guidelines, one could simply focus on the functional aspects of the disability, such as the impairment, activity limitations, and participation restrictions. As defined by WHO, “an impairment is a problem in body function or structure; an activity limitation is a difficulty encountered by an individual in executing a task or action; while a participation restriction is a problem experienced by an individual in involvement in life situations.” Thus, the disability could be the difficulty understanding speech, regardless of the underlying cause, and it is more important to identify the consequences of this impairment in terms of activity limitations or participation restrictions than to determine the underlying causes. That is, from a functional perspective, one could argue that it does not matter whether the underlying factor(s) producing activity limitation in an older adult can be validly and reliably identified as peripheral, central auditory, or cognitive, and it is more important that the activity limitation is appropriately addressed and remediated. This would be especially true if the ultimate intervention for remediation was the same regardless of the underlying contributing factors. However, this does not appear to be entirely the case. For example, consider both an invalid diagnosis of a central auditory deficit in an older adult, one which is really due to the inaudibility effects of the peripheral hearing loss on the speech-based test measures of central auditory function, and a valid diagnosis of a central auditory deficit impacting auditory brainstem function. If both are diagnosed as central auditory deficits, the prognosis for hearing aid benefit would be poor. However, in the case of the invalid diagnosis attributable to peripheral inaudibility, amplification would likely be a very successful intervention, one that might not even be attempted for this individual given the presumed involvement of central auditory factors. Ultimately, it is the task force’s belief that validly and reliably establishing the underlying anatomical locus (or loci) of an older adult’s speech-understanding difficulties will lead to better and appropriately tailored intervention. Until this can be appropriately addressed in a valid and reliable manner, however, it is not possible to evaluate the validity of this assumption. Ultimately, even if an anatomical or structural approach to evaluating the existing literature proves to be unnecessarily restrictive, it still represents a reasonable framework or taxonomy for the organization and evaluation of the existing research literature on central presbycusis.
With the foregoing presentation of general issues in mind, the next section provides an overview of the methods used by the task force to conduct this review. This is followed by the presentation of the results of the review.
PROCEDURES OF THE REVIEW
In June of 2009, the Academy Board of Directors (BOD), in response to a request from President-Elect Patricia Kricos, approved a Task Force on Central Presbycusis to be chaired by the first author. The task force’s charge was to review the body of evidence surrounding the existence of age-related declines in central auditory processes and the consequences of any such declines for everyday communication and function. If the evidence warranted, the task force was also to review approaches to the identification and treatment of such age-related declines in central processes and to make recommendations in that regard.
In November 2009, following clarification of the task force charge and the Academy’s requirements for the composition of such task forces, the coauthors of this report were recruited by the chair to serve on the task force and were approved by the Academy BOD. From November 2009 through February 2010, the task force reviewed the charge and proceeded to identify the research literature that could be used to meet this charge. The task force constrained its search of the literature to primary research articles, rather than reviews, book chapters, or books, involving human subjects and published in English in peer-reviewed journals after 1988. Because, as noted, a comprehensive and thorough review of the related literature had been published by a working group from CHABA of the National Research Council in 1988 (CHABA, 1988), it was agreed that this task force would focus on the literature published after 1988. Although the evidence base to be considered for detailed review was restricted to studies of human subjects in primary research articles appearing in peer-reviewed journals, the general information garnered from animal studies or from existing reviews, including book chapters, was used by the task force in completing its charge and in preparing this report. Indeed, such material, such as the concepts of CEPP and CEBA noted above, for example, was used for general background information but was not part of the evidence base used to address the task force’s charge.
Task force members contributed reference citations to the task force chair via e-mail, and a composite listing of all references was compiled. The initial draft of the composite reference list was circulated and edited as needed by task force members. A total of 200 articles were included in the initial list of compiled references. Each of these articles was made available to the task force via a secured Web site hosted by the Audiology Research Laboratory at Indiana University. Dana Kinney, a research audiologist at Indiana University, was instrumental in gathering these materials, organizing them into topical categories with task force guidance, and then posting them on the secure Web site for use by task force members. Task force members were assigned by the chair to read various sets of research articles, according to their categorization by topic, such that each article was reviewed by two to three task force members and each task force member was assigned to approximately 45 articles. This task was completed prior to the first face-to-face meeting of the group. At the initial face-to-face meeting of the task force in March 2010, in Scottsdale, Arizona, the task force immediately sought to define central presbycusis. After discussion at that meeting, and subsequent follow-up electronic communications among task force members, the definition presented previously in this report was developed.
Also at this initial face-to-face meeting, after review of the 200 articles compiled and the elimination of duplications and review articles, a total of 165 articles remained. The task force then developed a set of subtopics to further organize the review of these materials. The 20 resulting subtopics are shown in Table 1. Next, the group discussed the appropriate features or attributes of each research article to be captured during the review process. After discussion, the task force agreed that the 12 features listed in Table 2 should be extracted from each article, if possible, and tabulated for subsequent review and synthesis. Thus, in the end, the next task of the group was the completion of a vast table, with each of the 165 articles organized into one of the 20 topical categories from Table 1, comprising the rows of the table, and the 12 aspects or features of each study from Table 2, comprising the columns of the table.
Table 1.
20 Topical Categories Used to Sort the 145 Laboratory-Based Research Articles Identified for This Review
| General Topic | Number of Research Articles Reviewed |
|---|---|
| Speech Understanding—Steady-State Noise | 5 (4) |
| Speech Understanding—Competing Speech (including babble) | 12 (11) |
| Speech Understanding—Fluctuating Noise (interrupted noise, modulated noise) | 2 (1) |
| Speech Understanding—Binaural Advantages (including MLDs, spatial release of informational masking) | 3 (2) |
| Speech Understanding—Dichotic Listening | 6 (5) |
| Speech Understanding—Informational Masking (including talker uncertainty effects) | 1 |
| Speech Understanding—Time-Compressed or Speeded Speech | 12 (11) |
| Speech Understanding—Reverberation | 4 (3) |
| Speech Understanding—Other | 27 |
| Nonspeech—Gap Detection | 17 |
| Nonspeech—Duration Discrimination | 2 |
| Nonspeech—Temporal Integration | 0 |
| Nonspeech—Temporal Order Tasks | 8 (7) |
| Nonspeech—Temporal Masking | 3 |
| Nonspeech—Other | 7 |
| * Electrophysiology—General | 3 |
| * Electrophysiology—Auditory Brainstem Response | 4 |
| * Electrophysiology—AM and FM “Early” and “Middle” Latency Responses | 3 |
| * Electrophysiology—Cortical and Event-Related Potentials | 12 |
| * Anatomy/Imaging Studies | 11 |
| * Deleted following further review | 7 |
| Total = 145 |
Note: This table does not include the 20 articles with multiple measures of auditory processing from large samples, designated by the task force as “test battery studies” and reviewed separately. The right column provides the number of articles identified in each category. Numbers in parentheses indicate the number of articles that contributed only to the topic in that category. AM = amplitude modulation; FM = frequency modulation; MLD = masking level difference.
Not reviewed in detail by task force.
Table 2.
Attributes or Features for Each of 165 Research Articles Reviewed (145 laboratory studies and 20 test battery studies)
|
Following review of the 165 articles by the task force, 132 articles with a focus on behavioral measures for either speech or nonspeech stimuli were considered to be most relevant to the task-force charge. A total of 22 studies examining electrophysiological changes and the 11 articles measuring anatomical changes or functional changes via neuroimaging in the central auditory system of older adults were also reviewed and provided informative background material. The measures used in these studies, however, were somewhat heterogeneous, often assessing different electrophysiological responses or central auditory structures across studies. As a result, due to the combination of a relatively small number of studies employing these approaches and considerable heterogeneity in the specific methods and measures obtained, a concise summary of the pattern of findings or trends in these data was not pursued. These observations alone, however, are noteworthy and may provide impetus for further research on the age-related changes in the central auditory system using electrophysiological, anatomical, or neuroimaging techniques. Importantly, many of the issues noted above with regard to behavioral measures, including the influence of peripheral or cognitive deficits, are also relevant for some electrophysiological studies. In addition, if such techniques are successful in documenting age-related changes in the central auditory structures or functions of older adults, it will also be important to demonstrate the relevance of such changes to the everyday function of older adults, especially their ability to communicate with others.
The 132 human behavioral studies, listed in Table S1 (supplemental to the online version of this article), were grouped into three main categories for further analysis: (1) smaller-scale (typically, N < 25) laboratory studies using speech stimuli (76 articles); (2) smaller-scale (N < 25) laboratory studies using nonspeech stimuli (36 articles); and (3) larger-scale (N > 25, typically N > 100) test battery studies obtaining multiple measures of auditory processing using speech stimuli only or speech and nonspeech stimuli (18 studies, 20 articles). In addition to differences in sample size, the majority of studies designated “smaller scale” also tended to focus on one dependent measure and between-group comparisons, whereas all of those designated “larger scale” made use of test batteries comprised typically of three or more central auditory measures and used correlational or regression techniques in the data analyses.
The information about each study in each of the designated categories was compiled and reviewed, along with a first draft of the report, at the final face-to-face meeting of the task force in Chicago in April 2011. Inconsistencies in the way information had been tabulated for the smaller-scale and larger-scale test battery studies became apparent and were resolved at this meeting. Consistent procedures for summarizing the key findings were established and applied by at least two task force members after the meeting. Importantly, it was decided to not only tabulate the significant effects of age, hearing loss, and cognition reported by the author(s) of each study reviewed but also to establish the number of studies reporting a significant age effect for those studies determined to be unconfounded by hearing loss by the task force members performing the review. Ideally, such an analysis also would have been performed for those studies unlikely to be confounded by age-related cognitive declines, but, as will become apparent, this would have eliminated the great majority of studies from review. This is not necessarily because of the presence of cognitive confounds but because so few studies included cognitive measures to exclude possible cognitive confounds.
To illustrate the process of tabulating studies reporting significant effects of age, hearing loss, or cognition, consider the following example. A hypothetical smaller-scale study of gap detection for moderate level (60 dB SPL) noise bands at two stimulus center frequencies, 500 and 4000 Hz, and in two age groups, young and older normal-hearing adults, is to be reviewed by the task force. No cognitive measures were obtained from the subjects in this study. In this hypothetical study, significant group differences in gap-detection thresholds are observed only at 4000 Hz, which the author reports as a significant effect of age. Although both groups were designated by the authors as “normal hearing,” the groups actually differed in high-frequency hearing sensitivity by more than 25 dB. In this hypothetical example, this study would have been tabulated by the task force as a study reporting significant effects of age, even though age effects were observed only at one of the two stimulus frequencies. Further, it would have been tabulated as a study not examining the effects of either hearing loss or cognition on gap-detection performance. Based on the likely confound of high-frequency hearing loss for the measurement of gap-detection thresholds at 4000 Hz and the absence of other control groups or statistical controls to minimize the influence of this potential confound, this hypothetical study would not have been designated as a study likely to be unconfounded by hearing loss. Finally, suppose that this same hypothetical smaller-scale study also had several other gap-detection conditions, such as random variations in gap location and fixed gap locations (for example, as in Harris et al [2010]). Since the fixed gap location represents the typical gap-detection measurement paradigm shared by the studies reviewed, the results for the less common randomly varying gap location would have been ignored for the purpose of tabulating effects of age, hearing loss, and cognition on typical or standard gap-detection thresholds.
All told, the task force had three face-to-face meetings scheduled for the entire group (with six to seven task force members attending and, for two of the three meetings, the rest participating via conference call). One meeting took place near the beginning of the work and two near the end. In addition, there was another face-to-face meeting of a subgroup of four members near the middle of the project. In addition, the task force had two conference calls and numerous e-mail communications. The task force worked on meeting its charge for approximately 24 mo, measured from the time of the Academy BOD’s approval of the task force membership and charge to the submission of the final draft of this report to the board.
RESULTS OF THE REVIEW
Table 3 provides a summary tabulation of the information extracted from the smaller-scale laboratory studies. Note that the topics listed in the far left column represent a subset of topics from Table 1 for which at least three research articles were reviewed. Two exceptions to this are the categories of “Speech Understanding—Other” and “Nonspeech—Other” from Table 1 with 27 and 7 tallies, respectively. Typically, the studies placed into each of these categories were singular in their focus on a unique topic of relevance to the general issue of central presbycusis. For example, there was typically one study in the area of speech understanding in older adults addressing each of the following topics: talker uncertainty, the influence of the immediately surrounding context on word recognition in sentences, the temporal word-gating paradigm, processing of prosodic information, serial recall, dual-task measures, and each of several other cognitive processes. The largest group of articles in the “other” category for speech understanding included nine articles dealing with speech amplified by hearing aids, several of which focused on the role of cognition and amplitude-compression time constants in hearing aids. This subgroup was homogeneous with regard to the general subtopic of “amplified speech” but sufficiently heterogeneous in the aspects of amplified speech addressed to warrant elimination from further consideration by the task force. In the area of “Nonspeech—Other,” examples of topics addressed by only one or two articles included frequency discrimination, intensity discrimination, and horizontal sound localization.
Table 3.
Summary of Findings from Behavioral Laboratory Studies for Speech and Nonspeech Stimuli for Topic Areas for Which at Least Three Research Articles Were Available and Reviewed (see Table 1)
| Topic | # of studies |
# of studies, N < 25 (older adults) |
# of studies, N ≥ 100 (older adults) |
Proportion of studies reporting age effects* |
Proportion of studies reporting hearing loss effects* |
Proportion of studies reporting cognitive effects* |
Proportion of studies reporting age effects w/o hearing loss confound** |
|---|---|---|---|---|---|---|---|
| Speech—Competing Speech | 12 | 3 | 4 | 6/10 | 4/7 | 1/2 | 4/6 |
| Speech—Steady-State Noise | 5 | 5 | 0 | 2/5 | 4/4 | 3/3 | NA |
|
|
|||||||
| 17 | 8 | 4 | 8/15 | 8/11 | 4/5 | ||
|
|
|||||||
| Speech—Time Compression | 11 | 10 | 0 | 9/10 | 5/5 | 1/2 | 6/7 |
| Speech—Reverberation | 4 | 4 | 0 | 3/4 | 4/4 | 0/0 | NA |
|
|
|||||||
| 15 | 14 | 0 | 12/14 | 9/9 | 1/2 | ||
|
|
|||||||
| Speech—Dichotic | 6 | 5 | 1 | 5/5 | 0/4 | 1/1 | 2/2 |
| Speech—Binaural release from masking/spatial separation | 3 | 3 | 0 | 2/3 | 0/0 | 0/0 | NA |
|
|
|||||||
| 9 | 8 | 1 | 7/8 | 0/4 | 1/1 | ||
|
|
|||||||
| Nonspeech—Gap Detection | 15 | 10 | 2 | 12/13 | 2/7 | 2/2 | 9/12 |
| Nonspeech—Duration, Gap, or IOI Discrimination | 6 | 6 | 0 | 6/6 | 0/6 | 0/0 | 6/6 |
| Nonspeech—Temporal Order Discrimination and Identification | 5 | 5 | 0 | 5/5 | 1/4 | 0/0 | 4/4 |
| Nonspeech—Temporal Masking | 3 | 3 | 0 | 2/3 | 0/0 | 0/0 | NA |
|
|
|||||||
| 29 | 24 | 2 | 25/27 | 3/17 | 2/2 | ||
Note: IOI = inter-onset-interval.
Numerator = # of studies in which author(s) reported significant effect of independent variable (age, hearing loss, or cognitive function); Denominator = # of studies examining this effect.
Numerator = # of studies unconfounded by inaudibility, according to task force review, that found a significant effect of age; Denominator = # of such unconfounded studies examining this effect.
Smaller-Scale Studies
Speech Stimuli
For the 76 smaller-scale studies of speech understanding in older adults, the three phenomena that have received the greatest attention over the past two decades are speech in competition (17 articles), temporally distorted speech (15 articles), and binaural speech perception (9 articles). For the 17 articles involving speech in competition (Table 3), 12 involved competing speech and 5 involved competing noise. For speech stimuli presented in competition (Table 3), about half (8 of 15 studies) of these studies reported significantly worse performance in older adults than in young adults. When tallying studies observing significant effects of a particular independent variable, in this case the effects of age, counts were tallied regardless of whether the study fully documented that the effect was attributable to age and not to a potentially confounding variable (hearing loss or cognition in this case). The use of this liberal criterion inflates the number of studies showing true effects of each independent variable tallied. In several of these studies (8 of 11 studies), when older adults with impaired hearing were included, significant effects of hearing loss were observed such that those with more hearing loss performed more poorly on the speech-understanding measures. It is also noteworthy from Table 3 that only five of these studies obtained cognitive measures from study participants and that most of these studies (4 of 5) found that those with low cognitive performance performed worse on the speech-understanding measures than those with high cognitive function. Finally, the far right column of Table 3 provides a more conservative estimate of the number of studies revealing significant effects of age on performance. This column shows the proportion of studies (4 of 6) showing significant age effects among those studies considered by the task force to be unconfounded by hearing loss. However, these studies may have suffered from residual confounding from other factors, such as education and cognitive function, or may represent only highly selected subjects. As a result, a high proportion (4 of 6) of studies, here and elsewhere, should not be interpreted as strong evidence of age effects.
Of the 15 articles reviewed on temporally degraded speech, the data in Table 3 indicate that 11 involved time-compressed speech and 4 involved reverberation. Given that the latter form of temporal degradation is encountered more frequently in everyday listening, at least if one distinguishes time-compressed speech from rapidly articulated speech, the relatively small proportion of studies examining performance for reverberant speech in comparison to those involving time-compressed speech is noteworthy. In general, the pattern observed from the data in Table 3 for temporally degraded speech is quite similar to that noted above for speech in competition. Specifically, most of the studies (12 of 14) reported significant effects of age, such that older adults performed worse than young adults. Moreover, when hearing loss was present in the older adults, it had a negative impact on speech-understanding performance in 9 of 9 studies of temporally degraded speech. Only 2 of the 15 studies of temporally degraded speech measured cognitive function, and one of those studies observed a significant effect of cognitive function on speech-understanding performance. Finally, of the 7 studies of time-compressed speech determined by the task force to be unconfounded by hearing loss, 6 reported significant effects of age.
Of the 9 smaller-scale studies reviewed regarding binaural speech perception, the data in Table 3 indicate that most of these (6 studies) involved dichotic listening under headphones. For the area of binaural speech perception, the pattern of outcomes was considerably different from that observed for speech with competition and temporally degraded speech. Specifically, almost all of the studies (7 of 8) in this area found that age had a significant effect on binaural speech-understanding performance, but none of the studies (0 of 4) reported a significant effect of hearing loss. It may seem somewhat surprising that only 4 of the 9 studies in this area examined associations with hearing loss. However, of the 5 studies not examining the role of hearing loss, 2 studies examined the effects of age in normal-hearing listeners, eliminating older adults with impaired hearing, and 3 concentrated their analyses on relative differences in performance, either the right-ear advantage for dichotic listening or binaural gain. Interestingly, despite the long history of discussion about the auditory/linguistic and cognitive contributions to dichotic-listening tasks (e.g., Cherry, 1953; Broadbent, 1954; Kimura, 1967), only 1 of the 6 studies of dichotic listening examined cognitive function, and this study found a positive association between working memory function and dichotic performance. Finally, 2 of the 6 small-scale studies of dichotic speech perception were considered by the task force to be unconfounded by hearing loss, and both of these studies reported significant effects of age.
Summary of Findings
For the 76 smaller-scale studies of speech understanding in older adults, the following findings emerged: (1) the three phenomena that received the greatest attention over the past two decades were speech in competition (17 articles), temporally distorted speech (15 articles), and binaural speech perception (especially dichotic listening conditions; 9 articles); (2) for speech in competition and temporally degraded speech, but not necessarily binaural speech perception, hearing loss was reported to have a significant negative effect on performance in most (≥70%) of the laboratory studies; (3) significant negative effects of age were reported in most (≥67%) of the studies of speech in competing speech, time-compressed speech, and binaural speech perception; and (4) the influence of cognitive processing on speech understanding has been examined much less frequently, but when included, significant positive associations of cognitive function with speech understanding were observed (primarily for speech in speech competition). In general, given the smaller sample sizes employed in these studies and the large percentage of studies showing potential confounds of hearing loss or cognitive function on performance, there is little evidence in support of central presbycusis from these studies, despite a relatively large number of studies of this type that has been conducted.
Nonspeech Stimuli
With regard to the 36 smaller-scale studies of the perception of nonspeech stimuli by older adults, three phenomena were studied most frequently: gap detection (15 articles), temporal discrimination of some type (e.g., duration discrimination, gap discrimination; 6 studies), and some form of temporal-order processing (5 articles). In fact, from review of Tables 1 and 3, temporal gap detection was the auditory-processing phenomenon studied most often among the 145 smaller-scale studies reviewed by the task force. For the gap-detection measure, the pattern that emerged from the tabulation of findings in Table 3 was that older adults performed worse than younger adults in almost all cases (12 of 13 studies), and hearing loss was seldom a contributing factor (2 of 7 studies). Hearing loss was not studied in 8 of the 15 studies of gap detection as the study samples were confined to normal-hearing participants differing in age only. Most, if not all, of these studies also carefully selected the stimulus parameters, including level and frequency, to minimize the influence of hearing loss on performance. Of the 12 studies considered by the task force to be unconfounded by hearing loss, 9 reported significant effects of age on performance.
A very similar pattern of findings was observed for the 6 studies of temporal discrimination and the 5 studies of temporal-order discrimination or identification for nonspeech stimuli (Table 3). Specifically, all 11 of these studies in these two temporal-processing categories demonstrated poorer performance in older adults compared to young adults, and only 1 of 10 observed an effect of hearing loss on performance. Most of these 11 studies (10 of 11) were considered by the task force to be unconfounded by hearing loss and all of them reported a significant effect of age on performance. Finally, the three studies of temporal masking with nonspeech stimuli also show a very similar pattern of findings (Table 3).
In addition to these general findings for nonspeech stimuli, it is noteworthy that only two of the 29 studies tabulated in Table 3 examined the contributions of cognitive function to performance. Both studies examined gap detection and observed significant effects of cognition on performance.
Summary of Findings
With regard to the 36 smaller-scale studies of the perception of nonspeech stimuli by older adults, the following findings emerged: (1) the three most frequently studied phenomena were gap detection (15 articles), some form of temporal discrimination (6 studies), and temporal-order processing (5 articles); and (2) hearing loss was seldom (≤20%) a significant factor, especially when stimuli were selected to be low-frequency or midfrequency sounds; and (3) age effects were almost always (≥90%) observed. Age was negatively associated with performance on these nonspeech tasks. Although the evidence for the existence of central presbycusis is stronger for the smaller-scale studies using nonspeech stimuli than those using speech stimuli, potential cognitive confounds have seldom been examined in these studies, the studies are cross-sectional in nature, typically examining extremes of the adult age continuum, and the samples may represent only highly selected volunteer subjects. As such, this cannot be considered to be strong evidence of age effects, or central presbycusis, on these nonspeech tasks.
Larger-Scale Test Battery Studies
The 18 test battery studies (20 articles) were first divided into those making use of speech stimuli (all 18 studies) and nonspeech stimuli (four studies). The details of these studies are summarized in Table 4. Details of these studies are presented here because these larger-scale studies were believed by the task force to be most important to the task force’s charge due, in large part, to the large numbers of subjects included. Four studies made use of both speech and nonspeech stimuli and were included in both tabulations. Then, the studies were again examined with regard to the influence of age, hearing loss, and cognitive function on performance for the measures of central auditory processing, as had been the case for the smaller-scale studies described above. Additional variables of potential interest, such as gender and sample population, were also tabulated. The task force was divided into three subgroups for the purpose of reviewing the studies in Table 4. One subgroup addressed the four studies with nonspeech stimuli. For the test battery studies making use of speech stimuli, the outcomes of each study were tabulated in two ways by two separate task-force subgroups: (1) by list of studies, focusing on type of central auditory measure (e.g., dichotic speech, speech in competing speech, etc.); and (2) by list of specific central auditory tests employed (e.g., DSI, SSI-ICM, Dichotic Digits Test [DDT], time compressed NU-6, etc.). In the end, the results of these two separate analyses of the same 18 studies were reconciled and combined and are presented below.
Table 4.
Summary of the Analyses of the 18 Large-Scale Test Battery Studies
| Test battery study # |
Study | Speech tests (listing by committee member) |
Subject sample (age, number, groups) |
Age effects/speech test |
Hearing loss effects/speech test |
Cognitive effects/speech test |
Possible confounding/ speech test |
Other observations |
|---|---|---|---|---|---|---|---|---|
| 1 | Jerger J, Jerger S, Oliver T, Pirozzolo F. (1989) Speech understanding in the elderly. Ear Hear 12:103–109. | PB 50 word test, SSI test, SPIN test, DSI test; presentation level for PB, SSI, and SPIN were 50 dB SL re babble threshold of each ear; for SSI, MCR = 0 dB, for SPIN, SBR = +8 dB, and DSI, intensity level to each ear was 50 dB HL. Abnormal result: PB-SSI > 20%; SPIN test abnormal if performance <2 SD below mean; DSI: difference between ears > 16%. | Tested 130 community-dwelling, nonclinical subjects, ranging in age from 51–91 years. | CAPD defined as abnormal performance on one or more of three speech measures, and this was 50%. Tested only elderly subjects so no comparison group. Presumably, positive CAPD results reflect age effects. Of 65 subjects with abnormal (abn) speech results, 16 had PB-SSI difference, 5 had abn SPIN, 12 had abn DSI, 6 had abn SPIN + PB-SSI, and 26 had abn DSI + SPIN and/or PB-SSI difference. Age as a covariate was not explicitly examined; the focus was on CAP and cognitive status. | Accommodated by presenting signal level at 50 dB SL re babble threshold and selecting subjects with PTA (.5, 1, 2 kHz) < 50 dB HL in either ear. Hearing sensitivity mild-to-moderate sloping sensorineural hearing loss. Thus, results could be confounded by significant high-frequency sensorineural loss. | Neuropsychological battery administered: MMPI, WAIS-R, Wechsler Memory Scale, Boston Naming Test, Spatial Orientation Memory Test, Burschke Selective Reminding Test, Simple Auditory and Simple RT tests, and 4-choice Visual RT test; subjects classified as normal or abnormal; In total, 41% classified as abnormal; of the 65 subjects with abnormal CAPD status, 35 also had abnormal cognitive status. | Of 130 tested, 23% classified as CAPD with normal cognitive status; however, these could have had some confounding due to high-frequency hearing loss. Not broken down by test; of 130 tested, 27% classified as CAPD with abnormal cognitive status—clearly had abnormal cognitive findings and also may have had hearing loss. | The largest category of abnormal findings resulting in a classification of CAPD is multiple findings (DSI + SPIN and/or PB-SSI difference). |
|
| ||||||||
| 2 | Stach B, Spretnjak M, Jerger J. (1990) The prevalence of central presbycusis in a clinical population. J Am Acad Audiol 1:109–115. | SSI and PB-50 word lists; established PI-PB functions in quiet, SSI presented at 0 dB MCR. Central presbycusis defined as: SSI rollover > 20%, PB scores - SSI scores > 20%, or absolute SSI score lower than normative boundary with same degree of hearing loss. | Retrospective analysis of data from 700 clinical patients, aged 50+ yr, with 100 subjects/half age decade (50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80+) and 138 nonclinical subjects (20/age group described above). | Prevalence of central presbycusis increased with age (17%, 50–54 yr to 95%, 80+ yr) (not broken down by test measure). | Because hearing increased with age, conducted a substudy to control for hearing loss with 20 subjects/half decade, matched for degree of hearing loss based on PTA; even when degree of hearing loss controlled, prevalence of central presbycusis increased with age. But greater prevalence of CAPD in hearing loss group compared to nonclinical group (suggests confound). | Not measured. | Hearing loss and cognitive decline. However, authors mention that their methods of identifying CAPD are relatively immune to the effects of hearing loss. Didn’t specify if subjects were native speakers of English. | Significance of individual measures—unknown. |
|
| ||||||||
| 3 | Cooper JC, Jr., Gates GA. (1991) Hearing in the elderly—the Framingham cohort, 1983–1985: Part II. Prevalence of central auditory processing disorders. Ear Hear 12:304–311. | PI/PB functions for CID W-22, SSI-ICM (SSI presented at 50 dB HL at MCRs of +10, 0, −10, −20 dB; SSW test at 50 dB SL re PTA. Abnormal results: PB rollover > 20%, PB-SSI > 20%; SSW: moderately and severely abnormal (and overcorrected) categories based on TEC analysis (total, ear, and condition error scores). | n = 1018 from the Framingham cohort who provided sufficient data. | 1.4% of subjects failed PI-PB rollover test (CAPD), 18.1% subjects failed PB-SSI difference and were classified as CAPD; 10.7% showed abnormal SSW results. Total prevalence of CAPD in this group: 22.6%; abnormal performance increased with age. Accounted for ~15% of the variance and not considered a dominant factor in etiology of CAPD. | Not assessed directly but assumed it was minimal. | Not assessed. | Cognitive decline, high-frequency hearing loss (not reported). Didn’t specify if subjects were native speakers of English. | Prevalence of CAPD in a nonclinical population was 23% among those >63 yr. |
|
| ||||||||
| 4 | Jerger J, Jerger S, Pirozzolo F. (1991) Correlational analysis of speech audiometric scores, hearing loss, age, and cognitive abilities in the elderly. Ear Hear 12:103–109. | Speech tests identical to those described for #1 above (Jerger et al, 1989), but derived five speech scores (PB, SSI, SPIN-high, SPIN-low, DSI) by averaging the individual ear scores. | n = 200 subjects, 50–91 yr (same as subject recruitment in #1). | For SSI, hearing loss and age significantly contributed to performance. For other measures, age was not a significant predictor variable contributing to performance. | Predictor variable of hearing loss was significantly associated with PB (58% variance accounted for, VAF) and SPIN-low scores (61% VAF), addition of other variables accounted for only 3–6% additional variance. For SSI, hearing loss (42% VAF) and age (another 12% VAF) significantly contributed to performance; for SPIN-high—only hearing loss (54% VAF). For DSI, hearing loss (30% VAF) and digit symbol score (speed of mental processing; 13% VAF) for 43% of total variance. | For DSI, hearing loss and digit symbol score (speed of mental processing) accounted for 43% of variance. | Appears that hearing loss was a variable contributing to most of performance among this group. | Considerable variance was not accounted for, for each of the tests. If younger subjects were tested to compare to the older subjects, might have been possible to state that there were age effects (so pure age effects could not really be assessed, because it was an older group of participants). Of data reported, degree of hearing loss was strongest predictor variable for the four monotic speech measures, but accounted for less variance for the dichotic speech measure (DSI). |
|
| ||||||||
| 5 | van Rooij JCGM, Plomp R. (1992) Auditive and cognitive factors in speech perception by elderly listeners III. Additional data and final discussion. J Acoust Soc Am 91:1028–1033. | Reduced battery to SRT in quiet (Q) and noise (N), memory span and processing speed. Tests administered on computer in subject’s home (NOT in the laboratory). | 85 subjects, 53–94 yr; healthy volunteers, otoscopically inspected. | None. | Canonical correlations: Thresholds - 63% VAF, HL slope - 11% VAF | Canonical correlations: Digit span and memory scanning - 10% VAF. | ||
|
| ||||||||
| 6 | Humes LE, Watson BU, Christensen LA, Cokely CG, Halling DC, Lee L. (1994) Factors associated with individual differences in clinical measures of speech recognition among the elderly. J Speech Hear Res 37:465–474. | CUNY NST, CID W-22 in unshaped (W-22U) and spectrally shaped (W-22S) noise, R-SPIN; presentation level for all speech materials 70 and 90 dB SPL; SNR = +7 dB (using speech-shaped noise) (total of 20 measures of speech recognition: 5 tests × 2 presentation levels × 2 noise conditions [Quiet and Noise]). | n = 50, 63–83 yr; air-conduction thresholds show a mild-to-moderately severe hearing loss (on average). | Canonical analyses revealed that age was part of the set of predictor variables to predict speech scores at the lower presentation level (although weight was weak). | Canonical analyses identified hearing loss as the largest predictor variable to account for variation in a set of criterion variables (esp. the first speech variable: Speech scores at lower presentation level) among the elderly subjects. Canonical correlations: HL, 70–75% VAF. | Canonical analyses showed that all cognitive variables were part of the set of predictor variables to predict speech scores (especially cognitive measures from WAIS-R), but low weights. Little or no additional variance accounted for by cognitive or auditory-processing measures. | Accounted for through PCA and canonical correlations—to examine contribution of these sources. Didn’t specify if subjects were native speakers of English. | PCA’s second speech component reflects speech perception in noise at a high signal level that was not accounted for by audibility (accounts for about 7% of variance); source not identified. |
|
| ||||||||
| 7 | Humes LE, Coughlin M, Talley L. (1996) Evaluation of the use of a new compact disc for auditory perceptual assessment in the elderly. J Am Acad Audiol 7:419–427. | Dichotic nonsense syllables (NS) (simultaneous and 90 msec lag), dichotic digits, DSI, dichotic consonant-vowel (CV) segments (voice in one ear, consonant in the other ear), binaural NU-6 with high- and low-pass filtering, NU-6 with 45 and 65% TC; all presented at 90 dB SPL. | n = 38 elderly subjects and 40 young adults; young adults had normal hearing; elderly subjects had hearing ranging from normal to moderate sloping high-frequency sensorineural hearing loss; elderly subjects divided into 2 subgroups: elderly normal hearing (ENH) (13) and elderly hearing impaired (EHI) (25). | Age effects observed on dichotic CVs (2 levels), vowels in 1 ear, consonants in the other, and NU-6 filtered. NOTE: little association between scores on dichotic digit and DSI with high-frequency PTA up to 50 dB HL; PCA showed that age was associated with dichotic-competition skills and auditory-pattern/temporal sequencing factor. | Hearing loss was significant for 5/10 tests: dichotic digits, DSI, NU-6 filtered, NU-6 45 and 65% TC; no effect of age was observed for dichotic digits, DSI, and NU-6 45% TC ratio. NOTE: the 3 measures involving NU-6 were strongly correlated with high-frequency PTA; DESPITE presentation level of 90 dB SPL. HFPTA strongly negatively associated with general speech understanding (PCA Factor 1). | Not assessed. | Didn’t specify if subjects were native speakers of English. | Observed questionable test-retest reliability for dichotic NS and DSI; recommend 2 speech tests for auditory perceptual evaluation of elderly (at 90 dB SPL): VIOECITO (vowels in one ear consonants in the other) and dichotic nonsense-syllables with 90 msec lag. |
|
| ||||||||
| 8a | Divenyi P, Haupt KM. (1997) Audiological correlates of speech understanding deficits in elderly listeners with mild-to-moderate hearing loss. I. Age and lateral asymmetry effects. Ear Hear 18(1):42–61. | SRT, CCT at 50 dB SL re PTA with contralateral white noise masking at +30 dB SNR, low-pass filtered speech at .75 and 1 kHz, rapid alternating speech (RAS), MRT in reverberation a t = 0, .45, .85, and 1.25 sec reverberation time, SSI-ICM SSW, Competing sentence test at −5 dB SNR - monaural and binaural, NU-6 with TC30 and TC60, SPIN at +4 dB SBR - monaural and 3 spatial conditions. | n = 45 elderly subjects (60–81 yr), pure-tone thresholds <50 dB HL at .5, 1, 2, and 4 kHz; and 16 young normal hearing control subjects. | Following ANCOVA to remove hearing loss effects, continued to see age effects for some tests, including RAS, CCT, TC30, RT .45 and .85 sec, and SPIN spatial separation measures. | Hearing loss effects seen on SSI, contralateral competing sentence tests, TC60, MRT without reverberation and in 1.2 sec reverberation and some of the SPIN spatial nonspatial measures. (i.e., different scores between young and old before ANCOVA and differences were not preserved after ANCOVA). | Not assessed. | Didn’t specify if subjects were native speakers of English. | Robust age effects for reverberant speech, SPIN in spatial separation, sentence context-based facilitation of speech intelligibility (Predictability High [PH] vs. Predictability Low [PL] SPIN) |
|
| ||||||||
| 8b | Divenyi P, Haupt KM. (1997) Audiological correlates of speech understanding deficits in elderly listeners with mild-to-moderate hearing loss. II. Correlation analysis. Ear Hear 18(2):100–113. | Same as 8a. | Same as 8a, without young control group. | Age was expressed as the linear combination of 4 predictor variables: pure tone slope, SPIN 36 monaural (SPIN 360° High + Low minus SPIN Monaural High + Low), SSI, and bilateral competing sentence test (but only accounted for 31.5% of variance); when hearing loss removed, age predicted by CCT, SPIN and auditory filter width (53.4% VAF) | Observed a high canonical r between hearing sensitivity measures and 6 measures of speech understanding in nonoptimal conditions (mostly SPIN measures with and without spatial sep) and speech in reverberation; and between hearing sensitivity and 7 measures of speech understanding in distortion or interference | Not assessed. | Didn’t specify if subjects were native speakers of English. | Hearing loss accounts for 2/3 of variance in speech measures; believe the remaining variance must be due to central mechanisms (esp. for babble-related and other interference measures and reverb); thus, ability to perceptually segregate one speech signal from another—still a factor in older people when auditory sensitivity is controlled. |
|
| ||||||||
| 8c | Divenyi P, Haupt KM. (1997) Audiological correlates of speech understanding deficits in elderly listeners with mild-to-moderate hearing loss. III. Factor representation. Ear Hear 18(3):189–201. | Same as 8a. | Same as 8a with control group. | PCA extracted 6 factors, with the largest factor interpreted as speech understanding with interference; second factor is hearing sensitivity (didn’t really talk about “age effects” per se). | Second factor extracted was hearing sensitivity in PCA. | Not assessed. | Didn’t specify if subjects were native speakers of English. | Re-affirmed importance of evaluating perceptual segregation of simultaneous speech stimuli. |
|
| ||||||||
| 9 | Dubno JR, Lee F-S, Matthews LJ, Mills JH. (1997) Age-related and gender-related changes in monaural speech recognition. J Speech Lang Hear Res 40:444–452. | NU-6, SSI, R-SPIN; generated PI functions for NU-6 and SSI; for SSI-ICM, MCR was 0 dB. SPIN presented in standard mode (+8 dB SNR); derived PB and PB maximum (max), SSI max, SPIN-PH, SPIN-PL, and SPIN HFS (% hearing for speech). | n = 129 people with sensorineural hearing loss (55–84 yr); selected people within 10 yr age ranges with equivalent thresholds (55–64, 65–74, 75–84); final sample was 125 people; 250 ears). | Found no significant age effects on any of the 6 speech measures, across the 3 elderly age groups; after score and age were adjusted for association with PTA, age effects on speech scores observed for males but not females (PB max, SSI max, SPIN-PH). | Covaried with the speech measures; hearing loss accounted for largest proportion of variance in speech recognition scores. | Not assessed. | Didn’t specify if subjects were native speakers of English. | Gender differences noted on decline in speech recognition that were not accounted for by hearing sensitivity. |
|
| ||||||||
| 10 | Jerger J, Chmiel R. (1997) Factor analytic structure of auditory impairment in elderly persons. J Am Acad Audiol 7:269–276. | PB-50 word lists, SSI, DSI, at 2–3 SPLs (60, 80, 100); DSI with free report (FR) and directed report (DR). | n = 180 elderly subjects >60 years with high-frequency sensitivity loss (at 1, 2, and 4 kHz) > 15 dB HL; normal score on MMSE (>24). | 1 factor was general speech-understanding ability (word recog in both ears—not strongly related to audibility); other factors - separate ear-specific dichotic performance interpreted as a central processing factor. No effects of age per se. | 2 hearing loss factors accounted for 40% variance in the data (low-frequency sensitivity loss and high-frequency sensitivity loss). | Assessed measures of cognitive function but not reported in this study. | Didn’t specify if subjects were native speakers of English. | |
|
| ||||||||
| 11 | Humes LE. (2005) Do ’auditory processing’ tests measure auditory processing in the elderly? Ear Hear 26:109–119. | PI functions, to determine PI-PB rollover, dichotic CVs with 90 msec lag, NU-6 at 45% TC ratio | 213 elderly subjects, 60–88 yr (males and females); bilateral, symmetrical sensorineural hearing loss (compared performance to young norms). | Older listeners performed much poorer on NU-6 TC and dichotic CVs than comparative norms (not examined directly, though); dichotic CV identification—age was a second significant predictor variable accounting for performance but multiple correlation was low. | NU-6 TC speech scores—accounted for by high-frequency hearing loss (nonverbal IQ also identified but not significant). | IQ was the first significant predictor variable for dichotic CV perf (although VAF was low); for TC speech—with hearing loss partialled out, increases in age and decreases in IQ associated with decreases in TC speech (associations were weak). | Didn’t specify if subjects were native speakers of English. | Most subjects showed no PI-PB rollover; results indicate that many measures of auditory processing in the elderly may reflect individual differences in cognitive function—but this needs to be verified with parallel tasks in different modalities. |
|
| ||||||||
| 12 | Golding M, Taylor A, Cupples L, Mitchell P. (2006) Odds of demonstrating auditory processing abnormality in the average older adult: the Blue Mountains Hearing Study. Ear Hear 27:129–138. | Macquarie SSI (MSSI), Macquarie DSI (MDSI) PB scores; derived test outcomes that were + for CAPD: poorer than expected perf for Right MSSI max, Left MSSI Max, Right MDSI, Left MDSI, MDSI Diff score, Right PB - MSSI max, Left PB-MSSI max. | n = 1192 participants, 54–99 yr, PTA < 50 dB HL and no ear asymmetry. | Effects of age seen for all tests except Right MDSI score. | Did not study hearing loss per se but excluded individuals with >50 dB HL at.5, 1, 2 kHz and any asymmetry in PTA > 30 dB. Did not include a measure of hearing as a factor because subsample had good hearing (PTA < 20 dB HL); also think that results from sentence-based CAP materials are resistant to influence of peripheral hearing loss; outcomes were corrected for hearing loss. | Screened for MMSE—looked for normal performance (>24); odds of demonstrating CAP abnormality increased by 24% with every 1-unit decrease in MMSE score (thus, strong r between cognition and CAP findings). | Possible that high-frequency hearing loss could have influenced performance on the speech measures; native language/dialect of participants not specified. | Recommend testing for CAP abnormality and cognitive screening routinely in auditory assessment of older adults; also observed gender differences in dichotic MDSI test (men higher odds of CAPD than women); also observed ear difference with age effects on dichotic measures seen for LE and less for RE. |
|
| ||||||||
| 13 | Vaughan N, Storzbach D, Furukawa I. (2006) Sequencing versus nonsequencing working memory in understanding of rapid speech by older listeners. J Am Acad Audiol 17:506–518. | IEEE sentences and anomalous sentences - time compressed at 0, 40, 50, 60, and 65% (TCR); also conducted extensive neuropsychological battery that included working memory tests, speed-of-processing tests, and tests of attention. | n = 176 speakers of American English, 50–75 yr; hearing thresholds in normal, mild, moderate, or moderate to severe range; screened for normal cognitive function for age. | Removed effects of age using ANCOVA; hence, age as a variable was not examined in relation to the speech recognition tests. | Did an ANCOVA to remove hearing loss effects, so hearing loss wasn’t considered a variable that could influence performance. | PCA showed that nonsequential working memory (WM) accounted for 22.4% of variance in speech scores; Processing speed accounted for 19.5% of variance, and sequential WM accounted for 19.2% of variance; after partialling out age, sequential WM had most robust assoc with speech recognition followed by nonsequential WM. | Didn’t conduct a multiple regression or factor analyses to examine how much of the variance in TC speech is due to hearing sensitivity, age, and cognition. | |
|
| ||||||||
| 14 | Cox LC, McCoy SL, Tun PA, Wingfield A. (2008) Monotic auditory processing disorder tests in the older adult population. J Am Acad Audiol 19:293–308. | Low-pass filtered speech (LPFS; cutoff 750 Hz), QuickSIN, SSI-ICM at MCRs of +10, 0, −10 and −20 dB, TC sentences and words—at 40 and 60% time compression; presentation level did not exceed 80 dB HL. | n = 45 older adults: 14 older normal, 15 with high-frequency hearing loss, 15 with low- and high-frequency hearing loss (PTA < 50 dB HL through 4000 Hz); 3 groups similar in cognitive measures. | Only TC Speech at 60% in the hearing loss group (hi/low) would be considered + for CAPD (scores < 2 SD from norms); age did not emerge as an important factor in APD performance. | Hearing was a significant ME for TC sentences and words, LPFS.; in multiple regression analysis, hearing measures significantly predicted LPFS, SSI (−10 MCR), TC sent and words (60% TCR); high-frequency hearing loss NOT a significant predictor. | Cognitive measures were negligible in analyses; verbal ability was a significant predictor for TC words only. | Hearing loss in the speech range played an important role in APD performance, but age had little effect; the only speech APD test that was not degraded by peripheral hearing loss was QuickSIN; BUT SSI-ICM, LPFS, and TC speech—influenced by peripheral hearing loss (but mostly when there is hearing loss in low + high frequency range in mild to moderate category). | |
|
| ||||||||
| 15 | Gates GA, Feeney MP, Mills D. (2008) Cross-sectional age-changes of hearing in the elderly. Ear Hear 29(6):865–874. | W-22 at 90 dB HL or max comfort level, SSI-ICM (0 dB MCR), DSI - free report, DDT - free report. SSI, DSI, and DDT presented at 50 dB SL re PTA. Tested until asymptotic performance | n = 241 subjects with normal cognitive abilities (based on screen), PTA < 47 dB HL, word recognition > 70%. | SSI-ICM scores adjusted for PTA declined from .85 to 1.6%/yr depending on ear and gender; DDT showed small age effect after adjustment for PTA in men (RE; −.3/yr) and women (LE; −.92/yr). | Not examined but adjusted age regressions for PTA. | Not examined, but subjects were screened for cognitive function. | Doesn’t indicate if native language was English. | Concludes that CAPD dysfunction, beyond changes in peripheral input, is a major component in presbycusis in people >70 yr. SSI-ICM showed more rapid decline with age than the two dichotic tests; thus, recommend routine clinical assessment of CAP with SSI-ICM test (but need to have adequate memory) |
|
| ||||||||
| 16 | Gates GA, Anderson ML, Feeney MP. McCurry SM, Larson EB. (2008) Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg 134:771–777. | CID W-22 at 90 dB HL, SSI-ICM (0 dB MCR), DSI, DDT (same as study #15). | n = 313 volunteers; 3 groups: controls without memory loss, individuals with mild memory loss without dementia; memory-impaired individuals with dementia; otherwise, criteria are same as in study #15. | Two memory groups were older than the control group; hence, age was factored out of analyses. | Two memory groups had poorer hearing than control group; hence, hearing was factored out of analyses. | Adjustment for pure tone thresholds and age was used in evaluating group scores; DSI test showed largest difference between the 3 groups: controls > mild memory > dementia; SSI-ICM showed largest difference between mild memory and dementia groups (SSI may be sensitive to progression in memory impairment). | Doesn’t indicate if native language was English. | Findings suggest strong association between memory loss and tests of central auditory function. Not a surprising result given DSI stresses memory and uses free report as the mode of response selection; tests of perception should minimize memory and motor components of the task. |
|
| ||||||||
| 17 | Vaughan N, Storzbach D, Furukawa I. (2008) Investigation of potential cognitive tests for use with older adults in audiology clinics. J Am Acad Audiol 19:533–541. | IEEE sentences and anomalous sentences—TC at 0, 40, 50, 60, and 65% TCR; also conducted extensive neuropsychological battery that included working memory tests, speed of proc tests, and tests of attention; presented at 90 dB SPL. | n = 225 native speakers of English, 50–75 yr, pure tone thresholds in mild range (low frequencies) and moderately severe range (high frequencies); normal performance on cognitive screening tests. | PCA results, not adjusted for age, showed 3 components: nonsequential WM, sequential WM, and processing speed (61% VAF); sentence PCA with 2 sentence types at 50 and 60% TC ratio, 1 component (80% VAF). | Hearing loss + age accounted for 28% of variance in compressed sentence performance. | Sequential WM—significantly correlated with performance on the compressed sentence tasks; highest correlations with compressed speech were for Letter-Number-Sequence test, full-scale IQ, and verbal IQ (when controlling for age and hearing loss); approximately 13% of total variance in compressed speech was attributable to cognitive variables, especially LNS. | Total variance accounted for by age, hearing loss, and cognitive measures was 41.6% (< half of sentence score variance). | |
|
| ||||||||
| 18 | Gates GA, Gibbons L, McCurry S, Crane P, Feeney MP, Larson E. (2010) Executive dysfunction and presbycusis in older persons with and without dementia. Cogn Behav Neurol 23:218–223. | SSI-ICM, DSI free report, DDT (as described in study #15). | n = 313 volunteers (71–96 yr); 3 groups: controls without memory loss, individuals with mild memory loss without dementia; memory-impaired individuals with dementia; otherwise, criteria are same as in study #15. | Among control group with normal cognitive function, observed abnormal central auditory results in 40–45%. Reported as controlled, but not assessed as a separate factor. | Derived an exec function score from neuropsych tests: trail making, clock drawing, Stroop color and word test; exec function score was associated with PTA after controlling for sex, age, and educ; executive function score was significantly associated with all 3 CAP speech tests; Executive function explained worse DSI, and 16% variance of worse DDT (lower for better ear). Trails B was most strongly associated with auditory outcomes | Confirm an association between CAPD in aging and cognition (CAP tests require short-term memory, task shifting, and attention to task); recommend that elderly patients with substantial CAPD be referred for neuropsych evaluation. | ||
Note: ANCOVA = analysis of covariance; CAP(D) = central auditory processing (disorder); CCT = California Consonant Test; CID W-22 = Central Institute for the Deaf word lists; CUNY NST = City University of New York Nonsense Syllable Test; MCR = message-to-competition ratio; MMPI = Minnesota Multiphasic Personality Inventory; MRT = Modified Rhyme Test; PB = phonetically balanced; PI = performance-intensity; PCA = principal-components analysis; PTA = pure-tone average; RT = reverberation time; SBR = signal-to-babble ratio; Simple RT = simple reaction time; SNR = signal-to-noise ratio; SPIN = Speech Perception in Noise test; SRT = speech recognition threshold; SSW = Staggered Spondaic Words; TC = time compression; VAF = variance accounted for; Visual RT = visual reaction time WAIS = Wechsler Adult Intelligence Scale (WAIS-R = Revised).
Speech-Based Tests
There were 19 different tests used for evaluating central auditory processing among older subjects in the 18 test battery studies (20 articles) reviewed. Although these tests are generally available in “standardized” versions (including specific speech stimuli, stimulus presentation levels, signal-to-noise ratios, presentation rates, etc.), they were not presented using standardized methods in many of the studies. Table 4 presents details of the speech tests presented, methods, categorization of results (when appropriate), findings, and key observations.
A general summary of the speech tests used and the findings are shown in Table 5. Only those speech tests used in two or more studies have been included in Table 5. This table indicates that the most common speech tests used to assess central auditory function were the SSI-ICM (13 studies), DSI (8 studies), time-compressed speech (8 studies), and Revised Speech Perception in Noise test [R-SPIN]/Quick Speech-in-Noise test [QuickSIN] tests (8 studies). The types of measures are also categorized broadly in Table 5, in a manner similar to that for the smaller-scale studies making use of speech stimuli (Table 3), to include monaural speech in competing speech, speech in steady-state noise, temporally distorted speech, dichotic speech, and a miscellaneous category of other monaural speech measures. Of these categories, speech in competing speech and dichotic speech appear to be the most common test conditions used in the past 25 yr.
Table 5.
Summary of Findings from Review of 18 Test Battery Studies (20 articles) Making Use of Speech-Based Measures of Central Auditory Processing
| Type of speech test | Test or measure | # studies using test |
Proportion of studies reporting significant age effects* |
Proportion of studies reporting significant effects of hearing loss* |
Proportion of studies reporting significant effects of cognition* |
Proportion of studies reporting significant age effects without hearing loss confound *** |
|---|---|---|---|---|---|---|
| Speech in competing speech | SSI-ICM (single-talker background) | 13 | 9/10 | 8/8 | 4/5 | 3/7 |
| SPIN and QuickSIN (multiple-talker background) | 8 | 6/7 | 5/7 | 0/4 | 3/6 | |
| Speech in steady-state noise | Various syllable, word, and sentence stimuli | 2 | 1/1 | 2/2 | 1/2 | 0/1 |
| Temporally distorted speech | Time-compressed speech | 8 | 4/7 | 7/7 | 4/4 | 3/7 |
| Dichotic speech | DSI | 8 | 1/4 | 3/4 | 5/5 | 0/1 |
| Dichotic Digits | 4 | 1/2 | 1/1 | 2/2 | 0/0 | |
| Dichotic Nonsense Syllables | 2 | 2/2 | 0/2 | 1/1 | 2/2 | |
| SSW | 4 | 2/4 | 3/3 | 0/0 | 0/3 | |
| Other | PI-PB/PI-SSI Rollover | 2 | 2/2 | 1/1 | 0/0 | 0/0 |
| PB-SSI difference | 4 | 3/3 | 1/2 | 1/2 | 0/0 | |
| Low-pass filtered speech | 5 | 3/5 | 5/5 | 0/1 | 0/4 |
Note: To be included in this summary table, a speech test or measure was required to be used in two or more of the 18 test battery studies. For definitions of abbreviations, see Table 4.
Numerator = # of studies in which author(s) reported significant effect of independent variable (age, hearing loss, or cognitive function); Denominator = # of studies examining this effect.
Numerator = # of studies unconfounded by inaudibility, according to the author(s), that found a significant effect of age; Denominator = # of such unconfounded studies examining this effect.
Numerator = # of studies unconfounded by inaudibility, according to the task force, that found a significant effect of age; Denominator = # of such unconfounded studies examining this effect.
The most prominent findings for each type of speech test were tabulated by the task force. The principal results concerned initial tabulations of reported significant effects of age, hearing loss, and cognition, regardless of a particular study’s control, or lack thereof, for other potentially confounding variables. In addition, as with the review of the smaller-scale studies, for each speech test reviewed, task force members identified those studies that appeared to be unconfounded by hearing loss and examined the effects of age for such studies. Statistical techniques to control for hearing loss or cognition when identifying age effects were implemented in some, but not all, investigations. Age effects were identified in many of the studies by comparing the performance of younger and older groups. Other studies exclusively tested an older subject sample to determine whether central auditory processing disorders were evident in the sample, typically employing analyses based on correlations of the speech-understanding measures with age, hearing loss, or cognition.
Unlike the smaller-scale studies reviewed previously, most larger-scale test battery studies (16 of 18) included some measure of cognitive function. In fact, 9 studies included at least one cognitive measure as a variable in the study, with the remaining 7 studies performing a cognitive screen using a gross cognitive assessment to exclude participants with dementia, such as the Mini Mental State Exam (MMSE; Folstein et al, 1975). The incorporation of cognitive screens or tests in most of these larger-scale test battery studies is another reason the task force placed greater weight on the results from these studies than from the smaller-scale studies.
Table 5 includes these summary data, although the entries in the table are somewhat subjective. For the most frequently used test, the SSI-ICM, only 7 of the 13 studies were considered to be unconfounded by hearing loss, and 3 of these reported significant effects of age on performance. For the DSI, the second most commonly used test in these 18 studies, only 1 of 8 studies using the DSI was considered to be unconfounded by hearing loss, and that study failed to observe a significant effect of age. For time-compressed speech, tied with the DSI as the second most frequently used speech-based test in these studies, 7 of 8 studies were considered to be unconfounded by hearing loss, and 3 of these demonstrated significant effects of age on performance. The remaining test tied as the second-most frequently used measure, R-SPIN/QuickSIN, included 6 studies unconfounded by hearing loss, half of which reported significant effects of age on performance. For every measure in Table 5, except dichotic nonsense syllables (2 studies), the proportion of studies reporting effects of hearing loss is very high (1 of 2 to 8 of 8). Likewise, for just about every measure in Table 5, the proportion of studies reporting significant effects of cognition on performance is very high (typically, 1 of 2 to 5 of 5), except for the R-SPIN/QuickSIN and low-pass filtered speech. In summary, regardless of the specific speech-based test employed in these large-scale test battery studies, although many reported significant effects of age that may be consistent with the presence of central presbycusis, most of these studies are confounded by hearing loss, cognitive function, or both. Further, one must keep in mind that many of the tests used in these studies, some showing significant age effects, are also found to have relatively poor reliability as typically administered (e.g., SSI-ICM, DSI).
Most of the test battery studies of speech-based tests did not examine the effects of gender on performance. In the two studies that did examine gender effects, however, it is notable that gender differences were observed for the SSI-ICM test and for the DSI. In both of the studies examining gender effects, males tended to show greater age effects than females (Dubno et al, 1997; Golding et al, 2006). Ear differences were also reported in one study using dichotic speech, in which significant age effects were observed for the left ear but not the right ear (Golding et al, 2006).
One variable that is known to influence performance on difficult speech tasks is the native language of the listener when the native language is not English (e.g., Mayo et al, 1997; von Hapsburg et al, 2004; Shi, 2010). The more recent test battery studies excluded participants whose native language was other than English, but many of the earlier studies did not exclude such individuals. The extent to which nonnative listeners’ performance on the speech measures influenced reported findings of age effects or central auditory processing disorders among these earlier investigations is unknown.
Nonspeech Tests
Table 6 summarizes the nonspeech measures included in four of the 18 test battery studies. Every study included at least one measure of temporal processing, and the most common test, employed in three of the four studies, involved the perception (either discrimination or identification) of the temporal order of pure tones differing in frequency. Three of the four tests made use of low-frequency or midfrequency stimuli, and these same three found no significant effects of hearing loss on performance. All four studies found significant effects of age with some control for the effects of hearing loss. Only two studies examined the effects of cognition, and one of these found a significant effect such that higher cognitive function yielded better performance on the test. Most of the measures used were demonstrated to have been reliable measures when used with older adults.
Table 6.
Summary of Findings from Review of 4 of 18 Test Battery Studies (20 articles) Making Use of Nonspeech Measures of Central Auditory Processing
| Test battery study # (from Table 4) |
Nonspeech measures included in study | Reported age effects? |
Reported hearing loss effects? |
Reported cognitive effects? |
Reported age effect with control for hearing loss? |
|---|---|---|---|---|---|
| 7 | Duration and frequency tone patterns | Yes | No | NA | Yes |
| 8 | Auditory filter width at 1000 Hz, broadband noise gap detection, interaural time difference (ITD) discrimination for clicks centered at 500 and 2000 Hz | Yes | Yes | NA | Yes |
| 11 | Temporal order for midfrequency pure tones, 1000 Hz pure-tone duration discrimination | Yes | No | Yes | Yes |
| 14 | Pitch Pattern Sequence (PPS) Test and Random Gap Detection Test (RGDT); RGDT data later excluded | Yes | No | No | Yes |
| Summary: # “Yes”/# of studies examining effect | 4/4 | 1/4 | 1/2 | 4/4 |
Summary of Findings
For the 18 studies (20 articles) that made use of test batteries and medium-to-large sample sizes, all 18 studies included speech-based measures of auditory processing; 4 of the 18 studies included nonspeech stimuli, with a primary focus on measures of temporal processing; and none of the studies were longitudinal in design. For the speech-based measures of auditory processing, the following findings emerged: (1) the most frequently investigated measures were monaural speech in a competing-speech background, dichotic speech, and monaural time-compressed speech; (2) the most frequently used tests were the SSI-ICM, time-compressed speech (various compression factors and materials), and the DSI test; (3) although many studies reported significant effects of age that may be consistent with the presence of central presbycusis, most of these studies are confounded by hearing loss, cognitive function, or both, regardless of the specific speech-based test employed. For the four studies of nonspeech auditory-processing measures, (1) measures of temporal processing were common to all with temporal-order discrimination or identification being the most common test; (2) cognitive confounds have been studied less frequently (2 of 4 studies), with mixed results; and (3) all four studies examined the effects of hearing loss on performance and, due to judicious selection of stimulus parameters in most of the studies, hearing loss was not considered to be a confounding factor.
CONCLUSIONS AND RECOMMENDATIONS
Based on the research reviewed by the task force and the findings presented in this report, the existence of central presbycusis in older adults, as historically and structurally defined by the task force, remains unsubstantiated. This is due primarily to the use of broadband speech-based behavioral measures of auditory processing that have been demonstrated to be influenced considerably by the presence of high-frequency hearing loss, age-related cognitive decline, or both. Moreover, many of the behavioral tests used in the studies reviewed by the task force were of questionable reliability, and very few of the studies were longitudinal or population-based in design. Thus, both the validity and reliability of the behavioral speech-based measures used in the study of central presbycusis are unclear. An additional issue is a lack of uniformity in the cognitive measures employed across studies. Tests used have varied from rough cognitive screening, such as using the MMSE to exclude participants with dementia, to the use of standard intelligence tests, to the use of laboratory tests of specific cognitive “fundamentals,” such as speed of processing, working memory, and components of executive function. The latter processes are known to show age effects (Miyake et al, 2000; Salthouse, 2010) and may play a role in speech understanding in competing stimuli by older adults.
In contrast, the view that emerges from this review of published research is depicted in the lower Venn diagram of Figure 1. Peripheral-auditory, central auditory, and cognitive factors are intertwined and difficult to disentangle using behavioral measures from older adults. The functional form of central presbycusis, as represented by the overlapping central auditory and cognitive function domains outlined by the dashed line in the lower Venn diagram of Figure 1, likely contributes to a very common problem reported by older adults: difficulty understanding speech in degraded listening conditions. Consistent with this intertwined representation of central auditory and cognitive processing, an emerging hypothesis considers that, for speech understanding in complex environments, central auditory processing may be dependent on components of executive function, which may, in turn, further blur the distinction between “auditory” and “cognitive” function (e.g., Rönnberg et al, 2011).
Recommendations for Research
Nonspeech (or appropriately band-limited speech) measures of temporal processing, especially measures of gap detection and temporal-order discrimination or identification demonstrated significant effects of age, with little or no influence of hearing loss or cognition on performance, although these studies also were not longitudinal or population-based. Nonetheless, these measures hold the most promise for assessing auditory processing in older adults, especially when the frequencies and amplitudes of the stimuli have been selected to minimize the impact of hearing loss on performance. Many of these tests, moreover, have been demonstrated to be reliable in older adults. Unfortunately, several issues require further investigation before recommending widespread use of these behavioral tests as measures of central presbycusis. First, tests making use of nonspeech stimuli have received much less investigation to date, especially in larger-scale studies of older adults. Second, if it is desirable that such measures of auditory processing relate to difficulties experienced by older adults in everyday speech communication, research establishing such a link is relatively sparse. Third, although for true age-related declines in auditory processing, it is desirable to avoid the potential confound of peripheral hearing loss by using low-frequency or midfrequency stimuli, such a strategy would likely miss the identification of deficits in the auditory portions of the central nervous system induced by the presence of a peripheral hearing loss (i.e., CEPP). Thus, those individuals with a peripheral hearing loss and a central auditory deficit (which may further limit access to the information in that frequency region by higher centers) may go undetected with tests exclusively comprised of low-frequency and midfrequency stimuli. Again, additional research on the development of frequency-specific high-frequency nonspeech tests is warranted. Perhaps, with further research on band-limited speech tests or tests using nonspeech stimuli, valid and reliable measures of auditory processing can be developed for use with older adults. This alone, however, would not be sufficient to establish the existence of central presbycusis. Rather, these tests must be used to gather data from large numbers of adults across the adult life span using both cross-sectional and longitudinal research designs. Such studies might also report results in sufficient detail to enable alternate analyses of results to be explored, perhaps including access to de-identified raw data, or, for studies making use of factor analysis, structural equation modeling, or multiple regression, at least publishing the correlation matrices that served as the input to these analyses.
In addition to further research, both cross-sectional and longitudinal, on behavioral tests using nonspeech or band-limited speech stimuli, investigations using nonbehavioral measures, such as electrophysiological or neuroimaging measures, are sorely needed to confirm the existence of central presbycusis as narrowly defined by the task force. Ideally, such studies would include behavioral, electrophysiological, and neuroimaging measures for nonspeech or band-limited speech stimuli in the same subjects to minimize potential confounds already established from decades of behavioral research. Given the intertwined nature of peripheral, central auditory, and cognitive factors to central presbycusis, significant strides in understanding the nature of central presbycusis will most likely be made by interdisciplinary research teams having expertise in audiology, auditory processing, electrophysiology, neuroimaging, and cognition, among others.
Recommendations for Clinical Practice
If an audiologist desires a behavioral assessment of central auditory function in older adults that is likely to be reliable and unconfounded by peripheral hearing loss, then a limited set of options is currently available. As noted previously, this includes several tests from the Test of Basic Auditory Capabilities (TBAC; Watson, 1987) and the Veterans Administration compact disc for auditory perceptual assessment (Noffsinger et al, 1994). Average data for some of these measures have been published for a group of 171 older adults (Humes, 2002), which may aid interpretation of performance. Even for these tests, however, it is unclear that poor performance on such measures provides conclusive evidence for the structural form of central presbycusis. For example, there is some evidence that performance on the reliable nonspeech measures from the TBAC may be influenced by cognitive function (Humes, 1996). To rule out cognitive decline as a contributing factor, audiologists should consider including brief, reliable assessments of cognitive function. These might include measures of speed of processing, working memory, or executive function.
With additional research, it may be possible to develop clinically efficient procedures that tap central auditory and cognitive processing capabilities during the same test. For example, Pichora-Fuller et al (1995) demonstrated that a simple clinical measure of speech recognition in noise can be adapted to measure both speech understanding and working memory. Briefly, the speech-recognition test, similar to those administered routinely in the audiology clinic during basic hearing evaluations, was paused periodically to allow the patient to recall the last N words presented, adding a working-memory component to the testing with only a slight increase in total test time required. With additional research, it may be possible to use similar strategies to develop valid, reliable, and clinically efficient measures that provide assessments of both central auditory and cognitive function in older adults. From the perspective of the functional form of central presbycusis, parsing central auditory from cognitive deficits may not be critical for the individual patient. Rather, the presence of declines in function beyond those attributed to elevated hearing thresholds (reduced audibility) may be sufficient to characterize central presbycusis and its negative impact on auditory perception and speech communication. From the published evidence reviewed in the task force report, various nonspeech measures of temporal processing would be most appropriate for assessment of general auditory perception; measures of perception of time-compressed speech or speech in competing speech backgrounds would be most appropriate for assessment of speech communication.
Concluding Comment
The charge of this task force was to review the evidence with regard to the existence of central presbycusis. As noted, the task force chose to define central presbycusis narrowly as age-related changes in the auditory portions of the central nervous system beyond the auditory periphery. As such, it was important to distinguish difficulties in auditory perception or speech communication attributable to peripheral or cognitive factors from those attributable to age-related changes in the auditory portions of the central nervous system. The task force found it difficult to find evidence for central presbycusis as an independent entity in the absence of hearing loss, cognitive deficits, or both. Nevertheless, the sensitivity of some measures of auditory processing to deficits in cognitive function might enable the early identification of cognitive decline with such measures, though much more research is needed to corroborate this potential use of auditory-processing tests (e.g., Gates et al, 2008, 2010, 2011). Such early identification is consistent with the functional form of “central presbycusis” including the decline of any processing beyond the auditory periphery in older adults that may negatively impact auditory perception and speech communication. Moreover, the task force’s review of the literature lends credibility to the likely existence of this more broadly defined form of central presbycusis. In addition, from an ecological standpoint, perhaps using reliable measures that incorporate broadband speech stimuli in speech competition is a desirable approach precisely because these measures are subject to peripheral, central auditory, and cognitive influences on performance.
Given the current inability to reliably and validly differentiate among the various hypothesized mechanisms underlying the speech-communication problems for a given patient, the intervention pursued will also be undifferentiated. Those individuals of a certain age, having a specified amount of hearing loss and, perhaps, a specified level of cognitive function, who perform “worse than expected” would likely receive the same intervention whether the factors underlying the poor performance were peripheral, central auditory, or cognitive in nature. Such interventions might include more intensive counseling, auditory training, or aural rehabilitation. The interventions would be designed to encourage maintenance of social interactions to counteract a potential slide into social isolation, further worsening cognitive declines that might exist. For those manifesting a peripheral hearing loss and using hearing aids, the intervention would most likely include ways to improve the speech-to-noise ratio beyond that experienced by other similar individuals, perhaps through the use of supplemental assistive technologies. Improving the speech-to-noise ratio is always warranted, regardless of the underlying cause of the individual’s speech-understanding difficulties. Further, those older adults with relatively good hearing and who are not wearing hearing aids, for whom the underlying cause of exaggerated speech-understanding difficulties is central auditory or cognitive in nature, most likely would also benefit from an improved speech-to-noise ratio, but it would need to be delivered via a device or technology other than a hearing aid.
Supplementary Material
Acknowledgments
This work was supported, in part, by Indiana University, both through development and maintenance of a shared secure Web site and through the personnel support of Ms. Dana Kinney as an assistant to the authors. The task force thanks Kathy Pichora-Fuller, Karen Helfer, and Dennis McFarland for their reviews of an earlier draft of this report.
Abbreviations
- ANSI
American National Standards Institute
- CEBA
central effects of biological aging
- CEPP
central effect of peripheral pathology
- CHABA
Committee on Hearing, Bioacoustics and Biomechanics
- DDT
Dichotic Digits Test
- DSI
Dichotic Sentence Identification
- ICM
Ipsilateral Competing Message
- MCI
mild cognitive impairment
- MMSE
Mini Mental Status Exam
- QuickSIN
Quick Speech-in-Noise test
- R-SPIN
Revised Speech Perception in Noise test
- SSI
Synthetic Sentence Identification
- TBAC
Test of Basic Auditory Capabilities
- WHO
World Health Organization
References
- Akeroyd M. Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol. 2008;47(Suppl. 2):S53–S71. doi: 10.1080/14992020802301142. [DOI] [PubMed] [Google Scholar]
- Alain C, McDonald KL, Ostroff JM, Schneider B. Aging: a switch from automatic to controlled processing of sounds? Psychol Aging. 2004;19:125–133. doi: 10.1037/0882-7974.19.1.125. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute. ANSI S3.5-1997. American National Standard Methods for the Calculation of the Speech Intelligibility Index. New York: American National Standards Institute; 1997. [Google Scholar]
- Bacon SP, Viemeister NF. Temporal modulation transfer functions in normal-hearing and hearing-impaired listeners. Audiology. 1985;24:117–134. doi: 10.3109/00206098509081545. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive function across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Lowe-Bell SS, Cullen JK, Jr, Thompson CL. Dichotic speech perception: an interpretation of right-ear advantage and temporal offset effects. J Acoust Soc Am. 1973;53:699–709. doi: 10.1121/1.1913381. [DOI] [PubMed] [Google Scholar]
- Bertoli S, Smurzynski J, Probst R. Temporal resolution in young and elderly subjects as measured by mismatch negativity and a psychoacoustic gap detection task. Clin Neurophysiol. 2002;113:396–406. doi: 10.1016/s1388-2457(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Bizley JK, King AJ. Visual influences on ferret auditory cortex. Hear Res. 2009;258:55–63. doi: 10.1016/j.heares.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocca E, Calearo C. Central hearing processes. In: Jerger J, editor. Modern Developments in Audiology. New York: Academic Press; 1963. pp. 337–370. [Google Scholar]
- Broadbent DE. The role of auditory localization in attention and memory span. J Exp Psychol. 1954;47:191–196. doi: 10.1037/h0054182. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Decision and Stress. Oxford, UK: Academic Press; 1971. [Google Scholar]
- Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res. 2009;258:16–27. doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Cacace AT, McFarland DJ. Central auditory processing disorder in school-aged children: a critical review. J Speech Lang Hear Res. 1998;41:355–373. doi: 10.1044/jslhr.4102.355. [DOI] [PubMed] [Google Scholar]
- Cacace AT, McFarland DJ. The importance of modality specificity in diagnosing central auditory processing disorder. Am J Audiol. 2005;14:112–123. doi: 10.1044/1059-0889(2005/012). [DOI] [PubMed] [Google Scholar]
- Canlon B, Illing RB, Walton J. Cell biology and physiology of the aging central auditory pathway. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. New York: Springer; 2010. pp. 39–74. [Google Scholar]
- Cappe C, Rouiller EM, Barone P. Multisensory anatomical pathways. Hear Res. 2009;258:28–36. doi: 10.1016/j.heares.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Cherry EC. Some experiments on the recognition of speech, with one and with two ears. J Acoust Soc Am. 1953;25:975–979. [Google Scholar]
- Christopherson LA, Humes LE. Some psychometric properties of the Test of Basic Auditory Capabilities (TBAC) J Speech Hear Res. 1992;35:929–935. doi: 10.1044/jshr.3504.929. [DOI] [PubMed] [Google Scholar]
- Cokely CG, Humes LE. Reliability of two measures of speech recognition in elderly people. J Speech Hear Res. 1992;35:654–660. doi: 10.1044/jshr.3503.654. [DOI] [PubMed] [Google Scholar]
- Committee on Hearing, Bioacoustics, and Biomechanics (CHABA) Speech understanding and aging. J Acoust Soc Am. 1988;83:859–895. [PubMed] [Google Scholar]
- Craik F. The role of cognition in age-related hearing loss. J Am Acad Audiol. 2007;18:539–547. doi: 10.3766/jaaa.18.7.2. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ. Epidemiology of age-related hearing impairment. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. New York: Springer; 2010. pp. 259–274. [Google Scholar]
- Dubno JR, Ahlstrom JB, Horwitz AR. Use of context by younger and older adults with normal hearing. J Acoust Soc Am. 2000;107:538–546. doi: 10.1121/1.428322. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD. Suggestions for optimizing reliability with the Synthetic Sentence Identification test. J Speech Hear Disord. 1983;48:98–103. doi: 10.1044/jshd.4801.98. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD. Factors affecting performance on psychoacoustic and speech-recognition tasks in the presence of hearing loss. In: Studebaker GA, Hochberg I, editors. Acoustical Factors Affecting Hearing-Aid Performance. Boston: Allyn and Bacon; 1993. pp. 235–253. [Google Scholar]
- Dubno JR, Dirks DD, Morgan DE. Effects of age and mild hearing loss on speech recognition. J Acoust Soc Am. 1984;76:87–96. doi: 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Word recognition in noise at higher-than-normal levels: decreases in scores and increases in masking. J Acoust Soc Am. 2005a;118:914–922. doi: 10.1121/1.1953107. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Recognition of filtered words in noise at higher-than-normal levels: decreases in scores with and without increases in masking. J Acoust Soc Am. 2005b;118:923–933. doi: 10.1121/1.1953127. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Spectral and threshold effects on recognition of speech at higher-than-normal levels. J Acoust Soc Am. 2006;120:310–320. doi: 10.1121/1.2206508. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Lee F-S, Matthews LJ, Mills JH. Age-related and gender-related changes in monaural speech recognition. J Speech Lang Hear Res. 1997;40:444–452. doi: 10.1044/jslhr.4002.444. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Hallowell B. Practice and list effects on the Synthetic Sentence Identification test in young and elderly listeners. J Speech Lang Hear Res. 2000;43:1160–1167. doi: 10.1044/jslhr.4305.1160. [DOI] [PubMed] [Google Scholar]
- Fletcher H. Speech and Hearing in Communication. New York: D. Van Nostrand & Co; 1953. [Google Scholar]
- Fletcher H, Galt RH. The perception of speech and its relation to telephony. J Acoust Soc Am. 1950;22:89–151. [Google Scholar]
- Fogerty D, Humes LE, Kewley-Port D. Auditory temporal-order processing of vowel sequences by young and elderly listeners. J Acoust Soc Am. 2010;127:2509–2520. doi: 10.1121/1.3316291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gatehouse S, Naylor G, Elberling C. Benefits from hearing aids in relation to the interaction between user and the environment. Int J Audiol. 2003;42(Suppl. 1):S77–S85. doi: 10.3109/14992020309074627. [DOI] [PubMed] [Google Scholar]
- Gatehouse S, Naylor G, Elberling C. Linear and nonlinear hearing aid fittings—patterns of benefit 1. Int J Audiol. 2006a;45:130–152. doi: 10.1080/14992020500429518. [DOI] [PubMed] [Google Scholar]
- Gatehouse S, Naylor G, Elberling C. Linear and nonlinear hearing aid fittings—patterns of candidature. Int J Audiol. 2006b;45:153–171. doi: 10.1080/14992020500429484. [DOI] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, Feeney MP, McCurry SM, Larson EB. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2008;134:771–777. doi: 10.1001/archotol.134.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2011;137:390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- Gates GA, Gibbons L, McCurry S, Crane P, Feeney MP, Larson E. Executive dysfunction and presbycusis in older persons with and without dementia. Cogn Behav Neurol. 2010;23:218–223. doi: 10.1097/WNN.0b013e3181d748d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George ELJ, Zekveld AA, Kramer SE, Goverts ST, Festen JM, Houtgast T. Auditory and nonauditory factors affecting speech reception in noise by older listeners. J Acoust Soc Am. 2007;121:2362–2375. doi: 10.1121/1.2642072. [DOI] [PubMed] [Google Scholar]
- Golding M, Taylor A, Cupples L, Mitchell P. Odds of demonstrating auditory processing abnormality in the average older adult: the Blue Mountains Hearing Study. Ear Hear. 2006;27:129–138. doi: 10.1097/01.aud.0000202328.19037.ff. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Selected cognitive factors and speech recognition performance among young and elderly listeners. J Speech Lang Hear Res. 1997;40:423–431. doi: 10.1044/jslhr.4002.423. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Sources of age-related recognition difficulty for time-compressed speech. J Speech Lang Hear Res. 2001;44:709–719. doi: 10.1044/1092-4388(2001/056). [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ, Friedman SA. Recognition of time-compressed and natural speech with selective temporal enhancements by young and elderly listeners. J Speech Lang Hear Res. 2007;50:1181–1193. doi: 10.1044/1092-4388(2007/082). [DOI] [PubMed] [Google Scholar]
- Hällgren M, Larsby B, Lyxell B, Arlinger S. Cognitive effects in dichotic speech testing in elderly persons. Ear Hear. 2001;22:120–129. doi: 10.1097/00003446-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Harris KC, Eckert MA, Ahlstrom JB, Dubno JR. Age-related differences in gap detection: effects of task difficulty and cognitive ability. Hear Res. 2010;264:21–29. doi: 10.1016/j.heares.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AR, Dubno JR, Ahlstrom JB. Recognition of low-pass-filtered consonants in noise with normal and impaired high-frequency hearing. J Acoust Soc Am. 2002;111:409–416. doi: 10.1121/1.1427357. [DOI] [PubMed] [Google Scholar]
- Humes LE. Spectral and temporal resolution by the hearing impaired. In: Studebaker GA, Bess FH, editors. The Vanderbilt Hearing Aid Report. Upper Darby, PA: Monographs in Contemporary Audiology; 1982. pp. 16–31. [Google Scholar]
- Humes LE. Speech understanding in the elderly. J Am Acad Audiol. 1996;7:161–167. [PubMed] [Google Scholar]
- Humes LE. Factors underlying the speech-recognition performance of elderly hearing-aid wearers. J Acoust Soc Am. 2002;112:1112–1132. doi: 10.1121/1.1499132. [DOI] [PubMed] [Google Scholar]
- Humes LE. Do ’auditory processing’ tests measure auditory processing in the elderly? Ear Hear. 2005;26:109–119. doi: 10.1097/00003446-200504000-00001. [DOI] [PubMed] [Google Scholar]
- Humes LE. The contributions of audibility and cognitive factors to the benefit provided by amplified speech to older adults. J Am Acad Audiol. 2007;18:590–603. doi: 10.3766/jaaa.18.7.6. [DOI] [PubMed] [Google Scholar]
- Humes LE. Issues in the assessment of auditory processing in older adults. In: Cacace AT, McFarland DJ, editors. Controversies in Central Auditory Processing Disorder. San Diego: Plural Publishing; 2009. pp. 121–150. [Google Scholar]
- Humes LE, Burk MH, Coughlin MP, Busey TA, Strauser LE. Auditory speech recognition and visual text recognition in younger and older adults: similarities and differences between modalities and the effects of presentation rate. J Speech Lang Hear Res. 2007;50:283–303. doi: 10.1044/1092-4388(2007/021). [DOI] [PubMed] [Google Scholar]
- Humes LE, Christopherson LA, Cokely CG. Central auditory processing disorders in the elderly: fact or fiction? In: Katz J, Stecker N, Henderson D, editors. Central Auditory Processing: A Trans-disciplinary View. Philadelphia: BC Decker; 1992. pp. 141–150. [Google Scholar]
- Humes LE, Coughlin M, Talley L. Evaluation of the use of a new compact disc for auditory perceptual assessment in the elderly. J Am Acad Audiol. 1996;7:419–427. [PubMed] [Google Scholar]
- Humes LE, Dubno JR. Factors affecting speech understanding in older adults. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. New York: Springer; 2010. pp. 211–258. [Google Scholar]
- Humes LE, Kewley-Port D, Fogerty D, Kinney D. Measures of hearing threshold and temporal processing across the adult lifespan. Hear Res. 2010;264:30–40. doi: 10.1016/j.heares.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Lee JH, Coughlin MP. Auditory measures of selective and divided attention in young and older adults using single-talker competition. J Acoust Soc Am. 2006;120:2926–2937. doi: 10.1121/1.2354070. [DOI] [PubMed] [Google Scholar]
- Humes LE, Roberts L. Speech-recognition difficulties of hearing-impaired elderly: the contributions of audibility. J Speech Hear Res. 1990;33:726–735. doi: 10.1044/jshr.3304.726. [DOI] [PubMed] [Google Scholar]
- Humes LE, Watson BU, Christensen LA, Cokely CA, Halling DA, Lee L. Factors associated with individual differences in clinical measures of speech recognition among the elderly. J Speech Hear Res. 1994;37:465–474. doi: 10.1044/jshr.3702.465. [DOI] [PubMed] [Google Scholar]
- International Standards Organization (ISO) Acoustics-Statistical distribution of hearing thresholds as a function of age, ISO-7029. Basel, Switzerland: ISO; 2000. [Google Scholar]
- Ison JR, Tremblay KL, Allen PD. Closing the gap between neurobiology and human presbycusis: behavioral and evoked potential studies of age-related hearing loss in animal models and humans. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. New York: Springer; 2010. pp. 75–110. [Google Scholar]
- Jerger J, Chmiel R, Allen J, Wilson A. Effects of age and gender on dichotic sentence identification. Ear Hear. 1994;15:274–286. doi: 10.1097/00003446-199408000-00002. [DOI] [PubMed] [Google Scholar]
- Jerger J, Jerger S, Oliver T, Pirozzolo F. Speech understanding in the elderly. Ear Hear. 1989;10:79–89. doi: 10.1097/00003446-198904000-00001. [DOI] [PubMed] [Google Scholar]
- Jerger J, Jerger S, Pirozzolo F. Correlational analysis of speech audiometric scores, hearing loss, age and cognitive abilities in the elderly. Ear Hear. 1991;12:103–109. doi: 10.1097/00003446-199104000-00004. [DOI] [PubMed] [Google Scholar]
- Kayser C, Petkov CK, Logothetis NK. Multisensory interactions in primate auditory cortex: fMRI and electrophysiology. Hear Res. 2009;258:80–88. doi: 10.1016/j.heares.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Kidd GR, Watson CS, Gygi B. Individual differences in auditory abilities. J Acoust Soc Am. 2007;122:418–435. doi: 10.1121/1.2743154. [DOI] [PubMed] [Google Scholar]
- Kimura D. Functional assymmetry of the brain in dichotic listening. Cortex. 1967;3:163–178. [Google Scholar]
- Lemus L, Hernandez A, Lina R, Zainos A, Romo R. Do sensory cortices process more than one sensory modality during perceptual judgments? Neuron. 2010;67:335–348. doi: 10.1016/j.neuron.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Lin FR, Metter J, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Mayo LH, Florentine M, Buus S. Age of second-language acquisition and perception of speech in noise. J Speech Lang Hear Res. 1997;40:686–693. doi: 10.1044/jslhr.4003.686. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Cacace AT. Modality specificity as a criterion for diagnosing central auditory processing disorders. Am J Audiol. 1995;4:36–48. doi: 10.1044/1059-0889(2005/012). [DOI] [PubMed] [Google Scholar]
- Meyer K, Kaplan JT, Essex R, Damasio H, Damasio A. Seeing touch with content-specific activity in primary somatosensory cortex. Cereb Cortex. 2011;21:2113–2121. doi: 10.1093/cercor/bhq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman MP, Emerson MJ, Witzki AH, Howerter A, Wagner TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Noffsinger D, Wilson RH, Musiek FE. Department of Veterans Affairs compact disc recording for auditory perceptual assessment: background and introduction. J Am Acad Audiol. 1994;5:231–235. [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M, Wingfield AJ. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31:12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK. Use of supportive context by younger and older adult listeners: balancing bottom-up and top-down information processing. Int J Audiol. 2008;47(Suppl. 2):S72–S82. doi: 10.1080/14992020802307404. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old listen to and remember speech in noise. J Acoust Soc Am. 1995;97:593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Singh G. Effects of age on auditory and cognitive processing: implications for hearing aid fitting and audiologic rehabilitation. Trends Amplif. 2006;10:29–59. doi: 10.1177/108471380601000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp R. Auditory handicap of hearing impairment and the limited benefit of hearing aids. J Acoust Soc Am. 1978;63:533–549. doi: 10.1121/1.381753. [DOI] [PubMed] [Google Scholar]
- Pollack I, Pickett JM. Stereophonic listening and speech intelligibility against voice babble. J Acoust Soc Am. 1958;30:131–133. [Google Scholar]
- Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J Neurol Neurosurg Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KC, Crandell CC, Griffiths SK. Reliability issues with the Synthetic Sentence Identification test. J Am Acad Audiol. 1998;9:227–233. [PubMed] [Google Scholar]
- Rabbitt PMA. Channel capacity, intelligibility and immediate memory. Q J Exp Psychol. 1968;20:241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol Suppl. 1990;476:167–176. doi: 10.3109/00016489109127274. [DOI] [PubMed] [Google Scholar]
- Rönnberg J, Rudner M, Lunner T. Cognitive hearing science: the legacy of Stuart Gatehouse. Trends Amplif. 2011 doi: 10.1177/1084713811409762. 10.117711084713811409762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. A Theory of Cognitive Aging. New York: Elsevier Science Publishing Company; 1985. [Google Scholar]
- Salthouse TA. Theoretical Perspectives on Cognitive Aging. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- Salthouse TA. Major Issues in Cognitive Aging. New York: Oxford University Press; 2010. [Google Scholar]
- Schacht J, Hawkins JE., Jr Sketches of otohistory. Part 9: presby(a)cusis. Audiol Neurootol. 2005;10:243–247. doi: 10.1159/000086524. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Longitudinal Studies of Adult Psychological Development. New York: Guilford Press; 1983. [Google Scholar]
- Schmiedt RA. The physiology of cochlear presbycusis. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. New York: Springer; 2010. pp. 9–38. [Google Scholar]
- Schneider BA, Daneman M, Murphy DR. Speech comprehension difficulties in older adults: cognitive slowing or age-related changes in hearing? Psychol Aging. 2005;20:261–271. doi: 10.1037/0882-7974.20.2.261. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual processing for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2. New York: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Schuknecht HF. Presbyacusis. In: Schuknecht HF, editor. Pathology of the Ear. Cambridge, MA: Harvard University Press; 1974. [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Shi L-F. Perception of acoustically degraded sentences in bilingual listeners who differ in age of English acquisition. J Speech Lang Hear Res. 2010;53:821–835. doi: 10.1044/1092-4388(2010/09-0081). [DOI] [PubMed] [Google Scholar]
- Skenes LL, Schear JM, Larson VD. Simulated hearing loss and phrase dictation. Int J Neurosci. 1989;47:287–293. doi: 10.3109/00207458908987441. [DOI] [PubMed] [Google Scholar]
- Sommers MS. Stimulus variability and spoken word recognition. II. The effects of age and hearing impairment. J Acoust Soc Am. 1997;101:2278–2288. doi: 10.1121/1.418208. [DOI] [PubMed] [Google Scholar]
- Souza PE, Boike KT, Witherell K, Tremblay K. Prediction of speech recognition from audibility in older listeners with hearing loss: effects of age, amplification, and background noise. J Am Acad Audiol. 2007;18:54–65. doi: 10.3766/jaaa.18.1.5. [DOI] [PubMed] [Google Scholar]
- Studebaker GA, Sherbecoe RL, McDaniel DM, Gwaltney CA. Monosyllabic word recognition at higher-than-normal speech and noise levels. J Acoust Soc Am. 1999;105:2431–2444. doi: 10.1121/1.426848. [DOI] [PubMed] [Google Scholar]
- Surprenant AM, Watson CS. Individual differences in the processing of speech and non-speech sounds by normal-hearing listeners. J Acoust Soc Am. 2001;110:2086–2095. doi: 10.1121/1.1404973. [DOI] [PubMed] [Google Scholar]
- Surprenant AM. Effects of noise on identification and serial recall of nonsense syllables in older and younger adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:126–143. doi: 10.1080/13825580701217710. [DOI] [PubMed] [Google Scholar]
- Tun PA, O’Kane G, Wingfield A. Distraction by competing speech in younger and older listeners. Psychol Aging. 2002;17:453–467. doi: 10.1037//0882-7974.17.3.453. [DOI] [PubMed] [Google Scholar]
- von Hapsburg D, Champlin CA, Shetty SR. Reception thresholds for sentences in bilingual (Spanish/English) and monolingual (English) listeners. J Am Acad Audiol. 2004;15:88–98. doi: 10.3766/jaaa.15.1.9. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of CBA mouse. J Neurosci. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-related alterations in the neural coding of envelope periodicities. J Neurophysiol. 2002;88:565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Watson CS. Uncertainty, informational masking, and the capacity of immediate auditory memory. In: Yost WA, Watson CS, editors. Auditory processing of complex sounds. Hillsdale, NJ: Lawrence Erlbaum; 1987. pp. 267–277. [Google Scholar]
- Weinstein B, Amsel L. Hearing loss and senile dementia in the institutionalized elderly. Clin Gerontol. 1986;4:3–15. [Google Scholar]
- Willott JF. Aging and the Auditory System: Anatomy, Physiology, and Psychophysics. San Diego, CA: Singular; 1991. [Google Scholar]
- Willott JF. Anatomic and physiologic aging: a behavioral neuroscience perspective. J Am Acad Audiol. 1996;7:141–151. [PubMed] [Google Scholar]
- Wingfield A, Aberdeen JS, Stine EAL. Word onset gating and linguistic context in spoken word recognition by young and elderly adults. J Gerontol. 1991;46:P127–P129. doi: 10.1093/geronj/46.3.p127. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Poon LW, Lombardi L, Lowe D. Speed of processing in normal aging: effects of speech rate, linguistic structure, and processing time. J Gerontol. 1985;40:579–585. doi: 10.1093/geronj/40.5.579. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Tun PA, Koh CK, Rosen MJ. Regaining lost time: adult aging and the effect of time restoration on recall of time-compressed speech. Psychol Aging. 1999;14:380–389. doi: 10.1037//0882-7974.14.3.380. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood: what it is and how it interacts with cognitive performance. Curr Dir Psychol Sci. 2005;14:144–148. [Google Scholar]
- Wingfield A, Tun PA, Rosen MJ. Age differences in veridical and reconstructive recall of syntactically and randomly segmented speech. J Gerontol B Psychol Sci Soc Sci. 1995;50:P257–P266. doi: 10.1093/geronb/50b.5.p257. [DOI] [PubMed] [Google Scholar]
- Zhan W, Cruickshanks KJ, Klein BEK, et al. Generational differences in the prevalence of hearing impairment in adults. Am J Epidemiol. 2010;171:260–266. doi: 10.1093/aje/kwp370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.