Abstract
Introduction
Iodine is essential for normal brain development. Moderate and severe fetal iodine deficiency results in substantial to serious developmental delay in children. Mild iodine deficiency in pregnancy is associated with neurodevelopmental deficits in the offspring, but evidence from randomised trials is lacking. The aim of the Swedish Iodine in Pregnancy and Development in Children study is to determine the effect of daily supplementation with 150 µg iodine during pregnancy on the offspring’s neuropsychological development up to 14 years of age.
Methods and analysis
Thyroid healthy pregnant women (n=1275: age range 18–40 years) at ≤12 weeks gestation will be randomly assigned to receive multivitamin supplements containing 150 µg iodine or non-iodine-containing multivitamin daily throughout pregnancy. As a primary outcome, IQ will be measured in the offspring at 7 years (Wechsler Intelligence Scale for Children-V). As secondary outcomes, IQ will be measured at 3.5 and 14 years, psychomotor development at 18 months and 7 years, and behaviour at 3.5, 7 and 14 years. Iodine status (urinary iodine concentration) will be measured during pregnancy and in the offspring at 3.5, 7 and 14 years. Thyroid function (thyroid hormones, thyroglobulin), and deiodinase type 2 polymorphisms will be measured during pregnancy and in the offspring at 7 and 14 years. Structural MRI or other relevant structural or functional brain imaging procedures will be performed in a subgroup of children at 7 and 14 years. Background and socioeconomic information will be collected at all follow-up times.
Ethics and dissemination
This study is approved by the Ethics Committee in Göteborg, Sweden (Diary numbers: 431-12 approved 18 June 2012 (pregnancy part) and 1089-16 approved 8 February 2017 (children follow-up)). According to Swedish regulations, dietary supplements are governed by the National Food Agency and not by the Medical Product Agency. Therefore, there is no requirement for a monitoring committee and the National Food Agency does not perform any audits of trial conduct. The trial will be conducted in accordance with the Declaration of Helsinki. The participating sites will be contacted regarding important protocol changes, both orally and in writing, and the trial registry database will be updated accordingly. Study results will be presented at relevant conferences, and submitted to peer-reviewed journals with open access in the fields of endocrinology, paediatrics and nutrition. After the appropriate embargo period, the results will be communicated to participants, healthcare professionals at the maternal healthcare centres, the public and other relevant groups, such as the national guideline group for thyroid and pregnancy and the National Food Agency.
Trial registration number
Keywords: iodine, pregnancy, child development, cognition, thyroid hormones
Strengths and limitations of this study.
Large interventional controlled trial on iodine supplementation during pregnancy, powered to detect a difference of three IQ points in children.
Long observational follow-up of the children, up to 14 years, with complex assessment of neurocognitive development.
Future implementation of the study is feasible, as the intervention tablet exists on the market.
Lack of pure iodine and pure placebo tablets implies careful interpretation of results.
Dropout rate may be high.
Background
Iodine deficiency as an international issue
Iodine is essential for the production of thyroid hormones and important for growth and brain development during fetal and early postnatal life1; a knowledge obtained after a long history of iodine deficiency (ID) associated disorders. For centuries, goitre with hypothyroidism, mental retardation and cretinism have been an entity. During the 1920s in the USA, Marine and Kimball performed the classic experiment of treating schoolgirls with iodine, leading to a dramatic reduction in the prevalence of goitre. Iodine prophylaxis was established in the USA in 1921. After some debate, iodine prophylaxis was introduced in Switzerland in 1922, and then worldwide over the subsequent decades. The combat against severe and moderate ID has been successful in reducing the number of children with ID-caused mental retardation. However, mild ID is widely apparent, especially during pregnancy,2 when dietary iodine demand increases from 150 to 250 µg/day.3
Iodine status in Sweden as the country for this study
Before iodination of table salt in 1936, ID was common in Sweden.4 Current iodine intake is sufficient in the general population5 6 and was considered adequate during pregnancy during the 1990s7 8; there is no recommendation on iodine supplementation during pregnancy. However, since the 1990s, the situation may have changed due to dairy product consumption in adults being lower; milk iodine levels are lower than before9 10; a reduction in salt intake is recommended for reducing the risk of hypertension; new salt forms (flake salt, gourmet salt) without iodine are popular; there is a reluctance to consume ‘food additives’; awareness of ID among the younger population is generally low; and the main proportion of total salt intake (≈80%), that is, from ready-made foods and dishes, does not provide iodine. Unless iodine is added to all salts used, the risk of decreased iodine intake is apparent, and arouses concerns, especially for pregnant women. Retrospective, local data on pregnancy highlight this assumption is realistic.11
ID during pregnancy: effects on the child’s development
Severe and moderate ID leads to lower serum thyroid hormone levels and thereby to lower availability of thyroid hormones in the brain. During fetal life and early years, the growing brain is vulnerable12 13 and severe ID results in mental retardation in the newborn, unless the thyroid hormone is replaced.14 In addition, an increased incidence of attention deficit hyperactivity disorders (ADHD) has been associated with mild to moderate ID.15
In mild ID, thyroid hormone levels are maintained, whereas thyroglobulin (TG) levels are increased as a biomarker of goitre. The brain’s use of thyroid hormones depends on the local conversion of inactive hormone thyroxine (T4) to active hormone triiodothyronine (T3), a process mediated by deiodinase type 2 (D2).16 D2 is found in the hippocampi and the cerebral cortex and its activity is increased by ID to maintain sufficient T3 levels.16 17 In the presence of normal thyroid hormone in blood, it is unclear how mild ID affects brain development. One theory is that this depends on deiodinases, which can change thyroid hormone signalling locally in different tissues, without affecting serum hormone concentration.16 18
Mild ID during pregnancy might have an impact on brain development, despite maintained normal thyroid hormone levels.19–22 In the UK, a longitudinal study19 found 8-year-old children have an increased risk of being in the lowest quartile of verbal IQ, if their mothers had mild ID in early pregnancy, than children of mothers with normal iodine nutrition. In a similar association study from Australia,20 mild ID was linked with lower cognitive performance in 9-year-old children. Results from an observational pilot study from Italy21 indicate mild to moderate ID during fetal life affects cognitive development, especially verbal abilities, even in absence of maternal thyroid insufficiency. In Norway, a large observational study22 found maternal iodine intake below the estimated average requirement during pregnancy was associated with reduced fine motor skills and verbal abilities and with more behaviour problems at the age of 3 years.
As the randomised controlled trial23 evaluating 150 µg iodine/placebo in pregnant women in an iodine sufficient country was small (n=86) and lacked cognitive assessment in children, there were many expectations about the MITCH study.24 In this trial, 832 women from Thailand and India were randomised to 200 µg iodine/placebo, and there was no difference in cognitive outcome in 5–6 year-old children. However, these results were ambiguous for several reasons. First, the women had entered Maternal Iodine Supplementation and Effects on Thyroid Function and Child Development (MITCH) study with urinary iodine concentration (UIC) as in mild ID, but they did have a normal TG, which indicated the iodine stores in prepregnancy may have been sufficiently filled, thus minimising any mental effects on the children. Second, some women were already iodine sufficient at baseline.25 Third, both intervention and placebo groups were iodine sufficient in the second and third trimesters. To prevent subnormal fetal brain development, many international authorities recommend 150 µg extra iodine/day during pregnancy, despite the lack of studies proving causality.26 27
Knowledge gaps and background to the Swedish Iodine in Pregnancy and Development in Children study
There is a substantial gap in knowledge about mild ID during pregnancy and its potential negative consequences on neuropsychological development. Therefore, there is a need for a placebo controlled trial that compares neuropsychological outcome in children exposed to mild ID during fetal life and children with normal iodine nutrition during pregnancy.
From 29 November 2012 to 1 June 2015, a pilot randomised placebo controlled trial involving 200 pregnant women receiving a daily supplementation with either a multivitamin containing 150 µg iodine/day or a multivitamin without iodine (placebo) was conducted by our group. This study (ClinicalTrials.gov identifier: NCT02378246) aimed to evaluate the effects of iodine supplementation on UIC and thyroid function. As the MITCH study had ambiguous results, the question if mild ID during pregnancy affects fetal brain development remains unanswered, and it was evident to us that our trial needed to be expanded to include a sufficient number of pregnant women, to enable a satisfactorily powered child follow-up regarding neuropsychological development.
There are indications28 that UIC level during pregnancy in Sweden is lower than detected in the MITCH study, and an elevated TG is detected in early pregnancy, implying a lower iodine status at start of study. Moreover, iodine status in the third trimester is clearly lower in a local Swedish study11 than in the placebo group in the MITCH study, indicating a different iodine situation in Sweden than in Thailand and India. Therefore, the Swedish Iodine in Pregnancy and Development in Children (SWIDDICH) study is conducted. The hypothesis is that the use of an iodine-containing multivitamin during pregnancy results in better cognitive development in the child than with a multivitamin without minerals (superiority trial) and this effect is stronger on verbal competence, which is in agreement with previous findings.19 21 22
Objectives
The primary aim is to assess whether cognition (especially verbal competence) in children whose mothers received 150 µg iodine daily in a multivitamin during pregnancy is higher than children whose mothers received placebo (a multivitamin without iodine) and probably remained in mild ID. The purpose is to determine whether all pregnant women who live in a country where the general population is iodine sufficient, but live in conditions that can result in mild ID during pregnancy, should be recommended extra iodine during pregnancy.
Methods
Design of the SWIDDICH study
This is a randomised placebo controlled study in which children are followed up as an observational cohort, separated into two groups by fetal iodine exposure.
Setting and participants
Pregnant women will be recruited from more than 10 maternal healthcare centres in Sweden with the aim of forming several clusters to facilitate child follow-up. The main study site will be in Gothenburg, with secondary sites in Umeå and Linköping, and other areas where maternal healthcare centres are recruited. At the first scheduled pregnancy visit, information about the study will be provided and written informed consent collected by the midwife. All procedures during pregnancy will be combined with routine pregnancy visits.
All informed consents and blood and urine for future analyses will be sent to the main study site in Gothenburg. To promote participant retention and a complete follow-up, a contact from the study coordinator will be taken after childbirth. In addition, information will be shared with participants on the homepage https://www.gu.se/swiddich.
Inclusion
The following inclusion criteria will apply: women aged 18–40 years, pregnant at 7–12 weeks, willing to refrain from iodine supplementation and take a multivitamin supplement instead, without current thyroid disease, not in another pregnancy or lactating less than 6 months before inclusion, and non-vegan.
Randomisation, allocation, concealment and blinding
Randomisation numbers with an allocation ratio 1:1 are prepared centrally and sent to each participating centre. Consecutive numbers are used and the information regarding the study group allocation of each number stored securely at the premises of the University of Gothenburg, Sweden. Mothers are provided with a random container of pills by either drawing a lot or blindly drawing a container. All containers are identical, with tasteless pills of the same size for both groups. Recruiting staff, study participants and those involved in laboratory work and developmental assessment are blinded to the group allocation. The code will only be broken by the central study team for data analyses before publications, but will still be blinded to all groups working with the follow-up. The code has been broken for the 200 women of the pilot study, but all (ie, study participants, psychologists and lab engineers) except the central study team are still blinded. No other interim analyses are planned.
Intervention
Women in the experimental group receive a daily multivitamin supplement containing 150 µg iodine and those in the control group receive a daily multivitamin supplement containing no iodine (the contents of the two supplements are presented in table 1). The intervention lasts throughout pregnancy until the day of delivery. Women in both groups are recommended, as are all pregnant women in Sweden, to take extra folic acid 400 µg/day during the first trimester,29 and even extra iron, when the haemoglobin status indicates it. Therefore, women in the placebo group will be on maximum 600 µg daily folic acid supplementation, which is safely below the tolerable upper level of 1000 µg/day.30 The folic acid and iron administrations do not interfere with the study tablet. However, the women are not permitted to take any other multivitamins besides the study supplement.
Table 1.
Multivitamin with iodine (intervention) and multivitamin without iodine (‘placebo’): table of contents
| Intervention (iodine 150 μg) Commercial name: MITT VAL VEGETARIAN |
Placebo (no iodine) Commercial name: ENOMDAN |
| B2 1.4 mg (87%)* B12 15 μg (750%) Iron 12 mg (30%) Zinc 12 mg (133%) Iodine 150 μg (85%) Selenium 50 μg (71%) Calcium 250 mg (28%) |
Vitamin A 400 μg (50%) Vitamin B1 1.4 mg (93%) Vitamin B2 1.7 mg (106%) Vitamin B6 1.8 mg (128%) Vitamin B12 3 μg (150%) Vitamin C 60 mg (70%) Vitamin D 5 μg (50%) Vitamin E 10 mg (100%) Niacin 19 mg (111%) Folic acid 200 μg (50%) |
*Numbers in parentheses denote % of Recommended Daily Intake (%RDI) during pregnancy.29
Compliance: discontinuation
Participants are asked to bring the container with the remaining pills to the visit in the third trimester. The container is weighted and the percentage of intended doses used is calculated. Mothers who no longer want to participate in the study during pregnancy will be regarded as dropouts and no further data will be collected. If there is discontinuation in the children follow-up, children can come to the next visit. If the discontinuation is permanent, a registry search will still be done.
Outcomes
Outcomes in mothers
Outcomes in mothers will be assessed in the first, second and third trimesters of pregnancy. UIC and thyroid hormones will be measured in all three trimesters, and thyroperoxidase (TPO) antibodies and TG in the first and third trimesters.
Primary outcome in children
Cognition measured by IQ (total IQ) with focus on the verbal compound (verbal IQ) at 7 years is the primary outcome (Wechsler Intelligence Scale for Children, WISC-V31).
Secondary outcomes in children
Cognition measured by IQ at 3.5 years (Wechsler Preschool and Primary Scale of Intelligence-IV32) and at 14 years (WISC-V31 or an equivalent adequate version at the time) are secondary outcomes, together with outcomes related to psychomotor development, behaviour and ADHD. Psychomotor assessment will be done by the parents at 18 months (The Ages and Stages Questionnaire-3)33 and by a physiotherapist at 7 years (Movement Assessment Battery for Children test).34 Behaviour will be assessed through parental questionnaires, the Child Behavior Checklist (CBCL); first at 3.5 years (CBCL 1–5),35 then at 7 and 14 years (CBCL 6–18).36 At 7 and 14 years, the Nordic questionnaire 5–1537 will be used to assess ADHD-related symptoms.
Parents also give their consent to a registry search at 3.5, 7 and 14 years regarding the inpatient and outpatient registries for collecting information on medical diagnoses, the drug registry, the medical birth registry, quality registries, maternal, child and school healthcare for medical and growth data, and educational registries.
In a subgroup of children (n=200), structural brain changes will be evaluated by MRI of the brain (with a 3T Philips MR scanner) at 7 and 14 years. Automatic segmentation of the whole brain will be with Freesurfer38 and Maper, multiatlas propagation with enhanced registration.39 Mediotemporal lobe (MTL) structures will be analysed through manual segmentation with custom software developed in previous projects.40 41 Subregional analyses directed at regions of neurogenesis will be included. Intracranial volume measured manually will enable reliable normalisation of MTL volumes. Other structural and/or functional brain imaging methods may supplement, or even replace, the described protocol, depending on the state of knowledge at the time of study.
Possible confounding variables and background information
In children, UIC will be measured from the 3.5-year visit and forward and dry blood spots will be collected for thyroid hormones, TG and deiodinases at 7 and 14 years. Background and confounding variables will be assessed at 18 months, and 3.5, 7 and 14 years.
Time frame for the study actions
Recruitment to the SWIDDICH study began in March 2017 and is planned to be completed in 2019. Currently, 75 of 1075 pregnant women have been included. Several strategies are used to reach target sample size: a study coordinator is employed to contact maternal healthcare centres, and a stepwise reimbursement model is applied to the maternal healthcare centres in case of high recruitment rates; the National Food Agency promotes study participation in their communication with maternal healthcare centres; and local paediatricians are involved to facilitate the children follow-up. The follow-up of children was also offered to the families participating in the pilot study (2012–2015), before the study extension was decided. The time points for all study actions are presented in table 2.
Table 2.
Summary of SWIDDICH study actions
| Time point | Pregnancy | Child follow-up | ||||||
| First pregnancy visit (<12 weeks) | Weeks 7–12 | Weeks 25–28 | Weeks 34–38 | 18 months | 3.5 years | 7 years | 14 years | |
| Enrolment | ||||||||
| Information given | X | |||||||
| Eligibility screen | X | |||||||
| Informed consent | X | |||||||
| Allocation | X | |||||||
| Intervention | ||||||||
| Iodine 150 µg or placebo in multivitamins |

|
|||||||
| Assessments | First pregnancy visit (<12 weeks) | Weeks 7–12 | Weeks 25–28 | Weeks 34–38 | 18 months | 3.5 years | 7 years | 14 years |
| Urinary iodine concentration | X | X | X | X | X | X | ||
| Thyroid function* | X | X | X | X | X | |||
| Milk iodine concentration | ||||||||
| Cognition IQ |
X WPPSI |
X WISC |
X WISC |
|||||
| Behaviour | X CBCL |
X CBCL Nordic 5–15 |
X CBCL Nordic 5–15 |
|||||
| Psychomotor development | X ASQ-3 |
X Mov ABC |
||||||
| Brain MRI (subgroup) | X | X | ||||||
| Background information | ||||||||
| EUthyroid SES questionnaire adults | X | X | X | X | ||||
| EUthyroid SES questionnaire children | X | |||||||
| Own questionnaire | X | X | X | X | X | X | X | |
*FT4 TSH, thyroglobulin: serum sampling during pregnancy and dry blood spot sampling during children follow-up.
ASQ, The Ages and Stages Questionnaire; CBCL, Child Behavior Checklist; EUthyroid SES questionnaire, Socioeconomic Status questionnaire, validated by EUthyroid foundation; Mov ABC, Movement Assessment Battery for Children; SWIDDICH, Swedish Iodine in Pregnancy and Development in Children; WISC, Wechsler Intelligence Scale for Children; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
Patient and public involvement statement
Pregnant women were not involved in the planning of the study.
Considerations
Considerations on the content of the intervention and the ‘Placebo’ tablets
The reason for choosing iodine-containing multivitamins instead of pure iodine tablets as the intervention is to ensure future implementation of the study is feasible. There are currently no pure iodine tablets available on the market. In the planning state of the study, discussions were initiated with pharmaceutical companies to provide pure iodine tablets and placebo, but interest was low. In the future, iodine in multivitamins will be the only available supplement source in most countries. Therefore, a multivitamin containing 150 µg iodine was chosen for the intervention and a multivitamin without minerals as the comparator.
Other components in the multivitamin products, besides iodine, may intervene with outcomes. It is proposed that vitamin B12 42 43 and iron44 can have positive effects on the brain, and iron and selenium influence thyroid hormone levels.45 46 Iron is found in TPO enzyme that couples iodine to TG. Selenium is found in deiodinases, such as D2, which converts T4 to T3, and is also an antioxidant of the thyroid gland. Sweden is a selenium-deficient country,47 but it is unclear whether selenium deficiency affects cognitive outcome in humans.48 B12 is higher in iodine-containing multivitamins where iron and selenium also are included. However, B12 content in both placebo and intervention tablets is, at least, equal to the recommended daily intake for B12; thus B12 deficiency is not anticipated in any of the groups. In addition, the iron content is low and many pregnant Swedish women take a separate 100 mg iron supplement, which makes the 12 mg iron in the intervention tablet negligible. Iron, B12 and selenium will be measured in a subpopulation to evaluate possible group differences and contributions to thyroid metabolism.
Considerations in choosing a realistic starting point for intervention
Fetal brain development during the first 12 weeks is dependent on maternal T4 levels. By initiating the intervention at pregnancy weeks 7–12, a substantial part of the first trimester is missed. Ideally, iodine supplements may be initiated before conception. Practically, the recruitment of women who plan a pregnancy is difficult, as these women are not known by healthcare providers before pregnancy. One way would be through advertising in the newspaper to recruit women who are planning pregnancy. However, this would be ineffective and create selection bias, as only 50% of those who fall pregnant have planned the pregnancy, and not every woman responds to an advertisement. Women are included at the earliest possible stage and this is still far earlier than in a recent publication by Casey et al 45 that included pregnant women in mean gestational weeks 16.6–18.0 and found negative results. The inclusion in the proposed study is similar to that in the MITCH study, where women were included in gestational weeks 10 and 11.24
Power calculation, data management, statistical considerations and authorship
The sample size needed, excluding dropouts, is calculated to 788 children (394 in each group) for an effect size of 3 IQ points with SD 15 and power 0.80. Currently, there are no similar randomised studies for power calculation. The smallest significant effect of 3 IQ points is in accordance with an observational study,19 where children of mothers with UIC<150 µg/L during pregnancy had a 3-point lower IQ at school age than children of mothers with normal UIC during pregnancy. This expected effect from iodine supplementation in mild ID is also suggested by Troendle,49 where statistical considerations are discussed for the possibility that the needed placebo controlled study is conducted. Assuming a dropout frequency of 22% during pregnancy (which is in accordance with preliminary data from the pilot study with 200 pregnant women28) and 20% during the children follow-up, 1263 pregnant women need to be recruited to the study. This sample size is in general agreement with Troendle,49 thus the decision was made to try and recruit 1275 pregnant women. The dropout frequency for the children follow-up could be lower than estimated, as there are two occasions for dropout and mothers who remain in the study after the first follow-up can be assumed willing to continue the study. The power calculation assumes the use of an unpaired t-test between groups; however, more advanced analyses could decrease variance, thus requiring a lower sample size.
The sample size will be reassessed by calculating the dropout frequency when 750 women are included and when half of the children from the first 200 included women have been invited to the 3.5-year neuropsychological evaluation. Sample size reassessment will be conducted without unblinding the study groups.
A 100% compliance to the study medication is assumed, as the results will be based on an intention-to-treat (ITT) analysis. Compliance is monitored to enable a per-protocol analysis (only the compliant participants included) to be added. However, the ITT approach reflects the real-life clinical situation, in which a certain number of patients are not compliant with the recommended treatment, and this will be the foundation for future recommendations on iodine supplementation to all pregnant women.
A separate power calculation for the MRI investigation has been done. This assumes the described protocol will be followed, and a previous study of 11-year-old children has been used for guidance.50 To detect a 5% difference with power 0.80, each group requires 60 children. As the variation in the hippocampal volumes in 7-year-old children could be slightly larger than in the previous study,50 and as dropout from the MRI at 14 years needs to be considered, 100 children will be included in each group.
Coded collected data will be entered into a database, with appropriate backup from the university servers. Key lists will be kept safe and transfer of data to the databases will be validated by random cross-checks with the original data set. UIC analyses will be duplicated to promote validity. For further details, see ethical applications (Diary numbers: 431-12 approved 18 June 2012 (pregnancy part) and 1089-16 approved 8 February 2017 (children follow-up)) and https://clinicaltrials.gov/. All authors will have access to all data and the statisticians will have access to the data needed.
The choice of methods for comparing the main outcome between the experimental and control groups will be guided by the data distributions. In case of deviation from normality assumptions, transformations of data may be done. Non-parametric tests will be used for non-normal and ordinal data. Possible confounders, such as socioeconomic factors, other background information, gestational age, thyroid hormones, TG, deiodinase polymorphisms and UIC, will be considered in the data analyses. Repeated measurements in a mixed model (where groups are compared repeatedly at 3.5, 7 and 14 years) and within-group analyses are planned. The models will also consider dropout frequency and recruitment from different maternal healthcare centres, which will be used as a factor in the analysis. For all dropouts, relevant background variables will be studied. Adjustments for bias may be performed. For non-informative dropouts, methods for multiple imputations will be considered. A multivariate analysis with total grey, total brain volume, intracranial volume, MTL volumes and possibly other measures of brain structure and function as independent variables will be conducted. The data analyses will be undertaken by an experienced statistician. Authorship will be decided according to the Declaration of Vancouver.
MRI considerations: where are changes from ID located?
T3 receptors are distributed among all brain areas with high levels in the hippocampi and the cerebellar cortex. Rodent data indicate T3 receptors are involved in the regulation of hippocampal structure and function.51 In the human cerebral cortex, thyroid receptors are already present in week 9 and concentrations increase up to 18 weeks of gestation.52 Considerable amounts of D2 are also found in the cerebral cortex.53 In the first half of pregnancy, the fetus is dependent on the mother’s supply of thyroid hormones. In mild ID, the mother maintains serum thyroid hormone levels through unknown compensatory mechanisms. In the second half of a mild ID pregnancy, when the fetus partly relies on its own thyroid hormone production, the fetus will be hypothyroid, as it has not developed compensatory mechanisms and there is a lack of sufficient iodine levels transferred by the mother.53
The description of neuropathology caused by ID is limited to few observations from adult cretins, ranging from severe cortical atrophy to almost normal appearance. In areas with endemic goitre, fetuses aborted in the second half of the pregnancy have a less differentiated cerebral cortex.53 In rats, transient periods of thyroid hormone insufficiency during periods of cortical development affect cortical and hippocampal cytoarchitecture.53
Human data from maternal hypothyroidism support an effect on the brain, specifically on the hippocampus.54 These data are in line with the recent publication by Korevaar et al,55 who conclude the relationship of IQ with FT4 (in peripheral blood) exhibits a U-shaped configuration with lower IQ levels in both ends of the normal range. FT4 in this study55 is also associated with total grey matter volume.
Considerations on the neuropsychological evaluation
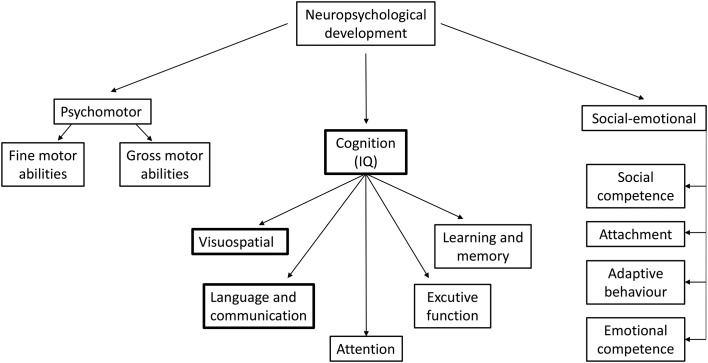
Neuropsychological development can be divided into three domains: psychomotor, cognitive (IQ) and socioemotional development (figure 1). There are five landmark studies in the iodine field evaluating neuropsychological development in the offspring that use neuropsychological tests: the Avon Longitudinal Study of Parents and Children (UK),19 Iodine Supplementation During Pregnancy and Infant Neuropsychological Development (INMA, Spain),56 Generation R (Netherlands),55 MITCH (India and Thailand)57 and Hynes et al (Australia).20 Verbal cognitive function appears to be the most susceptible subdomain for ID. In SWIDDICH, verbal cognitive function and total IQ were chosen (as the latter is the best understood and requested) as primary outcome measurements. As cognitive testing is less valid at younger ages, verbal IQ at 7 years was chosen as the primary evaluation time point, and all three domains of neuropsychological development will be evaluated at several follow-up times.
Figure 1.
Neuropsychological domains. Verbal cognitive function, as a subdomain of cognition, appears the most susceptible subdomain for iodine deficiency and is the primary outcome of the Swedish Iodine in Pregnancy and Development in Children (SWIDDICH) study at the age of 7 years.
Implications for society and the individual
Impaired child development increases economic burdens for society. Lowered IQ is associated with worse economic outcomes and lower lifetime earnings. Small decrements in IQ around the mean are linked to lower incomes.58 59 IQ may be the easiest factor to quantify, but may not be the factor with the most serious consequence for a ‘good life’. Environmental factors, including ID, that place the nervous system at risk may affect executive functions, such as planning and initiating ideas, and result in attention problems, impulsive behaviour and inability to handle stress and disappointment, and can impede success in school and in life and possibly lead to antisocial behaviour.60
If the average IQ of a population drops, the IQ distribution shifts and the number of individuals with low IQ (eg, below 75 or 85, classified as intellectually disabled) increases. In turn, this will also decrease the number of gifted and exceptionally gifted people with high IQ (eg, above 130), who may have major positive impacts on the immediate future for a company or a country. A cost-benefit analysis of iodine supplementation in mild to moderate ID has recently proved positive.61
Based on the dollar value in 1987 in the USA, the cost in terms of reduced income for a 1 point IQ reduction has been calculated to nearly US$20.7 billion per year.62 A 3-point decline in IQ also impacts social costs in the USA60 and increases the risk of: poverty by 20% during the first 3 years; low birth weight by 12%; being a recipient of welfare by 18%; and high school dropout by 28%. Even though a decline of a few IQ points may be small for the individual, the societal effects are considerable. As a small general risk reduction entails a large social benefit, iodine supplementation could be a cost-effective action if the main hypothesis of this study holds true.
Considerations on possible adverse effects of iodine or placebo
Iodine supplementation may increase the frequency of postpartum thyroiditis (PPT), as iodine affects autoimmunity63: 10%–15% of women already have PPT and this number may increase slightly with iodine supplementation. As PPT is not a dangerous condition and most cases resolve spontaneously, we consider the reduced risk for subnormal brain development in a child motivates accepting the risk for PPT. In Denmark, PPT was evaluated in a placebo controlled trial in mild to moderate ID, and treatment did not increase or worsen PPT.63
Excess iodine intake in the mother may block thyroid function in the fetus, leading to hypothyroidism and goitre, and is associated with poorer mental and psychomotor development or behaviour problems in children.22 56 64 However, the risk for adverse effects of iodine supplementation is higher in cases of preconception ID due to sudden increase of iodine intake, and should therefore not be the case in Sweden where the normal population is iodine sufficient.65
The placebo group is at risk of ID during pregnancy. However, as there are no current recommendations for iodine supplementation during pregnancy in Sweden, this group follows normal management.
The intervention and the comparator are dietary supplements, and the total intake of nutrients depends on the diet. Information on adverse reactions is not collected.
Conclusion
The aim of this paper is to describe the study protocol for the SWIDDICH research project and the considerations that led to its design. The study attempts to further understand the consequences of mild ID during pregnancy and to test whether treatment of the mothers with iodine-containing multivitamins improves outcome in the children. As the study is the largest of its kind, it offers the potential for influencing future recommendations on iodine supplementation with multivitamins to pregnant women living in conditions of mild ID.
Supplementary Material
Acknowledgments
The authors thank Elisabeth Gramatkovski,
Michael Hoppe and Therese Karlsson for their invaluable help as coordinators of the study.
Footnotes
Contributors: SM, BJ, AC, JE, KG, CJT, RE, HM, LH, MD and HNF contributed to the design of the SWIDDICH study. HNF wrote the first version of the manuscript and SM was responsible for pushing the work forward together with the other coworkers. All coauthors critically reviewed and approved the final version of the manuscript. SM is the guarantor. The primary sponsor is HNF (principal investigator), Sahlgrenska Academy and University of Göteborg and Sahlgrenska University Hospital, Göteborg, Sweden. The main study site is in Göteborg with additional sites in Umeå and Linköping.
Funding: This work was supported by the ALF agreement (grant numbers ALFGBG 58777, ALFGBG 717311); Regional FOU (grant number VGFOUREG 664301); Lilla barnets fond (grant number 20160917); Svenska Läkarsällskapet (grant number SLS 688891); Lars Hiertas Minne Foundation (grant number FO2016 0016); Formas grant (grant number 2017 0095); and a grant from the General Maternity Hospital Foundation 2017. Multivitamins for the first 200 women were provided by Recip Medical, Solna, Sweden, but they are not involved in the study design and they do not contribute in any other way. The National Food Agency is a stakeholder in this trial. The authors of this manuscript solely contributed to the design, management, future analyses with the support of unbound statisticians, interpretation of data, writing the manuscript and decisions on where to submit. The maternal healthcare centres are reimbursed for the collection of patients by the principal investigator (HNF).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Bioethics Committee of Gothenburg.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Zimmermann MB, Jooste PL, Pandav CS, et al. Iodine-deficiency disorders. Lancet 2008;372:1251–62. 10.1016/S0140-6736(08)61005-3 [DOI] [PubMed] [Google Scholar]
- 2. Zimmermann MB, Gizak M, Abbott K, et al. Iodine deficiency in pregnant women in Europe. Lancet Diabetes Endocrinol 2015;3:672–4. 10.1016/S2213-8587(15)00263-6 [DOI] [PubMed] [Google Scholar]
- 3. WHO. United Nations Children’s Fund & International Council for the Control of Iodine Deficiency Disorders, Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers. 3rd edn Geneva, 2007. [Google Scholar]
- 4. Nyström HF, Jansson S, Berg G. Incidence rate and clinical features of hyperthyroidism in a long-term iodine sufficient area of Sweden (Gothenburg) 2003-2005. Clin Endocrinol 2013;78:768–76. 10.1111/cen.12060 [DOI] [PubMed] [Google Scholar]
- 5. Andersson M, Berg G, Eggertsen R, et al. Adequate iodine nutrition in Sweden: a cross-sectional national study of urinary iodine concentration in school-age children. Eur J Clin Nutr 2009;63:828–34. 10.1038/ejcn.2008.46 [DOI] [PubMed] [Google Scholar]
- 6. Filipsson Nyström H, Andersson M, Berg G, et al. Thyroid volume in Swedish school children: a national, stratified, population-based survey. Eur J Clin Nutr 2010;64:1289–95. 10.1038/ejcn.2010.162 [DOI] [PubMed] [Google Scholar]
- 7. Elnagar B, Eltom A, Wide L, et al. Iodine status, thyroid function and pregnancy: study of Swedish and Sudanese women. Eur J Clin Nutr 1998;52:351–5. 10.1038/sj.ejcn.1600563 [DOI] [PubMed] [Google Scholar]
- 8. Eltom A, Elnagar B, Elbagir M, et al. Thyroglobulin in serum as an indicator of iodine status during pregnancy. Scand J Clin Lab Invest 2000;60:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Lindmark Månsson H. the Swedish Milk composition Svensk Mjölk (Swedish milk, 2010. [Google Scholar]
- 10. Lindmark Månsson H. Den svenska mejerimjölkens sammansättning 2009, Svensk Mjök (Swedish Milk), 2012. [Google Scholar]
- 11. Granfors M, Andersson M, Stinca S, et al. Iodine deficiency in a study population of pregnant women in Sweden. Acta Obstet Gynecol Scand 2015;94:1168–74. 10.1111/aogs.12713 [DOI] [PubMed] [Google Scholar]
- 12. Zimmermann MB. The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: a review. Thyroid 2007;17:829–35. 10.1089/thy.2007.0108 [DOI] [PubMed] [Google Scholar]
- 13. Taylor PN, Okosieme OE, Dayan CM, et al. Therapy of endocrine disease: Impact of iodine supplementation in mild-to-moderate iodine deficiency: systematic review and meta-analysis. Eur J Endocrinol 2014;170:R1–R15. 10.1530/EJE-13-0651 [DOI] [PubMed] [Google Scholar]
- 14. Stanbury JB, Ermans AM, Hetzel BS, et al. Endemic goitre and cretinism: public health significance and prevention. WHO Chron 1974;28:220–8. [PubMed] [Google Scholar]
- 15. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab 2004;89:6054–60. 10.1210/jc.2004-0571 [DOI] [PubMed] [Google Scholar]
- 16. Peeters R, Fekete C, Goncalves C, et al. Regional physiological adaptation of the central nervous system deiodinases to iodine deficiency. Am J Physiol Endocrinol Metab 2001;281:E54–E61. 10.1152/ajpendo.2001.281.1.E54 [DOI] [PubMed] [Google Scholar]
- 17. Markova N, Chernopiatko A, Schroeter CA, et al. Hippocampal gene expression of deiodinases 2 and 3 and effects of 3,5-diiodo-L-thyronine T2 in mouse depression paradigms. Biomed Res Int 2013;2013:1–14. 10.1155/2013/565218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Courtin F, Zrouri H, Lamirand A, et al. Thyroid hormone deiodinases in the central and peripheral nervous system. Thyroid 2005;15:931–42. 10.1089/thy.2005.15.931 [DOI] [PubMed] [Google Scholar]
- 19. Bath SC, Steer CD, Golding J, et al. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013;382:331–7. 10.1016/S0140-6736(13)60436-5 [DOI] [PubMed] [Google Scholar]
- 20. Hynes KL, Otahal P, Hay I, et al. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013;98:1954–62. 10.1210/jc.2012-4249 [DOI] [PubMed] [Google Scholar]
- 21. Moleti M, Trimarchi F, Tortorella G, et al. Effects of maternal iodine nutrition and thyroid status on cognitive development in offspring: a pilot study. Thyroid 2016;26:296–305. 10.1089/thy.2015.0336 [DOI] [PubMed] [Google Scholar]
- 22. Abel MH, Caspersen IH, Meltzer HM, et al. Suboptimal maternal iodine intake is associated with impaired child neurodevelopment at 3 years of age in the norwegian mother and child cohort study. J Nutr 2017;147:1314–24. 10.3945/jn.117.250456 [DOI] [PubMed] [Google Scholar]
- 23. Brucker-Davis F, Panaïa-Ferrari P, Gal J, et al. Iodine Supplementation throughout Pregnancy Does Not Prevent the Drop in FT4 in the second and third trimesters in women with normal initial thyroid function. Eur Thyroid J 2013;2:187–94. 10.1159/000350882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gowachirapant S, Jaiswal N, Melse-Boonstra A, et al. Effect of iodine supplementation in pregnant women on child neurodevelopment: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:853–63. 10.1016/S2213-8587(17)30332-7 [DOI] [PubMed] [Google Scholar]
- 25. Bath SC. Iodine supplementation in pregnancy in mildly deficient regions. Lancet Diabetes Endocrinol 2017;5:840–1. 10.1016/S2213-8587(17)30331-5 [DOI] [PubMed] [Google Scholar]
- 26. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017;27:315–89. 10.1089/thy.2016.0457 [DOI] [PubMed] [Google Scholar]
- 27. De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2543–65. 10.1210/jc.2011-2803 [DOI] [PubMed] [Google Scholar]
- 28. Pacini F, Nygaard B. Abstracts. European thyroid journal 2016;5:57–176.27099840 [Google Scholar]
- 29. Nordic Council of Ministers, Nordic Nutrition Recommendations 2012: integrating nutrition and physical activity, Nordic Council of Minsters. Copenhagen, 2014. [Google Scholar]
- 30. European Food Safety Authority. Folic Acid: an update on scientific developments. 2009. https://www.livsmedelsverket.se/globalassets/matvanor-halsa-miljo/kostrad-matvanor/gravida/folic-acid-an-update-on-scientific-developments.-rapport.-efsa-european-food-safety-authority.-2009.pdf?amp;epslanguage=sv.
- 31. Wechsler D. Wechsler Intelligence Scale for Children –5th edn (WISC V), Swedish version. Stockholm, Sweden, 2016. [Google Scholar]
- 32. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence – 4th edn (WPPSI IV) (Swedish version). Stockholm, Sweden, 2014. [Google Scholar]
- 33. Squires J TE, Bricker D, Potter L. ASQ-3 User’s Guide. 3rd edn Baltimore MD, US, 2009. [Google Scholar]
- 34. Henderson SE, Sugden DA, Barnett AL. Movement assessment battery for children [examiner’s manual. 2nd edn London, UK, 2007. [Google Scholar]
- 35. Achenbach TM RL. Manual for the ASEBA Preschool Forms & Profiles, University of Vermont, Research Center for Children, Youth, and Families, Burlington, Vermont, US, 2000. [Google Scholar]
- 36. Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles, University of Vermont, Research Center for Children, Youth and Families, Burlington, Vermont, US, 2001. [Google Scholar]
- 37. Kadesjö B, Janols LO, Korkman M, et al. The FTF (Five to Fifteen): the development of a parent questionnaire for the assessment of ADHD and comorbid conditions. Eur Child Adolesc Psychiatry 2004;13:iii3–iii13. 10.1007/s00787-004-3002-2 [DOI] [PubMed] [Google Scholar]
- 38. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- 39. Heckemann RA, Keihaninejad S, Aljabar P, et al. Improving intersubject image registration using tissue-class information benefits robustness and accuracy of multi-atlas based anatomical segmentation. Neuroimage 2010;51:221–7. 10.1016/j.neuroimage.2010.01.072 [DOI] [PubMed] [Google Scholar]
- 40. Eckerström C, Olsson E, Borga M, et al. Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: the Göteborg MCI study. J Neurol Sci 2008;272(1-2):48–59. 10.1016/j.jns.2008.04.024 [DOI] [PubMed] [Google Scholar]
- 41. Olsson E, Eckerström C, Berg G, et al. Hippocampal volumes in patients exposed to low-dose radiation to the basal brain. A case-control study in long-term survivors from cancer in the head and neck region. Radiat Oncol 2012;7:202 10.1186/1748-717X-7-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhate V, Deshpande S, Bhat D, et al. Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food Nutr Bull 2008;29:249–54. 10.1177/156482650802900401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull 2008;29(2 Suppl):S126–S131. 10.1177/15648265080292S117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev 2014;72:267–84. 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- 45. Ventura M, Melo M, Carrilho F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int J Endocrinol 2017;2017:1–9. 10.1155/2017/1297658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hu S, Rayman MP. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017;27:597–610. 10.1089/thy.2016.0635 [DOI] [PubMed] [Google Scholar]
- 47. Selinus O. Medical geology: Iodine - a classical element (jod ett klassiskt element), Medical geololgy (Medicinsk Geologi), Authors and Studentlitteratur, 2010:387–400. [Google Scholar]
- 48. Skröder HM, Hamadani JD, Tofail F, et al. Selenium status in pregnancy influences children’s cognitive function at 1.5 years of age. Clin Nutr 2015;34:923–30. 10.1016/j.clnu.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 49. Troendle JF. Statistical design considerations applicable to clinical trials of iodine supplementation in pregnant women who may be mildly iodine deficient. Am J Clin Nutr 2016;104:924S–7. 10.3945/ajcn.115.110403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bunketorp Käll L, Malmgren H, Olsson E, et al. Effects of a Curricular Physical Activity Intervention on Children’s School Performance, Wellness, and Brain Development. J Sch Health 2015;85:704–13. 10.1111/josh.12303 [DOI] [PubMed] [Google Scholar]
- 51. Guadaño-Ferraz A, Benavides-Piccione R, Venero C, et al. Lack of thyroid hormone receptor alpha1 is associated with selective alterations in behavior and hippocampal circuits. Mol Psychiatry 2003;8:30–8. 10.1038/sj.mp.4001196 [DOI] [PubMed] [Google Scholar]
- 52. Ferreiro B, Bernal J, Goodyer CG, et al. Estimation of nuclear thyroid hormone receptor saturation in human fetal brain and lung during early gestation. J Clin Endocrinol Metab 1988;67:853–6. 10.1210/jcem-67-4-853 [DOI] [PubMed] [Google Scholar]
- 53. de Escobar GM, Obregón MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr 2007;10:1554–70. 10.1017/S1368980007360928 [DOI] [PubMed] [Google Scholar]
- 54. Willoughby KA, McAndrews MP, Rovet JF. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid 2014;24:576–84. 10.1089/thy.2013.0215 [DOI] [PubMed] [Google Scholar]
- 55. Korevaar TI, Muetzel R, Medici M, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol 2016;4:35–43. 10.1016/S2213-8587(15)00327-7 [DOI] [PubMed] [Google Scholar]
- 56. Rebagliato M, Murcia M, Alvarez-Pedrerol M, et al. Iodine supplementation during pregnancy and infant neuropsychological development. INMA Mother and Child Cohort Study. Am J Epidemiol 2013;177:944–53. 10.1093/aje/kws333 [DOI] [PubMed] [Google Scholar]
- 57. Melse-Boonstra A, Gowachirapant S, Jaiswal N, et al. Iodine supplementation in pregnancy and its effect on child cognition. J Trace Elem Med Biol 2012;26(2-3):134–6. 10.1016/j.jtemb.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 58. Schwartz J. Societal benefits of reducing lead exposure. Environ Res 1994;66:105–24. 10.1006/enrs.1994.1048 [DOI] [PubMed] [Google Scholar]
- 59. Salkever DS. Updated estimates of earnings benefits from reduced exposure of children to environmental lead. Environ Res 1995;70:1–6. 10.1006/enrs.1995.1038 [DOI] [PubMed] [Google Scholar]
- 60. Muir T, Zegarac M. Societal costs of exposure to toxic substances: economic and health costs of four case studies that are candidates for environmental causation. Environ Health Perspect 2001;109:885–903. 10.1289/ehp.01109s6885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Monahan M, Boelaert K, Jolly K, et al. Costs and benefits of iodine supplementation for pregnant women in a mildly to moderately iodine-deficient population: a modelling analysis. Lancet Diabetes Endocrinol 2015;3:715–22. 10.1016/S2213-8587(15)00212-0 [DOI] [PubMed] [Google Scholar]
- 62. Schwartz J. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res 1994;65:42–55. 10.1006/enrs.1994.1020 [DOI] [PubMed] [Google Scholar]
- 63. Nøhr SB, Jørgensen A, Pedersen KM, et al. Postpartum thyroid dysfunction in pregnant thyroid peroxidase antibody-positive women living in an area with mild to moderate iodine deficiency: is iodine supplementation safe? J Clin Endocrinol Metab 2000;85:3191–8. 10.1210/jcem.85.9.6799 [DOI] [PubMed] [Google Scholar]
- 64. Murcia M, Rebagliato M, Iñiguez C, et al. Effect of iodine supplementation during pregnancy on infant neurodevelopment at 1 year of age. Am J Epidemiol 2011;173:804–12. 10.1093/aje/kwq424 [DOI] [PubMed] [Google Scholar]
- 65. Moleti M, Di Bella B, Giorgianni G, et al. Maternal thyroid function in different conditions of iodine nutrition in pregnant women exposed to mild-moderate iodine deficiency: an observational study. Clin Endocrinol 2011;74:762–8. 10.1111/j.1365-2265.2011.04007.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.