Summary
Clinically important rates of glaucoma progression (worsening) are ones that put a patient at risk of future functional impairment or reduction of vision-related quality of life. Rates of progression can be evaluated through measuring structural or functional changes of the optic nerve. Most treated eyes do not progress at rates that will lead to future visual impairment, but there are a significant proportion (3-17%) of eyes, that are at risk of impairment even under clinical care. While very fast rates of progression (e.g. MD progression of -1.5 dB/year) are generally problematic, much slower rates also may be deleterious for young patients, particularly those diagnosed with late disease. As a result, it is important to consider life expectancy, disease severity and vision-related quality of life based treatment targets to estimate future prognosis when evaluating whether a rate of glaucoma progression can be clinically relevant.
Keywords: Glaucoma, quality of life, progression rates, standard automated perimetry, spectral domain optical coherence tomography, confocal scanning laser ophthalmoscopy, life expectancy
Glaucoma is a chronic progressive disease and a leading cause of blindness, affecting more than 70 million people worldwide [1]. The main goal of glaucoma treatment is to not only prevent blindness but to prevent progression of the disease from reaching a stage that adversely affects the patient’s vision-related quality of life (VRQoL). Progression (worsening) in glaucoma patients is typically measured using a combination of structural and functional methods. Methods of glaucoma progression detection can further be divided between event and trend analysis approaches. Event analyses compare baseline measurements to future follow-up measurements to define whether that measurement is significantly worse, while trend analyses use serial measurements to determine rate of progression. Although event analysis methods are useful in determining whether patients are getting worse, they do not provide information about the velocity of vision loss or whether there is significant risk that these rates of loss will affect their vision-related quality of life. For this reason, this review focuses on rates of glaucomatous progression measured using trend analysis. We define a clinically significant rate of progression as a rate that can lead to permanent visual disability or loss of VRQoL. In evaluating whether a rate of loss is clinically important, three factors require consideration: 1) the current stage (or severity) of disease; 2) the life expectancy of the patient; and 3) the severity/stage of disease at which VRQoL would be affected.
There are several structural and functional methods that can be used to measure rates of glaucoma progression. Despite being patient-dependent and subject to considerable test-retest variability [2-5], visual field (VF) testing using standard automated perimetry remains a gold standard for measuring glaucoma progression. However, increasingly sophisticated imaging technologies, such spectral domain and swept-source optical coherence tomography (OCT), are being utilized to qualitatively and quantitatively assess the rate of structural change [6-14].
Rates of visual field loss
Measuring rates of VF progression provides a direct measure of optic nerve function and how it is changing over time. Trend analyses using VFs often involve linear regression of summary statistics such as Mean Deviation (MD) and, more recently, the Visual Field Index (VFI). Despite the fact that rates of VF worsening are not necessarily constant over time in reality (e.g. treatment intensity and adherence could change for instance), rates of VF loss are typically expressed as linear rates of change of dB/year in practice. Interestingly, past studies investigating rates of linear loss using these summary statistics in patients with glaucoma tend to show apparently slow progression rates. For instance, the of the Early Manifest Glaucoma Trial found that the median rate of MD loss in untreated eyes of 118 glaucoma patients was -0.4 dB/year [15]. However, median rates of loss of glaucoma patients in treated clinical practice vary widely among studies, ranging from -0.05dB/year to -0.62dB/year [16-19]. Others have reported average rates of VFI loss in glaucoma patients to be between -1.1 and -1.5%/year [20-22]. Placing this in perspective, rates of VF sensitivity decline due to aging have been estimated to be -0.06dB/year [23]. Moreover, rates for the most pessimistic study suggest it would take 20 years for the “average” patient with a mean constant VF progression rate of -0.6dB/year to progress from 0dB to -12dB, which is commonly defined as severe VF loss [24], and a further 17 years to reach -22dB, a standard for statutory blindness[25], so it is unlikely for the average patient to suffer glaucoma-related impairment during their lifetime, particularly as glaucoma tends to affect older individuals [26-29].
However, there is large variability in rates of VF loss among patients. Distributions of rates of loss in various studies suggest that though most do not progress very quickly, a sizeable proportion of eyes do, as reflected by the fact that the mean rates of VF loss in studies are systematically more pessimistic than medians [15-17]. The reported proportion of glaucomatous eyes progressing at faster than -1.5 dB/year have varied from 3 to 17% in some previous studies [16, 17, 19, 20, 30-35], while others found that 15-20% of eyes seem to progress at rates of VFI loss greater than 5% per year [21, 36]. Such rates of loss would lead to perimetric blindness in 20 years assuming rates of loss remain constant. These numbers echo those of a number of older studies estimating the number of patients going blind from glaucoma that range between 6 to 13% [37-39]. Hence, glaucoma management, then, should be directed at differentiating patients who are in danger of progressing to stages of visual impairment and/or blindness from those that are not. One diagnostic paradigm that has been proposed to achieve this is frequent early testing [40].
However, this raises an important question: how should “fast progression” be defined? Some definitions of fast progression have been fairly arbitrary with round numbers such as a VF MD rate of -1.5 dB/year [30, 33]or -2 dB/year [18, 40], commonly chosen. Without doubt, these rates would almost always have a deleterious impact on a patient’s visual health. However, an irreversible impact on a patient’s VRQoL could occur even at a much slower rate than this depending on their life expectancy and severity of disease.
Rates of structural loss
Neuroretinal rim area measured using confocal scanning laser ophthalmoscopy and retinal nerve fiber layer (RNFL) measurements measured with Optical Coherence Tomography (OCT) have tended to be the most commonly used metrics to measure structural change. As with VF-based measurements, progression is often expressed linearly in the form of change per year, despite the fact that the relationship between clinically utilised functional and structural measurements is not linear [41], so they cannot both progress at a constant over time simultaneously. Although, the true relationship between structure and function may well actually be linear, the fact that commonly-used measurements are not (due to the fact VF sensitivity measurements are log-transformed) is still an important consideration when thinking about structure-function correspondence [41]. In glaucoma patients, the rates of global neuroretinal rim area loss measured using confocal scanning laser ophthalmoscopy (CSLO) have been found to range between -0.018 and -0.0072 mm2/year [7, 11, 12, 22, 42-44]while average rates of global SD-OCT based RNFL thinning have been estimated to range between -0.76 and 1.5 μm/year [8-11, 45-47]. Faster rates of rim area using CSLO and RNFL loss have been reported in ocular hypertensive or suspect eyes developing glaucoma (rim area loss: -0.01 mm2/yr, RNFL loss: -2.02 μm/year) compared to those that did not (-rim area loss: 0.003 mm2/yr, RNFL loss: -0.93 μm/year) [10, 12, 48]. Typically rates of rim area loss have been found to be between 3.7 and 4.6 times faster in eyes progressing compared to those not [12, 44, 46]. Moreover, faster rates of CSLO measured rim area loss and OCT-based RNFL thinning are associated with an increased risk of developing VF damage [10, 48]. One relatively new OCT measure suggested for monitoring structural change is the Bruch’s membrane opening minimum rim width. Some early studies have suggested that this may be a more sensitive method of detecting structural change independent of aging [11] with greater correlation to RNFL thickness and MD than rim area measurements measured using CSLO [49]. Few studies have been conducted using this approach, but average rates of change have been recorded to be -2.18 μm/year in glaucoma patients [11]. However, there is some evidence that minimum rim width may not be as sensitive to monitoring progression as change in RNFL thickness [50]. Estimating the loss in retinal ganglion cells has also been suggested, with studies suggesting losses of 10,000-11,000 ganglion cells per year on average in glaucoma patients and suspects [13, 51]. In glaucoma suspects, these rates are noticeably faster in eyes showing VF progression during follow-up (-18,987 cells per year) than those that not (-8,808 cells per year) [51].
However, there are challenges in evaluating whether rates of structural progression are clinically significant for a particular patient. Most importantly, it is difficult to differentiate between deterioration as a result of aging and that caused by glaucoma as there is little longitudinal information on how the structure of the retina changes in normal subjects over time. Without this context, the clinical implications of any measured structural change are unclear. Estimates of significant mean RNFL rates of loss in healthy eyes of between -0.33 μm/year (cross-sectional analysis) and -0.52 μm/year (longitudinal analysis) have been reported [8]. However, RNFL thickness in healthy eyes can vary widely; RNFL thicknesses have been measured between 114.6 to 182.2 μm in the inferior hemifield of the optic disc and 83.4 to 166.1 μm in the superior hemifield [41]. To put these numbers into context, mean rates of superior and inferior loss in healthy eyes were reported as -1.35 and -1.25 μm/year respectively [8].
Another difficulty in evaluating the significance of rates in structural measurements is that patients tend to have vision well beyond the dynamic range of many structural measurements; this measurement “floor effect” for structural measurements typically occurs earlier than for corresponding VF measurements [41, 52] (although the usefulness of VF thresholds beyond the range of structural measurements has been disputed[53]). The importance of the dynamic range lies in the fact that it represents how well a measurement reflects the severity of the disease of the patient through the full range of disease. “Floor effects” lead to measurements becoming uninformative at a stage of disease while patients still have measurable vision remaining worth evaluating. As a result, poor dynamic range resulting from “floor effects” mean that rates of vision loss cannot be accurately measured in late disease.
It is further difficult to reconcile measures of structural loss to what a patient can see as research in this area is scarce. Notably, it has been found that RNFL loss is associated with longitudinal reduction in quality of life, even after adjustment for progressive visual field loss [54]. RNFL loss is also related to real world metrics of functional impairment such as reaction time in driving [55]. However, it is not yet possible to come up with some universal standard for what rates of progression are clinically important and more research of how structural changes relate to changes in VRQoL is needed.
What stage of vision loss affects vision-related quality of life?
Whether progression is “fast” or not needs to be considered in the context of a patient’s VRQoL, yet research of this has been sparse on the matter of considering what levels of VF or structural loss are associated with a substantial reduction in VRQoL [56]. However, the effect of glaucoma on quality of life is difficult to measure quantitatively, especially as patients use two eyes; loss of vision in one eye will inevitably not affect quality of life as much as when both eyes are affected. Further, patterns of VF loss in each eye tend to vary from patient to patient suggesting that patients with pairs of eyes with similar MDs may have very different experiences of vision loss.
One obvious milestone to prevent glaucoma patients from reaching is statutory blindness as defined by the US Social Security Administration (US SSA). The US SSA generally defines statutory blindness in terms of acuity; however, if the widest diameter of the visual field is found to subtend at an angle less than 20 degrees then this can also qualify as a measure for statutory blindness[25]. An MD of -22 dB in the better eye is said to correspond to this definition of blindness and has been used in other studies [19, 57], so this is a significant landmark. However, other visual activities are likely affected well before this stage of disease, with evidence that quality-of-life differs between patients with the disease and without the disease even at early stages of visual field loss [58]. Furthermore, more severe disease has also been linked with increased reduction in both general health and VRQoL evaluated using questionnaire responses [59-62]. Unfortunately, there is little evidence demonstrating what disease stages are associated with impairment of various day-to-day activities.
Three of the most important vision related tasks are mobility, reading and driving [63-65], so treatment targets related to these activities make sense. Reading tends to be most affected by damage to the central-most part of the VF [66, 67]; this part of the VF tends to be preserved until the end-stage of the disease [68], but studies have shown that the central field can also be damaged in early disease too [69]. Determining the severity of VF loss that inhibits driving safety has proven difficult due to the complexity of the task with many studies even struggling to find evidence that individuals with glaucoma are unsafe to drive [70-72]. Driving simulations have perhaps generated some of the most interesting insights into aspects of vision used when driving and provide promise for identifying more robust means of evaluating fitness to drive based on the VF in the future [55, 73-78]. Mobility is the area that has been most tested with Assessment of Disability Related to Vision testing showing some weak correlation to VF measurements [79]. Loss of balance [80, 81] and risk of falls [82] have also been shown to be associated with VF health.
Overall, it is important to know the severity of disease that needs to be prevented in order to define what rates of loss are rapid enough to risk future visual impairment and more work needs to be done to achieve this aim. Most current treatment targets are either based on intraocular pressure measurements or arbitrary functional or structural measurements not based on clinical evidence, which means that rates of loss thresholds defined as “fast” or even “catastrophic” in many studies are arbitrary.
Predicting future patient status according to their life expectancy and baseline measurements
In evaluating what rates of progression put patients at risk of reduced VRQoL, it is also essential to consider how much VF loss the patient has already experienced and how long they are likely to progress at their current rate of loss. For instance, a patient that already has substantial VF loss does not have to lose vision that quickly to become blind. This is especially important given links between disease severity at diagnosis and future visual impairment [19, 83, 84].
In addition, the life expectancy of the patient is also important to consider, as older patients will usually have less time to lose their vision than younger patients with the same level of disease severity [85]. For example, assuming for simplicity that VF loss remains constant over time, a patient losing 0.5dB of VF MD per year will lose 10dB if they live an additional 20 years and 20dB if they live another 40 years. In spite of the importance of this issue, few studies have actively attempted to incorporate what is known about the life expectancy of the patient into risk of VF progression [19, 85]. One of the main challenges is that predicting life expectancy of a given patient is even more difficult than predicting future rates of glaucoma progression. Previous studies have suggested that medical worker predictions can lack accuracy in estimating remaining life expectancy, with a bias towards underestimating the years a patient has to live left [86-89]. As a result, clinicians need to be conservative and make clinical management decisions as if their patients will exceed their expected life expectancy.
We have provided a schematic, again assuming a constant rate of change in VF loss over time, to help illustrate whether a specific progression rate is fast for an individual patient. For instance, the Figure A illustrates that for an individual patient with a starting VF MD of -7dB (commonly defined as moderate VF loss) would need an MD of -1dB/year to reach a severe VF loss of -12dB if the patient lives for five years, but this lowers to just -0.1dB/year if the patient lives fifty years. Similarly, the Figure B shows that a patient already blind in one eye who will likely live another 15 years requires a rate of loss of -1.4dB/year to reach statutory blindness if diagnosed early (MD = -1 dB), but this rate goes down to -0.7 dB/year if this patient already has severe disease (MD = -12dB).
Figure 1.
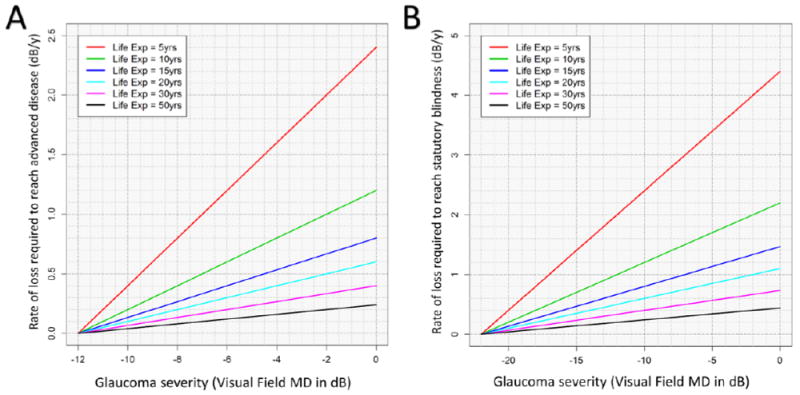
Incorporating severity of disease and life expectancy to identify clinically important rates of progression for an individual patient. The baseline visual field mean deviation (MD) is on the x-axis. The y-axis represents the linear rate of visual field loss that will result in advanced disease (Figure A) and blindness (Figure B). Advanced disease is defined as MD ≤ -12dB in Figure A, while Figure B uses an MD ≤ -22dB to represent statutory blindness. By estimating the patients remaining life expectancy, a critical rate of visual field loss to avoid can be defined. Each colored line corresponds to a different life expectancy (Life Exp) remaining.
It is worth bearing in mind that, in practice, it is unrealistic to expect rates of loss to remain constant over time. For a start, changes in treatment intensity and its adherence, as well as risk factors for progression may change over time, which would in turn affect rates of both structural and VF change. There is further evidence that natural progression of the disease can be better fitted using exponential or other non-linear models [90-93], which is supported by studies that suggest that rates of progression become faster with age [15]. However, given the complexity of the task of modelling rate of VF progression over long periods of time, there is not unanimous evidence that non-linear models are substantially more useful in practice than assuming rates of change are constant [94]. For these reasons, an argument can be made for repeatedly evaluating rate-of-change over shorter trends (say using the last 8 tests or evaluations in the last 10 years) [95] as a practical approach for estimating rates of change for clinical management decisions. This is particularly important in the context of changing treatment during follow-up; one study has shown, for instance, that the rate of MD loss is reduced by 56% after trabeculectomy [96]. Failing to account for events such as surgery and change in medication risks over-estimating an eye’s current progression rate.
Given the difficulty in evaluating rates of progression, it is useful to use concurrent risk factors when evaluating patient risk of progression rate of patients; for instance, it is known that higher intraocular pressure is a major risk factor for faster disease [33, 97], so a patient with less controlled pressure is more likely to progress quickly in the future. Rates of change alongside considering other risk factors such as intraocular pressure, race and family history of glaucoma, and change in treatment and surgery should therefore be used in deciding whether progression could lead to decline in VRQoL.
Expert Commentary
Addressing the question of what rates of progression in glaucoma are clinically relevant is highly nuanced. At first glance, rates of -1.5 or -2 dB visual field MD change per year or equivalently around 5 to 10% of VFI per year are fast and should always be avoided, if possible. However, far slower rates of loss may be significant for other patients. What rates of loss are clinically important depends on the life expectancy of the patient, the severity of disease, risk factors, and what daily activities need to be preserved. This means that a catastrophic rate of loss for one patient (e.g. a newly-diagnosed 60 year-old female progressing at a rate of -1dB/year from a baseline MD of -10 dB) may not be clinically important for another (e.g. a newly-diagnosed 90 year-old male progressing at a rate of -1dB/year from a baseline of -2dB). These examples clearly show that it is not only important to determine whether a glaucoma patient is progressing, but it is also critical to estimate the rate of progression to determine how aggressively to treat the patient. To do this, regular monitoring of patients is essential in glaucoma management.
Five Year View
The future of clinical treatment in glaucoma could one day be based on individualised progression rate-based treatment targets to prevent functional impairment and blindness based on the age/life expectancy, risk factors and severity of disease of each patient. As more is known about how new structural changes in the retina impact on what patients can actually see, one would expect structural measurements to play an increasing role in glaucoma management; not only in the context of detecting progression, but evaluating future prognosis for the patient. New technology such as OCT-angiography may further elucidate the disease process and allow better links to be made between how the eye is changing and how it functions [98-100]. Further, changes in test algorithms [101, 102], stimulus size [103, 104] and location [105-107] as well as analysis methods [108-112] of visual field measurements may help deal with the noise that currently obfuscates interpretation of visual function measurements. For example, an analytical method called ANSWERS has showed particular promise in this regard, allowing increased test-retest variability with disease severity to be taken into consideration when measuring rates of progression [110, 111]. Further, new objective technologies such as pupil-based perimetry [113-115] or electrophysiological testing [116, 117]may reduce variability caused by human error that currently plights perimetry [116, 117].
Key Issues.
A clinically significant rate of progression in glaucoma is one that could lead to a high risk of future visual impairment for the patient.
Only trend-based analyses can be used to evaluate rates of change.
Most eyes do not suffer disease progression at rates that may lead to future visual impairment.
However, between 3 and 17% of eyes progress at rates of loss that could lead to perimetric blindness within 20 years.
A key challenge in glaucoma management is differentiating between patients that are at risk of blindness and those that are not.
It is important, but difficult, to consider age-related changes in structural measurements (e.g. neuroretinal rim area and retinal nerve fiber layer thickness) when evaluating glaucoma progression.
Regular testing of patients is required to accurately evaluate rate of disease progression.
What rates of loss are clinically important depends on the life expectancy of the patient, the severity of disease and what daily activities need to be preserved.
Risk factors should also be utilised, alongside direct measurements of rates of loss, to evaluate risk of future patient progression to visual impairment.
Acknowledgments
The authors were supported by grants received by NIH: P30EY022589, R01011008, R01EY019869, R01EY021818, R01EY025056 and by research to prevent blindness. L.M. Zangwill has received financial support from Carl Zeiss Meditech, Heidelberg Engineering, Optovue, Topcon Medical Systems and Quark Pharmaceuticals and has received research support from Carl Zeiss and Optovue. F.A Medeiros has received financial support from Carl Zeiss Meditech, Heidelberg Engineering, Topcon, Ametek, Bausch +Lomb, Allergan and Sensimed. F.A Medeiros is a consultant for Carl Zeiss Meditech, Heidelberg Engineering, Ametek, Alcon and Allergan and has received research support from Carl Zeiss Meditech. R.N Weinreb has received financial support from Heidelberg Engineering, Carl Zeiss Meditech, Genentech, Konan, National Eye Institute, Neurovision, Optovue, Quark, Reichert, Tomey and Topcon; is a consultant for Alcon, Allergan, Ametek, Bausch + Lomb, Carl Zeiss Meditec, Forsight, Topcon and Valeant and has received research support from Carl Zeiss Meditec.
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artes PH, et al. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Invest Ophthalmol Vis Sci. 2002;43(8):2654–9. [PubMed] [Google Scholar]
- 3.Henson DB, et al. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41(2):417–21. [PubMed] [Google Scholar]
- 4.Russell RA, et al. The relationship between variability and sensitivity in large-scale longitudinal visual field data. Invest Ophthalmol Vis Sci. 2012;53(10):5985–90. doi: 10.1167/iovs.12-10428. [DOI] [PubMed] [Google Scholar]
- 5.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989;108(2):130–5. doi: 10.1016/0002-9394(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 6.Abe RY, Gracitelli CP, Medeiros FA. The Use of Spectral-Domain Optical Coherence Tomography to Detect Glaucoma Progression. Open Ophthalmol J. 2015;9:78–88. doi: 10.2174/1874364101509010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alencar LM, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci. 2010;51(7):3531–9. doi: 10.1167/iovs.09-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung CK, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012;119(4):731–7. doi: 10.1016/j.ophtha.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Liu T, et al. Rates of Retinal Nerve Fiber Layer Loss in Contralateral Eyes of Glaucoma Patients with Unilateral Progression by Conventional Methods. Ophthalmology. 2015;122(11):2243–51. doi: 10.1016/j.ophtha.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miki A, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121(7):1350–8. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vianna JR, et al. Importance of Normal Aging in Estimating the Rate of Glaucomatous Neuroretinal Rim and Retinal Nerve Fiber Layer Loss. Ophthalmology. 2015;122(12):2392–8. doi: 10.1016/j.ophtha.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Zangwill LM, et al. The rate of structural change: the confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study. Am J Ophthalmol. 2013;155(6):971–82. doi: 10.1016/j.ajo.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gracitelli CP, et al. Estimated rates of retinal ganglion cell loss in glaucomatous eyes with and without optic disc hemorrhages. PLoS One. 2014;9(8):e105611. doi: 10.1371/journal.pone.0105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros FA, et al. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. 2012;154(5):814–824 e1. doi: 10.1016/j.ajo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heijl A, et al. Natural history of open-angle glaucoma. Ophthalmology. 2009;116(12):2271–6. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan BC, et al. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci. 2014;55(7):4135–43. doi: 10.1167/iovs.14-14643. [DOI] [PubMed] [Google Scholar]
- 17.Heijl A, et al. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol. 2013;91(5):406–12. doi: 10.1111/j.1755-3768.2012.02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirwan JF, et al. Portsmouth visual field database: an audit of glaucoma progression. Eye (Lond) 2014;28(8):974–9. doi: 10.1038/eye.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders LJ, et al. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci. 2014;55(1):102–9. doi: 10.1167/iovs.13-13006. [DOI] [PubMed] [Google Scholar]
- 20.Aptel F, et al. Progression of visual field in patients with primary open-angle glaucoma - ProgF study 1. Acta Ophthalmol. 2015 doi: 10.1111/aos.12788. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson B, V, Patella M, Heijl A. Prediction of glaucomatous visual field loss by extrapolation of linear trends. Arch Ophthalmol. 2009;127(12):1610–5. doi: 10.1001/archophthalmol.2009.297. [DOI] [PubMed] [Google Scholar]
- 22.Leung CK, et al. Evaluation of retinal nerve fiber layer progression in glaucoma a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology. 2011;118(8):1551–7. doi: 10.1016/j.ophtha.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Spry PG, Johnson CA. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001;78(6):436–41. doi: 10.1097/00006324-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Hodapp E, Parrish RKI, Anderson DR. Clinical decisions in glaucoma. St Louis, Missouri: The CV Mosby Co; 1993. [Google Scholar]
- 25.Disability Evaluation Under Social Security. [2015 14th December];2011 [Google Scholar]
- 26.Chauhan BC, et al. Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–6. doi: 10.1001/archopht.126.8.1030. [DOI] [PubMed] [Google Scholar]
- 27.Gordon MO, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–20. doi: 10.1001/archopht.120.6.714. discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 28.Leske MC, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Mukesh BN, et al. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002;109(6):1047–51. doi: 10.1016/s0161-6420(02)01040-0. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan BC, et al. Canadian Glaucoma Study: 3. Impact of risk factors and intraocular pressure reduction on the rates of visual field change. Arch Ophthalmol. 2010;128(10):1249–55. doi: 10.1001/archophthalmol.2010.196. [DOI] [PubMed] [Google Scholar]
- 31.De Moraes CG, et al. Glaucoma with early visual field loss affecting both hemifields and the risk of disease progression. Arch Ophthalmol. 2009;127(9):1129–34. doi: 10.1001/archophthalmol.2009.165. [DOI] [PubMed] [Google Scholar]
- 32.Kim KE, et al. Long-term follow-up in preperimetric open-angle glaucoma: progression rates and associated factors. Am J Ophthalmol. 2015;159(1):160–8 e1-2. doi: 10.1016/j.ajo.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Medeiros FA, et al. Incorporating risk factors to improve the assessment of rates of glaucomatous progression. Invest Ophthalmol Vis Sci. 2012;53(4):2199–207. doi: 10.1167/iovs.11-8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prata TS, et al. Factors affecting rates of visual field progression in glaucoma patients with optic disc hemorrhage. Ophthalmology. 2010;117(1):24–9. doi: 10.1016/j.ophtha.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Teng CC, et al. Beta-Zone parapapillary atrophy and the velocity of glaucoma progression. Ophthalmology. 2010;117(5):909–15. doi: 10.1016/j.ophtha.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145(2):343–53. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 37.Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110(4):726–33. doi: 10.1016/S0161-6420(02)01974-7. [DOI] [PubMed] [Google Scholar]
- 38.Hattenhauer MG, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105(11):2099–104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- 39.Kwon YH, et al. Rate of visual field loss and long-term visual outcome in primary open-angle glaucoma. Am J Ophthalmol. 2001;132(1):47–56. doi: 10.1016/s0002-9394(01)00912-6. [DOI] [PubMed] [Google Scholar]
- 40.Chauhan BC, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92(4):569–73. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JW, et al. The Fast Component of Visual Field Decay Rate Correlates With Disc Rim Area Change Throughout the Entire Range of Glaucomatous Damage. Invest Ophthalmol Vis Sci. 2015;56(10):5997–6006. doi: 10.1167/iovs.15-17006. [DOI] [PubMed] [Google Scholar]
- 43.Poli A, et al. Analysis of HRT images: comparison of reference planes. Invest Ophthalmol Vis Sci. 2008;49(9):3970–5. doi: 10.1167/iovs.08-1764. [DOI] [PubMed] [Google Scholar]
- 44.See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009;116(5):840–7. doi: 10.1016/j.ophtha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Chauhan BC, et al. Bruch’s Membrane Opening Minimum Rim Width and Retinal Nerve Fiber Layer Thickness in a Normal White Population: A Multicenter Study. Ophthalmology. 2015;122(9):1786–94. doi: 10.1016/j.ophtha.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammel N, et al. Comparing the Rates of Retinal Nerve Fiber Layer and Ganglion Cell Layer Loss in Healthy and Glaucoma Eyes. Ophthalmology. 2015 doi: 10.1016/j.ajo.2017.03.008. In Press. [DOI] [PubMed] [Google Scholar]
- 47.Wessel JM, et al. Longitudinal analysis of progression in glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(5):3613–20. doi: 10.1167/iovs.12-9786. [DOI] [PubMed] [Google Scholar]
- 48.Medeiros FA, et al. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma. Ophthalmology. 2014;121(1):100–9. doi: 10.1016/j.ophtha.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gardiner SK, et al. A method to estimate the amount of neuroretinal rim tissue in glaucoma: comparison with current methods for measuring rim area. Am J Ophthalmol. 2014;157(3):540–9 e1-2. doi: 10.1016/j.ajo.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardiner SK, et al. Structural Measurements for Monitoring Change in Glaucoma: Comparing Retinal Nerve Fiber Layer Thickness With Minimum Rim Width and Area. Invest Ophthalmol Vis Sci. 2015;56(11):6886–91. doi: 10.1167/iovs.15-16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meira-Freitas D, et al. Predicting progression in glaucoma suspects with longitudinal estimates of retinal ganglion cell counts. Invest Ophthalmol Vis Sci. 2013;54(6):4174–83. doi: 10.1167/iovs.12-11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mwanza JC, et al. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol. 2015;99(6):732–7. doi: 10.1136/bjophthalmol-2014-305745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardiner SK, et al. Assessment of the reliability of standard automated perimetry in regions of glaucomatous damage. Ophthalmology. 2014;121(7):1359–69. doi: 10.1016/j.ophtha.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gracitelli CP, et al. Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma. JAMA Ophthalmol. 2015;133(4):384–90. doi: 10.1001/jamaophthalmol.2014.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tatham AJ, et al. Glaucomatous retinal nerve fiber layer thickness loss is associated with slower reaction times under a divided attention task. Am J Ophthalmol. 2014;158(5):1008–17. doi: 10.1016/j.ajo.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glen FC, Crabb DP, Garway-Heath DF. The direction of research into visual disability and quality of life in glaucoma. BMC Ophthalmol. 2011;11:19. doi: 10.1186/1471-2415-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heijl A, Aspberg J, Bengtsson B. The effect of different criteria on the number of patients blind from open-angle glaucoma. BMC Ophthalmol. 2011;11:31. doi: 10.1186/1471-2415-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viswanathan AC, et al. Severity and stability of glaucoma: patient perception compared with objective measurement. Arch Ophthalmol. 1999;117(4):450–4. doi: 10.1001/archopht.117.4.450. [DOI] [PubMed] [Google Scholar]
- 59.Chan EW, et al. Impact of glaucoma severity and laterality on vision-specific functioning: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2013;54(2):1169–75. doi: 10.1167/iovs.12-10258. [DOI] [PubMed] [Google Scholar]
- 60.Goldberg I, et al. Assessing quality of life in patients with glaucoma using the Glaucoma Quality of Life-15 (GQL-15) questionnaire. J Glaucoma. 2009;18(1):6–12. doi: 10.1097/IJG.0b013e3181752c83. [DOI] [PubMed] [Google Scholar]
- 61.McKean-Cowdin R, et al. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941–948 e1. doi: 10.1016/j.ophtha.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Gestel A, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond) 2010;24(12):1759–69. doi: 10.1038/eye.2010.133. [DOI] [PubMed] [Google Scholar]
- 63.Bhargava JS, et al. Views of glaucoma patients on aspects of their treatment: an assessment of patient preference by conjoint analysis. Invest Ophthalmol Vis Sci. 2006;47(7):2885–8. doi: 10.1167/iovs.05-1244. [DOI] [PubMed] [Google Scholar]
- 64.Aspinall PA, et al. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49(5):1907–15. doi: 10.1167/iovs.07-0559. [DOI] [PubMed] [Google Scholar]
- 65.Mangione CM, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116(2):227–33. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 66.Tabrett DR, Latham K. Important areas of the central binocular visual field for daily functioning in the visually impaired. Ophthalmic Physiol Opt. 2012;32(2):156–63. doi: 10.1111/j.1475-1313.2012.00892.x. [DOI] [PubMed] [Google Scholar]
- 67.Whittaker SG, Lovie-Kitchin J. Visual requirements for reading. Optom Vis Sci. 1993;70(1):54–65. doi: 10.1097/00006324-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Ramulu PY, et al. Glaucoma and reading speed: the Salisbury Eye Evaluation project. Arch Ophthalmol. 2009;127(1):82–7. doi: 10.1001/archophthalmol.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Traynis I, et al. Prevalence and nature of early glaucomatous defects in the central 10 degrees of the visual field. JAMA Ophthalmol. 2014;132(3):291–7. doi: 10.1001/jamaophthalmol.2013.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burg A. Vision and driving: a report on research. Hum Factors. 1971;13(1):79–87. doi: 10.1177/001872087101300110. [DOI] [PubMed] [Google Scholar]
- 71.McCloskey LW, et al. Motor vehicle collision injuries and sensory impairments of older drivers. Age Ageing. 1994;23(4):267–73. doi: 10.1093/ageing/23.4.267. [DOI] [PubMed] [Google Scholar]
- 72.Owsley C, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279(14):1083–8. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 73.Crabb DP, et al. Exploring eye movements in patients with glaucoma when viewing a driving scene. PLoS One. 2010;5(3):e9710. doi: 10.1371/journal.pone.0009710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glen FC, Smith ND, Crabb DP. Impact of superior and inferior visual field loss on hazard detection in a computer-based driving test. Br J Ophthalmol. 2015;99(5):613–7. doi: 10.1136/bjophthalmol-2014-305932. [DOI] [PubMed] [Google Scholar]
- 75.Medeiros FA, et al. Driving simulation as a performance-based test of visual impairment in glaucoma. J Glaucoma. 2012;21(4):221–7. doi: 10.1097/IJG.0b013e3182071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prado Vega R, et al. Obstacle avoidance, visual detection performance, and eye-scanning behavior of glaucoma patients in a driving simulator: a preliminary study. PLoS One. 2013;8(10):e77294. doi: 10.1371/journal.pone.0077294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tatham AJ, et al. Relationship Between Motor Vehicle Collisions and Results of Perimetry, Useful Field of View, and Driving Simulation in Drivers With Glaucoma. Transl Vis Sci Technol. 2015;4(3):5. doi: 10.1167/tvst.4.3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gracitelli CP, et al. Predicting Risk of Motor Vehicle Collisions in Patients with Glaucoma: A Longitudinal Study. PLoS One. 2015;10(10):e0138288. doi: 10.1371/journal.pone.0138288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richman J, et al. Relationships in glaucoma patients between standard vision tests, quality of life, and ability to perform daily activities. Ophthalmic Epidemiol. 2010;17(3):144–51. doi: 10.3109/09286581003734878. [DOI] [PubMed] [Google Scholar]
- 80.Kotecha A, et al. Balance control in glaucoma. Invest Ophthalmol Vis Sci. 2012;53(12):7795–801. doi: 10.1167/iovs.12-10866. [DOI] [PubMed] [Google Scholar]
- 81.Diniz-Filho A, et al. Evaluation of Postural Control in Patients with Glaucoma Using a Virtual Reality Environment. Ophthalmology. 2015;122(6):1131–8. doi: 10.1016/j.ophtha.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Black AA, Wood JM, Lovie-Kitchin JE. Inferior field loss increases rate of falls in older adults with glaucoma. Optom Vis Sci. 2011;88(11):1275–82. doi: 10.1097/OPX.0b013e31822f4d6a. [DOI] [PubMed] [Google Scholar]
- 83.Kotecha A, et al. Avoidable sight loss from glaucoma: is it unavoidable? Br J Ophthalmol. 2012;96(6):816–20. doi: 10.1136/bjophthalmol-2012-301499. [DOI] [PubMed] [Google Scholar]
- 84.Sinclair A, Hinds A, Sanders R. Ten years of glaucoma blindness in Fife 1990-99 and the implications for ophthalmology, optometry and rehabilitation services. Ophthalmic Physiol Opt. 2004;24(4):313–8. doi: 10.1111/j.1475-1313.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- 85.Wesselink C, Stoutenbeek R, Jansonius NM. Incorporating life expectancy in glaucoma care. Eye (Lond) 2011;25(12):1575–80. doi: 10.1038/eye.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarke MG, et al. How accurate are doctors, nurses and medical students at predicting life expectancy? Eur J Intern Med. 2009;20(6):640–4. doi: 10.1016/j.ejim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Walz J, et al. Clinicians are poor raters of life-expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 2007;100(6):1254–8. doi: 10.1111/j.1464-410X.2007.07130.x. [DOI] [PubMed] [Google Scholar]
- 88.Wilson JR, et al. The assessment of patient life-expectancy: how accurate are urologists and oncologists? BJU Int. 2005;95(6):794–8. doi: 10.1111/j.1464-410X.2005.05403.x. [DOI] [PubMed] [Google Scholar]
- 89.Wirth R, Sieber CC. Health care professionals underestimate the mean life expectancy of older people. Gerontology. 2012;58(1):56–9. doi: 10.1159/000327656. [DOI] [PubMed] [Google Scholar]
- 90.Azarbod P, et al. Validation of point-wise exponential regression to measure the decay rates of glaucomatous visual fields. Invest Ophthalmol Vis Sci. 2012;53(9):5403–9. doi: 10.1167/iovs.12-9930. [DOI] [PubMed] [Google Scholar]
- 91.Pathak M, Demirel S, Gardiner SK. Nonlinear, multilevel mixed-effects approach for modeling longitudinal standard automated perimetry data in glaucoma. Invest Ophthalmol Vis Sci. 2013;54(8):5505–13. doi: 10.1167/iovs.13-12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caprioli J, et al. A method to measure and predict rates of regional visual field decay in glaucoma. Invest Ophthalmol Vis Sci. 2011;52(7):4765–73. doi: 10.1167/iovs.10-6414. [DOI] [PubMed] [Google Scholar]
- 93.Lee JM, et al. Comparison of regression models for serial visual field analysis. Jpn J Ophthalmol. 2014;58(6):504–14. doi: 10.1007/s10384-014-0341-5. [DOI] [PubMed] [Google Scholar]
- 94.Bryan SR, et al. Robust and censored modeling and prediction of progression in glaucomatous visual fields. Invest Ophthalmol Vis Sci. 2013;54(10):6694–700. doi: 10.1167/iovs.12-11185. [DOI] [PubMed] [Google Scholar]
- 95.Gardiner SK, et al. Series length used during trend analysis affects sensitivity to changes in progression rate in the ocular hypertension treatment study. Invest Ophthalmol Vis Sci. 2013;54(2):1252–9. doi: 10.1167/iovs.12-10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bertrand V, et al. Rates of visual field loss before and after trabeculectomy. Acta Ophthalmol. 2014;92(2):116–20. doi: 10.1111/aos.12073. [DOI] [PubMed] [Google Scholar]
- 97.Medeiros FA, et al. The Relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116(6):1125–33 e1-3. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jia Y, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322–32. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015;133(9):1045–52. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2015;253(9):1557–64. doi: 10.1007/s00417-015-3095-y. [DOI] [PubMed] [Google Scholar]
- 101.Bengtsson B, Heijl A. SITA Fast, a new rapid perimetric threshold test. Description of methods and evaluation in patients with manifest and suspect glaucoma. Acta Ophthalmol Scand. 1998;76(4):431–7. doi: 10.1034/j.1600-0420.1998.760408.x. [DOI] [PubMed] [Google Scholar]
- 102.Saunders LJ, Russell RA, Crabb DP. Measurement precision in a series of visual fields acquired by the standard and fast versions of the Swedish interactive thresholding algorithm: analysis of large-scale data from clinics. JAMA Ophthalmol. 2015;133(1):74–80. doi: 10.1001/jamaophthalmol.2014.4237. [DOI] [PubMed] [Google Scholar]
- 103.Wall M, et al. The repeatability of mean defect with size III and size V standard automated perimetry. Invest Ophthalmol Vis Sci. 2013;54(2):1345–51. doi: 10.1167/iovs.12-10299. [DOI] [PubMed] [Google Scholar]
- 104.Wall M, et al. Repeatability of automated perimetry: a comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Invest Ophthalmol Vis Sci. 2009;50(2):974–9. doi: 10.1167/iovs.08-1789. [DOI] [PubMed] [Google Scholar]
- 105.Chen S, McKendrick AM, Turpin A. Choosing two points to add to the 24-2 pattern to better describe macular visual field damage due to glaucoma. Br J Ophthalmol. 2015;99(9):1236–9. doi: 10.1136/bjophthalmol-2014-306431. [DOI] [PubMed] [Google Scholar]
- 106.Aoyama Y, et al. A method to measure visual field sensitivity at the edges of glaucomatous scotomata. Invest Ophthalmol Vis Sci. 2014;55(4):2584–91. doi: 10.1167/iovs.13-13616. [DOI] [PubMed] [Google Scholar]
- 107.Chong LX, Turpin A, McKendrick AM. Targeted spatial sampling using GOANNA improves detection of visual field progression. Ophthalmic Physiol Opt. 2015;35(2):155–69. doi: 10.1111/opo.12184. [DOI] [PubMed] [Google Scholar]
- 108.Medeiros FA, et al. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci. 2011;52(8):5794–803. doi: 10.1167/iovs.10-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Russell RA, et al. Improved estimates of visual field progression using bayesian linear regression to integrate structural information in patients with ocular hypertension. Invest Ophthalmol Vis Sci. 2012;53(6):2760–9. doi: 10.1167/iovs.11-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu H, et al. More Accurate Modeling of Visual Field Progression in Glaucoma: ANSWERS. Invest Ophthalmol Vis Sci. 2015;56(10):6077–83. doi: 10.1167/iovs.15-16957. [DOI] [PubMed] [Google Scholar]
- 111.Zhu H, et al. Detecting changes in retinal function: Analysis with Non-Stationary Weibull Error Regression and Spatial enhancement (ANSWERS) PLoS One. 2014;9(1):e85654. doi: 10.1371/journal.pone.0085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murata H, Araie M, Asaoka R. A new approach to measure visual field progression in glaucoma patients using variational bayes linear regression. Invest Ophthalmol Vis Sci. 2014;55(12):8386–92. doi: 10.1167/iovs.14-14625. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, et al. Rapid pupil-based assessment of glaucomatous damage. Optom Vis Sci. 2008;85(6):471–81. doi: 10.1097/OPX.0b013e318177ec02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hong S, Narkiewicz J, Kardon RH. Comparison of pupil perimetry and visual perimetry in normal eyes: decibel sensitivity and variability. Invest Ophthalmol Vis Sci. 2001;42(5):957–65. [PubMed] [Google Scholar]
- 115.Kardon RH. Pupil perimetry. Curr Opin Ophthalmol. 1992;3(5):565–70. doi: 10.1097/00055735-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 116.Bach M, Poloschek CM. Electrophysiology and glaucoma: current status and future challenges. Cell Tissue Res. 2013;353(2):287–96. doi: 10.1007/s00441-013-1598-6. [DOI] [PubMed] [Google Scholar]
- 117.Graham SL, Klistorner AI, Goldberg I. Clinical application of objective perimetry using multifocal visual evoked potentials in glaucoma practice. Arch Ophthalmol. 2005;123(6):729–39. doi: 10.1001/archopht.123.6.729. [DOI] [PubMed] [Google Scholar]


