Abstract
Objective
Informal caregivers—i.e., close family and friends providing unpaid emotional or instrumental care—of patients admitted to intensive care units (ICUs) are at risk for posttraumatic stress disorder (PTSD). As a first step toward developing interventions to prevent PTSD in ICU caregivers, we examined the predictive validity of psychosocial risk screening during admission for caregiver PTSD at three and six months post-hospitalization.
Design
An observational, prospective study.
Participants
Ninety-nine caregivers were recruited as part of a longitudinal research program of patient-caregiver dyads in a neuroscience intensive care unit (Neuro-ICU).
Intervention
None
Measurements and Main Results
Caregiver PTSD symptoms were assessed during admission (baseline), three months, and six months post-hospitalization. We (1) characterized prevalence of clinically significant symptoms at each time point; (2) calculated sensitivity and specificity of baseline PTSD screening in predicting PTSD at three and six months; and (3) used recursive partitioning to select potential baseline factors and examine the extent to which they helped predict clinically significant PTSD symptoms at each time point. Rates of caregiver PTSD remained relatively stable over time (16-22%). Screening for PTSD at baseline predicted PTSD at three and six months with moderate sensitivity (75-80%) and high specificity (92-95%). Screening for PTSD at baseline was associated with caregiver anxiety, mindfulness (i.e., ability to be aware of one’s thoughts and feelings in the moment), and bond with patient. Moreover, baseline PTSD screening was the single most relevant predictor of PTSD at three and six months, such that other baseline factors did not significantly improve predictive ability.
Conclusions
Screening Neuro-ICU caregivers for clinically significant PTSD symptoms during admission is the single most important way to identify the majority of those likely to suffer from chronic PTSD following discharge. Addressing early PTSD symptoms and their psychosocial correlates during admission may help prevent chronic PTSD in these at-risk caregivers.
Keywords: Post-traumatic stress, PTSD, caregivers, neurocritical care, resilience, prevention
Introduction
Informal caregivers—i.e., close family members and friends providing unpaid emotional or instrumental care—of patients admitted to intensive care units (ICUs) are at risk for posttraumatic stress disorder (PTSD) due to the sudden onset of their loved ones’ medical trauma, which may include risk of death or profound disability, uncertainty about patient prognosis during hospitalization, and involvement in complex medical decision-making (1). Following discharge, caregivers often continue to provide emotional and instrumental support for their loved ones. Cross-sectional studies suggest one in five informal caregivers exhibit clinically significant symptoms of PTSD during patient admission (2), with variable rates observed post-hospitalization (3). Chronic PTSD in caregivers—defined as clinically significant PTSD symptoms persisting for at least three months (4)—hinders patients’ recovery by interfering with caregivers’ ability to provide quality care (5,6) and also increases caregivers’ own risks for morbidity (7,8) and mortality (9). As such, interventions to prevent chronic PTSD in caregivers are imperative to optimize outcomes for both patients and caregivers.
To date, our limited ability to identify caregivers at risk for PTSD is a significant barrier to developing and implementing interventions to prevent chronic PTSD in ICU caregivers. Early interventions are critical given that PTSD over time may become more difficult to treat as maladaptive symptom networks are consolidated (10). Although many caregivers experience acute symptoms of PTSD around the time of an ICU admission (11), the extent to which these symptoms transition to chronic PTSD post-hospitalization has not been well studied. Numerous prospective studies of PTSD in emergency settings have followed adult patients themselves (12,13), but limited studies have focused on caregivers. Among existing studies with ICU caregivers, several measured PTSD symptoms either during admission (2) or several months later (3,14–18), but did not perform repeated assessments of these symptoms across the hospitalization-recovery continuum. Of those with multiple longitudinal measurements of caregiver PTSD, some assessed PTSD symptoms following discharge but not during ICU admission (19), while others measured symptoms through three months post-admission but not beyond (20–22). Longitudinal studies over a longer period are needed to track how acute symptoms of PTSD observed during the ICU admission may persist following discharge, and which caregivers are most at risk for chronic PTSD over time. From a health resources perspective, such information would allow efficient and timely delivery of targeted interventions to those in greatest need. Examining acute PTSD symptoms and other modifiable factors that predict chronic PTSD in these caregivers could illuminate opportunities for early detection and subsequent intervention (23).
This present study, part of an ongoing longitudinal research program of patient-caregiver dyads in a neuroscience intensive care unit (Neuro-ICU), aimed to determine whether screening caregivers for clinically significant PTSD symptoms and other factors during ICU admission could help identify those most likely to qualify for chronic PTSD following hospitalization. Specifically, we sought to: (a) characterize prevalence of clinically significant PTSD symptoms in Neuro-ICU informal caregivers during admission (baseline), three months, and six months post-hospitalization; (b) determine sensitivity and specificity of baseline PTSD screening in predicting PTSD at three and six months post-hospitalization; and (c) use recursive partitioning to select potential baseline factors and examine the extent to which they predict clinically significant PTSD symptoms at baseline and/or chronic PTSD. Findings are expected to help identify Neuro-ICU caregivers at risk for chronic PTSD and guide intervention development to prevent chronic PTSD in these individuals who play a key role in patient care and recovery.
Materials and Methods
Sample and Procedures
This study drew on longitudinal data from a prospective study of adult patients admitted to the Neuro-ICU and their informal caregivers. Initial recruitment of patient-caregiver dyads occurred at a major medical center in Boston, MA, between June 2015 and July 2016. The institutional review board at Massachusetts General Hospital in Boston, MA, approved all study procedures, summarized elsewhere (2). Inclusion criteria for caregivers were: (1) 18 or more years of age, (2) English fluency and literacy, and (3) self-identification as the patient’s primary caregiver. Eligible caregivers were first approached by a research assistant based on the medical team’s recommendation of patients who would also be suitable for participation, excluding those with medical or cognitive barriers (e.g. critical condition with anticipated death within the ICU, chronic aphasia, limited decision-making capacity). Most caregivers were recruited within two days of patient admission, and all completed paper-based measures prior to patient discharge. Caregivers were then contacted by email at three and six months post-discharge to complete follow-up assessments via a free secure web-based data collection system (REDCap) (24) hosted at Partners HealthCare in Boston, MA.
Measures
PTSD
PTSD symptoms were assessed at baseline and again at three and six months post-hospitalization using the 17-item Post-Traumatic Checklist-Specific Stressor (PCL-S) (25). Caregivers rated how bothered they were by each symptom in the past month using a 5-point Likert scale (1=not at all, 5=extremely). The PCL-S yields a total score ranging from 17-85, with higher scores indicating greater PTSD symptoms. For main analyses, a scoring algorithm consistent with the Diagnostic and Statistical Manual of Mental Disorders-IV-TR (DSM-IV) (4) was used to identify individuals who screened in for PTSD (i.e., whose symptoms met criteria for clinical significance) at each time point.
Caregiver demographic variables
At baseline, caregivers reported their age, gender, race/ethnicity, education, employment status, and marital status.
Caregiver psychosocial variables
At baseline, caregivers also completed self-report scales on anxiety and depression symptoms (26), history of anxiety and/or depression, caregiving self-efficacy (27), satisfaction with health care (28), coping (29), mindfulness (30), and bond with patient (31). Detailed information on these scales, including number of items with scoring procedures, is provided in Supplementary Materials.
Patient medical variables
Patient medical and treatment characteristics were obtained from patients’ electronic medical record, including patient diagnosis, status at discharge, and whether the patient was intubated during hospitalization.
Analyses
Using SPSS Version 20 (IBM Corp, 2011), caregiver characteristics were summarized using relevant descriptive statistics (e.g. proportion, mean). Then, summary statistics of caregivers with clinically significant PTSD symptoms were tabulated for each time point using all available data. Second, 2×2 contingency tables were used to compare proportions of caregivers screening for PTSD at baseline with PTSD cases at three- and six-month follow-ups. McNemar’s tests of correlated proportions using a binomial distribution were used to further test whether these proportions differed significantly. Sensitivity was calculated as percentage of caregivers who screened in at baseline, out of those meeting criteria for PTSD at each follow-up. Specificity was calculated as percentage of caregivers who did not screen in at baseline, out of those not meeting criteria for PTSD at each follow-up.
Third, recursive partitioning analyses were conducted using the rpart package (32) in R (33) to derive a set of baseline predictors for PTSD at each time point. For clinically significant PTSD symptoms at baseline, all caregiver demographic, psychosocial, and patient medical variables were entered as potential predictors into a variable selection model. For PTSD at three and six months, these variables were entered along with clinically significant PTSD symptoms at baseline. Outcome PTSD variables were specified as dichotomous (“class”) in order to generate a classification tree. Default settings were used to produce parsimonious trees, though full tree generation followed by statistical pruning was also examined as an alternative. Predictors identified for inclusion were then entered simultaneously into a multivariate logistic regression to predict clinically significant PTSD symptoms at each time point. Significance of individual predictors was evaluated using standard criteria (p<.05) and Nagelkerke’s R2 was used to evaluate variance in PTSD explained by the included set of predictor(s). Positive predictive validity (i.e., percentage of PTSD cases at each time point correctly predicted by the baseline variable(s)) was also calculated.
Results
Characteristics of Caregivers
Of 151 eligible caregivers who were approached, 115 consented to participate (76%) and 99 (86%) completed the PCL-S at baseline (Table 1), while 79 (69%) caregivers (two without baseline PCL-S data) completed the PCL-S at three- and six-month follow-ups. Overall, caregivers were middle-aged, approximately two-thirds were female, and most were non-Hispanic white, highly educated, and married. The most prevalent diagnoses causing patients to be hospitalized were stroke/brain hemorrhage (35%) and brain tumor (25%). Approximately three-quarters of patients were intubated during the course of treatment, and approximately two-thirds of the patients were discharged home following hospitalization. The majority (66%) of caregivers were the patients’ romantic partner.
Table 1.
Caregiver Demographic and Psychosocial Variables and Patient Medical Characteristics of Neuro-ICU Informal Caregivers (N= 99)
| Baseline Variables | N (%) | M (SD) |
|---|---|---|
| Demographic Variables | ||
| Age | — | 53.34 (13.99) |
| Gender (male) | 37 (37.8%) | — |
| Race/Ethnicity (non-Hispanic white) | 85 (87.6%) | — |
| Education (some college or more) | 79 (80.6%) | — |
| Employed (full time) | 56 (57.7%) | — |
| Marital status (married/cohabitating) | 80 (82.5%) | — |
| Relationship to patient | ||
| Spouse/partner | 65 (65.7%) | — |
| Parent | 15 (15.2%) | — |
| Child | 15 (15.2%) | — |
| Sibling | 4 (4.0%) | — |
| Psychosocial Variables | ||
| Depressive symptoms | — | 5.37 (2.84) |
| Anxiety symptoms | — | 9.07 (4.15) |
| History of depression diagnosis | 7 (7.1%) | — |
| History of anxiety diagnosis | 3 (3.0%) | — |
| Caregiving self-efficacy | — | 84.10 (15.17) |
| Satisfaction with healthcare | — | 3.86 (0.28) |
| Life stress | — | 4.53 (3.28) |
| Coping | — | 32.89 (9.04) |
| Mindfulness | — | 33.96 (5.08) |
| Bond with patient | — | 58.44 (11.79) |
| Patient Medical Variables | ||
| Patients’ Diagnosis | ||
| Cerebrovascular | ||
| Stroke/Hemorrhage | 35 (35.4%) | — |
| Brain aneurysm | 3 (3.0%) | — |
| Other | ||
| Tumor | 25 (25.3%) | — |
| Lesion/Brain mass | 10 (10.1%) | — |
| Traumatic brain injury | 8 (8.1%) | — |
| Seizures | 5 (5.1%) | — |
| Other/More than 1 diagnosis | 6 (6.1%) | — |
| Discharge status | ||
| Discharge to home | 64 (64.6%) | — |
| Discharge to rehabilitation facility | 32 (32.3%) | — |
| Patient died during hospitalization | 3 (3.0%) | — |
| Intubated (yes) | 74 (74.7%) | — |
Note. Depressive symptoms = HADS-D; Anxiety symptoms = HADS-A; Caregiving self-efficacy = CSES; Satisfaction with healthcare = CPQ; Coping = MOCS-A; Mindfulness = CAMS-R; Bond with patient = IBM. Further details on these measures and corresponding references are provided in Supplementary Materials.
PTSD among Caregivers at Baseline, Three, and Six Months
At baseline, 16% of caregivers screened in for clinically significant PTSD symptoms, while 18% met criteria for PTSD at three-month follow-up and 22% at six-month follow-up (Table 2). Table 3 provides contingency tables comparing those who screened for PTSD at baseline with PTSD cases at three and six months. Over time, 12 caregivers stayed “stable” (i.e., screened in at baseline and through to six months); three “resolved” (i.e., screened in at baseline but not by six months), while three “emerged” (i.e., did not screen in at baseline but did by six months). Overall case proportions at three months or six months did not differ significantly from those at baseline, indicating relative stability over time.
Table 2.
Prevalence of PTSD Diagnoses and Symptom Severity by Time Point
| Assessment Time Points | Meeting Clinical Significance N (%) | Symptom Severity M (SD) |
|---|---|---|
| Baseline (N=99) | 16 (16.2%) | 28.52 (12.11) |
| 3-Month Post-Hospitalization (N=79) | 14 (17.7%) | 30.41 (12.11) |
| 6-Month Post-Hospitalization (N=79) | 17 (21.5%) | 30.03 (14.59) |
Note. Clinical significance for PTSD as determined on the Post-Traumatic Checklist-Specific Stressor (PCL-S) by symptoms rated “moderately” or above for at least: one B cluster item (questions 1-5); three C cluster items (questions 6-12); and two D cluster items (questions 13-17).
Table 3.
Contingency Tables of Baseline PTSD Screening with PTSD at 3- and 6-Month Post-Hospitalization
| Assessment Time Points | 3-Month Post-Hospitalization | 6-Month Post-Hospitalization | ||
|---|---|---|---|---|
|
| ||||
| PTSD | No PTSD | PTSD | No PTSD | |
| PTSD at Baseline (n) | 9 | 5 | 12 | 3 |
| No PTSD at Baseline (n) | 3 | 60 | 3 | 59 |
| McNemar’s X2 | 0.13 (p=n.s.) | 0.17 (p=n.s.) | ||
Note. Clinical significance for PTSD as determined on the Post-Traumatic Checklist-Specific Stressor (PCL-S) by symptoms rated “moderately” or above for at least: one B cluster item (questions 1-5); three C cluster items (questions 6-12); and two D cluster items (questions 13-17). McNemar’s X2 was calculated with Yate’s continuity correction.
Of caregivers who met criteria for clinically significant PTSD symptoms at three months and six months, 75% and 80% screened in at baseline (sensitivity), respectively. Among caregivers not meeting criteria at three months and six months, 92% and 95% did not screen in at baseline (specificity), respectively. Follow-up contingency table analyses indicated that 93% of caregivers who met criteria for PTSD at three months continued to meet criteria at six months, and that case proportions did not significantly differ between three and six months (p=.63).
Early Predictors of Baseline and/or Later Caregiver PTSD
For baseline PTSD screening, algorithm results for predictor selection are depicted in Figure 1. A three-split classification tree was identified as the most appropriate default solution. Caregiver baseline anxiety was the initial most relevant correlate, such that those who had higher anxiety scores (10 or higher) were more likely to screen for PTSD at baseline. Among caregivers with higher baseline anxiety scores, bond with patient was the next most relevant correlate, such that caregivers with lower bonds with the patient (scale score < 68) were more likely to screen in for PTSD. Among caregivers with higher anxiety and lower bonds with patient, mindfulness was the third and last relevant correlate, such that caregivers with lower levels of mindfulness (scale score < 32) were more likely to screen in for PTSD. When these selected variables were simultaneously entered into a multivariate logistic regression predicting screen-in for baseline PTSD, caregiver anxiety (B=0.44, p=.004) and bond with patient (B=-0.08, p=.03) were both significant correlates, accounting for unique variance, while an insignificant but consistent trend emerged for mindfulness (B=-0.15, p=.07). Together, these variables accounted for 47% of variance in baseline PTSD screen-in, and yielded a positive predictive validity of 71%.
Figure 1. Recursive Partitioning Decision Tree for Baseline PTSD Screening.
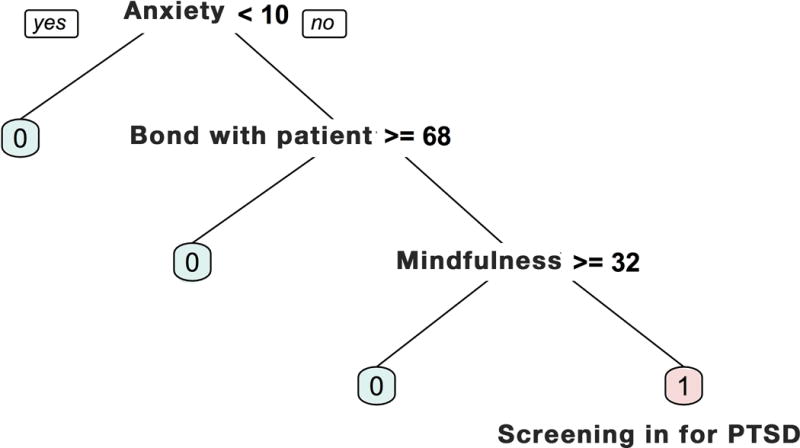
Anxiety was measured using the Hospital Anxiety and Depression Scale (HADS; possible scores 0-21); bond with patient was measured using the Intimate Bond Measure (IBM; possible scores 0-72); and mindfulness was measured using the Cognitive and Affective Mindfulness Scale-Revised (CAMS-R; possible scores 12-48). Further details on these measures and corresponding references are provided in Supplementary Materials. Cut-offs shown for each variable were determined empirically through recursive partitioning for optimal classification of baseline PTSD.
Separate recursive partitioning models revealed that only caregiver screening for PTSD at baseline was relevant for predicting PTSD at three and six months post-hospitalization, with no other predictors selected for inclusion. In logistic regressions, this single baseline predictor accounted for 47% of variance in three-month PTSD and 61% in six-month PTSD, and including other baseline predictors in these models did not substantially account for further unique variance. The positive predictive validity of baseline PTSD screen-in for PTSD at three and six months was 64% and 80%, respectively.
Discussion
This study of patients and their informal caregivers in the Neuro-ICU is the first to prospectively track PTSD symptoms in ICU caregivers across the hospitalization-recovery continuum. Literature from other trauma-exposed populations has suggested PTSD rates can decrease over time (34), but this was not observed in our sample of Neuro-ICU caregivers. Our observed rates of caregiver PTSD (16-22%) are slightly lower than in other ICU studies where prior estimates ranged from 35% to 51% (18,20–22). This may be due to our sampling of caregivers of ICU patients with relatively higher levels of functioning. Moreover, we used a scoring algorithm for clinically significant PTSD that requires a particular number of symptoms to be endorsed in each symptom cluster, which may be more stringent than an approach based on absolute cut-offs. Indeed, most prior studies used a version of the Impact of Event Scale (IES) whereas we used the PTSD Checklist (PCL), which maps onto established diagnostic criteria. Our findings are more similar to another study (16,17) that used the PCL and found rates of 14% at six months, though the latter did not assess symptoms longitudinally.
Without intervention, rates of PTSD in Neuro-ICU caregivers increased slightly but remained relatively stable over time, with the majority of those who initially screened in continuing to show clinically significant PTSD symptoms six months later. Results suggest a unique subset of Neuro-ICU caregivers are vulnerable to PTSD symptoms immediately after a loved one’s medical trauma and do not subsequently recover post-hospitalization. PTSD symptoms may persist in these caregivers because of ongoing physical sequelae in patients, which could serve as reminders of the traumatic event, and/or own deficits in coping and resiliency skills, which are important in the recovery process. Early intervention is needed to interrupt an otherwise stable course of PTSD in these caregivers following hospitalization.
Screening for PTSD at baseline emerged as the single most relevant predictor for later caregiver PTSD, demonstrating reasonable sensitivity and specificity for PTSD at three and six months. This suggests that Neuro-ICU caregivers at greatest risk for chronic PTSD can be identified with relative accuracy from their acute symptom presentation during patient admission. Results align with evidence from military and motor vehicle accident populations indicating that acute stress reactions are key to predicting later PTSD (35,36), but extends findings to caregivers of medical ICU patients. Screening Neuro-ICU caregivers when clinical follow-ups are available and providing targeted early intervention to those demonstrating clinically significant symptoms of PTSD may be an efficient way to prevent long-term PTSD.
Further opportunities for early intervention involve targeting psychosocial correlates identified in this study. Caregiver anxiety was most strongly associated with clinically significant symptoms of PTSD at baseline, suggesting a fearful/anxious profile during admission is an important marker. Additional psychosocial factors might increase precision of baseline risk assessment and serve as potential treatment targets. Specifically, caregivers’ bonds with their loved one may uniquely influence subjective experiences of patient hospitalization and related demands, such that a strong positive relationship may buffer early PTSD symptoms for caregivers, while a negative one may exacerbate them. Furthermore, caregiver mindfulness—ability to be aware of one’s thoughts and feelings in the present moment—was marginally inversely associated with baseline PTSD symptoms, suggesting that active resiliency skills employed by caregivers to respond to stressors during patient hospitalization may protect against PTSD risk (2). Interventions to teach and/or bolster mindfulness skills in acutely distressed caregivers, and to support use of these skills following hospitalization, may be critical for preventing chronic PTSD and enhancing caregiver resilience, i.e., adaptive functioning and/or absence of expected morbidities in the face of traumatic medical events (37,38).
Several methodological limitations prompt caution in interpreting the results. First, because of its longitudinal design, this study recruited a relatively modest sample of caregivers, some of whom were lost to follow-up over time. This precludes more complex modeling and may affect stability of our statistical findings, and thus should be replicated in a larger sample. Second, given the dyadic nature of the parent study, ICU caregivers were excluded if loved ones were medically contraindicated to participate or who had died. Thus, we likely captured caregivers with relatively lower distress, whereas overall rates of PTSD may be higher and/or less stable over time if we sampled a full range of caregivers. Results thus may not automatically generalize to all caregivers within the Neuro-ICU, but highlight that even caregivers of ICU patients with relatively good prognoses face notable risk for PTSD and require attention. We also sampled caregivers from a Neuro-ICU; while we expect findings may be relevant to other ICU caregiver populations, future studies should explore potential differences based on types of medical trauma, and may also benefit from targeted assessment of patient neurocognitive functioning, which may uniquely impact caregivers within Neuro-ICUs. Third, we derived predictors for PTSD at three and six months post-hospitalization with basic classification tree methods using a selection of potential factors. Future research should more thoroughly test predictive models for chronic PTSD, applying algorithm-based methods to larger datasets to derive and validate clinically relevant predictors to guide sequential decision-making (39). Although we were able to predict chronic PTSD relatively well from baseline symptoms at hospitalization, there were several cases that resolved and others that developed between baseline and subsequent time points. Future studies should also focus on identifying reliable prognostic markers that can distinguish between chronic and transient PTSD and facilitate preventive interventions for caregivers at greatest long-term risk. Finally, while we measured PTSD symptoms at baseline and assessed those who showed clinically significant symptoms, these symptoms reflect acute post-traumatic stress (10) rather than PTSD since full diagnostic criteria include a minimum of three months of such symptoms. Furthermore, these symptoms were self-reported and are not equivalent to official diagnoses provided by a trained clinician.
Despite its limitations, this study holds promising clinical implications. Using prospective data across the hospitalization-recovery continuum, we have examined potential predictors for chronic PTSD among Neuro-ICU caregivers. Assessing acute post-traumatic reactions in caregivers could help identify caregivers before they transition to chronic PTSD and while they are still accessible for early intervention. Given that anxiety, interpersonal bonds, and mindfulness were associated with early PTSD risk and are also modifiable, early interventions could incorporate cognitive-behavioral therapy (CBT) to target caregivers’ vulnerability to anxiety symptoms (40) while also improving caregiver-patient communication and teaching mindfulness-based stress management (41) to bolster psychological and behavioral resiliency while reducing distress.
Conclusions
In conclusion, this study suggests it is possible to prospectively identify Neuro-ICU caregivers who will exhibit PTSD at three and six months post-hospitalization, largely based on their acute PTSD symptoms during hospitalization. Without intervention, caregivers who exhibit clinically significant early PTSD symptoms will likely progress into chronic PTSD. By further identifying early and modifiable factors associated with baseline PTSD risk, our study provides preliminary yet tractable insights for preventing chronic PTSD in Neuro-ICU caregivers.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke Clinical Trials Methodology Course (5R25NS088248-02) to Dr. Vranceanu, and by an American Heart Association Grant (16GRNT31300008) to Dr. Vranceanu. Dr. Shaffer’s work on this manuscript was supported by the NCI (T32 CA009461 – PI: Jamie Ostroff; NIH/NCI Cancer Center Support Grant P30 CA008748 – PI: Craig Thompson). We thank Mary Guanci and the dedicated nursing team in the Neuro-ICU for their help with recruitment, as well as Michelle Jacobo and Eric Riklin.
Ms. Choi received support for article research from the National Institutes of Health (NIH). Dr. Shaffer’s institution received funding from National Cancer Institute - T32 CA009461 and the NIH/National Cancer Institute Cancer Center Support Grant P30 CA008748. Dr. Rosand’s institution received funding from American Heart Association, and he disclosed work for hire.
Footnotes
Disclosures: None
Copyright form disclosure: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: A literature review. Crit Care. 2015;20(1) doi: 10.1186/s13054-016-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer KM, Riklin E, Jacobs JM, Rosand J, Vranceanu A-M. Mindfulness and coping are inversely related to psychiatric symptoms in patients and informal caregivers in the neuroscience ICU: Implications for clinical care. Crit Care Med. 2016;44(11):2028–2036. doi: 10.1097/CCM.0000000000001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carek V, Norman P, Barton J. Cognitive appraisals and posttraumatic stress disorder symptoms in informal caregivers of stroke survivors. Rehabil Psychol. 2010;55(1):91–96. doi: 10.1037/a0018417. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. Washington, D.C.: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.Beach SR, Schulz R, Williamson GM, Miller LS, Weiner MF, Lance CE. Risk factors for potentially harmful informal caregiver behavior. J Am Geriatr Soc. 2005;53(2):255–261. doi: 10.1111/j.1532-5415.2005.53111.x. [DOI] [PubMed] [Google Scholar]
- 6.Bakas T, Burgener SC. Predictors of emotional distress, general health, and caregiving outcomes in family caregivers of stroke survivors. Top Stroke Rehabil. 2002;9(1):34–45. doi: 10.1310/GN0J-EXVX-KX0B-8X43. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: A prospective study. Am J Prev Med. 2003;24(2):113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 8.Ji J, Zoller B, Sundquist K, Sundquist J. Increased risks of coronary heart disease and stroke among spousal caregivers of cancer patients. Circulation. 2012;125(14):1742–1747. doi: 10.1161/CIRCULATIONAHA.111.057018. [DOI] [PubMed] [Google Scholar]
- 9.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. JAMA. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 10.Bryant RA, Creamer M, O’Donnell M, et al. Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: A network analysis. JAMA Psychiatry. 2017;74(2):135–142. doi: 10.1001/jamapsychiatry.2016.3470. [DOI] [PubMed] [Google Scholar]
- 11.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: Postintensive care syndrome–family. Crit Care Med. 2012;40(2):618–624. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 12.Shalev AY, Freedman S, Peri T, et al. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155(5):630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- 13.Harvey AG, Bryant RA. The relationship between acute stress disorder and posttraumatic stress disorder: A 2-year prospective evaluation. J Consult Clin Psychol. 1999;67(6):985–988. doi: 10.1037/0022-006X.67.6.985. [DOI] [PubMed] [Google Scholar]
- 14.van den Born-van Zanten SA, Dongelmans DA, Dettling-Ihnenfeldt D, Vink R, van der Schaaf M. Caregiver strain and posttraumatic stress symptoms of informal caregivers of intensive care unit survivors. Rehabil Psychol. 2016;61(2):173–178. doi: 10.1037/rep0000081. [DOI] [PubMed] [Google Scholar]
- 15.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 16.Gries CJ, Engelberg RA, Kross EK, et al. Predictors of symptoms of posttraumatic stress and depression in family members after patient death in the ICU. Chest. 2010;137(2):280–287. doi: 10.1378/chest.09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kross EK, Engelberg RA, Gries CJ, Nielsen EL, Zatzick D, Curtis JR. ICU care associated with symptoms of depression and posttraumatic stress disorder among family members of patients who die in the ICU. Chest. 2011;139(4):795–801. doi: 10.1378/chest.10-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson WG, Arnold RM, Angus DC, Bryce CL. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med. 2008;23(11):1871–1876. doi: 10.1007/s11606-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fumis RRL, Ranzani OT, Martins PS, Schettino G. Emotional disorders in pairs of patients and their family members during and after icu stay. PLOS ONE. 2015;10(1):e0115332. doi: 10.1371/journal.pone.0115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrinec AB, Mazanec PM, Burant CJ, Hoffer A, Daly BJ. Coping strategies and posttraumatic stress symptoms in post-icu family decision makers. Crit Care Med. 2015;43(6):1205–1212. doi: 10.1097/CCM.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdam JL, Dracup KA, White DB, Fontaine DK, Puntillo KA. Symptom experiences of family members of intensive care unit patients at high risk for dying. Crit Care Med. 2010;38(4):1078–1085. doi: 10.1097/CCM.0b013e3181cf6d94. [DOI] [PubMed] [Google Scholar]
- 22.Hartog CS, Schwarzkopf D, Riedemann NC, et al. End-of-life care in the intensive care unit: A patient-based questionnaire of intensive care unit staff perception and relatives’ psychological response. Palliat Med. 2015;29(4):336–345. doi: 10.1177/0269216314560007. [DOI] [PubMed] [Google Scholar]
- 23.Zohar J, Juven-Wetzler A, Sonnino R, Cwikel-Hamzany S, Balaban E, Cohen H. New insights into secondary prevention in post-traumatic stress disorder. Dialogues Clin Neurosci. 2011;13(3):301–309. doi: 10.31887/DCNS.2011.13.2/jzohar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behav Res Ther. 1996;34(8):669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Steffen AM, McKibbin C, Zeiss AM, Gallagher-Thompson D, Bandura A. The revised scale for caregiving self-efficacy: Reliability and validity studies. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):74–86. doi: 10.1093/geronb/57.1.p74. [DOI] [PubMed] [Google Scholar]
- 28.Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5(3):233–237. doi: 10.1016/0149-7189(82)90074-x. [DOI] [PubMed] [Google Scholar]
- 29.Antoni MH. Stress management and psychoneuroimmunology in HIV infection. CNS Spectr. 2003;8(1):40–51. doi: 10.1017/s1092852900023440. [DOI] [PubMed] [Google Scholar]
- 30.Feldman G, Hayes A, Kumar S, Greeson J, Laurenceau J-P. Mindfulness and emotion regulation: The development and initial validation of the cognitive and affective mindfulness scale-revised (CAMS-R) J Psychopathol Behav Assess. 2007;29(3):177. doi: 10.1007/s10862-006-9035-8. [DOI] [Google Scholar]
- 31.Wilhelm K, Parker G. The development of a measure of intimate bonds. Psychol Med. 1988;18(1):225–234. doi: 10.1017/s0033291700002051. [DOI] [PubMed] [Google Scholar]
- 32.rpart: Recursive Partitioning and Regression Trees. https://cran.r-project.org/web/packages/rpart/index.html. Accessed September 14, 2017.
- 33.R Core Team. A language and environment for statistical computer: R Foundation for Statistical Computing. R-Project; http://www.R-project.org/. Accessed June 14, 2017. [Google Scholar]
- 34.Santiago PN, Ursano RJ, Gray CL, et al. A systematic review of ptsd prevalence and trajectories in DSM-5 defined trauma exposed populations: Intentional and non-intentional traumatic events. In: Coyne J, editor. PLoS ONE. 4. Vol. 8. 2013. p. e59236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon Z. Trajectories of PTSD: A 20-year longitudinal study. Am J Psychiatry. 2006;163(4):659. doi: 10.1176/appi.ajp.163.4.659. [DOI] [PubMed] [Google Scholar]
- 36.Koren D, Arnon I, Klein E. Long term course of chronic posttraumatic stress disorder in traffic accident victims: A three-year prospective follow-up study. Behav Res Ther. 2001;39(12):1449–1458. doi: 10.1016/s0005-7967(01)00025-0. [DOI] [PubMed] [Google Scholar]
- 37.Windle G, Bennett KM. The Social Ecology of Resilience. Springer; New York, NY: 2012. Caring relationships: How to promote resilience in challenging times; pp. 219–231. [DOI] [Google Scholar]
- 38.Bonanno G, Mancini A. Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychol Trauma Theory Res Pract Policy. 2012;4(1):74–83. [Google Scholar]
- 39.Kononenko I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif Intell Med. 2001;23(1):89–109. doi: 10.1016/s0933-3657(01)00077-x. [DOI] [PubMed] [Google Scholar]
- 40.Ellard KK, Deckersbach T, Sylvia LG, Nierenberg AA, Barlow DH. Transdiagnostic treatment of bipolar disorder and comorbid anxiety with the unified protocol: A clinical replication series. Behav Modif. 2012;36(4):482–508. doi: 10.1177/0145445512451272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


