Abstract
Stress-induced gene expression in barley (Hordeum vulgare cv Salome) leaves has been correlated with temporally changing levels of octadecanoids and jasmonates, quantified by means of gas chromatography/mass spectrometry-single ion monitoring. Application of sorbitol-induced stress led to a low and transient rise of jasmonic acid (JA), its precursor 12-oxophytodienoic acid (OPDA), and the methyl esters JAME and OPDAME, respectively, followed by a large increase in their levels. JA and JAME peaked between 12 and 16 h, about 4 h before OPDA and OPDAME. However, OPDA accumulated up to a 2.5-fold higher level than the other compounds. Dihomo-JA and 9,13-didehydro-OPDA were identified as minor components. Kinetic analyses revealed that a transient threshold of jasmonates or octadecanoids is necessary and sufficient to initiate JA-responsive gene expression. Although OPDA and OPDAME applied exogenously were metabolized to JA in considerable amounts, both of them can induce gene expression, as evidenced by those genes that did not respond to endogenously formed JA. Also, coronatine induces JA-responsive genes independently from endogenous JA. Application of deuterated JA showed that endogenous synthesis of JA is not induced by JA treatment. The data are discussed in terms of distinct signaling pathways.
In the last decade, jasmonic acid (JA) and its methyl ester (JAME) (Fig. 1), collectively named “jasmonates,” were recognized as plant growth regulators with signaling properties in various developmentally and environmentally induced changes in gene expression (Creelman and Mullet, 1997; Wasternack and Parthier, 1997). Based on the biosynthetic pathway elucidated by Vick and Zimmerman (1983), a lipid-based signaling pathway was proposed in which JA and JAME are formed from α-linolenic acid (α-LeA). The sequential action of a lipoxygenase (LOX), an allene oxide synthase (AOS), and an allene oxide cyclase (AOC) lead to the formation of 12-oxophytodienoic acid (OPDA). This compound is further modified by a reductase and three β-oxidation steps, leading to JA. For all C18 compounds, such as OPDA, the term “octadecanoid” is used. First described in wounded tomato leaves, convincing data were collected on the occurrence of this so-called octadecanoid pathway in plant defense by: (a) detection of the induced formation of JA and its precursors and by inhibitor studies (Peña-Cortés et al., 1993; Farmer et al., 1994; O'Donnell et al., 1996), (b) isolation of mutants (Howe et al., 1996; McConn and Browse, 1996), (c) overexpression of an AOS (Harms et al., 1995), or (d) antisense expression of a chloroplastic LOX (Bell et al., 1995). The application of JA, JAME, or various external stimuli such as wounding (Farmer and Ryan, 1992; O'Donnell et al., 1996), UV light (Conconi et al., 1996), burning (Herde et al., 1996), oligosaccharides (Doares et al., 1995), electric current application (Herde et al., 1996), or sorbitol stress (Lehmann et al., 1995) all lead to an endogenous rise of jasmonates. These were accompanied by an up-regulation and a down-regulation of the expression of specific genes. Therefore, jasmonates act as a “master switch” (Wasternack and Parthier, 1997).
Figure 1.
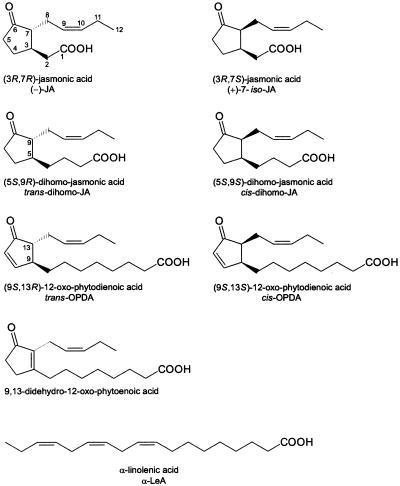
Structures of octadecanoids and jasmonates accumulating in sorbitol-stressed barley leaves.
OPDA and its molecular mimic, coronatine, a phytotoxin produced by several pathovars of Pseudomonas syringae, were found to be highly active in several JA-responsive events (Kutchan, 1993; Weiler et al., 1994). Furthermore, various JA amino acid conjugates and their synthetic analogs were more active than JA (Krumm et al., 1995) or exhibited activity without having to be cleaved (Kramell et al., 1997), suggesting that amino acid conjugates of JA are naturally occurring signaling compounds. Finally, a new OPDA derivative originating from a C16 fatty acid (16:3) was identified in extracts of wounded potato and Arabidopsis leaves (Weber et al., 1997). This is in agreement with the recently observed diversity of signaling properties among various JA-like compounds (Wasternack et al., 1998b). In numerous cell suspension cultures, a transient rise in OPDA followed by a transient rise in JA was found upon elicitation (Parchmann et al., 1997). For one of the most sensitive octadecanoid/jasmonate responses, the tendril coiling of Bryonia dioica, OPDA and JA were found to function as independent signals, with preferential activity exhibited by the former (Stelmach et al., 1998; Blechert et al., 1999). The preferential accumulation of distinct octadecanoids or compounds derived from them in a given plant led to the suggestion that each plant may have a distinct pattern designated as the “oxylipin signature” (Weber et al., 1997).
In barley, treatment of leaves with substituted, structurally deleted, or stereospecifically altered JA revealed that the naturally occurring activity of (+)-7-iso-JA was most active in altering gene expression (Miersch et al., 1999b). However, the signaling activity of a compound applied exogenously or produced endogenously may be different. Sorbitol treatment is known to increase endogenous jasmonates, followed by the synthesis of proteins. These so-called jasmonate-induced proteins (JIPs) have molecular masses of 6, 23, 37, and 60 kD (JIP-6, JIP-23, JIP-37, and JIP-60, respectively; Lehmann et al., 1995). The endogenous rise in the level of jasmonates also induces the expression of jrg1 (jasmonate-responsive gene 1), which codes for a protein with homology to a rice root protein (Lee et al., 1996). In contrast, LOX2:Hv:1 (Vörös et al., 1998), a LOX form of 100 kD, jrg5, coding for a caffeic acid O-methyl transferase (COMT), jrg10, and jrg12 (Lee et al., 1996) were exclusively inducible by exogenous JA.
In the work reported here, we identified and quantified various octadecanoids and jasmonates whose levels were increased endogenously by sorbitol treatment. Their activity in the induction of gene expression was assessed after exogenous application. The signaling pathway of exogenously applied jasmonates/octadecanoids was suggested to differ from that appearing upon endogenous accumulation of these compounds. Furthermore, octadecanoids were found to switch on the expression of some genes without being converted into jasmonates.
RESULTS
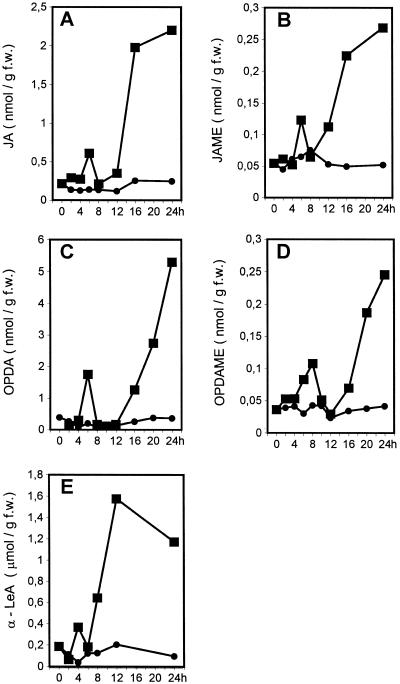
Levels of α-LeA, Jasmonates, and Octadecanoids in Stressed Barley Leaves
The synthesis of JIPs and the expression of jrgs upon sorbitol treatment of barley leaf tissue (Lehmann et al., 1995; Lee et al., 1996) prompted us to record endogenous formation of LeA, jasmonates, and octadecanoids. Barley leaf segments were floated on water (control) or a 1 m solution of sorbitol for different time periods and subjected to a gas chromatography (GC)-based method to estimate α-LeA, whereas JA and OPDA and their corresponding methyl esters were quantified by GC/mass spectrometry-selected ion monitoring (MS-SIM) analysis. The amounts of α-LeA per gram fresh weight were found to be in the micromolar range (Fig. 2E), in contrast to the nanomolar range found for JA, JAME, OPDA, and OPDAME (Fig. 2, A–D). The kinetics exhibited a transient increase of α-LeA at about 4 h, followed by a drastic rise between 5 and 12 h on about 1.5 μmol g−1 fresh weight, whereas the average of water-treated leaves was in the range of about 0.15 μmol g−1 fresh weight. In water-floated leaves, JA was found at about 130 pmol g−1 fresh weight (Fig. 2A). In sorbitol-treated leaf tissues, the JA level exhibited a transient rise that peaked at 6 h and was followed by a sharp increase between 12 and 16 h up to 2.2 nmol g−1 fresh weight at 24 h. A similar time course was found for JAME at a level 1 order of magnitude lower than that of JA (Fig. 2B). The GC/MS data indicated the parallel occurrence of both (−)-JA and (+)-7-iso-JA. For quantitative analysis, the data on the isomers were co-integrated, because the extraction procedure and the GC/MS conditions favor a rearrangement of 7-iso-JA (cis-configurated side chains) to the thermodynamically more stable JA (trans-configurated side chains) (Fig. 1) by isomerization of the pentenyl side chain based on keto/enol tautomerism.
Figure 2.
Accumulation of JA (A), JAME (B), OPDA (C), OPDAME (D), and α-LeA (E) in barley leaf segments after floating on 1 m sorbitol solution (▪) or distilled water (●) for various times. At each time point, 1 g fresh weight of leaf segments was taken and subjected to GC/MS-SIM analysis (A–D) or GC analysis (E), as described in “Materials and Methods.”
For quantitative analysis of OPDA and OPDAME, their trans- and cis-isomers (Fig. 1) were obtained as a sum in the GC/MS analysis. In water-treated leaves the endogenous level of OPDA was below 40 pmol g−1 fresh weight without significant changes during incubation time. In sorbitol-stressed leaves, OPDA accumulated, after a transient peak at about 6 h, mainly between 16 and 20 h, reaching 5 nmol g−1 fresh weight at 24 h of sorbitol treatment (Fig. 2C). OPDAME accumulated with similar kinetics up to about 250 pmol after 24 h of sorbitol treatment (Fig. 2D). The OPDAME levels of water-treated leaves were in the range of 30 to 50 pmol g−1 fresh weight. In addition to the SIM technique, the identity of OPDA and OPDAME was proven by full-scale scan measurements.
Dihomo-JA and Didehydro-OPDA Are Detectable in Sorbitol-Stressed Barley Leaves
An immunoassay of the neutral and acidic fraction extracted from 24-h-treated segments led to the detection of the following compounds (Fig. 1): (−)-JA, (+)-7-iso-JA, trans-OPDA, cis-OPDA, trans-dihomo-JA, and cis-dihomo-JA. The first four compounds, as well as the corresponding methyl esters and α-LeA, accumulated in amounts sufficient for quantification by GC/MS.
Both stereoisomeric forms of dihomo-JA were found in a minute amount by an immunological screen of the acidic fractions obtained from DEAE-Sephadex. These substances co-eluted in the reversed phase (RP)-HPLC with dihomo-JA. The identity was definitively confirmed by GC/MS spectra showing the molecular ion M+ at m/z 252 and ions at m/z 234, and m/z 184, which are in agreement with fragments published for a jasmonate derivative containing a butyric acid side chain at the pentacyclic ring (Vick and Zimmerman, 1983). Dihomo-JA was previously found to accumulate JIP23 mRNA if applied exogenously (Wasternack et al., 1998a).
Another signal detected by GC/MS was characteristic for a C18 component, a molecular ion M+ at m/z 306. This signal shifted as a GC peak to a longer retention time (tR = 14.64 min). The mass spectra provided an intensive ion signal at m/z 217 [M-(CH2)2- COOCH3-2H]+, m/z 177 [M-(CH2)5COOCH3]+, and at m/z 149 [M-(CH2)7COOCH3]+, originating from cleavage of the octanoic acid side chain by elimination. These fragments are characteristic of substituted cyclopentenes carrying the ring double bond between both side chains, as occurs in 9,13-didehydro-OPDA and its methyl ester (Vick and Zimmerman, 1984). Because of these data, the C18 compound could be confidently identified as 9,13-didehydro-OPDA. This compound was biologically inactive if applied exogenously to barley leaf segments and checked in terms of JIP-23 mRNA accumulation (data not shown).
Effect of Exogenously Applied Octadecanoids and Jasmonates on mRNA Steady-State Levels
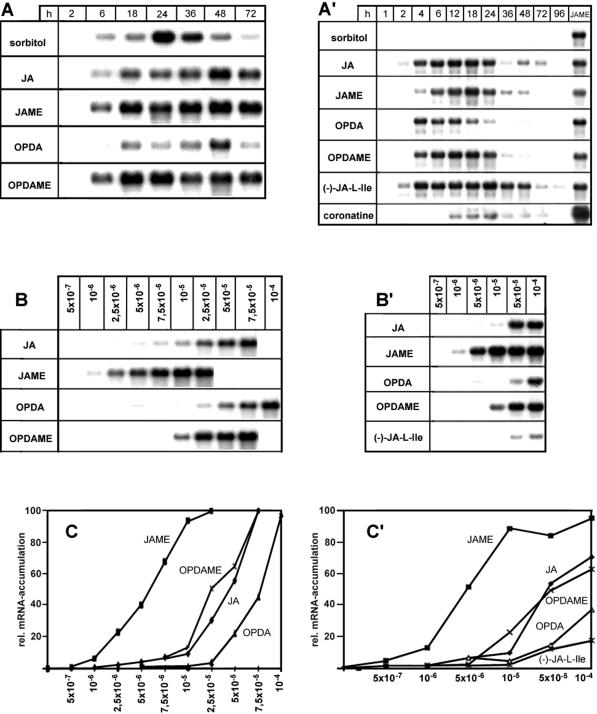
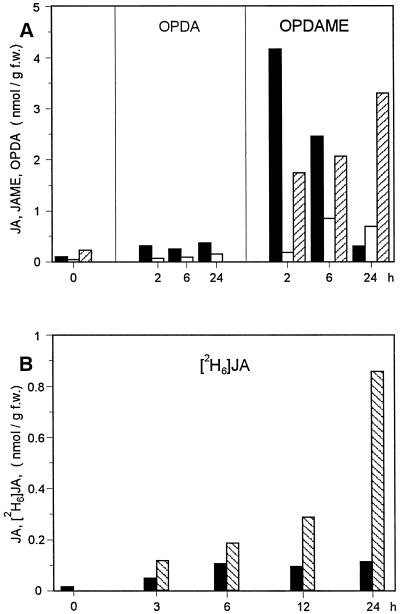
To compare the activity of JA, JAME, OPDA, and OPDAME in inducing gene expression, we recorded the accumulation kinetics and dose-response curves of two different classes of mRNA species after exogenous application of these compounds. One class of mRNAs accumulates upon exogenous application and endogenous induction of jasmonates, whereas the other class responds exclusively to exogenously applied jasmonates. Data sets for mRNAs of the same class were similar, and those for jip23 and jrg5 were selected as representatives and are shown here. Dose-response relationships, in terms of accumulation of both mRNAs, were recorded 24 h after treatment, since most of the compounds led to high mRNA steady-state levels at this time (Fig. 3, A and A′). If leaf segments were treated continuously with the different compounds, the amount of accumulated JIP-23 mRNA differed as follows: JAME > OPDAME ≈ JA > OPDA (Fig. 3, B and C). After OPDA and OPDAME treatment, a considerable amount of the portion taken up was found to be metabolized to JA and JAME (Fig. 4A). After OPDAME treatment, a level of 4 nmol JA g−1 fresh weight was already found after 2 h. This level declined dramatically to a similar level found after OPDA treatment during 24 h (Fig. 4A). Much less JA formation was found after OPDA treatment.
Figure 3.
Northern-blot analysis of mRNA accumulation of jip23 (A–C) and jrg5 (A′–C′) in barley leaf segments treated with 50 μm each of JA, JAME, OPDA, OPDAME, or JA-l-Ile and 10 nm coronatine either for various times (A and A′) or with different concentrations for 24 h (B and B′). Total RNA (10 μg per lane) was used for electrophoresis followed by northern-blot analysis (A and B) and quantification (C and C′) via the phosphor imager system described in “Materials and Methods.”
Figure 4.
Formation of jasmonates from octadecanoids (A) and endogenous levels of free JA upon treatment with deuterated JA (B) in barley leaf segments. A, 50 μm OPDAME or 50 μm OPDA was applied, and the formation of JA (black columns), JAME (white columns), and OPDA (hatched columns) were measured. B, 45 μm [2H6]JA was applied, and its portion taken up (hatched columns) and the endogenous levels of JA (black columns) were determined.
The considerable amount of JA formed upon OPDA/OPDAME treatment suggests that JA may cause jip23 mRNA to accumulate, because jip23 is a gene that responds to endogenous JA. This would mean that for both octadecanoids, the dose-response curves do not reflect the activity in inducing jip23 mRNA accumulation. Therefore, we analyzed the response in terms of accumulation of mRNAs of jrg5 (Fig. 3, A′–B′), LOX2:Hv:1, jrg10, and jrg12 (data not shown). These genes do not respond to endogenous increases in jasmonates, as indicated by the lack of mRNA accumulation after sorbitol stress (Lee et al., 1996; Vörös et al., 1998). However, following treatment with JA, JAME, OPDA, OPDAME, or the l-Ile conjugate of JA, a marked mRNA accumulation occurred, with similar kinetics for all compounds (Fig. 3A′). This mRNA accumulation corresponds to that observed for jip23 (Fig. 3, B versus B′) and jrg1, jip6, jip37, and jip60 (data not shown). This indicates that OPDA and OPDAME are able to switch on the expression of jrg5 and related genes, independently of the endogenous formation of JA, during treatment with OPDA and OPDAME, respectively (Fig. 4A). This is substantiated by the fact that coronatine induces jrg5 mRNA to accumulate (Fig. 3A′). Coronatine is a molecular mimic of OPDA (Weiler et al., 1994) and does not change the endogenous levels of jasmonates (Kramell et al., 1997). To exclude the possibility that even JA itself is able to induce a rise in endogenous JA upon treatment with JA, we analyzed endogenous amounts independently from the applied compound by using treatment with deuterated JA containing dihydro-JA as internal standard. As indicated in Figure 4B, there was no rise of endogenous JA upon treatment with deuterated JA within 24 h.
Accumulation of mRNAs in Stressed Barley Leaves
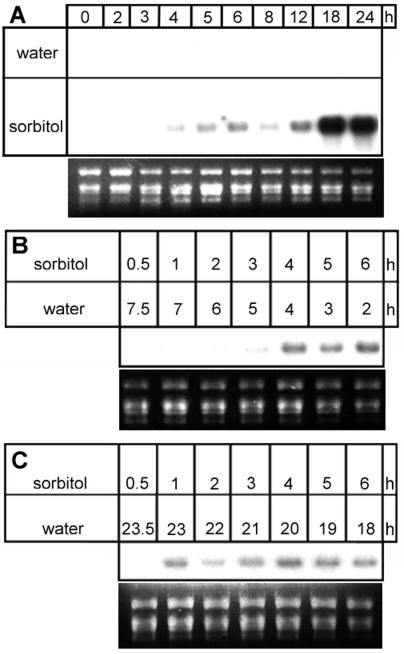
To determine whether the stress-induced accumulation of jasmonates and octadecanoids correlates temporally with the accumulation of distinct mRNAs, different regimes of sorbitol treatments were performed (Fig. 5). Upon continuous treatment (Fig. 5A), a transient JIP-23 mRNA accumulation, first detectable at about 3 h and peaking at about 6 h after the beginning of treatment, was followed by a continuous strong accumulation after 12 h. In these kinetics the transcripts accumulated in parallel with the accumulation of jasmonates and octadecanoids, as shown in Figure 2.
Figure 5.
Kinetics of mRNA accumulation of JIP-23 during treatment of barley leaf segments with 1 m sorbitol solution. In A, leaf segments were floated continuously on sorbitol. In B and C, leaf segments were floated on sorbitol for indicated times followed by floating on water up to 8 h (B) or 24 h (C). Total RNA (10 μg per lane) was used for northern-blot analysis as described in “Materials and Methods.” Each loading control is given by ethidium bromide staining.
If leaf segments were floated on sorbitol solution for increasing periods of time, followed by floating on water in the remaining intervals up to a total time of 8 or 24 h, first transcripts were detectable at 1 h after sorbitol treatment (Fig. 5C). However, after a 1-h treatment with sorbitol, the final response measured at 24 h was much less than that occurring upon continuous sorbitol treatment (Fig. 5, C versus A). This suggests that a threshold of jasmonate functions as a signal and its level may determine the response, which is analogous to a dose-response relationship following application of a compound. To test this hypothesis, α-LeA and jasmonates were quantified after different lengths of sorbitol treatment (data not shown). α-LeA was found to accumulate 3-, 4-, and 5-fold after sorbitol treatment for 1, 2, and 3 h, respectively. For jasmonates a similar level of increase was detected. This accumulation was transient and only a sorbitol treatment of >4 h led to a steady increase of the jasmonate level. However, treatment of up to 6 h followed by 18 h of water treatment did not lead to the threshold reached upon continuous treatment of 24 h. This may have been because the amount of JIP-23 mRNA accumulated at 6 h and shown in Figure 5C does not correspond to that of 24 h in Figure 5A.
DISCUSSION
The ubiquitously occurring plant growth regulators JA and JAME are believed to act as signals in various stress responses and developmentally regulated processes (Creelman and Mullet, 1997; Wasternack and Parthier, 1997). Recently, intermediates of JA biosynthesis, such as OPDA and the newly found octadecanoid dinor-OPDA, a C16 fatty acid-derived compound, as well as JA amino acid conjugates were shown to accumulate upon wounding or other environmental stimuli (Kramell et al., 1995; Parchmann et al., 1997; Weber et al., 1997; Stelmach et al., 1998). This suggests that: (a) more than one JA-like signal may exist and that (b) different plants may have a distinct pattern of octadecanoid- and JA-like compounds designated as an the “oxylipin signature” (Weber et al., 1997), which may function as a signal.
Octadecanoids and Jasmonates Accumulate in Stressed Barley Leaves
To prove signaling properties of a compound, dose-response relationships are usually recorded as first indications, as was done for JA-like compounds (Weiler et al., 1994; Krumm et al., 1995; Kramell et al., 1997; Wasternack et al., 1998b; Miersch et al., 1999b; Blechert et al., 1999). However, a response to exogenously applied JA might be different from that occurring upon its endogenous increase. Therefore, we compared alterations in mRNA accumulation pattern in response to exogenous application with those occurring upon endogenous increase in these compounds following stress. First, we investigated quantitatively the kinetics of accumulation of jasmonates and octadecanoids in barley leaves stressed by sorbitol treatment.
OPDA accumulated to 2.5-fold higher levels than JA, whereas the corresponding methyl esters reached 1 order of magnitude lower levels (Fig. 2). Each time course exhibited a weak transient peak at about 6 h followed by a more than a 5-fold increase over the basal levels of water-treated leaves. This rise occurred in the case of JA and JAME about 4 h earlier than that of OPDA and OPDAME (Fig. 2, A and B versus C and D). Since barley leaves did not release the volatile JAME under the conditions used (W. Boland, personal communication), the detected amounts are indicative of the total response of the tissue. Both the stereoisomeric pairs (−)-JA/(+)-7-iso-JA and trans-OPDA/cis-OPDA were found at a ratio of 9:1, indicating a higher stability of the trans configuration that is formed during the isolation procedure. Furthermore, the AOC was found to be the only enzymatic step of JA biosynthesis leading exclusively to the naturally cis-(+)diastereomeric product (Hamberg and Fahlstadius, 1990). Regarding these data, it is difficult to conceive of a parallel pathway originating from an 18:2 fatty acid and leading to dihydro-JA (Ziegler et al., 1997; Gundlach and Zenk, 1998). This was supported recently by substrate specificity tests for the AOC (Ziegler et al., 1999). Since JA and JAME levels balance in a ratio of 10:1 throughout the kinetics, there seems to be a constant equilibrium between both of them during sorbitol stress. A similar ratio was found upon treatment of barley leaves with deuterated JA and JAME—distinct amounts of each were detected, indicating that ester cleavage and esterification contributed to the equilibrium between JA and JAME. In contrast, JA amino acid conjugates induced JA-responsive genes without having to be cleaved (Kramell et al., 1997).
The accumulation of 9,13-didehydro-12-oxo-phytoenoic acid is surprising. Previously, this compound was reported as a constituent of the Japanese moss Dicranum majus (Ichikawa et al., 1984). The detection of both enantiomeric forms of dihomo-JA as minor components of stressed barley leaves indicates that this biogenetic intermediate of JA synthesis (Vick and Zimmerman, 1984) can also accumulate, although less abundantly than OPDA and OPDAME. Trace amounts of dihomo-JA were also detected in the fungal culture media of Botryodiplodia theobromae (Miersch et al., 1987) and Fusarium oxysporum (Miersch et al., 1999a).
Different Signaling Pathway for JA-Responsive Gene Expression
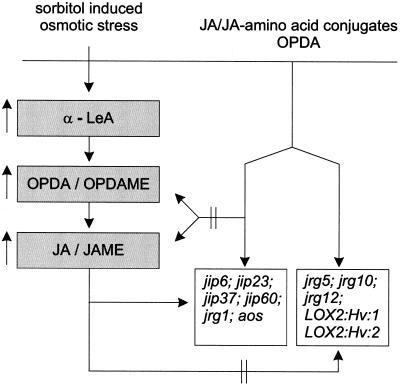
In barley leaves there are at least two sets of JA-responsive genes (Fig. 6). One group responds to exogenously applied and endogenously elevated levels of jasmonates, including genes (jip6, jip23, jip37, jip60, and jrg1). Genes of another group, such as that occurring in sorbitol-treated leaves, are not expressed after endogenous accumulation of jasmonates, but do respond to exogenously applied jasmonates. Among these genes are LOX2:Hv:1, which codes for a LOX form of 100 kD (Vörös et al., 1998); jrg5, which codes for a COMT (Lee et al., 1997); jrg10; and jrg12 (Lee et al., 1996). After treatment of barley leaves with octadecanoids and jasmonates, all of them were biologically active and exhibited the following sequence of activity in terms of accumulation of the corresponding mRNAs: JAME > OPDAME ≈ JA > OPDA. The higher activity of the methyl esters compared with the respective free acids may reflect a more efficient uptake of the former.
Figure 6.
Schematic presentation of different signaling pathways and sets of genes responding differentially to endogenous and exogenous octadecanoids and jasmonates in barley. Compounds that increased upon sorbitol treatment are indicated by an arrow.
The existence of two different sets of JA-responsive genes in barley implies different signaling pathways for exogenously applied and endogenous jasmonates and octadecanoids. However, this can only be the case if the endogenous formation of jasmonates is not induced by JA treatment within 24 h. The unchanged level of JA upon treatment with deuterated JA (Fig. 3B) argues against such an induction. These data are somewhat surprising, since the expression of the gene coding for the biosynthetic enzyme AOS of barley was shown to be transcriptionally up-regulated within 3 h after the onset of JA treatment (Maucher et al., 2000), which is similar to the situation in Arabidopsis (Laudert and Weiler, 1998). It will be interesting to see whether this up-regulation contributes to elevated α- and γ-ketol formation.
The data shown here support the existence of different signaling pathways for exogenous applied and endogenously produced octadecanoids/jasmonates as outlined schematically (Fig. 6). As shown for jrg5 expression upon OPDA or OPDAME treatment their activity seems to occur per se: (a) jrg5 is unable to respond to endogenous JA accumulation occurring during sorbitol treatment (Fig. 4A′), and consequently cannot respond to JA formed upon OPDA/OPDAME treatment (Fig. 3A), (b) coronatine, the molecular mimic of OPDA induces jrg5 expression (Fig. 4A′), (c) JA amino acid conjugates, which are active without having to be cleaved (Kramell et al., 1997), led to jrg5 expression.
Such different signaling pathways may reflect a general principle of plant cells. Separate pathways have been repeatedly described, e.g. for JA and salicylate signaling in which extracellular signals are differentially transduced leading to the expression of different but partially overlapping sets of genes (Thomma et al., 1998). It is tempting to speculate that different signaling pathways for exogenous and endogenous jasmonates (compounds) may reflect another part of the signaling network of a plant cells functioning spatially and temporally. Cross-talk between different pathways may attribute to optimize responses to various environmental stimuli (Genoud and Métraux, 1999).
The “Oxylipin-Signature” of Stressed Barley Leaves
Another question in JA/OPDA-mediated signaling in stressed barley leaves is the relative activity of these compounds in inducing expression of JA-responsive genes. There is a similar time-course of accumulation for all four compounds, suggesting that there is no preferential activity of anyone compound, at least within the time sequence. Although jasmonates increase steadily about 4 h earlier than octadecanoids this may not reflect preferential activity. As shown in Figure 5, a distinct threshold of compounds accumulating much earlier suggests that the transient rise of all compounds at about 6 h is necessary and sufficient to induce JA-responsive gene expression. Even the 2.5-fold-higher accumulation of OPDA is not necessarily indicative of a preferential biological activity, since octadecanoids release JA upon treatment (Fig. 3A). In this case, JAME instead of OPDAME may induce jip23 or other genes responding to an endogenous rise in jasmonates.
In contrast, however, the endogenous rise of OPDA was found to correlate kinetically to the onset of tendril coiling (Stelmach et al., 1998). Furthermore, octadecanoids and jasmonates were identified by structure activity tests in the tendril coiling response of B. dioica to function as independent groups of signals with preferential activity of the former (Blechert et al., 1999). Also, a transient rise in OPDA preceding that of JA was shown for numerous elicited cell suspension cultures (Parchmann et al., 1997). Together with results from accompanying papers (Gundlach et al., 1992; Blechert et al., 1995), these data suggest that OPDA is a preferential signal of phytoalexin synthesis and tendril coiling. In contrast, in barley, both groups of compounds exhibit a more similar activity; however, two or more sets of genes have to be distinguished that respond differentially to exogenous and endogenous signal (Fig. 6).
This obvious diversity of signals and signaling pathways among jasmonates and octadecanoids, as reflected in T. Farmer's term “oxylipin signature” (Weber et al., 1997), may be an advantage. Such a modular action of different signaling molecules allows the plants to respond to diverse environmental factors in a specific manner, as it becomes clear there is a concerted action of salicylate, jasmonates, and ethylene in response to pathogens (Pieterse and van Loon, 1999) and of jasmonate and ethylene in response to wounding (O'Donnell et al., 1996).
MATERIALS AND METHODS
Substances
(±)-JA (Fig. 1) was prepared by alkaline hydrolysis of racemic JAME purchased from Firmenich (Geneva); α-LeA and γ-LeA were obtained from Sigma (Deisenhofen, Germany). (±)-[10-2H,11-2H2,12-2H3]JA ([2H6J]JA) was synthesized as described previously (Miersch, 1991). OPDA was obtained from α-LeA using a crude enzyme extract from flax seeds (Zimmerman and Feng, 1978). OPDA-C2H3 ester was prepared by reaction of OPDA with deuterium-labeled methanol (C2H3OH) in the presence of catalytic amounts of p-toluene sulfonic acid for 12 h at an ambient temperature. Thereafter, the reaction mixture was concentrated by evaporation in vacuo, sodium bicarbonate solution was added, and the OPDA-C2H3 ester was extracted with chloroform. [2H5]OPDA was prepared according to the method of Zimmerman and Feng (1978) using [17-2H2,18-2H3]LeA and a flax seed extract. The standard was purified by HPLC. The labeled LeA was prepared from its methyl ester (Cambridge Isotope Laboratories, Andover, MA) by saponification with 1 m NaOH. 3-Oxo-2-(2Z-pentenyl) cyclopentane-1-butyric acid (dihomo-JA, OPC-4) was electrochemically prepared via Kolbe synthesis according to the method of Hamberg et al. (1988).
Plant Material and Incubation Conditions
Seedlings of barley (Hordeum vulgare cv Salome) were grown under greenhouse conditions with a 16-h/8-h light/dark cycle (130 μmol m−2 s−1) at 24°C and 70% relative humidity. Primary leaf segments from 7-d-old seedlings were floated on water (control), 1 m sorbitol, or aqueous solutions of JA, JAME, [2H6]JA, OPDA, and OPDAME at the concentrations indicated at 25°C and continuous white light for different time periods as described previously (Lehmann et al., 1995).
Immunological Detection of Jasmonates
The immunoreactive material was monitored using an antiserum raised against (−)-JA linked to hemocyanine (Knöfel et al., 1990). Based on the procedure described by Weiler (1986), ELISA was performed as described previously (Lehmann et al., 1995). Aliquots obtained from the RP-HPLC fractions were methylated prior to jasmonate estimation.
RP-HPLC
The chromatography was performed using a HPLC (Knauer, Berlin) fitted with a RP-18 column (250 × 4 mm, 5 μm; Eurospher 100, Knauer). The isocratic elution was carried out at a flow rate of 1 mL min−1 as follows: mobile phase 1, MeOH/0.2% (v/v) HOAc in H2O = 60:40 (v/v); mobile phase 2, MeOH/0.2% (v/v) HOAc in H2O = 80:20 (v/v). The UV absorbency was monitored at 210 nm (JA) or 225 nm (OPDA).
GC/MS-SIM
The GC/MS system was equipped with a quadruple mass spectrometer (model 5970B, Hewlett-Packard, Palo Alto, CA) combined with a gas chromatograph (model HP 5890, Hewlett-Packard) using a HP 9000/300-9133 computer set: DB5-MS column (15 m × 0.25 mm, film thickness 0.25 μm, helium gas as carrier [0.8 mL min−1], 70 eV electron impact). The temperature programs were as follows: JAME, from 80°C (3 min) to 110°C (25°C min−1); from 110°C to 125°C (10°C min−1), from 125°C (8 min) to 290°C (30°C min−1); OPDAME, from 6°C (1 min) to 190°C (15°C min−1); from 190°C to 220°C (5°C min−1); and from 220°C to 290°C (25°C min−1). Methyl esters were obtained by treating the samples with ethereal diazomethane for 10 min (JA) and 30 min (OPDA), and were analyzed by GC/MS-SIM. Retention times were as follows: [2H6]JAME, 7.87 min; JAME, 8.01 min; dihydro-JA, 8.24 min; 7-iso-JAME, 8.35 min; trans-dihomo-JAME, 9.78 min; cis-dihomo-JAME, 10.06 min; OPDA-C2H3, 13.66 min; trans-OPDAME, 13.78 min; cis-OPDAME, 14.14 min; 9,13-didehydro-12-oxo-OPAME, 14.64 min; trans-OPDAME + H2, 14.59 min; cis-OPDAME + CH2, 15.07 min; trans-(2H5) OPDAME + CH2, 14.09 min; and cis-[2H5]OPDAME + CH2, 14.95 min.
The content of JA and JAME was calculated on the basis of a calibration curve recorded with methylated [2H6]JA as an internal standard. The intensities of the molecular ions at m/z 230 for the deuterated compound and m/z 224 for the non-labeled substances were compared. Ions m/z 230, 226, and 224 were monitored when plants were treated with [2H6]JA. Endogenous levels of JA and [2H6]JA were distinguished by calculation based on fragment m/z 226, which comes from the internal standard dihydro-JA. OPDA and [2H5]OPDA could be calculated by their permethylated stable products using M+ for quantitation (m/z 320 and 325, respectively; compare with Hamberg and Fahlstadius, 1990). The estimation of the OPDA methyl ester used a calibration curve based on molecular peaks at m/z 309 for the OPDA-C2H3 ester and m/z 306 for the non-labeled compound.
Extraction, Isolation, and Quantification of Jasmonates and Octadecanoids
Barley leaf segments (1 g fresh weight) harvested at the indicated time periods were frozen in liquid nitrogen and stored at −80°C prior to homogenation and extraction with 5 mL of 80% (v/v) methanol.
For quantitation of JA and JAME, appropriate amounts of [2H6]JA were added as a supplement to the plant extract. Separation was performed by ion-exchange chromatography on DEAE-Sephadex A-25 cartridges filled with 4 mL of gel in the acetate form using a discontinuous gradient of acetic acid in methanol (7 mL of methanol, 4 mL of 0.04 m acetic acid, and 7 mL of 1 m acetic acid) (Gräbner et al., 1976). The acidic jasmonate fraction obtained was concentrated in vacuo and the residue subjected to a cartridge (500 mg, LiChrolut RP-18, Merck, Darmstadt), which was equilibrated with 20% (v/v) methanol in 0.2% (v/v) aqueous acetic acid. Subsequently, the gel was washed with 7 mL of this solvent and jasmonates were eluted at 80% (v/v) methanol in 0.2% (w/v) acetic acid/water. The concentrated eluate was finally purified by RP-HPLC using mobile phase 1. The fractions corresponding to authentic JA (7–9 min) and dihomo-JA (13–14 min) were concentrated in vacuo and stored at −20°C.
For quantitation of endogenous JA, JAME, or [2H6]JA after treatment of plants with 10−4 m [2H6]JA, dihydro-JA was added as an internal standard before homogenization of the plant material. Purification was done as described before, but GC/MS-SIM of the methylated samples was achieved after separation on C18 cartridges.
The neutral methanolic fraction containing JAME was supplemented with the [2H6]JA standard and saponified with 3.5 mL of 1 m sodium hydroxide at ambient temperature overnight. After evaporation of the methanol, the aqueous phase was adjusted to pH 3.0 and extracted five times with 1 mL of chloroform. The combined organic phases were concentrated in vacuo. Further purification was carried out by chromatography on a C18 cartridge and RP-HPLC as described above. Finally, methylated JA fractions were analyzed by GC/MS-SIM.
For analysis of OPDA and OPDAME, plant extracts with appropriate amounts of OPDA-C2H3 and [2H5]OPDA as internal standards were subjected to chromatography on DEAE-Sephadex A-25 cartridges filled with 4 mL of gel in the methanolic acetate form. The elution was performed with 7 mL of methanol, 4 mL of 0.04 m acetic acid and 7 mL of 1 m acetic acid. The neutral methanolic fraction was diluted with an equal volume of water and subjected to a C18 cartridge pretreated with 40% (v/v) methanol in 0.2% (v/v) aqueous acetic acid. After washing with 7 mL of solvent, the phytodienoate ester fraction was eluted with 7 mL of 80% methanol in 0.2% aqueous acetic acid. After concentration, the final purification was carried out by RP-HPLC with mobile phase 2. Fractions of identical retention times as the authentic OPDAME (13–15 min) were combined, concentrated, and stored at −20°C prior to GC/MS analysis.
The acidic OPDA fraction obtained from DEAE-Sephadex with 1 m acetic acid was concentrated in vacuo and subsequently purified on a C18 cartridge, similar to the procedure given for the ester fraction. Finally, the RP-HPLC fractions of identical retention volume as authentic OPDA (8–10 min) were combined, concentrated, and methylated prior to GC/MS-SIM analysis. Quantification of jasmonates and octadecanoids was performed via the corresponding internal standard added before extraction of the plant material. Due to variations in the biological material with respect to absolute amounts, an average of data at one time point of different kinetics was not be calculated. However, the kinetics shown in Figure 2 were performed with one batch of biological material. Therefore, all time points in Figure 2 are comparable in quantitative terms. One set of data of three independent replicates showing an identical time coarse is shown in Figure 2.
To isolate free LeA, aliquots (1/100) of the methanolic extracts containing the internal standard γ-LeA were purified on 500-mg RP-18 cartridge using a discontinuous gradient of methanol in 0.2% (v/v) aqueous acetic acid. Eluates with 80% to 90% methanol were concentrated in vacuo, and the remaining residue was derivatized with pentafluorobenzylbromide (Mueller and Brodschelm, 1994). The GC was performed on a gas chromatograph (HRGC 5160, Carlo Erba, Milan) using a fused silica SE30 column (25 m × 0.32 mm) with the following settings: film thickness 0.31 μm; nitrogen 2 mL min−1; makeup 30 mL min−1; split 1:15; detector: ECD; and column temperature 230°C. Retention times were: α-LeA-PFBE, 10.72 min and γ-LeA-PFBE, 9.86 min.
Isolation of RNA- and Northern-Blot Analysis
Total RNA of leaves was extracted by phenol-chloroform-isoamyl alcohol treatment, as described by Chirgwin et al. (1979) using modifications described by Andresen et al. (1992). RNA electrophoresis (5 μg of total RNA per lane if not otherwise indicated; RNA loading was checked by ethidium bromide staining) and northern-blot analysis were performed according to the method of Sambrook et al. (1989) using the following cDNA probes isolated from JAME-treated barley leaves: jip23, jip37, jip60, jrg1, jrg5, jrg10, jrg12, and LOX2:Hv:1. After transfer of RNA onto nitrocellulose BA85 (Schleicher & Schüll, Darmstadt, Germany), filters were hybridized as described previously (Leopold et al., 1996) with cDNA inserts labeled with [α-32P]dATP. Quantification via the phosphor imaging system was performed as described recently (Miersch et al., 1999b).
ACKNOWLEDGMENTS
We would like to thank S. Vorkefeld, M. Krohn, and B. Ortel for excellent technical assistance, C. Kuhnt for GC/MS-SIM measurements, Dr. B. Hause, Dr. I. Feussner, and M. Fuller for critical reading of the manuscript, C. Dietel for typing the manuscript, and C. Kaufmann for drawing the graphics.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft, Bonn (grant no. SFB 363/C5).
LITERATURE CITED
- Andresen I, Becker W, Schlüter K, Burges J, Parthier B, Apel K. The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum vulgare) Plant Mol Biol. 1992;19:193–204. doi: 10.1007/BF00027341. [DOI] [PubMed] [Google Scholar]
- Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert S, Bockelmann C, Füsslein M, v Schrader T, Stelmach B, Niesel U, Weiler EW. Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta. 1999;207:470–479. [Google Scholar]
- Blechert S, Brodschelm W, Hölder S, Kammerer L, Kutchan TM, Mueller MJ, Xia ZQ, Zenk MH. The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA. The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature. 1996;383:826–829. doi: 10.1038/383826a0. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Caldelari D, Pearce G, Walker-Simmons K, Ryan CA. Diethylthiocarbamic acid inhibits the octadecanoid signaling pathway for the wound induction of proteinase inhibitors in tomato leaves. Plant Physiol. 1994;106:337–342. [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud T, Métraux J-P. Cross-talk in plant cell signalling: structure and function of the genetic network. Trends Plant Sci. 1999;4:503–507. doi: 10.1016/s1360-1385(99)01498-3. [DOI] [PubMed] [Google Scholar]
- Gräbner R, Schneider G, Sembdner G. Fraktionierung von Gibberellinen, Gibberellinkonjugaten und anderen Phytohormonen durch DEAE-Sephadex-Chromatographie. J Chromatogr. 1976;121:110–115. doi: 10.1016/s0021-9673(00)82310-9. [DOI] [PubMed] [Google Scholar]
- Gundlach H, Müller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H, Zenk MH. Biological activity and biosynthesis of pentacyclic oxylipins: the linoleic acid pathway. Phytochemistry. 1998;47:527–537. [Google Scholar]
- Hamberg M, Fahlstadius P. Allene oxide cyclase: a new enzyme in plant lipid metabolism. Arch Biochem Biophys. 1990;276:518–526. doi: 10.1016/0003-9861(90)90753-l. [DOI] [PubMed] [Google Scholar]
- Hamberg M, Miersch O, Sembdner G. Absolute configuration of 12-oxo-10,15(Z)-phytodienoic acid. Lipids. 1988;23:521–524. [Google Scholar]
- Harms K, Atzorn R, Brash A, Kühn H, Wasternack C, Willmitzer L, Peña-Cortés H. Expression of a flax allene oxide synthase cDNA leads to increased endogenous jasmonic acid (JA) levels in transgenic potato plants but not to a corresponding activation of JA-responding genes. Plant Cell. 1995;7:1645–1654. doi: 10.1105/tpc.7.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde O, Atzorn R, Fisahn J, Wasternack C, Willmitzer L, Peña-Cortés H. Localized wounding by heat initiates the accumulation of proteinase inhibitor II in abscisic acid-deficient plants by triggering jasmonic acid biosynthesis. Plant Physiol. 1996;112:853–860. doi: 10.1104/pp.112.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Yamada K, Namikawa M, Sakai K, Kondo K. New cyclopentenonyl fatty acids from Japanese mosses. J Hattori Bot Lab. 1984;56:209–213. [Google Scholar]
- Knöfel HD, Brückner C, Kramell R, Sembdner G, Schreiber K. Radioimmunoassay for the natural plant growth regulator (−)-jasmonic acid. Biochem Physiol Pflanzen. 1990;186:387–394. [Google Scholar]
- Kramell R, Atzorn R, Schneider G, Miersch O, Brückner C, Schmidt J, Sembdner G, Parthier B. Occurrence and identification of jasmonic acid and its amino acid conjugates induced by osmotic stress in barley leaf tissue. J Plant Growth Regul. 1995;14:29–36. [Google Scholar]
- Kramell R, Miersch O, Hause B, Ortel B, Parthier B, Wasternack C. Amino acid conjugates of jasmonic acid induce jasmonate-responsive gene expression in barley (Hordeum vulgare L.) leaves. FEBS Lett. 1997;414:197–202. doi: 10.1016/s0014-5793(97)01005-3. [DOI] [PubMed] [Google Scholar]
- Krumm T, Bandemer K, Boland W. Induction of volatile biosynthesis in the lima bean (Phaseolus lunatus) by leucine- and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signalling pathway. FEBS Lett. 1995;377:523–529. doi: 10.1016/0014-5793(95)01398-9. [DOI] [PubMed] [Google Scholar]
- Kutchan TM. 12-Oxo-phytodienoic acid induces accumulation of berberine bridge enzyme transcript in a manner analogues to methyl jasmonate. J Plant Physiol. 1993;142:502–505. [Google Scholar]
- Laudert D, Weiler EW. Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signalling. Plant J. 1998;15:675–684. doi: 10.1046/j.1365-313x.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Parthier B, Löbler M. Jasmonate signalling can be uncoupled from abscisic acid signalling in barley: identification of jasmonate-regulated transcripts which are not induced by abscisic acid. Planta. 1996;199:625–632. doi: 10.1007/BF00195196. [DOI] [PubMed] [Google Scholar]
- Lee J, Vogt T, Hause B, Löbler M. Methyl jasmonate induces an O-methyltransferase in barley. Plant Cell Physiol. 1997;38:851–862. doi: 10.1093/oxfordjournals.pcp.a029244. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Atzorn R, Brückner C, Reinbothe S, Leopold J, Wasternack C, Parthier B. Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta. 1995;197:156–162. [Google Scholar]
- Leopold J, Hause B, Lehmann J, Graner A, Parthier B, Wasternack C. Isolation, characterization and expression of a cDNA coding for a jasmonate-inducible protein of 37 kDa in barley leaves. Plant Cell Environ. 1996;19:675–684. [Google Scholar]
- Maucher H, Hause B, Feussner I, Ziegler J, Wasternack C. Allene oxide synthases of barley (Hordeum vulgare cv. Salome): tissue specific regulation in seedling development. Plant J. 2000;2:199–213. doi: 10.1046/j.1365-313x.2000.00669.x. [DOI] [PubMed] [Google Scholar]
- McConn M, Browse J. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell. 1996;8:403–416. doi: 10.1105/tpc.8.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O. Synthesis of (±)-(10-2H,11-2H2,12-2H3) jasmonic acid. Z Naturforsch. 1991;46b:1724–1729. [Google Scholar]
- Miersch O, Bohlmann H, Wasternack C. Jasmonates and related compounds form Fusarium oxysporum. Phytochemistry. 1999a;50:517–523. [Google Scholar]
- Miersch O, Kramell R, Parthier B, Wasternack C. Structure-activity relations of substituted, deleted or stereospecifically altered jasmonic acid in gene expression of barley leaves. Phytochemistry. 1999b;50:353–361. [Google Scholar]
- Miersch O, Preiss A, Sembdner G, Schreiber C. (+)-7-iso-Jasmonatic acid and related compounds from Botryodiplodia theobromae. Phytochemistry. 1987;26:1037–1039. [Google Scholar]
- Mueller MJ, Brodschelm W. Quantification of jasmonic acid by capillary gas chromatography-negative chemical ionization-mass spectrometry. Anal Biochem. 1994;218:425–435. doi: 10.1006/abio.1994.1202. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Parchmann S, Gundlach H, Mueller MJ. Induction of 12-oxophytodienoic acid in wounded plants and elicited plant cell cultures. Plant Physiol. 1997;115:1057–1064. doi: 10.1104/pp.115.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta. 1993;191:123–128. [Google Scholar]
- Pieterse CMJ, van Loon C. Salicylic acid-independent plant defence pathways. Trends Plant Sci. 1999;4:52–56. doi: 10.1016/s1360-1385(98)01364-8. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Mannual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Stelmach BA, Müller A, Hennig P, Laudert D, Andert L, Weiler EW. Quantitation of the octadecanoid 12-oxophytodienoic acid, a signalling compound in plant mechanotransduction. Phytochemistry. 1998;47:539–546. doi: 10.1016/s0031-9422(97)00547-5. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun. 1983;111:470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC. Biosynthesis of jasmonic acid by several plant species. Plant Physiol. 1984;75:458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vörös K, Feussner I, Kühn H, Lee J, Graner A, Löbler M, Parthier B, Wasternack C. Characterization of methyljasmonate-inducible lipoxygenase from barley (Hordeum vulgare cv. Salome) leaves. Eur J Biochem. 1998;251:36–44. doi: 10.1046/j.1432-1327.1998.2510036.x. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Miersch O, Kramell R, Hause B, Ward J, Beale M, Boland W, Parthier B, Feussner I. Jasmonic acid: biosynthesis, signal transduction, gene expression. Fett Lipid. 1998a;100:139–146. [Google Scholar]
- Wasternack C, Ortel B, Miersch O, Kramell R, Beale M, Greulich F, Feussner I, Hause B, Krumm T, Boland W, Parthier B. Diversity in octadecanoid-induced gene expression of tomato. J Plant Physiol. 1998b;152:345–352. [Google Scholar]
- Wasternack C, Parthier B. Jasmonate-signalled plant gene expression. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- Weber H, Vick BA, Farmer EE. Dinor-oxophytodienoic acid: a new hexadecanoid signal in the jasmonate family. Proc Natl Acad Sci USA. 1997;94:10473–10478. doi: 10.1073/pnas.94.19.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler EW. Plant hormone immunoassays based on monoclonal and polyclonal antibodies. In: Linsken HF, Jackson JF, editors. Immunoassays in Plant Sciences: Modern Methods of Plant Analysis, New Series. Vol. 4. New York: Springer-Verlag; 1986. pp. 1–17. [Google Scholar]
- Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Niesel U, Bublitz F. The Pseudomonas phytotoxin coronatine mimics octadecanoid signalling molecules of higher plants. FEBS Lett. 1994;345:9–13. doi: 10.1016/0014-5793(94)00411-0. [DOI] [PubMed] [Google Scholar]
- Ziegler J, Hamberg M, Miersch O, Parthier B. Purification and characterization of allene oxide cyclase from dry corn seeds. Plant Physiol. 1997;114:565–573. doi: 10.1104/pp.114.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J, Wasternack C, Hamberg M. On the specificity of allene oxide cyclase. Lipids. 1999;34:1005–1015. doi: 10.1007/s11745-999-0451-z. [DOI] [PubMed] [Google Scholar]
- Zimmerman DC, Feng P. Characterization of a prostaglandin-like metabolite of linolenic acid produced by a flax seed extract. Lipids. 1978;13:313–316. [Google Scholar]