Abstract
The epidemic of Type 2 diabetes mellitus necessitates development of novel therapeutic and preventative strategies to attenuate expansion of this debilitating disease. Evidence links the circadian system to various aspects of diabetes pathophysiology and treatment. The aim of this review will be to outline the rationale for therapeutic targeting of the circadian system in the treatment and prevention of Type 2 diabetes mellitus and consequent metabolic comorbidities.
Pathophysiology of Type 2 Diabetes Mellitus
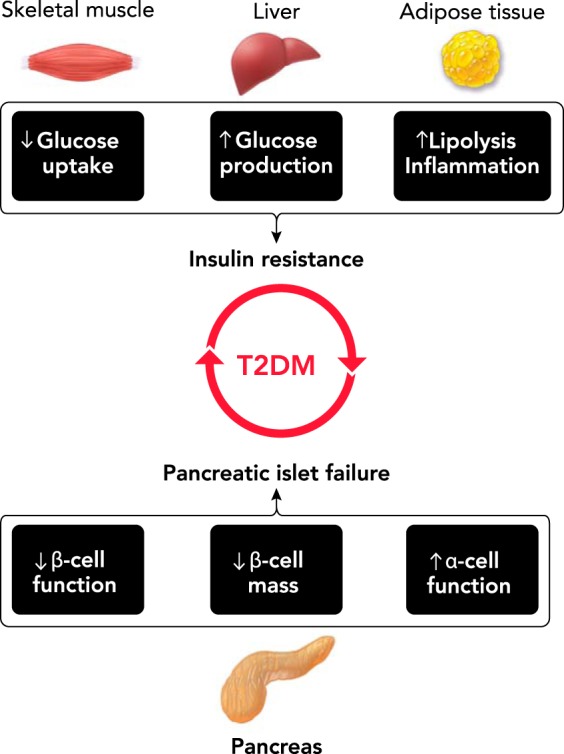
The prevalence of Type 2 diabetes mellitus (T2DM) has reached epidemic proportions and is estimated to afflict over 400 million people worldwide (174). Moreover, the incidence of diabetes is expected to continue to rise and, in the U.S. alone, is projected to affect nearly one in three people by the year 2050 (8). These alarming projections suggest that there is an urgent need for the development and implementation of novel preventative and treatment strategies to combat the rise in T2DM prevalence worldwide. T2DM manifests through the development of fasting and postprandial hyperglycemia, which is the primary contributor to the induction of numerous life-threatening complications and co-morbidities (153). The etiology of hyperglycemia in T2DM is a complex multifactorial process (127). However, it can be distilled to progressive impairments in insulin sensitivity (i.e., insulin resistance) and a corresponding failure of pancreatic islets to maintain appropriate insulin output to compensate for the decline in insulin sensitivity (i.e., islet failure) (55) (FIGURE 1). Pathophysiological manifestations of insulin resistance and islet failure are evidenced early in the evolution of the disease and are imperative for its full development (28).
FIGURE 1.
Pathophysiology of Type 2 diabetes mellitus
Type 2 diabetes (T2DM) is a complex metabolic disease in which the pathophysiology involves an interaction between genetic predisposition and environmental triggers. Hyperglycemia develops as a result of pancreatic islet failure in lieu of systemic insulin resistance. Islet failure in T2DM is associated with a deficit in β-cell mass and function and increased glucagon secretion. Insulin resistance in T2DM primarily manifests at the level of skeletal muscle, liver, and adipose tissue, and is characterized by impaired insulin-stimulated glucose disposal, failure to suppress hepatic glucose production, and elevated adipose tissue lipolysis and inflammation.
Insulin resistance manifests as a reduction in insulin’s ability to activate the cellular insulin signaling cascade and consequently stimulate insulin-mediated cellular processes. The pathophysiology of T2DM is primarily driven by induction of skeletal muscle, hepatic, and adipose tissue insulin resistance (27). Since skeletal muscle is the major organ responsible for postprandial glucose disposal, insulin resistance in skeletal muscle severely restricts the capacity for glucose clearance in patients with T2DM (102). At the cellular level, muscle insulin resistance expresses due to 1) impaired insulin-mediated recruitment of GLUT4 glucose transporter proteins to the plasma membrane, 2) attenuated capacity for glycogen storage, 3) reduction in glucose oxidation, and 4) impaired mitochondrial function (27). In the liver, insulin resistance is associated with excessive rates of hepatic glucose production during fasting, attributed in part to failed insulin-mediated suppression of gluconeogenesis (84). Liver insulin resistance is also associated with failure to suppress hepatic glucose production in the postprandial state due to impaired suppression of gluconeogenesis and glycogenolysis (127). Finally, adipose tissue insulin resistance is characterized by defective insulin-mediated glucose transport, a decreased capacity for lipid uptake, and a failure to suppress lipolysis and inflammation, resulting in elevated plasma free fatty acids (FFAs) and cytokines (145).
At the cellular level, induction of insulin resistance is largely attributed to ectopic lipid accumulation in insulin-sensitive tissues (i.e., liver, skeletal muscle, and adipose tissue) (133, 145). In muscle and liver, ectopic lipid deposition due to obesity-induced intracellular accumulation and consequent trafficking of lipid signaling intermediates (i.e., ceramides and diacylglycerols) plays a major contributory role in impaired activation of the cellular insulin signaling cascade (133, 145). Specifically, intracellular diacylglycerols and ceramides contribute to insulin resistance through deleterious effects on activation of insulin signaling molecules such as insulin receptor substrate 1 and 2 (IRS-1 and -2). This process is mediated through activation of atypical serine/threonine kinases such as protein kinase C θ (133, 145). In addition, obesity in T2DM is also associated with impaired adipocyte metabolism resulting in 1) excessive lipolysis and consequent increase in plasma free fatty acid levels, and 2) excessive production and secretion of pro-inflammatory cytokines (i.e., TNF-α, Il-6, etc.), which are thought to originate from activated adipose tissue macrophages (51). Thus aberrant adipose tissue metabolism in T2DM directly contributes to insulin resistance in target tissues through an increase in lipid accumulation or indirectly through cytokine-mediated disruption of the insulin signaling cascade in the liver and skeletal muscle (51) (FIGURE 1).
Pancreatic islet failure is a characteristic pathology in T2DM and, together with insulin resistance, is required for the establishment of hyperglycemia (18, 24) (FIGURE 1). The primary manifestation of islet failure in T2DM patients is the loss (or inappropriate activation) of glucose-stimulated insulin secretion and impaired suppression of glucagon release (55, 72). Impaired glucose-stimulated insulin secretion is attributed to the induction of β-cell secretory dysfunction and loss of β-cell numbers (i.e., β-cell mass) (55, 88). Loss of β-cell mass in T2DM patients has been attributed to increased β-cell apoptosis and the development of β-cell dedifferentiation (10, 157). The etiology of β-cell secretory dysfunction and loss is highly complex. Cumulative evidence points to induction of intracellular oxidative and/or endoplasmic reticulum stress brought on by increased exposure to toxicity associated with hyperglycemia/hyperlipidemia and/or islet amyloid peptide oligomers (IAPP) (42, 114, 136). Mechanisms underlying impaired suppression of glucagon release are not well understood, with limited evidence pointing toward an increase in α-cell numbers and alterations in α-cell function in diabetes (72, 91, 139).
T2DM is a complex metabolic disease driven by interactions among diverse environmental and genetic susceptibilities (148). Although genetic factors clearly play a contributory role in the pathogenesis of T2DM, environmental and epigenetic factors appear to be the primary contributors to the recent rise in T2DM prevalence (35). In this regard, more recent evidence suggests that environmental conditions associated with disruptions of normal circadian rhythms are associated with increased incidences of T2DM. Specifically, the incidence of T2DM is substantially increased in individuals experiencing night work and shift work; an observation consistent across a variety of professional industries (64, 65, 75, 99, 111, 154). Similarly, acute circadian disruption induces alterations in glucose metabolism, which promotes a diabetogenic state within days/weeks of exposure to circadian misalignment under a variety of clinical laboratory settings (11, 21, 74, 95, 119, 135, 141). In addition, genetic polymorphisms and variants in key circadian transcripts are associated with metabolic disorders and an increased incidence of obesity, hyperglycemia, and T2DM (37, 81, 140, 168).
In summary, there is an urgent need for the development of novel therapeutic and preventative strategies to attenuate expansion in T2DM prevalence worldwide. In this regard, there is an impressive amount of consistently growing evidence from epidemiological, clinical, and animal-based research linking the circadian system to various aspects of T2DM pathophysiology and treatment. The aim of the current review will be to describe emerging physiological and molecular insights into the role of the circadian system in the pathophysiology of metabolic abnormalities associated with the development of T2DM. In addition, a particular emphasis will be placed on the discussion of the therapeutic potential of targeting key components of the circadian system for the treatment and prevention of metabolic dysfunction.
Circadian Organization, Molecular Structure, and Entrainment
The majority of organisms have evolved optimal physiological functionality under environmental conditions created by daily changes in the light-dark (LD) cycle. The driving force behind biological adaptation to changes in LD cycle is precipitated by the evolutionary development of the circadian (circa diem, about a day) system. The primary utility of the circadian system is to promote adaptation and organismal fitness in response to 24-h environmental cycles (e.g., wake/sleep, fasting/feeding, etc). A key aspect of circadian physiology is its intrinsic nature, suggesting the presence of an internal cell-specific temporal program that anticipates and regulates behavioral, physiological, genetic, and metabolic cycles appropriate for the outside environment (112). Thus evolutionary origins of the circadian system appear to be selected to promote organismal adaptation to diurnal changes in oxidative environment, UV-mediated DNA damage, as well as optimal diurnal regulation of cellular energetics and metabolism (112, 158). Indeed, a functional circadian system has been shown to provide a clear fitness advantage to organisms from diverse living phyla such as plants (29), bacteria (167), yeast (19), and mammals (26, 63).
Circadian System Organization in Mammals
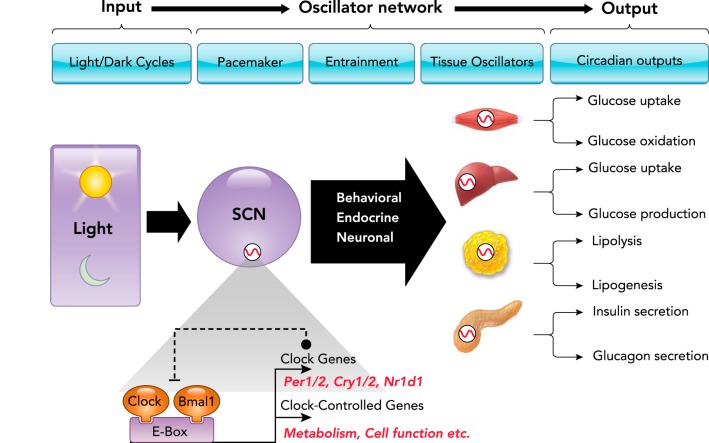
Given the fundamental importance of the circadian system in mammalian physiology, circadian rhythmicity permeates all levels of mammalian organization from behavior to hormonal secretion, temperature regulation, as well as molecular regulation of gene transcription and translation (124, 155, 156). To provide efficient and precise coordination of circadian timing throughout the body, the circadian system is organized as a multi-level hierarchical oscillator network (FIGURE 2). The pacemaker or “master clock” is localized to a subset of bilateral neurons in the suprachiasmatic nucleus (SCN) of the hypothalamus (56, 97, 123, 151). Indeed, SCN neurons appear to determine the period of an organism’s circadian rhythms and are essential for the generation of circadian rhythms in most physiological and behavioral functions (56, 97). Light is the most salient stimulus responsible for the entrainment of the SCN clock to daily changes in LD cycles (47). Entrainment of the SCN clock to environmental cycles is mediated via direct retinal projections from the specialized melanopsin-enriched photoreceptors (4). It is important to note that light-mediated circadian neuronal pathways are distinct from conventional phototransduction mediated by rods and cones, which further emphasizes the unique nature of the circadian system in mammalian physiology (36).
FIGURE 2.
Organization and molecular structure of the mammalian circadian system
To provide efficient coordination of circadian timing, the mammalian circadian system is organized as a multi-level hierarchical oscillator network. Changes in the LD cycle are perceived by specialized ganglion cells in the retina, synchronizing the central pacemaker of the circadian system in the SCN to the solar day. The main function of the SCN clock appears to coordinate and synchronize cell-autonomous circadian oscillators present in a wide array of peripheral tissues (e.g., skeletal myocytes, hepatocytes, adipocytes, and pancreatic islet cell subtypes) via a combination of neuronal, humoral, and behavioral cues, thus integrating a complex multi-level hierarchical oscillator network. The molecular make up of circadian oscillators consists of the transcriptional activators CLOCK and its heterodimer BMAL1, along with repressor genes that encode period (PER1, 2) and cryptochrome (CRY1, 2) proteins. This regulatory mechanism ensures the generation of 24-h cycles of transcription and translation. Specifically, the CLOCK: BMAL1 heterodimer is essential for the generation of circadian rhythms of transcription through DNA binding to conserved promoter regions (E-boxes) of numerous clock-controlled genes critical for regulation of diverse cellular functions and metabolic pathways.
Self-sustainable and cell-autonomous circadian oscillators are also present in a wide array of peripheral tissues/cells including tissues critical for metabolic control such as skeletal myocytes, hepatocytes, adipocytes, and pancreatic islet cell subtypes (132) (FIGURE 2). These cell-autonomous oscillators are capable of sustaining 24-h circadian rhythms in cell-specific functions without continuous input from the SCN (170). However, the main function of the SCN clock appears to be to coordinate and synchronize tissue circadian oscillators via a combination of neuronal, humoral, and behavioral cues, thus integrating a complex multi-level hierarchical oscillator network (FIGURE 2).
To date, the exact physiological mechanism by which the SCN synchronizes peripheral oscillators remains an area of active investigation. However, accumulating data shows that the SCN engages multiple biological routes to regulate phase entrainment and circadian physiology of peripheral tissue oscillators. Specifically, both parasympathetic and sympathetic branches of the autonomic nervous systems mediate SCN-dependent entrainment of circadian rhythms (57, 97, 162). For example, circadian rhythms in hepatic glucose production and ambient glycemia rely on SCN-mediated autonomic control of the hepatic circadian clock (66). The SCN also regulates entrainment of peripheral oscillators indirectly through modulation of fasting/feeding cycles, which is particularly apparent in cell types responsive to daily nutritional challenges (e.g., pancreatic islets and hepatocytes) (25, 121). Although the exact mechanisms mediating feeding-induced circadian entrainment of peripheral oscillators are unknown, combinations of gut-related hormonal factors and food-related metabolites have been proposed (98). Finally, the SCN also synchronizes circadian rhythms through regulation of temperature and hormonal rhythms (e.g., glucocorticoids) (68, 137). Overall, the SCN plays a central role in orchestrating the entrainment of peripheral oscillators while utilizing redundant signaling mechanisms to fine tune the temporal regulation of diverse circadian rhythms in mammals (FIGURE 2).
Molecular Mechanisms of Cell-Autonomous Circadian Clocks
The molecular makeup of the SCN and peripheral circadian oscillators is essentially identical and consists of a cell-autonomous transcriptional-translational feedback loop regulated by a set of core “clock” genes (FIGURE 2). Pioneering work by Hardin, Hall, and Rosbash first identified the Period (Per) gene as a core component of the circadian oscillator loop in Drosophila, thus providing early mechanistic insights into the function of the circadian clock (44, 45). In mammals, the core of the circadian clock comprises the transcription factors CLOCK and its heterodimer BMAL1 (also known Arntl or Mop3), and repressor genes that encode period (PER 1,2) and cryptochrome (CRY 1,2) proteins (155, 156). Secondary regulatory loops involving the nuclear receptors Rev-erbα/β and RORα/β provide additional molecular stabilization and control by acting as respective transcriptional repressors and activators of Bmal1 (115). Together, this regulatory mechanism ensures the generation of robust 24-h cycles of transcription and translation with varying phases of expression. Specifically, the CLOCK-BMAL1 heterodimer activates transcription of target genes via DNA binding to conserved promoter regions (E-boxes). This includes recruitment and interaction with histone acetyltransferases (e.g., p300), methyltransferases (e.g., MLL1), histone demethylases (Jarid1a), and cell-specific enhancers (e.g., PDX1 in β-cells) to enhance chromatin accessibility and thus promote transcriptional activation of target genes (155). Indeed, up to 20% of the genome in the majority of studied cell types display some form of regulatory oversight by the circadian clock, which includes regulation of transcription, translation, mRNA degradation, micro-RNA turnover, and splicing (155). Notably, a great proportion of clock-controlled genes are involved in the regulation of cellular metabolism (31, 67). Thus alterations in glucose homeostasis and the propensity for obesity and diabetes were one of the earliest phenotypical observations noted in clock gene mutant mouse models (128, 161).
Circadian Clocks as Cell-Specific Regulators of Glucose Homeostasis
Role of Skeletal Muscle Circadian Clocks in Glucose Homeostasis
Both in vitro and in vivo studies have clearly demonstrated the presence of robust self-sustainable and autonomous circadian oscillators in skeletal myocytes (43, 104). Most notably, robust circadian rhythmicity exists for nearly every aspect of skeletal muscle physiology critical for proper regulation of glucose homeostasis. This includes circadian variations in skeletal muscle GLUT4 glucose transporter expression and translocation (30), glucose uptake and oxidation (32, 73), glycogen deposition (73), mitochondrial oxidative capacity (163), as well as protein (17) and lipid metabolism (78). For example, peak physiological activity in skeletal muscle glucose uptake and oxidation coincides with the onset of active/feeding circadian cycles in both mouse and human experiments, which is consistent with the primary role of the circadian system to enhance metabolic flexibility (73, 163). Importantly, circadian rhythms in skeletal muscle functions (e.g., glucose uptake) persist in isolated muscle cells in vitro, thereby suggesting transcriptional control by the endogenous circadian clock mechanism (32, 104). Moreover, T2DM is associated with disruptions in circadian control of muscle glucose uptake and oxidation, thus implicating impairments in the circadian clock as a contributory factor to the pathophysiology of skeletal muscle insulin resistance in diabetes (5, 32, 82).
The most compelling evidence for the key role of the biological clock in the regulation of skeletal muscle physiology comes from studies in Bmal1 knockout mouse models. Bmal1 is a critical clock gene essential for proper functionality of the core circadian clock (9). Indeed, Bmal1 is the only non-redundant clock gene deletion that results in ablation of behavioral, physiological, and molecular circadian rhythms (63, 90). Significantly, whole body deletion of Bmal1 is characterized by a striking reduction in skeletal muscle mass, function, and mitochondrial density, which is reversed upon muscle-specific transgenic rescue (2, 63, 90). In recent years, skeletal muscle-specific Bmal1 knockout mouse models were developed to delineate its role in the regulation of glucose metabolism. Interestingly, both embryonic and adult muscle-specific Bmal1 knockouts primarily exhibit profound loss in glucose and insulin tolerance attributed largely to impaired insulin-stimulated glucose uptake akin to pathology common to insulin-resistant patients with T2DM (30, 46, 50).
At the cellular level, loss of glucose uptake due to Bmal1 deletion is associated with the reduction in the expression and plasma membrane translocation of GLUT4 glucose transporters mediated through decreased expression of TBC1D1, a key protein involved in GLUT4 translocation from the cytoplasm to the plasma membrane (30, 46). Notably, diminished glucose transport is also seen in skeletal muscle of T2DM patients (102). Furthermore, muscle Bmal1 deletion also attenuates the expression and enzymatic activity of key metabolic enzymes essential for glucose metabolism (e.g., hexokinase) and subsequent glucose oxidation (e.g., pyruvate dehydrogenase), which diverts cellular metabolism toward greater lipid utilization and storage. This further recapitulates the phenotypical changes observed in skeletal muscle of patients with T2DM (30, 46). Overall, there is strong experimental evidence that the skeletal muscle circadian clock is a key regulator of insulin-mediated glucose transport, uptake, storage, and oxidation, and when disrupted, contributes to the development of T2DM.
Role of Hepatic Circadian Clocks in Glucose Homeostasis
The liver is one of the main organs responsible for circadian control of daily glucose homeostasis (106). Under fasting conditions, diurnal fluctuations in the rate of hepatic glucose production dictate circadian variations in blood glycemia. Thus ablation of the SCN (or liver-specific) circadian clock abolishes circadian rhythms in blood glucose concentrations and results in disrupted glucose homeostasis (1, 58). The liver also contributes to circadian control of postprandial glucose metabolism since circadian changes in the rate of hepatic glucose production are also key determinants of diurnal cycles in insulin sensitivity and meal tolerance (6, 22, 130). Moreover, key metabolic and enzymatic determinants of hepatic glucose production (i.e., rates of gluconeogenesis, glycogenolysis, and glycogen storage) all have been shown to exhibit robust circadian variations in humans and a variety of animal models (60, 73, 82, 110).
At the cellular level, hepatocytes exhibit distinct circadian oscillations in transcription, translation, and morphological features such as cell size and volume (3, 89, 100, 146, 166). Specifically, hepatocytes exhibit circadian expression patterns for metabolic genes essential for regulation of hepatic glucose uptake and storage (e.g., Glut2 and Gck), hepatic glucose production (e.g., Pepck and Gcgr), as well as cellular processes involved in hepatic lipid oxidation and storage (e.g., Cpt1 and Lipin1) (67, 100). In addition, the hepatic proteome (both nuclear and cytoplasmic) is also subjected to significant diurnal regulations, particularly in biological processes involved in DNA repair, proliferation, ribosomal biogenesis, and protein intracellular transport (166). It is important to point out that fasting-feeding cycles appear to be the main timing signals regulating many daily rhythms in hepatic function and related diurnal oscillations in hepatic transcriptome and proteome (3, 89, 152, 164). Thus, when assessing mechanisms responsible for the generation of circadian rhythms in the liver, a distinction should be made between clock-dependent and clock-independent (i.e., feeding-driven) circadian rhythms, both of which contribute to the generation of diurnal oscillations in the liver.
Chromatin immunoprecipitation combined with deep sequencing studies (ChIP-Seq) have shed some light on potential molecular targets and mechanisms guiding clock-controlled transcriptional regulation in hepatocytes (62, 126). Specifically, Rey and colleagues (126) identified a genome-wide map corresponding to over 2,000 BMAL1 binding sites in the liver. Importantly, the most prominent binding sites were enriched for promoter regions of genes regulating hepatic glucose (e.g., Glut2 and G6pc) and lipid (e.g., Agpat6 and Dgat2) metabolism, thus further implicating hepatic clocks as key mediators of glucose homeostasis (126). In accordance with this finding, liver-specific deletion of Bmal1 in mice results in the loss of circadian regulation of genes essential for regulation of hepatic glucose metabolism, such as glucose transporter Glut2, leading to dysregulation in systemic glycemia and glucose tolerance (67). More recently, Jacobi and colleagues (53) extended these findings to demonstrate that hepatic Bmal1 orchestrates intracellular mitochondrial dynamics (fission and mytophagy) and plays an essential role in the regulation of hepatic oxidative capacity. Consequently, liver-specific deletion of Bmal1 recapitulates hepatic pathology in T2DM characterized by increased oxidative stress, loss of insulin signaling, ectopic lipid accumulation, and hepatic insulin resistance (53).
Hepatic clocks also orchestrate the regulation of cellular metabolism through “indirect” pathways mediated through activation of transcription factors and other transcriptional and translational regulators (12, 40, 138, 171, 172). ChIP-Seq studies show “transcriptional regulation” as the most enriched annotated functional cluster of BMAL1 binding sites in the liver, which includes 82 DNA transcription factors and 18 liver-expressed nuclear receptors (126). Specifically, hepatic clocks regulate lipid and cholesterol metabolism through BMAL1:CLOCK-mediated transactivation of Klf10 (Kruppel-like transcription factor) and nuclear receptor Pparα (peroxisome proliferator-activated receptor α) (12, 40). Interestingly, Klf10 and Pparα also regulate Bmal1 promoter activation, thus underscoring a complex interrelationship between the circadian clock and hepatic metabolism (12, 40). Finally, studies also show that circadian clock repressor proteins (e.g., CRY 1 and 2, and PER2) coordinate circadian control over hepatic glucose metabolism through posttranslational regulation of cAMP signaling and nuclear receptor function (138, 171). For example, Zhang et al. (171) eloquently demonstrated that circadian expression of CRY1 inhibits activation of key gluconeogenic enzymes through direct binding and inhibition of the hepatic glucagon receptor Gsα subunit, whereas hepatic overexpression of Cry1 resulted in attenuated gluconeogenesis and lowered glycemia in diabetic mice. Overall, accumulating data highlights hepatic clocks as essential transcriptional/translational regulators of hepatic glucose production, uptake, and insulin sensitivity, thus underscoring their potential as therapeutic targets in T2DM (160).
Role of Adipocyte Circadian Clocks in Glucose Homeostasis
White adipose tissue (WAT) serves a dual purpose in the regulation of glucose homeostasis through its role as both an energy store and through its diverse endocrine-related functions. WAT are central energy stores harboring triglycerides (TGs), which are utilized through the process of lipolysis [i.e., the breakdown of TGs into free fatty acids (FFAs) and glycerol]. Thus proper regulation of lipolysis and its counterpart, lipogenesis, is critical in maintaining normal glucose homeostasis, as excess circulating FFAs contribute to ectopic lipid accumulation and consequent development of insulin resistance in T2DM (145). Indeed, circadian variations in plasma free fatty acids concentrations have long been described in humans (14), a phenomenon recently ascribed to circadian changes in the activation of adipose tissue insulin signaling cascade (13).
Due to robust diurnal changes in mammalian energy demands, adipose tissue processes including lipolysis, adipogenesis, and metabolic inflammation have been shown to be regulated by the circadian clock (142, 143, 169). Consistently, induction of obesity due to excessive levels of plasma triglycerides and free fatty acids are characteristically observed in Clock and/or Bmal1 mutant mice (128, 161). Furthermore, studies done in both rodents and humans have identified rhythmically expressing clock-controlled genes in various adipose tissue depots (77, 143, 175), revealing circadian expression profiles for thousands of genes critical for proper regulation of adipocyte metabolism (175). For example, in one study, the release of FFAs and glycerol was shown to be clock-regulated due to transcriptional regulation of two key lipolysis pacemaker enzymes (e.g., Atgl and Hsl) by the BMAL1 and CLOCK heterodimer (144). Moreover, Bmal1-deficient mice demonstrated a lower capacity of adipocytes to synthesize polyunsaturated fatty acids due to lower expression levels of two key genes involved in FFA elongation and desaturation, Elovl6 and Scd1, which were shown to be also transcriptionally controlled by Bmal1 (101).
In addition to serving as an energy depot, adipose tissue has been considered one of the largest endocrine organs in the body due to its unlimited growth potential and capacity to secrete various hormones and cytokines. The number of adipokines known to be secreted by adipocytes in obesity and diabetes has expanded to include leptin, adiponectin, resistin, visfatin, and omentin, in addition to inflammatory cytokines such as TNF-α, IL-6, and MCP-1, among others (83). One of the most well-regarded sources of proinflammatory cytokines in obesity-induced insulin resistance comes from adipose tissue macrophages (ATMs) (51, 79). Under diabetic conditions, ATMs are shown to undergo a switch from anti-inflammatory M2 macrophages to proinflammatory M1 macrophages, which are known producers of proinflammatory cytokines such as TNF-α, IL-6, and IL-12 (79). Although the role of the circadian system in controlling adipose tissue inflammation is not well understood, accumulating evidence suggests that perturbations in circadian pathways can elicit inflammatory responses. For example, obese circadian-disrupted mice showed enhanced adiposity associated with increased expression of key adipose inflammatory markers such as macrophage-1 antigen (MAC1) and TNF-α in WAT (33). Furthermore, clock-disrupted (Per2 mutant) macrophages demonstrated enhanced proinflammatory activation under LPS conditions and increased M1 macrophage polarization in WAT (169). Interestingly, these findings were further recapitulated in obese mice with Per1/2-disrupted myeloid cells mediated through macrophage downregulation of PPARγ, a key clock-regulated transcription factor involved in M2 macrophage polarization (169). Overall, compelling evidence suggests that multiple aspects of adipose tissue biology (e.g., insulin sensitivity, lipolysis, lipogenesis, inflammatory pathways, etc.) are controlled by the circadian clock, disruption of which leads to enhanced adiposity, insulin resistance, and an increased susceptibility for the development of T2DM.
Role of Islet Circadian Clocks in Glucose Homeostasis
In humans, circadian variation in glucose and meal tolerance has long been recognized as a hallmark feature of glucose homeostasis (14, 69, 130). Indeed, enhanced glucose tolerance manifests at the onset of the active circadian cycle and is associated with a greater insulin secretory response to glucose and β-cell-specific secretagogues (14). In support of these early observations, classic studies by Boden and colleagues utilized a 72-h glucose clamp protocol to demonstrate robust feeding and glycemia-independent circadian insulin secretory patterns in humans (7). Furthermore, circadian rhythms in glucose-stimulated insulin secretion persist in vitro in cultured isolated human (and rodent) pancreatic islets as well as in purified β-cell populations, suggesting regulatory oversight by the cell-autonomous circadian clock (103, 105, 108). Although circadian regulation of the α-cell glucagon response is much less understood, studies also show circadian variations in glucagon secretion in vivo, in cultured islets, and in purified α-cells in vitro (85, 129, 130).
The presence and function of the cell-autonomous circadian clock in pancreatic islets has been explored and confirmed by multiple groups utilizing a method to track islet cell bioluminescence in vitro with clock gene luciferase fusion constructs (e.g., Per1:LUC and Per2:LUC) (87, 107, 108, 116–118, 121). Collectively, these studies show that islet cells 1) exhibit robust autonomous circadian rhythms in clock gene expression with a distinct ~24-h period, phase, and amplitude of circadian oscillations, and 2) the SCN appears to entrain the phase of circadian clocks in islets via modulation of the feeding behavior (121). It was also demonstrated that the function of islet circadian clocks is perturbed on exposure to circadian disruption induced by changes in the photoperiod and/or exposure to obesogenic diets (117, 118). Finally, more recently, Petrenko and colleagues have employed a novel triple reporter mouse model to show the presence of robust cell-autonomous circadian oscillators in purified populations of β- and α-cells, which were notably characterized by distinct phases in transcriptional and functional oscillations (108).
At the cellular level, islet cell clocks orchestrate circadian oscillations in the transcriptome akin to other metabolically active cell types (i.e., liver and skeletal muscle) (103, 108, 122). Indeed, a large proportion of transcripts in mouse islets (up to a third) exhibit statistically significant circadian profiles (103). Circadian-expressed transcripts in islets cells are predominantly enriched for biological pathways regulating insulin secretion and exocytosis (e.g., SNARE interactions, vesicular transport, and exocytosis), mitochondrial function, ER protein processing and transport, as well as transcripts involved in the regulation of cell turnover (e.g., DNA replication and DNA damage and repair response) (103, 108, 122). Importantly, purified populations of islet cell subtypes have been recently shown to display a subset of α- and β-cell-specific circadian transcripts with respective peak expression during either the inactive/fasting or active/feeding circadian cycle corresponding to peak secretion of glucagon and insulin (108).
At the molecular level, recent work by Perelis et al. (103) eloquently demonstrated that circadian control of insulin secretion and islet transcriptional regulation is orchestrated by rhythmic DNA binding of the CLOCK:BMAL1 heterodimer with the key islet transcription factor, PDX-1, along with concurrent recruitment of islet cell-specific enhancers. Consequently, given the widespread importance of circadian clocks for regulation of islet transcription and hormonal secretion, multiple groups have now shown induction of glucose intolerance, hyperglycemia, and impaired glucose-stimulated insulin secretion in pancreas and β-cell-specific clock gene mutants (71, 87, 103, 120, 131). Moreover, β-cell-specific clock disruption also compromises the β-cell’s replicative capacity and promotes DNA damage-induced apoptosis, likely attributed to an increased intracellular oxidative environment and induction of ER stress (70, 120). Taken together, islet circadian clocks appear to play an essential role in the regulation of β- and α-cell function and turnover. Consequently, clock-disrupted islets recapitulate both functional and morphological abnormalities associated with islet failure in T2DM.
Circadian System as a Novel Therapeutic Target in T2DM
Growing evidence suggests that therapeutic strategies designed to enhance circadian clock function may be beneficial for the prevention and treatment of metabolic disorders such as T2DM. First, as outlined in the current review, cell-autonomous circadian clocks orchestrate the regulation of physiological functions required for maintenance of normal glucose homesosis (e.g., skeletal muscle glucose uptake, hepatic glucose production, adipose tissue lipolysis, and islet cell function) (30, 46, 53, 103, 142). Correspondingly, phenotypical characteristics of many clock gene mutant mice recapitulate the pathophysiology commonly observed in patients with T2DM, such as insulin resistance, excessive hepatic glucose production, ectopic fat accumulation, impaired insulin secretion, and β-cell loss (30, 46, 53, 103, 120, 142). Second, pathogenesis of obesity and T2DM per se is linked with impairments in circadian regulation both at the level of central and peripheral circadian oscillators (16, 39, 61, 92, 134). Thus development of obesity and T2DM in humans is evidenced by disrupted circadian physiology (5, 54, 69, 82, 86, 125, 150). Indeed, obese diabetic rodents are characterized by disrupted behavioral and metabolic circadian rhythms associated with suppression of CLOCK-BMAL1 transactivation and impaired function of circadian oscillators in peripheral tissue (e.g., liver, skeletal muscle and islets) (48, 61, 118). Moreover, ectopic expression of CLOCK and BMAL1 in insulin-sensitive tissues has been shown to reverse insulin resistance in skeletal muscle and liver, reduce lipolysis in adipocytes, and improve glucose tolerance in mouse models of T2DM (76, 142, 173).
The first circadian-based therapeutic tested for antidiabetic efficacy was melatonin, a hormone synthesized and secreted from the endocrine cells in the pineal gland (93). Circadian melatonin production and secretion is driven through direct neuronal inputs from the SCN (94), and thus circadian secretion of melatonin has been described as a hormonal output of the central circadian clock (109). Indeed, exogenous melatonin administration exerts direct effects on the SCN and peripheral circadian oscillators through ubiquitously expressed melatonin receptors (38). Subsequently, timed melatonin supplementation is effective in enhancing global circadian rhythms in both rodents and humans (15, 147). Importantly, melatonin supplementation in rodents has proven to be effective in reducing adiposity and attenuating both hepatic and skeletal muscle insulin resistance by direct enhancement of the cellular insulin signaling cascade (41, 96). Furthermore, in isolated T2DM islets, activation of melatonin signaling has been shown to attenuate induction of the oxidative and endoplasmic reticulum stress and improve glucose-stimulated insulin secretion and β-cell survival (23, 159). Although some initial clinical studies in humans reported beneficial effects of melatonin treatment for glycemic control in patients with T2DM, additional well-controlled clinical trials are required to elucidate the potential efficacy of melatonin for the prevention and treatment of T2DM in humans (34).
In recent years, an increased emphasis has been placed on the development of small-molecule chemical enhancers of circadian system with goals of augmenting clock-regulated physiological outputs (20, 165). Subsequently, a number of chemical compounds specifically targeting the core circadian oscillator have been developed with documented efficacy in modulating metabolic function in pre-clinical studies. Specifically, Nobiletin (a naturally occurring flavonoid) has been recently identified as a clock amplitude enhancer, actions of which are mediated through ROR nuclear receptor activation (48). Importantly, chronic administration of Nobiletin in two distinct obese/T2DM mouse models resulted in restoration of oscillatory patterns in circadian and metabolic gene expression in the liver and corresponding phenotypical improvements in glycemia, glucose tolerance, insulin resistance, and ectopic fat accumulation (48). Another recently discovered chemical modulator of the circadian system, Rev-ERB-α/β agonist, also has been shown to modulate circadain metabolic gene expression and improve glycemia, lipidemia, and ectopic fat accumulation in mice (149). Moreover, Hirota and colleagues identified a small molecule CRY activator (KL001) that interferes with ubiquitin-mediated CRY degradation, resulting in CRY stabilization and lengthening of the circadain period (49). Importantly, KL001 administration was shown to attenuate glucagon-induced gluconeogenic flux in isolated hepatocytes in vitro and also to improve glucose intolerance in obese insulin-resistant mice in vivo (49, 52). Finally, beneficial metabolic effects ascribed to chemical modulators of Rev-ERB-α/β and CRY were not associated with enhanced circadian clock amplitude, which underscores that more studies are needed to fully understand mechanisms underlying physiological effects of clock-modifying chemical compounds (49, 52).
Concluding Remarks
Mammalian circadian physiology is highlighted by exceptional stability in time-based regulation of numerous biological functions displaying a near-24-h temporal resolution period with minimal coefficient of variance (112). However, physiological benefits of the circadian system are diminished when external environmental cycles deviate from the norm (i.e., 24 h) as is becoming more and more prevalent in today’s society (80). This produces the state often characterized as circadian misalignment or disruption (26, 59, 113), a condition strongly associated with metabolic dysfunction and development of obesity and T2DM (11, 21, 64, 65, 74, 75, 95, 99, 111, 119, 135, 141, 154). The current review highlights extensive clinical and pre-clinical data supporting the essential role of circadian system in the regulation of metabolic function and glucose homeostasis. This work also provides strong rationale for therapeutic targeting of circadian system in the treatment and prevention of T2DM and consequent metabolic comorbidities. Future studies are urgently needed to identify and characterize mechanisms of action of novel chemical and endogenous modifiers of mammalian circadian system, with potential to augment clock-regulated metabolic and glucose-responsive physiological processes.
Acknowledgments
Funding for this work was provided by National Institutes of Health Grants DK-098468 (to A.V.M.) and T32-HL-105355 (to N.J.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: N.J. and A.V.M. conceived and designed research; N.J. and A.V.M. drafted manuscript; N.J. and A.V.M. edited and revised manuscript; N.J. and A.V.M. approved final version of manuscript; A.V.M. prepared figures.
References
- 1.Ando H, Ushijima K, Shimba S, Fujimura A. Daily fasting blood glucose rhythm in male mice: a role of the circadian clock in the liver. Endocrinology 157: 463–469, 2016. doi: 10.1210/en.2015-1376. [DOI] [PubMed] [Google Scholar]
- 2.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 107: 19090–19095, 2010. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atger F, Gobet C, Marquis J, Martin E, Wang J, Weger B, Lefebvre G, Descombes P, Naef F, Gachon F. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc Natl Acad Sci USA 112: E6579–E6588, 2015. doi: 10.1073/pnas.1515308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070–1073, 2002. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 5.Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes 48: 2182–2188, 1999. doi: 10.2337/diabetes.48.11.2182. [DOI] [PubMed] [Google Scholar]
- 6.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 45: 1044–1050, 1996. doi: 10.2337/diab.45.8.1044. [DOI] [PubMed] [Google Scholar]
- 7.Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol 271: E246–E252, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8: 29, 2010. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017, 2000. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 11.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4: 129ra43, 2012. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol 20: 1715–1727, 2006. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- 13.Carrasco-Benso MP, Rivero-Gutierrez B, Lopez-Minguez J, Anzola A, Diez-Noguera A, Madrid JA, Lujan JA, Martínez-Augustin O, Scheer FA, Garaulet M. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J 30: 3117–3123, 2016. doi: 10.1096/fj.201600269RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll KF, Nestel PJ. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes 22: 333–348, 1973. doi: 10.2337/diab.22.5.333. [DOI] [PubMed] [Google Scholar]
- 15.Cassone VM, Natesan AK. Time and time again: the phylogeny of melatonin as a transducer of biological time. J Biol Rhythms 12: 489–497, 1997. doi: 10.1177/074873049701200602. [DOI] [PubMed] [Google Scholar]
- 16.Challet E, van Reeth O, Turek FW. Altered circadian responses to light in streptozotocin-induced diabetic mice. Am J Physiol Endocrinol Physiol 277: E232–E237, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Chang SW, Yoshihara T, Machida S, Naito H. Circadian rhythm of intracellular protein synthesis signaling in rat cardiac and skeletal muscles. Biochem Biophys Rep 9: 153–158, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab 6: 943–957, 2017. doi: 10.1016/j.molmet.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Odstrcil EA, Tu BP, McKnight SL. Restriction of DNA replication to the reductive phase of the metabolic cycle protects genome integrity. Science 316: 1916–1919, 2007. doi: 10.1126/science.1140958. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Yoo SH, Takahashi JS. Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu Rev Pharmacol Toxicol 58: 231–252, 2018. doi: 10.1146/annurev-pharmtox-010617-052645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colwell CS, Matveyenko AV. Timing is everything: implications for metabolic consequences of sleep restriction. Diabetes 63: 1826–1828, 2014. doi: 10.2337/db14-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR, Willems van Dijk K, Romijn JA, Meijer JH. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 62: 1102–1108, 2013. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costes S, Boss M, Thomas AP, Matveyenko AV. Activation of Melatonin Signaling Promotes β-Cell Survival and Function. Mol Endocrinol 29: 682–692, 2015. doi: 10.1210/me.2014-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costes S, Langen R, Gurlo T, Matveyenko AV, Butler PC. β-Cell failure in type 2 diabetes: a case of asking too much of too few? Diabetes 62: 327–335, 2013. doi: 10.2337/db12-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol 16: R914–R916, 2006. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37: 667–687, 1988. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 29.Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AA. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309: 630–633, 2005. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 30.Dyar KA, Ciciliot S, Wright LE, Biensø RS, Tagliazucchi GM, Patel VR, Forcato M, Paz MI, Gudiksen A, Solagna F, Albiero M, Moretti I, Eckel-Mahan KL, Baldi P, Sassone-Corsi P, Rizzuto R, Bicciato S, Pilegaard H, Blaauw B, Schiaffino S. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab 3: 29–41, 2013. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev 93: 107–135, 2013. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feneberg R, Lemmer B. Circadian rhythm of glucose uptake in cultures of skeletal muscle cells and adipocytes in Wistar-Kyoto, Wistar, Goto-Kakizaki, and spontaneously hypertensive rats. Chronobiol Int 21: 521–538, 2004. doi: 10.1081/CBI-200026958. [DOI] [PubMed] [Google Scholar]
- 33.Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 154: 3817–3825, 2013. doi: 10.1210/en.2013-1121. [DOI] [PubMed] [Google Scholar]
- 34.Forrestel AC, Miedlich SU, Yurcheshen M, Wittlin SD, Sellix MT. Chronomedicine and type 2 diabetes: shining some light on melatonin. Diabetologia 60: 808–822, 2017. doi: 10.1007/s00125-016-4175-1. [DOI] [PubMed] [Google Scholar]
- 35.Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science 354: 69–73, 2016. doi: 10.1126/science.aaf5094. [DOI] [PubMed] [Google Scholar]
- 36.Freedman MS, Lucas RJ, Soni B, von Schantz M, Muñoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284: 502–504, 1999. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 37.Garaulet M, Corbalán-Tutau MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, Ordovas JM. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc 110: 917–921, 2010. doi: 10.1016/j.jada.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillette MU, McArthur AJ. Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res 73: 135–139, 1996. doi: 10.1016/0166-4328(96)00085-X. [DOI] [PubMed] [Google Scholar]
- 39.Grosbellet E, Dumont S, Schuster-Klein C, Guardiola-Lemaitre B, Pevet P, Criscuolo F, Challet E. Circadian phenotyping of obese and diabetic db/db mice. Biochimie 124: 198–206, 2016. doi: 10.1016/j.biochi.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 40.Guillaumond F, Gréchez-Cassiau A, Subramaniam M, Brangolo S, Peteri-Brünback B, Staels B, Fiévet C, Spelsberg TC, Delaunay F, Teboul M. Kruppel-like factor KLF10 is a link between the circadian clock and metabolism in liver. Mol Cell Biol 30: 3059–3070, 2010. doi: 10.1128/MCB.01141-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ha E, Yim SV, Chung JH, Yoon KS, Kang I, Cho YH, Baik HH. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J Pineal Res 41: 67–72, 2006. doi: 10.1111/j.1600-079X.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 42.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev 29: 303–316, 2008. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen J, Timmers S, Moonen-Kornips E, Duez H, Staels B, Hesselink MK, Schrauwen P. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci Rep 6: 35047, 2016. doi: 10.1038/srep35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardin PE, Hall JC, Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci USA 89: 11711–11715, 1992. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343: 536–540, 1990. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- 46.Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 6: 12, 2016. doi: 10.1186/s13395-016-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295: 1065–1070, 2002. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab 23: 610–621, 2016. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, Doyle FJ III, Schultz PG, Kay SA. Identification of small molecule activators of cryptochrome. Science 337: 1094–1097, 2012. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, Schroder EA, Esser KA. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 5: 17, 2015. doi: 10.1186/s13395-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 52.Humphries PS, Bersot R, Kincaid J, Mabery E, McCluskie K, Park T, Renner T, Riegler E, Steinfeld T, Turtle ED, Wei ZL, Willis E. Carbazole-containing sulfonamides and sulfamides: Discovery of cryptochrome modulators as antidiabetic agents. Bioorg Med Chem Lett 26: 757–760, 2016. doi: 10.1016/j.bmcl.2015.12.102. [DOI] [PubMed] [Google Scholar]
- 53.Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, Unluturk U, Li X, Kong X, Hyde AL, Gangl MR, Mair WB, Lee CH. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab 22: 709–720, 2015. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadono M, Nakanishi N, Yamazaki M, Hasegawa G, Nakamura N, Fukui M. Various patterns of disrupted daily rest-activity rhythmicity associated with diabetes. J Sleep Res 25: 426–437, 2016. doi: 10.1111/jsr.12385. [DOI] [PubMed] [Google Scholar]
- 55.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383: 1068–1083, 2014. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms 21: 458–469, 2006. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 57.Kalsbeek A, Perreau-Lenz S, Buijs RM. A network of (autonomic) clock outputs. Chronobiol Int 23: 521–535, 2006. doi: 10.1080/07420520600651073. [DOI] [PubMed] [Google Scholar]
- 58.Kalsbeek A, Yi CX, La Fleur SE, Fliers E. The hypothalamic clock and its control of glucose homeostasis. Trends Endocrinol Metab 21: 402–410, 2010. doi: 10.1016/j.tem.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA 108: 1657–1662, 2011. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kida K, Nishio T, Yokozawa T, Nagai K, Matsuda H, Nakagawa H. The circadian change of gluconeogenesis in the liver in vivo in fed rats. J Biochem 88: 1009–1013, 1980. doi: 10.1093/oxfordjournals.jbchem.a133051. [DOI] [PubMed] [Google Scholar]
- 61.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349–354, 2012. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20: 1868–1873, 2006. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koopman ADM, Rauh SP, van ’t Riet E, Groeneveld L, van der Heijden AA, Elders PJ, Dekker JM, Nijpels G, Beulens JW, Rutters F. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn Study. J Biol Rhythms 32: 359–368, 2017. doi: 10.1177/0748730417713572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol 165: 175–183, 2007. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 66.La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol 11: 643–652, 1999. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 67.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105: 15172–15177, 2008. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 20: 7128–7136, 2001. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes 41: 750–759, 1992. doi: 10.2337/diab.41.6.750. [DOI] [PubMed] [Google Scholar]
- 70.Lee J, Liu R, de Jesus D, Kim BS, Ma K, Moulik M, Yechoor V. Circadian control of β-cell function and stress responses. Diabetes Obes Metab 17, Suppl 1: 123–133, 2015. doi: 10.1111/dom.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J, Moulik M, Fang Z, Saha P, Zou F, Xu Y, Nelson DL, Ma K, Moore DD, Yechoor VK. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol Cell Biol 33: 2327–2338, 2013. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee YH, Wang MY, Yu XX, Unger RH. Glucagon is the key factor in the development of diabetes. Diabetologia 59: 1372–1375, 2016. doi: 10.1007/s00125-016-3965-9. [DOI] [PubMed] [Google Scholar]
- 73.Leighton B, Kowalchuk JM, Challiss RA, Newsholme EA. Circadian rhythm in sensitivity of glucose metabolism to insulin in rat soleus muscle. Am J Physiol 255: E41–E45, 1988. [DOI] [PubMed] [Google Scholar]
- 74.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63: 1860–1869, 2014. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin YC, Hsiao TJ, Chen PC. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int 26: 740–755, 2009. doi: 10.1080/07420520902929029. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Zhou B, Yan M, Huang R, Wang Y, He Z, Yang Y, Dai C, Wang Y, Zhang F, Zhai Q. CLOCK and BMAL1 Regulate Muscle Insulin Sensitivity via SIRT1 in Male Mice. Endocrinology 157: 2259–2269, 2016. doi: 10.1210/en.2015-2027. [DOI] [PubMed] [Google Scholar]
- 77.Loboda A, Kraft WK, Fine B, Joseph J, Nebozhyn M, Zhang C, He Y, Yang X, Wright C, Morris M, Chalikonda I, Ferguson M, Emilsson V, Leonardson A, Lamb J, Dai H, Schadt E, Greenberg HE, Lum PY. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med Genomics 2: 7, 2009. doi: 10.1186/1755-8794-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loizides-Mangold U, Perrin L, Vandereycken B, Betts JA, Walhin JP, Templeman I, Chanon S, Weger BD, Durand C, Robert M, Paz Montoya J, Moniatte M, Karagounis LG, Johnston JD, Gachon F, Lefai E, Riezman H, Dibner C. Lipidomics reveals diurnal lipid oscillations in human skeletal muscle persisting in cellular myotubes cultured in vitro. Proc Natl Acad Sci U S A 114: E8565–E8574, 2017. doi: 10.1073/pnas.1705821114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, Stevens RG, Turek FW, Vermeulen R, Carreón T, Caruso CC, Lawson CC, Thayer KA, Twery MJ, Ewens AD, Garner SC, Schwingl PJ, Boyd WA. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 607-608: 1073–1084, 2017. doi: 10.1016/j.scitotenv.2017.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41: 82–88, 2009. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Macauley M, Smith FE, Thelwall PE, Hollingsworth KG, Taylor R. Diurnal variation in skeletal muscle and liver glycogen in humans with normal health and Type 2 diabetes. Clin Sci (Lond) 128: 707–713, 2015. doi: 10.1042/CS20140681. [DOI] [PubMed] [Google Scholar]
- 83.MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab 6: 159–161, 2007. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 84.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 90: 1323–1327, 1992. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malmgren S, Ahrén B. Evidence for time dependent variation of glucagon secretion in mice. Peptides 76: 102–107, 2016. doi: 10.1016/j.peptides.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 86.Mäntele S, Otway DT, Middleton B, Bretschneider S, Wright J, Robertson MD, Skene DJ, Johnston JD. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS One 7: e37123, 2012. doi: 10.1371/journal.pone.0037123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627–631, 2010. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab 10, Suppl 4: 23–31, 2008. doi: 10.1111/j.1463-1326.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mauvoisin D, Wang J, Jouffe C, Martin E, Atger F, Waridel P, Quadroni M, Gachon F, Naef F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc Natl Acad Sci USA 111: 167–172, 2014. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McDearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL, Wilsbacher LD, Song EJ, Hong HK, Bradfield CA, Takahashi JS. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314: 1304–1308, 2006. doi: 10.1126/science.1132430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meier JJ, Kjems LL, Veldhuis JD, Lefèbvre P, Butler PC. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 55: 1051–1056, 2006. doi: 10.2337/diabetes.55.04.06.db05-1449. [DOI] [PubMed] [Google Scholar]
- 92.Mendoza J, Pévet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol 586: 5901–5910, 2008. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milcou SM, Vrejoin G, Marcean R, Nanu L. [Effect of a hypoglycemic pineal hormone on the endocrine pancreas in alloxanized animals; morphological study]. Ann Endocrinol (Paris) 18: 621–627, 1957. [PubMed] [Google Scholar]
- 94.Moore RY. Neural control of the pineal gland. Behav Brain Res 73: 125–130, 1996. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- 95.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA 112: E2225–E2234, 2015. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nogueira TC, Lellis-Santos C, Jesus DS, Taneda M, Rodrigues SC, Amaral FG, Lopes AM, Cipolla-Neto J, Bordin S, Anhê GF. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology 152: 1253–1263, 2011. doi: 10.1210/en.2010-1088. [DOI] [PubMed] [Google Scholar]
- 97.Okamura H. Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb Symp Quant Biol 72: 551–556, 2007. doi: 10.1101/sqb.2007.72.033. [DOI] [PubMed] [Google Scholar]
- 98.Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. Am J Physiol Regul Integr Comp Physiol 308: R337–R350, 2015. doi: 10.1152/ajpregu.00322.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8: e1001141, 2011. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. doi: 10.1016/S0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 101.Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med 18: 1768–1777, 2012. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pendergrass M, Bertoldo A, Bonadonna R, Nucci G, Mandarino L, Cobelli C, Defronzo RA. Muscle glucose transport and phosphorylation in type 2 diabetic, obese nondiabetic, and genetically predisposed individuals. Am J Physiol Endocrinol Metab 292: E92–E100, 2007. doi: 10.1152/ajpendo.00617.2005. [DOI] [PubMed] [Google Scholar]
- 103.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350: aac4250, 2015. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perrin L, Loizides-Mangold U, Skarupelova S, Pulimeno P, Chanon S, Robert M, Bouzakri K, Modoux C, Roux-Lombard P, Vidal H, Lefai E, Dibner C. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab 4: 834–845, 2015. doi: 10.1016/j.molmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peschke E, Peschke D. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia 41: 1085–1092, 1998. doi: 10.1007/s001250051034. [DOI] [PubMed] [Google Scholar]
- 106.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13: 572–587, 2017. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrenko V, Gosmain Y, Dibner C. High-resolution recording of the circadian oscillator in primary mouse α- and β-cell culture. Front Endocrinol (Lausanne) 8: 68, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petrenko V, Saini C, Giovannoni L, Gobet C, Sage D, Unser M, Heddad Masson M, Gu G, Bosco D, Gachon F, Philippe J, Dibner C. Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev 31: 383–398, 2017. doi: 10.1101/gad.290379.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris 105: 170–182, 2011. doi: 10.1016/j.jphysparis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 110.Phillips LJ, Berry LJ. Circadian rhythm of mouse liver phosphoenolpyruvate carboxykinase. Am J Physiol 218: 1440–1444, 1970. [DOI] [PubMed] [Google Scholar]
- 111.Pietroiusti A, Neri A, Somma G, Coppeta L, Iavicoli I, Bergamaschi A, Magrini A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med 67: 54–57, 2010. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 112.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol 55: 16–54, 1993. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 113.Pittendrigh CS, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci USA 69: 1537–1539, 1972. doi: 10.1073/pnas.69.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 29: 351–366, 2008. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260, 2002. doi: 10.1016/S0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 116.Pulimeno P, Mannic T, Sage D, Giovannoni L, Salmon P, Lemeille S, Giry-Laterriere M, Unser M, Bosco D, Bauer C, Morf J, Halban P, Philippe J, Dibner C. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia 56: 497–507, 2013. doi: 10.1007/s00125-012-2779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes 62: 3469–3478, 2013. doi: 10.2337/db12-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qian J, Yeh B, Rakshit K, Colwell CS, Matveyenko AV. Circadian disruption and diet-induced obesity synergize to promote development of β-cell failure and diabetes in male rats. Endocrinology 156: 4426–4436, 2015. doi: 10.1210/en.2015-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci 73: 2467–2475, 2003. doi: 10.1016/S0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- 120.Rakshit K, Hsu TW, Matveyenko AV. Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia 59: 734–743, 2016. doi: 10.1007/s00125-015-3859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rakshit K, Qian J, Colwell CS, Matveyenko AV. The islet circadian clock: entrainment mechanisms, function and role in glucose homeostasis. Diabetes Obes Metab 17, Suppl 1: 115–122, 2015. doi: 10.1111/dom.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rakshit K, Qian J, Ernst J, Matveyenko AV. Circadian variation of the pancreatic islet transcriptome. Physiol Genomics 48: 677–687, 2016. doi: 10.1152/physiolgenomics.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 247: 975–978, 1990. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 124.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 125.Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, Van Cauter E. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care 36: 2523–2529, 2013. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol 9: e1000595, 2011. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 59: 2697–2707, 2010. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2: e377, 2004. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 52: 1709–1715, 2003. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 130.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61: 2691–2700, 2012. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 54: 120–124, 2011. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saini C, Suter DM, Liani A, Gos P, Schibler U. The mammalian circadian timing system: synchronization of peripheral clocks. Cold Spring Harb Symp Quant Biol 76: 39–47, 2011. doi: 10.1101/sqb.2011.76.010918. [DOI] [PubMed] [Google Scholar]
- 133.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 148: 852–871, 2012. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sans-Fuentes MA, Díez-Noguera A, Cambras T. Light responses of the circadian system in leptin deficient mice. Physiol Behav 99: 487–494, 2010. doi: 10.1016/j.physbeh.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 135.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106: 4453–4458, 2009. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev 29: 317–333, 2008. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Sinturel F, Gosselin P, Gerber A, Fleury-Olela F, Rando G, Demarque M, Franken P. Clock-talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb Symp Quant Biol 80: 223–232, 2015. doi: 10.1101/sqb.2015.80.027490. [DOI] [PubMed] [Google Scholar]
- 138.Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev 24: 345–357, 2010. doi: 10.1101/gad.564110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schrader H, Menge BA, Breuer TG, Ritter PR, Uhl W, Schmidt WE, Holst JJ, Meier JJ. Impaired glucose-induced glucagon suppression after partial pancreatectomy. J Clin Endocrinol Metab 94: 2857–2863, 2009. doi: 10.1210/jc.2009-0826. [DOI] [PubMed] [Google Scholar]
- 140.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes 32: 658–662, 2008. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 141.Sharma A, Laurenti MC, Dalla Man C, Varghese RT, Cobelli C, Rizza RA, Matveyenko A, Vella A. Glucose metabolism during rotational shift-work in healthcare workers. Diabetologia 60: 1483–1490, 2017. doi: 10.1007/s00125-017-4317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 102: 12071–12076, 2005. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shostak A, Husse J, Oster H. Circadian regulation of adipose function. Adipocyte 2: 201–206, 2013. doi: 10.4161/adip.26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 62: 2195–2203, 2013. doi: 10.2337/db12-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371: 1131–1141, 2014. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 146.Sinturel F, Gerber A, Mauvoisin D, Wang J, Gatfield D, Stubblefield JJ, Green CB, Gachon F, Schibler U. Diurnal oscillations in liver mass and cell size accompany ribosome assembly cycles. Cell 169: 651–663.e14, 2017. doi: 10.1016/j.cell.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem 43: 344–353, 2006. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 148.Smushkin G, Vella A. Genetics of type 2 diabetes. Curr Opin Clin Nutr Metab Care 13: 471–477, 2010. doi: 10.1097/MCO.0b013e32833a558d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485: 62–68, 2012. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sridhar GR, Madhu K. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res Clin Pract 23: 183–186, 1994. doi: 10.1016/0168-8227(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 151.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 153.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321: 405–412, 2000. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suwazono Y, Dochi M, Oishi M, Tanaka K, Kobayashi E, Sakata K. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiol Int 26: 926–941, 2009. doi: 10.1080/07420520903044422. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179, 2017. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9: 764–775, 2008. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150: 1223–1234, 2012. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tauber E, Last KS, Olive PJ, Kyriacou CP. Clock gene evolution and functional divergence. J Biol Rhythms 19: 445–458, 2004. doi: 10.1177/0748730404268775. [DOI] [PubMed] [Google Scholar]
- 159.Thomas AP, Hoang J, Vongbunyong K, Nguyen A, Rakshit K, Matveyenko AV. Administration of melatonin and metformin prevents deleterious effects of circadian disruption and obesity in male rats. Endocrinology 157: 4720–4731, 2016. doi: 10.1210/en.2016-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tseng HL, Yang SC, Yang SH, Shieh KR. Hepatic circadian-clock system altered by insulin resistance, diabetes and insulin sensitizer in mice. PLoS One 10: e0120380, 2015. doi: 10.1371/journal.pone.0120380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci 2: 1051–1053, 1999. doi: 10.1038/15973. [DOI] [PubMed] [Google Scholar]
- 163.van Moorsel D, Hansen J, Havekes B, Scheer FA, Jörgensen JA, Hoeks J, Schrauwen-Hinderling VB, Duez H, Lefebvre P, Schaper NC, Hesselink MK, Staels B, Schrauwen P. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab 5: 635–645, 2016. doi: 10.1016/j.molmet.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA 106: 21453–21458, 2009. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wallach T, Kramer A. Chemical chronobiology: toward drugs manipulating time. FEBS Lett 589: 1530–1538, 2015. doi: 10.1016/j.febslet.2015.04.059. [DOI] [PubMed] [Google Scholar]
- 166.Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, Sizzano F, Palini A, Kussmann M, Waridel P, Quadroni M, Dulić V, Naef F, Gachon F. Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Cell Metab 25: 102–117, 2017. doi: 10.1016/j.cmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol 14: 1481–1486, 2004. doi: 10.1016/j.cub.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 168.Woon PY, Kaisaki PJ, Bragança J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA 104: 14412–14417, 2007. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu H, Li H, Woo SL, Kim SM, Shende VR, Neuendorff N, Guo X, Guo T, Qi T, Pei Y, Zhao Y, Hu X, Zhao J, Chen L, Chen L, Ji JY, Alaniz RC, Earnest DJ, Wu C. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J Biol Chem 289: 16374–16388, 2014. doi: 10.1074/jbc.M113.539601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16: 1152–1156, 2010. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zheng Z, Kim H, Qiu Y, Chen X, Mendez R, Dandekar A, Zhang X, Zhang C, Liu AC, Yin L, Lin JD, Walker PD, Kapatos G, Zhang K. CREBH couples circadian clock with hepatic lipid metabolism. Diabetes 65: 3369–3383, 2016. doi: 10.2337/db16-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, Wang Y, Hu Y, Wu J, Dai C, Wang H, Tu Y, Peng X, Wang Y, Zhai Q. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 59: 2196–2206, 2014. doi: 10.1002/hep.26992. [DOI] [PubMed] [Google Scholar]
- 174.Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 12: 616–622, 2016. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 175.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55: 962–970, 2006. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]