Significance
β-Arrestin regulates G protein-coupled receptor (GPCR) signaling by interacting with two regions of agonist-activated receptors—the phosphorylated C terminus and the seven transmembrane helix bundle. The phosphorylation pattern on GPCRs is thought to be the primary driver of β-arrestin binding affinity and functional consequences. To more effectively delineate the relative contributions of these two interactions, we present an innovative strategy to homogeneously phosphorylate purified GPCRs—enzymatic ligation of a synthetic phosphopeptide. This approach unexpectedly revealed that different receptors with identical phosphorylation patterns exhibit dramatic variability in their ability to couple to β-arrestin through the transmembrane core. These differences could play an important role in tuning the balance of G protein- and β-arrestin–mediated cellular signaling pathways stimulated by each GPCR.
Keywords: G protein-coupled receptor, β-arrestin, sortase, allostery, phosphorylation
Abstract
The ability of G protein-coupled receptors (GPCRs) to initiate complex cascades of cellular signaling is governed by the sequential coupling of three main transducer proteins, G protein, GPCR kinase (GRK), and β-arrestin. Mounting evidence indicates these transducers all have distinct conformational preferences and binding modes. However, interrogating each transducer’s mechanism of interaction with GPCRs has been complicated by the interplay of transducer-mediated signaling events. For example, GRK-mediated receptor phosphorylation recruits and induces conformational changes in β-arrestin, which facilitates coupling to the GPCR transmembrane core. Here we compare the allosteric interactions of G proteins and β-arrestins with GPCRs’ transmembrane cores by using the enzyme sortase to ligate a synthetic phosphorylated peptide onto the carboxyl terminus of three different receptors. Phosphopeptide ligation onto the β2-adrenergic receptor (β2AR) allows stabilization of a high-affinity receptor active state by β-arrestin1, permitting us to define elements in the β2AR and β-arrestin1 that contribute to the receptor transmembrane core interaction. Interestingly, ligation of the identical phosphopeptide onto the β2AR, the muscarinic acetylcholine receptor 2 and the μ-opioid receptor reveals that the ability of β-arrestin1 to enhance agonist binding relative to G protein differs substantially among receptors. Furthermore, strong allosteric coupling of β-arrestin1 correlates with its ability to attenuate, or “desensitize,” G protein activation in vitro. Sortase ligation thus provides a versatile method to introduce complex, defined phosphorylation patterns into GPCRs, and analogous strategies could be applied to other classes of posttranslationally modified proteins. These homogeneously phosphorylated GPCRs provide an innovative means to systematically study receptor–transducer interactions.
G protein-coupled receptors (GPCRs), a large family of plasma membrane receptors coupled to guanine nucleotide regulatory proteins, represent one of the most important mechanisms for transducing extracellular signals into specific cellular responses. Their important role in regulating many physiological processes makes them a common therapeutic target. Despite their ability to recognize a vast array of ligands (1), GPCRs have a highly conserved mechanism of action. Ligand binding to the extracellular orthosteric pocket induces conformational changes within the receptor transmembrane (TM) region (2), leading to the sequential intracellular coupling of three main transducer proteins: G protein, GPCR kinase (GRK), and β-arrestin (βarr) (3). More specifically, GPCR-dependent activation of the heterotrimeric G protein leads to the dissociation of the α-subunit from the βγ-subunits, resulting in modulation of second messenger systems, such as cAMP (4). Subsequent GRK phosphorylation of specific serine/threonine residues within the receptor third intracellular loop (ICL3) or carboxyl (C)-terminal tail recruits βarr (5). The binding of βarr desensitizes GPCR signaling by sterically blocking G protein coupling and promoting receptor internalization through interactions with AP2 and clathrin (6). Additionally, βarr can directly modulate cell signaling through G protein-independent pathways (7).
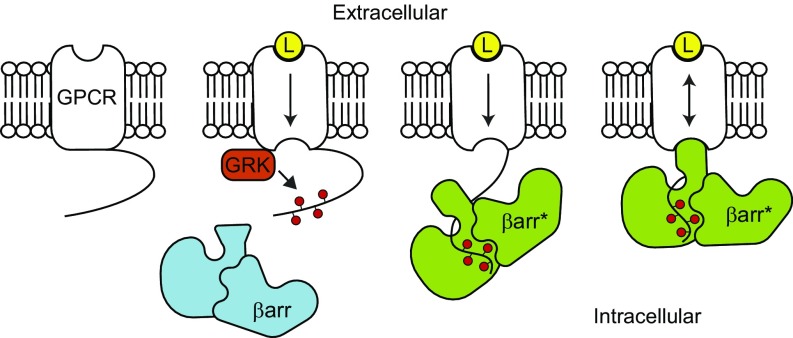
It is now well established that “biased” GPCR ligands can disproportionately regulate particular branches of receptor signaling, a phenomenon known as biased agonism (8). The selective activation of signaling pathways indicates that, although all three transducers specifically interact with agonist-bound GPCRs, their conformational specificities are not identical. However, the fundamental mechanisms underlying this differential coupling remain obscure, largely because events mediated by different transducers are intricately intertwined. In particular, βarr binds to receptors through a two-step process, initially interacting with GRK-phosphorylated residues and then coupling to the agonist-activated GPCR TM core (Fig. 1) (9). Biochemical and structural studies have demonstrated that binding to GPCRs’ phosphorylated tails induces extensive conformational changes in βarr, including the extension of several loops implicated in βarr’s interaction with GPCRs’ TM bundle (10).
Fig. 1.
Illustration showing the two-step binding mode of β-arrestin. Ligand (L) binding to the extracellular orthosteric binding pocket leads to conformational changes within the GPCR transmembrane region to influence intracellular transducer binding. The phosphorylation (red circles) of the receptor C terminus by GPCR kinase (GRK) initiates the recruitment of β-arrestin (βarr). Conformational changes induced in βarr (βarr*) as a result of binding to the phosphorylated C terminus promotes coupling to the GPCR transmembrane core, which allosterically enhances ligand affinity.
Engagement of βarr with GPCRs’ TM cores is believed to mediate particular functions of βarr, such as receptor desensitization, but efforts to understand the nature and consequences of this interaction have been hampered by its low affinity and its dependence on GRK phosphorylation. Obtaining uniformly phosphorylated receptors in a cellular context or in vitro has proven to be challenging. Here we present a method to generate homogeneously phosphorylated GPCRs by enzymatically ligating a synthetic phosphorylated peptide, removing the confounding variable of phosphorylation so that the effects of βarr’s coupling to the TM cores can be isolated. This allows us to compare systematically how agonists allosterically influence the interactions of multiple transducers with multiple GPCRs, revealing unexpected diversity that may influence the balance of cellular signaling responses.
Results
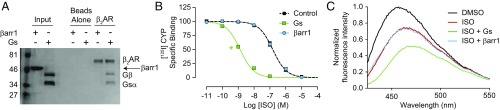
The binding of βarr to GPCRs is mainly initiated through an interaction with the phosphorylated receptor C terminus, and conformational changes induced in βarr by this interaction promote coupling to the receptor TM core (Fig. 1). Coimmunoprecipitation experiments confirm that heterotrimeric Gs protein, but not βarr1, can interact with purified nonphosphorylated β2-adrenergic receptor (β2AR) (Fig. 2A).
Fig. 2.
Nonphosphorylated β2AR interacts with Gs heterotrimer but not β-arrestin1. (A) Coomassie-stained gel showing the coimmunoprecipitation of Gs heterotrimer (Gs) or β-arrestin1 (βarr1) with isoproterenol (ISO)-bound FLAG-β2AR. Loading controls represent 10% of input. (B) Competition binding experiments using radiolabeled [125I]-cyanopindolol (CYP). Gs increases ISO affinity for β2AR HDLs (log IC50: −8.88 ± 0.03) compared with no transducer (log IC50: −6.82 ± 0.03), but βarr1 does not (log IC50: −6.81 ± 0.02). Data shown are the mean of three independent experiments, with error bars representing SE. The green asterisk (*) indicates a log IC50 value significantly different from the control curve (P < 0.05, one-way ANOVA). (C) The fluorescence emission spectrum of bimane-labeled β2AR HDLs shows a rightward shift and decrease in fluorescence upon addition of ISO, indicative of receptor activation. The effects of ISO are enhanced by Gs but not βarr1. Data shown are representative of three independent experiments.
To verify that this apparent lack of interaction with βarr is not simply due to poor complex stability, we employed two assays capable of detecting complex formation in situ. First, we used competition radioligand binding to measure the allosteric effects of transducers on ligand binding to the receptor. As described by the ternary complex model, first for G proteins and later for βarrs, ligand-induced changes in receptor conformation enhance the binding and affinity of transducers, which reciprocally increase ligand affinity by stabilizing an active receptor state (11, 12). When we reconstitute wild-type (WT) β2AR in high-density lipoprotein (HDL) particles to mimic a cellular membrane environment (13), G protein enhances the affinity of the full agonist isoproterenol (ISO) for nonphosphorylated HDL-β2AR by nearly 1,000-fold, as expected, but βarr1 has no effect even at micromolar concentrations (Fig. 2B).
Second, to directly monitor β2AR conformational changes associated with activation, we labeled C265 at the cytoplasmic end of TM6 with monobromobimane, an environmentally sensitive fluorophore. Receptor activation leads to an outward movement of TM6 that places the bimane label in a more solvent-exposed position, causing a decrease in fluorescence and a shift in λmax (14). Indeed, isoproterenol reduces β2AR-bimane fluorescence compared with control (DMSO), and addition of Gs but not βarr1 further attenuates fluorescence (Fig. 2C). Taken together, these data clearly indicate that nonphosphorylated β2AR fails to form a productive interaction with βarr.
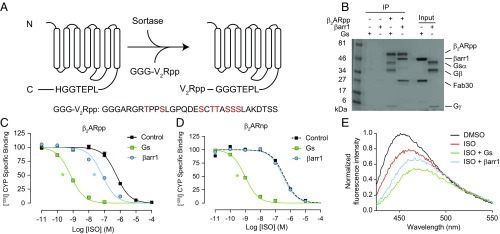
We induced phosphorylation of the β2AR by using the prokaryotic enzyme sortase to ligate a synthetic phosphorylated peptide onto the receptor C terminus (Fig. 3A and Fig. S1). This strategy quantitatively yields receptor with a defined, homogeneous phosphorylation pattern, which is difficult to achieve or validate with either in cellulo or in vitro GRK phosphorylation. We ligated a phosphopeptide (pp) derived from the C terminus of the vasopressin-2-receptor (V2R), given our previous crystallographic and biophysical data (10), which indicate that V2Rpp binds to βarr with high affinity and effectively primes it for interaction with GPCRs’ TM core. In contrast to WT β2AR (Fig. 2A), phosphorylated β2AR (β2ARpp) can immunoprecipitate both Gs and βarr1 (Fig. 3B). βArr1 enhances isoproterenol affinity for the β2ARpp by 9-fold, compared with 800-fold by Gs (Fig. 3C and Fig. S2A). However, as for Gs, βarr1 does not increase the binding of the antagonist ICI-118,551 (Fig. S2B). The βarr1-mediated increase in agonist affinity requires phosphorylation of β2ARpp, since ligation of a nonphosphorylated V2R peptide or phosphatase treatment abrogates βarr1’s allosteric effect (Fig. 3D and Fig. S2C). While βarr1 augments isoproterenol’s decrease in the fluorescence of β2ARpp-bimane, its effects are less profound than those of G protein (Fig. 3E), consistent with the ∼100-fold difference in the cooperativity between G protein and βarr1 observed by radioligand binding (Fig. 3C). These findings suggest that despite binding to a similar pocket, G protein and βarr differ substantially in the strength of their allosteric interactions with the β2AR TM core.
Fig. 3.
Sortase ligation of a phosphopeptide onto the β2AR restores its allosteric interaction with β-arrestin1. (A) Cartoon schematic of sortase ligation method. A synthetic phosphopeptide (pp) derived from the vasopressin-2-receptor (V2R) with three N-terminal glycine residues (GGG-V2Rpp) is ligated onto receptors containing a C-terminal LPETGGH recognition motif. In the sequence of GGG-V2Rpp below the schematic, phosphorylated residues are highlighted in red. (B) Coomassie-stained gel showing the coimmunoprecipitation of heterotrimeric Gs and β-arrestin1 (βarr1) with isoproterenol (ISO)-bound, phosphopeptide-ligated FLAG-β2AR (β2ARpp). Fab30 binds specifically to V2Rpp-bound βarr1 (10). Loading controls represent 10% of input. (C and D) Competition binding experiments using radiolabeled [125I]-cyanopindolol (CYP) with HDLs containing (C) β2ARpp or (D) β2AR ligated to a nonphosphorylated version of the V2R peptide (β2ARnp). Gs increases the affinity of ISO for both β2ARpp and β2ARnp HDLs (log IC50: −9.15 ± 0.03, −9.02 ± 0.04, respectively) compared with no transducer (log IC50: −6.24 ± 0.04, −6.42 ± 0.09, respectively), but βarr1 only increases ISO affinity for β2ARpp HDLs (log IC50: −7.14 ± 0.07) and not β2ARnp HDLs (log IC50: −6.49 ± 0.04). Data shown in C and D are the mean of at least three independent experiments, with error bars representing SE, and asterisks (*) indicate a log IC50 value significantly different from the control curve (P < 0.05, one-way ANOVA). (E) The effects of ISO on the HDL-β2ARpp-bimane fluorescence emission spectrum are enhanced by Gs and βarr1. Data shown are representative of three independent experiments.
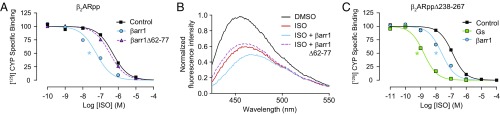
This defined system allows us to rigorously assess the contributions of specific regions within each protein that have been implicated in mediating the TM core/βarr interaction. For example, the “finger loop” region of βarr1 is extended upon βarr’s binding to phosphorylated receptors and is believed to insert into the TM core. We previously reported that this region was essential to observe an engaged conformation of βarr1 with the TM core of in cellulo phosphorylated β2AR, as assessed by negative stain electron microscopy using a βarr1 finger loop-deleted mutant (15). This same mutant, βarr1Δ62–77, fails to stabilize an active state of β2ARpp by competition radioligand binding (Fig. 4A) and bimane fluorescence (Fig. 4B), consistent with our previous findings.
Fig. 4.
The allosteric interaction between phosphorylated β2AR and β-arrestin1 requires the finger loop of β-arrestin1 but does not require the third intracellular loop of the β2AR. (A) In competition radioligand binding with β2ARpp HDLs as described in Fig. 3C, a finger loop deletion mutant of β-arrestin1 (βarr1) (Δ62–77) has minimal effect on isoproterenol (ISO) binding (log IC50s: no transducer, −6.30 ± 0.05; βarr1, −7.25 ± 0.07; βarr1 Δ62–77, −6.48 ± 0.03). (B) βarr1Δ62–77 does not intensify the effects of ISO on the fluorescence spectrum of β2ARpp-bimane HDLs. Data shown are representative of three independent experiments. (C) In competition radioligand binding with β2ARpp HDLs containing a deletion of the third intracellular loop (Δ238–267), both Gs (log IC50: −8.85 ± 0.03) and βarr1 (log IC50: −7.63 ± 0.06) retain their ability to increase isoproterenol (ISO) affinity (no transducer, log IC50: −6.91 ± 0.04). Data shown in A and C are the mean of at least three independent experiments, with error bars representing SE, and asterisks (*) indicate a log IC50 value significantly different from the control curve (P < 0.05, one-way ANOVA).
On the receptor side, it has been suggested that ICL3 of the β2AR is critical for engagement of βarr1 with the TM core (16). The phosphopeptide-ligated version of a previously reported deletion mutant, β2ARppΔ238–267, retains a normal affinity for the agonist isoproterenol when reconstituted in HDL particles (Fig. 4C). Surprisingly, agonist affinity increases (∼10-fold) in the presence of βarr1 (Fig. 4C), quite comparable to βarr1’s effect on WT β2ARpp (Fig. 3C). Together these data indicate that the finger loop of βarr, but not the β2AR ICL3, is required for the TM core interaction.
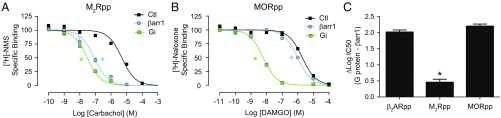
For the β2AR, the TM core’s allosteric communication with G protein is substantially stronger than it is with βarr. To determine whether this is a conserved phenomenon among other GPCRs, we investigated the allosteric coupling of G protein and βarr at the muscarinic acetylcholine receptor 2 (M2R) and μ-opioid receptor (MOR). Using the sortase ligation strategy described above for the β2AR, we ligated the V2Rpp onto the C termini of purified M2R (M2Rpp) and MOR (MORpp) (Fig. S3A), reconstituted the receptors into HDL particles, and measured the allosteric coupling of their cognate G protein (Gi heterotrimer) and βarr using competition radioligand binding. We selected competitor ligands that are full agonists and have similar affinities for their respective receptors as isoproterenol does for the β2AR. As observed for the β2AR, G protein induces more than a 100-fold increase in agonist affinity for both M2Rpp (carbachol, Fig. 5A) and MORpp (DAMGO, Fig. 5B), consistent with previous reports (17, 18). βArr1 enhances agonist affinity for both M2Rpp and MORpp in a phosphorylation-dependent manner (Fig. 5 A and B and Fig. S3 B and C), but interestingly, βarr1 increases carbachol affinity for M2Rpp by 57-fold compared with only 2- and 9-fold for MORpp and β2ARpp, respectively (Fig. 5 A and B and Fig. S3D). A summary of transducer allosteric binding at each receptor is shown in Fig. 5C, where we observe a 100-fold difference between G protein and βarr effects on agonist affinity for the β2ARpp and MORpp but less than a 3-fold difference with M2Rpp. The comparable effects of G protein and βarr at the M2Rpp are not carbachol specific but are also observed with the agonist iperoxo (Fig. S3 E and F). βArr1’s effects at the M2Rpp also appear to be dependent on the transmembrane core interaction, as deletion of the finger loop eliminates its allosteric coupling (Fig. S3G). Therefore, even for GPCRs which preferentially couple to the same G protein isoform, such as the M2R and the MOR, allosteric communication with G protein does not always vary proportionally to allosteric communication with βarr.
Fig. 5.
The allosteric enhancement of agonist binding induced by β-arrestin1 varies among different receptors. (A) Competition binding experiments with sortase-ligated M2Rpp HDLs, using [3H]-N-methyl-scopolamine (NMS) as the tracer. Heterotrimeric Gi (100 nM, log IC50: −7.51 ± 0.06) and β-arrestin1 (βarr1) (1 μM, log IC50: −7.06 ± 0.08) increase the affinity of the agonist carbachol to a similar extent (no transducer, log IC50: −5.31 ± 0.09). (B) Competition binding experiments with sortase-ligated MORpp HDLs, using [3H]-naloxone as the tracer. Gi (1 μM, log IC50: −8.22 ± 0.05) increases the affinity of the agonist DAMGO to a far greater extent than βarr1 (1 μM, log IC50: −6.02 ± 0.05) (no transducer, log IC50: −5.71 ± 0.06). Data in A and B are the mean of three independent experiments, with error bars representing SE, and asterisks (*) indicate a log IC50 value significantly different from the control curve (P < 0.05, one-way ANOVA). (C) Comparison of the difference in agonists’ log IC50 values in the presence of their cognate G proteins versus βarr1 for sortase-ligated β2ARpp (Fig. 3C), M2Rpp (A), and MORpp (B) HDLs.
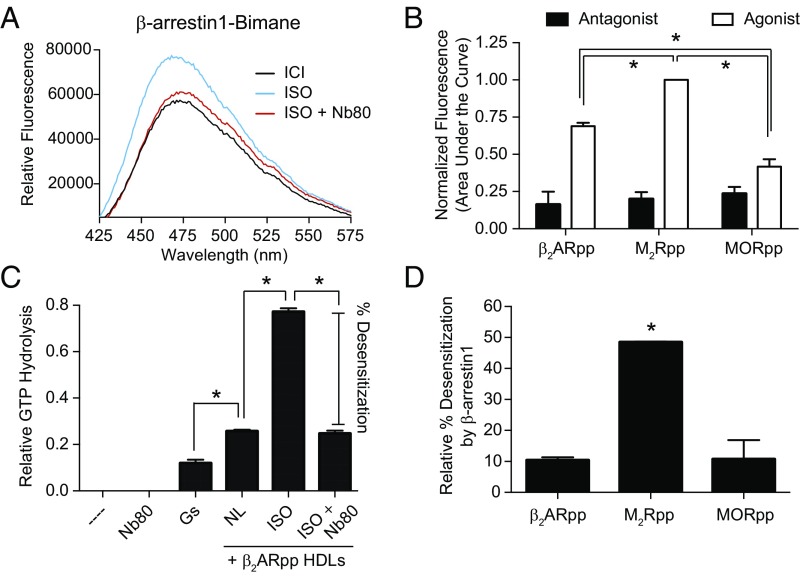
We then asked how this broad range of allostery between GPCRs and βarr might affect the stability and function of these complexes. The ternary complex model posits that the observed enhancement of agonist affinity in the presence of βarr must be reciprocated by an equivalent increase in βarr’s affinity for the receptor transmembrane core (11). Thus, the strength of βarr engagement with the receptor core would be expected to follow the same rank order of allosteric cooperativity among the three receptors tested. We assessed the degree of βarr1 engagement by site-specifically labeling its finger loop with monobromobimane (βarr1-bimane); coupling to a receptor’s TM core results in an increase in fluorescence due to reduced solvent exposure of the label (19, 20). As expected, βarr1-bimane fluorescence increases for β2ARpp stimulated with isoproterenol compared with the antagonist ICI-118,551 (Fig. 6A and Fig. S4A). Importantly, the single domain antibody Nb80, which binds to agonist-activated β2AR in the same region as G protein, competitively blocks the agonist-induced increase in fluorescence (Fig. 6A). This confirms that the agonist effects on βarr1-bimane are indeed mediated through interaction with β2ARpp’s TM core. Comparison of βarr1-bimane’s response to agonist stimulation of β2ARpp, M2Rpp, and MORpp shows that M2Rpp displays the highest level of agonist-induced βarr1-bimane engagement (Fig. 6B and Fig. S4), consistent with the observed allosteric cooperativities of these receptors.
Fig. 6.
The extent to which β-arrestin1 engages receptors’ transmembrane cores varies among different receptors. (A) Fluorescence spectra of β-arrestin1 (βarr1) labeled with monobromobimane at residue 70 in the finger loop. Activation of HDL-β2ARpp by the agonist isoproterenol (ISO) increases βarr1-bimane fluorescence, which is blocked by Nb80 binding to the receptor TM core. Data shown are representative of three independent experiments. (B) Comparison of βarr1-bimane fluorescence by agonist activation of β2ARpp, M2Rpp, and MORpp HDLs. The area under the fluorescence emission spectra were determined and normalized to M2Rpp plus iperoxo (the maximum signal) in each experiment (see Fig. S4 for representative spectra). All three receptors are significantly different from one another (*P < 0.05), and β2ARpp and M2Rpp are significantly different from their respective antagonist controls (not indicated, P < 0.05). (C) An in vitro GTPase assay measuring GTP hydrolysis as a readout of G protein activation. The basal level of GTP hydrolysis induced by G protein is robustly increased by HDL-β2ARpp HDLs in the presence of ISO compared with no ligand (NL) (*P < 0.05), which is blocked (desensitization) by the addition of Nb80 (no significant difference between NL and ISO + Nb80). (D) Inhibition (percentage desensitization) of G protein activation by βarr1 is strongest at M2Rpp and significantly different from β2ARpp and MORpp HDLs (*P < 0.05) (see also Fig. S5). Data in B–D are the mean of at least three independent experiments, with error bars representing SE; P values were determined by one-way ANOVA.
One mechanism by which βarr desensitizes receptors’ activation of G protein signaling is steric occlusion of the TM receptor core. We hypothesized that βarr might more efficiently desensitize GPCRs such as the M2R—those for which βarr has similar allosteric binding properties as G protein—compared with GPCRs with very divergent transducer coupling, such as the β2AR and the MOR. To test this, we utilized an in vitro GTPase activity assay that can quantitatively measure agonist-induced receptor activation of G protein. As seen in Fig. 6C, addition of isoproterenol to the β2AR enhances G protein activation as measured by an increase in GTP hydrolysis, which is blocked by competitive binding of Nb80. A similar agonist-induced increase in GTPase activity is observed for both M2Rpp and MORpp (Fig. S5). Desensitization, or inhibition of GTP hydrolysis, by βarr is significantly elevated for M2Rpp compared with MORpp and β2ARpp (Fig. 6D and Fig. S5). Taken together, we find that the efficiency of βarr-mediated receptor desensitization in vitro correlates with the strength of the receptor’s allosteric interaction with βarr relative to G protein.
Discussion
Extensive biochemical and biophysical data indicate that βarr interacts with two distinct GPCR epitopes—a relatively high-affinity interaction with GRK-phosphorylated residues in the receptor C-terminal tail (and/or ICL3) and a much lower affinity interaction with the agonist-activated TM core (reviewed in ref. 21). A number of studies have demonstrated that the former interaction alone induces conformational changes in βarr sufficient to allow it to carry out some of its canonical receptor-dependent functions (10, 15, 16). These include interactions that regulate intracellular signaling cascades (e.g., Src kinase) and facilitate receptor internalization (e.g., AP2 and clathrin). The precise functional contribution of βarr’s interaction with the receptor TM core has been more difficult to define due to its dependence on the phosphorylation-dependent conformational changes in βarr. However, multiple lines of evidence argue for its central role in desensitizing G protein-mediated signaling by interacting with the TM core in a mutually exclusive manner (22).
In this study, we circumvented the variable of phosphorylation by using the sortase enzyme to ligate a synthetic phosphopeptide onto the C termini of receptors. Chemical and enzymatic ligation methods have long been pursued as routes to incorporate chemically defined, homogeneous posttranslational modifications into proteins (23, 24), and the sortase enzyme in particular has been used to introduce several types of common modifications, including lipids (25) and glycans (26). Our results demonstrate that sortase has untapped potential to define the contributions of complex posttranslational modification patterns for a variety of protein classes, including phosphoproteins. Our proof-of-principle experiments with three receptors—the β2AR, the M2R, and the MOR—suggest that sortase ligation will be a versatile, generally applicable method to introduce defined phosphorylation patterns into GPCRs and to form GPCR–βarr complexes. Interestingly, the M2R’s native C terminus ends immediately after helix 8 and lacks phosphorylatable residues; all potential GRK phosphorylation sites are found in its particularly large ICL3. Nevertheless, appending the V2R-derived phosphopeptide to the M2R C terminus is sufficient to promote βarr’s interaction with the TM core as assessed by pharmacological, biophysical, and functional measures. In addition, the relative magnitudes of allosteric cooperativity of sortase-ligated M2Rpp and β2ARpp with βarr match well with those previously observed for in cellulo phosphorylated native receptors in membranes (12). This suggests that, at least in some cases, the proximity and activation state of βarr may be more crucial than its precise orientation in promoting its binding to GPCRs’ TM core.
Verifying the efficiency and pattern of phosphorylation for GPCRs is technically challenging. As a result, it has been difficult to ascertain whether particular structural elements directly affect GPCRs’ interactions with βarr or indirectly influence them at the level of GRK phosphorylation. Sortase-ligated receptors provide a tool to separate these variables, enabling independent manipulation of phosphorylation state and βarr binding to clarify some of these outstanding questions. For example, our observation that an ICL3 deletion mutant of β2ARpp still efficiently couples to βarr1 in both radioligand binding and bimane assays conflicts with a previous report that this mutant’s interaction with βarr1 is severely impaired (16). However, as the earlier work utilized in cellulo, GRK-phosphorylated receptor, this discrepancy suggests that the ICL3’s primary effect might occur at the level of GRK recognition and phosphorylation of agonist-bound receptors.
Mass spectrometry analysis of several receptors has demonstrated that GPCR phosphorylation in cells is quite heterogeneous (27–29). Variation in the stoichiometry and pattern of phosphorylation is observed as a function of external factors such as cell type and the nature of the stimulus, but even individual receptors in the same cell can exhibit different phosphorylation. Since the degree and positioning of phosphorylated residues may affect βarr’s activation state, previously described as the “barcode” hypothesis (30), phosphorylation provides a potential mechanism for diversifying a receptor’s signaling outcomes. Ultimately we envision using sortase to ligate various phosphopeptides to systematically analyze how specific phosphorylation patterns affect βarr’s active conformation, its allosteric interactions with GPCRs, and its interplay with G proteins and downstream signaling effectors.
In this initial study, we ligated the same well-characterized phosphopeptide onto three different receptors, allowing us to detect differences in how βarr interacts with the TM core when activated in the same manner. These three receptors exhibited large variations in their allosteric cooperativity with βarr1, spanning two orders of magnitude, but very similar cooperativities with their cognate G proteins. This observation accords well with the theory that G proteins and βarrs have distinct conformational preferences, providing mechanistic grounds for biased signaling. In the future, sortase-ligated GPCRs could provide a valuable tool to dissect the molecular basis of how biased agonists differentially couple to transducers.
Our data indicate that βarr cannot harness the full allosteric potential of all GPCRs; receptors’ conformational ensembles can inherently bias them toward or against βarr coupling. These differences might be important for the physiological function of GPCRs. Indeed, we found that βarr1 could inhibit G protein activation induced by M2Rpp in vitro more effectively than that induced by β2ARpp or MORpp, correlating with the strength of the allosteric coupling of the two transducers at each receptor. This variance occurred even under conditions where βarr1 was activated by the same phosphopeptide and was present at a very high effective concentration, due to its high affinity for the ligated C terminus.
It is interesting to speculate how βarr’s ability to interact with the GPCR TM core factors into the complex picture of transducer regulation in the cell. In addition to sterically blocking G protein binding, βarrs regulate G protein-mediated receptor signaling by interacting with AP2 and clathrin to promote receptor internalization. Internalized receptors can either be recycled to the plasma membrane or targeted to lysosomes for degradation (22). The choice of pathways is governed by the receptor’s phosphorylation, with weaker “class A” patterns that transiently bind βarr favoring recycling and “class B” patterns that stably bind βarr favoring degradation pathways (31). A more recent twist is that G protein activation from intracellular compartments, such as endosomes and Golgi, has now been demonstrated for several receptors, including the β2AR (32–34). One could hypothesize that the relative strengths of βarr’s interactions with the phosphorylated tail and TM core could be sufficient to direct the receptor’s fate at multiple junctions. Receptors with class A phosphorylation and weak TM core interactions would desensitize the most slowly, perhaps primarily through negative feedback mechanisms resulting from G protein-mediated pathways. Class A receptors with stronger TM core interactions would be desensitized primarily by βarr’s blockade of the G protein binding site. Class B receptors with weak TM core interactions could continue to activate G proteins even during endocytosis since βarr would not effectively compete, but those with stronger TM core interactions would be silent in G protein signaling as they transit to lysosomes.
It should be noted that most cell-based assays used to study the interaction between βarr and GPCRs are recruitment assays, which will primarily reflect GRK phosphorylation rather than βarr’s full engagement of the receptor through the TM core. In fact, the C termini of GPCRs are often replaced with those of class B receptors to enhance the signal. Caution may need to be exercised in using such systems to draw conclusions about the deeply interwoven regulation of GPCRs by G proteins, GRKs, and βarrs. A further understanding of the interaction of βarr with the receptor TM core and its physiological role in GPCR regulation may reveal additional levels of bias which can be targeted in the next generation of GPCR drugs.
Materials and Methods
Complete details and descriptions of molecular biology methods, protein expression and purification, HDL reconstitution, sortase ligation reactions, bimane fluorescence assays, coimmunoprecipitation experiments, radioligand binding assays, and GTPase assays are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Darrell Capel and Xinrong Jiang for technical assistance and Donna Addison, Joanne Bisson, Katherine Harley, Quivetta Lennon, and Victoria Ronk for administrative assistance. Support was provided by the American Heart Association (predoctoral fellowship to M.C.), the Smith Family Foundation (A.C.K.), and the National Institutes of Health (Grant HL16037 to R.J.L.). R.J.L. is an investigator with the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: D.P.S., L.M.W., and R.J.L. are co-inventors on a patent application that covers methods described in this manuscript.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722336115/-/DCSupplemental.
References
- 1.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 2.Manglik A, Kruse AC. Structural basis for G protein-coupled receptor activation. Biochemistry. 2017;56:5628–5634. doi: 10.1021/acs.biochem.7b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture) Angew Chem Int Ed Engl. 2013;52:6366–6378. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- 4.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 5.Benovic JL, et al. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: Potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson YK, Luttrell LM. The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev. 2017;69:256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin. Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J Biol Chem. 1993;268:11628–11638. [PubMed] [Google Scholar]
- 10.Shukla AK, et al. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 12.Gurevich VV, Pals-Rylaarsdam R, Benovic JL, Hosey MM, Onorato JJ. Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem. 1997;272:28849–28852. doi: 10.1074/jbc.272.46.28849. [DOI] [PubMed] [Google Scholar]
- 13.Denisov IG, Sligar SG. Nanodiscs for structural and functional studies of membrane proteins. Nat Struct Mol Biol. 2016;23:481–486. doi: 10.1038/nsmb.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao XJ, et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc Natl Acad Sci USA. 2009;106:9501–9506. doi: 10.1073/pnas.0811437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill TJ, 3rd, et al. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci USA. 2017;114:2562–2567. doi: 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari P, et al. Functional competence of a partially engaged GPCR-β-arrestin complex. Nat Commun. 2016;7:13416. doi: 10.1038/ncomms13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruse AC, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W, et al. Structural insights into µ-opioid receptor activation. Nature. 2015;524:315–321. doi: 10.1038/nature14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer ME, Farrens DL, McDowell JH, Weber LA, Smith WC. Dynamics of arrestin-rhodopsin interactions: Loop movement is involved in arrestin activation and receptor binding. J Biol Chem. 2007;282:25560–25568. doi: 10.1074/jbc.M702155200. [DOI] [PubMed] [Google Scholar]
- 20.Hanson SM, et al. Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci USA. 2006;103:4900–4905. doi: 10.1073/pnas.0600733103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JS, Rajagopal S. The beta-arrestins: Multifunctional regulators of G protein-coupled receptors. J Biol Chem. 2016;291:8969–8977. doi: 10.1074/jbc.R115.713313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajagopal S, Shenoy SK. GPCR desensitization: Acute and prolonged phases. Cell Signal. 2018;41:9–16. doi: 10.1016/j.cellsig.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witte K, Sears P, Martin R, Wong CH. Enzymatic glycoprotein synthesis: Preparation of ribonuclease glycoforms via enzymatic glycopeptide condensation and glycosylation. J Am Chem Soc. 1997;119:2114–2118. [Google Scholar]
- 24.Muir TW, Sondhi D, Cole PA. Expressed protein ligation: A general method for protein engineering. Proc Natl Acad Sci USA. 1998;95:6705–6710. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antos JM, Miller GM, Grotenbreg GM, Ploegh HL. Lipid modification of proteins through sortase-catalyzed transpeptidation. J Am Chem Soc. 2008;130:16338–16343. doi: 10.1021/ja806779e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samantaray S, Marathe U, Dasgupta S, Nandicoori VK, Roy RP. Peptide-sugar ligation catalyzed by transpeptidase sortase: A facile approach to neoglycoconjugate synthesis. J Am Chem Soc. 2008;130:2132–2133. doi: 10.1021/ja077358g. [DOI] [PubMed] [Google Scholar]
- 27.Nobles KN, et al. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zindel D, et al. Identification of key phosphorylation sites in PTH1R that determine arrestin3 binding and fine-tune receptor signaling. Biochem J. 2016;473:4173–4192. doi: 10.1042/BCJ20160740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butcher AJ, et al. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J Biol Chem. 2011;286:11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 32.Wehbi VL, et al. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc Natl Acad Sci USA. 2013;110:1530–1535. doi: 10.1073/pnas.1205756110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godbole A, Lyga S, Lohse MJ, Calebiro D. Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. Nat Commun. 2017;8:443. doi: 10.1038/s41467-017-00357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.