Significance
Long-term potentiation (LTP) is the most compelling cellular and molecular model for learning and memory. Both AMPARs and KARs, two separate classes of glutamate receptor with very limited homology, express normal LTP in pyramidal neurons. However, the general underlying molecular mechanism remains a mystery. Here, with the strategy of single-cell molecular replacement we show that the PDZ-binding domains of AMPAR/TARP and KAR/Neto receptor complexes are essential for basal synaptic transmission and LTP. Our work suggests that the glutamate receptors share the same postsynaptic requirement for LTP.
Keywords: long-term potentiation, glutamate receptor, auxiliary protein, PDZ-binding domain
Abstract
Long-term potentiation (LTP) is a persistent strengthening of synaptic transmission in the brain and is arguably the most compelling cellular and molecular model for learning and memory. Previous work found that both AMPA receptors and exogenously expressed kainate receptors are equally capable of expressing LTP, despite their limited homology and their association with distinct auxiliary subunits, indicating that LTP is far more promiscuous than previously thought. What might these two subtypes of glutamate receptor have in common? Using a single-cell molecular replacement strategy, we demonstrate that the AMPA receptor auxiliary subunit TARP γ-8, via its PDZ-binding motif, is indispensable for both basal synaptic transmission and LTP. Remarkably, kainate receptors and their auxiliary subunits Neto proteins share the same requirement of PDZ-binding domains for synaptic trafficking and LTP. Together, these results suggest that a minimal postsynaptic requirement for LTP is the PDZ binding of glutamate receptors/auxiliary subunits to PSD scaffolding proteins.
One of the most remarkable properties of excitatory synapses in the brain is their ability to undergo long-lasting functional changes, referred to as long-term potentiation (LTP). Most glutamatergic excitatory synaptic transmission is mediated by AMPA receptors (AMPARs) and NMDA receptors (NMDARs). It is generally accepted that LTP requires the activation of NMDARs, which, via CaMKII and a series of poorly understood steps, results in the rapid accumulation of AMPARs at the activated synapses (1–5). Contrary to existing dogma, recent work has demonstrated that LTP is surprisingly promiscuous and any AMPAR subunit expresses normal LTP (6). Even more remarkable, kainate receptors (KARs), a separate class of glutamate receptor with very limited homology to AMPARs that are normally not expressed at these synapses, express normal LTP (6). Furthermore, in addition to their limited homology, AMPARs and KARs assemble with distinct auxiliary subunits; transmembrane AMPAR regulatory proteins (TARPs) assemble with AMPARs (7–9) and Neto proteins assemble with KARs (9, 10). What is the basis for the promiscuity? What might these two classes of receptor have in common? Providing answers to these questions might shed light on fundamental underlying steps in LTP.
TARPs comprise a family of proteins, which share considerable sequence homology, including the presence of a C-terminal PDZ-binding motif (11), and therefore would be expected to have similar properties. Although it is well established that TARPs selectively bind to AMPAR subunits and that their PDZ-binding motifs are essential and specific for the synaptic trafficking of AMPARs in cerebellar granule cells, they are widely expressed throughout the brain and this model has been difficult to apply to other synapses (12–15). In the hippocampus, γ-8 is the highest expressed TARP; however, the presence of other TARPs has obscured its role in basal synaptic transmission and synaptic plasticity in pyramidal neurons (15, 16). Here we have used a single-cell molecular replacement strategy to demonstrate that both basal synaptic transmission and LTP require the PDZ-binding motif of the AMPAR auxiliary subunit TARP γ-8. Remarkably, kainate receptors and their auxiliary subunits Netos share the same requirement of PDZ-binding domains for synaptic trafficking and LTP. These results suggest that the PDZ binding of glutamate receptors/auxiliary subunits to PSD scaffolding proteins is the minimal postsynaptic requirement for LTP.
Results
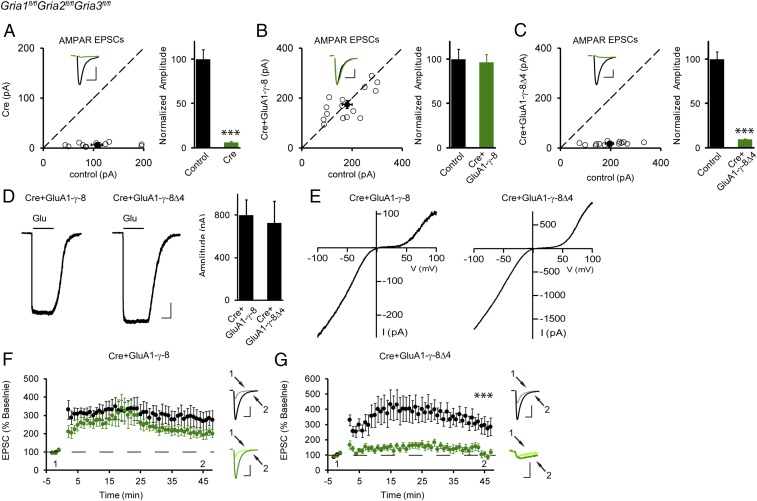
Our understanding of AMPAR trafficking in the hippocampus has been hampered by the fact that pyramidal cells express multiple TARPs with seemingly redundant roles (15–20). To circumvent this redundancy, we developed a strategy to replace the endogenous AMPARs with GluA1-TARP tethered receptors (Fig. S1). We used in utero electroporation in triple-floxed mice (Gria1fl/flGria2fl/flGria3fl/fl) followed by recording from acute slices at postnatal days 17–21 (P17–P21). This allowed us to examine, in isolation, the properties of the tethered receptor, using simultaneous dual whole-cell recordings from a transfected neuron and a neighboring control neuron (6, 21, 22). Consistent with our previous studies (21), we verified that expressing Cre alone in CA1 neurons deleted all AMPAR function (Fig. 1A). We next coexpressed GluA1 that was tethered to the full-length γ-8 (GluA1-γ-8) to replace the endogenous AMPARs. In this case we recorded a full rescue of the synaptic responses (Fig. 1B). Most importantly, when we expressed GluA1 tethered to the γ-8 mutant lacking the PDZ-binding motif (GluA1-γ-8Δ4), there was no rescue of synaptic currents (Fig. 1C). These results indicate that the PDZ-binding motif is essential for synaptic targeting and, in addition, the presence of the tethered γ-8 prevented any of the endogenous TARPs from displacing it from the receptor. One might argue that the receptor is not being expressed and/or trafficked to the cell surface. We therefore pulled somatic outside-out patches and applied glutamate. The size of the AMPAR currents in cells expressing GluA1-γ-8Δ4 was the same as those in neurons expressing GluA1-γ-8 (Fig. 1D). Additionally, we performed voltage ramps to measure rectification of the currents. If we had successfully replaced all endogenous AMPARs with our tethered constructs, all receptors should be homomeric GluA1 and fully rectifying. This was, indeed, the case (Fig. 1E). Moreover, in all of these experiments the NMDAR synaptic currents were of normal size (Fig. S2).
Fig. 1.
PDZ-binding motif-mediated interaction is required for basal synaptic trafficking and LTP of AMPARs. Approximate E15.5 Gria1-3fl/fl mice embryos were electroporated in utero with indicated constructs, and the endogenous AMPARs were deleted by Cre (A, n = 15) or replaced with the tethered receptors GluA1-γ-8 (B, n = 15) or GluA1-γ-8Δ4 (C, n = 16). Then P17–P21 acute slices were prepared, and simultaneous dual whole-cell recordings were applied to a transfected CA1 pyramidal neuron and a neighboring wild-type one. Open and filled circles represent amplitudes of AMPAR-EPSCs for single pairs and mean ± SEM, respectively. (Insets) Sample current traces from control (black trace) and experimental (green trace) cells. (Scale bars: 50 pA and 25 ms for representative traces.) Bar graphs show normalized EPSC amplitudes (mean ± SEM) (A, 6.02 ± 0.72% control, ***P < 0.0001; B, 96.53 ± 8.64% control, P > 0.05; C, 9.42 ± 0.67% control, ***P < 0.0001) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (D) Sample traces and bar graphs showing the amplitude of glutamate-evoked AMPAR-mediated currents (mean ± SEM) in the presence of 100 μM cyclothiazide, of outside-out patches from Gria1-3fl/fl organotypic hippocampal slices biolistically transfected with Cre+GluA1-γ-8 (n = 9, 799.44 ± 142.36 pA) and Cre+GluA1-γ-8Δ4 (n = 7, 726.71 ± 203.17 pA). (Scale bars: 250 pA and 2 s for representative traces.) Mann–Whitney U test was used for the statistical analysis and P > 0.05. (E) Sample traces showing voltage ramps applied to outside-out patches in D. (F and G) Dual whole-cell paired-LTP recordings were performed from a control CA1 neuron and a neighboring cell expressing either Cre+GluA1-γ-8 (F, n = 9) or Cre+GluA1-γ-8Δ4 (G, n = 6) in P17–P21 Gria1-3fl/fl acute slices. LTP of GluA1-γ-8 replacement neurons (G) is similar to that of wild-type cells, while LTP of GluA1-γ-8Δ4 tethered receptor (F) is impaired (***P < 0.001, 45 min). The data are shown as the percentage of the respective baseline before LTP induction (mean ± SEM). Sample traces show EPSCs before and 30 min after LTP induction in paired control (black) and experimental neurons (green). (Scale bars: 100 pA/25 ms and 15 pA/25 ms, respectively.) All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test.
We next examined LTP with these tethered receptors. Simultaneous recordings were made from a transfected cell and a neighboring control cell. As might be expected, cells expressing GluA1-γ-8 showed normal LTP (Fig. 1F). We then examined cells expressing GluA1-γ-8Δ4. Since the extrasynaptic pool of AMPARs is normal (Fig. 1D), we might expect to see a large rescue of the AMPAR synaptic currents following LTP. However, LTP was absent in cells expressing only GluA1-γ-8Δ4 receptors (Fig. 1G). All these results suggest that the PDZ-binding motif-mediated postsynaptic interaction is required for both basal synaptic trafficking and LTP of AMPARs. It is interesting to note that the small residual EPSC (excitatory postsynaptic current) observed in Fig. 1 A, C, and G, which has slow kinetics, is primarily due to NMDARs, the same observation as in our previous study (23). LTP of NMDA EPSCs is small and variable (24). Previous studies using either slice culture (19) or knock-in mice (18) have found that phosphor-null mutations in the C-terminal of stargazin (19) or TARP γ-8 (18) impair LTP. When we expressed the tethered TARP γ-8 containing the phosphor-null mutations (18), basal synaptic transmission was not fully rescued (Fig. S3A), but LTP in these neurons was normal (Fig. S3B). These results suggest that these sites are not essential for the expression of LTP. Perhaps the differences in results stem from different experimental strategies. Another possibility is that, while the phosphorylation of the TARP C-tail might contribute to LTP of heteromeric AMPARs, it may play a minimal role during LTP of GluA1 homomeric receptors, as we report in this study.
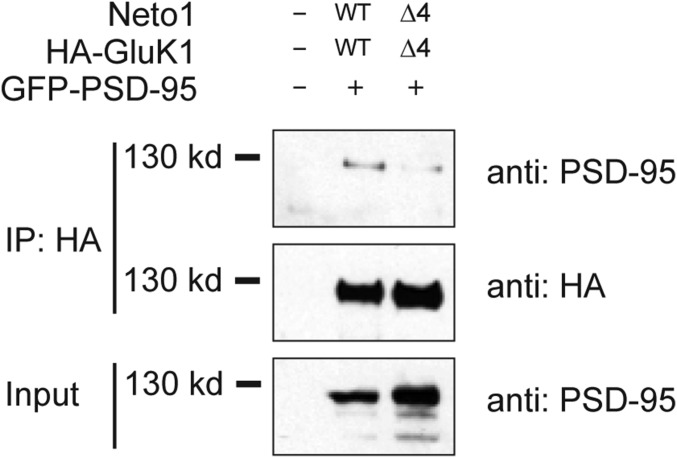
One of the most surprising recent results in the LTP field is the finding that the CA1 neurons, in which the endogenous AMPARs were replaced with KAR/Netos (GluK1/Neto2) that have limited homology with AMPARs and do not bind to TARPs, exhibit normal LTP expression (6). What might these two subclasses of glutamate receptors have in common? Might the critical role of the PDZ-binding domain of γ-8 provide insight into the seemingly promiscuous nature of LTP? It has been found that GluK2 binds to the postsynaptic scaffolding protein PSD-95 through the last four amino acids EMTA (25). Our previous studies have shown that cotransfection of Neto1 or Neto2 proteins is essential for GluK1 synaptic targeting (26), suggesting that pyramidal cells do not express appreciable amounts of either Neto1 or Neto2. Therefore, Neto1 or Neto2 was coexpressed with GluK1 to replace the endogenous AMPARs for later electroporation and electrophysiological experiments. It should be noted that the last four amino acids of GluK1 (ETVA) and Neto1 (TTRV) share homology with the type 1 PDZ-binding motif. We therefore first examined whether the GluK1/Neto1 receptor complex can bind to PSD-95. When PSD-95 was expressed together with wild-type HA-tagged GluK1 and Neto1 in HEK cells, we were able to immunoprecipitate PSD-95 with the HA antibody (Fig. 2). In contrast, when the mutants without the last four amino acids (HA-GluK1Δ4 and Neto1Δ4) were used for coexpression, the level of coimmunoprecipitated PSD-95 was greatly reduced (Fig. 2), indicating that the last four amino acids in GluK1 and Neto1 are functional PDZ-binding motifs mediating their interaction with PSD-95. These results suggest that the PDZ-binding motifs in the GluK1/Neto1 receptor complex might also be necessary for the receptors’ interaction with postsynaptic scaffolding proteins and synaptic trafficking, similar to the requirement of PDZ domain interaction for AMPAR/TARPs.
Fig. 2.
GluK1/Neto1 receptor complex interacts with PSD-95 through PDZ-binding motifs. HEK293T cells were transfected with expression plasmids of GFP-tagged PSD-95 together with wild-type HA-tagged GluK1 and Neto1 or respective mutants deleting the last for amino acids (HA-GluK1Δ4 and Neto1Δ4) as indicated. Western blot was performed to analyze the levels of PSD-95 and GluK1 in the HA immunoprecipitates (IP) and whole-cell lysate (Input) with indicated antibodies.
As an initial test of this hypothesis, we overexpressed these constructs in slice cultures on a wild-type background. As previously reported (26), both Neto1 (Fig. S4 A and E) and Neto2 (Fig. S4 B and E) promoted GluK1 receptor targeting to synapses, leading to a large enhancement of synaptic currents mediated by GluK1. As predicted, exogenous coexpression of mutated GluK1Δ4 and Neto1Δ4 failed to enhance synaptic transmission (Fig. S4 C and E). Similarly, expression of GluK1Δ4 together with Neto2Δ4 substantially reduced the enhancement (Fig. S4 D and E). It is important to note that deleting the last four amino acids in either Neto1 or Neto 2 alone, while keeping the last four amino acids in GluK1 intact, had no effect (26), suggesting functional redundancy.
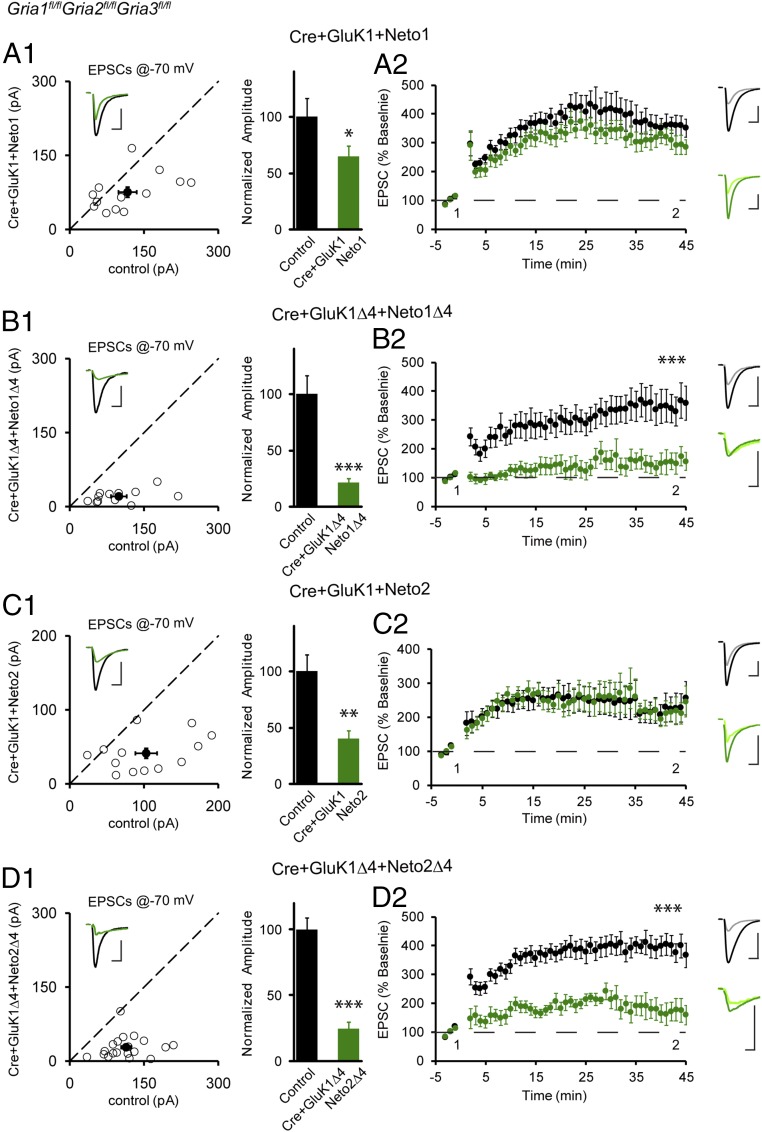
We next returned to in utero electroporation to replace endogenous AMPARs with these same constructs (Fig. 3). Coexpression of GluK1 and Neto1 under these conditions resulted in a substantial rescue of synaptic currents (Fig. 3A1). Cells coexpressing GluK1 and Neto1 also exhibited normal LTP (Fig. 3A2). In striking contrast, the coexpression of GluK1Δ4 and Neto1Δ4 failed to rescue synaptic currents (Fig. 3B1 and Fig. S4F), and LTP was absent (Fig. 3B2). We repeated these experiments with Neto2. In agreement with previous results (6), coexpression of GluK1 and Neto2 caused a partial rescue of synaptic currents (Fig. 3C1), and LTP was normal (Fig. 3C2). In a final series of experiments, we coexpressed GluK1Δ4 together with Neto2Δ4. This resulted in minimal rescue of synaptic currents (Fig. 3D1 and Fig. S4F), and LTP was severely reduced (Fig. 3D2). Importantly, in all of the experiments, the NMDAR synaptic currents were unaltered (Fig. S5). Thus, any impairment in LTP cannot be attributed to the loss of NMDARs. Taken together, these results indicate that the PDZ domains of the GluK1/Neto receptor complex are necessary for both basal synaptic transmission and LTP.
Fig. 3.
PDZ-binding motif-mediated interaction is required for synaptic trafficking and plasticity of KARs. (A1, B1, C1, and D1) The same experimental procedure as in Fig. 1B except that GluK1/Neto1 (A1, n = 13), GluK1Δ4/Neto1Δ4 (B1, n = 12), GluK1/Neto2 (C1, n = 13), or GluK1Δ4/Neto2Δ4 (D1, n = 19) were electroporated to replace endogenous AMPARs. Open and filled circles represent amplitudes of EPSCs for single pairs and mean ± SEM, respectively. (Insets) Sample current traces from control (black) and experimental (green) cells. (Scale bars: 50 pA and 25 ms for representative traces.) Bar graphs show normalized EPSC amplitudes (mean ± SEM) (A1, 64.66 ± 8.90% control, *P < 0.05; B1, 21.02 ± 3.71% control, ***P < 0.0001; C1, 40.14 ± 6.60% control, **P < 0.005; D1, 24.75 ± 4.57% control, ***P < 0.0001) presented in scatter plots. All of the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed-rank sum test. (A2, B2, C2, and D2) The same experimental procedure as in Fig. 1F except that the LTP of the following replaced KARs were examined: GluK1/Neto1 (A2, n = 11), GluK1Δ4/Neto1Δ4 (B2, n = 10), GluK1/Neto2 (C2, n = 10), or GluK1Δ4/Neto2Δ4 (D2, n = 13). The LTP of GluK1/Neto1 or GluK1/Neto2 replacement neurons is normal while the LTP of GluK1Δ4/Neto1Δ4 or GluK1Δ4/Neto2Δ4 receptors is impaired (***P < 0.001, 45 min). The data are shown as the percentage of the respective baseline before LTP induction (mean ± SEM). Sample traces show EPSCs before and 30 min after LTP induction in paired control (black) and replacement neurons (green). [Scale bars: 100 pA/25 ms and 50 pA/25 ms (A2 and C2); 200 pA/25 ms and 50 pA/25 ms (B2 and D2).] All of the statistical analyses are compared with respective control neurons by the two-tailed Wilcoxon signed-rank sum test.
Discussion
Despite many decades of investigation, the rules governing AMPAR trafficking and LTP remain confusing. In the present study, we took a single-cell genetic approach that allowed us to study the behavior of a defined AMPAR/TARP complex in isolation. With this approach we established that the PDZ ligand of TARP γ-8 is essential for the translocation of surface AMPARs to the synapse. Furthermore, this PDZ ligand is also essential for LTP. Using γ-8Δ4 knock-in mice, it was previously found that the PDZ-binding motif of TARP γ-8 is involved in constitutive synaptic transmission, but not required for synaptic plasticity (17). This seemingly inconsistent result may be caused by the redundancy of other TARPs expressed in pyramidal cells. Most importantly, our findings provided a clue as to why exogenously expressed KARs, with minimal homolog to AMPARs, exhibit normal LTP. These receptors, together with their auxiliary Neto subunits, also contain PDZ ligands, which we demonstrate are essential both for basal synaptic targeting and for LTP.
Previous studies directly compared the properties of TARP γ-8 to that of TARP γ-2 in stargazin cerebellar granule cells (27). Both TARP γ-8Δ4 and TARP γ-2Δ4 trafficked to the surface the same as their respective wild type but both profoundly reduced the frequency of miniature EPSCs (mEPSCs), confirming the importance of the PDZ-binding motif for both TARPs in synaptic targeting. Interestingly, although the amplitude of the few remaining mEPSCs in TARP γ-2Δ4 expressing cells was reduced, a significant reduction was not observed with TARP γ-8Δ4, perhaps suggesting a minor PDZ-binding independent component to trafficking. However, our present results in hippocampal pyramidal cells indicate that the PDZ-binding domain of TARP γ-8 can fully account for its synaptic targeting. Furthermore, our findings suggest that the PDZ-domain interactions between glutamate receptors/auxiliary subunits and PSD scaffolding proteins are a general and minimal postsynaptic requirement for LTP. However, it is conceivable that the PDZ-domain interactions that deliver the receptors to the PSD are a prerequisite for the induction of LTP, which is a downstream step.
Along with previous results (28–32), we propose a model for LTP in which PDZ-domain–containing synaptic scaffolding proteins, such as PSD-95, are masked under basal conditions, but become unmasked during LTP, thus allowing the synaptic capture of mobile surface glutamate receptors via their PDZ ligands. An intriguing candidate for a masking protein is synGAP (33, 34), which binds with high affinity to PSD-95 (35, 36). Upon CaMKII phosphorylation of synGAP, the binding affinity decreases, and synGAP dissociates from PSD-95 (36), freeing PDZ domains to which AMPAR/TARP complexes can bind. It has long been proposed that LTP directly targets AMPARs and that the PSD plays a passive role. The present results support a fundamentally different model that focuses on the PSD as the target for modification during LTP, with the glutamate receptor/auxiliary subunit complexes playing passive roles.
Materials and Methods
Experimental Constructs.
The GluA1-TARP tethered constructs were made as previously described (22) and were subcloned into the pCAGGS vector for biolistic transfection and in utero electroporation.
Mouse Genetics.
Animals were housed according to the Institutional Animal Care and Use Committee Guidelines at the University of California, San Francisco and the Institutional Review Board of Kunming Institute of Zoology, Chinese Academy of Sciences. Gria1fl/flGria2fl/flGria3fl/fl mice were generated and genotyped as previously described (21).
Electrophysiology.
Voltage-clamp recordings were taken from CA1 pyramidal neurons in acute hippocampal slices or organotypic slice cultures. For acute slices, 300-μm transverse slices were cut using a Microslicer DTK-Zero1 (Ted Pella) in chilled high-sucrose cutting solution containing the following (in mM): 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 7 D-glucose, 210 sucrose, and 1.3 ascorbic acid. The slices were then incubated for 30 min at 34 °C in artificial CSF (ACSF) containing the following (in mM): 119 NaCl, 2.5 KCl, 26.2 NaHCO3, 1 NaH2PO4, and 11 D-glucose. For acute slices, 2.5 mM CaCl2 and 1.3 mM MgSO4 were added to the ACSF, which was bubbled with 95% O2 and 5% CO2 to maintain pH, and the slices were allowed to recover at room temperature for 30 min to 1 h before recording at room temperature. For organotypic slice cultures, 4 mM CaCl2 and 4 mM MgSO4 were added. For recording, slices were transferred to a perfusion stage on an Olympus BX51WI upright microscope and perfused at 2.5 mL/min with ACSF containing 0.1 mM picrotoxin. Synaptic responses were evoked by stimulating with a bipolar metal electrode in stratum radiatum of CA1. To ensure stable recording, membrane holding current, input resistance, and pipette series resistance were monitored throughout the recording. Data were gathered through a MultiClamp 700B amplifier (Axon Instruments), filtered at 2 kHz, and digitized at 10 kHz.
Whole-Cell Synaptic Recordings and LTP.
Simultaneous dual whole-cell recordings were made between GFP- and mCherry-positive experimental cells as identified by epifluorescence and neighboring nontransfected control cells. The internal recording solution contained (in mM): 135 CsMeSO4, 8 NaCl, 10 Hepes, 0.3 EGTA, 5 QX314-Cl, 4 Mg-ATP, 0.3 Na-GTP, and 0.1 spermine. Osmolarity was adjusted to 290–295 mOsm, and pH was buffered at 7.3–7.4. AMPAR-mediated responses were isolated by voltage-clamping the cell at −70 mV, whereas NMDAR-mediated responses were recorded at +40 mV, with amplitudes measured 100 ms after stimulation to avoid contamination by AMPAR current. LTP was induced by stimulating at 2 Hz for 90 s while clamping the cell at 0 mV, after recording a stable 3- to 5-min baseline, but not more than 6 min after breaking into the cell. To minimize run-up of baseline responses during LTP, slices were stimulated for ∼10 min before breaking in, and both cells were held as cell-attached for 2–5 min before breaking into the whole-cell mode. Before breaking in, stimulation intensity was calibrated just below the threshold required to elicit an action potential from the wild-type control neuron.
Immunoblotting.
HEK293T cells were maintained and transfected as previously described (37). For coimmunoprecipitation, cells were washed in PBS and lysed in a TBS buffer containing 1 mM EDTA (Sigma), protease inhibitors (Roche), and 1% Triton X-100 (Fisher Scientific). Of the total whole-cell lysates, 2.5% was saved as input, and the rest was then incubated with ∼10 μL of monoclonal anti-HA agarose antibody-conjugated beads (Sigma) at 4 °C overnight. The following day, the immunoprecipitations were washed in TBS buffer containing 150 mM NaCl, 50 mM Tris⋅HCl, 5 mM EDTA, protease inhibitors, and 0.1% Triton X-100. The antibody-conjugated beads were resuspended in SDS/PAGE sample buffer and subjected to Western blotting. Antibodies used in the study were PSD-95 (MS; Neuromab) and HA (Rat; Roche).
Neuronal Transfection.
Organotypic hippocampal slice cultures were made from P6–P8 mice as previously described (38). Biolistic transfections were carried out on day 2 after culturing using a Helios Gene Gun (Bio-Rad) with 1-μm DNA-coated gold particles. When expressing two plasmids, gold particles were coated with equal amounts of each plasmid expressing different fluorescent markers. Observed frequency of coexpression was nearly 100%. Slices were maintained at 34 °C with media changes every other day.
For in utero electroporations, ∼E15.5 pregnant Gria1-3fl/fl mice were anesthetized with 2.5% isoflurane in O2 and injected with buprenorphine for analgesic. Embryos within the uterus were temporarily removed from the abdomen and injected with 2 μL of mixed plasmid DNA into the left ventricle via a beveled micropipette. FUGW-Cre:mCherry and pCAGGS-GluA1-TARP:EGFP plasmids were typically diluted to final concentration of approximate 0.5 μg/μL each. Each embryo was electroporated with 5 × 50 ms, 35 V pulses. The positive electrode was placed in the lower right hemisphere, and the negative electrode was placed in the upper left hemisphere (39). After electroporation, the embryos were sutured into the abdomen and killed on P17–P21 for dual whole-basal synaptic transmission or LTP recording (6).
Outside-Out Patches.
Outside-out patches were taken from CA1 pyramidal neurons of organotypic hippocampal slices. Initially, cells were recorded in whole-cell mode at −70 mV with a 4- to 5-MΩ patch pipette. The pipette was then slowly pulled away from the soma until a high-resistance seal reformed. The external Hepes-ACSF contained (in mM): 140 NaCl, 5 KCl, 1.4 MgCl2, 1 CaCl2, 5 EGTA, 10 Hepes, 1 NaH2PO4, 10 D-glucose, with pH adjusted to 7.4. Glutamate currents were evoked by perfusion over the tip of the pipette of the same solution with the addition of 1 mM L-glutamic acid, 0.1 mM D-APV, 0.1 mM picrotoxin, 0.1 mM cyclothiazide, and 0.5 mM tetrodotoxin. A ValveLink 8 (AutoMate Scientific) was used for fast perfusion of control and glutamate-containing Hepes-ACSF. During outside-out patch experiments, experimental cells were interleaved with nontransfected control cells.
Statistical Analysis.
Significance of evoked dual whole-cell recordings compared with controls was determined using the two-tailed Wilcoxon signed-rank sum test. For all experiments involving unpaired data, including all outside-out patch data, a Mann–Whitney U test with Bonferroni correction for multiple comparisons was used. Data analysis was carried out in Igor Pro (Wavemetrics), Excel (Microsoft), and GraphPad Prism (GraphPad Software).
Supplementary Material
Acknowledgments
We thank M. Cerpas and D. Qin for technical assistance and all members of the R.A.N. laboratory for discussion of and comments on the manuscript. This work was funded by grants from the National Institute of Mental Health (to R.A.N.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB13000000), the National Natural Science Foundation of China (31741055), and the Chinese Academy of Sciences Pioneer Hundred Talents Program (to N.S.). Y.S.S. is supported by National Natural Science Foundation of China Grants (31371061 and 31571060), Ministry of Science and Technology of China Grants (2014CB942804 and 2015BAI08B02), and Natural Science Foundation of Jiangsu Province Grant (BK20140018).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800719115/-/DCSupplemental.
References
- 1.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 2.Murakoshi H, Yasuda R. Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci. 2012;35:135–143. doi: 10.1016/j.tins.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 5.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 6.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493:495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greger IH, Watson JF, Cull-Candy SG. Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron. 2017;94:713–730. doi: 10.1016/j.neuron.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol. 2012;22:488–495. doi: 10.1016/j.conb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copits BA, Swanson GT. Dancing partners at the synapse: Auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci. 2012;13:675–686. doi: 10.1038/nrn3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita S, et al. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 13.Tomita S, et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 14.Menuz K, O’Brien JL, Karmizadegan S, Bredt DS, Nicoll RA. TARP redundancy is critical for maintaining AMPA receptor function. J Neurosci. 2008;28:8740–8746. doi: 10.1523/JNEUROSCI.1319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menuz K, Kerchner GA, O’Brien JL, Nicoll RA. Critical role for TARPs in early development despite broad functional redundancy. Neuropharmacology. 2009;56:22–29. doi: 10.1016/j.neuropharm.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouach N, et al. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- 17.Sumioka A, et al. PDZ binding of TARPγ-8 controls synaptic transmission but not synaptic plasticity. Nat Neurosci. 2011;14:1410–1412. doi: 10.1038/nn.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J, et al. CaMKII phosphorylation of TARPγ-8 is a mediator of LTP and learning and memory. Neuron. 2016;92:75–83. doi: 10.1016/j.neuron.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Lu W, Milstein AD, Nicoll RA. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron. 2009;62:633–640. doi: 10.1016/j.neuron.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díaz-Alonso J, et al. Subunit-specific role for the amino-terminal domain of AMPA receptors in synaptic targeting. Proc Natl Acad Sci USA. 2017;114:7136–7141. doi: 10.1073/pnas.1707472114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicoll RA. A brief history of long-term potentiation. Neuron. 2017;93:281–290. doi: 10.1016/j.neuron.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Garcia EP, et al. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 26.Sheng N, Shi YS, Lomash RM, Roche KW, Nicoll RA. Neto auxiliary proteins control both the trafficking and biophysical properties of the kainate receptor GluK1. eLife. 2015;4:e11682. doi: 10.7554/eLife.11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milstein AD, Nicoll RA. TARP modulation of synaptic AMPA receptor trafficking and gating depends on multiple intracellular domains. Proc Natl Acad Sci USA. 2009;106:11348–11351. doi: 10.1073/pnas.0905570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opazo P, Sainlos M, Choquet D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr Opin Neurobiol. 2012;22:453–460. doi: 10.1016/j.conb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci USA. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penn AC, et al. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549:384–388. doi: 10.1038/nature23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herring BE, Nicoll RA. Long-term potentiation: From CaMKII to AMPA receptor trafficking. Annu Rev Physiol. 2016;78:351–365. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Tao-Cheng JH, Reese TS, Dosemeci A. SynGAP moves out of the core of the postsynaptic density upon depolarization. Neuroscience. 2011;192:132–139. doi: 10.1016/j.neuroscience.2011.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dosemeci A, Weinberg RJ, Reese TS, Tao-Cheng JH. The postsynaptic density: There is more than meets the eye. Front Synaptic Neurosci. 2016;8:23. doi: 10.3389/fnsyn.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araki Y, Zeng M, Zhang M, Huganir RL. Rapid dispersion of SynGAP from synaptic spines triggers AMPA receptor insertion and spine enlargement during LTP. Neuron. 2015;85:173–189. doi: 10.1016/j.neuron.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walkup WG, et al. A model for regulation by SynGAP-α1 of binding of synaptic proteins to PDZ-domain ‘Slots’ in the postsynaptic density. eLife. 2016;5:e16813. doi: 10.7554/eLife.16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bemben MA, et al. CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat Neurosci. 2014;17:56–64. doi: 10.1038/nn.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnell E, et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci USA. 2008;105:20953–20958. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.