Abstract
Rationale
Depression is a leading cause of disease burden and disability for older adults; thus, prevention is a priority. Biologic and observational data support potential mental health benefits of vitamin D and omega-3 fatty acids; however, it is unclear whether these supplements can prevent late-life depression.
Design
We describe the novel methodology of a large-scale study: VITAL-DEP (VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention), an ancillary to the VITAL trial. Primary Aims of VITAL-DEP are to determine effects on prevention of depression and on trajectory of mood symptoms of long-term (mean=5 years) supplementation with vitamin D (vitamin D3 [cholecalciferol], 2000 IU/day) and marine omega-3 fatty-acids (eicosapentaenoic acid + docosahexaenoic acid, 1 g/day), in a 2x2 factorial design, among 25,874 older adults. Secondary Aims will evaluate: vitamin D’s effects among African-Americans (an at-risk group for vitamin D deficiency); both agents’ effects among those with high-risk factors or sub-syndromal depression in a sub-set of ~1,000 participants with detailed examinations at baseline and 2-year follow-up; whether baseline nutrient levels influence depression risk and/or modify agents’ effects. Additional planned analyses will use pre-randomization blood samples available in ~17,000 participants to address whether key biomarkers and factors influence long-term mood and depression risk and/or the agents’ effects.
Conclusion
VITAL-DEP applies all modalities of state-of-the-art prevention research – universal, selective and indicated. VITAL-DEP will clarify effects of supplemental vitamin D and/or omega-3 on mood, and inform clinical care and public health guidelines on the use of these agents for prevention of depression in mid-life and older adults.
Keywords: geriatric, depression, mood, prevention, cholecalciferol, fish oil, omega-3
1. INTRODUCTION
Late-life depression is common, disabling and associated with higher healthcare utilization and costs1,2. Residual symptoms and dysfunction frequently occur in late-life depression, even with appropriate treatment3,4. Furthermore, among older minorities there are increased challenges of under-diagnosis, under-treatment5 and greater dysfunction from depression6. Thus, prevention of late-life depression is a public health priority. In response, the VITAL-DEP (VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention) study will address depression prevention among diverse mid-life and older adults using two agents – vitamin D3 (cholecalciferol) and marine omega-3 fatty acids – with credible evidence for mental health benefits. VITAL-DEP is an ancillary study to VITAL, a double-blind, placebo-controlled, randomized clinical trial (RCT) of primary prevention of cardiovascular disease and cancer7.
Vitamin D is initially obtained as vitamin D2 or D3 from diet or supplements, or as D3 from synthesis in skin after sunlight exposure8; it is further synthesized to an active hormone that binds to vitamin D receptors (VDRs) found in more than 30 cell types8. Vitamin D deficiency is influenced by several factors including older age9–11, darker skin pigmentation12–18, northern latitude17 and genetics8. There are strong biologic links of vitamin D to mood19–21: VDRs are widely distributed in the brain22, including limbic structures involved in mood/affect, and VDR genetic variation may influence late-life depression susceptibility23. Observational data24–26 generally support associations between low vitamin D and depression; however, few investigations focused on older people19,27. Recent systematic reviews and/or meta-analyses27–29 showed mixed findings. Overall, Rejnmark et al.28 concluded that, due to design issues, existing RCT data are not sufficient to disprove hypotheses of non-skeletal health effects of vitamin D. Key critiques are small samples, insufficient dose/duration and/or lack of biomarkers. Spedding29 found that, compared to RCTs without biological design flaws (e.g., lack of – or failure to demonstrate meaningful change in – vitamin D biomarkers), RCTs without such flaws showed benefits of vitamin D in depression. Another review27 identified only 3 RCTs30–32 of vitamin D that both included older adults at-risk for mood problems and featured large samples, high doses (≥800 IU/d) and long-term treatment (≥1 year); the study31 with the most robust and specific depression measure found evidence of benefit.
Strong evidence exists for mood benefits of long-chain omega-3 fatty acids, eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), which are abundant in fish and seafood and linked to health benefits33,34. Proposed biologic mechanisms include increased membrane fluidity and serotonergic transmission35,36 and reduction of inflammatory cytokines37,38 associated with depression39. Most large observational studies40–46, but not all47–50, support inverse relations of fish/omega-3 to depression. Also most trials, reviews and RCT meta-analyses51–55 suggest antidepressant effects of omega-3 supplementation, particularly as adjunctive therapy51. Inconsistencies in results may have resulted from: small samples; incomplete randomization and/or blinding; short follow-up; variable compliance; differences in protocols (e.g., monotherapy vs. augmentation, formulations/doses) or study populations (clinic vs. community)56. Thus, benefits for mood have not been uniformly observed57, and the role of omega-3 in prevention must be clarified. Finally, synergies between vitamin D and omega-3 may exist (Figure 1), but this possibility remains untested.
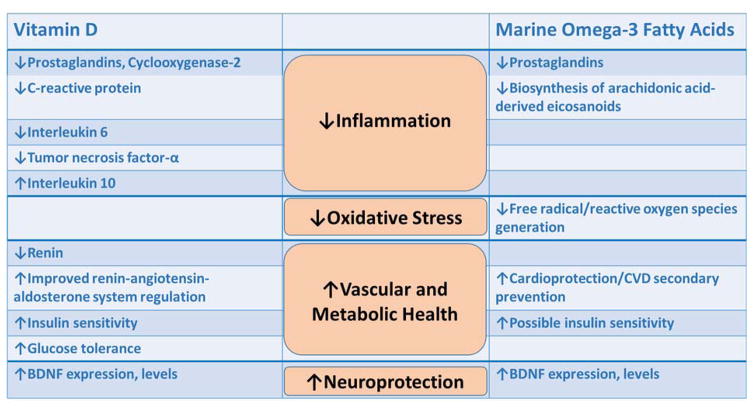
Figure 1. Mechanisms by which vitamin D and/or fish oil may lower depression risk.
Four hypothesized mechanistic areas are illustrated: inflammation, oxidative stress, vascular/metabolic health and neuroprotection. Vitamin D may lower depression risk through: more favorable profiles of inflammatory indicators, vascular (renin-angiotensin-aldosterone system) and metabolic (improved insulin sensitivity and glucose tolerance) factors, and increased expression and levels of brain-derived neurotrophic factor (BDNF). Marine omega-3 fatty acids (fish oil) may lower depression risk through: more favorable profiles of inflammatory indicators, lower levels of free radicals and reactive oxygen species formation, improved protection against cardiovascular disease (CVD), and increased expression and levels of BDNF.
2. MATERIALS AND METHODS
2.1. Overview of study design
VITAL-DEP is a late-life depression prevention ancillary study, separately designed to coordinate with the National Institutes of Health (NIH)-funded VITAL trial58 (U01 CA138962 and R01 CA138962), a 2x2 factorial RCT of vitamin D3 (2000 IU/d) and omega-3 fatty acids (1 g/d of EPA and DHA) in the primary prevention of cancer and cardiovascular disease (CVD)
The final study population at randomization in VITAL includes 12,793 men aged 50+ years and 13,081 women aged 55+ years; the average duration of randomization will be 5 years. The mean participant age-at-randomization is 67.1 years (SD=7.1, range=50–100); over 60% of participants are aged 65+ years, and 13% are aged 75+ years59. In addition, nearly 17,000 participants provided baseline blood samples, and approximately 6,000 follow-up blood samples are expected. Extensive details on the design of VITAL are provided elsewhere7,59. Therefore, in this report we provide detail on those elements of VITAL that are relevant to the conduct of the VITAL-DEP ancillary study.
2.2. Aims
Primary Aims of VITAL-DEP are to utilize a universal prevention paradigm to test whether long-term (average of 5 years) vitamin D3 or marine omega-3 fatty acid supplementation: 1) reduces risk for incident or recurrent depression (a composite endpoint of any clinically significant depressive syndrome60); 2) yields better mood scores on symptom questionnaires, over 5 years, among the full VITAL cohort. These Primary Aims will be undertaken in the full, nation-wide cohort of 25,874 VITAL participants.
There are three Secondary Aims in VITAL-DEP. First, as African-Americans are disproportionately affected by low biochemical vitamin D levels, we will address impact of vitamin D supplementation on depression risk and mood scores among African-Americans, who represent 20% (n=5,107) of the full VITAL cohort. Second, among a deeply-phenotyped sub-cohort of ~1,000 VITAL participants who underwent detailed baseline and 2-year follow-up examinations at our local NIH-funded Clinical and Translational Science Center (CTSC), we will address selective and indicated prevention. Specifically, we will address whether supplementation with vitamin and omega-3 reduces depression risk and yields lower depression scores among persons with high-risk factors for late-life depression (selective prevention) or with subsyndromal depressive symptoms (indicated prevention). Third, we will assess whether baseline plasma levels of vitamin D and omega-3 fatty acids are related to depression outcomes over 5 years, and whether they modify the study agents’ effects within the full VITAL cohort. Thus, with the above complementary set of Primary and Secondary Aims, VITAL-DEP is a first-of-its-kind study that simultaneously addresses universal, selective and indicated prevention61 paradigms in late-life depression.
Additional analyses are planned as part of VITAL-DEP. Planning of these analyses was facilitated by the fact that further funding became available in VITAL-DEP to accrue depression outcomes data for an additional two years beyond the projected end of the randomized phase of VITAL. Thus, the overall goal of these additional analyses is to address: 1) longer-term effect (average of 7 years) of the study agents on mood trajectories, and long-term relations of baseline biochemical nutrient levels of vitamin D and omega-3 to mood trajectories of 7+ years; 2) potential biological mediators and moderators of response to the study agents’ as it pertains to mood outcomes. Regarding biological mediators and moderators, details are provided below (Section 2.7) on the rationale for their selection and potential to inform biological paths involved in mood responses to vitamin D and fish oil. Briefly, because VITAL-DEP prioritizes the theme of racial/ethnic differences in long-term study agent effects, we will examine relations of bioavailable vitamin D to depression, with attention to race differences in outcomes, in a sub-set of ~2,000 persons in the full VITAL cohort (sub-set will be selected to include at least 50% participants who are black). Further, to test whether changes in mechanistically relevant biomarkers may explain variation in mood response to the study agents, we will conduct pre- and post-randomization serum brain-derived neurotrophic factor (BDNF) assays and plasma metabolite profiling in the sub-set of VITAL-DEP CTSC participants at risk for depression at baseline. Finally, we will conduct exploratory analyses to assess whether there are additive and/or synergistic effects of the agents in depression, and we will test effect modification by age, gender, baseline nutrient levels, baseline medical comorbidity, geographic latitude (for vitamin D), and physical activity (for vitamin D).
2.3. Sponsors
VITAL-DEP (R01 MH091448) is supported by the National Institute of Mental Health (NIMH). VITAL-DEP Human Subjects’ procedures were developed with input from an independent ethics consultation by the Harvard Ethics Team, which is supported by the Harvard Catalyst/Harvard CTSA (UL1 TR001102). The study protocol was approved by the Partners Human Research Committees, the Institutional Review Board of Brigham and Women’s Hospital (BWH). A Data and Safety Monitoring Board (DSMB) has been assembled for the review of the parent trial and ancillaries, including VITAL-DEP. The VITAL-DEP study is registered with clinicaltrials.gov (NCT01696435). The VITAL parent trial has received IND approval from the FDA and has been registered at clinicaltrials.gov (NCT01169259). A study website with information pertaining to VITAL and ancillaries is maintained at www.vitalstudy.org.
2.4. Summary of eligibility, recruitment, and enrollment
Because VITAL-DEP is an ancillary study to VITAL, the eligibility criteria for VITAL-DEP reflect those of VITAL, as detailed elsewhere7. Briefly, participants are U.S. men aged ≥50y and women aged ≥55y, who are required to: have no history of cancer or cardiovascular disease; limit consumption of vitamin D from all supplemental sources to under 800 IU/d; limit consumption of supplemental calcium to under 1200 mg/d; forego the use of fish-oil supplements; for safety reasons, be free of renal failure or dialysis, hypercalcemia, hypo- or hyperparathyroidism, cirrhosis, or sarcoidosis or other granulomatous diseases, allergy to soy (which is in the vitamin D placebo) or fish. Participants were also required to show adequate adherence to placebo pills (defined as taking ≥2/3 of the pills) during a 3 month run-in period, which served to identify a group of excellent compliers for long-term participation, minimizing loss-to-follow-up62. VITAL achieved a final randomized population of 25,874 participants. Study enrollment began in November 2011 and proceeded on a rolling basis until all randomization cells were closed in March 2014.
VITAL-DEP participants are members of the full VITAL cohort who satisfy additional eligibility criteria for a depression prevention study. Specifically, eligibility for testing of the Primary Aims in VITAL-DEP requires absence of: current significant depressive symptoms by PHQ-8 (Patient Health Questionnaire-8) score; core major depressive disorder symptoms for a period of two or more weeks in the past two years; history of alcohol and/or substance abuse disorder active in the past 12 months, schizophrenia or other primary psychotic disorder, bipolar disorder, post-traumatic stress disorder or obsessive-compulsive disorder; psychiatric hospitalization in the past 2 years; current psychotherapy or current use of psychotropics (including non-prescription agents for the treatment of mood disorders), except for limited use of mild sedatives/hypnotics; history of major neurologic disorder or delirium episode in the past 12 months; history of clinical (i.e., overt and not sub-clinical) hypothyroidism diagnosis. Also see the ClinicalTrials.gov listing for VITAL-DEP (NCT01696435).
VITAL established a sub-cohort of 1,054 participants from the New England Region to be evaluated during in-person assessments at the NIH-sponsored Harvard Catalyst Clinical and Translational Science Center (CTSC) conducted at baseline and 2 years later. Those enrolled met the same eligibility criteria as in the full VITAL trial; in addition, persons with factors that could significantly impact patient safety or compliance (e.g., transportation problems resulting in transit time of >3 hours to/from the CTSC) were excluded from participating in the VITAL CTSC. All 1,054 members of the VITAL CTSC sub-cohort were invited to take part in the 45-minute VITAL-DEP neurobehavioral assessment, and 1,041 completed these assessments at baseline. Participants were eligible for the VITAL-DEP CTSC component if they did not have: 1) unstable psychiatric symptoms during evaluation (e.g., suicidality, homicidality, psychosis); 2) cognitive performance below impairment cutoff on a validated screener; 3) any of the following, as determined by structured psychiatric interview: current depression (e.g., major depressive disorder (MDD) or episode), alcohol or substance abuse or dependence in the past 12 months, schizophrenia/primary psychotic disorder, bipolar disorder, dementia, OCD or PTSD; 4) significant handicap that would interfere with testing procedures (e.g., severe visual or hearing loss). Thus, a key objective of the VITAL-DEP CTSC protocol was to facilitate careful testing of one of the Secondary Aims of VITAL-DEP – the study agents’ impact on depression risk among those with high-risk factors (selective prevention)63,64, as well as individuals with sub-syndromal depressive symptoms64–69 but not major depression (indicated prevention)61,70.
2.5. Interventions
Interventions tested in VITAL are vitamin D3 (2000 IU/d of cholecalciferol) and active fish oil (Omacor® fish oil, a 1 g/d capsule containing 840 mg of marine omega-3 fatty acids as 465 mg of EPA and 375 mg of DHA) and matching inert placebos7,59. VITAL procedures permit participants intake of up to 800 IU/d of additional vitamin D supplementation and 1200 mg/d of elemental calcium from all sources, in accordance with safety limits indicated by the Institute of Medicine (now National Academy of Medicine (NAM))71. Participants also agree to forgo consumption of non-study fish-oil supplements during the trial. The doses of vitamin D3 and omega-3 fatty acids tested in VITAL were chosen after comprehensive review of the available literature to achieve optimal efficacy and safety. Both active and placebo capsules have similar flavoring, and the use of the high-quality Omacor® preparation provides uniform dosing and removes concerns of a fishy after-taste, reducing risk of accidental unblinding72,73.
2.6. Blood collection
Fasting blood samples in the full cohort of the parent VITAL trial were collected during run-in (i.e., before randomization) from a subset of 16,954 participants. Among this subset who provided baseline blood samples within the full cohort of VITAL, follow-up samples are being obtained from a randomly selected group of ~6,000 participants. The baseline and follow-up blood samples from a random subset of VITAL participants will allow assessment of (a) pill-taking compliance, (b) changes in biomarkers, including safety measures, with the study agents, and (c) in the placebo group, effects of changing trends in background fortification with vitamin D and omega-3 fatty acids. Fasting bloods are also collected at baseline and 2-year follow-up among the 1,054 VITAL participants who are attending CTSC visits. As detailed below under “2.7 Study assessments,” VITAL-DEP will utilize the blood samples collected among both the full VITAL cohort and VITAL-CTSC to complete several study aims and analyses.
2.7 Study assessments
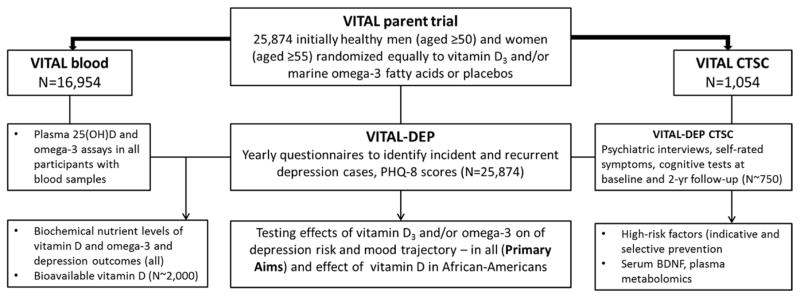
To test the effects of daily supplemental vitamin D and/or omega-3 fatty acids on depression risk and mood (Primary Aims) as well as to address Secondary Aims and other planned analyses in VITAL-DEP, a comprehensive list of assessments were conducted in both the full VITAL cohort and the VITAL-CTSC sub-cohort. In addition, several biomarkers were obtained to test hypotheses in VITAL-DEP; these are enumerated below, along with the scientific rationale for their inclusion. Figure 2 illustrates the sources of study participants for assessments and different planned analyses in VITAL-DEP.
Figure 2.
Sources of study participants for assessments and analyses in VITAL-DEP.
2.7.1. Main VITAL study questionnaires
All VITAL participants receive questionnaires that collect information on a wide variety of demographic, health, lifestyle, and medication variables, as well as intakes of vitamin D and marine omega-3 fatty acids from dietary or non-study supplemental sources. These variables are ascertained on VITAL questionnaires administered at baseline (pre-randomization) and annually thereafter. A validated, self-administered, semi-quantitative food frequency questionnaire (FFQ) is also collected prior to randomization. Participants are also asked to complete a follow-up FFQ at 4 years, to monitor background dietary changes during VITAL.
2.7.2. VITAL-DEP assessment measures in the full VITAL cohort
The measures selected for VITAL-DEP are used to detect within the full cohort of VITAL participants the Primary Aim outcomes of: 1) incidence and recurrence of depression over 5 years and 2) 5-year trajectories of repeated mood scores. The categorical depression endpoint (“depression”) in VITAL-DEP was any depressive syndrome60, which includes both major and minor depression, as both can have significant impact on morbidity in later-life74,75. The patterns of responses on each questionnaire are used to determine whether participants are currently experiencing or at risk for depression incidence (no current or prior depression) or recurrence (prior diagnosis of depression but no active symptoms or ongoing treatment in past 2 years). Thus, the following are obtained via questionnaires annually in the full cohort of VITAL-DEP participants over the average study period of 5 years:
1) Patient Health Questionnaire-8
The PHQ-8 was developed for detection of depression in primary care and community-based settings. It is identical to the PHQ-976 with the exception of the item on suicidal ideation and behaviors77,78, but elimination of this item has no impact on the ability of the PHQ-8 to classify depression and identical scoring thresholds for depression can be used79. The PHQ-8 is validated for detection of clinical depressive syndrome77,80; other strengths of the PHQ include: 1) use of continuous PHQ scores (range=0–24) as a severity measure81; 2) sensitivity to change82; 3) lack of evidence of differential item functioning among African-Americans and other minorities83,84 – which has been detected with other instruments85–87; 4) brevity and low participant burden.
Noteworthy additional considerations also influenced selection of the PHQ-8 vs. PHQ-9. VITAL features mailings of paper questionnaires to tens of thousands of participants around the country, and the time lag between questionnaire send-date and post-receipt data processing is usually several weeks to months. Thus, we would not be able to identify a positive response to the PHQ-9 suicide item in a reasonable period. However, including this item could well create a false expectation of a rapid response, as many participants may reasonably expect that investigators would not make such an inquiry unless they intended to respond immediately. Consequently, safety could actually be compromised, as participants could be induced to wait months for an investigator response, rather than contacting local health providers or supportive persons promptly with such symptoms. To best protect participant safety, we obtained independent consultation from a research ethicist and consulted with senior staff at our Institutional Review Board in order to devise the enhanced PHQ-8 follow-up procedures that are approved for use in our study and described in Section 2.8.2.
2) Other self-reported depression measures
These include the gating items from the Diagnostic Interview Schedule (DIS)88, as well global questions re: prior clinical diagnosis and/or treatment of depression and core symptoms of MDD.
3) Supplemental information on depression outcomes
To supplement these self-reports, we will access information in Centers for Medicare & Medicaid Services data queries funded by the parent VITAL trial and partly by VITAL-DEP and other ancillaries, with assistance of a CMS contractor using Privacy Act (HIPAA)-compliant data release policies and procedures; data for retrieval include codes consistent with diagnosis of and/or treatment for a depressive disorder as antidepressant prescriptions.
4) Use of assessments to identify depression endpoints for Primary Aims in VITAL-DEP
For identification of depression endpoints for VITAL-DEP Primary Aim 1, we employ a strategy similar to existing methods for case-finding that have been successfully used in high-quality studies60,89,90 involving large samples of community-dwelling elders (see Figure 3). Briefly, a participant is considered an incident case the first occasion any of the following occurs: 1) PHQ-8 score consistent with MDD, 2) self-report of clinician diagnosis of depression, 3) self-report of DIS MDD symptoms, 4) initiation of regular use of prescription antidepressants, 5) presence of new ICD-9/ICD-10 code for depression. A recurrent case is counted the first occasion any of the following occurs: 1) PHQ-8 score consistent with MDD, 2) self-report of DIS MDD symptoms, 3) presence of a new mental health visit with linked ICD-9/ICD-10 code for depression, 4) new use of prescription antidepressants. Of note, no “double-counting” of cases (incident cases occurring can remit and then recur during the subsequent follow-up period) is permitted, in order to preserve original sets of participants from the time of the randomization. Finally, a Depression Endpoint Committee, consisting of psychiatrists Drs. Okereke, Chang, Reynolds and Mischoulon, determines diagnosis and event date by consensus for all cases with conflicting reports from 2 or more sources. For Primary Aim 2 in VITAL-DEP, the outcome is the trajectory, over 5 years, of PHQ-8 scores on the annually mailed questionnaires in the full VITAL cohort.
2.7.3. VITAL-DEP assessment measures in the VITAL CTSC sub-cohort
Participants in the VITAL-DEP CTSC protocol are administered diagnostic interviews to determine the agents’ impact on risk of depression among high-risk participants – e.g., individuals with anxiety symptoms, physical/functional impairment, medical comorbidity, problem drinking, poor social support, or high caregiving burden (selective prevention)63,64 – and individuals with sub-syndromal depression64–69 but not meeting criteria for current MDD or dysthymia (indicated prevention)61,70. Thus, these assessments, obtained at baseline and at 2-year follow-up, are used to conduct one of the Secondary Aims of VITAL-DEP (see Section 2.2, Aims).
The Mini-International Neuropsychiatric Interview (MINI) for the DSM-IV91 is used to achieve valid, time-efficient90 determinations of safety and diagnostic status for both eligibility and outcome purposes. Within the CTSC, administration of the MINI, along with the non-item version of the PHQ, allows for real-time assessments of high-risk symptoms (i.e., suicidality, manic symptoms, psychotic symptoms). Study psychiatrists immediately evaluate persons presenting with such symptoms to determine safety and ability to participate in VITAL-DEP.
Self-report instruments are used to assess late-life depression risk factors relevant to indicated and selective prevention63,64,92 among CTSC participants. These include: PHQ-976 (depressive symptoms); GAD-793 (Generalized Anxiety Disorder-7; anxiety symptoms); AUDIT-C94 (Alcohol Use Disorders Identification Test-Concise; problem/at-risk drinking); Duke Social Support Index95,96 (social support/network status); OARS (Older Americans Resources and Services) questionnaire97,98 (activities of daily living (ADLs)/functional impairments); HHIE-S99 (Hearing Handicap Inventory for the Elderly-Screening; hearing impairments); Zarit 12-item Survey100 (caregiver burden); STIDA101 (Structured Telephone Interview for Dementia Assessment) items (subjective cognitive impairments).
Detailed neuropsychological assessment. Neuropsychological testing is conducted to perform exclusions and to identify persons with the late-life depression risk factor of mild cognitive impairment (e.g., dysexecutive features102,103). Tests included: 1) Modified-Mini-Mental State (3MS, which has validated age-, gender-, education- and race-corrected norms)104,105; 2) immediate and delayed recall trials of a word list and the East Boston Memory Test paragraph106; 3) trail-making tests107 (to assess psychomotor speed and executive function, respectively); 4) two category fluency tests, which are similarly sensitive to deficits of frontal networks108 and also capture language.
2.7.4. Biochemical measures
Biomarker studies are critical to determine, ultimately, how vitamin D and fish oil may work to prevent depression and for whom. As such understanding is essential to devising and deploying optimal strategies for late-life depression prevention, measures of potential biologic mediators and moderators of agent response have been tightly integrated into VITAL-DEP. Both vitamin D and fish oil may activate expression pathways for, and increase measured levels of, brain-derived neurotrophic factor (BDNF)109–114, which is regarded as a key peripheral marker of depression and depressive symptoms in humans115. Similarly, both may lower inflammation116–118, which is now recognized as a major factor in depression and persistence of depressive symptoms39,119–123. Furthermore, vitamin D and fish oil appear to influence several other potential etiologic targets in depression: vascular health, oxidative stress, and insulin sensitivity and glucose metabolism34,124–131. Finally, there has been a recent discovery that differences in bioavailable (i.e., directly available for in vivo biological activity132) vitamin D – determined by both blood 25(OH)D levels as well as albumin and vitamin D binding protein (DBP) – may not only be the more relevant biomarker for addressing health influences of vitamin D, it also may be critical to addressing race differences in vitamin D with respect to health outcomes133–135. Thus, the selection of assays for VITAL-DEP was driven by the potential of these biomarkers to inform mediation and moderation.
1) Biochemical nutrient levels
The VITAL parent trial has funded measurement of biochemical levels of the study agents – plasma 25(OH)D and fatty acids (total, omega-3, omega-6) – at baseline among all participants with blood samples (n=16,954). In addition, VITAL-DEP has funded collection of other key biomarkers to address mediator and moderator aims, as detailed below.
2) Brain-derived neurotrophic factor (BDNF)
BDNF has strong relations to mood136–138 and blood levels correlate strongly (r>0.8) with brain levels139. Serum BDNF will be assayed in baseline and follow-up CTSC blood samples using a commercial ELISA assay kit (R&D Systems, Inc., Minneapolis, MN) among those VITAL CTSC participants who are found at baseline to be at risk for depression. As described in Section 2.4, a total of 1,041 participants of the 1,054-member VITAL CTSC sub-cohort were administered baseline neurobehavioral assessments; based on high follow-up rates in the CTSC (>90%), it is projected that there will be ~750 participants with paired samples for baseline and 2-year follow-up BDNF assays.
3) Mass spectrometry based metabolomics
Metabolomics offers a powerful, modern approach to examine potential mediators and moderators in depression and to identify novel biosignatures in late-lifedepression140. Approaches will focus on known metabolites relevant to mood, such as amino acid (serotonin, tryptophan, kynurenine141), lipid-inflammatory (eicosanoids)142, oxidative stress143–145 (glutathione metabolism146), and insulin147,148 (branched-chain amino acids149,150) pathway metabolites. As with the above-described BDNF assays, plasma metabolite profiling will be performed among the richly-phenotyped CTSC cohort using paired baseline and 2-year follow-up samples of participants at risk for depression (n~750). Metabolite profiling in targeted and untargeted modes will be conducted using a state-of-the-art, high-throughput mass spectroscopy (MS)-based analytical system in the laboratory of Mohit Jain151, University of California San Diego. Robustness and reproducibility of these MS methods were internally assessed for previously collected and stored human plasma control samples, and the methods used are appropriate for application in large, population-scale studies involving hundreds or thousands of samples151.
4) Bioavailable vitamin D (bVD)
Emerging data have shown that variations in bVD levels may be more relevant to racial/ethnic differences in related health outcomes than traditional 25(OH)D levels. African-Americans are at increased risk for vitamin D deficiency, as darkly pigmented skin generates less vitamin D in response to sunlight (UVB) exposure152,153. However, recent studies have reported that bioavailable (free and albumin-bound fraction) vitamin D may be more strongly correlated with health outcomes than total 25(OH)D134,154,155. From the full set of VITAL trial participants who provided baseline blood samples (n=16,954), we will select 2,000 participants for assays of bVD; to ensure power to examine key race differences in bVD, samples will be selected for assay such that black participants comprise at least 50%). Values of bVD will be calculated from total 25-OH D, albumin and vitamin D binding protein; albumin and DBP will be measured using an ELISA method (R&D Systems, Inc., Minneapolis, MN).
2.8. Data management
2.8.1. Overview
Data management and follow-up procedures are coordinated centrally by the VITAL parent trial for VITAL and ancillary studies such as VITAL-DEP. As detailed separately, the VITAL trial ensures that participants are mailed follow-up questionnaires and a re-supply of study pills at 6 months and at randomization anniversary; compliance is monitored primarily via yearly questionnaires, as well as through blood samples collected from a random subset of participants.7
All data collected in VITAL-DEP are collected directly by the Brigham and Women’s Hospital (BWH) Division of Preventive Medicine, which leads the parent VITAL trial, or at the CTSC and then transferred to the Division of Preventive Medicine for review, verification, and data checks to maximize accuracy. By-hand review of all raw data collected in the CTSC, including MINI interview packets and raw results from neuropsychological testing, is conducted on-site by research staff at the Division of Preventive Medicine as an additional level of data quality review. Extensive computerized data management and security systems are in place for data integrity assurance and effective data storage. The computing system tracks each person’s stage and level of participation. Questionnaire data are optically scanned into the computer using TELEform and Alchemy (Cardiff Software) programs; data are maintained on a UNIX server. For the CTSC sub-cohort, personnel collect data from the VITAL-DEP visits using optically scannable forms, which are sent to the Division for verification and archival. All psychiatric diagnoses from the MINI assessments conducted in the CTSC are individually adjudicated by one of the study psychiatrists. Physician adjudications are collected on structured code forms and the information is optically scanned for secure storage at the Division of Preventive Medicine. Only study personnel who communicate with participants are able to access necessary information. An independent Data and Safety Monitoring Board (DSMB) has been assembled by VITAL and reviews all data on study endpoints and adverse events for the VITAL trial, including the VITAL-DEP ancillary study. The randomized trial phase of VITAL ended as scheduled on December 31, 2017; endpoint completion will continue through 2018. Post-randomization observational follow-up of participants will continue for at least two years after the end of the randomized phase.
2.8.2. Enhanced follow-up procedures implemented in VITAL-DEP
To provide further assurance of participant safety, VITAL-DEP has uniquely instituted enhanced follow-up procedures for those with elevated PHQ-8 scores77,81. First, the study questionnaires include links to information on depression, and participants are directly educated via text on the questionnaire that if any of the items raise their level of awareness or concern about depression or mood, they should promptly contact their health providers. Second, we contact participants with elevated PHQ-8 scores (≥10 algorithm cutoff) at baseline and follow-up (6.2.c.) by sending mailed letters, where there is no self-report by the participant of both recent diagnosis and treatment of depression on the questionnaire. Among all participants who score PHQ-8 ≥15, we will send these same letters, regardless of recent self-reported diagnosis or treatment for depression. The purpose of this enhanced procedure is to ensure that participants are educated about the need to discuss their mood with their local providers and, thus, initiate a path for ongoing evaluation and/or treatment as necessary; thus, among participants who are randomized and continue in the study, these letters are only sent when participants first report above-threshold scores. As part of the VITAL informed consent procedure, participants have already been notified that study investigators may need to contact them directly with regard to development of endpoints and also to obtain releases to contact their PCPs. VITAL-DEP leads the efforts in the Division of Preventive Medicine to obtain contact information of persons with high PHQ-8 scores – working with data programmers to send out the letters, performing tracking of the letters, as well as fielding and tracking all comments, questions or responses from participants in response to the letters.
2.9. Analysis plan
2.9.1. Analysis plan for Primary Aims in VITAL-DEP
First, we will confirm that balance was achieved by the randomization. Baseline characteristics (e.g., age, gender, race/ethnicity, physical activity, medical conditions, vitamin D and omega-3 intakes) will be compared by randomized groups using 2-sample t or Wilcoxon rank sum tests for continuous variables and χ2 statistics for categorical variables.
Then, for Primary Aim 1, we will examine the main effects of intention-to-treat with vitamin D3 and/or with fish oil on incident and recurrent depression over 5 years. Kaplan-Meier survival curves will be used to determine cumulative depression incidence and recurrence for each study agent group and its corresponding placebo group; the log-rank test will compare the curves of agent vs. placebo. We will use the Cox proportional hazards model to estimate the hazard ratios (HRs) and confidence intervals (CIs) for each intervention using indicators for study agent and controlling for design variables (the other intervention, age, and gender)156. Participants will be followed until the occurrence of the depression endpoint, death, loss to follow-up, or the end of the trial, whichever comes first. Thus, person-time is counted from randomization until event or censoring; person-time ends after the first depression during follow-up and any subsequent depression detected during follow-up will not be used to count person-time. We will test the proportionality assumption (i.e., that of non-changing hazards ratios over time) both analytically and graphically. Finally, given potential concerns of participant non-adherence, drop-out, or case misclassification, compliance sensitivity analyses will be carried out. We will address effects of compliance by censoring follow-up when a participant reports taking less than two-thirds of study medications over the previous year.
For Primary Aim 2, we will examine the main effects of intention-to-treat with vitamin D3 and/or with fish oil on depression scores in the full cohort over the 5-year follow-up period. A mixed-effects model will be used for the repeated PHQ-8 measures obtained at baseline and annually thereafter. This model will include fixed effects for study agent, time, and interaction between study agent and time, and will account for the correlations between repeated measures; mean differences and 95% CIs will be estimated.
All analyses will be conducted with SAS (SAS Institute, Cary, NC). A 2-sided test with α=0.05 will be used.
2.9.2. Analysis plan for Secondary Aims
The first of the Secondary Aims addresses the effect of long-term (5-year) vitamin D among on depression risk and mood trajectory among African-Americans in the full VITAL cohort. Survival analyses for the categorical outcome and mixed-effects models for the repeated measures mood score outcome will be conducted, as above, within this group; a multiplicative interaction term for race will be used to perform a formal statistical test of interaction.
Secondary Aim 2 addresses selective and indicated prevention within the enriched sub-cohort of CTSC participants seen at baseline and 2-year follow-up. As with the primary aim analyses, survival methods will be used to test whether there are significant differences in risk of the composite depression endpoint in the high-risk group (i.e., persons with ≥1 high-risk factors), and of MDD in the sub-syndromal group (i.e., persons with sub-syndromal but not clinical depression); we will again use mixed-effects models with mood (PHQ-8) scores as the dependent variables. We will estimate 95% CIs and P values for the two intervention main effects for change in depression scores between baseline and 2 years for each group. Given higher participant burden of in-person assessments, the CTSC sub-cohort may be slightly more affected by loss-to-follow-up than the full cohort. Thus, if follow-up is less than complete, we will conduct sensitivity analyses to estimate the effect under intention-to-treat157,158.
The third of the Secondary Aims addresses whether baseline plasma levels of vitamin D and omega-3 fatty acids are related to depression outcomes over 5 years and whether they modify the study agents’ effects, within the full VITAL cohort. Specifically, we will examine whether having low biochemical levels of the nutrients (defined as <50 nmol/L (<20 ng/mL) for plasma 25(OH)D8,159,160 and as the bottom 25th percentile of the distribution for plasma omega-3 fatty acids) is significantly related to depression risk or mood trajectory over 5 years. Multiplicative interaction terms will be used to conduct formal statistical tests of interaction between low biochemical nutrient levels and the effects of study agents on mood outcomes.
2.9.3. Additional planned analyses
Among all participants in the full VITAL cohort with available blood samples n=16,954), we will examine whether randomized study agent assignment as well as biochemical nutrient levels influence long-term mood over extended follow-up of 7+ years (i.e., including the post-randomized phase observational follow-up period that will last at least 2 years). We will use mixed-effects models to examine whether there are significant relations of study agents and of blood nutrient levels to paths of the annually repeated PHQ-8 scores over the extended follow-up.
In a random sub-set (including 50% who are African-American) of ~2,000 participants in the full VITAL cohort with blood samples, we will use linear mixed-effects models with interaction terms to test whether low baseline bioavailable vitamin D (bottom quartile) relates to worse mood scores over 7+ years, including separately among African-Americans.
We will conduct moderator and mediator analyses to assess whether levels of novel biomarkers influence study agent response among the ~750 participants in the VITAL CTSC sub-cohort who are at-risk for depression at baseline. We will use regression models to determine whether: 1) baseline serum BDNF or targeted plasma metabolite levels modify the 2-year change in mood symptom (PHQ-9) scores in active vs. placebo groups; 2) 2-year changes in BDNF or metabolite levels mediate the mood response over 2 years in active vs. placebo groups. For hypothesis testing, baseline BDNF or metabolite levels will be dichotomized at the median; multiplicative interaction terms will be used to test moderator variables. We will use pathway analytic approaches to test whether 2-year changes in biomarkers mediate 2-year changes in mood scores.
We will conduct exploratory analyses of effect modification by health and lifestyle variables. Using multiplicative interaction terms, we will determine whether effects for each study agent differ by: the other agent (i.e., additive vs. synergistic effects); age; gender; medical comorbidity161–163; and geographic region/latitude and physical activity (for D3). Parameters will be estimated with the Proc MIXED procedure of SAS, using a 2-sided test with α=0.05.
2.10. Statistical power
2.10.1. Power for VITAL-DEP Primary Aims
All power calculations assume 80% compliance with the study agents. Calculations are based on the log-rank test with a significance level of 0.05, and are adjusted appropriately for design features, such as arbitrary time-to-event distribution, nonproportional hazards, non-uniform rates of entry, loss to follow-up, and changes from allocated group164. Estimates of lifetime and current depression prevalence rates among similar age male and female adults were used to determine the numbers of participants eligible for incidence and recurrence; these numbers were included in the power analysis. Based on these rates, we estimated a population at risk for incident or recurrent depression of n=18,200 participants. Of note, although data cleaning is still in progress, we project that we will exceed this target with n~18,400 at risk for incident or recurrent depression in VITAL-DEP. Power calculation results for the specified reductions in relative risk (RR) of incident and recurrent depression (single agent effects) are displayed in tables 1 and 2, respectively.
Table 1.
Power for effects of a single agent on incident depression in VITAL-DEP, over 5 years of follow-up.
| RR† | Total | Women | Men | Non-Hispanic White | Minority | African-American |
|---|---|---|---|---|---|---|
| 15,470 ‡ | 7,244 ‡ | 8,226 ‡ | 10,071 ‡ | 5,399 ‡ | 3,868 ‡ | |
| 0.90 | 52.6% | 33.5% | 24.9% | 37.3% | 22.3% | 17.2% |
| 0.85 | 86.7% | 64.3% | 49.5% | 69.9% | 44.3% | 33.6% |
| 0.80 | 98.6% | 88.1% | 74.8% | 91.8% | 68.9% | 54.6% |
| 0.75 | >99.9% | 97.8% | 91.5% | 98.9% | 87.4% | 74.8% |
| 0.70 | >99.9% | 99.8% | 98.1% | >99.9% | 96.5% | 89.1% |
| 0.65 | >99.9% | >99.9% | 99.8% | >99.9% | 99.4% | 96.5% |
| 0.60 | >99.9% | >99.9% | >99.9% | >99.9% | >99.9% | 99.2% |
Expected RR;
Expected total number of eligible participants.
Table 2.
Power for effects of a single agent on recurrent depression in VITAL-DEP, over 5 years of follow-up.
| RR† | Total | Women | Men | Non-Hispanic White | Minority | African-American |
|---|---|---|---|---|---|---|
| 2,730 ‡ | 1,856 ‡ | 874 ‡ | 1,777 ‡ | 953 ‡ | 683 ‡ | |
| 0.90 | 38.2% | 30.8% | 12.7% | 26.7% | 16.4% | 12.9% |
| 0.85 | 70.9% | 59.6% | 23.5% | 52.6% | 31.6% | 24.0% |
| 0.80 | 92.1% | 84.1% | 38.5% | 77.7% | 51.3% | 39.2% |
| 0.75 | 98.9% | 96.1% | 55.7% | 92.9% | 70.9% | 56.5% |
| 0.70 | >99.9% | 99.5% | 72.2% | 98.6% | 85.9% | 72.9% |
| 0.65 | >99.9% | >99.9% | 85.2% | 99.8% | 94.7% | 85.6% |
| 0.60 | >99.9% | >99.9% | 93.4% | >99.9% | 98.5% | 93.6% |
Expected RR;
Expected total number of eligible participants.
When calculating power for continuous outcomes165,166, explicitly, a 25% difference in PHQ-8 scores, we used the SD of change in PHQ scores from two 1-year-apart assessments in non-depressed adults81. We will have >99% power for detecting differences of this size between randomized groups.
2.10.2. Power for VITAL-DEP Secondary Aims
As illustrated in Table 1, there is 73–75% power to detect 25% RR reduction and 85–89% power to detect a 30% RR reduction in depression among African-American participants alone. As with the full cohort of VITAL participants, there will be >99% power for detecting differences by randomized group assignment in PHQ-8 scores over 5 years, due to the large number of black participants in the study.
For depression prevention among high-risk participants, power calculations were based on an expected 2-year incidence of 25%167 in the placebo group and an expected n~500 CTSC participants with ≥1 high-risk factors. Of note, investigators have achieved RR reductions of 60% (i.e., an RR of 0.4) in prevention trials among high-risk persons90. Thus, we calculate 80% and >95% power to detect RRs for a single agent of 0.6 and 0.5, respectively. For MDD prevention among with sub-syndromal depression, power was calculated based on a 2-year risk of 35% in the placebo group and an expected n~200 with sub-syndromal depression; we will have 80% and >90% power to detect respective RRs of 0.5 and 0.4. If 2-year risk is higher (e.g., 45%), there will be 80% and >90% power to detect respective RRs of 0.6 and 0.5. Regarding the continuous outcome measure, we calculate >99% power to detect a 30% difference in PHQ-8 scores in the high-risk group, and 90% power to detect a 30% difference among sub-syndromal participants.
Finally, to address relation of low biochemical nutrient levels, as well as interactions of low biochemical nutrient levels with the study agents, to mood outcomes over the 5-year randomized follow-up period, power is calculated using the procedures of Cook and Rosner168 for clinical trials with longitudinal continuous outcomes. The outcome of interest is the mean difference in the change, or Δ-Δ. Power was calculated over a broad range of estimates of Δ-Δ, and possible r values (correlation between baseline and follow-up). All estimates assume ≥90% cohort follow-up, which is likely to be conservative since multiple recent similar randomized trials in the Division of Preventive Medicine have had ≥95% follow-up62,169–173. Assuming the expected prevalence of deficient levels of vitamin D, as measured by 25(OH)D, we have >99% power for comparing even small mean differences in change in mood scores (i.e., 0.25 points on PHQ-8) over 5 years’ follow-up in deficient vs. sufficient groups; a similar computation can be performed for or plasma omega-3 FAs, where the bottom 10th percentile is considered low. To determine power to test effect modification (i.e., interaction of active vs. placebo assignment with deficient/low vs. non-deficient baseline biochemical level), we consider the contrast between two sets of mean differences in change in PHQ-8 scores: that is, the differences in two sets of Δ-Δs, where (Δ-Δ)1 is the mean difference in change comparing active vs. placebo groups among nutrient-deficient persons, and (Δ-Δ)2 is the mean difference in change comparing active vs. placebo groups among nutrient-sufficient persons. When this contrast is conservatively estimated at only 25% of a meaningful clinical difference (1 point on the PHQ-8), power is >90% for all values of r.
2.10.3. Power for additional planned analyses in VITAL-DEP
1) Extended follow-up
Because of having additional follow-up time with 2 more repeated measures of PHQ-8 scores, power for evaluating the relations of the study agents and biochemical nutrient levels to mood outcomes in extended follow-up (average 7 years) will be >99%.
2) Bioavailable vitamin D
With a sample size of 2,000, and a bottom 10% threshold for low bVD, we have >99% power for comparing mean differences in mood scores over 7 years in low vs. sufficient bVD groups for all values of r. Among black participants (who will comprise at least 50% of the ~2,000 in this subset), power for testing moderation of vitamin D’s effects by bVD is >80% for all values of r.
3) Novel biochemical mediators and moderators
Calculation of power to detect mediator effects was based on the proportion of the treatment effect explained (PTE) by the mediator, using the pathway analytic approach174,175. Among ~750 CTSC participants with baseline and 2-year biomarker and mood (PHQ-9) measures, we will perform analyses of mediation of study agent effects by serum BDNF and plasma metabolites, which will be log-normal standardized variables. Estimates for the effect of an agent on the mediator of 1 to 1.25 standard units are based on available preliminary data for differences in plasma metabolites among persons with deficient vs. sufficient vitamin D levels in a separate cohort176; comparable effects have been noted for serum BDNF in depression clinical studies177,178. Assuming a conservative 67% 2-year follow-up, simulations174 were run with 10,000 replicates of size n=500. If effect sizes on PHQ-9 scores are ≥0.5, we have ≥80% power to detect PTEs of 30% or greater179. Power for testing moderation by baseline low vs. high BDNF and metabolite levels (i.e., dichotomized at the median) is >80% for all values of r.
4) Power for exploratory analyses of additive and synergistic effects of the agents
Assuming a 2-sided α of 0.05, we calculated power of single agent effects ranging from 0.90 to 0.75, and RRs for interactions from 1.00 (i.e., additive) to 0.60. If both agents are effective, but act independently, power tends to decrease, due to a smaller number of events. If the agents interact, power will be affected by the extent of the interaction. We determine that there will be ≥80% power to detect important interactions (e.g., additional risk reduction of ≥30% for single agent RRs from 0.90 to 0.75).
3. DISCUSSION
VITAL-DEP is a unique large-scale, long-term ancillary study of late-life depression prevention in a highly diverse sample of randomized trial participants. The innovative design of this study allows for testing of long-term outcomes using universal as well as selective and indicated depression prevention paradigms simultaneously. Two promising agents for prevention of late-life depression are being examined, and several important scientific gaps will be addressed. First, strong evidence supports use of omega-3 for mood disorders51, yet there is limited knowledge on how these data can be applied toward prevention. Second, numerous large-scale observational studies suggest mood benefits of vitamin D, but many of these have been cross-sectional, in addition to having problems of confounding can only be solved by a randomization strategy. Third, there have been several barriers to conducting large-scale depression prevention research, such as lack of appropriate agents92, adequate doses and intervention duration, or sufficient sample size and statistical power180. Finally, VITAL-DEP includes important features of modern prevention study and clinical trial design, such as extensive integration of plausible biological moderators and mediators of effects.
Strengths of VITAL-DEP include the large sample size, the fact that depression prevention is being addressed both in high-risk groups and in populations for whom depression treatment disparities have been observed, the racial/ethnic diversity among participants, the inclusion of high-quality covariate data, the use of novel blood biomarkers, and the efficient, creative leveraging of infrastructure and resources of a large randomized trial. As noted above, a key innovation of simultaneous assessment of universal, selective and indicated prevention of depression is a highly unique implementation of the NAM (formerly, IOM) theoretical model of prevention into actual experimental design61. To achieve this, we employ a highly efficient strategy that may serve as an example for future study designs – embedding local CTSC psychiatric exams in the context of a larger national trial to assemble a well-characterized sub-cohort with deep-phenotyping, including biomarkers. Notably, the extended follow-up of this cohort – up to 7 years for many participants – has substantial value by facilitating prospective evaluation of the extended influence on mood of supplemental intake of vitamin D3 and EPA+DHA, as well as of baseline intake and blood levels of these nutrients in the placebo group.
Finally, VITAL-DEP includes careful consideration of participant safety issues in a large-scale study. We recognized the need for these enhanced safety measures in the unique context of this study: many participants might not recognize symptoms of depression as warranting further evaluation – in the way they would recognize the importance of symptoms such as chest pain or bleeding, for example; on the other hand, many participants with elevated mood scores may not actually have depression and could be unduly alarmed or feel stigmatized by being treated differently due to these symptoms compared to any number of other potential health concerns. Following careful consultation with local Human Subjects committee leaders and an independent research ethics team, VITAL-DEP developed a unique procedure to ensure participants are provided timely information and education about mood and paths for evaluation and treatment, while minimizing issues of stigmatization or exceptionalization.
This study also has limitations. First, these doses of vitamin D and omega-3 are fixed within the VITAL parent trial. However, these doses were determined for use in VITAL after careful consideration of potential benefits for reducing risk of CVD and cancer, plausible health effects and participant safety. Specifically, advantageous 25(OH)D levels for health effects appear to begin at 75 nmol/L, with optimal levels between 90 and 100 nmol/L159. However, a non-linear relation between intake and 25(OH)D has been identified181 and suggests that 2000 IU/d of vitamin D3 would be needed to reach 90–100 nmol/L. Thus, this dose was chosen for the active vitamin D arm of the VITAL trial – a dose that is expected to yield a sufficient difference in vitamin D status between the active agent and placebo groups to test efficacy. Similarly, the 1 g/d dose for omega-3 FAs was carefully selected for the VITAL trial based on plausible cardiovascular benefits, and the dose is within currently recommended health and safety guidelines. An advantage of the 1 g/d dose is that it is also highly plausible for mood outcomes182,183 to be addressed by the VITAL-DEP ancillary study. Second, the depression endpoint (“depression”) in VITAL-DEP is defined as any clinically significant depressive syndrome60, which encompasses Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)184 diagnoses major and minor depression. This may be regarded as a limitation, as the endpoint is not specific exclusively to major depressive disorder. However, as depressive syndromes – even those that do not meet criteria for MDD – can have significant impact on morbidity74,75 among older adults, we chose not to restrict our endpoint to DSM-IV-defined MDD. Use of such composite endpoints of highly-related outcomes is common in large prevention trials169–171,185, and has also been utilized in studies of incident late-life depression186. In addition, while the DSM-5 has been published since initiation of the VITAL-DEP study, our endpoints remain consistent with updated diagnoses. Third, use of the PHQ-8 instead of the PHQ-9 in the full trial cohort – the rationale for which was extensively described above – does not permit characterization of suicidality, an important psychiatric outcome. Nevertheless, administration of the suicidality module of the MINI as well as PHQ-9 among the CTSC sub-cohort of VITAL-DEP participants ensures that this information is at least captured within this deeply-phenotyped subset. Fourth, about 15% of our sample will not have CMS data due to age under 65 years187; thus, questionnaire responses on symptoms, diagnoses and treatments for those participants cannot be supplemented by CMS data. However, occurrences of un-diagnosed depression are likely to be low on this basis and, more importantly, will be evenly distributed in each randomized group188. Finally, results from participants may not generalize fully to all US older adults or to younger adults. Because of the eligibility requirements of VITAL (e.g., being free of cardiovascular disease and cancer at baseline), study participants may be healthier than those of similar ages in the larger population; thus, findings will be interpreted with caution. Nonetheless, older people have among the highest levels of depression-related morbidity, and a depression prevention study in this group is of clear public health and social importance.
In addition to its anticipated scientific contributions to the testing of agents for depression prevention, VITAL-DEP may afford the scientific community with broader lessons regarding the conduct of prevention research. Specifically, the design and implementation of VITAL-DEP has highlighted the importance of: 1) simultaneous use of multiple modalities of prevention where possible; 2) incorporation of mechanistic biomarkers and integration of potential mediators and moderators into study aims; 3) identification of creative opportunities for preventive interventions that are scalable; 4) efficient and synergistic leveraging of large-scale study infrastructures; 5) consideration of unique ethical considerations in large-scale clinical research.
In summary, the high degree of morbidity associated with late-life depression suggests that a safe and effective, yet low-cost, preventive strategy will yield a significant impact on the public health. The design employed by VITAL-DEP is highly novel and detailed while being resource-efficient. Furthermore, there is significant potential for added value in its design, as long-term post-trial observation of VITAL-DEP participants will make it possible to ascertain whether and how long any observed mood effects of vitamin D3 and omega-3 persist after discontinuation of the VITAL trial. This ongoing, large-scale, study of depression prevention and mood enhancement will yield new and major advances by: 1) informing the scientific community regarding the direct impacts of vitamin D and fish oil for depression prevention and longitudinal mood symptoms, 2) providing high-quality, long-term follow-up of mood outcomes within what has become a rich and unprecedented dataset, and 3) addressing multiple and novel mechanistic biomarkers in a large and racially/ethnically diverse group of older adults, in order to inform how vitamin D and fish oil supplements may work to impact mood and for whom.
Acknowledgments
We are indebted to the 25,874 VITAL participants and to the entire VITAL staff for their dedicated and conscientious collaboration, with special appreciation for Ms. Alison Weinberg and Ms. Ankura Singh for their assistance with the VITAL-DEP ancillary study. VITAL-DEP is supported by R01 MH091448 from NIMH. VITAL is supported by grants U01 CA138962 and R01 CA138962, which includes support from the National Cancer Institute; National Heart, Lung and Blood Institute (NHLBI); Office of Dietary Supplements; National Institute of Neurological Disorders and Stroke; and the National Center for Complementary and Alternative Medicine of National Institutes of Health (NIH). The VITAL ancillary studies and CTSC component are supported by grants DK088078 and R01 DK088762 from the National Institute of Diabetes and Digestive and Kidney Diseases; R01 HL101932 and R01 HL102122 from NHLBI; R01 AG036755 from the National Institute on Aging (NIA); R01 AR059086 and R01 AR060574 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; and R01 MH091448 from the National Institute of Mental Health (NIMH). Dr. Reynolds’ participation is also supported by P30 MH090333 from NIMH, and the University of Pittsburgh Medical Center Endowment in Geriatric Psychiatry. Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma (BASF) of Norway (Omacor® fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. VITAL-DEP has been approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital, and the VITAL study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration. Voting members of the Data and Safety Monitoring Board for VITAL and ancillary studies include Lawrence S. Cohen, MD; Theodore Colton, ScD; Mark A. Espeland, PhD; Craig Henderson, MD; Alice H. Lichtenstein, ScD; Rebecca A. Silliman, MD, PhD; and Nanette Wenger, MD (chair). Ex-officio members include Josephine Boyington, PhD, MPH; Rebecca Costello, PhD; Cindy Davis, PhD; Peter Greenwald, MD; Gabriela Riscuta, MD; and Harold Seifried, PhD. VITAL and VITAL-DEP are registered at clinicaltrials.gov (VITAL: NCT01169259; VITAL-DEP: NCT01696435). The VITAL website is www.vitalstudy.org.
Footnotes
DISCLOSURES
Dr. Buring’s spouse is on the Scientific Advisory Board of Pharmavite LLC.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409–416. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Beekman AT, Geerlings SW, Deeg DJ, et al. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59(7):605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- 4.Lenze EJ, Sheffrin M, Driscoll HC, et al. Incomplete response in late-life depression: getting to remission. Dialogues Clin Neurosci. 2008;10(4):419–430. doi: 10.31887/DCNS.2008.10.4/jlenze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. Mental health: Culture, race, and ethnicity--A supplement to mental health: A report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services; 2001. [PubMed] [Google Scholar]
- 6.Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64(3):305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 9.Cherniack EP, Levis S, Troen BR. Hypovitaminosis D: a widespread epidemic. Geriatrics. 2008;63(4):24–30. [PubMed] [Google Scholar]
- 10.Gloth FM, 3rd, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 11.LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281:1505–1511. doi: 10.1001/jama.281.16.1505. [DOI] [PubMed] [Google Scholar]
- 12.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76:470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 14.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 15.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 16.Yanoff LB, Parikh SJ, Spitalnik A, et al. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf) 2006;64:523–529. doi: 10.1111/j.1365-2265.2006.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103:620–625. doi: 10.1016/j.jsbmb.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 18.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. 2009;67(8):481–492. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69(6):1316–1319. doi: 10.1016/j.mehy.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Cherniack EP, Troen BR, Florez HJ, Roos BA, Levis S. Some new food for thought: the role of vitamin D in the mental health of older adults. Curr Psychiatry Rep. 2009;11(1):12–19. doi: 10.1007/s11920-009-0003-3. [DOI] [PubMed] [Google Scholar]
- 22.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RG, van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466–473. doi: 10.1016/j.neurobiolaging.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722–729. doi: 10.1136/jnnp.2008.165720. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 26.Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508–512. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- 27.Okereke OI, Singh A. The role of vitamin D in the prevention of late-life depression. J Affect Disord. 2016;198:1–14. doi: 10.1016/j.jad.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rejnmark L, Bislev LS, Cashman KD, et al. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS One. 2017;12(7):e0180512. doi: 10.1371/journal.pone.0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6(4):1501–1518. doi: 10.3390/nu6041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 31.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. Journal of internal medicine. 2008;264(6):599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 32.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011;198(5):357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Ascherio A, Hu FB, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–164. doi: 10.1161/01.CIR.0000152099.87287.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris WS. International recommendations for consumption of long-chain omega-3 fatty acids. J Cardiovasc Med (Hagerstown) 2007;8(Suppl 1):S50–S52. doi: 10.2459/01.JCM.0000289274.64933.45. [DOI] [PubMed] [Google Scholar]
- 35.Block ER, Edwards D. Effect of plasma membrane fluidity on serotonin transport by endothelial cells. Am J Physiol. 1987;253(5 Pt 1):C672–C678. doi: 10.1152/ajpcell.1987.253.5.C672. [DOI] [PubMed] [Google Scholar]
- 36.Kodas E, Galineau L, Bodard S, et al. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89(3):695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- 37.Logan AC. Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern Med Rev. 2003;8(4):410–425. [PubMed] [Google Scholar]
- 38.Logan AC. Omega-3 fatty acids and major depression: a primer for the mental health professional. Lipids Health Dis. 2004;3:25. doi: 10.1186/1476-511X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maes M, Smith RS. Fatty acids, cytokines, and major depression. Biol Psychiatry. 1998;43(5):313–314. doi: 10.1016/s0006-3223(97)00401-0. [DOI] [PubMed] [Google Scholar]
- 40.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 41.Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamaki H. Fish consumption, depression, and suicidality in a general population. Arch General Psychiatry. 2001;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- 42.Tanskanen A, Hibbeln JR, Tuomilehto J, et al. Fish consumption and depressive symptoms in the General population in Finland. Psychiatric Services. 2001;52:529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 43.Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Rasanen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- 45.Tiemeier H, Van Tuijl RH, Hofman A, Kiliaan AJ, Breteler MMB. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 46.Mischoulon D, Fava M. Docosahexanoic acid and omega-3 fatty acids in depression. Psychiatr Clin North Am. 2000;23(4):785–794. doi: 10.1016/s0193-953x(05)70197-0. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki S, Akechi T, Kobayashi M, et al. Daily omega-3 fatty acid intake and depression in Japanses patients with newly diagnosed lung cancer. Br J Cancer. 2004;90:787–793. doi: 10.1038/sj.bjc.6601621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mamalakis G, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose polyunsaturated fatty acids in the survivors of the seven countries study population of Crete. Prostaglandins Leukot Essent Fatty Acids. 2004;70:495–501. doi: 10.1016/j.plefa.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Jacka FN, Pasco JA, Kotowicz MA, Nicholson GC, Berk M. Dietary omega-3 fatty acids and depression in a community sample. Nutritional Neuroscience. 2004;7:101–106. doi: 10.1080/10284150410001710438. [DOI] [PubMed] [Google Scholar]
- 50.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lonnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry. 2004;161:567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- 51.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 52.Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr. 2006;84(6):1308–1316. doi: 10.1093/ajcn/84.6.1308. [DOI] [PubMed] [Google Scholar]
- 53.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 54.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 55.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163(6):969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- 56.Richardson AJ. n-3 Fatty acids and mood: the devil is in the detail. Br J Nutr. 2008;99(2):221–223. doi: 10.1017/S0007114507824123. [DOI] [PubMed] [Google Scholar]
- 57.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2008;88(3):706–713. doi: 10.1093/ajcn/88.3.706. [DOI] [PubMed] [Google Scholar]
- 58.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2011 Oct 2; doi: 10.1016/j.cct.2011.09.009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL) Contemp Clin Trials. 2016;47:235–243. doi: 10.1016/j.cct.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luijendijk HJ, van den Berg JF, Dekker MJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394–1401. doi: 10.1001/archpsyc.65.12.1394. [DOI] [PubMed] [Google Scholar]
- 61.Committee on Prevention of Mental Disorders: Institute of Medicine. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. National Academy Press; 1994. [PubMed] [Google Scholar]
- 62.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 63.Smits F, Smits N, Schoevers R, Deeg D, Beekman A, Cuijpers P. An epidemiological approach to depression prevention in old age. Am J Geriatr Psychiatry. 2008;16(6):444–453. doi: 10.1097/JGP.0b013e3181662ab6. [DOI] [PubMed] [Google Scholar]
- 64.Schoevers RA, Smit F, Deeg DJ, et al. Prevention of late-life depression in primary care: do we know where to begin? Am J Psychiatry. 2006;163(9):1611–1621. doi: 10.1176/ajp.2006.163.9.1611. [DOI] [PubMed] [Google Scholar]
- 65.Pincus HA, Davis WW, McQueen LE. ‘Subthreshold’ mental disorders. A review and synthesis of studies on minor depression and other ‘brand names’. Br J Psychiatry. 1999;174:288–296. doi: 10.1192/bjp.174.4.288. [DOI] [PubMed] [Google Scholar]
- 66.Rapaport MH, Judd LL. Minor depressive disorder and subsyndromal depressive symptoms: functional impairment and response to treatment. J Affect Disord. 1998;48(2–3):227–232. doi: 10.1016/s0165-0327(97)00196-1. [DOI] [PubMed] [Google Scholar]
- 67.Rowe SK, Rapaport MH. Classification and treatment of sub-threshold depression. Curr Opin Psychiatry. 2006;19(1):9–13. doi: 10.1097/01.yco.0000194148.26766.ba. [DOI] [PubMed] [Google Scholar]
- 68.Lyness JM, Heo M, Datto CJ, et al. Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Ann Intern Med. 2006;144(7):496–504. doi: 10.7326/0003-4819-144-7-200604040-00008. [DOI] [PubMed] [Google Scholar]
- 69.Lyness JM, Kim J, Tang W, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15(3):214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 70.Robinson RG, Jorge RE. Prevention of first-episode depression: progress and potential. Br J Psychiatry. 2009;194(4):296–297. doi: 10.1192/bjp.bp.108.059873. [DOI] [PubMed] [Google Scholar]
- 71.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 72.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 73.Damico KE, Stoll AL, Marangell LB, Cohen BM. How blind is double-blind? A study of fish oil versus placebo. Prostaglandins Leukot Essent Fatty Acids. 2002;66(4):393–395. doi: 10.1054/plef.2002.0364. [DOI] [PubMed] [Google Scholar]
- 74.Korten A, Henderson S. The Australian National Survey of Mental Health and Well-Being. Common psychological symptoms and disablement. Br J Psychiatry. 2000;177:325–330. doi: 10.1192/bjp.177.4.325. [DOI] [PubMed] [Google Scholar]
- 75.Hybels C, Blazer D, Pieper C. Toward a threshold for subthreshold depression: an analysis of correlates of depression by severity of symptoms using data from an elderly community survey. Gerontologist. 2001;41:357–365. doi: 10.1093/geront/41.3.357. [DOI] [PubMed] [Google Scholar]
- 76.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 77.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 78.McGuire LC, Strine TW, Allen RS, Anderson LA, Mokdad AH. The Patient Health Questionnaire 8: current depressive symptoms among U.S. older adults, 2006 Behavioral Risk Factor Surveillance System. Am J Geriatr Psychiatry. 2009;17(4):324–334. doi: 10.1097/JGP.0b013e3181953bae. [DOI] [PubMed] [Google Scholar]
- 79.Kroenke K, Spitzer RL. The PHQ-9: a new depression and diagnostic severity measure. Psychiatric Ann. 2002;32:509–521. [Google Scholar]
- 80.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 81.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21(6):547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hepner KA, Morales LS, Hays RD, Edelen MO, Miranda J. Evaluating differential item functioning of the PRIME-MD mood module among impoverished black and white women in primary care. Womens Health Issues. 2008;18(1):53–61. doi: 10.1016/j.whi.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Iwata N, Turner RJ, Lloyd DA. Race/ethnicity and depressive symptoms in community-dwelling young adults: a differential item functioning analysis. Psychiatry Res. 2002;110(3):281–289. doi: 10.1016/s0165-1781(02)00102-6. [DOI] [PubMed] [Google Scholar]
- 86.Cole SR, Kawachi I, Maller SJ, Berkman LF. Test of item-response bias in the CES-D scale. experience from the New Haven EPESE study. J Clin Epidemiol. 2000;53(3):285–289. doi: 10.1016/s0895-4356(99)00151-1. [DOI] [PubMed] [Google Scholar]
- 87.Yang FM, Jones RN. Center for Epidemiologic Studies-Depression Scale (CES-D) item response bias found with Mantel-Haenszel method was successfully replicated using latent variable modeling. J Clin Epidemiol. 2007;60(11):1195–1200. doi: 10.1016/j.jclinepi.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 89.Smit F, Ederveen A, Cuijpers P, Deeg D, Beekman A. Opportunities for cost-effective prevention of late-life depression: an epidemiological approach. Arch Gen Psychiatry. 2006;63(3):290–296. doi: 10.1001/archpsyc.63.3.290. [DOI] [PubMed] [Google Scholar]
- 90.van’t Veer-Tazelaar PJ, van Marwijk HW, van Oppen P, et al. Stepped-care prevention of anxiety and depression in late life: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(3):297–304. doi: 10.1001/archgenpsychiatry.2008.555. [DOI] [PubMed] [Google Scholar]
- 91.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 92.Lyness JM, Yu Q, Tang W, Tu X, Conwell Y. Risks for depression onset in primary care elderly patients: potential targets for preventive interventions. Am J Psychiatry. 2009;166(12):1375–1383. doi: 10.1176/appi.ajp.2009.08101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 94.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C), an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 95.Koenig HG, Westlund RE, George LK, Hughes DC, Blazer DG, Hybels C. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34(1):61–69. doi: 10.1016/S0033-3182(93)71928-3. [DOI] [PubMed] [Google Scholar]
- 96.Landerman R, George LK, Campbell RT, Blazer DG. Alternative models of the stress buffering hypothesis. Am J Community Psychol. 1989;17(5):625–642. doi: 10.1007/BF00922639. [DOI] [PubMed] [Google Scholar]
- 97.Fillenbaum GG, Smyer M. The development, validity and reliability of the OARS Multidimensional Functional Assessment Questionnaire. Journal of Gerontology. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 98.Fillenbaum GG. Multidimensional Function Assessment of Old Adults: The Duke Older Americans Resources and Services Procedures. Hillsdale, NJ: Lawrance Erlbaum Associations, Publishers; 1988. [Google Scholar]
- 99.Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259(19):2875–2878. [PubMed] [Google Scholar]
- 100.Higginson IJ, Gao W, Jackson D, Murray J, Harding R. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J Clin Epidemiol. 2010;63(5):535–542. doi: 10.1016/j.jclinepi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 101.Go RC, Duke LW, Harrell LE, et al. Development and validation of a Structured Telephone Interview for Dementia Assessment (STIDA): the NIMH Genetics Initiative. J Geriatr Psychiatry Neurol. 1997;10(4):161–167. doi: 10.1177/089198879701000407. [DOI] [PubMed] [Google Scholar]
- 102.Román GC. Vascular depression: An archetypal neuropsychiatric disorder. Biol Psychiatry. 2006;60(12):1306–1308. doi: 10.1016/j.biopsych.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1295–1298. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Brown LM, Schinka JA, Mortimer JA, Graves AB. 3MS normative data for elderly African Americans. J Clin Exp Neuropsychol. 2003;25(2):234–241. doi: 10.1076/jcen.25.2.234.13643. [DOI] [PubMed] [Google Scholar]
- 105.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 106.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s Disease. Intern J Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 107.Reitan R. Trail Making Test. Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychological Laboratory; [Google Scholar]
- 108.Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 109.Naveilhan P, Neveu I, Baudet C, et al. 1,25-Dihydroxyvitamin D3 regulates the expression of the low-affinity neurotrophin receptor. Brain Res Mol Brain Res. 1996;41(1–2):259–268. doi: 10.1016/0169-328x(96)00103-9. [DOI] [PubMed] [Google Scholar]
- 110.Kiraly SJ, Kiraly MA, Hawe RD, Makhani N. Vitamin D as a neuroactive substance: review. ScientificWorldJournal. 2006;6:125–139. doi: 10.1100/tsw.2006.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21(10):1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 112.Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport. 1996;7(13):2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- 113.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155(3):751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 115.Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484) Mol Psychiatry. 2014;19(7):791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- 116.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197(1):12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 117.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 118.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6(6):461–467. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 119.Maes M, Scharpe S, Meltzer HY, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49(1):11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 120.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 121.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatry. 2013;18(1):15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Friedrich MJ. Research on psychiatric disorders targets inflammation. JAMA. 2014;312(5):474–476. doi: 10.1001/jama.2014.8276. [DOI] [PubMed] [Google Scholar]
- 124.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 125.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Weber KT, Simpson RU, Carbone LD. Vitamin D and calcium dyshomoeostasis-associated heart failure. Heart. 2008;94:540–541. doi: 10.1136/hrt.2007.126359. [DOI] [PubMed] [Google Scholar]
- 128.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79(6):935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 129.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Q J Med. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 130.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 131.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 132.Ekins RP, Jackson T, Sinha A, Edwards P. Principles of free hormone measurement. Journal of endocrinological investigation. 1986;9(Suppl 4):3–15. [PubMed] [Google Scholar]
- 133.Holick MF. Bioavailability of vitamin D and its metabolites in black and white adults. The New England journal of medicine. 2013;369(21):2047–2048. doi: 10.1056/NEJMe1312291. [DOI] [PubMed] [Google Scholar]
- 134.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England journal of medicine. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. The New England journal of medicine. 2014;370(9):880–881. doi: 10.1056/NEJMc1315850. [DOI] [PubMed] [Google Scholar]
- 136.Wolkowitz OM, Wolf J, Shelly W, et al. Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1623–1630. doi: 10.1016/j.pnpbp.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 138.Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 139.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328(3):261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 140.Diniz BS, Sibille E, Ding Y, et al. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry. 2014 Aug 5; doi: 10.1038/mp.2014.76. doi:2010.1038/mp.2014.2076. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oxenkrug GF. Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Ann N Y Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- 142.Sinclair AJ, Begg D, Mathai M, Weisinger RS. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac J Clin Nutr. 2007;16:391–397. [PubMed] [Google Scholar]
- 143.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68(4):261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 144.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 145.Stafford L, Berk M. The use of statins after a cardiac intervention is associated with reduced risk of subsequent depression: proof of concept for the inflammatory and oxidative hypotheses of depression? J Clin Psychiatry. 2010 Dec 14; doi: 10.4088/JCP.09m05825blu. Epub ahead of print. [DOI] [PubMed]
- 146.Soga T, Baran R, Suematsu M, et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281(24):16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 147.Ryan JP, Sheu LK, Critchley HD, Gianaros PJ. A neural circuitry linking insulin resistance to depressed mood. Psychosom Med. 2012;74(5):476–482. doi: 10.1097/PSY.0b013e31824d0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pan A, Lucas M, Sun Q, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170(21):1884–1891. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids. 2012;43(1):171–181. doi: 10.1007/s00726-011-1088-7. [DOI] [PubMed] [Google Scholar]
- 150.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Watrous JD, Henglin M, Claggett B, et al. Visualization, Quantification, and Alignment of Spectral Drift in Population Scale Untargeted Metabolomics Data. Analytical chemistry. 2017;89(3):1399–1404. doi: 10.1021/acs.analchem.6b04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 153.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135(10):2478–2485. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- 154.Bhan I, Powe CE, Berg AH, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney international. 2012;82(1):84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(7):1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 157.Cook NR. Imputation strategies for blood pressure data nonignorably missing due to medication use. Clin Trials. 2006;3:411–420. doi: 10.1177/1740774506070802. [DOI] [PubMed] [Google Scholar]
- 158.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 159.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 160.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 161.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 162.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 163.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 164.Barthel FM, Babiker A, Royston P, Parmar MK. Evaluation of sample size and power for multi-arm survival trials allowing for non-uniform accrual, non-proportional hazards, loss to follow-up and cross-over. Stat Med. 2006;25:2521–2542. doi: 10.1002/sim.2517. [DOI] [PubMed] [Google Scholar]
- 165.Muller KE, LaVange LM, Ramey SL, Ramey CT. Power calculations for general linear multivariable models including repeated measures applications. Journal of the American Statistical Association. 1992;87:1209–1226. doi: 10.1080/01621459.1992.10476281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Castelloe JM. Sample size computations and power analysis with the SAS System. Proceedings of the Twenty-Fifth Annual SAS Users Group International Conference; Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 167.Sriwattanakomen R, Ford AF, Thomas SB, et al. Preventing depression in later life: translation from concept to experimental design and implementation. Am J Geriatr Psychiatry. 2008;16(6):460–468. doi: 10.1097/JGP.0b013e318165db95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cook NR, Rosner BA. Sample size estimation for clinical trials with longitudinal measures: application to studies of blood pressure. Journal of Epidemiology and Biostatistics. 1997;2(3):65–74. [Google Scholar]
- 169.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 171.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 172.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–1880. doi: 10.1001/jama.2012.14641. Erratum in: JAMA. 2014 Aug 1876;1312(1875):1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(17):1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effec. Psychol Sci. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Christopher K, Rogers A, Baron R, et al. Metabolome alteration in critical illness according to vitamin D status: a prospective cohort study. Critical Care Medicine. 2014;42(12):A1443. Poster Session: Endocrine/Nutrition 1443. [Google Scholar]
- 177.Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 178.Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11(8):1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 179.Vittinghoff E, Sen S, McCulloch CE. Sample size calculations for evaluating mediation. Stat Med. 2009;28(4):541–557. doi: 10.1002/sim.3491. [DOI] [PubMed] [Google Scholar]
- 180.Cuijpers P. Examining the effects of prevention programs on the incidence of new cases of mental disorders: the lack of statistical power. Am J Psychiatry. 2003;160(8):1385–1391. doi: 10.1176/appi.ajp.160.8.1385. [DOI] [PubMed] [Google Scholar]
- 181.Aloia JF, Patel M, Dimaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–1958. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 182.Mischoulon D, Best-Popescu C, Laposata M, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol. 2008;18(9):639–645. doi: 10.1016/j.euroneuro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 183.Mischoulon D, Papakostas GI, Dording CM, et al. A double-blind randomized controlled trial of ethyl-eicosapentaenoate (EPA-E) for major depressive disorder. J Clin Psychiatry. 2009;70(12):1636–1644. doi: 10.4088/JCP.08m04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. 4. Washington, DC: APA; 1994. [Google Scholar]
- 185.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Paĺsson SP, Ostling S, Skoog I. The incidence of first-onset depression in a population followed from the age of 70 to 85. Psychol Med. 2001;31(7):1159–1168. doi: 10.1017/s0033291701004524. [DOI] [PubMed] [Google Scholar]
- 187.Prince JD, Akincigil A, Kalay E, et al. Psychiatric rehospitalization among elderly persons in the United States. Psychiatr Serv. 2008;59(9):1038–1045. doi: 10.1176/ps.2008.59.9.1038. [DOI] [PubMed] [Google Scholar]
- 188.Wacholder S, Rothman N, Caporaso N. Population stratification in epidemiologic studies of common genetic variants and cancer: quantification of bias. J Natl Cancer Inst. 2000;92:1151–1158. doi: 10.1093/jnci/92.14.1151. [DOI] [PubMed] [Google Scholar]