Abstract
In an attempt to understand the process mediating K+ transport into roots, we examined the contribution of the NH4+-sensitive and NH4+-insensitive components of Rb+ transport to the uptake of Rb+ in barley (Hordeum vulgare L.) plants grown in different ionic environments. We found that at low external Rb+ concentrations, an NH4+-sensitive component dominates Rb+ uptake in plants grown in the absence of NH4+, while Rb+ uptake preferentially occurs through an NH4+-insensitive pathway in plants grown at high external NH4+ concentrations. A comparison of the Rb+-uptake properties observed in roots with those found in heterologous studies with yeast cells indicated that the recently cloned HvHAK1 K+ transporter may provide a major route for the NH4+-sensitive component. HvHAK1 failed to complement the growth of a yeast strain defective in NH4+ transport, suggesting that it could not act as an NH4+ transporter. Heterologous studies also showed that the HKT1 K+/Na+-cotransporter may act as a pathway for high-affinity Rb+ transport sensitive to NH4+. However, we found no evidence of an enhancement of Rb+ uptake into roots due to Na+ addition. The possible identity of the systems contributing to the NH4+-insensitive component in barley plants is discussed.
K+ plays unique and important roles in all living cells. The high K+ concentrations required by plants to sustain growth convert the uptake of this element by roots in a critical process in nutrient-poor environments, where K+ availability may be a limiting factor for plant productivity (Clarkson, 1985). K+ nutrition, particularly at low external K+ concentrations, is frequently impaired by an excess of Na+ or NH4+ in the solution bathing the roots (Rufty et al., 1982; Flowers and Läuchli, 1983). Reciprocally, the maintenance of an adequate K+ concentration inside the cells is thought to play a protective role against the detrimental effects of high external Na+ and NH4+ concentrations (Cao et al., 1993; Zhu et al., 1998). A protective role for K+ during the development of water deficit has been also proposed (Gupta et al., 1989). Thus, understanding the processes involved in the movement of K+ toward, between, and within plant cells is a central issue in studies of the resistance of higher plants to a wide panoply of environmental stresses.
Early kinetic studies performed with barley roots demonstrated that the uptake of K+ from low external K+ concentrations can be described at a phenomenological level in terms of the Michaelis-Menten equation (Epstein et al., 1963). Later kinetic studies with other plant species (Epstein, 1973) and alternative approaches (Kochian and Lucas, 1982; Maathuis and Sanders, 1994) confirmed the universal presence of this transport mechanism usually referred to as “mechanism 1” or the “high-affinity transport system.” Although the precise nature of the molecular systems underlying “mechanism 1” remained elusive for more than 30 years, recent studies have suggested that members of three families of alkali cation transporters are likely to be involved in the transport of K+ into the root symplasm from micromolar K+ concentrations: AKT1 (Sentenac et al., 1992), HKT1 (Schachtman and Schroeder, 1994; Rubio et al., 1995), and the HAK-Kup transporters HvHAK1 and AtKup1 (Santa-María et al., 1997; Fu and Luan, 1998; Kim et al., 1998).
Recently, an insertional mutant line for AKT1 has been identified in Arabidopsis, which exhibits a conditional capacity to grow at micromolar K+ concentrations (Hirsch et al., 1998). This finding indicates that, at least in some environments, the AKT1 inward-rectifier K+ channel could be involved in the transport of K+ from low K+ concentrations in Arabidopsis. Interestingly, akt1 plants are unable to grow at low external K+ concentrations only if millimolar NH4+ concentrations are present in the growth medium; this is an indication that other parallel NH4+-sensitive pathways of K+ transport exist. Evidence for an inhibitory effect of NH4+ on this transport process has been earlier offered for other plant species on the basis of short-term radiometric studies suggesting that NH4+-sensitive and -insensitive components could be present (Deane-Drummond and Glass, 1983; Scherer et al., 1984; Vale et al., 1987, 1988; Wang et al., 1996). However, the relevance of these components to long-term K+ nutrition and the molecular systems contributing to each of them remain essentially unknown.
While there is some controversy regarding the role of the HKT1 K+/Na+ cotransporter in the movement of K+ from the external solution into root cells (Maathuis et al., 1996; Rubio et al., 1996; Walker et al., 1996b; Wang et al., 1998), the HvHAK1 transporter exhibits some of the hallmarks expected for a major contributor to high-affinity K+ transport in roots (Santa-María et al., 1997). Preliminary evidence indicated that the K+-transport activity of HvHAK1 is strongly affected by the presence of NH4+. A detailed exploration of the effect of NH4+ on the transport properties of HvHAK1 and HKT1 may offer further evidence on the involvement of these transporters in the NH4+-sensitive and -insensitive pathways of K+ and Rb+ transport.
To date, a functional characterization in planta of the structural and regulatory elements involved in K+ uptake has been partially done only in Arabidopsis (Wu and Zhu, 1996; Hirsch et al., 1998; Zhu et al., 1998; Spalding et al., 1999). No comparative studies have been made to support the idea that Arabidopsis could be a universal model system to understand the physiology of K+ uptake in other photosynthetic organisms, particularly in monocotyledonous plants. Consequently, intensive studies are necessary to elucidate the nature of the systems contributing to the transport of K+ in this major class of angiosperms. To explore the relative contribution of NH4+-sensitive and -insensitive components to K+ transport from diluted K+ solutions in terms of the putative underlying molecular mechanisms already known in monocotyledonous plants, we have examined the characteristics of Rb+ uptake in roots of barley plants grown at combined levels of NH4+ and K+ supply and compared them with the intrinsic properties of the HvHAK1 and HKT1 transporters as displayed in a heterologous yeast background. We found that the ionic environment encountered by barley roots during growth exerts a strong influence on the activity of the components participating in Rb+ transport at low Rb+ concentrations. We also present evidence indicating that HvHAK1 may be involved in the NH4+-sensitive pathway of high-affinity Rb+ transport in roots.
RESULTS
NH4+ Inhibits Barley Growth in the Absence of K+
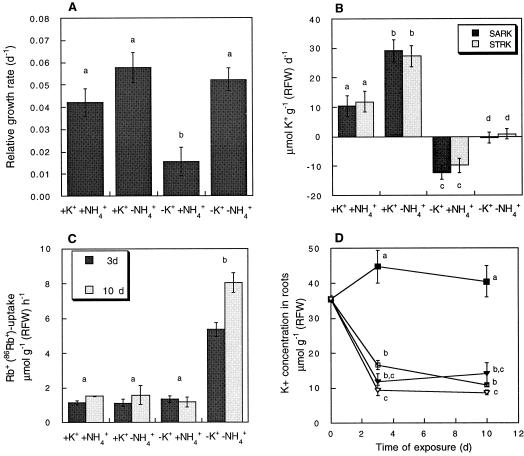
With the aim of providing an overall framework for interpreting the nature of the effects of NH4+ on K+ transport, we first studied the long-term effect of combined levels of K+ and NH4+ supply on growth and K+ accumulation. In several plant species, growth is severely inhibited by NH4+ in the absence of K+ (Barker et al., 1967; Cao et al., 1993), while the addition of small quantities of the latter cation suppress some of the toxic effects (Cao et al., 1993). Figure 1A shows that a synergistic effect of K+ starvation and high NH4+ supply is operative in barley as well, since we found that growth in this plant species is negatively affected after 10 d of exposure to 5 mm NH4+ in the absence of K+, while for plants grown in the presence of just 100 μm K+ the growth rate was similar to that measured in plants not exposed to high NH4+ concentrations. In addition to this known effect on plant growth, the presence of 5 mm NH4+ strongly interfered with the absorption of K+ by roots as well as with K+ transport to shoots (Fig. 1B).
Figure 1.
Long-term exposure to high external NH4+ concentrations interferes with growth, K+ accumulation, and Rb+ uptake in barley. The effect of combined levels of K+ and NH4+ on the whole plant relative growth rate (RGR) (A), the specific absorption and translocation rates of K+ (SARK and STRK, respectively) (B), Rb+ uptake into roots 3 and 10 d after the beginning of the treatments (C), and the concentration of K+ in roots (D). RGR, SARK, and STRK were determined from the data corresponding to the period between 0 and 10 d after the beginning of the treatments. The external K+ concentration used for growth was 0 or 100 μm (white and black symbols in D, respectively), while the external NH4+ concentration was 0 or 5 mm (squares and inverted triangles in D, respectively). Measurements of Rb+ uptake were made at 100 μm Rb+ in a solution of the same composition as used for growth, except for the absence of K+. Results are average values of an experiment consisting of four independent replicates. The presence of the same letter indicates the absence of significant differences among treatments at P = 0.05.
Next, we investigated the effect of combined levels of K+ and NH4+ on the uptake of Rb+(86Rb) in barley roots measured in the same conditions as used for growth, except for the exclusion of K+ from the loading solution. Figure 1C shows that after 3 or 10 d of exposure to combined levels of NH4+ and K+, the uptake of Rb+(86Rb) in roots of K+-starved plants not exposed to high external NH4+ concentrations was higher than that measured for plants grown in the presence of 100 μm K+ in either the absence or presence of NH4+. Remarkably, the high uptake of Rb+ observed in K+-starved plants was not observed when 5 mm NH4+ was included in the culture solution. This effect was not linked with the bulk concentration of K+ in roots, which declined markedly for K+-starved plants grown in the presence or the absence of NH4+ (Fig. 1D).
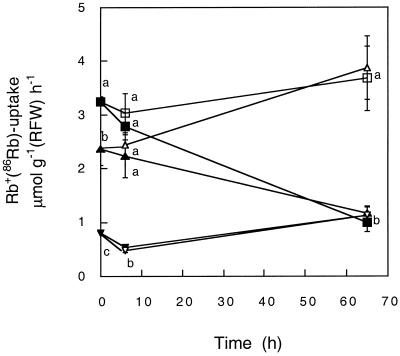
To explore to what extent the pattern described above depends on the time of exposure and the external supply of NH4+, the time course of Rb+(86Rb) uptake by 14-d-old plants suddenly exposed to 0, 0.15, or 5 mm NH4+ in the presence or the absence of K+ was also studied. Figure 2 shows that while a low NH4+ concentration did not exert a strong effect, a high NH4+ concentration immediately inhibited the transport of Rb+. The inhibition due to 5 mm NH4+ was also evident 6 h after exposure to the new external K+ and NH4+ concentrations. However, the long-term effect of NH4+ on Rb+ transport depended on whether K+ was included in the growth medium. For K+-starved plants, high external concentrations of NH4+ resulted in a low uptake of Rb+ within the first 3 d of treatment. However, for plants grown in the presence of 100 μm K+, the inhibitory effect of external NH4+ was almost nil at the end of this period. These results support the notion that plants grown in the presence of high external NH4+ concentrations are able to overcome the initial inhibitory effect. Note that for plants always kept in a constant ionic environment, the uptake of Rb+ declined along the developmental window studied. A decline in K+ uptake during plant ontogeny has been previously observed in other Triticeae species (Kuhlmann and Barraclough, 1987) and is likely to be the result of a regulatory process superimposed on that studied here.
Figure 2.
NH4+ effect on Rb+ uptake depends on the time of exposure as well as on the presence of K+ in the growth medium. Time course of Rb+(86Rb) uptake in plants exposed to the presence (black symbols) or the absence (white symbols) of 100 μm K+ at different NH4+ concentrations: squares, 0 mm; triangles, 0.15 mm; and inverted triangles, 5 mm NH4+. Measurements were made at 100 μm Rb+ 0, 6, and 66 h after the beginning of the treatments. The composition of the loading solution was the same as used for each growth condition, except for the exclusion of K+. Results are from an experiment consisting of three independent replicates. The presence of the same letter for a given time of exposure to different treatments indicates the absence of significant differences at P = 0.05.
Long-Term Exposure to High NH4+ Levels Elicits a Component of Rb+ Uptake Insensitive to NH4+
The results above suggest the possibility that different systems may participate in the transport of Rb+ into roots after a long-term exposure to different external concentrations of K+ and NH4+. To test this hypothesis, we studied the effect of Na+ and NH4+ on the uptake of Rb+ in 17-d-old plants exposed for the last 3 d to combined levels of K+ and NH4+. Preliminary experiments showed that the inclusion of Na+ in the growth medium did not affect markedly the pattern of Rb+ uptake described above (data not shown). The inclusion of 100 μm Na+ in the growth treatments allowed the possibility that some transport systems might require the presence of this cation to be operative.
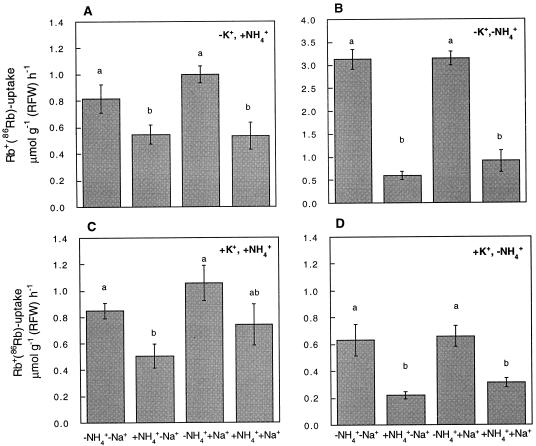
Figure 3A shows that in plants exposed for 3 d to the presence of NH4+ and the absence of K+, the uptake of Rb+ measured in the absence of NH4+ did not reach the high values observed in plants grown in the absence of both K+ and NH4+ (Fig. 3B), which indicates that long-term NH4+ nutrition interferes with a pathway responsible for the high rate of Rb+ transport observed after K+ starvation. Aside from this absolute difference, the relative effect of NH4+ on Rb+ uptake was also dependent on the external concentrations of K+ and NH4+ to which the roots had been exposed. Figure 3, B and D, shows that for plants grown in the absence of NH4+, Rb+ uptake was sharply inhibited by the inclusion of 5 mm NH4+ in the loading solution, while Na+ did not exert any significant effect. The inhibitory effect of NH4+ was more pronounced in plants grown in the absence than in the presence of K+ (Fig. 3B). A separate experiment showed that Rb+ uptake by plants grown in the absence of NH4+ was reduced to 58% and 60% by addition of 100 μm K+ to K+-starved and K+-sufficient plants, respectively (data not shown).
Figure 3.
NH4+ exerts a differential effect on the transport of Rb+ in barley plants grown in different ionic environments. The short-term effect of 5 mm NH4+, in combination with the presence or the absence of 100 μm Na+, on the uptake of Rb+ was determined at 100 μm Rb+ for plants previously exposed for 3 d to the absence of K+ in the presence of 5 mm NH4+ (A), the absence of both K+ and NH4+ (B), 100 μm K+ in the presence of 5 mm of NH4+ (C), and 100 μm K+ in the absence of NH4+ (D). All growth solutions contained 100 μm Na+. Note the use of a different scale in B. Results given are the average values of two separate experiments consisting of three and five independent replicates. For each growth condition, the presence of the same letter on two columns indicates the absence of significant differences at P = 0.05.
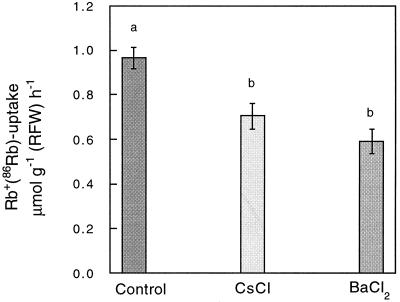
Conversely, in plants grown in the presence of 5 mm NH4+, the presence of 5 mm NH4+ in the loading solution led to a relatively small decrease of Rb+ uptake, and Na+ did not produce a significant effect (Fig. 3, A and C). Thus, for plants grown in the absence of NH4+, Rb+ uptake is dominated by a component sensitive to NH4+, whereas in plants grown in the presence of NH4+, the bulk uptake of Rb+ is mediated by a component able to operate in the presence of high NH4+ concentrations. Complementary experiments showed that Rb+ uptake for plants grown at 5 mm NH4+ in the presence or absence of K+ was reduced to 78% and 94%, respectively, by the addition of 100 μm K+ (data not shown). To obtain some additional information on the systems contributing to Rb+ uptake in plants grown in the presence of NH4+, we also studied the effect of CsCl and BaCl2 on Rb+ uptake. While the addition of 100 μm CsCl produced a 27% inhibition, the addition of 1 mm BaCl2 resulted in a 39% inhibition of Rb+ transport into roots (Fig. 4).
Figure 4.
Cs+ and Ba2+ inhibit Rb+ uptake in plants grown in the absence of K+ and the presence of 5 mm NH4+. Relative effect of CsCl (100 μm) and BaCl2 (1 mm) on the uptake of Rb+(86Rb) in plants exposed for 3 d to the absence of K+ and the presence of both 5 mm NH4+ and 100 μm Na+. Rb+(86Rb) uptake was measured at 100 μm Rb+ in the absence of NH4+ and the presence of 100 μm Na+. On the left, the uptake of Rb+(86Rb) measured in the absence of inhibitors is shown. Results are from an experiment consisting of five independent replicates. The presence of the same letter on two columns indicates the absence of significant differences at P = 0.05.
Yeast Cells Expressing HKT1 Exhibit an Enhanced Resistance to High External NH4+ Concentrations Compared with Those Expressing HvHAK1
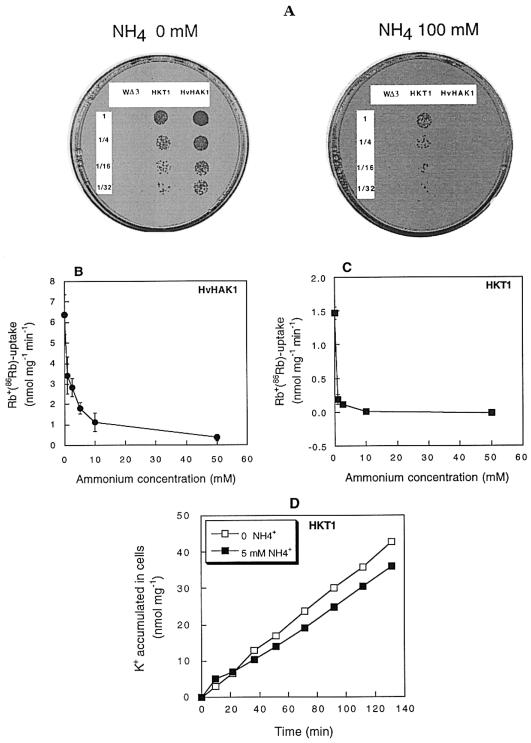
Yeast cells of the strain WΔ3, which are defective in high-affinity K+ transport, were used to explore the effect of external NH4+ on the transport of Rb+ mediated by the HKT1 and HvHAK1 transporters. Figure 5A shows that WΔ3 cells did not grow at low external K+ concentrations regardless of the external NH4+ concentration supplied to the medium. Transformation of these cells with HKT1 or HvHAK1 restored growth both at 10 (Fig. 5A) and 100 (data not shown) μm K+ in the absence of NH4+. A progressive inhibition of growth was observed for HvHAK1-expressing cells at increasing levels of NH4+, more so at 10 than at 100 μm K+. At 10 μm K+ in the presence of 50 or 100 mm NH4+, these cells were unable to grow or grew poorly. However, under the same conditions, the growth of yeast cells expressing HKT1 was only slightly reduced. These results indicate that the expression of HKT1 conferred on WΔ3 cells an enhanced capacity to grow at very high external NH4+ concentrations relative to that exhibited by yeast cells expressing HvHAK1; furthermore, the inhibition of growth in yeast cells expressing HvHAK1 depended markedly on the balance between the external concentrations of K+ and NH4+. To explain these findings in terms of the kinetic properties of HKT1 and HvHAK1, we examined the dependence of Rb+(86Rb) uptake on the external NH4+ concentration displayed by these transporters. Figure 5B shows that the uptake of Rb+(86Rb) mediated by HvHAK1 decreased with the increase in external NH4+ concentrations, with a half inhibition at 2.7 mm NH4+. At 5 mm NH4+, Rb+(86Rb) uptake was inhibited to 27% of that measured in the absence of NH4+. Interestingly, the uptake of Rb+(86Rb) in yeast cells expressing HKT1 was sharply inhibited by external NH4+ concentrations considerably lower than those required to inhibit Rb+(86Rb) uptake mediated by HvHAK1 to the same extent; it was almost nil at 5 mm NH4+ (Fig. 5C).
Figure 5.
HKT1 confers on yeast cells an enhanced capacity for growth in the presence of high external NH4+ concentrations. A, Growth of WΔ3 yeast cells, defective in K+ uptake, and the same cells transformed with HvHAK1 or HKT1 on AP medium supplied with K+ 10 μm at two levels of NH4+ addition; 10 μL of progressive dilutions of a 0.1-optical density cell suspension were inoculated on the medium. B, Rb+(86Rb) uptake mediated by WΔ3 cells expressing HvHAK1 at 100 μm Rb+ at increasing levels of NH4+. C, As in B, but for HKT1-expressing WΔ3 cells. In B and C, results are the average values of not less than three experiments. D, Results of a representative experiment showing the accumulation of K+ measured as K+ removal from a 100 μm K+ solution mediated by HKT1-expressing cells in the presence (white symbols) or absence (black symbols) of 5 mm NH4+.
When the results obtained in the growth and Rb+(86Rb)-uptake experiments are compared, a consistent pattern emerges for HvHAK1, but a contradiction is apparent for HKT1. This anomaly can be explained if the uptake of Rb+ does not mirror the uptake of K+ mediated by HKT1 in the presence of high external NH4+ concentrations. Figure 5D shows that the accumulation of K+ by HKT1-expressing cells was only slightly lower in the presence of 5 mm NH4+ than in the absence of this cation. This pattern contrasts with that observed in HvHAK1-expressing cells, for which the K+ accumulation was sharply reduced by 5 mm NH4+ (Santa-María et al., 1997). Thus, HKT1 is able to mediate the uptake of K+ even at high external NH4+ concentrations, whereas K+ uptake mediated by HvHAK1 is only marginal under these conditions. The slow growth rate and slow net K+ uptake of HKT1-expressing yeast cells can be explained by the strong depolarization induced by HKT1 in yeast (Madrid et al., 1998).
NH4+ Exerts a Mixed Inhibitory Effect on HvHAK1-Mediated Rb+ Transport
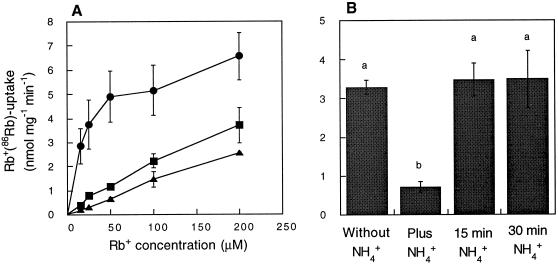
Because of the possibility that HvHAK1 is involved in an NH4+-sensitive component of Rb+ transport in roots, we further examined the effect of the former cation on the kinetic parameters of Rb+ transport mediated by HvHAK1, as determined in WΔ3 yeast cells (Fig. 6A). Estimations of Vmax and apparent Km from the data shown in Figure 6A yielded the following values: 6.54, 4.97, and 3.11 nmol mg−1 min−1 and 29.4, 140, and 213 μm Rb+ at 0, 2.5, and 5.0 mm NH4+, respectively. This repeated effect on both Vmax and Km values suggests the presence of a mixed inhibition kinetics. Repeated attempts to complement the growth of mep1Δ mep2Δ mep3Δ yeast cells, which are defective in NH4+ transport, with the HvHAK1 cDNA failed. We were also unable to observe any increase in methylamine uptake between mep1Δ mep2Δ mep3Δ cells and the cells expressing HvHAK1 at 1 mm methylamine (data not shown). These results indicate that HvHAK1 could not transport NH4+ and that Rb+ uptake inhibition by NH4+ could not imply competition between these ions for the entry into the pore domain. Equally importantly, we observed that this inhibition was reversed by NH4+ removal (Fig. 6B).
Figure 6.
Rb+ transport mediated by HvHAK1 is reversibly affected by NH4+ through a mixed mode inhibition. A, Uptake of Rb+(86Rb) by WΔ3 cells expressing HvHAK1 in the absence (●) or presence of 2.5 (▪) or 5 (▴) mm NH4+. B, Immediate effect of NH4+, and the effect of NH4+ removal after a 15-or 30-min exposure to 5 mm NH4+ (right side) on Rb+ uptake measured from a 100 μm Rb+ solution; on the left, the rate of Rb+ measured in cells never exposed to NH4+ is shown. Results shown in A and B are the average values of five and three experiments, respectively. The presence of the same letter on two columns indicates the absence of significant differences at P = 0.05.
A previous report (Santa-María et al., 1997) showed that the uptake of Rb+ by WΔ3 cells expressing HvHAK1 is inhibited by K+ (Ki = 27 μm) and Na+ (Ki = 15 mm). In addition to exploring the inhibitory effect of NH4+, we studied the effect of other monovalent cations on Rb+ uptake mediated by HvHAK1. We found that CsCl inhibited the uptake of Rb+ (Ki = 80 μm), while the addition of LiCl did not affect this transport process (data not shown). Thus, the inhibitory effect of monovalent cations on Rb+ uptake mediated by HvHAK1 follows the sequence K+>Cs+> NH4+≫Na+, with Li+ exerting no effect, at least within the range of external concentrations studied here. We also examined the effect of Ba2+ on the transport of Rb+ mediated by HvHAK1 at 100 μm Rb+, and found that concentrations of BaCl2 much higher than those inhibiting Rb+ uptake in plants grown at 5 mm NH4+ were necessary to produce a detectable inhibition of Rb+ uptake in HvHAK1-expressing yeast cells, with a half inhibition at 16 mm (data not shown).
DISCUSSION
The identity and contribution of the systems involved in the transport of K+ from dilute K+ solutions have been extensively discussed in the recent literature (Schachtman and Liu, 1999; Rodríguez-Navarro, 2000). Because of the difficulties derived from the use of 42K+, most of the studies referring to the unidirectional fluxes of K+ in roots have been done with Rb+(86Rb) instead of K+(42K) or with 86Rb as a tracer for K+. It is worth noting that Rb+ can be used as a K+ analog only under certain circumstances (Rodríguez-Navarro, 2000). For some transport systems, even such simple characteristics as the NH4+ sensitivity of the uptake can be different for Rb+ and K+, as clearly illustrated in Figure 5. In this report, we have used Rb+ to characterize some of the root-expressed transporters of monocotyledonous plants, depending on whether they mediate NH4+-sensitive or -insensitive Rb+ uptake. Because of the existence of differences in the rates of K+ and Rb+ transport, extrapolation of Rb+ measurements to the transport of K+ in roots should be done with caution.
The notion that plant roots are furnished with NH4+-sensitive and -insensitive components for the transport of K+ and Rb+ from diluted solutions into the root symplasm of higher plants was developed previously (Deane-Drummond and Glass, 1983; Scherer et al., 1984; Vale et al., 1987, 1988). However, the contribution of these components to plant K+ nutrition has not been deeply explored. Here we present evidence that the contribution made by these components to the uptake of Rb+ depends on the external concentration of K+ and NH4+ to which the roots were exposed during growth (Figs. 1–3). We observed that for barley plants grown in the absence of NH4+ with an adequate provision of nitrogen, Rb+ uptake was extremely sensitive to NH4+. This result is consistent with those previously reported by Wang et al. (1996) for rice plants grown in the absence of or at low external NH4+ concentrations in a medium containing 1.5 mm NO3−. In addition, we found that in barley plants grown at high NH4+ concentrations, Rb+ uptake is preferentially mediated by a system (or systems) insensitive to NH4+.
It has recently been argued that in Arabidopsis, the NH4+-sensitive component of K+ transport can be attributed to HKT1 and/or Kup-HAK transporters (Spalding et al., 1999). However, no critical information has been available to test this hypothesis properly, while the NH4+-insensitive component is thought to correspond to the AKT1 K+ channel (Hirsch et al., 1998). Whether these systems are also involved in the transport of Rb+ from micromolar Rb+ concentrations in monocotyledonous plants has not been examined. A comparison of the properties exhibited by the main types of root-expressed Rb+ transporters in heterologous systems with those observed in roots helped us to make an assessment of the identity of the systems involved in each component of Rb+ transport in barley, and also to test the relevance of HKT1 and HAK1 transporters in the NH4+-sensitive component.
NH4+-Sensitive Systems of Rb+ Transport
Several lines of evidence obtained in this work converge in assigning to HvHAK1 a major role in high-affinity Rb+ transport in roots of plants grown in the absence of K+ and NH4+. The absence of an effect of Na+ and the inhibitory effect of external K+ and NH4+ on Rb+ transport in roots of plants grown under these conditions (Epstein et al., 1963; Vale et al., 1987, 1988; Fig. 3B) and HvHAK1-expressing yeast cells (Santa-María et al., 1997; Fig. 5B) offer evidence supporting this possibility. In the same way, the mixed inhibitory effect of NH4+ on Rb+ transport kinetics, as well as recovery of transport after NH4+ removal, has been observed in maize and tobacco roots (Breteler, 1977; Scherer et al., 1984) and in HvHAK1-expressing yeast cells (Fig. 6). Furthermore, the inhibitory effect of monovalent cations on the transport of Rb+ mediated by HvHAK1 reported here follows a sequence similar to that previously determined in barley roots and maize shoots of plants grown in the absence of NH4+ (Epstein et al., 1963; Smith and Epstein, 1964). The intrinsic properties of HvHAK1 observed in WΔ3 yeast cells seem to be consistent with a major role of this transporter in the NH4+-sensitive component of high-affinity Rb+ transport in plants grown under limiting external K+ concentrations in the absence of or at low external NH4+ concentrations. Interestingly, our results indicate that HvHAK1 does not act as an NH4+ transporter.
The second possible candidate thought to be involved in the NH4+-sensitive component of Rb+ transport is HKT1. Results shown in Figure 5C support this possibility. However, the absence of a stimulatory effect of Na+ on the transport of Rb+ (when measured in the absence of NH4+) observed in plants grown at different pH in the presence or absence of Na+ (Maathuis et al., 1996), as well as for plants grown at different external concentrations of K+ and NH4+ (Fig. 3), indicate that HKT1 does not contribute, or contributes only as a minor system, to the total uptake of Rb+ into barley roots regardless of the ionic conditions prevailing in the external solution in which plants had been grown. Remarkably, while HKT1 acts as an NH4+-sensitive pathway of high-affinity Rb+ transport, it operates as an NH4+-insensitive pathway of K+ accumulation inside the yeast cells (Fig. 5). In akt1 Arabidopsis plants, changes in membrane potential associated with the transport of K+ were greatly enhanced by Na+ and reduced by NH4+ addition (Spalding et al., 1999). This result and those reported here indicate that no inhibition of K+ accumulation should be observed after NH4+ addition if the HKT1 identified in the Arabidopsis genome behaves as its wheat counterpart in yeast cells. It should be mentioned that because of the existence of differences in the rates of K+ and Rb+ transport mediated by HKT1 (Gassmann et al., 1996), the possibility that HKT1 is a contributor to the NH4+-insensitive component of K+ accumulation in barley roots could be not rejected.
The NH4+-Insensitive Pathway of Rb+ Transport
The heterologous studies reported above clearly indicate that neither HKT1 nor HvHAK1 were involved in the component of Rb+ transport insensitive to NH4+. Thus, the next question refers to the identity of the systems involved in that pathway. Studies made with yeast and insect cells expressing the inward-rectifier K+ channel AKT1 have shown that it is about 10- to 20-fold more permeable to K+ than to NH4+ (Bertl et al., 1997; C. Horeau, personal communication). These observations and those derived from the null mutant of AKT1 in Arabidopsis (Hirsch et al., 1998) suggest the possibility that a barley AKT1 could be responsible for the NH4+-insensitive component observed here. The presence of AKT1 in monocot genomes has been previously established (Hoth et al., 1997; accession no. Y07632.1), and electrophysiological studies suggest the involvement of an AKT1-like channel in low-affinity K+ transport into barley roots (Amtmann et al., 1999). However, compelling evidence indicates that passive movement of K+ from the external solution into the cytosol of epidermal and cortical root cells of this plant species only takes place when the external K+ concentration is higher than 0.5 mm (Walker et al., 1996a), which rules out the involvement of K+ channels in the transport of K+ from dilute K+ solutions (Kochian and Lucas, 1993; Maathuis and Sanders, 1993, 1994).
To date, no information is available to determine if a similar thermodynamic impediment also applies to plants grown in the presence of high external NH4+ concentrations. Thus, the only way to make an assessment of the possible contribution of AKT1 to the NH4+-insensitive component is to compare the properties exhibited by heterologous systems expressing AKT1 with those observed in barley plants. The absence of an effect of Na+ on Rb+ transport in roots is consistent with the selectivity properties displayed by both HvHAK1 and AKT1, while the weak effect of K+ on Rb+ transport could be explained either by a high Rb+ Km and/or a high K+ Ki. Therefore, these two properties serve to characterize the NH4+-insensitive component, but are not useful to explore the possible involvement of AKT1 in this pathway. However, studies with yeast cells expressing AKT1 have shown that inward K+ currents mediated by this channel are strongly blocked by Ba2+ and are abolished by very low Cs+ concentrations (Bertl et al., 1997). In the present study, we found that while the effect of 1 mm BaCl2 could be consistent with a role of AKT1 in this process, the effect of 100 μm CsCl on Rb+ transport was much lower than expected if this hypothesis were correct (Fig. 4). In fact, the slight reduction of Rb+ transport observed in barley roots upon the addition of CsCl could be explained by the inhibitory effect exerted by Cs+ on other transporters (such as HvHAK1) potentially involved in the small NH4+-sensitive component of Rb+ transport of NH4+-grown plants. Thus, none of the transport systems expressed in roots to date characterized exhibit intrinsic properties entirely consistent with those displayed by the NH4+-insensitive component of Rb+ transport in barley, and, consequently, the identity of the systems contributing to this pathway could be not resolved. Therefore, the possibility that other non-characterized root-expressed K+ transporters (Rubio et al., 2000) participate in this kinetic pathway could not be excluded.
The idea that several overlapping or partially redundant systems participate in the transport of K+ or Rb+ into the root symplasm from micromolar K+ or Rb+ concentrations was initially discussed by Walker et al. (1996b) and Rubio et al. (1996) and received further support for dicotyledonous plants from the disruption of AKT1 in Arabidopsis (Hirsch et al., 1998). Our results provide evidence that this concept may be applied for the transport of Rb+ to monocotyledonous plants, although the identity of the major systems contributing to the NH4+-sensitive and -insensitive pathways could be different in Arabidopsis and barley.
MATERIALS AND METHODS
Plant Growth
Seeds of barley (Hordeum vulgare cv Golden promise) were sown on moistened filter paper and kept in the dark for 48 h. Seedlings were then transferred to an acrylic ring, and placed on a 0.8-L plastic pot containing a nutrient solution of the following composition: 1.0 mm Ca(NO3)2, 0.5 mm MgSO4, 0.5 mm H3PO4, 50 μm FeEDTA, 50 μm CaCl2, 25 μm H3BO3, 2 μm ZnSO4, 2 μm MnSO4, 0.5 μm CuSO4, 0.5 μm molybdic acid, 2.5 mm 2-(N-morpholino)-ethanesulfonic acid (MES), and 100 μm KCl. The pH was brought to 6.00 ± 0.05 by the addition of Ca(OH)2. To avoid the possibility that the low external K+ concentrations supplied could be limiting for plant growth, the growth rate was reduced by manipulating the photon flux density at the plant level. It was set at 70 μmol m−2 s−1 over a photoperiod of 16 h. The temperature in the growth chamber was set to 22°C (day) and 18°C (night). Relative humidity was kept at 85%, day and night. The nutrient solution, permanently aerated, was renewed every 3 d during the first week and then every 2 d until the beginning of the experiments on d 14 after germination. During the afternoon of d 13, the solution was again renewed and the experiments started 3 h after the beginning of the light period of d 14. During the course of the experiments the solution was renewed daily. For measurements of growth and chemical analysis, four plants were harvested before and after each treatment, divided into shoots and roots, and weighed. Subsequently, samples were placed in plastic vials and treated with HCl 0.5 n to allow the release of free cations. The concentration of K+ was determined in dilutions of the extracts with atomic absorption spectrophotometer on emission mode (Shimadzu, Columbia, MD). The plant relative growth rate and the specific absorption and translocation rates of K+ were estimated according to methods previously described (Santa-María and Cogliatti, 1998).
Measurements of Rb+(86Rb) Uptake in Plants
Because some plant K+ transporters strongly discriminate K+ over Rb+, we used 86Rb as a tracer for the uptake of Rb+, excluding K+ from the loading solution. Thus, for measurements of Rb+(86Rb) uptake, roots of intact plants were transferred for 5 min to a pot containing the complete nutrient solution described above without K+. This step was performed to allow the elution of the K+ contained in the root apoplast. Following this treatment, plants were transferred to plastic pots containing 0.14 L of the same solution without K+, vigorously aerated, to which Rb+(86Rb) had been previously added to reach a 100 μm Rb+ concentration. The loading period was set at 20 min, and was followed by two wash outs with a solution of the same ionic composition as used for loading without 86Rb for a total of 6 min. An exception to this procedure was made when the effect of CsCl or BaCl2 was studied; in these experiments, the wash-out solutions did not include the inhibitors. Plants were harvested as previously described and the radioactivity in the samples was determined in a liquid scintillation counter (model 1414, Wallac, Turku, Finland).
Yeast Growth Complementation Tests and Uptake Experiments
Studies of complementation of WΔ3 yeast cells (trk1Δ trk2Δ) were done in a mineral solid medium with Arg as the basic nitrogen source, and supplemented with different amounts of K+ and NH4+ provided as chloride salts. WΔ3 cells were transformed either with a pYPGE15 plasmid containing the HvHAK1 cDNA under the control of the PGK1 promoter (Santa-María et al., 1997) or with a pDR195 plasmid containing the wheat HKT1 cDNA under the control of the PMA1 promoter (Rubio et al., 1995). For complementation studies, 10 μL of a series of dilutions of 0.1 absorbance cell suspensions of WΔ3 cells and WΔ3 cells expressing HvHAK1 or HKT1 were placed in each plate. For Rb+(86Rb) uptake experiments, cells were grown overnight in Arg medium plus 40 mm KCl, and were K+ starved for 4 h before the start of the experiments. These experiments were conducted on MES/Ca2+, pH 6.0, for HvHAK1-expressing or MES/Ca2+ plus 0.5 mm NaCl for HKT1-expressing yeast cells. Other conditions, as well as the procedure followed in experiments done to study K+ removal by HKT1 expressing yeast cells from mineral medium supplemented with 100 μm K+, were the same as described elsewhere (Santa-María et al., 1997).
Complementation studies with the yeast strain 31019 (mep1Δ mep2Δ::LEU2 mep3Δ::KanMX2 ura3; Marini et al., 1997), which is defective in NH4+ transport, were in a mineral medium, without Arg and containing Na2HPO4/NaH2PO4 (pH 6.0). The medium was supplemented with 20 μm KCl at increasing NH4Cl concentrations as the sole source of nitrogen. Uptake of methylamine (14CH3NH2) in these cells was measured following a similar experimental procedure as described above for the uptake of Rb+.
ACKNOWLEDGMENTS
We would like to express our thanks to Melina Arena, Carla Caputo, Gonzalo Estabillo, and Augusto Vallejo for assistance during Rb+ uptake experiments in plants; to Dr. Federico Gullace for his technical assistance with AAS measurements; and to Dr. Antonio Diaz-Paleo for supplying barley seed. We express gratitude to Prof. Alonso Rodríguez-Navarro and Dr. Francisco Rubio (Escuela Técnica Superior de Ingenieros Agrónomos, Universidad Politécnica de Madrid) for helpful discussions and comments on the manuscript, and to Dr. Christelle Horeau and Prof. Hervé Sentenac (Laboratoire de Biochimie et Physiologie Végétales, Ecole Nationale Supérieure Agronomique (Montpellier), Institut National de la Recherche Agronomique, Montpellier) for kindly sharing unpublished aspects of their work. We also express gratitude to Dr. Rodolfo Ugalde for his support in many aspects during our research. Yeast strains WΔ3, mep1Δ mep2Δ mep3Δ, as well as the HKT1 cDNA were kindly provided by Dr. Rosario Haro (Universidad Politécnica de Madrid), Prof. Alonso Rodríguez-Navarro, and Dr. F. Rubio, respectively.
Footnotes
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PEI 38/97 to G.E.S.-M.).
LITERATURE CITED
- Amtmann A, Jelitto TC, Sanders D. K+-Selective inward-rectifying channels and apoplastic pH in barley roots. Plant Physiol. 1999;119:331–338. doi: 10.1104/pp.120.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AV, Maynard DN, Lachman WH. Induction of tomato stem and leaf lesions, and potassium deficiency, by excessive ammonium nutrition. Soil Sci. 1967;103:319–327. [Google Scholar]
- Bertl A, Reid JD, Sentenac H, Slayman CL. Functional comparison of plant inward-rectifier channels expressed in yeast. J Exp Bot. 1997;48:405–413. doi: 10.1093/jxb/48.Special_Issue.405. [DOI] [PubMed] [Google Scholar]
- Breteler H. Ammonium-rubidium interaction in excised maize roots. In: Thellier M, editor. Transmembrane Ionic Exchanges in Plants. Paris: Centre Nationale de la Recherche Scientifique; 1977. pp. 185–191. [Google Scholar]
- Cao Y, Glass ADM, Crawford NM. Ammonium inhibition of Arabidopsis thaliana root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiol. 1993;102:983–989. doi: 10.1104/pp.102.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT. Factors affecting mineral nutrient acquisition. Annu Rev Plant Physiol. 1985;36:77–115. [Google Scholar]
- Deane-Drummond CE, Glass ADM. Short term studies of nitrate uptake into barley plants using ion-specific electrodes and 36ClO3−1. Plant Physiol. 1983;73:105–110. doi: 10.1104/pp.73.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Mechanisms of ion transport through plant cell membranes. Int Rev Cytol. 1973;34:123–168. [Google Scholar]
- Epstein E, Rains DW, Elzam OE. Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA. 1963;49:684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers TJ, Läuchli A. Sodium versus potassium: substitution and compartmentation. In: Läuchli A, Bieleski RL, editors. Inorganic Plant Nutrition. Berlin: Springer-Verlag; 1983. pp. 651–681. [Google Scholar]
- Fu H-H, Luan S. AtKup1: a dual-affinity K+ transporter from Arabidopsis thaliana. Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996;10:869–882. doi: 10.1046/j.1365-313x.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- Gupta AS, Berkowitz GA, Pier PA. Maintenance of photosynthesis at low leaf water potential in wheat: role of potassium status and irrigation history. Plant Physiol. 1989;89:1358–1365. doi: 10.1104/pp.89.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Hoth S, Dreyer I, Dietrich P, Becker D, Müller-Röber B, Hedrich R. Molecular basis of plant-specific acid activation of K+ uptake channels. Proc Natl Acad Sci USA. 1997;94:4806–4816. doi: 10.1073/pnas.94.9.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKup1: an Arabidopsis thaliana gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10:51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. Potassium transport in corn roots: resolution of kinetics into a saturable and linear components. Plant Physiol. 1982;70:1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. Can K+ channels do it all? Plant Cell. 1993;5:720–721. doi: 10.1105/tpc.5.7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann H, Barraclough PB. Comparison between the seminal and nodal root systems of winter wheat in their activity for N and K uptake. Z Pflanzenernähr Bodenk. 1987;150:24–30. [Google Scholar]
- Maathuis FJM, Sanders D. Energization of potassium uptake in Arabidopsis thaliana. Planta. 1993;191:302–307. [Google Scholar]
- Maathuis FJM, Sanders D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci USA. 1994;91:9272–9276. doi: 10.1073/pnas.91.20.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Verlin D, Smith A, Sanders D, Fernández JA, Walker NA. The physiological relevance of Na+-coupled K+-transport. Plant Physiol. 1996;112:1609–1616. doi: 10.1104/pp.112.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Gómez MJ, Ramos J, Rodríguez-Navarro A. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J Biol Chem. 1998;273:14838–14844. doi: 10.1074/jbc.273.24.14838. [DOI] [PubMed] [Google Scholar]
- Marini AM, Soussi-Boudekou S, Vissers S, André B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4282–4293. doi: 10.1128/mcb.17.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A. Potassium transport in fungi and plants. Biochim Biophys Acta. 2000;146:1–30. doi: 10.1016/s0304-4157(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. High-affinity potassium uptake in plants. Science. 1996;273:977–979. doi: 10.1126/science.273.5277.978. [DOI] [PubMed] [Google Scholar]
- Rubio F, Santa-María GE, Rodríguez-Navarro A (2000) Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in plant root and shoot cells. Physiol Plant (in press)
- Rufty TW, Jackson WA, Raper CD. Inhibition of nitrate assimilation in roots in the presence of ammonium: the moderating influence of potassium. J Exp Bot. 1982;33:1122–1137. [Google Scholar]
- Santa-María GE, Cogliatti DH. The regulation of zinc uptake in wheat plants. Plant Sci. 1998;137:1–12. [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A. The HAK1 gene of barley belongs to a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman D, Liu W. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999;4:281–287. doi: 10.1016/s1360-1385(99)01428-4. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Scherer HW, Mackown CT, Legget JE. Potassium-ammonium uptake interactions in tobacco seedlings. J Exp Bot. 1984;35:1060–1070. [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Smith RC, Epstein E. Ion absorption by shoot tissue: kinetics of potassium and rubidium absorption by corn leaf tissue. Plant Physiol. 1964;39:992–996. doi: 10.1104/pp.39.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD. Potassium uptake supporting plant growth in the absence of AKT1 channel activity. J Gen Physiol. 1999;113:1–10. doi: 10.1085/jgp.113.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale FR, Jackson WA, Volk RJ. Potassium influx into maize root systems: influence of root potassium concentration and ambient ammonium. Plant Physiol. 1987;84:1416–1420. doi: 10.1104/pp.84.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale FR, Volk RJ, Jackson WA. Simultaneous influx of ammonium and potassium into maize roots: kinetics and interactions. Planta. 1988;173:424–431. doi: 10.1007/BF00401031. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ. Potassium homeostasis in vacuolated plant cells. Proc Natl Acad Sci USA. 1996a;93:10510–10514. doi: 10.1073/pnas.93.19.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NA, Sanders D, Maathuis FJM. High-affinity potassium uptake in plants. Science. 1996b;273:977–979. doi: 10.1126/science.273.5277.977. [DOI] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MY, Glass ADM. Interactions between K+ and NH4+: effects on ion uptake by rice roots. Plant Cell Environ. 1996;19:1037–1046. [Google Scholar]
- Wang T-B, Gassmann W, Rubio F, Schroeder JI, Glass AD. Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol. 1998;118:651–659. doi: 10.1104/pp.118.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-J, Zhu J-K. SOS1, a genetic locus essential for salt tolerance and potassium accumulation. Plant Cell. 1996;8:617–627. doi: 10.1105/tpc.8.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K, Liu J, Xiong L. Genetic analysis of salt tolerance in Arabidopsis thaliana: evidence for a critical role of potassium nutrition. Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]