Abstract
Ascorbic acid is synthesized from galactono-γ-lactone (GL) in plant tissues. An improved extraction procedure involving ammonium sulfate precipitation of membrane proteins from crude leaf homogenates yielded a simple, quick method for determining tissue activities of galactono-γ-lactone dehydrogenase (GLDH). Total foliar ascorbate and GLDH activity decreased with leaf age. Subcellular fractionation experiments using marker enzymes demonstrated that 80% of the total GLDH activity was located on the inner mitochondrial membrane, and 20% in the microsomal fraction. Specific antibody raised against potato (Solanum tuberosum L.) tuber GLDH recognized a 56-kD polypeptide in extracts from the mitochondrial membranes but failed to detect the equivalent polypeptide in microsomes. We demonstrate that isolated intact mitochondria synthesize ascorbate in the presence of GL. GL stimulated mitochondrial electron transport rates. The respiration inhibitor antimycin A stimulated ascorbate biosynthesis, while cyanide inhibited both respiration and ascorbate production. GL-dependent oxygen uptake was observed in isolated intact mitochondria. This evidence suggests that GLDH delivers electrons to the mitochondrial electron transport chain between complexes III and IV.
Ascorbic acid is an abundant antioxidant in plant tissues that is found in millimolar concentrations in leaves and storage organs (for review, see Smirnoff 1996; Noctor and Foyer, 1998). It reacts directly with O2·−, ·OH, and 1O2 (Buettner and Jurkiewicz, 1996), recycles the lipid-soluble α-tocopherol by reduction of its oxidized form (Sharma and Buettner, 1993), and is essential for the protection of enzymes with prosthetic transition metal ions (Padh, 1990). It is involved in the regulation of the fundamental cellular processes of photoprotection and regulation of photosynthesis (Foyer and Harbinson, 1994), root elongation, cell vacuolarization, and cell expansion (for review, see Smirnoff, 1996). Ascorbic acid is also involved in the cell cycle (Kerk and Feldman, 1995) and in other important enzyme reactions in plant tissues (i.e. ethylene biosynthesis).
The pathway of ascorbate biosynthesis in plants is different from that in animals. It has remained unresolved until recently, when convincing evidence in support of a novel putative pathway was presented (Wheeler et al., 1998). Although many steps remain to be completely elucidated, the last step in the pathway involves the conversion of l-galactono-γ-lactone (GL) to ascorbic acid, a reaction catalyzed by the membrane-bound enzyme l-galactono-γ-lactone dehydrogenase (GLDH) (Ostergaard et al., 1997). GLDH, with a molecular mass of 56 kD, is highly specific for GL and requires cytochrome c (Cyt c) as a second substrate that is reduced as GL is converted to ascorbic acid (Ôba et al., 1995; Ostergaard et al., 1997; Imai et al., 1998). GL is synthesized from l-Gal by a novel enzyme, l-Gal dehydrogenase (Wheeler et al., 1998), and is rapidly converted to ascorbic acid when applied to different plant tissues (Conklin et al., 1996; Arrigoni et al., 1997). The ascorbate pool in leaves represents over 10% of the soluble carbohydrates (Noctor and Foyer, 1998), and concentrations of 20 to 50 mm have been found in spinach chloroplasts and cytosol (Foyer et al., 1983). Even when ascorbic acid is present at high concentrations, there are no data on the concentration of GL in plant tissues, and the integration of ascorbate synthesis, regeneration, and degradation is still not completely resolved.
The requirement of mitochondria for the biosynthesis of ascorbic acid from GL was reported in early studies (Mapson and Breslow, 1958). By Suc-density gradient fractionation, GLDH activity was localized in mitochondria of white potato (Solanum tuberosum L.) tubers and spinach leaves (Ôba et al., 1994; Mut-suda et al., 1995). Mitochondria from fava bean embryos and maize seedlings were used as a source of GLDH for studies on the in vivo conversion of GL into ascorbic acid (Arrigoni et al., 1997; De Gara et al., 1997). Very recently, the presence of GLDH on the mitochondrial inner membrane from kidney bean hypocotyls has been reported (Siendones et al., 1999). Although GLDH activity was also found in microsomes from potato tubers (Ôba et al., 1994), no fur-ther studies have been carried out to establish unequivocally the localization of this enzyme in other subcellular compartments.
An accurate assay of GLDH activity in crude homogenates has not been reported until now because of the specificity of this enzyme for Cyt c as an electron acceptor and the high interference of competing reactions. The use of mitochondrial preparations is therefore a prerequisite for the measurement of GLDH activity, precluding experiments that require a rapid extraction of plant tissues. The present study describes a new method for the quick, reproducible measurement of GLDH activity in crude leaf homogenates. We demonstrate that isolated intact mitochondria synthesize ascorbate in the presence of the precursor GL. The localization of GLDH on the inner mitochondrial membrane and its specificity for Cyt c as an electron acceptor are confirmed. The localization of GLDH activity in potato leaf microsomes and the involvement of GLDH in mitochondrial electron transport are also discussed.
RESULTS
At the outset of this study it was clear that an improved extraction method for the assay of GLDH activity in leaf homogenates was needed. By precipitation of crude leaf homogenates with 20% (w/v) (NH4)2SO4, two fractions, designated as membrane-bound and soluble proteins, were obtained (Table I). GLDH activity was found to be associated mostly with the membrane-enriched fraction and had a distribution similar to that of NADPH-Cyt c reductase, which is used as a membrane-bound protein marker. This simple, rapid step allows full recovery of GLDH activity and allows easy analysis of GLDH activity in plant tissues.
Table I.
GLDH and NADPH-CCR activities in potato leaf extracts after precipitation of protein membranes with 20% (NH4)2SO4
| (NH4)2SO4 Fraction | GLDH | NADPH-Cyt c Reductase |
|---|---|---|
| nmol min−1 mg−1 protein | ||
| Pellet | 31.6 ± 2.4 (97.5%) | 4.65 ± 0.5 (94%) |
| Supernatant | 0.8 ± 0.14 (2.5%) | 0.3 ± 0.01 (6%) |
Values in parentheses are the percentage of enzyme activities. Values are means of three different experiments ± sd.
The relationship between GLDH and ascorbate content was studied in young, mature, and senescent potato leaves (Table II). Total foliar ascorbate concentration decreased dramatically with leaf age, being lowest in senescent leaves. The ascorbate/dehydroascorbate ratio also decreased significantly in senescent leaves. GLDH activity was highest in young leaves and decreased with age, being lowest in senescent leaves. Furthermore, when potato leaves from each leaf age were incubated in saturating (50 mm) GL for 3 h, GLDH activity was lower in mature and senescent leaves than in young leaves.
Table II.
Biosynthesis of ascorbate in potato leaves at different stages of development
| Leaf Age | Total Ascorbate Content | Indirect GLDH Activity | GLDH Activity | ||
|---|---|---|---|---|---|
| nmol disc−1 | nmol mg−1 fresh wt | nmol ascorbate disc−1 | nmol ascorbate mg−1 fresh wt | nmol min−1 mg−1 protein | |
| Young | 88 ± 1 (10%) | 4.51 ± 0.05 | 77.5 ± 6.5 | 4.0 ± 0.3 | 62.7 ± 5 |
| Mature | 53.2 ± 4 (21%) | 3.15 ± 0.2 | 55.6 ± 3 | 3.3 ± 0.2 | 29.2 ± 1.4 |
| Senescent | 8.9 ± 0.7 (68%) | 0.62 ± 0.05 | 10.2 ± 3 | 0.7 ± 0.2 | 15.2 ± 1.3 |
Indirect GLDH activity is measured as the difference in ascorbate content in whole leaves after incubation in 50 mm GL for 3 h. Values in parentheses indicate the percentage of DHA. Values represent the mean of three different experiments ± sd.
Subcellular fractionation of potato leaves yielded intact chloroplasts, microsomal and cytosolic fractions, as well as a fraction enriched in intact mitochondria and peroxisomes. These were characterized by the distribution of enzyme marker activities and chlorophyll contents (Table III). GLDH activity was mainly associated with the mitochondrial- and peroxisomal-enriched fraction, although over 20% of the enzyme activity was found in microsomes. The purity of the microsomal fraction was estimated by measuring NADPH-Cyt c reductase and Cyt c oxidase activities as microsomal and mitochondrial markers, respectively. Although there was a low contamination of microsomes by mitochondria, the rest of the GLDH activity was associated with the microsomal fraction. The GLDH activity found in chloroplasts and in cytosol was of the same order as the level of contamination. Therefore, GLDH was not present in these fractions (Table III).
Table III.
Distribution of GLDH activity in subcellular fractions of potato leaves
| Subcellular Fraction | GLDH | NADPH-Cyt c Reductase | Cyt c Oxidase | Chlorophylls |
|---|---|---|---|---|
| μmol min−1 mg−1 protein | mg mg−1 protein | |||
| Chloroplasts | 1.6 ± 1.0 | 0.3 ± 0.3 | 0.16 ± 0.02 | 390 ± 10 |
| (2,200g pellet) | (4.5%) | (5.47%) | (6%) | (80.7%) |
| Mitochondrial- and peroxisomal-enriched | 25.3 ± 3.2 | 0 | 2.3 ± 0.7 | 83.5 ± 5 |
| fraction (14,000g pellet) | (71.2%) | (83.48%) | (17%) | |
| Microsomes | 8.4 ± 1.2 | 5.0 ± 0.4 | 0.2 ± 0.01 | 8.3 ± 0.5 |
| (100,000g pellet) | (23.6%) | (91.24%) | (7.26%) | (1.7%) |
| Cytosol | 0.25 ± 0.13 | 0.18 ± 0.15 | 0.09 ± 0.03 | 1.4 ± 0.5 |
| (100,000g supernatant) | (0.7%) | (3.28%) | (3.27%) | (3.5%) |
Values between parentheses indicate a percentage of enzyme activities. Values are means of three different experiments ± sd.
Mitochondria and peroxisomes were separated in Percoll-density gradients, and the purity of each fraction was determined by measuring Cyt c oxidase and hydroxypyruvate reductase activities as marker enzymes of mitochondria and peroxisomes, respectively (Table IV). GLDH activity was found in mitochondria; the activity in peroxisomes was attributed to mitochondrial contamination.
Table IV.
Distribution of GLDH activity in intact mitochondria and peroxisomes from potato leaves
| Fraction | GLDH | Hydroxypyruvate Reductase | Cyt c Oxidase |
|---|---|---|---|
| nmol min−1 mg−1 protein | μmol min−1 mg−1 protein | ||
| Mitochondria | 22 ± 2 | nd | 1.7 ± 2 |
| Peroxisomes | 0.9 ± 0.1 | 76.3 ± 8 | 0.15 ± 0.05 |
Values are means of three different experiments ± sd. nd, Non-detected.
The localization of GLDH was further studied by comparing the latency of the enzyme together with the enzyme markers Cyt c oxidase and malate dehydrogenase (Table V). In intact mitochondria, GLDH and Cyt c oxidase activities were barely detectable and their latencies were over 85%, reflecting the high yield of intact organelles isolated by Percoll-density gradient. After disruption of the intact mitochondria with 0.15% (w/v) Triton X-100, GLDH and Cyt c oxidase latencies decreased to a similar extent, while malate dehydrogenase activity remained high, suggesting that GLDH localization is similar to Cyt c oxidase (Table V).
Table V.
Latency of GLDH, CCO, and MDH in mitochondria from potato leaves
| Fraction | GLDH | Cyt c Oxidase | Malate Dehydrogenase |
|---|---|---|---|
| % | |||
| Intact mitochondria | 95 ± 4 | 86 ± 3 | 88 ± 4 |
| HT mitochondria | 52 ± 4 | 41 ± 6 | 80 ± 6 |
Enzyme latencies are expressed as a percentage and calculated as described in “Materials and Methods.” HT, Mitochondria after swelling in hypotonic medium. Values are means of three different experiments ± sd.
Inner- and outer-membrane enriched fractions were prepared from intact leaf mitochondria, and Cyt c oxidase and antimycin A-resistant Cyt c reductase activities were used as inner and outer mitochondrial membrane markers, respectively (Table VI). By measuring GLDH/Cyt c oxidase and GLDH/antimycin A-resistant Cyt c reductase activity ratios, GLDH was found to be largely associated with the inner-membrane-enriched fraction.
Table VI.
GLDH activity in mitochondrial inner- and outer-membrane enriched fractions from potato leaves
| Fraction | CCO | A-CCR | GLDH | GLDH/CCO | GLDH/A-CCR |
|---|---|---|---|---|---|
| nmol Cyt c min−1 mg−1 protein | |||||
| Inner-membrane enriched | 260 ± 22 | 143 ± 60 | 14 ± 4 | 0.054 ± 0.01 | 0.11 ± 0.01 |
| Outer-membrane enriched | 108 ± 13 | 3100 ± 775 | 7 ± 3 | 0.065 ± 0.01 | 0.004 ± 0.001 |
Cyt c oxidase (CCO) and antimycin A-resistant Cyt c reductase (A-CCR) were used as inner and outer membrane markers, respectively. Values are means of three different experiments ± sd.
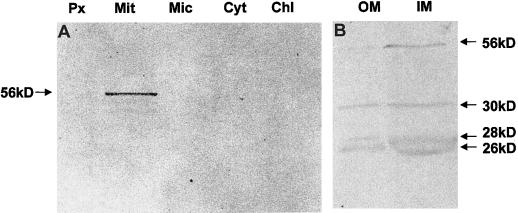
The localization of the GLDH protein was also determined by western-blot analysis using a specific antibody against GLDH from potato (Fig. 1). A polypeptide of 56 kD was detected only in the mitochondrial fraction with this antibody (Fig. 1A). Although GLDH activity was detected in microsomes, the antibody did not recognize polypeptides in this fraction. Similarly, the antibody did not detect polypeptides in chloroplasts, peroxisomes, or cytosol. Western analysis of mitochondrial inner- and outer-membrane-enriched fractions revealed that GLDH is localized in the mitochondrial inner membrane (Fig. 1B). The antibody detected proteins with molecular masses of 56, 30, 28, and 26 kD.
Figure 1.
Western blot of GLDH in potato leaf subcellular fractions (A) and mitochondrial inner- and outer-membrane-enriched fractions (B). Fifty micrograms of protein was loaded onto 12% (w/v) denaturing acrylamide gels, transferred to nitrocellulose membranes, and blotted against a specific antibody against GLDH from potato. Px, Peroxisomes; Mit, mitochondria; Mic, microsomes; Cyt, cytosol; Chl, chloroplasts; OM, mitochondrial outer-membrane-enriched fraction; IM, mitochondrial inner-membrane-enriched fraction.
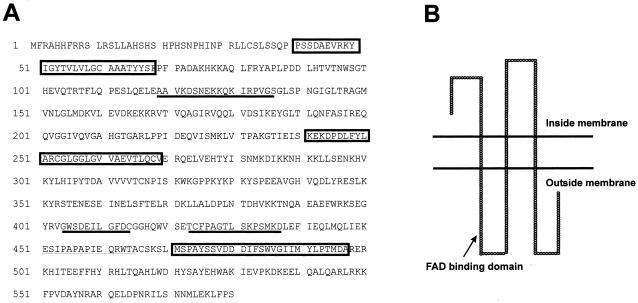
The predicted GLDH transmembrane regions and orientation in the inner mitochondrial membrane are shown in Figure 2. The analysis predicted three spanning membrane regions and a FAD-binding domain on the outside of the inside membrane. This suggests that the catalytic site of GLDH faces the outside of the inner mitochondrial membrane. Analysis of absorption spectra suggested the presence of a flavin prosthetic group associated with purified sweet potato GLDH (Imai et al., 1998). The amino acid sequence of polypeptides generated after partial digestion with V8-protease predicts a similar binding domain.
Figure 2.
Predicted inner mitochondrial membrane-spanning region of GLDH. GLDH transmembrane regions and orientation were predicted using the Tmpred program. A, GLDH protein sequence from sweet potato (Imai et al., 1998). Areas included in boxes are membrane-spanning regions, while those underlined are peptides generated after digestion with a V8 protease. The Leu at position 137 indicates the normal position for the FAD-binding domain, and is located on the outside of the inner mitochondrial membrane. B, Predicted inner mitochondrial membrane-spanning region of GLDH.
The localization of GLDH in the mitochondrial inner membrane and its specificity for Cyt c suggested the possible involvement of GLDH in the mitochondrial respiratory electron transport chain. To explore this possibility, respiration rates and ascorbate production were estimated in intact mitochondria isolated from potato tubers (Table VII). Potato tubers were selected for these assays because they could be isolated in large numbers, are free of chlorophylls, and contain a similar pattern of localization of GLDH to potato leaves (data not shown). Respiration rates in the presence of ADP and NADH were high, but they were increased over 20% when 5 mm GL was present in the respiration medium. While the production of ascorbate by intact mitochondria was undetectable in control conditions, the presence of GL in the medium provoked a marked increase in ascorbate production, indicating that isolated mitochondria are able to synthesize ascorbate at a high rate through GLDH activity (Table VII).
Table VII.
Effect of GL and mitochondrial respiratory chain inhibitors on respiration rate and ascorbate production in isolated mitochondria from white potato tubers
| Treatment | Oxygen Uptake | Respiratory Control | Ascorbate Production |
|---|---|---|---|
| % of control | nmol mg−1 protein | ||
| Control | 100 | 2.12 ± 0.04 | 0 |
| + GL | 122 | 2.04 ± 0.04 | 453 ± 41 |
| + GL + Antimycin A | 25 | nd | 571 ± 32 |
| + GL + KCN | 10 | nd | 0 |
Respiration was measured after incubation of intact mitochondria in buffer containing 5 mm GL and 5 μg/mL antimycin A or 1 mm KCN for 90 min. Values represent the mean of three different experiments ± sd. nd, Non-detected.
The respiratory inhibitors antimycin A and KCN provoked a decrease of respiration in the absence or presence of GL. However, ascorbate production was stimulated by antimycin A, an inhibitor of Cyt c reductase at the level of complex III. Rotenone, an inhibitor of complex I, did not decrease ascorbate production, although the presence of other NADH dehydrogenases resistant to rotenone resulted in only a partial inhibition of mitochondrial respiration (data not shown). In contrast, KCN, a potent inhibitor of Cyt c oxidase in complex IV, provoked complete inhibition of ascorbate synthesis. The inhibition of ascorbate production by KCN was not due to a decrease of GLDH activity, since KCN did not inhibit this enzyme in the in vitro assay (data not shown).
To confirm that GL is an electron donor to the mitochondrial respiration, oxygen uptake was measured in intact mitochondria incubated with 5 mm GL without ADP and NADH (Table VIII). In the absence of ADP and NADH, GL provoked an approximately 35% increase in the respiration rate in all experiments.
Table VIII.
Mitochondrial respiration in the presence of GL as the only source of electrons in isolated mitochondria from white potato tubers
| Treatment | Mitochondrial Respiration |
|---|---|
| nmol O2 min−1 mg−1 protein | |
| Control (no ADP, no NADH) | 8.6 ± 0.85 |
| + GL | 13.4 ± 1.0 |
| + GL + ADP + NADH | 43.0 ± 6.1 |
Respiration was measured in intact mitochondria in the presence of 5 mm GL. Respiratory control = 2.15. Values represent the mean of three different experiments.
DISCUSSION
The mitochondrial inner membrane is one of the most complex biological membranes. It is a highly specialized system for oxidative phosphorylation and energy-linked ion translocation (Douce, 1985). Energy capture, transduction, and utilization are achieved in a number of reactions that are energetically interlinked and involve at least several hundred polypeptides. In the present study we analyzed the function of GLDH, a protein bound to the inner mitochondrial membrane, and its interaction with the respiratory electron transport chain. We also demonstrated that isolated intact mitochondria produce ascorbate, as suggested by the early studies on crude mitochondria preparations (Mapson and Breslow, 1958).
Foliar ascorbate and GLDH activity decrease with leaf age, with the lowest levels found in senescent leaves. The turnover of ascorbate in leaves is clearly regulated by developmental triggers. The loss of GLDH activity with leaf age implies that ascorbate synthesis is decreased as leaves mature and senesce. Similarly, the increase in the amount of oxidized ascorbate with age implies decreased capacity for reduction. Whether the loss of GLDH is due to decreased transcription/translation or increased protein turnover is unknown; however, age-dependent losses in GLDH and ascorbate will render the leaf less able to combat oxidation. Therefore, senescent leaves are more susceptible to oxidative stress.
GLDH activity was found in mitochondria and microsomes isolated from potato leaves and tubers. Our results on the localization of GLDH in mitochondria are in agreement with previous studies, such as the pioneering work of Mapson and Breslow (1958), who reported the requirement of these organelles for the biosynthesis of ascorbate, and with Ôba et al. (1994), Mutsuda et al. (1995), and Arrigoni et al. (1997), who demonstrated the presence of GLDH in mitochondria from potato tubers, spinach leaves, bean embryos, and maize seedlings, respectively. GLDH was found to be associated with the mitochondrial inner membrane of kidney bean hypocotyls (Siendones et al., 1999).
The data presented in this manuscript confirm the localization of GLDH activity on the inner mitochondrial membrane and provide corroborative evidence by detection of the protein on western blots. Bands of low Mr detected by the antibody could be products of GLDH degradation, as reported by Imai et al. (1998). Since the antibody did not recognize polypeptides in the microsomes, even though 20% of the total foliar activity was localized with this fraction, it would appear that an epitopically distinct GLDH form resides in the microsomes. The antibody did not detect polypeptides in membranes of other subcellular compartments, confirming that GLDH is absent from other sites in the plant cell. The natural electron acceptor for the microsomal GLDH form is unknown, but the involvement of other cytochromes present in microsomal fractions suggests that electron donation may not be limited to Cyt c. Microsomal GLDH may use l-gulono-γ-lactone as a substrate. This would explain why purified GLDH is specific for GL and does not catalyze the oxidation of l-gulono-γ-lactone (Ôba et al., 1995), while intact tissues can oxidize GL and l-gulono-γ-lactone. This was demonstrated in Arabidopsis cell suspension cultures incubated with a range of potential biosynthetic precursors of ascorbic acid (Davey et al., 1999). It is interesting that cytosolic expression of rat liver l-gulono-γ-lactone oxidase in potato (T. Imai, A. Kingston-Smith, and C.H. Foyer, unpublished results), lettuce, and tobacco plants results in increased ascorbate biosynthesis (Jain and Nessler, 2000). This enzyme must have access to an alternative electron acceptor, since there has been no attempt to target this enzyme to the inner mitochondrial membrane in these studies.
GL may diffuse through the outer mitochondrial membrane, which is highly permeable to solutes such as Suc, nucleotides, and NAD+, while a specific ascorbate transporter may be required for transport across the inner membrane, which is impermeable to Suc but selectively permeable to a limited number of anions, depending on the orientation of GLDH. While the transport of dehydroascorbate is mediated by Glc transporters in animals, ascorbate is transported across cellular membranes by a different mechanism, as demonstrated in Xenopus laevis oocytes (Rumsey et al., 1997). While analogous systems have yet to be characterized in plants, several transport systems have been described (Horemans et al., 1999). According to the predicted localization, GLDH spans the inner mitochondrial membrane, but no transporters would be required, since the catalytic site of GLDH faces the intermembrane space.
Plant mitochondria are considerably more complex than animal mitochondria. Apart from producing ATP through an electron transport chain, plant mitochondria interact with chloroplasts to produce a wealth of primary and secondary metabolites (some specifically in response to stress), and participate in photorespiration (for review, see Douce, 1985; Mackenzie and McIntosh, 1999). Respiration involves the transfer of electrons from organic molecules to oxygen through four respiratory complexes located in the inner mitochondrial membrane (Fig. 3). Unlike animals, respiratory oxygen consumption in plants is not completely inhibited by cyanide because of the presence of a cyanide-resistant, alternative respiratory pathway that drives electrons from ubiquinone to oxygen in the inner mitochondrial membrane, by-passing the cytochromes in complexes III and IV. Respiration via the alternative pathway only proceeds in the presence of high concentrations of respiratory substrates (Lambers, 1982), and is up-regulated by many types of stress, including exposure to cold, pathogen attack, drought, and wounding (Mackenzie and McIntosh, 1999). A principal function may be prevention of excessive production of superoxide and hydrogen peroxide (Wagner and Moore, 1997).
Figure 3.
Hypothetical model of the interaction between GLDH and the mitochondrial electron transport chain. GLDH feeds electrons into Cyt c between complexes III and IV, while GL is converted into ascorbate. The inhibition of Cyt c oxidase by KCN in complex IV blocks ascorbate production, possibly by the accumulation of electrons in Cyt c. AOX, Alternative oxidase; DH, dehydrogenase; UQ, ubiquinone.
The presence of GLDH in the inner mitochondrial membrane and its capacity to donate electrons to the mitochondrial electron transport chain during ascorbate biosynthesis contribute to the complexity of plant mitochondrial respiration (Fig. 3). GL is able to function as an alternative electron donor, allowing GLDH to reduce Cyt c between complexes III and IV. GLDH is not inhibited by KCN, but ascorbate synthesis is completely blocked when this inhibitor is added to mitochondria. This suggests that the inhibition of Cyt c oxidase activity by KCN inhibits ascorbate synthesis in situ due to a lack of an available electron acceptor. Therefore, intact mitochondria cannot synthesize ascorbate when the electron acceptor Cyt c is fully reduced.
A pivotal question concerns the relative rates of flux of electron transport driven by ascorbate synthesis and that driven by the activity of the tricarboxylic acid cycle. Since GLDH may not be the rate-limiting step of ascorbate synthesis, the supply of GL may frequently be limiting. However, in situations of elicit rapid ascorbate turnover, GLDH activity may be high enough to impact on the rate of respiration.
MATERIALS AND METHODS
Plant Material
Potato (Solanum tuberosum L. cv Solara) plants were grown for 4 weeks in a growth chamber at 20°C with a 16-h photoperiod (photosynthetic photon flux density of 300 μmol m−2 s−1). Plants were placed in 5-L pots containing a soil mixture. Leaves were selected at three developmental stages: young (50% expansion); mature (full expansion); and senescent (yellowish expanded). White potato tubers, obtained from a local store, were used for mitochondrial respiration studies.
Subcellular Fractionation
The following procedures were carried out at 4°C. Mature potato leaves were homogenized in extraction medium (30 mm 3-[N-morpholino]-propanesulfonic acid [MOPS], pH 7.5, 0.2% [w/v] bovine serum albumin [BSA], 4 mm Cys, 0.35 m mannitol, 1% [w/v] PVP-40, 25 mm Na4P2O7, and 2 mm EDTA) at a ratio of 1 g of tissue per 5 mL of extraction medium, in a homogenizer. The homogenate was filtered through four layers of cotton cloth and centrifuged at 2,200g for 5 min to obtain the chloroplast fraction. Chloroplasts were resuspended in 10 mL of washing medium (20 mm MOPS, pH 7.2, 0.2% BSA [w/v], 0.3 m mannitol, and 1 mm EDTA), and centrifuged at the same speed once more. The first 2,200g supernatant was centrifuged at 14,000g for 15 min to yield a fraction enriched in mitochondria and peroxisomes. This pellet was resuspended in washing medium, and centrifuged again at 14,000g before being loaded onto a gradient consisting of a bottom layer of 15 mL of 28% (w/v) Percoll in 10 mm MOPS, pH 7.2, 0.3 m Suc, and 0.1% (w/v) BSA, and a top layer of 21 mL of the same solution with mannitol instead of Suc (Jiménez et al., 1997). After centrifugation at 41,400g for 35 min, intact mitochondria and peroxisomes were washed twice by a 10-times dilution in washing medium and centrifugation at 17,400g for 15 min. Mitochondria from white potato tubers were isolated using the same method as for leaf mitochondria.
For isolation of microsomal and cytosolic fractions, the first 14,000g supernatant was centrifuged at 100,000g for 1 h.
Isolation of Mitochondrial Outer and Inner Membranes
Outer- and inner-membrane-enriched fractions from potato leaf mitochondria were isolated as described by Mannella (1987). In this method, intact mitochondria are exposed to successively greater hypotonic shocks, which increases succinate-Cyt c oxidoreductase and malate dehydrogenase activities as the outer and inner membranes are being disrupted, respectively. Intact mitochondria isolated from potato leaves were swollen in 12 mm mannitol for 30 min, a concentration suitable to separate the outer membrane without disrupting the inner membrane, and then loaded in a 0.6 to 0.9 m Suc-step gradient, which was then centrifuged in a swinging bucket rotor at 40,000g for 60 min. Outer membranes were recovered from the supernatant fraction between the bottom and the Suc interphase. Inner membranes and remaining intact mitochondria were collected from the bottom of the tube. Both fractions were diluted at least three times in a 20 mm MOPS, pH 7.2, and 0.3 m mannitol, and centrifuged at 60,000g for 1.5 h. Pellets consisting of mitochondrial outer- and inner-membrane enriched fractions were resuspended in small volumes of the same medium.
Ascorbate Determination
Ascorbate was measured as described by Foyer et al. (1983). Fifty milligrams of leaf tissues were homogenized in 0.5 mL of 1 m HClO4 with mortar and pestle in an ice bath and centrifuged at 13,000g for 5 min at 4°C. 0.1 m 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)/KOH buffer, pH 7.0, was added to the supernatants at a ratio of 1:5 (buffer:extract), neutralized with 1 m K2CO3 to pH 5.6, and centrifuged again as above. Ascorbate was measured as the difference of A265 before and after the addition of ascorbate oxidase.
Enzyme Assays
GLDH (EC 1.3.2.3) activity was assayed by the reduction of Cyt c at 550 nm (ε = 21 mm−1 cm−1) in a medium consisting of 50 mm Tris-HCl, pH 8.0, 60 μm Cyt c, 0.15% (w/v) Triton X-100, and 25 to 50 μg of sample protein (Ostergaard et al., 1997). GLDH activity was also estimated indirectly by incubating two leaf discs in 50 mm GL or in water as a control for 3 h. Indirect GLDH activity was calculated as the difference in ascorbate content between GL-treated and control leaf discs.
Cyt c oxidase (EC 1.9.3.1) activity was measured by incubating 5 to 10 μg of protein of each sample in 30 mm MOPS, 0.3 m mannitol, and 50 μm reduced Cyt c, and following the oxidation of Cyt c (Tolbert et al., 1968).
NADPH-Cyt c reductase (EC 1.6.2.4) activity was determined by the reduction of Cyt c in a reaction mixture containing 0.3 m potassium phosphate buffer, pH 7.6, 20 μm Cyt c, 0.1 mm NADPH, and 10 to 50 μg of sample protein (Simontachi et al., 1992).
Antimycin A-resistant Cyt c reductase (EC 1.6.99.3) activity was estimated by the reduction of Cyt c in a reaction mixture containing 20 mm MOPS, pH 7.0, 1 mm KCN, 1 mm NADH, 1 μg/mL antimycin A, 50 μm Cyt c, and 10 to 25 μg of sample protein (Mannella, 1987).
Malate dehydrogenase (EC 1.1.1.82) activity was measured as the oxidation of NADH at 340 nm (ε = 6.22 mm−1 cm−1) in a medium consisting of 20 mm MOPS, pH 7.2, 1 mm oxalacetate, 1 mm KCN, 0.2 mm NADH, and 10 to 50 μg of sample protein (Moller et al., 1987).
Hydroxypyruvate reductase (EC 1.1.1.29) activity was determined by the oxidation of NADH in a reaction mixture containing 69 mm potassium phosphate buffer, pH 6.2, 0.2 mm NADH, 1 mm KCN, 2.0 mm hydroxypyruvate, and 10 to 50 μg of sample protein (Schwitzguébel and Siegenthaler, 1984).
Enzyme Latency Determination
The enzyme latency was determined in intact mitochondria in a reaction mixture containing 0.3 m mannitol by assaying the enzyme activities before and after the addition of 0.025% (v/v) Triton X-100. The latency was calculated with the following formula (Burgess et al., 1985):
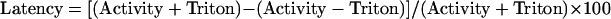 |
Improved Extraction and Assay for GLDH Activity
An improved method for the assay of GLDH activity in potato leaf crude homogenates was devised: 10 g of potato leaves were ground in 50 mL of extraction buffer, filtered through four layers of cheesecloth, and centrifuged at 2,200g for 5 min. Twenty percent (w/v) (NH4)2SO4 was added to the 2,200g supernatants, incubated at 4°C for 30 min under continuous stirring, and centrifuged at 10,000g for 20 min. The pellet containing membrane-bound proteins was resuspended in 1 mL of washing medium without BSA. GLDH activity was measured in the resuspended pellet after a semi-purification step through Sephadex G-25.
SDS-PAGE and Western Blotting
Fifty-milligram protein samples of each subcellular fraction were incubated at 95°C for 4 min in sample buffer containing 62.5 mm Tris-HCl, pH 6.8, 10% (v/v) glycerol, 2% (w/v) SDS, 5% (v/v) 2-β-mercaptoethanol, and 0.0025% (w/v) bromophenol blue. Samples were loaded on a 12% (w/v) denaturing polyacrylamide gel and electrophoresed at 25 mA/gel for 1.5 h. For western blotting, proteins were electrotransferred to a nitrocellulose membrane at 70 V for 1 h. Blots were blocked in 5% (w/v) non-fat dry milk dissolved in PBS (10 mm phosphate buffer, pH 7.4, 2.7 mm KCl, and 137 mm NaCl) for 2 h, and probed with a polyclonal antibody against GLDH from potato tubers for 1 h. After washing the membrane three times for 10 min each with PBS-T (PBS plus 0.05% [w/v] Tween 20) blots were incubated with a secondary antibody against goat anti-rabbit IgG conjugated to alkaline phosphatase for 1 h, washed with PBS-T, and developed with a colorimetric assay consisting of 0.15 mg/mL 5-bromo-4-chloro-3-indolyl phosphate, 0.3 mg/mL nitroblue tetrazolium, 100 mm Tris-HCl buffer, pH 9.5, and 5 mm MgCl2 (Sigma, Dorset, UK).
Ascorbate Production by Whole Leaves and Intact Mitochondria
Whole leaves were incubated in 50 mm GL for 3 h using water as a control. Intact mitochondria were incubated in the medium used for respiration studies consisting of 20 mm MOPS, pH 8.0, 0.3 m mannitol, 1 mm MgCl2, 5 mm K2HPO4, 10 mm KCl, plus 2 mm GL for 15 to 90 min at room temperature. The reaction was stopped by the addition of HClO4, and the ascorbate content was assayed immediately.
Measurements of Mitochondrial Respiration Rate
Respiration experiments were carried out in intact mitochondria isolated from white potato tubers for an accurate assay of respiratory rates without the interference of chlorophylls. Oxygen uptake was recorded with an oxygen electrode (Hansatech, King's Lynn, UK). Mitochondrial respiration was measured at pH 8.0, since lower pHs inhibit GLDH activity. Oxygen uptake was estimated in a medium containing 20 mm MOPS, pH 8.0, 0.3 m mannitol, 1 mm MgCl2, 5 mm K2HPO4, and 10 mm KCl, followed by the addition of 2 mm NADH and 0.1 mm ADP. Rates of respiration in mitochondria from potato tubers were similar to those reported in the literature. The possible interaction of GLDH with the mitochondrial respiratory electron transport chain was studied using 5 μg/mL antimycin A and 1 mm KCN as inhibitors of complexes III and IV, respectively.
Chlorophyll and Protein Determinations
Chlorophylls and proteins were measured according to the methods of Arnon (1949) and Bradford (1976), respectively.
Analysis of GLDH Structure
GLDH transmembrane regions and orientation were predicted with the program TMpred (Hofmann and Stoffel, 1993), which predicts protein membrane-spanning regions and their orientation. The algorithm is based on the statistical analysis of TMbase, a database of naturally occurring transmembrane proteins. The prediction is made using a combination of several weight matrices for scoring. The prediction of GLDH was based in the protein sequence deduced from GLDH cDNA from sweet potato (Imai et al., 1998; accession no. AB017357).
ACKNOWLEDGMENTS
The authors wish to thank Drs. M. Nishikimi and T. Imai for the antibody against GLDH. The authors are indebted to Guy Kiddle, Prof. Verrier, and the BioInformatics Department at IACR-Rothamsted for invaluable assistance in the computer analysis of GLDH orientation within the inner mitochondrial membrane.
Footnotes
C.B. acknowledges financial support from the British Council, Consejo Nacional de Investigaciones Científicas y Técnicas, and Fundacion Antorchas at IACR-Rothamsted.
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris L. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni O, De Gara L, Paciolla C, Evidente A, de Pinto MC, Liso R. Lycorine: a powerful inhibitor of l-galactono-γ-lactone dehydrogenase activity. J Plant Physiol. 1997;150:362–364. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buettner GR, Jurkiewicz BA. Chemistry and biochemistry of ascorbic acid. In: Cadenas E, Packer L, editors. Handbook of Antioxidants. New York: Marcel Dekker; 1996. pp. 91–115. [Google Scholar]
- Burgess N, Beakes G, Thomas D. Separation of mitochondria from microbodies of Pisum sativum L. cv. Alaska cotyledons. Planta. 1985;166:151–155. doi: 10.1007/BF00397341. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M, Gilot C, Persiau G, Østergaard J, Han Y, Bauw G, Van Montagu M. Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol. 1999;121:535–543. doi: 10.1104/pp.121.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gara L, de Pinto MC, Arrigoni O. Ascorbate synthesis and ascorbate peroxidase activity during the early stage of wheat germination. Physiol Plant. 1997;100:894–900. [Google Scholar]
- Douce R. Mitochondria in Higher Plants. American Society of Plant Physiologists Monograph Series. London: Academic Press; 1985. Functions of plant mitochondrial membranes; pp. 77–153. [Google Scholar]
- Foyer CH, Harbinson J. Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer C, Mullineaux P, editors. Causes of Photooxidative Stresses and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 1–42. [Google Scholar]
- Foyer CH, Rowell J, Walker D. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Stoffel W. TM-base: a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler. 1993;347:166–172. [Google Scholar]
- Horemans N, Foyer C, Anard H. The functions of ascorbate and ascorbate transport systems in plant membranes. In: Denke A, Dornisch K, Fleischmann F, Grassman J, Heiser I, Hippeli S, Osswald W, Schempp H, editors. Different Pathways through Life. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 217–238. [Google Scholar]
- Imai T, Karita S, Shiratori G, Hattori M, Nunome T, Ôba K, Hirai M. l-Galactono-γ-lactone dehydrogenase from sweet potato: purification and cDNA sequence analysis. Plant Cell Physiol. 1998;39:1350–1358. doi: 10.1093/oxfordjournals.pcp.a029341. [DOI] [PubMed] [Google Scholar]
- Jain A, Nessler C. Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol Breed. 2000;6:73–78. [Google Scholar]
- Jiménez A, Hernández JA, del Río LA, Sevilla F. Role of ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1997;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk N, Feldman L. A biochemical model for initiation and maintenance of the quiescent centre: implications for organisation of root meristems. Plant Dev. 1995;121:2825–2833. [Google Scholar]
- Lambers H. Cyanide resistance respiration: a nonphosphorylating electron transport pathway acting as an energy overflow. Plant Physiol. 1982;55:478–485. [Google Scholar]
- Mackenzie S, McIntosh L. Higher plant mitochondria. Plant Cell. 1999;11:571–585. doi: 10.1105/tpc.11.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA. Isolation of the outer membrane of plant mitochondria. Methods Enzymol. 1987;148:453–464. [Google Scholar]
- Mapson LW, Breslow E. Biological synthesis of ascorbic acid: l-galactono-γ-lactone dehydrogenase. Biochem J. 1958;68:395–406. doi: 10.1042/bj0680395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller IM, Liden AC, Ericson I, Gardeström Isolation of submitochondrial particles with different polarities. Methods Enzymol. 1987;148:442–452. [Google Scholar]
- Mutsuda M, Ishikawa T, Takeda T, Shigeoka S. Subcellular localization and properties of l-galactono-γ-lactone dehydrogenase in spinach leaves. Biosci Biotechnol Biochem. 1995;59:1983–1984. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Ôba K, Fukui M, Imai Y, Iriyama S, Nogaru K. l-Galactono-γ-lactone dehydrogenase: partial characterization, induction of activity and role in synthesis of ascorbic acid in wounded white potato tuber tissue. Plant Cell Physiol. 1994;35:473–478. [Google Scholar]
- Ôba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T. Purification and properties of l-galactono-γ-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis, from sweet potato roots. J Biochem. 1995;117:120–124. doi: 10.1093/oxfordjournals.jbchem.a124697. [DOI] [PubMed] [Google Scholar]
- Ostergaard J, Persiau G, Davey MW, Bauw G, Van Montagu M. Isolation of a cDNA coding for l-galactono-γ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. J Biol Chem. 1997;272:30009–30016. doi: 10.1074/jbc.272.48.30009. [DOI] [PubMed] [Google Scholar]
- Padh H. Cellular functions of ascorbic acid. Biochem Cell Biol. 1990;68:1166–1173. doi: 10.1139/o90-173. [DOI] [PubMed] [Google Scholar]
- Rumsey S, Kwon O, Xu G, Burant C, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997;272:18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- Schwitzguébel JP, Siegenthaler PA. Purification of peroxisomes and mitochondria from spinach leaf by Percoll-density gradient centrifugation. Plant Physiol. 1984;75:670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MK, Buettner GR. Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radic Biol Med. 1993;14:649–653. doi: 10.1016/0891-5849(93)90146-l. [DOI] [PubMed] [Google Scholar]
- Siendones E, González-Reyes J, Santos-Ocaña C, Navas P, Córdoba F. Biosynthesis of ascorbic acid in kidney bean: l-galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiol. 1999;120:907–912. doi: 10.1104/pp.120.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simontachi M, Puntarulo S. Oxygen radical generation by isolated microsomes from soybean seedlings. Plant Physiol. 1992;100:1263–1268. doi: 10.1104/pp.100.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78:661–669. [Google Scholar]
- Tolbert NE, Oeser A, Kisaki T, Hageman RH, Yamazaki RK. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968;243:5179–5184. [PubMed] [Google Scholar]
- Wagner A, Moore A. Structure and function of the plant alternative oxidase: its putative role in the oxygen defense mechanism. Biosci Rep. 1997;17:319–333. doi: 10.1023/a:1027388729586. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–368. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]