Abstract
The fourth member of the leucine-rich repeat–containing GPCR family (LGR4, frequently referred to as GPR48) and its cognate ligands, R-spondins (RSPOs) play crucial roles in the development of multiple organs as well as the survival of adult stem cells by activation of canonical Wnt signaling. Wnt/β-catenin signaling acts to regulate breast cancer; however, the molecular mechanisms determining its spatiotemporal regulation are largely unknown. In this study, we identified LGR4 as a master controller of Wnt/β-catenin signaling–mediated breast cancer tumorigenesis, metastasis, and cancer stem cell (CSC) maintenance. LGR4 expression in breast tumors correlated with poor prognosis. Either Lgr4 haploinsufficiency or mammary-specific deletion inhibited mouse mammary tumor virus (MMTV)-PyMT- and MMTV-Wnt1–driven mammary tumorigenesis and metastasis. Moreover, LGR4 down-regulation decreased in vitro migration and in vivo xenograft tumor growth and lung metastasis. Furthermore, Lgr4 deletion in MMTV-Wnt1 tumor cells or knockdown in human breast cancer cells decreased the number of functional CSCs by ∼90%. Canonical Wnt signaling was impaired in LGR4-deficient breast cancer cells, and LGR4 knockdown resulted in increased E-cadherin and decreased expression of N-cadherin and snail transcription factor -2 (SNAI2) (also called SLUG), implicating LGR4 in regulation of epithelial–mesenchymal transition. Our findings support a crucial role of the Wnt signaling component LGR4 in breast cancer initiation, metastasis, and breast CSCs.—Yue, Z., Yuan, Z., Zeng, L., Wang, Y., Lai, L., Li, J., Sun, P., Xue, X., Qi, J., Yang, Z., Zheng, Y., Fang, Y., Li, D., Siwko, S., Li, Y., Luo, J., Liu, M. LGR4 modulates breast cancer initiation, metastasis, and cancer stem cells.
Keywords: mammary tumorigenesis, tumor progression, epithelial–mesenchymal transition, Wnt
Breast cancer is a severe health problem in industrialized nations, with 2015 having an estimated 232,000 new cases in the United States (1) and ∼268,600 new cases in China (2). Effective therapies are available for some subtypes of breast cancer, but after metastatic spread, clinical options are more limited. Efforts to develop new therapies have been hampered by our incomplete understanding of cancer progression and metastasis.
Wingless type (Wnt)-1 was initially identified as an oncogene in mice (3–5), and activating mutations in Wnt pathway members occur in up to 50% of patients with breast cancer (6, 7). Furthermore, Wnt signaling is implicated in maintenance of mammary stem cells (8, 9) and breast cancer stem cells (CSCs) (10, 11). The Wnt pathway is complex, with different effects, depending on the cellular context (12), and the pathways upstream of Wnt activation in breast cancer are still to be elucidated.
The leucine-rich repeat containing GPCR-4 (LGR4, or GPR48) has been implicated in numerous physiologic processes, including kidney (13), eye (14, 15), prostate (16), bone (17, 18), gall bladder (19), and reproductive organ (20, 21) development, macrophage inflammation (22), electrolyte balance (23), energy balance (24), and liver metabolism (25). As a classic GPCR, LGR4 is able to activate the Gαs/PKA/cAMP response element binding protein (CREB) pathway following binding of an as-yet-unidentified ligand (14, 23, 26). In addition, LGR4, together with its family members LGR5 and -6, binds to R-spondins (RSPOs)-1–4 and results in Wnt signaling potentiation (27, 28). Crucially, mice lacking LGR4 have disrupted mammary ductal morphogenesis (29) and defective mammary stem cells (30), suggesting a key role for LGR4 in mammary gland development.
In addition, LGR4 (as well as LGR5 and -6) is implicated in multiple cancers and has a potential role in cancer progression. LGR4 promotes invasion and metastasis in colorectal, prostate, and cervical cancer cell lines (31–34). Overexpression of the LGR4 ligand RSPO-3 is frequent in Kelch-like ECH-associated protein-1–deficient lung adenocarcinomas and predicts poor survival (35, 36). Curiously, humans carrying a nonsense mutation of LGR4 are at elevated risk for bile duct cancer and skin squamous cell carcinoma (37), suggesting a tumor-suppressive role for LGR4 in those organs. However, the function of LGR4 in breast cancer has not been evaluated, and its role in early tumorigenesis of various cancers is unknown. Meanwhile, whether LGR4 activation of the Gαs/CREB pathway or the Wnt pathway is the crucial molecular mechanism in tumorigenesis and progression remains unresolved.
We investigated the effect of Lgr4 loss on mammary tumorigenesis, metastasis, and breast CSCs. High LGR4 expression in human breast cancers was correlated with poorer patient survival. Using 2 Lgr4-deficient mouse models [Lgr4 mammary-specific conditional knockout (CKO) and whole-body Lgr4 heterozygosity], we found that loss of Lgr4 expression delayed mammary tumor occurrence, progression, and metastasis formation. LGR4 positively regulated in vitro migration and invasion in seven different breast cancer cell lines. LGR4 knockdown reduced MDA-MB-231 xenograft tumor size and proliferation and impaired lung metastasis formation in nude mice. Molecular analysis demonstrated that Wnt/β-catenin signaling was abrogated by LGR4 knockdown, with decreased expression of epithelial–mesenchymal transition (EMT) markers. Finally, LGR4 down-regulation or loss resulted in a ∼90% decline in the number of CSCs in both MDA-MB-231 human breast cancer cells and the mouse mammary tumor virus (MMTV)-Wnt1 transgenic mouse model. Our findings support a crucial role for LGR4 in human breast cancer progression, metastasis, and CSCs.
MATERIALS AND METHODS
Animal studies
All experiments using mice were approved by the East China Normal University Animal Care and Use Committee. MMTV-driven polyoma middle T antigen (PyMT) mice (FVB/N background) (38) and MMTV-Wnt1 mice (FVB/N background) (39) have been described. MMTV-Cre mice (FVB/N background) were purchased from the Nanjing University Model Animal Research Center. Female Lgr4 knockout mice (129 × C57BL/6 background) (14), which had a gene trap vector (consisting of a splice acceptor, CD4 transmembrane domain, β-galactosidase, internal ribosome entry site, and placental alkaline phosphatase coding sequence) inserted into intron-1 of the mouse Lgr4 gene, were backcrossed 10 generations to FVB/N mice. Lgr4fl/fl mice (C57BL/6 background) (18) were backcrossed 6 generations to FVB/N mice. PyMT genotyping was performed according to the protocol published by Jackson Laboratories (Bar Harbor, ME, USA). Female mice were palpated weekly for mammary tumors. The volume of tumors was determined through the equation (length × width2)/2. The volumes of palpable tumors were calculated in 8-wk-old mice (Lgr4 haploinsufficiency or Lgr4-CKO). Fifteen (Lgr4 haploinsufficiency)- or 12 (Lgr4-CKO)-wk-old mice were euthanized, and the lungs were isolated for detecting the metastatic foci.
Mammary gland whole mount analysis
For whole mount analysis, after euthanasia the 4th mammary glands were fixed for 4 h in a jar containing Carnoy’s fixative (60% ethanol, 30% chloroform, and 10% glacial acetic acid) at room temperature. The glands were then rehydrated and stained in carmine alum (1 g carmine, 2.5 g aluminum potassium sulfate in 500 ml distilled water, boiled for 20 min) overnight at room temperature. Whole-gland β-galactosidase (LacZ) staining was performed as has been described in Luo et al. (18).
Cell culture
MDA-MB-231 cells (luciferase-labeled) were obtained from Caliper Life Sciences (Waltham, MA, USA). MDA-MB-453, T47D, MCF-7, MCF-10A, BT-474, BT549, and MDA-MB-468 cell lines were purchased from the Chinese Academy of Sciences Committee Type Culture Collection Cell Bank (Shanghai, China) with authentication. Cell culture was performed according to the methods recommended by American Type Culture Collection (Manassas, VA, USA). MCF10A-ErbB2-14,3,3 cells were a present from Dihua Yu (M. D. Anderson Cancer Center, Houston, TX, USA) and were cultured as described in Lu et al. (40).
Real-time quantitative PCR
Cells were lysed in Trizol (Thermo Fisher Scientific, Waltham MA, USA) and RNA was extracted. Real-time quantitative (q)PCR was performed as described in the operating instructions of SYBR premix Ex Taq (Takara, Kyoto, Japan). Primers were designed with the Primer premier 5.0 software package. The primers of human LGR4 and mouse Lgr4 primers are from 2 publications (18, 41). Primers for candidate downstream target analysis are listed in Supplemental Table 1.
Immunoblot analysis
Cells were lysed in SDS loading buffer and proteins separated using SDS-PAGE. Proteins were then transferred from the gel to nitrocellulose membranes (Millipore-Sigma, Billerica, MA, USA). The membranes were then blocked in 5% non–fat-dried milk diluted in PBS (w/v) for 1 h at room temperature, after which the membrane incubation was performed at 4°C with indicated primary antibodies overnight. The membranes were washed 3 times in PBS-Tween, incubated with the secondary antibody for 1 h, the membrane was washed and imaged by the Odyssey System (Li-Cor Biosciences, Lincoln, NE, USA). All of the antibodies used are listed in Supplemental Table 2.
Plasmids, small interfering RNA, transfection
The nontargeting control short hairpin RNA (shRNA) (control, 5′-GTTCTCCGAACGTGTCACGTT-3′) and 2 LGR4 shRNA sequences (shLGR4 #1, 5′-GCGTAATCAAATCTACCAAAT-3′; shLGR4 #2, 5′-GGTACTGCTGATGCAGCAAAT-3′) targeting LGR4 mRNA were inserted into the lentiviral vector pLKO.1-puro cut by AgeI/EcoRI sites. One small interfering RNA (siRNA) sequence targeting human LGR4 was adopted from a previous publication (27) (siLGR4 #1, 5′-GAAAGUAAACUGUGGUCAAUU-3′, 5′-UUGACCACAGUUUACUUUCUU-3′), the other was self-designed targeting the 3′UTR (siLGR4 #2, 5′-GUAGAAACCUGAUACAUGATT-3′, 5′-UCAUGUAUCAGGUUUCUACAG-3′). The cDNA of human LGR4 was cloned from our previously reported pcDNA3.1 plasmid construct (14) and inserted into the EcoRI/BamHI sites of pLVX-IRES-zsGreen1. Lentivirus preparation and lentiviral infections were performed according to a previously described (30). For transient transfection, cells were transfected with Lipofectamine 2000 (Thermo Fisher Scientific). For viral infection, MDA-MB-231, BT549 and MCF-10A were infected with control shRNA and LGR4 shRNA. For generating stable cell lines, the infected cells were cultured in 1 μg/ml puromycin (Millipore-Sigma) for 7 d. After selection, polyclonal cell populations were tested for decreased LGR4 expression levels before being used in experiments.
Cell migration, invasion, and wound-healing assays
For cell migration, 50,000 cells (suspended in 100 μl/well) were plated into the upper Boyden chambers in serum-free DMEM with the lower chamber containing complete DMEM (10% fetal bovine serum). For cell invasion, 10% Matrigel (BD Bioscience, Franklin Lakes, NJ, USA) and cells were mixed and plated into the upper chamber. Six hours later, the whole chamber was fixed after the cells remaining in the upper chamber were removed, stained with 0.05% Crystal violet and imaged by an Olympus IX71 microscope (Olympus, Tokyo, Japan). The migrated cells were counted. For the wound-healing assay, the experiment was performed according to a published protocol (42). In brief, cells were seeded at 400,000 cells/well (in 6-well plates) and cultured until 90% confluent, after which they were changed to serum-free medium for 12 h. The starved cell monolayer was scratched by a pipette tip, incubated for 12–24 h, and fixed with paraformaldehyde solution. The number of cells was counted, and the number of migrating cells refers to dividing the total cell number in the region flanking the scratch.
Three-dimensional morphogenesis
The 3-dimensional (3-D) culture of MCF10A-ErbB2-14,3,3 cells on basement membrane was performed as described in Debnath et al. (43). In brief, cells were resuspended (2500 cell/100 μl) in assay medium and then mixed with an equal volume of assay medium with 5% Matrigel and 10 ng/ml epidermal growth factor. Afterward, 100 μl/well of the cell mixture was plated into 96-well plates. The assay medium containing 2.5% Matrigel, as well as 5 ng/ml EGF was changed every 4 d. The colonies were photographed and quantitated after 8–12 d.
Immunofluorescence staining
MDA-MB-231 cells (10,000/well) were plated on glass coverslips pretreated with 0.1% gelatin in 24-well plates, and fixed in 4% paraformaldehyde. Cells were permeabilized for 6 min with 0.1% Triton X-100, blocked for 30 min in 0.2% bovine serum albumin, and incubated with rhodamine-linked phalloidin or indicated primary antibodies for 1 h. The cells were then stained with DAPI (D9542; Millipore-Sigma) or incubated with secondary antibody, respectively. Visualization of F-actin was achieved by laser scanning confocal microscopy (Leica Microsystems, Buffalo Grove, IL, USA), and vinculin was visualized with a Leica microscope (DM 4000 B LED). The antibodies used are listed in Supplemental Table 2.
Orthotopic and subcutaneous xenograft tumor model
Luciferase-labeled MDA-MB-231 cells that stably expressed control shRNA or shLGR4 were injected into the 4th mammary fat pad (1.5 × 106 cells) or subcutaneous flank (3 × 106 cells) of female BALB/c nude mice. The growing tumor size was measured by the equation (length × width2)/2 twice a week. After 49 d (orthotopic injection) or 31 d (subcutaneous injection) of tumor growth, the mice were photographed and euthanized. Tumors were weighed, sectioned, stained with hematoxylin and eosin (H&E), and the tumor volume was analyzed.
Lung metastasis model
Luciferase-labeled cells (2 × 105 MDA-MB-231) that were lentivirus infected with control shRNA or shLGR4 were intravenously injected into the tail vein in female BALB/c nude mice (6/shRNA). The lung bioluminescence was assayed and photographed using the Ivis System (Caliper Life Sciences). After euthanasia, the lungs were excised, fixed, and stained with H&E, and metastatic foci were quantitated by visual inspection.
Immunohistochemistry
Isolated tumors were excised, fixed, and embedded in paraffin. Sectioning into 4 μm slices was followed by deparaffinization and rehydration in a gradient of xylene and alcohol baths. After antigen retrieval, the blocked sections were rocked overnight at 4°C in the indicated primary antibodies, diluted in blocking buffer, and rewarmed for 40 min at room temperature the subsequent day. The sections were rinsed in PBS, incubated with secondary antibody for 20–30 min, developed with diaminobenzidine (D8001; Millipore-Sigma), and counterstained with hematoxylin. Photomicrographs were taken with a microscope (DM 4000 B LED; Leica). The antibodies used are listed in Supplemental Table 2. The human breast cancer tumor array was purchased from Shanghai Outdo Biotech (OD-CT_RpBre03-004; Shanghai, China) and authenticated by immunohistochemistry. The expression of LGR4 protein was assessed by using the multiplicative quick score method (44).
Tumorsphere assay
Primary mammary tumors were digested into single-cell suspensions. Ten thousand cells from the suspension were plated in nonadherent conditions and then cultured in sphere culture medium to observe tumorsphere formation. After 18 d, spheres larger than 50 μm in diameter were quantitated as tumorspheres.
Limiting dilution tumorigenesis
Two-month-old FVB/NJ (for primary mammary tumor cell injection) or nude female recipient mice (for MDA-MB-231 cell injection) were anesthetized by isoflurane. Primary tumor cells or MDA-MB-231 breast cancer cells were suspended at different dilutions into 50 µl of DMEM with 10% FBS and then combined with 50 µl of Matrigel (BD Biosciences). Injection of the cell mixture into the 4th mammary gland of female recipient mice was performed. The injected mice were monitored for tumor development twice a week by palpation, and the estimated number of tumor-initiating cells was calculated (45).
Bioinformatic analysis
The distant metastasis-free survival (DMFS), relapse-free survival (RFS), postprogression survival (PPS), and overall survival (OS) probability in patients with breast cancer was analyzed through Kaplan–Meier Plotter (K-M Plot) (http://www.kmplot.com) analysis (46). The Gene Expression Omnibus (GEO, National Center for Biotechnology Information, Bethesda, MD, USA; https://www.ncbi.nlm.nih.gov/geo/) (affymetrix microarrays only; Thermo Fisher Scientific), The Cancer Genome Atlas (TCGA), and European Genome-Phenome Archive (EGA) databases were used, with 5143 patients with breast cancer containing basal (n = 879), luminal A (n = 2504), luminal B (n = 1425), and HER2+ (n = 335) breast cancer subtypes; the samples were divided into 2 cohorts based on gene expression. Comparison of the 2 patient groups was performed with K-M Plot [(% confidence intervals (CI)] and the log-rank P value was analyzed. The gene expression profiling of 45 human breast epithelial or breast cancer cell lines was downloaded from the GEO database (GSE10890) with a Human Genome U133P 2.0 Array.
Statistical analysis
Experiments were conducted with more than 3 biologic replicates, and the results are presented as means ± sd. Unless otherwise indicated, data were analyzed by the 2-tailed Student’s t test, and the cutoff for statistical significance was set at P ≤ 0.05. Prism software v.5.01 (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis.
RESULTS
Expression of LGR4 in breast cancer is clinically correlated with progression, metastasis, and poor prognosis
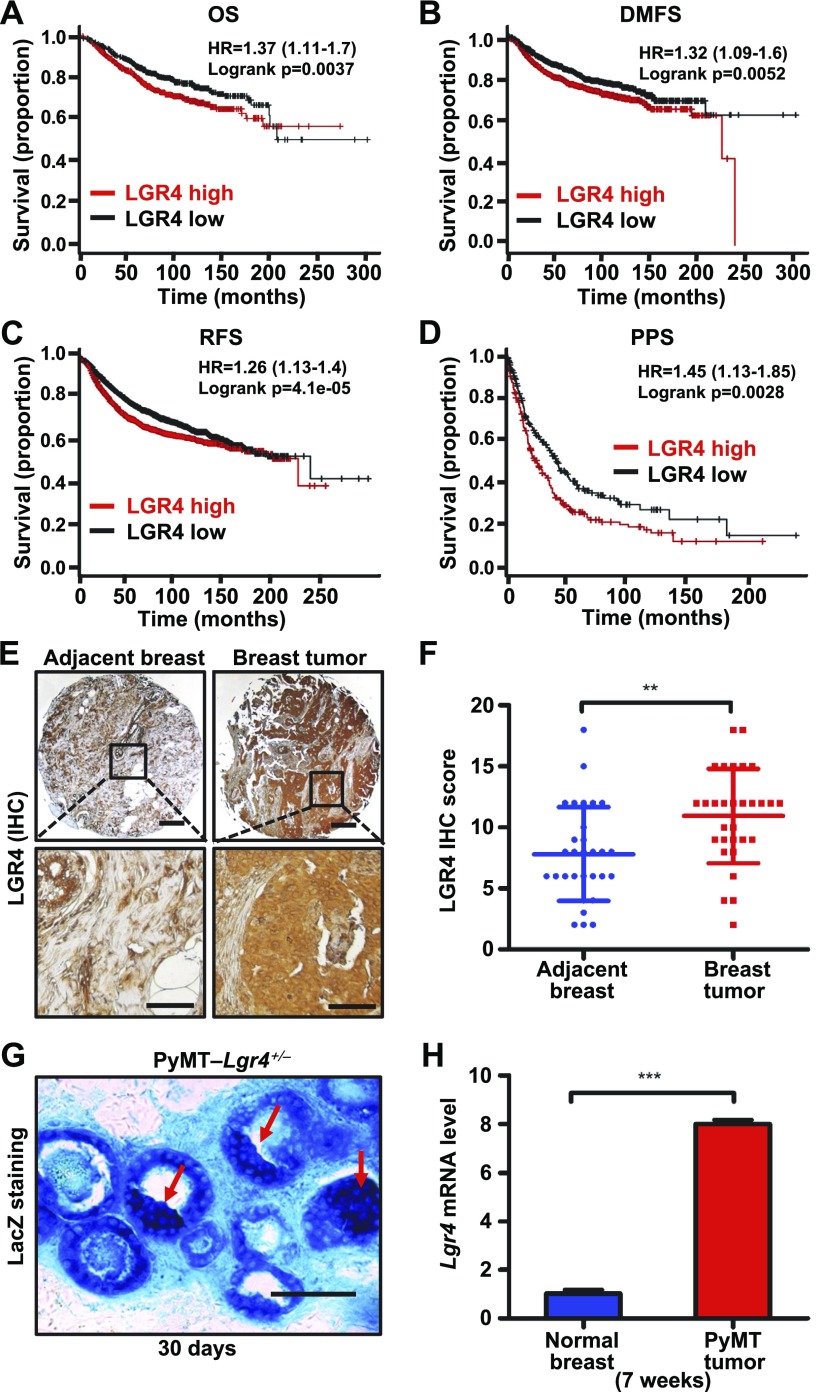
There are 8 subtypes (LGR1–8) in the LGR family within the rhodopsin GPCR superfamily. LGR1 (FSH receptor), LGR2 (LH receptor), and LGR3 (TSH receptor) are involved in breast cancer growth, metastasis, and poor prognosis (47, 48), but the effect of the remaining LGRs (LGR4–8) in breast cancer is largely unknown. To evaluate whether the expression of LGR family members (LGR4–8) correlates with breast cancer, we used four separate approaches. First, we examined whether expression of LGR4–8 in breast cancer has prognostic value. We found by Kaplan–Meier survival analysis (data from the EGA, GEO, and TCGA datasets) that LGR4 mRNA expression was most correlated with poor patient prognosis, including OS, DMFS, RFS, and PPS (Fig. 1A–D and Supplemental Fig. 1), suggesting that high LGR4 expression correlates significantly with breast cancer metastasis and poor patient prognosis. Second, using bioinformatic analysis, LGR4 had a higher expression compared with other LGRs in a panel of 44 breast cancer cell lines and 1 nontransformed breast epithelial cell line (Supplemental Fig. 2A). LGR4 expression was not associated with a particular breast cancer subtype in the different breast cancer cell lines examined (Supplemental Fig. 2A). Third, we assessed the invasive potential of 6 widely used breast cancer cell lines by Transwell (Corning, Corning, NY, USA) invasion assay (Supplemental Fig. 2B). The expression of LGR4 correlated much more closely with the relative invasive ability of these cell lines (Supplemental Fig. 2B and data not shown). Finally, we compared the expression of LGR4 protein in human breast cancer tissue (n = 30) to normal breast tissue from the same patients (n = 30). As expected, significantly higher levels of LGR4 protein were detected in breast tumors than in adjacent breast tissue in these patients (Fig. 1E, F). Together, these results indicate that LGR4 expression is most associated with human breast cancer progression.
Figure 1.
LGR4 is a poor prognostic factor and is overexpressed in breast tumor tissues. A–D) Kaplan-Meier plot of survival of patients with breast cancer stratified according to LGR4 mRNA expression OS (nhigh = 558, nlow = 559); DMFS (nhigh = 803, nlow = 806); RFS (nhigh = 1771, nlow = 1783); and PPS (nhigh = 175, nlow = 176). E, F) LGR4 protein expression levels were significantly up-regulated in human breast cancer tissues. Representative immunohistochemistry staining images of LGR4 in human breast tumor tissues (n = 30) or matched adjacent breast tissues (n = 30) (left). Scores of the breast tissue array staining (right). Error bars are means ± sd. **P < 0.01. Scale bars, 250 µm (top); 10 µm (bottom). G) LGR4 expression in mouse mammary tissue by LacZ staining of PyMT-Lgr4+/− mice at 30 d. Representative images (n = 3, 3 images taken from each mice) of LacZ staining results of mammary sections. Blue: LacZ staining of β-gal expression driven by the LGR4 promoter. Red arrows: neoplastic abnormal hyperplasia sites. Scale bar, 50 µm. H) qPCR analysis to detect the expression of Lgr4 in 7-wk-old wild-type mouse normal mammary tissue and MMTV-PyMT mouse tumor tissue. Error bars are means ± sd. ***P < 0.001.
Expression of Lgr4 correlates with tumor progression in MMTV-PyMT transgenic mice
To address whether LGR4 plays a functional role in mammary tumor formation, or metastasis, or both, we used the widely used MMTV-PyMT transgenic breast cancer mouse model featuring expression of the PyMT antigen oncogene specifically in mammary epithelial cells. We first investigated the expression of Lgr4 in MMTV-PyMT breast tumors. Because the β-galactosidase transcript had been knocked into the Lgr4 locus (14), Lgr4 expression can be assessed by β-galactosidase activity (lacZ expression). At 30 d of age, we found abundant β-gal expression in the mammary gland and sites of abnormal neoplastic hyperplasia (Fig. 1G). We also found that Lgr4 mRNA levels were elevated in tumors of MMTV-PyMT mice as compared to normal mammary epithelial tissue (Fig. 1H), further supporting an increase in LGR4 expression that accompanies tumor progression.
Lgr4 loss impedes mammary tumor occurrence, progression, and metastasis in MMTV-PyMT transgenic mice
We have reported that LGR4 plays an essential role in mammary gland development (30); in the current study, we sought to determine whether LGR4 gene function is necessary in breast cancer. We initially crossed Lgr4+/− mice with MMTV-PyMT mice to examine the effect of Lgr4 deficiency (Supplemental Fig. 3A) on mammary tumor development. Nine-week-old wild-type MMTV-PyMT (PyMT- Lgr4+/+) mice showed frequent ductal carcinoma in situ (DCIS) or invasive ductal carcinoma (IDC), whereas MMTV-PyMT Lgr4−/− (PyMT-Lgr4−/−) mice had largely normal mammary architecture with occasional hyperplasia or DCIS and rare instances of IDC (Supplemental Fig. 3B, C). MMTV-PyMT Lgr4+/− (PyMT-Lgr4+/−) mice showed an intermediate delay in tumor development, suggesting haploinsufficiency in Lgr4 heterozygous mice. At 15 wk, most PyMT-Lgr4+/+ mammary glands examined had frank invasive carcinoma, whereas glands from PyMT-Lgr4−/− mice had DCIS or lower grade IDC (Supplemental Fig. 3B, C). PyMT-Lgr4+/+ tumors were also more proliferative (Supplemental Fig. 3D), suggesting a potential role for LGR4 in regulating cell proliferation. Therefore, Lgr4 loss delayed the development of PyMT-driven early lesions.
We next examined the effect of Lgr4 heterozygosity on MMTV-PyMT tumor-free survival. Although there were no significant developmental defects in PyMT-Lgr4+/– mice, as compared to PyMT-Lgr4+/+ (Supplemental Fig. 4A), tumor-free survival was lengthened in PyMT-Lgr4+/− mice, as compared to that in PyMT-Lgr4+/+ mice (Supplemental Fig. 4B; P = 0.0352). Tumor load per mouse and tumor volume were also reduced in Lgr4 heterozygous mice (Supplemental Fig. 4C, D), further supporting reduced tumorigenesis after Lgr4 loss.
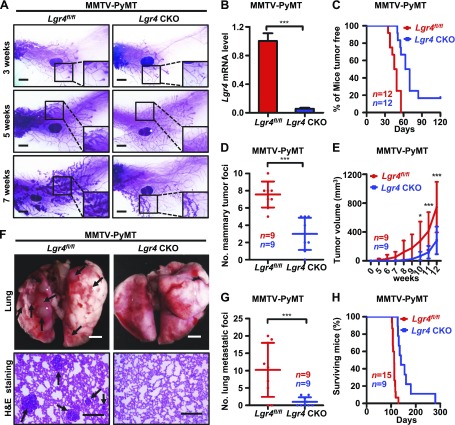
LGR4 has diverse functions in multiple tissues and organs. To avoid the potential complication of systemic effects related to Lgr4 loss, we also generated mice with mammary epithelium–specific conditional deletion of Lgr4 by crossing Lgr4flox/flox mice with MMTV-Cre mice. We then mated the resulting MMTV-Cre;Lgr4flox/flox mice with MMTV-PyMT mice to generate MMTV-PyMT;MMTV-Cre;Lgr4flox/flox (PyMT-Lgr4-CKO) mice, and examined the knockout efficiency (Fig. 2B). We detected a marked delay in mammary tumor formation in PyMT-Lgr4-CKO mice, as compared to PyMT-Lgr4fl/fl mice (Fig. 2A, C). Tumor load per mouse and tumor volume were also reduced in PyMT-Lgr4-CKO mice (Fig. 2D, E), further supporting a key role for LGR4 in mammary tumorigenesis.
Figure 2.
Lgr4 loss impairs MMTV-PyMT tumor formation and metastasis. A) Mammary gland–specific Lgr4-deficiency delays MMTV-PyMT tumor formation. Carmine alum staining of the fourth mammary glands of PyMT-Lgr4 CKO and -Lgr4fl/fl mice of the indicated ages. Scale bars, 2 mm. B) Lgr4 mRNA levels in mammary tumors from PyMT-Lgr4 CKO and -Lgr4fl/fl mice at 12 wk. Error bars are means ± sd. ***P < 0.001 (log rank test). C) Kaplan-Meier plot of palpable tumor incidence up to 17 wk (about 120 d) in PyMT-Lgr4 CKO and -Lgr4fl/fl mice (n = 12/group). D) The number of mammary tumors per mouse at 12 wk in PyMT-Lgr4 CKO and -Lgr4fl/fl mice. Error bars are means ± sd (n = 9/group). ***P < 0.001. E) The volume of primary tumors within 12 wk after detection in PyMT-Lgr4 CKO and -Lgr4fl/fl mice. Error bars are means ± sd (n = 9/group). *P < 0.05, ***P < 0.001. F, G) Lgr4-deficiency in mammary gland decreased lung metastatic tumor foci. The indicated mice were euthanized at 12 wk of age, lungs were excised (F, top; 2 images taken from each mouse), sectioned, and processed with H&E staining (F, bottom; 3 images taken from each mouse), and the number of tumor foci was counted (G). Error bars are means ± sd (n = 9/group). ***P < 0.001 (log-rank test). Scale bars, 2 mm (top); 200 µm (bottom). H) Kaplan–Meier survival curves of PyMT-Lgr4 CKO (n = 9) and -Lgr4fl/fl mice (n = 15).
Finally, we sought to determine whether Lgr4 loss affected MMTV-PyMT tumor metastasis in vivo. We examined the lungs for the presence of metastatic foci. We found a sharp reduction in the frequency of lung metastases in both PyMT-Lgr4+/− and PyMT-Lgr4-CKO mice (Fig. 2F, G and Supplemental Fig. 4E, F). As a result, Lgr4-deficient tumor-bearing mice had significantly prolonged survival (Fig. 2H). These in vivo data for MMTV-PyMT mice suggest that loss of Lgr4 delays mammary tumor initiation, progression, and metastasis.
LGR4 knockdown inhibits breast cancer xenograft tumor growth
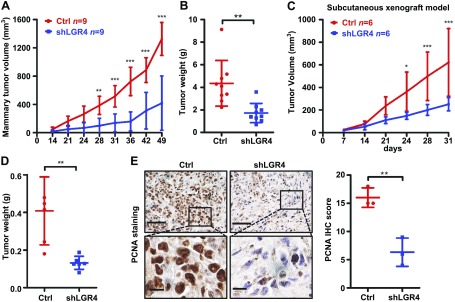
To specifically evaluate the effects of LGR4 loss on breast cancer cells, we next examined whether LGR4 down-regulation affects human breast cancer cell tumor formation. MDA-MB-231 cells selected to stably express either shLGR4 or nonspecific control shRNA were orthotopically or subcutaneously injected into nude mice, and the resulting tumor growth was measured. shLGR4 MDA-MB-231 xenografts grew at a significantly slower rate (Fig. 3A, C). After 31 (subcutaneously injected) or 49 (orthotopically injected) days, recipient mice were euthanized, and xenograft tumors were analyzed. Mean xenograft tumor weight of shLGR4 MDA-MB-231-injected mice was reduced more than 50% compared with control tumors (Fig. 3B, D). We also noted a decrease in cell proliferation in shLGR4 cell tumors as demonstrated by proliferating cell nuclear antigen (PCNA) staining (Fig. 3E). These results indicate that LGR4 functions directly in human breast cancer cells to promote tumor growth, apart from any systemic or hormonal disruptions resulting from whole-body LGR4 loss.
Figure 3.
LGR4 regulates tumorigenicity in human breast cancer cells. A, B) shLGR4 MDA-MB-231 or control (ctrl) MDA-MB-231 cells were injected orthotopically into 9 mice per group. The tumor volumes were monitored until 7 wk (A). The tumor weights were measured at 49 d when the mice were euthanized (B). Error bars are means ± sd. **P < 0.01, ***P < 0.001. C, D) shLGR4 MDA-MB-231 or ctrl MDA-MB-231 cells were injected subcutaneously into 6 nude mice per group. The tumor volumes were recorded up to 31 d (C). The tumor weights were recorded at 31 d when the mice were euthanized (D). Error bars are means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001. E) LGR4 knockdown decreased PCNA expression in xenograft tumors. The tumors from panel C were excised and sectioned for PCNA staining. Representative images are shown (3 images from each of 3 mice). Scale bars, 50 µm (top); 10 µm (bottom).
LGR4 knockdown impairs human breast cancer cell migration, invasion, and metastatic capacity
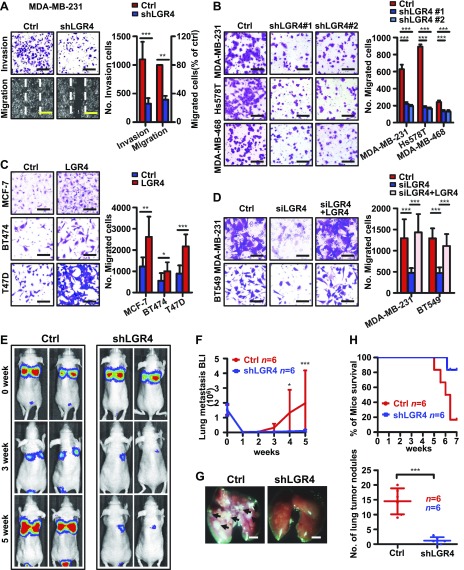
The metastasis decrease observed in PyMT-Lgr4+/− and PyMT-Lgr4-CKO mice may be a consequence of the delay in initial tumor formation, or it could be a separate, direct result of Lgr4 loss affecting metastatic properties of tumor cells. To further examine LGR4 function in breast cancer metastasis, we generated MDA-MB-231 sublines stably expressing shRNA targeting LGR4 (shLGR4) or control, nonspecific shRNA (control) (Supplemental Fig. 4G) and sublines of MCF-7, BT474 and T47D cells stably transfected with either LGR4 overexpression vector or zsGREEN control vector (Supplemental Fig. 4I). LGR4 knockdown had little effect on breast cancer cell proliferation in the same time frame (data not shown). The in vitro properties related to metastasis were tested. First, we found that LGR4 knockdown inhibited MDA-MB-231 cell wound healing and invasion (Fig. 4A), an effect that was also observed with a second shRNA sequence in 3 different breast cancer cell lines (MDA-MB-231, Hs578T, and MDA-MB-468) (Fig. 4B and Supplemental Fig. 4H). In contrast, LGR4 overexpression in MCF-7, BT474, and T47D cells significantly increased cell migration (Fig. 4C). Moreover, exogenous expression of LGR4 lacking the siRNA target sequence in MDA-MB-231 and BT549 (Supplemental Fig. 4J) siLGR4 cells restored cell migration to baseline levels (Fig. 4D). Therefore, LGR4 (and not off-target siRNA effects) is responsible for the observed changes in breast cancer cell invasion and migration in vitro. Finally, we examined LGR4 in an in vivo metastasis model. MDA-MB-231 cells stably expressing luciferase and either shLGR4 or control shRNA were injected into the tail veins of nude mice, and metastatic involvement was measured by in vivo imaging. LGR4 knockdown MDA-MB-231 cells generated fewer metastases in vivo (Fig. 4E–G), suggesting that LGR4 functions in breast cancer cells to support one or more steps in the metastatic cascade. However, we cannot rule out the possibility that in the shLGR4 group, cancer cells became dormant and were not able to form tumor nodules. In any event, mice injected with LGR4 knockdown cells had significantly longer survival (Fig. 4H). Together, our data support the conclusion that LGR4 functions to promote breast cancer cell invasion and metastasis in vivo.
Figure 4.
Knockdown of LGR4 impedes human breast cancer cell migration, invasion, and metastasis. A) Knockdown of LGR4 in MDA-MB-231 human breast cancer cells reduced cell migration by wound-healing assay and invasion by Boyden chamber invasion assay. Photographs (left) and quantitation (right). Graph shows the means ± sd of 3 independent experiments. Scale bars, 200 µm. **P < 0.01, ***P < 0.001 (unpaired, 2-tailed Student t test). B) LGR4 stable knockdown by 2 different shRNAs (shLGR4 #1 and #2) reduced MDA-MB-231, Hs578T, and MDA-MB-468 cell well migration in a Boyden chamber migration assay. The migrated cells were stained (left) and tallied (right). Graph shows the means ± sd of 3 independent experiments. Scale bars, 100 µm. ***P < 0.001 (unpaired, 2-tailed Student’s t test). C) Overexpression of LGR4 in MCF7, BT474, and T47D human breast cancer cells increased migration properties by Boyden chamber migration assay. The migrated cells were photographed (left), and quantitated (right). Graph indicates the means ± sd of 3 independent experiments. Scale bars, 100 µm. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired, 2-tailed Student t test). D) Loss of migration ability induced by knockdown of endogenous LGR4 (siLGR4) was rescued through re-expression of LGR4 (siLGR4+LGR4) in MDA-MB-231 and BT549 breast cancer cells. The migrated cells were photographed (left) and quantitated (right). Graph shows the means ± sd of 3 independent experiments. Scale bar, 100 µm. ***P < 0.001 (unpaired, 2-tailed Student’s t test). E, F) shLGR4 luciferase labeled MDA-MB-231 or Ctrl cells were injected into the tail veins of mice (n = 6 nude mice/group). The same color scale was used in bioluminescent images of representative mice in each experimental group at the indicated days after injection (1 image taken from each mouse) (E). Quantitative analysis of lung metastatic cell bioluminescence (means ± sd) is shown (F). *P < 0.05, ***P < 0.001. G) LGR4 knockdown in human breast cancer cells decreased the number of lung metastatic tumor foci. The mouse lungs were excised (left; two images taken from each mouse) and the tumor nodules were counted (right) within 7 wk. Scale bars, 2 mm. Error bars are means ± sd. ***P < 0.001 (n = 6/group). H) Kaplan-Meier survival curves of mice in E–G. P = 0.028 (by log-rank test).
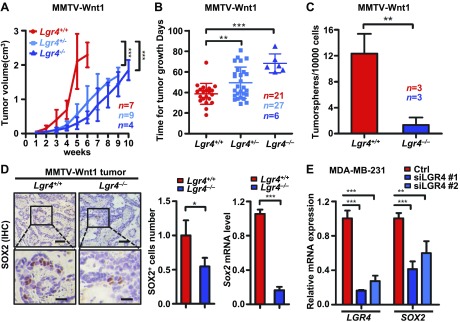
Lgr4 loss impairs breast CSCs
CSCs constitute a distinct tumor cell subpopulation able to both self-renew and generate the different subpopulations present in a tumor. Because we have demonstrated that LGR4 loss decreases the pool of normal stem cells in both the mouse mammary gland (30) and prostate (16), we sought to determine whether LGR4 functions in regulation of breast CSCs. To address this question, we crossed the MMTV-Wnt1 mice with Lgr4+/− mice and examined tumor formation. We found that tumor growth rates were significantly slowed in both Wnt1-Lgr4+/− and Wnt1-Lgr4−/− groups compared with Wnt1-Lgr4+/+ mice (Fig. 5A, B). To initially examine whether Lgr4 loss affects CSCs, we compared the tumorsphere formation ability of primary cells from Wnt1-Lgr4+/+ and Wnt1-Lgr4−/− tumors. We observed a significant decrease in tumorsphere formation in cells from Wnt1-Lgr4−/− tumors (Fig. 5C), suggesting a potential loss of CSCs in tumors lacking Lgr4. The most definitive assay for CSC function is tumor formation in vivo. Therefore, we performed limiting dilution transplantation of Wnt1-Lgr4+/+ and Wnt1-Lgr4−/− primary tumor cells into FVB/N hosts. We observed an 87.5% decrease in the number of tumor-initiating cells in Wnt1-Lgr4−/− tumors (Table 1), suggesting an impairment in CSCs in the absence of Lgr4. We then evaluated whether LGR4 functions similarly in human breast cancer cells. We observed similar results when we injected MDA-MB-231 cells into nude mice, where we also found a significant decrease in tumor-initiating cells after LGR4 knockdown (Table 2). The data further support a meaningful role for LGR4 in breast CSC function. Finally, we examined LGR4 targets potentially mediating a role in breast CSC maintenance. Sex determining region Y-box-(SOX)-2 is a Yamanaka transcription factor that, when expressed, induces pluripotency in differentiated cells, and has been implicated in breast CSCs (49, 50). Therefore, we analyzed whether Lgr4 affects Sox2 expression. The percentage of SOX2-expressing cells was decreased in tumors from Wnt1-Lgr4−/− mice (Fig. 5D), as well as in LGR4 knockdown MDA-MB-231 cells (Fig. 5E), compared with control cells. In summary, our findings support the hypothesis that LGR4 sustains breast CSC function, in part through regulating SOX2 expression.
Figure 5.
Lgr4 loss impairs breast CSCs. A) The volume of primary tumors until 10 wk from initial tumor detection by palpation in Wnt1-Lgr4+/+ (n = 7), -Lgr4+/ – (n = 9), and -Lgr4−/− (n = 4) mice. B) The time from initial detection to tumor reaching 1.5 cm diameter in MMTV-Wnt1 mice of indicated Lgr4 genotypes. Wnt1-Lgr4+/+ (n = 21), -Lgr4+/− (n = 27), and -Lgr4−/− (n = 6). C) Primary mammary tumor cells from indicated mice were plated under nonadherent conditions for 18 d, and resulting tumorspheres were quantitated (n = 3). D) The protein expression of SOX2 was determined by immunohistochemistry (left) in Wnt1-Lgr4+/+and -Lgr4−/−mammary tumors. Scale bars, 50 µm (top); 20 μm (bottom). Graphs show quantitation of SOX2+ cells (middle) and the SOX2 mRNA level determined by quantitative PCR (right). E) LGR4 and SOX2 mRNA expression in MDA-MB-231 expressing control (ctrl) or indicated siRNA. Error bars are means ± sd. * P < 0.05, **P < 0.01, ***P < 0.001.
TABLE 1.
Effect on CSCs of MMTV-Wnt1;Lgr4−/− mammary tumors
| Cells injected | 102 | 103 | 104 | 105 | CSC frequency |
|---|---|---|---|---|---|
| MMTV-Wnt1;Lgr4+/+ | 0/7 | 3/11 | 21/22 | 10/10 | 1/2758 (1546–4919) |
| MMTV-Wnt1;Lgr4−/− | 0/8 | 1/12 | 11/22 | 9/10 | 1/21,963 (12,059–40,001) |
Data shown as tumor outgrowths/mice injected with primary tumor cells. Calculated frequency of cancer stem cells and 95% confidence interval is denoted in the last column. P = 3.42e−07.
TABLE 2.
Effect on CSCs of LGR4 knockdown in human cancer cells
| Cells injected | 103 | 104 | 105 | 106 | CSC frequency |
|---|---|---|---|---|---|
| Control | 0/6 | 2/6 | 5/6 | 6/6 | 1/45,903 (18,996–110,925) |
| shLGR4 | 0/6 | 0/6 | 1/6 | 5/6 | 1/569,691 (235,901–1,375,781) |
Data shown as tumor outgrowths/mice injected with MDA-MB-231 cells. Calculated frequency of cancer stem cells and 95% confidence interval is denoted in the last column. P = 9.8e−05.
LGR4 induces Wnt/ β-catenin-mediated EMT
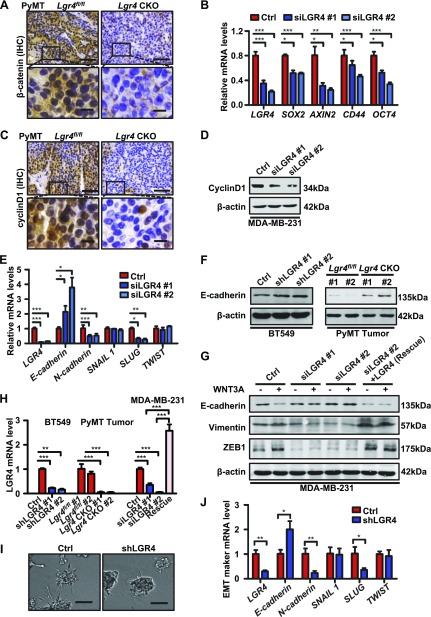
LGR4 potentiates Wnt signaling through binding RSPO family members. Wnt/β-catenin signaling has been shown to promote EMT and maintain survival of CSCs (51–53). We therefore examined whether LGR4 regulates breast cancer through canonical Wnt/β-catenin signaling. Both total and nuclear β-catenin was higher in tumors of PyMT-Lgr4fl/fl mice than in PyMT-Lgr4-CKO mice (Fig. 6A), indicating impaired canonical Wnt/β-catenin signaling after Lgr4 knockout. In addition, LGR4 deficiency decreased expression of multiple Wnt/β-catenin target genes including AXIN2, CD44, SOX2, OCT4 (Fig. 6B), and cyclin D1 in MDA-MB-231 cells (Fig. 6D), as well as cyclin D1 levels in tumors of PyMT-Lgr4-CKO mice (Fig. 6C), further supporting LGR4-mediated Wnt/β-catenin signaling. To investigate potential pathways underlying the decreased invasion and metastasis after LGR4 down-regulation, we examined markers of EMT. We found that LGR4 knockdown attenuated EMT maker gene mRNA expression in MDA-MB-231 (Fig. 6E) and elevated E-cadherin protein expression in BT549 and PyMT-Lgr4-CKO tumors (Fig. 6F, H). Furthermore, treatment with WNT3A decreased expression of E-cadherin and increased expression of vimentin and zinc finger E-box–binding homeobox (ZEB)-1 (Fig. 6G), indicating promotion of EMT after WNT3A treatment. In contrast, siLGR4 expression resulted in an increase in E-cadherin and a decrease in vimentin and ZEB1 levels under WNT3A stimulation, suggesting a partial reversal of EMT in MDA-MB-231 cells upon LGR4 knockdown (Fig. 6G, H). Significantly, mesenchymal marker expression was rescued by exogenous expression of LGR4 lacking the siRNA target sequence in MDA-MB-231 cells. Therefore, LGR4 is necessary for Wnt/β-catenin signaling and may support EMT in human breast cancer cells.
Figure 6.
LGR4 acts through Wnt/β-catenin signaling to promote EMT and invasion. A) Expression of β-catenin decreased in mammary tumors of PyMT-Lgr4 CKO mice. Sections of mammary tumors were subjected to immunohistochemical staining by an anti-β-catenin antibody (n = 9 images taken in total, 3 images taken from each of 3 mice). Scale bars, 50 µm (top); 10 µm (bottom). B) Relative expression of Wnt target gene mRNA level in MDA-MB-231 cells transfected as indicated. Experiments performed 3 times using biologic replicates. Representative graph shows the means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001. C) Sections of indicated mammary tumors were subjected to immunohistochemistry staining (n = 9 images taken in total, 3 images taken from each of 3 mice) using an anti-cyclin D1 antibody. Scale bars, 50 µm (top); 10 µm (bottom). D) LGR4 knockdown decreased cyclin D1 protein expression in breast cancer cells. LGR4 was knocked down by siRNA in MDA-MB-231 cells, and the cells were lysed and subjected to Western blot analysis with the indicated antibodies. Images are representative of 3 independent experiments. Expression of Wnt target gene cyclin D1 decreased in PyMT-Lgr4-CKO mammary tumors. E) Relative expression of indicated EMT genes was determined by qPCR in MDA-MB-231 cells transfected as indicated. Experiments independently repeated 3 times. Graph shows the means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001. F) Western blot of E-cadherin in control or shLGR4 (#1 and #2) expressing BT549 cells (left) and PyMT-Lgr4fl/fl tumors tissues from 12-wk-old mice (right). Representative blot of 3 independent experiments. G) Western blot of EMT genes (E-cadherin, vimentin, and ZEB1) in MDA-MB-231 cells transfected and treated as indicated. MDA-MB-231 control (ctrl), siLGR4 #1, siLGR4 #2 and siLGR4+LGR4 cells were treated with vehicle control or WNT3A (80 ng/ml) for 24 h before harvesting. Representative blots of 3 independent experiments. H) LGR4 knockdown/knockout efficiency in BT549 or PyMT-Lgr4fl/fl tumors related to panel F and LGR4 rescue efficiency in MDA-MB-231 related to (G) identified by qPCR. The experiment was independently repeated 3 times, and representative graphs show the means ± sd. **P < 0.01, ***P < 0.001. I) LGR4 regulated EMT. MCF10A-ErbB2-14,3,3 cells stably expressing ctrl shRNA or shRNA targeting LGR4 (shLGR4) were seeded in Matrigel. Colonies were photographed 9 d after plating (top). Scale bars, 50 µm. J) Colonies of indicated MCF10A-ErbB2-14,3,3 cells were harvested, and mRNA levels of indicated EMT genes were determined by qPCR. Experiments was repeated 2 times. Error bars are means ± sd. *P < 0.05, **P < 0.01.
To further evaluate whether LGR4 functions in EMT, we used MCF10A cells transformed by exogenous expression of Her2 and 14-3-3ζ (10A-ErbB2-14,3,3 cells). When grown in 3-D Matrigel culture, 10A-ErbB2-14,3,3 cells expressing control shRNA formed colonies with large, invasive protrusions, whereas cells expressing shLGR4 had extremely limited ability to invade the surrounding matrix (Fig. 6I). Analysis of EMT gene expression in these 3-D colonies indicated that LGR4 knockdown in 10A-ErbB2-14,3,3 cells increased epithelial marker expression (E-cadherin) at the expense of mesenchymal markers (N-cadherin) and furthermore decreased expression of the EMT-inducing transcription factor snail family zinc finger (SNAI)-2 (also called SLUG) (Fig. 6J), consistent with a role for LGR4 in promoting EMT and invasiveness in breast cancer cells.
LGR4 induces focal adhesion kinase/steroid receptor coactivator–mediated cytoskeletal reorganization
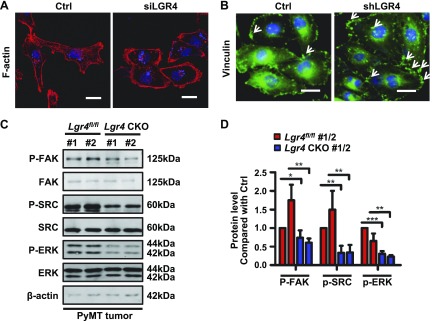
Our data support a key role for LGR4 in regulating breast cancer invasion and metastasis. In addition to LGR4 potentiation of canonical Wnt signaling, the RSPO3-LGR4- IQ motif–containing GTPase-activating protein-1 pathway regulates focal adhesion assembly in lung cancer cells (54). We therefore examined whether LGR4 may modulate invasion by regulating F-actin–mediated cytoskeletal organization. MDA-MB-231 cells transiently transfected with siLGR4 displayed modest changes in shape from a spindle-shaped mesenchymal morphology toward a more rounded, epithelial-like cell morphology and increased levels of cortical F-actin, but without loss of actin stress fibers (Fig. 7A). Similarly, shRNA-mediated LGR4 knockdown in MDA-MB-231 cells increased focal adhesion plaques (Fig. 7B), suggesting that LGR4 regulates vinculin anchoring of F-actin to the cell membrane.
Figure 7.
LGR4 regulates the actin cytoskeleton by modulating the FAK/SRC pathway. A) Actin stress fiber distribution was changed in LGR4 knockdown cells. MDA-MB-231 cells were transfected with control or LGR4 siRNA, then the cells were fixed for actin staining by phalloidin. Scale bar, 20 μm. The experiment was repeated 3 times. B) LGR4 knockdown increased focal adhesion. Control or shLGR4 MDA-MB-231 cells were fixed for focal adhesion plaque staining by vinculin (white arrows: focal adhesions). Scale bars, 20 μm. The experiment was repeated 3 times. C, D) LGR4-deficiency in mouse decreased FAK/SRC/ERK signaling. Western blot of PyMT-Lgr4 CKO and PyMT-Lgr4fl/fl tumors from 12-wk-old mice. C) Representative blot. D) The quantitative histogram represents the means ± sd of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Because focal adhesion kinase (FAK) is associated with cellular adhesion and spreading processes, we next examined the FAK/steroid receptor coactivator (SRC)/ERK signaling pathway in MMTV-PyMT tumors. Lgr4 haploinsufficiency significantly decreased the phosphorylation levels of FAK, SRC, and ERK in MMTV-PyMT tumors (Fig. 7C, D). Overall, these results suggest that LGR4 regulates the FAK-SRC pathway that coordinates actin dynamics and modulates cell adhesion, ultimately promoting breast cancer cell migration. Because LGR4 activates the Gαs/PKA/CREB pathway in many physiologic functions, we also examined the adenosine cAMP level and CREB phosphorylation level in MMTV-PyMT tumors (Supplemental Fig. 5A, B) and also treated MDA-MB-231 knockdown LGR4 cells with forskolin (Supplemental Fig. 5C). The results of all 3 experiments suggest that LGR4 regulates breast cancer independent of the cAMP/CREB signaling pathway.
DISCUSSION
Our studies provide evidence for a key role of LGR4 in breast cancer progression, metastasis, and CSCs. Lgr4 haploinsufficiency or mammary gland–specific Lgr4 deletion significantly delayed PyMT-driven mammary cancer development and lung metastasis in vivo. Although at present we cannot exclude the possibility that delayed tumor formation contributed to the absence of metastases in Lgr4-CKO mice, manipulating LGR4 expression in human breast cancer cells inhibited cell migration and invasion, further supporting an essential requirement for LGR4 in these processes, in line with reported LGR4 functions in other tumors (31, 32, 34, 35, 54). Furthermore, LGR4 knockdown impaired both MDA-MB-231 cell xenograft tumor growth and metastasis formation, and human patients with tumors expressing higher LGR4 levels were more prone to relapse, strongly suggesting that LGR4 functions in human breast cancer progression and metastasis.
Lgr4 has been implicated in a broad array of biologic processes. Whole-body Lgr4 deletion is frequently embryonic lethal (55), with survivors bearing a host of developmental defects affecting bone (17, 18, 56–58), blood (22, 26), brain (13, 59), eye (14, 15, 60), intestines (61, 62), kidneys (63–65), bile duct (19), and reproductive organs (29, 66–71). Several of these phenotypes were reported in humans bearing a nonsense mutation LGR4 allele (37). Lgr4 plays key roles in maintaining electrolyte (23) and energy balance (24, 25, 72–74), while modulating feeding behavior (75). As a potentiator of Wnt signaling, Lgr4 regulates prostate stem cell differentiation (16), as well as intestinal and mammary stem cell maintenance (30, 76), functions which are consistent with our findings that the number of CSCs is depleted in mammary tumors of Lgr4-deficient mice. Furthermore, our results implicating Lgr4 in invasion/metastasis, potentially through regulation of EMT, are supported by similar roles in prostate (33, 77) and other cancers (32, 35). Our data suggesting regulation of actin dynamics through control of FAK/Src signaling, similar to the findings of Snyder and coworkers (78), opens a new area of Lgr4 function in need of further research.
EMT has been proposed as a crucial process in tumor metastasis. Both cells at the tumor invasive front and circulating breast tumor cells express EMT markers (77, 78), and inhibition of EMT impairs metastasis in mouse models (81). Among the transcription factors potentially driving EMT in breast cancer, we observed a significant decrease in SLUG and ZEB1 expression after LGR4 knockdown in vitro. High SLUG expression correlates with metastasis and poor patient prognosis (82, 83), and SLUG expression together with SOX2 is sufficient to induce a mammary stem cell state (83).
Wnt/β-catenin signaling is strongly implicated in breast cancer, with mutations in this pathway occurring in up to half of human breast cancers (6, 7). Downstream targets of canonical Wnt signaling include the EMT-inducing transcription factor SLUG, and the LGR4 homolog LGR5, suggesting that Wnt signaling down-regulation upon LGR4 loss may be reinforced by subsequent reduction of LGR5 expression (51, 53). Curiously, LGR5 was recently reported to correlate with breast cancer survival (84), in contrast to our finding of no significant correlation, possibly because of databases containing different patient populations and scope (Asian vs. predominantly Caucasian). Wnt signaling is essential in mammary stem cells (8), and, given the striking decrease in mammary stem cells of mice lacking Lgr4 (30), our findings support an important role for LGR4 in breast CSC maintenance that may underlie LGR4 function in breast cancer progression. This connection is an area in need of further research.
In summary, we provide evidence supporting an essential role for LGR4 in regulating CSCs and breast cancer metastasis through Wnt/β-catenin signaling and induction of EMT. Furthermore, LGR4 correlation with patient prognosis supports its role as a potential breast cancer prognostic marker. Therefore, our findings suggest that LGR4 is an attractive therapeutic target for inhibition of breast cancer metastasis and likely has prognostic value in patients with breast cancer.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dihua Yu [M. D. Anderson Cancer Center (MDACC), Houston, TX, USA] for the gift of the MCF10A-ErbB2-14,3,3 cells; Hans Clevers (Hubrecht Institute for Developmental Biology and Stem Cell Research, Utrecht, The Netherlands) for the gift of the LGR4 expression plasmid; Qingyun (Jim) Liu (University of Texas Health Science Center, Houston, TX, USA) for the gift of the anti-LGR4 antibody; Hong Zhang (MDACC) for pathological consultation; and Yanqing Huang (Methodist Research Institute, Houston, TX, USA) for technical advice and assistance. This work was supported by National Key Research and Development Program of China Grant 2016YFC0902102 (to J.L.); the National Natural Science Foundation of China Grants 81722020, 81472048, and 81272911 (to J.L.), and 81330049 (to M.L.); the Innovation Program of Shanghai Municipal Education Commission Grant 14ZZ051 (to J.L.) and 2017-01-07-00-05-E00011 (to M.L.); Science and Technology Commission of Shanghai Municipality Grant 12ZR1447900 (to J.L.); U.S. National Institutes of Health, National Cancer Institute Grant R01CA204926 (to Y.L.); U.S. Department of Defense–Congressionally Directory Medical Research Program (DOD–CDMRP) Grant BC160240 (to Y.L. and M.L.); and the Fundamental Research Funds for the Central Universities (to J.L.). The authors declare no conflicts of interest.
Glossary
- 3-D
three dimensional
- CREB
cAMP response element binding
- CKO
conditional knockout
- CSC
cancer stem cell
- DCIS
ductal carcinoma in situ
- DMFS
distant metastasis free survival
- EMT
epithelial–mesenchymal transition
- FAK
focal adhesion kinase
- LGR
G protein-coupled receptor
- H&E
hematoxylin and eosin
- IDC
invasive ductal carcinoma
- MMTV
mouse mammary tumor virus
- OS
overall survival
- PCNA
proliferating cell nuclear antigen
- PPS
postprogression survival
- PyMT
polyoma middle T antigen
- RFS
relapse-free survival
- RSPO
R-spondin
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SOX2
sex determining region Y-box 2
- SRC
steroid receptor coactivator
- Wnt
wingless type
- ZEB
zinc finger E-box binding homeobox
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Z. Yue, Z. Yuan, L. Lai designed the study, performed the experiments and the data analysis, and wrote the manuscript; L. Zeng Y. Wang, J. Li, P. Sun, X. Xue, and J. Qi performed the experiments and the data analysis; Z. Yang interpreted the results, performed the data analysis, and revised the manuscript; Y. Zheng performed the bioinformatic analysis; Y. Fang performed cell migration experiments and the data analysis; D. Li performed the data analysis; S. Siwko performed the data analysis and wrote the manuscript; Y. Li interpreted the results, and provided reagents; and J. Luo and M. Liu supervised the study, designed the experiments, interpreted results, and wrote the manuscript.
REFERENCES
- 1.DeSantis C. E., Fedewa S. A., Goding Sauer A., Kramer J. L., Smith R. A., Jemal A. (2016) Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J. Clin. 66, 31–42 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Baade P. D., Zhang S., Zeng H., Bray F., Jemal A., Yu X. Q., He J. (2016) Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Nusse R., Varmus H. E. (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31, 99–109 10.1016/0092-8674(82)90409-3 [DOI] [PubMed] [Google Scholar]
- 4.Lee F. S., Lane T. F., Kuo A., Shackleford G. M., Leder P. (1995) Insertional mutagenesis identifies a member of the Wnt gene family as a candidate oncogene in the mammary epithelium of int-2/Fgf-3 transgenic mice. Proc. Natl. Acad. Sci. USA 92, 2268–2272 10.1073/pnas.92.6.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S., Fantini D., Merrill B., Bagchi S., Raychaudhuri P. (2017) DDB2 activates Rnf43 and regulates Wnt/β-catenin signaling in colorectal cancer cells. FASEB J. 31, 775.22 [Google Scholar]
- 6.Nagahata T., Shimada T., Harada A., Nagai H., Onda M., Yokoyama S., Shiba T., Jin E., Kawanami O., Emi M. (2003) Amplification, up-regulation and over-expression of DVL-1, the human counterpart of the Drosophila disheveled gene, in primary breast cancers. Cancer Sci. 94, 515–518 10.1111/j.1349-7006.2003.tb01475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khramtsov A. I., Khramtsova G. F., Tretiakova M., Huo D., Olopade O. I., Goss K. H. (2010) Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 176, 2911–2920 10.2353/ajpath.2010.091125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shackleton M., Vaillant F., Simpson K. J., Stingl J., Smyth G. K., Asselin-Labat M. L., Wu L., Lindeman G. J., Visvader J. E. (2006) Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 10.1038/nature04372 [DOI] [PubMed] [Google Scholar]
- 9.Wang D., Cai C., Dong X., Yu Q. C., Zhang X. O., Yang L., Zeng Y. A. (2015) Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517, 81–84 10.1038/nature13851 [DOI] [PubMed] [Google Scholar]
- 10.Monteiro J., Gaspar C., Richer W., Franken P. F., Sacchetti A., Joosten R., Idali A., Brandao J., Decraene C., Fodde R. (2014) Cancer stemness in Wnt-driven mammary tumorigenesis. Carcinogenesis 35, 2–13 10.1093/carcin/bgt279 [DOI] [PubMed] [Google Scholar]
- 11.Chakrabarti R., Wei Y., Hwang J., Hang X., Andres Blanco M., Choudhury A., Tiede B., Romano R. A., DeCoste C., Mercatali L., Ibrahim T., Amadori D., Kannan N., Eaves C. J., Sinha S., Kang Y. (2014) DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat. Cell Biol. 16, 1004–1015, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green J. L., La J., Yum K. W., Desai P., Rodewald L. W., Zhang X., Leblanc M., Nusse R., Lewis M. T., Wahl G. M. (2013) Paracrine Wnt signaling both promotes and inhibits human breast tumor growth. Proc. Natl. Acad. Sci. USA 110, 6991–6996 10.1073/pnas.1303671110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi T., Weng J., Siwko S., Luo J., Li D., Liu M. (2014) Lgr4/Gpr48 inactivation leads to aniridia-genitourinary anomalies-mental retardation syndrome defects. J. Biol. Chem. 289, 8767–8780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng J., Luo J., Cheng X., Jin C., Zhou X., Qu J., Tu L., Ai D., Li D., Wang J., Martin J. F., Amendt B. A., Liu M. (2008) Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc. Natl. Acad. Sci. USA 105, 6081–6086 10.1073/pnas.0708257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato S., Mohri Y., Matsuo T., Ogawa E., Umezawa A., Okuyama R., Nishimori K. (2007) Eye-open at birth phenotype with reduced keratinocyte motility in LGR4 null mice. FEBS Lett. 581, 4685–4690 10.1016/j.febslet.2007.08.064 [DOI] [PubMed] [Google Scholar]
- 16.Luo W., Rodriguez M., Valdez J. M., Zhu X., Tan K., Li D., Siwko S., Xin L., Liu M. (2013) Lgr4 is a key regulator of prostate development and prostate stem cell differentiation. Stem Cells 31, 2492–2505 10.1002/stem.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo J., Zhou W., Zhou X., Li D., Weng J., Yi Z., Cho S. G., Li C., Yi T., Wu X., Li X. Y., de Crombrugghe B., Höök M., Liu M. (2009) Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 136, 2747–2756 10.1242/dev.033571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J., Yang Z., Ma Y., Yue Z., Lin H., Qu G., Huang J., Dai W., Li C., Zheng C., Xu L., Chen H., Wang J., Li D., Siwko S., Penninger J. M., Ning G., Xiao J., Liu M. (2016) LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 22, 539–546 10.1038/nm.4076 [DOI] [PubMed] [Google Scholar]
- 19.Yamashita R., Takegawa Y., Sakumoto M., Nakahara M., Kawazu H., Hoshii T., Araki K., Yokouchi Y., Yamamura K. (2009) Defective development of the gall bladder and cystic duct in Lgr4-hypomorphic mice. Dev. Dyn. 238, 993–1000 10.1002/dvdy.21900 [DOI] [PubMed] [Google Scholar]
- 20.Qian Y., Liu S., Guan Y., Pan H., Guan X., Qiu Z., Li L., Gao N., Zhao Y., Li X., Lu Y., Liu M., Li D. (2013) Lgr4-mediated Wnt/β-catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development 140, 1751–1761 10.1242/dev.093641 [DOI] [PubMed] [Google Scholar]
- 21.Sone M., Oyama K., Mohri Y., Hayashi R., Clevers H., Nishimori K. (2013) LGR4 expressed in uterine epithelium is necessary for uterine gland development and contributes to decidualization in mice. FASEB J. 27, 4917–4928 10.1096/fj.13-232215 [DOI] [PubMed] [Google Scholar]
- 22.Du B., Luo W., Li R., Tan B., Han H., Lu X., Li D., Qian M., Zhang D., Zhao Y., Liu M. (2013) Lgr4/Gpr48 negatively regulates TLR2/4-associated pattern recognition and innate immunity by targeting CD14 expression. J. Biol. Chem. 288, 15131–15141 10.1074/jbc.M113.455535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Li X., Ke Y., Lu Y., Wang F., Fan N., Sun H., Zhang H., Liu R., Yang J., Ye L., Liu M., Ning G. (2012) GPR48 increases mineralocorticoid receptor gene expression. J. Am. Soc. Nephrol. 23, 281–293 10.1681/ASN.2011040351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Liu R., Wang F., Hong J., Li X., Chen M., Ke Y., Zhang X., Ma Q., Wang R., Shi J., Cui B., Gu W., Zhang Y., Zhang Z., Wang W., Xia X., Liu M., Ning G. (2013) Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nat. Cell Biol. 15, 1455–1463 10.1038/ncb2867 [DOI] [PubMed] [Google Scholar]
- 25.Wang F., Zhang X., Wang J., Chen M., Fan N., Ma Q., Liu R., Wang R., Li X., Liu M., Ning G. (2014) LGR4 acts as a link between the peripheral circadian clock and lipid metabolism in liver. J. Mol. Endocrinol. 52, 133–143 10.1530/JME-13-0042 [DOI] [PubMed] [Google Scholar]
- 26.Song H., Luo J., Luo W., Weng J., Wang Z., Li B., Li D., Liu M. (2008) Inactivation of G-protein-coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathway. J. Biol. Chem. 283, 36687–36697 10.1074/jbc.M800721200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., Stange D. E., van Es J. E., Guardavaccaro D., Schasfoort R. B., Mohri Y., Nishimori K., Mohammed S., Heck A. J., Clevers H. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- 28.Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452–11457 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyama K., Mohri Y., Sone M., Nawa A., Nishimori K. (2011) Conditional knockout of Lgr4 leads to impaired ductal elongation and branching morphogenesis in mouse mammary glands. Sex Dev. 5, 205–212 10.1159/000329476 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Dong J., Li D., Lai L., Siwko S., Li Y., Liu M. (2013) Lgr4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2. Stem Cells [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y., Kitagawa K., Hiramatsu Y., Kikuchi H., Isobe T., Shimada M., Uchida C., Hattori T., Oda T., Nakayama K., Nakayama K. I., Tanaka T., Konno H., Kitagawa M. (2006) Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res. 66, 11623–11631 10.1158/0008-5472.CAN-06-2629 [DOI] [PubMed] [Google Scholar]
- 32.Gao Y., Shan Z. Y., Wang H., Zhang H. M., Teng W. P. (2009) Inhibitory effect of shRNA targeting GPR48 on invasion and metastasis of human cervical carcinoma cell line HeLa. Chin. J. Cancer 28, 104–107 [PubMed] [Google Scholar]
- 33.Liang F., Yue J., Wang J., Zhang L., Fan R., Zhang H., Zhang Q. (2015) GPCR48/LGR4 promotes tumorigenesis of prostate cancer via PI3K/Akt signaling pathway. Med. Oncol. 32, 49 10.1007/s12032-015-0486-1 [DOI] [PubMed] [Google Scholar]
- 34.Wu J., Xie N., Xie K., Zeng J., Cheng L., Lei Y., Liu Y., Song L., Dong D., Chen Y., Zeng R., Nice E. C., Huang C., Wei Y. (2013) GPR48, a poor prognostic factor, promotes tumor metastasis and activates beta-catenin/TCF signaling in colorectal cancer. Carcinogenesis 34, 2861–2869 [DOI] [PubMed] [Google Scholar]
- 35.Gong X., Yi J., Carmon K. S., Crumbley C. A., Xiong W., Thomas A., Fan X., Guo S., An Z., Chang J. T., Liu Q. J. (2015) Aberrant RSPO3-LGR4 signaling in Keap1-deficient lung adenocarcinomas promotes tumor aggressiveness. Oncogene 34, 4692–4701 10.1038/onc.2014.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu C. Y., Liang G. B., Du P., Liu Y. H. (2013) Lgr4 promotes glioma cell proliferation through activation of Wnt signaling. Asian Pac. J. Cancer Prev. 14, 4907–4911 10.7314/APJCP.2013.14.8.4907 [DOI] [PubMed] [Google Scholar]
- 37.Styrkarsdottir U., Thorleifsson G., Sulem P., Gudbjartsson D. F., Sigurdsson A., Jonasdottir A., Jonasdottir A., Oddsson A., Helgason A., Magnusson O. T., Walters G. B., Frigge M. L., Helgadottir H. T., Johannsdottir H., Bergsteinsdottir K., Ogmundsdottir M. H., Center J. R., Nguyen T. V., Eisman J. A., Christiansen C., Steingrimsson E., Jonasson J. G., Tryggvadottir L., Eyjolfsson G. I., Theodors A., Jonsson T., Ingvarsson T., Olafsson I., Rafnar T., Kong A., Sigurdsson G., Masson G., Thorsteinsdottir U., Stefansson K. (2013) Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature 497, 517–520 10.1038/nature12124 [DOI] [PubMed] [Google Scholar]
- 38.Cho S. G., Wang Y., Rodriguez M., Tan K., Zhang W., Luo J., Li D., Liu M. (2011) Haploinsufficiency in the prometastasis Kiss1 receptor Gpr54 delays breast tumor initiation, progression, and lung metastasis. Cancer Res. 71, 6535–6546 10.1158/0008-5472.CAN-11-0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukamoto A. S., Grosschedl R., Guzman R. C., Parslow T., Varmus H. E. (1988) Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55, 619–625 10.1016/0092-8674(88)90220-6 [DOI] [PubMed] [Google Scholar]
- 40.Lu J., Guo H., Treekitkarnmongkol W., Li P., Zhang J., Shi B., Ling C., Zhou X., Chen T., Chiao P. J., Feng X., Seewaldt V. L., Muller W. J., Sahin A., Hung M. C., Yu D. (2009) 14-3-3zeta operates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell 16, 195–207 10.1016/j.ccr.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu P., Dang Y., Wang L., Liu X., Ren X., Gu J., Liu M., Dai X., Ye X. (2016) Lgr4 is crucial for skin carcinogenesis by regulating MEK/ERK and Wnt/β-catenin signaling pathways. Cancer Lett. 383, 161–170 10.1016/j.canlet.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi Z. F., Cho S. G., Zhao H., Wu Y. Y., Luo J., Li D., Yi T., Xu X., Wu Z., Liu M. (2009) A novel peptide from human apolipoprotein(a) inhibits angiogenesis and tumor growth by targeting c-Src phosphorylation in VEGF-induced human umbilical endothelial cells. Int. J. Cancer 124, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Debnath J., Muthuswamy S. K., Brugge J. S. (2003) Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 10.1016/S1046-2023(03)00032-X [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Gu Y., Han Y., Zhang Q., Jiang Z., Zhang X., Huang B., Xu X., Zheng J., Cao X. (2016) Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 30, 243–256 10.1016/j.ccell.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 45.Hu Y., Smyth G. K. (2009) ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347, 70–78 [DOI] [PubMed] [Google Scholar]
- 46.Lanczky A., Nagy A., Bottai G., Munkacsy G., Szabo A., Santarpia L., Gyorffy B. (2016) miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. 160, 439–446 [DOI] [PubMed] [Google Scholar]
- 47.Zhou J., Chen Y., Huang Y., Long J., Wan F., Zhang S. (2013) Serum follicle-stimulating hormone level is associated with human epidermal growth factor receptor type 2 and Ki67 expression in post-menopausal females with breast cancer. Oncol. Lett. 6, 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Govindaraj V., Arya S. V., Rao A. J. (2014) Differential action of glycoprotein hormones: significance in cancer progression. Horm. Cancer 5, 1–10 10.1007/s12672-013-0164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leis O., Eguiara A., Lopez-Arribillaga E., Alberdi M. J., Hernandez-Garcia S., Elorriaga K., Pandiella A., Rezola R., Martin A. G. (2012) Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene 31, 1354–1365 10.1038/onc.2011.338 [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 51.Wu Z. Q., Li X. Y., Hu C. Y., Ford M., Kleer C. G., Weiss S. J. (2012) Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic breast cancer 1, early onset (BRCA1) repression. Proc. Natl. Acad. Sci. USA 109, 16654–16659 10.1073/pnas.1205822109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yook J. I., Li X. Y., Ota I., Hu C., Kim H. S., Kim N. H., Cha S. Y., Ryu J. K., Choi Y. J., Kim J., Fearon E. R., Weiss S. J. (2006) A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 8, 1398–1406 10.1038/ncb1508 [DOI] [PubMed] [Google Scholar]
- 53.DiMeo T. A., Anderson K., Phadke P., Fan C., Perou C. M., Naber S., Kuperwasser C. (2009) A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 69, 5364–5373 10.1158/0008-5472.CAN-08-4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmon K. S., Gong X., Yi J., Thomas A., Liu Q. (2014) RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc. Natl. Acad. Sci. USA 111, E1221–E1229 10.1073/pnas.1323106111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazerbourg S., Bouley D. M., Sudo S., Klein C. A., Zhang J. V., Kawamura K., Goodrich L. V., Rayburn H., Tessier-Lavigne M., Hsueh A. J. (2004) Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol. Endocrinol. 18, 2241–2254 10.1210/me.2004-0133 [DOI] [PubMed] [Google Scholar]
- 56.Shi G. X., Zheng X. F., Zhu C., Li B., Wang Y. R., Jiang S. D., Jiang L. S. (2017) Evidence of the role of R-Spondin 1 and its receptor Lgr4 in the transmission of mechanical stimuli to biological signals for bone formation. Int. J. Mol. Sci. 18, 564 10.3390/ijms18030564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu C., Zheng X. F., Yang Y. H., Li B., Wang Y. R., Jiang S. D., Jiang L. S. (2016) LGR4 acts as a key receptor for R-spondin 2 to promote osteogenesis through Wnt signaling pathway. Cell. Signal. 28, 989–1000 10.1016/j.cellsig.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 58.Yamakami Y., Kohashi K., Oyama K., Mohri Y., Hidema S., Nishimori K. (2016) LGR4 is required for sequential molar development. Biochem. Biophys. Rep. 8, 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guan X., Duan Y., Zeng Q., Pan H., Qian Y., Li D., Cao X., Liu M. (2014) Lgr4 protein deficiency induces ataxia-like phenotype in mice and impairs long term depression at cerebellar parallel fiber-Purkinje cell synapses. J. Biol. Chem. 289, 26492–26504 10.1074/jbc.M114.564138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin C., Yin F., Lin M., Li H., Wang Z., Weng J., Liu M., Da Dong X., Qu J., Tu L. (2008) GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Invest. Ophthalmol. Vis. Sci. 49, 4245–4253 10.1167/iovs.08-1860 [DOI] [PubMed] [Google Scholar]
- 61.Kinzel B., Pikiolek M., Orsini V., Sprunger J., Isken A., Zietzling S., Desplanches M., Dubost V., Breustedt D., Valdez R., Liu D., Theil D., Müller M., Dietrich B., Bouwmeester T., Ruffner H., Tchorz J. S. (2014) Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev. Biol. 390, 181–190 10.1016/j.ydbio.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 62.Liu S., Qian Y., Li L., Wei G., Guan Y., Pan H., Guan X., Zhang L., Lu X., Zhao Y., Liu M., Li D. (2013) Lgr4 gene deficiency increases susceptibility and severity of dextran sodium sulfate-induced inflammatory bowel disease in mice. J. Biol. Chem. 288, 8794–8803, discussion 8804 10.1074/jbc.M112.436204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dang Y., Liu B., Xu P., Zhu P., Zhai Y., Liu M., Ye X. (2014) Gpr48 deficiency induces polycystic kidney lesions and renal fibrosis in mice by activating Wnt signal pathway. PLoS One 9, e89835 10.1371/journal.pone.0089835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kato S., Matsubara M., Matsuo T., Mohri Y., Kazama I., Hatano R., Umezawa A., Nishimori K. (2006) Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron, Exp. Nephrol. 104, e63–e75 10.1159/000093999 [DOI] [PubMed] [Google Scholar]
- 65.Mohri Y., Oyama K., Sone M., Akamatsu A., Nishimori K. (2012) LGR4 is required for the cell survival of the peripheral mesenchyme at the embryonic stages of nephrogenesis. Biosci. Biotechnol. Biochem. 76, 888–891 10.1271/bbb.110834 [DOI] [PubMed] [Google Scholar]
- 66.Mendive F., Laurent P., Van Schoore G., Skarnes W., Pochet R., Vassart G. (2006) Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev. Biol. 290, 421–434 10.1016/j.ydbio.2005.11.043 [DOI] [PubMed] [Google Scholar]
- 67.Mohri Y., Umezu T., Hidema S., Tomisawa H., Akamatsu A., Kato S., Nawa A., Nishimori K. (2010) Reduced fertility with impairment of early-stage embryos observed in mice lacking Lgr4 in epithelial tissues. Fertil. Steril. 94, 2878–2881 10.1016/j.fertnstert.2010.05.050 [DOI] [PubMed] [Google Scholar]
- 68.Hoshii T., Takeo T., Nakagata N., Takeya M., Araki K., Yamamura K. (2007) LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol. Reprod. 76, 303–313 10.1095/biolreprod.106.054619 [DOI] [PubMed] [Google Scholar]
- 69.Pan H., Cui H., Liu S., Qian Y., Wu H., Li L., Guan Y., Guan X., Zhang L., Fan H. Y., Ma Y., Li R., Liu M., Li D. (2014) Lgr4 gene regulates corpus luteum maturation through modulation of the WNT-mediated EGFR-ERK signaling pathway. Endocrinology 155, 3624–3637 10.1210/en.2013-2183 [DOI] [PubMed] [Google Scholar]
- 70.Kida T., Oyama K., Sone M., Koizumi M., Hidema S., Nishimori K. (2014) Lgr4 is required for endometrial receptivity acquired through ovarian hormone signaling. Biosci. Biotechnol. Biochem. 78, 1813–1816 10.1080/09168451.2014.936353 [DOI] [PubMed] [Google Scholar]
- 71.Koizumi M., Oyama K., Yamakami Y., Kida T., Satoh R., Kato S., Hidema S., Oe T., Goto T., Clevers H., Nawa A., Nishimori K. (2015) Lgr4 controls specialization of female gonads in mice. Biol. Reprod. 93, 90 10.1095/biolreprod.114.123638 [DOI] [PubMed] [Google Scholar]
- 72.Planas-Paz L., Orsini V., Boulter L., Calabrese D., Pikiolek M., Nigsch F., Xie Y., Roma G., Donovan A., Marti P., Beckmann N., Dill M. T., Carbone W., Bergling S., Isken A., Mueller M., Kinzel B., Yang Y., Mao X., Nicholson T. B., Zamponi R., Capodieci P., Valdez R., Rivera D., Loew A., Ukomadu C., Terracciano L. M., Bouwmeester T., Cong F., Heim M. H., Forbes S. J., Ruffner H., Tchorz J. S. (2016) The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat. Cell Biol. 18, 467–479 10.1038/ncb3337 [DOI] [PubMed] [Google Scholar]
- 73.Sun Y., Hong J., Chen M., Ke Y., Zhao S., Liu W., Ma Q., Shi J., Zou Y., Ning T., Zhang Z., Liu R., Wang J., Ning G. (2015) Ablation of Lgr4 enhances energy adaptation in skeletal muscle via activation of Ampk/Sirt1/Pgc1α pathway. Biochem. Biophys. Res. Commun. 464, 396–400 10.1016/j.bbrc.2015.06.066 [DOI] [PubMed] [Google Scholar]
- 74.Zou Y., Ning T., Shi J., Chen M., Ding L., Huang Y., Kauderer S., Xu M., Cui B., Bi Y., Liu S., Hong J., Liu R., Ning G., Wang J. (2017) Association of a gain-of-function variant in LGR4 with central obesity. Obesity (Silver Spring) 25, 252–260 10.1002/oby.21704 [DOI] [PubMed] [Google Scholar]
- 75.Li J. Y., Chai B., Zhang W., Fritze D. M., Zhang C., Mulholland M. W. (2014) LGR4 and its ligands, R-spondin 1 and R-spondin 3, regulate food intake in the hypothalamus of male rats. Endocrinology 155, 429–440 10.1210/en.2013-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mustata R. C., Van Loy T., Lefort A., Libert F., Strollo S., Vassart G., Garcia M. I. (2011) Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep. 12, 558–564 10.1038/embor.2011.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo W., Tan P., Rodriguez M., He L., Tan K., Zeng L., Siwko S., Liu M. (2017) Leucine-rich repeat-containing G protein-coupled receptor 4 (Lgr4) is necessary for prostate cancer metastasis via epithelial-mesenchymal transition. J. Biol. Chem. 292, 15525–15537 10.1074/jbc.M116.771931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snyder J. C., Rochelle L. K., Marion S., Lyerly H. K., Barak L. S., Caron M. G. (2015) Lgr4 and Lgr5 drive the formation of long actin-rich cytoneme-like membrane protrusions. J. Cell Sci. 128, 1230–1240 10.1242/jcs.166322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu M., Bardia A., Wittner B. S., Stott S. L., Smas M. E., Ting D. T., Isakoff S. J., Ciciliano J. C., Wells M. N., Shah A. M., Concannon K. F., Donaldson M. C., Sequist L. V., Brachtel E., Sgroi D., Baselga J., Ramaswamy S., Toner M., Haber D. A., Maheswaran S. (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Wever O., Pauwels P., De Craene B., Sabbah M., Emami S., Redeuilh G., Gespach C., Bracke M., Berx G. (2008) Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem. Cell Biol. 130, 481–494 10.1007/s00418-008-0464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang J., Mani S. A., Donaher J. L., Ramaswamy S., Itzykson R. A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R. A. (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 10.1016/j.cell.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 82.Liu T., Zhang X., Shang M., Zhang Y., Xia B., Niu M., Liu Y., Pang D. (2013) Dysregulated expression of Slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal-like breast cancer. J. Surg. Oncol. 107, 188–194 10.1002/jso.23240 [DOI] [PubMed] [Google Scholar]
- 83.Guo W., Keckesova Z., Donaher J. L., Shibue T., Tischler V., Reinhardt F., Itzkovitz S., Noske A., Zürrer-Härdi U., Bell G., Tam W. L., Mani S. A., van Oudenaarden A., Weinberg R. A. (2012) Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–1028 10.1016/j.cell.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L., Tang H., Kong Y., Xie X., Chen J., Song C., Liu X., Ye F., Li N., Wang N., Xie X. (2015) LGR5 promotes breast cancer progression and maintains stem-like cells through activation of Wnt/β-catenin signaling. Stem Cells 33, 2913–2924 10.1002/stem.2083 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.