Abstract
Objective
To assess if manual therapy (MT) in the treatment of plantar fasciitis (PF) patients improves pain and function more effectively than other interventions.
Methods
A systematic review of all randomized control trials (RCTs) investigating the effects of MT in the treatment of human patients with PF, plantar fasciosis, and heel pain published in English on PubMed, CINAHL, Cochrane, and Web of Science databases was conducted. Research quality was appraised utilizing the PEDro scale. Cohen’s d effect sizes (ES) and associated 95% confidence intervals (CI) were calculated between treatment groups.
Results
Seven RCTs were selected that employed MT as a primary independent variable and pain and function as dependent variables. Inclusion of MT in treatment yielded greater improvement in function (6 of 7 studies, CI that did not cross zero in 14 of 25 variables, ES = 0.5–21.5) and algometry (3 of 3 studies, CI that did not cross zero in 9 of 10 variables, ES = 0.7–3.0) from 4 weeks to 6 months when compared to interventions such as stretching, strengthening, or modalities. Though pain improved with the inclusion of MT, ES calculations favored MT in only 2 of 6 studies (3 of 13 variables) and was otherwise equivalent in effectiveness to comparison interventions.
Discussion
MT is clearly associated with improved function and may be associated with pain reduction in PF patients. It is recommended that clinicians consider use of both joint and soft tissue mobilization techniques in conjunction with stretching and strengthening when treating patients with PF.
Level of Evidence
Treatment, level 1a.
Keywords: Mobilization, manipulation, heel pain, soft tissue, aponeurosis, plantar fascia
Introduction
Plantar heel pain is a common musculoskeletal complaint that affects an estimated 1–2 million people per year in the United States (US)[1–3] and approximately 10% of the population at some point during their lives [4]. Among the potential etiologies of plantar heel pain, plantar fasciitis (PF) is the most common [5–7]. PF is a clinical condition marked with complaints of sharp pain in the heel starting from the medial border of the plantar fascia continuing to its insertion at the medial tuberosity of the calcaneus. Pain is often provoked with loading and with the initial few steps following periods of inactivity, such as rising from sleep in morning, and often increase toward the end of the day [5,7,8].
The symptoms associated with PF are frequently attributed to inflammation of the plantar fascia. Other evidence has suggested an alternative mechanism to the onset of PF [9]. In plantar fasciosis, degenerative changes and microscopic tearing [9] may lead to thickening of the plantar fascia [10,11]. For this manuscript, PF and plantar fasciosis will be encompassed under the same diagnostic umbrella. Evidence suggests that intrinsic and extrinsic risk factors, both modifiable and non-modifiable, influence the outcome of PF [12,13]. These elements consist of factors such as prolonged standing, inappropriate footwear, previous injury, limited dorsiflexion of the ankle, hyperpronation of the foot, weak calf musculature, aging, and increased Body Mass Index [12,13]. Alteration of ankle-foot biomechanics resulting from soft tissue or joint limitation is postulated to contribute to the development of PF [7,14–16] and may be remedied from treatments such as manual therapy (MT).
More than 1 million ambulatory patient care visits are made annually for assessment and treatment of PF in the US [3,17]. It is important for clinicians to be able to treat these patients comprehensively using evidence-based interventions. Recommendations for using MT, such as soft tissue mobilization and joint mobilization or manipulation, in conservative treatment have recently been reported. In a 2008 clinical practice guideline (CPG) put forth by the Orthopaedic Section of the American Physical Therapy Association, MT received a recommendation grading of ‘E,’ indicating theoretical or foundational evidence to support the use of this intervention in the treatment of PF patients [18]. In just 6 years, the updated and most recent CPG published in 2014 now recommends MT in the care of PF patients with a grade of ‘A,’ indicating a strong recommendation based on a multitude of level I and II studies in the literature [19]. Utilization of MT by physical therapists in the care of patients with PF has progressively increased in recent years and appears to result in decreased cost and length of care [3]. The mechanism of effectiveness of MT is multifactorial and encompasses mechanical, neurophysiological, and psycho-emotional effects [20], all of which may benefit patients with PF. Despite growth of evidence for the use of MT in the care of patients with PF, the authors are unaware of any systematic reviews that have compared MT to other interventions in this patient population. The purpose of this systematic review was to compare randomized control trials (RCTs) of MT, to include soft tissue mobilization and joint mobilization or manipulation, with control interventions on the outcomes of patient-reported pain, patient-reported function, and pressure-pain thresholds (PPT) measured by algometry in patients with PF.
Methods
This systematic review was registered in PROSPERO (CRD42016038379) and can be accessed at https://goo.gl/f296V2.
Search strategy
A medical research librarian assisted in the development of a systematic search of PubMed, CINAHL, Cochrane, and Web of Science databases utilizing the search terms: ((groups[TIAB] OR trial[TIAB]) OR randomly[TIAB] OR placebo[TIAB] OR randomized[TIAB] OR Controlled clinical trial[pt] OR Randomized controlled trial[pt]) AND (((((‘Fasciitis’[Mesh] AND ‘Foot Diseases’[Mesh]) OR (‘planter fasciitis’[All Fields] OR ‘plantar fasciosis’[All Fields] OR ‘Fasciitis, Plantar’[Mesh] OR plantar fascia[text word] OR plantar fasciae[text word] OR plantar fascias[text word] OR plantar fasciopathy[text word] OR plantar fascitis[text word]) OR (calcaneodynia[text word] OR ‘calcaneal periostitis’[text word] OR enthesopathy[text word] OR ‘heel spur’[text word])) OR ((pain[text word] OR inflammation[text word] OR inflammatory[text word] OR inflame[text word] OR inflamed[text word]) AND (plantar[text word] OR (heel[text word] OR heels[text word]) OR foot[text word] OR feet[text word] OR arch[text word] OR arches[text word]))) AND ((manual[tw] OR physical[tw] OR manipulate[tw] OR manipulation[tw] AND therapy[tw] OR therapies[tw] OR therapeutic[tw] OR physiotherapy[tw]) OR ((joint[text word] OR mobility[text word] OR mobile[text word] OR mobilization[text word] OR ‘joints’[MeSH Terms] OR ‘joints’[All Fields] OR soft tissue[tw]) AND (manipulate[tw] OR manipulation[tw])))) AND ‘humans’[MeSH Terms]) AND English[lang].
Study selection criteria
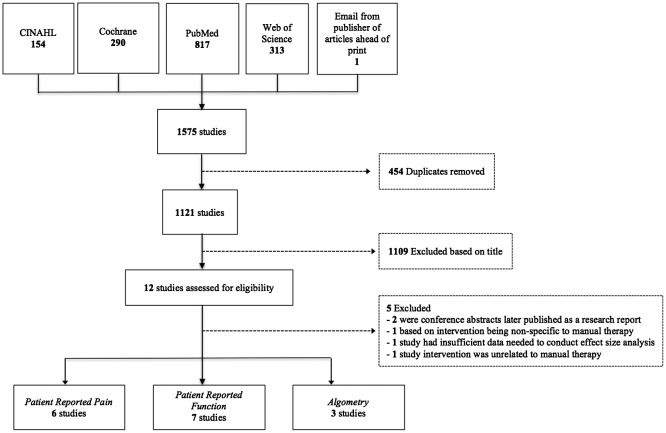
Studies were included if they were an RCT that employed a form of MT in the experimental group for the treatment of patients with PF. Inclusion criteria was non-specific for the treatment setting, the type of MT utilized, the discipline of the treating clinician, or the comparison intervention utilized in the design. MT interventions, which included both soft tissue mobilization and joint mobilization or manipulation, were often employed conjunctively with another treatment such as self-stretching exercises. To be included in this review, MT had to be a focal independent variable in the study design. Outcome measures of interest included patient-reported pain, PPT during algometric testing, and patient self-reported function. In the case of studies that did not provide statistical measures of mean and variance, the corresponding author was contacted. Studies were excluded if the corresponding author was unable to provide this information. See Figure 1 for details of the study selection process.
Figure 1.
Study selection process and search results with outcome measures of concern.
Assessment of methodological quality
The methodological quality of the included studies was assessed using the PEDro scale, a 10 item assessment of quality of RCTs, with a score of 10 representative of the highest quality study and 0 representative of the lowest [21]. Three of the authors scored the included studies independently and came to a consensus on the final PEDro score for each study. In the event a consensus could not be achieved, the fourth and most senior author would independently make the final determination of quality for the disputed study.
Data extraction and statistical analysis
Study design, sample population, setting, experimental and comparison interventions, and group means and standard deviations for patient-reported pain and function and algometric PPT were extracted for each reported time point in the included studies (Table 1). Post-intervention means and standard deviations were calculated for studies that reported baseline means, pre-post change scores, and variance. Statistical analysis was performed by calculating Cohen’s d effect sizes (ES) and associated 95% confidence intervals (CI) [22]. ES were interpreted using the scheme proposed by Cohen [22]:<0.2 equates to a trivial ES, 0.2–0.49 small, 0.5–0.79 moderate, and ≥0.8 large. When the ES point estimates and 95% CI were plotted, the treatment effect was interpreted as being conclusively advantageous over the other when the 95% CI did not cross zero. Meta-analysis was not performed due to the heterogeneity of MT and comparison interventions and outcome measures used across the reviewed studies.
Table 1.
Characteristics of the seven Randomized Control Trials (RCT) comparing manual therapeutic interventions with control interventions in patients with plantar fasciitis.
| Author Year Design | Sample population | Inclusion criteria | Groups/intervention | Experimental mean, SD | Control mean, SD | Outcomes and assessment time points | PEDRO score |
|---|---|---|---|---|---|---|---|
| Ajimsha 2013 RCT | Adults (n = 67; 49 females/17 males) with unilateral heel pain treated at a nonprofit research foundation clinic | Insidious onset of sharp pain under the plantar heel surface upon weight bearing after a period of non- weight bearing, Plantar heel pain that increases in the morning with the first steps after waking up, and symptoms decreasing with slight levels of activity | Manual therapy group (n = 33): Myofascial release of the gastrocnemius (gastroc) X 5-min, soleus X 5-min, plantar fascia 2 X 5-min, 3/wk X 4-wks | FFI: BL 63.01 ± 4.44 4wks17.39 ± 4.02 12wks 24.81 ± 3.98 | FFI: BL 61.38 ± 5.22 4wks 56.85 ± 6.91 12wks 60.15 ± 8.11 | FFI, Algometry: 4wks, 12wks | 9 |
| Control group (n = 32): Sham ultrasound | PPT: Gastroc BL 1.8 ± 0.44 4wks 2.9 ± 0.82 12wks 2.6 ± 0.54 Soleus BL 2.0 ± 0.48 4wks 3.1 ± 0.91 12wks 2.7 ± 0.65 Calcaneus BL 2.1 ± 0.38 4wks3.4 ± 0.95 12wks 3.1 ± 0.78 | PPT: Gastroc BL 2.0 ± 0.22 4wks 2.2 ± 0.51 12wks2.1 ± 0.32 Soleus BL 2.2 ± 0.52 4wks 2.2 ± 0.31 12wks 2.1 ± 0.72 Calcaneus BL 2.3 ± 0.77 4wks 2.5 ± 0.67 12wks 2.4 ± 0.48 | |||||
| Celik 2015 RCT | Adults (n = 43; 23 females, 20 males) with PF treated in an ortho and trauma clinic | Patients with a point of maximal tenderness on clinical examination over the medial tubercle of the calcaneus, pain with palpation of the proximal insertion of the plantar fascia, heel pain during weight-bearing activity, a negative tarsal tunnel test, and a positive windlass test | Manual therapy group (n = 19): Grade I & II subtalar traction and lateral glide; talocrural post glide; 1st TMT dorsal glide; gastroc and plantar fascia stretching 3/wk X 3-wks | VAS: BL 7.8 (1.6), 3wk 5.4 (2.8), 6wk 5.0 (2.3), 12wk 4.9 (2.4) 12mo 2.7 (3.2) | VAS: BL 7.7 (1.5), 3wk 1.8 (2.1), 6wk 1.2 (1.4), 12wk 1.5 (1.9) 12mo 3.3 (3.2) | VAS, FAAM; 3wks, 6wks, 3mos, 12 mos | 7 |
| Injection group (n = 20): Administered 1 cc 40 mg methyl-prednisolone & 4 mL 2% prilocaine to the PF | FAAM: BL 55.2 (18.4), 3wk 60.6 (14.4), 6wk 70.2 (17.5), 12wk 69.4 (16.8), 12mo 86.7 (21.9) | FAAM: BL 45.5 (17.6), 3wk 80.7 (19.4), 6wk 85.7 (11.2), 12wk 83.5 (14.6), 12mo 83.4 (17.3) | |||||
| Cleland 2009 RCT | Adults (n = 60; 42 females, 18 males) with primary heel pain in 2 outpt ortho clinics | Heel pain, LEFS ≤ 65 | Manual therapy group (n = 30): Soft tissue mobilization of triceps surae and plantar fascia, foot X 5-min, Grade III-V ankle, knee, hip mobilizations as needed) and exercise 2/wk X 2-wks 1/wk X 2wks | NPRS: BL: 4.8 (1.9) Mean change (CI) 4wks: −1.4 (−0.8, −2.2) 6wks: −2.8 (−1.9, −3.7) | NPRS: BL: 4.6 (1.6) Mean change (CI) 4wks: −1.4 (−0.8, −2.2) 6wks: −2.8 (−1.9, −3.7) | NPRS, LEFS, FAAM; 4wks, 6mos | 8 |
| Control group (n = 30): Electrophysiological agents and exercise | LEFS: BL: 47.8 (14.3) Mean change (CI) 4wks: 21.0 (15.1, 26.9) 6wks:22.8 (15.6, 30.1) | LEFS: BL: 51.1 (10.8) Mean change (CI) 4wks: 7.5 (3.1, 12.0) 6wks:12.9 (7.8, 18) | |||||
| FAAM: 57.2 (16.4) Mean change (CI) 4wks: 22.2 (15.1, 29.4) 6wks: 31.6 (22.2, 41.1) | FAAM: 57.3 (12.2) Mean change (CI) 4wks: 8.9 (3.6, 14.3) 6wks: 17.9 (12.9, 23.1) | ||||||
| Ghafoor 2016 RCT | Adults (n = 60; 48 females, 12 males) referred from orthopedics with plantar heel pain | Diagnosis of plantar fasciitis, LEFS ≤ 65 | Manual therapy group (n = 30): Soft tissue mobilization of triceps surae and plantar fascia, Gr II & IV rearfoot mobilizations X 5-min, strengthening, stretching, ultrasound X 8-visits | NPRS: BL: 5.1 (1.3) Mean change 3wks: 1.9 (0.2), 6wks: 3.5 (0.1) | NPRS: BL: 4.8 (1.7) Mean change 3wks: 0.4 (0.2), 6wks: 1.6 (0.2) | NPRS, LEFS, FAAM; 3wks, 6wks | 6 |
| FAAM: BL: 54.4 (3.0) Mean change 3wks: 15.1 (0.7), 6wks: 26.6 (0.6) | FAAM: BL: 54.0 (5.0) Mean change 3wks: 1.8 (1.2), 6wks: 6.2 (1.2) | ||||||
| Control group (n = 30): Strengthening, stretching, ultrasound | LEFS: BL: 54.9 (6.2) Mean change 3wks: 11.7 (0.9), 6wks: 17.0 (0.9) | LEFS: BL: 50.1 (4.7) Mean change 3wks: 1.5 (0.8), 6wks: 9.3 (0.8) | |||||
| Renan-Ordine 2011 RCT | Adults (n = 60; 45 females/15 males) with a dx of plantar heel pain treated in a PT clinic | Primary report of unilateral plantar heel pain of insidious onset, sharp pain under the plantar heel surface upon weight bearing after a period of non-weight bearing, pain that increases in the morning with the first steps after waking up and symptoms decreasing with slight levels of activity | Manual therapy group (n = 30): Trigger point manual therapy to gastroc 3 X 90-s, self-stretching protocol, 4/wk X 4-wks | PF: BL 44.3 ± 16.86 4wks 65.2 ± 12.2 Mean Change 20.9 (CI16.5, 25.2) | PF: BL 41.2 ± 16.2 4wks 52.8 ± 19.4 Mean Change 11.6 (CI 8, 15) | Physical function and bodily pain domains of the SF-36 questionnaire; Algometry: Baseline, 4wks | 6 |
| PR: BL 30.3 ± 31.6 4wks 63.5 ± 27.63 Mean Change 3.2 (CI 22.2, 44.1) | PR: BL 29.6 ± 34.7 4wks 50.9 ± 32.9 Mean Change 21.3 (CI 8.2, 34.3) | ||||||
| BP: BL 35.3 ± 18.25 4wks 56.1 ± 13.8 Mean Change 20.8 (CI 16.6, 25.0) | BP: BL 31.7 ± 18.4 4wks 44.7 ± 17.5 Mean Change 13 (CI 9.4, 16.5) | ||||||
| GH: BL 54.6 ± 17.3 4wks 60.8 ± 12.2 Mean Change 6.2 (CI 2.1, 10.3) | GH: BL 54.1 ± 15.9 4wks 54.9 ± 16.2 Mean Change .8 (−2.6, 4.2) | ||||||
| Vit: BL 1.1 ± 18.4 4wks 52.1 ± 15.7 Mean Change 11.0 (CI 2.7, 13.3) | Vit: BL 36.5 ± 18.5 4wks 44.1 ± 19 Mean Change 7.6 (3.7, 11.4) | ||||||
| Control group (n=30): Instruction in in a self-stretching protocol | SF: BL 52.7 ± 24.6 4wks 68.3 ± 18.8 Mean Change 15.6 (CI 9.2, 22.0) | SF: BL 46.2 ± 28.5 4wks 57 ± 17.8 Mean Change 10.8 (2.9, 18.6) | |||||
| ER: BL 47.6 ± 36.7 4wks 78.6 ± 27.5 Mean Change 31.0 (CI 18.3, 43.6) | ER: BL 40.8 ± 39.6 4wks 51.9 ± 32.5 Mean Change 11.1 (0.8, 21.5) | ||||||
| MH: BL 55.3 ± 18.0 4wks 62.0 ± 19.8 Mean Change 6.7 (CI 1.2, 12.3) | MH: BL 51.1 ± 25.7 4wks 60.1 ± 22.2 Mean Change 9 (3.3, 14.9) | ||||||
| Algometry: Gastrocnemius BL: 1.3 ± 0.5 4wks 2.7 ± 0.6; Soleus BL: 1.9 ± 0.6, 4wks: 3.0 ± 0.9; Calcaneus BL 1.7 ± 0.8, 4wks: 3.2 ± 1.3 | Algometry: Gastrocnemius BL: 1.8 ± 0.7 4wks 2.3 ± 0.5; Soleus BL: 2.1 ± 0.5, 4wks: 2.4 ± 0.5; Calcaneus BL 2.3 ± 1.1, 4wks: 2.6 ± 0.9 | ||||||
| Saban 2014 RCT | Adults (n = 69) with plantar heel pain treated in an outpt PT clinic | Plantar heel pain with increased pain on initial weight bearing after a period of rest, lessening with continued activity | Manual therapy group (n = 36): Deep massage X 10-min to posterior calf muscles and neural mobilization with a self-stretch exercise program X 8 visits | VAS: BL: 6.8 (3.0) Mean change (CI) 4–6wks: −2.4 (−1.4, −3.4) | VAS: 7.0 (2.7) Mean change (CI) 4–6wks: −2.5 (−1.4, −3.8) | VAS, FS: 4–6wks | 7 |
| Control group (n = 33): Ultrasound therapy to the painful heel area with the same self-stretch exercises | FS: BL: 47 (13) Mean change (CI) 4–6wks: 15 (9, 21) | FS: BL: 50 (13) Mean change (CI) 4–6wks: 6 (1, 11) | |||||
| Shashua 2015 RCT | Adults (n = 55; 35 females/15 males) with plantar pain | Pain at the bottom of the heel generated by pressure, and an increase in pain (NPRS, greater than 3) in the morning on taking a few steps or after prolonged non–weight bearing | Manual therapy group (n = 25): Anterior/posterior talocrural (weight-bearing and non–weight-bearing), subtalar eversion/inversion, and midtarsal pronation/supination joint mobilizations X 1–1.5-min each, midfoot stretching, exercises, and ultrasound X 8 sessions) | NPRS: BL: 7.76 ± 2.03 2wks: 7.16 ± 2.36 4wks 5.6 ± 3.3 10wks: 4.68 ± 3.38 | NPRS: BL: 8.12 ± 1.77 2wks 6.68 ± 1.89 4wks: 5.28 ± 2.88 10wks 4.76 ± 3.41 | NPRS, LEFS 2wks, 4wks, 10wks | 8 |
| Control group (n = 25): stretching exercises and ultrasound | LEFS: BL: 40.00 ± 16.48 2wks: 43.12 ± 18.47 4wks: 47.6 ± 19.38 10wks: 55.96 ± 19.45 | LEFS: BL 48.16 ± 17.06 2wks 51.88 ± 17.35 4wks 52.32 ± 19.69 10wks: 57.88 ± 18.03 | |||||
| Algometry: BL: 423.17 ± 176.43 4wks: 461.74 ± 184.98 | Algometry Pain: BL 365.52 ± 200.66 4wks: 395.92 ± 198.94 | Algometry: 4wks |
Outpt = Outpatient, PT = Physical Therapy, Ortho = Orthopaedic, Dx = Diagnosis, Post = Posterior, BL = Baseline, wks = weeks, mos = months, VAS = Visual Analogue Scale, FAAM = Foot and Ankle Ability Measure, NPRS = Numeric Pain Rating Scale, LEFS = Lower Extremity Functional Scale, FS = Functional Status of the Foot & Ankle Computerized Adaptive Test, PF = Physical Function scale of the SF-36, PR = Physical Role scale of the SF-36, BP = Bodily pain scale of the SF-36, GH = General Health scale of the SF-36, Vit = Vitality scale of the SF-36, SF = Social Function scale of the SF-36, ER = Emotion role scale of the SF-36, MH = Mental Health scale of the SF-36, FFI = Foot Function Index, PPT = Pressure-pain thresholds.
Results
Our search strategy yielded seven RCTs [23–29] that compared MT interventions to comparative interventions. The details of the subject characteristics, treatment rendered, and assessment time points are summarized in Table 1. Details of the methodological quality assessment are provided in Table 2. PEDro scores for the included studies ranged from 6 to 9. The most common PEDro items that were not addressed involved blinding of the patient or the treating clinician.
Table 2.
PEDro scoring for studies included in analysis.
| Ajimsha (2013) | Celik (2015) | Cleland (2009) | Ghafoor (2016) | Renan-Ordine (2011) | Saban (2014) | Shashua (2015) | |
|---|---|---|---|---|---|---|---|
| 1. Eligibility criteria? | Y | Y | Y | Y | Y | Y | Y |
| 2. Random allocation? | Y | Y | Y | Y | Y | Y | Y |
| 3. Allocation concealed? | N | Y | Y | N | N | Y | Y |
| 4. Groups similar? | Y | Y | Y | Y | Y | Y | Y |
| 5. Subject blinding? | Y | N | N | N | Y | N | N |
| 6. Therapist blinding? | Y | N | N | N | N | N | N |
| 7. Assessor blinding? | Y | N | Y | N | Y | Y | Y |
| 8. 85% subjects completed? | Y | Y | Y | Y | N | N | Y |
| 9. Allocation maintained or intention to treat? | Y | Y | Y | Y | N | Y | Y |
| 10. Between group statistical comparisons? | Y | Y | Y | Y | Y | Y | Y |
| 11. Point and variability measures reported? | Y | Y | Y | Y | Y | Y | Y |
| PEDro Score | 9/10 | 7/10 | 8/10 | 6/10 | 6/10 | 7/10 | 8/10 |
Patient-reported pain
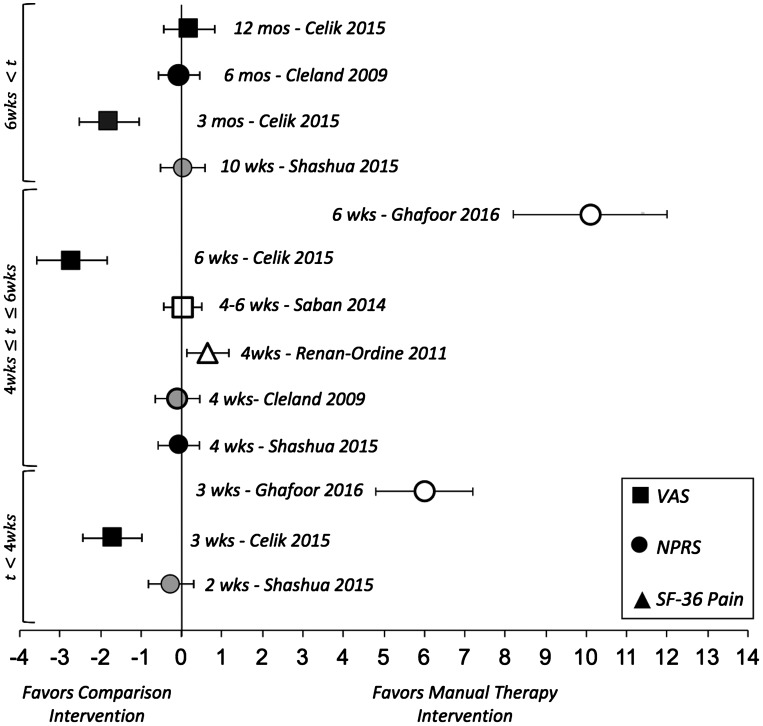
Six studies reported patient-reported pain as an outcome [23–26,28,29]. Of these studies, two utilized the visual analog scale (VAS) [26,28], three utilized the Numeric Pain Rating Scale (NPRS) [23,24,29], and one utilized the Bodily Pain subscale of the SF-36 [25] to assess patient-reported pain. ES point estimates and the associated 95% CI for comparisons of treatment effect on patient-reported pain are illustrated in Figure 2. With the exception of three studies [25,28,29], there were no conclusive differences in patient-reported pain between MT and the comparison groups at 2 weeks through 6 months post treatment. A large and conclusive ES favoring MT and routine care (consisting of stretching, strengthening, and ultrasound) over routine care alone for the NPRS at 3 and 6 week time points [29]. Patients who received MT, in addition to self-stretching, demonstrated moderate ES with 95% CI that did not cross zero on the SF-36 Bodily Pain subscale at 4 weeks post treatment [25]. In a comparison of corticosteroid injection with Grade I-II joint mobilizations and calf and plantar fascia stretching, patients who received the injection had better outcomes, as demonstrated by large ES, at 3 weeks, 6 weeks, and 3 month time points, but fared no better at 12 months [28].
Figure 2.
Effect sizes and 95% CIs of patient-reported outcome measures of pain comparing manual therapy with control interventions in patients with plantar fasciitis.
Algometry
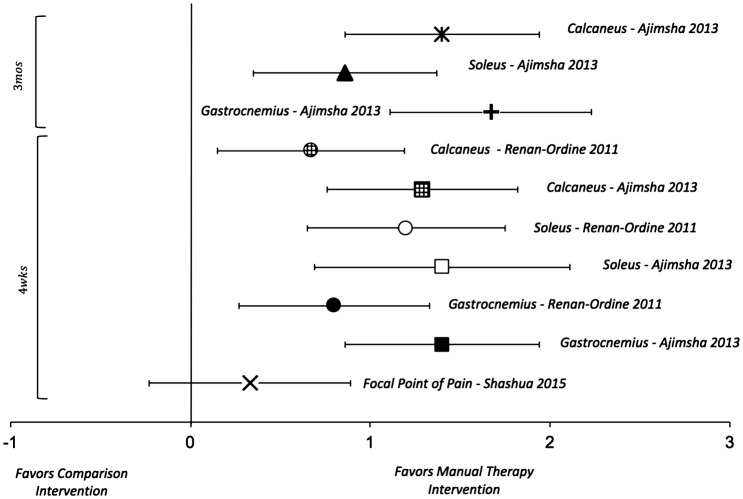
Three studies reported algometric PPT as an outcome [23,25,27]. Of these studies, one utilized the location of the most tender spot on the plantar foot to assess the PPT [23]. The other two studies utilized three standardized points on gastrocnemius, soleus, and the posterior calcaneus to assess PPT [25,27]. The details of the subject characteristics, treatment rendered, and assessment time points are summarized in Table 1. ES point estimates and 95% CI for comparisons of PPT are illustrated in Figure 4. When assessed with algometry, patients treated with MT had conclusively better outcomes than controls at 4 weeks and 3 months with large ES in two studies [25,27], but were equivalent at 4 weeks in the third study [23]. The trend of the ES point estimates for algometry appears to favor groups treated with MT.
Figure 4.
Effect sizes and 95% CIs of algometry/pressure-pain thresholds comparing manual therapy with control interventions in patients with plantar fasciitis.
Patient-reported function
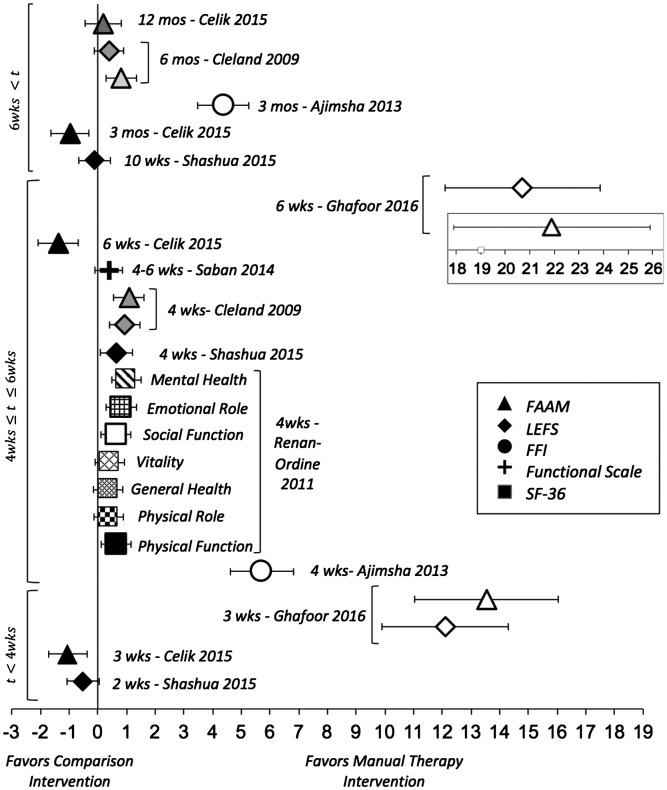
Seven studies reported patient-reported function as an outcome [23–29]. Of these, three studies utilized the Lower Extremity Functional Scale (LEFS) [23,24,29], three utilized the Foot and Ankle Ability Measure (FAAM) [24,28,29], one utilized the functional subscales of the SF-36 [25], one utilized the Functional Scale derived from Foot & Ankle Computerized Adaptive Test (FS) [26], and one utilized the Foot Function Index (FFI) [27] to assess patient-reported function. The details of the subject characteristics, treatment rendered, and assessment time points are summarized in Table 1. ES point estimates and 95% CI for comparisons of patient-reported function are illustrated in Figure 3. There was a trend of improved function that favored patients who received MT from 3 weeks to 6 months with moderate to large ES. Patients who received a corticosteroid injection to the plantar fascia had improved function with large ES from 3 weeks to 3 months, but no better than those treated with MT at 12 months (Figure 4).
Figure 3.
Effect sizes and 95% CIs of patient-reported outcome measures of function comparing manual therapy with control interventions in patients with plantar fasciitis.
Discussion
Patients who received MT interventions in combination with stretching or strengthening exercises generally had greater improved self-reported function and PPT thresholds during algometric assessment when compared to patients treated with stretching, strengthening, or modalities alone. It is important to qualify that group means for reported pain in the included studies improved following treatment, regardless of the intervention received.
Only one study demonstrated large ES that favored the inclusion of MT (joint and soft tissue mobilization) in routine care (stretching, extrinsic plantarflexion and intrinsic foot strengthening, and ultrasound) over routine care alone in reducing both self-reported pain and improving function [29]. While the superior improvements observed in the MT group are likely attributed to the multimodal treatment approach utilized in this study, these results should be interpreted with caution. Because this study did not employ any blinding (patient, treating clinician, or assessor administering the outcome measures), there is a risk of bias that may have influenced the outcomes. Administration bias is a concern when utilizing patient-reported outcome measures, especially in MT research and practice [30].
The effect of MT on self-reported pain was equivalent to comparison interventions in two studies despite improvements in self-reported function at the same time points [23,24]. It is likely that patients who had improvement in self-reported function as a response to treatment may also have increased pain associated with increased activity. One study demonstrated moderate ES that favored MT for patient-reported pain was assessed utilizing the bodily pain scale (BPS) of the SF-36 [25]. The SF-36 BPS is a two-item scale that asks the patient to not only rate pain intensity, but also how pain impacts function. It is plausible that the NPRS and VAS, both of which do not have qualifiers of impact of pain on function or quality of life, may not have the same responsiveness as the SF-36 BPS in detecting change in symptoms in patients with PF. Another plausible explanation may be attributed to differences in effectiveness between type of MT intervention provided to these patients. This was the only study to utilize trigger point MT as an intervention [25]. The application of focused manual force over a painful, taut band of muscle may have palliative effects that other milder interventions, such as massage, may not elicit.
Underlying mechanical disruption or inflammation of the plantar fascia may sensitize local cutaneous receptors and contribute to symptom severity. Basic research has demonstrated decreased cutaneous hypersensitization following ankle joint mobilization as a result of spinal level neurochemical mechanisms [31,32]. Methodological differences in studies utilizing PPT outcomes may explain the observed results. Specifically, the equivalent ES estimate found in the Shashua (2015) study [ES = 0.33, 95% CI (−0.23, 0.89)] is likely associated with the proximity of the algometric test site to the mechanical or inflammatory pain generator. Patients who were administered MT, specifically joint mobilization of the talocrural, subtalar, and midfoot joints, demonstrated equivalent ES at 4 weeks post treatment when PPTs were measured at the most tender spot on the plantar foot [23]. PPT utilizing a site that is most painful is more likely an assessment of tissue reactivity, compared to a measure of central sensitization. Hence, discretion should be used when interpreting these results.
Large ES for PPTs were observed at 4 weeks and 3 months post intervention in studies of PF patients treated with myofascial release [27] or trigger point MT [25] when algometric PPT was measured at standardized test sites on the calcaneus, soleus, and gastrocnemius. It is possible that greater effects of MT in these studies are a result of intervention and algometric assessment in regions remote to the pain generator, but share common cutaneous innervation. PPT testing remote to a pain generator has previously been recommended as a method of assessing spinal level sensitization [33]. The areas assessed in these studies are innervated by branches of the tibial nerve and dermatomes L5-S2, the same as the plantar fascia. Pain generation in the PF may facilitate central sensitization of the afferent fibers of the tibial nerve and therefore have a secondary hypersensitization effect in the sural nerve as well. Interestingly, the improvement in PPTs in the study conducted by Ajimsha and colleagues [27] persisted for at least 3 months post treatment. These findings are surprising for a neurophysiologic response to MT. Aboodarda and colleagues [34] found improvements in PPT in the triceps surae following local and non-local massage, but that the effects were transient and short-lived.
The observed ES may be attributed to heterogeneity of control interventions studied. Shashua and colleagues [23] prescribed stretching exercises and therapeutic ultrasound for their control group. This is in stark contrast to the placebo ultrasound utilized with the control group in the study conducted by Ajimsha and colleagues [27]. Regardless, Renan-Ordine and colleagues [25] also observed large ES when MT and self-stretching was compared to self-stretching alone.
Patients who received a corticosteroid injection to the plantar fascia demonstrated more immediate improvements in self-reported pain and function up to 3 months, but not at 12 months when compared to patients treated with stretching and MT [28]. Compared to stretching and joint mobilization, patients may benefit from PF injection earlier in the treatment course. Decreased pain associated with PF injection may allow patients to tolerate stretching and strengthening exercises earlier in the rehabilitation course. There are risks associated with PF injection, such as rupture of the fascia [35]. Clinicians must weigh the short-term benefit of PF injection with the risks associated with the intervention.
Regarding clinical effectiveness, it is unclear whether there is any one MT technique that is superior in improving pain and function in patients with PF. It is recommended that clinicians consider use of both joint mobilization of the ankle and foot and soft tissue mobilization techniques to include trigger point therapy, deep massage, and myofascial release in conjunction with stretching and strengthening when treating patients with PF. Clinicians should continue to exercise sound clinical judgment and provide MT intervention based on physical examination findings. For future research, the authors encourage more superiority trials where MT combined with standard care, such as stretching and strengthening exercises, is compared to standard care. Parallel group RCTs that compare different types of MT interventions would also be of great value in determining clinical effectiveness of specific techniques in the treatment of patients with PF.
Limitations
Heterogeneity of design, specifically delimitations and experimental and control interventions employed, may amplify or mute the observed treatment effects. While all reviewed RCTs employed plantar heel pain as an inclusion criterion, there was a wide range of delimitations utilized. Multiple potential pain generators contribute to symptoms in PF [5,7,8], which adds complexity when diagnosing and managing this condition. While less stringent inclusion criteria may improve generalizability of study findings, intervention group response to treatment may be muted due to variability in this heterogeneous condition. While most studies compared MT in conjunction with exercise to exercise alone, Ajimsha and colleagues [27] used sham ultrasound as a control intervention. This study also demonstrated much larger effect sizes for patient reported function than other studies illustrated in Figure 3 [27]. Heterogeneity of outcome measures, differences in instrument responsiveness, and study design in MT may also contribute to bias. Differences in MT techniques utilized across the reviewed studies preclude us from making a recommendation to any one specific form of MT. It is expected that this limitation will resolve as the body of evidence grows and encompasses trials comparing similar forms of MT.
Conclusion
Based on the seven RCTs that met our criteria for review, we conclude that inclusion of MT in a treatment plan improves PPT and function more effectively than comparison interventions in patients with PF. The inclusion of MT interventions in a comprehensive rehabilitation plan of care appears to yield greater improved function from 3 weeks to 6 months and PPT when compared to interventions such as stretching and strengthening exercises or modalities. MT techniques for the ankle-foot complex utilized in the studies included both joint mobilizations (Grade V proximal tibiofibular anterior glide, Grade III–IV posterior fibular glides, Grade I–V rearfoot distraction, Grade I–IV subtalar lateral glides, Grade I–V talocrural posterior glides in non-weight-bearing and weight-bearing, Grade V cuboid dorsal glide, Grade III–IV intertarsal mobilizations, Grade I–II first tarsometatarsal dorsal glides, and Grade II & IV unspecified rearfoot mobilizations) and soft tissue techniques (trigger point mobilization of the gastrocnemius, deep massage to the triceps surae, myofascial release to the gastrocnemius, soleus, and plantar fascia, and unspecified soft tissue mobilizations to the plantar fascia) applied for 1.5–10-min in 6–16 treatment sessions. Based on the low risk and the potential benefits of improved self-reported and clinically measured pain and function, it is recommended that MT be included in a comprehensive rehabilitation program, including stretching and exercise, in the treatment of patients with PF.
Disclosure statement
No potential conflict of interest was reported by the authors.
Disclosures
The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. Lieutenant Commander John J. Fraser is a military service member and this work was prepared as part of his official duties. Title 17, USC, §105 provides that ‘Copyright protection under this title is not available for any work of the U.S. Government.’ Title 17, USC, §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Notes on contributors
John J Fraser, Lieutenant Commander, PT, MS, OCS is a board-certified orthopedic physical therapist in the United States Navy and a PhD candidate in the Kinesiology – Sports Medicine program at the University of Virginia. His clinical and research interests include biomechanical and sensorimotor function in conditions of the foot and ankle and clinical effectiveness of manual therapeutic interventions.
Revay Corbett, MS, ATC, is a PhD student in the Kinesiology – Sports Medicine program at the University of Virginia and clinical research coordinator for the Foot and Ankle Division, Department of Orthopedic Surgery. Her research interests include Lateral Ankle Instability and it’s effects on quality of life.
Chris Donner, MEd, ATC, is an assistant athletic trainer at Lindsey Wilson College where he provides medical coverage for the Blue Raider men’s soccer, men’s and women’s swimming, and baseball programs.
Jay Hertel, PhD, ATC, is the Joe H Gieck professor of Sports Medicine at the University of Virginia where he holds appointments in the Department of Kinesiology and the Department of Orthopedic Surgery.
Hertel’s research focuses on the prevention and management of lower extremity injuries with particular emphasis on the foot and ankle.
Acknowledgements
Kelly Near, RN, MSN, MLS for her assistance in development of the review search strategy.
References
- [1].Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676–682. [PubMed] [Google Scholar]
- [2].Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20:214–221. 10.1177/107110079902000402 [DOI] [PubMed] [Google Scholar]
- [3].Fraser JJ, Glaviano NR, Hertel J. Utilization of physical therapy intervention among patients with plantar fasciitis in the United States. J Orthop Sports Phys Ther. 2017;47:49–55. 10.2519/jospt.2017.6999 [DOI] [PubMed] [Google Scholar]
- [4].DeMaio M, Paine R, Mangine RE, et al. Plantar fasciitis. Orthopedics. 1993;16:1153–1163. [DOI] [PubMed] [Google Scholar]
- [5].Tu P, Bytomski JR. Diagnosis of heel pain. Am Fam Physician. 2011;84:909–916. [PubMed] [Google Scholar]
- [6].Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25:303–310. 10.1177/107110070402500505 [DOI] [PubMed] [Google Scholar]
- [7].Yi TI, Lee GE, Seo IS, et al. Clinical characteristics of the causes of plantar heel pain. Ann Rehabil Med. 2011;35:507–513. 10.5535/arm.2011.35.4.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lareau CR, Sawyer GA, Wang JH, et al. Plantar and medial heel pain. J Am Acad Orthop Surg. 2014;22:372–380. 10.5435/JAAOS-22-06-372 [DOI] [PubMed] [Google Scholar]
- [9].Lemont H, Ammirati KM, Usen N. Plantar fasciitis. J Am Podiatr Med Assoc. 2003;93:234–237. 10.7547/87507315-93-3-234 [DOI] [PubMed] [Google Scholar]
- [10].Berkowitz JF, Kier R, Rudicel S. Plantar fasciitis: MR imaging. Radiology. 1991;179:665–667. 10.1148/radiology.179.3.2027971 [DOI] [PubMed] [Google Scholar]
- [11].Fabrikant JM, Park TS. Plantar fasciitis (fasciosis) treatment outcome study: plantar fascia thickness measured by ultrasound and correlated with patient self-reported improvement. Foot Edinb Scotl. 2011;21:79–83. [DOI] [PubMed] [Google Scholar]
- [12].Irving DB, Cook JL, Young MA, et al. Obesity and pronated foot type may increase the risk of chronic plantar heel pain: a matched case-control study. BMC Musculoskelet Disord. 2007;8:41. 10.1186/1471-2474-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Riddle DL, Pulisic M, Pidcoe P, et al. Risk factors for plantar fasciitis. J Bone Joint Surg-Am Vol. 2003;85(5):872–877. 10.2106/00004623-200305000-00015 [DOI] [PubMed] [Google Scholar]
- [14].Bolívar YA, Munuera PV, Padillo JP. Relationship between tightness of the posterior muscles of the lower limb and plantar fasciitis. Foot Ankle Int. 2013;34:42–48. 10.1177/1071100712459173 [DOI] [PubMed] [Google Scholar]
- [15].Wearing SC, Smeathers JE, Urry SR, et al. The pathomechanics of plantar fasciitis. Sports Med. 2006;36:585–611. 10.2165/00007256-200636070-00004 [DOI] [PubMed] [Google Scholar]
- [16].Bolgla LA, Malone TR. Plantar fasciitis and the windlass mechanism: a biomechanical link to clinical practice. J Athl Train. 2004;39:77–82. [PMC free article] [PubMed] [Google Scholar]
- [17].Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25:303–310. 10.1177/107110070402500505 [DOI] [PubMed] [Google Scholar]
- [18].McPoil TG, Martin RL, Cornwall MW, et al. Heel pain—plantar fasciitis. J Orthop Sports Phys Ther. 2008;38:A1–A18. 10.2519/jospt.2008.0302 [DOI] [PubMed] [Google Scholar]
- [19].Martin RL, Davenport TE, Reischl SF, et al. Heel pain—plantar fasciitis: revision 2014. J Orthop Sports Phys Ther. 2014;44:A1–A33. 10.2519/jospt.2014.0303 [DOI] [PubMed] [Google Scholar]
- [20].Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–538. 10.1016/j.math.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- [22].Cohen J. Statistical power analysis for the behavioral sciences. New York (NY): Academic Press; 2013. [Google Scholar]
- [23].Shashua A, Flechter S, Avidan L, et al. The effect of additional ankle and midfoot mobilizations on plantar fasciitis: a randomized controlled trial. J Orthop Sports Phys Ther. 2015;45:265–272. 10.2519/jospt.2015.5155 [DOI] [PubMed] [Google Scholar]
- [24].Cleland JA, Abbott JH, Kidd MO, et al. Manual physical therapy and exercise versus electrophysical agents and exercise in the management of plantar heel pain: a multicenter randomized clinical trial. J Orthop Sports Phys Ther. 2009;39:573–585. 10.2519/jospt.2009.3036 [DOI] [PubMed] [Google Scholar]
- [25].Renan-Ordine R, Alburquerque-SendÍn F, Rodrigues De Souza DP, et al. Effectiveness of myofascial trigger point manual therapy combined with a self-stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41:43–50. 10.2519/jospt.2011.3504 [DOI] [PubMed] [Google Scholar]
- [26].Saban B, Deutscher D, Ziv T. Deep massage to posterior calf muscles in combination with neural mobilization exercises as a treatment for heel pain: a pilot randomized clinical trial. Man Ther. 2014;19:102–108. 10.1016/j.math.2013.08.001 [DOI] [PubMed] [Google Scholar]
- [27].Ajimsha MS, Binsu D, Chithra S. Effectiveness of myofascial release in the management of plantar heel pain: a randomized controlled trial. Foot. 2014;24:66–71. 10.1016/j.foot.2014.03.005 [DOI] [PubMed] [Google Scholar]
- [28].Celik D, Kuş G, Sırma SÖ. Joint mobilization and stretching exercise vs steroid injection in the treatment of plantar fasciitis a randomized controlled study. Foot Ankle Int. 2015;37:150–1561071100715607619. [DOI] [PubMed] [Google Scholar]
- [29].Ghafoor I, Ahmad A, Gondal JI. Effectiveness of routine physical therapy with and without manual therapy in treatment of plantar fasciitis. Rawal Med J. 2016;41:2–6. [Google Scholar]
- [30].Cook C. Mode of administration bias. J Man Manip Ther. 2010;18:61–63. 10.1179/106698110X12640740712617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martins DF, Mazzardo-Martins L, Gadotti VM, et al. Ankle joint mobilization reduces axonotmesis-induced neuropathic pain and glial activation in the spinal cord and enhances nerve regeneration in rats. Pain. 2011;152:2653–2661. 10.1016/j.pain.2011.08.014 [DOI] [PubMed] [Google Scholar]
- [32].Martins DF, Bobinski F, Mazzardo-Martins L, et al. Ankle joint mobilization decreases hypersensitivity by activation of peripheral opioid receptors in a mouse model of postoperative pain. Pain Med. 2012;13:1049–1058. 10.1111/j.1526-4637.2012.01438.x [DOI] [PubMed] [Google Scholar]
- [33].Nijs J, Van Houdenhove B, Oostendorp RAB. Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice. Man Ther. 2010;15:135–141. 10.1016/j.math.2009.12.001 [DOI] [PubMed] [Google Scholar]
- [34].Aboodarda S, Spence A, Button DC. Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskelet Disord [Internet]. 2015. [cited [cited 2016 Mar 24]];16 Available from: http://www.biomedcentral.com/1471-2474/16/265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19:91–97. 10.1177/107110079801900207 [DOI] [PubMed] [Google Scholar]