Abstract
BACKGROUND
The integration of biological, psychological, and social factors in medicine has benefited from increasingly precise stress response biomarkers. Mitochondria, a sub-cellular organelle with its own genome, produce the energy required for life and generate signals that enable stress adaptation. An emerging concept proposes that mitochondria sense, integrate, and transduce psychosocial and behavioral factors into cellular and molecular modifications. Mitochondrial signaling might in turn contribute to the biological embedding of psychological states.
METHODS
A narrative literature review was conducted to evaluate evidence supporting this model implicating mitochondria in the stress response, and its implementation in behavioral and psychosomatic medicine.
RESULTS
Chronically, psychological stress induce metabolic and neuroendocrine mediators that cause structural and functional recalibrations of mitochondria, which constitutes mitochondrial allostatic load (MAL). Clinically, primary mitochondrial defects affect the brain, endocrine, and immune systems that play a role in psychosomatic processes, suggesting a shared underlying mechanistic basis. Mitochondrial function and dysfunction also contribute to systemic physiological regulation through the release of mitokines and other metabolites. At the cellular level, mitochondrial signaling influences gene expression and epigenetic modifications, and modulates the rate of cellular aging.
CONCLUSIONS
This evidence suggests that MAL represents a potential sub-cellular mechanism for transducing psychosocial experiences and the resulting emotional responses – both adverse and positive – into clinically meaningful biological and physiological changes. The associated article in this issue of Psychosomatic Medicine presents a systematic review of the effects of psychological stress on mitochondria. Integrating mitochondria into biobehavioral and psychosomatic research opens new possibilities to investigate how psychosocial factors influence human health and well-being across the lifespan.
Keywords: psychosomatic medicine, mitochondrion, psychoneuroendocrinology, mind-body, mitochondrial allostatic load
Introduction
Foundational work in psychosomatic medicine first documented the main effects of psychological states such as fear and anger, and social stressors including social isolation, on health outcomes and mortality. The field then rapidly advanced by exploring the underlying behavioral, biological, and physiological pathways in search of modifiable mechanisms that would explain these associations, and for which interventions could be targeted. Early on, this effort was guided by Engel’s biopsychosocial model (1), the psychoneuroimmunology (PNI) framework (2), and more recently by the allostatic load (AL) model of chronic stress (3). These and associated models have enabled significant strides towards identifying mechanisms by which ‘psyche’ and ‘soma’ are functionally linked, as originally envisioned by the founders of Psychosomatic Medicine (4). Investigators have since focused upon biological mediators of the stress-disease cascade including specific molecular changes, hormones, metabolites, and cytokines that reflect cellular activity. Identifying hard-wired mechanisms linking psychosomatic processes to elements of the biopsychosocial model and to the current biomedical framework has thus contributed to a deeper understanding of interrelated psychological and somatic processes.
Work in psychosomatic medicine is driven by the collective vision ‘to integrate biological, psychological and social factors in medicine’. Past success indicates that this endeavor is facilitated by the identification of biological intersection points – where psychosocial and biological factors “meet”, interact, and trigger measurable cellular and health effects (5). The immune system represents such an intersection point (6). Immune cells respond to neuroendocrine substrates of psychological states, interact with biological entities such as the HIV virus, which in turn alter immune responses impacting wound healing, AIDS progression, and clinical outcomes (7). The brain is also a notable example, responding acutely and chronically to emotional and environmental perturbations, interacting broadly with other neuroendocrine and immune systems, and undergoing changes in both structure and function that impact health throughout human development (8, 9). The success of PNI and brain remodeling research highlights the notion that identifying biological intersection points is a productive endeavor for psychosomatic medicine research.
This article examines the mitochondrion, a multifunctional life-sustaining organelle, as a potential biological intersection point in psychosomatic medicine. The first section begins with an introduction about the origin and functions of mitochondria for the non-expert. A summary of the evidence that mitochondrial function and dysfunction contribute to systemic physiological (dys)regulation is then presented. We then discuss progress in mitochondrial medicine and psychosomatic research demonstrating that the systemic pathophysiological states triggered by either mitochondrial defects or chronic stress exposure significantly overlap, implicating similar cellular mechanisms. This overlap primarily involves the brain, neuroendocrine processes, and the immune system, suggesting that disease-causing psychosomatic processes could in part act via mitochondrial dysfunction. A systematic review of experimental evidence from animal studies and preliminary work in humans presented in the associated article (10) indicates that chronic and acute stress exposure alter specific aspects of mitochondrial structure and function. This highlights the accumulation of adaptive and maladaptive recalibrations in mitochondria in response to stress, here described as mitochondrial allostatic load (MAL). Finally, the last section discusses molecular and physiological processeralles that link MAL to accelerated cell aging and increased disease risk. This article concludes with highlighting knowledge gaps and opportunities for psychosomatic mitochondrial research to advance our understanding of the stress-disease cascade.
1. Mitochondrial sensing, integration, and signaling
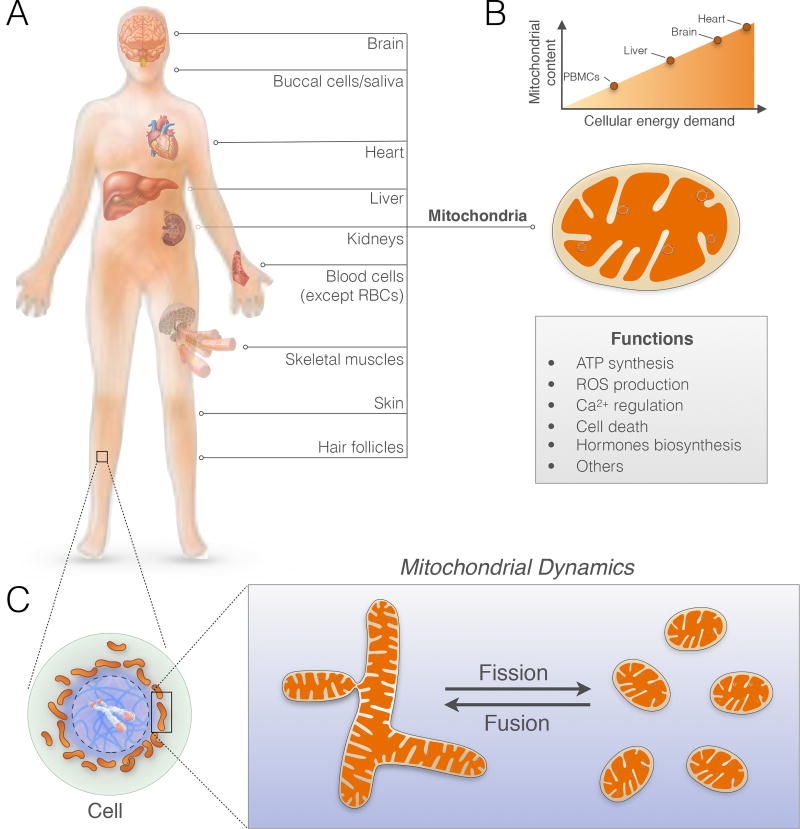
The mitochondrion (plural, mitochondria) is an intracellular organelle that evolved from an endosymbiotic relationship. About 2 billion years ago, an oxygen-consuming bacterium (to become the mitochondrion) was engulfed by a host cell (to become today’s mammalian cell) (11). Complex multicellular life – humans included – emerged from this symbiosis (12). As a result, mitochondria now sustain human life via energy production and intracellular signaling. Each cell of the body, with the exception of red blood cells that transport oxygen, contain hundreds to thousands of mitochondria (Figure 1A). They are the only organelles to house their own genome. The mitochondrial genome, also known as mitochondrial DNA (mtDNA), contains genes that are critical to the flow of energy through the electron transport chain. The electron transport chain, or respiratory chain, enables mitochondria to use oxygen and food substrates to generate a charge called the mitochondrial membrane potential (ΔΨm). In the same way that batteries store electrical charges that can subsequently be used to power various devices, organisms essentially breathe and eat to charge their mitochondria. This charge is then used to produce energy in the form of adenosine triphosphate (ATP) to power neural activity, the heartbeat, muscle contraction, digestion, and every other cellular activities that occur under resting conditions and during stress. Cells that need more energy typically have more mitochondria (Figure 1B). But mitochondria are more than the powerhouse. They sense, integrate, and signal information about their environment.
Figure 1. Mitochondrial content and function in humans.
(A) Hundreds of mitochondria are present within various cells and organs across the body. (B) Mitochondrial content varies according to energy demand in different organs, where they perform multiple functions ranging from energy transformation, signaling, and hormone biosynthesis. (C) General schematic of a cell, its cytoplasm (green), nucleus (blue), and mitochondria (brown). Mitochondria are dynamic and undergo changes in shape through fusion and fission within minutes in response to external biochemical and energetic signals.
Mitochondria sense stress mediators. Of relevance for psychosomatic research, mitochondria evolved to be sensitive to a wide variety of environmental, metabolic, and neuroendocrine stressors and stress mediators, including glucocorticoids (13, 14), estrogen (ER) (15, 16), angiotensin (17), and cannabinoids (18). Metabolic stress, including high blood glucose and lipids, also influence dynamic processes of fusion and fission that remodel mitochondrial shape within the cytoplasm (Figure 1C) (19, 20).
Mitochondria dynamically interact with each other and respond to stressors. There are specialized inter-mitochondrial junctions that resemble synapses in the brain (21) and thin tubular connections (22) through which mitochondria exchange information among each other. In response to environmental signals, mitochondria also undergo dynamic morphological and functional changes. Chronically, these alterations of mitochondrial structure and function can lead to functional recalibrations (23) and to the accumulation of mtDNA damage (24). The accumulation of mtDNA defects impairs bioenergetics, is generally long-lasting, and may be amplified over time (25).
Mitochondria generate signals of adaptation. Within the cell, mitochondria are in close proximity to the cell nucleus (see Figure 1C). Changes in mitochondrial functions modify cellular bioenergetics (23), leading to the production of biochemical signals to which the cell and its plastic (epi)genome have evolved molecular sensitivity (26–29). Mitochondria speak the language of the epigenome and multiple mechanisms link their functions to fundamental aspects of cellular health. In fact, the majority of the human genome is under mitochondrial regulation (30). Systemically, mitochondria in lower organisms produce signals – mitokines – with broad actions throughout the organism (31, 32). It is also interesting to note in the context of stress regulation that all steroid hormones, including glucocorticoids and sex hormones, are synthesized in a process that is regulated by, and occurs in mitochondria (33, 34), further linking mitochondrial biology to stress signaling.
As discussed in the subsequent sections, the evolutionary-acquired critical role of mitochondria in complex life and stress responses helps to rationalize why mitochondria – a sub-cellular organelle – regulate whole-body physiological functions including the nervous, endocrine, and immune systems. This also helps to explain how genetic mitochondrial defects influence complex whole-body physiological processes such as the stress response (35), the aging process, and multiple complex diseases that challenge modern medicine (36).
2. The rise of mitochondria in medicine
Across all areas of medicine, mitochondrial research is on the rise. The number of medical publications related to mitochondria has increased at a faster pace than for other organelles, including the nucleus and genomic studies, which have steadily declined in the “post-genomic era” (37). The rising interest for mitochondria in medical research likely stems, on one hand, from more accessible tools to grasp the complexity of mitochondria functions (38), and on the other hand, from a growing number of medical conditions now recognized to be caused or promoted by mitochondrial defects (36).
The discovery in the 1980’s by Wallace and colleagues that the mtDNA is maternally inherited (39) and that defects on the mtDNA (i.e., point mutations and deletions of mtDNA fragments) caused serious diseases (40) was a breakthrough for molecular medicine. It is now established that over 200 inherited genetic defects cause a broad range of neurological, endocrine, immune and cardiovascular symptoms (41, 42). In milder forms, mitochondrial disorders cause metabolic disease and progressive age-related multisystemic disorders associated with morbidity and increased mortality, while in most severe cases they cause death in the first years of life (43). Like psychosocial stress and trauma (44–46), mitochondrial defects influence various physiological functions and physical conditions at multiple developmental stages across the lifespan.
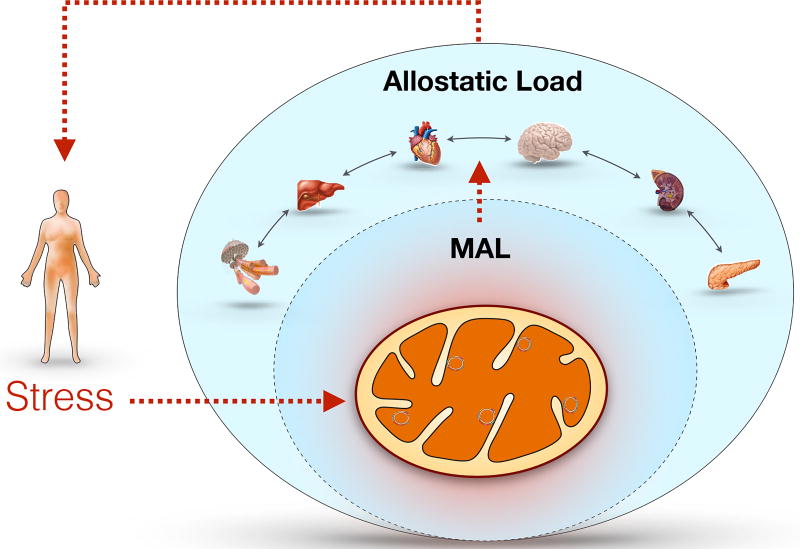
With the historical role attributed to mitochondria as the cell’s powerhouse, it was naturally believed that mitochondrial disorders were caused by energy deficiency. However, the recent recognition of non-energetic roles of mitochondrial sensing, communication, and signaling has revealed a new paradigm where multiple mechanisms cooperate to translate abnormal mitochondrial function into pathophysiology. Of particular interest for psychosomatic medicine, mitochondrial dysfunctions affects most deeply the nervous, endocrine, and immune systems, which are understood to play central roles in allostasis and stress pathophysiology (47). The subsequent sections outline evidence that mitochondria regulate key stress-related physiological systems, which contribute to systemic allostatic load as originally defined. Furthermore, we discuss the concept of mitochondrial allostatic load (MAL), which also develops intracellularly at the level of mitochondria and may contribute to systemic allostatic load (Figure 2).
Figure 2.
Model of mitochondrial allostatic load (MAL) as a source of systemic allostatic load. Mitochondrial allostasis is the active process of responding to challenges including the demand for ATP and other biomolecules to maintain cell function and survival, as well as providing signaling molecules (e.g., limited amount of ROS). MAL is defined as the dysregulation mitochondrial functions resulting from the structural and functional changes that mitochondria undergo in response to stressors. Challenges that overwhelm the capacity to respond and produce an imbalance contribute, over time, to impaired cell function, senescence, and even cell death. Clinical cases of inherited mitochondrial disorders demonstrate the direct influence of mitochondrial dysfunction on multiple organ systems. Because mitochondria are intrinsic partners and participants in systemic allostasis, MAL is a nested construct that contributes to systemic allostatic load and overload.
3. Mitochondria, allostasis, allostatic load, and overload
Allostasis is the active process of the body adapting to stress via mediators such as cortisol and the autonomic, metabolic, and immune systems that act together to maintain homeostasis (48). Allostatic load refers to the cumulative effect of multiple stressors as well as the dysregulation of the non-linear network of allostasis (e.g., too much or too little cortisol, or adrenalin, or prolonged inflammatory response to a challenge). Allostatic overload refers to the cumulative pathophysiological changes that can result from this dysregulation, both at the cellular and organ level. The concepts of allostasis and allostatic load and overload emphasize that the same systems that help the body and brain adapt to experiences also contribute to pathophysiology when the same mediators are overused or dysregulated among themselves (49). Moreover, health promoting and health damaging behaviors that often accompany stressful experiences and, more generally, living in stressful social and physical environments all contribute to allostatic load and overload (50).
Mitochondria contribute to allostasis and allostatic load of the whole individual while having their own forms of allostasis and allostatic load within cells. The sequence of events from the stressor to mitochondrial recalibrations can be conceptualized as follows: i) systemic stress mediators cause mitochondrial structural and functional adaptation (e.g., activation of hormonal receptors, fusion/fission shape changes, reactive oxygen species (ROS) production); ii) cumulative effects of stressors eventually damage the mtDNA (e.g., mutations, deletions) and/or induce lasting changes in mitochondrial content and energy production capacity; and iii) production of signals, including mitokines, that influence cellular and systemic pathophysiological processes involving traditional allostatic load biomarkers such as lipids and glucose (51), but also other allostatic changes including gene dysregulation, oxidative stress, inflammation, and senescence (Figure 3). Collectively, the structural and functional changes that mitochondria undergo in response to chronic stressors is thus referred to as mitochondrial allostatic load – MAL (52). The systemic recalibrations caused by mitochondrial dysfunction may further feedforward and sustain MAL, as discussed in the section “Mitochondrial regulation of stress reactivity systems: HPA, SAM and ANS”.
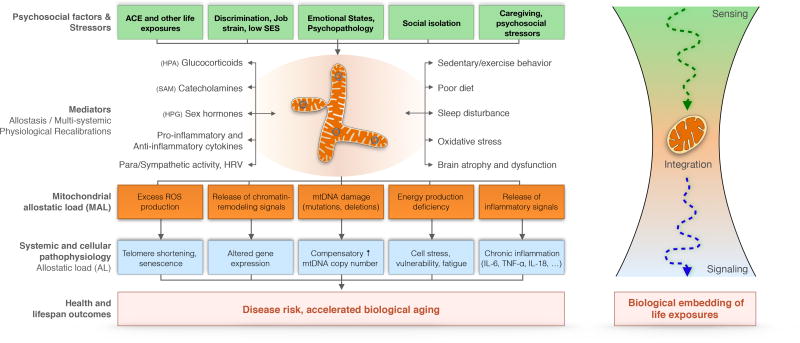
Figure 3. Mitochondrial stress transduction.
(Left) Conceptual model of mitochondrial stress pathophysiology outlining the effects of psychosocial factors on health and disease risk via mitochondria. Mitochondria interact bi-directionally with stress mediators of allostasis, contributing to physiological stress responses and multi-systemic recalibrations. Chronic activation of these systems leads to mitochondrial allostatic load (MAL), which is transduced through molecular signals into systemic pathophysiology, allostatic load (AL), and molecular changes within cells. Relationships between psychosocial factors (green boxes) and pathophysiological measures (blue boxes) can be modeled statistically as a direct effect with mitochondria as mediator/moderator. (Right) Schematic representation of mitochondrial sensing, integration, and signaling of life exposures, including psychosocial stressors and emotional (negative and positive) states. Three main testable corollary hypotheses arise from this model: 1) Exposure to psychosocial factors and stressors induce MAL; 2) MAL and primary mitochondrial defects cause systemic dysregulation and adverse health outcomes; 3) The end effect and biological embedding of the same exposure will differ based on the mitochondrial health of the system/individual. Bi-directional relationships exist between some biobehavioral and psychosocial factors, but are not depicted here for parsimony.
MAL is operationalized as the multi-factorial alterations of mitochondrial biology induced by chronic stressors and may involve multiple functional and molecular indicators (see Table 1). MAL involves both quantitative changes in specific parameters (e.g., ATP synthesis, ROS production), and qualitative alterations in their physiological functions (e.g., fusion/fission dynamics, preference for fat or carbohydrate substrates, production of specific signaling molecules). Both changes in the quantity of mitochondria per cell and the quality of each mitochondrion may represent MAL, depending upon the stressor and the cell type.
Table 1.
Potential markers of mitochondrial allostatic load – MAL.
| Mitochondrial features | Examples | Physiological effects |
|---|---|---|
| Mitochondrial content | Decreased or increased mitochondrial number, mitochondrial size | Energy production capacity, metabolic regulation |
| Molecular damage | Oxidized mtDNA, proteins, and lipids | Multiple effects |
| Molecular composition | Lipid and protein composition | Multiple effects |
| Dynamics, morphology, and ultrastructure | Fragmented, elongated, or “donut” morphology; Reduced number or abnormal cristae | Energy production capacity, oxidative stress, apoptosis, systemic metabolic regulation |
| Genetic (mtDNA) | mtDNA copy number per cell, mtDNA mutations and deletions | Energy production capacity, aging, systemic metabolic regulation |
| Respiration and OXPHOS | ETC enzymatic activity, oxygen consumption rate, ATP synthesis | Energy production capacity, multiple functions (gene expression, endocrine, metabolic) |
| Other mitochondrial functions | ROS production, calcium uptake and release, decreased membrane potential, biosynthesis | Multiple functions (gene expression, endocrine, metabolic, tissue repair) |
| Mitokine production and metabolite signaling | ccf-mtDNA release, mtDNA-encoded proteins, Krebs cycle metabolic intermediates | Paracrine and endocrine effects on multiple organ systems |
A key distinction between MAL and the traditional allostatic load index must be noted. Whereas allostatic load biomarkers are individual molecular entities, such as circulating proteins (i.e., cytokines) or metabolites, mitochondria are living symbiotic microorganisms. A single protein such as a secreted interleukin is best characterized by its abundance: it can be higher or lower in concentration. In contrast, living systems, regardless of their size, are generally best characterized by dynamic measures of their rhythms and functions (53). Based on this principle, stress-induced changes in mitochondrial energy production capacity, or ROS production, will be more accurately reflected by enzymatic activities measured over a period of time, rather than by the fixed amount of specific mitochondrial proteins. In keeping with the definition of MAL as the collective changes in structure and functions, MAL should be more precisely quantified with a combination of measures that reflect and integrate multiple dynamic functions – or MAL indices. Because mitochondria are different between cell types, tissue or cell-type specific MAL indices may gain in specificity and sensitivity. Furthermore, it is conceivable that certain MAL indices will be most specific and/or sensitive to certain types or duration of stressors, a question that remains to be explored.
In the same way that the allostatic load index has evolved substantially since its inception (3, 54), MAL measurements are bound to evolve and become more precise. This evolution will be driven by three main factors: i) our increasing understanding of various facets of mitochondrial biology, ii) of their relevance to stress physiology and psychosomatic medicine, and iii) by technical developments that will permit an increasing number of suitable mitochondrial measures from accessible biological samples (e.g., plasma, leukocytes, buccal cells, hairs).
Developing robust MAL measures will enable researchers to address two major questions: 1) What are the effects of psychosocial stress and emotional states on mitochondrial functions? 2) What are the physiological and health consequences of MAL. Because mitochondria are present in every cell and organ of the body, MAL can theoretically engender organ-specific effects, as seen in mitochondrial diseases. The presented framework positions mitochondria as an integrating element of stress-disease cascade (Figure 3, right), lying at the interface of the psychosocial and behavioral factors and the organism. Research is needed to better define the existing relationships between mitochondria and the various systems involved in psychosomatic processes, and the resulting systemic but also organ-specific effects of MAL. Moreover, stress-induced mtDNA and other damage could represent a mechanism for the biological embedding (9) of stressful experiences at the mitochondrial level. However, empirical research using longitudinal and prospective study designs will be necessary to evaluate this possibility, and if proven, to evaluate its reversibility.
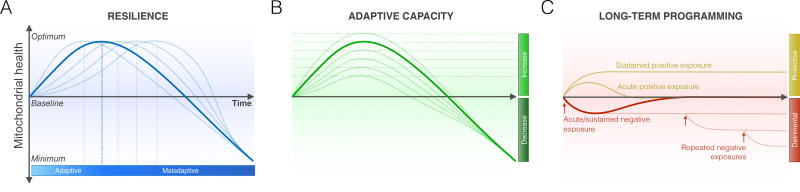
In mapping the relationships between psychosocial exposures across one’s lifetime and MAL, the kinetics of mitochondrial responses to various stressors should be considered. Mitochondrial responses to stress may be bi-phasic. For example, low levels of glucocorticoids may enhance mitochondrial calcium buffering, but at high doses result in decreased calcium buffering capacity, excess free radicals generation, and sensitization to cell death (14). Thus, molecular and functional recalibrations in mitochondria may be best characterized by inverted U-shaped responses that vary either in kinetics or amplitude, reflecting resilience and adaptive (in)capacity of the system (Figure 4). From a research design perspective, this underscores the value to measure mitochondrial outcomes at multiple time points, and to monitor stress duration, intensity, and type, which may synergize to cause specific MAL patterns. The next three sections consider the specific influence of mitochondria on normal and abnormal functions of the brain, the neuroendocrine system, and immune regulation.
Figure 4. Possible inverted U-shaped mitochondrial responses to chronic stress.
(A) Bi-phasic mitochondrial stress responses as a form of mitohormesis (186), as induced by glucocorticoids (e.g., (14)). The transition from adaptive to maladaptive is marked by the inversion of the curve at the top. Each line depicts a cellular or physiological system with a different degree of resilience, as indicated by the duration for which it can sustain an adaptive response to the stressor before undergoing a decline in mitochondrial health below baseline. (B) The adaptive capacity of mitochondria can vary, as indicated by the degree to which they can generate an adaptive increase in function during stress, depicted hereby the height of curves. (C) Certain stressors, particularly during sensitive developmental windows, can also have long-term programming effects that establish lasting set points (detrimental or protective) for mitochondrial health. For example, anti-retroviral pharmacotherapy for HIV-AIDS may lead to the permanent accumulation of mtDNA defects that undermine respiratory chain function and decrease baseline function (187). Subsequent exposures (arrows) may have additive effects and further decrease function (below baseline), as a form of detrimental embedding. On the other hand, positive behavioral exposures such as exercise training can increase basal mitochondrial function (above baseline), as a form or protective embedding.
4. Mitochondria impact brain structure and function
Mitochondrial DNA defects cause mitochondrial disease and multiple neurological symptoms that may preferentially affect the brain (55, 56). In addition, other developmental and age-related neurological disorders not believed to be of primary mitochondrial origin also present with underlying mitochondrial dysfunction, including autism spectrum disorder (57, 58), and neurodegenerative conditions like Alzheimer’s and Parkinson’s diseases (59, 60). In diseased brain tissue, mitochondrial disorders and neurodegenerative conditions share common gene expression signatures, also suggesting a mechanistic overlap (30, 61). In an animal model of Alzheimer’s disease, cognitive and neuropathological symptoms progression have been prevented with mitochondria-targeted antioxidant therapy (62), providing direct evidence that mitochondrial defects likely play a primary role in the etiology and progression of neurodegenerative conditions (63).
Structural changes within the brain, such as those induced by chronic stress on the hippocampus, may be indicative of mitochondrial dysfunction. Structurally, patients with primary mitochondrial disorders frequently present with atrophy of cerebrocortical, cerebellar and brainstem regions (64, 65), as well as cerebrovascular disease (66). Genetic studies in animal models have also shown that mtDNA mutations can influence brain development (67). Likewise, some but not all (68, 69) studies of the human hippocampus have demonstrated shrinkage of the hippocampus in mild cognitive impairment and Alzheimer’s disease (70), chronic major depression (71), Cushing’s disease where glucocorticoids are produced in excess (72), and post-traumatic stress disorder (PTSD) (73). The hippocampus is not the only brain region affected. Amygdala enlargement and overactivity, as well as hippocampal and prefrontal cortical shrinkage has been reported in a number of mood disorders (71, 74). Moreover, psychosomatic conditions that do not qualify as “disease”, such as chronic (several years) stress exposure (75), systemic inflammation associated with metabolic syndrome and underlying oxidative stress (76, 77), lack of physical activity (78), and jet lag (79), have been associated with smaller hippocampal or temporal lobe volumes have been reported. Although it remains unclear from available research if mitochondria contribute to these effects on the human brain, evidence that cerebral atrophy is a neurological feature common to both psychopathology and primary mitochondrial disorders is consistent with a mitochondrial etiology of stress-induced brain atrophy.
Experiencing chronic stress evokes in the brain an array of adaptive responses consisting of neuronal atrophy in the hippocampus and medial prefrontal cortex, and expansion of dendrite of neurons in basolateral amygdala (80). These are adaptive in the face of danger as they increase vigilance, but at the cost of some cognitive acuity (81). In a striking similarity to mitochondrial biogenesis (i.e., the formation of new mitochondria), neurogenesis is inhibited by chronic stress and enhanced by exercise (81, 82). In fact, exercise-induced neuronal stem cell expansion, a prerequisite for neurogenesis within the brain, requires normal mitochondrial dynamics and involves expansion of mitochondrial content in the hippocampus (83), underscoring a primary role of mitochondrial functions in brain plasticity. In line with this, hippocampal atrophy and memory decline occur in aging but are prevented by physical activity (84), similar to the age-related decline in mitochondrial function that is prevented by physical activity (85). Together, this further highlights the potential parallel between exercise/physical activity-induced systemic changes in mitochondria and those that occur within the brain.
Stress vulnerability is also evident in relation to changes in neural activity, which entail variations in mitochondrial energy supply and demand. For instance, dangerous elevations of glucose for extended periods as in diabetes type I and II represents a form of metabolic stress that fragments mitochondria and promote mtDNA damage (20). In the human brain, excess glucose accelerates age-related brain atrophy, neurovascular damage, and impair neurogenesis (86–88). In animals, reduced energy demand during hibernation is also associated with hippocampal CA3 shrinkage (89, 90). Over-activation of the hippocampus in seizures and ischemia along with elevated glucocorticoid increases vulnerability to permanent damage, referred to as “glucocorticoid endangerment”, whereas reduction of glucocorticoids under these conditions is protective (91–93). In that connection, there are biphasic effects of glucocorticoids on mitochondrial calcium buffering, with high glucocorticoid levels leading to a failure of the calcium buffering mechanism and cell death (94).
In addition to structural remodeling, mitochondria also dynamically impact brain function and cognition via specific molecular and cellular mechanisms (95). In particular, smaller synapses and impaired working memory in non-human primates has been linked to abnormal presynaptic mitochondrial shape, such as the conspicuous donut-shaped mitochondria (96). Likewise, anxiety-related behavior (97), as well as circadian rhythm (i.e., physiological processes that occur within a 24-hour cycle) in mice and hippocampal-dependent spatial memory are affected by the mtDNA (98). Thus, both morphological and genetic mitochondrial anomalies are emerging causes of brain dysfunction. These are thought to operate at least in part by promoting the maladaptive structural and functional changes that the brain undergoes in response to adverse psychosocial environments throughout development (8). In future work, it will be important to determine if changes in mitochondrial function and MAL precede brain changes in humans, and if promoting mitochondrial health may have salutary effects on the brain and cognition.
5. Mitochondrial regulation of stress reactivity systems: HPA, SAM and ANS
Connected and downstream from the brain are the hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenal-medullary (SAM) axes, which produce hormones required for the normal stress response (99). Preliminary evidence suggests that mtDNA genetic variants can alter stress-reactive corticosterone (CORT) production in mice (35, 97), and adrenal cortex dysfunction and hypocortisolemia was observed in individuals with an inherited condition causing mitochondrial oxidative stress (100). In patients with a genetic defect impairing mitochondrial ATP transport, resting circulating catecholamine levels are also elevated to about double the concentration in healthy controls (101). These clinical data indicate that both HPA and SAM axes activities may be modulated by mitochondrial function. This question was recently examined experimentally in mouse models with genetic mitochondrial defects exposed to restraint stress, a model of psychological stress in rodents (35). This work demonstrated that mitochondria influence all aspects of the stress response investigated, including cortisol and catecholamine levels, positioning mitochondria as stress response modulators (35).
Likewise, cardiorespiratory and neuroendocrine responses, which are in part driven by a combination of sympathetic (SNS) and parasympathetic (PNS) autonomic nervous system inputs, are altered during exercise in patients with mtDNA disorders (102). This may partly be due to decreased vagus nerve activity both at rest and during exercise as evidenced by increased resting heart rate and decreased high frequency power RR interval variability (RRV) in patients with mitochondrial disease (103). Furthermore, individuals with mtDNA disorders have elevated stress-reactive epinephrine and norepinephrine release by the SAM axis during an exercise challenge (104), possibly because mitochondrial defects alter the physiological and possibly the psychological/affective perception of the stressor.
Whether these mitochondria-driven abnormal neuroendocrine responses to exercise also translate into abnormal responses to psychosocial stress remains unclear. But evidence in healthy individuals suggest that those who exhibit stronger HPA axis responses to physical activity also show stronger cortisol release to psychological stress (105), suggesting that a common biological mechanism regulates the magnitude of responses to stressors of different nature (physical and psychological). Interestingly, inter-individual differences in neuroendocrine responses to psychosocial stressors also exist between racial and ethnic groups (106). Because mtDNA genes vary with ethnicity (107), differences in mitochondrial function could also in part account for inter-individual differences in stress responses. Overall, more research is needed to examine if differences in mitochondrial functions are at the origin of known inter-individual differences in HPA, SAM, and ANS responses to psychological stress in humans.
6. Mitochondrial control of immunity and inflammation
The immune system exhibits particular sensitivity to psychological states and related neuroendocrine mediators including catecholamines and glucocorticoids (2). Immune cells are also a major source of inflammatory mediators (108), which feed back onto the brain and autonomic nervous system, and vice-versa (109, 110). Pro-inflammatory gene expression at the cellular level (e.g., NF-kB), and systemic release of pro-inflammatory cytokines (e.g., interleukin 6, [IL-6]) are thus thought to contribute to effects of acute and chronic emotional states, both positive and negative, on health outcomes and other psychosomatic processes (111–113). Interestingly, discoveries over the last decade have positioned mitochondria within canonical cellular processes related to both innate and adaptive immunity (114). The role of mitochondria on the immune system and inflammation can be divided into three main categories.
i) Mitochondria trigger inflammation
Because mitochondria are of bacterial origin, their circular mtDNA and resultant proteins (n-forlmyl peptides) are recognized as foreign by the immune system. These mitochondrial immunogenic molecules, termed “alarmins” or damage-associated molecular patterns (DAMPs), are released under conditions of mitochondrial stress, particularly in response to oxidative stress (115). The release of mitochondria-derived DAMPs triggers the innate immune system through the intracellular DNA-sensing system cGAS (116), and systemically via toll-like receptors (TLRs) (117). In macrophages, activation of these systems by mtDNA engages the inflammasome (118), cytokine release, and pro-inflammatory gene expression (119).
In animal models, mitochondria-induced inflammation results in cardiovascular lesions (120) and has been associated with neurodegeneration in humans (121). Interestingly, the anti-inflammatory signal of acetylcholine may prevent stress-induced release of mtDNA possibly via binding of a mitochondrial nicotinic acetylcholine receptor (115). Mitochondria-localized proteins encoded in the nuclear genome such as heat shock protein 60 (Hsp60) can also be released from cells and become detectable in circulating plasma (122). From a psychosomatic perspective, a noteworthy study conducted among disease-free workers from the Whitehall II cohort revealed that circulating Hsp60 levels were correlated with psychological distress, job demand, and low emotional support, as well as with cholesterol levels (122), indicating a potential link between psychosocial factors, mitochondrial stress, and cardiovascular disease indicators.
Another recently described mitochondria-derived signaling molecule (mitokine) is circulating cell-free mtDNA (ccf-mtDNA), which consists of mtDNA circulating in the liquid fraction of blood. Such circulating mitochondrial genome (CMG) is either passively released from cellular damage or necrotic death, or actively extruded via active secretion from mitochondria; however, the exact origin of CMG fragments is unclear. Ccf-mtDNA is found at detectable levels in human plasma and serum (123), where it exists as small genomic fragments (124). Serum levels are significantly higher than plasma levels, suggesting that mtDNA release is enhanced during coagulation (125). Ccf-mtDNA is particularly abundant in inflammatory diseases (123), as well as in cancers, myocardial infarction, and sepsis where it is a prognostic indicator of disease and mortality (126–128). Strikingly, in hospitalized critically ill individuals, high ccf-mtDNA levels was associated with a 4–8 fold increased risk in mortality compared to individuals with normal ccf-mtDNA levels (126). In suicide attempters, ccf-mtDNA was also found to be dramatically elevated compared to controls and partially correlated to cortisol levels post dexamethasone suppression test, suggesting that the psychological state associated with suicidality might promote the extrusion of ccf-mtDNA into the blood. In relation to inflammation, mtDNA amplifies tumor necrosis factor α (TNF-α) release by lipopolysaccharide (LPS)-stimulated primary human monocytes, indicating the immunogenicity of circulating mtDNA in human leukocytes (129). Besides stimulating inflammation, the role of mtDNA release as a paracrine or endocrine signals remains to be determined. Overall, mitokines like ccf-mtDNA are emerging as a source chronic systemic inflammation, suggesting potential new biomarkers of early-stage inflammatory processes known to be related to indicators of psychosocial stress (112).
ii) Mitochondria are essential to innate immunity
In the cytoplasm of infected immune cells, energized mitochondria (i.e., with an active membrane potential) recruit the mitochondrial anti-viral signaling (MAVS) protein, which aggregates on the mitochondrial outer membrane and initiate signaling (130). This process enables the cellular anti-viral response by downstream activation of NF-kB and interferon regulatory factors (IRFs), which translocate to the nucleus to induce the expression of type I interferons and pro-inflammatory cytokines genes (130). Ablating mitochondrial membrane potential inhibits this response (131), whereas mitochondrial ROS potentiate it (132), illustrating bi-modal mitochondrial regulation. Fuethermore, as mentioned above, mtDNA that “leaks” into the cytoplasm can also activate intracellular DNA receptors such as cGAS and directly trigger innate immune response genes independently of any infection or external stressor (116).
Possibly as a result of the influence of mitochondrial function on innate immunity, mtDNA variants – which vary according to ethic origin (107) – influence not only mitochondrial function, but also correlate with metabolic and immune parameters during anti-retroviral therapy (133, 134), as well as HIV/AIDS progression and mortality (135). This modulatory effect of mtDNA on infectious disease progression is believed to result from biochemical differences in mitochondrial respiratory capacity (136), and is reminiscent of the modulatory effect of both positive and negative psychosocial factors, such as social support and depressive symptoms, on HIV/AIDS disease (137). Mitochondrial regulation of innate immune responses may thus interact with psychosocial factors to impact inflammatory responses and vulnerability to infectious diseases.
iii) Mitochondrial metabolism regulates immune cell differentiation and inflammatory phenotype
Activation and quiescence of immune cells involve metabolic reprogramming where mitochondrial content and function are altered (138). For instance, upon injury undifferentiated monocytes actively differentiate into either pro-inflammatory (M1) or anti-inflammatory (M2) macrophages. M1 pro-inflammatory cells rely mainly on glycolysis for energy production; whereas M2 anti-inflammatory cells show mitochondrial proliferation and upregulation of oxidative metabolism (139). The same is true of lymphocytes, which cannot adopt specific effector functions (T-regulatory vs memory) without adopting the correct metabolism (114). Not unexpectedly then, mitochondrial dysfunction influences immune phenotypes. For instance, mice with different mitochondrial genomes exhibit differential susceptibility to experimental autoimmune encephalomyelitis (140), and infectious complications are a significant clinical concern in patients with mitochondrial disorders (141).
In relation to stress, mitochondrial immune modulation may also involve glucocorticoids. Glucocorticoid signaling via the GR can significantly inhibit pro-inflammatory responses (142), and an important feature of the immune-endocrine system is glucocorticoid receptor resistance (GCR) that develops with chronic stress (e.g., (143)). Given that glucocorticoids induce GR translocation into mitochondria where it affects mtDNA gene expression and mitochondrial functions (14), and that the mitochondria are central to immune modulation, GR-mediated suppression of immune cell activation may involve (non-genomic) mitochondrial mechanisms. The role of mitochondria in GCR, their role in the chronic low-grade inflammatory state that characterizes chronic stress and aging, as well as their contribution to psychological stress-induced inflammatory responses, all remain to be examined. Conclusively resolving these questions at a mechanistic level will require the combination of experimental approaches and population-based studies with repeated measurements of mitochondrial functions in parallel with psychosocial and neuroendocrine factors queried prospectively.
Beyond but related to the immune system, mitochondrial may also link inflammation to metabolic dysregulation and depression. There is a specific short-chain lipid molecule, acetyl-L-carnitine (LAC), which enhances mitochondrial oxidation of substrates and may protect and enhance mitochondrial function (144). LAC acts as an acetyl donor for metabolism and for epigenetic modification of histones (145) and for mitochondrial proteins in a biphasic manner (146, 147). In animal models, LAC deficiency is associated with metabolic dysregulation, including insulin resistance, elevated triglycerides and leptin that, like the depressive-like behavior, is rapidly corrected by LAC treatment (148). Together, this evidence demonstrates that mitochondrial metabolism can rapidly remodel both immune and neural activities, which together influence the activation/deactivation of stress-response systems at the interface of psycho-somatic processes.
7. Mitochondrial dysfunction and cellular aging
Forty years ago, mitochondrial dysfunction, more specifically mtDNA damage, was postulated to represent the biological “aging clock” (149). Other aging clocks have also been proposed including telomere length (150) and DNA methylation (151). Only recently has evidence unequivocally demonstrated that mitochondria influence the rate of aging in mammals. With age, the mtDNA accumulates low mutation levels (152). To study this process experimentally, mice with a faulty proof-reading mtDNA polymerase gamma (PolG) that introduces random mtDNA mutations were generated. These “mutator” mice accumulate higher-than normal mtDNA mutations with concomitant mitochondrial oxidative stress (153), and as a result, age prematurely, living only up to about half the lifespan of their counterparts with normal mtDNA mutation levels (154, 155). Affected animals exhibit multiple signs of advanced human aging including muscle and brain atrophy, exercise intolerance, whitening of hair, and kyphosis (154, 155). Interestingly, a study demonstrated that this progeroid phenotype is entirely avoided by exercise (156), suggesting the potential for profound modulation of mitochondrial functions and of the deleterious physiological consequences by behavioral factors.
One possible mechanism by which mitochondria accelerate the aging process is by mitochondria-derived oxidative stress directly promoting telomere erosion. Mitochondrial ROS can cause telomere instability and shortening in vitro (157), and preliminary evidence suggests that individuals with mtDNA mutations causing mitochondrial disease may have abnormally short telomeres in affected tissues (158). A recent study where cultured cells were depleted of their mitochondria demonstrated that cell senescence, including the senescence-associated secretory phenotype (SASP), was prevented in the absence of mitochondria (159). Such evidence is consistent with the notion that mtDNA defects and MAL, via the production of signals that reach the cell nucleus, can trigger organismal aging and senescence, possibly via telomere dysfunction and other mechanisms.
As biology rarely operates unidirectionally, mitochondria and telomeres are in fact bi-directionally linked. Telomere dysfunction resulting from telomerase (hTert) deficiency in mice effectively decreases mitochondrial content and function, involving the downregulation of PGC-1α signaling and mitochondrial biogenesis (160). Accordingly, in human blood cells, mtDNA copy number and telomere length are moderately correlated (r = 0.12 to 0.56) (161, 162), with the strength of the association being stronger in those having experienced ACE and with a history of psychopathology (162). Should this association be replicated in longitudinal and prospective studies, resolving the mechanisms underlying this specificity in relation to psychological stress would yield important insight into the role of mito-nuclear signaling in humans.
One common factor proposed to contribute to both telomere shortening and acceleration of the aging process is inflammation (163). In one study, genetically enhancing mitochondrial respiratory chain complex I activity in cultured cells simultaneously increased cellular ATP levels and decreased ROS production. This mitochondrial phenotype was associated with a concomitant reduction in LPS-stimulated IL-6 production while suppressing cell senescence (164). In another study, individuals previously exposed to early life adversity had their leukocyte mitochondrial respiration measured in parallel with inflammatory cytokines (165). Results revealed that the basal mitochondrial respiration relative to their maximal capacity was positively correlated to IL-6, TNF-α, and IL-1β (165). This evidence suggests that cellular metabolism in general, and mitochondrial respiratory capacity in particular, may influence both inflammation and cell aging. If proven true in humans, strategies to improve mitochondrial function could represent an effective upstream countermeasure to prevent the deleterious health effects of psychosocial adversity and inflammation.
In the same way that telomere shortening is considered to indicate biological age, decreasing mtDNA copy number and increasing tissue mtDNA damage are aging biomarkers (166, 167). mtDNA copy number measured in whole blood (including all cell populations and contaminating platelets) decreases with advancing age, starting around 50 years (168). Also starting after the fifth decade of life, the amount of immunogenic plasma ccf-mtDNA is elevated in older age groups, in parallel with increases in pro-inflammatory cytokines TNF-α, IL-6, and IL-1 receptor a (IL-1ra), suggesting a role of ccf-mtDNA in ‘inflammaging’ (129).
In muscle of elderly populations, mtDNA copy number and respiratory capacity also decline with age (169), but these recalibrations may be attributed to physical inactivity since exercise training restores several, albeit not all, parameter to levels of young individuals (170). The accumulation of mtDNA deletions in post-mitotic (i.e., non-dividing, such as the brain, heart, muscles) tissues is robust (166), representing a form of MAL. Moreover, as discussed above, accumulation of mtDNA defects is promoted by metabolic stress (20, 171). Metabolic stress in the form of hyperglycemia and hyperlipidemia is also promoted by the action of glucocorticoids and catecholamines released during psychosocial stress and life adversity. As a result, stress-induced metabolic stress leading to mtDNA damage and MAL is a potential mechanism by which adverse psychosocial experiences and chronic negative emotional states may impair cell energetics and possibly contribute to the age-related functional decline and increased vulnerability to disease (52). In blood cells, mtDNA deletions have also been detected in individuals with coronary artery calcification and cardiovascular disease (172), representing cross-sectional evidence that MAL is associated with downstream organ-specific pathology. Thus, mtDNA defects and particularly mtDNA deletions, may represent MAL markers linked to mitochondrial dysfunction and disease risk.
The study of exposure to chronic stressors and their effects on aging and lifespan have mainly been guided by three general models, namely cumulative risk (173, 174), stress sensitization (175, 176), and stress buffering (177, 178). Table 2 outlines the parallel between each model in the context of psychosomatic medicine, with parallel facets of mitochondrial biology. These include the accumulation of mtDNA damage over time, the stress-sensitizing effects of mitochondrial dysfunction causing abnormal HPA and SAM axes responses to challenge, and the role of energetic capacity in buffering against certain types of stressors. In relation to aging and geroscience, mitochondrial dysfunction is thus considered one of the “hallmarks” or “pillars” of aging (179), which interact broadly with other biological factors involved in the aging process.
Table 2.
Parallel comparison between theoretical models guiding psychosocial investigation of stress pathophysiology, and biological concepts relevant to mitochondrial function.
| PSYCHOSOCIAL – Theoretical models | MITOCHONDRIAL – Biological concepts |
|---|---|
| Cumulative risk | |
| Damage accumulates over time, eventually reaching a threshold where dysfunction and/or senescence compromises physiological regulation. | Mitochondria accumulate mtDNA defects with age and metabolic stress, eventually reaching a functional, threshold where energy production and other bioenergetic functions become compromised. |
| Stress sensitization | |
| Stressors becomes increasingly likely to cause damage or dysregulation under specific adverse circumstances or over time. | Organisms with inherited or acquired mitochondrial defects exhibit exaggerated and abnormal responses to subsequent stressors. |
| Stress buffering | |
| Psychosocial resources (e.g., social support, self-efficacy) and behavior (e.g., exercise) confer protection against the deleterious effects of chronic stress. | Upregulation of mitochondrial content, mitochondrial networking, and mitochondrial anti-oxidant defenses increases overall mitochondrial function, and as a result promotes cellular resilience to insults. |
8. CONCLUSION
It is with the vision to re-integrate psyche and soma that the first edition of Psychosomatic Medicine was published in 1939 (4). The field evolved from the concept of “milieu intérieur” (Claude Bernard – 1813–1878), subsequently built from understanding how emotional states led to physiological perturbations (Walter Cannon – 1871–1945), and the discovery that chronic stress could alter organ structure and their function (Hans Selye – 1907–1982). With time, statistical methods have become increasingly refined while larger and more sophisticated study designs have been elaborated to extract causal relationships between variables. More recently, increasingly precise biomarkers have been identified and applied to extend the reach of mind-body research into the cellular-molecular domain that is the core foundation of current biomedical training and practice.
Historically, it has been noted that progress within individual fields of investigation can be hindered by a lack of understanding of the relationships across fields (180). Conversely, identifying and studying intersection points between fields, focusing on different levels of function ranging from molecule to systems, has contributed to further the development of psychosomatic medicine. This is exemplified by foundational work in PNI and the discovery of intersection points between the immune system and the brain (99). Subsequent work on glucocorticoid hormones, catecholamines, and inflammatory cytokines, which carry information between various organ systems, considerably expanded our understanding of the inter-relations between physiological systems, their activation by stress, and the role of brain remodeling in disease (8, 112, 181). The allostatic load model describing the impact of chronic stress and resultant health behaviors has been particularly productive in providing a quantifiable (182) and integrative perspective of multi-systemic physiological dysregulation in response to chronic stress and adversity (183). At the cellular level, the discoveries that the rate of telomere shortening is modified by stress (184) and that gene expression is subject to social modulation (185) have spurred new depth into the biology of subjective experiences.
Importantly for the general mandate of psychosomatic medicine, these molecular findings resonate with the sub-cellular focus of the biomedical model. Discoveries of novel biomarkers that dynamically respond to emotional states help to establish a common semantic and conceptual basis for discussion among psychosocial and medical scholars. This contributes to building common transdisciplinary knowledge and is of value to all scientists invested in constructing a holistic understanding of human health and disease processes. While keeping in mind the whole individual, identifying increasingly refined biological intersections points, including mitochondria, should therefore continue to uncover new layers of complexity onto which we can observe, measure, quantify the cross-talk between ‘psyche’ and ‘soma’.
One of the objectives for psychosomatic research is to understand the basis for stress pathophysiology. How do adverse and positive life experiences leave biological marks, and influence trajectories of aging and disease risk? In considering the role of mitochondria in this process, evidence discussed above and summarized in Figure 3 indicate that mitochondria represent a potential biological intersection point that could contribute to multiple facets of stress pathophysiology. The proposed framework suggests that chronic psychosocial stressors and related emotional states lead to dysfunctional mitochondria and MAL, which in turn contribute to stress pathophysiology via multiple mechanisms including changes in gene expression and the epigenome, alterations of brain structure and functions, abnormal stress reactivity, inflammation, and by promoting cellular aging. More research is needed to test various elements of this model, particularly in humans. Furthermore, although compelling evidence reviewed here positions mitochondria as a nexus for various stress- and aging-related processes, future research should consider the dynamic bi-directional interactions between mitochondria and other important physiological systems.
Mitochondrial biology is deeply interlaced with the basic molecular and physiological principles onto which lies medical practice and education. Exploring different facets of mitochondrial function and MAL in psychosomatic research should therefore provide new insights directly linked to biomedical knowledge, and hopefully contribute to the bridging enterprise with medicine. To do so, mitochondrial functions and related aspects of cellular bioenergetics should be investigated with the proper methodology, using prospective and longitudinal study designs when possible, in parallel with established neuroendocrine and molecular biomarkers known to be responsive to stress and other psychosocial factors. This integration of concepts and approaches will insure a synergy with existing research and methodologies, promote collaboration, and enable us to map new pathways by which stress “gets under the skin”, all the way into the genome.
Integrating the many rich concepts and approaches from psychosomatic medicine, PNI, and PNE with mitochondrial biology under a common ‘psycho-mito-somatic’ framework will enable us to map the effects of psychological stress and other psychological states on mitochondria. The resulting mitochondrial recalibrations and their downstream correlates should then inform us on the effects of acquired mitochondrial defects and MAL on important physiological, behavioral, and health outcomes. Ultimately, the successful integration of mitochondria in psychosomatic research should foster a more comprehensive understanding of the forces that influence our health across the lifespan, and of the factors that hinder our ability to heal from disease. Psycho-mito-somatic studies will hopefully illuminate novel mechanisms for mind-body interactions, and form the empirical foundation to develop higher-level health promoting interventions based on the principles of allostasis and bioenergetics.
Acknowledgments
The authors are grateful to Claudia Trudel-Fitzgerald for comments and thoughtful edits to this manuscript.
Support for this work was provided by the Wharton Fund, NIH grants R35GM119793 R21MH113011 (M.P.), and Hope for Depression Research Foundation (B.S.M.).
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- Ach
Acetylcholine
- AMPK
Adenosine monophosphate activated protein kinase
- ANS
Autonomic nervous system
- ATP
Adenosine triphosphate
- ccf-mtDNA
Circulating cell-free mitochondrial DNA
- cGAS
Cyclic GMP-AMP (cGAMP) synthase
- CMG
circulating mitochondrial genome
- DAMPs
damage-associated molecular patterns
- ER
Estrogen receptor
- ELA
Early life adversity
- GCR
glucocorticoid receptor resistance
- GR
Glucocorticoid receptor
- HSP60
heat shock protein 60
- HIV
human immunodeficiency virus
- HPA
Hypothalamic-pituitary-adrenal
- IL-6
Interleukin 6
- IRFs
interferon regulatory factors
- LAC
acetyl-L-carnitine
- MAL
Mitochondrial allostatic load
- MAVS
mitochondrial anti-viral signaling protein
- mtDNA
Mitochondrial DNA
- mtDNA deletion
Deletion of a mtDNA segment
- Nf-kB
nuclear factor kappa B
- PGC-1α
peroxisome proliferator activated receptor gamma co-activator 1 alpha
- PNE
Psychoneuroendocrinology
- PNI
Psychoneuroimmunology
- PolG
Mitochondrial DNA polymerase gamma
- PTSD
post-traumatic stress disorder
- ROS
Reactive oxygen species
- SAM
Sympathetic-adrenal-medullary
- SES
Socioeconomic status
- RRV
RR interval variability
- SASP
senescence-associated secretory phenotype
- TLRs
toll-like receptors
Footnotes
The authors have no conflict of interest to report.
References
- 1.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–36. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and psychosomatic medicine: back to the future. Psychosom Med. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 4.Introductory Statement. Psychosom Med. 1939;1:3–5. [Google Scholar]
- 5.Picard M. Pathways to aging: the mitochondrion at the intersection of biological and psychosocial sciences. J Aging Res. 2011;2011:814096. doi: 10.4061/2011/814096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pert CB, Ruff MR, Weber RJ, Herkenham M. Neuropeptides and their receptors: a psychosomatic network. J Immunol. 1985;135:820s–6s. [PubMed] [Google Scholar]
- 7.Cole SW. Psychosocial influences on HIV-1 disease progression: neural, endocrine, and virologic mechanisms. Psychosom Med. 2008;70:562–8. doi: 10.1097/PSY.0b013e3181773bbd. [DOI] [PubMed] [Google Scholar]
- 8.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 9.Halfon N, Larson K, Lu M, Tullis E, Russ S. Lifecourse health development: past, present and future. Maternal and child health journal. 2014;18:344–65. doi: 10.1007/s10995-013-1346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picard M, McEwen BS. Psychological stress and mitochondria: A systematic review. Psychosom Med. doi: 10.1097/PSY.0000000000000545. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margulis L, Bermudes D. Symbiosis as a mechanism of evolution: status of cell symbiosis theory. Symbiosis. 1985;1:101–24. [PubMed] [Google Scholar]
- 12.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–34. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 13.Psarra AM, Sekeris CE. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells: role of the mitochondrial glucocorticoid receptor. Biochim Biophys Acta. 2011;1813:1814–21. doi: 10.1016/j.bbamcr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. 2009;106:3543–8. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psarra AM, Solakidi S, Sekeris CE. The mitochondrion as a primary site of action of steroid and thyroid hormones: presence and action of steroid and thyroid hormone receptors in mitochondria of animal cells. Mol Cell Endocrinol. 2006;246:21–33. doi: 10.1016/j.mce.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol. [Research Support, N.I.H., Extramural] 2012;24:236–48. doi: 10.1111/j.1365-2826.2011.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849–54. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, Pagano Zottola AC, Delamarre A, Cannich A, Vincent P, Varilh M, Robin LM, Terral G, Garcia-Fernandez MD, Colavita M, Mazier W, Drago F, Puente N, Reguero L, Elezgarai I, Dupuy JW, Cota D, Lopez-Rodriguez ML, Barreda-Gomez G, Massa F, Grandes P, Benard G, Marsicano G. A cannabinoid link between mitochondria and memory. Nature. 2016;539:555–9. doi: 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- 19.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard M, Turnbull DM. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes. 2013;62:672–8. doi: 10.2337/db12-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard M. Mitochondrial synapses: intracellular communication and signal integration. Trends in neurosciences. 2015;38:468–74. doi: 10.1016/j.tins.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Vincent A, Turnbull DM, Eisner V, Hajnoczky G, Picard M. Mitochondrial Nanotunnels. Trends Cell Biol. 2017 doi: 10.1016/j.tcb.2017.08.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol. 2013;304:R393–406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–9. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picard M, Vincent AE, Turnbull DM. Expanding Our Understanding of mtDNA Deletions. Cell Metab. 2016;24:3–4. doi: 10.1016/j.cmet.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Shaughnessy DT, McAllister K, Worth L, Haugen AC, Meyer JN, Domann FE, Van Houten B, Mostoslavsky R, Bultman SJ, Baccarelli AA, Begley TJ, Sobol RW, Hirschey MD, Ideker T, Santos JH, Copeland WC, Tice RR, Balshaw DM, Tyson FL. Mitochondria, energetics, epigenetics, and cellular responses to stress. Environ Health Perspect. 2014;122:1271–8. doi: 10.1289/ehp.1408418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–7. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502:489–98. doi: 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- 29.Chandel NS. Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015;22:204–6. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Picard M, Zhang J, Hancock S, Derbeneva O, Golhar R, Golik P, O'Hearn S, Levy S, Potluri P, Lvova M, Davila A, Lin CS, Perin JC, Rappaport EF, Hakonarson H, Trounce IA, Procaccio V, Wallace DC. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc Natl Acad Sci U S A. 2014;111:E4033–42. doi: 10.1073/pnas.1414028111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab. 2013;24:222–8. doi: 10.1016/j.tem.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao LW, Niu R, Liu Y. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 2016;26:1182–96. doi: 10.1038/cr.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose HS, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- 34.Midzak A, Papadopoulos V. Adrenal Mitochondria and Steroidogenesis: From Individual Proteins to Functional Protein Assemblies. Front Endocrinol (Lausanne) 2016;7:106. doi: 10.3389/fendo.2016.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A. 2015;112:E6614–23. doi: 10.1073/pnas.1515733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace DC. A mitochondrial bioenergetic etiology of disease. J Clin Invest. 2013;123:1405–12. doi: 10.1172/JCI61398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–16. doi: 10.1016/j.mito.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBride HM. Open questions: seeking a holistic approach for mitochondrial research. BMC biology. 2015;13:8. doi: 10.1186/s12915-015-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980;77:6715–9. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, 2nd, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–30. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 41.Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med. 2012;366:1132–41. doi: 10.1056/NEJMra1012478. [DOI] [PubMed] [Google Scholar]
- 42.Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–70. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 45.Trudel-Fitzgerald C, Chen Y, Singh A, Okereke OI, Kubzansky LD. Psychiatric, Psychological, and Social Determinants of Health in the Nurses' Health Study Cohorts. Am J Public Health. 2016;106:1644–9. doi: 10.2105/AJPH.2016.303318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puterman E, Epel E. An intricate dance: Life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Personal Psychol Compass. 2013;6:807–25. doi: 10.1111/j.1751-9004.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 48.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dial in Clin Neurosci: Stress. 2006;8:367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 50.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Picard M, Juster RP, Sloan RP, McEwen BS. Mitochondrial Nexus to Allostatic Load Biomarkers. Psychosom Med. 2017;79:114–7. doi: 10.1097/PSY.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the 'gluc' back in glucocorticoids. Nat Rev Endocrinol. 2014;10:303–10. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- 53.Kurz FT, Kembro JM, Flesia AG, Armoundas AA, Cortassa S, Aon MA, Lloyd D. Network dynamics: quantitative analysis of complex behavior in metabolism, organelles, and cells, from experiments to models and back. Wiley interdisciplinary reviews Systems biology and medicine. 2017:9. doi: 10.1002/wsbm.1352. [DOI] [PubMed] [Google Scholar]
- 54.Seeman TE, Singer BH, Rowe J, Horwitz RI, McEwen B. Price of adaptation - allostatic load and its health consequences. Arch Intern Med. 1997;157:2259–68. [PubMed] [Google Scholar]
- 55.McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2011;9:829–40. doi: 10.1016/S1474-4422(10)70116-2. [DOI] [PubMed] [Google Scholar]
- 56.Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M, Toscano A, Musumeci O, Valentino ML, Caporali L, Lamperti C, Tallaksen CM, Duffey P, Miller J, Whittaker RG, Baker MR, Jackson MJ, Clarke MP, Dhillon B, Czermin B, Stewart JD, Hudson G, Reynier P, Bonneau D, Marques W, Jr, Lenaers G, McFarland R, Taylor RW, Turnbull DM, Votruba M, Zeviani M, Carelli V, Bindoff LA, Horvath R, Amati-Bonneau P, Chinnery PF. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133:771–86. doi: 10.1093/brain/awq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goh S, Dong Z, Zhang Y, DiMauro S, Peterson BS. Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA psychiatry. 2014;71:665–71. doi: 10.1001/jamapsychiatry.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Picard M, Gu Z. Genetic Evidence for Elevated Pathogenicity of Mitochondrial DNA Heteroplasmy in Autism Spectrum Disorder. PLoS Genet. 2016;12:e1006391. doi: 10.1371/journal.pgen.1006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, McHugh P, Swerdlow RH. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer's disease. J Alzheimers Dis. 2011;27:483–90. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta. 2012;1820:553–64. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper-Knock J, Kirby J, Ferraiuolo L, Heath PR, Rattray M, Shaw PJ. Gene expression profiling in human neurodegenerative disease. Nature reviews Neurology. 2012;8:518–30. doi: 10.1038/nrneurol.2012.156. [DOI] [PubMed] [Google Scholar]
- 62.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703–15. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–53. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devaux-Bricout M, Grevent D, Lebre AS, Rio M, Desguerre I, De Lonlay P, Valayannopoulos V, Brunelle F, Rotig A, Munnich A, Boddaert N. [Aspect of brain MRI in mitochondrial respiratory chain deficiency. A diagnostic algorithm of the most common mitochondrial genetic mutations]. Rev Neurol (Paris) 2014;170:381–9. doi: 10.1016/j.neurol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Cordeiro M, Scaglia F, Lopes Da Silva S, Garcia P, Grazina M, Moura C, Diogol L. The brain-heart connection in mitochondrial respiratory chain diseases. The neuroradiology journal. 2009;22:558–63. doi: 10.1177/197140090902200508. [DOI] [PubMed] [Google Scholar]
- 66.Lax NZ, Pienaar I, Reeve A, Hepplewhite PD, Jaros E, Taylor RE, Kalaria R, Turnbull DM. Microangiopathy in the cerebellum of patients with mitochondrial DNA disease. Brain. 2012 doi: 10.1093/brain/aws110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ross JM, Stewart JB, Hagstrom E, Brene S, Mourier A, Coppotelli G, Freyer C, Lagouge M, Hoffer BJ, Olson L, Larsson NG. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature. 2013;501:412–5. doi: 10.1038/nature12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fink G. Stress controversies: post-traumatic stress disorder, hippocampal volume, gastroduodenal ulceration*. J Neuroendocrinol. 2011;23:107–17. doi: 10.1111/j.1365-2826.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- 69.Brown ES, Hughes CW, McColl R, Peshock R, King KS, Rush AJ. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology. 2014;39:770–9. doi: 10.1038/npp.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisniewski HM. Frequency of hippocampus atrophy in normal elderly and Alzheimer's disease patients. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 71.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiat. 2003;54:338–52. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 72.Starkman MN, Giordani B, Gebrski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiat. 1999;46:1595–602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 73.Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. BiolPsychiatry. 1996;40:1091–9. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drevets WC, Raichle ME. PET Imaging Studies of Human Emotional Disorders. PET Studies of Emotion. 1994:1–46. [Google Scholar]
- 75.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindlau A, Widmann CN, Putensen C, Jessen F, Semmler A, Heneka MT. Predictors of hippocampal atrophy in critically ill patients. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2015;22:410–5. doi: 10.1111/ene.12443. [DOI] [PubMed] [Google Scholar]
- 77.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiat. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–9. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho K. Chronic 'jet lag' produces temporal lobe atrophy and spatial cognitive deficits. Nature Neurosci. 2001;4:567–8. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 80.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 82.Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014;111:15810–5. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steib K, Schaffner I, Jagasia R, Ebert B, Lie DC. Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci. 2014;34:6624–33. doi: 10.1523/JNEUROSCI.4972-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–42. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, Bollen EL, Middelkoop HA, van Buchem MA, van der Grond J. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997–1002. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- 87.Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Munch G, Wood AG, Forbes J, Greenaway TM, Pearson S, Srikanth V. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–42. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–9. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 89.Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci USA. 2006;103:18775–80. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- 91.Armanini M, Hutchings C, Stein B, Sapolsky R. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- 92.Sapolsky R, Pulsinelli W. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–9. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 93.McLaughlin J, Roozendaal B, Dumas T, Gupta A, Ajilore O, Hsieh J, Ho D, Lawrence M, McGaugh JL, Sapolsky R. Sparing of neuronal function postseizure with gene therapy. Proc Natl Acad Sci U S A. 2000;97:12804–9. doi: 10.1073/pnas.210350097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA. 2009;106:3543–8. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Picard M, McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci U S A. 2014;111:7–8. doi: 10.1073/pnas.1321881111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A. 2014;111:486–91. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gimsa U, Kanitz E, Otten W, Ibrahim SM. Behavior and stress reactivity in mouse strains with mitochondrial DNA variations. Ann N Y Acad Sci. 2009;1153:131–8. doi: 10.1111/j.1749-6632.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- 98.Sharpley MS, Marciniak C, Eckel-Mahan K, McManus M, Crimi M, Waymire K, Lin CS, Masubuchi S, Friend N, Koike M, Chalkia D, MacGregor G, Sassone-Corsi P, Wallace DC. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell. 2012;151:333–43. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weiner H. Perturbing the Organism: The Biology of Stressful Life Experience. Chicago: University of Chicago Press; 1992. [Google Scholar]
- 100.Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nurnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, King PJ, Clark AJ, Metherell LA. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44:740–2. doi: 10.1038/ng.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strauss KA, DuBiner L, Simon M, Zaragoza M, Sengupta PP, Li P, Narula N, Dreike S, Platt J, Procaccio V, Ortiz-Gonzalez XR, Puffenberger EG, Kelley RI, Morton DH, Narula J, Wallace DC. Severity of cardiomyopathy associated with adenine nucleotide translocator-1 deficiency correlates with mtDNA haplogroup. Proc Natl Acad Sci U S A. 2013;110:3453–8. doi: 10.1073/pnas.1300690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126:413–23. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- 103.Bates MG, Newman JH, Jakovljevic DG, Hollingsworth KG, Alston CL, Zalewski P, Klawe JJ, Blamire AM, MacGowan GA, Keavney BD, Bourke JP, Schaefer A, McFarland R, Newton JL, Turnbull DM, Taylor RW, Trenell MI, Gorman GS. Defining cardiac adaptations and safety of endurance training in patients with m.3243A>G-related mitochondrial disease. Int J Cardiol. 2013;168:3599–608. doi: 10.1016/j.ijcard.2013.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jeppesen TD, Orngreen MC, Van Hall G, Vissing J. Lactate metabolism during exercise in patients with mitochondrial myopathy. Neuromuscul Disord. 2013;23:629–36. doi: 10.1016/j.nmd.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 105.Singh A, Petrides JS, Gold PW, Chrousos GP, Deuster PA. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J Clin Endocrinol Metab. 1999;84:1944–8. doi: 10.1210/jcem.84.6.5746. [DOI] [PubMed] [Google Scholar]
- 106.DeSantis AS, Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Racial and ethnic differences in diurnal cortisol rhythms: are they consistent over time? Psychosom Med. 2015;77:6–15. doi: 10.1097/PSY.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 107.Kenney MC, Chwa M, Atilano SR, Falatoonzadeh P, Ramirez C, Malik D, Tarek M, Del Carpio JC, Nesburn AB, Boyer DS, Kuppermann BD, Vawter MP, Jazwinski SM, Miceli MV, Wallace DC, Udar N. Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: implications for population susceptibility to diseases. Biochim Biophys Acta. 2014;1842:208–19. doi: 10.1016/j.bbadis.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lacy P. Editorial: secretion of cytokines and chemokines by innate immune cells. Frontiers in immunology. 2015;6:190. doi: 10.3389/fimmu.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunological reviews. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang EV, Glaser R. Stress-induced immunomodulation and the implications for health. Int Immunopharmacol. 2002;2:315–24. doi: 10.1016/s1567-5769(01)00182-5. [DOI] [PubMed] [Google Scholar]
- 111.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature reviews Immunology. 2011;11:625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76:181–9. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 113.Trudel-Fitzgerald C, Qureshi F, Appleton AA, Kubzansky LD. A healthy mix of emotions: underlying biological pathways linking emotions to physical health. Curr Opin Behav Sci. 2017:15. [Google Scholar]
- 114.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, Joshi S, Wang H, Andersson U, Chavan SS, Tracey KJ. Alpha 7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol Med. 2014 doi: 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–7. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2011;12:222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–14. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mathew A, Lindsley TA, Sheridan A, Bhoiwala DL, Hushmendy SF, Yager EJ, Ruggiero EA, Crawford DR. Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J Alzheimers Dis. 2012;30:617–27. doi: 10.3233/JAD-2012-120145. [DOI] [PubMed] [Google Scholar]
- 122.Shamaei-Tousi A, Steptoe A, O'Donnell K, Palmen J, Stephens JW, Hurel SJ, Marmot M, Homer K, D'Aiuto F, Coates AR, Humphries SE, Henderson B. Plasma heat shock protein 60 and cardiovascular disease risk: the role of psychosocial, genetic, and biological factors. Cell stress & chaperones. 2007;12:384–92. doi: 10.1379/CSC-300.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boyapati RK, Tamborska A, Dorward DA, Ho GT. Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Res. 2017;6:169. doi: 10.12688/f1000research.10397.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang R, Nakahira K, Guo X, Choi AM, Gu Z. Very Short Mitochondrial DNA Fragments and Heteroplasmy in Human Plasma. Scientific reports. 2016;6:36097. doi: 10.1038/srep36097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia P, Radpour R, Zachariah R, Fan AX, Kohler C, Hahn S, Holzgreve W, Zhong XY. Simultaneous quantitative assessment of circulating cell-free mitochondrial and nuclear DNA by multiplex real-time PCR. Genet Mol Biol. 2009;32:20–4. doi: 10.1590/S1415-47572009000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Mc Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, Choi AM. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577. doi: 10.1371/journal.pmed.1001577. discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li L, Hann HW, Wan S, Hann RS, Wang C, Lai Y, Ye X, Evans A, Myers RE, Ye Z, Li B, Xing J, Yang H. Cell-free circulating mitochondrial DNA content and risk of hepatocellular carcinoma in patients with chronic HBV infection. Scientific reports. 2016;6:23992. doi: 10.1038/srep23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 129.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T, Franceschi C, Cossarizza A. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for "inflamm-aging". European journal of immunology. 2014;44:1552–62. doi: 10.1002/eji.201343921. [DOI] [PubMed] [Google Scholar]
- 130.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nature reviews Immunology. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- 132.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–80. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hulgan T, Stein JH, Cotter BR, Murdock DG, Ritchie MD, Dube MP, Gerschenson M, Haas DW, Torriani FJ, Aids Clinical Trials Group A, Dacs 252 Study T Mitochondrial DNA variation and changes in adiponectin and endothelial function in HIV-infected adults after antiretroviral therapy initiation. AIDS Res Hum Retroviruses. 2013;29:1293–9. doi: 10.1089/aid.2013.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grady BJ, Samuels DC, Robbins GK, Selph D, Canter JA, Pollard RB, Haas DW, Shafer R, Kalams SA, Murdock DG, Ritchie MD, Hulgan T, Actg, Teams DS. Mitochondrial genomics and CD4 T-cell count recovery after antiretroviral therapy initiation in AIDS clinical trials group study 384. J Acquir Immune Defic Syndr. 2011;58:363–70. doi: 10.1097/QAI.0b013e31822c688b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hendrickson SL, Hutcheson HB, Ruiz-Pesini E, Poole JC, Lautenberger J, Sezgin E, Kingsley L, Goedert JJ, Vlahov D, Donfield S, Wallace DC, O'Brien SJ. Mitochondrial DNA haplogroups influence AIDS progression. AIDS. 2008;22:2429–39. doi: 10.1097/QAD.0b013e32831940bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, Diez-Sanchez C, Montoya J, Lopez-Perez MJ, Ruiz-Pesini E. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet. 2010;19:3343–53. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- 137.Ironson G, Hayward H. Do positive psychosocial factors predict disease progression in HIV-1? A review of the evidence. Psychosom Med. 2008;70:546–54. doi: 10.1097/PSY.0b013e318177216c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–43. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cellular signalling. 2014;26:192–7. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 140.Yu X, Gimsa U, Wester-Rosenlof L, Kanitz E, Otten W, Kunz M, Ibrahim SM. Dissecting the effects of mtDNA variations on complex traits using mouse conplastic strains. Genome research. 2009;19:159–65. doi: 10.1101/gr.078865.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garone C, Tadesse S, Hirano M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2011;134:3326–32. doi: 10.1093/brain/awr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–41. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 143.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–9. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosca MG, Lemieux H, Hoppel CL. Mitochondria in the elderly: Is acetylcarnitine a rejuvenator? Adv Drug Deliv Rev. 2009;61:1332–42. doi: 10.1016/j.addr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, Battaglia G, Mathe AA, Pittaluga A, Lionetto L, Simmaco M, Nicoletti F. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci U S A. 2013;110:4804–9. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kerner J, Yohannes E, Lee K, Virmani A, Koverech A, Cavazza C, Chance MR, Hoppel C. Acetyl-L-carnitine increases mitochondrial protein acetylation in the aged rat heart. Mech Ageing Dev. 2015;145:39–50. doi: 10.1016/j.mad.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hirschey MD, Shimazu T, Huang JY, Schwer B, Verdin E. SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:267–77. doi: 10.1101/sqb.2011.76.010850. [DOI] [PubMed] [Google Scholar]
- 148.Bigio B, Mathe AA, Sousa VC, Zelli D, Svenningsson P, McEwen BS, Nasca C. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proc Natl Acad Sci U S A. 2016;113:7906–11. doi: 10.1073/pnas.1603111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–7. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 150.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–42. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sondheimer N, Glatz CE, Tirone JE, Deardorff MA, Krieger AM, Hakonarson H. Neutral mitochondrial heteroplasmy and the influence of aging. Hum Mol Genet. 2011;20:1653–9. doi: 10.1093/hmg/ddr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kolesar JE, Safdar A, Abadi A, MacNeil LG, Crane JD, Tarnopolsky MA, Kaufman BA. Defects in mitochondrial DNA replication and oxidative damage in muscle of mtDNA mutator mice. Free Radic Biol Med. 2014;75:241–51. doi: 10.1016/j.freeradbiomed.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 154.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 155.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 156.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–40. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TB, von Zglinicki T. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oexle K, Zwirner A. Advanced telomere shortening in respiratory chain disorders. Hum Mol Genet. 1997;6:905–8. doi: 10.1093/hmg/6.6.905. [DOI] [PubMed] [Google Scholar]
- 159.Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A, Rushton MD, Charles M, Jurk D, Tait SW, Czapiewski R, Greaves L, Nelson G, Bohlooly YM, Rodriguez-Cuenca S, Vidal-Puig A, Mann D, Saretzki G, Quarato G, Green DR, Adams PD, von Zglinicki T, Korolchuk VI, Passos JF. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35:724–42. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–65. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pieters N, Janssen BG, Valeri L, Cox B, Cuypers A, Dewitte H, Plusquin M, Smeets K, Nawrot TS. Molecular responses in the telomere-mitochondrial axis of ageing in the elderly: A candidate gene approach. Mech Ageing Dev. 2015;145:51–7. doi: 10.1016/j.mad.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 162.Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, Ridout KK, Price LH. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. 2015;66:17–20. doi: 10.1016/j.exger.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schauer M, Kottek T, Schonherr M, Bhattacharya A, Ibrahim SM, Hirose M, Kohling R, Fuellen G, Schmitz U, Kunz M. A mutation in the NADH-dehydrogenase subunit 2 suppresses fibroblast aging. Oncotarget. 2015;6:8552–66. doi: 10.18632/oncotarget.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boeck C, Koenig AM, Schury K, Geiger ML, Karabatsiakis A, Wilker S, Waller C, Gundel H, Fegert JM, Calzia E, Kolassa IT. Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion. 2016;30:197–207. doi: 10.1016/j.mito.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 166.Meissner C, Bruse P, Oehmichen M. Tissue-specific deletion patterns of the mitochondrial genome with advancing age. Exp Gerontol. 2006;41:518–24. doi: 10.1016/j.exger.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 167.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992;2:324–9. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 168.Mengel-From J, Thinggaard M, Dalgard C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 2014;133:1149–59. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lanza IR, Sreekumaran Nair K. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol (Oxf) 2010;199:529–47. doi: 10.1111/j.1748-1716.2010.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liang P, Hughes V, Fukagawa NK. Increased prevalence of mitochondrial DNA deletions in skeletal muscle of older individuals with impaired glucose tolerance: possible marker of glycemic stress. Diabetes. 1997;46:920–3. doi: 10.2337/diab.46.5.920. [DOI] [PubMed] [Google Scholar]
- 172.Botto N, Berti S, Manfredi S, Al-Jabri A, Federici C, Clerico A, Ciofini E, Biagini A, Andreassi MG. Detection of mtDNA with 4977 bp deletion in blood cells and atherosclerotic lesions of patients with coronary artery disease. Mutat Res. 2005;570:81–8. doi: 10.1016/j.mrfmmm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 173.Slopen N, Non A, Williams DR, Roberts AL, Albert MA. Childhood adversity, adult neighborhood context, and cumulative biological risk for chronic diseases in adulthood. Psychosom Med. 2014;76:481–9. doi: 10.1097/PSY.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Puterman E, Gemmill A, Karasek D, Weir D, Adler NE, Prather AA, Epel ES. Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. Proc Natl Acad Sci U S A. 2016;113:E6335–E42. doi: 10.1073/pnas.1525602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med. 2010;40:1647–58. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–84. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 177.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–57. [PubMed] [Google Scholar]
- 178.Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Flanders-Dunbar H. Emotions and bodily changes: A survey of literature on psychosomatic interrelationships 1910–1953. New York, NY: Arno Press; 1976. [Google Scholar]
- 181.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–45. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bizik G, Picard M, Nijjar R, Tourjman V, McEwen BS, Lupien SJ, Juster RP. Allostatic load as a tool for monitoring physiological dysregulations and comorbidities in patients with severe mental illnesses. Harv Rev Psychiatry. 2013;21:296–313. doi: 10.1097/HRP.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 183.McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Slavich GM, Cole SW. The Emerging Field of Human Social Genomics. Clinical psychological science: $b a journal of the Association for Psychological Science. 2013;1:331–48. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2016;98:123–30. doi: 10.1016/j.freeradbiomed.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 187.Payne BA, Wilson IJ, Hateley CA, Horvath R, Santibanez-Koref M, Samuels DC, Price DA, Chinnery PF. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet. 2011;43:806–10. doi: 10.1038/ng.863. [DOI] [PMC free article] [PubMed] [Google Scholar]