Abstract
Background
Preoperative portal vein embolization (PVE) is increasingly used as a preparation for major hepatectomy in patients with inadequate liver remnant volume or function. However, whether segment 4 (S4) portal veins should be embolized is controversial. The effect of S4 portal vein embolization on the volume gain of segments 2 and 3 (S2+3) was examined.
Methods
Among 73 patients with uninjured liver who underwent right PVE (RPVE, n = 15) or RPVE extended to S4 portal veins (RPVE+4, n = 58), volume changes in S2+3 and S4 after embolization were compared. Clinical outcomes and PVE complications were assessed.
Results
After a median of 27 days, the S2+3 volume increased significantly after both RPVE and RPVE+4, but the absolute increase was significantly higher for RPVE+4 (median, 106 ml vs. 141 ml, P = 0.044), as was the hypertrophy rate (median, 26% vs. 54%, P = 0.021). There was no significant difference between RPVE and RPVE+4 in the absolute S4 volume increase (52 ml for RPVE vs. 55 ml for RPVE+4, P = 0.61) or the hypertrophy rate of S4 (30% for RPVE vs. 26% for RPVE+4, P = 0.45). Complications of PVE occurred in 1 patient (7%) after RPVE and 6 (10%) after RPVE+4 (P > 0.99). No PVE complication precluded subsequent resection. Curative hepatectomy was performed in 13 patients (87%) after RPVE and 40 (69%) after RPVE+4 (P = 0.21).
Conclusions
RPVE+4 significantly improves S2+3 hypertrophy compared to RPVE alone. Extending RPVE to S4 does not increase PVE-associated complications.
INTRODUCTION
Preoperative portal vein embolization (PVE) was proposed by Makuuchi et al in 19901 to induce hypertrophy of the liver remnant. The procedure is increasingly used at major hepatobiliary centers and has contributed to expanded use of major hepatectomy in patients with initially insufficient liver remnant volume or function.2-6
For patients with advanced cholangiocarcinoma in whom extended right hepatectomy is required, Nagino et al7 first introduced the concept of percutaneous transhepatic PVE with extension to the segment 4 (S4) portal veins (RPVE+4). The authors advocated this approach to optimize the hypertrophy of segments 2 and 3 (S2+3). We previously reported low mortality and morbidity (0.8% and 30.7% respectively) after extended hepatectomy (resection of 5 or more liver segments) in a series of 127 patients, 31 of whom underwent RPVE+4 prior to resection.8 However, the appropriateness of RPVE+4 is controversial. Recently, Capussotti et al9 reported a similar volume increase in patients treated with RPVE and those treated with RPVE+4. These authors concluded that RPVE+4 should not be routinely performed because it is associated with a higher risk of migration of embolization materials to left portal vein branches.
Only a few previous studies have compared the change in volume of S2+3 after RPVE and RPVE+4.7,9 The aim of this study was to determine the effect of adding S4 embolization to RPVE on the gain in volume of S2+3 in the homogeneous subset of patients with normal liver. We examined this issue through retrospective review of a large series of patients who underwent PVE at our institution over the past 9 years.
PATIENTS AND METHODS
This study was approved by the Institutional Review Board. Between October 1998 and August 2007, a total of 144 patients underwent preoperative RPVE (n = 41) or RPVE+4 (n = 103) at The University of Texas M.D. Anderson Caner Center (Fig 1). Eight patients for whom volumes were not calculated separately for segments 2, 3, and 4 were excluded, leaving 136 patients for study. In order to assure a homogenous study population of patients with uninjured liver, 27 patients with histologically proven cirrhosis or steatosis that involved more than 30% of the liver parenchyma and 36 patients who underwent sequential transcatheter arterial chemoembolization followed by PVE10 or 2-stage hepatectomy11 were excluded. The remaining 73 patients were the subjects of this study. Fifteen of these patients underwent RPVE, and 58 underwent RPVE+4. In patients who received chemotherapy, chemotherapy was terminated at least 2 weeks before PVE.
Fig 1.
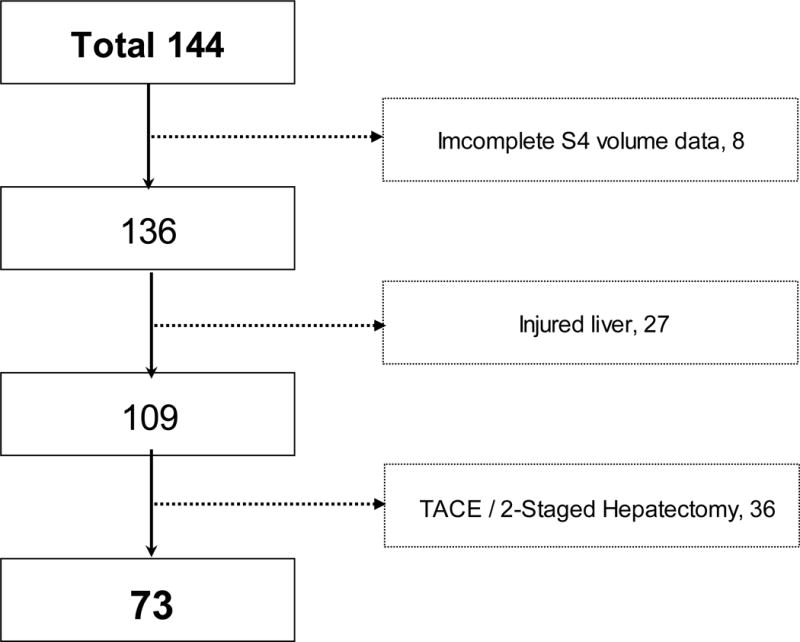
Flow chart of the selection of patients for this study. TACE, transcatheter arterial chemoembolization; Hx, hepatectomy.
During the period covered by this study, PVE was considered indicated if computed tomography (CT) volumetry revealed that the future liver remnant (FLR) volume was 20% or less of the standardized total liver volume (sTLV) in patients with normal liver.6,8 This criterion was established on the basis of our previous experiences showing that hepatectomy in patients with FLR of 20% or less of the sTLV was associated with increased postoperative morbidity2. The sTLV was calculated using a formula based on body surface area.12 This formula has recently been independently validated especially for its predictive accuracy of total liver volume in adults.13 This standardized method of measurement of the FLR (ratio of FLR/sTLV) is preferred to the classic method of measurement (ratio of FLR/radiologically measured total liver volume – tumor volume) because the latter does not provide a fixed estimation for total liver volume before and after PVE, is associated with the cumulative error associated with the measurement of multiple tumors, and does not adequately reflect the liver function in patients with chronic liver disease.8,12
All embolizations were performed using an ipsilateral percutaneous transhepatic approach as previously described.14,15 In brief, the right portal vein branch was accessed percutaneously under sonographic and fluoroscopic guidance. Seldinger technique was used to place a 5- to 6-F vascular sheath, and portography was then performed following injection of contrast medium into the main portal vein. In patients who underwent RPVE+4, embolization of S4 was done first. A 3-F microcatheter was placed selectively in each S4 portal branch, and the branches were embolized with tris-acryl microspheres or polyvinyl alcohol particles and subsequently with microcoils placed within the proximal portion. Embolization of the right portal vein was then performed in the same manner after the 3-F catheter was exchanged for a 5-F reverse-curve catheter. Final portography was performed with the flush catheter placed in the main portal vein (Fig 2). Embolization of portal vein branches were confirmed by CT images of portal venous phase (Fig. 3).
Fig 2.
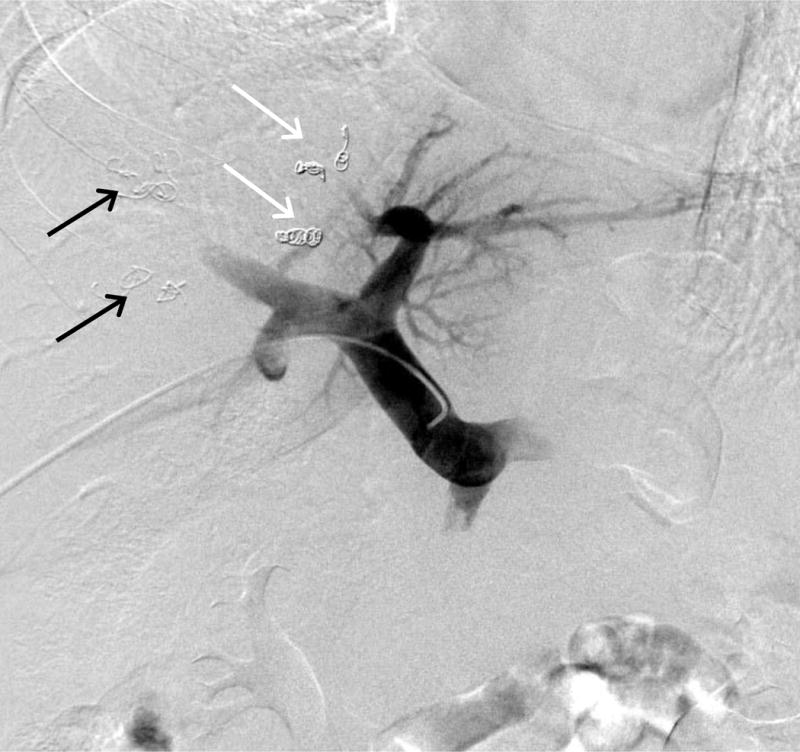
Portography after RPVE+4. White arrows, coils used to embolize S4 portal vein branches; black arrows, coils used to embolize right portal vein branches.
Fig 3.
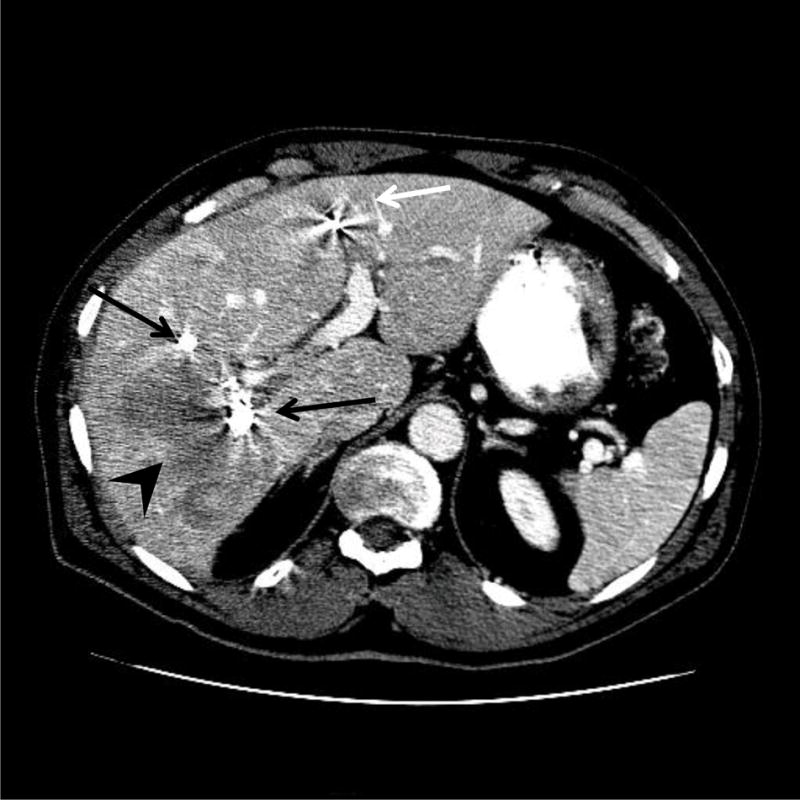
CT image after RPVE+4. Note that major S4 branches, in addition to right portal vein branches, were distally embolized (white arrow). Black arrows, coils used to embolized right portal vein branches. Black arrowhead, tumor
Volumes of each liver segment before and after embolization were calculated from helical CT data using integrated software techniques that use density threshold seeding.16 Although several patients had 2 to 5 CT series done before sufficient hypertrophy of the FLR was confirmed, the findings from the first CT series after embolization were used for analysis to compare between the 2 groups.
Volumetry results were reviewed, and both the absolute volume and hypertrophy rate (the ratio of volume change before and after PVE) of S2+3 and S4 were compared between the RPVE and RPVE+4 groups. Complications resulting from PVE have been reported from our previous series of 112 patients with both normal liver and underlying liver disease6. We specifically report the analysis of S4 volumetry and complication in patients with normal liver.
Statistical analysis
Continuous data were expressed as median and range and compared using Mann-Whitney U test. Categorical data were compared using chi-square test or Fisher’s exact test as appropriate. P values < 0.05 were considered statistically significant.
RESULTS
Clinical characteristics
Clinical characteristics of the patients in the RPVE and RPVE+4 groups are summarized in Table 1. The 2 groups did not differ significantly with respect to age, sex, body mass index, body surface area, frequency of diabetes, types of malignancy, or interval from embolization to first CT for volume evaluation.
Table 1.
Clinical characteristics of patients in the RPVE and RPVE+4 groups
| Characteristic | RPVE | RPVE+4 | P Value |
|---|---|---|---|
| Age, yr | 62 (31-73) | 58 (41-78) | 0.346 |
|
| |||
| Sex, M:F | 11:4 | 47:13 | 0.728 |
|
| |||
| Body height, cm | 176 (158-89) | 175 (150-193) | 0.589 |
|
| |||
| Body weight, kg | 87 (55-116) | 85.2 (49-157) | 0.543 |
|
| |||
| Body mass index, kg/m2 | 28 (21-41) | 27 (19-48) | 0.445 |
|
| |||
| Body surface area, m2 | 2.1 (1.6-2.4) | 2.0 (1.4-2.8) | 0.754 |
|
| |||
| Diabetes mellitus, % of patients | 13 | 19 | 0.611 |
|
| |||
| Etiology, no (%) of patients | 0.068 | ||
| Colorectal mets | 9 (60) | 23 (40) | |
| ICC, HBD, GB | 3 (20) | 18 (31) | |
| HCC | 3 (20) | 4 (7) | |
| Other mets | 0 | 13 (22) | |
|
| |||
| Median interval between PVE and CT (range), days | 27 (14-47) | 27 (9-70) | 0.951 |
mets, metastases; ICC, intrahepatic cholangiocarcinoma; HBD, hilar bile duct cholangiocarcinoma; GB, gallbladder cancer; HCC, hepatocellular carcinoma.
Volume analysis
The changes in absolute S2+3 and S4 volume for each patient are shown in Fig 4. In both the RPVE and RPVE+4 groups, the S2+3 and S4 volumes increased significantly after embolization. For S2+3 (Fig 4a), none of the 15 patients in the RPVE group had a volume increase of more than 70%, whereas 17 of the 58 patients in the RPVE+4 group had a volume increase of more than 70%, and 5 of these patients had an increase of more than 100% (0/15 vs. 17/58, P = 0.017). For S4 (Fig 4b), 1 of the 15 patients in the RPVE group had a volume decrease (from 169.5 ml to 166.6 ml), and 13 of the 58 patients in the RPVE+4 group had a volume decrease (1/15 vs. 13/58, P = 0.167). The proportion of patients with an S4 volume increase of more than 20% was similar in the RPVE group (9/15, 60%) and the RPVE+4 group (33/58, 57%, P = 0.828).
Fig 4.
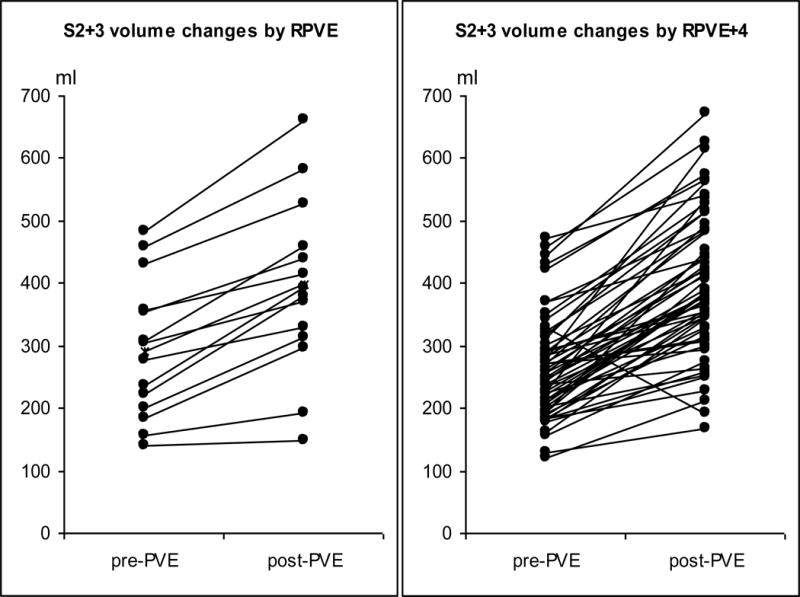
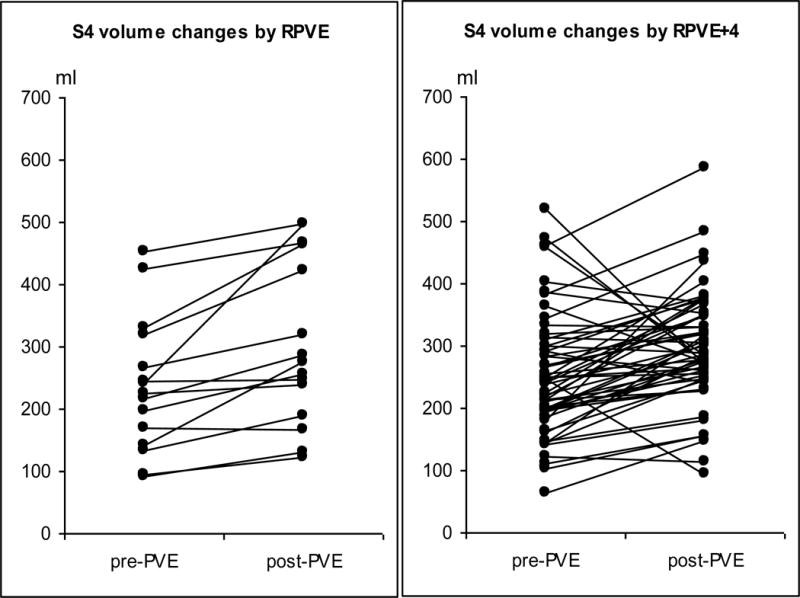
Changes in S2+3 volume (a) and S4 volume (b) in each patient as a result of RPVE or RPVE+4.
Changes in liver segment volumes for the RPVE and RPVE+4 groups are summarized in Table 2. The increase in the absolute S2+3 volume was significantly higher in the RPVE+4 group, as was the increase in the hypertrophy rate of S2+3. There was no significant difference between the RPVE and RPVE+4 groups in the change in the absolute volume and hypertrophy rate of S4.
Table 2.
Results of volume analysis in each group*
| Liver-Segment Volumes and Volume Changes | RPVE | RPVE+4 | P Value |
|---|---|---|---|
| S2+3 | |||
| Volume before PVE, ml | 291 (139-483) | 250 (120-473) | 0.306 |
| Volume after PVE, ml | 394 (149-660) | 384.2 (213-673) | 0.978 |
| Change, ml | 106 (10-178) | 141 (22-335) | 0.044 |
| Change, % | 26 (7-70) | 54 (8-135) | 0.021 |
| S4 | |||
| Volume before PVE, ml | 225 (91-453) | 242 (63-519) | 0.548 |
| Volume after PVE, ml | 274 (123-498) | 283 (95-587) | 0.972 |
| Change, ml | 52 (-3-255) | 55 (-250-255) | 0.613 |
| Change, % | 30 (-2-105) | 26 (-62-141) | 0.446 |
Values for each group are presented as median (range).
Clinical outcome
Complications related to embolization occurred in 1 patient (7%) after RPVE and 6 patients (10%) after RPVE+4 (P > 0.999). The single complication after RPVE was coil migration to the left portal vein. The 6 complications after RPVE+4 were partial portal vein thrombosis in 3 patients, complete main portal vein thrombosis in 1 patient, coil migration to the left portal vein in 1 patient, and subcapsular hematoma in 1 patient. In 2 of the 3 patients with partial portal vein thrombosis, the thrombus was removed surgically, but the other patient could not undergo hepatic resection owing to progression of disease. In the patient with complete portal vein thrombosis, local thrombolytic infusion and mechanical thrombectomy resolved the thrombus and subsequent successful resection was performed 41 days after PVE. In both cases of coil migration to the left portal vein, curative hepatectomy was performed, resulting in long postoperative survival times. Among the 71 patients who were excluded from this study, 1 patient could not proceed to hepatectomy owing to complications after RPVE+4 —esophageal bleeding secondary to occult preexisting portal hypertension aggravated by PVE.
Curative hepatectomy was performed in 13 (87%) of the 15 patients who underwent RPVE and 40 (69%) of the 58 patients who underwent RPVE+4 (P = 0.211). After surgery, the maximum serum total bilirubin value exceeded 5.0 mg/dl in no patients in the RPVE group and 9 patients in the RPVE+4 group, 2 of whom died of hepatic failure 10 and 38 days after extended right hepatectomy. In total, 28 of 41 patients (68%) underwent curative hepatic resection after RPVE and 68 of 103 (66%) underwent curative hepatic resection after RPVE+4 (P = 0.794). The postoperative 90-day mortality rate was 4% (1 patient) in the RPVE group and 6% (4 patients) in the RPVE+4 group (P > 0.999).
DISCUSSION
In this study, we found that the magnitude of the increase in S2+3 volume was significantly greater after RPVE+4 than after RPVE alone. Previous studies have shown that morphologic increase in liver volume is correlated with enhanced liver function in the form of increased biliary indocyanine green excretion,17 bilirubin clearance,18 and uptake of technetium-99m-galactosyl human serum albumin19,20 in the unembolized liver. In addition, in a previous study, we found a negative association between the size of the liver remnant and postoperative peak values for alkaline phosphatase, prothrombin time, and bilirubin.16 Therefore, it is rational to maximize hypertrophy of the FLR, and the results of the current study support extending embolization to the S4 portal veins.
In this study, the change in S4 volume as a result of embolization did not differ significantly between the RPVE and RPVE+4 groups. Although S4 volume decreased in 22% of the patients in the RPVE+4 group, 57% of the patients in that group had an S4 volume increase of more than 20%. This finding may be due to the technical difficulty of S4 embolization. Similar to our study, the study of Nagino et al,7 which showed an unchanged mean S4 volume after RPVE+4 (153 cm3 before embolization and 149 cm3 afterwards), also included several patients with increased S4 volume. The instances of increased S4 volume were considered to be due to difficulty (or undue risk) in achieving complete embolization of all the S4 portal vein branches. Usually, embolization of S4 requires catheterization of several branches arising from the umbilical portion of the left portal vein.7,14 Although the S4 portography were not reviewed in detail, the result suggests that embolization of S4 was incomplete to assure safety and to avoid inadvertent embolization of branches that might feed segment 2 or 3. Technological advances may overcome this problem. One such advance is C-arm CT, which is available in many angiography suites and allows simultaneous acquisition of 3-dimensional CT images.21 Use of C-arm CT would contribute to confirmation of the distribution of each portal vein branch. Alternatively recanalization of S4 segments may account for some of our findings regarding S4. The median interval of 27 days between PVE and CT evaluation in our study was longer than the mean 15.5-day interval in Nagino et al’s report.7 It is therefore possible that S4 regenerated during this 1-month period because of the remaining portal flow.
Despite the finding that S4 frequently increases in volume after RPVE+4, the S2+3 hypertrophy is significantly greater when S4 is embolized with the right portal vein vs. RPVE alone. This finding differs from that of Capussotti et al,9 who reported similar S2+3 volume increases after RPVE and RPVE+4. In their study, the number of patients was only 26, lower than the number of Nagino et al’s study (n = 57)7 or ours (n = 73). In addition, Capussotti et al included patients with a damaged liver, such as those with greater than 20% steatosis. These factors might have contributed to their negative findings.
As for the risks associated with embolization of S4 portal veins, Capussotti et al reported only 1 case of migration of embolizing materials in S2+3 after RPVE+4. More aggressive embolization of S4 branches could lead to a higher incidence of unintentional embolization of S2+3. However, this can happen not only after RPVE+4 but also after RPVE. In fact, coil migration in 1 of 2 patients of our series occurred after RPVE. In both of these 2 patients, coil migration in S2+3 did not preclude subsequent hepatic resection with a satisfactory outcome. Portal vein ligation has also been described as an another option for obtaining hypertrophy of the FLR, and partial ligation of S4 portal veins following right portal vein ligation has been described.22 However, dissection of the hepatic hilum leads to adhesions, which may render a second operation more difficult. In the study of Broering et al., PVE extended to S4 resulted in better regeneration of the FLR than combined portal vein ligation of main right portal vein and S4 portal vein branches.22
Our study was designed to evaluate the impact of S4 embolization on regeneration of the remnant liver parenchyma, but other potential impacts of S4 embolization should also be considered. One study23 showed that the growth of hepatocellular carcinomas was accelerated in embolized liver after PVE, and another study showed that the growth of metastatic tumors of colorectal origin was accelerated in nonembolized liver after PVE.24 Both reports were based on very small numbers of patients with growth reported in unembolized tumor-bearing liver (S4) in several patients. The approach we use is to embolize the entire tumor-bearing liver in an attempt to avoid the risk of accelerated tumor growth. In a previously published detailed analysis of tumor volume in 80 patients before and after PVE, we found no significant changes in tumor volume.6 In patients with bilateral liver metastases involving the FLR, we have recently reported a 4-step sequential approach: (1) systemic chemotherapy, (2) partial hepatic resection of metastatic tumors in the FLR, (3) PVE of the remaining tumor-bearing liver, and (4) second-stage major hepatectomy.11 Among 21 of the 30 patients in whom this planned approach was completed, the 3-year survival rate from the time of first hepatectomy was 86%.11
In conclusion, right PVE extended to S4 does not increase the morbidity of PVE and is associated with improved hypertrophy of segment 2+3. Further, more effective radiological techniques are needed to improve the efficacy of S4 embolization, which may in turn further optimize S2+3 hypertrophy.
ABBREVIATIONS
- CT
computed tomography
- FLR
future liver remnant
- PVE
portal vein embolization
- RPVE
right portal vein embolization
- RPVE+4
right portal vein embolization extended to the segment 4 portal veins
- sTLV
standardized total liver volume
- S2
segment 2
- S2+3
segments 2 and 3
- S4
segment 4
References
- 1.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–7. [PubMed] [Google Scholar]
- 2.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–80. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 3.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–17. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covey AM, Tuorto S, Brody LA, Sofocleous CT, Schubert J, von Tengg-Kobligk H, et al. Safety and efficacy of preoperative portal vein embolization with polyvinyl alcohol in 58 patients with liver metastases. AJR Am J Roentgenol. 2005;185:1620–6. doi: 10.2214/AJR.04.1593. [DOI] [PubMed] [Google Scholar]
- 5.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–72. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–94. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 7.Nagino M, Kamiya J, Kanai M, Uesaka K, Sano T, Yamamoto H, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155–60. doi: 10.1067/msy.2000.101273. [DOI] [PubMed] [Google Scholar]
- 8.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–32. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capussotti L, Muratore A, Ferrero A, Anselmetti GC, Corgnier A, Regge D. Extension of right portal vein embolization to segment IV portal branches. Arch Surg. 2005;140:1100–3. doi: 10.1001/archsurg.140.11.1100. [DOI] [PubMed] [Google Scholar]
- 10.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–8. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 11.Chun YS, Vauthey JN, Ribero D, Donadon M, Mullen JT, Eng C, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg. 2007;11:1498–504. doi: 10.1007/s11605-007-0272-2. [DOI] [PubMed] [Google Scholar]
- 12.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–40. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: A meta-analysis. Liver Transpl. 2005;11:1481–93. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
- 14.Madoff DC, Abdalla EK, Gupta S, Wu TT, Morris JS, Denys A, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–25. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 15.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness–study in 26 patients. Radiology. 2003;227:251–60. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 16.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–9. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 17.Uesaka K, Nimura Y, Nagino M. Changes in hepatic lobar function after right portal vein embolization. An appraisal by biliary indocyanine green excretion. Ann Surg. 1996;223:77–83. doi: 10.1097/00000658-199601000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ijichi M, Makuuchi M, Imamura H, Takayama T. Portal embolization relieves persistent jaundice after complete biliary drainage. Surgery. 2001;130:116–8. doi: 10.1067/msy.2001.115358. [DOI] [PubMed] [Google Scholar]
- 19.Hirai I, Kimura W, Fuse A, Suto K, Urayama M. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495–506. doi: 10.1067/msy.2003.138. [DOI] [PubMed] [Google Scholar]
- 20.Nanashima A, Yamaguchi H, Shibasaki S, Morino S, Ide N, Takeshita H, et al. Relationship between CT volumetry and functional liver volume using technetium-99m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization before major hepatectomy: a preliminary study. Dig Dis Sci. 2006;51:1190–5. doi: 10.1007/s10620-006-8031-x. [DOI] [PubMed] [Google Scholar]
- 21.Madoff DC, Abdalla EK, Wallace MJ, Ng CS, Ribero D, Vauthey JN. Portal vein embolization: a preoperative approach to improve the safety of major hepatic resection. Current Medical Imaging Reviews. 2006;2:385–404. [Google Scholar]
- 22.Broering DC, Hillert C, Krupski G, Fischer L, Mueller L, Achilles EG, et al. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg. 2002;6:905–13. doi: 10.1016/s1091-255x(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721–7. doi: 10.1080/02841850701424514. [DOI] [PubMed] [Google Scholar]
- 24.Elias D, De Baere T, Roche A, Ducreux M, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–8. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]


