Abstract
Protein kinase C (PKC) isozymes belong to a family of Ser/Thr kinases whose activity is governed by reversible release of an autoinhibitory pseudosubstrate. For conventional and novel isozymes, this is effected by binding the lipid second messenger, diacylglycerol, but for atypical PKC isozymes, this is effected by binding protein scaffolds. PKC shot into the limelight following the discovery in the 1980s that the diacylglycerol-sensitive isozymes are ‘receptors’ for the potent tumor-promoting phorbol esters. This set in place a concept that PKC isozymes are oncoproteins. Yet three decades of cancer clinical trials targeting PKC with inhibitors failed and, in some cases, worsened patient outcome. Emerging evidence from cancer-associated mutations and protein expression levels provide a reason: protein kinase C isozymes generally function as tumor suppressors and their activity should be restored, not inhibited, in cancer therapies. And whereas not enough activity is associated with cancer, variants with enhanced activity are associated with degenerative diseases such as Alzheimer’s disease. This review describes the tightly controlled mechanisms that ensure PKC activity is perfectly balanced and what happens when these controls are deregulated. PKC isozymes serve as a paradigm for the wisdom of Confucius: “to go beyond is as wrong as to fall short.”
Keywords: Protein kinase C, pseudosubstrate, phorbol esters, diacylglycerol, phosphorylation, tumor suppressor, cancer, Alzheimer’s disease
Introduction
The discovery of protein kinase C solved a 25-year long mystery of how agonists that promote lipid hydrolysis alter cell physiology (Figure 1). In a story that involves a transatlantic boat crossing carrying test tubes of 32P-labeled samples (Hokin LE 1987), Hokin and Hokin unearthed the first clue that cells use lipids to transduce information. Their finding that cholinergic stimulation of pancreatic slices promoted the incorporation of 32P from radiolabeled ATP into phospholipids (Hokin MR and Hokin 1953) initiated a flurry of research into stimulus-dependent lipid turn-over. It became quickly apparent that the phosphoinositides were hydrolyzed in response to a plethora of extracellular signals to generate two critical second messengers, the lipid backbone diacylglycerol and the water-soluble headgroup inositol trisphosphate (IP3), which was eventually shown to directly elevate intracellular Ca2+ (Michell 1975; Streb et al. 1983). However, the effector enzyme coupled to this lipid signaling pathway remained elusive.
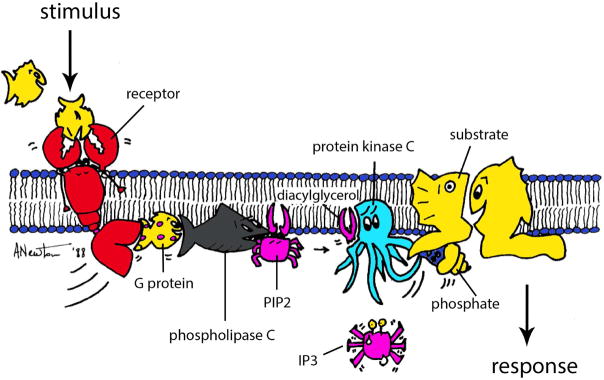
Figure 1. Cartoon illustrating activation of conventional protein kinase C.
Receptor-mediated hydrolysis of phosphatidyl-inositol-4,5,-bisphosphate (PIP2) generates inositol trisphosphate (IP3), which mobilizes intracellular Ca2+ and diacylglycerol, the allosteric activator of conventional and novel PKC. Binding of these second messengers localizes PKC in an active conformation at the plasma membrane, where it phosphorylates diverse substrates, including membrane proteins such as transporters (e.g. drug transporters) and receptors (e.g. receptor tyrosine kinases).
PKC Family and Regulation
The path from an unknown activity in brain fractions to a branch on the kinome
Protein kinase C was discovered by Yasutomi Nishizuka and colleagues at Kobe University as a histone and protamine phosphorylating activity in fractions of bovine and rat brain. Because the only requirement for activity was Mg2+, the new kinase was initially named protein kinase M (PKM) (Inoue et al. 1977; Takai et al. 1977). With the realization that PKM could be generated by Ca2+-dependent proteolysis, the race was on to identify the pro-enzyme. Two years later, the Kobe group discovered that the activity of the parent enzyme was stimulated by extracts of brain phospholipids, namely phosphatidylserine (Takai, Kishimoto, Iwasa, Kawahara, Mori, Nishizuka 1979), and a ‘trace impurity’ which was very quickly thereafter identified as diacylglycerol (Takai, Kishimoto, Kikkawa, et al. 1979). This conclusively identified the new kinase as the missing link from agonist-evoked lipid hydrolysis to alterations in cell physiology (Takai, Kishimoto, Kikkawa, et al. 1979). The pro-enzyme was named Ca2+-phospholipid-dependent protein kinase, or protein kinase C (PKC) (Takai, Kishimoto, Iwasa, Kawahara, Mori, Nishizuka 1979; Takai, Kishimoto, Iwasa, Kawahara, Mori, Nishizuka, et al. 1979). Huang and coworkers then showed that this brain-isolated PKC resolved into three distinct species on hydroxylapatite columns, which they named Types I, II, and III, introducing the idea that PKC comprised multiple isozymes (Huang et al. 1986). In 1986, PKCα, β, and γ (corresponding to Types III, II, and I, respectively) were cloned and shown to have a similar domain structure, consisting of conserved domains 1, 2, 3, and 4 (C1, C2, C3, and C4) interspersed with variable regions (Coussens et al. 1986; Knopf et al. 1986; Parker et al. 1986); this domain structure defines the subfamily of conventional PKC isozymes. Shortly thereafter, a class of isozymes with a slightly different domain composition were cloned: this class was named the novel PKC isozymes (Ohno et al. 1988) and represents the Ca2+-independent but diacylglycerol-dependent isozymes (δ, ε, η, θ). At the same time, PKC isozymes whose activity depended on neither Ca2+ nor diacylglycerol, but was stimulated by phosphatidylserine (Nakanishi H and Exton 1992), were cloned (Ono et al. 1988; Ono et al. 1989); this class was named the atypical PKC isozymes and comprises PKCζ and PKCι/λ (human/mouse) (Nishizuka 1992). By 1992, there were nine mammalian PKC genes (Nishizuka 1992), which evolved from the single Pkc1 (corresponding not to PKC, but to the closely related PKN in mammals (Roelants et al. 2017)) in Saccharomyces cerevisiae (Levin et al. 1990; Watanabe et al. 1994). Determination of the human kinome established that nine was, indeed, the total number of genes in the PKC family and verified that protein kinase D (PKD) (Valverde et al. 1994), which had also been named PKCμ (Johannes et al. 1994), was not part of the family (Manning et al. 2002). The PKC isozymes belong to the AGC super family of eukaryotic protein kinases (Hanks and Hunter 1995) and are perched at the tip of a branch that contains Akt, S6 kinase, and PDK-1 (Figure 2A). Splice variants, including the common C-terminal splice variants, PKCβI and βII, and a brain-specific variant that encodes only the catalytic domain of PKCζ, PKMζ, increase the number of isoforms (Ono et al. 1987; Hernandez et al. 2003; Patel et al. 2006).
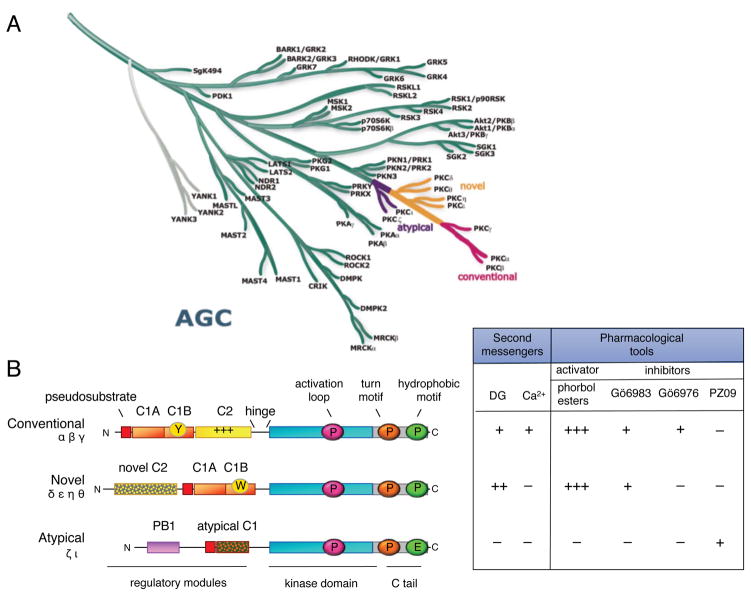
Figure 2. PKC isozymes are AGC kinases with N-terminal modules that control activity.
A. The AGC branch of the human kinome (reproduced from www.cellsignal.com/reference/kinase and courtesy of Gerard Manning) showing the position of the PKC isozymes. This branch includes Akt, p70S6 kinase and PDK-1. Most closely related to the PKC isozymes are the PKN family members that diverge first from the branch, followed by the atypical PKC isozymes (purple), the novel PKC isozymes (orange), and finally, the conventional PKC isozymes (pink), which are at the tip of the branch. B. Domain composition of PKC family members showing pseudosubstrate (red rectangle), C1 domain (orange rectangle; Y/W switch that dictates affinity for diacylglycerol-containing membranes indicated by circle in C1B domain), C2 domain (yellow rectangle; basic patch that drives binding to PIP2 indicated by ++ on domain), connecting hinge segment, kinase domain (cyan), and carboxyl-terminal tail (CT, grey rectangle). Also shown are the three priming phosphorylations: the activation loop in the kinase domain (magenta circle) and the turn motif (orange circle) and hydrophobic motif (green circle) in the carboxyl-terminal tail (note atypical PKC isozymes have Glu at phospho-acceptor position of hydrophobic motif). Table shows dependence of protein kinase C family members on second messengers (diacyglycerol (DG) and Ca2+) and pharmacological tools to activate (phorbol esters) or inhibit (Gö 6983, Gö 6976, and PZ09) PKC; +, ++, and +++ indicate relative affinity for C1 domain ligands.
Domain Composition of PKC Isozymes
All PKC isozymes share a common architecture of an N-terminal regulatory moiety (approximately 35 kDa) linked by a hinge region to a C-terminal kinase domain (approximately 45 kDa) (Figure 2B). The regulatory moiety of all PKC isozymes contains an autoinhibitory pseudosubstrate segment whose position in or out of the substrate-binding cavity is controlled by second messenger-binding or protein-binding modules specific to each PKC subclass.
Pseudosubstrate
The pseudosubstrate is a key molecular switch in the regulation of protein kinase C isozymes. It comprises a stretch of basic amino acids resembling the consensus substrates sequence but with an Ala at the position of the phosphoacceptor site (House and Kemp 1990). The affinity of isolated peptides based on this sequence for the kinase domain is relatively weak (0.1–1 μM range (House and Kemp 1987)), but they are effective for autoinhibition in the context of the full-length protein because their interaction with the kinase domain is intramolecular. Because of this relatively low affinity, pseudosubstrate peptides are not effective inhibitors of PKC in cells, even when myristoylated (Wu-Zhang et al. 2012). Additionally, there is little selectivity amongst PKC isozymes for pseudosubstrate sequences amongst family members, further invalidating the use of pseudosubstrate-based peptides for isozyme-specific studies in cells (Kazanietz M. G. et al. 1993; Nishikawa et al. 1997). This contrasts with PKI, the inhibitory peptide for protein kinase A (PKA), which binds its cognate kinase with high specificity and nM affinity and thus can be used to effectively inhibit PKA in cells (Scott et al. 1986).
C1 domains
All PKC isozymes contain either one or two C1 domains with no (atypical PKC isozymes), low (conventional PKC isozymes), or high (novel PKC isozymes) affinity for diacylglycerol. Conventional and novel PKC isozymes have tandem C1 domains (C1A-C1B). Whereas isolated domains both bind diacylglycerol, only one domain binds in the context of the full-length protein. This was established by Scatchard plot analyses which revealed that the stoichiometry of binding of diacylglycerol, or its functional analogues, the phorbol esters, is one mole ligand per mole PKC (Kikkawa et al. 1983; König et al. 1985). Additionally, bisfunctional phorbols are ineffective at engaging both domains, consistent with one C1 domain binding ligand in a physiological context (Giorgione J et al. 2003). For the conventional PKCβII and the novel PKCδ, mutagenesis studies have revealed that the primary diacylglycerol sensor is the C1B domain (Szallasi et al. 1996; Pu et al. 2009; Antal et al. 2014). Of note, PKD also has tandem C1 domains with the C1B domain being the relevant diacylglycerol sensor in the context of the full-length protein (Iglesias et al. 1998). The C1B domain of novel PKC isozymes binds diacylglycerol with two orders of magnitude higher affinity than the C1B of conventional PKC isozymes, allowing novel PKC isozymes to respond to agonist-evoked increases in diacylglycerol alone, whereas conventional PKC isozymes also need increases in intracellular Ca2+ (Giorgione JR et al. 2006). This differential affinity is tuned by a single amino acid in one of the ligand-binding loops of the C1B domain: when present as a Tyr (conventional isozymes), diacylglycerol affinity is low and when present as a Trp (novel isozymes), diacylglycerol affinity is high (Dries et al. 2007). NMR studies have recently revealed that Trp restricts the dynamics of the ligand-binding loops of the domain, with the more closed pocket favouring binding to diacylglycerol; Tyr permits increased movement and disfavours binding of the small diacylglycerol ligand (Stewart and Igumenova 2017). The larger phorbol ester is not influenced as much by these loop dynamics. Note that a Trp is present in this affinity-toggling position of all PKC C1A domains, the module that appears to be masked in the full-length protein. Atypical PKC isozymes have just one C1 domain and it is not a diacylglycerol sensor; a ring of basic residues surrounding the binding cleft precludes ligand binding (Kazanietz Marcelo G. et al. 1994; Pu et al. 2006). The C1 domain of atypical PKC isozymes also immediately follows the pseudosubstrate segment and functions as part of the autoinhibitory segment (Graybill et al. 2012).
C2 domain
Conventional PKC isozymes have a Ca2+-sensing C2 domain. Binding of Ca2+ to an Asp-lined binding pocket localizes the domain to the plasma membrane via 1] bridging of the C2-bound Ca2+ to anionic phospholipids and 2] interaction of a basic face in the C2 domain with phosphatidylinositol-4,5-bisphosphate (PIP2), a lipid localized to plasma membrane (Nalefski E. A. et al. 2001; Kohout et al. 2002; Corbalan-Garcia et al. 2003; Sanchez-Bautista et al. 2006; Igumenova 2015; Morales et al. 2016). Novel PKC isozymes have a ‘novel’ C2 domain that does not serve as a Ca2+ or plasma membrane sensor: it lacks acidic residues that coordinate Ca2+ and basic residues that bind PIP2. Interestingly, novel C2 domains contain the same β-strand fold as conventional C2 domains, but it is circularly permutated, such that the N- and C- termini are at different positions in the fold (Nalefski Eric A. and Falke 1996). The C2 domain has also been identified to function as a phospho-Tyr binding module in PKCδ (Benes et al. 2005).
PB1 domain
Atypical PKC isozymes contain a PB1 domain that mediates binding to protein scaffolds. Engagement to binding partners such as p62 and Par6 serves the same function as diacylglycerol binding to the C1 domain of conventional and novel PKC isozymes: the interaction disengages the pseudosubstrate from the substrate-binding cavity, resulting in kinase activation. Thus, atypical PKC isozymes are constitutively active when bound to protein scaffolds via PB1 domain interactions (Graybill et al. 2012; Tsai et al. 2015; Tobias and Newton 2016).
Kinase domain
The kinase domain of PKC isozymes is structurally similar to that of the archetypal kinase, protein kinase A (PKA), containing all the hallmark motifs, regulatory spines, and community networks that tune the catalytic step (Taylor and Kornev 2011). The catalytic rate of PKCβII is a few reactions per second (Johnson et al. 1997), comparable to the ‘average’ of 10 reactions per second for enzymes (Bar-Even et al. 2011) and slightly lower than that of PKA (20 reactions per second (Zhou and Adams 1997)); atypical PKC isozymes are exceptionally slow enzymes, catalyzing approximately 0.1 reactions per second (Tobias et al. 2016). Compared with its close cousins PKA and Akt, PKC isozymes do not have a strong consensus phosphorylation motif beyond the requirement for basic residues. Studies using an oriented peptide library to determine optimal peptide sequences phosphorylated by protein kinase C have confirmed the importance of basic residues at either amino- or carboxyl-terminal ends of the phosphoacceptor site, showing particular importance of a basic residue at the P-3 position (Songyang et al. 1994). In addition, a hydrophobic residue at the P+1 position markedly enhances recognition by protein kinase C. Nonetheless, the selectivity for specific residues is modest and differences in preferences between isozymes are generally subtle. Thus, as noted above under Pseudosubstrate, peptide substrates based on the pseudosubstrate segment of one isozyme are generally effective substrates for other isozymes in vitro (Kazanietz M. G. et al. 1993; Songyang et al. 1994). Although the similarity in active site architecture has made it difficult to develop specific inhibitors, the bisindolylmaleimides Gö6983, which inhibits conventional and novel PKC isozymes, and Gö6976, which inhibits conventional PKC isozymes (but also protein kinase D), are effective tools for cellular studies (Wu-Zhang and Newton 2013). Note that PKC isozymes bound to protein scaffolds are generally refractory to ATP competitive inhibitors such as Gö6983 and Gö6976, but are effectively inhibited by BisIV, an uncompetitive inhibitor with respect to substrates (Hoshi et al. 2010). PZ09 is a small molecule inhibitor that effectively inhibits atypical, but not conventional or novel, PKC isozymes (Trujillo et al. 2009; Tobias and Newton 2016).
C-terminal tail
The C-terminal tail is a key regulatory element of AGC kinases, serving both as a tether to structure determinants in the kinase domain and as a docking surface for regulatory proteins (Kannan et al. 2007). It wraps from the bottom of the C-terminal lobe up and over the N-terminal lobe (see inset in Figure 3), serving to structure the catalytic core, facilitate ATP binding, and promote substrate binding (Kannan et al. 2007). One of the key structural features of the C-terminal tail is a conserved PXXP motif which interacts with a conserved Tyr in the αE-helix of the kinase domain to create a binding surface for Hsp90 and its co-chaperone Cdc37, an interaction that is required for PKC to undergo priming phosphorylations (Gould et al. 2009). The C-terminal tail contains two highly conserved phosphorylation sites, the turn motif and hydrophobic motif, whose phosphorylation is required for the stability of PKC (Bornancin and Parker 1997; Edwards and Newton 1997). In addition, it serves as the docking site for the upstream kinase PDK-1 (Gao T et al. 2001), the prolyl isomerase Pin1 (Abrahamsen et al. 2012), the heat shock protein Hsp70 (Gao T and Newton 2006), and the mTORC2 component, Sin1 (Cameron et al. 2011). Deletion of the C-terminal tail prevents the generation of functional PKC (Yeong et al. 2006). Although the C-terminal tail is intrinsically disordered as an isolated peptide in solution, it has a propensity to adopt a helical structure upon interaction with detergent:lipid mixed micelles, leading Igumenova and colleagues to propose that the C-terminal tail serves as a membrane tether during the maturation process of PKC (Yang and Igumenova 2013).
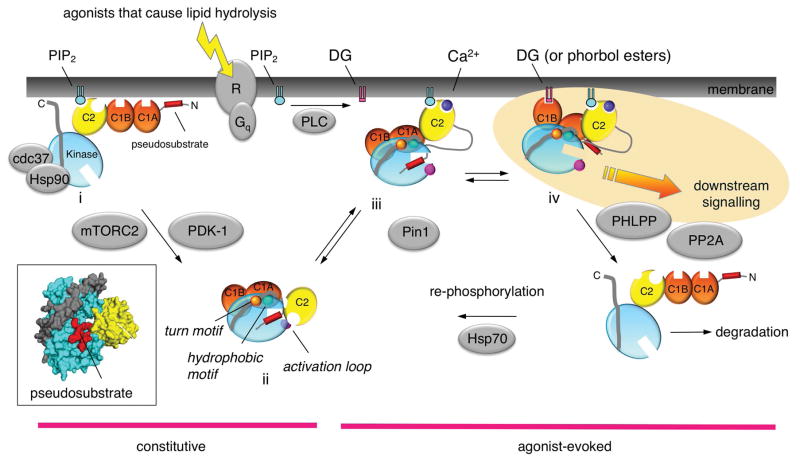
Figure 3. Cartoon showing the life cycle of a conventional PKC.
Following its biosynthesis, PKC is in an open and degradation-sensitive conformation in which all its regulatory modules are unmasked (species i). It is then processed by three ordered phosphorylations that depend on the binding of Hsp90 and Cdc37 (to a conserved PXXP motif in the kinase domain), the kinase complex mTORC2, and the activation loop kinase, PDK-1. Phosphorylation at these priming sites, the activation loop (magenta circle), the turn motif (orange circle) and the hydrophobic motif (green circle), promote PKC to adopt an autoinhibited conformation. Specifically, the Ca2+-sensing C2 domain (yellow) clamps the autoinhibitory pseudosubstrate segment (red) in the substrate-binding cavity of the kinase domain (cyan), and the diacyglycerol-sensing C1 domains (orange) become masked (species ii). The masking of the C1 domains, in particular, effectively prevents basal signaling in the absence of agonists. Agonists that bind Gq-coupled receptors (R) cause phospholipase C (PLC)-catalyzed hydrolysis of PIP2 generating diacylglycerol and Ca2+. This promotes Ca2+-dependent recruitment of PKC to the plasma membrane via engagement of the Ca2+-bound C2 domain (species iii), where PKC binds its membrane-embedded ligand, diacylglycerol, via primarily the C1B domain (species iv). This active PKC phosphorylates downstream substrates, with one function being to suppress oncogenic signaling via its inactivating phosphorylation of proteins such as Ras and the EGF receptor. PKC returns to the autoinhibited conformation following the decay of its second messengers. The kinetics of activation mirror those for the rise in intracellular Ca2+ and the kinetics of inactivation mirror those for the decay in diacylglycerol. Note the membrane-bound conformation of PKC is sensitive to dephosphorylation, with the first dephosphorylation event on the hydrophobic motif catalyzed by PHLPP; subsequent dephosphorylation by PP2A produces a fully dephosphorylated PKC that is degraded via a proteasomal pathway (species v). However, binding of Hsp70 to the dephosphorylated turn motif allows PKC to become rephosphorylated to sustain the signaling lifetime of the enzyme. Phorbol esters bind the C1B domain with two-orders of magnitude higher affinity than diacylglycerol and are not readily metabolized, trapping PKC in the open, phosphatase-sensitive conformation and resulting in chronic loss, or down-regulation, of PKC. Novel PKC isozymes are regulated by similar mechanisms except their C2 domain does not function as a Ca2+ or plasma membrane sensor, resulting in the localization of novel PKC isozymes primarily to the more abundant and diacylglycerol-rich Golgi membranes. Atypical PKC isozymes are activated upon binding to specific protein scaffolds that tether the pseudosubstrate out of the substrate-binding cavity. Proteins indicated in grey are key regulators of the steady-state levels of PKC: Hsp70, Hsp90, mTORC2, and PDK-1 function to increase the steady-state levels of PKC by permitting/catalyzing processing phosphorylations; Pin1 and the phosphatases PHLPP and PP2A function to decrease the steady-state levels of PKC by permitting/catalyzing the dephosphorylation of PKC. Targeting any of these proteins will disrupt the balance of PKC signaling. Inset shows the structure of the autoinhibited kinase domain of PKCβII, showing autoinhibitory pseudosubstrate locked in the substrate-binding cavity of the kinase domain (cyan) by the C2 domain (yellow); C-terminal tail is indicated in grey.
Maturation of PKC
All PKC isozymes are processed by a series of ordered phosphorylations that occur shortly after biosynthesis to yield a stable, autoinhibited enzyme that is primed to respond to second messengers. Studies with the conventional PKCβII have established that newly-synthesized enzyme is in an open conformation in which the membrane-targeting modules are exposed and the pseudosubstrate is not engaged in the kinase domain (Figure 3). The molecular chaperone Hsp90 binds this open PKC via a conserved PXXP motif in the C-terminal tail (see above) to allow PKC to become phosphorylated (Gould et al. 2009). The phosphoinositide-dependent kinase, PDK-1 binds the C-terminus of newly-synthesized and ‘open’ PKC (Newton 2010) to phosphorylate a highly-conserved Thr (Thr500 in PKCβII) on the activation loop, a segment near the entrance to the active site to position key determinants that control catalysis (Parker and Parkinson 2001; Taylor and Kornev 2011). This phosphorylation triggers two tightly-coupled phosphorylations in the C-terminal tail, the turn motif (containing the segment LTP in conventional and atypical PKC isozymes and LS/TX in novel isozymes; Thr641 in PKCβII) and the hydrophobic motif (with the consensus motif FXXFSF/Y; Ser660 in PKCβII). Pulse chase experiments reveal that the C-terminal modifications, which result in distinct electrophoretic mobility shifts, occur with a half-time of approximately 15 minutes after biosynthesis (Borner et al. 1989; Keranen et al. 1995; Sonnenburg et al. 2001). The C-terminal modifications depend on 1] the intrinsic catalytic activity of PKC, which in vitro, autophosphorylates by an intramolecular reaction at the hydrophobic motif (Behn-Krappa and Newton 1999), and 2] the kinase complex mTORC2 (Sarbassov et al. 2004; Ikenoue et al. 2008). The fully-phosphorylated enzyme undergoes conformational transitions to adopt an autoinhibited conformation in which the pseudosubstrate is now tucked in the substrate-binding cavity and the C1 and C2 domains become masked. The crystal structure of full-length PKCβII (Leonard et al. 2011) revealed an additional function of the C2 domain in clamping over the kinase domain of autoinhibited PKC, locking the pseudosubstrate segment in the substrate-binding cavity and ensuring minimal signaling in the absence of activators (Antal, Callender, et al. 2015). Effective autoinhibition also depends on intact C1 domains, in particular the C1A, which immediately follows the pseudosubstrate segment (Sommese et al. 2017). In this autoinhibited conformation, the phosphorylation sites are protected from dephosphorylation and the enzyme is relatively resistant to degradation. Disruption of any of the mechanisms that allow phosphorylation, such as reducing the binding of Hsp90, inhibiting mTORC2, removing PDK-1, or preventing autophosphorylation by kinase-inactivating mutations in PKC, results in the degradation of PKC; thus, enzyme does not accumulate in cells. PKC matured by these phosphorylation events is the species that accounts for almost all the PKC in the cell, and it is this species that is activated by lipid hydrolysis to transduce signals.
Novel PKC isozymes are also phosphorylated by PDK-1 at the activation loop (Cenni et al. 2002), but only PKCε requires mTORC2 for processing phosphorylations (Facchinetti et al. 2008; Ikenoue et al. 2008). Thus, PKCδ, PKCη, and PKCθ mature into fully-phosphorylated and stable enzymes in cells lacking mTORC2, in striking contrast to conventional PKC isozymes and PKCε which do not become phosphorylated and thus are degraded (Facchinetti et al. 2008; Ikenoue et al. 2008). PKCδ is unusual in that it is the only PKC reported to be active when expressed in bacteria, albeit with low activity (Stempka et al. 1997); whether this is a result of not requiring mTORC2 for processing remains to be determined.
Atypical PKC isozymes are also constitutively phosphorylated, but their mechanism of phosphorylation differs in a few key aspects from that of the diacylglycerol-sensitive isozymes (Tobias et al. 2016). Similar to what occurs for their close cousin Akt (Facchinetti et al. 2008), atypical PKC isozymes are co-translationally phosphorylated at the turn motif by ribosome-associated mTORC2. This phosphorylation at the turn motif is followed by constitutive phosphorylation at the activation loop by PDK-1. These are the only known processing phosphorylations on atypical PKC isozymes as they have a Glu at the position of hydrophobic motif phosphorylation site of the other PKC isozymes.
In addition to the priming phosphorylations at the activation loop and C-terminal sites, it is clear from unbiased mass spectrometric analyses that PKC has an abundance of other post-translational modifications (Freeley et al. 2011; Hornbeck et al. 2012). These include Tyr phosphorylation sites, first noted by Nishizuka and coworkers (Konishi et al. 1997), additional Ser/Thr phosphorylation sites, and acetylation and ubiquitination sites. Whether any of these function as ‘priming’ events remains to be established.
Reversible Activation of PKC
Once processed by phosphorylation, PKC localizes in the cytosol in an autoinhibited and stable conformation that is poised to respond to second messengers. Importantly, masking of the C1 domains provides an effective mechanism to suppress signaling in the absence of agonists (Antal et al. 2014). PIP2 hydrolysis to generate Ca2+ and diacylglycerol results in a two-step activation for conventional PKC isozymes. First, binding of Ca2+ to the C2 domain recruits these isozymes primarily to the plasma membrane: Ca2+ binds an Asp-lined mouth in the C2 domain such that upon a diffusion-controlled encounter with the plasma membrane, a bridge with anionic phospholipids is formed, thus selecting a conformation in which the C2 is displaced from the kinase domain. This exposes a PIP2-binding basic face that is masked in the autoinhibited conformation. The interaction of this basic face with PIP2, a lipid restricted to plasma membrane, localizes conventional PKC isozymes to plasma membrane. Second, the C1B domain then engages its membrane-embedded ligand, diacylglycerol, prompting a second conformational change that expels the pseudosubstrate from the substrate-binding cavity (Orr et al. 1992; Newton and Johnson 1998). This membrane translocation is a hallmark of PKC (see video in Supplemental Figure 1), with the on-rate reflecting the kinetics of the Ca2+ rise and the off-rate dictated by the kinetics of diacylglycerol decay (Gallegos et al. 2006). Pioneering technologies by Roger Tsien and colleagues led to the development of fluorescence resonance energy transfer (FRET) reporters to measure the spatiotemporal dynamics of PKC signaling in live cells (Violin et al. 2003).
Novel PKC isozymes are activated by diacylglycerol alone, allowing them to be activated by phospholipase C-catalyzed hydrolysis of lipids other than PIP2. Agonist stimulation causes their translocation to a variety of intracellular locations, including plasma membrane, Golgi, mitochondria, and, in the case of PKCδ, the nucleus. Because they lack a plasma membrane sensor, they favor binding to the more abundant Golgi membranes, with the kinetics of translocation mirroring the kinetics of diacylglycerol increases at this location. The sustained elevation of diacylglycerol at Golgi results in sustained signaling of novel PKC isozymes (Gallegos et al. 2006).
Atypical PKC isozymes are regulated by neither Ca2+ nor diacylglycerol. Rather, they are regulated by binding of their PB1 domain to the PB1 domains of protein scaffolds such as p62 and Par6. Scaffold binding not only positions these PKC isozymes near their substrates, but also relieves autoinhibitory constraints by tethering the pseudosubstrate away from the substrate-binding cavity (Drummond and Prehoda 2016). In the case of the interaction with p62, the pseudosubstrate of PKCζ is tethered to an acidic surface on the PB1 domain of this scaffold, stabilizing the open and active conformation of the kinase (Tsai et al. 2015). Similarly, PKCζ is maintained in an open conformation when bound to Par6 (Graybill et al. 2012; Tobias and Newton 2016). Colocalization of atypical PKC isozymes and substrates on protein scaffolds ensures efficient phosphorylation given the exceptionally slow catalytic rate of these isozymes.
In addition to second messenger-dependent regulation, several post-translational modifications regulate the function of PKC isozymes in an agonist-dependent manner (Steinberg 2004; Reyland 2007, 2009). Notably, Src-dependent phosphorylation at positions in the regulatory moiety, including the C2 domain, drives PKCδ into the nucleus, where it can be cleaved by caspases to commit to an irreversible activation (Humphries et al. 2008). Additionally, this isozyme can be converted to a constitutively active enzyme following oxidative stress-induced phosphorylation of Tyr311 in the hinge region, forming a docking site for its C2 domain: interaction between the hinge and C2 not only disrupts autoinhibitory constraints, but promotes the dephosphorylation of Ser357 in the kinase domain, a residue whose phosphorylation state tunes selectivity between Ser and Thr (Gong et al. 2016). Phosphorylation sites immediately preceding the pseudosubstrate of PKCδ have also been identified and shown to enhance lipid-independent activity (Gong et al. 2016). Additionally, PKCβII has been shown to autophosphorylate at sites preceding its pseudosubstrate (Flint et al. 1990), suggesting a general mechanism for PKC isozymes to sustain signaling. PKCε similarly autophosphorylates at several sites in vitro, and interestingly, conventional PKC isozymes have been reported to control these modifications in cells (Sommese et al. 2017). In the invertebrate Aplysia, autophosphorylation of novel PKCs in the C2 domain is important for increased lipid binding and membrane translocation (Pepio and Sossin 2001). This role of autophosphorylation in the C2 domain of novel PKCs may be conserved through vertebrates as PKCη also has autophosphorylation sites that regulate lipid interactions (Littler et al. 2006). Autophosphorylation sites serve as a marker for PKC activation, and an autophosphorylation site in the C2 domain of PKCα has been used as a dynamic marker in human cancer tissues (Ng et al. 1999). These sites contrast from the processing sites (see Maturation of PKC) that are constitutive and not a marker of activity, Thus, in addition to reversible activation by binding second messengers, reversible phosphorylation may fine tune the function of individual isozymes.
Down-regulation of PKC
Given that PKC is only transiently activated following lipid hydrolysis, it spends most of its life in a stable and autoinhibited conformation. Furthermore, at least in the case of PKCα, even sustained stimulation by repeated additions of short-chained diacylglycerols to cells does not promote any significant turn-over of the protein (Lum et al. 2016). However, potent C1 domain ligands, such as phorbol esters or bryostatins (see next section), lock PKC in an open (active) conformation on the membrane. This results in their dephosphorylation and subsequent degradation, a process referred to as down-regulation (Figure 4C) (Hansra et al. 1999).
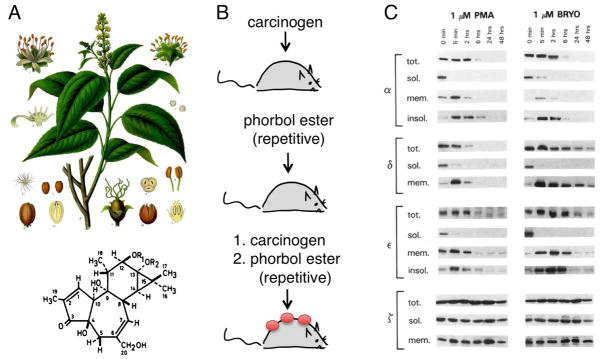
Figure 4. Phorbol esters: potent tumor promoters.
A. Drawing of the plant Croton tiglium, whose milky white sap contains phorbol esters (from Franz Eugen Köhler, Köhler’s Medizinal-Pflanzen (1887)). Also shown is the structure of phorbol ester, whose potency depends on the esterified group (R) at positions 12 and 13: phorbol-12-myristate-13-acetate (PMA) and phorbol-12, 13-dibutyrate (PDBu) are relatively water soluble phorbol esters used in cell biology (Courtesy of P. Blumberg, NIH.). B. Skin model of tumor promotion: painting a subthreshold amount of either a carcinogen (such as DMBA) or phorbol esters (such as PMA) alone on the skin of nude mouse does not result in papilloma formation. However, painting a subthreshold amount of the carcinogen followed by repetitive treatment with phorbol esters causes papillomas to form, which eventually develop into carcinomas. C. Treatment of NIH-3T3 fibroblasts with either PMA or bryostatin 1 causes endogenous conventional and novel PKC redistribution from the cytosolic fraction (soluble; sol.) to the detergent-solubilized membrane fraction (membrane; mem) and detergent-insoluble fraction (insoluble; insol.) within minutes, followed by down-regulation of the protein (apparent as loss of total (tot) PKC). PKCα, δ, and ε have different kinetics of down-regulation following PMA vs bryostatin 1 treatment, but all are effectively depleted following 48 hours of treatment. The atypical PKCζ is not down-regulated by these C1 ligands. Reproduced courtesy of P. Blumberg from (Szallasi et al. 1994).
Studies with the conventional PKCβII have revealed that the first step in phorbol ester-induced down-regulation is dephosphorylation at the hydrophobic motif, a reaction that is catalyzed by the PH domain Leucine-rich repeat Protein Phosphatase, PHLPP (Gao T et al. 2008). This destabilizes the kinase domain, promoting subsequent dephosphorylation at the turn motif and activation loop by PP2A phosphatases, followed by ubiquitination, and proteasomal degradation. For conventional PKC isozymes, a Pin1-catalyzed isomerization of the phospho-Thr-Pro peptidyl bond of the turn motif converts these isozymes into a species that can be readily dephosphorylated, ubiquitinated, and degraded (Abrahamsen et al. 2012). Sumoylation also controls the lifetime of PKC: mature (phosphorylated) PKCα is sumoylated on a conserved Lys in the kinase domain (Lys65). Dephosphorylation at the priming sites reduces sumoylation, which then promotes ubiquitination and thus down-regulation (Wang Y et al. 2016). Note that the molecular chaperone Hsp70 binds the dephosphorylated turn motif, an event that stabilizes PKC and promotes its rephosphorylation and re-entry into the pool of signaling-competent enzymes. In contrast, atypical PKC isozymes are neither down-regulated by phorbol esters (they do not have a ligand-binding C1 domain), nor are they dephosphorylated by PHLPP (they have a Glu at the hydrophobic motif phoshoacceptor site).
Downstream Substrates of PKC
Diacylglycerol-sensitive PKC isozymes are activated at membranes and it is thus not surprising that a large number of their substrates are membrane proteins. A major function of PKC-catalyzed phosphorylations of transmembrane receptors is to suppress their steady-state levels. One of the earliest identified substrates of PKC was the EGF receptor, whose inhibitory phosphorylation on Thr654 was identified by Hunter and colleagues over 30 years ago (Hunter Tony et al. 1984). This phosphorylation reduces EGFR tyrosine kinase activity, decreases ligand-binding affinity, and promotes internalization of the receptor (Hunter T. et al. 1984; Livneh et al. 1987; Livneh et al. 1988; Santiskulvong and Rozengurt 2007). PKC also phosphorylates and promotes the internalization of the proto-oncogene HER2 (Ouyang et al. 1998).
PKC isozymes also phosphorylate and desensitize an abundance of G-protein coupled receptors such as the β-adrenergic (Pitcher et al. 1992), muscarinic (Hosey et al. 1995), dopamine (Namkung and Sibley 2004), and histamine (Fujimoto et al. 1999) receptors, among many others. Linking their control of both receptor tyrosine kinases and GPCRs, PKCα has recently been shown to mediate a feedback inhibition of the EGF receptor transactivation induced by Gq-coupled receptor agonists (Santiskulvong and Rozengurt 2007). PKC isozymes also control the levels of metabotropic glutamate receptors, with PKCε regulating the surface expression of mGluR5 (Schwendt and Olive 2017).
In addition to receptors, a large number of transporters, including ABC transporters such as P-glycoprotein and solute-like carriers such as the organic cation transporter OCT1 have been reported to be phosphorylated by PKC to regulate transport activity, substrate specificity, and/or plasma membrane localization. Acute phorbol ester treatment induces the internalization of a large number of these transporters, revealing a role in controlling their steady-state levels (Mayati et al. 2017). Atypical PKC isozymes also regulate insulin-stimulated glucose transport (Bandyopadhyay et al. 1997; Kotani et al. 1998; Bandyopadhyay et al. 2000; Bandyopadhyay et al. 2002; Sajan et al. 2006; Farese et al. 2007).
In addition to suppressing signaling by receptor tyrosine kinases, PKC phosphorylates a number of other oncoproteins to suppress their activity. One such target is K-Ras, one of the most frequently mutated genes in cancer. PKC was first reported to phosphorylate K-Ras on Ser181 in the basic segment of a farnesoyl-electrostatic switch, causing the protein to disengage from the plasma membrane (Bivona et al. 2006). Although the role of this specific phosphorylation in tumors is unclear (Barcelo et al. 2014), McCormick and colleagues subsequently reported that activation of PKC suppresses K-Ras signaling in cancer (Wang MT et al. 2015). Strikingly, oral administration to mice of a phorbol ester of very weak potency (prostratin (Szallasi and Blumberg 1991)) promoted K-Ras phosphorylation and repressed growth in orthotopic models of human pancreatic cancer. PKCα catalyzes an inhibitory phosphorylation of another frequently mutated oncoprotein, the catalytic subunit of phosphatidylinositol-3-kinase (PI3K), thus suppressing signaling downstream of the PI3K/Akt cell survival leg of growth factor signaling (Sipeki et al. 2006). It also inactivates Akt by inducing its PP2A-mediated dephosphorylation (Tanaka et al. 2003). Thus, a major function of PKC may be to keep oncoproteins in check.
Atypical PKC isozymes phosphorylate a number of substrates co-localized on protein scaffolds. Notably, their coordination on the signaling platform Par6 positions them near substrates such as the polarity regulators Par3 and Lgl (Lin et al. 2000; Hirose et al. 2002; Nagai-Tamai et al. 2002; Betschinger et al. 2003; Plant et al. 2003; Yamanaka et al. 2003; Soriano et al. 2016).
Phorbol Esters, Tumor Promotion, and Down-regulation
Phorbol esters
The milky sap exuded by plants of the Euphorbiaceae family is a potent irritant that has been used over the millennia for diverse purposes, including poison arrows. The oil from one such species, the Croton tiglium plant (see Figure 4A), was used medicinally as a counter-irritant and cathartic (Hecker 1968). Classic studies starting in the 1940s established that croton oil is a tumor promoter (Berenblum and Shubik 1947): painting a sub-threshold amount of a carcinogen such as 7,12-Dimethylbenz[a]anthracene (DMBA) on the skin of mice did not result in papilloma formation; however, papillomas developed if the carcinogen treatment was followed by repetitive application of croton oil (reviewed in (Griner and Kazanietz 2007)) (Figure 4B). Indeed in humans, the common use of Croton flavens, one species of Euphorbia, for ‘bush tea’ in Curaçao was proposed to be causally related with the high incidence of esophageal cancer on this Caribbean island (Weber and Hecker 1978). In the late 1960s, the active ingredient was identified as a family of diesters of the tetracyclic diterpene phorbol, with varying acyl chains at the C-12 and C-13 positions (Hecker et al. 1966; Hecker 1978) (Figure 4A). The most potent compound was phorbol 12-myristate 13-acetate (PMA, also referred to as TPA for 12-0-tetradecanoyl phorbol 13-acetate). The highly lipophilic properties of these molecules resulted in their nonspecific intercalation into cell membranes, impeding the identification of their receptor. A breakthrough was made by Blumberg and colleagues, who reasoned that a more water-soluble molecule that still retained the pharmacophore would facilitate identification of the receptor. Their synthesis of 3H-phorbol 12,13-dibutyrate (PDBu) revealed specific and saturable binding to cell membranes (Driedger and Blumberg 1980; Blumberg et al. 1984). Shortly thereafter, PKC was identified as a major receptor for phorbol esters (Castagna et al. 1982). To this day, PDBu remains one of the most commonly used tools to modulate signaling pathways in cells.
Phorbol esters bind diacylglycerol-sensitive C1 domains in a competitive manner with respect to the physiological ligand, diacylglycerol (Sharkey et al. 1984). Thus, they bind all conventional and novel, but not atypical, PKC isozymes, as well as PKD, Ras GRPs, and other C1 domain-containing proteins (Kazanietz M. G. 2000; Toker 2005). However, unlike diacylglycerol, phorbol esters are not readily metabolized and consequently lock PKC in an open conformation that is sensitive to dephosphorylation, ubiquitination, and degradation. Thus, while phorbol esters cause the acute activation of PKC, this is followed by the chronic loss, or down-regulation, of all conventional and novel PKC isozymes (Figure 4C) (Jaken et al. 1981; Szallasi et al. 1994). As a result, overnight treatment with phorbol esters was a common and effective method to deplete cells of conventional and novel PKC isozymes in the era preceding genetic knockdown.
Bryostatins
Bryostatins are macrocylic lactones found in marine bryozoans that compete with phorbol esters for binding the C1 domain and activating PKC (Kraft et al. 1986; Wender et al. 1988). By locking PKC in the open conformation, they also cause the down-regulation of diacylglycerol-sensitive PKC isozymes (see Figure 4). However, their biological effects are more variable, and although they can effectively block some of the effects of phorbol esters, they do not always mimic phorbol esters (Kraft et al. 1986).
Molecular dynamics simulations reveal that bryostatin 1 and PDBu differentially position the PKCδ C1B domain in membranes: bryostatin 1 favors both a shallow and deep penetration in the membrane, whereas PDBu only has one free energy minimum and that is with the C1B domain embedded deeply in the membrane (Ryckbosch et al. 2017). The differential membrane interaction likely contributes to the differences in biological effects between bryostatins and phorbol esters.
In the paradigm described above for carcinogen-induced tumor promotion, the repetitive application of phorbol esters would be expected to cause a loss of PKC. Indeed, prolonged infusion with bryostatin 1, a marine natural product that, like phorbol esters, also bind the C1 domain with exceptionally high affinity and down-regulate PKC (de Vries et al. 1988), resulted in a significant reduction in the levels of PKCα, PKCε, and PKCη in peripheral blood monocytes of patients with advanced metastatic cancers (Marshall et al. 2002). This begs the question as to whether the tumor-promoting effects of TPA in the skin carcinogenesis models could result from the chronic loss of PKC. Experiments with transgenic mice overexpressing specific PKC isozymes suggest that loss of PKC could contribute to the tumor-promoting properties of phorbol esters. PKCδ overexpression in the epidermis of mice protects them from chemically induced development of squamous carcinomas (Reddig et al. 1999; Aziz et al. 2006); PKCε overexpression in the epidermis reduced papilloma burden by 95% compared to wild-type controls, but enhanced carcinomas (Reddig et al. 2000). Mice overexpressing PKCα in their epidermis were reported not to affect susceptibility to skin tumor promotion (Wang HQ and Smart 1999). Such experiments suggest that in the skin tumor carcinogenesis models, the loss (rather than activation) of at least one isozyme, PKCδ, promotes papilloma formation.
Further evidence that phorbol ester-dependent loss of PKC could be driving tumorigenesis came from studies by Altman and coworkers that showed that long-term treatment of a metastatic fibrosarcoma cell line with PDBu, resulting in a 4-fold reduction in PKC activity, increased lung tumor formation following intravenous injection of the cells in mice (Isakov et al. 1991; Isakov 2017). In contrast, a single short-term exposure to PDBu, shown to acutely activate PKC, decreased the cell’s ability to form lung metastasis.
PKC and Cancer
The discovery that PKC is the major receptor for phorbol esters was the genesis of the notion that PKC isozymes are oncogenes. At a time when more and more kinases were being shown to drive survival pathways, attributing tumor promotion to the hyperactivity of PKC was unsurprising. Thus, inhibitors for PKC activity entered clinical trials. Three decades later, it became apparent that not only were they not working as anti-cancer drugs, but in some cases, PKC inhibitors were worsening patient outcome. Most striking, a meta-analysis of five clinical trials for non-small cell lung carcinomas revealed worsened patient outcome when PKC inhibitors (enzastaurin, an ATP competitive inhibitor, or aprinocarsen, a PKCα antisense oligonucleotide) were combined with chemotherapy compared with chemotherapy alone (Zhang et al. 2015). Thus, clinical trials unveiled a disconnect between the biology of phorbol esters and the presumed function of PKC.
LOF Mutations
The first clue that PKC function may be lost in human cancers was the identification by Joubert and colleagues, over 20 years ago, of an inactivating mutation in PKCα in human pituitary tumors (Prevostel et al. 1995; Prevostel et al. 2000). This mutation, D294G, occurred in the hinge region separating the regulatory and catalytic moieties and abolished the targeting of the enzyme to cell-cell contacts, effectively reducing its function (Vallentin et al. 2001). It took the advent of whole genome sequencing of tumor and pair-matched control samples to realize that this loss-of-function (LOF) mutation in a human cancer was not the exception, but rather the rule. Yet numerous studies were consistent with at least this isozyme being a tumor suppressor: as early as the 1990s, Black and workers established a role for PKCα in suppressing cell growth (Saxon et al. 1994; Frey et al. 1997).
There are now well over 1,000 unique cancer-associated somatic mutations in the PKC family annotated in cBioPortal (Gao J et al. 2013). They occur in all PKC isozymes and throughout their domain structure. Introduction of approximately 50 of these point mutations, throughout the entire PKC family, revealed that two thirds of these cancer-associated mutations resulted in LOF of PKC, as assessed using live-cell imaging assays of PKC activity (Antal, Hudson, et al. 2015). Strikingly, not a single activity-enhancing (i.e. gain-of-function (GOF)) mutation was identified (Antal, Hudson, et al. 2015). Inactivation occurred by disabling regulatory inputs to process or activate PKC or by disabling catalytic mechanisms. Thus, one class of mutations prevented processing phosphorylations, resulting in decreased steady-state levels of the mutant PKC. Another class impeded second messenger binding, either by mutations in the C1 domains or in the C2 domain. A third class impaired catalysis, most commonly by mutation of highly conserved motifs required for catalytic activity such as the hallmark amino acid segments HRD, DFG, or APE (Meharena et al. 2013). The recently developed software KinView, which annotates cancer-associated protein kinase mutations, identifies many additional LOF mutations in the kinase domain of PKC, one of which was validated experimentally (McSkimming et al. 2016). The peppering of mutations throughout the domain structure is characteristic of tumor suppressors: in the case of PKC, there abundant mechanisms to tune its activity and thus an abundance of ways to disrupt function. This contrasts with mutations in oncogenes, which typically target one or two key residues to cause constitutive activity. It is noteworthy that mutations that render PKC constitutively active destroy the protective advantage of autoinhibition, so such mutations are destabilizing and result in PKC degradation and LOF.
In addition to the LOF mutations, neomorphic mutations that disrupt normal signaling by redirecting PKC away from physiological substrates, thus potentially engaging novel signaling pathways, may also contribute to cancer. Indeed, the mislocalizing mutation identified in PKCα may provide such a function (see above; (Prevostel et al. 1995; Prevostel et al. 2000)). Additionally, a mutation in PKCγ that alters the substrate specificity of the kinase has been identified in lung cancer (Creixell et al. 2015). Also of note, a number of fusion proteins in PKC have also been identified in human cancers (Stransky et al. 2014) and analysis of one such fusion in PKCε in a thyroid cancer cell line reveals that its impaired function protects thyroid cells from apoptosis (Knauf et al. 1999). Lastly, numerous truncating mutations or indels (insertions or deletions) in each of the PKC isozymes have also been identified. It is now clear that the identification in 1995 of a LOF mutation in a PKCα was generally representative of the mutational status of PKC isozymes in cancer.
PKCβII: haploinsufficient and dominant-negative mutations
Genome editing in a colon cancer cell line harboring a mutant PKCβ allele (A509T) revealed that this isozyme suppresses anchorage-independent growth in agar and tumor growth in a xenograft model, and is haploinsufficient towards these functions. This mutation occurred in a mutational ‘warm spot’ in the kinase domain, the APE motif of the activation loop: the Glu in this motif forms a highly conserved salt bridge in the eukaryotic protein kinase structure whose loss is associated with a number of diseases (Torkamani et al. 2008; McClendon et al. 2014). Clonal cell lines made homozygous for wild-type PKCβ had greatly reduced anchorage-independent growth, a hallmark of cancer, compared to the parental cell line, which was heterozygous for the PKCβ A509T mutation. Furthermore, clonal cell lines made hemizygous for PKCβ (by deletion of the A509T mutant allele) displayed increased anchorage-independent growth compared to the same cell line made homozygous for PKCβ. Thus, one allele of wild-type PKC is considerably less effective than two alleles in suppressing anchorage-independent growth, revealing haploinsufficiency; and the presence of a mutant allele is even less effective in suppressing growth, revealing the mutant PKCβ is dominant negative. Most strikingly, correction of the mutant allele to wild-type effectively suppressed tumor growth in a xenograft murine model (Antal, Hudson, et al. 2015). This cell line also harbored an oncogenic mutation in K-Ras, underscoring the dominating tumor suppressive role of PKC, even in the context of one of the most potent driver oncogenes.
Many of the inactivating mutations characterized for PKC are dominant negative towards the global signaling output of other PKC isozymes (Antal, Hudson, et al. 2015). This dominant negative effect on the activity of other PKC isozymes may result from the mutant PKC interfering with the phosphorylation of other PKC isozymes, because their phosphorylation requires common titratable components (see Figure 3). In support of this, expression of a mutant PKC isozyme that was not processed by phosphorylation was shown to impede the accumulation of other PKC isozymes both in overexpression studies (Garcia-Paramio et al. 1998) and by comparison of PKCα levels in cell lines harboring one allele of PKCβ A509T vs two wild-type alleles (Antal, Hudson, et al. 2015). One potential candidate for this dominant-negative effect is PDK-1, required for the priming phosphorylations of all PKC isozymes and reported to be present at 10 nM in HeLa cells, considerably below the sum concentration of all the PKC isozymes (>100 nM) (Hein et al. 2015). PDK-1 binds with high affinity to the C-tail of unprocessed PKC (Gao T et al. 2001), potentially sequestering it from its other functions. Thus, LOF mutations may exert particularly broad effects on suppressing oncogenic signaling because of global disruption of signaling by multiple PKC isozymes. The consensus emerging from analysis of human cancers is that PKC acts as the brakes to oncogenic function: its levels not only tune its signaling output, but inactivating mutations are dominant-negative with respect to other PKC isozymes.
Reduced PKC Protein Levels in Human Tumors
A general tumor-suppressive function of PKC begs the question of whether higher PKC levels may serve as a predictor for patient survival. Indeed, analysis of clinical data support this possibility. Low levels of PKCα expression (both mRNA and protein) have recently been shown to predict poor outcome in T-cell Acute Lymphoblastic Leukemia (T-ALL) (Milani et al. 2014). Similarly, low levels of PKCβII protein predict poor outcome in colorectal cancer (Dowling et al. 2016). And PKCβI, PKCβII, and PKCδ protein levels have been reported to be lower in high-grade and late-stage bladder cancer compared with normal, low-grade, or early-stage tissue (Koren et al. 2000; Langzam et al. 2001; Varga et al. 2004). Low levels of PKCη in hepatocellular carcinomas have also been shown to correlate with poor survival in liver cancer (Lu et al. 2009). Recent compilation of a pathology atlas of the human cancer transcriptome (www.proteinatlas.org/pathology), reveals that high expression of PKC isozymes, in general, correlates with better survival in multiple cancers (Uhlen et al. 2017). Specifically, high expression levels of each of the conventional PKC isozymes correlate with increased survival in colon, breast, and prostate cancer; high expression levels of PKCδ correlate with high survival in liver cancer, high levels of PKCε correlate with better survival in lung and renal cancer (but not endometrial), and high levels of PKCη are favorable in head and neck cancers. High expression levels of both atypical PKC isozymes track with better survival in renal cancer (but high levels of PKCι are unfavorable in endometrial, pancreatic, and liver cancers). Thus, in general, high levels of either mRNA or protein expression for PKC isozymes serve as predictors of better survival for diverse cancers.
If a major function of PKC is to keep oncoproteins in check (see Downstream Substrates of PKC), oncogenic mutations may not confer a significant survival advantage to cells unless PKC signaling is disabled. In the classic skin carcinogenesis studies, subthreshold amounts of a carcinogen may be ineffective because of the strong ‘brakes’ applied by PKC; repetitive treatment with phorbol esters would promote the loss of PKC and thus allow unchecked activity of oncogenes such as K-Ras.
Could PKC Function as an Oncoprotein in Certain Contexts?
The PRKCI gene is part of the 3q amplicon and considerable evidence supports a role for PKCι as an oncoprotein (Parker et al. 2014). Notably, Fields and coworkers have identified an unambiguous oncogenic role for PKCι in lung cancer: in lung squamous cell carcinomas, PKCι was shown to phosphorylate SOX2, a master transcriptional regulator of stemness, thus allowing the expression of Hedgehog acetyl transferase to permit growth in soft agar (Justilien et al. 2014; Ali et al. 2016). Furthermore, PKCι was shown to promote a tumor-initiating phenotype in K-Ras-mediated lung adenocarcinomas by phosphorylating ELF-3 to control Notch expression (Ali et al. 2016). Glioblastoma may also be a cancer in which atypical PKC isozymes function as oncoproteins: Ghosh and coworkers showed that high atypical PKC immunoreactivity, primarily PKCι, correlated with poor disease prognosis in patients with glioblastoma and that an atypical PKC inhibitor reduced tumor growth in a mouse model of glioblastoma (Kusne et al. 2014). Nonetheless, no GOF mutations have yet been identified in atypical PKC isozymes. In contrast, LOF mutations have been identified in PKCζ and mutations of the highly conserved APE motif have been identified in both PKCζ (E421K in a breast cancer) and PKCι (E423D in lung cancer) (Galvez et al. 2009; Antal, Hudson, et al. 2015). Additionally, a frequently observed mutation in PKCι is neomorphic: mutation of R471C in PKCι changes its substrate specificity (Linch et al. 2013). As noted above, low levels of PKCζ correlate with poor patient outcome in colon cancer, and functional studies in intestinal cells reveal that loss of PKCζ promotes metabolic reprogramming by two mechanisms – regulating the activity of a key metabolic enzyme, 3-phosphoglycerate dehydrogenase, and regulating the nuclear translocation of the transcription factors YAP and β-catenin (Ma et al. 2013; Llado et al. 2015). PKCι has also been proposed to have a tumor-suppressive function in the intestine: this isozyme is lost in the intestinal epithelium of patients with Crohn’s disease, a pathology associated with high risk of cancer, and mice lacking PKCι in their intestinal epithelium have increased inflammation and tumorigenesis (Nakanishi Y et al. 2016). Taken together, atypical PKC isozymes have oncogenic functions in certain contexts and tumor suppressive functions in other contexts.
There is one cancer, Adult T-Cell Leukemia (ATLL), in which recurrent activity-enhancing mutations in PKCβ have been identified. Whole genome and whole exome sequencing has revealed frequent (33% of patients) mutations in PKCβ, with a hotspot at Asp427 in the kinase domain and a warm spot in the pseudosubstrate segment (Kataoka et al. 2015). Overexpression studies indicate that mutation of Asp427 increases the activity of PKCβ as assessed by several cellular readouts, including accelerated phorbol ester-dependent membrane translocation and enhanced NF-κB transcription. These activating effects are, however, so great that it rasies the question as to whether this enhanced open conformation of PKC may promote the degradation of the mutants. Indeed, mutation of the pseudosubstrate to decrease autoinhibition destabilizes PKC. Analysis of the steady-state levels of the mutant PKCβ in these patients will be important.
No GOF mutations in cancer have yet to be indentified in novel PKC isozymes, however numerous reports suggest they may function as oncoproteins in certain contexts. Kazanietz and coworkers have recently shown that PKCε overexpression in mice, which alone causes the development of preneoplastic lesions (Garg et al. 2014), cooperates with loss of PTEN in development of prostate cancer in a mouse model (Garg et al. 2017). Conversely, genetic ablation of PKCε in a transgenic mouse model of prostate adenocacinoma inhibits prostate cancer development and metastasis (Hafeez et al. 2011). PKCδ, which has roles both in survival and apoptotic pathways (Brodie and Blumberg 2003; Griner and Kazanietz 2007; Reyland 2007; Basu and Pal 2010), has been reported to promote tumor progression in pancreatic cancer (Mauro et al. 2010), and mice deficient in this isozyme have an increased incidence of lung tumors (Symonds et al. 2011). Reyland and colleagues have shown that elevated PKCδ mRNA levels negatively correlate with prognosis in Erb2-positive breast cancer, with mouse models suggesting that it is required for ErbB2-driven mammary gland tumorigenesis (Allen-Petersen et al. 2014). Elevated PKCδ mRNA has also been reported to correlate with poor survival outcome in estrogen receptor-positive breast cancer (McKiernan et al. 2008; Gyorffy et al. 2010). Establishing whether PKC isozymes may play oncogenic roles in specific contexts in specific cancers, such as breast cancer, awaits functional characterization of mutations in PKC isozymes in these cancers (Garg et al. 2014).
PKC LOF Germline Mutations in Cancer
One hallmark for a bona fide tumor suppressor is the presence of germline mutations that cause human proliferative disorders (Payne and Kemp 2005). PKCδ meets this criterion: LOF germline mutations are causal in juvenile systemic lupus erythematosus (JSLE) and autoimmune lymphoproliferative syndrome (Belot et al. 2013; Kuehn et al. 2013; Salzer et al. 2013; Kiykim et al. 2015). These LOF mutations result in increased proliferation and resistance to apoptosis in immune cells. As such, JSLE patients often develop B-cell lymphomas (Bernatsky et al. 2005). Four LOF mutations have been identified in the disease (Salzer et al. 2016): an invariant Gly (G248) on one of the ligand-binding loops of the C1B domain is mutated to Ser in one patient with a JSLE-like disorder, a biallelic splice-site mutation causing the absence of protein product was identified in a patient with severe autoimmunity (Salzer et al. 2013), an invariant Gly (G510) in the highly conserved activation loop of AGC kinases is mutated to Ser in three siblings with JSLE, and an Arg in a segment preceding the conserved PXXP motif of the C-terminal tail is mutated to Trp in a patient with autoimmune lymphoproliferative syndrome (Kuehn et al. 2013). Somatic mutations in the latter residue (including to Trp) have also been identified in three different colorectal tumors (cBioPortal; (Gao J et al. 2013)). Whether germline mutations in other PKC isozymes are causal in proliferative disorders remains to be determined.
PKC in Degenerative Disease: GOF mutations
In striking contrast to the LOF mutations associated with cancer, a number of germline mutations that subtly enhance the activity of PKC have been identified in degenerative diseases. These mutations do not cause constitutive activation of PKC, which would have the paradoxical effect of down-regulating the enzyme, rather they facilitate or enhance the activation of the enzyme.
Spinocerebellar Ataxia
Over 30 germline mutations have been identified in spinocerebellar ataxia type 14 (SCA14) in PKCγ (Adachi et al. 2008; Verbeek et al. 2008; Takahashi et al. 2015), an isozyme whose expression is restricted to the brain in normal physiology (Ding et al. 2005). Curiously, almost all mutations occur in the C1B domain, but not to a specific position, suggesting a general role in perturbing the structure of the domain. These mutations seem to have the same effect of loosening autoinhibitory constraints to facilitate the ligand-induced ‘open’ conformation of PKCγ. Presumably, autoinhibitory constraints are loosened enough to enhance activation, but not so much as to promote the down-regulation of PKC.
Alzheimer’s Disease
Mutations that enhance the activation of another conventional PKC, PKCα, track with affected, but not unaffected, individuals in some families with Alzheimer’s disease (Alfonso et al. 2016). This isozyme of PKC is required for the synaptic depression caused by amyloid-β (Aβ), a cytotoxic peptide associated with Alzheimer’s disease. Three Alzheimer’s disease-associated mutations have been identified, and they increase the agonist-evoked activity of PKC by a relatively small amount, too little to promote the down-regulation of the enzyme. However a lifetime of slightly enhanced signaling may sensitize individuals to the detrimental effects of Aβ. One of these variants involves mutation of Met489 in the activation loop to a smaller Val, resulting in no changes in autoinhibitory contacts but increasing the catalytic rate by approximately 30% (Callender et al. 2018). This provides an effective mechanism to enhance activity while evading down-regulation. A role of enhanced PKC activity in Alzheimer’s disease is supported by a recent phosphoproteomics analysis of postmortem brains showing that elevation of PKC signaling is one of the earliest events in Alzheimer’s disease (Tagawa et al. 2015).
Stroke
A polymorphism in the kinase domain of PKCη (V374I) is associated with increased risk for cerebral infarction (stroke) (Kubo et al. 2007), increased risk of arthritis (Takata et al. 2007), and severe gastric atrophy (Goto et al. 2010). In vitro kinase assays reveal that the mutation enhances the catalytic activity of PKC, as assessed by increased autophosphorylation and substrate phosphorylation (Kubo et al. 2007; Zurgil et al. 2014). Assuming autoinhibitory constraints are unchanged (as is the case for the activity-enhancing PKCα M489V variant in Alzheimer’s disease), the enhanced catalytic activity would serve as an effective mechanism to allow enhanced activity without compromising the stability of the mutant.
The increasing annotation of disease-associated single nucleotide polymorphisms (SNPs) will likely unveil an abundance of variants of PKC that predispose to specific diseases, from heart disease to drug and alcohol addiction (Olive and Messing 2004). Both conventional (PKCα and βII) and novel (PKCδ and PKCε) have been shown to affect cardiac function (Palaniyandi et al. 2009), so variants that cause small changes in activity may either protect or predispose to heart disease. Behavioural studies with knock out mice reveal that lack of PKCγ causes decreased anxiety (Bowers et al. 2000) and a high ethanol drinking phenotype (Ron and Messing 2013), whereas mice lacking PKCε have a low ethanol drinking phenotype (Ron and Messing 2013). Diseases causally linked to PKC variants with enhanced PKC activity could thus benefit from inhibitors to adjust PKC signaling to lower levels.
Should PKC be Inhibited in Degenerative Disease?
The enhanced signaling by PKC in degenerative diseases such as Alzheimer’s disease contrasts with the reduced signaling that is associated with cancer, suggesting opposing roles in survival versus degenerative diseases. Indeed, a recent meta-analysis of nine independent studies reveals that Alzheimer’s disease patients exhibit an overall 45% decreased risk of cancer compared with the general population (Shi et al. 2015), consistent with earlier reports that Alzheimer’s disease and cancer display an inverse association (Roe et al. 2005; Driver et al. 2012). Perhaps repurposing PKC inhibitors for Alzheimer’s disease may prevent the effects of Aβ on synapses and thereby mitigate loss of cognitive function. In this regard, bryostatin 1, which causes the loss of PKC and failed in cancer therapies (Nezhat et al. 2004), is in phase II clinical trials for Alzheimer’s disease (Nelson et al. 2017), with mouse studies showing promising results in improving learning deficits in an Alzheimer’s disease mouse model (Russo et al. 2015; Schrott et al. 2015). It is noteworthy that targeting PKC in degenerative diseases may be particularly feasible as its activity would only need to be brought down from supraphysiological to physiological levels, essentially adjusting the balance to regain homeostasis.
Conclusion
The paradoxical acute activation, followed by chronic loss, of diacylglycerol-sensitive PKC isozymes by phorbol esters confounded understanding the role of PKC in cancer. However, the recent availability of whole genome sequencing of tumors and patient populations, coupled to growing databases analyzing survival curves as a function of protein or mRNA expression for specific cancers, converge on a new understanding of the biological function of PKC isozymes: they generally suppress survival signaling, functioning as the brakes to oncogenic signaling. Furthermore, these isozymes frequently have LOF mutations, rather than gene deletions, allowing the defective PKC to exert dominant-negative effects more broadly on the signaling output of other PKC isozymes. This new understanding of PKC function highlights the need to restore, rather than inhibit PKC function for cancer therapies. Additionally, mechanistic insight into how PKC isozymes are regulated suggests caution in targeting pathways that control levels of PKC. For example, the use of inhibitors for the kinase mTOR (Don and Zheng 2011) or Hsp90 (Neckers and Workman 2012), both currently in use in the clinic, may have the detrimental effect of inhibiting PKC processing, in turn decreasing the steady-state levels of PKC (Guertin et al. 2006; Gould et al. 2009). With better patient survival generally associated with higher PKC levels, careful consideration should be given to selecting therapies that do not remove PKC.
Acknowledgments
I thank Gerard Manning for providing a figure of the AGC branch of kinome, Gema Lorden for assistance in drafting, and members of the Newton lab for helpful comments.
Abbreviations
- Ab
amyloid-β
- GOF
gain-of-function
- JSLE
juvenile systemic lupus erythematosus
- LOF
loss-of-function
- mTORC
mammalian target of rapamycin complex
- PB1
Phox and Bem1p
- PDBu
phorbol 12,13-dibutyrate
- PDK-1
phosphoinositide-dependent kinase-1
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PKA
protein kinase A
- PKB
protein kinase B
- PKC
protein kinase C
- PKN
protein kinase N
- PMA
phorbol 12-myristate 13-acetate
Footnotes
Declaration of interest:
The author has no competing interests. This work was supported by NIH R35 GM122523 and the Cure Alzheimer’s Fund.
References
- Abrahamsen H, O’Neill AK, Kannan N, Kruse N, Taylor SS, Jennings PA, Newton AC. Peptidyl-prolyl Isomerase Pin1 Controls Down-regulation of Conventional Protein Kinase C Isozymes. The Journal of biological chemistry. 2012;287(16):13262–13278. doi: 10.1074/jbc.M112.349753. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Kobayashi T, Takahashi H, Kawasaki T, Shirai Y, Ueyama T, Matsuda T, Seki T, Sakai N, Saito N. Enzymological analysis of mutant protein kinase Cgamma causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. The Journal of biological chemistry. 2008;283(28):19854–19863. doi: 10.1074/jbc.M801492200. [DOI] [PubMed] [Google Scholar]
- Alfonso SI, Callender JA, Hooli B, Antal CE, Mullin K, Sherman MA, Lesne SE, Leitges M, Newton AC, Tanzi RE, et al. Gain-of-function mutations in protein kinase Calpha (PKCalpha) may promote synaptic defects in Alzheimer’s disease. Science signaling. 2016;9(427):ra47. doi: 10.1126/scisignal.aaf6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SA, Justilien V, Jamieson L, Murray NR, Fields AP. Protein Kinase Ciota Drives a NOTCH3-dependent Stem-like Phenotype in Mutant KRAS Lung Adenocarcinoma. Cancer cell. 2016;29(3):367–378. doi: 10.1016/j.ccell.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Petersen BL, Carter CJ, Ohm AM, Reyland ME. Protein kinase Cdelta is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene. 2014;33(10):1306–1315. doi: 10.1038/onc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Callender JA, Kornov AP, Taylor SS, Newton AC. Intramolecular C2 Domain-Mediated Autoinhibition of Protein Kinase CbII. Cell reports. 2015 doi: 10.1016/j.celrep.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari FB, et al. Cancer-Associated Protein Kinase C Mutations Reveal Kinase’s Role as Tumor Suppressor. Cell. 2015;160(3):489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Violin JD, Kunkel MT, Skovso S, Newton AC. Intramolecular Conformational Changes Optimize Protein Kinase C Signaling. Chemistry & biology. 2014 doi: 10.1016/j.chembiol.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MH, Wheeler DL, Bhamb B, Verma AK. Protein kinase C delta overexpressing transgenic mice are resistant to chemically but not to UV radiation-induced development of squamous cell carcinomas: a possible link to specific cytokines and cyclooxygenase-2. Cancer research. 2006;66(2):713–722. doi: 10.1158/0008-5472.CAN-05-2684. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141(11):4120–4127. doi: 10.1210/endo.141.11.7766. eng. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Lea-Currie R, Sen A, Farese RV. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. The Journal of clinical endocrinology and metabolism. 2002;87(2):716–723. doi: 10.1210/jcem.87.2.8252. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. The Journal of biological chemistry. 1997;272(4):2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- Bar-Even A, Noor E, Savir Y, Liebermeister W, Davidi D, Tawfik DS, Milo R. The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry. 2011;50(21):4402–4410. doi: 10.1021/bi2002289. eng. [DOI] [PubMed] [Google Scholar]
- Barcelo C, Paco N, Morell M, Alvarez-Moya B, Bota-Rabassedas N, Jaumot M, Vilardell F, Capella G, Agell N. Phosphorylation at Ser-181 of oncogenic KRAS is required for tumor growth. Cancer research. 2014;74(4):1190–1199. doi: 10.1158/0008-5472.CAN-13-1750. [DOI] [PubMed] [Google Scholar]
- Basu A, Pal D. Two faces of protein kinase Cdelta: the contrasting roles of PKCdelta in cell survival and cell death. TheScientificWorldJournal. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behn-Krappa A, Newton AC. The hydrophobic phosphorylation motif of conventional protein kinase C is regulated by autophosphorylation. Current biology : CB. 1999;9(14):728–737. doi: 10.1016/s0960-9822(99)80332-7. [DOI] [PubMed] [Google Scholar]
- Belot A, Kasher PR, Trotter EW, Foray AP, Debaud AL, Rice GI, Szynkiewicz M, Zabot MT, Rouvet I, Bhaskar SS, et al. Protein kinase cdelta deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 2013;65(8):2161–2171. doi: 10.1002/art.38008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121(2):271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Berenblum I, Shubik P. The role of croton oil applications, associated with a single painting of a carcinogen, in tumour induction of the mouse’s skin. British journal of cancer. 1947;1(4):379–382. doi: 10.1038/bjc.1947.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S, Boivin JF, Joseph L, Rajan R, Zoma A, Manzi S, Ginzler E, Urowitz M, Gladman D, Fortin PR, et al. An international cohort study of cancer in systemic lupus erythematosus [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Arthritis Rheum. 2005;52(5):1481–1490. doi: 10.1002/art.21029. eng. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422(6929):326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Bivona TG, Quatela SE, Bodemann BO, Ahearn IM, Soskis MJ, Mor A, Miura J, Wiener HH, Wright L, Saba SG, et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Molecular cell. 2006;21(4):481–493. doi: 10.1016/j.molcel.2006.01.012. eng. [DOI] [PubMed] [Google Scholar]
- Blumberg PM, Jaken S, Konig B, Sharkey NA, Leach KL, Jeng AY, Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochemical pharmacology. 1984;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. eng. [DOI] [PubMed] [Google Scholar]
- Bornancin F, Parker PJ. Phosphorylation of protein kinase C-alpha on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase- resistant state [published erratum appears in J Biol Chem 1997 May 16;272(20):13458] The Journal of biological chemistry. 1997;272(6):3544–3549. doi: 10.1074/jbc.272.6.3544. [DOI] [PubMed] [Google Scholar]
- Borner C, Filipuzzi I, Wartmann M, Eppenberger U, Fabbro D. Biosynthesis and posttranslational modifications of protein kinase C in human breast cancer cells. The Journal of biological chemistry. 1989;264(23):13902–13909. [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet. 2000;30(2):111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8(1):19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- Callender JA, Yang Y, Stephenson N, Brognard J, Newton AC. Protein Kinase Cα (PKCα) Gain-of-Function Variant in Alzheimer’s Disease Displays Enhanced Catalysis by Mechanism that Evades Down-Regulation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2018 doi: 10.1073/pnas.1805046115. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AJ, Linch MD, Saurin AT, Escribano C, Parker PJ. mTORC2 targets AGC kinases through Sin1-dependent recruitment. The Biochemical journal. 2011;439(2):287–297. doi: 10.1042/BJ20110678. [DOI] [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct Activation of Calcium-activated, Phospholipid-dependent Protein Kinase by Tumor-promoting Phorbol Esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- Cenni V, Doppler H, Sonnenburg ED, Maraldi N, Newton AC, Toker A. Regulation of novel protein kinase C epsilon by phosphorylation. The Biochemical journal. 2002;363(Pt 3):537–545. doi: 10.1042/0264-6021:3630537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Garcia-Garcia J, Rodriguez-Alfaro JA, Gomez-Fernandez JC. A new phosphatidylinositol 4,5-bisphosphate-binding site located in the C2 domain of protein kinase Calpha [Research Support, Non-U.S. Gov’t] The Journal of biological chemistry. 2003;278(7):4972–4980. doi: 10.1074/jbc.M209385200. eng. [DOI] [PubMed] [Google Scholar]
- Coussens L, Parker PJ, Rhee L, Yang-Feng TL, Chen E, Waterfield MD, Francke U, Ullrich A. Multiple, Distinct Forms of Bovine and Human Protein Kinase C Suggest Diversity in Cellular Signaling Pathways. Science. 1986;233:859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Creixell P, Schoof EM, Simpson CD, Longden J, Miller CJ, Lou HJ, Perryman L, Cox TR, Zivanovic N, Palmeri A, et al. Kinome-wide decoding of network-attacking mutations rewiring cancer signaling. Cell. 2015;163(1):202–217. doi: 10.1016/j.cell.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries DJ, Herald CL, Pettit GR, Blumberg PM. Demonstration of sub-nanomolar affinity of bryostatin 1 for the phorbol ester receptor in rat brain. Biochemical pharmacology. 1988;37(21):4069–4073. doi: 10.1016/0006-2952(88)90097-4. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Xiang CX, Chen ZF. Generation and characterization of the PKC gamma-Cre mouse line. Genesis. 2005;43(1):28–33. doi: 10.1002/gene.20151. [DOI] [PubMed] [Google Scholar]
- Don AS, Zheng XF. Recent clinical trials of mTOR-targeted cancer therapies. Reviews on recent clinical trials. 2011;6(1):24–35. doi: 10.2174/157488711793980147. [DOI] [PubMed] [Google Scholar]
- Dowling CM, Phelan J, Callender JA, Cathcart MC, Mehigan B, McCormick P, Dalton T, Coffey JC, Newton AC, O’Sullivan J, et al. Protein kinase C beta II suppresses colorectal cancer by regulating IGF-1 mediated cell survival. Oncotarget. 2016;7(15):20919–20933. doi: 10.18632/oncotarget.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger PE, Blumberg PM. Specific Binding of Phorbol Ester Tumor Promoters. Proc Natl Acad Sci U S A. 1980;77:567–571. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries DR, Gallegos LL, Newton AC. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. The Journal of biological chemistry. 2007;282(2):826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Kiel DP, Lu KP, Seshadri S, Wolf PA. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. Bmj. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond ML, Prehoda KE. Molecular Control of Atypical Protein Kinase C: Tipping the Balance between Self-Renewal and Differentiation. J Mol Biol. 2016;428(7):1455–1464. doi: 10.1016/j.jmb.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AS, Newton AC. Phosphorylation at conserved carboxyl-terminal hydrophobic motif regulates the catalytic and regulatory domains of protein kinase C. The Journal of biological chemistry. 1997;272(29):18382–18390. doi: 10.1074/jbc.272.29.18382. [DOI] [PubMed] [Google Scholar]
- Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] The EMBO journal. 2008;27(14):1932–1943. doi: 10.1038/emboj.2008.120. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, et al. Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. The Journal of clinical investigation. 2007;117(8):2289–2301. doi: 10.1172/JCI31408. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AJ, Paladini RD, Koshland DE., Jr Autophosphorylation of Protein Kinase C at Three Separated Regions of Its Primary Sequence. Science. 1990;249:408–411. doi: 10.1126/science.2377895. [DOI] [PubMed] [Google Scholar]
- Freeley M, Kelleher D, Long A. Regulation of Protein Kinase C function by phosphorylation on conserved and non-conserved sites [Review] Cell Signal. 2011;23(5):753–762. doi: 10.1016/j.cellsig.2010.10.013. eng. [DOI] [PubMed] [Google Scholar]
- Frey MR, Saxon ML, Zhao X, Rollins A, Evans SS, Black JD. Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. The Journal of biological chemistry. 1997;272(14):9424–9435. doi: 10.1074/jbc.272.14.9424. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Ohta K, Kangawa K, Kikkawa U, Ogino S, Fukui H. Identification of protein kinase C phosphorylation sites involved in phorbol ester-induced desensitization of the histamine H1 receptor. Molecular pharmacology. 1999;55(4):735–742. [PubMed] [Google Scholar]
- Gallegos LL, Kunkel MT, Newton AC. Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. The Journal of biological chemistry. 2006;281:30947–30956. doi: 10.1074/jbc.M603741200. [DOI] [PubMed] [Google Scholar]
- Galvez AS, Duran A, Linares JF, Pathrose P, Castilla EA, Abu-Baker S, Leitges M, Diaz-Meco MT, Moscat J. Protein kinase Czeta represses the interleukin-6 promoter and impairs tumorigenesis in vivo. Molecular and cellular biology. 2009;29(1):104–115. doi: 10.1128/MCB.01294-08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Science signaling. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. The Journal of biological chemistry. 2008;283(10):6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- Gao T, Newton AC. Invariant Leu preceding turn motif phosphorylation site controls the interaction of protein kinase C with Hsp70. The Journal of biological chemistry. 2006;281(43):32461–32468. doi: 10.1074/jbc.M604076200. eng. [DOI] [PubMed] [Google Scholar]
- Gao T, Toker A, Newton AC. The carboxyl terminus of protein kinase c provides a switch to regulate its interaction with the phosphoinositide-dependent kinase, pdk-1. The Journal of biological chemistry. 2001;276(22):19588–19596. doi: 10.1074/jbc.M101357200. [DOI] [PubMed] [Google Scholar]
- Garcia-Paramio P, Cabrerizo Y, Bornancin F, Parker PJ. The broad specificity of dominant inhibitory protein kinase C mutants infers a common step in phosphorylation. The Biochemical journal. 1998;333( Pt 3):631–636. doi: 10.1042/bj3330631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG. Protein kinase C and cancer: what we know and what we do not. Oncogene. 2014;33(45):5225–5237. doi: 10.1038/onc.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Blando JM, Perez CJ, Abba MC, Benavides F, Kazanietz MG. Protein Kinase C Epsilon Cooperates with PTEN Loss for Prostate Tumorigenesis through the CXCL13-CXCR5 Pathway. Cell reports. 2017;19(2):375–388. doi: 10.1016/j.celrep.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgione J, Hysell M, Harvey DF, Newton AC. Contribution of the C1A and C1B Domains to the Membrane Interaction of Protein Kinase C. Biochemistry. 2003;42(38):11194–11202. doi: 10.1021/bi0350046. [DOI] [PubMed] [Google Scholar]
- Giorgione JR, Lin JH, McCammon JA, Newton AC. Increased membrane affinity of the C1 domain of protein kinase Cdelta compensates for the lack of involvement of its C2 domain in membrane recruitment. The Journal of biological chemistry. 2006;281(3):1660–1669. doi: 10.1074/jbc.M510251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Holewinski RJ, Van Eyk JE, Steinberg SF. A novel phosphorylation site at Ser130 adjacent to the pseudosubstrate domain contributes to the activation of protein kinase C-delta. The Biochemical journal. 2016;473(3):311–320. doi: 10.1042/BJ20150812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Hishida A, Matsuo K, Tajima K, Morita E, Naito M, Wakai K, Hamajima N. PRKCH gene polymorphism is associated with the risk of severe gastric atrophy. Gastric Cancer. 2010;13(2):90–94. doi: 10.1007/s10120-009-0542-7. [DOI] [PubMed] [Google Scholar]
- Gould CM, Kannan N, Taylor SS, Newton AC. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. The Journal of biological chemistry. 2009;284(8):4921–4935. doi: 10.1074/jbc.M808436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill C, Wee B, Atwood SX, Prehoda KE. Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. The Journal of biological chemistry. 2012;287(25):21003–21011. doi: 10.1074/jbc.M112.360495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nature reviews Cancer. 2007;7(4):281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Developmental cell. 2006;11(6):859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast cancer research and treatment. 2010;123(3):725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- Hafeez BB, Zhong W, Weichert J, Dreckschmidt NE, Jamal MS, Verma AK. Genetic ablation of PKC epsilon inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer research. 2011;71(6):2318–2327. doi: 10.1158/0008-5472.CAN-10-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Hunter T. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. The FASEB Journal. 1995;9:576–596. [PubMed] [Google Scholar]
- Hansra G, Garcia-Paramio P, Prevostel C, Whelan RD, Bornancin F, Parker PJ. Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes [In Process Citation] The Biochemical journal. 1999;342(Pt 2):337–344. [PMC free article] [PubMed] [Google Scholar]
- Hecker E. Cocarcinogenic principles from the seed oil of Croton tiglium and from other Euphorbiaceae. Cancer research. 1968;28(11):2338–2349. [PubMed] [Google Scholar]
- Hecker E. Co-carcinogens or modulators of carcinogenesis. New aspects of the etiology of human tumors and of the molecular mechanisms of carcinogenesis. Naturwissenschaften. 1978;65(12):640–648. doi: 10.1007/BF00401906. [DOI] [PubMed] [Google Scholar]
- Hecker E, Kubinyi H, Bresch H, von Szczepanski C. The chemical structure of a cocarcinogen and of phorbol isolated from croton oil. J Med Chem. 1966;9(2):246–247. doi: 10.1021/jm00320a024. [DOI] [PubMed] [Google Scholar]
- Hein MY, Hubner NC, Poser I, Cox J, Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F, et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell. 2015;163(3):712–723. doi: 10.1016/j.cell.2015.09.053. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein Kinase M{zeta} Synthesis from a Brain mRNA Encoding an Independent Protein Kinase C{zeta} Catalytic Domain: IMPLICATIONS FOR THE MOLECULAR MECHANISM OF MEMORY. The Journal of biological chemistry. 2003;278(41):40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, Suzuki Y, Yamanaka T, Suzuki A, Mizuno K, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. Journal of cell science. 2002;115(Pt 12):2485–2495. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- Hokin LE. The road to the phosphoinositide-generated second messengers. Trends in pharmacological sciences. 1987;8:53–56. [Google Scholar]
- Hokin MR, Hokin LE. Enzyme Secretion and the Incorporation of P32 into Phospholipids of Pancreas Slices. J Biol Chem. 1953;203:967–977. [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse [Research Support, N.I.H., Extramural] Nucleic acids research. 2012;40(Database issue):D261–270. doi: 10.1093/nar/gkr1122. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey MM, Benovic JL, DebBurman SK, Richardson RM. Multiple mechanisms involving protein phosphorylation are linked to desensitization of muscarinic receptors. Life sciences. 1995;56(11–12):951–955. doi: 10.1016/0024-3205(95)00033-3. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 Modifies the Cellular Pharmacology of PKC. Molecular cell. 2010;37(4):541–550. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C, Kemp BE. Protein Kinase C Contains a Pseudosubstrate Prototope in Its Regulatory Domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- House C, Kemp BE. Protein Kinase C Pseudosubstrate Prototope: Structure-Function Relationships. Cell Sig. 1990;2:187–190. doi: 10.1016/0898-6568(90)90022-3. [DOI] [PubMed] [Google Scholar]
- Huang K-P, Nakabayashi H, Huang FL. Isozymic Forms of Rat Brain Ca2+-Activated and Phospholipid-Dependent Protein Kinase. Proc Natl Acad Sci U S A. 1986;83:8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME. Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene. 2008;27(21):3045–3053. doi: 10.1038/sj.onc.1210967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Ling N, Cooper JA. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Hunter T, Ling N, Cooper JA. Protein Kinase C Phosphorylation of the EGF Receptor at a Threonine Residue Close to the Cytoplasmic Face of the Plasma Membrane. Nature. 1984;311:480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Iglesias T, Matthews S, Rozengurt E. Dissimilar phorbol ester binding properties of the individual cysteine-rich motifs of protein kinase D. FEBS letters. 1998;437(1–2):19–23. doi: 10.1016/s0014-5793(98)01189-2. [DOI] [PubMed] [Google Scholar]
- Igumenova TI. Dynamics and Membrane Interactions of Protein Kinase C. Biochemistry. 2015;54(32):4953–4968. doi: 10.1021/acs.biochem.5b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] The EMBO journal. 2008;27(14):1919–1931. doi: 10.1038/emboj.2008.119. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a Cyclic Nucleotide-Independent Protein Kinase and Its Proenzyme in Mammalian Tissues. J Biol Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- Isakov N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Seminars in cancer biology. 2017 doi: 10.1016/j.semcancer.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Isakov N, Gopas J, Priel E, Segal S, Altman A. Effect of protein kinase C activating tumor promoters on metastases formation by fibrosarcoma cells. Invasion Metastasis. 1991;11(1):14–24. [PubMed] [Google Scholar]
- Jaken S, Tashjian AH, Jr, Blumberg PM. Characterization of phorbol ester receptors and their down-modulation in GH4C1 rat pituitary cells [Research Support, U.S. Gov’t, P.H.S.] Cancer research. 1981;41(6):2175–2181. eng. [PubMed] [Google Scholar]
- Johannes F-j, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu Is a Novel, Atypical Member of the Protein Kinase C Family. J Biol Chem. 1994;269:6140–6148. [PubMed] [Google Scholar]
- Johnson JE, Edwards AS, Newton AC. A putative phosphatidylserine binding motif is not involved in the lipid regulation of protein kinase C. The Journal of biological chemistry. 1997;272(49):30787–30792. doi: 10.1074/jbc.272.49.30787. [DOI] [PubMed] [Google Scholar]
- Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer cell. 2014;25(2):139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N, Haste N, Taylor SS, Neuwald AF. The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1272–1277. doi: 10.1073/pnas.0610251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, Totoki Y, Chiba K, Sato-Otsubo A, Nagae G, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nature genetics. 2015;47(11):1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- Kazanietz MG. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Molecular carcinogenesis. 2000;28(1):5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kazanietz MG, Areces LB, Bahador A, Mischak H, Goodnight J, Mushinski JF, Blumberg PM. Characterization of ligand and substrate specificity for the calcium- dependent and calcium-independent protein kinase C isozymes. Molecular pharmacology. 1993;44(2):298–307. [PubMed] [Google Scholar]
- Kazanietz MG, Bustelo XR, Barbacid M, Kolch W, Mischak H, Wong G, Pettit GR, Bruns JD, Blumberg PM. Zinc Finger Domains and Phorbol Ester Pharmacophore: Analysis of binding to mutated form of protein kinase C z and the vav and c-raf proto-oncogene products. J Biol Chem. 1994;269:11590–11594. [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Current biology : CB. 1995;5(12):1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Takai Y, Tanaka Y, Miyake R, Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. The Journal of biological chemistry. 1983;258(19):11442–11445. [PubMed] [Google Scholar]
- Kiykim A, Ogulur I, Baris S, Salzer E, Karakoc-Aydiner E, Ozen AO, Garncarz W, Hirschmugl T, Krolo A, Yucelten AD, et al. Potentially Beneficial Effect of Hydroxychloroquine in a Patient with a Novel Mutation in Protein Kinase Cdelta Deficiency. J Clin Immunol. 2015;35(6):523–526. doi: 10.1007/s10875-015-0178-9. [DOI] [PubMed] [Google Scholar]
- Knauf JA, Elisei R, Mochly-Rosen D, Liron T, Chen XN, Gonsky R, Korenberg JR, Fagin JA. Involvement of protein kinase Cepsilon (PKCepsilon) in thyroid cell death. A truncated chimeric PKCepsilon cloned from a thyroid cancer cell line protects thyroid cells from apoptosis. The Journal of biological chemistry. 1999;274(33):23414–23425. doi: 10.1074/jbc.274.33.23414. [DOI] [PubMed] [Google Scholar]
- Knopf JL, Lee M-H, Sultzman LA, Kriz RW, Loomis CR, Hewick RM, Bell RM. Cloning and Expression of Multiple Protein Kinase C cDNAs. Cell. 1986;46:491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ. C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry. 2002;41(38):11411–11424. doi: 10.1021/bi026041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B, DiNitto PA, Blumberg PM. Stoichiometric Binding of Diacylglycerol to the Phorbol Ester Receptor. J Cell Biochem. 1985;29:37–44. doi: 10.1002/jcb.240290105. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren R, Langzam L, Paz A, Livne PM, Gal R, Sampson SR. Protein kinase C (PKC) isoenzymes immunohistochemistry in lymph node revealing solution-fixed, paraffin-embedded bladder tumors. Applied immunohistochemistry & molecular morphology : AIMM / official publication of the Society for Applied Immunohistochemistry. 2000;8(2):166–171. doi: 10.1097/00129039-200006000-00013. [DOI] [PubMed] [Google Scholar]
- Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, et al. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Molecular and cellular biology. 1998;18(12):6971–6982. doi: 10.1128/mcb.18.12.6971. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AS, Smith JB, Berkow RL. Bryostatin, an activator of the calcium phospholipid-dependent protein kinase, blocks phorbol ester-induced differentiation of human promyelocytic leukemia cells HL-60. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(5):1334–1338. doi: 10.1073/pnas.83.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Hata J, Ninomiya T, Matsuda K, Yonemoto K, Nakano T, Matsushita T, Yamazaki K, Ohnishi Y, Saito S, et al. A nonsynonymous SNP in PRKCH (protein kinase C eta) increases the risk of cerebral infarction. Nature genetics. 2007;39(2):212–217. doi: 10.1038/ng1945. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Niemela JE, Rangel-Santos A, Zhang M, Pittaluga S, Stoddard JL, Hussey AA, Evbuomwan MO, Priel DA, Kuhns DB, et al. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121(16):3117–3125. doi: 10.1182/blood-2012-12-469544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusne Y, Carrera-Silva EA, Perry AS, Rushing EJ, Mandell EK, Dietrich JD, Errasti AE, Gibbs D, Berens ME, Loftus JC, et al. Targeting aPKC disables oncogenic signaling by both the EGFR and the proinflammatory cytokine TNFalpha in glioblastoma. Science signaling. 2014;7(338):ra75. doi: 10.1126/scisignal.2005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langzam L, Koren R, Gal R, Kugel V, Paz A, Farkas A, Sampson SR. Patterns of protein kinase C isoenzyme expression in transitional cell carcinoma of bladder. Relation to degree of malignancy [Comparative Study Research Support, Non-U.S. Gov’t] Am J Clin Pathol. 2001;116(3):377–385. doi: 10.1309/1VKK-HWH7-YVJN-7UF7. eng. [DOI] [PubMed] [Google Scholar]
- Leonard TA, Rozycki B, Saidi LF, Hummer G, Hurley JH. Crystal structure and allosteric activation of protein kinase C betaII. Cell. 2011;144(1):55–66. doi: 10.1016/j.cell.2010.12.013. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE, Fields FO, Kunisawa R, Bishop JM, Thorner J. A Candidate Protein Kinase C Gene, PKC1, Is Required for the S. cerevisiae Cell Cycle. Cell. 1990;62:213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nature cell biology. 2000;2(8):540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- Linch M, Sanz-Garcia M, Soriano E, Zhang Y, Riou P, Rosse C, Cameron A, Knowles P, Purkiss A, Kjaer S, et al. A cancer-associated mutation in atypical protein kinase Ciota occurs in a substrate-specific recruitment motif. Science signaling. 2013;6(293):ra82. doi: 10.1126/scisignal.2004068. [DOI] [PubMed] [Google Scholar]
- Littler DR, Walker JR, She YM, Finerty PJ, Jr, Newman EM, Dhe-Paganon S. Structure of human protein kinase C eta (PKCeta) C2 domain and identification of phosphorylation sites [Research Support, Non-U.S. Gov’t] Biochemical and biophysical research communications. 2006;349(4):1182–1189. doi: 10.1016/j.bbrc.2006.08.160. eng. [DOI] [PubMed] [Google Scholar]
- Livneh E, Dull TJ, Berent E, Prywes R, Ullrich A, Schlessinger J. Release of a phorbol ester-induced mitogenic block by mutation at Thr-654 of the epidermal growth factor receptor. Molecular and cellular biology. 1988;8(6):2302–2308. doi: 10.1128/mcb.8.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh E, Reiss N, Berent E, Ullrich A, Schlessinger J. An insertional mutant of epidermal growth factor receptor allows dissection of diverse receptor functions. The EMBO journal. 1987;6(9):2669–2676. doi: 10.1002/j.1460-2075.1987.tb02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF, Yajima T, Campos A, Aza-Blanc P, Leitges M, et al. Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of beta-Catenin and Yap by PKCzeta. Cell reports. 2015 doi: 10.1016/j.celrep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Chou FP, Yeh KT, Chang YS, Hsu NC, Chang JG. Analysing the expression of protein kinase C eta in human hepatocellular carcinoma. Pathology. 2009;41(7):626–629. doi: 10.3109/00313020903273076. [DOI] [PubMed] [Google Scholar]
- Lum MA, Barger CJ, Hsu AH, Leontieva OV, Black AR, Black JD. Protein Kinase Calpha (PKCalpha) Is Resistant to Long Term Desensitization/Down-regulation by Prolonged Diacylglycerol Stimulation. The Journal of biological chemistry. 2016;291(12):6331–6346. doi: 10.1074/jbc.M115.696211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, Castilla EA, Chen J, Yajima T, Porollo A, et al. Control of nutrient stress-induced metabolic reprogramming by PKCzeta in tumorigenesis. Cell. 2013;152(3):599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. eng. [DOI] [PubMed] [Google Scholar]
- Marshall JL, Bangalore N, El-Ashry D, Fuxman Y, Johnson M, Norris B, Oberst M, Ness E, Wojtowicz-Praga S, Bhargava P, et al. Phase I study of prolonged infusion Bryostatin-1 in patients with advanced malignancies. Cancer biology & therapy. 2002;1(4):409–416. doi: 10.4161/cbt.1.4.17. [DOI] [PubMed] [Google Scholar]
- Mauro LV, Grossoni VC, Urtreger AJ, Yang C, Colombo LL, Morandi A, Pallotta MG, Kazanietz MG, Bal de Kier Joffe ED, Puricelli LL. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer [Research Support, Non-U.S. Gov’t] Pancreas. 2010;39(1):e31–41. doi: 10.1097/MPA.0b013e3181bce796. eng. [DOI] [PubMed] [Google Scholar]
- Mayati A, Moreau A, Le Vee M, Stieger B, Denizot C, Parmentier Y, Fardel O. Protein Kinases C-Mediated Regulations of Drug Transporter Activity, Localization and Expression. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon CL, Kornev AP, Gilson MK, Taylor SS. Dynamic architecture of a protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(43):E4623–4631. doi: 10.1073/pnas.1418402111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan E, O’Brien K, Grebenchtchikov N, Geurts-Moespot A, Sieuwerts AM, Martens JW, Magdolen V, Evoy D, McDermott E, Crown J, et al. Protein kinase Cdelta expression in breast cancer as measured by real-time PCR, western blotting and ELISA. British journal of cancer. 2008;99(10):1644–1650. doi: 10.1038/sj.bjc.6604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSkimming DI, Dastgheib S, Baffi TR, Byrne DP, Ferries S, Scott ST, Newton AC, Eyers CE, Kochut KJ, Eyers PA, et al. KinView: a visual comparative sequence analysis tool for integrated kinome research. Mol Biosyst. 2016;12(12):3651–3665. doi: 10.1039/c6mb00466k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharena HS, Chang P, Keshwani MM, Oruganty K, Nene AK, Kannan N, Taylor SS, Kornev AP. Deciphering the structural basis of eukaryotic protein kinase regulation. PLoS Biol. 2013;11(10):e1001680. doi: 10.1371/journal.pbio.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell RH. Inositol phospholipids and cell surface receptor function. Biochimica et biophysica acta. 1975;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Milani G, Rebora P, Accordi B, Galla L, Bresolin S, Cazzaniga G, Buldini B, Mura R, Ladogana S, Giraldi E, et al. Low PKCalpha expression within the MRD-HR stratum defines a new subgroup of childhood T-ALL with very poor outcome. Oncotarget. 2014;5(14):5234–5245. doi: 10.18632/oncotarget.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales KA, Yang Y, Cole TR, Igumenova TI. Dynamic Response of the C2 Domain of Protein Kinase Calpha to Ca(2+) Binding. Biophys J. 2016;111(8):1655–1667. doi: 10.1016/j.bpj.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7(11):1161–1171. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Exton JH. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. The Journal of biological chemistry. 1992;267(23):16347–16354. [PubMed] [Google Scholar]
- Nakanishi Y, Reina-Campos M, Nakanishi N, Llado V, Elmen L, Peterson S, Campos A, De SK, Leitges M, Ikeuchi H, et al. Control of Paneth Cell Fate, Intestinal Inflammation, and Tumorigenesis by PKClambda/iota. Cell reports. 2016;16(12):3297–3310. doi: 10.1016/j.celrep.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Prot Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski EA, Wisner MA, Chen JZ, Sprang SR, Fukuda M, Mikoshiba K, Falke JJ. C2 domains from different Ca2+ signaling pathways display functional and mechanistic diversity [Comparative Study Research Support, U.S. Gov’t, P.H.S.] Biochemistry. 2001;40(10):3089–3100. doi: 10.1021/bi001968a. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Sibley DR. Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. The Journal of biological chemistry. 2004;279(47):49533–49541. doi: 10.1074/jbc.M408319200. [DOI] [PubMed] [Google Scholar]
- Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TJ, Sun MK, Lim C, Sen A, Khan T, Chirila FV, Alkon DL. Bryostatin Effects on Cognitive Function and PKCvarepsilon in Alzheimer’s Disease Phase IIa and Expanded Access Trials. Journal of Alzheimer’s disease : JAD. 2017;58(2):521–535. doi: 10.3233/JAD-170161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298(3):E395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC, Johnson JE. Protein kinase C: a paradigm for regulation of protein function by two membrane-targeting modules. Biochimica et biophysica acta. 1998;1376(2):155–172. doi: 10.1016/s0304-4157(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Nezhat F, Wadler S, Muggia F, Mandeli J, Goldberg G, Rahaman J, Runowicz C, Murgo AJ, Gardner GJ. Phase II trial of the combination of bryostatin-1 and cisplatin in advanced or recurrent carcinoma of the cervix: a New York Gynecologic Oncology Group study. Gynecologic oncology. 2004;93(1):144–148. doi: 10.1016/j.ygyno.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Ng T, Squire A, Hansra G, Bornancin F, Prevostel C, Hanby A, Harris W, Barnes D, Schmidt S, Mellor H, et al. Imaging protein kinase Calpha activation in cells. Science. 1999;283(5410):2085–2089. doi: 10.1126/science.283.5410.2085. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272(2):952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ohno S, Akita Y, Konno Y, Imajoh S, Suzuki K. A Novel Phorbol Ester Receptor/Protein Kinase, nPKC, Distantly Related to the Protein Kinase C Family. Cell. 1988;53:731–741. doi: 10.1016/0092-8674(88)90091-8. [DOI] [PubMed] [Google Scholar]
- Olive MF, Messing RO. Protein kinase C isozymes and addiction. Mol Neurobiol. 2004;29(2):139–154. doi: 10.1385/mn:29:2:139. [DOI] [PubMed] [Google Scholar]
- Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. The Journal of biological chemistry. 1988;263(14):6927–6932. [PubMed] [Google Scholar]
- Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Protein Kinase C z Subspecies From Rat Brain: Its Structure, Expression, and Properties. Proc Natl Acad Sci USA. 1989;86:3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Kikkawa U, Ogita K, Fujii T, Kurokawa T, Asaoka Y, Sekiguchi K, Ase K, Igarashi K, Nishizuka Y. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987;236(4805):1116–1120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- Orr JW, Keranen LM, Newton AC. Reversible exposure of the pseudosubstrate domain of protein kinase C by phosphatidylserine and diacylglycerol. The Journal of biological chemistry. 1992;267(22):15263–15266. [PubMed] [Google Scholar]
- Ouyang X, Gulliford T, Epstein RJ. The duration of phorbol-inducible ErbB2 tyrosine dephosphorylation parallels that of receptor endocytosis rather than threonine-686 phosphorylation: implications for the physiological role of protein kinase C in growth factor receptor signalling. Carcinogenesis. 1998;19(11):2013–2019. doi: 10.1093/carcin/19.11.2013. [DOI] [PubMed] [Google Scholar]
- Palaniyandi SS, Sun L, Ferreira JC, Mochly-Rosen D. Protein kinase C in heart failure: a therapeutic target? Cardiovascular research. 2009;82(2):229–239. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker PJ, Coussens L, Totty N, Rhee L, Young S, Chen E, Stabel S, Waterfield MD, Ullrich A. The Complete Primary Structure of Protein Kinase C-the Major Phorbol Ester Receptor. Science. 1986;233:853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Parker PJ, Justilien V, Riou P, Linch M, Fields AP. Atypical protein kinase Ciota as a human oncogene and therapeutic target. Biochemical pharmacology. 2014;88(1):1–11. doi: 10.1016/j.bcp.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker PJ, Parkinson SJ. AGC protein kinase phosphorylation and protein kinase C. Biochemical Society transactions. 2001;29(Pt 6):860–863. doi: 10.1042/0300-5127:0290860. [DOI] [PubMed] [Google Scholar]
- Patel NA, Song SS, Cooper DR. PKCdelta alternatively spliced isoforms modulate cellular apoptosis in retinoic acid-induced differentiation of human NT2 cells and mouse embryonic stem cells. Gene Expr. 2006;13(2):73–84. doi: 10.3727/000000006783991890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne SR, Kemp CJ. Tumor suppressor genetics [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Carcinogenesis. 2005;26(12):2031–2045. doi: 10.1093/carcin/bgi223. eng. [DOI] [PubMed] [Google Scholar]
- Pepio AM, Sossin WS. Membrane translocation of novel protein kinase Cs is regulated by phosphorylation of the C2 domain. J Biol Chem. 2001;276(6):3846–3855. doi: 10.1074/jbc.M006339200. [DOI] [PubMed] [Google Scholar]
- Pitcher J, Lohse MJ, Codina J, Caron MG, Lefkowitz RJ. Desensitization of the isolated beta 2-adrenergic receptor by beta-adrenergic receptor kinase, cAMP-dependent protein kinase, and protein kinase C occurs via distinct molecular mechanisms. Biochemistry. 1992;31(12):3193–3197. doi: 10.1021/bi00127a021. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nature cell biology. 2003;5(4):301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Prevostel C, Alice V, Joubert D, Parker PJ. Protein kinase C(alpha) actively downregulates through caveolae- dependent traffic to an endosomal compartment. Journal of cell science. 2000;113(Pt 14):2575–2584. doi: 10.1242/jcs.113.14.2575. [DOI] [PubMed] [Google Scholar]
- Prevostel C, Alvaro V, de Boisvilliers F, Martin A, Jaffiol C, Joubert D. The natural protein kinase C alpha mutant is present in human thyroid neoplasms. Oncogene. 1995;11(4):669–674. [PubMed] [Google Scholar]
- Pu Y, Garfield SH, Kedei N, Blumberg PM. Characterization of the differential roles of the twin C1a and C1b domains of protein kinase C-delta. The Journal of biological chemistry. 2009;284(2):1302–1312. doi: 10.1074/jbc.M804796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Peach ML, Garfield SH, Wincovitch S, Marquez VE, Blumberg PM. Effects on ligand interaction and membrane translocation of the positively charged arginine residues situated along the C1 domain binding cleft in the atypical protein kinase C isoforms. The Journal of biological chemistry. 2006;281(44):33773–33788. doi: 10.1074/jbc.M606560200. [DOI] [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Ahrens H, Simsiman R, Tseng CP, Zou J, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase Cdelta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer research. 1999;59(22):5710–5718. [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Zou J, Bourguignon SE, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase C epsilon in their epidermis exhibit reduced papilloma burden but enhanced carcinoma formation after tumor promotion. Cancer research. 2000;60(3):595–602. [PubMed] [Google Scholar]
- Reyland ME. Protein kinase Cdelta and apoptosis. Biochemical Society transactions. 2007;35(Pt 5):1001–1004. doi: 10.1042/BST0351001. [DOI] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death [Review] Front Biosci. 2009;14:2386–2399. doi: 10.2741/3385. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology. 2005;64(5):895–898. doi: 10.1212/01.WNL.0000152889.94785.51. [DOI] [PubMed] [Google Scholar]
- Roelants FM, Leskoske KL, Martinez Marshall MN, Locke MN, Thorner J. The TORC2-Dependent Signaling Network in the Yeast Saccharomyces cerevisiae. Biomolecules. 2017;7(3) doi: 10.3390/biom7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P, Kisialiou A, Lamonaca P, Moroni R, Prinzi G, Fini M. New Drugs from Marine Organisms in Alzheimer’s Disease. Marine drugs. 2015;14(1) doi: 10.3390/md14010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckbosch SM, Wender PA, Pande VS. Molecular dynamics simulations reveal ligand-controlled positioning of a peripheral protein complex in membranes. Nature communications. 2017;8(1):6. doi: 10.1038/s41467-016-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajan MP, Rivas J, Li P, Standaert ML, Farese RV. Repletion of atypical protein kinase C following RNA interference-mediated depletion restores insulin-stimulated glucose transport. The Journal of biological chemistry. 2006;281(25):17466–17473. doi: 10.1074/jbc.M510803200. eng. [DOI] [PubMed] [Google Scholar]
- Salzer E, Santos-Valente E, Keller B, Warnatz K, Boztug K. Protein Kinase C delta: a Gatekeeper of Immune Homeostasis. J Clin Immunol. 2016;36(7):631–640. doi: 10.1007/s10875-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer E, Santos-Valente E, Klaver S, Ban SA, Emminger W, Prengemann NK, Garncarz W, Mullauer L, Kain R, Boztug H, et al. B-cell deficiency and severe autoimmunity caused by deficiency of protein kinase C delta. Blood. 2013;121(16):3112–3116. doi: 10.1182/blood-2012-10-460741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bautista S, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. The C2 domain of PKCalpha is a Ca2+ -dependent PtdIns(4,5)P2 sensing domain: a new insight into an old pathway [Research Support, Non-U.S. Gov’t] J Mol Biol. 2006;362(5):901–914. doi: 10.1016/j.jmb.2006.07.093. eng. [DOI] [PubMed] [Google Scholar]
- Santiskulvong C, Rozengurt E. Protein kinase Calpha mediates feedback inhibition of EGF receptor transactivation induced by Gq-coupled receptor agonists [Research Support, N.I.H., Extramural] Cell Signal. 2007;19(6):1348–1357. doi: 10.1016/j.cellsig.2007.01.006. eng. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current biology : CB. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Saxon ML, Zhao X, Black JD. Activation of protein kinase C isozymes is associated with post-mitotic events in intestinal epithelial cells in situ. The Journal of cell biology. 1994;126(3):747–763. doi: 10.1083/jcb.126.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott LM, Jackson K, Yi P, Dietz F, Johnson GS, Basting TF, Purdum G, Tyler T, Rios JD, Castor TP, et al. Acute oral Bryostatin-1 administration improves learning deficits in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Current Alzheimer research. 2015;12(1):22–31. doi: 10.2174/1567205012666141218141904. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Olive MF. Protein kinase Cvarepsilon activity regulates mGluR5 surface expression in the rat nucleus accumbens. Journal of neuroscience research. 2017;95(4):1079–1090. doi: 10.1002/jnr.23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Glaccum MB, Fischer EH, Krebs EG. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(6):1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey NA, Leach KL, Blumberg PM. Competitive Inhibition by Diacylglycerol of Specific Phorbol Ester Binding. Proc Natl Acad Sci U S A. 1984;81:607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HB, Tang B, Liu YW, Wang XF, Chen GJ. Alzheimer disease and cancer risk: a meta-analysis. Journal of cancer research and clinical oncology. 2015;141(3):485–494. doi: 10.1007/s00432-014-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipeki S, Bander E, Parker PJ, Farago A. PKCalpha reduces the lipid kinase activity of the p110alpha/p85alpha PI3K through the phosphorylation of the catalytic subunit. Biochemical and biophysical research communications. 2006;339(1):122–125. doi: 10.1016/j.bbrc.2005.10.194. [DOI] [PubMed] [Google Scholar]
- Sommese RF, Ritt M, Swanson CJ, Sivaramakrishnan S. The Role of Regulatory Domains in Maintaining Autoinhibition in the Multidomain Kinase PKCalpha. The Journal of biological chemistry. 2017;292(7):2873–2880. doi: 10.1074/jbc.M116.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Current Biology. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Sonnenburg ED, Gao T, Newton AC. The phosphoinositide-dependent kinase, PDK-1, phosphorylates conventional protein kinase C isozymes by a mechanism that is independent of phosphoinositide 3-kinase. The Journal of biological chemistry. 2001;276(48):45289–45297. doi: 10.1074/jbc.M107416200. [DOI] [PubMed] [Google Scholar]
- Soriano EV, Ivanova ME, Fletcher G, Riou P, Knowles PP, Barnouin K, Purkiss A, Kostelecky B, Saiu P, Linch M, et al. aPKC Inhibition by Par3 CR3 Flanking Regions Controls Substrate Access and Underpins Apical-Junctional Polarization. Dev Cell. 2016;38(4):384–398. doi: 10.1016/j.devcel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. The Biochemical journal. 2004;384(Pt 3):449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempka L, Girod A, Müller H-J, Rincke G, Marks F, Gschwendt M, Bossemeyer D. Phosphorylation of protein kinase Cd (PKCd) at threonine 505 is not a prerequisite for enzymatic activity. J Biol Chem. 1997;272:6805–6811. doi: 10.1074/jbc.272.10.6805. [DOI] [PubMed] [Google Scholar]
- Stewart MD, Igumenova TI. Toggling of Diacylglycerol Affinity Correlates with Conformational Plasticity in C1 Domains. Biochemistry. 2017;56(21):2637–2640. doi: 10.1021/acs.biochem.7b00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nature communications. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA, Reyland ME. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer research. 2011;71(6):2087–2097. doi: 10.1158/0008-5472.CAN-10-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi Z, Blumberg PM. Prostratin, a nonpromoting phorbol ester, inhibits induction by phorbol 12-myristate 13-acetate of ornithine decarboxylase, edema, and hyperplasia in CD-1 mouse skin. Cancer research. 1991;51(19):5355–5360. [PubMed] [Google Scholar]
- Szallasi Z, Bogi K, Gohari S, Biro T, Acs P, Blumberg PM. Non-equivalent roles for the first and second zinc fingers of protein kinase Cdelta. Effect of their mutation on phorbol ester-induced translocation in NIH 3T3 cells. The Journal of biological chemistry. 1996;271(31):18299–18301. doi: 10.1074/jbc.271.31.18299. [DOI] [PubMed] [Google Scholar]
- Szallasi Z, Smith CB, Pettit GR, Blumberg PM. Differential Regulation of Protein Kinase C Isozymes by Bryostatin 1 and Phorbol 12-Myristate 13-Acetate in NIH 3T3 Fibroblasts. J Biol Chem. 1994;269:2118–2124. [PubMed] [Google Scholar]
- Tagawa K, Homma H, Saito A, Fujita K, Chen X, Imoto S, Oka T, Ito H, Motoki K, Yoshida C, et al. Comprehensive phosphoproteome analysis unravels the core signaling network that initiates the earliest synapse pathology in preclinical Alzheimer’s disease brain. Human molecular genetics. 2015;24(2):540–558. doi: 10.1093/hmg/ddu475. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Adachi N, Shirafuji T, Danno S, Ueyama T, Vendruscolo M, Shuvaev AN, Sugimoto T, Seki T, Hamada D, et al. Identification and characterization of PKCgamma, a kinase associated with SCA14, as an amyloidogenic protein. Human molecular genetics. 2015;24(2):525–539. doi: 10.1093/hmg/ddu472. [DOI] [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a Cyclic Nucleotide-independent Protein Kinase and Its Proenzyme in Mammalian Tissues. J Biol Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. Calcium-dependent Activation of a Multifunctional Protein Kinase by Membrane Phospholipids. J Biol Chem. 1979;254:3692–3695. [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y, Tamura A, Fujii T. A role of membranes in the activation of a new multifunctional protein kinase system. J Biochem. 1979;86(2):575–578. doi: 10.1093/oxfordjournals.jbchem.a132557. [DOI] [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Unsaturated Diacylglycerol as a Possible Messenger for the Activation of Calcium-activated, Phospholipid-dependent Protein Kinase System. Biochem Biophys Res Comm. 1979;91:1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Takata Y, Hamada D, Miyatake K, Nakano S, Shinomiya F, Scafe CR, Reeve VM, Osabe D, Moritani M, Kunika K, et al. Genetic association between the PRKCH gene encoding protein kinase Ceta isozyme and rheumatoid arthritis in the Japanese population. Arthritis Rheum. 2007;56(1):30–42. doi: 10.1002/art.22262. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. The Journal of biological chemistry. 2003;278(36):33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends in biochemical sciences. 2011;36(2):65–77. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias IS, Kaulich M, Kim PK, Simon N, Jacinto E, Dowdy SF, King CC, Newton AC. Protein kinase Czeta exhibits constitutive phosphorylation and phosphatidylinositol-3,4,5-triphosphate-independent regulation. The Biochemical journal. 2016;473(4):509–523. doi: 10.1042/BJ20151013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias IS, Newton AC. Protein scaffolds control localized protein kinase C zeta Activity. The Journal of biological chemistry. 2016 doi: 10.1074/jbc.M116.729483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. The biology and biochemistry of diacylglycerol signalling. Meeting on molecular advances in diacylglycerol signalling. EMBO Rep. 2005;6(4):310–314. doi: 10.1038/sj.embor.7400378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamani A, Kannan N, Taylor SS, Schork NJ. Congenital disease SNPs target lineage specific structural elements in protein kinases. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(26):9011–9016. doi: 10.1073/pnas.0802403105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo JI, Kiefer JR, Huang W, Thorarensen A, Xing L, Caspers NL, Day JE, Mathis KJ, Kretzmer KK, Reitz BA, et al. 2-(6-Phenyl-1H-indazol-3-yl)-1H-benzo[d]imidazoles: design and synthesis of a potent and isoform selective PKC-zeta inhibitor. Bioorganic & medicinal chemistry letters. 2009;19(3):908–911. doi: 10.1016/j.bmcl.2008.11.105. [DOI] [PubMed] [Google Scholar]
- Tsai LC, Xie L, Dore K, Xie L, Del Rio JC, King CC, Martinez-Ariza G, Hulme C, Malinow R, Bourne PE, et al. Zeta Inhibitory Peptide Disrupts Electrostatic Interactions That Maintain Atypical Protein Kinase C in Its Active Conformation on the Scaffold p62. The Journal of biological chemistry. 2015;290(36):21845–21856. doi: 10.1074/jbc.M115.676221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352) doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- Vallentin A, Lo TC, Joubert D. A single point mutation in the V3 region affects protein kinase Calpha targeting and accumulation at cell-cell contacts. Molecular and cellular biology. 2001;21(10):3351–3363. doi: 10.1128/MCB.21.10.3351-3363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(18):8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A, Czifra G, Tallai B, Nemeth T, Kovacs I, Kovacs L, Biro T. Tumor grade-dependent alterations in the protein kinase C isoform pattern in urinary bladder carcinomas. European urology. 2004;46(4):462–465. doi: 10.1016/j.eururo.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Verbeek DS, Goedhart J, Bruinsma L, Sinke RJ, Reits EA. PKC gamma mutations in spinocerebellar ataxia type 14 affect C1 domain accessibility and kinase activity leading to aberrant MAPK signaling. Journal of cell science. 2008;121(Pt 14):2339–2349. doi: 10.1242/jcs.027698. [DOI] [PubMed] [Google Scholar]
- Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. The Journal of cell biology. 2003;161(5):899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HQ, Smart RC. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. Journal of cell science. 1999;112( Pt 20):3497–3506. doi: 10.1242/jcs.112.20.3497. [DOI] [PubMed] [Google Scholar]
- Wang MT, Holderfield M, Galeas J, Delrosario R, To MD, Balmain A, McCormick F. K-Ras Promotes Tumorigenicity through Suppression of Non-canonical Wnt Signaling. Cell. 2015;163(5):1237–1251. doi: 10.1016/j.cell.2015.10.041. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Zhang H, Gao Y, Huang C, Zhou A, Zhou Y, Li Y. Sequential posttranslational modifications regulate PKC degradation. Molecular biology of the cell. 2016;27(2):410–420. doi: 10.1091/mbc.E15-09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Chen C-Y, Levin DE. Saccharomyces cerivisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J Biol Chem. 1994;269:16829–16836. [PubMed] [Google Scholar]
- Weber J, Hecker E. Cocarcinogens of the diterpene ester type from Croton flavens L. and esophageal cancer in Curacao. Experientia. 1978;34(6):679–682. doi: 10.1007/BF01947253. [DOI] [PubMed] [Google Scholar]
- Wender PA, Cribbs CM, Koehler KF, Sharkey NA, Herald CL, Kamano Y, Pettit GR, Blumberg PM. Modeling of the bryostatins to the phorbol ester pharmacophore on protein kinase C. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(19):7197–7201. doi: 10.1073/pnas.85.19.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Zhang AX, Newton AC. Protein kinase C pharmacology: refining the toolbox. The Biochemical journal. 2013;452(2):195–209. doi: 10.1042/BJ20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC. Cellular Pharmacology of Protein Kinase Mzeta (PKMzeta) Contrasts with Its in Vitro Profile: IMPLICATIONS FOR PKMzeta AS A MEDIATOR OF MEMORY. The Journal of biological chemistry. 2012;287(16):12879–12885. doi: 10.1074/jbc.M112.357244. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Current biology : CB. 2003;13(9):734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Igumenova TI. The C-terminal V5 domain of Protein Kinase Calpha is intrinsically disordered, with propensity to associate with a membrane mimetic. PloS one. 2013;8(6):e65699. doi: 10.1371/journal.pone.0065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong SS, Zhu Y, Smith D, Verma C, Lim WG, Tan BJ, Li QT, Cheung NS, Cai M, Zhu YZ, et al. The last 10 amino acid residues beyond the hydrophobic motif are critical for the catalytic competence and function of protein kinase Calpha. The Journal of biological chemistry. 2006;281(41):30768–30781. doi: 10.1074/jbc.M511278200. eng. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Cao FF, Wang Y, Meng FL, Zhang Y, Zhong DS, Zhou QH. The protein kinase C (PKC) inhibitors combined with chemotherapy in the treatment of advanced non-small cell lung cancer: meta-analysis of randomized controlled trials. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2015;17(5):371–377. doi: 10.1007/s12094-014-1241-3. [DOI] [PubMed] [Google Scholar]
- Zhou J, Adams JA. Participation of ADP dissociation in the rate-determining step in cAMP-dependent protein kinase. Biochemistry. 1997;36(50):15733–15738. doi: 10.1021/bi971438n. eng. [DOI] [PubMed] [Google Scholar]
- Zurgil U, Ben-Ari A, Atias K, Isakov N, Apte R, Livneh E. PKCeta promotes senescence induced by oxidative stress and chemotherapy. Cell death & disease. 2014;5:e1531. doi: 10.1038/cddis.2014.481. [DOI] [PMC free article] [PubMed] [Google Scholar]