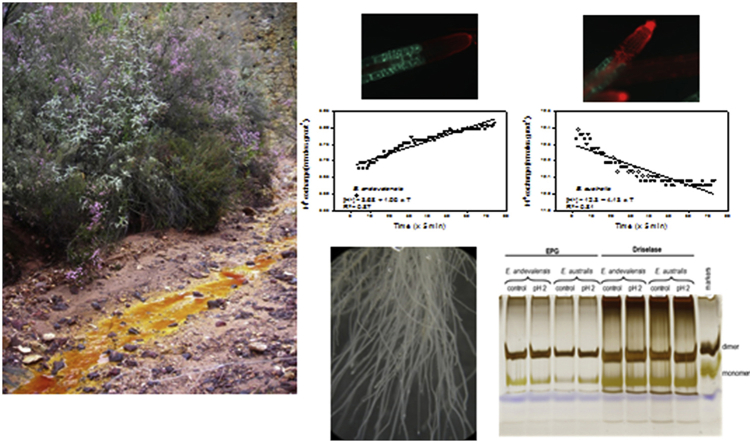
Abstract
Background and aims
Tolerance to soil acidity was studied in two species of Ericaceae that grow in mine-contaminated soils (S Portugal, SW Spain) to find out if there are interspecific variations in H+ tolerance which might be related to their particular location.
Methods
Tolerance to H+ toxicity was tested in nutrient solutions using seeds collected in SW Spain. Plant growth and nutrient contents in leaves, stems and roots were determined. Viability tests and proton exchange were studied in roots exposed, short-term, to acidic conditions. Membrane ATPase activity and the cell-wall pectic polysaccharide domain rhamnogalacturonan-II (RG-II) were analysed to find out interspecific differences.
Results
Variation in survival, growth and mineral composition was found between species. The H+-tolerant species (Erica andevalensis) showed greater concentration of nutrients than E. australis. Very low pH (pH 2) produced a significant loss of root nutrients (K, P, Mg) in the sensitive species. Root ATPase activity was slightly higher in the tolerant species with a correspondingly greater H+ efflux capacity. In both species, the great majority of the RG-II domains were in their boron-bridged dimeric form. However, shifting to a medium of pH 2 caused some of the boron bridges to break in the sensitive species.
Conclusions
Variation in elements linked to the cell wall-membrane complex and the stability of their components (RG-II, H+-ATPases) are crucial for acid stress tolerance. Thus, by maintaining root cell structure, active proton efflux avoided toxic H+ build-up in the cytoplasm and supported greater nutrient acquisition in H+-tolerant species.
Keywords: Erica andevalensis, E. australis, Acidity tolerance, Boron, Calcium, Magnesium, Mineral nutrition, H+ toxicity, RG-II
Graphical abstract
Highlights
-
•
Why some species grow in low pH soils with but only one in riverbanks of an acidic river?
-
•
There are interspecific differences in response to proton toxicity.
-
•
Tolerance to acid stress related to stability of root wall components (RG-IIs) and proton extrusion.
1. Introduction
The heathers Erica andevalensis Cabezudo & Rivera and E. australis L. (Ericaceae) are common species in the Riotinto (SW Spain) and São Domingo (S Portugal) areas where they grow under very harsh climatic conditions and in soils contaminated by past mining activities. The soils present very low nutrient availability, low pH (pH 3.3–4.9) and high concentration of potentially phytotoxic elements like As, Cu, Fe, Pb and Zn (Soldevilla et al., 1992; Rodríguez et al., 2007; Monaci et al., 2011). Elements like Al and Mn are present at low or normal concentrations (Rodríguez et al., 2007; Monaci et al., 2011). Both species behave as excluders of metals like Cu, Zn and Pb but accumulate significant concentrations of Mn (Soldevilla et al., 1992; Monaci et al., 2011). Tolerance to high heavy metal concentration is quite similar in both species (Rossini Oliva et al., 2009a, 2012; Trigueros Vera, 2011; Pérez-López et al., 2014). They grow in mixed communities or in mono-specific patches in heavily polluted mining spots of the Iberian Pyrite Belt (Abreu et al., 2008; Monaci et al., 2011). However, the endemic species (E. andevalensis) seems to thrive best in the vicinity of water courses carrying highly acidic waters (pH 2.3) (Rodríguez et al., 2007; Rossini Oliva et al., 2009b; Monaci et al., 2011). Soil pH was a distinctive trait associated with the distribution of the species in South Portugal (E. australis not found at pH values below 3.5) (Rodríguez et al., 2007; Abreu et al., 2008). However, in a different survey in the SW of Spain no significant association was found between soil acidity and species distribution (Monaci et al., 2011). Might tolerance to proton toxicity explain their differential distribution? To our knowledge, no attempt to determine the pH tolerance in both species has been carried out. The tolerance to acid soils and high concentration of metals makes both species ideal for re-vegetation of contaminated areas and phyto-stabilization of contaminated soils (Abreu et al., 2008; Rodríguez et al., 2007; Rossini Oliva et al., 2009a; Pérez-López et al., 2014). The remarkable adaptation of these species to soils with very low nutrient contents (Rodríguez et al., 2007; Monaci et al., 2011) makes these Ericaceae interesting subjects of research to provide information on nutrient acquisition under severe acidity and metal toxicity stresses.
Plant tolerance to acid soils involves a series of traits to cope with H+ and Al toxicity, deficiency of main nutrients either by low availability or inhibition of uptake (e.g. Ca, Mg, K), or decreased solubility (P, Mo) (Marschner, 1991). Interestingly, primary limitations to plant growth in acid soils have emphasized Al and Mn toxicities and low P levels (Kochian et al., 2004). However, H+ toxicity may be equally crucial for the adaptation of some species to particular acid soils (Kidd and Proctor, 2001). Both Erica andevalensis and E. australis tolerate Al and acidity (Abreu et al., 2008; Monaci et al., 2011) and grow at very low soil contents of Ca and P (Soldevilla et al., 1992; Monaci et al., 2011). Apart from the observation of a possible soil pH-based discrimination on predominance of one or another species (Abreu et al., 2008), no attempt has been made to test such hypothesis. The acidity tolerance might rely on physiological (acclimation) mechanisms leading to the avoidance of H+ toxicity and/or on adaptation to nutrient limitations or metal excesses (Marschner, 1991). Proton toxicity may directly inhibit root (and plant) growth by complex mechanisms affecting cell wall and plasma membrane Ca, inhibiting K uptake and disturbing cytosol pH regulation (Schubert et al., 1990; Yan et al., 1992; Kinraide, 1998; Koyama et al., 2001). Growth adaptation to low pH leads to an increase in plant root H+ ATPase (Yan et al., 1998; Zhu et al., 2009) but when external pH falls, H+ release may be inhibited by an increased membrane H+ permeability (Yan et al., 1998). Frequently, Ca, Mg and K deficiencies develop at acidic pH and the main symptoms in plants are root growth inhibition and water stress (Marschner, 1991). Additional effects of H+ toxicity are the reduction of apoplastic Ca (Kinraide, 1998) and the disruption in the structure and stability of cell wall polysaccharides (Koyama et al., 2001). Low pH levels are also associated with inhibited cation uptake, whereas anion uptake may be only slightly influenced or unaffected (Marschner, 1995).
Plants utilize a variety of mechanisms to cope with the adverse soil factors and successful adaptation will depend on the plasticity of the species. Mechanical and biochemical malfunctioning of root cell walls, plasma membranes and wall–membrane attachment caused by acid pH might be attributable to a failure of polysaccharide or glycolipid fractions to bind boron and/or calcium. In particular, the cell-wall pectic polysaccharide domain rhamnogalacturonan-II (RG-II) is noted for participating in boron bridges (Ishii et al., 2001; O’Neill et al., 2001; Chormova et al., 2014), and glycolipids of the glycosylinositol phosphorylceramide (GIPC) class may bond through boron bridges to RG-II (Voxeur and Fry, 2014).
The objective of the present work was to study both species under controlled conditions to test their ability to grow at different pH values and to determine the possible mechanisms of acidity tolerance.
2. Materials and methods
2.1. Plant materials
Seeds of Erica andevalensis and E. australis were collected in the mining area of Riotinto (Huelva) and sown in Rockwool for germination and further cultivation in fully aerated nutrient solutions at pH 4 (Rossini Oliva et al., 2009b). Basically, the nutrient solution contains (in mM): K, 4 mM; Ca, 2mM; Mg, 1 mM; NH4+, 1mM; NO3−, 5; SO42−, 1; H2PO4−, 1; and (in μM): B, 50; Fe, 100; Mn, 10; Cu, 1; Zn, 1; Mo, 0.5. Plants used for root membrane extraction and isolation of root cell wall components were cultivated in a 1/10th diluted version (0.1x) of the same nutrient solution.
2.2. Determination of pH tolerance
When the plantlets reached approximately 5 cm height were transferred to nutrient solutions at different pH (1.5, 2, 3, 4 and 6). The different pH treatments were obtained by adding NaOH (1 M) or diluted H2SO4 (1:10) into the nutrient solution. The experiment was carried out in a growth chamber with cycles of 26-22 °C (day-night temperature) and 16 h light/8 h darkness during 2 months. The solution was continuously aerated with an aquarium air pump and changed every week to maintain nutrient concentration relatively constant.
An additional test of tolerance was performed assaying root cell viability (Koyama et al., 2001) by transferring seedlings growing in control solution (pH 4) to a nutrient solution pH 2 and sampling roots after 0, 2, 6 and 12 h of treatment. Roots were then stained with a plant cell viability assay kit (Sigma, Missouri, USA). Fluorescence images were obtained with a Zeiss fluorescence microscope (Axioskop 2) equipped with filter cubes for green fluorescence (Chroma 41017 Endow GFP (exciter HQ470/40; dichroic Q495LP; emitter HQ525/50) and red fluorescence (HQ-Filterset for Texas Red (exciter HQ560/55; dichroic Q595LP; emitter HQ645/75), acquired with AxioVision 4 and merged with Image J.
2.3. Effect of Ca and Mg in pH tolerance
To test the effect of increasing Ca and Mg in the tolerance to low pH (Marschner, 1995), an experiment was carried out by adding additional Ca (10 or 20 mM Ca as CaCl2) or Mg (20 mM as MgSO4) to nutrient solutions with pH 2. Every 10 days, the culture medium was renewed and the fresh weight of plants was recorded. After two months, the plants were harvested and processed for chemical analysis as described below.
2.4. Effect of low pH on root H+ release or uptake and nutrients leakage
Proton release by roots was measured in intact plants similarly as described by Yan et al. (1998) using a pH meter with continuous recording of pH changes (pH 301, Hanna Instruments) in a solution containing 1 mM K (KCl), 1 mM Ca (CaCl2) and 1 mM Na (NaCl). Roots from plants cultivated at pH 3were rinsed with the solution and set into the measuring system providing continuous aeration and temperature control. When the pH of the bathing solution was stable, its pH was lowered to pH 2 by adding concentrated H2SO4 and the pH changes were recorded during 6 h.
The effect of acidity on root nutrient leakage was tested using plants set in distilled H2O with pH adjusted to 2.0 with concentrated H2SO4 and a control (distilled H2O, pH 6.0). At different time intervals (0, 1, 2 4, 8, 16 and 24 h), samples were taken from the solution for the analysis of roots released elements by inductively coupled plasma atomic emission spectroscopy (ICP/AES). Root chemical contents before and after acidic treatment were analysed as described below.
2.5. ATPase activity in root membranes
Isolation of root membranes was as described by Vera-Estrella et al. (1999) and microsomes composed of plasma membranes and tonoplasts were used for studying ATP hydrolytic activities. Approximately 5–10 g roots were chopped, placed in a pre-cooled glass jar and homogenized in 100–200 ml of an ice-cold medium containing 400 mM mannitol, 10% (v/v) glycerol, 5% (w/v) polyvinylpyrrolidone (PVP-10), 0.5% (w/v) BSA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 30 mM Tris, 2 mM DTT, 5 mM EGTA, 5 mM MgSO4, 0.5 mM butylated hydroxytoluene, 0.25 mM dibucaine, 1 mM benzamidine, and 26 mM K+-metabisulfite, adjusted to pH 7.5 with H2SO4. The homogenate was filtered through four layers of Miracloth and centrifuged at 10,000g 20 min and the pellet discarded. The supernantants were centrifuged at 100,000g for 45 min. The pellet was carefully resuspended in a buffer containing 400 mM mannitol, 10% glycerol, 6 mM Tris/Mes pH 7.5 and 2 mM DTT. All operations were performed at 4 °C. Root tonoplast (V) and plasma membrane (PM) ATPases were measured by the release of Pi according to the method of Ames (1966) as previously described by Vera-Estrella et al. (1999). Estimates of V-ATPase and PM-ATPase were made by assaying Pi release using buffers of different pH (pH 8 and 6.5 respectively) and inclusion of specific inhibitors (bafilomycin, vanadate, and Na azide). The values are presented as μmol Pi released (mg membrane protein)−1 h−1.
2.6. Analysis of rhamnogalacturonan-II from roots
The preparation of alcohol-insoluble residue (AIR; i.e. cell wall-enriched fraction) from roots was performed essentially as described by Chormova et al. (2014). Roots were rinsed with deionized water, chopped and left stirring in 75% ethanol for 6 h and the process was repeated twice. Then the AIR was saponified with 1 M Na2CO3 for 16 h at 4 °C, neutralised with acetic acid, rinsed with deionized water to remove salts, and freeze-dried. An aliquot of root AIR was used for ICP measurement of mineral elements as indicated below. Another aliquot, used for RG-II analysis, was digested with endopolygalacturonase (EPG; Megazyme, Ireland) or Driselase (Chormova et al., 2014). One milligram of AIR was incubated for 16 h at room temperature with 100 μl EPG solution (2.25 U/ml) in 50 mM acetate buffer (Na+, pH 4.8) supplemented with 0.5% (w/v) chlorobutanol. For the Driselase digestion, AIR was incubated at 37 °C for 16 h in 0.02% enzyme (purified as described by Fry (2000)) in the same buffer/chlorobutanol mixture. In both cases, the enzymic digest was analysed by polyacrylamide gel electrophoresis as described by Chormova et al. (2014). Authentic monomeric and (boron-bridged) dimeric RG-II for use as markers were obtained from Rosa cell-suspension cultures (Chormova et al., 2014).
2.7. Plant analyses
At the end of the experiment, plants were harvested and separated into roots, stems and leaves, weighed and the chemical analysis performed on each organ separately. To assess plant growth, changes in biomass weight every 10 days were used and biomass production was calculated as the difference between initial and final fresh weight. Water content in shoots and roots was calculated as 100*(fresh weight-dry weight)/fresh weight. Plants samples were dried in oven at 70 °C for 48 h, powered and digested with HNO3 and H2O2. Concentration of elements was measured by ICP/AES and accuracy of digestion and analytical procedures was checked by routine determination of element concentration in standard reference materials (Monaci et al., 2011).
2.8. Data analysis
All treatments had four replicates and the experiments were run two months. The statistical significance of the differences between the treatments and species was tested using a factorial design and if the F-value showed significant differences (p < 0.05), the means were compared by Tukey post-hoc test. When variables did not fit a normal distribution, means were compared by Kruskal-Wallis and Mann-Whitney non-parametric tests. Data were analyzed using a statistical package (Statsoft package v6.12).
3. Results
3.1. Effect of low pH in plant growth
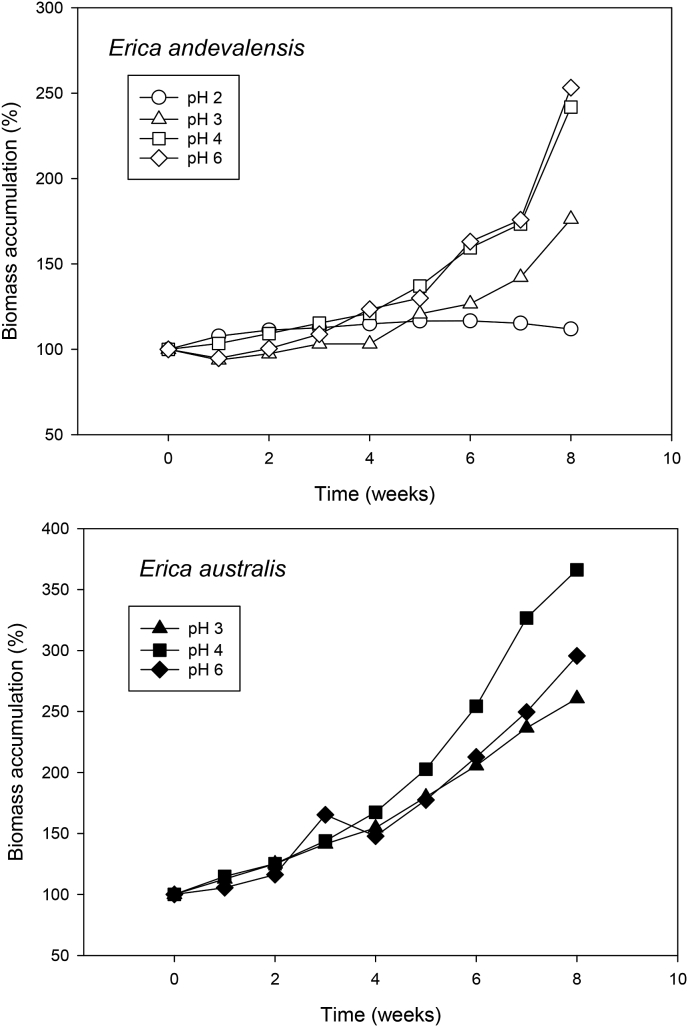
Neither species survived at pH 1.5 but Erica australis died even at pH 2, quickly developing initial toxicity symptoms (shoot wilting) and reaching 100% mortality after 3 weeks. The species Erica andevalensis recovered from an initial wilting and survived at pH 2 but remained quiescent (Fig. 1). The low pH also led to a reduction in shoot and root water content in comparison with higher pHs (Table 1). No significant differences in growth were found for E. andevalensis among pH treatments above pH 2. However, E. australis showed a significantly greater growth rate at pH 4 than at lower or higher pH (p < 0.05, Table 1). In E. andevalensis, the low pH (pH 2) also modified the shoot/root ratio (p = 0.001) as result of the significant inhibition of root growth (Table 1). No differences were observed for these parameters at higher pH (p > 0.05).
Fig. 1.
Change in plant biomass (shoots + roots) of Erica andevalensis and E. australis grown in nutrient solutions with different pH treatments (relative values based in initial fresh weight, 100%). Values at pH 2 for E. australis are not shown as plants were dead at the third week of treatment.
Table 1.
Shoot and root biomass, root/shoot ratio and shoot water contents in two species of Erica after 60 days of growth in nutrient solutions of different pH. Different letters following means ± standard error indicate statistically significant differences in data group (p < 0.05).
| Species | pH | Shoot biomass (g dry weight) | Root biomass (g dry weight) | Shoot/root ratio | Shoot water content (%) |
|---|---|---|---|---|---|
| E. andevalensis | 2 | 0.71 ± 0.20a | 0.30 ± 0.08a | 2.34 ± 0.58a | 61.6 ± 2.8a |
| 3 | 0.88 ± 0.37a | 1.05 ± 0.46b | 0.89 ± 0.25b | 79.9 ± 7.0b | |
| 4 | 0.91 ± 0.40a | 0.77 ± 0.40b | 1.23 ± 0.17b | 80.7 ± 5.3b | |
| 6 | 1.13 ± 0.58a | 0.91 ± 0.44b | 1.21 ± 0.29b | 76.4 ± 8.0b | |
| E. australis | 21 | – | – | – | – |
| 3 | 3.03 ± 1.16a | 1.58 ± 0.56a | 1.97 ± 0.54a | 72.4 ± 1.2ab | |
| 4 | 6.17 ± 1.70b | 4.44 ± 1.60b | 1.45 ± 0.32a | 70.0 ± 1.0ab | |
| 6 | 3.02 ± 1.44a | 1.63 ± 0.90a | 1.90 ± 0.51a | 73.9 ± 2.0a | |
--1, All E. australis plants died at pH 2.
The differential tolerance to H+ toxicity between species was also found when plants were transferred to acidic solution (pH 2) for a short-term treatment. The species E. australis showed greater root sensitivity to acidity than E. andevalensis with root cell death in the elongation zone and mature roots (Fig. S1).
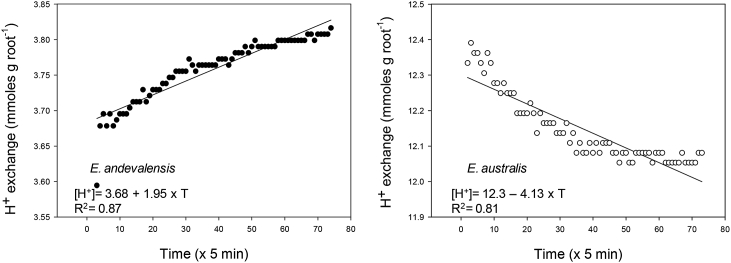
The capacity of root cell membranes to adapt to high H+ concentration was assayed by measuring net proton exchange. Significant differences were found between roots of both species exposed to low pH (Fig. 2). The roots of E. australis showed a net uptake of H+ while those from E. andevalensis presented a continuous H+ efflux during the short-treatment in acidic conditions (Fig. 2). The activity of root plasma membrane ATPases, responsible of H+ efflux, showed small differences between species (Table S1). However, the ATPase activity in the tolerant species (E. andevalensis) was consistently higher and correlated with its greater capacity for H+-efflux in acidic conditions (Fig. 2). The species presented higher ATP hydrolytic activity when plants were grown at two different pHs (Table S1).
Fig. 2.
Proton exchange by roots of Erica andevalensis and E. australis when incubated in minimal medium (1 K: 1 Na: 1 Ca, in mM) at low pH (pH = 2) during 6 h.
In both of these Erica spp., the pH of the medium influenced the monomer:dimer ratio of the pectic polysaccharide domain, RG-II (Fig. S2). At pH 4.0, both species showed more monomer and slightly less dimer than at pH 3.0. On a purely chemical basis, this was unexpected as a low pH tends to monomerise dimeric RG-II (O'Neill et al., 1996) [but for complete monomerisation in the laboratory, pH 1.0 is usually employed]. The more acid-tolerant E. andevalensis appears less capable of keeping its RG-II in the dimeric form, exhibiting a higher proportion of RG-II monomer than the acid-susceptible E. australis; this result was also unexpected as it is generally assumed that maintaining RG-II–boron–RG-II bridges is beneficial for plants. The above observations were made on plants grown in media containing low (5 μM) boric acid. On the other hand, supplementation of the pH 4.0 medium with 1 mM boric acid converted most of the RG-II to its (boron-bridged) dimer.
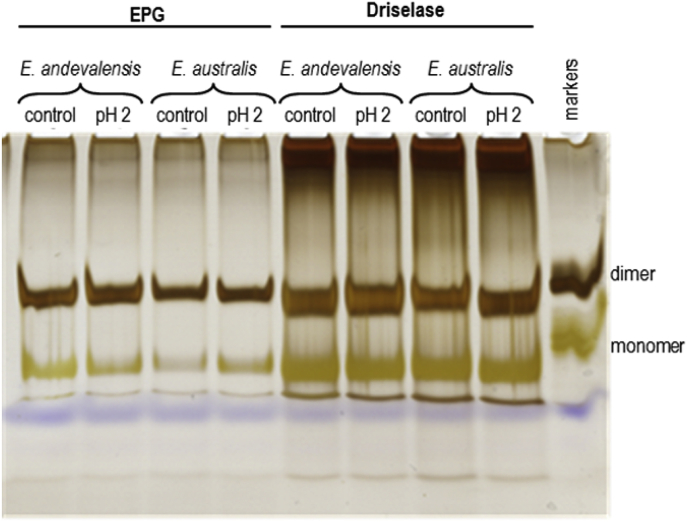
Similar behaviour was observed, especially in gels of EPG digests, in plants grown at pH 3.0 and then transferred for 6 h to pH 2.0 (both media containing a low boric acid concentration, 5 μM; Fig. 3). Prior to the pH shift, E. andevalensis roots exhibited a higher monomer:dimer ratio in their RG-II than did E. australis, as observed in Fig. S2. However, after the shift to an extremely acidic medium, E. australis increased in monomer:dimer ratio, indicating a partial cleavage of boron bridges, whereas E. andevalensis did not (Fig. 3). Since it is generally assumed that RG-II dimerisation (i.e. maintenance of boron bridging) is beneficial for plants (Brown et al., 2002; Brown and Hu, 1997; Dell and Huang, 1997; Findeklee and Goldbach, 1996; Fleischer et al., 1999; Ghanati et al., 2001; Goldbach, 1997; Holdaway-Clarke et al., 2003; Hu and Brown, 1994; Ishii et al., 2001; Loomis and Durst, 1992; Matoh et al., 2000; O'Neill et al., 2001; Reiter el at. 1997; Ryden et al., 2003), this observation tallies with the observed acid-susceptibility of E. australis.
Fig. 3.
Effect of a highly acidified medium on the boron-bridging of endogenous RG-II in roots of two Erica spp. Electrophoresis of root RG-II from Erica andevalensis and E. australis after digestion with endo-polygalacturonase (EPG; left panel) or Driselase (right panel). Plants were grown in 0.1 × nutrient solutions (i.e. containing 5 μM boric acid) at pH 3.0 (control) and then in some cases transferred to a highly acidified medium (pH 2) for 6 h.
The alcohol insoluble residues (AIR) used for the isolation of RG-II species showed a variation in the concentration of B and Ca between species (Table S2) that may be correlated with their actual concentration in whole roots (Fig. 4, Fig. 5).
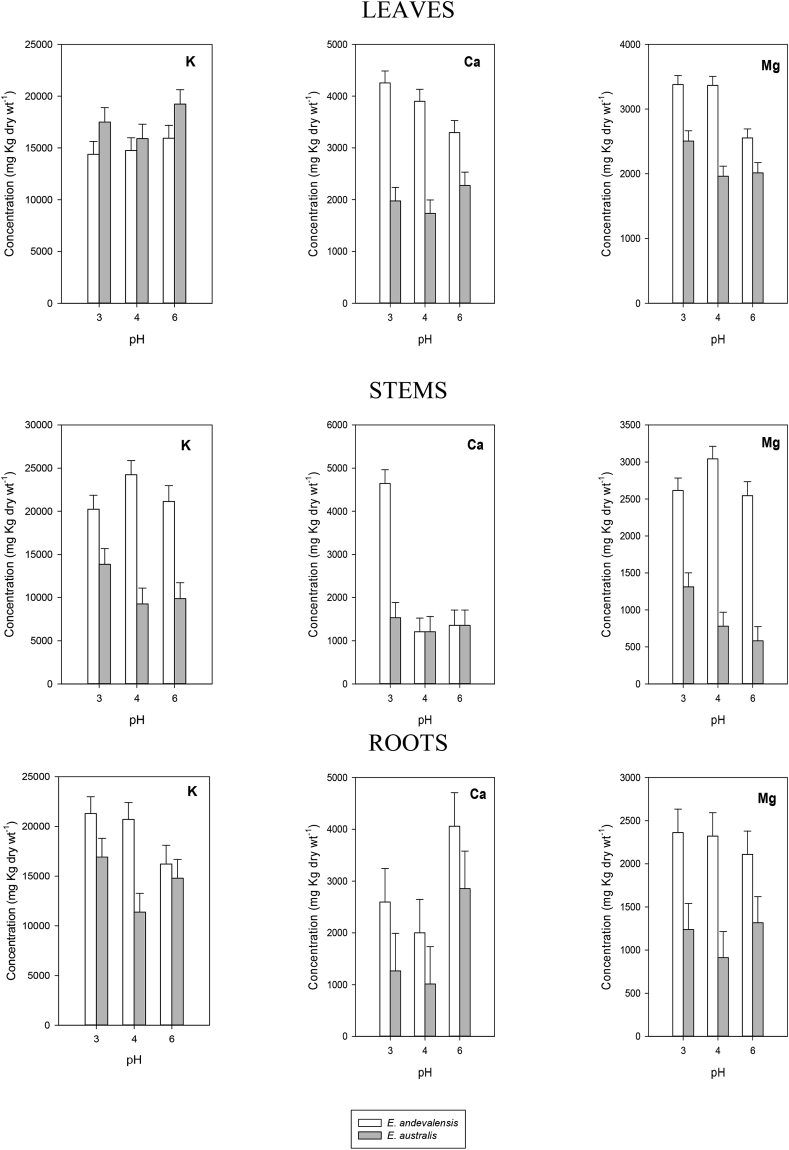
Fig. 4.
Concentration of K, Ca and Mg in leaves, stems and roots of Erica andevalensis and E. australis grown in full nutrient solutions at different pH. Bars on columns indicate standard error of the mean.
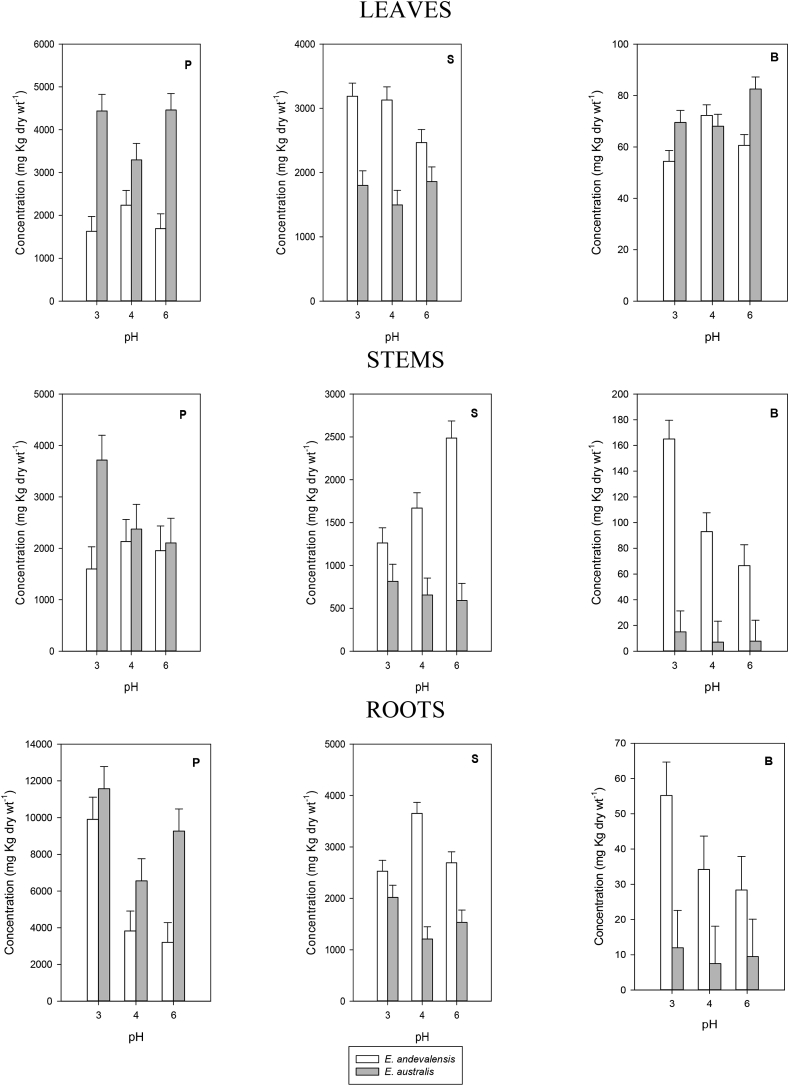
Fig. 5.
Concentration of P, S and B in leaves, stems and roots of Erica andevalensis and E. australis grown in full nutrient solutions at different pH. Bars on columns indicate standard error of the mean.
Maintenance of root cell membrane selectivity under acidic conditions was also determined by measuring leakage of nutrients. When roots were treated with acidified water a massive leakage of K and P was recorded in the roots from E. australis (Fig. S3) while in roots from E. andevalensis nutrient losses were lower. Meanwhile, the release of Fe and Ca induced by H+-stress was significant but similar between species (Fig. S3). The release of other nutrients into the incubation medium remained fairly stable or they were in the detection limit for ICP system used (data not shown). The analysis of roots after the acid treatment revealed greater retention of most nutrients (K, Ca, Mg, B, Mn, Zn) in E. andevalensis than in E. australis with the exception of Fe (Table S3).
3.2. Plant nutrient contents as affected by pH and species
The solution pH affected the root concentration of Ca, P, Fe, Cu and Zn as well as the concentration of Mg and Mn in the leaves and the accumulation of S, B and Fe in the stems (Table 2). When comparing both species significant differences were found in the concentration in the mineral contents of their leaves, stems and roots (Table 2, Fig. 4, Fig. 5, Fig. 6). The acid-tolerant species (E. andevalensis) showed significantly greater concentration of K, Ca, Mg, S, B and Mn in the roots. A significant inter-specific variation also existed in the macro- and micronutrient concentration of leaves and stems (Table 2). While the roots of E. andevalensis displayed greater concentration in the elements indicated above (Fig. 4, Fig. 5, Fig. 6), the roots and leaves from E. australis showed significantly greater concentration of P (Fig. 4). Among other differential features of E. andevalensis was the greater leaf concentration of macronutrients like Ca, Mg, S (Fig. 4, Fig. 5) and micronutrients as Mn (Fig. 6).
Table 2.
Results of 2-way ANOVA for nutrient concentration in leaves, stems and roots from E. andevalensis and E. australis plants grown in nutrients solutions at pH 3, 4 and 6.
| K | Ca | Mg | P | S | B | Fe | Mn | Cu | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | ||||||||||
| pH (P) | ns | ns | P < 0.001 | ns | ns | ns | ns | P < 0.05 | ns | ns |
| Species (S) | P < 0.05 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| P x S | ns | P < 0.05 | P < 0.05 | P < 0.05 | ns | P < 0.05 | ns | P < 0.01 | ns | P < 0.01 |
| Stems | ||||||||||
| pH (P) | ns | ns | ns | ns | P < 0.05 | P < 0.01 | P < 0.01 | ns | ns | ns |
| Species (S) | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.05 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| P x S | ns | ns | P < 0.05 | ns | P < 0.01 | P < 0.05 | ns | P < 0.05 | ns | ns |
| Roots | ||||||||||
| pH (P) | ns | P < 0.05 | ns | P < 0.001 | ns | ns | P < 0.001 | ns | P < 0.01 | P < 0.001 |
| Species (S) | P < 0.01 | P < 0.05 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.01 | P < 0.05 | P < 0.001 | ns | P < 0.001 |
| P x S | ns | ns | ns | ns | P < 0.001 | ns | ns | ns | P < 0.01 | P < 0.001 |
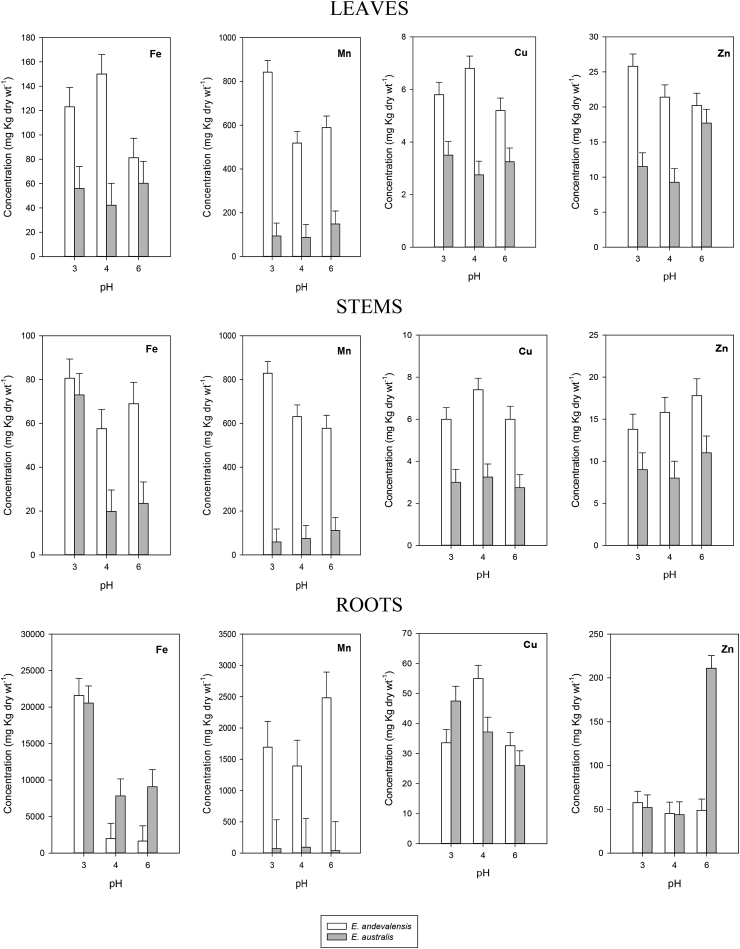
Fig. 6.
Variation in the concentration of Fe, Mn, Cu and Zn in leaves, stems and roots of Erica andevalensis and E. australis grown in full nutrient solutions at different pH. Bars on columns indicate standard error of the mean.
3.3. Effect of Ca and Mg availability on pH tolerance
Increasing Ca2+ levels in the nutrient solution did not improve the survival E. australis at low pH and all plants of this species had died by the end of the experiment. Additional Mg2+ in the nutrient solution did not enhance acid tolerance either in E. australis and a similar mortality (100%) as in the control treatment was recorded. In E. andevalensis, growth was not improved at low pH either by additional Ca2+ or Mg2+ (Table S4) and plants showed similar growth rates in the control solution and in the solutions supplemented with bivalent cations. However, the addition of Ca2+ significantly increased B and K concentration (and depressed Cu concentration) in E. andevalensis roots at low pH (Table S5). Increasing Mg2+ concentration had no significant effect either in K or B concentration in roots but significantly inhibited the concentration of Cu and Zn (Table S5).
4. Discussion
Very acidic conditions produced an early shoot wilting from which only plants of E. andevalensis recovered but showed stunted growth afterwards. Meanwhile, E. australis, more sensitive to low pH, died at pH 2 but it showed higher growth rates at higher pH. Both species showed marked phenotypic responses in nutrient accumulation at different pH. Root cells of the sensitive species showed damage soon after exposure to low pH stress as the membranes became disrupted by external H+ and leaked nutrients required for cell structure, turgor and metabolism.
The differential behaviour between species recorded under controlled conditions in the present report is in agreement with the one observed in highly acid soils of South Portugal (Abreu et al., 2008). The survival in highly acidic media was not improved by increasing the concentration of divalent cations (Ca2+, Mg2+) even though it was reported these ions may improve root growth in acidity-sensitive species when affected by low pH stress (Tan et al., 1992; Marschner, 1995). In acid soils, a major constrain to plant growth is the reduced concentration of metal cations like Ca, Mg or K (Marschner, 1991). In fact, low nutrient concentration in the rhizosphere was one of the factors associated with mono-specific patches of E. andevalensis (Monaci et al., 2011). We found significant inter-specific differences in the concentration of macro and micronutrients in all plant organs (Table 2). The effects of solution pH were significant only for Mg and Mn in leaves, for S, B and Fe in stems, and for Ca, P, Fe, Cu and Zn in roots (Table 2). With few exceptions (e.g. P and Fe) the acid tolerant species (E. andevalensis) showed greater root nutrient concentration than the sensitive one (Fig. 4, Fig. 5, Fig. 6). No significant differences were found in the root concentration of Cu between species (Table 2) but leaf and stem Cu concentration was significantly higher in E. andevalensis (Table 2, Fig. 6). Particularly distinctive traits between species were the concentrations of B and Mn in roots and stems which were significantly greater in E. andevalensis than in E. australis (Fig. 5, Fig. 6). Fine roots of both species grown in acidic soils of Riotinto similarly showed significant differences in B concentration (F. Monaci, personal communication) with E. andevalensis holding the higher root B concentration.
Species in very poor soils grow slowly and have a low nutrient uptake rate but maximize nutrient intake by developing a greater root system (a lower shoot/root ratio) (Chapin, 1987; Lambers and Poorter, 2004). Thus, a greater concentration of nutrients in the roots in the acid-tolerant species might be explained by differences in growth rate and shoot/root ratio (Table 1, Table S6). When the nutrient demand for growth is low, intake exceeds the demand and nutrients are accumulated. The differences in requirements for growth might result in the greater accumulation of many nutrients in E. andevalensis (Fig. 4, Fig. 5, Fig. 6). In fact, other reports with data from field-grown plants show differences between both species in leaf concentration of Ca, Mg, Mn and P (Soldevilla et al., 1992; Monaci et al., 2011; Trigueros Vera, 2011) showing the same tendency as the observed in the present report. Our results provide the first evidence of the differential plant nutrient concentration between these species when the elements are at the same concentration. One might speculate that long term adaptation to nutrient poor and acidic soils pH led to the recorded variation in element concentration as a strategy for survival.
A short-term root treatment with very low pH (pH 2), which might reflect an inherent acid tolerance, affected roots differently in both species. The sensitive species showed greater damage in its roots with loss of viability in the elongation zone and mature roots (Fig. S1). Additionally, the pH-sensitive species (E. australis) showed a net root proton uptake while the tolerant one (E. andevalensis) was able to maintain a continuous root H+ efflux (Fig. 2). In maize, the adaptation to low pH resulted in the increase in plasmalemma H+-ATPase providing a greater proton extrusion ability (Yan et al., 1998). The ATP hydrolytic activity of root membranes was slightly greater in the H+ tolerant species and it might be related to its greater H+ extrusion. A very low medium pH (pH 2) stunted plant growth and inhibited root growth (Table 1).
Nutrients like Ca, Mg and B are linked to cell wall stability and maintenance of membrane selective properties (Wimmer and Goldbach, 1999; Koyama et al., 2001; Brown et al., 2002). Boron is the required element for the formation of cross-links between RG-II domains, providing stability to plant cell walls and their biophysical properties (Ishii et al., 2001; O’Neill et al., 2001; Chormova et al., 2014). We have shown in the present work that the RG-II–B–RG-II bridges are more resistant to extremely acidic growth media (pH 2) in E. andevalensis than in E. australis. We suggest that this correlation may be related to the enhanced ability of E. andevalensis to survive in highly acidic soils. The bridging of RG-II domains through a boron atom is well established to be necessary for the normal functioning of the primary cell walls in healthy vascular plants, especially in terms of wall growth, strength and porosity (Hirsch and Torrey, 1980; Hu and Brown, 1994; Findeklee and Goldbach, 1996; Fleischer et al., 1999; Ishii et al., 2001). The differential capacity to keep greater contents of B and Ca in the H+-tolerant species was observed even in the alcohol insoluble residues used for RG-II isolation (Table S2).
Decrease in root growth is one of the main symptoms of proton toxicity occurring during adaptation (Schubert et al., 1990; Yan et al., 1992; Kidd and Proctor, 2001; Shavrukov and Hirai, 2016). In the short-term, membranes are depolarized and proton pumps work for re-establishing gradients to permit nutrient uptake (Shavrukov and Hirai, 2016). Some species, root medium acidity produces irreversible root damage which is reduced by increasing Ca and B concentration (Koyama et al., 2001). However, increasing the concentration of bivalent cations (Ca2+, Mg2+) at low pH neither increased survival in E. australis nor diminished the acid-induced growth inhibition in E. andevalensis (see Table S4). Additional supply of Ca2+ and Mg2+ in acidic solutions (pH 2) only partially recovered nutrient uptake in the tolerant species (Table S5).
The significant loss of nutrients induced after treating roots with very low pH (Fig. S3, Table S3) may be associated to the disruption of membrane selective properties and cell wall structure induced by high H+ concentrations. The differential amount of nutrients retained after a short-term acid treatment (Table S3) may be among the particular features providing the H+-tolerant species (E. andevalensis) with a greater tolerance to acid stress. The sensitive species (E. australis) showed a greater loss of nutrients in acidified water (Table S3). The greater root concentration of Ca, Mg and B in the acid-tolerant species (Fig. 4, Fig. 5) might buffer the acidity-induced loss of structural cell wall elements and thereby keep root cell viability for a longer term. Among the rapid root responses to low pH, the loss of K and P were the most significant changes (Fig. S3). The roots of acid-sensitive species (E. australis) released both nutrients at a much greater rate that the tolerant one (Fig. S3). Massive K leakage from roots is a general response to a variety of abiotic and biotic stresses (Demidchik et al., 2014) and has been described in roots under H+ toxicity long time ago (Marschner et al., 1966). The loss of root K, a main osmoticum for cell turgor and root osmotic water uptake (Marschner, 1995), may have led to the rapid dehydration of shoots observed after acidic treatment. Shoot wilting produced by soil acidity has been described in other H+ sensitive-species (Kidd and Proctor, 2001). While E. andevalensis recovered from wilting and maintained a quiescent state, E. australis was unable to re-establish root functionality and restore shoot water contents and the plants died shortly after. It is noteworthy that E. andevalensis stems also hold significantly greater B concentration and it might be an additional feature providing greater tolerance in the species.
Proton-impermeable membranes, channels of reduced pores, efficient proton-efflux systems and uptake of cations in excess to inhibit H+ influx are among adaptations developed by acidophile organisms, to grow at pH less than 3 (Baker-Austin and Dopson, 2007). Not being an acidophile sensu stricto but an acid-tolerant species, E. andevalensis might have evolved acquiring plasma membrane properties to keep H+ at bay (Messerli et al., 2005), complex cell wall structures to tolerate low pH (Baker-Austin and Dopson, 2007) and greater cation uptake (Fig. 5). Adaptation to the harsh conditions in acid root environment by significant accumulation of nutrients like Ca, Mg and B might provide E. andevalensis roots with a greater resistance to the structural disturbing effects of H+ excess on cell walls and membranes. The greater tolerance of E. andevalensis to H+ toxicity found in in this work may explain its adaptation to grow in very acid soils and the formation of monospecific communities (Abreu et al., 2008; Monaci et al., 2011). The extremely low soil pH and periodic flooding with the acid river waters might be too hostile for E. australis establishment. Considering the significant differences in root nutrient concentration, the species might also cope with the poor nutrient availability found in the acid soils (Monaci et al., 2011). Other factors like the intrinsic lower growth rate in the tolerant species or interactions with the soil microbiota (Marschner, 1991) which also might play a role in the plant adaptation to this extreme environment.
The maintenance of root cell-wall–membrane structure in the tolerant species through a greater stability of the RG-II dimers kept a functional plasma membrane H+-ATPase leading to cytoplasmic H+ toxicity avoidance by active H+ efflux and providing the driving force for nutrient uptake.
5. Conclusions
The significant differences in pH tolerance between Erica andevalensis and E. australis were mostly associated with major variation in the concentration of nutrients like Ca, Mg and B required for the maintenance of cell wall structure and membrane selective properties. Indeed, differences in boron bridging of the pectic polysaccharide domain, RG-II, were noted. The interspecific variation in pH sensitivity might certainly explain the differential distribution of the Erica species in the contaminated acid soils and margins of highly acid rivers in South Portugal and SW Spain.
In the soils affected by past mining activities (e.g. Riotinto, SW Spain), complex environments evolved and at present are occupied by species highly adapted to situations of heavy metal contamination and low nutrient availability. However, in conditions of very low pH some species like E. andevalensis may thrive more successfully than others because of their intrinsic root tolerance to H+ toxicity.
Author contributions
S.R.O. and E.O. Leidi, performed and designed research; M.D. Mingorance, performed chemical analyses; D. Sanhueza and S.C. Fry, performed research on RGII; E.O.L. and S.C.F. wrote the paper.
Compliance with ethical standards
The authors comply with the Ethical Standards in the COPE statement and declare that they have no conflict of interest.
Acknowledgements
The authors are grateful to F. Monaci (University of Siena, Italy) for sharing his data from Erica spp. plants grown in acid soils from Riotinto (Huelva, Spain). This work was partially granted by MICINN contract CGL2006/02860 and by Fundación Areces.
SCF thanks the BBSRC (UK; grant reference BB/H000690/1) and DS thanks the Comisión Nacional de Investigación Científica y Tecnológica (Conicyt; Chile) for financial support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.plaphy.2018.02.029.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abreu M.M., Tavares M.T., Batista M.J. Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos. Portugal. J Geochem Expl. 2008;96:210–222. [Google Scholar]
- Ames B.N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8 115–111. [Google Scholar]
- Baker-Austin C., Dopson M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007;15:165–171. doi: 10.1016/j.tim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Brown P.H., Hu H. Does boron play only a structural role in the growing tissues of higher plants. Plant Soil. 1997;196:211–215. [Google Scholar]
- Brown P.H., Bellaloui N., Wimmer M.A., Bassil E.S., Ruiz J., Hu H., Pfeffer H., Dannel F., Römheld V. Boron in plant biology. Plant Biol. 2002;4:205–223. [Google Scholar]
- Chapin F.S., III . Adaptations and physiological responses of wild plants to nutrient stress. In: Gabelman H.W., Loughman B.C., editors. Genetic Aspects of Plant Mineral Nutrition. Martinus Nijhoff; Dordrecht: 1987. pp. 15–25. [Google Scholar]
- Chormova D., Messenger D.J., Fry S.C. Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion. Plant J. 2014;77:534–546. doi: 10.1111/tpj.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell B., Huang L.B. Physiological responses of pants to low boron. Plant Soil. 1997;193:103–120. [Google Scholar]
- Demidchik V., Straltsova D., Medvedev S.S., Pozhvanov G.A., Sokolik A., Yurin V. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014;65:1259–1270. doi: 10.1093/jxb/eru004. [DOI] [PubMed] [Google Scholar]
- Findeklee P., Goldbach H.E. Rapid effects of boron deficiency on cell wall elasticity modulus in Curcubita pepo roots. Bot. Acta. 1996;109:463–465. [Google Scholar]
- Fleischer A., O'Neill M.A., Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S.C. The Blackburn Press; Caldwell, New Jersey: 2000. The Growing Plant Cell Wall: Chemical and Metabolic Analysis. Reprint Edition. Pp. xviii + 333 [ISBN 1-930665-08-3] [Google Scholar]
- Ghanati F., Morita A., Yokota H. Selection and partial characterization of a boron-tolerant tobacco cell line. Soil Sci. Plant Nutr. 2001;47:405–410. [Google Scholar]
- Goldbach H.E. A critical review of current hypotheses concerning the role of boron in higher plants: suggestions for further research and methodological requirements. J. Trace Microprobe Tech. 1997;15:51–91. [Google Scholar]
- Hirsch A.M., Torrey J.G. Ultrastructural changes in sunflower root cells in relation to boron deficiency and added auxin. Can. J. Bot. 1980;58:856–866. [Google Scholar]
- Holdaway-Clarke T.L., Weddle N.M., Kim S., Robi A., Parris C., Kunkel J.G., Hepler P.K. Effect of extracellular calcium, pH and borate on growth oscillations in Lillium formosanum pollen tubes. J. Exp. Bot. 2003;54:65–72. doi: 10.1093/jxb/erg004. [DOI] [PubMed] [Google Scholar]
- Hu H., Brown P.H. Localization of boron cell wall squash and tobacco and its association with pectin. Plant Physiol. 1994;105:681–689. doi: 10.1104/pp.105.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Matsunaga T., Hayashi N. Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol. 2001;126:1698–1705. doi: 10.1104/pp.126.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P.S., Proctor J. Why plants grow poorly on very acid soils: are ecologists missing the obvious? J. Exp. Bot. 2001;52:791–799. doi: 10.1093/jexbot/52.357.791. [DOI] [PubMed] [Google Scholar]
- Kinraide T.B. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 1998;118:513–520. doi: 10.1104/pp.118.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L.V., Hoekenga O.A., Piñeros M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annu. Rev. Plant Biol. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Koyama H., Toda T., Hara T. Brief exposure to low-pH stress causes irreversible damage to the growing root in Arabidopsis thaliana: pectin-Ca interaction may play an important role in proton rhizotoxicity. J. Exp. Bot. 2001;52:361–368. [PubMed] [Google Scholar]
- Lambers H., Poorter H. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv. Ecol. Res. 2004;34:283–362. [Google Scholar]
- Loomis W.D., Durst R.W. Chemistry and biology of boron. Biofactors. 1992;3:229–239. [PubMed] [Google Scholar]
- Marschner H. Mechanisms of adaptation of plants to acid soils. Plant Soil. 1991;134:1–20. [Google Scholar]
- Marschner H. Academic Press, Harcourt Brace & Company, Publishers; 1995. Mineral Nutrition of Higher Plants. [Google Scholar]
- Marschner H., Handley R., Overstreet R. Potassium loss and changes in the fine structure of corn root tips induced by H-ion. Plant Physiol. 1966;41:1725–1735. doi: 10.1104/pp.41.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh T., Takasaki M., Kobayashi M., Takabe K. Boron nutrition of cultured tobacco BY-2 cells: III. Chraracterization of the boron-rhamnogalacturonan II complex in cells acclimated to low levels of boron. Plant Cell Physiol. 2000;41:363–366. doi: 10.1093/pcp/41.3.363. [DOI] [PubMed] [Google Scholar]
- Messerli M.A., Amaral-Zettler L.A., Zettler E., Jung S.K., Smith P.J.S., Sogin M.L. Life at acidic pH imposes an increased energetic cost for a eukaryotic acidophile. J. Exp. Biol. 2005;208:2569–2579. doi: 10.1242/jeb.01660. [DOI] [PubMed] [Google Scholar]
- Monaci F., Leidi E.O., Mingorance M.D., Valdés B., Rossini Oliva S., Bargagli R. The selective uptake of major and trace elements in Erica andevalensis, an endemic species to extreme habitats in the Iberian Pyrite Belt. J. Environ. Sci. 2011;23:444–452. doi: 10.1016/s1001-0742(10)60429-9. [DOI] [PubMed] [Google Scholar]
- O'Neill M.A., Warrenfeltz D., Kates K., Pellerin P., Doco T., Darvill A.G., Albersheim P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. J. Biol. Sci. 1996;271:22923–22930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- O'Neill M.A., Eberhard S., Albersheim P., Darvill A.G. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- Pérez-López R., Márquez-García B., Abreu M.M., Nieto J.M., Córdoba F. Erica andevalensis and Erica australis growing in the same extreme environments: phytostabilization potential of mining areas. Geoderma. 2014;230-231:194–203. [Google Scholar]
- Reiter W.D., Chapple C., Somerville C.R. Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J. 1997;12:335–345. doi: 10.1046/j.1365-313x.1997.12020335.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez N., Amils R., Jiménez-Ballesta R., Rufo L. Heavy metal content in Erica andevalensis: an endemic plant from the extreme acidic environment of Tinto River and its soils. Arid Land Res. Manag. 2007;21:51–65. [Google Scholar]
- Rossini Oliva S., Valdés B., Leidi E.O. Accumulation and in vivo tissue distribution of pollutant element in Erica andevalensis. Sci. Total Environ. 2009;407:1929–1936. doi: 10.1016/j.scitotenv.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Rossini Oliva S., Bargagli R., Monaci F., Valdés B., Mingorance M.D., Leidi E.O. Stress responses of Erica andevalensis Cabezudo & Rivera plants induced by polluted water from Tinto River (SW Spain) Ecotoxicol. 2009;18:1058–1067. doi: 10.1007/s10646-009-0366-6. [DOI] [PubMed] [Google Scholar]
- Rossini Oliva S., Mingorance M.D., Leidi E.O. Tolerance to high Zn in the metallophyte Erica andevalensis Cabezudo & Rivera. Ecotoxicol. 2012;21:2012–2021. doi: 10.1007/s10646-012-0953-9. [DOI] [PubMed] [Google Scholar]
- Ryden P., Sugimoto-Shirasu K., Smith A.C., Findlay K., Reiter W.D., McCann M.C. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 2003;132:1033–1040. doi: 10.1104/pp.103.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S., Schubert E., Mengel K. Effect of low pH of the root medium on proton release, growth, and nutrient uptake of field beans (Vicia faba) Plant Soil. 1990;124:239–244. [Google Scholar]
- Shavrukov Y., Hirai Y. Good and bad protons: genetic aspects of acidity stress responses in plants. J. Exp. Bot. 2016;67:15–30. doi: 10.1093/jxb/erv437. [DOI] [PubMed] [Google Scholar]
- Soldevilla M., Marañón T., Cabrera F. Heavy metal content in soil and plants from a pyrite mining area in Southwest Spain. Commun. Soil Sci. Plant Anal. 1992;23:1301–1319. [Google Scholar]
- Tan K., Keltjens W.G., Findenegg G.R. Acid soil damage in sorghum genotypes: role of magnesium deficiency and root impairment. Plant Soil. 1992;139:149–155. [Google Scholar]
- Trigueros Vera D. University of Seville; 2011. Respuesta de dos especies arbustivas (Erica australis y Nerium oleander) frente a la contaminación derivada de la actividad minera en Riotinto. PhD Thesis. [Google Scholar]
- Vera-Estrella R., Barkla B.J., Bohnert H.J., Pantoja O. Salt stress in Mesembryanthemum crystallinum L. cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta. 1999;207:426–435. doi: 10.1007/s004250050501. [DOI] [PubMed] [Google Scholar]
- Voxeur A., Fry S.C. Glycosylinositol phosphorylceramides (GIPCs) from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. Plant J. 2014;79:139–149. doi: 10.1111/tpj.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer M.A., Goldbach H.E. Influence of Ca2+ and pH on the stability of different boron fractions in intact roots of Vicia faba L. Plant Biol. 1999;1:632–637. [Google Scholar]
- Yan F., Schubert S., Mengel K. Effect of low root medium pH on net proton release, root respiration, and root growth of corn (Zea mays L.) and broad bean (Vicia faba L.) Plant Physiol. 1992;99:415–421. doi: 10.1104/pp.99.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Feuerle R., Schäffer S., Fortmeier H., Schubert S. Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiol. 1998;117:311–319. doi: 10.1104/pp.117.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Di T., Xu G., Chen X., Zeng H., Yan F., Shen Q. Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ. 2009;32:1428–1440. doi: 10.1111/j.1365-3040.2009.02009.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.