Abstract
Bacteria initiate translation using a modified amino acid, N-formylmethionine (fMet), adapted specifically for this function. Most proteins are processed co-translationally by peptide deformylase (PDF) to remove this modification. Although PDF activity is essential in WT cells and is the target of the antibiotic actinonin, bypass mutations in the fmt gene that eliminate the formylation of Met-tRNAMet render PDF dispensable. The extent to which the emergence of fmt bypass mutations might compromise the therapeutic utility of actinonin is determined, in part, by the effects of these bypass mutations on fitness. Here, we characterize the phenotypic consequences of an fmt null mutation in the model organism Bacillus subtilis. An fmt null mutant is defective for several post-exponential phase adaptive programmes including antibiotic resistance, biofilm formation, swarming and swimming motility and sporulation. In addition, a survey of well-characterized stress responses reveals an increased sensitivity to metal ion excess and oxidative stress. These diverse phenotypes presumably reflect altered synthesis or stability of key proteins involved in these processes.
Keywords: translation, tRNA, peptide deformylase, formyl-Met (fMet), Bacillus subtilis
Introduction
The protein translation machinery of bacteria differs significantly from that of eukaryotes. These differences allow the selective inhibition of bacterial growth in a eukaryotic host and are the basis for many commonly used antibiotics [1]. While the overall structure of the ribosome is conserved across the three domains of life, the differences are nevertheless sufficient to allow some ribosome-targeting antibiotics to inhibit bacterial cells with relatively little if any effect on the host translational apparatus [2]. Other antibiotics have been developed to target distinctive features of the bacterial translation machinery such as the use of a formylated initiator tRNA to initiate translation [3].
In bacteria, translation initiates with a formylated methionyl tRNA (fMet-tRNAfMet) produced by methionyl-tRNA formyltransferase (FMT). The resulting fMet-tRNAfMet is loaded onto the 30S subunit of the ribosome bound to mRNA to form the 30S initiation complex [4]. Subsequent addition of the 50S (large) ribosome subunit and completion of loading of the fMet-tRNAfMet into the P site (with release of the initiation factors) primes the process of translation initiation. Translation leads to the production of a polypeptide bearing an N-terminal fMet residue, which is usually co-translationally processed by peptide deformylase (PDF) [5]. In many cases, depending in large part on the size of the second amino acid [6], the resulting N-terminal methionine is subsequently removed by methionine aminopeptidase (MAP). Both PDF and MAP are essential metalloenzymes, and PDF in particular has been considered an excellent target for the development of antibiotics [7–11]. The reasons why PDF and MAP are essential are not entirely clear, but the presumption is that a failure to remove the formyl group leads to a dysregulation of either protein function or stability and blocks MAP activity, which is required because some essential proteins require removal of the N-terminal Met residue in order to function [12].
We are interested in Bacillus subtilis as a model system for both antibiotic resistance and metal ion homeostasis. These interests have converged around the process of translation initiation since B. subtilis, somewhat unusually, has two functionally redundant PDF enzymes [13] and also encodes two MAP enzymes [14]. It is unclear why the cell retains these apparently redundant metalloenzymes, but one possibility is that they differ in their required metal cofactors and perhaps differ in their resistance to metal limitation or oxidative stress.
PDF is inhibited by the antibiotic actinonin [7], and it has been targeted for development of new antibacterials by multiple pharmaceutical companies [9, 15, 16]. One complication in targeting PDF with antibacterials is that resistance can arise. Although several mechanisms have been described, one of the most frequent is the emergence of so-called Fmt bypass mutations [16–19]. In these bypass strains, translation initiates using unformylated Met-tRNAMet and PDF is therefore no longer needed (Fig. 1). Bypass mutations in folD and glyA, which are required for synthesis of the FMT substrate 10-formyltetrahydrofolate, were also identified in B. subtilis [18]. A second complication relates to the presence of PDF in mitochondria, which initiates translation with fMet like bacteria. Indeed, impairment of this pathway due to mitochondrial mutations affecting FMT leads to a variety of human diseases associated with bioenergetic defects [20–22].
Fig. 1.
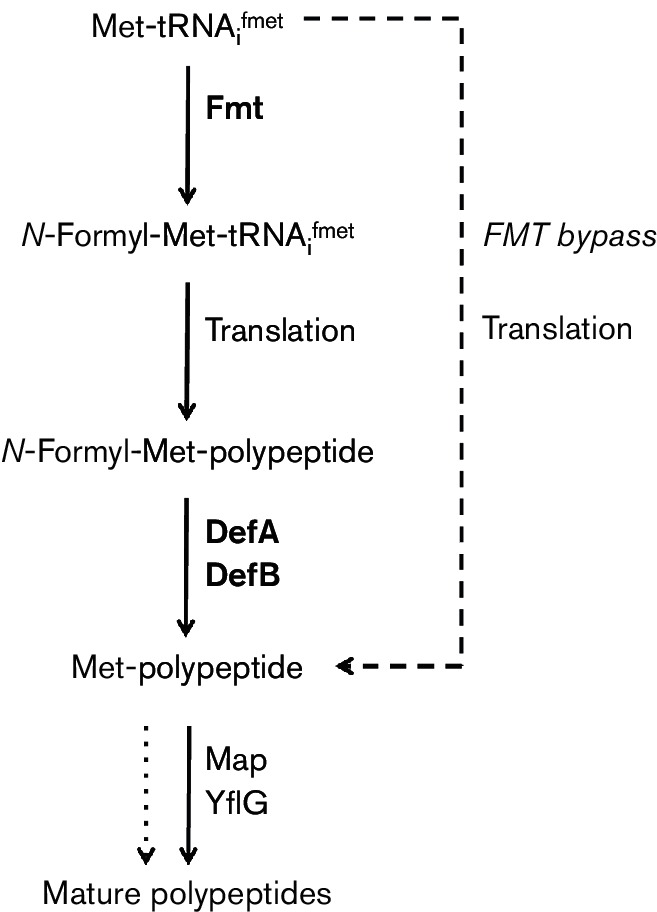
Schematic diagram illustrating the roles of FMT (methionyl formylation) and the two redundant PDF enzymes (polypeptide deformylation) during bacterial translation (DefA and DefB). The dashed line indicates the use of unmodified Met-tRNAfMet in initiation in the FMT bypass mutant strains. Note that some polypeptides are considered mature in the absence of removal of the initiating Met residue (dotted line).
Studies in Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and B. subtilis have provided initial insights into the effects of fmt mutations on bacterial physiology. In P. aeruginosa, cells lacking Fmt were reported to have a modest growth rate defect [23]. In contrast, an E. coli fmt null mutant was severely growth impaired [24]. In Staph. aureus, an fmt mutant had a significant fitness defect in vitro and reduced virulence as monitored in mouse models of infection [19, 25]. Suppressor mutations in the agrC gene restored better growth in vitro for reasons not entirely clear [26]. B. subtilis fmt null strains were also generated as part of a study of actinonin resistance. The fmt null cells were insensitive to actinonin and had a significant fitness defect, but a detailed phenotypic characterization was not reported [18].
Here, we present a survey of the phenotypic consequences arising from inactivation of fmt in B. subtilis. The fmt null strain is defective for several post-exponential phase adaptive programmes including biofilm formation, both swarming and swimming motility and sporulation. In addition, a survey of stress responses reveals an increased sensitivity to antibiotics, metal ion excess and oxidative stress. These diverse phenotypes presumably reflect altered synthesis or stability of key proteins involved in these processes.
Methods
Bacterial strains and growth conditions
B. subtilis strains are derivatives of strain CU1065 and undomesticated strain NCIB 3610 [27] and are shown in Table S1 (available in the online Supplementary Material). E. coli strain DH5α was used for standard cloning procedures. Bacteria were grown in LB medium; where indicated, LBC medium (LB medium amended with 1 g l−1 of citrate trisodium dihydrate; 3.4 mM) was used. Minimal medium contained 40 mM MOPS (pH 7.4), 2 mM potassium phosphate buffer (pH 7.0), glucose (2 %, w/v), (NH4)2SO4 (2 g l−1), MgSO4.7H2O (0.2 g l−1), potassium glutamate (1 g l−1), tryptophan (10 mg l−1) and 80 nM MnCl2. Ampicillin (100 µg ml−1) was used to select E. coli transformants. For B. subtilis, antibiotics used for selection were spectinomycin (100 µg ml−1), kanamycin (15 µg ml−1), chloramphenicol (10 µg ml−1), tetracycline (5 µg ml−1) and macrolide lincosoamide/streptogramin B (contains 1 µg ml−1 erythromycin and 25 µg ml−1 lincomycin). For iron intoxication experiments, 100 mM FeSO4 stocks were prepared in 0.1 N HCl, and iron was added to the indicated concentrations as described [28]. OD600 readings were taken on a Spectronic 21 spectrophotometer.
The defA :: erm and defB :: erm mutants were acquired from the Bacillus Knockout Erythromycin collection (B. M. Koo and C. A. Gross, University of California, San Francisco, unpublished results) maintained by the Bacillus Genetic Stock Center and transformed into CU1065 background. To convert the defA :: erm null mutant to an unmarked, in-frame ΔdefA mutant, the strain was transformed with pDR244 (Bacillus Genetic Stock Center) which encodes the Cre recombinase. The suppressed ΔdefA defB :: erm double mutant strains were constructed by transformation using standard techniques [29] and were confirmed by PCR to exclude strains that recovered an intact copy of defA by congression. PCR products were amplified for the fmt, folD and glyA genes and sequenced to identify possible suppressor mutations. An fmt :: kan null mutant was generated by replacing the coding region with a kanamycin resistance cassette using long flanking homology PCR followed by DNA transformation as previously described [30]. Primer pairs used for PCR amplification were 6891/6892 for fmt (Table S2). The fmt complemented strains were constructed by using vector pPL82 [31]. PCR products were amplified from B. subtilis CU1065 chromosomal DNA, digested with endonucleases and cloned into pPL82. pPL82 contains a chloramphenicol resistance cassette, a multiple cloning site downstream of the Pspac(hy) promoter and the lacI gene between the upstream and downstream fragments of the amyE gene. The sequences of the inserts were verified by DNA sequencing.
Measurement of stress sensitivity
Disc diffusion assays were performed as described [32]. Briefly, strains were grown to an OD600 of 0.4. A 100 µl aliquot of these cultures was mixed with 4 ml of 0.75 % LB soft agar (kept at 50 °C) and directly poured onto LB plates (containing 15 ml of 1.5 % LB agar). The plates were dried for 10 min in a laminar airflow hood. Filter paper (6.5 mm) discs containing the chemicals to be tested were placed on the top of the agar, and the plates were incubated at 37 °C overnight. The overall diameter of the inhibition zones was measured along two orthogonal lines. Plates were imaged using a Chemi DocTM MP Imaging System (Bio-Rad) with white transillumination. For metal intoxication, 10 µl of the following chemicals was added to the filter paper disc: 1 M FeSO4, 1 M FeCl3, 100 mM MnCl2, 100 mM ZnCl2, 100 mM CoCl2 and 100 mM NiCl2. For streptonigrin sensitivity tests, FeSO4 or FeCl3 was added to both the soft agar and the plates to a concentration of 100 µM, and 5 µl streptonigrin (5 mg ml−1) solution in DMSO was added to the filter paper discs. For sensitivity tests, antibiotics used in this study included cefuroxime (6 µg), ampicillin (20 µg), fosfomycin (250 µg), oxacillin (20 µg), vancomycin (50 µg), lysozyme (200 µg), d-cycloserine (1 mg), penicillin G (100 µg), lincomycin (250 µg), erythromycin (10 µg), spectinomycin (1 mg), tetracycline (50 µg) and methylglyoxal (MG, 50 µg). In addition, hydrogen peroxide (~2.4 µmol H2O2) and paraquat (2.5 µmol) were used to test the sensitivity to reactive oxygen species.
Actinonin sensitivity was measured by growth curve analysis in a Bioscreen microplate reader. Strains were grown to an OD600 of 0.4 in MH medium (Sigma-Aldrich). Two microlitre aliquots were inoculated in 200 µl MH medium containing 0, 0.5, 5, 10, 50 or 500 µg ml−1 actinonin in a Bioscreen 100-well microtitre plate. Growth was measured spectrophotometrically (OD600) every 15 min for 24 h using a Bioscreen C incubator (Growth Curves USA) at 37 °C with continuous shaking.
Growth measurements
To test metal dependence of growth, 10 µM FeCl3 or 5 µM MnCl2 was added to the minimal medium containing 40 mM MOPS (pH 7.4), 2 mm potassium phosphate buffer (pH 7.0), 20 g l−1 glucose, 2 g l−1 (NH4)2SO4, 0.2 g l−1 MgSO4.7H2O, 1 g l−1 potassium glutamate, 10 mg l−1 tryptophan and 80 nm MnCl2. To test the iron sensitivity of the mutant, strains were grown to an OD600 of 0.4 in LB medium. Two microlitre aliquots were inoculated to 200 µl LBC medium in a Bioscreen 100-well microtitre plate. Growth was measured spectrophotometrically (OD600) every 15 min for 48 h using a Bioscreen C incubator (Growth Curves USA) at 37 °C with continuous shaking.
Swarming and swimming motility assays
Swimming and swarming motility were monitored using standard assays [33, 34]. LB plates containing 0.7 and 0.3 % agar were dried in a laminar flow hood for 30 min and then spotted in the centre with 5 µl LB precultures (OD600 ~0.4). The plates were then dried for another 15 min and incubated overnight at 37 °C.
Biofilm and pellicle formation
To monitor pellicle formation [35], 50 µl LB preculture (OD600 ~0.4) was inoculated into 10 ml minimal MSgg medium [5 mM potassium phosphate (pH 7), 100 mM morpholinepropanesulfonic acid (pH 7), 2 mM MgCl2, 700 µM CaCl2, 50 µM MnCl2, 50 µM FeCl3, 1 µM ZnCl2, 2 µM thiamine, 0.5 % glycerol (v/v), 0.5 % glutamate, 50 µg ml−1 tryptophan, 50 µg ml−1 phenylalanine and 50 µg ml−1 threonine] and incubated at 22 °C [35]. For colony architecture analysis, 5 µl of LB precultures (OD600 ~0.4) was spotted onto minimal MSgg agar plates (dried for 30 min in a laminar airflow prior to spotting) and incubated at 30 °C.
Monitoring sporulation progress and efficiency
For imaging sporulation, starvation was induced by resuspension [36]. Microscopy was performed using an Olympus BX61 epifluorescence microscope. Images were acquired using Cooke SensiCam and Slidebook software (Intelligent Imaging). For the sporulation assay, 0.5 ml of sporulating cells was pelleted and resuspended in 0.11 ml of the original culture medium; 2 µl of this cell suspension was placed on a slide and mixed with 1 µl of a stain mix containing 5 µg ml−1 FM 4-64 and 30 µg ml−1 MitoTracker Green FM (Molecular Probes) diluted in sporulation salts [37]. For quantitative sporulation assays, cells were grown after resuspension for 24 h with shaking at 37 °C. For spore measurements, 500 µl of cell culture was added to a new tube and heated 30 min at 80 °C and 3 µl aliquots from serial 10-fold dilutions were spotted on LB plates. The plates were incubated at 37 °C for overnight (WT) or 48 h (fmt mutant) to monitor sporulation efficiency.
Results
Isolation and characterization of fmt null mutants
In ongoing work, we have sought to understand the rationale behind the presence of two, apparently redundant, PDFs in B. subtilis. PDF activity is required to remove the formyl group introduced into proteins by FMT (Fig. 1). The presence of such paralogous pairs of metalloenzymes is a common theme in bacterial physiology and is often associated with utilization of distinct metal cofactors [38]. In the course of these studies, we used genomic transformation to generate a defA defB double mutant strain (ΔdefA defB :: erm). After excluding those transformants that had recovered an intact copy of defA by congression, a small number of double mutants were recovered. We reasoned that this low frequency was likely indicative of a requirement for a suppressor mutation to allow growth in the absence of PDF. Since prior work had indicated that inactivation of the fmt, folD and glyA genes can lead to actinonin resistance in B. subtilis [18], these three loci were sequenced in three isolates. One strain contained a frameshift mutation in fmt (fmt1), one had multiple base changes in glyA and the third appeared to be WT at all three loci and may therefore contain a mutation in a different locus (Table S3).
We quickly appreciated that the ΔdefA defB :: erm fmt1 strain is pleiotropic (Figs S1–S3). For example, this strain grows more slowly than WT and is more sensitive than WT to H2O2, paraquat and iron intoxication. Since PDF is not required in the absence of Fmt activity, we reasoned that these phenotypes likely arise from the fmt1 mutation and are probably unrelated to the loss of PDF. To date, there has only been one prior study of a B. subtilis fmt null mutant (a large in-frame deletion) recovered in a selection for actinonin resistance [18]. The mutant had a doubling time roughly twice as long as WT in MH medium and an even greater impairment in minimal media. Some changes were also noted at both the transcriptome and proteome levels, but the details were not reported. These observations all suggest that cells lacking Fmt activity are viable, but growth impaired. However, since this strain arose as a suppressor, and the whole-genome sequence was not determined (sequencing was limited to four candidate loci), we cannot exclude the possibility that some or all of these effects may have resulted from changes at other loci. Moreover, no complementation studies were performed in this prior study.
To further explore the physiological consequences of inactivating Fmt, we generated an fmt :: kan null mutant (designed fmt) in an otherwise WT background using allelic replacement. Next, we complemented this mutation by expression of fmt from a plasmid integrated at the amyE locus (fmt Pspac(hy)-fmt). We then used this mutant strain together with its isogenic complemented strain for further physiological characterization.
An fmt null mutant is sensitive to H2O2, paraquat and methylglyoxal
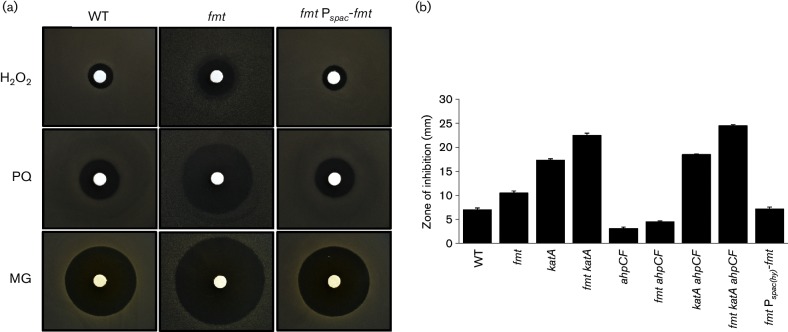
The fmt null mutant has a significantly increased zone of growth inhibition when exposed to H2O2 or to paraquat, a redox-cycling dye that generates reactive oxygen species. Importantly, these phenotypes are complemented by ectopic expression of fmt from the Pspac(hy) promoter (Fig. 2a). We hypothesized that alterations in the process of translation initiation in the fmt strain might have reduced production of the major vegetative catalase (KatA), known to be both highly abundant and a primary H2O2 resistance determinant [39]. However, epistasis studies suggest that the effects of Fmt and KatA on H2O2 resistance are additive, and therefore, the effect of Fmt cannot be due simply to decreased catalase activity (Fig. 2b). Next, we tested the hypothesis that the fmt mutation might affect expression of alkylhydroperoxide reductase, which is also likely to play a role in the detoxification of H2O2 [40]. As reported previously [41], an ahpC null mutant was more resistant than WT to H2O2, and this was shown previously to result from elevated expression of the PerR regulon including catalase. This effect of an ahpC mutation was still seen in the fmt strain (Fig. 2b), suggesting that this strain can produce elevated levels of catalase even in the absence of Fmt. Finally, we note that the highly H2O2-sensitive katA ahpC fmt triple mutant is more sensitive than the katA ahpC double mutant, indicating that loss of Fmt affects peroxide sensitivity independently of both KatA and AhpC (Fig. 2b). Collectively, these results demonstrate that the fmt mutant is sensitive to H2O2, this defect can be complemented and it is not due to an inability of the cell to synthesize protective levels of catalase.
Fig. 2.
An fmt mutant has elevated sensitivity to hydrogen peroxide, paraquat and MG stress. (a) Representative photograph (from at least six replicates) of disc diffusion assays with WT (CU1065), an fmt mutant (HB21006) and an fmt Pspac(hy)-fmt complemented strain (HB21016) on LB plates. The discs were spotted with hydrogen peroxide (H2O2, 2.4 µmol), paraquat (PQ, 2.5 µmol) or MG (27.5 µmol) as indicated. (b) Sensitivity of WT and various mutant strains to hydrogen peroxide. The results are expressed as the diameter of the inhibition zone (mm) minus the diameter of the filter paper disc (6.5 mm). The mean±se from at least three biological replicates is reported.
The fmt null strain also displays a dramatically increased sensitivity to the endogenously produced reactive electrophile MG (Fig. 2a). This phenotype is also complemented by the ectopic expression of fmt. Previous results have defined the major resistance pathways for MG which include both bacillithiol-dependent and bacillithiol-independent detoxification enzymes [42]. We used epistasis analysis to determine whether the increased MG sensitivity could be linked to decreased expression of a specific MG resistance determinant. However, introduction of an fmt null mutation further increased the MG sensitivity of every tested strain, including strains lacking multiple resistance pathways (Fig. S4). We therefore conclude that the sensitivity to MG in the fmt null strain is likely to be multifactorial and/or is independent of altered expression of known resistance pathways.
An fmt null mutant has altered metal ion homeostasis
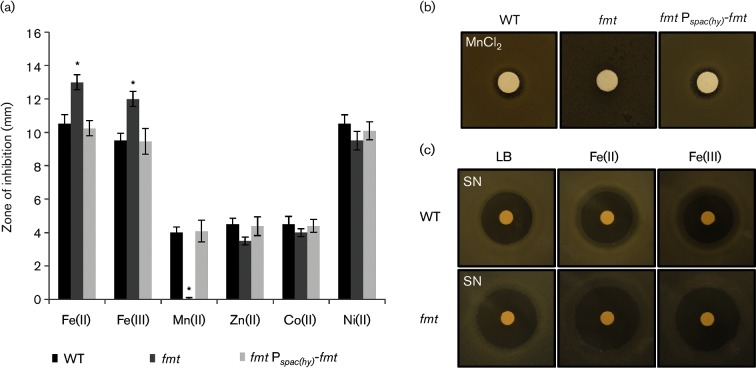
The major molecular mechanisms of H2O2 toxicity result from cytosolic Fenton chemistry catalysed by Fe(II) [43]. As a result, mutations that elevate intracellular Fe levels may result in an increased sensitivity to H2O2 [28, 44]. To determine if the fmt mutation leads to alterations in metal ion homeostasis, we tested metal ion sensitivity using a disc diffusion assay. The fmt mutant displayed a significantly increased sensitivity to both Fe(II) and Fe(III), which can be complemented by expression of fmt from the Pspac(hy) promoter (Fig. 3a). In contrast with Fe, there was no statistically significant change for the fmt null strain in sensitivity to Zn(II), Co(II) or Ni(II). However, the fmt null strain was dramatically increased in resistance to Mn(II) and this phenotype was reversed upon ectopic expression of fmt (Fig. 3b).
Fig. 3.
An fmt mutant is sensitive to iron and has an increased resistance to manganese. (a) The sensitivity of WT (CU1065, black bars), an isogenic fmt :: kan mutant (HB21006, dark grey bars) and an fmt Pspac(hy)-fmt complemented strain (HB21016, light grey bars) to metal ion stress as monitored using a disc diffusion assay. The results are expressed as the diameter of the inhibition zone (mm) minus the diameter of the filter paper disc (6.5 mm). The mean±se from at least three biological replicates is reported. Significant differences from WT and fmt mutant as determined by two-tailed t-test are indicated: *P<0.01. The discs were spotted with 10 µl of 1 M FeSO4, 1M FeCl3, 100 mM MnCl2, 100 mM ZnCl2, 100 mM CoCl2 and 100 mM NiCl2 separately. (b) An fmt mutant is more resistant to Mn(II) intoxication than WT. Representative photographs (from three replicates) of a disc diffusion assay with WT, fmt and fmt Pspac(hy)-fmt strains on LB plates containing a disc spotted with 10 µl of 100 mM MnCl2. (c) An fmt null mutant displays increased sensitivity to streptonigrin (SN). Representative photographs (from three replicates) of a disc diffusion assay with WT, fmt and fmt Pspac(hy)-fmt strains on LB plates containing either no supplement, 100 µM FeSO4 or FeCl3. Each disc was spotted with 5 µl of 5 mg ml−1 (25 µg) SN.
The molecular basis for these alterations in metal ion homeostasis in the fmt null mutant is not yet clear. However, iron toxicity in efflux defective cells is strongly mitigated by Mn(II) [28], and Mn(II) toxicity in efflux defective E. coli cells has been linked to a dysregulation of iron homeostasis [45]. Therefore, we speculate that the Mn(II) resistance noted here is due to elevated intracellular Fe(II) levels. To determine if the fmt mutation leads to an elevation of intracellular Fe pools, we monitored sensitivity to streptonigrin, a quinone antibiotic whose activity is correlated with intracellular iron availability [46]. Under all conditions tested, the fmt null strain was more sensitive to streptonigrin, consistent with a defect in iron homeostasis and an elevation of intracellular Fe pools (Fig. 3c). The reason why iron levels are elevated is presently unclear, but it has previously been shown that a null mutation in pfeT, encoding an Fe(II) efflux ATPase, leads to a similar phenotype [28]. It is therefore possible that synthesis or stability of PfeT is compromised in the fmt null mutant strain.
An fmt null mutant is sensitive to citrate intoxication
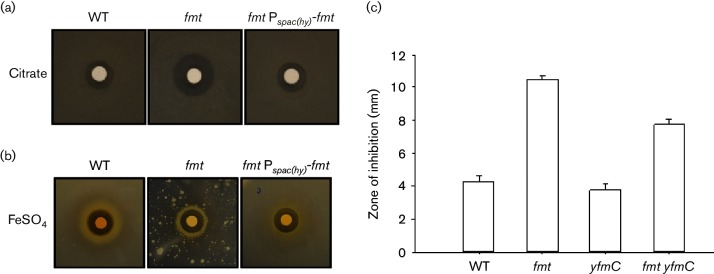
While investigating iron sensitivity, we tried to grow the fmt strain using LB medium amended with 1 g l−1 of citrate to increase iron solubility (as reported previously [28]) but discovered, unexpectedly, that this strain is very sensitive to growth inhibition by citrate (Fig. 4a). This citrate sensitivity phenotype is also apparent as a greatly reduced plating efficiency on citrate-containing medium (LBC plates [28]) relative to WT in studies to test Fe(II) sensitivity, and this could be reversed by ectopic expression of fmt (Fig. 4b). Sensitivity to carboxylic acids was specific for citrate when monitored by disc diffusion assay (Fig. 4c) and was not observed with fumarate or succinate (data not shown). It had been noted previously that an fmt mutant strain was less able to grow using carboxylic acids as carbon sources, but the growth inhibition reported here is specific to citrate and is observed on rich medium, suggesting that this is not due to a defect in citrate catabolism.
Fig. 4.
An fmt null mutant is sensitive to citrate. (a) Representative photograph (from at least six replicates) of a disc diffusion assay with WT (CU1065), fmt mutant (HB21006) and fmt Pspac(hy)-fmt (HB21016) strains on LB plates. The discs were spotted with 10 µl of 1 M trisodium citrate (Na3C6H5O7) as indicated. (b) Growth of WT, fmt mutant and fmt Pspac(hy)-fmt (HB21016) strains on LBC plates (LB supplemented with 1g l−1 of sodium citrate) at 37 °C for 36 h. In this experiment, the discs were spotted with 10 µl of 1 M FeSO4. Note that the fmt mutant had a severe growth defect in this medium and could not form a lawn. Note also that there is better growth near the Fe-containing filter, suggesting that citrate may impose an Fe limitation. (c) Quantitation of sensitivity of WT and mutant strains to citrate using disc diffusion assay. The results are expressed as the diameter of the inhibition zone (mm) minus the diameter of the filter paper disc (6.5 mm).
Since citrate is known to be a good chelator of iron and other metals, we hypothesized that citrate sensitivity might be related to altered iron homeostasis. Citrate could act by altering metal availability in the medium or by being imported into the cell where high levels of citrate could perturb metal homeostasis. To determine if citrate toxicity required import into the cell, we tested the effect of various mutations known to affect metal homeostasis. The only mutation that reduced citrate sensitivity was yfmC, which inactivates an operon encoding an importer specific for iron citrate [47]. This uptake system is Fur regulated and normally expressed under iron limitation. This suggests that citrate toxicity occurs, at least in part, intracellularly. Toxicity was not fully suppressed, but citrate can also be imported through alternative citrate uptake systems including CitM and CitH [48].
An fmt null mutant is altered in sensitivity to antibiotics
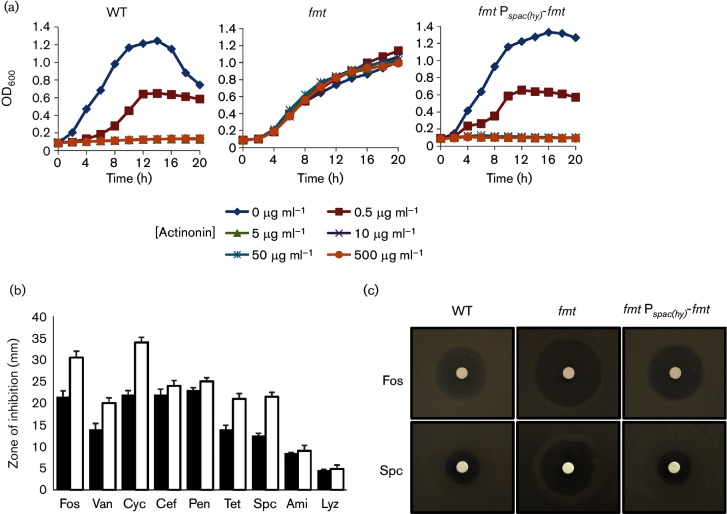
We next examined the effects of the fmt mutation on susceptibility to antibiotics. As previously reported [18], the fmt mutant is markedly more resistant to the PDF inhibitor actinonin (Fig. 5a), since PDF is dispensable in the absence of Fmt (Fig. 1). As expected, actinonin sensitivity was restored by ectopic expression of fmt. Furthermore, the fmt mutant exhibited significantly increased sensitivity to the peptidoglycan synthesis inhibitors fosfomycin, d-cycloserine and vancomycin, but not to the β-lactam cefuroxime or penicillin (Fig. 5b). The fmt mutant was also increased in sensitivity to the translation inhibitors tetracycline and spectinomycin (Fig. 5b), as well as lincomycin and erythromycin (data not shown). As shown for fosfomycin and spectinomycin, the antibiotic sensitivity could be complemented by ectopic expression of fmt (Fig. 5c). The genetic determinants of fosfomycin resistance have been previously studied in B. subtilis and the major resistance determinant is the FosB bacillithiol S-transferase, which inactivates fosfomycin by covalent modification [49–51]. We therefore hypothesized that, perhaps, the fmt mutation led to a significant decrease in expression of either FosB or the enzymes required for the synthesis of the required bacillithiol cofactor [50]. However, epistasis studies did not support this hypothesis and indicate that other factors are involved (Fig. S5). The basis for these phenotypic differences awaits further investigation.
Fig. 5.
An fmt mutant displays altered antibiotic sensitivity. (a) Sensitivity of WT, isogenic fmt :: kan mutant (HB21006) and the fmt :: kan Pspac-fmt (HB21016) strains to actinonin as measured by growth curve assays. Actinonin concentrations used are noted below the graph. The data shown are representative of at least three independent experiments. (b) Sensitivity of WT (black bars) and an isogenic fmt :: kan mutant (HB21006, white bars) to antibiotic as monitored using a disc diffusion assay on LB plates. Antibiotics and cell envelope active agents used were fosfomycin (Fos, 250 µg), vancomycin (Van, 50 µg), d-cycloserine (Cyc, 1 mg), cefuroxime (Cef, 6 µg), penicillin G (Pen, 100 µg), tetracycline (Tet, 50 µg), spectinomycin (Spc, 0.5 mg), amitriptyline (Ami, 250 µg) and lysozyme (Lyz, 200 µg), The mean±se from at least three biological replicates is reported. (c) Representative photographs (from at least six replicates) of a disc diffusion assay with WT or an fmt mutant (HB21006) and an fmt Pspac(hy)-fmt complemented strain (HB21016) cell on LB plates. The discs were spotted with fosfomycin (250 µg) or spectinomycin (0.5 mg) as indicated.
Fmt is required for swarming motility
From the analyses above, it is clear that the fmt mutant is pleiotropic and has affected cellular physiology and growth in diverse ways. Next, we turned our attention to the ability of the fmt mutant to engage in the various complex post-exponential responses that have been well characterized in B. subtilis. These include the activation of motility (both swimming and swarming), formation of complex structured biofilms and formation of endospores [52].
Bacteria can exhibit swimming motility in liquid media or swarming motility on surfaces. Swimming requires flagellar rotation, whereas swarming requires both flagella and the ability to form multicellular rafts [53]. To test whether Fmt is involved in swimming and swarming motility, we inoculated Petri plates containing 0.3 and 0.7 % agar (‘swim plates’ and ‘swarm plates’, respectively) [33]. For this assay, we used the undomesticated NCIB 3610 background, since the standard 168 laboratory strain does not exhibit significant motility on solid surfaces [54]. After 18 h, the WT 3610 was able to completely colonize the surface of the 0.7 % agar swarm plate, whereas the growth of the fmt mutant was restricted to the centre of the plate (Fig. 6), suggesting a loss of swarming motility, which requires both flagellar-based motility and an ability of cells to bundle and form motile rafts. The fmt mutant clearly retains flagellar-based motility as seen by light microscopy (data not shown), suggesting that the defect may be in other factors required for swarming motility. On 0.3 % agar (‘swim plates’), NCIB 3610 expands rapidly and completely through the agar within 18 h. In contrast, the fmt mutant exhibits complex pattern formation with branching dendrites, a very unusual phenotype for a ‘swim plate’ assay (Fig. 6). Both the swarming and swimming defects of the fmt mutant can be complemented by expression of fmt from an ectopic locus.
Fig. 6.
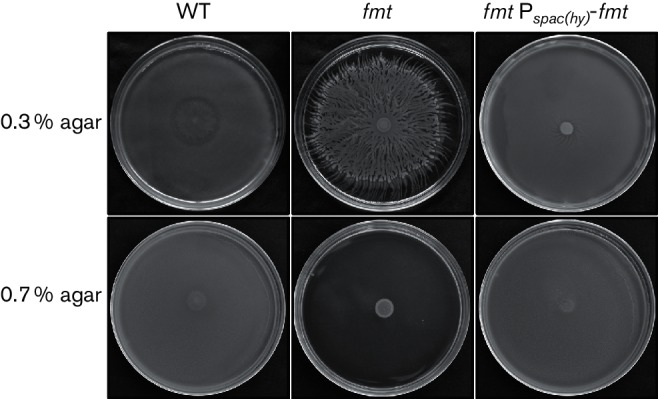
An fmt mutant displays a swimming defect and a loss of swarming motility. Representative photographs of the NCIB 3610 WT (NCIB 3610), an isogenic fmt mutant (HB21009) and an fmt Pspac(hy)-fmt complemented strain (HB21017) inoculated on LB plates. A 5 µl culture from the mid logarithmic growth stage (OD600 ~0.4) was spotted on LB plates containing 0.3 or 0.7 % agar and incubated at 37 °C for 18 h.
Fmt is necessary for formation of complex structured biofilms
Bacteria often exist in the environment as cell aggregates called biofilms and B. subtilis is a model organism to study biofilm formation [55, 56]. To assess the contribution of Fmt to this multicellular process, we monitored colony morphology on MSgg plates and pellicle formation in MSgg liquid medium. These studies were also conducted using NCIB 3610, as it forms more robust biofilms and complex colony morphology in comparison to the standard 168 laboratory strain [35]. As expected, the WT NCIB 3610 strain forms complex colony patterns on MSgg plates and robust, wrinkled pellicles on MSgg liquid medium after 2 days. In contrast, the fmt mutant displays reduced biofilm architecture and an unstructured pellicle in comparison to the WT strain, even after 9 days (Fig. 7).
Fig. 7.
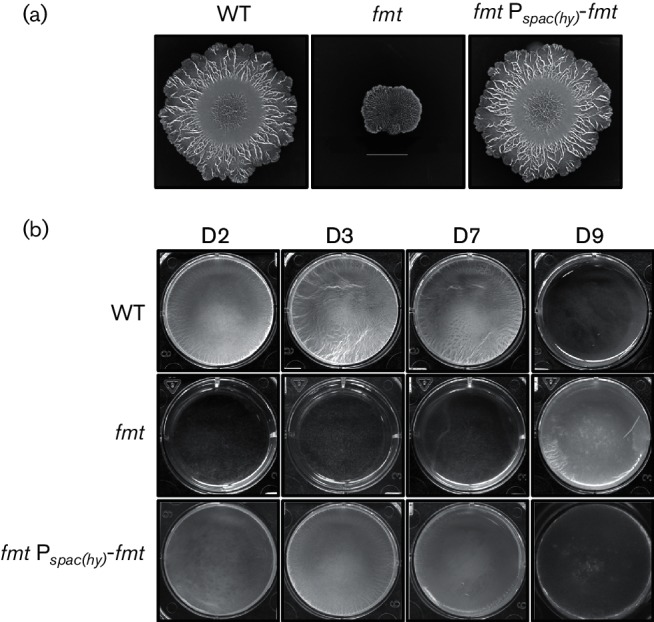
An fmt mutant is defective in biofilm architecture and pellicle formation. (a) Representative images showing colony architecture morphology after 8 days of growth at 30 °C on MSgg plates. The WT (NCIB 3610), fmt (HB21009) and fmt Pspac(hy)-fmt (HB21017) strains are shown (scale bar=1 cm). (b) The pellicle formation monitored by growth at 22 °C in MSgg liquid medium at 2, 3, 7 and 9 days. The WT (NCIB 3610), fmt (HB21009) and fmt Pspac(hy)-fmt (HB21017) strains are shown.
Fmt is necessary for efficient sporulation
Spore formation is a highly regulated, complex developmental process, which allows B. subtilis to survive many environmental insults [57]. To test whether Fmt is involved in sporulation, the sporulation efficiency of WT and an isogenic fmt mutant was compared. After resuspension in sporulation-inducing medium [36] and incubation for 24 h (37 °C with shaking), non-sporulating cells were heat killed by incubation at 80 °C for 30 min. The surviving spores were enumerated after serial dilution on LB plates incubated at 37 °C (Fig. 8a). After 24 h of incubation, WT cells had a sporulation efficiency of 85±0.2 %, compared to a sporulation efficiency of 4.1±0.8 % for the fmt mutant, suggesting that the fmt mutant is oligosporogenous.
Fig. 8.
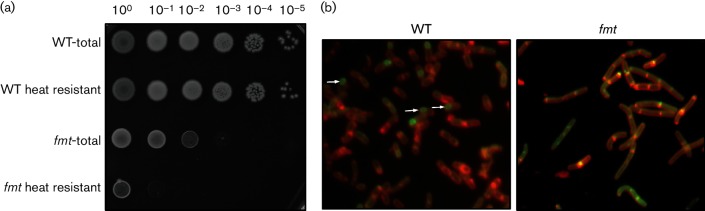
The fmt mutant is oligosporogenous and is defective in membrane fusion. (a) The selected strains were induced to sporulate using a resuspension protocol. Efficiency of spore formation for WT (CU1065) and an isogenic fmt mutant (HB21006) was monitored by plating 3 µl of 10-fold cell dilutions (left to right; 100 to 10−5) with and without treatment at 80 °C for 30 min to inactivate vegetative cells and was then incubated at 37 °C for 24 h. The photograph is representative of three biological replicates. Additional growth is seen on the fmt plate after 48 h, but there is still a nearly 100-fold reduction in colonies after heating as before, consistent with a low frequency of successful sporulation. (b) Sporulation progress for the WT and fmt mutant as monitored 4 h after resuspension using a membrane fusion assay with staining with FM 4-64 and MitoTracker Green [37]. The arrows (left panel) indicate cells that have completed engulfment and membrane fusion, as indicated by the green staining. In the right panel, cells stain with both dyes, indicative of a block at the membrane fusion step (similar results were seen after 24 h, with cells that had engulfed the forespore but had been blocked in membrane fusion).
To identify the role of Fmt mutant in spore maturation, we monitored spore development by microscopy 4 and 24 h after initiation of sporulation by resuspension in sporulation medium. B. subtilis sporulation has seven stages (Stages I–VII) [58]. An early step in the B. subtilis sporulation pathway, asymmetric cell division, creates the small forespore and larger mother cell (Stage II). During the next stage of sporulation, engulfment (Stage III), the mother cell membranes move up and around the forespore. Completion of engulfment corresponds with membrane fusion resulting in the release of the forespore into the mother cell cytoplasm. After engulfment (Stages IV and V), the spore completes development within the mother cell and is released by mother cell lysis (Stages VI and VII). Cells were visualized by staining with FM 4-64 and MitoTracker Green FM [37]. Since FM 4-64 binds the cell membrane but is nonpermeable, both the membrane of the mother cell and forespore are accessible to FM 4-64 during engulfment. After engulfment is complete, only the mother cell membrane stains with FM 4-64 and the membrane-permeable MitoTracker Green FM selectively stains green the fully engulfed forespore after membrane fusion (Fig. 8b, arrows). We monitored sporulation of over 1000 cells at 4 and 24 h following the onset of sporulation. In WT, membrane fusion and completion of engulfment was evident after 4 h of sporulation (Table 1, Fig. 8) and the majority of the cells (84 %) had completed sporulation after 24 h. In contrast, the fmt mutant failed to complete membrane fusion and engulfment after 4 h. Furthermore, after 24 h, 87 % of the fmt mutant cells had initiated membrane migration, yet only a small fraction (6 %) were able to complete engulfment, indicating that an fmt mutant is defective, rather than delayed, for sporulation. In total, these data suggest that, in the absence of Fmt, the final stage of engulfment (membrane fusion) is inefficient.
Table 1. Sporulation phenotypes of WT versus fmt mutant.
Scoring of different sporulation phenotypes (number of cells) of WT and fmt at 4 and 24 h after initiation of sporulation by resupension. Membrane migration associating with engulfment of the forespore to generate sporangia (a cell in the process of sporulation) was monitored by membrane staining. Polar septa (%) indicates the percentage of polar septa relative to total cells.
| Phenotype | No. of cells | |||
|---|---|---|---|---|
| WT 4 h | fmt 4 h | WT 24 h | fmt 24 h | |
| Flat polar septa | 177 | 240 | 0 | 89 |
| Engulfing | 400 | 76 | 0 | 0 |
| Membrane migration complete | 181 | 186 | 163 | 1078 |
| Engulfment complete | 89 | 0 | 857 | 76 |
| Not sporulating | 228 | 638 | 0 | 0 |
| Total sporangia | 847 | 502 | 1020 | 1243 |
| Total cells | 1075 | 1140 | 1020 | 1243 |
| Polar septa (%) | 12 | 16 | 0 | 7 |
| Engulfing (%) | 37 | 7 | 0 | 0 |
| Complete migration (%) | 17 | 16 | 16 | 87 |
| Complete engulfment (%) | 8 | 0 | 84 | 6 |
Discussion
Roles of PDF and FMT in bacterial translation
Translation in bacteria differs from that of the eukaryal cytosol in several key features [4]. Of particular relevance to this study, initiation begins with a specialized tRNA, the fMet-tRNAfMet. This initiator tRNA is first aminoacylated by the methionyl-tRNA synthetase and then the methionyl residue is formylated by FMT. The majority of polypeptides are subsequently deformylated by PDF in a co-translational process. Inhibition of PDF leads to cell death [7], but suppressors often emerge that have inactivated FMT (FMT bypass; Fig. 1) [19]. These findings suggest that cells are viable, albeit growth impaired, if forced to initiate translation with Met-tRNAfMet (no methionyl formylation), but are inviable if proteins are synthesized with an N-terminal fMet residue that cannot be deformylated.
Use of a formylated initiator tRNA is thought to confer substantial benefits on the efficiency of the translation initiation reaction. Specifically, formylation increases the interaction of fMet-tRNAfMet with initiation factor 2 while blocking recognition by EF-Tu, and it facilitates loading into the ribosomal P site [4]. Protein N-terminal formylation may also play a regulatory role by serving as a signal for protein degradation [12]. Finally, it has been suggested that the use of fMet for initiation may help to coordinate translation activity with the overall metabolic status of the cell as reflected in the activity of the methionyl biosynthesis enzymes and the status of the tetrahydrofolate pool [4]. In the case of mitochondria, where the endogenously synthesized proteome is comparatively limited, formylmethionine has been shown to be required for the efficient incorporation of COX1 into respiratory complexes [20]. An analogous critical role for formylation has not been demonstrated for bacterial proteins, and formylation cannot be essential as evidenced by the viability of FMT bypass mutations.
Since PDF has been aggressively pursued as a target for new antibacterials (e.g. LBM415 from Novartis and GSK1322322 from Glaxo SmithKline), possible bypass mutations, and their implications for antibiotic efficacy, have been well studied in pathogenic organisms. In both Staph. aureus and Streptococcus pyogenes, fmt null mutations were the most frequent causes of resistance to PDF inhibitors [15, 19]. However, emergence of suppressor strains lacking fmt activity might not affect antibiotic efficacy if the resulting strains have a significant fitness defect or are compromised in virulence. Another major mechanism of resistance is either amplication of the def gene encoding PDF, as seen in Haemophilus influenzae [16], or the emergence of resistant mutations in def, as noted in Staph. aureus and Streptococcus pneumoniae [15, 59].
Diverse effects of fmt inactivation in bacteria
The consequences of inactivating fmt appear to vary significantly between strains. E. coli fmt mutants were reported to be severely compromised for growth [24], whereas P. aeruginosa had a more modest defect [23]. Staph. aureus fmt mutants were also growth defective [19] and, intriguingly, severely affected in the expression of pathogenicity determinants [25]. The origins of these effects are largely unexplained but presumably result from altered translation initiation leading to reduced expression of specific proteins, although why some proteins might be less able to initiate using Met instead of fMet is not understood.
As might be expected for mutations leading to a growth defect, fmt null strains frequently generate suppressed strains that have increased fitness. In Salmonella enterica, suppression was mediated in some strains by an amplification of the genes encoding the methionyl-initiator tRNA [60]. This increase in gene dosage was postulated to help facilitate translation initiation using unmodified Met-tRNAMet in place of the fMet-tRNAMet that is normally used. Alternatively, mutations can arise in initiation factor 2 that increase the ability of translation to initiate using unmodified tRNA [61]. Mutations that increased fitness of Staph. aureus fmt null mutants were identified in agrC encoding an accessory gene regulator involved in quorum sensing and virulence [26]. In P. aeruginosa, mutation of fmt led to upregulation of the MexXY efflux pump [17]. These observations all suggest that cells lacking Fmt activity are viable, but growth impaired due to defects in translation activity and possible effects on protein stability. In many of these studies, the analysed fmt mutations arose as suppressors that give rise to resistance to PDF inhibitors. In general, it is unclear whether or not the fmt mutation was the only mutation in the resulting strains, and with one exception [62], complementation studies were not reported.
Effects of an fmt null mutation on B. subtilis physiology
We have sought to define the physiological consequences of a lack of methionyl formylation in B. subtilis. The effect of an fmt mutation in an actinonin-resistant B. subtilis isolate was addressed in a previous report, which noted general growth defects in a variety of media and effects on the transcriptome, but did not unambiguously link these phenotypes to the loss of FMT [18]. In principle, transcriptomics is a potentially useful approach since the appearance of specific stress signatures might provide clues to the consequences of translational dysregulation in cells obligately initiating translation with unmodified Met. However, the reported results were interpreted as indicating no significant transcriptome differences (as stated in the Abstract of [18]) with, paradoxically, more than 300 genes altered by more than twofold. However, no details were included as to the precise identity of these genes [18]. One can also imagine using a global proteomics approach to monitor the effects of an fmt mutation, as reported in a study of an Staph. aureus fmt bypass strain [63]. However, this approach is also limited since there are changes in the absolute levels of numerous proteins, and it is not obvious how these changes can be linked to physiologically significant variations.
Here, we chose instead to survey a variety of growth and stress-related phenotypes, including post-exponential phase adaptive processes such as motility, biofilm formation and sporulation. By focusing on well-studied but complex processes, we reasoned that it might be possible to link defects due to a loss of FMT activity to altered translation of specific protein targets and thereby provide insights into why FMT is more important for some processes (and for some organisms) than for others. Our results clearly indicate that cells lacking FMT are able to sustain growth but are defective in their resistance to stress and in their ability to engage in a variety of adaptive processes. While we have sought to link these phenotypes to specific proteins, our epistasis results to date imply that the effects are, perhaps not surprisingly, multifactorial. Nevertheless, we suggest that further investigation of these processes may yet reveal well-defined and specific examples of proteins whose function is specifically impaired in the absence of the normal fMet-dependent initiation process. The effects of the of the fmt mutation may be largely due to differences in the relative amounts of specific protein products, rather than due to an absence of any specific product. The ability of B. subtilis to grow in the face of a globally altered proteomic landscape is a testament to the robust nature of bacterial metabolism. However, the dramatic impairment noted in motility, biofilm formation and sporulation suggests that these processes are sensitive to the presumably altered levels and ratios of their many participant proteins.
Funding information
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers GM047446 and GM059323 (J. D. H) and by a grant from the National Natural Science Foundation of China (41471214) to Y. C.
Acknowledgements
We thank H. Pi for technical advice and Dr E. Angert (Cornell University) for sharing her microscope and for detailed advice and guidance on analysing sporulation-related phenotypes.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: FMT, methionyl-tRNA formyltransferase; MAP, methionine aminopeptidase; MG, methylglyoxal; PDF, peptide deformylase.
Five supplementary figures and three supplementary tables are available with the online Supplementary Material.
Edited by: J. Stulke and T. Msadek
References
- 1.Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol. 2014;12:35–48. doi: 10.1038/nrmicro3155. [DOI] [PubMed] [Google Scholar]
- 2.Arenz S, Wilson DN. blast from the past: reassessing forgotten translation inhibitors, antibiotic selectivity, and resistance mechanisms to aid drug development. Mol Cell. 2016;61:3–14. doi: 10.1016/j.molcel.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Brandi L, Fabbretti A, La Teana A, Abbondi M, Losi D, et al. Specific, efficient, and selective inhibition of prokaryotic translation initiation by a novel peptide antibiotic. Proc Natl Acad Sci USA. 2006;103:39–44. doi: 10.1073/pnas.0507740102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gualerzi CO, Pon CL. Initiation of mRNA translation in bacteria: structural and dynamic aspects. Cell Mol Life Sci. 2015;72:4341–4367. doi: 10.1007/s00018-015-2010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandikci A, Gloge F, Martinez M, Mayer MP, Wade R, et al. Dynamic enzyme docking to the ribosome coordinates N-terminal processing with polypeptide folding. Nat Struct Mol Biol. 2013;20:843–850. doi: 10.1038/nsmb.2615. [DOI] [PubMed] [Google Scholar]
- 6.Hirel PH, Schmitter MJ, Dessen P, Fayat G, Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci USA. 1989;86:8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DZ, Patel DV, Hackbarth CJ, Wang W, Dreyer G, et al. Actinonin, a naturally occurring antibacterial agent, is a potent deformylase inhibitor. Biochemistry. 2000;39:1256–1262. doi: 10.1021/bi992245y. [DOI] [PubMed] [Google Scholar]
- 8.Olaleye OA, Bishai WR, Liu JO. Targeting the role of N-terminal methionine processing enzymes in Mycobacterium tuberculosis. Tuberculosis. 2009;89:S55–S59. doi: 10.1016/S1472-9792(09)70013-7. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, Khuller GK, Sharma S. Peptide deformylase – a promising therapeutic target for tuberculosis and antibacterial drug discovery. Expert Opin Ther Targets. 2009;13:753–765. doi: 10.1517/14728220903005590. [DOI] [PubMed] [Google Scholar]
- 10.Helgren TR, Wangtrakuldee P, Staker BL, Hagen TJ. Advances in bacterial methionine aminopeptidase inhibition. Curr Top Med Chem. 2016;16:397–414. doi: 10.2174/1568026615666150813145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leeds JA, Dean CR. Peptide deformylase as an antibacterial target: a critical assessment. Curr Opin Pharmacol. 2006;6:445–452. doi: 10.1016/j.coph.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Piatkov KI, Vu TT, Hwang CS, Varshavsky A. Formyl-methionine as a degradation signal at the N-termini of bacterial proteins. Microb Cell. 2015;2:376–393. doi: 10.15698/mic2015.10.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas M, Beyer D, Gahlmann R, Freiberg C. YkrB is the main peptide deformylase in Bacillus subtilis, a eubacterium containing two functional peptide deformylases. Microbiology. 2001;147:1783–1791. doi: 10.1099/00221287-147-7-1783. [DOI] [PubMed] [Google Scholar]
- 14.You C, Lu H, Sekowska A, Fang G, Wang Y, et al. The two authentic methionine aminopeptidase genes are differentially expressed in Bacillus subtilis. BMC Microbiol. 2005;5:57. doi: 10.1186/1471-2180-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min S, Ingraham K, Huang J, McCloskey L, Rilling S, et al. Frequency of spontaneous resistance to peptide deformylase inhibitor GSK1322322 in Haemophilus influenzae, Staphylococcus aureus, Streptococcus pyogenes, and Streptococcus pneumoniae. Antimicrob Agents Chemother. 2015;59:4644–4652. doi: 10.1128/AAC.00484-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean CR, Narayan S, Richards J, Daigle DM, Esterow S, et al. Reduced susceptibility of Haemophilus influenzae to the peptide deformylase inhibitor LBM415 can result from target protein overexpression due to amplified chromosomal def gene copy number. Antimicrob Agents Chemother. 2007;51:1004–1010. doi: 10.1128/AAC.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caughlan RE, Sriram S, Daigle DM, Woods AL, Buco J, et al. Fmt bypass in Pseudomonas aeruginosa causes induction of MexXY efflux pump expression. Antimicrob Agents Chemother. 2009;53:5015–5021. doi: 10.1128/AAC.00253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duroc Y, Giglione C, Meinnel T. Mutations in three distinct loci cause resistance to peptide deformylase inhibitors in Bacillus subtilis. Antimicrob Agents Chemother. 2009;53:1673–1678. doi: 10.1128/AAC.01340-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margolis PS, Hackbarth CJ, Young DC, Wang W, Chen D, et al. Peptide deformylase in Staphylococcus aureus: resistance to inhibition is mediated by mutations in the formyltransferase gene. Antimicrob Agents Chemother. 2000;44:1825–1831. doi: 10.1128/aac.44.7.1825-1831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinttala R, Sasarman F, Nishimura T, Antonicka H, Brunel-Guitton C, et al. An N-terminal formyl methionine on COX 1 is required for the assembly of cytochrome c oxidase. Hum Mol Genet. 2015;24:4103–4113. doi: 10.1093/hmg/ddv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha A, Köhrer C, Weber MH, Masuda I, Mootha VK, et al. Biochemical characterization of pathogenic mutations in human mitochondrial methionyl-tRNA formyltransferase. J Biol Chem. 2014;289:32729–32741. doi: 10.1074/jbc.M114.610626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tucker EJ, Hershman SG, Köhrer C, Belcher-Timme CA, Patel J, et al. Mutations in MTFMT underlie a human disorder of formylation causing impaired mitochondrial translation. Cell Metab. 2011;14:428–434. doi: 10.1016/j.cmet.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton DT, Creuzenet C, Mangroo D. Formylation is not essential for initiation of protein synthesis in all eubacteria. J Biol Chem. 1999;274:22143–22146. doi: 10.1074/jbc.274.32.22143. [DOI] [PubMed] [Google Scholar]
- 24.Guillon JM, Mechulam Y, Schmitter JM, Blanquet S, Fayat G. Disruption of the gene for Met-tRNA(fMet) formyltransferase severely impairs growth of Escherichia coli. J Bacteriol. 1992;174:4294–4301. doi: 10.1128/jb.174.13.4294-4301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewandowski T, Huang J, Fan F, Rogers S, Gentry D, et al. Staphylococcus aureus formyl-methionyl transferase mutants demonstrate reduced virulence factor production and pathogenicity. Antimicrob Agents Chemother. 2013;57:2929–2936. doi: 10.1128/AAC.00162-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorzet A, Andersen JM, Nilsson AI, Møller NF, Andersson DI. Compensatory mutations in agrC partly restore fitness in vitro to peptide deformylase inhibitor-resistant Staphylococcus aureus. J Antimicrob Chemother. 2012;67:1835–1842. doi: 10.1093/jac/dks168. [DOI] [PubMed] [Google Scholar]
- 27.McLoon AL, Guttenplan SB, Kearns DB, Kolter R, Losick R. Tracing the domestication of a biofilm-forming bacterium. J Bacteriol. 2011;193:2027–2034. doi: 10.1128/JB.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Argüello JM, et al. PfeT, a P1B4-type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol Microbiol. 2015;98:787–803. doi: 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harwood CR, Cutting SM, editors. Molecular Biological Methods for Bacillus. Chichester: John Wiley and Sons; 1990. [Google Scholar]
- 30.Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- 31.Quisel JD, Burkholder WF, Grossman AD. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J Bacteriol. 2001;183:6573–6578. doi: 10.1128/JB.183.22.6573-6578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascher T, Hachmann AB, Helmann JD. Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function sigma factors. J Bacteriol. 2007;189:6919–6927. doi: 10.1128/JB.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearns DB, Losick R. Swarming motility in undomesticated Bacillus subtilis. Mol Microbiol. 2003;49:581–590. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- 34.Morales-Soto N, Anyan ME, Mattingly AE, Madukoma CS, Harvey CW, et al. Preparation, imaging, and quantification of bacterial surface motility assays. J Vis Exp. 2015;98 doi: 10.3791/52338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Branda SS, González-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci USA. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterlini JM, Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969;113:29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp MD, Pogliano K. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc Natl Acad Sci USA. 1999;96:14553–14558. doi: 10.1073/pnas.96.25.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merchant SS, Helmann JD. Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv Microb Physiol. 2012;60:91–210. doi: 10.1016/B978-0-12-398264-3.00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuber P. Management of oxidative stress in Bacillus. Annu Rev Microbiol. 2009;63:575–597. doi: 10.1146/annurev.micro.091208.073241. [DOI] [PubMed] [Google Scholar]
- 40.Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bsat N, Chen L, Helmann JD. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J Bacteriol. 1996;178:6579–6586. doi: 10.1128/jb.178.22.6579-6586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandrangsu P, Dusi R, Hamilton CJ, Helmann JD. Methylglyoxal resistance in Bacillus subtilis: contributions of bacillithiol-dependent and independent pathways. Mol Microbiol. 2014;91:706–715. doi: 10.1111/mmi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faulkner MJ, Helmann JD. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal. 2011;15:175–189. doi: 10.1089/ars.2010.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin JE, Waters LS, Storz G, Imlay JA. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet. 2015;11:e1004977. doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeowell HN, White JR. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982;22:961–968. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD. Role of the Fur regulon in iron transport in Bacillus subtilis. J Bacteriol. 2006;188:3664–3673. doi: 10.1128/JB.188.10.3664-3673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boorsma A, van der Rest ME, Lolkema JS, Konings WN. Secondary transporters for citrate and the Mg2+-citrate complex in Bacillus subtilis are homologous proteins. J Bacteriol. 1996;178:6216–6222. doi: 10.1128/jb.178.21.6216-6222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao M, Bernat BA, Wang Z, Armstrong RN, Helmann JD. FosB, a cysteine-dependent fosfomycin resistance protein under the control of sigma(W), an extracytoplasmic-function sigma factor in Bacillus subtilis. J Bacteriol. 2001;183:2380–2383. doi: 10.1128/JB.183.7.2380-2383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaballa A, Newton GL, Antelmann H, Parsonage D, Upton H, et al. Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli. Proc Natl Acad Sci USA. 2010;107:6482–6486. doi: 10.1073/pnas.1000928107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamers AP, Keithly ME, Kim K, Cook PD, Stec DF, et al. Synthesis of bacillithiol and the catalytic selectivity of FosB-type fosfomycin resistance proteins. Org Lett. 2012;14:5207–5209. doi: 10.1021/ol302327t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovács ÁT. Bacterial differentiation via gradual activation of global regulators. Curr Genet. 2016;62:125–128. doi: 10.1007/s00294-015-0524-8. [DOI] [PubMed] [Google Scholar]
- 53.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kearns DB, Chu F, Rudner R, Losick R. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol Microbiol. 2004;52:357–369. doi: 10.1111/j.1365-2958.2004.03996.x. [DOI] [PubMed] [Google Scholar]
- 55.Cairns LS, Hobley L, Stanley-Wall NR. Biofilm formation by Bacillus subtilis: new insights into regulatory strategies and assembly mechanisms. Mol Microbiol. 2014;93:587–598. doi: 10.1111/mmi.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mielich-Süss B, Lopez D. Molecular mechanisms involved in Bacillus subtilis biofilm formation. Environ Microbiol. 2015;17:555–565. doi: 10.1111/1462-2920.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan IS, Ramamurthi KS. Spore formation in Bacillus subtilis. Environ Microbiol Rep. 2014;6:212–225. doi: 10.1111/1758-2229.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Errington J. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol. 2003;1:117–126. doi: 10.1038/nrmicro750. [DOI] [PubMed] [Google Scholar]
- 59.Margolis P, Hackbarth C, Lopez S, Maniar M, Wang W, et al. Resistance of Streptococcus pneumoniae to deformylase inhibitors is due to mutations in defB. Antimicrob Agents Chemother. 2001;45:2432–2435. doi: 10.1128/AAC.45.9.2432-2435.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nilsson AI, Zorzet A, Kanth A, Dahlström S, Berg OG, et al. Reducing the fitness cost of antibiotic resistance by amplification of initiator tRNA genes. Proc Natl Acad Sci USA. 2006;103:6976–6981. doi: 10.1073/pnas.0602171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zorzet A, Pavlov MY, Nilsson AI, Ehrenberg M, Andersson DI. Error-prone initiation factor 2 mutations reduce the fitness cost of antibiotic resistance. Mol Microbiol. 2010;75:1299–1313. doi: 10.1111/j.1365-2958.2010.07057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mader D, Liebeke M, Winstel V, Methling K, Leibig M, et al. Role of N-terminal protein formylation in central metabolic processes in Staphylococcus aureus. BMC Microbiol. 2013;13:7. doi: 10.1186/1471-2180-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plikat U, Voshol H, Dangendorf Y, Wiedmann B, Devay P, et al. From proteomics to systems biology of bacterial pathogens: approaches, tools, and applications. Proteomics. 2007;7:992–1003. doi: 10.1002/pmic.200600925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.