Abstract
Short telomeres are associated with atherosclerosis. However, the temporal relation between atherosclerosis and telomere length is unclear. The objective of this work was to examine the temporal formation and progression of carotid atherosclerotic plaques in relation to telomere dynamics.
In a longitudinal study, comprising 154 French men and women (aged 31–76 years at baseline), carotid plaques were quantified by echography and telomere length on leucocytes was measured by Southern blots at baseline and follow-up examinations.
Telomere attrition rates during the 9.5-year follow-up period were not different in individuals with plaques at both baseline and follow-up examinations (23.3 ± 2.0 bp/year) than in individuals who developed plaques during the follow-up period (26.5 ± 2.0 bp/year) and those without plaques at either baseline or follow-up examinations (22.5 ± 2.3 bp/year, p = 0.79). At baseline, telomere length was associated with presence of carotid plaques (p = 0.02) and with the number of regions with plaques (p = 0.005). An interaction (p = 0.03) between age and the presence of plaques was observed, such that the association between plaques and telomere length was more pronounced at a younger age.
In conclusion, carotid atherosclerosis is not associated with increased telomere attrition over a 9.5 years follow-up period. Short telomere length is more strongly associated with early-onset than late-onset carotid atherosclerosis. Our results support the thesis that heightened telomere attrition during adult life might not explain the short telomeres observed in subjects with atherosclerotic disease. Rather, short telomeres antecedes the clinical manifestation of the disease.
Keywords: Leucocyte telomere length, telomere attrition, carotid atherosclerotic plaque, atherosclerotic cardiovascular disease
Introduction
Two recent reviews and meta-analyses showed that short leukocyte telomere length (LTL) is associated with atherosclerotic cardiovascular disease (ACVD)1,2, which is largely aging-related. Short LTL is also associated with aortic stiffness3, an aging phenotype, and with diminished survival4. With some exceptions5,6, previous research has shown that individuals with atherosclerotic plaques of the carotid artery had comparatively short LTL7–11.
Indolent inflammation and oxidative stress are key determinants in aging-related vascular injury, contributing to atherosclerosis and arterial stiffness12,13. For this reason, the prevailing view on the association of short LTL with ACVD has been that during adult life14–16 chronic inflammation and oxidative stress heighten the pace of age-dependent LTL shortening in tandem with the development of ACVD. Clinical studies have emphasized this concept by linking short LTL with atherosclerosis risk factors such as smoking, sedentary lifestyle and high BMI17–19. However, recent findings suggest that the contribution of LTL attrition during adult life to the variation in LTL across the population is comparatively small to the inter-individual variation in LTL at birth20 and LTL attrition during the first two decades of life21,22. Since short telomeres may precede the clinical manifestation of atherosclerosis, telomere length, as expressed in LTL, may be an active determinant in rather than a passive marker of arterial aging, perhaps because short telomeres might diminish replicative potential and compromise vascular repair23.
The Strong Heart Family Study reported that individuals with short LTL had a higher risk of developing carotid atherosclerosis over a period of 5.5 years9, while the Bruneck Study observed that short LTL was associated with progression to advance stages of carotid atherosclerosis over a 5-year period11. However, no study has performed sequential LTL measurements over a relatively long follow-up period to establish whether the rate of LTL attrition is higher in individuals with carotid atherosclerosis than in their peers. The present longitudinal study was designed to fill this gap, focusing on the relation between LTL dynamics and carotid atherosclerotic plaques (CAPs).
Subjects and Methods
Subjects
This research draws on the Evolution de la Rigidité Artérielle (ERA) study, which examined determinants of arterial aging24. The ERA study was approved by the research ethics committee, and all participants signed an informed consent form. Participants were from a Parisian cohort that had been followed at the Centre d’Investigations Préventives et Cliniques. Among these, 156 participants had sequential LTL measurements. The follow-up examination was performed 9.5 years later (2007–2008). Two subjects were excluded because of technical problems related to the echographic quantification of CAPs at the follow-up examination. Thus, the study comprised 154 participants (31% women), whose age range was 31–76 years (58 ± 10 years; mean ± SD) at baseline examination (1998–1999).
Carotid artery measurements
Carotid artery echography was performed at baseline and follow-up examinations. The same device (Aloka SSD-650, with a 7.5-MHz linear array transducer, processing and storage of B-mode images with the software M’ATHS, Metris, France) was used on both occasions25. Intima-media thickness (IMT) and presence of carotid atherosclerotic plaques (CAP) were measured as previously described7. Presence of CAP was examined in 2 regions (common carotid and bifurcation) of the right and left carotid arteries; the combined CAP score for these regions therefore ranged from 0 (no CAP in either left or right carotid artery) to 4 (CAPs in two regions of the left and right carotid arteries).
The relationship between LTL dynamics and carotid atherosclerosis was studied by stratifying the cohort into 3 CAP groups: Gr no/no, participants without CAP at both baseline and follow-up examinations; Gr no/yes, participants with CAP only at the follow-up visit, i.e., individuals who developed CAP during the follow-up period; and Gr yes/yes, participants with CAP at both baseline and follow-up examinations.
Participants were further grouped based on the number of carotid regions with CAPs (Gr 0 = no CAP; Gr 1 = CAP in one region; Gr ≥ 2 = CAPs in two or more regions). This CAP grouping applied either to the baseline or to the follow-up examination.
In both baseline and follow-up examinations, supine BP was measured in the right arm using a manual sphygmomanometer. After a 10-min rest period, systolic and diastolic blood pressure (SBP and DBP) were measured three times with a 5-min interval between measurements, and the average of the last two measurements was used for the statistical analyses. Height and weight parameters were used for BMI measurements. Smoking status (current and ex-smokers vs. non-smokers) was determined using a questionnaire.
LTL measurements
LTL measurements were performed from blood drawn at baseline and follow-up examinations. White blood cell DNA was extracted from whole blood after red cell osmotic lysis by a salting out method as previously described26. All DNA samples were tested for integrity using a 1% (wt/vol) agarose gel. LTL was measured by Southern blots of the terminal restriction fragments (TRFs), as previously described27. Briefly, DNA samples were digested (37°C) overnight with restriction enzymes Hinf I and Rsa I (Roche Diagnostics GmbH, Germany). Digested DNA samples and DNA ladders were resolved on 0.5% (wt/vol) agarose gels. After 23 h, the DNA was depurinated, denatured, neutralized and transferred onto a positively charged nylon membrane (Roche) using a vacuum blotter (Biorad, Hercules, CA). Membranes were hybridized at 65°C with the DIG-labeled telomeric probe after which the probe was detected by the DIG luminescent detection procedure (Roche) and exposed on CCD camera (Las 4000, Fuji). Measurements were performed in duplicate on separate gels with analyses based on the average of these two measurements. The baseline and follow-up samples from each individual were run in adjacent lanes. The inter-assay coefficient of variation for the duplicate measurements (on different gels) was 1.2%.
Statistical analysis
For variables presenting a normal distribution, descriptive values are expressed as mean ± SD, or otherwise, as median, interquartile range (IQR) and percentages. LTL attrition was calculated from the difference between LTL at baseline and LTL at follow-up, divided by the duration of the follow-up.
The relationships of LTL attrition with LTL at baseline, LTL at follow-up, age, BMI, SBP, DBP, HR and IMT were determined using Pearson’s correlation coefficients. The effect of age (a continuous variable) on LTL-CAP association was tested with the interaction term age × presence of CAPs. This applied to age in which the CAP has been first detected, i.e. at the baseline examination for Gr yes/yes and at the follow-up examination for Gr no/yes. For Gr no/no, we used the age and LTL at the baseline examination for this analysis.
ANOVA and ANOVA trend tests were used for categorical variables (sex, smoking status and CAP groups). Significance was tested using the post hoc tests between groups were Tukey-Kramer or Kruskal-Wallis test depending on the distribution (continuous variables) and Chi2 (discrete variables). A p-value < 0.05 was considered statistically significant. Statistical analyses were carried out using the NCSS 9 statistical software package (NCSS, Kaysville, UT).
Results
Cardiovascular characteristics
Between the baseline and follow-up examinations, participants showed an increase in BMI (p = 0.03), carotid IMT (p = 0.02) and heart rate (HR) (p < 0.0001), a drop in mean diastolic blood pressure (DBP) (p = 0.008) but no change in mean systolic blood pressure (SBP) (Table 1). Gr yes/yes was older than Gr no/no (p < 0.001) and showed higher baseline carotid artery IMT (p < 0.001), BMI (p < 0.001), SBP (p < 0.001) and DBP (p < 0.01). Gr no/yes showed intermediate values (trend test) for carotid IMT (p < 0.0001), SBP (p < 0.0001), DBP (p < 0.002) and BMI (p < 0.001). No differences in smoking and in HR were observed across the three CAP groups.
Table 1.
Characteristics at baseline and changes over the 9.5-year follow-up period in the entire population and in the 3 subgroups according to the presence of carotid atherosclerotic plaques at baseline/follow-up visits
| Parameter | Whole population | Presence of CAPs (Baseline/Follow-up) | |||
|---|---|---|---|---|---|
| no/no | no/yes | yes/yes | p (trend ANOVA) | ||
| Number of subjects | 154 | 56 | 56 | 42 | |
| Sex (Women %) | 31% | 32% | 32% | 26% | 0.77 |
| Age at BL (years) | 58.4 ± 9.6 | 53.3 ± 9.4 | 60.5 ± 8.8‡ | 62.5 ± 8.0‡ | < 0.0001 |
| FU duration (years) | 9.5 ± 0.5 | 9.5 ± 0.5 | 9.7 ± 0.5 | 9.3 ± 0.5 | 0.017 |
| Smoking at BL (%) | 47% | 46% | 50% | 46% | 0.89 |
| Smoking at FU (%) | 47% | 46% | 50% | 46% | 0.89 |
| BMI at BL (kg/m2) | 25.4 (23.8 – 28.3) | 24.2 (22.5 – 26.8) | 25.7 (24.8 – 28.0)* | 28.1 (24.6 – 31.5) ‡ | 0.0002 |
| Δ BMI (kg/m2) | 0.30 (−0.62 – 1.22)† | 0.74 (−0.39 – 1.54) | 0.00 (−0.68 – 0.90) | 0.28 (−1.01 – 1.33) | 0.12 |
| SBP at BL (mmHg) | 141 ± 19 | 130 ± 16 | 145 ± 18‡ | 151 ± 17‡ | < 0.0001 |
| Δ SBP (mmHg) | 2.0 ± 17.1 | 4.7 ± 14.1 | 1.7 ± 17.9 | −1.0 ± 19.3 | 0.27 |
| DBP at BL (mmHg) | 87 ± 10 | 84 ± 10 | 89 ± 9* | 90 ± 9† | 0.002 |
| Δ DBP (mmHg) | −2.1 ± 9.8† | 0.9 ± 9.7 | −3.6 ± 9.8* | −4.1 ± 9.0* | 0.02 |
| HR at BL (bpm) | 66 ± 9 | 66 ± 8 | 66 ± 10 | 67 ± 8 | 0.92 |
| Δ HR (bpm) | 3.6 ± 9.2† | 2.6 ± 7.7 | 3.2 ± 10.6 | 5.5 ± 9.1 | 0.28 |
| Carotid IMT at BL (mm) | 0.71 (0.66 – 0.81) | 0.68 (0.60 – 0.74) | 0.72 (0.67 – 0.83) † | 0.80 (0.70 – 0.85)*** | < 0.0001 |
| Δ carotid IMT (mm) | 0.01 (−0.04 – 0.08)† | 0.02 (−0.02 – 0.06) | 0.01 (−0.06 – 0.11) | 0.01 (−0.02 – 0.13) | 0.98 |
| Number of regions with CAPs at BL | 0 (0 – 1) | 0 | 0 | 1 (1 – 2) | - |
| Δ number of regions with CAPs | 1 (0 – 2)† | 0 | 2 (1 – 2) ‡ | 1 (1 – 2) ‡ | < 0.0001 |
| LTL at BL (kb) | 6.46 ± 0.56 | 6.64 ± 0.60 | 6.46 ± 0.51 | 6.20 ± 0.46‡ | 0.0004 |
| LTL at FU (kb) | 6.23 ± 0.53 | 6.39 ± 0.57 | 6.21 ± 0.50 | 6.02 ± 0.42‡ | 0.0009 |
| LTL attrition (bp/year) | 24.2 ± 16.0† | 26.1 ± 19.4 | 26.0 ± 14.9 | 19.5 ± 10.7 | 0.08 |
BL, baseline; FU, follow-up; BMI, body mass index; Δ, changes between baseline and follow-up visits; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; IMT, intima media thickness; CAP, carotid atherosclerotic plaque; LTL, leukocyte telomere length; kb, kilobase pairs; bp, base pair; no/no: absence of CAP in both baseline and follow-up examinations; no/yes: presence of CAP only at the follow-up examination; yes/yes: presence of CAP in both baseline and follow-up examinations.
Values are mean ± SD, median (IQR) or percentages (%);
p < 0.05;
p < 0.01;
p < 0.001 Tukey-Kramer’s or Kruskal-Wallis post hoc test and Chi2 vs. Gr no/no.
: Follow-up – baseline significantly different from 0
Relationship between LTL dynamics and CAP at baseline and follow-up examinations
For the entire cohort, mean LTL was 6.46 ± 0.56 kb at baseline and 6.23 ± 0.53 kb at follow-up (p < 0.001). The average rate of LTL attrition over the 9.5 follow-up period was 24.2 ± 16.0 base pairs (bp) per year (Table 1). Among the 154 participants, 149 displayed LTL shortening, while 5 (3 %) displayed LTL lengthening.
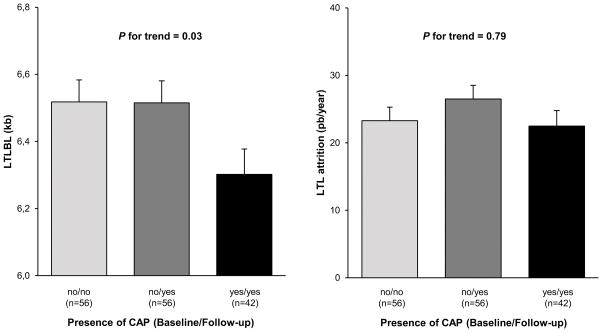
There were no differences in LTL attrition across the three CAP groups (p < 0.24, Table 1). This finding held after adjusting for age, sex and baseline LTL: Gr yes/yes = 23.3 ± 2.0 bp/year; Gr no/yes = 26.5 ± 2.0 bp/year; Gr no/no = 22.5 ± 2.3 bp/year (p = 0.79, Figure 1, right).
Figure 1. Baseline leukocyte telomere length (left) and leukocyte telomere attrition during the follow-up period (right) versus the presence of carotid atherosclerotic plaques during the baseline/follow-up visits.
Adjusted baseline LTL (kb) versus the presence of CAP at baseline and follow-up (left); Adjusted LTL attrition (bp/year) versus the presence of CAP at baseline and follow-up (right).
Values are mean ± SEM. BL, baseline; CAP, carotid atherosclerotic plaque; LTL, leukocyte telomere length, kb, kilo base pairs; bp, base pair; no/no: absence of CAP in both baseline and follow-up examinations; no/yes: presence of CAP only at the follow-up examination; yes/yes: presence of CAP in both baseline and follow-up examinations.
BL LTL is adjusted to age and sex; LTL attrition is adjusted to age, sex and baseline value of LTL.
Across the three CAP groups, LTL was shorter at both baseline and follow-up examinations in Gr yes/yes than Gr no/yes, which, in turn, was shorter than Gr no/no (i.e. LTL, Gr yes/yes < LTL, Gr no/yes < LTL, Gr no/no; p = 0.0004 for baseline LTL, and p = 0.0009 for follow-up LTL) (Table 1). This finding held for baseline examination after adjusting for age and sex, i.e., LTL for Gr yes/yes = 6.30 ± 0.08 kb, as compared to the two other groups: LTL, Gr no/yes = 6.52 ± 0.07 kb, and LTL, Gr no/no = 6.51 ± 0.07 kb (Figure 1 left panel, trend ANOVA, p = 0.03 and ANOVA Gr yes/yes vs. 2 other groups p = 0.02,).
After adjustments (for baseline LTL, age, sex), LTL attrition over the follow-up period was not associated with SBP, DBP, HR, carotid IMT, BMI or smoking.
Relationship between LTL dynamics and number of CAPs
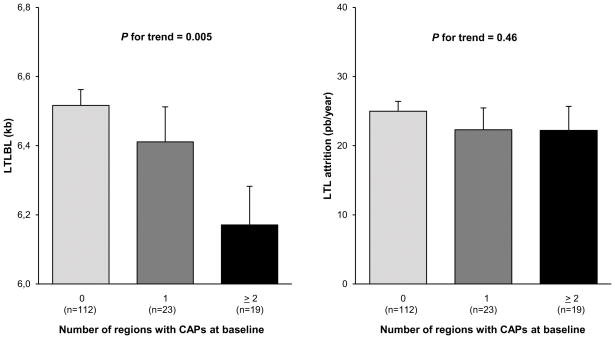
LTL at baseline was inversely correlated with the number of regions with CAPs at baseline examination (adjusted for age and sex, trend ANOVA p = 0.005, Figure 2, left panel).
Figure 2. Baseline leukocyte telomere length (left) and leukocyte telomere attrition during the follow-up period (right) versus the number of regions with carotid atherosclerotic plaques at baseline.
Values are mean ± SEM. BL, baseline; CAP, carotid atherosclerotic plaque; LTL, leukocyte telomere length; kb, kilo base pairs; bp, base pair.
BL LTL is adjusted to age and sex; LTL attrition is adjusted to age, sex and baseline value of LTL.
LTL attrition rates during the follow-up period were not correlated with the number of plaques at baseline: Gr 0 = 25.0 ± 1.4 bp/year; Gr 1 = 22.3 ± 3.2 bp/year; Gr ≥ 2 = 22.3 ± 3.5 bp/year (p = 0.46, Figure 2, right panel). LTL at baseline and LTL attrition rates during the follow-up period were not correlated with the number of plaques at the follow-up examination (data not shown).
The age effect on LTL-CAP correlation
We further analyzed the association of CAP with LTL as a function of participant’s age when CAPs were detected. LTL was negatively associated with age (p < 0.001) and the presence of CAPs (p < 0.02) with an interaction (age × presence CAPs; p = 0.03) indicating that the younger the participant’s age of the detection of the plaque the stronger is the association between LTL and CAPs.
Discussion
The key finding of this longitudinal study is that compared with participants with no CAPs at baseline and follow-up examinations, LTL attrition was not higher in participants with CAPs at baseline or in those who developed CAPS during the follow-up period. This finding challenges the convention that the association of short LTL with ACVD principally reflects a heightened LTL attrition due to increased burdens of inflammation and oxidative stress15,16. Although some studies showed that in adults, cardiovascular risks associated with increased inflammation and oxidative stress are also associate with short LTL6,28, the influence of such factors on LTL might be small compared with those that define LTL prior to adulthood.
Previous studies observed that short LTL was principally associated with severe forms of carotid artery atherosclerosis8,11 or with presence of CAPs in young individuals9. In one study, the CAPs-short LTL association was limited to young women5. These findings are largely compatible with our findings; they infer that individuals with early-onset atherosclerosis and those with severe forms of atherosclerosis display comparatively short LTL29.
In practice, having carotid artery atherosclerosis is a question of “when”, and “how severe” since most individuals develop some degree of atherosclerosis if they live long enough. For instance, at least some buildup of CAPs is found in: a) 48% of men and 36% of women younger 45 years; b) 71% of men and 54% of women between the ages of 50–54 years; and c) 94% of men and 93% of women 80 years or older30. Thus, having shorter LTL might not apply to all individuals with carotid artery atherosclerosis. Our results go along with this hypothesis suggesting that short LTL largely denotes early onset and more severe manifestation of carotid artery atherosclerosis.
Our findings do not support the view that a higher pace of LTL attrition during adulthood explains the association of short LTL with ACVD and that LTL is a “biomarker” of the aging of the vasculature. They do support the competing view, which ascribes an active role of telomere length, as expressed in LTL, in the development of ACVD. This view is based on the following series of observations: a) LTL is highly heritable (~ 65%)31–33, a finding already observed at birth34; b) alleles associated with short LTL are over-represented in individuals with clinical manifestation of ACVD35–37, thus largely excluding reverse causality, i.e., that ACVD accelerates LTL attrition; c) the wide LTL variation is already observed across newborns (SD of about 0.7 kb)34, suggesting that having short or long LTL is primarily determined prior to adulthood; and d) individuals who enter adult life with short or long LTL typically display long or short LTL for the rest of their adult life course38,39. Individually, each of these observations hardly infers causality, but collectively they suggest an active role of telomeres in ACVD.
Study strengths and limitations
The key strengths of study are the relatively long follow-up duration, which is critical for obtaining valid data in longitudinal studies40, and the precise LTL measurements by Southern blots27,41. The modest sample size can be considered as a limitation of the study. However, given the longitudinal nature of the study, each participant’s LTL at follow-up examination is scaled to his/her LTL at baseline examination. Thus, there may not be a need for a large sample size to answer the critical question: Is LTL attrition in individuals with CAPs from the outset different that of participants who developed CAPs during the 9.5 years of follow-up and that of participants with no evidence of CAPS (no/no). Using a relative long follow-up period, our approach circumvents the high inter-individual variation in LTL across participants. However, the temporal relation between CAPs and LTL across the population warrants replication in large-scale studies.
Conclusions and Perspectives
This study shows that carotid atherosclerosis was not associated with increased LTL attrition over a 9.5 years follow up period, yet carotid atherosclerosis was associated with short LTL, principally in younger participants. Based on these findings, the concept of precedence, i.e., LTL is primarily determined prior to adulthood22,38,39, the high LTL heritability31–33 and recent genetic studies35–37, we infer that telomere length might be actively involved in the development of ACVD. In the final analysis, atherosclerosis and other aging-related degenerative diseases may arise from a progressive increase in the imbalance between injury and repair42,43. Perhaps the vascular repair potential of individuals with ACVD is compromised because of their shorter telomeres. Given that LTL is a complex genetic trait, elucidating more LTL gene variants and learning their potential role in the development of ACVD will take us a long way towards understanding the role of telomere biology in ACVD and human aging in general.
Novelty and Significance.
What Is New?
LTL attrition rate over a relatively long follow-up period in a population of adults was not associated with the presence and magnitude of carotid atherosclerotic plaques
Short LTL predicted development of carotid atherosclerosis, as expressed by increased number of anatomic regions with carotid atherosclerotic plaques.
The association between short LTL and carotid atherosclerotic plaques applies principally to individuals with an early and/or more severe form of carotid atherosclerosis.
What Is Relevant?
Shorter LTL observed in individuals with carotid atherosclerosis is not due to a higher LTL attrition as compared to controls.
Short LTL ostensively precedes the development of carotid atherosclerosis and predicts its early onset and progression.
Summary
This longitudinal study shows no increased LTL attrition in subjects with atherosclerotic plaques, suggesting that short LTL precedes the manifestations of atherosclerosis.
Acknowledgments
We thank Mr Pierre Pothier for his critical review and language corrections.
Sources of Funding
This study has been supported by the French National Research Agency (ANR), Translationnelle: N°ID RCB: 2014-A00298-39: 2014-2017, by the French National Programme Hospitalier de Recherche Clinique (PHRC) and by a public grant overseen by the French National Research Agency (ANR) as part of the second “Investissements d’Avenir” programme RHU FIGHT-HF (reference: ANR-15-RHUS-0004). A. Aviv research is currently supported by the NIH grants R01HL116446, R01HD071180 and R01HL13840.
Footnotes
Conflict of interest: None declared
Bibliography
- 1.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Mello MJJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 3.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(2 Pt 2):381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 4.Deelen J, Beekman M, Codd V, et al. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. Int J Epidemiol. 2014;43:878–886. doi: 10.1093/ije/dyt267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Meyer T, Rietzschel ER, De Buyzere ML, Langlois MR, De Bacquer D, Segers P, Van Damme P, De Backer GG, Van Oostveldt P, Van Criekinge W, Gillebert TC, Bekaert S Asklepios Study Investigators. Systemic telomere length and preclinical atherosclerosis: the Asklepios Study. Eur Heart J. 2009;30:3074–3081. doi: 10.1093/eurheartj/ehp324. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Alvira JM, Fuster V, Dorado B, Soberón N, Flores I, Gallardo M, Pocock S, Blasco MA, Andrés V. Short Telomere Load, Telomere Length, and Subclinical Atherosclerosis: The PESA Study. J Am Coll Cardiol. 2016;67:2467–2476. doi: 10.1016/j.jacc.2016.03.530. [DOI] [PubMed] [Google Scholar]
- 7.Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 8.Huzen J, Peeters W, de Boer RA, Moll FL, Wong LSM, Codd V, de Kleijn DPV, de Smet BJGL, van Veldhuisen DJ, Samani NJ, van Gilst WH, Pasterkamp G, van der Harst P. Circulating leukocyte and carotid atherosclerotic plaque telomere length: interrelation, association with plaque characteristics, and restenosis after endarterectomy. Arterioscler Thromb Vasc Biol. 2011;31:1219–1225. doi: 10.1161/ATVBAHA.110.217158. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Lin J, Matsuguchi T, Blackburn E, Yeh F, Best LG, Devereux RB, Lee ET, Howard BV, Roman MJ, Zhao J. Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: the Strong Heart Family Study. Aging. 2014;6:414–427. doi: 10.18632/aging.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panayiotou AG, Nicolaides AN, Griffin M, Tyllis T, Georgiou N, Bond D, Martin RM, Hoppensteadt D, Fareed J, Humphries SE. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis. 2010;211:176–181. doi: 10.1016/j.atherosclerosis.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Willeit P, Willeit J, Brandstätter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(5, Supplement):S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 14.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 15.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10:274–283. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 16.Yeh J-K, Wang C-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes. 2016:7. doi: 10.3390/genes7090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 18.Du M, Prescott J, Kraft P, Han J, Giovannucci E, Hankinson SE, De Vivo I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am J Epidemiol. 2012;175:414–422. doi: 10.1093/aje/kwr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huzen J, Wong LSM, van Veldhuisen DJ, et al. Telomere length loss due to smoking and metabolic traits. J Intern Med. 2014;275:155–163. doi: 10.1111/joim.12149. [DOI] [PubMed] [Google Scholar]
- 20.Graakjaer J, Pascoe L, Der-Sarkissian H, Thomas G, Kolvraa S, Christensen K, Londoño-Vallejo J-A. The relative lengths of individual telomeres are defined in the zygote and strictly maintained during life. Aging Cell. 2004;3:97–102. doi: 10.1111/j.1474-9728.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- 21.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8:e1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benetos A, Adamopoulos C, Bureau J-M, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 25.Touboul PJ, Prati P, Scarabin PY, Adrai V, Thibout E, Ducimetière P. Use of monitoring software to improve the measurement of carotid wall thickness by B-mode imaging. J Hypertens Suppl Off J Int Soc Hypertens. 1992;10:S37–41. [PubMed] [Google Scholar]
- 26.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, Harley CB, Aviv A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 28.Cafueri G, Parodi F, Pistorio A, Bertolotto M, Ventura F, Gambini C, Bianco P, Dallegri F, Pistoia V, Pezzolo A, Palombo D. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PloS One. 2012;7:e35312. doi: 10.1371/journal.pone.0035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt SC, Kark JD, Aviv A. Association between shortened leukocyte telomere length and cardio-metabolic outcomes. Circ Cardiovasc Genet. 2015;8:4–7. doi: 10.1161/CIRCGENETICS.114.000964. [DOI] [PubMed] [Google Scholar]
- 30.Boulos NM, Gardin JM, Malik S, Postley J, Wong ND. Carotid Plaque Characterization, Stenosis, and Intima-Media Thickness According to Age and Gender in a Large Registry Cohort. Am J Cardiol. 2016;117:1185–1191. doi: 10.1016/j.amjcard.2015.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broer L, Codd V, Nyholt DR, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet EJHG. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honig LS, Kang MS, Cheng R, Eckfeldt JH, Thyagarajan B, Leiendecker-Foster C, Province MA, Sanders JL, Perls T, Christensen K, Lee JH, Mayeux R, Schupf N. Heritability of telomere length in a study of long-lived families. Neurobiol Aging. 2015;36:2785–2790. doi: 10.1016/j.neurobiolaging.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hjelmborg JB, Dalgård C, Möller S, Steenstrup T, Kimura M, Christensen K, Kyvik KO, Aviv A. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52:297–302. doi: 10.1136/jmedgenet-2014-102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, Kimura M, Wapner R, Aviv A. Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics. 2016:137. doi: 10.1542/peds.2015-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. 427–432. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheller Madrid A, Rode L, Nordestgaard BG, Bojesen SE. Short Telomere Length and Ischemic Heart Disease: Observational and Genetic Studies in 290 022 Individuals. Clin Chem. 2016;62:1140–1149. doi: 10.1373/clinchem.2016.258566. [DOI] [PubMed] [Google Scholar]
- 37.Haycock PC, Burgess S, Nounu A, et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017 Feb 23; doi: 10.1001/jamaoncol.2016.5945. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J, Srinivasan SR, Berenson GS, Labat C, Aviv A. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12:615–621. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhulst S, Dalgård C, Labat C, Kark JD, Kimura M, Christensen K, Toupance S, Aviv A, Kyvik KO, Benetos A. A short leucocyte telomere length is associated with development of insulin resistance. Diabetologia. 2016;59:1258–1265. doi: 10.1007/s00125-016-3915-6. [DOI] [PubMed] [Google Scholar]
- 40.Steenstrup T, Hjelmborg JVB, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum--artifact or biology? Nucleic Acids Res. 2013;41:e131. doi: 10.1093/nar/gkt370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aviv A, Kark JD, Susser E. Telomeres, Atherosclerosis, and Human Longevity. Epidemiol Camb Mass. 2015;26:295–299. doi: 10.1097/EDE.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone RC, Horvath K, Kark JD, Susser E, Tishkoff SA, Aviv A. Telomere Length and the Cancer-Atherosclerosis Trade-Off. PLoS Genet. 2016;12:e1006144. doi: 10.1371/journal.pgen.1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]