Abstract
Background
The aim of the present systematic review was to evaluate the hypothesis of an association between periodontitis and the development of cancer.
Methods
Two reviewers independently screened electronic and manual sources for pertinent articles. Primary outcome measures were the occurrence of neoplasm diagnosis in exposed and non-exposed groups, reported to evaluate association between cancer and periodontitis.
Results
Of the 490 initially retrieved papers 10 were included in the qualitative synthesis and eight in the quantitative synthesis; the eight papers covered six studies. Considering hazard ratios, a statistically significant association was found for all cancers studied (1.14; CI 95%: 1.04, 1.24), digestive tract cancer (1.34; CI 95%: 1.05, 1.72), pancreatic cancer (1.74; CI 95%: 1.21, 2.52), prostate cancer (1.25; CI 95%: 1.04, 1.51), breast cancer (1.11; CI 95%: 1.00, 1.23), corpus uteri cancer (2.20; CI 95%: 1.16, 4.18), lung cancer (1.24; CI 95%: 1.06, 1.45), hematological cancer (1.30; CI 95%: 1.11, 1.53), esophagus / oropharyngeal cancer pooled together (2.25; CI 95%: 1.30, 3.90) and Non-Hodgkin lymphoma (1.30; CI 95%: 1.11, 1.52).
Conclusions
Despite the sparse scientific evidence and considering the low statistical power of the results, this systematic review revealed a substantial lack of studies with standardized and comparable methods to speculate about the association between periodontitis and cancer; more studies are need in order to explore further the scientific evidence of such correlation.
Introduction
Periodontal diseases and, in particular, periodontitis is reported to be potentially associated with some systemic diseases and conditions such as cardiovascular disease, the impairment of glycemic control in patients with diabetes and preterm births or low-birth weight [1–6]. Such correlation could be due to several mechanisms: 1) the spread of bacteria from the oral cavity could cause tissue damage to various organs [7, 8]; 2) the increase in inflammatory systemic burden [4, 9, 10], that may augment the susceptibility of atheromatous plaque formation [7]; 3) an autoimmune response which could be triggered by bacterial epitopes from oral bacterial species [7].
Following the publication of some primary reports [11, 12], the authors hypothesized that periodontitis could be an independent risk factor for cancer development (both locally and at a distance) due to the long-standing chronic inflammatory status of the periodontal tissues [13, 14]. Some mechanisms were advocated explaining the potential basis of such association. Published studies demonstrated a role of viruses such as Human Papilloma virus (HPV) and Epstein-Barr virus (EBV), that could be detected in periodontal pockets, as suspected agents for oral cancer through the activation of specific oncogenes (such as E6 and E7 for HPV) [15–17]. Specific pathogens, such as P. gingivalis, were demonstrated to prevent, after invading the epithelium, cell apoptosis, thus favoring cancer initiation [18–20]. These pathogens could be found in carcinomas of the gingiva [18], but could also be associated with distant tumors [21].
Indirect mechanisms for a link between periodontitis and cancer were mainly related to the known association between the inflammatory process itself and cancer [22–24]. Indeed, it was demonstrated that periodontitis may induce a significant increase in inflammatory markers and molecules that enhances the inflammatory reaction. This condition causes the release of reactive oxygen species and other metabolites that could promote cancer initiation [22, 24]. Moreover, the stimulation of the inflammatory process and the presence of cell-stimulating signals may create an optimal environment for cell proliferation and differentiation [22, 24]. Such mechanism could act both locally and at a distance [22, 24]. Furthermore, other authors hypothesized that a para-inflammation mechanism (a low-grade inflammation that could be associated to periodontitis [25]) can be involved in cancer development [26].
Published systematic reviews of the literature have investigated the association between periodontitis and oral cancer [23, 27]. Even though a positive correlation was found in one meta-analysis, the validity of the results was limited by the criteria adopted for periodontal assessment in the included studies [28]. Another systematic review of the literature, published by Fitzpatrick and Katz in 2010, found a positive association between periodontitis and any type of cancer, although this was only a qualitative analysis of the included papers [13]. To our knowledge, a comprehensive review of the literature with meta-analysis is missing in the literature and, for this reason, the present study was carried out.
The aim of the present systematic review of the literature was to evaluate if, in humans (P), having periodontitis (I) (compared to being periodontally healthy (C)) implies a higher risk of neoplasms (O).
Materials and methods
The study protocol was approved by the Review Board of the Center for Research in Oral Implantology of the “Università degli Studi di Milano” in Milan, Italy in January 2016. The protocol was registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO) before the beginning of the research with the number CRD42016036061.
The study was reported following the instructions of the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement [29] and was conducted according to the Cochrane Handbook [30].
PICO question
In human subjects, does having periodontitis (compared to being periodontally healthy) increase the risk of neoplasms initiation (P: human subjects; I (Indicator): Periodontitis; C: No periodontitis; O: Cancer)?
Search strategy
An electronic search of the following databases was conducted: MEDLINE / PubMed, Scopus, ISI Web of Science, Cochrane Central and EMBASE using an ad hoc created search string obtained combining pertinent keywords with the use of boolean operators “OR” and “AND”. The search string for PubMed was: ("periodont*"[All Fields] OR "periodontal disease*"[All Fields]) AND ("cancer*"[All Fields] OR "oncolog*"[All Fields] OR "leukoplakia"[All Fields] OR "eritroplakia"[All Fields]). Grey literature was also searched (Greylit, OpenGrey). The reference list of the included papers and the table of contents of Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, Journal of Dentistry, Journal of Dental Research, CA—A Cancer Journal for Clinicians, Nature Reviews Cancer, The Lancet Oncology, Journal of Clinical Oncology, Annals of Oncology, Clinical Cancer Research, and European Journal of Cancer were manually searched beginning from 2000. The last electronic search was performed September, 20th 2017.
Selection criteria
Two authors (SC, MDF) independently screened titles and abstracts and then full texts evaluating them for potential inclusion on the basis of the following selection criteria:
Studies on human subjects
Case-control and prospective cohort studies
Studies in which data about cases (subjects who developed neoplasms) and controls (subjects who did not develop neoplasms) could be distinguished and extrapolated
Clear definition of periodontitis
Description of how confounders were controlled in the analysis (adjustments)
The level of concordance, calculated through Cohen’s kappa, between the two reviewers was 0.92 for titles and abstracts and 0.98 for full texts.
In case of disagreement in the article selection process a third reviewer (LF) was asked to decisively solve the discussion.
Data extraction
Two reviewers (SC, LF) independently collected the following data from the studies included:
Author names, year of publication, country of examination, sample characteristics (ethnicity, mean age, smoking status, alcohol consumption), definition of periodontal disease (periodontitis), type of neoplasia, outcome measure (the diagnosis of a neoplasm in the exposed and in non-exposed group) and parameters for adjustment.
If the information provided in the paper was insufficient, the corresponding author of the article would have been contacted for the missing data. However, all studies provided sufficient information about outcomes.
Quality assessment for included studies
The quality assessment of the included study was performed using the tool for quality assessment of case-control and cohort studies elaborated by the National Institute of Health—National Heart, Lung, and Blood Institute (https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort). The tool was designed to assess the research question, the characteristics of study population, the recruitment procedure and sample size justification, if the exposure was assessed prior to outcome measurement, if the timeframe was sufficient, the characteristics of the exposure, how outcomes were recorded, the follow-up rate and the statistical analysis. Such tool was independently used for assessment by the two reviewers (SC, RW) (k = 0.93).
Studies that scored “No” for one or no items were judged having good quality, those that score “No” for more than one but less than three items were judged having fair quality. Other studies were judged of poor quality and were excluded from the quantitative analysis.
Summary measures, synthesis of the results and additional analysis
The synthesis of the results was performed with the use of the software RevMan (Review Manager Version 5.3, 2014; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark).
For studies presenting data on the same cohort with different follow-up, only the report with the longer time of observation was considered for the quantitative synthesis. The most adjusted model (and outcome measure) was used when more models were presented in the same study.
The measure of the association between cancer and periodontitis (hazard ratio (HR) which is the hazard in the exposed groups divided by the hazard in the non-exposed groups and relative 95% confidence interval (CI) (an interval estimator)) was extrapolated from the papers presenting it. In studies in which such parameter was not reported it would have been calculated through appropriate method. However, all included papers presented HRs.
In the meta-analysis, in order to estimate the association between periodontitis and cancer in adult subjects, the method of inverse variance was used combining the results using the DerSimonian and Laird’s random-effect model [31] and the Mantel-Haenszel fixed-effect model [32]. The analysis was performed using as the summary measure pooled HR. For each measure, pooled estimate of 95% CI was calculated. Standard error (SE) was computed as follows: SE = ((ln (Upper CI) / ln (Lower CI)) / 3.92).
The consistency of the results was measured using Cochran’s test considering it significant if P < 0.1. The quantification of such heterogeneity was computed through I2 statistics, that served to describe the total variation across studies that was due to heterogeneity rather than to chance. If I2 was found to be less than 40% the heterogeneity was negligible, if it was found from 40% to 60% it signified a moderate heterogeneity, if from 60% to 90% it signified a substantial heterogeneity while it showed a considerable heterogeneity if it was from 75% to 100% [30].
One single analysis was performed for each type of cancer as it was defined in the included studies. In case one single study was present for one type of cancer, a meta-analysis could not be performed and the results of the study were extracted and presented.
Sensitivity analysis was performed by substituting one paper with a less recent one when belonging to the same study, or substituting one outcome value in one study with another one with less adjustments.
Results
Study selection
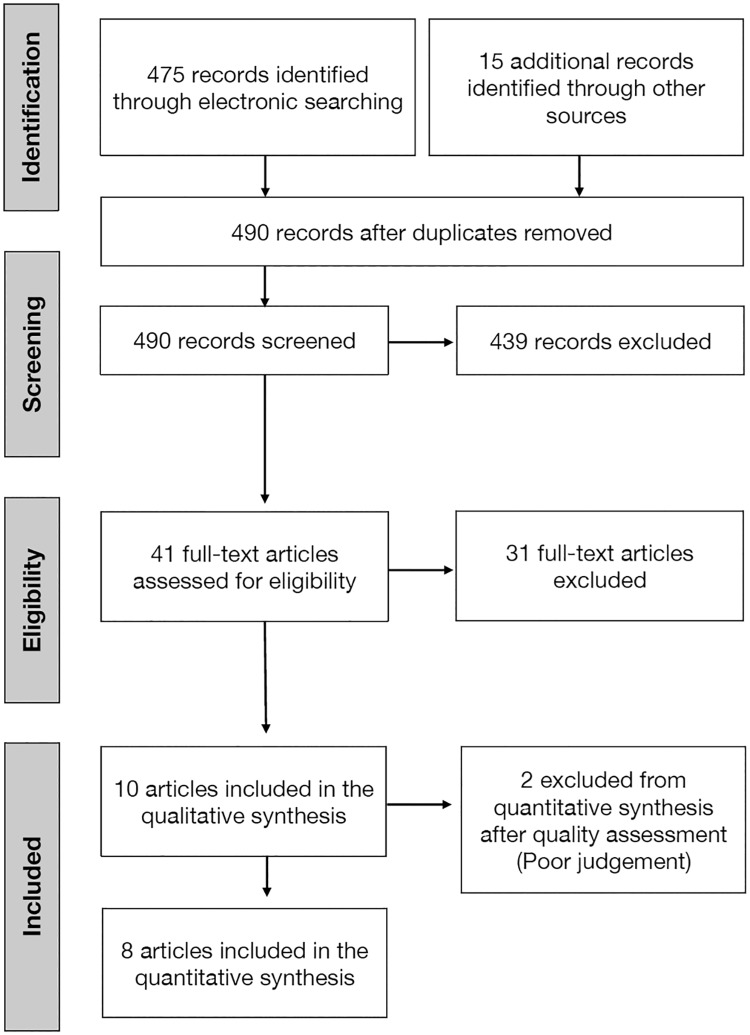
The flowchart of article selection process is shown in Fig 1. The literature search resulted in the identification of 475 papers from electronic databases and 15 from a manual search. Of the 490 titles and abstracts retrieved, 439 papers were excluded as they were inconsistent with the aims of the review. A total of 41 full texts were assessed for eligibility; after screening for inclusion criteria 31 papers were excluded (the reasons for exclusion are presented in Table 1) and 10 papers (referring to 6 studies) were finally considered in the review.
Fig 1. Diagram of article selection process.
Table 1. Excluded papers and reasons for exclusion.
| Studies | Reason for exclusion |
|---|---|
| Abnet et al. 2001, Abnet et al. 2005a, Abnet et al. 2005b, Abnet et al. 2008, Ansai et al. 2013, Bundgaard et al. 1995, Divaris et al. 2010, Fernandez-Garrote et al. 2010a, Hiraki et al. 2008, Marshall et al. 1992, Stolzenberg et al. 2003, Talamini et al. 2000, Tu et al. 2007, Watabe et al. 1998, Wei et al. 2005, Zheng et al. 1990 | No periodontitis but tooth loss |
| Ahn et al. 2012 | Narrative review |
| Cabrera et al. 2005 | Association between poor oral health and cardiovascular disease |
| Chang et al. 2016 | Association between all periodontal diseases (including gingivitis) and pancreatic cancer |
| Demirer et al. 1990, Guha et al. 2007, Rosenquist et al. 2005, Sepehr et al. 2005, Talamini et al. 2000 | Association between oral health in general and cancer |
| Hujoel et al. 2003 | Association between periodontitis and mortality for cancer |
| Mai et al. 2015 | Association between periodontal pathogens and cancer |
| Mai et al. 2016, Salazar et al. 2012, Soder et al. 2011, Tezal et al. 2005, Tezal et al. 2007 | Unclear / No definition of periodontitis |
| Yen et al. 2014 | Other association |
a No periodontitis but tooth loss and gingival bleeding
Study characteristics
The main characteristics of the six included studies are presented in Table 2. One study was set up in Sweden [33], and five in the United States [34–42]. With regard to characteristics of the population, one study was composed of twins from the Swedish Twin Registry [33] and one was composed on people from nine medical facilities in the area of Boston [35]. Two studies were case-control [35, 38] and this should be considered as a potential source of methodological heterogeneity among the included papers.
Table 2. General characteristics of the included studies.
| Study ID | Authors | Year | Country | Population characteristics | Cancer type | Periodontal assessment |
|---|---|---|---|---|---|---|
| #1 | Arora et al. | 2010 | Sweden | 55% female; Twins from Swedish Twin Registry (n = 15,333) | Any cancer; Digestive tract; Colorectal; Pancreas; Stomach; Bladder; Prostate; Breast; Corpus Uteri; Lung | Self-assessed in 1963 through a questionnaire: “Have you noticed that some of your own teeth have come loose or fallen out on their own?” |
| #2 | Eliot et al. | 2013 | USA | Nine medical facilities in the Boston (USA) area (n = 1,080) | Oral cavity; Pharynx; Larynx | Self-assessed and self-reported |
| #3 | Mai et al. | 2014 | USA | Womens’s Health Initiative Observational Study; Postmenopausal women; Mean age 48.3 years; (n = 93,676) | Lung | Self-assessed asking the question: “Has a dentist or dental hygienist ever told you that you had periodontal or gum disease?” [42] |
| Freudenheim et al. | 2015 | Breast | ||||
| #4 | Michaud et al. | 2007 | USA | Health Professionals Follow-up Study (57.6% dentists) (n = 51,529) | Pancreas | Self-assessed asking the question: “Have you had periodontal disease with bone loss?” [43, 44] |
| Michaud et al. | 2008 | Any cancer; Lung; Oropharynx; Esophagus; Stomach; Pancreas; Colon-Rectus; Kidney; Bladder; Prostate; Hematopoietic; Brain; Melanoma | ||||
| Michaud et al. | 2016 | Any cancer; Prostate; Colon-Rectus; Melanoma; Bladder; Lung; Kidney; Esophagus and oropharynx; Pancreas | ||||
| Bertrand et al. | 2017 | Non-Hodgkin lymphoma | ||||
| #5 | Momen-Heravi et al. | 2017 | USA | Nurses’ Health Study (n = 77,443) | Colorectal cancer | Self-reported asking the question “Have you had periodontal bone loss diagnosed by a physician?” |
| #6 | Mazul et al. | 2017 | USA | Carolina Head and Neck Cancer Study (n = 492) | Head and Neck Squamous Cell Carcinoma | History of “gum disease diagnosed by a dentist” |
Two of the included articles [36, 37] reported data from the Women’s Health Initiative Observational Study (WHIOS), that enlisted 93,676 postmenopausal women. Four papers [34, 39–41] reported data from the Health Professionals Follow-up Study (HPFS) that is composed of 51,529 men in health professions (57.6% dentists). One article [42] was based on data from the Nurses’ Health Study (NHS) and one [38] from the Caroline Head and Neck Cancer Study (CHNCS). HR was used as outcome measure in eight papers [33–37, 40–42], OR was used in two papers [35, 38] and RR in one paper [39]. Outcome measures and adjustments are shown in Table 3. Two studies examined population that was composed exclusively by women (WHIOS and NHS), one exclusively by men (HPFS) and two studied a population composed both by men and women (CHNCS and Swedish Twin Registry). Differences in the studied population could be considered as a further source of heterogeneity.
Table 3. Outcomes and adjustments.
| Study ID | Authors | Year | Outcomes | Adjustments |
|---|---|---|---|---|
| #1 | Arora et al. | 2010 | HR | Gender, age, education, employment, number of siblings, smoking status, smoking status of partner, alcohol status, diabetes, body mass index |
| #2 | Eliot et al. | 2013 | OR | Age, Gender, Race, Smoking, Alcohol status, education, annual household income |
| #3 | Mai et al. | 2014 | HR | Unadjusted; Age; MODEL A: Age, smoking status, pack-years; MODEL B: MODEL A + education, race, BMI, alcohol status, hormone use, dental visits, physical activity, region of residence, aspirin use, secondhand smoke |
| Freudenheim et al. | 2015 | Age; MODEL 1: Age, Education, Race, BMI, Age at menarche, Age at menopause, Parity, Age at first birth, Hormone use, Alcohol status, Physical activity, NS Anti-Inflammatory Drugs; MODEL 2: MODEL 1 + Smoking status, pack-years | ||
| #4 | Michaud et al. | 2007 | RR | Age; MODEL A: Age, smoking history, profession, race, geographic location, history of diabetes, BMI, height, history of cholecystectomy, Nonsteroideal anti-inflammatory drug use, multivitamin use, baseline teeth numbers; MODEL B: dietary intakes of fruits and vegetables, vitamin D, calcium, sucrose, and total calories |
| Michaud et al. | 2008 | HR | MODEL A: Age, race, physical activity, diabetes, alcohol status, BMI, geographical location, height, calcium intake, red-meat intake, fruit and vegetables intake, vitamin D score; MODEL B: MODEL A + smoking history, pack-years | |
| Michaud et al. | 2016 | Age, Race, Alcohol status, physical activity, diabetes, BMI, geographical location, height, NSAID use | ||
| Bertrand et al. | 2017 | Age, Race, Diabetes history, BMI at baseline, geographical location, smoking, NSAID use | ||
| #5 | Momen-Heravi et al. | 2017 | HR | Age, race, smoking, history of colorectal cancer in a parent or sibling, history of sigmoidoscopy / colonscopy, current physical activity, regular aspirin use, multivitamin use, type 2 diabetes, alcohol consumption, adult BMI, energy-adjusted intake of total calcium, vitamin D, folate, red meat and processed meat and postmenopausal hormone use |
| #6 | Mazul et al. | 2017 | OR | Age, race, sex, alcohol use, socioeconomic status (income, insurance, education) |
HR: Hazard Ratio; OR: Odds Ratio; COPD: Chronic Obstructive Pulmonary Disease; BMI: Body Mass Index; NSAID: Non-Steroideal Anti-Inflammatory Drug
With regard to the methods of assessing the presence of periodontitis, in one paper periodontal status was assessed once in 1963 by a question [33], it was self-reported and self-assessed in another study [35] but none of the questionnaires used for these studies were validated before. In one study, periodontal status was explored asking if a “gum disease” was ever diagnosed by a dentist [38]. In the WHIOS the questionnaire used to evaluate the presence of periodontal disease was previously validated by LaMonte and coworkers in 2014 [43]. In the HPFS, the questionnaire used was validated both in non-professionals [44] and in dental professionals of the same cohort [45]. In one paper [42] the same questionnaire of HPFS was used but it was not specifically validated for the NHS cohort.
Cancer types were classified according to the International Classification of Diseases (ICD) Ninth Edition in most of the included papers [33, 35–37, 42]. In one study cases with cancer were selected from Swedish National Cancer Register [33]. In other studies cancer type was assessed through a questionnaire that was then confirmed by medical records [34, 36, 37, 39–42].
Quality assessment
A summary of the results of the quality assessment of the included studies is presented in Table 4. Two papers were judged, on the basis of the considered parameters, to be of poor quality and were excluded from the quantitative synthesis [35, 38]; both of them did not assess if the exposure (periodontitis) was present before the development of cancer.
Table 4. Summary of quality assessment.
| Study ID | Authors | Year | Quality rating | Reason for downgrading |
|---|---|---|---|---|
| #1 | Arora et al. | 2010 | Fair | Definition / assessment of periodontitis not validated Periodontal conditions measured once |
| #2 | Eliot et al. | 2013 | Poor | No sample size justification Definition / assessment of periodontitis not validated Periodontal conditions measured once |
| #3 | Mai et al. | 2014 | Good | - |
| Freudenheim et al. | 2015 | Good | - | |
| #4 | Michaud et al. | 2007 | Good | - |
| Michaud et al. | 2008 | Good | - | |
| Michaud et al. | 2016 | Good | - | |
| Bertrand et al. | 2017 | Good | - | |
| #5 | Momen-Heravi et al. | 2017 | Fair | No sample size justification Definition / assessment of periodontitis not validated |
| #6 | Mazul et al. | 2017 | Poor | No sample size justification Definition / assessment of periodontitis not validated Periodontal conditions measured once |
Results of the quantitative analysis
The results of the meta-analysis are summarized in Table 5. A statistically significant association was found considering all cancers, digestive tract cancer (evaluated in the study by Arora and coworkers; HR = 1.34 [1.05, 1.72] [33]), pancreatic cancer, prostate cancer, breast cancer, corpus uteri cancer (evaluated in the study by Arora and coworkers; HR = 2.20 [1.16, 4.18] [33]), lung cancer, hematological cancer (evaluated in the study by Michaud and coworkers published in 2008; HR = 1.30 [1.11, 1.53] [41]), and esophagus / oropharyngeal cancer (evaluated in the study by Michaud and coworkers in 2016; HR = 2.25 [1.30, 3.90] [40]) pooled together and Non-Hodgkin lymphoma (evaluated by Bertrand and coworkers; HR = 1.30 [1.11, 1.52] [34]). The heterogeneity was negligible or its evaluation was not applicable if only one study was included in the meta-analysis.
Table 5. Summary of the results.
| Cancer | Outcome | N° of Studies | Value [95% CI] | Test for overall effect P | I2 | |
|---|---|---|---|---|---|---|
| Any cancer | ||||||
| HR | 2 | 1.14 [1.04, 1.24] | 0.004 | 0% | ||
| Colon—rectus | ||||||
| Fair quality | HR | 2 | 0.90 [0.73, 1.11] | 0.42 | 0% | |
| Good quality | HR | 1 | 1.03 [0.76, 1.40] | 0.85 | N/A | |
| All | HR | 3 | 0.94 [0.79, 1.12] | 0.49 | 0% | |
| Pancreas | ||||||
| HR | 2 | 1.74 [1.21, 2.52] | 0.003 | 0% | ||
| Stomach | ||||||
| HR | 2 | 1.03 [0.71, 1.48] | 0.90 | 0% | ||
| Bladder | ||||||
| HR | 2 | 1.31 [0.93, 1.84] | 0.12 | 0% | ||
| Prostate | ||||||
| HR | 2 | 1.25 [1.04, 1.51] | 0.02 | 16% | ||
| Breast | ||||||
| HR | 2 | 1.11 [1.00, 1.23] | 0.04 | 0% | ||
| Lung | ||||||
| Fair quality | HR | 1 | 1.41 [0.81, 2.46] | 0.23 | N/A | |
| Good quality | HR | 2 | 1.22 [1.04, 1.44] | 0.58 | 0% | |
| All | HR | 3 | 1.24 [1.06, 1.45] | 0.007 | 0% |
CI: Confidence Interval; HR: Hazard Ratio; N/A: Not applicable
Sensitivity analysis
Sensitivity analysis did not find any changes in the evaluation of the association between periodontitis and cancers. One exception was found in the association between periodontitis and esophageal cancer, as it was evaluated in the paper by Michaud and co-workers published in 2008 [41]. Considering the HR obtained after some adjustments (model A, see Table 3) a significant association was found while it was not significant using other adjustments (model B).
Discussion
The present systematic review of the literature found a small, but statistically significant association, between the diagnosis of periodontitis and the presence of cancer. The meta-analysis performed considering HRs as outcomes found an association between periodontitis and the presence of any type of cancer as well as the presence of specific neoplasms such as digestive tract cancer, pancreatic cancer, prostate cancer, breast cancer, corpus uteri cancer, lung cancer, hematological cancer, and esophagus / oropharyngeal cancer pooled together and Non-Hodgkin lymphoma.
In order to interpret adequately the validity of the obtained results, several limitations of the study should be considered.
One important limitation is the criteria of selection of the included papers. In order to remain adherent to the aim of the review we included only papers evaluating patients with periodontitis. Studies that correlate cancer with tooth loss, to the amount of attachment loss or to other clinical measures were excluded because such parameters could be modified also by clinical conditions other than periodontitis. As an example, tooth loss could be caused by a number of factors such as caries, root fractures, infection of endodontic origin, and dental or maxillary trauma [46–48]. Tooth loss could also be associated to low socioeconomic status that is also considered as an important risk factor for the development of cancer in general and oral cancer particularly [49–52]. Then, considering the definitions and classification schemes used for periodontitis in included studies, several considerations should be made. The use of self-reported periodontitis, even though validated [43–45], could be considered a significant bias because they are based substantially on subjective perception. However, it should be considered that one of the used questionnaire had a 0.78 and 0.76 positive predictive values (respectively among dentists and non-dentist health professionals) [44, 45] and another one showed a moderate accuracy to characterize periodontal disease prevalence [43]. In general, most of the included studies provided an insufficient description of the methods used for classification, when performed by a dental specialist. In one study the authors classified cases of periodontal diseases using the distance between the cemento-enamel junction and the bone crest, as evaluated through periapical radiographs [53]. The absence of a validation of this method and of the description of which threshold was use to define cases causes the exclusion of the paper from the present review. Another study by Tezal and colleagues related oral cancer to the presence of sites with clinical attachment loss higher than 1.5 mm [12]. One cross-sectional study found a positive correlation between the level of bleeding on probing and gastric precancerous lesions [54].
Another issue to be considered is the outcome measure. Hazard ratios were used in most of the considered epidemiological studies even though a criticism was raised about the value of HR as an indicator of a causal relationship between two conditions [51]. Other studies used ORs as outcome measures, aiming at measuring the association between exposure and outcome. Differently from HRs, which represent a point estimate, ORs derive from post hoc calculation. Considering this, the two outcomes could not be pooled in the meta-analysis, thus reducing the number of papers available for each comparison. However, none of the studies included in the quantitative synthesis presented the results as ORs.
The relatively strict inclusion criteria used in the present study have considerably limited the number of studies available for the meta-analysis and this was because we chose to select only studies that were comparable. Indeed, for two cancer types (colon-rectus, and lung) meta-analysis included three papers, for six types (all cancers, stomach, bladder, prostate, pancreas and breast) the meta-analysis included two papers, and for other types just one paper for each was available. Although some authors it was stated that two papers could be sufficient to perform a meta-analysis [55], we should consider the number of available studies as a limitation, and consequently the results have a relatively low statistical power. Another limitation could appear the different ethnicity of the populations studies that could be considered as a further source of heterogeneity.
Considering these limitations, the results of the present systematic review should be considered with caution as compared to the available literature, in particular previously published systematic reviews of the literature. One narrative review of the literature published in 2010 by Fitzpatrick and Katz provided evidence of a significant association between periodontal disease and oral cancer while the evidence of a link to other types of cancer was questionable and controversial [13]. However, such conclusions were based on a narrative, although exhaustive, interpretation of the results without any attempt of meta-analysis, including studies relating cancer and tooth loss, thus incurring in the limitation exposed above.
With regard to narrative reviews, they reported a correlation between periodontitis (or periodontal diseases in general, since a heterogeneity among assessment of periodontal status was reported in the included papers) and head and neck cancer, even though the lack of a meta-analysis (due to the narrative nature of these papers) and the choice of inclusion criteria (broader that those used in the present review) could have limited the validity of the results [27, 56].
Other published meta-analyses on the same topic should be considered carefully when comparing to the results obtained in the present study. One paper reported the outcomes of one meta-analysis attempting to relate the prevalence rate of P. gingivalis and the development of cancer [21]. This study found that the prevalence of such bacteria increased the chance of cancer development by 1.36 times. It has to be considered that the reported CI (95%) for such OR was 0.47–3.07 based on a total of four studies, two supporting the association and two not supporting. Moreover, the test for overall effect was missing. So, the interpretation of the OR value should be made with extreme caution. The absence of a strong association between the presence of some bacteria and cancer was confirmed also by a paper published in 2016 reporting the result of a prospective study on postmenopausal women [57].
With regard to the sole oral cancer, the association with periodontitis was evaluated in a meta-analysis published by Yao and co-workers in 2014 [28]. A significant association was reported (OR = 3.53, 95% CI (1.52–8.23); P = 0.003) even though a substantial heterogeneity among the studies was found in all comparisons and this was probably due to the differences in the assessment methods, which were significantly different. This result confirmed the one obtained in another meta-analysis published by Zeng and colleagues in 2013 [58], albeit showing a consistent heterogeneity among the studies included in the quantitative synthesis. In fact, the hypothesis that local risk factors could be the cause of a relationship between periodontitis and oropharyngeal cancer than between other cancer types is still in need of a scientific support.
With regard to the relation between periodontitis and cancer mortality, one study that was not included in the present review, found some correlation with mortality for lung cancer, but the effect of potential confounders should be furtherly explored [59].
Considering the limitations, the present study found a low but statistically significant association between periodontitis and different types of cancer (both alone and pooled together). Despite the statistical significance, the clinical value (external validity) and the statistical power of this relationship were significantly limited by the low number of papers included in the quantitative synthesis, and we can speculate that the evidence of such correlation needs more support to be hypothesized and it is far to be considered conclusive.
In order to better understand the mechanisms and to explore the existence and the strength of the association between cancer and periodontitis more studies are needed, with standardized methods for periodontal evaluation, assessment and classification, and representative samples.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Corbella S, Taschieri S, Del Fabbro M, Francetti L, Weinstein R, Ferrazzi E. Adverse pregnancy outcomes and periodontitis: A systematic review and meta-analysis exploring potential association. Quintessence international. 2016;47(3):193–204. doi: 10.3290/j.qi.a34980 [DOI] [PubMed] [Google Scholar]
- 2.Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontology 2000. 2013;62(1):271–86. doi: 10.1111/prd.12007 [DOI] [PubMed] [Google Scholar]
- 3.Faggion CM Jr., Cullinan MP, Atieh M. An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control. Journal of periodontal research. 2016. [DOI] [PubMed] [Google Scholar]
- 4.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. Journal of clinical periodontology. 2013;40 Suppl 14:S8–19. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt A, Carra MC, Boutouyrie P, Bouchard P. Periodontitis and arterial stiffness: a systematic review and meta-analysis. Journal of clinical periodontology. 2015;42(11):977–87. doi: 10.1111/jcpe.12467 [DOI] [PubMed] [Google Scholar]
- 6.Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. The Cochrane database of systematic reviews. 2015;11:CD004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budzynski J, Wisniewska J, Ciecierski M, Kedzia A. Association between Bacterial Infection and Peripheral Vascular Disease: A Review. The International journal of angiology: official publication of the International College of Angiology, Inc. 2016;25(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papapanou PN. Systemic effects of periodontitis: lessons learned from research on atherosclerotic vascular disease and adverse pregnancy outcomes. International dental journal. 2015;65(6):283–91. doi: 10.1111/idj.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. Journal of clinical periodontology. 2008;35(4):277–90. doi: 10.1111/j.1600-051X.2007.01173.x [DOI] [PubMed] [Google Scholar]
- 10.Sahingur SE, Yeudall WA. Chemokine function in periodontal disease and oral cavity cancer. Frontiers in immunology. 2015;6:214 doi: 10.3389/fimmu.2015.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125(12):1327–36. doi: 10.1080/00016480510012273 [DOI] [PubMed] [Google Scholar]
- 12.Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? Journal of periodontology. 2005;76(3):406–10. doi: 10.1902/jop.2005.76.3.406 [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. Journal of dentistry. 2010;38(2):83–95. doi: 10.1016/j.jdent.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 14.Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer causes & control: CCC. 2008;19(9):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hormia M, Willberg J, Ruokonen H, Syrjanen S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. Journal of periodontology. 2005;76(3):358–63. doi: 10.1902/jop.2005.76.3.358 [DOI] [PubMed] [Google Scholar]
- 16.Saygun I, Kubar A, Ozdemir A, Slots J. Periodontitis lesions are a source of salivary cytomegalovirus and Epstein-Barr virus. Journal of periodontal research. 2005;40(2):187–91. doi: 10.1111/j.1600-0765.2005.00790.x [DOI] [PubMed] [Google Scholar]
- 17.Slots J, Saygun I, Sabeti M, Kubar A. Epstein-Barr virus in oral diseases. Journal of periodontal research. 2006;41(4):235–44. doi: 10.1111/j.1600-0765.2006.00865.x [DOI] [PubMed] [Google Scholar]
- 18.Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. International journal of oral science. 2011;3(4):209–15. doi: 10.4248/IJOS11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuboniwa M, Hasegawa Y, Mao S, Shizukuishi S, Amano A, Lamont RJ, et al. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes and infection / Institut Pasteur. 2008;10(2):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cellular microbiology. 2007;9(8):1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayehmiri F, Sayehmiri K, Asadollahi K, Soroush S, Bogdanovic L, Jalilian FA, et al. The prevalence rate of Porphyromonas gingivalis and its association with cancer: A systematic review and meta-analysis. International journal of immunopathology and pharmacology. 2015;28(2):160–7. doi: 10.1177/0394632015586144 [DOI] [PubMed] [Google Scholar]
- 22.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gondivkar SM, Gondivkar RS, Gadbail AR, Chole R, Mankar M, Yuwanati M. Chronic periodontitis and the risk of head and neck squamous cell carcinoma: facts and figures. Experimental oncology. 2013;35(3):163–7. [PubMed] [Google Scholar]
- 24.Mantovani A, Pierotti MA. Cancer and inflammation: a complex relationship. Cancer letters. 2008;267(2):180–1. doi: 10.1016/j.canlet.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 25.Barton MK. Evidence accumulates indicating periodontal disease as a risk factor for colorectal cancer or lymphoma. CA Cancer J Clin. 2017. [DOI] [PubMed] [Google Scholar]
- 26.Aran D, Lasry A, Zinger A, Biton M, Pikarsky E, Hellman A, et al. Widespread parainflammation in human cancer. Genome Biol. 2016;17(1):145 doi: 10.1186/s13059-016-0995-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javed F, Warnakulasuriya S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Critical reviews in oncology/hematology. 2016;97:197–205. doi: 10.1016/j.critrevonc.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 28.Yao QW, Zhou DS, Peng HJ, Ji P, Liu DS. Association of periodontal disease with oral cancer: a meta-analysis. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(7):7073–7. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Green S. Cochrane Handbook for Systematic Review of Interventions: The Cochrane Collaboration; 2011. www.cochrane-handbook.org. [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 32.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 33.Arora M, Weuve J, Fall K, Pedersen NL, Mucci LA. An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. American journal of epidemiology. 2010;171(2):253–9. doi: 10.1093/aje/kwp340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand KA, Shingala J, Evens A, Birmann BM, Giovannucci E, Michaud DS. Periodontal disease and risk of non-Hodgkin lymphoma in the Health Professionals Follow-Up Study. International journal of cancer. 2017;140(5):1020–6. doi: 10.1002/ijc.30518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eliot MN, Michaud DS, Langevin SM, McClean MD, Kelsey KT. Periodontal disease and mouthwash use are risk factors for head and neck squamous cell carcinoma. Cancer causes & control: CCC. 2013;24(7):1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freudenheim JL, Genco RJ, LaMonte MJ, Millen AE, Hovey KM, Mai X, et al. Periodontal Disease and Breast Cancer: Prospective Cohort Study of Postmenopausal Women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mai X, LaMonte MJ, Hovey KM, Nwizu N, Freudenheim JL, Tezal M, et al. History of periodontal disease diagnosis and lung cancer incidence in the Women’s Health Initiative Observational Study. Cancer causes & control: CCC. 2014;25(8):1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazul AL, Taylor JM, Divaris K, Weissler MC, Brennan P, Anantharaman D, et al. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2017;123(1):71–80. doi: 10.1002/cncr.30312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. Journal of the National Cancer Institute. 2007;99(2):171–5. doi: 10.1093/jnci/djk021 [DOI] [PubMed] [Google Scholar]
- 40.Michaud DS, Kelsey KT, Papathanasiou E, Genco CA, Giovannucci E. Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the Health Professionals Follow-up Study. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. The Lancet Oncology. 2008;9(6):550–8. doi: 10.1016/S1470-2045(08)70106-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Momen-Heravi F, Babic A, Tworoger SS, Zhang L, Wu K, Smith-Warner SA, et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. International journal of cancer. 2017;140(3):646–52. doi: 10.1002/ijc.30486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaMonte MJ, Williams AM, Genco RJ, Andrews CA, Hovey KM, Millen AE, et al. Association between metabolic syndrome and periodontal disease measures in postmenopausal women: the Buffalo OsteoPerio study. Journal of periodontology. 2014;85(11):1489–501. doi: 10.1902/jop.2014.140185 [DOI] [PubMed] [Google Scholar]
- 44.Joshipura KJ, Douglass CW, Garcia RI, Valachovic R, Willett WC. Validity of a self-reported periodontal disease measure. J Public Health Dent. 1996;56(4):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshipura KJ, Pitiphat W, Douglass CW. Validation of self-reported periodontal measures among health professionals. J Public Health Dent. 2002;62(2):115–21. [DOI] [PubMed] [Google Scholar]
- 46.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. Journal of dental research. 2015;94(5):650–8. doi: 10.1177/0022034515573272 [DOI] [PubMed] [Google Scholar]
- 47.Salehrabi R, Rotstein I. Epidemiologic evaluation of the outcomes of orthograde endodontic retreatment. Journal of endodontics. 2010;36(5):790–2. doi: 10.1016/j.joen.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 48.van der Velden U, Amaliya A, Loos BG, Timmerman MF, van der Weijden FA, Winkel EG, et al. Java project on periodontal diseases: causes of tooth loss in a cohort of untreated individuals. Journal of clinical periodontology. 2015;42(9):824–31. doi: 10.1111/jcpe.12446 [DOI] [PubMed] [Google Scholar]
- 49.Bernabe E, Sheiham A. Tooth loss in the United Kingdom—trends in social inequalities: an age-period-and-cohort analysis. PloS one. 2014;9(8):e104808 doi: 10.1371/journal.pone.0104808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conway DI, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LM. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. International journal of cancer. 2008;122(12):2811–9. doi: 10.1002/ijc.23430 [DOI] [PubMed] [Google Scholar]
- 51.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommer I, Griebler U, Mahlknecht P, Thaler K, Bouskill K, Gartlehner G, et al. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC public health. 2015;15:914 doi: 10.1186/s12889-015-2227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mai X, LaMonte MJ, Hovey KM, Freudenheim JL, Andrews CA, Genco RJ, et al. Periodontal disease severity and cancer risk in postmenopausal women: the Buffalo OsteoPerio Study. Cancer causes & control: CCC. 2016;27(2):217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salazar CR, Francois F, Li Y, Corby P, Hays R, Leung C, et al. Association between oral health and gastric precancerous lesions. Carcinogenesis. 2012;33(2):399–403. doi: 10.1093/carcin/bgr284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35(2):215–47. [Google Scholar]
- 56.Han YW, Houcken W, Loos BG, Schenkein HA, Tezal M. Periodontal disease, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Advances in dental research. 2014;26(1):47–55. doi: 10.1177/0022034514528334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mai X, Genco RJ, LaMonte MJ, Hovey KM, Freudenheim JL, Andrews CA, et al. Periodontal Pathogens and Risk of Incident Cancer in Postmenopausal Females: The Buffalo OsteoPerio Study. Journal of periodontology. 2016;87(3):257–67. doi: 10.1902/jop.2015.150433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng XT, Deng AP, Li C, Xia LY, Niu YM, Leng WD. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PloS one. 2013;8(10):e79017 doi: 10.1371/journal.pone.0079017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association. Annals of epidemiology. 2003;13(5):312–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.