Abstract
Allene oxide synthase (AOS) and fatty acid hydroperoxide lyase (HPL) are plant-specific cytochrome P450s that commit fatty acid hydroperoxides to different branches of oxylipin metabolism. Here we report the cloning and characterization of AOS (LeAOS) and HPL (LeHPL) cDNAs from tomato (Lycopersicon esculentum). Functional expression of the cDNAs in Escherichia coli showed that LeAOS and LeHPL encode enzymes that metabolize 13- but not 9-hydroperoxide derivatives of C18 fatty acids. LeAOS was active against both 13S-hydroperoxy-9(Z),11(E),15(Z)-octadecatrienoic acid (13-HPOT) and 13S-hydroperoxy-9(Z),11(E)-octadecadienoic acid, whereas LeHPL showed a strong preference for 13-HPOT. These results suggest a role for LeAOS and LeHPL in the metabolism of 13-HPOT to jasmonic acid and hexenal/traumatin, respectively. LeAOS expression was detected in all organs of the plant. In contrast, LeHPL expression was predominant in leaves and flowers. Damage inflicted to leaves by chewing insect larvae led to an increase in the local and systemic expression of both genes, with LeAOS showing the strongest induction. Wound-induced expression of LeAOS also occurred in the def-1 mutant that is deficient in octadecanoid-based signaling of defensive proteinase inhibitor genes. These results demonstrate that tomato uses genetically distinct signaling pathways for the regulation of different classes of wound responsive genes.
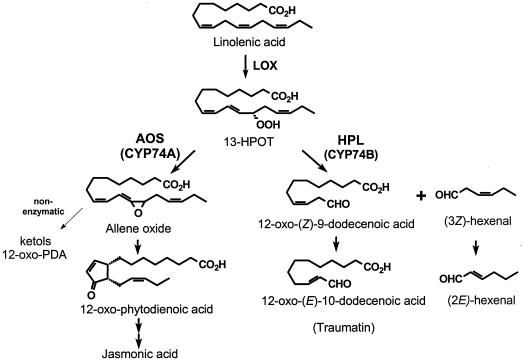
Fatty acid hydroperoxides produced by 13-lipoxygenases are important intermediates in the oxylipin pathway of fatty acid oxygenation in plants. In one branch of oxylipin metabolism often referred to as the octadecanoid pathway, allene oxide synthase (AOS) commits 13S-hydroperoxy-9(Z),11(E),15(Z)-octadecatrienoic acid (13-HPOT) to the formation of jasmonic acid (JA) and related cyclopenta(e)nones (Creelman and Mullet, 1997) (Fig. 1). Products of the AOS pathway are essential signals for plant defense against pest attack (Staswick and Lehman, 1999), mechanical responses (Weiler et al., 1993), and some developmental processes (McConn and Browse, 1996). An alternative pathway for 13-HPOT metabolism is initiated by fatty acid hydroperoxide lyase (HPL; Fig. 1). Short chain aldehyde products of HPL, together with their corresponding reduced alcohols, are important volatile constituents of the characteristic odor of fruits, vegetables, and green leaves (Gardner, 1991; Hatanaka, 1993). C6 aldehydes produced by HPL are also reported to act as phytoalexins against protozoa, bacteria, and fungi (for review, see Blée, 1998), and may be signals for gene regulation (Bate and Rothstein, 1998). The C12 oxo-acid product of HPL is the precursor of the previously identified “wound signal” known as traumatin (Zimmerman and Coudron, 1979). 13-HPOT is metabolized by other plant enzymes including lipoxygenase (Salch et al., 1995), peroxygenase (Blée et al., 1993), and divinyl ether synthase (Grechkin et al., 1995; Hamberg, 1998), and may be subject to degradation by non-specific alkyl hydroperoxide reductases (Baier and Dietz, 1999).
Figure 1.
Cyt P450-dependent metabolism of 13-HPOT. AOS (CYP74A) commits 13-HPOT to the production of JA and related cyclopenta(e) nones. In the absence of allene oxide cyclase (AOC), the epoxide product of AOS undergoes spontaneous hydrolysis to α- and γ-ketols and racemic 12-OPDA. HPL (CYP74B) cleaves 13-HPOT to produce C6 and C12 products that are further metabolized as shown.
AOS and HPL comprise an unusual class of cytochrome (Cyt) P450s that is specialized for the rearrangement of fatty acid hydroperoxides. Unlike typical P450 monoxygenases, AOS and HPL demonstrate low affinity for carbon monoxide and do not require O2 or NADPH-dependent Cyt P450 reductase for their activity (Song and Brash, 1991; Shibata et al., 1995a, 1995b). Identification of cDNA sequences encoding AOS and HPL has provided additional insight into the relationship between these two enzymes, and their divergence from classical P450s (Song et al., 1993; Pan et al., 1995; Laudert et al., 1996; Matsui et al., 1996; Bate et al., 1998). Based on the amino acid sequence identity between AOS and HPL (approximately 38%), the two enzymes are classified as subfamilies CYP74A and CYP74B, respectively, within the CYP74 family of P450s (Nelson, 1999).
The importance of oxylipins as signals for plant stress responses has prompted interest in understanding the mechanisms by which their synthesis is regulated (Creelman and Mullet, 1997; Farmer et al., 1998). JA accumulation, for example, is stimulated by mechanical wounding and herbivory (Creelman et al., 1992; Blechert et al., 1995; Conconi et al., 1996), pathogen attack (Penninckx et al., 1996), treatment with elicitors (Gundlach et al., 1992; Doares et al., 1995), and water or nutrient deprivation (Creelman and Mullet, 1995; Lehmann et al., 1995). Similarly, mechanical injury and some plant-pathogen interactions lead to the production of HPL products (Hatanaka et al., 1987; Gardner, 1991; Croft et al., 1993). Formation of AOS- and HPL-derived oxylipins is controlled in large part by the availability of hydroperoxide substrates that are generated from lipase/acyl hydrolase-mediated release of fatty acids from membrane lipids, followed by lipoxygenase-catalyzed conversion to 9- and 13-hydroperoxides (Galliard et al., 1977; Hatanaka, 1993; Mueller et al., 1993; Narváez-Vásquez et al., 1999). Nonenzymatic lipid peroxidation, such as that associated with the initial stages of plant-pest interactions, may also contribute to the pool of hydroperoxides available to AOS and HPL (Gardner, 1989; Hammond-Kosack and Jones, 1996).
In addition to substrate availability, fatty acid hydroperoxide metabolism may also be influenced by the spatial and temporal expression of enzymes that utilize these substrates. For example, the localization of both AOS and HPL to the chloroplast (Vick and Zimmerman, 1987; Song et al., 1993; Blée and Joyard, 1996; Laudert et al., 1996; Bate et al., 1998; Froehlich et al., 1999) suggests that these enzymes utilize a common pool of hydroperoxide substrates. Recent studies indicate that AOS expression is positively regulated by wounding, as well as by terminal products of the AOS pathway (Laudert and Weiler, 1998). These results, together with transgenic studies showing that AOS is a rate-limiting step in JA biosynthesis (Harms et al., 1995), indicate that up-regulation of AOS activity during the wound response may provide a mechanism to amplify the octadecanoid signaling pathway. On the other hand, others have shown that exogenous methyl JA stimulates oxylipin metabolism through the HPL pathway, and thus may shift oxylipin metabolism away from JA biosynthesis (Avdiushko et al., 1995; Kohlmann et al., 1999).
The aim of the present work was to gain an understanding of the molecular basis of Cyt P450-dependent metabolism of fatty acid hydroperoxides in tomato (Lycopersicon esculentum). Owing to the wealth of knowledge of plant-pest interactions in tomato, this system is likely to provide a good model for assessing the role of oxylipins in plant defense. The importance of HPL-derived volatiles in determining the flavor and aroma of fruits and vegetables provides additional incentive for investigating oxylipin metabolism in tomato (Kazeniac and Hall, 1970; Buttery and Ling, 1993). Toward this goal, we report here the isolation of cDNAs that encode functional members of the CYP74A (AOS) and CYP74B (HPL) subfamilies of P450 enzymes in tomato. The results of expression studies in Escherichia coli suggest a role for AOS and HPL in the commitment of 13-HPOT to the JA and C6 aldehyde/traumatin pathways, respectively. We also report findings relevant to the developmental and defense-related expression of these two genes in planta. The significance of these results for understanding the regulation of fatty acid hydroperoxide metabolism is discussed.
RESULTS
cDNA Isolation and Sequence Analysis
An AOS-encoding cDNA from Arabidopsis (Laudert et al., 1996) was used to screen for related sequences in a tomato cDNA library. The longest clone obtained (designated as LeAOS) contained a 1,533-bp open reading frame, a 57-bp 5′-untranslated region (UTR), and a 111-bp 3′-UTR excluding the poly(A) tail. The open reading frame was predicted to encode a 510-amino acid protein having a calculated molecular mass of 57,202 D. The presence of an in-frame stop codon (UAA) 30 nucleotides upstream of the putative AUG start codon indicated that LeAOS contained the full-length coding sequence. The deduced amino acid sequence of LeAOS was approximately 61% identical to AOS from flax (Song et al., 1993), guayule (Pan et al., 1995), and Arabidopsis (Laudert et al., 1996) (Table I). Thus, LeAOS is classified as a new member of the CYP74A subfamily of Cyt P450s. The N-terminal region of LeAOS displayed features of a typical chloroplast targeting peptide including an enrichment of hydroxylated amino acids. Conclusive evidence that LeAOS is localized to the chloroplast was recently obtained (Froehlich et al., 1999). These findings indicate that LeAOS is more similar to AOS from flax and Arabidopsis, which also reside in the chloroplast (Song et al., 1993; Harms et al., 1995; Laudert et al., 1996), than it is to the cytosolic AOS from guayule (Pan et al., 1995).
Table I.
Percent amino acid and nucleotide identity between different AOSs and HPLs
| LuAOS | AtAOS | PaAOS | LeAOS | LeHPL | CaHPL | AtHPL | |
|---|---|---|---|---|---|---|---|
| LuAOS | 59.9 | 65.0 | 61.1 | 36.1 | 36.0 | 34.9 | |
| AtAOS | 55.7 | 60.5 | 62.2 | 38.2 | 37.2 | 36.3 | |
| PaAOS | 56.2 | 55.6 | 60.1 | 36.5 | 36.1 | 34.8 | |
| LeAOS | 53.1 | 57.5 | 55.9 | 35.6 | 35.3 | 35.9 | |
| LeHPL | 36.3 | 38.7 | 38.0 | 36.0 | 87.6 | 55.3 | |
| CaHPL | 36.3 | 38.5 | 38.7 | 36.5 | 85.5 | 53.2 | |
| AtHPL | 37.8 | 38.9 | 36.6 | 36.3 | 51.2 | 49.1 |
Values on the upper right diagonal of the matrix indicate the percentage of amino acid identity between different family members. Values on the lower left of the matrix indicate the percentage of nucleotide identity. Percentage identity within the open reading frame of each pair of sequences was calculated using DNA Star software (Clustal method). AOS sequences were from flax (Linum usitatissimum), guayule (Parthenium argentatum), Arabidopsis, and tomato. HPL sequences were from pepper, Arabidopsis, and tomato. GenBank accession nos. for the sequences are given in Figure 2.
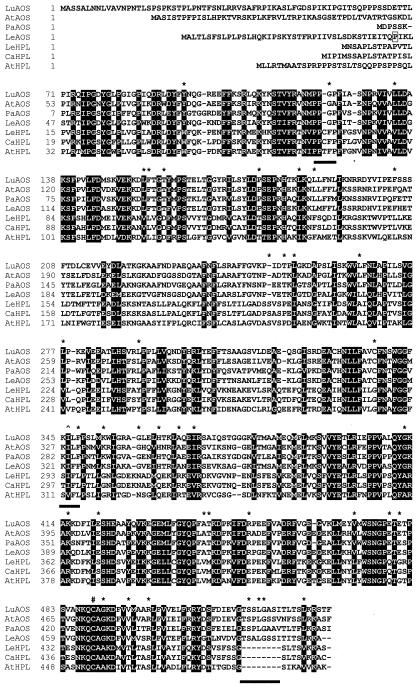
A cDNA encoding HPL from bell pepper (Capsicum annum) was used to screen a tomato cDNA library for related sequences. Among the 15 positive clones identified, the longest cDNA (designated LeHPL) contained a 1,431-bp open reading frame that was predicted to specify a 476-amino acid protein with a molecular mass of 53,542 D. LeHPL contained a 169-bp 5′-UTR, and a 210-bp 3′-UTR excluding poly(A) residues. The presence of an in-frame stop codon (UGA) 93 nucleotides upstream of the putative initiator AUG codon indicated that LeHPL encoded the entire protein. This was confirmed by DNA sequence analysis of RACE products derived from the 5′ end of LeHPL transcripts (“Materials and Methods”). The deduced amino acid sequence of LeHPL was 88% and 55% identical to the published sequence of HPL from bell pepper (Matsui et al., 1996) and Arabidopsis (Bate et al., 1998), respectively (Table I). This establishes LeHPL as a new member of the CYP74B subfamily of Cyt P450s. Unlike HPL from Arabidopsis (Bate et al., 1998), LeHPL does not appear to contain a typical chloroplast targeting sequence at the N terminus of the protein (Fig. 2).
Figure 2.
Comparison of cDNA-deduced protein sequences of plant AOS and HPL genes. LeAOS and LeHPL sequences were aligned, using the ClustalW 1.7 program available at http://mbcr.bcm.tmc.edu/searchlauncher. AOS sequences were from flax (Song et al., 1993; accession no. U00428), guayule (Pan et al., 1995; accession no. X78166), and Arabidopsis (Laudert et al., 1996; accession no. Y12636). HPL sequences were from bell pepper (Matsui et al., 1996; accession no. U51674) and Arabidopsis (Bate et al., 1998; accession no. AF087932). Black boxes indicate amino acid residues that are conserved between all seven CYP74 members. Subfamily-specific substitutions are indicated with an asterisk. The three subfamily-specific motifs discussed in the text are underlined by the black bars. The symbol denotes the T → (I/V) change within the I helix that is a hallmark of CYP74 enzymes. The conserved Cys within the heme-binding domain is marked by a # symbol. The boxed residue (Pro-43) at the N terminus of LeAOS denotes the site where the His-tag was added in the pQE-AOS expression construct.
Comparisons between the primary sequences of the seven known CYP74 members (four AOSs and three HPLs) revealed 182 positions (38%) that were conserved in all members of both subfamilies (Fig. 2). Many conserved residues were clustered at the N and C termini, and may be important for functions common to HPL and AOS (e.g. heme or substrate binding). We also noted subfamily-specific amino acid differences that might play a role in distinguishing AOS function from that of HPL. Specifically, there were 39 positions at which an amino acid was invariant among all AOSs, and was substituted to a different residue in all HPLs (Fig. 2). One HPL-specific motif was PPxFP, which represents a variation of the N-terminal PPGP tetrapeptide that is important for stability and catalysis in many P450s (Szczesna-Skorpa et al., 1993). A hallmark of all CYP74 enzymes, including LeAOS and LeHPL, is a T → (V/I) substitution within the I helix that, in most P450s, participates in O2 binding (Song et al., 1993). Sequences surrounding this site show a subfamily-specific character, with the AOS and HPL consensus sequences being KI(L/F)F and (S/T)IFL, respectively. Several other subfamily-specific signatures were located near the C-terminal heme-binding domain. The most striking of these was an eight-amino acid insertion in AOS sequences relative to HPL sequences, at the extreme C-terminal end of the protein (Fig. 2).
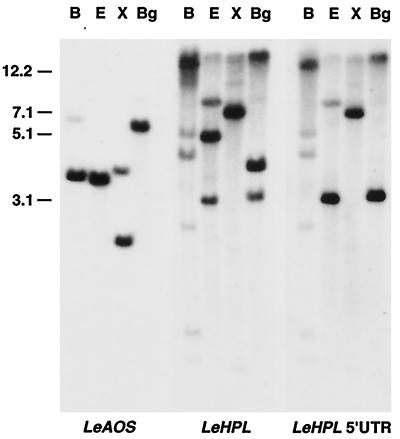
Genomic DNA-blot analysis using the LeAOS cDNA as a probe revealed a simple hybridization pattern for each of the restriction enzymes tested (Fig. 3). This result indicates that LeAOS is derived from a single copy gene. However, detection of additional hybridizing bands (data not shown) under conditions of reduced stringency leaves open the possibility of related sequences in the genome. The results of hybridization analysis using a LeHPL cDNA probe revealed a more complex pattern of weakly and strongly hybridizing bands (Fig. 3). Use of a probe derived from the 5′-UTR of LeHPL reduced the complexity of the hybridization pattern as expected, but nevertheless still detected two or more bands for each restriction digest tested (Fig. 3, right panel). These results indicate that LeHPL is one member of a family of highly related genes that may be clustered as tandem repeats in one region of the genome.
Figure 3.
Southern-blot analysis of LeAOS and LeHPL. Genomic DNA from tomato was digested with restriction enzymes BamHI (B), EcoRV (E), XbaI (X), or BglII (Bg). DNA blots were hybridized to labeled probes derived from the open reading frame of LeAOS (left), LeHPL (middle), or the LeHPL 5′-UTR (right). Blots were hybridized in 5× SSPE at 65°C and washed in 0.5× SSPE at the same temperature, as described in “Materials and Methods.” Molecular mass standards (in kb) are indicated on the left.
Functional Expression of LeAOS and LeHPL in E. coli
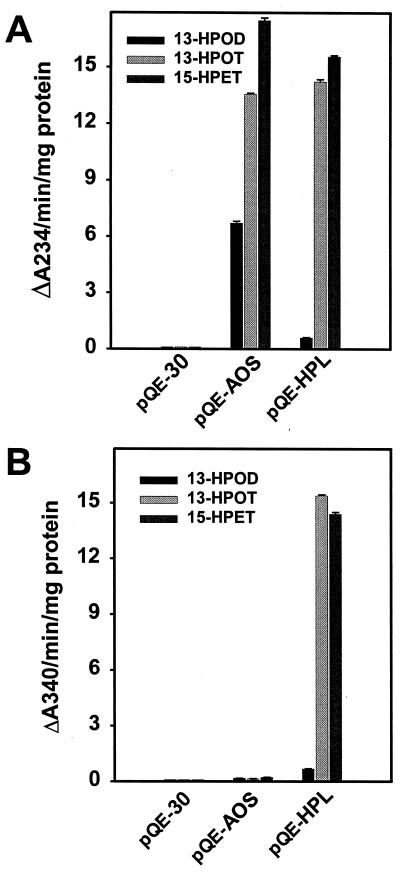
To confirm that LeAOS and LeHPL encode the expected P450 enzymes, the cDNAs were subcloned into the pQE-30 expression vector to yield pQE-AOS and pQE-HPL, respectively, and transformed into an appropriate E. coli host. Bacterial cultures induced to express the constructs accumulated high levels of the recombinant proteins as determined by SDS-PAGE of bacterial lysates (data not shown). Crude lysates from cells expressing either pQE-AOS or pQE-HPL efficiently degraded 13-HPOT (Fig. 4A) but did not metabolize 9-hydroperoxide derivatives of linolenic or linoleic acid (data not shown). Recombinant LeAOS and LeHPL metabolized the C20 hydroperoxide 15S-hydroperoxy-11(Z),13(E),17(Z)-eicosatrienoic acid at a rate comparable to that observed with 13-HPOT (Fig. 4A), indicating that both enzymes can accommodate a range of fatty acid hydroperoxide substrates. However, the two enzymes differed in their ability to metabolize 13S-hydroperoxy-9(Z), 11(E)-octadecadienoic acid (13-HPOD), a common C18 hydroperoxide derived from linoleic acid. Whereas LeAOS utilized 13-HPOD at about one-half the rate observed with 13-HPOT, the rate of breakdown of 13-HPOD by LeHPL was less than 5% of that observed for 13-HPOT. Similar results were obtained using a coupled enzyme assay (Vick, 1991) to measure aldehyde production in the in vitro reactions. As expected from the known products of AOS and HPL (Fig. 1), aldehyde production was associated with reactions catalyzed by LeHPL but not by LeAOS (Fig. 4B). This assay also confirmed the strong preference of LeHPL for 13-HPOT over 13-HPOD.
Figure 4.
Activity of LeAOS and LeHPL expressed in E. coli. Total lysates of E. coli cells expressing pQE-AOS, pQE-HPL, or the empty vector (pQE-30) were tested for their ability to metabolize C18 (13-HPOD and 13-HPOT) and C20 (15-HPET) fatty acid hydroperoxides. Activity was measured either directly as a decrease in absorbance of the substrate at A234 (A) or indirectly as the production of aldehydes using a NADH-coupled assay (B). Error bars represent the mean and sd of activity determined from three enzyme preparations of each culture.
Gas chromatography-mass spectometry (GC-MS) was used to identify the trimethylsilyl (TMS) derivatives of metabolites produced upon incubation of 13-HPOT with lysates from bacteria that expressed either pQE-AOS, pQE-HPL, or the pQE-30 mock control. In the case of pQE-AOS, three prominent peaks (A, B, and C) that were not present among the products of the mock reaction were observed. The relative abundance of these compounds, as estimated by integration of the GC peak areas, was 22% (peak A), 100% (peak B), and 13% (peak C) (values normalized to peak B). The retention time (11 min 9 s), molecular ion ([M]+. at m/z 364), and fragmentation pattern of peak A were identical to that of an authentic 12-oxo-phytodienoic (12-OPDA) standard (Cayman Chemical, Ann Arbor, MI). Peak B eluted at 12 min 37 s and gave the following mass spectrum as m/z (% relative intensity, ion structure): 526 (18%, [M]+.), 511 (13%, [M − CH3]+.), 457 (100%, [M − C5H9]+.), 367 (8%), 221 (5%), 179 (4%), 147 (12%), and 73 (28%, TMS+). This fragmentation pattern was consistent with identification of the compound as the tri-TMS derivative of the α-ketol compound 12-oxo-13-hydroxy-9(Z),15(Z)-octadecadienoic acid (enolization of the 12-oxo group provided an additional hydroxyl for derivatization). The major fragment at m/z = 457 ([M − C5H9]+.) indicated that this compound represented the α-ketol rather than the γ-ketol (12-oxo-9-hydroxy-10[E],15(Z)-octadecadienoic acid). Peak C eluted with a retention time of 13 min 10 s and produced a mass spectrum identical to that of peak B, indicating probable double bond isomerization during derivatization or GC analysis. α-Ketol and 12-OPDA, together with minor amounts of γ-ketol, are known to arise by spontaneous hydrolysis of the unstable epoxide product of AOS (Song and Brash, 1991) (Fig. 1). The products of the pQE-HPL-catalyzed reaction were analyzed by GC-MS as the oxime, TMS derivatives. The major product eluted with a retention time of 8 min 59 s. A molecular ion, m/z 371 [M+.], was observed for the di-TMS derivative of this product, and the fragmentation pattern was identical to that of an authentic 12-oxo-trans-10-dodecenoic acid standard, the expected product of HPL (Fig. 1). These results confirmed the identity of LeAOS and LeHPL as functional members of the CYP74A and CYP74B subfamilies of P450 enzymes, respectively.
Developmental Expression of LeAOS and LeHPL
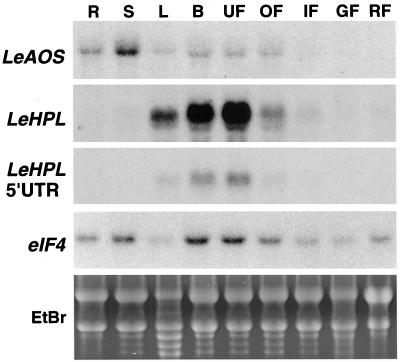
RNA-blot analysis was used to investigate the distribution of LeHPL and LeAOS mRNA in different organs of tomato (Fig. 5). LeHPL transcripts accumulated to high levels in developing flowers, and decreased during flower maturation. LeHPL mRNA levels were also relatively high in leaf tissue, with greater expression detected in younger leaves compared to older leaves from the same plant (see Fig. 7). Very low levels of LeHPL mRNA were detected in stems and immature green fruit, whereas roots and mature green and red fruit lacked detectable transcripts. Hybridization probes derived from either the full-length LeHPL cDNA or the LeHPL 5′-UTR revealed a similar organ-specific expression pattern (Fig. 5). This result showed that LeHPL transcripts detected by RNA-blot analysis are derived from a single LeHPL gene, or highly related LeHPL genes that have a similar developmental expression pattern.
Figure 5.
Expression of LeAOS and LeHPL genes in different organs of tomato. Total RNA was extracted from roots (R), stems (S), leaves (L), developing flower buds (B), mature unopened flowers (UF), mature opened flowers (OF), small (<0.5 cm) immature green fruit (IF), mature green fruit (GF), or mature red fruit (RF). Ten-microgram samples of RNA were subjected to RNA-blot analysis. Specific transcripts were detected by hybridization of blots to probes corresponding to full-length LeAOS, full-length LeHPL, the 5′-UTR of LeHPL, or an eIF4A probe used as a loading control. Also shown is a photograph of an ethidium bromide-stained gel of the RNA used for the experiment (EtBr).
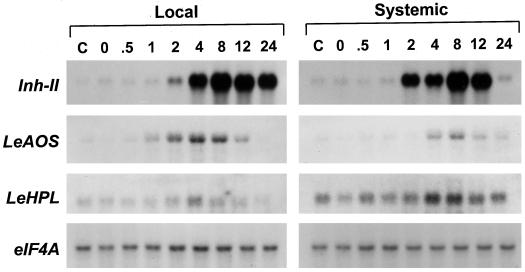
Figure 7.
Accumulation of LeAOS, LeHPL, and Inh-II mRNAs in tomato plants in response to herbivory. Tobacco hornworm larvae (third instar) were placed onto the lower leaf of 3-week-old cv Micro-Tom plants and allowed to feed for 5 to 10 min. During this period, approximately 5% to 10% of the area of the attacked leaf was consumed by the larvae. Leaf tissue was harvested for extraction of total RNA immediately after removal of the larvae (0 point) or at the times indicated (in h). RNA was prepared separately from the lower damaged leaf (Local response) and from the third leaf (counted from the base of the plant) (Systemic response). RNA was also prepared from a set of control plants that received no damage (C). Duplicate RNA blots containing 5 μg of RNA per sample were hybridized to cDNA probes for proteinase inhibitor II (Inh-II), LeAOS, LeHPL, and eIF4A as a loading control.
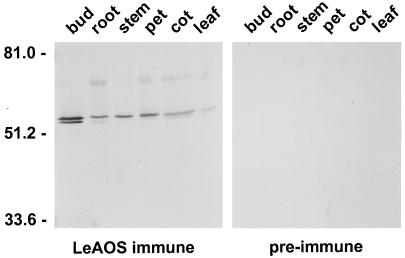
LeAOS mRNA was broadly distributed among all organs examined (Fig. 5). LeAOS transcript levels were relatively low in fruit, and appeared to decrease during fruit development. Polyclonal antibodies raised against recombinant LeAOS, but not preimmune serum from the same rabbit, reacted with a polypeptide in the membrane fraction of extracts prepared from different organs (Fig. 6). The estimated mass of the cross-reacting polypeptide as judged by SDS-PAGE was 55 kD, which is consistent with that expected for the LeAOS gene product. Furthermore, the distribution of this polypeptide in different organs correlated with the distribution of LeAOS mRNA. Taken together, these results indicate that LeAOS is expressed in all tomato organs with the possible exception of ripe fruit. A second cross-reacting polypeptide of slightly lower Mr was often observed in immunoblot experiments, particularly in extracts derived from flowers (Fig. 6). This band could represent a polypeptide that shares common epitopes with LeAOS, or a post-translationally modified form of LeAOS.
Figure 6.
Accumulation of LeAOS protein in different organs of tomato. Fifteen-microgram samples of membrane protein prepared from young flower buds (buds), roots (root), stems (stem), petioles (pet), cotyledons (cot), and leaves (leaf) were separated by SDS-PAGE. Protein was transferred to Immobilon-P membranes and probed with either antiserum raised against LeAOS (left) or an equivalent amount of preimmune serum (right). The numbers on the left of the figure indicate the position of Mr standards.
Wound-Inducible Expression of LeAOS Is Mediated by a Def-1-Independent Signaling Pathway
The importance of oxylipin metabolism for wound-inducible defense gene expression in tomato prompted us to examine the effect of wounding on LeAOS and LeHPL gene expression. Damage inflicted to tomato leaves by Manduca sexta larvae resulted in a modest (approximately 2-fold) increase in LeHPL mRNA accumulation (Fig. 7). Wound-induced accumulation of LeHPL mRNA was more apparent in the lower damaged leaf than it was in the younger undamaged leaf. This is likely to reflect the higher constitutive expression of LeHPL in younger leaves. Wound-induced accumulation of LeAOS transcripts was much more apparent, and thus became the focus of additional experiments. The time course and amplitude of LeAOS expression differed in several ways from that of the well-characterized proteinase inhibitor II (Inh-II) gene (Fig. 7). Whereas the maximum level of induction of LeAOS in local and systemic leaves was approximately 9- and 5-fold, respectively, Inh-II mRNA levels in these tissues increased by at least 60-fold. Wound-inducible accumulation of LeAOS mRNA was also more transient than that of Inh-II. These results indicated that the mechanism controlling wound-inducible expression of LeAOS might be different from that regulating Inh-II gene expression.
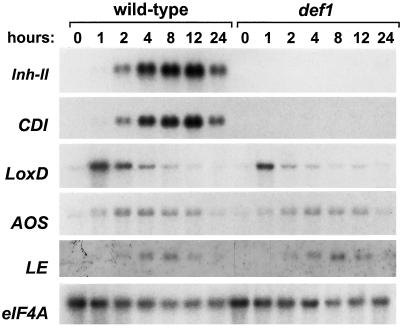
To further test this idea, we examined LeAOS expression in the tomato def-1 mutant that is deficient in the octadecanoid-based signaling pathway that mediates the expression of Inh-II and other defense-related genes (Howe et al., 1996). Previous characterization of def-1 showed that it is deficient in JA accumulation in response to wounding and other elicitors (Howe et al., 1996). Genetic mapping studies have shown that the def-1 phenotype does not result from a defect in the LeAOS gene (A. Itoh and G.A. Howe, unpublished data). Moreover, direct measurements of 12-OPDA levels in def-1 and wild-type plants indicated that the mutant has both AOS and allene oxide cyclase activity (B. Stelmach, E. Weiler, G.A. Howe, unpublished data). Taken together, the available evidence suggests that the Def-1 gene product plays a role in the regulation of a late step in the biosynthesis of JA, or in the further metabolism of JA (e.g. transport or stability). A dramatic aspect of the def-1 phenotype is the lack of wound-induced accumulation of defensive proteinase inhibitor genes such as Inh-II and cathepsin D inhibitor (CDI) (Fig. 8). In contrast to this, the pattern of wound-inducible LeAOS mRNA accumulation in def-1 plants was comparable to that in wild-type plants. This effect was not specific for LeAOS, as other transcripts, including those for lipoxygenase (LoxD) (Heitz et al., 1997) and a senescence-induced RNase (LE) (Lers et al., 1998), were also induced by wounding in both mutant and wild-type plants. These results demonstrate that wound-induced expression of LeAOS, LoxD, and LE mRNA is Def-1 independent.
Figure 8.
Analysis of wound-induced gene expression in wild-type and def-1 mutant plants. Fifteen-day-old wild-type (cv Castlemart) and def-1 mutant seedlings were mechanically wounded at the distal end of the terminal leaflet of the lower leaf. Undamaged tissue on the same leaflet was harvested for RNA extraction at the indicated times after wounding. RNA blots were hybridized to cDNA probes for proteinase inhibitor II (Inh-II), cathepsin D inhibitor (CDI), TomLoxD (LoxD), LeAOS (AOS), LE RNase (LE), and eIF4A as a loading control.
DISCUSSION
Fatty acid hydroperoxides derived from lipoxygenase are precursors for an array of oxylipins that function in diverse aspects of plant growth and development. In this paper we report the isolation and characterization of tomato cDNAs encoding AOS and HPL, two similar P450 enzymes that commit 13-HPOT to different branches of oxylipin metabolism. The LeAOS and LeHPL proteins are 36% identical at the amino acid level, and are classified as members of the CYP74A and CYP74B subfamilies of Cyt P450s, respectively. Identification of AOS and HPL genes in tomato brings the total number of reported AOS and HPL sequences to seven. In comparing the primary sequences of these, we noted 39 positions at which all AOS members contained one common amino acid and all HPLs contained a different residue. The significance of these subfamily-specific substitutions will become more or less apparent as additional CYP74 genes are identified. Subfamily-specific substitutions might reflect differences in the catalytic properties or substrate specificity of the two classes of enzymes. The facile expression of recombinant forms of AOS and HPL in E. coli should facilitate studies aimed at understanding the structure-function relationship that defines the catalytic identity of these unusual P450s.
A major difference between the predicted amino acid sequence of LeAOS and LeHPL was the presence of a typical chloroplast targeting sequence at the N terminus of LeAOS (Fig. 2). Previous studies indicate that chloroplast targeting peptides are present on AOS from flax and Arabidopsis (Song et al., 1993; Harms et al., 1995; Laudert et al., 1996), as well as HPL from Arabidopsis (Bate et al., 1998). A plastid location for AOS and HPL is consistent with biochemical studies demonstrating that AOS and HPL activity is associated with chloroplasts (Vick and Zimmerman, 1987; Gardner et al., 1991; Blée and Joyard, 1996; Zhuang et al., 1996). Recently, we have shown that LeAOS is imported into chloroplasts where it specifically targets to the inner membrane of the chloroplast envelope (Froehlich et al., 1999). This finding suggests that LeAOS obtains its hydroperoxide substrates from one or both of the plastid-localized lipoxygenases (TomLoxC and TomLoxD) that have been described in tomato (Heitz et al., 1997). In contrast to LeAOS, the deduced N terminus of LeHPL lacked a typical transit peptide. That the N-terminal sequence of LeHPL is very similar to that of bell pepper HPL (Fig. 2) suggests that these proteins share a similar subcellular location. Given the preponderance of evidence indicating that HPL activity is associated with the chloroplast, additional experiments aimed at determining the subcellular location of LeHPL are clearly warranted.
Characterization of LeAOS
Expression of LeAOS in E. coli showed that the open reading frame encodes an authentic CYP74A enzyme (LeAOS) that metabolizes 13- but not 9-hydroperoxides of linoleic and linolenic acids. LeAOS expression was detected in all organs of the plant except mature red fruit. Similar expression patterns were observed for AOS in Arabidopsis and flax (Harms et al., 1998; Laudert and Weiler, 1998). Accumulation of LeAOS mRNA and protein in flowers is consistent with previous studies in Arabidopsis showing that AOS promoter activity is high in flowers, particularly in pollen sacs and pollen grains (Kubigsteltig et al., 1999). It is presently not known whether AOS-derived products are required for pollen development in tomato, as they are in Arabidopsis (McConn and Browse, 1996). Detection of LeAOS mRNA and protein in leaves supports previous reports of AOS activity (Caldelari and Farmer, 1997) and inducible JA synthesis in tomato leaves (Peña-Cortés et al., 1993; Doares et al., 1995; Conconi et al., 1996). The relatively high accumulation of LeAOS mRNA in stems (Fig. 5) compared to that in leaves suggests that LeAOS is expressed in vascular bundles, as was reported to be the case in wounded leaves of Arabidopsis (Kubigsteltig et al., 1999). LeAOS mRNA and protein were also detected in tomato roots. Given that root development appears normal in JA-deficient mutants of Arabidopsis (McConn and Browse, 1996), this result suggests that AOS-derived oxylipins serve a non-developmental role in roots. LeAOS mRNA expression in green fruit, while being relatively low, is consistent with previous studies showing increased levels of cis-JA during the early stages of tomato fruit ripening (Fan et al., 1998). However, our results do not exclude the possibility that JA synthesis in tomato fruit involves a different AOS-encoding gene that is undetectable by high stringency nucleic acid hybridization.
Characterization of LeHPL
Expression of LeHPL in E. coli confirmed that this cDNA encodes a functional member of the CYP74B subfamily of enzymes. LeHPL was similar to LeAOS in its ability to use 13- but not 9-hydroperoxides of C18 fatty acids. However, LeHPL was clearly distinguishable from LeAOS in its strong preference for 13-HPOT over 13-HPOD. This feature is shared by HPL isolated from other sources, including bell pepper (Shibata et al., 1995b), tea leaves (Matsui et al., 1991), and Arabidopsis (Bate et al., 1998). Fauconnier et al. (1997) reported the purification from tomato leaves of an HPL that, like recombinant LeHPL, did not utilize 9-hydroperoxides and showed a strong preference for 13-HPOT over 13-HPOD. However, the purified enzyme displayed a molecular mass (73 kD) much greater than that predicted for LeHPL (53.5 kD). Additional experiments are needed to clarify the relationship between LeHPL and this purified form of tomato leaf HPL.
LeHPL mRNA was most abundant in developing flowers. This finding suggests that HPL-derived products might have a role in the production of floral scent. Relatively high levels of LeHPL mRNA were also detected in leaves. The overlapping expression pattern of LeAOS and LeHPL in leaves is consistent with the idea that these two enzymes compete for the same pool of substrate (Avdiushko et al., 1995; Blée and Joyard, 1996; Blée, 1998). However, additional studies aimed at determining the subcellular and tissue-specific location of both enzymes are needed to substantiate this hypothesis. The paucity of LeHPL mRNA accumulation in mature green and red fruit was surprising since cis-3-hexenal, derived from the action of HPL on 13-HPOT, is a prominent volatile component of the aroma and flavor of tomato fruit (Buttery and Ling, 1993). A possible explanation for these results is that LeHPL plays only a minor, if any, role in the production of C6 volatiles during tomato fruit ripening. Additional insight into the contribution of LeHPL to fruit aroma and flavor might be gained by altering LeHPL expression in transgenic plants.
Wound-Inducible Expression of LeAOS Is Mediated by a Def-1-Independent Signaling Pathway
Damage inflicted to tomato leaves by hornworm larvae triggered the accumulation of LeAOS mRNA both in the damaged leaf and in the upper undamaged leaves of the plant (Fig. 7). Similar changes in LeAOS expression occurred in plants subjected to mechanical wounding (Fig. 8; data not shown). The transient accumulation of LeAOS transcripts in these experiments was a consequence of the limited damage inflicted to the plant (e.g. 5–10 min of feeding by the insect). It is likely that sustained feeding by herbivores, such as that occurring in natural and agricultural ecosystems, would result in much greater increases in LeAOS expression. Increased expression of AOS, and possibly other octadecanoid pathway enzymes, could serve to amplify the JA signaling cascade as a means of enhancing the induced resistance response. Wound-inducible increases in AOS mRNA, protein, and activity have been documented in Arabidopsis (Laudert and Weiler, 1998; Kubigsteltig et al., 1999) and flax (Harms et al., 1998). LeHPL transcript levels also appeared to increase in response to insect attack (Fig. 7) and mechanical wounding (data not shown), but only by about 2- fold relative to unwounded controls. Additional studies are needed to determine whether the expression of LeHPL and LeAOS in tomato leaves is affected by other defense signals, or by interactions with pathogens.
The wound-inducible expression pattern of LeAOS differed in several respects from that of proteinase inhibitor genes. First, the time course of LeAOS mRNA accumulation was more transient than that of the inhibitor genes. Second, the amplitude of LeAOS mRNA accumulation in both damaged and systemic leaves was 10- to 20-fold lower than that of Inh-II mRNAs. Finally, wound-inducible expression of LeAOS was observed in the def-1 mutant, while that of the Inh-II and CDI genes was not. Two additional genes, LoxD and LE, were also wound inducible in the def-1 background. These results demonstrate the existence of two classes of genes whose requirements for wound induction in tomato can be defined as being either Def-1 dependent or Def-1 independent. Given the involvement of Def-1 in wound-inducible JA accumulation (Howe et al., 1996), we suggest that endogenous JA is a signal for Def-1-dependent, but not Def-1-independent, wound responses. It is noteworthy that some genes exhibiting Def-1-independent expression, such as LeAOS and LoxD, are inducible by exogenous JA (Heitz et al., 1997; G.I. Lee and G.A. Howe, unpublished data). This raises the possibility that genes whose expression is altered by exogenous JA might not be under the control of the JA signaling pathway as it operates in planta. Alternatively, LeAOS and LoxD may be controlled by both JA-dependent and -independent wound-response pathways. This interpretation is consistent with the observation that wound-induced accumulation of LeAOS and LoxD mRNA in def-1 plants was slightly less than that in wild-type plants (Fig. 8). Regulation of AOS and LOX activities by both JA-dependent and -independent signaling pathways might allow amplification or increased sensitization of wound responsiveness under different conditions. This notion is consistent with other studies showing that wound and defense responses in tomato involve multiple signaling pathways (Chao et al., 1999; Ryan, 2000). In Arabidopsis, JA-dependent and -independent wound responses have been shown to be differentially regulated by Ca2+/calmodulin, as well as by reversible protein phosphorylation events (Titarenko et al., 1997; León et al., 1998; Rojo et al., 1998). Thorough analysis of mutants such as def-1 or those that are suppressed in the action of systemin (Howe and Ryan, 1999) may provide further insight into the role of oxylipins in wound and defense signaling pathways in tomato.
MATERIALS AND METHODS
Plant Material and Growth Conditions
cv Micro-Tom seed (Lycopersicon esculentum cv Micro-Tom) was obtained from Dr. Avraham Levy (Weizmann Institute, Rehovot, Israel). Seed for the tomato def-1 mutant was collected from a def-1/def-1 homozygous line that had been back-crossed four times to L. esculentum cv Castlemart, the wild-type parent of def-1. Seedlings were grown in Jiffy peat pots (Hummert International, St. Louis) in a growth chamber maintained under 17 h of light (300 μE m−2 s−1) at 28°C and 7 h of dark at 18°C. Flowers and fruit were collected from plants maintained in a greenhouse.
cDNA Cloning and Sequencing
A 1.1-kb XhoI fragment derived from the coding region of an Arabidopsis AOS cDNA (Laudert et al., 1996) was labeled with [α-32P]dCTP and used to screen a tomato cDNA library constructed from tomato plants that overexpress the prosystemin gene, as described by Heitz et al. (1997). Duplicate filters were hybridized at 42°C in a solution containing 5× SSPE, 50% (v/v) formamide, 5× Denhardt's reagent, 0.5% (w/v) SDS, and 50 μg/mL denatured salmon sperm DNA. Filters were washed at 42°C in a solution containing 5× SSPE and 0.5% (w/v) SDS, followed by an additional wash at 65°C. Four positive clones were obtained among approximately 4 × 105 plaque-forming units screened. Following excision of the cDNA from the phagmid, DNA sequence analysis showed that all clones were identical with the exception of minor differences in the length of the 5′ end. The longest cDNA insert, designated LeAOS, was sequenced completely on both strands using a primer walking approach. The sequence of the LeAOS cDNA was deposited to GenBank (accession no. AF230371).
A bell pepper (Capsicum annum) cDNA encoding HPL was isolated using a reverse-transcription PCR (RT-PCR) kit (Life Technologies/Gibco-BRL, Cleveland) and total RNA isolated from bell pepper as a template. Two gene-specific primers were designed from the cDNA sequence reported by Matsui et al. (1996) (GenBank accession no. U51674). The sequence of the forward and reverse primers used for RT-PCR was 5′-(GTG-GAT-CCA-TTC-ATA-AAA-CAA-CAA-CTA-C)-3′ and 5′-(GTG-AAT-TCA-GCA-ACC-TTT-AGT-ACC-TAC-C)-3′, respectively. An amplified 1,477-bp product was subcloned into pBluescript SK(−) (Stratagene, La Jolla, CA) and sequenced to confirm its identity to the published sequence (Matsui et al., 1996). This clone was used to screen a tomato leaf cDNA library as described above but with the following modifications. Filters were washed at 65°C in a solution containing 5× SSPE and 0.5% (w/v) SDS. Fifteen positive plaques were identified among 3 × 105 plaque-forming units screened. DNA sequence analysis of eight cDNA inserts showed that all cDNAs were identical except for minor differences in the length of the 5′ end and the number of poly(A) residues at the 3′ end. The longest clone, designated LeHPL, was subcloned into smaller fragments and sequenced in its entirety on both strands. The sequence of the LeHPL cDNA was deposited to GenBank (accession no. AF230372). A RACE procedure (Life Technologies/Gibco-BRL) was used to obtain additional sequence information at the 5′ end of LeHPL. First strand cDNA was synthesized from total RNA prepared from either tomato leaves or flowers as a template. The sequence of the gene-specific primer used for this reaction was 5′-(ACT-TCC-TTG-GCT-TCA-TTT-T)-3′. PCR amplification of the dC-tailed cDNA was performed using the manufacturer's abridged anchor primer and a gene-specific primer having the sequence 5′-(AGC-GCC-GAG-GAT-AGT-GAG-GGA-GAA)-3′. PCR products were re-amplified using the manufacturer's abridged universal amplification primer and a nested gene-specific primer having the sequence: 5′-(TGG-AGT-GCA-GGA-AGA-AGA-GAA-G)-3′. Amplified PCR products were subcloned into pBluescript SK(−). DNA sequencing of 5′ RACE products derived from both leaf and flower mRNA confirmed the structure of the 5′-UTR of LeHPL, including the presence of the in-frame stop codon upstream of the initiator Met.
Expression of LeAOS and LeHPL in Escherichia coli
A PCR strategy was employed to subclone the LeAOS cDNA into the E. coli expression vector pQE-30 (Qiagen USA, Valencia, CA). Forward and reverse primers were designed to contain BglII and PstI restriction sites, respectively. The sequence of the forward primer was 5′-(GCT-AGA-TCT-CCT-ATA-AAA-TTA-TCT-ACC-AGG)-3′ and that of the reverse primer 5′-(GTT-CTG-CAG-CCG-ATA-GTG-ACA-GTG-TAG-ACC)-3′. Using the LeAOS cDNA as a template, the PCR-amplified product was cut with BglII and PstI and cloned into BamHI and PstI sites of pQE-30. The resulting expression vector was called pQE-AOS. This strategy removed the first 42 amino acids from the N terminus of LeAOS, and added the sequence MRGSHHHHHHGS to Pro 43 of LeAOS (Fig. 2). A similar strategy was used to construct a vector for expression of LeHPL. Forward and reverse primers were designed to contain BamHI and SstI sites, respectively. The sequence of the forward primer was 5′-(CGG-GAT-CCC-CGA-TAA-TGA-ATT-CTG-CTC)- 3′ and that of the reverse primer 5′-(GCG-AGC-TCT-CAT-AAG-TCA-GAA-CAG)-3′. PCR products obtained using the LeHPL cDNA as a template were digested with BamHI and SstI, and cloned into BamHI and SacI sites of pQE-30 to give pQE-HPL. This strategy added the sequence MRGSHHHHHHGSPI to the deduced initiator Met of LeHPL.
Expression constructs pQE-AOS and pQE-HPL were transformed into E. coli strain M15. Bacteria grown under standard conditions (37°C in Luria-Bertani medium) and induced with isopropylthio-β-galactoside produced recombinant protein that was associated with inclusion bodies (data not shown). Induction of cultures using the following procedure significantly enhanced the recovery of active enzyme in the soluble fraction of lysed cells. Bacterial cultures (50 mL) were grown in Terrific Broth medium at 37°C to logarithmic phase (A600 of 0.6), at which time the culture was induced by the addition of isopropylthio-β-galactoside to a final concentration of 0.5 mm. Cultures were incubated for an additional 8 h at 26°C with gentle shaking (150 rpm). Bacteria were harvested by centrifugation and resuspended in 5 mL of a solution containing 50 mm potassium phosphate (pH 7.5) and 5% (v/v) glycerol. Following one freeze-thaw cycle, cells were broken by sonication and centrifuged at 10,000g for 20 min. SDS-PAGE analysis of supernatant protein from induced culture extracts showed the presence of the recombinant protein, migrating with the expected molecular mass (data not shown).
Enzyme Assays and Preparation of Fatty Acid Hydroperoxides
The hydroperoxide-degrading activity of recombinant LeAOS and LeHPL was measured spectrophotometrically using two methods described by Vick (1991). One assay, which does not distinguish between AOS and HPL activity, involved monitoring the decrease in A234 that results from disruption of the conjugated diene bond in the substrate. The second method was specific for HPL and involved an NADH-coupled assay for detection of aldehyde reaction products. The protein content of cell extracts was determined by the Bradford assay. Fatty acid hydroperoxide substrates (9- and 13-substituted) were prepared using soybean lipoxygenase (Sigma-Aldrich, St. Louis) or corn seed lipoxygenase as described (Vick, 1991). Fatty acids for these reactions were obtained from Nu-Chek-Prep, Inc (Elysian, MN). Substrate specificity results were confirmed using purified hydroperoxides (9-HPOD, 9-HPOT, 13-HPOD, 13-HPOT) purchased from Cayman Chemical.
Identification of Metabolites
Two micromoles of 13-HPOT, dissolved in 30 mL of 50 mm potassium phosphate (pH 7.0), was mixed with 1 mg of soluble protein (enzyme source) obtained from E. coli cells expressing either pQE-AOS, pQE-HPL, or the pQE-30 vector control. The reaction was allowed to proceed for 10 min at room temperature and then stopped by acidification to pH 4.0 with 1 m citrate. Products were extracted twice with diethyl ether and dried under N2 gas. TMS derivatives of pQE-AOS reaction products were prepared by treatment of the extract with 30 μL of BSTFA (bis[TMS] trifluoroacetamide/trimethylchlorosilane) (99:1, v/v) (Supelco, Bellefonte, PA) and 10 μL of pyridine for 1 h at 80°C. Oxime TMS derivatives of pQE-HPL products were prepared by first reacting enzyme products with hydroxylamine hydrochloride at 80°C for 1 h, followed by treatment with BSTFA and pyridine as described above. One to 2 μL of the derivatized compounds was used for GC-MS analyses, which were carried out on an AX 505H double focusing mass spectrometer (JEOL, Peabody, MA) equipped with a 5890 gas chromatograph (Hewlett-Packard, Palo Alto, CA). GC separations employed a DB-1 methyl silicone capillary column (30 m × 0.25 mm i.d.) (J&W Scientific, Folsom, CA) interfaced directly to the ion source via a heated transfer block. The temperature program was initiated at 50°C and ramped to 225°C at 20°C min−1. The temperature was then increased to 270°C at 2°C min−1. The ion source was operated at 70 eV with the scan rate of the instrument set to approximately 1 spectra s−1.
Antibody Production and Western-Blot Analysis
A 0.5-L culture of E. coli was induced for the expression of pQE-AOS as described above, except that induced cells were grown at 37°C for 4 h in Luria-Bertani medium. Cells were harvested by centrifugation and resuspended in 1/10 volume of lysis buffer (50 mm sodium-phosphate, pH 8.0, 10 mm imidazole, 300 mm NaCl, and 0.25% [v/v] Emulgen 911 [Kayo Corporation, Tokyo]). Following one freeze-thaw cycle, cells were broken by two passes through a French press calibrated at 17,000 pounds per square inch. Insoluble inclusion bodies containing LeAOS were recovered by centrifugation for 20 min at 8,000g. The pellet was washed twice with 40 mL of lysis buffer and recovered by centrifugation. Washed pellets were solubilized at 4°C in 35 mL of a solution containing 6 m guanidine HCl, 100 mm sodium-phosphate, pH 8.0, and 10 mm Tris (tris[hydroxymethyl]aminomethane) HCl, pH 8.0. The mixture was sonicated for 10 min at 4°C to facilitate solubilization, and then centrifuged at 12,000g for 20 min. Recombinant LeAOS in the supernatant was purified by nickel affinity chromatography as described by the manufacturer (Qiagen). His-tagged LeAOS, which eluted from the nickel column at pH 4.5, was dialyzed twice for 4 h against 100 volumes of 50 mm Tris, and 0.2% (v/v) Emulgen 911. Approximately 0.5 mg of protein was solubilized in Laemmli sample buffer and further purified by preparative SDS-PAGE (12% [w/v] gel). Acrylamide gel slices containing His-tagged LeAOS were stained with Coomassie Brilliant Blue and macerated through a syringe as described (Harlowe and Lane, 1988). For the initial immunization, 100 μg of antigen in the mashed gel slice was mixed with Freund's complete adjuvant and injected at multiple subcutaneous and intramuscular sites of a New Zealand white rabbit. Four boosts, each consisting of 50 μg of antigen mixed with Freund's incomplete adjuvant, were administered over the course of a 90-d immunization schedule.
Protein extracts for western-blot analysis were prepared from fresh plant tissue that was extracted with a mortar and pestle at 4°C in a buffer containing 50 mm sodium-phosphate, pH 7.0. The buffer to tissue ratio (w/w) was about 2:1. Crude cellular debris was removed by centrifugation at 2,000g for 10 min. The membrane fraction of the resulting supernatant was recovered by centrifugation at 100,000g for 15 min at 2°C. Pelleted membranes were washed with 1 m NaCl, and recovered by centrifugation as described above. Membrane material equivalent to 15 μg of total protein was solubilized in Laemmli sample buffer, boiled for 5 min, and separated by SDS-PAGE (10% [w/v] gels). Separated proteins were electrophoretically transferred to Immobilon-P membranes (Millipore, Bedford, MA) in a solution consisting of 25 mm Tris, 192 mm Gly, and 20% (v/v) methanol, using standard procedures (Harlowe and Lane, 1988). Membranes were probed with anti-LeAOS antibodies used at a 1:2,000 dilution in a Tris-buffered saline solution containing 1% (w/v) bovine serum albumin as a blocking agent and 0.05% (w/v) Tween 20 to reduce non-specific binding. Antigen-antibody complexes were detected with the use of an alkaline phosphatase-conjugated second antibody as described by the manufacturer (Kirkegaard and Perry, Gaithersburg, MD).
Southern-Blot Analysis
Genomic DNA from young leaves of cv Micro-Tom plants was purified as described by Rogers and Bendich (1985). Ten-microgram aliquots of DNA were digested with restriction enzymes, electrophoresed on a 0.8% (w/v) agarose gel, and blotted to Duralon-UV membranes (Stratagene) as suggested by the manufacturer. Blots were pre-hybridized at 65°C in a solution containing 5× SSPE, 5× Denhardt's solution, 100 μg/mL denatured salmon sperm DNA, and 0.5% (w/v) SDS. Blots were hybridized at 65°C and washed at the same temperature in a solution containing 0.5× SSPE and 0.5% (w/v) SDS. DNA probes were prepared using a T7 Quickprime Kit (Pharmacia Biotech, Piscataway, NJ). The following cDNA fragments were labeled for use as probes: a 1.7-kb EcoRI-XhoI fragment containing full-length LeAOS; a 1.4-kb EcoRI-HinDIII fragment containing the coding region of LeHPL; and a 0.2-kb EcoRI-EcoRI fragment containing the LeHPL 5′-UTR.
Wounding Experiments
Manducta sexta larvae were reared on artificial diet as described by the vendor (Carolina Biological Supply, Burlington, NC) from which the eggs were purchased. One larva (third instar) was placed on the terminal leaflet of the oldest leaf of a 3-week-old cv Micro-Tom plant that contained three fully expanded leaves. Larvae were allowed to feed on the leaf for 5 to 10 min, during which time 5% to 10% of the area of the leaf was consumed. Plants were sampled for RNA analysis at different times after the challenge. Leaf tissue from six to eight plants per time point was pooled prior to RNA extraction. Mechanical wounding of plants was performed using a hemostat as described previously (Howe et al., 1996).
RNA Gel-Blot Analysis
Total RNA was isolated from tomato tissue and analyzed by RNA-blot hybridization as previously described (Howe et al., 1996), except that Duralon-UV membranes were used in place of nitrocellulose. All gels were run in duplicate, with one set stained with ethidium bromide to ensure equal loading of the samples and intactness of the RNA. Hybridization signals were visualized by autoradiography using Kodak XAR-5 film, or were measured using a Phosphorimager (Molecular Dynamics). These signals were normalized to signals obtained using a probe for translation initiation factor eIF4A mRNA (Taylor et al., 1993). Hybridization and subsequent washing of eIF4A-probed blots was performed at 60°C in 2× SSPE. DNA probes were prepared as described above.
NOTE ADDED IN PROOF
Another allene oxide synthase-encoding cDNA from tomato, displaying 69% amino acid identity to the LeAOS sequence reported here, was recently described (S. Sivasankar, B. Sheldrick, S. J. Rothstein [2000] Plant Physiol 122: 1335–1342).
ACKNOWLEDGMENTS
We acknowledge Kathy Hubley for assistance with the isolation of LeAOS cDNAs, Dr. Douglas Gage for assistance with GC-MS analysis, and Drs. Pamela Green and Miguel Perez-Amador for help with hybridization experiments involving eIF4A and LE. We are also indebted to Drs. Elmar Weiler, Brady Vick, and Avraham Levy for providing the Arabidopsis AOS cDNA, a 12-oxo-trans-10-dodecenoic acid standard, and cv Micro-Tom seed, respectively.
Footnotes
This work was supported by the U.S. Department of Agriculture/National Research Initiative Program (grant no. 9801335) and by an All University Research Initiation Grant from Michigan State University.
LITERATURE CITED
- Avdiushko S, Croft KPC, Brown GC, Jackson DM, Hamilton-Kemp TR, Hildebrand D. Effect of volatile methyl jasmonate on the oxylipin pathway in tobacco, cucumber, and Arabidopsis. Plant Physiol. 1995;109:1227–1230. doi: 10.1104/pp.109.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Dietz K-J. Alkyl hydroperoxide reductases: the way out of the oxidative breakdown of lipids in chloroplasts. Trends Plant Sci. 1999;4:166–168. doi: 10.1016/s1360-1385(99)01398-9. [DOI] [PubMed] [Google Scholar]
- Bate NJ, Rothstein SJ. C6-Volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998;16:561–569. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- Bate NJ, Sivasankar S, Moxon C, Riley JMC, Thompson JE, Rothstein SJ. Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiol. 1998;117:1393–1400. doi: 10.1104/pp.117.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert S, Brodschelm W, Holder S, Kammerer L, Kutchan TM, Mueller MJ, Xia Z-Q, Zenk MH. The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blée E. Phytooxylipins and plant defense reactions. Prog Lipid Res. 1998;37:33–72. doi: 10.1016/s0163-7827(98)00004-6. [DOI] [PubMed] [Google Scholar]
- Blée E, Joyard J. Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol. 1996;110:445–454. doi: 10.1104/pp.110.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blée E, Wilcox AL, Marnett LJ, Schuber F. Mechanism of reaction of fatty acid hydroperoxides with soybean peroxygenase. J Biol Chem. 1993;268:1708–1715. [PubMed] [Google Scholar]
- Buttery RG, Ling LC. Volatile components of tomato fruits and plant parts. In: Teranishi R, Buttery RG, Sugisawa H, editors. Bioactive Volatile Compounds in Plants. Washington, DC: American Chemical Society; 1993. pp. 23–34. [Google Scholar]
- Caldelari D, Farmer EE. A rapid assay for the coupled cell free generation of oxylipins. Phytochemistry. 1997;47:599–604. [Google Scholar]
- Chao WS, Gu Y-Q, Pautot V, Bray EA, Walling LL. Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol. 1999;120:979–992. doi: 10.1104/pp.120.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conconi A, Miquel M, Browse JA, Ryan CA. Intracellular levels of free linolenic and linoleic acids increase in tomato leaves in response to wounding. Plant Physiol. 1996;111:797–803. doi: 10.1104/pp.111.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft KPC, Juttner F, Slusarenko AJ. Volatile products of the lipoxygenase pathway evolved from Phaseolus vulgaris (L.) leaves inoculated with Pseudomonas syringae pv phaseolicola. Plant Physiol. 1993;101:13–24. doi: 10.1104/pp.101.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defense genes through the octadecanoid pathway. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Mattheis JP, Fellman JK. A role for jasmonates in climacteric fruit ripening. Planta. 1998;204:444–449. [Google Scholar]
- Farmer EE, Weber H, Vollenweider S. Fatty acid signaling in Arabidopsis. Planta. 1998;206:167–174. doi: 10.1007/s004250050388. [DOI] [PubMed] [Google Scholar]
- Fauconnier M-L, Perez AG, Sanz C, Marlier M. Purification and characterization of tomato leaf (Lycopersicon esculentum Mill.) hydroperoxide lyase. J Agric Food Chem. 1997;45:4232–4236. [Google Scholar]
- Froehlich JE, DeRocher A, Howe GA. Allene oxide synthase is imported and targeted to the inner envelope membrane of pea and tomato chloroplasts (abstract no. 317) Plant Biology '99. 1999;1999:85. [Google Scholar]
- Galliard T, Matthew JA, Wright AJ, Fishwick MJ. The enzymatic breakdown of lipids to volatile and non-volatile carbonyl fragments in disrupted tomato fruits. J Sci Food Agric. 1977;28:863–868. [Google Scholar]
- Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- Gardner HW. Recent investigations into the lipoxygenase pathway of plants. Biochim Biophys Acta. 1991;1084:221–239. doi: 10.1016/0005-2760(91)90063-n. [DOI] [PubMed] [Google Scholar]
- Gardner HW, Weisleder D, Plattner RD. Hydroperoxide lyase and other hydroperoxide-metabolizing activity in tissues of soybean, Glycine max. Plant Physiol. 1991;97:1059–1072. doi: 10.1104/pp.97.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grechkin AN, Fazliev FN, Mukhtarova LS. The lipoxygenase pathway in garlic (Allium sativum L.) bulbs: detection of the divinyl ether oxylipins. FEBS Lett. 1995;371:159–162. doi: 10.1016/0014-5793(95)00895-g. [DOI] [PubMed] [Google Scholar]
- Gundlach H, Muller MJ, Kutchan T, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M. A pathway for biosynthesis of divinyl ether fatty acids in green leaves. Lipids. 1998;33:1061–1071. doi: 10.1007/s11745-998-0306-7. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlowe E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Harms K, Atzorn R, Brash A, Kuhn H, Wasternack C, Willmitzer L, Peña-Cortés H. Expression of a flax allene oxide synthase cDNA leads to increased endogenous jasmonic acid (JA) levels in transgenic potato plants but not to a corresponding activation of JA-responding genes. Plant Cell. 1995;7:1645–1654. doi: 10.1105/tpc.7.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms K, Ramirez I, Peña-Cortés H. Inhibition of wound-inducible accumulation of allene oxide synthase transcripts in flax leaves by aspirin and salicylic acid. Plant Physiol. 1998;118:1057–1065. doi: 10.1104/pp.118.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka A. The biogeneration of green odor by green leaves. Phytochemistry. 1993;34:1201–1218. [Google Scholar]
- Hatanaka A, Kajiwara T, Sekiya J. Biosynthetic pathway for C6-aldehyde formation from linolenic acid in green leaves. Chem Phys Lipids. 1987;44:341–361. [Google Scholar]
- Heitz T, Bergey DR, Ryan CA. A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol. 1997;114:1085–1093. doi: 10.1104/pp.114.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Ryan CA. Suppressors of systemin signaling identify genes in the tomato wound response pathway. Genetics. 1999;153:1411–1421. doi: 10.1093/genetics/153.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazeniac SJ, Hall RM. Flavor chemistry of tomato volatiles. J Food Sci. 1970;35:519–530. [Google Scholar]
- Kohlmann M, Bachmann A, Weichert H, Kolbe A, Balkenhohl T, Wasternack C, Feussner I. Formation of lipoxygenase-pathway-derived aldehydes in barley leaves upon methyl jasmonate treatment. Eur J Biochem. 1999;260:885–895. doi: 10.1046/j.1432-1327.1999.00231.x. [DOI] [PubMed] [Google Scholar]
- Kubigsteltig I, Laudert D, Weiler EW. Structure and regulation of the Arabidopsis thaliana allene oxide synthase gene. Planta. 1999;208:463–471. doi: 10.1007/s004250050583. [DOI] [PubMed] [Google Scholar]
- Laudert D, Pfannschmidt U, Lottspeich F, Hollander-Czytko H, Weiler EW. Cloning, molecular and functional characterization of Arabidopsis thaliana allene oxide synthase (CYP 74), the first enzyme of the octadecanoid pathway to jasmonates. Plant Mol Biol. 1996;31:323–335. doi: 10.1007/BF00021793. [DOI] [PubMed] [Google Scholar]
- Laudert D, Weiler EW. Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signaling. Plant J. 1998;15:675–684. doi: 10.1046/j.1365-313x.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Atzorn R, Brückner C, Reinbothe S, Leopold J, Wasternack C, Parthier B. Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta. 1995;197:156–162. [Google Scholar]
- León J, Rojo E, Titarenko E, Sánchez-Serrano JJ. Jasmonic acid-dependent and -independent wound signal transduction pathways are differentially regulated by Ca2+/calmodulin in Arabidopsis thaliana. Mol Gen Genet. 1998;258:412–419. doi: 10.1007/s004380050749. [DOI] [PubMed] [Google Scholar]
- Lers A, Khalchitski A, Lomaniec E, Burd S, Green PJ. Senescence-induced RNases in tomato. Plant Mol Biol. 1998;36:439–449. doi: 10.1023/a:1005993024161. [DOI] [PubMed] [Google Scholar]
- Matsui K, Shibutani M, Hase T, Kajiwara T. Bell pepper fruit fatty acid hydroperoxide lyase is a cytochrome P450 (CYP74B) FEBS Lett. 1996;394:21–24. doi: 10.1016/0014-5793(96)00924-6. [DOI] [PubMed] [Google Scholar]
- Matsui K, Toyota H, Kajiwara T, Kakuno T, Hatanaka A. A fatty acid hydroperoxide cleaving enzyme, hydroperoxide lyase, from tea leaves. Phytochemistry. 1991;30:2109–2113. [Google Scholar]
- McConn M, Browse J. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell. 1996;8:403–416. doi: 10.1105/tpc.8.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MJ, Brodschelm W, Spannagl E, Zenk MH. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA. 1993;90:7490–7494. doi: 10.1073/pnas.90.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narváez-Vásquez J, Florin-Christensen J, Ryan CA. Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell. 1999;11:2249–2260. doi: 10.1105/tpc.11.11.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR. Cytochrome P450 and the individuality of species. Arch Biochem Biophys. 1999;369:1–10. doi: 10.1006/abbi.1999.1352. [DOI] [PubMed] [Google Scholar]
- Pan Z, Durst F, Wreck-Reichhart D, Gardner HW, Camara B, Cornish K, Backhaus RA. The major protein of guayule rubber particles is a cytochrome P450. J Biol Chem. 1995;15:8487–8494. doi: 10.1074/jbc.270.15.8487. [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Albert T, Prat S, Weiler EW, Willmitzer L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta. 1993;191:123–128. [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, Samblanx GWD, Buchala A, Metraux J-P, Manners JM, Broekaert WF. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SO, Bendich AJ. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissue. Plant Mol Biol. 1985;5:69–76. doi: 10.1007/BF00020088. [DOI] [PubMed] [Google Scholar]
- Rojo E, Titarenko E, León J, Berger S, Vancanneyt G, Sánchez-Serrano JJ. Reversible protein phosphorylation regulates jasmonic acid-dependent and -independent wound signal transduction pathways in Arabidopsis thaliana. Plant J. 1998;13:153–165. doi: 10.1046/j.1365-313x.1998.00020.x. [DOI] [PubMed] [Google Scholar]
- Ryan CA. The systemin signaling pathway: differential activation of plant defensive genes. Biochem Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- Salch YP, Grove MJ, Takamura H, Gardner HW. Characterization of a C-5,13-cleaving enzyme of 13(S)-hydroperoxide of linolenic acid by soybean seed. Plant Physiol. 1995;108:1211–1218. doi: 10.1104/pp.108.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Matsui K, Kajiwara T, Hatanaka A. Fatty acid hydroperoxide lyase is a heme protein. Biochem Biophys Res Commun. 1995a;207:438–443. doi: 10.1006/bbrc.1995.1207. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Matsui K, Kajiwara T, Hatanaka A. Purification and properties of fatty acid hydroperoxide lyase from green bell pepper fruits. Plant Cell Physiol. 1995b;36:147–156. [Google Scholar]
- Song W-C, Brash AR. Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science. 1991;252:781–784. doi: 10.1126/science.1876834. [DOI] [PubMed] [Google Scholar]
- Song W-C, Funk CD, Brash AR. Molecular cloning of an allene oxide synthase: a cytochrome P450 specialized for the metabolism of fatty acid hydroperoxides. Proc Natl Acad Sci USA. 1993;90:8519–8523. doi: 10.1073/pnas.90.18.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Lehman CC. Jasmonic acid-signaled responses in plants. In: Agrawal AA, Tazun S, Bent E, editors. Induced Plant Defenses against Pathogens and Herbivores. American Phytopathological Society Press, St. Paul. 1999. pp. 117–136. [Google Scholar]
- Szczesna-Skorpa E, Straul P, Kemper B. Deletion of a conserved tetrapeptide, PPGP, in P450 2C2 results in loss of enzymatic activity without a change in its cellular location. Arch Biochem Biophys. 1993;304:170–175. doi: 10.1006/abbi.1993.1335. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Bariola PA, Delcardayré SB, Raines RT, Green PJ. RNS2: a senescence-associated RNase of Arabidopsis that diverged from the S-RNase before speciation. Proc Natl Acad Sci USA. 1993;90:5118–5122. doi: 10.1073/pnas.90.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titarenko E, Rojo E, León J, Sánchez-Serrano JJ. Jasmonic acid-dependent and -independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol. 1997;113:817–826. doi: 10.1104/pp.115.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick BA. A spectrophotometric assay for hydroperoxide lyase. Lipids. 1991;26:315–320. [Google Scholar]
- Vick BA, Zimmerman DC. Pathways of fatty acid hydroperoxide metabolism in spinach leaf chloroplasts. Plant Physiol. 1987;85:1073–1078. doi: 10.1104/pp.85.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler EW, Albrecht T, Groth B, Xia Z-Q, Luxem M, Lib H, Andert L, Spengler P. Evidence for the involvement of jasmonates and their octadecanoid precursors in the tendril coiling response of Bryonia dioica. Phytochemistry. 1993;32:591–600. [Google Scholar]
- Zhuang H, Hamilton-Kemp TR, Andersen RA, Hildebrand DF. The impact of alteration of polyunsaturated fatty acid levels on C6-aldehyde formation of Arabidopsis thaliana leaves. Plant Physiol. 1996;111:805–812. doi: 10.1104/pp.111.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D, Coudron CA. Identification of traumatin, a wound hormone, as 12-oxo-trans-dodecenoic acid. Plant Physiol. 1979;63:536–541. doi: 10.1104/pp.63.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]