Abstract
Free radicals derived from molecular oxygen and nitrogen are highly reactive metabolites called reactive oxygen species (ROS). Cells continuously produce free radicals and ROS as part of the metabolic process. They are involved in the various functions of the reproductive system. Antioxidants are enzymes or compounds that scavenge and reduce the presence of free radicals. Normally, a balance exists between concentrations of reactive oxygen species and antioxidant scavenging systems. The disruption of the delicate balance between pro‐ and antioxidants results in oxidative stress. Oxidative stress has been implicated in embryo fragmentation, DNA damage, apoptosis and poor pregnancy outcome. It has also been implicated in a large number of gynecologic diseases, such as endometriosis, pre‐eclampsia and maternal diabetes. The use of antioxidants may be beneficial in combating the harmful effects of oxidative stress in many of these diseases. The present review outlines the importance of these species in the pathology of various gynecologic diseases. (Reprod Med Biol 2004; 3: 177 – 199)
Keywords: antioxidants, DNA damage, oxidative stress, pregnancy, reactive oxygen species
INTRODUCTION
FREE RADICALS ARE involved in the physiology of reproduction. Free radicals may be defined as any molecule that has one or more unpaired electrons. Oxygen radicals, such as the superoxide anion (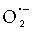 ), the hydroxyl radical (OH·), hydrogen peroxide and hypochlorite radical (OHCl·) and the peroxyl radical (ROO·), comprise the highly reactive group of oxygen species called the reactive oxygen species (ROS).
1
,
2
,
3
,
4
,
5
,
6
,
7
,
8
Also included in this group are the reactive nitrogen radicals, such as nitric oxide (NO·) and nitric dioxide (
), the hydroxyl radical (OH·), hydrogen peroxide and hypochlorite radical (OHCl·) and the peroxyl radical (ROO·), comprise the highly reactive group of oxygen species called the reactive oxygen species (ROS).
1
,
2
,
3
,
4
,
5
,
6
,
7
,
8
Also included in this group are the reactive nitrogen radicals, such as nitric oxide (NO·) and nitric dioxide ( ) free radicals.
) free radicals.
Oxygen toxicity is an inherent challenge to aerobic life. Antioxidants defense mechanisms include a variety of enzymes, the most important of which are superoxide dismutase (SOD), which produces hydrogen peroxide from superoxide radicals, and catalase and glutathione (GSH) related enzymes, which decompose hydrogen peroxide. In addition, there are lypophilic and hydrophilic low‐molecular‐weight antioxidants, such as vitamin C, uric acid, GSH and vitamin E, which act directly with various ROS (Table 1). 9 Oxidative stress is caused by an imbalance between production of ROS and the antioxidant capacity. 1 , 6 , 7 , 10 , 11 , 12 , 13
Table 1.
Enzymatic and non‐enzymatic antioxidants and their reproductive functions
| Antioxidant | Localization | Remarks |
|---|---|---|
| Enzymatic antioxidants | ||
| Superoxide dismutase | Cytoplasmic (Cu, Zn‐SOD). Mitochondrial (Mn‐SOD), endometrial glandular cells | Neutralizes intra‐ and extracellular superoxide anions. Promotes an increase in the proportion of zygotes undergoing at least one cleavage. Improves cleavage past the 2‐cell block, increases blastocyst development. Decreased concentrations in pregnancies complicated by preclamsia. Elevated levels of Cu, Zn‐SOD elicits a protective effect against diabetes‐associated embryopathy. |
| Catalase | Tubal fluid | Neutralizes intra‐ and extracellular hydrogen peroxide, Promotes an increase in the proportion of zygotes undergoing at least one cleavage. |
| Glutathione peroxidase | Glandular epithelium in endometrium | Reduction of hydrogen peroxide and lipid peroxides and other peroxides to water or alcohol. Eliminates hydroxyl radical. |
| Nitric oxide synthase | Endometrial glandular cells and surface epithelia | Endothelial eNOS most marked in mid‐secretory phase. |
| Non‐enzymatic antioxidants | ||
| Vitamin E | Ovary or follicular fluid | Major chain breaking antioxidant in membranes, directly neutralizes superoxide anion, hydrogen peroxide, and hydroxyl radical. Increases number of embryos developing to the expanded blastocysts. Increases viability of embryos exposed to heat shock. Increases survival of explanted rat conceptuses in vitro, Supplementation reduces the risk of ovulating aneuploid and diploid oocytes in aged female mice. |
| Vitamin C | Ovary | Chain breaking antioxidant, competitively protects the lipoproteins from peroxyl radicals and recycles vitamin E. Diverse antioxidant function. Reduces presence of sulfahydryls. Inhibit embryo development at higher concentrations. Supplementation reduces the risk of ovulating aneuploid and diploid oocytes in aged female mice. Characteristic of infertility. Deficiency produces ovarian atrophy, extensive follicular artresia. Supplementation inhibits follicular apoptosis. |
| Glutathione | Tubal fluid, oocyte/embryo | Neutralizes superoxide anion, reduced glutathione metabolizes hydrogen peroxide and hydroxyl radical, Diverse antioxidant function. Improves the development of zygotes through the 2‐cell block to the morula or blastocyst stage. Protects embryos against ROS. Improves bovine embryo production. Prevents oxygen‐induced embryonic malformation. Depletion causes increase in H2O2 concentration. Depletion in embryonic cells during the critical periods of organogenesis plays a role in hyperglycemia‐induced embryopathy. |
| N‐acetyl‐L‐ cysteine | May act as a precursor of glutathione and thus facilitates its biogenesis. Inhibits upstream events leading to NF‐kappaB activation. Inhibits phospholipids metabolism, proinflammatory cytokine release, and NF‐kappaB DNA‐binding in human fetal membranes. | |
| Carotene | Chain breaking radical | |
| Ascorbate | Follicular fluid | Blocks DNA damage and supports cytoplasmic maturation in porcine oocytes. |
| Cysteamine | Follicular fluid | Precursor of hypotaurine, maintains redox status in oocytes. Maintains GSH content. Increases synchronous pronuclear formation. Improves normal embryo development. |
| EDTA | Chelating agent. Necessary to overcome the 2‐cell block. Enhances in vitro embryonic development. | |
| Taurine | Tubal fluid, follicular fluid | Chelating agent, neutralization of cytotoxic aldehydes, improves embryo development. |
| Hypotaurine | Tubal fluid, follicular fluid | Neutralizes hydroxyl radicals, precursor of taurine. |
| Transferrin | Tubal fluid | Metal chelation, necessary to overcome the 2‐cell block. |
| Thioredoxin | Protects embryo against oxidative stress and embryopathic effects. | |
| Dithiothreitol | Improves fertilization. Prevents age‐associated decrease in oocyte viability. | |
| Cu, Zn‐SOD, Cu‐Zn‐superoxide dismutase; EDTA, ethylenediaminetetraacetic acid; Mn‐SOD, manganese‐superoxide dismutase; NOS, nitric oxide synthase; ROS, reactive oxygen species; SOD, superoxide dismutase. | ||
While the controlled generation of ROS may have physiological functions as signaling molecules (second messenger) in many different cell types, their uncontrolled production is considered as an important factor in embryo toxicity and teratogenecity during prenatal life. Lipid peroxidation is a normal phenomenon that occurs continuously at low levels in all humans. Uncontrolled peroxidation can damage enzymes, lipids, proteins and cell membranes, and results in cell injury and death. During pregnancy, excess lipid peroxidation is associated with pre‐eclampsia. Oxidative stress has been implicated in aging, diet, health, and in the etiology of pathological conditions, such as maternal diabetes, myocardial infarction, cataract or rheumatoid arthritis. 14 , 15
OXIDATIVE STRESS AND INFERTILITY
Production and deleterious effects of ROS on embryos
MANY INVESTIGATORS BELIEVE that the concentrations of free radicals also play a major role in the implantation of fertilized eggs. A low superoxide environment is an important determinant in the development of fertilized eggs, and optimal concentrations of NO are necessary for the implantation of fertilized eggs into mice. 16 , 17 Glutathione peroxidase (GPX) is involved in the elimination of the hydroxyl radicals, which is one of the most active free radicals and can damage intracellular DNA and the cell membrane.
Numerous endogenous and exogenous conditions can induce oxidative stress on the embryos. 18 , 19 Various metabolic pathways and enzymes can produce endogenous ROS, including oxidative phosphorylation, β‐nicotinamide adenine dinucleotide phosphate reduced (β‐NADPH) oxidase and xanthine oxidase (XO). 20 , 21 , 22 , 23 , 24 , 25 Several exogenous factors can contribute to ROS production by embryos, such as oxygen concentration, metallic cations, visible light, amine oxidases,and spermatozoa. 26 , 27 , 28 , 29 , 30 , 31 , 32 ROS can alter most types of cellular molecules, such as lipids, proteins, DNA, mitochondrial alterations, embryo cell block, adenosine triphosphate depletion, apoptosis and fragmentation, and freeze‐thaw survival. 2 , 4 , 16 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 In addition, embryos may have different sensitivities to ROS at different stages, for example, bovine embryos at the 9–12 cell stage are more resistant to exogenous H2O2 compared with zygotes and blastocysts. These differences in sensitivities are related to variations in thresholds of the defense mechanism. 42
Antioxidants and embryo development
Embryo 2‐cell block is associated with an oxidative burst, suggesting that the embryo may be particularly vulnerable to ROS at this stage. At this time, the embryo in vivo is present in the oviduct, which provides various radical scavengers. 43 , 44 Female reproductive tract fluid contains high concentrations of certain amino acids, suggesting that they may play a role in preimplantation development. 45 , 46 , 47 , 48 , 49
Several defense mechanisms against ROS are present both in embryos and their surroundings. In vivo embryos are protected against oxidative stress by oxygen scavengers present in follicular and oviductal fluids. Oxidative stress can be avoided by preventing against ROS formation, interception by antioxidants and repair. Metal chelation is a major means of controlling lipid peroxidation and DNA fragmentation, and metal‐binding proteins such as transferrin are important in controlling potential radical‐generating reactions. Numerous non‐enzymatic compounds, such as vitamin A, ascorbate and pyruvate have antioxidant function. 42 , 43 , 50 , 51 , 52 , 53 , 54 , 55 Other sulfur compounds such as GSH, taurine, hypotaurine and cysteamine are equally important in protecting the embryos against ROS. 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 Enzymatic defense mechanism protecting oocytes and embryos include SOD, catalase or GPX. 44 , 58 , 67 , 71 , 72 , 73
Repair systems in oxidative stress and apoptosis
Both embryos and spermatozoa possess repair mechanisms to counteract the toxic effects of ROS. Newly fertilized eggs are capable of repairing the damaged DNA of spermatozoa. 74 Both in vitro and in vivo repair of damaged spermatozoa or injected DNA is known to occur in oocytes. 75 In addition, the pronuclei of oxidative‐damaged embryos can be rescued partly by transferring into normal cytoplasm. 76 However, the ability of mitochondrial DNA to auto‐repair leads to a progressive decline in mitochondrial function. 77
Oxidative stress must be controlled during in vitro culture to optimize in vitro embryo production. Pro‐oxidant/antioxidant balance within the follicular microenvironment is implicated in the maturation of the oocytes. 78 , 79 Both oocytes and embryos must be protected by reducing oxygen concentrations in the gaseous environment, by a radical buffering system produced by co‐cultured cells, and/or by adding supplements to the culture medium. 3 DNA damage beyond the capacity to repair will result in apoptosis and fragmentation of the early embryo or morbidity in later life. 80 , 81 This may be one of the causes of fragmentation in human embryos seen in in vitro fertilization (IVF) programs.
Extensive evidence has demonstrated that cellular redox status modulates various aspects of cellular function. Glutathione (GSH) has been shown to modulate many important aspects of oogenesis, fertilization and development. 82 , 83 , 84 , 85 Redox status of the cell is a key factor mediating the apoptotic pathway, and GSH presumably plays a critical role in mediating apoptosis by influencing the redox status. 86 Oxidative stress induces aneuploidy during oocyte development, and has been linked to maternal aging‐associated infertility. 82 , 83
Normal cells balance the redox system and defense systems to protect against ROS. If such defenses fail or if there is excess ROS formation, oxidative stress and cell death may occur via necrosis or apoptosis. Necrosis typically arises from acute pathological stimuli, while apoptosis typically is associated with mild toxic stimulation over prolonged periods of time. Apoptosis plays an important role in development and differentiation. 87 Evidence suggests that some oocytes may undergo apoptosis or degenerate during development and aging. 82 , 88 Oxidation of cellular sulfhydryl (SH) groups has been implicated in the induction of apoptosis and in the disturbance of the meiotic spindles of murine oocytes during aging. 89 , 90 Altering thiol‐redox status in zygotes and blastocysts may result in cell cycle arrest, apoptosis and/or cell death.
Fragmented embryos have limited development potential and rarely result in implantation. 91 , 92 Apoptotic configurations in fragmented embryos have been reported at a stage prior to blastocyst formation. 93 Significantly higher concentrations of H2O2 and apoptosis have been reported in fragmented embryos compared to non‐fragmented and unfertilized human oocytes, and embryos. 94 Although the implantation rate is not significantly different, the ability to develop to blastocyst and the development of live fetuses is reduced in DNA damaged spermatozoa. Therefore, when there is a genetic damage, though some embryos may reach to the blastocyst stage, natural selection ensures that most of them would abort and not go to term. 95 , 96 , 97
DNA DAMAGE AND PREGNANCY OUTCOME
IN NATURAL CONCEPTION, sperm DNA status is an essential prerequisite to the achievement of a successful pregnancy. 98 , 99 Several studies have attempted to establish a correlation between sperm DNA integrity, cleavage rates and embryo quality. 100 , 101 , 102 DNA damage can occur as a result of defective sperm chromatin packaging, apoptosis and oxidative stress. 1 , 6 , 7 , 11 , 13 , 81 , 103 , 104 , 105 , 106 , 107 Whether DNA‐damaged spermatozoa can impair the process of embryo development remains unclear.
Damage to sperm DNA may be linked to an increase in early embryo death. 102 However, a greater miscarriage rate was reported in intracytoplasmic sperm injection (ICSI), possibly reflecting the use of genomically compromised spermatozoa. 108 The number of embryos that develop to the blastocyst stage have been shown to negatively correlate with both the blasytocyst number and quality in cases of male infertility. 109 , 110 , 111 Zygotes resulting from IVF have a higher blastocyst development rate over ICSI. 112 , 113 Sperm from infertile men have an elevated rate of DNA abnormalities. These abnormalities may lead to abnormal blastocyst development, failed implantations and spontaneous miscarriages. 98 , 101 , 103 , 114 , 115 , 116 , 117 , 118 , 119 The deleterious consequences of fragmented paternal DNA become evident when the embryonic genome is activated. DNA damage has been correlated with pregnancy outcome both in vivo and in vitro (Table 2). 38 , 81 , 116 , 118 , 120 , 121 , 122
Table 2.
Correlation of DNA damage and outcome of various assisted reproductive procedures
| References | Study population | ART procedure | DNA assay | Pregnancy outcome |
|---|---|---|---|---|
| 81 | 143 | IVF | TUNEL | DNA fragmentation negatively correlated with fertilization and embryo cleavage rate. |
| 102 | IVF ICSI | Chromomycin A3Nick translation | Lower rates. | |
| 125 | 150 | ICSI | TUNEL | Fertilization rate negatively correlated with DNA fragmentation. |
| 98 | 165 | SCSA | ||
| 95 | TUNEL | Embryonic development related to high pregnancy loss when DNA damage >8%. | ||
| 116 | 24 | ICSI | SCSA | DNA denaturation lower in men that initiated pregnancy. No pregnancies when DNA fragmentation >27%. |
| 126 | 75 | IVF ICSI | Negative correlation with proportion of oocytes fertilized. | |
| 127 | 140 | IVF | In situ nick translation Chromomycin staining | No association of DNA damage with fertilization rate. Pregnant patients had significantly lower NT levels. |
| 99 | 215 | SCSA | Decline in fecundibility as the percentage of cells with abnormal chromatin are >40%. | |
| 128 | 15 | IVF | Comet | Head and tail DNA parameters useful in predicting embryo quality. |
| 100 | 40 | IVF | Comet | |
| 101 | 60 | IVF | Comet | In ICSI cycles DNA damage associated with impairment of post fertilization embryo cleavage. |
| 121 | 119 | IUI | TUNEL | No pregnancies if fragmentation >12%. |
| 129 | 33 | IUI IVF ICSI | SCSA | Both fertilization and embryo development negatively correlated with DNA denaturation. |
| 120 | 50 54 | IVF ICSI | TUNEL | No significant difference in fertilization and embryo quality in IVF and ICSI. In ICSI pregnancy resulted when DNA fragmentation <10%. No pregnancy when DNA fragmentation >20%. |
| 124 | 34 | IVF ICSI | SCSA | No differences seen in SCSA parameters between patients who got pregnant to those who did not in either or conventional IVF. |
| 123 | 249 | IVF ICSI | SCSA | IVF and ICSI fertilization rates comparable between high‐ and low – DFI groups. HDS ≥15% resulted in lower fertilization rates. Blastocyst rates or pregnancy outcome not affected by HDS. ICSI may be indicated in men with HDS ≥15%. Men with ≥30% DFI at greater risk for low blastocyst rates and failure to initiate an ongoing pregnancy. |
| 130 | 306 | IUI IVF ICSI | SCSA | Higher chances of pregnancy and delivery in IUI with DFI ≤27% and HDS ≤10%. No differences in outcome of IVF or IFCSI when DFI ≤27%. ICSI significantly better than IVF when DFI ≥27%. |
ART, assisted reproductive techniques; DFI, DNA fragmentation index; HDS, high DNA stainability; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; IVF, in vitro fertilization; NT, in situ nick translation assay; SCSA, sperm chromatin structure assay; TUNEL, terminal deoxynucleotidyl transferase‐mediated dUTP nick end labeling.
Using the sperm chromatin structure assay (SCSA), men with DNA fragmentation ≥30% are at greater risk for low blastocyst rates and failure to initiate an ongoing pregnancy. In addition, the high DNA stainability (≥15%) also is indicative of poor IVF fertilization rates. The extent of DNA damage can provide valuable prognostic information to the physicians counseling couples before IVF and ICSI. 122 , 123 , 124
OXIDATIVE STRESS IN OTHER TISSUES AND FLUIDS
Oviduct
GENETIC EXPRESSION OF antioxidant enzymes involved in the mechanisms protecting embryos against ROS has been reported in human and mouse oviducts. 8 Different expression profiles of transcripts encoding for gamma glutamylcysteine synthetase (GCSD), GPX, Cu‐Zn‐superoxide dismutase (Cu‐Zn‐SOD), manganese‐superoxide dismutase (Mn‐SOD) and catalase have been reported in the mouse model, whereas in the human, only transcripts encoding for GPX, Cu‐Zn‐SOD and catalase have been detected. Differences in the gene expression patterns of these antioxidant enzymes may reflect the variations in the ability of embryos to develop in vivo and in vitro.
Umblical cord
Umbilical cord lipid peroxide concentrations reflect the extent of cell membrane damage by ROS. Persistent intrauterine asphyxia may result in ischemia to the organs, leading to permanent damage, especially to the brain. Oxygen free radicals activity in the neonate at birth and its relationship to umbilical cord acid‐base status has been investigated in singleton deliveries. Lipoperoxides levels, cord blood pH and base excess were significantly related with singleton deliveries. 131
In cases of uncomplicated labor followed by spontaneous vaginal delivery, significantly higher lipid peroxide concentrations were seen compared with those delivered following elective Caesarean section. Especially noted was an increase in the levels of malondialdehyde (MDA) (105%), while the hydroperoxide increase of 27% and base excess increase of 78% were seen. 132 , 133 High levels of free oxygen radical activity in the fetus are a function of the labor process as are the changes in acid‐base balance.
Follicles and follicular fluid
Ascorbic acid deficiency characteristically produces ovarian atrophy, along with extensive follicular artresia and premature resumption of meiosis. 134 Ascorbic acid can be depleted both by oxidant scavenging, as well as by cellular secretion. 135 In the preovulatory follicles, ascorbic acid is depleted by the presence of luteinizing hormone and by incubating the follicle with agents known to generate ROS. Isolation of the oocyte also results in ascorbic acid depletion. 133
Transferrin suppresses ROS generation and is important for the successful development of follicles. Transferrin messenger ribonucleic acid (mRNA) expression has been demonstrated in granulosa cells of the human and mouse ovary but not in the oocyte. 136 In the follicular fluid, transferrin levels have been found to be highly correlated with those in the serum, suggesting that the small contribution made by its localized synthesis in the granulose cell may be important for some as yet unknown mechanism in follicle maturation.
In women undergoing IVF, significantly higher levels of ROS have been reported in the follicular fluid of women who got pregnant compared with those who did not. Developmental competence of oocytes retrieved during IVF‐embryo transfer procedure is not related to increased levels of oxidative stress in the follicular milieu they originate from. 137 , 138 Both lipid peroxidation and total antioxidant capacity were positively correlated with the pregnancy rate. Follicular fluid ROS and lipid peroxidation levels might be potential markers for predicting success in IVF patients. 138 , 139
Amniotic fluid
The presence of ROS has been demonstrated in the amniotic fluid collected both at second and third trimester. Antioxidant capacity is reported to correlate with gestational age and estimated fetal weights or neonatal birthweights. However, ROS levels may not necessarily be influenced by gestational age, estimated fetal or neonatal weights. 140
Fetal membranes
The production of ROS, prostaglandins, proinflammatory cytokines and proteases has been implicated in the pathogenesis of term and preterm labor. NFkappaB activated pathways and subsequent phospholipid metabolism, pro‐inflammatory cytokine release and N‐acetyl‐cysteine (NAC), through its ROS scavenging affect, inhibits protease activity in human fetal membranes. 141
Preterm premature rupture of membranes (PPROM) results initially from ROS damage to collagen in the chorioamnion, leading to tear/rupture in the membrane as demonstrated by epidemiological and by in‐vitro studies. 142 In addition, clinical studies also show that chorioamnion and amniotic fluid samples, obtained from PPROM patients, exhibit excessive collagen degradation and antioxidants protect the chorioamnion from ROS damage.
OXIDATIVE STRESS AND SMOKING IN WOMEN
OVER 20% OF the women of reproductive age in Europe and the USA regularly smoke cigarettes. 143 Tobacco is a major source of exogenous pro‐oxidants; ROS and free radical generators are present in both its gas and particulate phase. 144 Active smoking affects the pro‐oxidant/antioxidant balance inside the Graffian follicle in women undergoing ovulation induction for IVF. Cigarette smoking is associated with increased intensity of lipid peroxidation inside the mature ovarian follicle, which is accompanied by the depletion of local antioxidant scavengers. 145 There is a depletion of both enzymatic and non‐enzymatic antioxidants in the follicular fluid of smokers. 78 , 146 The decreased antioxidant capacity of follicular fluid in the smokers is, most likely, a secondary phenomenon caused by the utilization of antioxidants in defense reactions neutralizing ROS originating from, or induced by, the tobacco smoke constituents. A relatively high diploidy has been reported in unfertilized oocytes originating from smoking IVF‐embryo transfer patients, suggesting a smoking‐related meiotic immaturity of the oocytes. 147 An increased risk of trisomy has been observed in the offspring of mothers who smoke cigarettes. 148 A shift of the pro‐oxidant/antioxidant balance inside the ovarian follicle towards oxidative stress may provide another possible explanation of impaired folliculogenesis in female smokers undergoing IVF‐embryo transfer.
OXIDATIVE STRESS AND PREGNANCY
CONCENTRATIONS OF LIPID peroxides in the placenta are higher than in blood. 149 Higher levels of lipid peroxides are reported in blood in pregnant than in non‐pregnant women. During gestation, elevated levels are seen in the second trimester and these taper off later in gestation, decreasing further after delivery. 150 Lipid peroxides are also produced in placenta, but their pattern of change over the course of pregnancy is unclear. 151
Lipid peroxidation levels in placental tissue follow a different pattern over gestation than levels in blood. 152 Both peroxidation and antioxidation reactions are enhanced during pregnancy. 153 , 154 , 155 Maternal levels of prostacyclin, thromboxane, vitamin E and lipid peroxides were measured throughout the gestation period. 156
During uncomplicated pregnancy, ROS levels are elevated, 157 however, they are counterbalanced by an increased activity of antioxidants. 158 Maternal levels of vitamin E and lipid peroxides are both increased in pregnancy compared with non‐pregnancy. The increase in the vitamin E levels may be a result of the physiological response to pregnancy, and there may be increased binding capacity of the blood for vitamin E. 155 Increased lipid peroxides during pregnancy may be related to the increase in spontaneous auto‐oxidation of serum lipids.
Placenta lipid peroxides apparently contribute to maternal circulating levels because plasma lipid peroxide levels decrease abruptly after delivery. 158 The uterine contractile activity may generate ROS through the process of repetitive ischemia and reperfusion during labor in healthy women at term. Concentrations of vitamin C are significantly lower in maternal plasma, amniotic fluid and fetal plasma of vaginal delivery, indicating that water soluble vitamin C scavenges ROS in the aqueous phase and recycles lipid‐soluble vitamin E to combat ROS‐induced tissue damage. 159
MULTIVITAMIN SUPPLEMENTATION AND DNA DAMAGE DURING PREGNANCY
THE EFFECT OF multivitamin/mineral supplementation during pregnancy on plasma levels of antioxidants and sister chromatid exchange (SCE) rate – an indicator of damage to DNA has been examined. 160 A significant increase in SCE rates were reported at 34 weeks, whereas in the supplemental group, although the SCE rates did not decrease, they prevented the increase induced by ROS generated from enhanced steroid hormones in the last trimester, suggesting that multivitamin/mineral‐supplementation during pregnancy may prevent DNA damage to the altered hormonal profile. 160
OXIDATIVE STRESS IN SPONTANEOUS ABORTION
SPONTANEOUS ABORTION IS accompanied by a profound disruption of the pro‐oxidant‐antioxidant homeostasis towards oxidative stress. 161 Administration of antioxidants confers partial protection from the consequences of surgically induced oxidative burden. 162
OXIDATIVE STRESS IN GYNECOLOGIC DISEASES
Oxidative stress in the pathophysiology of endometriosis
ENDOMETRIOSIS IS A common gynecologic disorder that affects 8 to 10% of all reproductive age women, and is found in up to 50% of asymptomatic women undergoing laparoscopic tubal ligation. 163 It is characterized by the implantation and growth of endometrial tissue outside the uterine cavity. It is a multifactorial disease associated with a general inflammatory response in the peritoneal cavity. It is the leading cause of infertility. 164 , 165 Pelvic pain or infertility caused by endometriosis often cannot be distinguished on clinical grounds. The pathophysiology of endometriosis and the mechanisms responsible for its complication, in particular infertility, remain largely unexplained. 166 Involvement of various immunologic anomalies has been suggested in the past as a cause of these disorders. 167 , 168 , 169
Oxidative stress has been proposed as a potential factor involved in the pathophysiology of the disease. ROS may be involved in the endometrial‐associated infertility and may play a role in the regulation of the expression of genes encoding immunoregulators, cytokines, and cell adhesions molecules implicated in the pathogenesis of endometriosis. 170 Increased production of hydroxyl radical and other free radicals in endometriosis is due to the activation of the immune system, and the various antigens in endometrial cells are excessively expressed. 167 , 169 , 171 , 172 , 173 However, other studies based on direct measurement of ROS, NO, lipid peroxides and total antioxidant status have failed to demonstrate significant differences between the peritoneal fluid obtained from women with endometriosis compared to fertile women. 174 , 175 , 176 , 177 , 178 The apparent discrepancy between these results may be due to the fact that only persistent markers of oxidative stress, such as enzymes or stable by‐products of oxidative reaction may still be detected when endometriosis is diagnosed. Another possible explanation is that oxidative stress occurs only locally, for example at the site of bleeding, and does not result in an increase in total peritoneal fluid concentrations.
Increased presence of iron and lipid peroxidation
Retrograde menstruation is likely to carry highly pro‐oxidant factors, such as heme and iron, into the peritoneal cavity, as well as apoptotic endometrial cells, which are well known inducers of oxidative stress. The role of increased iron levels in the peritoneal fluid of patients with endometriosis however, does not show altered levels of MDA, even though iron concentrations correlated with the severity of the disease. These results indicate that elevated levels of iron in these patients may not be involved in catalyzing free‐radical reactions as judged by the degree of lipid peroxidation. 179
Nitric oxide and oxidative stress
The role of NO and oxidative stress in the pathogenesis of endometriosis‐associated infertility has been examined. No increase in total antioxidant status (TAS) or the concentration of products of NO metabolism were reported in women with endometriosis during the early follicular phase, suggesting that NO or TAS may not be the major cause in the pathology of endometriosis. 175 Persistently greater levels of endothelial NO synthase expression have been reported in endometriosis compared to controls throughout the menstrual cycle. 168 , 180
Glutathione peroxidase, superoxide dismutase and xanthine oxidase in endometrial tissues
Phase‐dependent changes of GPX expression are seen in the surface and glandular epithelia in the eutopic endometrium during the menstrual cycle in the fertile control. However, this is lost in the eutopic endometrium in endometriosis. The aberrant expression of GPX in the eutopic endometrium throughout the cycle suggests a pathological role in endometriosis and adenomyosis. 181 In endometriosis, expression of SOD is pronounced in the endometrium throughout the menstrual cycle, suggesting that superoxide plays a key role in infertility in endometriosis. 168 There is an exaggerated expression of Cu, Zn‐SOD and Mn‐SOD in eutopic endometrium in endometriosis and adenomyosis. The distribution of XO in eutopic and ectopic endometrium in endometriosis and adenomyosis has also been examined. 178
Oxidatively modified lipids in the peritoneal fluid
Evidence of oxidative stress in the peritoneal fluid of women with endometriosis and the presence of oxidatively modified lipids in the peritoneal fluid has been demonstrated. 5 Oxidation‐specific epitopes and macrophages are present in the endometrium and in endometriosis. 182 Lipid peroxides interact with proteins, resulting in oxidatively modified proteins that are antigenic. Autoantibodies to oxidatively modified proteins have been detected in subjects with coronary artery disease, pre‐eclamptic pregnancy and other vascular disorders. 183 , 184 The detection of increased anti‐oxidized modified low‐density lipoproteins autoantibody titer is thought to represent a biologic marker of enhanced low‐density lipoprotein oxidation in vivo. 183 , 185
A significant increase is seen in the autoantibodies to markers of oxidative stress were in women with surgically confirmed endometriosis, indicating that these women have enhanced oxidative stress. 186 It is not known whether inhibition of oxidation decreases the severity of the symptoms of endometriosis. Conversely, if antioxidant treatment of endometriosis decreases the titer of the antibodies, this might indicate the source of oxidative stress.
Macrophages through scavenger receptor(s) play an important role in the uptake and removal of modified proteins, which can be, formed as a result of inflammatory processes. 187 Scavenger receptors also known as oxidized low‐density lipoprotein receptors do not recognize native low‐density lipoprotein. 188 Oxidatively modified lipid‐proteins constitute ligands for scavenger receptor(s). The presence of macrophages expressing scavenger receptor(s) in the peritoneal cavity of women with endometriosis may indicate oxidative damage to cellular or acellular components of the peritoneum.
Staining with antibodies to oxidatively modified lipid proteins (HNE‐2 and MDA2) in both endometrium and endometriosis tissues containing stromal cells, in contrast to the controls, strongly indicates the occurrence of oxidative stress in endometriotic tissue. Although macrophages themselves are capable of inducing oxidation, a variety of other cells share this capacity. Ectopic endometrium may be a possible source of oxidized lipid proteins in the peritoneal cavity, also it can be speculated that endometriotic implants could promote a pro‐oxidant environment in the peritoneal cavity of women with endometriosis. 182
Several hypotheses have been proposed to explain why oxidative stress is induced in cases of pelvic endometriosis. Erythrocytes, apoptotic endometrial tissues, and cell debris transplanted into peritoneal cavity by menstrual reflux and macrophages have been implicated as potential inducers of oxidative stress. 173 , 183 Available data do not clearly indicate whether oxidative stress associated with pelvic endometriosis results from increased production of ROS, defective antioxidant defense or both conditions. Information is lacking on how the peritoneal environment, and particularly mesothelial cells, responds to the presence of blood, which is repeatedly carried into peritoneal cavity by retrograde menstruation, ovulation and bleeding lesions. The expression of antioxidant enzymes by endometrial cells appears to be enhanced in patients with developing endometriosis. The occurrence of oxidative stress suggests that despite this increase in antioxidant defenses, detoxifying mechanisms might be overloaded in endometriosis.
OXIDATIVE STRESS IN PRE‐ECLAMPTIC PREGNANCY
PRE‐ECLAMPSIA IS A leading cause of maternal and neonatal mortality and morbidity. It is a complex syndrome of undetermined etiologic origin, usually diagnosed during the second half of pregnancy, with clinical features of hypertension, proteinurea and edema. It is characterized by endothelial cell dysfunction and lipid peroxidation, and alterations of immune responses may be involved in the pathogenesis. 190 , 191 , 192 Currently, there is no cure for pre‐eclampsia except premature delivery.
Oxidative stress
There is increasing evidence that oxidative stress may be an important contributing factor to the pathogenesis of pre‐eclampsia.
193
,
194
,
195
,
196
,
197
,
198
Pregnant women affected by pre‐eclampsia may have abnormal ROS production, particularly NO· and 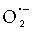 , abnormal levels of antioxidant defenses and increased placental lipid peroxidation.
199
In severe pre‐eclampsia, the balance between ROS and antioxidants is disturbed due to an increase in oxidants and compromise of antioxidants.
192
,
194
,
195
,
196
,
197
, abnormal levels of antioxidant defenses and increased placental lipid peroxidation.
199
In severe pre‐eclampsia, the balance between ROS and antioxidants is disturbed due to an increase in oxidants and compromise of antioxidants.
192
,
194
,
195
,
196
,
197
Neutrophil activation and pre‐eclampsia
Activated neutrophils have been implicated in the pathophysiology of pre‐eclampsia and may play a significant role in the diverse manifestation of the disease, such as vascular endothelial damage and dysfunction associated with pre‐eclampsia. 204 , 205 Increased production of ROS is associated with neutrophil activation in pre‐eclampsia. Neutrophils isolated from women with pre‐eclampsia during the third trimester showed increased sensitivity to agonist stimulation and produce significantly more ROS than age‐matched normotensive controls. This highlights the role of neutrophils in the oxidative stress and associated endothelial dysfunction that are characteristic of pre‐eclampsia. 206
Endothelium‐derived nitric oxide
Data suggest that endothelium‐derived NO may be increased in women with pre‐eclampsia.
207
An increase in XO activity has been proposed as a possible cause of oxidative stress in pre‐eclampsia.
208
Besides causing direct inactivation of NO· as a vasodilator, XO‐derived 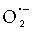 reacts with NO· to form a potent peroxynitrite (ONOO·–), which has been detected both within the vasculature and in the vessel walls of the placenta after pre‐eclampsia.
209
,
210
Increased endothelial NO synthase, decreased SOD, and increased nitrotyrosine staining in the maternal vasculature of women with pre‐eclampsia have also been reported, indicating increased peroxynitrite formation. Decreased bioavailability of endothelium‐derived NO, due to oxidative destruction of NO by ROS, might contribute to the pathology of pre‐eclampsia, a phenomenon in which antioxidant vitamins may play a beneficial role.
reacts with NO· to form a potent peroxynitrite (ONOO·–), which has been detected both within the vasculature and in the vessel walls of the placenta after pre‐eclampsia.
209
,
210
Increased endothelial NO synthase, decreased SOD, and increased nitrotyrosine staining in the maternal vasculature of women with pre‐eclampsia have also been reported, indicating increased peroxynitrite formation. Decreased bioavailability of endothelium‐derived NO, due to oxidative destruction of NO by ROS, might contribute to the pathology of pre‐eclampsia, a phenomenon in which antioxidant vitamins may play a beneficial role.
Endothelial cell dysfunction
Pre‐eclampsia is characterized by endothelial cell dysfunction and lipid peroxidation. Activation of cell‐meditated immunity caused by the alteration of immune response, especially caused by T‐cell activation, may be involved in the pathogenesis of pre‐eclampsia and release of endothelial ROS. Total antioxidants concentration has been shown to be negatively correlated with fibronectin, indicating endothelial cell damage and positively with creatinine clearance. 211 These changes are early events in the development of pre‐eclampsia, which may cause endothelial cell dysfunction in pre‐eclampsia.
Another leading theory of the pathophysiology of pre‐eclampsia is that oxidative stress induces vascular endothelial cell dysfunction. 212 Advanced glycation end products (AGE) are circulatory molecules that can generate ROS and vascular dysfunction through an association with cell surface receptors (RAGE). Insulin resistance and obesity, as well as conditions that would increase RAGE levels, can induce pathophysiologic changes similar to those observed in women with pre‐eclampsia. 212
Vascular–cellular adhesion molecule and inflammatory reactions
Pro‐inflammatory cytokines, bacterial endotoxins, ROS and lipid peroxides can stimulate vascular cellular adhesion molecule‐1 (VCAM‐1) over expression on the surface of the cell. Increased concentrations of soluble VCAM‐1 A as well as over expression of the percentage of CD49d+ peripheral blood and CD49d+ decidual lymphocytes have been reported in pre‐eclamptic women. 213 Immunological mechanisms similar to inflammatory reaction may therefore be involved in pathogenesis of pre‐eclampsia in peripheral blood as well as locally inside maternal–fetal interface.
Lipid peroxidation
The rate of lipid peroxidation in the placenta is abnormally high in the serum and the plasma of women with pre‐eclampsia. 214 , 215 , 216 As normal pregnancy advances, the antioxidant activity of vitamin E and the vasodilating actions of prostacyclins are progressively favored with advancing gestation. However, this is not true in pathological pregnancies such as pre‐eclampsia, where there is a reversal in these ratios to favor the vasoconstrictive actions of thromboxane and the toxic action of lipid peroxides. 214 , 217 Both enhanced lipid peroxidation and alteration of immune responses are involved in endothelial cell dysfunction in pre‐eclampsia. 218 , 219 , 220 , 221
The source of pre‐eclampsia is unknown, but poorly perfused placental tissue may trigger free radical process and initiation of generalized lipid peroxidation. 152 Increasing evidence supports the role of abnormal lipid metabolism and circulating modified lipids in the pathophysiologic mechanism of pre‐eclampsia. In particular, lipids peroxides and blood oxidative imbalance are proposed as part of the cytotoxic mechanisms leading to endothelial cell injury. 222 Enhanced lipid peroxidation may result from excessive production of reactive oxygen species by neutrophil activation in pre‐eclampsia. 211 The increase in antioxidants is probably of a compensatory nature responding to the increased peroxide load in pre‐eclampsia and may reflect the severity of the disease.
β‐Nicotinamide adenine dinucleotide phosphate reduced oxidase and reactive oxygen species production
Pre‐eclamptic women have circulating agonistic antibodies (AT1‐AA) directed at the angiotension (Ang) II receptor (AT1). 223 , 224 The effect of AT1‐AA on ROS, NADPH oxidase expression and NFkappaB activation in women with pre‐eclampsia has been studied. 223
Genetic factors
Genetic factors may contribute to the release of ROS and consequently the pathophysiology of the disease. Increased neutrophil ROS may be an important in mediating the endothelial damage seen in pre‐eclampsia. Superoxide can initiate lipid peroxidation, resulting in endothelial cell lysis. 225 There is evidence in the literature to suggest that pre‐eclamptic cells are ‘primed’ by some factors in the plasma, rendering them more sensitive to agonists stimulation with increased ROS production. 226 Tumor necrosis factor‐α, platelet‐activating factor and syncytiotrophoblast microvesicles are among the factors that may contribute. 227 , 228 , 229 Women with pre‐eclampsia may be genetically predisposed to have an NADPH oxidase and therefore to increased oxidative stress. 230
Using lymphoblasts from pre‐eclamptic women, significant elevation in the agonist stimulated (phorbol ester) NADPH oxidase‐mediated ROS production have been reported. 231 Enhancement of ROS generation might be important in mediating the endothelial dysfunction seen in pre‐eclampsia. Tyrosine‐dependent mechanisms may be implicated in control of the NADPH oxidase‐associated increased ROS production with pre‐eclampsia. Therefore, it is important to investigate the identification of low abundance‐specific proteins that could be tyrosine phosphorylated, involvement of these proteins in the signal transduction pathways in pre‐eclampsia, and their investigations as targets for pharmacological manipulation. There may be some underlying genetic predisposition. Oxidative stress has been suggested as a link between the two‐stage model of the pre‐eclampsia syndrome; maternal factors cause reduced placental perfusion (stage 1) and stage 2 involves activation of the maternal endothelium with multisystem disorders. 198
OXIDATIVE STRESS IN PREGNANCY INDUCED HYPERTENSION
HYPERTENSION COMPLICATES 6–20% of all pregnancies and ranks among the four most common causes of maternal/perinatal morbidity. Gestational hypertension is hypothesized to be due to pre‐existing maternal disorders, such as obesity, insulin resistance, hypertension or renal disease. 232 All these different causes converge, resulting in pre‐eclampsia by a common mechanism of endothelial cell activation and injury. 233 Pregnancy‐induced hypertension (PIH) is an endothelial dysfunction with an impaired effect of NO, the main regulator of the fetoplacental blood flow, which causes a maternal systemic vasodilatation during normal pregnancy. 234
Oxidative stress has been demonstrated in the plasma samples of patients with hypertension by the elevated levels of uric acid and lipid peroxidation products. Activation of XO system may be a potential source of free radicals in PIH. 235 Significantly lower levels of reduced GSH concentrations and increased levels of peroxides have been reported in patients with PIH compared with results in normal pregnancies. PIH has been associated with overproduction of lipid peroxides and impaired antioxidant defense. 236
Both an increase in ROS and lipid peroxides, as well as a compromised antioxidant status, has been implicated in the pathophysiology of severe pre‐eclampsia. Higher levels of antioxidant vitamins E and uric acid, and lower levels of vitamin C have been reported in women with PIH and pre‐eclampsia compared with normal pregnant and non‐pregnant women. 237
Lipid peroxidation has been suggested as a causative factor in PIH. 202 , 238 , 239 In addition, low levels of plasma antioxidant activity and plasma levels of antioxidant vitamins (vitamins E and C) are seen in these women. 200 , 207
Pregnancy‐induced hypertension complicates 5–10% of pregnancies, and a distinction must be made between pre‐eclampsia and gestational hypertension, a usually more benign form of hypertension in pregnancy. Some classifications of pre‐eclampsia require the presence of proteinurea, whereas others do not. 240 , 241 The presence of proteinurea is a strong indicator of women at risk for perinatal complications and a poor pregnancy outcome. However, a small proportion of women with non‐proteinuric gestational hypertension have similar perinatal results as patients with pre‐eclampsia as defined by hypertension and proteinurea. 242
Significantly increased levels of lipid peroxide, both in serum and placental tissue, and significantly decreased levels of vitamin E in serum, have been reported in women with severe gestational hypertension and pre‐eclampsia compared with controls. 215 Similar values of lipid peroxides or vitamin E as controls have been reported in the group with mild gestational hypertension or chronic hypertension. Percent increase in lipid peroxide levels in women with severe gestational hypertension and pre‐eclampsia appears to be higher in placental tissue than in serum. This correlates with the notion that the placenta acts as a source of lipid peroxides and that these changes are a primary event in the pathophysiology of endothelial lesions. 222 The severity of hypertension is inversely correlated with concentration of vitamin E. 217
Use of antioxidants in pre‐eclampsia treatment
Supplementation with vitamins C and E may be beneficial in the prevention of pre‐eclampsia in women at increased risk of the disease. 243 The potential benefits of antioxidant supplementation on markers of endothelial and placental function, using vitamin C (1000 mg/day), vitamin E 400 IU/day) or placebo at 16–20 week's gestation, have been examined. In normal pregnancy, the ratio of plasma markers of endothelial activation −plasminogen‐activator inhibitor 1 (PAI‐1) and placental dysfunction (PAI‐2) is decreased, but is high in pre‐eclampsia owing to endothelial‐cell activation and placental insufficiency. Vitamin supplementation was associated with a 21% decrease in PAI‐1/PA II ratio during gestation. 243 Larger multicenter trials of high‐risk women from different populations are needed to further validate the benefits of multivitamin supplementation. This could be a relevant target for future clinical trials.
OXIDATIVE STRESS IN MATERNAL DIABETES
CONGENITAL ANOMALIES AMONG infants of diabetic mothers occur at a rate of 6–10%. This represents a two‐ to fivefold increase over that observed in the general population. These congenital malformations most commonly involve the central nervous system and the heart, and account for approximately 40% of the perinatal mortality and morbidity seen in pregnancies complicated by pregestational diabetes. Maternal diabetes leads to a higher frequency of congenital malformations in offspring compared with offspring of non‐diabetic mothers. 244 The teratological processes in diabetic pregnancy are not completely understood. 245 There are excellent reviews on the role of free radicals in the etiology of diabetes, role of altered antioxidant defenses in the development of complications, role of oxidation of plasma lipids and lipoproteins in the development of artherosclerosis in diabetes and role of oxidative stress in the development of complications in diabetes. 246 , 247 , 248 , 249
Alterations in prostaglandin metabolism and role of cyclooxygenases
Diabetes‐induced alterations of several metabolites, such as arachidonic acid and prostaglandins, in particular prostaglandins E2 (PGE2), have teratological capacity. 250 , 251 , 252 , 253 , 254
One of the mechanisms for diminished PGE2 production in the embryo may be decreased cyclooxygenase (COX)‐2 activity, 255 , 256 this constitutes a possible link between the functional state of this enzyme and embryonic dysmorphogenesis. Inhibition of COX, a rate‐limiting enzyme of prostaglandin biosynthesis causes developmental disturbances analogous to those seen in embryos exposed to high glucose concentrations. 250 The addition of either arachidonic acid or PGE2 to the culture medium with COX inhibitors in low glucose corrected the embryonic development, and PGE2 supplementation also normalized the development of embryos cultured with COX inhibitors in high glucose concentration. Both SOD and NAC were able to diminish the dysmorphogenesis induced by the COX inhibitors, at doses previously shown to diminish glucose‐induced embryo damage in the same in vitro culture system. The addition of NAC normalized the morphology and 8‐epi‐PGF2α concentration of the embryos exposed to high glucose without normalizing the COX‐2 expression in embryos and membranes. The PGE concentration of day 10 embryos and membranes was reported to decrease after exposure to high glucose in vitro or diabetes in vivo. The addition of NAC in vitro to high glucose cultures largely rectified morphology and restored PGE2 concentration.
Hyperglycemia/diabetes induced down regulation of embryonic COX‐2 gene expression may be a primary event in diabetic embryopathy, leading to lowered PGE2 levels and dysmorphogenesis. Antioxidant treatment does not prevent the decrease of COX‐2 mRNA levels but restores the PGE2 concentrations, indicating that diabetes‐induced oxidative stress aggravates the loss of COX‐2 activity, thereby partly explaining the antiteratogenic affect of antioxidant treatment.
Role of reactive oxygen species in the induction of dysmorphogenesis
The direct demonstration of ROS production has been controversial, in both short‐term incubations of embryonic cells and in vivo measurements. 257 , 258 , 259 A putative excess of ROS has been observed in studies during which diabetes‐induced embryopathy was blocked by antioxidants in vitro and in vivo. 250 , 258 , 260 , 261 , 262 , 263 , 264 , 265 , 266
Both increased rates of lipid peroxidation and protein carbonylation was reported both in the mother and fetus in experimentally induced diabetic pregnancy. 267 Also, increasing evidence has indicated a role for ROS in the development of diabetes‐induced malformations in the rodent models. 260 , 268
Further evidence that ROS is involved in diabetes‐induced dysmorphogenesis is provided by studies where antioxidant treatment with butylated hydroxytoluene, vitamin E or C resulted in a decline in the occurrence of gross malformations from approximately 25% to less than 8%. 269 Increased concentrations of lipid peroxides as a result of ROS damage and reduced concentration of antioxidant vitamin E provides a rationale for developing antiteratogenic treatments for pregnant women with diabetes mellitus. Furthermore, diabetes per se is a state of oxidative stress. Low‐density lipoprotein from pregnant diabetic women is more susceptible to oxidation. Embryonic mitochondria have been implicated in mediating ROS‐induced teratogenic effect in a diabetic environment, as a consequence of increased production of ROS, decreased capacity to scavenge ROS or both. 257 , 260 , 261 , 270 , 271 Administering antioxidants to embryos in a diabetic environment, both in vivo and in vitro, significantly reduces the developmental damage. 257 , 258 , 260 , 261 , 262 , 263 , 265
Reactive oxygen species and hyperglycemia
Embryos cultured under hyperglycemic conditions show increased formation of ROS and depletion of GSH contents, as well as reduced synthesis of GSH. 257 The addition of free radical scavenging enzymes (e.g. SOD, catalase, GPX and N‐acetylcysteine, butylated hydroxytoluene) to culture media reduced the incidence of embryonic malformation after hyperglycemia. 265 , 268 Hyperglycemia‐induced embryonic malformations may be due to increased ROS formation and depletion of intracellular GSH in embryonic tissues. 272 GSH depletion and impaired responsiveness of GSH‐synthesizing enzyme to oxidative stress during organogenesis may have important roles in the development of embryonic malformations in diabetes.
Pregnancy complicated by poor control of diabetes is associated with a high risk of embryopathies, spontaneous abortions and perinatal mortality attributed to excessive oxidative stress. 273 Both lipid peroxidation and scavenging enzyme activities may be valuable, sensitive indices of fetal/neonatal threat in diabetic pregnancy in humans. Adequate measurements at the time of birth would significantly contribute to clarifying the fetal/neonatal status in a medical and legal context, and may be even of value in altering therapy in newborn infants.
Dysmorphogenesis caused by maternal diabetes is correlated with ROS‐induced inhibition of glyceraldehydes‐3‐phosphate dehydrogenase (GAPDH) in embryos indicating that inhibition of GAPDH plays a causal role in diabetic embryopathy. 274
The offspring of women with diabetes mellitus are at increased risk for congenital defects. 275 , 276 , 277 , 278 , 279 , 280 , 281 The incidence and severity of the defects are related to glycemia within the first weeks of pregnancy, during which organogenesis is initiated. 282 , 283 , 284 , 285
Maternal diabetes also inhibits the expression of PAX‐3, an embryonic gene required for neural tube closure caused by increased levels of oxidative stress. Alpha‐tocopherol blocks these defects. 286
Role of antioxidants
Reactive oxygen species are increased in embryos exposed to excess glucose. 257 , 270 , 287 This could be due both to increased oxidative metabolism and superoxide generation, or a result of the relative immaturity of the free radical scavenging pathways. 257 , 270
Concentration of antioxidant vitamins is decreased both in the diabetic experimental animals and humans. 288 , 289 Studies have indicated a protective effect of orally administered antioxidants, such as vitamin E and buytylated hydroxytoluene in pregnant diabetic rats, 258 , 261 further implicating the generation of ROS or decreased antioxidant status in the teratogenic process of diabetic pregnancy. Hyperglycemia‐induced oxidative stress plays a causal role in diabetes‐induced congenital defects. This is confirmed by studies utilizing antioxidants such as vitamin E, vitamin C, butylated hydroxytoluene, GSH precursor, N‐acetylcysteine or transgenic over expression of copper/zinc superoxide dismutase. 250 , 257 , 258 , 262 , 263 , 264 , 271 , 287 In vitro, embryonic dysmorphogenesis has been shown to be significantly diminished, either by restricting the influx of oxidative substrates, such as pyruvates, to the embryonic mitochondria or by improving the embryonic capacity to scavenge‐free oxygen radicals. 260 , 268
Vitamin E supplementation to the diabetic mother can correct both decreased vitamin E levels and substantial mitochondrial high‐amplitude swelling in the embryos. 258 , 290 Antioxidants have been shown to protect the offspring of diabetic rats against malformations. 258 , 261 , 262 , 264 , 291 , 292 Elevated levels of copper zinc SOD, catalase, GPX and SOD elicit a protective effect against diabetes‐associated embryopathy. 268 , 271
CONCLUDING REMARKS
STUDIES ON THE implications of oxidative stress in gynecologic disorders have not received adequate attention, and literature on the molecular mechanism(s) underlying these diseases is sporadic. Oxidative stress in infertility involves poor embryo quality, DNA fragmentation, and poor pregnancy outcome in assisted reproductive programs. Maintaining adequate pro‐oxidant‐antioxidant equilibrium is also important in other gynecologic diseases, such as endometriosis, pre‐eclampsia, maternal diabetes, embryopathies and pregnancy. The rise in antioxidants is probably of a compensatory nature responding to the increased peroxide load and reflects severity of the disease. Multicenter clinical trials with large number of patients are needed, and supplementation with vitamins in all pregnant women with pre‐eclampsia should be considered. Clinical studies, aiming at characterization of the maternal and fetal oxidative stress in relation to the fetal outcome, should also be conducted.
From our review of the existing literature, we conclude that the importance of oxidative stress in reproduction is just beginning to be appreciated and studied.
REFERENCES
- 1. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 2003; 79: 829–843. [DOI] [PubMed] [Google Scholar]
- 2. Halliwell B, Gutteridge JMC. The chemistry of oxygen radicals and other derived species. In: Halliwell B, Gutteridge JMC, eds. Free Radicals in Biology and Medicine, 2nd edn Oxford: Clarendon Press, 1989; 22–85. [Google Scholar]
- 3. Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre‐implantation embryo and its surroundings. Hum Reprod Update 2001; 7: 175–189. [DOI] [PubMed] [Google Scholar]
- 4. Johnson MH, Nasr‐Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays 1994; 16: 31–38. [DOI] [PubMed] [Google Scholar]
- 5. Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T‐lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab 1998; 83: 2110–2113. [DOI] [PubMed] [Google Scholar]
- 6. Agarwal A, Saleh RA. Role of oxidants in male infertility: rationale, significance, and treatment. Urol Clin North Am 2002; 29: 817–827. [DOI] [PubMed] [Google Scholar]
- 7. Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology 1996; 48: 835–850. [DOI] [PubMed] [Google Scholar]
- 8. El Mouatassim S, Guerin P, Menezo Y. Mammalian oviduct and protection against free oxygen radicals: expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol 2000; 89: 1–6. [DOI] [PubMed] [Google Scholar]
- 9. Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online 2004; 8: 616–627. [DOI] [PubMed] [Google Scholar]
- 10. Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem 2001; 8: 851–862. [DOI] [PubMed] [Google Scholar]
- 11. Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction 2001; 122: 497–506. [DOI] [PubMed] [Google Scholar]
- 12. Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl 2002; 23: 737–752. [PubMed] [Google Scholar]
- 13. Saleh RA, Agarwal A, Nelson DR et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril 2002; 78: 313–318. [DOI] [PubMed] [Google Scholar]
- 14. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 1993; 90: 7915–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fantel AG. Reactive oxygen species in developmental toxicity: review and hypothesis. Teratology 1996; 53: 196–217. [DOI] [PubMed] [Google Scholar]
- 16. Noda Y, Matsumoto H, Umaoka Y, Tatsumi K, Kishi J, Mori T. Involvement of superoxide radicals in the mouse two‐cell block. Mol Reprod Dev 1991; 28: 356–360. [DOI] [PubMed] [Google Scholar]
- 17. Biswas S, Kabir SN, Pal AK. The role of nitric oxide in the process of implantation in rats. J Reprod Fertil 1998; 114: 157–161. [DOI] [PubMed] [Google Scholar]
- 18. Ornoy A, Kimyagarov D, Yaffee P, Abir R, Raz I, Kohen R. Role of reactive oxygen species in diabetes‐induced embryotoxicity: studies on pre‐implantation mouse embryos cultured in serum from diabetic pregnant women. Isr J Med Sci 1996; 32: 1066–1073. [PubMed] [Google Scholar]
- 19. Tarin JJ. Potential effects of age‐associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod 1996; 2: 717–724. [DOI] [PubMed] [Google Scholar]
- 20. Loutradis D, John D, Kiessling AA. Hypoxanthine causes a 2‐cell block in random‐bred mouse embryos. Biol Reprod 1987; 37: 311–316. [DOI] [PubMed] [Google Scholar]
- 21. Downs SM, Dow MP. Hypoxanthine‐maintained two‐cell block in mouse embryos: dependence on glucose and effect of hypoxanthine phosphoribosyltransferase inhibitors. Biol Reprod 1991; 44: 1025–1039. [DOI] [PubMed] [Google Scholar]
- 22. Nasr‐Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 1991; 113: 551–560. [DOI] [PubMed] [Google Scholar]
- 23. Manes C, Lai NC. Nonmitochondrial oxygen utilization by rabbit blastocysts and surface production of superoxide radicals. J Reprod Fertil 1995; 104: 69–75. [DOI] [PubMed] [Google Scholar]
- 24. Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL. Oxidative phosphorylation‐dependent and ‐independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod 2000; 62: 1866–1874. [DOI] [PubMed] [Google Scholar]
- 25. Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil 2000; 118: 47–55. [PubMed] [Google Scholar]
- 26. Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med 1993; 15: 69–75. [DOI] [PubMed] [Google Scholar]
- 27. Nasr‐Esfahani MH, Johnson MH. How does transferrin overcome the in vitro block to development of the mouse preimplantation embryo? J Reprod Fertil 1992; 96: 41–48. [DOI] [PubMed] [Google Scholar]
- 28. Nasr‐Esfahani MH, Winston NJ, Johnson MH. Effects of glucose, glutamine, ethylenediaminetetraacetic acid and oxygen tension on the concentration of reactive oxygen species and on development of the mouse preimplantation embryo in vitro. J Reprod Fertil 1992; 96: 219–231. [DOI] [PubMed] [Google Scholar]
- 29. Nasr‐Esfahani M, Johnson MH, Aitken RJ. The effect of iron and iron chelators on the in‐vitro block to development of the mouse preimplantation embryo: BAT6 a new medium for improved culture of mouse embryos in vitro. Hum Reprod 1990; 5: 997–1003. [DOI] [PubMed] [Google Scholar]
- 30. Beehler BC, Przybyszewski J, Box HB, Kulesz‐Martin MF. Formation of 8‐hydroxydeoxyguanosine within DNA of mouse keratinocytes exposed in culture to UVB and H2O2 . Carcinogenesis 1992; 13: 2003–2007. [DOI] [PubMed] [Google Scholar]
- 31. Parchment RE, Lewellyn A, Swartzendruber D, Pierce GB. Serum amine oxidase activity contributes to crisis in mouse embryo cell lines. Proc Natl Acad Sci USA 1990; 87: 4340–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alvarez JG, Minaretzis D, Barrett CB, Mortola JF, Thompson IE. The sperm stress test: a novel test that predicts pregnancy in assisted reproductive technologies. Fertil Steril 1996; 65: 400–405. [DOI] [PubMed] [Google Scholar]
- 33. Bilodeau JF, Blanchette S, Gagnon C, Sirard MA. Thiols prevent H2O2‐mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 2001; 56: 275–286. [DOI] [PubMed] [Google Scholar]
- 34. Hyslop PA, Hinshaw DB, Halsey WA Jr et al. Mechanisms of oxidant‐mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem 1988; 263: 1665–1675. [PubMed] [Google Scholar]
- 35. Pierce GB, Parchment RE, Lewellyn AL. Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation 1991; 46: 181–186. [DOI] [PubMed] [Google Scholar]
- 36. Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 1992; 13: 232–241. [PubMed] [Google Scholar]
- 37. Nasr‐Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 1990; 109: 501–507. [DOI] [PubMed] [Google Scholar]
- 38. Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 1998; 13: 896–900. [DOI] [PubMed] [Google Scholar]
- 39. Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 1999; 26: 463–471. [DOI] [PubMed] [Google Scholar]
- 40. Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta 1999; 1410: 103–123. [DOI] [PubMed] [Google Scholar]
- 41. Blondin P, Coenen K, Sirard MA. The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J Androl 1997; 18: 454–460. [PubMed] [Google Scholar]
- 42. Morales H, Tilquin P, Rees JF, Massip A, Dessy F, Van Langendonckt A. Pyruvate prevents peroxide‐induced injury of in vitro preimplantation bovine embryos. Mol Reprod Dev 1999; 52: 149–157. [DOI] [PubMed] [Google Scholar]
- 43. Guerin P, Menezo Y. Hypotaurine and taurine in gamete and embryo environments: de novo synthesis via the cysteine sulfinic acid pathway in oviduct cells. Zygote 1995; 3: 333–343. [DOI] [PubMed] [Google Scholar]
- 44. El Mouatassim S, Guerin P, Menezo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod 1999; 5: 720–725. [DOI] [PubMed] [Google Scholar]
- 45. Brinster RL. Uptake and incorporation of amino acids by the preimplantation mouse embryo. J Reprod Fertil 1971; 27: 329–338. [DOI] [PubMed] [Google Scholar]
- 46. Kaye PL, Schultz GA, Johnson MH, Pratt HP, Church RB. Amino acid transport and exchange in preimplantation mouse embryos. J Reprod Fertil 1982; 65: 367–380. [DOI] [PubMed] [Google Scholar]
- 47. Lewis AM, Kaye PL. Characterization of glutamine uptake in mouse two‐cell embryos and blastocysts. J Reprod Fertil 1992; 95: 221–229. [DOI] [PubMed] [Google Scholar]
- 48. Chatot CL, Tasca RJ, Ziomek CA. Glutamine uptake and utilization by preimplantation mouse embryos in CZB medium. J Reprod Fertil 1990; 89: 335–346. [DOI] [PubMed] [Google Scholar]
- 49. Gardner DK, Lane M, Spitzer A, Batt PA. Enhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: amino acids, vitamins, and culturing embryos in groups stimulate development. Biol Reprod 1994; 50: 390–400. [DOI] [PubMed] [Google Scholar]
- 50. Schweigert FJ, Zucker H. Concentrations of vitamin A, beta‐carotene and vitamin E in individual bovine follicles of different quality. J Reprod Fertil 1988; 82: 575–579. [DOI] [PubMed] [Google Scholar]
- 51. Pascoe GA, Fariss MW, Olafsdottir K, Reed DJ. A role of vitamin E in protection against cell injury. Maintenance of intracellular glutathione precursors and biosynthesis. Eur J Biochem 1987; 166: 241–247. [DOI] [PubMed] [Google Scholar]
- 52. Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA 1991; 88: 11003–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hansen C, Srikandakumar A, Downey BR. Presence of follicular fluid in the porcine oviduct and its contribution to the acrosome reaction. Mol Reprod Dev 1991; 30: 148–153. [DOI] [PubMed] [Google Scholar]
- 54. Paszkowski T, Clarke RN. The Graafian follicle is a site of L‐ascorbate accumulation. J Assist Reprod Genet 1999; 16: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J Androl 1992; 13: 379–386. [PubMed] [Google Scholar]
- 56. Guerin P, Guillaud J, Menezo Y. Hypotaurine in spermatozoa and genital secretions and its production by oviduct epithelial cells in vitro. Hum Reprod 1995; 10: 866–872. [DOI] [PubMed] [Google Scholar]
- 57. Takahashi M, Nagai T, Hamano S, Kuwayama M, Okamura N, Okano A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol Reprod 1993; 49: 228–232. [DOI] [PubMed] [Google Scholar]
- 58. Gardiner CS, Reed DJ. Synthesis of glutathione in the preimplantation mouse embryo. Arch Biochem Biophys 1995; 318: 30–36. [DOI] [PubMed] [Google Scholar]
- 59. Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod 1993; 49: 89–94. [DOI] [PubMed] [Google Scholar]
- 60. Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol 1988; 125: 181–186. [DOI] [PubMed] [Google Scholar]
- 61. De Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development effect of beta‐mercaptoethanol, cysteine and cystine. Theriogenology 2000; 53: 761–771. [DOI] [PubMed] [Google Scholar]
- 62. Luvoni GC, Keskintepe L, Brackett BG. Improvement in bovine embryo production in vitro by glutathione‐containing culture media. Mol Reprod Dev 1996; 43: 437–443. [DOI] [PubMed] [Google Scholar]
- 63. Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J 1988; 256: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Van Winkle LJ, Dickinson HR. Differences in amino acid content of preimplantation mouse embryos that develop in vitro versus in vivo: in vitro effects of five amino acids that are abundant in oviductal secretions. Biol Reprod 1995; 52: 96–104. [DOI] [PubMed] [Google Scholar]
- 65. Barnett DK, Bavister BD. Hypotaurine requirement for in vitro development of golden hamster one‐cell embryos into morulae and blastocysts, and production of term offspring from in vitro‐fertilized ova. Biol Reprod 1992; 47: 297–304. [DOI] [PubMed] [Google Scholar]
- 66. Dumoulin JC, Evers JL, Bras M, Pieters MH, Geraedts JP. Positive effect of taurine on preimplantation development of mouse embryos in vitro. J Reprod Fertil 1992; 94: 373–380. [DOI] [PubMed] [Google Scholar]
- 67. Li J, Foote RH, Simkin M. Development of rabbit zygotes cultured in protein‐free medium with catalase, taurine, or superoxide dismutase. Biol Reprod 1993; 49: 33–37. [DOI] [PubMed] [Google Scholar]
- 68. Guyader‐Joly C, Guerin P, Renard JP, Guillaud J, Ponchon S, Menezo Y. Precursors of taurine in female genital tract: effects on developmental capacity of bovine embryo produced in vitro. Amino Acids 1998; 15: 27–42. [DOI] [PubMed] [Google Scholar]
- 69. Grupen CG, Nagashima H, Nottle MB. Cysteamine enhances in vitro development of porcine oocytes matured and fertilized in vitro. Biol Reprod 1995; 53: 173–178. [DOI] [PubMed] [Google Scholar]
- 70. De Matos DG, Furnus CC, Moses DF, Baldassarre H. Effect of cysteamine on glutathione level and developmental capacity of bovine oocyte matured in vitro. Mol Reprod Dev 1995; 42: 432–436. [DOI] [PubMed] [Google Scholar]
- 71. Lapointe S, Sullivan R, Sirard MA. Binding of a bovine oviductal fluid catalase to mammalian spermatozoa. Biol Reprod 1998; 58: 747–753. [DOI] [PubMed] [Google Scholar]
- 72. Forsberg H, Borg LA, Cagliero E, Eriksson UJ. Altered levels of scavenging enzymes in embryos subjected to a diabetic environment. Free Radic Res 1996; 24: 451–459. [DOI] [PubMed] [Google Scholar]
- 73. Paynton BV, Bachvarova R. Polyadenylation and deadenylation of maternal mRNAs during oocyte growth and maturation in the mouse. Mol Reprod Dev 1994; 37: 172–180. [DOI] [PubMed] [Google Scholar]
- 74. Matsuda Y, Tobari I. Repair capacity of fertilized mouse eggs for X‐ray damage induced in sperm and mature oocytes. Mutat Res 1989; 210: 35–47. [DOI] [PubMed] [Google Scholar]
- 75. Ashwood‐Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod 1996; 2: 46–51. [DOI] [PubMed] [Google Scholar]
- 76. Liu L, Keefe DL. Cytoplasm mediates both development and oxidation‐induced apoptotic cell death in mouse zygotes. Biol Reprod 2000; 62: 1828–1834. [DOI] [PubMed] [Google Scholar]
- 77. Barritt JA, Brenner CA, Cohen J, Matt DW. Mitochondrial DNA rearrangements in human oocytes and embryos. Mol Hum Reprod 1999; 5: 927–933. [DOI] [PubMed] [Google Scholar]
- 78. Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta 1995; 236: 173–180. [DOI] [PubMed] [Google Scholar]
- 79. Sabatini L, Wilson C, Lower A, Al‐Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. Fertil Steril 1999; 72: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 80. Ji BT, Shu XO, Linet MS et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst 1997; 89: 238–244. [DOI] [PubMed] [Google Scholar]
- 81. Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997; 56: 602–607. [DOI] [PubMed] [Google Scholar]
- 82. Tarin JJJ, Vendrell FJ, Cano A. Dithiothreitol prevents age‐associated decrease in oocyte/conceptus viability in vitro. Hum Reprod 1998; 13: 381–386. [DOI] [PubMed] [Google Scholar]
- 83. Tarin JJ, Ten Vendrell FJJ, Cano A. Antioxidant therapy counteracts the disturbing effects of diamide and maternal ageing on meiotic division and chromosomal segregation in mouse oocytes. Mol Hum Reprod 1998; 4: 281–288. [DOI] [PubMed] [Google Scholar]
- 84. Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod 1997; 57: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 85. Gardiner CS, Reed DJ. Status of glutathione during oxidant‐induced oxidative stress in the preimplantation mouse embryo. Biol Reprod 1994; 51: 1307–1314. [DOI] [PubMed] [Google Scholar]
- 86. Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl‐2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993; 75: 241–251. [DOI] [PubMed] [Google Scholar]
- 87. Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell 1997; 88: 347–354. [DOI] [PubMed] [Google Scholar]
- 88. Takase K, Ishikawa M, Hoshiai H. Apoptosis in the degeneration process of unfertilized mouse ova. Tohoku J Exp Med 1995; 175: 69–76. [DOI] [PubMed] [Google Scholar]
- 89. Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod 1997; 56: 1088–1096. [DOI] [PubMed] [Google Scholar]
- 90. Liu L, Trimarchi JR, Keefe DL. Thiol oxidation‐induced embryonic cell death in mice is prevented by the antioxidant dithiothreitol. Biol Reprod 1999; 61: 1162–1169. [DOI] [PubMed] [Google Scholar]
- 91. Plachot M, Mandelbaum J. Oocyte maturation, fertilization and embryonic growth in vitro. Br Med Bull 1990; 46: 675–694. [DOI] [PubMed] [Google Scholar]
- 92. Erenus M, Zouves C, Rajamahendran P, Leung S, Fluker M, Gomel V. The effect of embryo quality on subsequent pregnancy rates after in vitro fertilization. Fertil Steril 1991; 56: 707–710. [DOI] [PubMed] [Google Scholar]
- 93. Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod 1996; 2: 93–98. [DOI] [PubMed] [Google Scholar]
- 94. Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod 1998; 13: 998–1002. [DOI] [PubMed] [Google Scholar]
- 95. Ahmadi A, Ng SC. Fertilizing ability of DNA‐damaged spermatozoa. J Exp Zool 1999; 284: 696–704. [DOI] [PubMed] [Google Scholar]
- 96. Ahmadi A, Ng SC. Destruction of protamine in human sperm inhibits sperm binding and penetration in the zona‐free hamster penetration test but increases sperm head decondensation and male pronuclear formation in the hamster‐ICSI assay. J Assist Reprod Genet 1999; 16: 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ahmadi A, Ng SC. Developmental capacity of damaged spermatozoa. Hum Reprod 1999; 14: 2279–2285. [DOI] [PubMed] [Google Scholar]
- 98. Evenson DP, Jost LK, Marshall D et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999; 14: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 99. Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril 2000; 73: 43–50. [DOI] [PubMed] [Google Scholar]
- 100. Tomsu M, Sharma V, Miller D. Embryo quality and IVF treatment outcomes may correlate with different sperm comet assay parameters. Hum Reprod 2002; 17: 1856–1862. [DOI] [PubMed] [Google Scholar]
- 101. Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod 2002; 17: 990–998. [DOI] [PubMed] [Google Scholar]
- 102. Sakkas D, Urner F, Bizzaro D et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod 1998; 13 (Suppl 4): 11–19. [DOI] [PubMed] [Google Scholar]
- 103. Twigg JP, Irvine DS, Aitken RJ. Oxidative damage to DNA in human spermatozoa does not preclude pronucleus formation at intracytoplasmic sperm injection. Hum Reprod 1998; 13: 1864–1871. [DOI] [PubMed] [Google Scholar]
- 104. McPherson S, Longo FJ. Chromatin structure‐function alterations during mammalian spermatogenesis: DNA nicking and repair in elongating spermatids. Eur J Histochem 1993; 37: 109–128. [PubMed] [Google Scholar]
- 105. Manicardi GC, Bianchi PG, Pantano S et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod 1995; 52: 864–867. [DOI] [PubMed] [Google Scholar]
- 106. Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod 1999; 4: 31–37. [DOI] [PubMed] [Google Scholar]
- 107. Kemal Duru N, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril 2000; 74: 1200–1207. [DOI] [PubMed] [Google Scholar]
- 108. Sanchez R, Stalf T, Khanaga O, Turley H, Gips H, Schill WB. Sperm selection methods for intracytoplasmic sperm injection (ICSI) in andrological patients. J Assist Reprod Genet 1996; 13: 228–233. [DOI] [PubMed] [Google Scholar]
- 109. Jones GM, Trounson AO, Lolatgis N, Wood C. Factors affecting the success of human blastocyst development and pregnancy following in vitro fertilization and embryo transfer. Fertil Steril 1998; 70: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 110. Janny L, Menezo YJ. Evidence for a strong paternal effect on human preimplantation embryo development and blastocyst formation. Mol Reprod Dev 1994; 38: 36–42. [DOI] [PubMed] [Google Scholar]
- 111. Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod 2001; 16: 918–924. [DOI] [PubMed] [Google Scholar]
- 112. Dumoulin JM, Coonen E, Bras M et al. Embryo development and chromosomal anomalies after ICSI. effect of the injection procedure. Hum Reprod 2001; 16: 306–312. [DOI] [PubMed] [Google Scholar]
- 113. Shoukir Y, Chardonnens D, Campana A, Sakkas D. Blastocyst development from supernumerary embryos after intracytoplasmic sperm injection: a paternal influence? Hum Reprod 1998; 13: 1632–1637. [DOI] [PubMed] [Google Scholar]
- 114. Banerjee S, Lamond S, McMahon A, Campbell S, Nargund G. Does blastocyst culture eliminate paternal chromosomal defects and select good embryos?: inheritance of an abnormal paternal genome following ICSI. Hum Reprod 2000; 15: 2455–2459. [DOI] [PubMed] [Google Scholar]
- 115. Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl 2000; 21: 33–44. [PubMed] [Google Scholar]
- 116. Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod 2000; 15: 1717–1722. [DOI] [PubMed] [Google Scholar]
- 117. Larson‐Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril 2003; 80: 895–902. [DOI] [PubMed] [Google Scholar]
- 118. Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 2003; 9: 331–345. [DOI] [PubMed] [Google Scholar]
- 119. Sakkas D, Urner F, Bianchi PG et al. Sperm chromatin anomalies can influence decondensation after intracytoplasmic sperm injection. Hum Reprod 1996; 11: 837–843. [DOI] [PubMed] [Google Scholar]
- 120. Benchaib M, Braun V, Lornage J et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod 2003; 18: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 121. Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002; 17: 3122–3128. [DOI] [PubMed] [Google Scholar]
- 122. Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl 2004; 6: 139–148. [PubMed] [Google Scholar]
- 123. Virro MR, Larson‐Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004; 81: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 124. Gandini L, Lombardo F, Paoli D et al. Full‐term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod 2004; 19: 1409–1417. [DOI] [PubMed] [Google Scholar]
- 125. Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor‐quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril 1998; 69: 528–532. [DOI] [PubMed] [Google Scholar]
- 126. Host E, Lindenberg S, Smidt‐Jensen S. The role of DNA strand breaks in human spermatozoa used for IVF and ICSI. Acta Obstet Gynecol Scand 2000; 79: 559–563. [PubMed] [Google Scholar]
- 127. Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, Sakkas D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod 2001; 16: 2160–2165. [DOI] [PubMed] [Google Scholar]
- 128. Raman RS, Chan PJ, Corselli JU et al. Comet assay of cumulus cell DNA status and the relationship to oocyte fertilization via intracytoplasmic sperm injection. Hum Reprod 2001; 16: 831–835. [DOI] [PubMed] [Google Scholar]
- 129. Saleh RA, Agarwal A, Nada EA et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 2003; 79 (Suppl 3): 1597–1605. [DOI] [PubMed] [Google Scholar]
- 130. Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod 2004; 19: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 131. Wang W, Pang CC, Rogers MS, Chang AM. Lipid peroxidation in cord blood at birth. Am J Obstet Gynecol 1996; 174: 62–65. [DOI] [PubMed] [Google Scholar]
- 132. Rogers MS, Mongelli JM, Tsang KH, Wang CC, Law KP. Lipid peroxidation in cord blood at birth: the effect of labour. Br J Obstet Gynaecol 1998; 105: 739–744. [DOI] [PubMed] [Google Scholar]
- 133. Guarnaccia MM, Takami M, Jones EE, Preston SL, Behrman HR. Luteinizing hormone depletes ascorbic acid in preovulatory follicles. Fertil Steril 2000; 74: 959–963. [DOI] [PubMed] [Google Scholar]
- 134. Kramer MM, Harmon MT, Brill AK. Disturbances of reproduction and ovarian changes in the guinea pig in relation to vitamin C deficiency. Am J Physiol 1933; 106: 611–622. [Google Scholar]
- 135. Musicki B, Kodaman PH, Aten RF, Behrman HR. Endocrine regulation of ascorbic acid transport and secretion in luteal cells. Biol Reprod 1996; 54: 399–406. [DOI] [PubMed] [Google Scholar]
- 136. Briggs DA, Sharp DJ, Miller D, Gosden RG. Transferrin in the developing ovarian follicle: evidence for de‐novo expression by granulosa cells. Mol Hum Reprod 1999; 5: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 137. Jozwik M, Wolczynski S, Szamatowicz M. Oxidative stress markers in preovulatory follicular fluid in humans. Mol Hum Reprod 1999; 5: 409–413. [DOI] [PubMed] [Google Scholar]
- 138. Attaran M, Pasqualotto E, Falcone T et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med 2000; 45: 314–320. [PubMed] [Google Scholar]
- 139. Pasqualotto EB, Agarwal A, Sharma RK et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril 2004; 81: 973–976. [DOI] [PubMed] [Google Scholar]
- 140. Burlingame JM, Esfandiari N, Sharma RK, Mascha E, Falcone T. Total antioxidant capacity and reactive oxygen species in amniotic fluid. Obstet Gynecol 2003; 101: 756–761. [DOI] [PubMed] [Google Scholar]
- 141. Lappas M, Permezel M, Rice GE. N‐Acetyl‐cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor‐kappaB deoxyribonucleic acid‐binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab 2003; 88: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 142. Woods JR Jr. Reactive oxygen species and preterm premature rupture of membranes‐a review. Placenta 2001; 22 (Suppl A): S38–S44. [DOI] [PubMed] [Google Scholar]
- 143. Brandt E. Smoking and reproductive health. In: Rosenberg J, ed. Smoking and Reproductive Health. Litterton: PSG Publications, 1987, 1–23. [Google Scholar]
- 144. Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J 1991; 277 (Pt 1): 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Paszkowski T, Clarke RN, Hornstein MD. Smoking induces oxidative stress inside the Graafian follicle. Hum Reprod 2002; 17: 921–925. [DOI] [PubMed] [Google Scholar]
- 146. Palan PR, Cohen BL, Barad DH, Romney SL. Effects of smoking on the levels of antioxidant beta carotene, alpha tocopherol and retinol in human ovarian follicular fluid. Gynecol Obstet Invest 1995; 39: 43–46. [DOI] [PubMed] [Google Scholar]
- 147. Zhou JF, Yan XF, Guo FZ, Sun NY, Qian ZJ, Ding DY. Effects of cigarette smoking and smoking cessation on plasma constituents and enzyme activities related to oxidative stress. Biomed Environ Sci 2000; 13: 44–55. [PubMed] [Google Scholar]
- 148. Yang Q, Sherman SL, Hassold TJ et al. Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population‐based case‐control study. Genet Med 1999; 1: 80–88. [DOI] [PubMed] [Google Scholar]
- 149. Takehara Y, Yoshioka T, Sasaki J. Changes in the levels of lipoperoxide and antioxidant factors in human placenta during gestation. Acta Med Okayama 1990; 44: 103–111. [DOI] [PubMed] [Google Scholar]
- 150. Little RE, Gladen BC. Levels of lipid peroxides in uncomplicated pregnancy: a review of the literature. Reprod Toxicol 1999; 13: 347–352. [DOI] [PubMed] [Google Scholar]
- 151. Walsh SW, Wang Y. Secretion of lipid peroxides by the human placenta. Am J Obstet Gynecol 1993; 169: 1462–1466. [DOI] [PubMed] [Google Scholar]
- 152. Diamant S, Kissilevitz R, Diamant Y. Lipid peroxidation system in human placental tissue: general properties and the influence of gestational age. Biol Reprod 1980; 23: 776–781. [DOI] [PubMed] [Google Scholar]
- 153. Ishihara M. Studies on lipoperoxide of normal pregnant women and of patients with toxemia of pregnancy. Clin Chim Acta 1978; 84: 1–9. [DOI] [PubMed] [Google Scholar]
- 154. Cranfield LM, Gollan JL, White AG, Dormandy TL. Serum antioxidant activity in normal and abnormal subjects. Ann Clin Biochem 1979; 16: 299–306. [DOI] [PubMed] [Google Scholar]
- 155. Jagadeesan V, Prema K. Plasma tocopherol and lipid levels in pregnancy and oral contraceptive users. Br J Obstet Gynaecol 1980; 87: 903–907. [DOI] [PubMed] [Google Scholar]
- 156. Wang YP, Walsh SW, Guo JD, Zhang JY. Maternal levels of prostacyclin, thromboxane, vitamin E, and lipid peroxides throughout normal pregnancy. Am J Obstet Gynecol 1991; 165: 1690–1694. [DOI] [PubMed] [Google Scholar]
- 157. Wickens D, Wilkins MH, Lunec J, Ball G, Dormandy TL. Free radical oxidation (peroxidation) products in plasma in normal and abnormal pregnancy. Ann Clin Biochem 1981; 18: 158–162. [DOI] [PubMed] [Google Scholar]
- 158. Uotila J, Tuimala R, Aarnio T, Pyykko K, Ahotupa M. Lipid peroxidation products, selenium‐dependent glutathione peroxidase and vitamin E in normal pregnancy. Eur J Obstet Gynecol Reprod Biol 1991; 42: 95–100. [DOI] [PubMed] [Google Scholar]
- 159. Woods JR Jr, Cavanaugh JL, Norkus EP, Plessinger MA, Miller RK. The effect of labor on maternal and fetal vitamins C and E. Am J Obstet Gynecol 2002; 187: 1179–1183. [DOI] [PubMed] [Google Scholar]
- 160. Park E, Wagenbichler P, Elmadfa I. Effects of multivitamin/mineral supplementation, at nutritional doses, on plasma antioxidant status and DNA damage estimated by sister chromatid exchanges in lymphocytes in pregnant women. Int J Vitam Nutr Res 1999; 69: 396–402. [DOI] [PubMed] [Google Scholar]
- 161. Lagod L, Paszkowski T, Sikorski R, Rola R. [The antioxidant‐prooxidant balance in pregnancy complicated by spontaneous abortion]. Ginekol Pol 2001; 72: 1073–1078. [PubMed] [Google Scholar]
- 162. Peiker G, Dawczynski H, Winnefeld K, Michels W, Seewald HJ. [Levels of antioxidants after cesarean section and administration of Multibionta N, Inzolen and selenase]. Med Klin (Munich) 1997; 92 (Suppl 3): 34–35. [PubMed] [Google Scholar]
- 163. Balasch J, Creus M, Fabregues F et al. Visible and non‐visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11: 387–391. [DOI] [PubMed] [Google Scholar]
- 164. Parazzini F, Di Cintio E, Chatenoud L, Moroni S, Mezzanotte C, Crosignani PG. Oral contraceptive use and risk of endometriosis. Italian Endometriosis Study Group. Br J Obstet Gynaecol 1999; 106: 695–699. [PubMed] [Google Scholar]
- 165. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997; 24: 235–258. [DOI] [PubMed] [Google Scholar]
- 166. Daya S. Endometriosis and spontaneous abortion. In: Coutinho EM, De Moura LH, eds. Progress in the Management of Endometriosis. New York: Parthenon, 1995; 61–68. [Google Scholar]
- 167. Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril 1981; 35: 696–698. [DOI] [PubMed] [Google Scholar]
- 168. Ota H, Igarashi S, Hatazawa J, Tanaka T. Immunohistochemical assessment of superoxide dismutase expression in the endometrium in endometriosis and adenomyosis. Fertil Steril 1999; 72: 129–134. [DOI] [PubMed] [Google Scholar]
- 169. Ota H, Igarashi S. Expression of major histocompatibility complex class II antigen in endometriotic tissue in patients with endometriosis and adenomyosis. Fertil Steril 1993; 60: 834–838. [DOI] [PubMed] [Google Scholar]
- 170. Van Langendonckt A, Casanas‐Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril 2002; 77: 861–870. [DOI] [PubMed] [Google Scholar]
- 171. Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990; 87: 1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gleicher N, El‐Roeiy A, Confino E, Friberg J. Is endometriosis an autoimmune disease? Obstet Gynecol 1987; 70: 115–122. [PubMed] [Google Scholar]
- 173. Zeller JM, Henig I, Radwanska E, Dmowski WP. Enhancement of human monocyte and peritoneal macrophage chemiluminescence activities in women with endometriosis. Am J Reprod Immunol Microbiol 1987; 13: 78–82. [DOI] [PubMed] [Google Scholar]
- 174. Arumugam K, Dip YC. Endometriosis and infertility: the role of exogenous lipid peroxides in the peritoneal fluid. Fertil Steril 1995; 63: 198–199. [DOI] [PubMed] [Google Scholar]
- 175. Ho HN, Wu MY, Chen SU, Chao KH, Chen CD, Yang YS. Total antioxidant status and nitric oxide do not increase in peritoneal fluids from women with endometriosis. Hum Reprod 1997; 12: 2810–2815. [DOI] [PubMed] [Google Scholar]
- 176. Wang Y, Sharma RK, Falcone T, Goldberg J, Agarwal A. Importance of reactive oxygen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil Steril 1997; 68: 826–830. [DOI] [PubMed] [Google Scholar]
- 177. Polak G, Koziol‐Montewka M, Tarkowski R, Kotarski J. [Peritoneal fluid and plasma 4‐hydroxynonenal and malonyldialdehyde concentrations in infertile women]. Ginekol Pol 2001; 72: 1316–1320. [PubMed] [Google Scholar]
- 178. Ota H, Igarashi S, Tanaka T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil Steril 2001; 75: 785–790. [DOI] [PubMed] [Google Scholar]
- 179. Arumugam K, Yip YC. De novo formation of adhesions in endometriosis: the role of iron and free radical reactions. Fertil Steril 1995; 64: 62–64. [PubMed] [Google Scholar]
- 180. Ota H, Igarashi S, Hatazawa J, Tanaka T. Endothelial nitric oxide synthase in the endometrium during the menstrual cycle in patients with endometriosis and adenomyosis. Fertil Steril 1998; 69: 303–308. [DOI] [PubMed] [Google Scholar]
- 181. Ota H, Igarashi S, Kato N, Tanaka T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil Steril 2000; 74: 313–318. [DOI] [PubMed] [Google Scholar]
- 182. Murphy AA, Palinski W, Rankin S, Morales AJ, Parthasarathy S. Evidence for oxidatively modified lipid‐protein complexes in endometrium and endometriosis. Fertil Steril 1998; 69: 1092–1094. [DOI] [PubMed] [Google Scholar]
- 183. Salonen JT, Yla‐Herttuala S et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 1992; 339: 883–887. [DOI] [PubMed] [Google Scholar]
- 184. Yamamoto T, Yoshimura S, Geshi Y et al. Measurement of antiphospholipid antibody by ELISA using purified beta 2‐glycoprotein I in preeclampsia. Clin Exp Immunol 1993; 94: 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Bergmark C, Wu R, De Faire U, Lefvert AK, Swedenborg J. Patients with early‐onset peripheral vascular disease have increased levels of autoantibodies against oxidized LDL. Arterioscler Thromb Vasc Biol 1995; 15: 441–445. [DOI] [PubMed] [Google Scholar]
- 186. Shanti A, Santanam N, Morales AJ, Parthasarathy S, Murphy AA. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil Steril 1999; 71: 1115–1118. [DOI] [PubMed] [Google Scholar]
- 187. Murphy AA, Palinski W, Rankin S, Morales AJ, Parthasarathy S. Macrophage scavenger receptor(s) and oxidatively modified proteins in endometriosis. Fertil Steril 1998; 69: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 188. Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 1993; 268: 11811–11816. [PubMed] [Google Scholar]
- 189. Murphy AA, Santanam N, Parthasarathy S. Endometriosis: a disease of oxidative stress? Semin Reprod Endocrinol 1998; 16: 263–273. [DOI] [PubMed] [Google Scholar]
- 190. Cunningham FG, Lindheimer MD. Hypertension in pregnancy. N Engl J Med 1992; 326: 927–932. [DOI] [PubMed] [Google Scholar]
- 191. Yoneyama Y, Sawa R, Suzuki S et al. Relationship between plasma malondialdehyde levels and adenosine deaminase activities in preeclampsia. Clin Chim Acta 2002; 322: 169–173. [DOI] [PubMed] [Google Scholar]
- 192. Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Medical 1999; 222: 222–235. [DOI] [PubMed] [Google Scholar]
- 193. Sikkema JM, Van Rijn BB, Franx A et al. Placental superoxide is increased in pre‐eclampsia. Placenta 2001; 22: 304–308. [DOI] [PubMed] [Google Scholar]
- 194. Myatt L, Rosenfield RB, Eis AL, Brockman DE, Greer I, Lyall F. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension 1996; 28: 488–493. [DOI] [PubMed] [Google Scholar]
- 195. Hubel CA, McLaughlin MK, Evans RW, Hauth BA, Sims CJ, Roberts JM. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am J Obstet Gynecol 1996; 174: 975–982. [DOI] [PubMed] [Google Scholar]
- 196. Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J Clin Endocrinol Metab 1995; 80: 1888–1893. [DOI] [PubMed] [Google Scholar]
- 197. Zusterzeel PL, Rutten H, Roelofs HM, Peters WH, Steegers EA. Protein carbonyls in decidua and placenta of pre‐eclamptic women as markers for oxidative stress. Placenta 2001; 22: 213–219. [DOI] [PubMed] [Google Scholar]
- 198. Roberts JM, Hubel CA. Is oxidative stress the link in the two‐stage model of pre‐eclampsia? Lancet 1999; 354: 788–789. [DOI] [PubMed] [Google Scholar]
- 199. Bilodeau JF, Hubel CA. Current concepts in the use of antioxidants for the treatment of preeclampsia. J Obstet Gynaecol Can 2003; 25: 742–750. [DOI] [PubMed] [Google Scholar]
- 200. Mikhail MS, Anyaegbunam A, Garfinkel D, Palan PR, Basu J, Romney SL. Preeclampsia and antioxidant nutrients: decreased plasma levels of reduced ascorbic acid, alpha‐tocopherol, and beta‐carotene in women with preeclampsia. Am J Obstet Gynecol 1994; 171: 150–157. [DOI] [PubMed] [Google Scholar]
- 201. Madazli R, Benian A, Gumustas K, Uzun H, Ocak V, Aksu F. Lipid peroxidation and antioxidants in preeclampsia. Eur J Obstet Gynecol Reprod Biol 1999; 85: 205–208. [DOI] [PubMed] [Google Scholar]
- 202. Uotila JT, Tuimala RJ, Aarnio TM, Pyykko KA, Ahotupa MO. Findings on lipid peroxidation and antioxidant function in hypertensive complications of pregnancy. Br J Obstet Gynaecol 1993; 100: 270–276. [DOI] [PubMed] [Google Scholar]
- 203. Chen G, Wilson R, Cumming G, Walker JJ, Smith WE, McKillop JH. Intracellular and extracellular antioxidant buffering levels in erythrocytes from pregnancy‐induced hypertension. J Hum Hypertens 1994; 8: 37–42. [PubMed] [Google Scholar]
- 204. Dekker GA, Kraayenbrink AA, Zeeman GG, Van Kamp GJ. Increased plasma levels of the novel vasoconstrictor peptide endothelin in severe pre‐eclampsia. Eur J Obstet Gynecol Reprod Biol 1991; 40: 215–220. [DOI] [PubMed] [Google Scholar]
- 205. Chen H, Wang Z, Lin M. The role of neutrophil activation in pathogenesis of preeclampsia. J Tongji Med University 2000; 20: 246–248. [DOI] [PubMed] [Google Scholar]
- 206. Lee VM, Quinn PA, Jennings SC, Ng LL. Neutrophil activation and production of reactive oxygen species in pre‐eclampsia. J Hypertens 2003; 21: 395–402. [DOI] [PubMed] [Google Scholar]
- 207. Davidge ST, Hubel CA, Brayden RD, Capeless EC, McLaughlin MK. Sera antioxidant activity in uncomplicated and preeclamptic pregnancies. Obstet Gynecol 1992; 79: 897–901. [PubMed] [Google Scholar]
- 208. Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol 1996; 174: 288–291. [DOI] [PubMed] [Google Scholar]
- 209. Roggensack AM, Zhang Y, Davidge ST. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension 1999; 33: 83–89. [DOI] [PubMed] [Google Scholar]
- 210. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 1996; 271: C1424–C1437. [DOI] [PubMed] [Google Scholar]
- 211. Shaarawy M, Aref A, Salem ME, Sheiba M. Radical‐scavenging antioxidants in pre‐eclampsia and eclampsia. Int J Gynaecol Obstet 1998; 60: 123–128. [DOI] [PubMed] [Google Scholar]
- 212. Cooke CL, Brockelsby JC, Baker PN, Davidge ST. The receptor for advanced glycation end products (RAGE) is elevated in women with preeclampsia. Hypertens Pregnancy 2003; 22: 173–184. [DOI] [PubMed] [Google Scholar]
- 213. Wilczynski JR, Glowacka E, Nowak M, Szpakowski A. [Serum concentration of soluble vascular‐cellular adhesion molecule‐1 (VCAM‐1) and expression of its receptor VLA‐4 on the surface of peripheral blood and decidual lymphocytes of preeclamptic women]. Ginekol Pol 2003; 74: 1335–1342. [PubMed] [Google Scholar]
- 214. Wang Y, Walsh SW, Kay HH. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. Am J Obstet Gynecol 1992; 167: 946–949. [DOI] [PubMed] [Google Scholar]
- 215. Gratacos E, Casals E, Deulofeu R, Cararach V, Alonso PL, Fortuny A. Lipid peroxide and vitamin E patterns in pregnant women with different types of hypertension in pregnancy. Am J Obstet Gynecol 1998; 178: 1072–1076. [DOI] [PubMed] [Google Scholar]
- 216. Mutlu‐Turkoglu U, Ademoglu E, Ibrahimoglu L, Aykac‐Toker G, Uysal M. Imbalance between lipid peroxidation and antioxidant status in preeclampsia. Gynecol Obstet Invest 1998; 46: 37–40. [DOI] [PubMed] [Google Scholar]
- 217. Wang YP, Walsh SW, Guo JD, Zhang JY. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol 1991; 165: 1695–1700. [DOI] [PubMed] [Google Scholar]
- 218. Mosmann TR, Sad S. The expanding universe of T‐cell subsets: Th1, Th2 and more. Immunol Today 1996; 17: 138–146. [DOI] [PubMed] [Google Scholar]
- 219. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999; 180: 499–506. [DOI] [PubMed] [Google Scholar]
- 220. Taylor RN, De Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol 1998; 16: 17–31. [DOI] [PubMed] [Google Scholar]
- 221. Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol 1999; 117: 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Walsh SW. Lipid peroxidation in pregnancy. Hypertens Pregnancy 1994; 13: 1–32. [Google Scholar]
- 223. Dechend R, Viedt C, Muller DN et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 2003; 107: 1632–1639. [DOI] [PubMed] [Google Scholar]
- 224. Wallukat G, Homuth V, Fischer T et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999; 103: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite‐induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 1991; 288: 481–487. [DOI] [PubMed] [Google Scholar]
- 226. Von Dadelszen P, Wilkins T, Redman CW. Maternal peripheral blood leukocytes in normal and pre‐eclamptic pregnancies. Br J Obstet Gynaecol 1999; 106: 576–581. [DOI] [PubMed] [Google Scholar]
- 227. Clark CJ, Boswell F, Greer IA, Lyall F. Treatment of endothelial cells with serum from women with preeclampsia: effect on neutrophil adhesion. J Soc Gynecol Invest 1997; 4: 27–33. [DOI] [PubMed] [Google Scholar]
- 228. Wang Y, Gu Y, Philibert L, Lucas MJ. Neutrophil activation induced by placental factors in normal and pre‐eclamptic pregnancies in vitro. Placenta 2001; 22: 560–565. [DOI] [PubMed] [Google Scholar]
- 229. Lal AS, Clifton AD, Rouse J, Segal AW, Cohen P. Activation of the neutrophil NADPH oxidase is inhibited by SB 203580, a specific inhibitor of SAPK2/p38. Biochem Biophys Res Commun 1999; 259: 465–470. [DOI] [PubMed] [Google Scholar]
- 230. Barden A, Ritchie J, Walters B et al. Study of plasma factors associated with neutrophil activation and lipid peroxidation in preeclampsia. Hypertension 2001; 38: 803–808. [DOI] [PubMed] [Google Scholar]
- 231. Lee VM, Quinn PA, Jennings SC, Ng LL. NADPH oxidase activity in preeclampsia with immortalized lymphoblasts used as models. Hypertension 2003; 41: 925–931. [DOI] [PubMed] [Google Scholar]
- 232. Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol 1996; 175: 1365–1370. [DOI] [PubMed] [Google Scholar]
- 233. Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989; 161: 1200–1204. [DOI] [PubMed] [Google Scholar]
- 234. Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol 1997; 272: R441–R463. [DOI] [PubMed] [Google Scholar]
- 235. Nemeth I, Talosi G, Papp A, Boda D. Xanthine oxidase activation in mild gestational hypertension. Hypertens Pregnancy 2002; 21: 1–11. [DOI] [PubMed] [Google Scholar]
- 236. Pyska W, Klejewski A, Karolkiewicz J, Szczesniak L, Szczesniak‐Chmielecka A, Nowak A. [Imbalance of pro‐oxidants‐antioxidants in blood of pregnant women with pregnancy induced hypertension]. Ginekol Pol 2002; 73: 14–18. [PubMed] [Google Scholar]
- 237. Zusterzeel PL, Steegers‐Theunissen RP, Harren FJ et al. Ethene and other biomarkers of oxidative stress in hypertensive disorders of pregnancy. Hypertens Pregnancy 2002; 21: 39–49. [DOI] [PubMed] [Google Scholar]
- 238. Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK. Lipid peroxidation in pregnancy: new perspectives on preeclampsia. Am J Obstet Gynecol 1989; 161: 1025–1034. [DOI] [PubMed] [Google Scholar]
- 239. Jain SK, Wise R. Relationship between elevated lipid peroxides, vitamin E deficiency and hypertension in preeclampsia. Mol Cell Biochem 1995; 151: 33–38. [DOI] [PubMed] [Google Scholar]
- 240. Australasian Society for the Study of Hypertension in Pregnancy. Management of hypertension in pregnancy: executive summary. Med J Aust 1993; 158: 700–702. [PubMed] [Google Scholar]
- 241. Consensus report. National high blood pressure education program Working Group report on high blood pressure in pregnancy. Am J Obstet Gynecol 1990; 163: 1689–1712. [DOI] [PubMed] [Google Scholar]
- 242. Brown MA, Buddie ML. The importance of nonprotenuric hypertension in pregnancy. Hypertens Pregnancy 1995; 14: 57–65. [Google Scholar]
- 243. Chappell LC, Seed PT, Briley AL et al. Effect of antioxidants on the occurrence of pre‐eclampsia in women at increased risk: a randomised trial. Lancet 1999; 354: 810–816. [DOI] [PubMed] [Google Scholar]
- 244. Pedersen LM, Tygstrup I, Pedersen J. Congenital malformations in newborn infants of diabetic women. Correlation with maternal diabetic vascular complications. Lancet 1964; 13: 1124–1126. [DOI] [PubMed] [Google Scholar]
- 245. Eriksson UJ, Borg LAH, Forsberg H, Simian CM, Suzuki N, Yang X. Can fetal loss be prevented? The biochemical basis of diabetic embryopathy. Diabetes 1996; 4: 49–69. [Google Scholar]
- 246. Oberley LW. Free radicals and diabetes. Free Radic Biol Med 1988; 5: 113–124. [DOI] [PubMed] [Google Scholar]
- 247. Godin GV, Wohaib SA. Reactive oxygen radical processes in diabetes In: Singal PK, ed. Oxygen Radicals in the Pathophysiology of the Heart Disease. Boston: Kluwer, 1988. [Google Scholar]
- 248. Daughterey A, Baynes JW, eds. The Role of Oxidation in the Pathophysiology. St. Louis: MedStrategy, 1991. [Google Scholar]
- 249. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991; 40: 405–412. [DOI] [PubMed] [Google Scholar]
- 250. Wentzel P, Eriksson UJ. Antioxidants diminish developmental damage induced by high glucose and cyclooxygenase inhibitors in rat embryos in vitro. Diabetes 1998; 47: 677–684. [DOI] [PubMed] [Google Scholar]
- 251. Baker L, Piddington R, Goldman A, Egler J, Moehring J. Myo‐inositol and prostaglandins reverse the glucose inhibition of neural tube fusion in cultured mouse embryos. Diabetologia 1990; 33: 593–596. [DOI] [PubMed] [Google Scholar]
- 252. Goto MP, Goldman AS, Uhing MR. PGE2 prevents anomalies induced by hyperglycemia or diabetic serum in mouse embryos. Diabetes 1992; 41: 1644–1650. [DOI] [PubMed] [Google Scholar]
- 253. Goldman AS, Baker L, Piddington R, Marx B, Herold R, Egler J. Hyperglycemia‐induced teratogenesis is mediated by a functional deficiency of arachidonic acid. Proc Natl Acad Sci USA 1985; 82: 8227–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Pinter E, Reece EA, Leranth CZ et al. Arachidonic acid prevents hyperglycemia‐associated yolk sac damage and embryopathy. Am J Obstet Gynecol 1986; 155: 691–702. [DOI] [PubMed] [Google Scholar]
- 255. Hulkower KI, Wertheimer SJ, Levin W et al. Interleukin‐1 beta induces cytosolic phospholipase A2 and prostaglandin H synthase in rheumatoid synovial fibroblasts. Evidence for their roles in the production of prostaglandin E2. Arthritis Rheum 1994; 37: 653–661. [DOI] [PubMed] [Google Scholar]
- 256. Reddy ST, Herschman HR. Ligand‐induced prostaglandin synthesis requires expression of the TIS10/PGS‐2 prostaglandin synthase gene in murine fibroblasts and macrophages. J Biol Chem 1994; 269: 15473–15480. [PubMed] [Google Scholar]
- 257. Trocino RA, Akazawa S, Ishibashi M et al. Significance of glutathione depletion and oxidative stress in early embryogenesis in glucose‐induced rat embryo culture. Diabetes 1995; 44: 992–998. [DOI] [PubMed] [Google Scholar]
- 258. Siman CM, Eriksson UJ. Vitamin E decreases the occurrence of malformations in the offspring of diabetic rats. Diabetes 1997; 46: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 259. Forsberg H, Eriksson UJ, Welsh N. Apoptosis in embryos of diabetic rats. Pharmacol Toxicol 1998; 83: 104–111. [DOI] [PubMed] [Google Scholar]
- 260. Eriksson UJ, Borg LA. Diabetes and embryonic malformations. Role of substrate‐induced free‐oxygen radical production for dysmorphogenesis in cultured rat embryos. Diabetes 1993; 42: 411–419. [DOI] [PubMed] [Google Scholar]
- 261. Eriksson UJ, Siman CM. Pregnant diabetic rats fed the antioxidant butylated hydroxytoluene show decreased occurrence of malformations in offspring. Diabetes 1996; 45: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 262. Viana M, Herrera E, Bonet B. Teratogenic effects of diabetes mellitus in the rat. Prevention by vitamin E. Diabetologia 1996; 39: 1041–1046. [DOI] [PubMed] [Google Scholar]
- 263. Sivan E, Reece EA, Wu YK, Homko CJ, Polansky M, Borenstein M. Dietary vitamin E prophylaxis and diabetic embryopathy: morphologic and biochemical analysis. Am J Obstet Gynecol 1996; 175: 793–799. [DOI] [PubMed] [Google Scholar]
- 264. Siman CM, Eriksson UJ. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia 1997; 40: 1416–1424. [DOI] [PubMed] [Google Scholar]
- 265. Wentzel P, Thunberg L, Eriksson UJ. Teratogenic effect of diabetic serum is prevented by supplementation of superoxide dismutase and N‐acetylcysteine in rat embryo culture. Diabetologia 1997; 40: 7–14. [DOI] [PubMed] [Google Scholar]
- 266. Yang X, Borg LA, Siman CM, Eriksson UJ. Maternal antioxidant treatments prevent diabetes‐induced alterations of mitochondrial morphology in rat embryos. Anat Rec 1998; 251: 303–315. [DOI] [PubMed] [Google Scholar]
- 267. Cederberg J, Basu S, Eriksson UJ. Increased rate of lipid peroxidation and protein carbonylation in experimental diabetic pregnancy. Diabetologia 2001; 44: 766–774. [DOI] [PubMed] [Google Scholar]
- 268. Eriksson UJ, Borg LA. Protection by free oxygen radical scavenging enzymes against glucose‐induced embryonic malformations in vitro. Diabetologia 1991; 34: 325–331. [DOI] [PubMed] [Google Scholar]
- 269. Siman M. Congenital malformations in experimental diabetic pregnancy: aetiology and antioxidative treatment. Mini review based on a doctoral thesis. Ups J Medical Sci 1997. ; 102. [DOI] [PubMed]
- 270. Yang X, Borg LA, Eriksson UJ. Altered metabolism and superoxide generation in neural tissue of rat embryos exposed to high glucose. Am J Physiol 1997; 272: E173–E180. [DOI] [PubMed] [Google Scholar]
- 271. Hagay ZJ, Weiss Y, Zusman I et al. Prevention of diabetes‐associated embryopathy by overexpression of the free radical scavenger copper zinc superoxide dismutase in transgenic mouse embryos. Am J Obstet Gynecol 1995; 173: 1036–1041. [DOI] [PubMed] [Google Scholar]
- 272. Sakamaki H, Akazawa S, Ishibashi M et al. Significance of glutathione‐dependent antioxidant system in diabetes‐induced embryonic malformations. Diabetes 1999; 48: 1138–1144. [DOI] [PubMed] [Google Scholar]
- 273. Kinalski M, Sledziewski A, Telejko B et al. Lipid peroxidation, antioxidant defence and acid‐base status in cord blood at birth: the influence of diabetes. Horm Metab Res 2001; 33: 227–231. [DOI] [PubMed] [Google Scholar]
- 274. Wentzel P, Ejdesjo A, Eriksson UJ. Maternal diabetes in vivo and high glucose in vitro diminish GAPDH activity in rat embryos. Diabetes 2003; 52: 1222–1228. [DOI] [PubMed] [Google Scholar]
- 275. Martinez‐Frias ML. Epidemiological analysis of outcomes of pregnancy in diabetic mothers: identification of the most characteristic and most frequent congenital anomalies. Am J Med Genet 1994; 51: 108–113. [DOI] [PubMed] [Google Scholar]
- 276. Mills JL, Baker L, Goldman AS. Malformations in infants of diabetic mothers occur before the seventh gestational week. Implications for treatment. Diabetes 1979; 28: 292–293. [DOI] [PubMed] [Google Scholar]
- 277. Cousins L. Congenital anomalies among infants of diabetic mothers. Etiology, prevention, prenatal diagnosis. Am J Obstet Gynecol 1983; 147: 333–338. [DOI] [PubMed] [Google Scholar]
- 278. Becerra JE, Khoury MJ, Cordero JF, Erickson JD. Diabetes mellitus during pregnancy and the risks for specific birth defects: a population‐based case‐control study. Pediatrics 1990; 85: 1–9. [PubMed] [Google Scholar]
- 279. Miodovnik M, Mimouni F, Tsang RC, Ammar E, Kaplan L, Siddiqi TA. Glycemic control and spontaneous abortion in insulin‐dependent diabetic women. Obstet Gynecol 1986; 68: 366–369. [DOI] [PubMed] [Google Scholar]
- 280. Kitzmiller JL, Cloherty JP, Younger MD et al. Diabetic pregnancy and perinatal morbidity. Am J Obstet Gynecol 1978; 131: 560–580. [DOI] [PubMed] [Google Scholar]
- 281. Mills JL. Malformations in infants of diabetic mothers. Teratology 1982; 25: 385–394. [DOI] [PubMed] [Google Scholar]
- 282. Langer O, Conway DL. Level of glycemia and perinatal outcome in pregestational diabetes. J Matern Fetal Med 2000; 9: 35–41. [DOI] [PubMed] [Google Scholar]
- 283. Suhonen L, Hiilesmaa V, Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia 2000; 43: 79–82. [DOI] [PubMed] [Google Scholar]
- 284. Schaefer‐Graf UM, Buchanan TA, Xiang A, Songster G, Montoro M, Kjos SL. Patterns of congenital anomalies and relationship to initial maternal fasting glucose levels in pregnancies complicated by type 2 and gestational diabetes. Am J Obstet Gynecol 2000; 182: 313–320. [DOI] [PubMed] [Google Scholar]
- 285. Aberg A, Westbom L, Kallen B. Congenital malformations among infants whose mothers had gestational diabetes or pre‐existing diabetes. Early Hum Dev 2001; 61: 85–95. [DOI] [PubMed] [Google Scholar]
- 286. Chang TI, Horal M, Jain SK, Wang F, Patel R, Loeken MR. Oxidant regulation of gene expression and neural tube development: insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia 2003; 46: 538–545. [DOI] [PubMed] [Google Scholar]
- 287. Wentzel P, Welsh N, Eriksson UJ. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase‐2 gene expression, and lowered prostaglandin E2 levels in rat embryos exposed to a diabetic environment. Diabetes 1999; 48: 813–820. [DOI] [PubMed] [Google Scholar]
- 288. Jain SK, Levine SN, Duett J, Hollier B. Reduced vitamin E and increased lipofuscin products in erythrocytes of diabetic rats. Diabetes 1991; 40: 1241–1244. [DOI] [PubMed] [Google Scholar]
- 289. Cunningham JJ, Ellis SL, McVeigh KL, Levine RE, Calles‐Escandon J. Reduced mononuclear leukocyte ascorbic acid content in adults with insulin‐dependent diabetes mellitus consuming adequate dietary vitamin C. Metabolism 1991; 40: 146–149. [DOI] [PubMed] [Google Scholar]
- 290. Yang X, Borg LA, Eriksson UJ. Altered mitochondrial morphology of rat embryos in diabetic pregnancy. Anat Rec 1995; 241: 255–267. [DOI] [PubMed] [Google Scholar]
- 291. Reece EA, Eriksson UJ. The pathogenesis of diabetes‐associated congenital malformations. Obstet Gynecol Clin North Am 1996; 23: 29–45. [DOI] [PubMed] [Google Scholar]
- 292. Wiznitzer A, Ayalon N, Hershkovitz R et al. Lipoic acid prevention of neural tube defects in offspring of rats with streptozocin‐induced diabetes. Am J Obstet Gynecol 1999; 180: 188–193. [DOI] [PubMed] [Google Scholar]


