Abstract
Cell delivery or cell killing processes often involve the crossing or disruption of cellular membranes. We review how, by modifying the composition and properties of membranes, membrane oxidation can be exploited to enhance the delivery of macromolecular cargos into live human cells. We also describe how membrane oxidation can be utilized to achieve efficient killing of bacteria by antimicrobial peptides. Finally, we present recent evidence highlighting how membrane oxidation is intimately engaged in natural biological processes such as antigen delivery in dendritic cells and in the killing of bacteria by human macrophages. Overall, the insights that have been recently gained in this area should facilitate the development of more effective delivery technologies and antimicrobial therapeutic approaches.
Keywords: Membrane oxidation, cell delivery, cell-penetrating peptides, antimicrobial peptides, photochemical internalization, lipid peroxidation, cell killing
1. INTRODUCTION
The plasma membrane of cells, whether eukaryotic or prokaryotic, acts as barriers that separate the intracellular milieu from its surrounding environment. Crossing or disrupting this membrane is the focus of many biotechnological applications. For instance, the delivery of macromolecules into the interior of human cells is often required in applications that aim at controlling or probing the intracellular circuitry.1 In contrast, achieving a temporal defect or a stable disruption of membrane function is desired in applications that aim at killing cells. This includes in particular strategies that use lytic agents to selectively kill pathogenic bacteria infecting human tissues.2 In this context, learning how to manipulate the physical properties of membranes with high selectivity and high efficiency remains an intense and vibrant area of research.
Cells are exposed to oxidative stress, the extent of which depends on experimental context (e.g. in vitro vs in vivo, 20% vs 2% oxygen atmosphere during culture, presence or absence of antioxidants, etc.).3 In addition, it is well documented that the components of cell membranes are the targets of oxidation chemistry.4, 5 On one hand, this can potentially lead to alterations in overall membrane properties, including stability and permeability. It is therefore possible that membrane oxidation may impact the activity of reagents that lyse or cross membranes. On the other hand, the oxidation of cellular membranes is challenging to measure and quantify experimentally. In particular, oxidation leads to highly heterogeneous mixtures.4, 6 Additionally, oxidized species may exist only transiently, either because of high chemical reactivity or because of degradation by cellular repair mechanisms.7 To date, the extent to which membrane oxidation influences cell lysis and cell delivery applications remains therefore unclear.
In this review, we discuss recent evidence that highlights membrane oxidation as a determining factor in several cell killing and cell delivery applications. In some instances, membrane oxidation provides new mechanistic insights on how biological or synthetic molecules interact with membranes. In other instances, membrane oxidation is a feature that is directly exploited to achieve enhanced translocation or lytic activities.
2. THE COMPLEX CHEMISTRY OF MEMBRANE OXIDATION
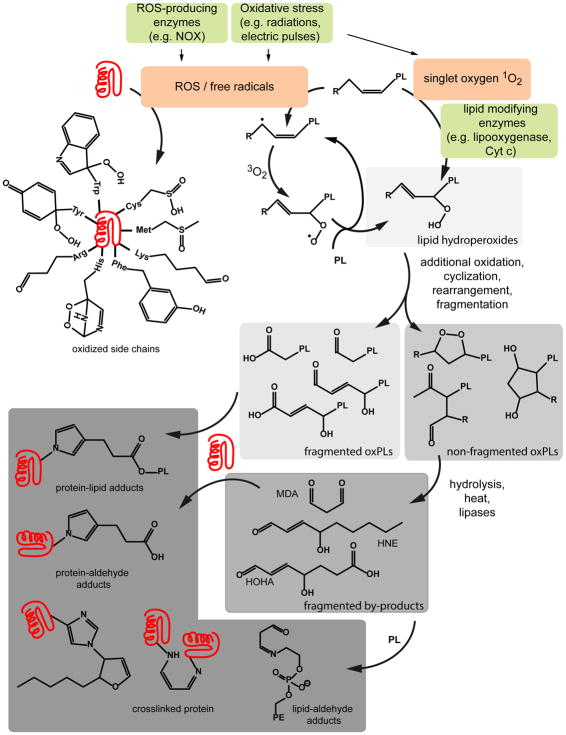
Because of its significance to biology and biochemistry, membrane oxidation has been the subject of considerable mechanistic and structural scrutiny, some of it previously reviewed8. Examples of reactions that modify membrane components, lipids and proteins, are provided in Figure 1. For instance, endogenous or exogenous factors can promote the formation of reactive oxygen species (ROS) that directly modify proteins and lipids to form products containing hydroxyl, carbonyl, or epoxide groups (Figure 1). Free-radical ROS can also abstract hydrogen from membrane lipids and proteins to form new radicals. Upon reaction with oxygen, products containing peroxides are formed, Notably, in the case of lipids, hydrogen abstraction of unsaturated acyl chains can induce a chain reaction that propagates to other lipids present in a bilayer. A single initiation event can thereby be amplified and lead to the formation of many lipid peroxides. Oxidation is often followed by a variety of chemical reactions, including cyclization, rearrangement, and cleavage. This in turn can lead to the formation of non-fragmented and fragmented species, especially new lipid species such as oxidized lipids containing carboxylic acid in one of their acyl chains. Some species will contain aldehydes that can further react with nearby proteins or lipids to form Schiff base adducts (Figure 1).
Figure 1. Examples of the chemical reactions and lipid or protein products potentially generated during membrane oxidation.
ROS generated in the vicinity of the membrane can target the fatty acyl chain or the head group of phospholipids, or the side chains of membrane proteins leading to a plethora of new functionality in the bilayer. The most prominent reaction is the self-propagating peroxidation of lipid unsaturations (in the figure, the structure of phospholipids is simplified, with PL= phospholipid, R= a fatty acid chain, one fatty acid unsaturation is shown but several can be present, as in the case of PUFAs). The lipid hydroperoxides formed in this reaction can in turn generate cyclic, truncated, or fragmented oxidized lipids (OxPLs) (MDA, malonyldialdehyde; HNE, hydroxynonenal; HOHA, 4-hydroxy-7-oxo-5-heptenoic acid). Cellular nucleophiles, including protein side chains and the polar head of certain lipids (e.g. phosphatidyl ethanol amine, PE) can react with some of these oxidized products, further increasing the diversity and complexity of the species present.
Many factors dictate the extent to which a membrane is oxidized. For instance, a lipophilic antioxidant such as vitamin E protects membranes from damage by limiting the propagation of lipid peroxidation. Other cellular components can provide protection, either by scavenging ROS or by degrading oxidized products.7, 9, 10 In contrast, presence of metals and relatively high levels of oxygen promote lipid peroxidation. It is also important to note that oxidation is favorable in a membrane because oxygen is more concentrated in the hydrophobic environment of lipid bilayers than in solution.8 This is also true for singlet oxygen, an excited form of oxygen that can be formed upon irradiation of chromophores referred to as photosensitizers. Singlet oxygen is highly reactive and it directly attacks lipids or proteins to form peroxides (Figure 1). If formed in a membrane, this ROS is unlikely to diffuse away from a bilayer. Instead, it will rapidly react with molecules in its vicinity.
Given the few examples provided, it should be evident that an overall effect of oxidation is the generation of a complex and heterogenous population of products. These products may be principally derived from polyunsaturated lipids (PUFAs). These lipids are indeed considered the main targets of membrane oxidation.11, 12 This is because bis-allylic methylene carbons present in polyunsaturated fatty acids (PUFAs) are more prone to hydrogen abstraction than the allylic carbon of monounsaturated fatty acids.13 Yet, monounsaturated aliphatic chains can also be oxidized, albeit under different oxidation conditions (as discussed further in the 4.1).14 In addition, nonsaponifiable lipids are also readily oxidizable (not shown in Figure 1).14 For instance, cholesterol, an important component of mammalian membranes, gives rise to multiple oxidized products.15
Overall, the accumulation of oxidized products can alter various membrane properties. For instance, in vitro, the inclusion of oxidized lipids into lipid bilayers induces a loss in permeability barrier function.16–18 In vivo, oxidatively truncated phospholipids, typically found in circulation, can enter cells, alter mitochondrial membrane function, and ultimately induce apoptosis.19 Below, we elaborate on the contribution of membrane oxidation in the context of delivering cargos to mammalian cells. We then transition into how this phenomenon relates to bacterial cell death.
3. RELEVANCE OF MAMMALIAN MEMBRANE OXIDATION IN INTRACELLULAR DELIVERY APPLICATIONS
3.1. Electroporation
Electroporation uses electric pulses of short duration to deliver macromolecules into cells.20 It is thought that these pulses create nanoscale pores in the cell membrane. These nanopores are stable enough during electropermeabilization to allow for the diffusion of biomolecules from the media to the interior of the cell. Notably, electroporation-mediated DNA transfer has been shown to involve endocytosis and endosomal escape,21–24 This is turn indicates that electropermeabilization can affect both cytoplasmic and endosomal membranes.
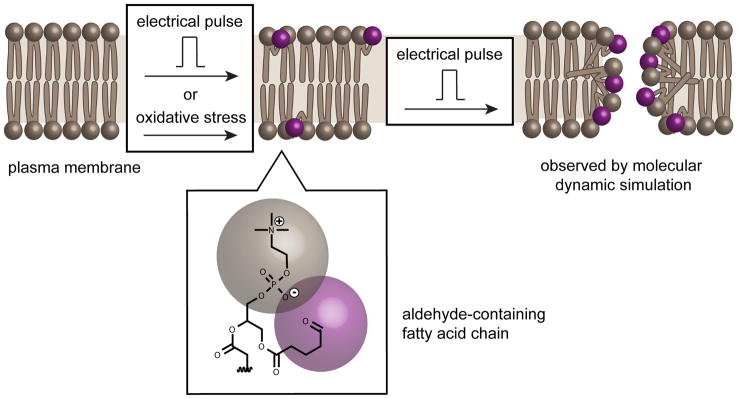
A link between membrane oxidation and electroporation has been established by Vernier and co-workers.25 In particular, Jurkat cells pre-oxidized with hydrogen peroxide (H2O2) and ferrous sulfate showed greater electropermeabilization than untreated cells, as measured by the cellular uptake of the fluorescent dye YO-PRO-1. This increase correlated positively with both the concentration of oxidizing agents and the duration of applied electric pulse. Notably, the oxidants did not lead to an enhancement in cargo delivery in the absence of electric pulse. The notion that electroporation targets oxidatively damaged area of cell membranes was also supported by molecular dynamic simulations. The propensity of pore formation in a lipid bilayer positively correlated with the concentration of oxidized lipids present in the bilayer. Poration was also observed at the area where oxidized lipids were located within the bilayer (Figure 2). Whether oxidation-dependent poration takes place at the plasma membrane or at the membrane of endosomes was not directly established. However, because these membranes systems are related in composition, they may both be involved in a non-exclusive manner.
Figure 2. Electroporation induces and targets oxidized lipids at the cell membrane.
Externally applied electric pulse as well as environmental oxidative stress can generate a variety of oxidized lipids, including aldehyde-containing species. Molecular dynamic simulations have suggested that additional electric pulsing leads these oxidized species to preferentially form pores within the lipid bilayer. Electroporation may therefore involve of a mechanism where oxidized lipids are both generated and directly utilized for permeation.
While oxidized defects in the lipid bilayer might be primary sites of nanopore formation during electroporation, it also appears that electric pulsing stimulates oxidation. For instance, electroporation leads to an increase in the level of oxidized lipids present in the membrane of human erythroleukemia K562.26 Additionally, ROS, detected using fluorescent and chemiluminescent probes, are generated in Chinese hamster ovary (CHO) cells subjected to electric pulses.27, 28 Given that ROS can readily oxidize the membrane of cells, it is possible that electric pulses initially generate oxidation-related defects in treated cells and, upon subsequent pulsing, induce poration at these membrane defect sites. (Figure 2).
3.2. Cell-penetrating peptides
Cell-penetrating peptides (CPPs) represent a class of peptides that have the ability to enter cells, albeit with various efficiencies. In some cases, CPPs directly translocate through the plasma membrane of cells.29–34 In other cases, CPPs are internalized via endocytic uptake mechanisms.35–37 CPPs trapped inside the lumen of endosomes can then escape into the cytosolic space.38, 39 Overall, CPPs have been utilized to successfully deliver a wide variety of molecular cargos, including nucleic acids, proteins or nanoparticles, in numerous cell types.40 Several studies have investigated the molecular underpinnings involved in cell penetration. Because CPPs are often rich in cationic arginine residues, interactions with negatively-charged membrane components are thought to play important roles. These species include heparin sulfate proteoglycans, as well as anionic lipids such as phosphatidylserine and bis(monoacylglycero)phosphate. 41–43 A better understanding of how CPPs interact with membranes has supported the development of reagents with enhanced penetration activities. Yet, inconsistencies in published results indicate a high variability in the performance of these reagents. For instance, the polyarginine CPP R9 penetrates cells efficiently in some investigations but remains primarily trapped inside endosomes in others. Paradoxically, contradictory results are observed even when experimental settings are similar (i.e. same cell type and peptide concentration).44, 45 Overall, this indicates that some important parameters involving peptide and cell membrane might not be accounted for, thereby leading to variability.
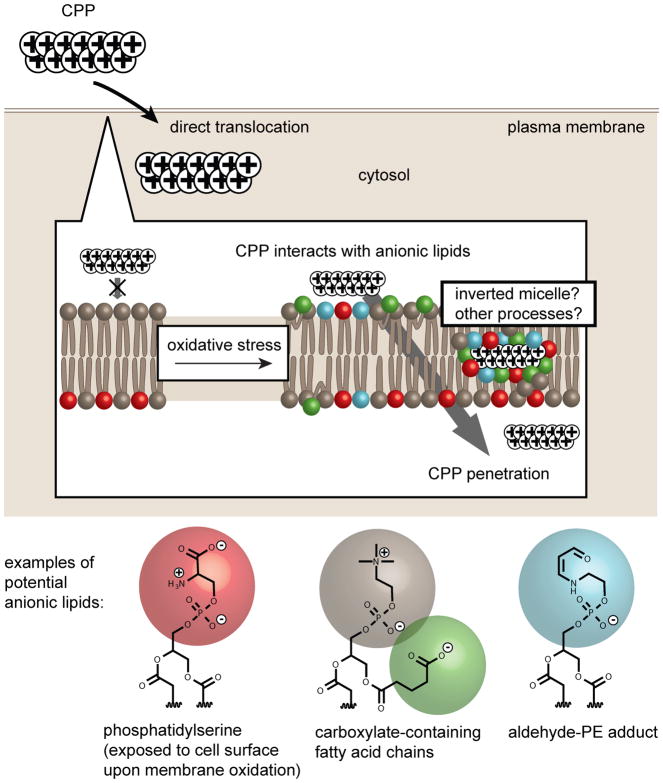
Recently, the cytosolic penetration of nona- or trideca-arginine CPPs (r9 and r13 where r is D-Arg, an unnatural arginine residue used to confer protease-resistance to the peptide) was abolished or significantly reduced, respectively, when cells were cultured at 2% oxygen as opposed to 20% oxygen. Cell penetration was also reduced when the cellular growth media was supplemented with antioxidants.46 Together, these results indicated that reducing oxidative cellular stress diminishes the transport of these CPPs into cells. Conversely, an increase in the oxidation state of the plasma membrane of cells led to an increase in cytosolic penetration. For instance, r13 efficiently entered cells pretreated with lipophilic oxidants (oxidation conditions were mild and did not affect cell viability and proliferation rates) and the penetration activity of the CPPs positively correlated with the level of lipid peroxides present in cellular membranes. Additionally, a cell line (MCH58) prone to cell penetration by TMR-r13 displayed higher endogenous levels of oxidized lipids than a cell line (HDF) more resistant to the translocation of TMR-r13. The involvement of oxidized lipids was further demonstrated with the use of E06, a monoclonal antibody that binds oxidized phosphatidylcholine (oxPC) lipids.47 In particular, E06 blocked the intracellular delivery of r13 after a simple pre-incubation with cells. Furthermore, extracellular addition of the anionic oxPC lipids, 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine (PGPC) or 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (PazePC), enhanced the cytosolic penetration of the CPPs. Notably, an in vitro partitioning assay established that both PGPC and PazePC can transport CPPs into a milieu of low dielectric constant.46 This, in turn, is consistent with the notion that CPPs and anionic oxPC species have the ability to form structures, most likely inverted micelles, that allow transfer of the hydrophilic peptide across a hydrophobic lipid bilayer. Interestingly, many anionic lipid species can in principle be generated during membrane oxidation (see Figure 1). Like PGPC and PazePC, these species might act in concert to interact with CPPs to allow or enhance cell penetration (Figure 3).
Figure 3. Oxidation-mediated plasma membrane translocation of CPP.
The CPP, rich in arginine residues, is cationic at physiological pH and is presented as a string of positive charges. In the absence of oxidative stress, the CPP displays no apparent membrane penetration. In contrast, oxidative stress leads to membrane damage and exposure of anionic lipids. This, in turn, enhances the recruitment of the peptide at the bilayer. The formation of inverted micelles between cationic CPP and the anionic lipids that may enable cell penetration has been speculated.46 However, this mechanism has not been formally demonstrated and other processes may be involved. Examples of anionic oxidized lipids potentially involved in CPP recruitment and penetration are presented.
3.3. Photochemical internalization
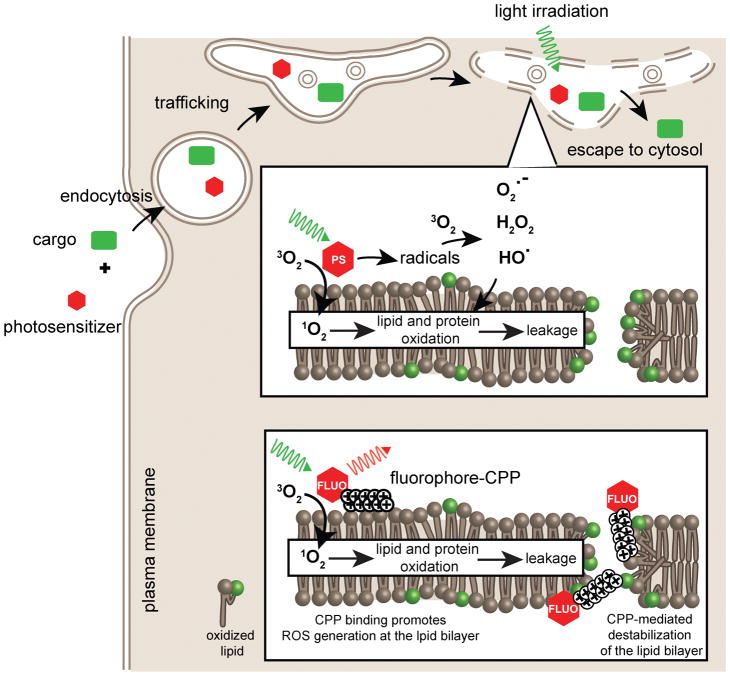
Photochemical internalization (PCI) is a strategy that combines photosensitizers and light to achieve intracellular delivery of macromolecules.48 ROS can be abundantly generated when exciting photosensitizers by light. When incubated with cells, photosensitizers can accumulate with macromolecular cargos inside endosomes.48 Light irradiation leads to oxidation of the endosomal membrane by the photosensitizers. Internalized macromolecules subsequently escape from the oxidized endosomes with high efficiencies. PCI has been successfully applied to the cytosolic delivery of small-molecule anti-cancer drugs, proteins, oligonucleotides and other therapeutic agents both in cellulo and in vivo.49 Photosensitizers such as sulfonated tetraphenylporphyrin (TPPS2a) and sulfonated aluminum phthalocynanine (AlPcS2a), can be used for PCI because these compounds are endocytosed by cells.49, 50 Notably, photosensitizers that lack the ability to accumulate inside endosomes can be conjugated to CPPs. This is turn enhances their targeting to endosomes and enables PCI-mediated delivery.51–54 Interestingly, fluorophores that typically produce significantly lower levels of ROS than photosensitizers can also cause photo-induced endosomal leakage upon conjugation to CPPs.55–60 For instance, fluorescein and tetramethylrhodamine, two commonly used fluorophores in microscopy applications, induce endosomal leakage when conjugated to the CPPs TAT or R9.58
PCI-mediated endosomal leakage is an oxidation dependent process (Figure 4). ROS generated during irradiation of the photosensitizer react with lipids and introduce oxygen-containing functional groups. Above a particular damage threshold, the integrity of the lipid bilayer is disrupted and the membrane becomes permeable. Damage has been observed in the form of hydrophobic and hydrophilic defects, as well as membrane swelling.61, 62 Notably, additional steps have been observed in the case of CPP conjugates. For instance, both the lipophilic photosensitizer Rose Bengal (RB) and the hydrophilic conjugates TMR-TAT or TMR-R9 cause leakage of liposomes when irradiated.63 However, liposomes photo-oxidized with RB do not aggregate while those oxidized with TMR-TAT/R9 do. Aggregation was not attributed to the ROS generator (TMR in this case), but with the CPPs instead. Similarly, red blood cells, used as a model system for the membrane of human cells, lyse when irradiated in the presence of RB or TMR-TAT.64 Yet, the morphology of the RB-treated cells does not change upon hemolysis. In contrast, red blood cells treated with TMR-TAT dramatically shrink upon light exposure, indicating that a significant loss of membrane surface area accompanies lysis. Interestingly, the photolytic activity of RB was also enhanced in the presence of unlabeled TAT or R9.64 Moreover, both the arginine content and the position of photosensitizer in the CPP significantly impact the membranolytic activity. Among different CPP constructs, a peptide containing nine arginine residues and a fluorophore positioned in the center of sequence showed the best light-dependent membranolytic activity.65 Together, these results indicated that the CPP moieties directly interact with oxidized lipid species and enhance the membrane destabilizing effects initiated by ROS.
Figure 4. Intracellular cargo delivery by PCI and light-triggered endosomal release.
A photosensitizer and a macromolecular cargo incubated with live cells through endocytic uptake followed by endosomal escape. Both the photosensitizer and cargo traffick along the endocytic pathway. Upon irradiating the photosensitizer, ROS are generated and the membrane of endocytic organelles is oxidized. Oxidation leads to membrane rupture and leakage of the cargo into the cytosol. To maximize the selective disruption of endosomal membranes (i.e. minimize cell death by plasma membrane disruption), the PCI process can be mediated by photosensitizers that are relatively hydrophilic or by fluorophore-CPP conjugates. In the latter case, the CPP is thought to target the ROS-generating fluorophore to the endosomal membrane and enhance the generation of ROS in the close vicinity of the lipid bilayer. In addition, the CPP interacts with oxidized species to facilitate membrane leakage.
3.4. Delivery by gas plasma
Gas plasma is a technology that utilizes electric fields to ionize an efflux of noble gas or ambient air so as to generate a gas plasma with low temperature. The permeability of cells exposed to gas plasma, either in vivo or in vitro, is increased and delivery of macromolecular cargos such as plasmid DNA can be achieved.66 For the past decades, several plasma generation methods have been developed, including atmospheric discharge plasma67, atmospheric helium plasma68–70, non-thermal plasma71, shielded sliding discharge plasma72, atmospheric-pressure plasma73, and plasma-activated air74. Using cold atmospheric plasma generated by a plasma jet system, Kong and co-coworkers recently established that ROS production is involved in plasma-induced cell permeabilization.75 ROS, including superoxide and hydrogen peroxide, are present in the plasma generated by this technique and transferred to the liquid culture media.76 In the presence of cells the levels of hydrogen peroxide, measured with the Amplex Red reagent, however decrease, suggesting that the oxidant is consumed by reacting with cells. Conversely, intracellular ROS levels, detected the unspecific ROS probe CM-H2DCFDA, increase following plasma treatment. This is accompanied by a simultaneous increase in membrane permeability, as measured by the influx of calcium from outside to inside cells. Moreover, DNA transfection and calcium influx were inhibited by sodium pyruvate, tiron, and mannitol, scavengers of hydrogen peroxide, superoxide, and the hydroxyl radical, respectively. Together, these results suggest that the ROS produced during exposure to plasma modulate the permeability of cells by oxidation of membrane components. Notably, the hydroxyl radical, which appears to play a role in modulating membrane permeability but which is not be directly present in plasma, is presumably generated upon exposure of superoxide and hydrogen peroxide to cells. Interestingly, this process, which may involve in situ catalysis by iron, was also implicated in the cytotoxicity induced by plasma treatment.76
3.5. Oxidation-regulated membrane permeation in biological transport processes
While the cell penetration processes described in the above text are related to delivery technologies, recent evidence suggests that oxidation might also be exploited by nature as a means of inducing membrane permeabilization. In particular, lipid oxidation has recently been proposed to mediate the delivery of antigens from the lumen of endosomes into the cytosolic space of dendritic cells.77 Dendritic cells (DCs) internalize foreign macromolecules by endocytosis and phagocytosis and subsequently present antigenic products on their surface for recognition by T-cells and immune activation. This process is known as cross-presentation.78 It is thought that the trafficking of antigens within dendritic cells involves their egress from the lumen of endosomes and their entry into the cytosol. However, how antigens escape endosomes remain a matter of debate.78
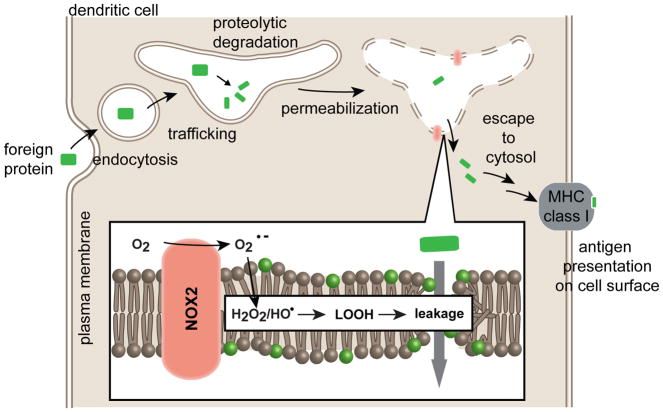
The enzyme complex NADPH oxidase (NOX2), located at the membrane of endosomes, produces superoxide (O2•−) upon stimulation.79 The acidic milieu of endosomes and the presence of iron can then lead to the formation of H2O2 and of the hydroxyl radical. Bogaart and co-workers have recently reported that the NOX2-generated ROS oxidize the lipids of endosomal membranes.77 This promotes membrane destabilization and enhances cytosolic antigen release (Figure 5). For instance, DCs produce an increased level of hydrogen peroxide when treated with lipopolysaccharide (LPS), a TLR4-ligand that stimulates NOX2. Lipid peroxidation, as detected by changes in the fluorescence of the oxidation-sensitive probe C11-BODIPY581/591, was also increased in the presence of LPS. Inversely, presence of the lipophilic antioxidant α-tocopherol (vitamin E) or knockdown of NOX2 by siRNA lead to a decrease in antigen cross presentation. Cross presentation was also reduced in DCs from chronic granulomatous disease patients containing dysfunctional NOX2. Notably, endosomal escape of antigens could be artificially promoted by using a PCI-inspired approach. In this assay, the genetically encodable photosensitizer protein KillerRed was targeted to endosomal compartments by fusion to the SNARE protein VAMP8. Excitation of KillerRed with light resulted in the leakage of antigens from endosomes. The authors concluded that PCI could be used to artificially enhance antigen cross-presentation in a manner that mimic NOX2 driven oxidation. Overall, while other non-mutually exclusive mechanisms of endosomal escape might be involved in antigen processing, these results highlight how oxidation-driven permeabilization, at the very least, facilitates this process.
Figure 5. Cytosolic delivery of antigens in dendritic cells.
An exogenous protein is endocytosed by dentritic cells. The internalized protein is degraded by endosomal proteases. Upon activation, NOX2, a NADPH oxidase complex present at the membrane of endosomes, generates superoxide anion (O2•−). Hydrogen peroxide (H2O2) and hydroxyl radical (•OH) are subsequently formed in the lumen of endosomes. These ROS lead to formation of lipid hydroperoxides (LOOH). Lipid peroxidation is propagated within the lipid bilayer and damage ultimately leads to membrane rupture and leakage of the protein fragments into the cytosol of cells. The intracellular protein fragments may be further degraded by the proteasome and are eventually exposed on the plasma membrane for presentation by MHC class I receptors.
4. RELEVANCE OF BACTERIAL MEMBRANE OXIDATION IN CELL KILLING APPLICATIONS
It is well understood that membrane oxidation is a mechanism that can be exploited to kill human cells. Technologies that utilize this process include, for instance, photodynamic therapy. The modes of action of these technologies have been characterized extensively and readers are referred to recent reviews.80 In contrast, the relevance of membrane oxidation in bacterial killing applications has received less attention. One possible reason for this is that the lipid components of bacterial membranes are expected to be less reactive towards oxidants than their mammalian counterparts. Moreover, the structural complexity of bacterial membranes and the inherent differences between Gram-positive and –negative bacteria add another layer of complication to the study of membrane oxidation in bacteria. All prokaryotes contain an inner (cytoplasmic) membrane – composed of a lipid bilayer and the embedded membrane proteins – and a peptidoglycan cell wall. In addition to these two layers, Gram-negative bacteria also contain an asymmetric outer membrane whose inner leaflet is composed largely of glycerophospholipids and an outer leaflet composed of lipopolysaccharides (LPS). While unsaturated fatty acids can be found in the inner leaflet, the lipid A in LPS is almost exclusively composed of saturated acyl chains.81 Thus, the overall susceptibility of bacterial membrane towards oxidation remains a matter of debate. Herein, we review recent articles that highlight how the oxidative damage of bacterial membrane is possible and how it is exploited in several antimicrobial technologies. Furthermore, the bilayers mentioned in this section refer to the cytoplasmic membrane – which contain glycerol-derived phospholipids – and should not be confused with the cell wall or the outer membrane lipopolysaccharides.
4.1. Membrane oxidation and Lipid Peroxidation in Bacteria: Fact or Fiction?
An argument supporting a relative resistance of bacterial membrane to ROS is based on membrane composition. For instance, bacterial lipid bilayers contain high levels of monounsaturated fatty acids (MUFAs) and do not tend to incorporate polyunsaturated fatty acids (PUFAs) in their membranes.82, 83 While PUFAs react readily with ROS, MUFAs are often considered virtually unreactive.12 Furthermore, even though many studies have highlighted how ROS-mediated degradation of cytoplasmic proteins and nucleic acids can be detected in bacteria, methods to detect lipid oxidation have been scarce.84, 85, 86
This paradigm is challenged by evidences in the literature suggesting that lipid peroxidation does in fact occur in bacteria as a result of exposure to ROS. In vitro studies support the possibility of MUFAs as substrate for lipid peroxidation.87, 88, 89 Detection of low- and high-molecular weight lipid peroxidation products was reported after oxidation of MUFA-containing phospholipid vesicles with Cu(II) and ascorbic acid. In particular, 17 different types of carbonylated products were detected from oxidation of oleic acid (18:1).14 Membrane oxidation has also been detected directly in the context of bacteria. For instance, treating an E. coli strain expressing high levels of antioxidant enzymes with H2O2 leads to the accumulation of malondialdehyde, a byproduct of lipid peroxidation.90 More recently, Mycobacterium tuberculosis (Mtb) has been shown to be extraordinarily susceptible to the pro-oxidant activity of ascorbic acid.91 Ascorbic acid treatment led to the detection of 2-hydroxylated long-chain fatty acids likely generated from oxidation of 2-alkenoic acids in the cytoplasmic membrane by hydroxyl radicals generated from ascorbic acid-induced Fenton reaction.91 While dismutases and catalases detoxify O2•− and H2O2, respectively, no known enzymes detoxify hydroxyl radicals (•OH), making this radical more lethal85. This coupled with the fact that Mtb has no biosynthetic machinery to generate hydroxylated fatty acids suggests that lipid oxidation was a direct result of ascorbic acid treatment. Together, these results suggest that MUFAs oxidation within bacterial membrane is possible. It should also be noted that membrane oxidation may involve lipid species other than MUFAs. For instance, the membrane of bacteria may contain isoprenoid quinones or unsaturated hopanoids.92 To the best of our knowledge, the reactivity of these lipids toward oxidation has not been tested. Yet, these quinones can in principle, be damaged by ROS, either via oxidative cleavage of the β,γ-unsaturation linking the isoprenoid unit to the quinone or possibly epoxidation of the quinone moiety. Both of these modifications can result in attenuation or loss of electron carrier capability of the isoprenoid quinones.93 The hopanoids on the other hand, can be hydroxylated during oxidative stress resulting in a significant change in hydrophobicity.94 Finally, it is also possible that oxidative damage targets membrane proteins.5 However, the extent to which protein damage relates to membrane disruption remains unclear.
4.2. Photodynamic inactivation: bacterial killing by membrane oxidation with photosensitizers
Photodynamic inactivation (PI) aims at killing cells by using photosensitizers, which together with a light source, generate a toxic burst of ROS. Originally developed to combat cancerous lesions,95 PI is emerging as method to fight infections.96, 97 Organic chromophores including methylene blue, rose Bengal, acridine orange, and various tetrapyrroles (e.g. porphyrins, and phthalocyanins) have been used as photosensitizers for PI.98–102 A report from Nisnevitch and co-workers shows a scanning electron microscope (SEM) image of E. coli cells that survived irradiation in the presence of methylene blue immobilized in polystyrene.103 These cells showed compromised membranes evidenced by large, spherical membrane protrusions, often referred to as blebs. Whether these “blebs” are structures enclosed by dual bilayers (both inner and outer membrane) remain unclear. This uncertainty can be addressed by a study of the activity of human defensin 5 (HD5) on E. coli, which showed temporal leakage of cytoplasmic GFP into blebs and other bleb-like structures,104 suggesting that in the context of a non-oxidative attack on the bacterial membrane, the blebs formed are composed of two intact bilayers. This redistribution of GFP occurs at the same time as influx of propidium iodide into the cell, suggesting that cells that developed blebs are likely dead. Whether blebs arising from oxidative and non-oxidative attack on bacteria are morphologically and functionally similar remains to be seen. However, given the high affinity that most photosensitizers have for lipid bilayers, oxidation-dependent membrane perturbation is likely the underlying cause of cell death achieved upon light irradiation.105 Two questions remain, first, why both oxidative and non-oxidative attack on membranes lead to formation of cell surface blebs. Second, whether specific cellular structures are responsible to the genesis of these membrane blebs.
4.3. Membrane oxidation and AMP bactericidal activity
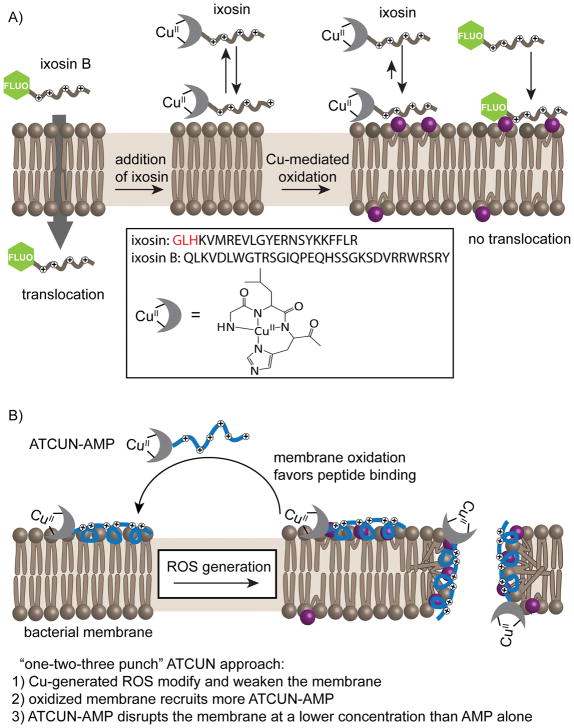
Recent reports suggest a complex relationship between membrane oxidation and the activity of AMPs. First, AMPs exhibit higher affinities to liposomes containing oxidized phospholipids.106 This might potentially involve changes in lipid packing, or electrostatic interactions; or covalent tethering by formation of Schiff bases between the primary amine groups of the peptide and the aldehyde moiety of the oxidized phospholipids.107 The relevance of such phenomenon was highlighted in a report that studied two antimicrobial peptides, ixosin and ixosin B, isolated from the tick Ixodes sinensis.108–110 The study demonstrated the change in the localization of ixosin B as a result of membrane oxidation by ixosin. Ixosin contains a sequence known as the amino-terminal copper and nickel binding (ATCUN) motif that binds copper ions with high affinity (GLHKVMREVLGYERNSYKKFFLR, Figure 6A).111 The copper-ixosin adduct is capable of generating ROS in the presence of oxygen. In contrast, ixosin B (QLKVDLWGTRSGIQPEQHSSGKSDVRRWRSRY) lacks a copper-binding sequence and does not produce ROS. In E. coli treated with ixosin B alone, the peptide penetrated the cell and localized in the cytosol. When bacteria were co-treated with ixosin and ixosin B, the latter localized to the E. coli membrane. The native ixosin B localization was restored when an ixosin derivative harboring a point mutation that abolishes copper-binding and ROS formation was used.110 Together, these results indicate that the oxidation mediated by ixosin leads to retention of ixosin B at the membrane. It is then possible that membrane oxidation promotes the lytic behavior of the peptide, and ultimately enhances its bactericidal activity. Using mixtures of ixosin and ixosin B, the fractional inhibitory concentration (FIC) index – an indicator of synergy – was determined. When tested against E. coli (MWF1 strain), a synergistic interaction between ixosin and ixosin B (FIC = 0.281) was observed. When mutants of ixosin lacking an ATCUN motif were used in combination with ixosin B, the synergistic interaction was abolished. These results indicate that the oxidative activity of the ATCUN motif is necessary to elicit a synergistic interaction between ixosin and ixosin B.
Figure 6. Inactivation of bacteria by ATCUN-AMP mediated membrane oxidation.
A) Model for the synergistic behavior between the tick salivary gland peptides ixosin and ixosin B. By itself, Ixosin B can cross E. coli outer and inner membranes and localize in the cytosolic space. However, when co-incubated with ixosin, ixosin B changes its localization and is observed to interact with the membrane. The change in localization is due to the oxidation of the bacterial membrane by ixosin, which contains a copper binding motif (shown in red in the peptide sequence) capable of generating oxidizing species. Overall, ixosin and ixosin B work in synergy to kill bacteria by membrane lysis. B) Appending Amino Terminal Copper and Nickel (ATCUN) binding motifs to AMP increases bactericidal activities. Once bound to copper ions, ATCUN-AMP produces reactive oxygen species that target the bacterial membrane. In turn, oxidation of the membrane causes the recruitment of more ATCUN-AMP, thereby generating a forward loop. Membrane lysis is then achieved by the combined effect of oxidized membrane components and of the AMP moiety. While the AMP can cause membrane lysis in the absence of oxidation, addition of the ATCUN sequence leads to a reduction in minimum inhibitory concentration.
Notably, the ATCUN motif can be added as a biological warhead to antimicrobial peptides that target bacterial membranes to produce peptides with membrane oxidizing activity.89, 112 As a proof of concept, the ATCUN motifs Asp-Ala-His, Gly-Gly-His, and Val-Ile-His were added to the antimicrobial peptides anoplin (GLLKRIKTLL-NH2) and (KLAKLAK)2, two well-characterized membrane targeting agents (Figure 6B).89 The presence of the three residues did not disturb the affinity of the peptides for the bacterial membranes, and the new hybrid peptides continued targeting the membranes. In the case of the ATCUN-anoplin peptides, a correlation between lipid peroxidation activity of the ATCUN derivatives and the ability of the ATCUN motif itself to produce ROS was found. The ATCUN-(KLAKLAK)2 hybrids showed better activity than the parent peptide; however, this activity did not correlate with the oxidation ability of the ATCUN motif. It is likely that the position of the ATCUN motif within the lipid bilayer is important for the lipid oxidation activity.
4.4. Bacterial killing by membrane oxidation with antimicrobial peptide-photosensitizer conjugates
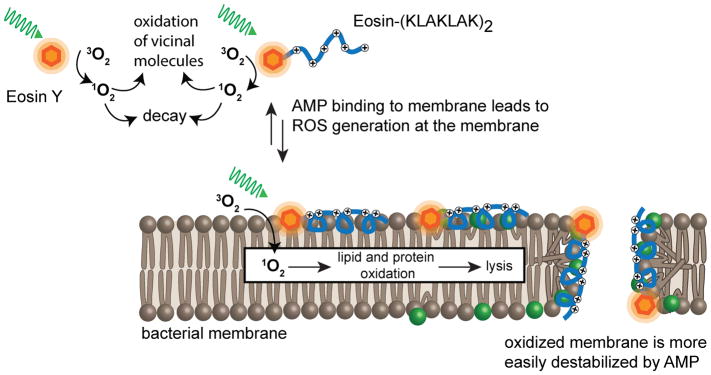
While lipophilic photosensitizers can maximize oxidative damage at bacterial membranes, they are often poorly selective. As a matter of fact, photosensitizers used for photodynamic inactivation are often also phototoxic to human cells. This therefore limits their therapeutic utility in the context of conditions such as infected wounds. Hydrophilic photosensitizers are, in contrast, poorly active especially towards Gram-negative strains. To circumvent some of these issues, photosensitizers have recently been conjugated to antimicrobial peptides (AMPs).113–116 AMPs associate with bacteria more readily than with human cells.117, 118 Consequently, AMPs were shown to act as carriers that can target PS to bacteria selectively over human cells. For instance, the conjugate eosin-(KLAKLAK)2 is capable of effectively killing both Gram-positive and Gram-negative bacteria without inducing damage to human erythrocytes (Figure 7).115 Notably, eosin alone, despite being capable of generating singlet oxygen and superoxide in high yields upon light irradiation, was relatively innocuous to bacteria in the absence or presence of light.68 The AMP alone is bactericidal, albeit only at high concentrations (e.g. 10 μM is required to kill 90% of 108 CFU E. coli). Yet, upon conjugation, eosin-(KLAKLAK)2 is highly effective, with 1 μM of the conjugate killing more than 99.99% of 108 CFU E. coli or S. aureus upon a 30 min irradiation with green light (230 J/cm2). These results are therefore consistent with the notion that the AMP, by associating with bacteria, brings eosin to the vicinity of oxidizable bacterial molecules.
Figure 7. Bacterial photo-inactivation by an AMP-photosensitizer conjugate.
The hydrophilic photosensitizer eosin Y generates ROS, including singlet oxygen, when irradiated with green light. By itself, eosin Y is relatively innocuous because it does not associate with bacterial cell walls. In contrast, eosin Y conjugated to the antimicrobial peptide (KLAKLAK)2 kills Gram negative and Gram positive bacteria effectively. The amphiphilic peptide moiety binds to bacterial membranes and promotes ROS generation in the vicinity of lipid bilayers. The bacterial membranes are destabilized by oxidation of membrane components and by action of the peptide. Overall, a synergistic relationship exists: the AMP renders the photosensitizer more effective at oxidizing membranes while the photosensitizer facilitates AMP-mediated membrane lysis.
The evidence that killing is achieved by membrane oxidation was provided by several means. Membrane damage during light irradiation has been observed in the form of the influx of membrane impermeable dye and formation of membrane blebs.114, 115 In addition, in the case of eosin-(KLAKLAK)2, the ROS-generating capacity of the photosensitizer was exploited to directly visualize the location of the peptide associated with bacteria. Using a photo-induced 3,3-diaminobenzidine (DAB) polymerization protocol and electron microscopy, eosin-(KLAKLAK)2 was shown to exclusively reside on the surface of both E. coli and S. aureus prior to cell killing.119 Severe defects in the cell membrane were observed at early time points of the photokilling process. In addition, the conjugate was shown to bind and disrupt liposomes mimicking the lipid composition of cytoplasmic membrane of Gram-negative bacteria (e.g. PE:PG:CA 75:20:5, lipids contain only MUFAs). Together, these results suggest that eosin-(KLAKLAK)2 is capable of directly damaging the lipids of bacterial membranes and subsequently affect the integrity of the bacterial cell wall. Notably, (KLAKLAK)2 appear to actively participate in this process. (KLAKLAK)2 enhanced the damage of liposomes pre-oxidized with the lipophilic oxidant Ce6. This in turn is indicative of a synergy between photosensitizer and peptide. More precisely, the AMP acts as a membrane targeting agent for the photosensitizer prior to irradiation (Figure 7). Upon irradiation, eosin generates ROS in the vicinity of the bilayer and this leads to lipid damage. The AMP then displays an additional activity by contributing to membrane destabilization. The detailed mechanisms underlying this activity have not been explored, however it may be analogous to the observed synergy between Ixosin and Ixosin B (vide supra).
4.5. Plasma-induced Bacterial Inactivation
In the context of physical sciences, plasma refers to a gaseous mixture of particles with opposite charges, often also containing excited atoms, molecules and free radicals.120 Atmospheric-pressure non-thermal plasma, first described by Laroussi in the 90s, is a technology that has attracted attention in biomedical applications including bacterial inactivation.121–124 The plasma generated contains singlet oxygen (1O2), atomic oxygen (O) and even ozone (O3) responsible for general oxidation of biomolecules. These ROS, along with several other reactive species is believed to give rise to the plasma’s bactericidal properties.120, 125 Furthermore, it was found that the greatest sterilization effect was achieved when “moistened oxygen” is used, from which hydroxyl radicals can be produced.120 Recently, Joshi et al. have evaluated the use of a plasma generating probe known as floating-electrode dielectric-barrier discharge (FE-DBD) capable of generating microsecond-long high-voltage-pulsed plasma between two electrodes against planktonic and biofilm forms of several bacterial strains.121, 122 Planktonic cultures and biofilms of E. coli, S. aureus and Methicillin-resistant Staphylococcus aureus (MRSA) were rapidly (< 60 seconds) and completely eradicated using this technique. FE-DBD plasma exposure of E. coli cells resulted in morphological changes from the typical bacillus form observed in healthy planktonic cultures to the coccoid form consistent with damage to the lipid membrane.121 In addition, E. coli cells were observed to lose their Gram stain-retaining capacities upon exposure to plasma suggesting a considerable deterioration of the outer membrane.121 Extensive lipid peroxidation was observed in intact E. coli cells upon exposure to 60 seconds of FE-DBD. The use of the antioxidant α-tocopherol reduced significantly the extent of lipid peroxidation. Antioxidants also prevented plasma-induced E. coli inactivation. Overall, this study indicated that the ROS produced during FE-DBD damage bacterial cell membranes and contribute to reducing viability.
5. CONCLUSION
It is becoming increasingly clear that oxidation impacts the behavior of a membrane (and the molecules that interact with it) in relation to processes such a translocation or lysis. Given that oxidation alters the structures of lipids and membrane proteins, the relevance of this phenomenon is logical. For techniques that directly produce ROS (e.g. gas plasma or photosensitizing approaches), it is apparent that differences in extent, timing and location of oxidative events can influence whether cell delivery or cell killing is achieved. However, in the case of other techniques, the precise role played by oxidation remains unclear. For instance, is membrane oxidation only enhancing the activities of CPPs or AMPs or is it actually required to achieve detectable permeation or lysis? Similarly, would electroporation work in the complete absence of oxidative events? These questions remain hard to answer for the following reasons. First, the chemical complexity associated with the oxidation renders the analysis of oxidized products a significant challenge. Consequently, the identity, prevalence, and extent of the oxidized products that contribute to changing the membrane behavior are aspects that are often not characterized. Second, because of degradation and repair mechanisms (as described in section 1), the effects of oxidation can be transient and possibly masked. Finally, it is possible that oxidized lipids might have profound effects while being present at only very low concentrations or densities. This is related to the notion that a small defect in a bilayer can have rather dramatic consequences in the barrier capacity of the membrane, in the same way as a single small pinhole can deflate a balloon just as effectively as many holes, albeit more slowly. Similarly, intracellular delivery or bacterial killing can in principle be mediated by membrane leakage involving only few oxidized molecules. This is especially plausible if oxidation reactions were to lead to a localized accumulation of oxidized products126 (e.g. high local concentration of oxidized lipids in the bilayer in proximity to an oxidant prior to lateral diffusion). Of equal importance is the behavior, or change in behavior, of membrane-active peptides or proteins interacting with oxidized lipids. As the physico-chemical characteristics of the membrane change upon oxidation, the thermodynamics and kinetics of the interaction between membranes and membrane-active species are affected.106, 127, 128 An exciting question for future research will be to correlate the thermodynamics and kinetics of peptide binding with the degree of lipid oxidation in membranes.
The technical challenges described above have prevented a full characterization of the extent to which membranes are naturally oxidized in vitro or in vivo. It can be argued that membrane oxidation in tissue culture studies is partially artifactual. In particular, it is clear that typical growth conditions (incubation in 20% oxygen and in media lacking antioxidants) favor oxidative stress. In contrast, bacteria infecting human wounds or cells in human tissues might be exposed to 2% oxygen or less. In such environments, cells might therefore be less prone to oxidation and in vitro studies of delivery or killing agents might not adequately predict in vivo activities. Yet, evidence of membrane and lipid oxidation in vivo has been reported. For example, airway tissues are exposed to 20% oxygen and oxidation of lung epithelium has already been established to play a role in tissue permeability.129 Oxidized lipids are also found in blood plasma and tissues of both healthy and diseased humans.130 Oxidative stress and oxidation of PUFAs are also implicated in several diseases including glaucoma.131 Overall, it would therefore seem that membrane oxidation is a phenomenon that can take place in vivo. The insights gained from the in vitro studies presented herein might therefore apply to more complex settings.
One of the important lesson learned from the studies presented herein is that membrane oxidation can be exploited to increase permeation and lysis. A key issue related to membrane oxidation however is that of its impact on physiological cellular processes. Elevated levels of oxidation can lead to extensive cellular damage, including membrane rupture, and cell death. Consistent with this notion, delivery techniques such as PCI, electroporation, or delivery by gas plasma can be quite toxic to human cells. Similarly, photodynamic bacterial inactivation can be poorly selective and lead to undesirable human cell toxicity. In practice, this means that membrane oxidation should ideally be achieved at a level high enough to positively affect permeability while low enough to avoid deleterious physiological responses or unwanted side effects. A possible solution to these issues is emerging in the form of synergy between oxidants and membrane active peptides. As highlighted in several studies, multifunctional peptides can for instance direct oxidants to selected membranes and lead to targeted damage: e.g. leakage of endosomes without leakage of plasma membrane for delivery approaches,55, 56, 63 lysis of bacteria without lysis of human cells for antibacterial killing.115 The peptides then assist in the membrane permeation process.114 This in turn reduces the amount of oxidation that is required to achieve delivery or killing and further promotes the selectivity of the process. Overall, these studies highlight the possibility of designing highly tunable, selective and efficient compounds that could achieve desirable outcomes with minimal side effects.
Acknowledgments
This work was supported by award R01GM110137 from the US National Institute of General Medical Sciences (to J.-P. P), the Lyme Disease Association and generous start-up funds from the University of Connecticut (to A. M. A.-B.).
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
T.-Y. W., M. D. J. L., A. M. A.-B., and J.-P. P. wrote, reviewed and approved the final version of the manuscript.
References
- 1.Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538:183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 2.Lewis K. Platforms for antibiotic discovery. Nat Rev Drug Discov. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomedical journal. 2014;37:99–105. doi: 10.4103/2319-4170.128725. [DOI] [PubMed] [Google Scholar]
- 4.Reis A, Spickett CM. Chemistry of phospholipid oxidation. Biochimica et biophysica acta. 2012;1818:2374–2387. doi: 10.1016/j.bbamem.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Davies MJ. Protein oxidation and peroxidation. The Biochemical journal. 2016;473:805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxidants & redox signaling. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 5. Oxford University Press; 2015. [Google Scholar]
- 9.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. Journal of lipid research. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 10.Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochemical and Biophysical Research Communications. 2005;338:668–676. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 11.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 12.Bielski BH, Arudi RL, Sutherland MW. A study of the reactivity of HO2/O2- with unsaturated fatty acids. J Biol Chem. 1983;258:4759–4761. [PubMed] [Google Scholar]
- 13.Blanksby SJ, Ellison GB. Bond dissociation energies of organic molecules. Accounts of chemical research. 2003;36:255–263. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 14.Milic I, Hoffmann R, Fedorova M. Simultaneous detection of low and high molecular weight carbonylated compounds derived from lipid peroxidation by electrospray ionization-tandem mass spectrometry. Analytical chemistry. 2013;85:156–162. doi: 10.1021/ac302356z. [DOI] [PubMed] [Google Scholar]
- 15.Murphy RC, Johnson KM. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. The Journal of biological chemistry. 2008;283:15521–15525. doi: 10.1074/jbc.R700049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holopainen JM, Lehtonen JY, Kinnunen PK. Evidence for the extended phospholipid conformation in membrane fusion and hemifusion. Biophysical journal. 1999;76:2111–2120. doi: 10.1016/S0006-3495(99)77367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatini K, Mattila JP, Megli FM, Kinnunen PK. Characterization of two oxidatively modified phospholipids in mixed monolayers with DPPC. Biophys J. 2006;90:4488–4499. doi: 10.1529/biophysj.105.080176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volinsky R, Cwiklik L, Jurkiewicz P, Hof M, Jungwirth P, Kinnunen PK. Oxidized phosphatidylcholines facilitate phospholipid flip-flop in liposomes. Biophys J. 2011;101:1376–1384. doi: 10.1016/j.bpj.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre TM. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: formation, targets, and inactivation. Biochim Biophys Acta. 2012;1818:2456–2464. doi: 10.1016/j.bbamem.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron S, Poast J, Rizzo D, McFarland E, Kieff E. Electroporation of antibodies, DNA, and other macromolecules into cells: a highly efficient method. J Immunol Methods. 2000;242:115–126. doi: 10.1016/s0022-1759(00)00242-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Yuan F. Membrane binding of plasmid DNA and endocytic pathways are involved in electrotransfection of mammalian cells. PloS one. 2011;6:e20923. doi: 10.1371/journal.pone.0020923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosazza C, Phez E, Escoffre JM, Cezanne L, Zumbusch A, Rols MP. Cholesterol implications in plasmid DNA electrotransfer: Evidence for the involvement of endocytotic pathways. International journal of pharmaceutics. 2012;423:134–143. doi: 10.1016/j.ijpharm.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Rosazza C, Buntz A, Riess T, Woll D, Zumbusch A, Rols MP. Intracellular tracking of single-plasmid DNA particles after delivery by electroporation. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:2217–2226. doi: 10.1038/mt.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosazza C, Deschout H, Buntz A, Braeckmans K, Rols MP, Zumbusch A. Endocytosis and Endosomal Trafficking of DNA After Gene Electrotransfer In Vitro. Mol Ther Nucleic Acids. 2016;5:e286. doi: 10.1038/mtna.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernier PT, Levine ZA, Wu YH, Joubert V, Ziegler MJ, Mir LM, Tieleman DP. Electroporating fields target oxidatively damaged areas in the cell membrane. PloS one. 2009;4:e7966. doi: 10.1371/journal.pone.0007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maccarrone M, Rosato N, Agro AF. Electroporation enhances cell membrane peroxidation and luminescence. Biochem Biophys Res Commun. 1995;206:238–245. doi: 10.1006/bbrc.1995.1033. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel B, Teissie J. Generation of reactive-oxygen species induced by electropermeabilization of Chinese hamster ovary cells and their consequence on cell viability. Eur J Biochem. 1994;223:25–33. doi: 10.1111/j.1432-1033.1994.tb18962.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonnafous P, Vernhes M, Teissie J, Gabriel B. The generation of reactive-oxygen species associated with long-lasting pulse-induced electropermeabilisation of mammalian cells is based on a non-destructive alteration of the plasma membrane. Biochimica et biophysica acta. 1999;1461:123–134. doi: 10.1016/s0005-2736(99)00154-6. [DOI] [PubMed] [Google Scholar]
- 29.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 30.Fretz MM, Penning NA, Al-Taei S, Futaki S, Takeuchi T, Nakase I, Storm G, Jones AT. Temperature-, concentration- and cholesterol-dependent translocation of L- and D-octa-arginine across the plasma and nuclear membrane of CD34+ leukaemia cells. The Biochemical journal. 2007;403:335–342. doi: 10.1042/BJ20061808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosuge M, Takeuchi T, Nakase I, Jones AT, Futaki S. Cellular internalization and distribution of arginine-rich peptides as a function of extracellular peptide concentration, serum, and plasma membrane associated proteoglycans. Bioconjugate chemistry. 2008;19:656–664. doi: 10.1021/bc700289w. [DOI] [PubMed] [Google Scholar]
- 32.Verdurmen WP, Thanos M, Ruttekolk IR, Gulbins E, Brock R. Cationic cell-penetrating peptides induce ceramide formation via acid sphingomyelinase: implications for uptake. Journal of controlled release: official journal of the Controlled Release Society. 2010;147:171–179. doi: 10.1016/j.jconrel.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Hirose H, Takeuchi T, Osakada H, Pujals S, Katayama S, Nakase I, Kobayashi S, Haraguchi T, Futaki S. Transient focal membrane deformation induced by arginine-rich peptides leads to their direct penetration into cells. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:984–993. doi: 10.1038/mt.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herce HD, Garcia AE, Cardoso MC. Fundamental molecular mechanism for the cellular uptake of guanidinium-rich molecules. Journal of the American Chemical Society. 2014;136:17459–17467. doi: 10.1021/ja507790z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Molecular therapy: the journal of the American Society of Gene Therapy. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Fischer R, Kohler K, Fotin-Mleczek M, Brock R. A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. The Journal of biological chemistry. 2004;279:12625–12635. doi: 10.1074/jbc.M311461200. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. Journal of controlled release: official journal of the Controlled Release Society. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Erazo-Oliveras A, Muthukrishnan N, Baker R, Wang T-Y, Pellois J-P. Improving the Endosomal Escape of Cell-Penetrating Peptides and Their Cargos: Strategies and Challenges. Pharmaceuticals. 2012;5:1177–1209. doi: 10.3390/ph5111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erazo-Oliveras A, Najjar K, Dayani L, Wang TY, Johnson GA, Pellois JP. Protein delivery into live cells by incubation with an endosomolytic agent. Nat Methods. 2014;11:861–867. doi: 10.1038/nmeth.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends in Molecular Medicine. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler A, Seelig J. Contributions of glycosaminoglycan binding and clustering to the biological uptake of the nonamphipathic cell-penetrating peptide WR9. Biochemistry. 2011;50:4650–4664. doi: 10.1021/bi1019429. [DOI] [PubMed] [Google Scholar]
- 42.Yang ST, Zaitseva E, Chernomordik LV, Melikov K. Cell-penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophys J. 2010;99:2525–2533. doi: 10.1016/j.bpj.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erazo-Oliveras A, Najjar K, Truong D, Wang TY, Brock DJ, Prater AR, Pellois JP. The Late Endosome and Its Lipid BMP Act as Gateways for Efficient Cytosolic Access of the Delivery Agent dfTAT and Its Macromolecular Cargos. Cell Chem Biol. 2016 doi: 10.1016/j.chembiol.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verdurmen WP, Bovee-Geurts PH, Wadhwani P, Ulrich AS, Hallbrink M, van Kuppevelt TH, Brock R. Preferential uptake of L- versus D-amino acid cell-penetrating peptides in a cell type-dependent manner. Chem Biol. 2011;18:1000–1010. doi: 10.1016/j.chembiol.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Traboulsi H, Larkin H, Bonin MA, Volkov L, Lavoie CL, Marsault E. Macrocyclic cell penetrating peptides: a study of structure-penetration properties. Bioconjugate chemistry. 2015;26:405–411. doi: 10.1021/acs.bioconjchem.5b00023. [DOI] [PubMed] [Google Scholar]
- 46.Wang TY, Sun Y, Muthukrishnan N, Erazo-Oliveras A, Najjar K, Pellois JP. Membrane Oxidation Enables the Cytosolic Entry of Polyarginine Cell-penetrating Peptides. J Biol Chem. 2016;291:7902–7914. doi: 10.1074/jbc.M115.711564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation, The Journal of biological chemistry. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 48.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad O, Kjolsrud S, Anholt H, Rodal GH, Rodal SK, Hogset A. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59:1180–1183. [PubMed] [Google Scholar]
- 49.Selbo PK, Weyergang A, Hogset A, Norum OJ, Berstad MB, Vikdal M, Berg K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. Journal of controlled release: official journal of the Controlled Release Society. 2010;148:2–12. doi: 10.1016/j.jconrel.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi Y, McCarthy JR, Weissleder R, Tung CH. Conjugation of a photosensitizer to an oligoarginine-based cell-penetrating peptide increases the efficacy of photodynamic therapy. ChemMedChem. 2006;1:458–463. doi: 10.1002/cmdc.200500036. [DOI] [PubMed] [Google Scholar]
- 52.Sibrian-Vazquez M, Jensen TJ, Hammer RP, Vicente MG. Peptide-mediated cell transport of water soluble porphyrin conjugates. J Med Chem. 2006;49:1364–1372. doi: 10.1021/jm050893b. [DOI] [PubMed] [Google Scholar]
- 53.Sibrian-Vazquez M, Hao E, Jensen TJ, Vicente MG. Enhanced cellular uptake with a cobaltacarborane-porphyrin-HIV-1 Tat 48–60 conjugate. Bioconjugate chemistry. 2006;17:928–934. doi: 10.1021/bc060047v. [DOI] [PubMed] [Google Scholar]
- 54.Wang JT, Giuntini F, Eggleston IM, Bown SG, MacRobert AJ. Photochemical internalisation of a macromolecular protein toxin using a cell penetrating peptide-photosensitiser conjugate. Journal of controlled release: official journal of the Controlled Release Society. 2012;157:305–313. doi: 10.1016/j.jconrel.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 55.Maiolo JR, 3rd, Ottinger EA, Ferrer M. Specific redistribution of cell-penetrating peptides from endosomes to the cytoplasm and nucleus upon laser illumination. Journal of the American Chemical Society. 2004;126:15376–15377. doi: 10.1021/ja044867z. [DOI] [PubMed] [Google Scholar]
- 56.Matsushita M, Noguchi H, Lu YF, Tomizawa K, Michiue H, Li ST, Hirose K, Bonner-Weir S, Matsui H. Photo-acceleration of protein release from endosome in the protein transduction system. FEBS letters. 2004;572:221–226. doi: 10.1016/j.febslet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 57.Endoh T, Sisido M, Ohtsuki T. Spatial regulation of specific gene expression through photoactivation of RNAi. Journal of controlled release: official journal of the Controlled Release Society. 2009;137:241–245. doi: 10.1016/j.jconrel.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasan D, Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Lim J, Simanek EE, Pellois JP. Conjugation to the cell-penetrating peptide TAT potentiates the photodynamic effect of carboxytetramethylrhodamine. PloS one. 2011;6:e17732. doi: 10.1371/journal.pone.0017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtsuki T, Miki S, Kobayashi S, Haraguchi T, Nakata E, Hirakawa K, Sumita K, Watanabe K, Okazaki S. The molecular mechanism of photochemical internalization of cell penetrating peptide-cargo-photosensitizer conjugates. Sci Rep. 2015;5:18577. doi: 10.1038/srep18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minamihata K, Maeda Y, Yamaguchi S, Ishihara W, Ishiwatari A, Takamori S, Yamahira S, Nagamune T. Photosensitizer and polycationic peptide-labeled streptavidin as a nano-carrier for light-controlled protein transduction. J Biosci Bioeng. 2015;120:630–636. doi: 10.1016/j.jbiosc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Kerdous R, Heuvingh J, Bonneau S. Photo-dynamic induction of oxidative stress within cholesterol-containing membranes: shape transitions and permeabilization. Biochimica et biophysica acta. 2011;1808:2965–2972. doi: 10.1016/j.bbamem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Sankhagowit S, Wu SH, Biswas R, Riche CT, Povinelli ML, Malmstadt N. The dynamics of giant unilamellar vesicle oxidation probed by morphological transitions. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbamem.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 63.Meerovich I, Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Pellois J-P. Photodamage of lipid bilayers by irradiation of a fluorescently labeled cell-penetrating peptide. Biochimica et Biophysica Acta (BBA) - General Subjects. 2014;1840:507–515. doi: 10.1016/j.bbagen.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Pellois J-P. Synergy Between Cell-Penetrating Peptides and Singlet Oxygen Generators Leads to Efficient Photolysis of Membranes. Photochemistry and Photobiology. 2013;89:625–630. doi: 10.1111/php.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muthukrishnan N, Donovan S, Pellois JP. The Photolytic Activity of Poly-Arginine Cell Penetrating Peptides Conjugated to Carboxy-tetramethylrhodamine is Modulated by Arginine Residue Content and Fluorophore Conjugation Site. Photochem Photobiol. 2014;90:9. doi: 10.1111/php.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakai Y, Khajoee V, Ogawa Y, Kusuhara K, Katayama Y, Hara T. A novel transfection method for mammalian cells using gas plasma. J Biotechnol. 2006;121:299–308. doi: 10.1016/j.jbiotec.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 67.Ogawa Y, Morikawa N, Ohkubo-Suzuki A, Miyoshi S, Arakawa H, Kita Y, Nishimura S. An epoch-making application of discharge plasma phenomenon to gene-transfer. Biotechnology and bioengineering. 2005;92:865–870. doi: 10.1002/bit.20659. [DOI] [PubMed] [Google Scholar]
- 68.Connolly RJ, Lopez GA, Hoff AM, Jaroszeski MJ. Plasma facilitated delivery of DNA to skin. Biotechnology and bioengineering. 2009;104:1034–1040. doi: 10.1002/bit.22451. [DOI] [PubMed] [Google Scholar]
- 69.Connolly RJ, Rey JI, Lambert VM, Wegerif G, Jaroszeski MJ, Ugen KE. Enhancement of antigen specific humoral immune responses after delivery of a DNA plasmid based vaccine through a contact-independent helium plasma. Vaccine. 2011;29:6781–6784. doi: 10.1016/j.vaccine.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connolly RJ, Chapman T, Hoff AM, Kutzler MA, Jaroszeski MJ, Ugen KE. Non-contact helium-based plasma for delivery of DNA vaccines. Enhancement of humoral and cellular immune responses, Hum Vaccin Immunother. 2012;8:1729–1733. doi: 10.4161/hv.21624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leduc M, Guay D, Leask RL, Coulombe S. Cell permeabilization using a non-thermal plasma. New Journal of Physics. 2009;11:115021. [Google Scholar]
- 72.Edelblute CM, Heller LC, Malik MA, Heller R. Activated air produced by shielded sliding discharge plasma mediates plasmid DNA delivery to mammalian cells. Biotechnology and bioengineering. 2015;112:2583–2590. doi: 10.1002/bit.25660. [DOI] [PubMed] [Google Scholar]
- 73.Kaneko T, Sasaki S, Hokari Y, Horiuchi S, Honda R, Kanzaki M. Improvement of cell membrane permeability using a cell-solution electrode for generating atmospheric-pressure plasma. Biointerphases. 2015;10:029521. doi: 10.1116/1.4921278. [DOI] [PubMed] [Google Scholar]
- 74.Edelblute CM, Heller LC, Malik MA, Bulysheva A, Heller R. Plasma-activated air mediates plasmid DNA delivery in vivo. Mol Ther Methods Clin Dev. 2016;3:16028. doi: 10.1038/mtm.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu D, Wang B, Xu Y, Chen Z, Cui Q, Yang Y, Chen H, Kong MG. Intracellular ROS mediates gas plasma-facilitated cellular transfection in 2D and 3D cultures. Scientific reports. 2016;6:27872. doi: 10.1038/srep27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu D, Liu D, Wang B, Chen C, Chen Z, Li D, Yang Y, Chen H, Kong MG. In Situ OH Generation from O2- and H2O2 Plays a Critical Role in Plasma-Induced Cell Death. PloS one. 2015;10:e0128205. doi: 10.1371/journal.pone.0128205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dingjan I, Verboogen DR, Paardekooper LM, Revelo NH, Sittig SP, Visser LJ, Mollard GF, Henriet SS, Figdor CG, Ter Beest M, van den Bogaart G. Lipid peroxidation causes endosomal antigen release for cross-presentation. Scientific reports. 2016;6:22064. doi: 10.1038/srep22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 79.Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- 80.Lucky SS, Soo KC, Zhang Y. Nanoparticles in Photodynamic Therapy. Chemical Reviews. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 81.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boylan JA, Lawrence KA, Downey JS, Gherardini FC. Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Mol Microbiol. 2008;68:786–799. doi: 10.1111/j.1365-2958.2008.06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perez JM, Arenas FA, Pradenas GA, Sandoval JM, Vasquez CC. Escherichia coli YqhD exhibits aldehyde reductase activity and protects from the harmful effect of lipid peroxidation-derived aldehydes. J Biol Chem. 2008;283:7346–7353. doi: 10.1074/jbc.M708846200. [DOI] [PubMed] [Google Scholar]
- 84.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 86.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loidl-Stahlhofen A, Kern W, Spiteller G. Gas chromatographic-electron impact mass spectrometric screening procedure for unknown hydroxyaldehydic lipid peroxidation products after pentafluorobenzyloxime derivatization. J Chromatogr B Biomed Appl. 1995;673:1–14. doi: 10.1016/0378-4347(95)00244-d. [DOI] [PubMed] [Google Scholar]
- 88.Reis A, Domingues MR, Amado FM, Ferrer-Correia AJ, Domingues P. Separation of peroxidation products of diacyl-phosphatidylcholines by reversed-phase liquid chromatography-mass spectrometry. Biomed Chromatogr. 2005;19:129–137. doi: 10.1002/bmc.429. [DOI] [PubMed] [Google Scholar]
- 89.Libardo MD, Cervantes JL, Salazar JC, Angeles-Boza AM. Improved bioactivity of antimicrobial peptides by addition of amino-terminal copper and nickel (ATCUN) binding motifs. ChemMedChem. 2014;9:1892–1901. doi: 10.1002/cmdc.201402033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semchyshyn H, Bagnyukova T, Storey K, Lushchak V. Hydrogen peroxide increases the activities of soxRS regulon enzymes and the levels of oxidized proteins and lipids in Escherichia coli. Cell Biol Int. 2005;29:898–902. doi: 10.1016/j.cellbi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 91.Vilcheze C, Hartman T, Weinrick B, Jacobs WR., Jr Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun. 2013;4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia Costas AM, Tsukatani Y, Rijpstra WIC, Schouten S, Welander PV, Summons RE, Bryant DA. Identification of the Bacteriochlorophylls, Carotenoids, Quinones, Lipids, and Hopanoids of “Candidatus Chloracidobacterium thermophilum”. Journal of Bacteriology. 2012;194:1158–1168. doi: 10.1128/JB.06421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nowicka B, Kruk J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim Biophys Acta. 2010;1797:1587–1605. doi: 10.1016/j.bbabio.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Talbot HM, Rohmer M, Farrimond P. Structural characterisation of unsaturated bacterial hopanoids by atmospheric pressure chemical ionisation liquid chromatography/ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1613–1622. doi: 10.1002/rcm.2997. [DOI] [PubMed] [Google Scholar]
- 95.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA: a cancer journal for clinicians. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections--state of the art. Photodiagnosis Photodyn Ther. 2009;6:170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malik Z, Ladan H, Nitzan Y, Ehrenberg B. The bactericidal activity of a deuteroporphyrin-hemin mixture on gram-positive bacteria. A microbiological and spectroscopic study. Journal of photochemistry and photobiology B, Biology. 1990;6:419–430. doi: 10.1016/1011-1344(90)85115-d. [DOI] [PubMed] [Google Scholar]
- 99.Nitzan Y, Balzam-Sudakevitz A, Ashkenazi H. Eradication of Acinetobacter baumannii by photosensitized agents in vitro. Journal of photochemistry and photobiology B, Biology. 1998;42:211–218. doi: 10.1016/s1011-1344(98)00073-6. [DOI] [PubMed] [Google Scholar]
- 100.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 101.Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier JE. Photosensitizing activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios. 1992;71:33–46. [PubMed] [Google Scholar]
- 102.Bertoloni G, Rossi F, Valduga G, Jori G, van Lier J. Photosensitizing activity of water- and lipid-soluble phthalocyanines on Escherichia coli. FEMS microbiology letters. 1990;59:149–155. doi: 10.1111/j.1574-6968.1990.tb03814.x. [DOI] [PubMed] [Google Scholar]
- 103.Nakonechny F, Pinkus A, Hai S, Yehosha O, Nitzan Y, Nisnevitch M. Eradication of Gram-Positive and Gram-Negative Bacteria by Photosensitizers Immobilized in Polystyrene. Photochemistry and Photobiology. 2013;89:671–678. doi: 10.1111/php.12022. [DOI] [PubMed] [Google Scholar]
- 104.Chileveru HR, Lim SA, Chairatana P, Wommack AJ, Chiang IL, Nolan EM. Visualizing attack of Escherichia coli by the antimicrobial peptide human defensin 5. Biochemistry. 2015;54:1767–1777. doi: 10.1021/bi501483q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bacellar IOL, Pavani C, Sales EM, Itri R, Wainwright M, Baptista MS. Membrane Damage Efficiency of Phenothiazinium Photosensitizers. Photochemistry and Photobiology. 2014;90:801–813. doi: 10.1111/php.12264. [DOI] [PubMed] [Google Scholar]
- 106.Mattila JP, Sabatini K, Kinnunen PK. Oxidized phospholipids as potential molecular targets for antimicrobial peptides. Biochim Biophys Acta. 2008;1778:2041–2050. doi: 10.1016/j.bbamem.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 107.Wang TY, Pellois JP. Peptide translocation through the plasma membrane of human cells: Can oxidative stress be exploited to gain better intracellular access? Communicative & Integrative Biology. 2016;9:e1205771. doi: 10.1080/19420889.2016.1205771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu D, Sheng Z, Xu X, Li J, Yang H, Liu Z, Rees HH, Lai R. A novel antimicrobial peptide from salivary glands of the hard tick, Ixodes sinensis. Peptides. 2006;27:31–35. doi: 10.1016/j.peptides.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 109.Liu Z, Liu H, Liu X, Wu X. Purification and cloning of a novel antimicrobial peptide from salivary glands of the hard tick, Ixodes sinensis. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 2008;149:557–561. doi: 10.1016/j.cbpb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Libardo MDJ, Gorbatyuk VY, Angeles-Boza AM. Central Role of the Copper-Binding Motif in the Complex Mechanism of Action of Ixosin: Enhancing Oxidative Damage and Promoting Synergy with Ixosin B. ACS Infectious Diseases. 2016;2:71–81. doi: 10.1021/acsinfecdis.5b00140. [DOI] [PubMed] [Google Scholar]
- 111.Harford C, Sarkar B. Amino Terminal Cu(II)- and Ni(II)-Binding Motif of Proteins and Peptides: Metal Binding, DNA Cleavage, and Other Properties. Acc Chem Res. 1997;30:123–130. [Google Scholar]
- 112.Libardo MDJ, Nagella S, Lugo A, Pierce S, Angeles-Boza AM. Copper-binding tripeptide motif increases potency of the antimicrobial peptide Anoplin via Reactive Oxygen Species generation. Biochemical and Biophysical Research Communications. 2015;456:446–451. doi: 10.1016/j.bbrc.2014.11.104. [DOI] [PubMed] [Google Scholar]
- 113.Liu F, Soh Yan Ni A, Lim Y, Mohanram H, Bhattacharjya S, Xing B. Lipopolysaccharide neutralizing peptide-porphyrin conjugates for effective photoinactivation and intracellular imaging of gram-negative bacteria strains. Bioconjugate chemistry. 2012;23:1639–1647. doi: 10.1021/bc300203d. [DOI] [PubMed] [Google Scholar]
- 114.Johnson GA, Ellis EA, Kim H, Muthukrishnan N, Snavely T, Pellois JP. Photoinduced membrane damage of E. coli and S. aureus by the photosensitizer-antimicrobial peptide conjugate eosin-(KLAKLAK)2. PloS one. 2014;9:e91220. doi: 10.1371/journal.pone.0091220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson GA, Muthukrishnan N, Pellois JP. Photoinactivation of Gram positive and Gram negative bacteria with the antimicrobial peptide (KLAKLAK)2 conjugated to the hydrophilic photosensitizer eosin Y. Bioconjugate chemistry. 2013;24:114–123. doi: 10.1021/bc3005254. [DOI] [PubMed] [Google Scholar]
- 116.Dosselli R, Gobbo M, Bolognini E, Campestrini S, Reddi E. Porphyrin-apidaecin conjugate as a new broad spectrum antibacterial agent. ACS medicinal chemistry letters. 2010;1:35–38. doi: 10.1021/ml900021y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 118.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2009;1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 119.Valduga G, Bertoloni G, Reddi E, Jori G. Effect of extracellularly generated singlet oxygen on gram-positive and gram-negative bacteria. Journal of photochemistry and photobiology B, Biology. 1993;21:81–86. doi: 10.1016/1011-1344(93)80168-9. [DOI] [PubMed] [Google Scholar]
- 120.Laroussi M. Nonthermal Decontamination of Biological Media by Atmospheric-Pressure Plasmas: Review, Analysis, and Prospects. IEEE Trans on Plasma Science. 2002;30:1409–1415. [Google Scholar]
- 121.Joshi SG, Cooper M, Yost A, Paff M, Ercan UK, Fridman G, Friedman G, Fridman A, Brooks AD. Nonthermal Dielectric-Barrier Discharge Plasma-Induced Inactivation Involves Oxidative DNA Damage and Membrane Lipid Peroxidation in Escherichia coli. Antimicrobial Agents and Chemotherapy. 2011;55:1053–1062. doi: 10.1128/AAC.01002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Joshi SG, Paff M, Friedman G, Fridman G, Fridman A, Brooks AD. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am J Infect Control. 2010;38:293–301. doi: 10.1016/j.ajic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 123.Ziuzina D, Patil S, Cullen PJ, Keener KM, Bourke P. Atmospheric cold plasma inactivation of Escherichia coli in liquid media inside a sealed package. Journal of Applied Microbiology. 2013;114:778–787. doi: 10.1111/jam.12087. [DOI] [PubMed] [Google Scholar]
- 124.Laroussi M. Sterilization of Contaminated Matter with an Atmospheric Pressure Plasma. IEEE Trans on Plasma Science. 1996;24:1188–1191. [Google Scholar]
- 125.Hermann HW, Henins I, Park J, Selwyn GS, Hicks RF. Decontamination of chemical and biological warfare (CBW) agents using an atmospheric pressure plasma jet. Phys Plasmas. 1998;6:2284–2289. [Google Scholar]
- 126.Runas KA, Malmstadt N. Low levels of lipid oxidation radically increase the passive permeability of lipid bilayers. Soft matter. 2015;11:499–505. doi: 10.1039/c4sm01478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hermetter A, Kinnunen P, Spickett C. Oxidized phospholipids-their properties and interactions with proteins. Biochim Biophys Acta. 2012;1818:2373. doi: 10.1016/j.bbamem.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 128.Wallgren M, Beranova L, Pham QD, Linh K, Lidman M, Procek J, Cyprych K, Kinnunen PK, Hof M, Grobner G. Impact of oxidized phospholipids on the structural and dynamic organization of phospholipid membranes: a combined DSC and solid state NMR study. Faraday Discuss. 2013;161:499–513. doi: 10.1039/c2fd20089a. discussion 563–489. [DOI] [PubMed] [Google Scholar]
- 129.Starosta V, Wu T, Zimman A, Pham D, Tian X, Oskolkova O, Bochkov V, Berliner JA, Birukova AA, Birukov KG. Differential regulation of endothelial cell permeability by high and low doses of oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine. Am J Respir Cell Mol Biol. 2012;46:331–341. doi: 10.1165/rcmb.2011-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clinical chemistry. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 131.Pemp B, Polska E, Karl K, Lasta M, Minichmayr A, Garhofer G, Wolzt M, Schmetterer L. Effects of antioxidants (AREDS medication) on ocular blood flow and endothelial function in an endotoxin-induced model of oxidative stress in humans. Invest Ophthalmol Vis Sci. 2010;51:2–6. doi: 10.1167/iovs.09-3888. [DOI] [PubMed] [Google Scholar]