Abstract
Chronic exposure to manganese (Mn) causes neurotoxicity, referred to as manganism, with common clinical features of parkinsonism. 17β-estradiol (E2) and tamoxifen (TX), a selective estrogen receptor modulator (SERM), afford neuroprotection in several neurological disorders, including Parkinson’s disease (PD). In the present study, we tested if E2 and TX attenuate Mn-induced neurotoxicity in mice, assessing motor deficit and dopaminergic neurodegeneration. We implanted E2 and TX pellets in the back of the neck of ovariectomized C57BL/6 mice two weeks prior to a single injection of Mn into the striatum. One week later, we assessed locomotor activity and molecular mechanisms by immunohistochemistry, real-time quantitative PCR, western blot and enzymatic biochemical analyses. The results showed that both E2 and TX attenuated Mn-induced motor deficits and reversed the Mn-induced loss of dopaminergic neurons in the substantia nigra. At the molecular level, E2 and TX reversed the Mn-induced decrease of (1) glutamate aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1) mRNA and protein levels; (2) transforming growth factor-α (TGF-α) and estrogen receptor-α (ER-α) protein levels; and (3) catalase (CAT) activity and glutathione (GSH) levels, and Mn-increased (1) malondialdehyde (MDA) levels and (2) the Bax/Bcl-2 ratio. These results indicate that E2 and TX afford protection against Mn-induced neurotoxicity by reversing Mn-reduced GLT1/GLAST as well as Mn-induced oxidative stress. Our findings may offer estrogenic agents as potential candidates for the development of therapeutics to treat Mn-induced neurotoxicity.
Keywords: manganese, 17β-estradiol, tamoxifen, GLT-1, GLAST, tyrosine hydroxylase
Introduction
Manganese (Mn) is an essential element for development, metabolism and antioxidant systems (Keen et al., 1999). However, chronic exposure to excessive Mn levels leads to its accumulation in the globus pallidus and other basal ganglia nuclei, culminating in a neurological disorder referred to as manganism (Barbeau, 1984; Calne et al., 1994). Manganism exhibits clinical symptoms analogous to Parkinson’s disease (PD), characterized by motor impairment, psychiatric and cognitive deficits (Calne et al., 1994; Guilarte, 2010). The symptoms of manganism and PD are associated with loss of dopaminergic neurons in the substantia nigra, resulting in dopaminergic dysregulation-related pathological symptoms.
Although Mn-induced pathological symptoms are well established, cellular/molecular mechanisms of Mn neurotoxicity are not completely understood. Among several mechanisms proposed for Mn-induced toxicity, oxidative stress and glutamate-mediated excitotoxicity appear critical and widely supported by numerous studies (Lee et al., 2017; Martinez-Finley et al., 2013), providing a putative target for developing therapeutics for Mn-induced neurotoxicity. Notably, excitotoxic neuronal death is associated with various neurodegenerative diseases, including multiple sclerosis, Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), PD, and manganism (Sheldon and Robinson, 2007). Glutamate transporters play a crucial role in maintaining optimal glutamate levels in the synaptic cleft to mitigate excitotoxic neuronal injury (Gegelashvili and Schousboe, 1997).
Among the five identified human excitatory amino acid transporters (EAATs), two main glutamate transporters responsible for preventing excitotoxic neuronal injury are EAAT1 and EAAT2, referred to as glutamate-aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) in rodents, respectively. Both transporters are predominantly expressed in astrocytes, accounting for >90% of synaptic glutamate clearance (Danbolt, 2001; Kim et al., 2011). Mn decreases expression as well as function of the astrocytic glutamate transporters, GLAST and GLT-1 (Erikson and Aschner, 2002; Gillessen et al., 2002; Karki et al., 2014a), resulting in glutamate accumulation in the synaptic clefts and the ensuing excitotoxic neuronal injury (Sidoryk-Wegrzynowicz et al., 2009).
The female sex hormone 17β-estradiol (E2) exerts neuroprotection in various neurological disease models, including PD (Ramirez et al., 2003). Notably, the incidence of PD is 1.5 times greater in men than those in women, suggesting that E2 might contribute to the lower incidence of the disease (Natrajan and Gambrell, 2002; Wooten et al., 2004). Several selective estrogen receptor modulators (SERMs) such as tamoxifen (TX) which has agonistic or antagonistic effects on the estrogen receptors (ERs) depending on tissue types, have shown to exert neuroprotection. Numerous clinical and animal studies have demonstrated the neuroprotective effects of E2 and SERMs including TX in various neurodegenerative diseases, including PD models (Bourque et al., 2009; Cyr et al., 2002; Dluzen et al., 2001; Kuo et al., 2003).
Anti-oxidative effects appear to be involved in E2- and TX-induced neuroprotection in several animal models of neurological disorders with ER-dependent and -independent mechanisms (Abdelhamid et al., 2011; Bourque et al., 2009; D'Astous et al., 2004; Wang et al., 2015; Zhang et al., 2007; Zou et al., 2015). E2 ameliorated light-induced retinal damage by antioxidant mechanism (Wang et al., 2015), as well as enhancing phase-2 antioxidant enzyme expression including catalase and superoxide dismutase by ER-dependent mechanisms in rats (Zhu et al., 2015). TX protected focal cerebral ischemia in rats via ER-independent antioxidant effects (Zhang et al., 2007). We have previously reported that E2 and TX attenuated Mn-induced oxidative stress in in vitro primary astrocytes (Lee et al., 2009).
The cell-type specificity of E2- and TX-neuroprotection has not been completely understood, but astrocytes appear to play a critical role in mediating this effect (Spence et al., 2011). E2 has shown to reduce the cortical lesion size induced by ibotenate, a glutamate analogue that activates N-methyl-d-aspartate (NMDA) in neonatal rats by astrocytic mechanism (Pansiot et al., 2016). Moreover, our previous study demonstrated that E2, TX and raloxifene (RX) attenuated Mn-induced impairment of astrocytic glutamate transporters (Lee et al., 2012; Lee et al., 2009). Given that astrocytic glutamate transporters is a critical regulator of synaptic glutamate levels, modulation of these transporters could be involved in E2/SERMs-induced protective effects in animal models of excitotoxic brain injury (Armagan et al., 2009; Ciriza et al., 2004; Mendelowitsch et al., 2001; O'Neill et al., 2004; Zhang et al., 2009).
In the present study, we determined if E2 and TX protect against Mn-induced neurotoxicity in vivo. We implanted E2 and TX pellets in the back of ovariectomized C57BL/6 mice two weeks prior to a single injection of Mn into the striatum lasting a week, followed by assessing locomotor activity and dopaminergic neuronal damage along with relevant molecular/biochemical assays. Our results showed that both E2 and TX afforded neuroprotection against Mn-induced dopaminergic neurotoxicity by reversing Mn-reduced locomotor activity, tyrosine hydroxylase (TH) mRNA/protein levels, astrocytic GLT-1/GLAST mRNA/protein levels, and antioxidant markers such as glutathione (GSH) and catalase (CAT).
Materials and methods
Experimental animals
All animal protocols were reviewed and approved by the Meharry Medical College Institutional Animal Care and Use Committee (Nashville, TN). The ovariectomized female C57BL/6 mice (12 weeks old; weight 18–20 g) were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed and maintained on a 12-h light/dark cycle at 25 ± 2°C and 60–70% relative humidity with food and water available ad libitum in the Animal Care Facility of Meharry Medical College (Nashville, TN).
Chemicals and reagents
Manganese chloride (MnCl2, Cat. # 244589) was obtained from Sigma-Aldrich (St. Louis, MO). Pellets of 17β-estradiol (E2, Cat. # E-121) and its placebo (Cat. # C-111), and tamoxifen citrate (TX, Cat. # E-351) and its placebo (Cat. # C-111) were obtained from Innovative Research of America (Sarasota, FL). EAAT1/GLAST (ab416) and EAAT2/GLT-1 (ab41621) antibodies were obtained from Abcam (Cambridge, MA). Antibodies for TH (sc-25269), transforming growth factor-α (TGF-α, sc-36), ER-α (sc-542), catalase (CAT, sc-271803), Bax (sc-7480), Bcl-2 (sc-7382) and β-actin (sc-47778) were acquired from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-mouse IgG H&L horseradish peroxidase (HRP)-conjugated (ab6728), Goat anti-rabbit IgG H&L HRP-conjugated (ab97051) and donkey anti-goat (ab97110) IgG H&L horseradish peroxidase (HRP)-conjugated and goat anti-mouse IgG Alexa Fluor® 568 (ab150119) secondary antibodies were obtained from Abcam. CAT activity colorimetric/fluorometric Kit (Cat. # K773-100) and glutathione (GSH) fluorometric assay kit (Cat. # K251-100) were obtained from BioVision Inc. (Milpitas, CA). Malondialdehyde (MDA)/lipid peroxidation/TBARS assay kit (Cat. # 10009055) was obtained from Cayman Chemical (Ann Arbor, MI).
Experimental procedure
Ovariectomized C57BL/6 mice were randomly assigned to one of the following eight groups (n = 7–8 per group): (1) Control (sham), (2) E2 placebo, (3) TX placebo, (4) Mn, (5) E2, (6) TX, (7) E2+Mn and (8) TX+Mn. E2 pellets (0.25 mg/pellet, 21-d release) and TX pellets (5 mg/pellet, 21-d release) along with their placebo pellets were used (Sasayama et al., 2017). All pellets (Innovative Research of America, Sarasota, FL, USA) were inserted in chloral hydrate-anesthetized (400 mg/kg i.p.) mice subcutaneously into a 0.5 cm incision made along the loose skin of the mouse’s neck (Pan et al., 1994). The pellets were inserted with tweezers into a small pocket, which was formed by bluntly dissecting caudolaterally (Subramanian et al., 2017). The incision and the closed suture (made with a wound clips) were performed under aseptic techniques to minimize the risk of infection. E2 and TX were administered as pellets for two weeks (Strom et al., 2008). MnCl2 (0.4 µl of 1 µmol/µl of MnCl2 in H2O) was administered into the right striatum by stereotaxic injection in each mouse in groups of Mn, Mn plus E2, Mn plus TX one week before animals sacrificed (Brouillet et al., 1993; Sloot et al., 1994; Zhao et al., 2009). Chloral hydrate-anesthetized (400 mg/kg, i.p.) mice from each treatment group were placed in a stereotaxic frame with a nose bar. For the stereotaxic injection, a hole was drilled and the following stereotaxic coordinates were aimed: anteroposterior (bregma) = +0.8 mm; mediolateral = +1.9 mm; dorsoventral = 3.4 mm, corresponding similar studies for right striatum and Allen Brain Atlas (Sloot et al., 1994; Zhao et al., 2009).
Open-field test
Behavioral data for total distance traveled was collected on day 21 in Seamless Open-field Activity Arenas using Activity Monitor 5 software (Med Associates, Fairfax, VT). Each animal went through an acclimation period (1 trial for 30 min, one day before injecting MnCl2 solution). On day 21, total distance traveled (cm) was recorded for groups of control, Mn, E2, Mn plus E2, TX, Mn plus TX, placebos of E2 and TX for 30 min. Data were collected in 1 min intervals over the 30 min test session.
Tissue preparation
After the open-field test, two animals per group were perfused transcardially with 4% (w/v) paraformaldehyde (PFA) in 0.1 M phosphate buffered saline (PBS) at pH 7.4 under anesthesia with chloral hydrate (400 mg/kg, i.p.). Brains were removed and prepared for cryosectioning and immunohistochemistry. For cryosectioning, brains were post-fixed in a fixative overnight and transferred into a 30% w/v sucrose solution for cryoprotection. Serial coronal sections of 30 µm thick were made with an HM525 NX Cryostat (Thermo Fisher Scientific, Waltham, MA). Coronal sections of substantia nigra, spanning from Bregma −2.90 to −3.65 mm, were prepared for immunohistochemistry.
Real-time quantitative PCR (qPCR) analysis
Tissue samples (3 samples/group) were extracted from striatal and cerebellar regions of C57BL/6 mice used in behavioral experiments. The mRNA levels of GLT-1, GLAST, and TH from these regions were analyzed. Total RNA was extracted from mouse brain tissue using RNeasy® Mini Kit (Qiagen, Valencia, CA). The purified RNA was transcribed to cDNA with high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). For real-time qPCR, the following primers were used: for GLT-1, 5’-GCC AAT ACA ACC AAG GCA GTC-3’ (forward) and 5’-TTC ATC CCG TCC TTG AAC TC-3’ (reverse); for GLAST, 5’-GAT CGG AAA CAT GAA GGA GC-3’ (forward) and 5’-CAA GAA GAG GAT GCC CAG AG-3’ (reverse); for TH, 5’-CAC TAT GCC CAC CCC CAG-3’ (forward) and 5’- CGC CGT CCA ATG AAC CTT-3’ (reverse); and for GAPDH, 5'-TCA ACG GGA AGC CCA TCA-3' (forward) and 5'-CTC GTG GTT CAC ACC CAT CA-3' (reverse). The total reaction volume (25 µl) contained 1 µl of cDNA template of each sample 0.4 µM of primers iQ™ SYBR® Green Supermix from Bio-Rad Laboratories, Inc. (Hercules, CA). The qPCR parameters were set for one cycle at 95°C for 10 min, 40 cycles at 95°C for 15 sec and 60°C for 1 min in the Bio-Rad CFX96 real-time PCR detection. GAPDH was used to normalize all samples. Data analysis was performed using the Bio-Rad CFX Manager version 3.1.
Western blot analysis
The harvested brain regions (e.g. striatal and cerebellar regions) were homogenized and used for protein extraction and further analysis. The protein concentration of homogenized tissue samples [3:1] ratio of radioimmunoprecipitation assay (RIPA) buffer with protease inhibitor cocktails over tissue sample was determined by bicinchoninic acid (BCA) assay. Ten µg of total protein per lane was run on 10% SDS-PAGE gels and transferred to nitrocellulose membrane for immunoblotting with relevant antibodies. All blots were developed using a Pierce chemiluminescence detection kit (Rockford, IL), followed by blot imaging and quantification with the Molecular Imager ChemiDoc™ XRS+ System (Bio-Rad).
Immunohistochemistry
TH expression from the coronal brain sections was analyzed in each experimental group. Briefly, frozen sections (30 µm) were washed three times with PBS for 5 min per wash. After washing, tissue sections outlined with hydrophobic PAP pen were incubated with 50 µL of blocking solution (PBS containing 0.01% Triton X100 and 2% normal goat serum) for 1 h at room temperature. Then, incubation of primary antibody was carried out overnight at 4 °C with TH antibody at 1:200 dilution. Tissues were washed three times with PBS for 5 min per wash. Then, sections were incubated with goat anti-mouse IgG Alexa Fluor® 568 secondary antibody in blocking solution in a light-protected humidity chamber for 1 h at room temperature. Tissues were then washed three times with PBS and once with ddH2O, followed by air drying. After air drying, coverslips were mounted onto glass slides with VectaShield® mounting medium with DAPI staining (Aqua-Poly/Mount, Polysciences, Inc). High-resolution diascopic and epi-fluorescence imaging were performed using Nikon Eclipse Ts2R-FL equipped with DS-Qi2 high-definition camera (Melville, NY).
Assays of catalase activity, glutathione level and lipid peroxidation
CAT activity, GSH levels, and lipid peroxidation were measured according to the manufacturer’s protocols. Endpoint product fluorescence was measured in each assay using the Spectramax® i3x Multi-Mode microplate reader from Molecular Devices (Sunnyvale, CA). Briefly, the harvested brain tissues were extracted with mammalian cell lysis buffer and further analyzed using the assay kits. CAT activity was calculated as the rate of breakdown in hydrogen peroxide (H2O2). OxiRed™ Probe reacts with unconverted H2O2 and measured at excitation/emission wavelength of 535/587 nm in the fluorescence plate reader. GSH levels were measured as the amount of o-phthalaldehyde (OPA) probe that reacts with GSH resulting in generation of fluorescence, which was determined at excitation/emission wavelength of 360/460 nm in the fluorescence plate reader using GSH standard. Lipid peroxidation was assessed in tissue homogenates by the measurement of MDA, an end-product of lipid peroxidation, which reacts with thiobarbituric acid to form a complex. The reaction product fluorescence was determined at excitation/emission wavelength of 530/550 nm in the fluorescence plate reader using MDA standard curve.
Statistical analysis
All data were expressed as the mean ± standard error of the mean (SEM). Multiple comparisons analyses were performed using a two-way analysis of variance (ANOVA) followed by Tukey's posthoc tests using the GraphPad Prism Software version 6.0 (San Diego, CA). A p-value less than 0.05 (p<0.05) was considered statistically significant.
Results
E2 and TX attenuate Mn-induced motor deficits
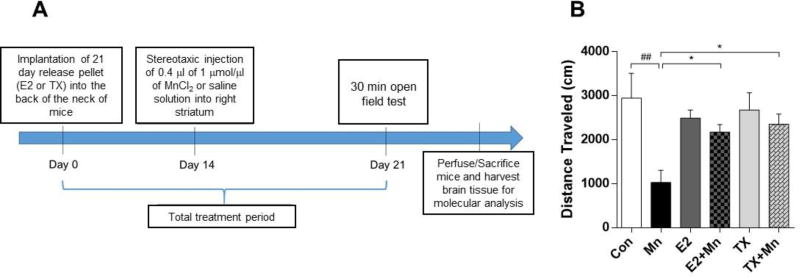
The ovariectomized C57BL/6 mice were implanted with E2- or TX-containing pellets in a slow-release mode for 21 days to eliminate the variation of E2 levels by menstrual cycles in mice. Mn (0.4 µl of 1 µmol/µl) was directly injected into the right striatum of mice (Brouillet et al., 1993) two weeks after pellets of E2 or TX implanted in the back of the neck of mice (Khan et al., 2015) (Fig. 1A). The general health status of the animals after the pellet implantation of E2 and TX, as well as Mn administration were well tolerated. All animals remained healthy during the behavioral studies and throughout the period prior to tissue collection. The placebo groups for E2 and TX showed no significant differences compared to the control sham group, and therefore, all data derived from the placebo groups are not shown. One week after a single injection of Mn into the striatum, open field locomotor activity was measured as total distances traveled in centimeters (cm). As shown in Fig. 1B, total distance traveled was significantly decreased in the Mn-exposed mice compared to the control group (p<0.01), whereas both the E2 plus Mn (p<0.05) and TX plus Mn (p<0.05) groups showed significant attenuation of the Mn-induced decrease in locomotor activity. Locomotor activity in the E2 and TX groups was indistinguishable from the control sham group.
Fig. 1.
Effects of E2 and TX on Mn-induced decrease in locomotor activity in mice. (A) Experimental paradigm. C57BL/6 ovariectomized female mice were implanted with E2 or TX pellets in the back of the neck of mice prior to striatal administration of Mn in the right side of mouse brain as described in the Methods section. (B) Locomotor activity was assessed in an open-field on 21 days after treatment of E2, TX, and Mn. Total distances traveled were measured in each group of mice. ##, p<0.01; *, p<0.05 (two-way ANOVA followed by Tukey’s post hoc test; n = 8).
E2 and TX attenuate Mn-induced loss of dopaminergic neurons in the substantia nigra of the mouse brain
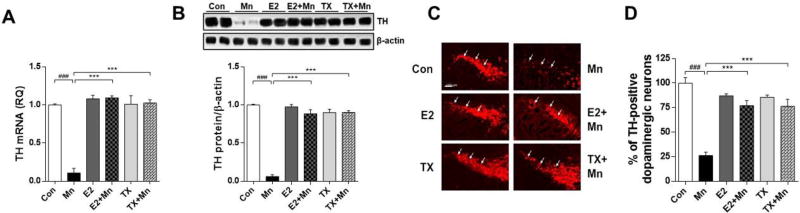
We examined whether dopaminergic dysregulation might mediate the Mn-induced motor deficits. Expression of TH as a dopaminergic neuronal marker in the substantia nigra were assessed (Guilarte, 2010; Vidal et al., 2005; Zhang et al., 2011; Zhao et al., 2009). As shown in Fig. 2A and 2B, Mn significantly decreased striatal TH mRNA (p<0.001) and protein (p<0.001) levels compared to the control group. E2 and TX reversed the Mn-induced reduction in TH mRNA and protein levels. Moreover, Mn significantly decreased the number of nigral TH-positive dopaminergic neurons in the ipsilateral side of the injection, while E2 and TX significantly attenuated the Mn-induced reduction (p<0.001) in dopaminergic neuron (Fig. 2C and 2D). E2 or TX alone did not alter the number of dopaminergic neurons compared with the control group. We also assessed whether striatal Mn injection influences on TH mRNA and proteins levels in the cerebellum to determine if these effects occur in other regions, and found that the effects were similar to those in striatum (data not shown).
Fig. 2.
Effects of E2 and TX on Mn-induced decrease in TH protein levels and dopaminergic neurons in the mouse brain. (A), (B) At the end of treatment with Mn, E2, and TX, striatal tissues were isolated from the ipsilateral side of mouse brain from each experimental group as described in the Methods section. Mouse brain tissues were analyzed for TH mRNA (A) and protein (B) levels. GAPDH and β-actin were used for loading controls of mRNA and protein, respectively. ###, p<0.001; ***, p<0.001 (two-way ANOVA followed by Tukey’s post hoc test; n = 3). (C) Coronal sections were used for immunohistochemistry to assess the level of TH-positive dopaminergic neurons in the substantia nigra pars compacta (white arrows), as described in the Methods section. Images are captured under 10× magnification (scale bar = 100 µm). (D) Quantification of TH-positive dopaminergic neurons are shown as a percentage of the control. The imaging data are representatives of 6 samples in each group and derived from two animals.
E2 and TX attenuate Mn-induced decrease in mRNA/protein levels of GLAST and GLT-1 in the mouse brain
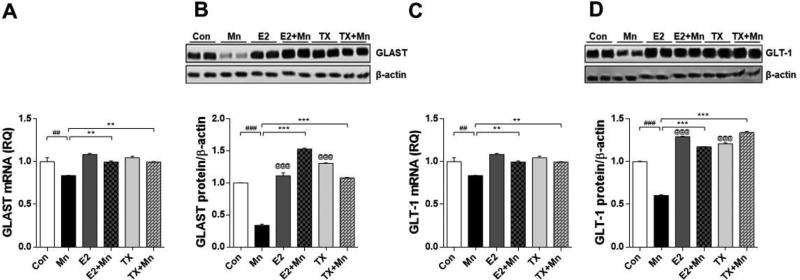
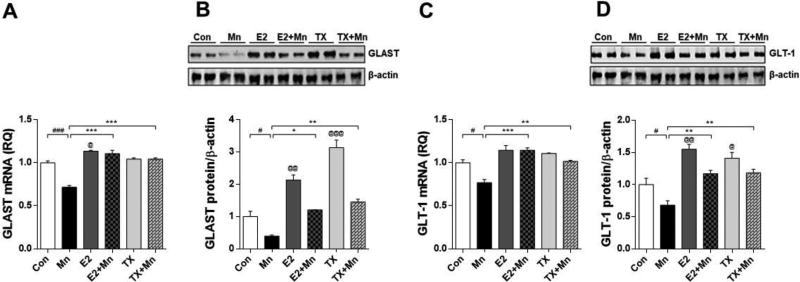
Next, we determined whether Mn decreased the expression of the astrocytic transporters (GLAST and GLT-1) concomitant with the dopaminergic toxicity as these transporters are dysregulated by Mn and directly associated with excitotoxic neuronal injury. Mn reduced GLAST and GLT-1 mRNA and protein levels in the striatal region where dopaminergic nerve terminals are located and Mn preferentially localized, compared to the control group (p<0.01), and both E2 and TX attenuated these effects (Fig. 3, p<0.01). E2 and TX alone significantly increased GLAST/GLT-1 protein levels (p<0.001) in the striatum, but GLAST/GLT-1 mRNA levels were indistinguishable from controls. Cerebellar regions were also examined to determine if Mn affected other brain regions secondary due to its diffusion from the injection site. Notably, one week after injection, Mn significantly decreased both GLAST and GLT-1 mRNA and protein levels in the cerebellum (p<0.05), while E2 and TX significantly attenuated this Mn effect (Fig. 4, p<0.05). Analogous to the striatal observations, E2 and TX alone significantly increased cerebellar GLAST (p<0.01) as well as GLT-1 protein levels (p<0.05), but no changes in GLAST/GLT-1 mRNA levels.
Fig. 3.
Effects of E2 and TX on Mn-induced decrease of GLAST and GLT-1 in the mouse striatum. At the end of treatment with Mn, E2, and TX, striatal tissues were isolated from the ipsilateral side of the brain from each experimental group. Tissues were analyzed for GLAST (A and B) and GLT-1 (C and D) mRNA and protein levels as described in the Methods section. GAPDH and β-actin were used for loading controls of mRNA and protein, respectively. ##, p<0.01; ###, p<0.001; **, p<0.01; ***, p<0.001; @, p<0.001 compared to control (two-way ANOVA followed by Tukey’s post hoc test; n = 3).
Fig. 4.
Effects of E2 and TX on Mn-induced decrease of GLAST and GLT-1 in the cerebellar region of the mouse brain. At the end of treatment with Mn, E2, and TX, cerebellar tissues were prepared from the mouse brain as described in the Methods section. Tissues were analyzed for mRNA and protein levels of GLAST (A and B) and GLT-1 (C and D). GAPDH and β-actin were used as loading controls of mRNA and protein, respectively. #, p<0.05; ###, p<0.001; *, p<0.05; **, p<0.01; ***, p<0.001; @, p<0.05;, p<0.01; @, p<0.001 compared to control (two-way ANOVA followed by Tukey’s post hoc test; n = 3).
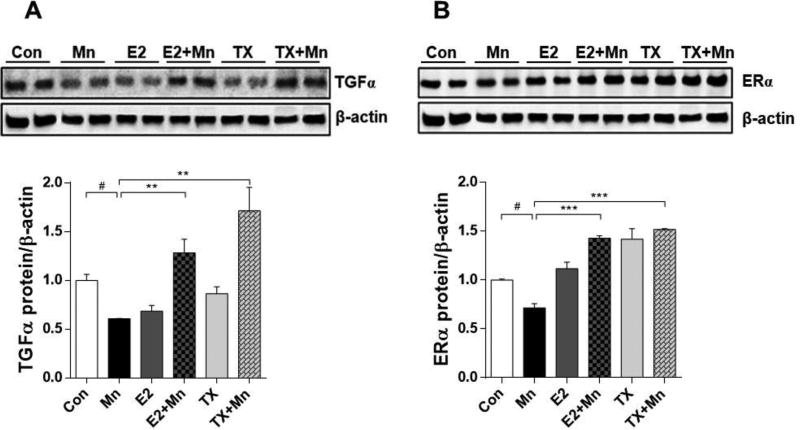
E2 and TX reverse the Mn-induced decrease in protein levels of TGF-α and ER-α
Previously we have shown that both E2 and TX reversed Mn-reduced GLT-1 expression and function via TGF-α in in vitro astrocytes (Lee et al., 2012). Thus, we tested if TGF-α protein levels are recapitulated in vivo as well. The results showed that Mn decreased TGF-α expression in the mouse brain (p<0.05), while co-treatment with E2 and TX reversed the Mn-reduced TGF-α protein levels (p<0.01, Fig. 5A), similar to those in in vitro study. Interestingly, E2 and TX alone decreased TGF-α protein levels in this experimental paradigm, indicating that TGF-α levels are dynamic and unsteady during the course of E2/TX treatment. Mn also decreased ER-α protein levels (p<0.05), while E2 and TX attenuated this Mn effect (p<0.001, Fig. 5B).
Fig. 5.
Effects of E2 and TX on Mn-induced decrease of TGF-α and ER-α in the mouse brain. At the end of treatment with Mn, E2, and TX, brain tissues were analyzed for TGF-α (A) and ER-α (B) protein levels. β-actin was used as a loading control. #, p<0.05; **, p<0.01; ***, p<0.001 (two-way ANOVA followed by Tukey’s post hoc test; n = 3).
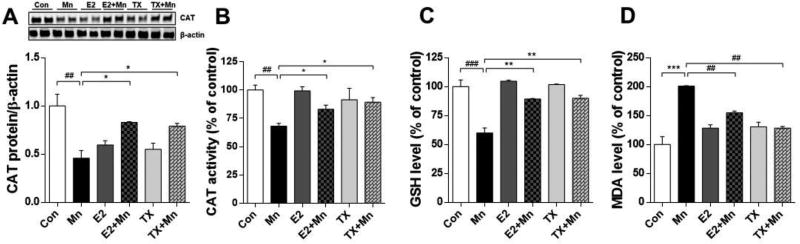
E2 and TX reverse Mn-induced oxidative stress
It is well established that Mn induces neurotoxicity via oxidative stress (Avila et al., 2008; Dobson et al., 2003; Erikson et al., 2004; Santos et al., 2012). E2 and TX are known to exert neuroprotection via anti-oxidant mechanisms by ER-dependent as well as ER-independent pathways (Abdelhamid et al., 2011; Colon and Miranda, 2016; D'Astous et al., 2004; Wang et al., 2015; Zhang et al., 2007; Zhu et al., 2015; Zou et al., 2015). Accordingly, we tested if E2/TX-attenuation of Mn-induced neurotoxicity was mediated via antioxidant mechanism. We have conducted several biochemical assays to determine the involvement of oxidative stress and antioxidant mechanisms in this process. We found that Mn decreased CAT protein levels and activity (p<0.01), while E2 and TX reversed this effect (Fig. 6A and 6B). Mn reduced GSH levels (p<0.001), which were reversed by E2 and TX (Fig. 6C). We also measured MDA, a product of lipid peroxidation (Romero et al., 1998). Mn increased brain MDA levels (p<0.001), while E2 and TX reversed this effect (Fig. 6D). These findings indicate that the effects of Mn on oxidative stress were attenuated by E2 and TX treatment. We also found that E2 and TX alone decreased CAT protein levels, although CAT activity remained unchanged from the control group, indicating other mechanisms involved in E2/TX regulation on CAT protein expression.
Fig. 6.
Effects of E2 and TX on Mn-induced oxidative stress in the mouse brain. At the end of treatment with Mn, E2, and TX, tissue samples were prepared from the mouse brain as described in the Methods section. Tissues were analyzed for CAT protein levels (A) and CAT activity (B), GSH levels (C) and MDA levels (D). ##, p<0.01; ###, p<0.001; * p<0.05; **, p<0.01; ***, p<0.001 (two-way ANOVA followed by Tukey’s post hoc test; n = 3).
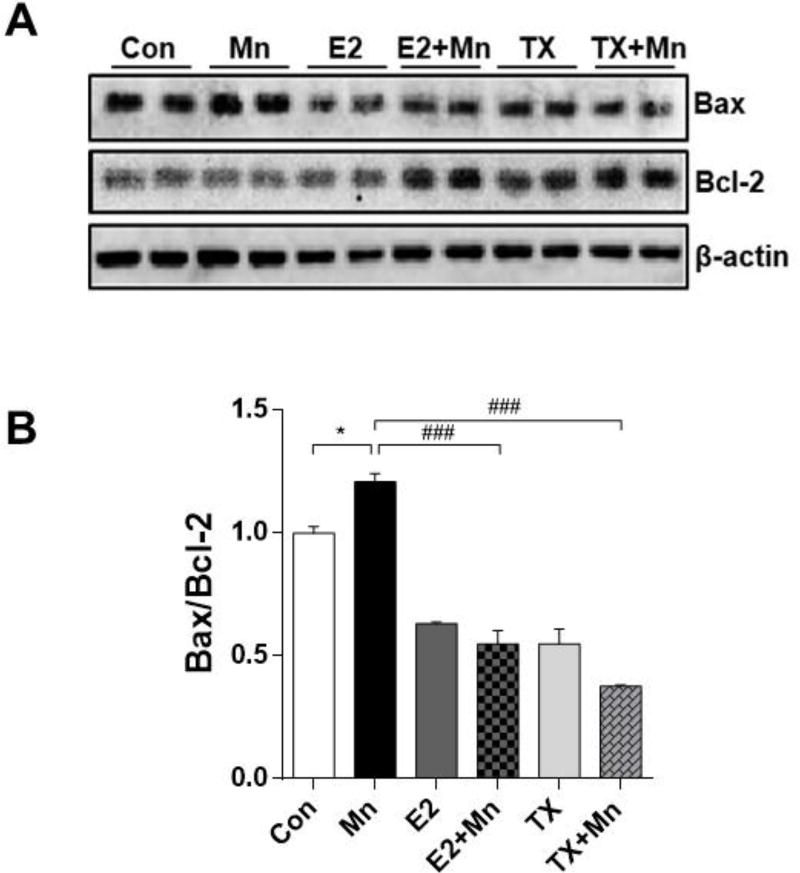
E2 and TX attenuate Mn-induced apoptosis
Since Mn induced dopaminergic neuronal injury as shown by reduced TH protein levels, next, we examined if apoptotic signals were involved in Mn-induced dopaminergic neuronal toxicity, and if E2/TX could attenuate this effect. We measured protein levels of Bax and Bcl-2 which are pro- and anti-apoptotic markers, respectively. Mn increased Bax and decreased Bcl-2 protein levels compared to the control (Fig. 7A), resulting in a significant increase of the ratio of Bax/Bcl-2 (p<0.05, Fig. 7B), which is commonly used to determine cell susceptibility to apoptosis (Khodapasand et al., 2015). In contrast, the Mn plus E2 and TX groups showed decreased Bax and increased Bcl-2 protein levels (Fig. 7A), and a significant decrease in the ratio of Bax/Bcl-2 compared to the Mn group alone (p<0.001, Fig. 7B).
Fig. 7.
Effects of E2 and TX on Mn-induced apoptosis in the mouse brain. At the end of treatment with Mn, E2, and TX, tissue samples were prepared from the mouse brain as described in the Methods section. Tissues were analyzed for pro-apoptotic Bax and antiapoptotic Bcl-2 protein (A), and the levels of Bax/Bcl-2 ratio (B). β-actin was used as a loading control. ###, p<0.001; *, p<0.05 (two-way ANOVA followed by Tukey’s post hoc test; n = 3).
Discussion
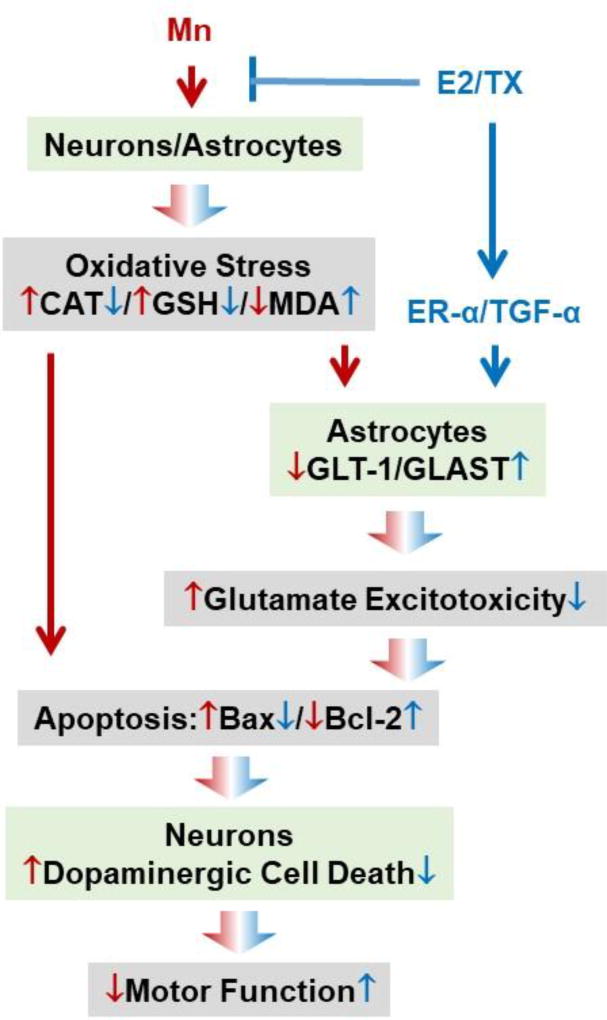
The present study demonstrates the in vivo efficacy of E2 and TX in attenuating dopaminergic neuronal injury in the substantia nigra of ovariectomized mice striatally injected with Mn. Exposure to Mn reduces mRNA/protein levels of GLT-1 and /GLAST, while E2 and TX reversed these Mn effects in the mouse brain. E2 and TX also attenuated Mn-induced oxidative stress and apoptosis. All these findings were summarized in Fig. 8. Our findings impart new information on potential strategies to therapeutically mitigate in vivo Mn-induced neurotoxicity by means of estrogenic compounds and neuroSERMs.
Fig. 8.
Schematic diagram of the proposed mechanism of neuroprotective effects of E2/TX against Mn-induced dopaminergic neurotoxicity in the mouse brain. E2 and TX increase expression of astrocytic glutamate transporters GLT-1/GLAST, and reverse Mn-reduced GLT-1/GLAST. E2/TX also attenuate Mn-induced oxidative stress by reversing Mn-reduced CAT protein levels, CAT activity and GSH levels, as well as reversing Mn-induced lipid peroxidation. These events may lead to impairment of GLT-1/GLAST as well as apoptosis, which might be directly associated with dopaminergic cell death and impairment of motor function. ↓↑ Mn effects, ↓⊥ E2/TX effects.
Although many studies have reported that E2 and TX exerted neuroprotective effects in several disease models with various mechanisms, this is the first report that E2 and TX induce neuroprotection against Mn-induced dopaminergic neurotoxicity in parallel with impairment of astrocytic glutamate transporters (GLT-1/GLAST) and oxidative stress in vivo mouse model. The findings suggest that Mn injection into the striatum can diffuse into other regions of the mouse brain as cerebellar regions also show that Mn decreased GLT-1/GLAST mRNA/protein levels, while E2/TX reversed these Mn effects. Cerebellum as one of two extrapyramidal motor systems along with basal ganglia critically regulates motor functions in the brain. Moreover, dopaminergic neurons are known to be innervated and functional in this region (Melchitzky and Lewis, 2000). This region may also contribute to E2/TX attenuation of Mn-induced motor function deficits.
It is well established that E2 exerts neuroprotection in various disease models, but its adverse effects from chronic treatment hampered its clinical usage. Accordingly, developing ideal neuroSERMs which induce neuroprotection only in the brain has been proposed (Zhao et al., 2005). To be able to achieve this goal, understanding mechanisms of SERMs-induced neuroprotection is imperative. Our findings that TX exerts neuroprotection is corroborating with many previously reported neurotoxicity models, including methamphetamine-induced dopaminergic neurotoxicity in mice (Dluzen et al., 2001). TX exerts neuroprotection not only via ER-dependent mechanisms, but also the ER-independent mechanisms as shown that TX induced neuroprotection by activating antioxidant mechanisms in the methamphetamine toxicity mouse model (Kuo et al., 2003), ischemic rat model (Zhang et al., 2007), and the spinal cord injury rat model (Salgado et al., 2015).
Astrocytes appear to play a critical role in mediating neuroprotective effects of E2 and TX in numerous experimental models (Dhandapani and Brann, 2003; Spence et al., 2011; Spence et al., 2013), implicating that astrocytic mechanism might be involved in E2/TX-induced protection against Mn neurotoxicity in the present in vivo study. The impairment of astrocytic GLT-1/GLAST is implicated in many excitotoxic neurodegenerative diseases (Jayakumar and Norenberg, 2016; Johnson et al., 2017; Karki et al., 2015). The importance of GLT-1 on dopaminergic neuronal survival is evidenced by the previous study that MPTP-induced dopaminergic neurodegeneration coincides with reduced GLT-1 protein levels and is reversed by ceftriaxone, a GLT-1 enhancer (Hsu et al., 2015). E2 and TX attenuated Mn-reduced expression and function of GLAST and GLT-1 in astrocyte cultures (Lee et al., 2009). The findings from the present study that E2 and TX also prevented Mn-induced dopaminergic neurodegeneration, might critically involve astrocytic GLT-1/GLAST mechanisms. Given that astrocytic glutamate transporters are critically regulating excitotoxic neuronal death, numerous studies demonstrated that SERMs exerted neuroprotection against excitotoxicity. The SERMs such as TX, RX and bazedoxifene prevented hippocampal neuronal damage induced by kainic acid in ovariectomized rats (Ciriza et al., 2004). RX induced neuroprotection against kainic acid-induced excitotoxicity along with oxidative stress in the rat brain cortex (Armagan et al., 2009). TX exerted neuroprotection against glutamate-induced excitotoxicity in rat primary neuronal cultures (O'Neill et al., 2004). TX induced neuroprotection against AMPA/NMDA receptor-mediated excitotoxicity caused by oxygen/glucose deprivation in ischemic rat brain (Zhang et al., 2009).
TGF-α might be an important mediator of E2 and TX protection against Mn-induced neurotoxicity. Our findings revealed that both attenuated Mn-reduced TGF-α protein levels in the mouse brain, corroborating with our previous in vitro studies (Karki et al., 2014b; Lee et al., 2012). Interestingly, E2 or TX alone downregulated TGF-α protein at three weeks of treatment. This indicates that E2/TX might regulate TGF-α expression dynamically rather than a steady expression pattern during the course of treatment. Nonetheless, under Mn exposure, E2 and TX clearly reversed Mn-reduced TGF-α levels, suggesting that E2 and TX might activate other mechanisms to increase TGF-α expression and protect tissues from the toxic insults such as Mn. Further investigation is warranted to better delineate the role of E2 and TX in modulating TGF-α protein expression in vivo with/without toxic insults. Modulation of ER-α protein levels by Mn as well as E2 and TX implicates the importance of ER-α in affording neuroprotection. ER-α has been shown to mediate the neuroprotective effects of E2 in experimental models, such as MPTP-induced PD (D'Astous et al., 2004). Although the mechanism of Mn-decreased ER-α protein expression is yet to be determined, our findings suggest that ER-α is modulated in the effects of E2 and TX on Mn-induced toxicity.
Mn-induced neuronal injuries are closely associated with oxidative stress (Avila et al., 2008; Dobson et al., 2003; Erikson et al., 2004; Milatovic et al., 2011; Santos et al., 2012; Yoon et al., 2011; Zhao et al., 2009). Our findings that E2 and TX attenuated Mn-induced reduction in CAT expression and activity indicates that free-radical scavenging activity might be involved in E2 and TX-induced neuroprotection. E2 and TX exert anti-oxidative effects in various neurological disorders such as spinal cord injury (Colon and Miranda, 2016) and brain ischemia (Zhang et al., 2007; Zou et al., 2015). Notably, E2 and TX alone reduced CAT protein levels, but CAT activity was unchanged from the control, possibly due to in vivo complexity of E2/TX effects. E2 and TX also reversed the Mn-reduced GSH levels, a vital intracellular and extracellular protective antioxidant, detoxifying certain xenobiotics and heavy metals. Lipid peroxidation as determined by MDA levels is another important oxidative stress indicator for Mn toxicity (Avila et al., 2008). Our findings that Mn-increased MDA, which was reversed by E2 and TX clearly indicate that oxidative stress mechanisms play a role in E2- and TX-protection against Mn toxicity. These antioxidative effects of E2 and TX could be ER-dependent (Lee et al., 2008) or ER– independent mechanisms (Zhang et al., 2007).
Apoptotic signaling also appears to be involved in the E2- and TX-afforded attenuation of Mn-induced toxicity. The anti-apoptotic Bcl-2 is a crucial regulator of mitochondrial-mediated apoptotic cell death, inducing neuroprotection against neurotoxic insults (Nilsen et al., 2006; Sun et al., 2012; Zhou et al., 2011), while the pro-apoptotic Bax promotes apoptosis by facilitating cytochrome c release from mitochondria (Kazi et al., 2011; Sun et al., 2012). Thus, a balance between Bax and Bcl-2 can determine the cellular fate, such as apoptosis. Our findings that E2 and TX attenuated Mn-increased ratio of Bax to Bcl-2 suggest that apoptotic signaling is involved in this effect, corroborating previous studies that E2 and TX possess anti-apoptotic activity in various neuronal injury models, including ischemia and AD (Miller et al., 2005; Nilsen et al., 2006; Sawada et al., 2000; Zou et al., 2015).
Taken together, the results from the current study demonstrate that E2 and TX attenuate Mn-induced dopaminergic neurotoxicity and movement deficits in mice possibly by several mechanisms including the modulation of astrocytic glutamate transporters (GLT-1/GLAST) and oxidative stress. We summarized the proposed mechanisms involved in this E2/TX protection against Mn-induced neurotoxicity in Fig. 8. Our findings provide novel information on development of neuroSERMs against Mn neurotoxicity as well as other neurological disorders associated with impairment of GLT-1/GLAST and oxidative stress.
Highlights.
17β-estradiol (E2) attenuates manganese (Mn)-induced loss of tyrosine hydroxylase (TH) expression in mouse brain
Tamoxifen (TX) also reverses Mn-induced motor deficits and TH expression
E2 and TX attenuate Mn-induced reduction of astrocytic glutamate transporters (GLT-1/GLAST)
Acknowledgments
The present study was supported in part by R01 ES024756 (EL), SC1 089630(EL), SC1 CA200519 (DS), R01 ES10563 (MA), 1R03 ES024849 (MA) and 1R21 ES025415 (MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- Abdelhamid R, Luo J, Vandevrede L, Kundu I, Michalsen B, Litosh VA, Schiefer IT, Gherezghiher T, Yao P, Qin Z, Thatcher GR. Benzothiophene Selective Estrogen Receptor Modulators Provide Neuroprotection by a novel GPR30-dependent Mechanism. ACS Chem Neurosci. 2011;2(5):256–268. doi: 10.1021/cn100106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armagan G, Kanit L, Terek CM, Sozmen EY, Yalcin A. The levels of glutathione and nitrite-nitrate and the expression of Bcl-2 mRNA in ovariectomized rats treated by raloxifene against kainic acid. Int J Neurosci. 2009;119(2):227–239. doi: 10.1080/00207450802330959. [DOI] [PubMed] [Google Scholar]
- Avila DS, Gubert P, Fachinetto R, Wagner C, Aschner M, Rocha JB, Soares FA. Involvement of striatal lipid peroxidation and inhibition of calcium influx into brain slices in neurobehavioral alterations in a rat model of short-term oral exposure to manganese. Neurotoxicology. 2008;29(6):1062–1068. doi: 10.1016/j.neuro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5(1):13–35. [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 2009;30(2):142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120(1):89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44(9):1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Azcoitia I, Lundeen SG, Garcia-Segura LM. Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J Neurobiol. 2004;61(2):209–221. doi: 10.1002/neu.20043. [DOI] [PubMed] [Google Scholar]
- Colon JM, Miranda JD. Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res. 2016;11(8):1208–1211. doi: 10.4103/1673-5374.189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Calon F, Morissette M, Di Paolo T. Estrogenic modulation of brain activity: implications for schizophrenia and Parkinson's disease. J Psychiatry Neurosci. 2002;27(1):12–27. [PMC free article] [PubMed] [Google Scholar]
- D'Astous M, Morissette M, Di Paolo T. Effect of estrogen receptor agonists treatment in MPTP mice: evidence of neuroprotection by an ER alpha agonist. Neuropharmacology. 2004;47(8):1180–1188. doi: 10.1016/j.neuropharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dhandapani K, Brann D. Neuroprotective effects of estrogen and tamoxifen in vitro: a facilitative role for glia? Endocrine. 2003;21(1):59–66. doi: 10.1385/endo:21:1:59. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL, Anderson LI. Tamoxifen diminishes methamphetamine-induced striatal dopamine depletion in intact female and male mice. J Neuroendocrinol. 2001;13(7):618–624. doi: 10.1046/j.1365-2826.2001.00675.x. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Weber S, Dorman DC, Lash LK, Erikson KM, Aschner M. Oxidative stress is induced in the rat brain following repeated inhalation exposure to manganese sulfate. Biol Trace Elem Res. 2003;93(1–3):113–126. doi: 10.1385/BTER:93:1-3:113. [DOI] [PubMed] [Google Scholar]
- Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23(4–5):595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52(1):6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Gillessen T, Budd SL, Lipton SA. Excitatory amino acid neurotoxicity. Adv Exp Med Biol. 2002;513:3–40. doi: 10.1007/978-1-4615-0123-7_1. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect. 2010;118(8):1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Hung CS, Chang HM, Liao WC, Ho SC, Ho YJ. Ceftriaxone prevents and reverses behavioral and neuronal deficits in an MPTP-induced animal model of Parkinson's disease dementia. Neuropharmacology. 2015;91:43–56. doi: 10.1016/j.neuropharm.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Norenberg MD. Glutamine Synthetase: Role in Neurological Disorders. Adv Neurobiol. 2016;13:327–350. doi: 10.1007/978-3-319-45096-4_13. [DOI] [PubMed] [Google Scholar]
- Johnson J, Jr, Pajarillo EAB, Taka E, Reams R, Son DS, Aschner M, Lee E. Valproate and sodium butyrate attenuate manganese-decreased locomotor activity and astrocytic glutamate transporters expression in mice. Neurotoxicology. 2017 doi: 10.1016/j.neuro.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Kim C, Smith K, Son DS, Aschner M, Lee E. Transcriptional Regulation of the Astrocytic Excitatory Amino Acid Transporter 1 (EAAT1) via NF-kappaB and Yin Yang 1 (YY1) J Biol Chem. 2015;290(39):23725–23737. doi: 10.1074/jbc.M115.649327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Johnson J, Jr, Lee K, Son DS, Aschner M, Lee E. Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol Cell Biol. 2014a;34(7):1280–1289. doi: 10.1128/MCB.01176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia. 2014b;62(8):1270–1283. doi: 10.1002/glia.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi A, Sun J, Doi K, Sung SS, Takahashi Y, Yin H, Rodriguez JM, Becerril J, Berndt N, Hamilton AD, Wang HG, Sebti SM. The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J Biol Chem. 2011;286(11):9382–9392. doi: 10.1074/jbc.M110.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, Clegg MS. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20(2–3):213–223. [PubMed] [Google Scholar]
- Khan MM, Wakade C, de Sevilla L, Brann DW. Selective estrogen receptor modulators (SERMs) enhance neurogenesis and spine density following focal cerebral ischemia. J Steroid Biochem Mol Biol. 2015;146:38–47. doi: 10.1016/j.jsbmb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19(2):69–75. doi: 10.6091/ibj.1366.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226(10):2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Chen HH, Shieh CC, Chuang KP, Cherng CG, Yu L. 4-Hydroxytamoxifen attenuates methamphetamine-induced nigrostriatal dopaminergic toxicity in intact and gonadetomized mice. J Neurochem. 2003;87(6):1436–1443. doi: 10.1046/j.1471-4159.2003.02089.x. [DOI] [PubMed] [Google Scholar]
- Lee E, Karki P, Johnson J, Jr, Hong P, Aschner M. Manganese Control of Glutamate Transporters' Gene Expression. Adv Neurobiol. 2017;16:1–12. doi: 10.1007/978-3-319-55769-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Yin Z, Webb A, Son DS, Aschner M. Transforming growth factor-alpha mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes. Glia. 2012;60(7):1024–1036. doi: 10.1002/glia.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem. 2009;110(2):530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Jung SC, Lee JH, Han HJ. Estradiol-17beta protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res. 2008;18(4):491–499. doi: 10.1038/cr.2008.42. [DOI] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Gavin CE, Aschner M, Gunter TE. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med. 2013;62:65–75. doi: 10.1016/j.freeradbiomed.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in the primate cerebellum. Evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology. 2000;22(5):466–472. doi: 10.1016/S0893-133X(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Mendelowitsch A, Ritz MF, Ros J, Langemann H, Gratzl O. 17beta-Estradiol reduces cortical lesion size in the glutamate excitotoxicity model by enhancing extracellular lactate: a new neuroprotective pathway. Brain Res. 2001;901(1–2):230–236. doi: 10.1016/s0006-8993(01)02359-9. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Montine TJ, Aschner M. Measurement of isoprostanes as markers of oxidative stress. Methods Mol Biol. 2011;758:195–204. doi: 10.1007/978-1-61779-170-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146(7):3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Natrajan PK, Gambrell RD., Jr Estrogen replacement therapy in patients with early breast cancer. Am J Obstet Gynecol. 2002;187(2):289–294. doi: 10.1067/mob.2002.125999. discussion 294–285. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill K, Chen S, Diaz Brinton R. Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer's disease. Exp Neurol. 2004;188(2):268–278. doi: 10.1016/j.expneurol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Pan WH, Chen NH, Lai YJ, Luoh HF. Differential effects of chloral hydrate and pentobarbital sodium on cocaine-induced electroencephalographic desynchronization at the medial prefrontal cortex in rats. Life Sci. 1994;54(23):PL419–424. doi: 10.1016/0024-3205(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Pansiot J, Pham H, Dalous J, Chevenne D, Colella M, Schwendimann L, Fafouri A, Mairesse J, Moretti R, Schang AL, Charriaut-Marlangue C, Gressens P, Baud O. Glial response to 17beta-estradiol in neonatal rats with excitotoxic brain injury. Exp Neurol. 2016;282:56–65. doi: 10.1016/j.expneurol.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Liu X, Menniti FS. Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology. 2003;77(4):223–231. doi: 10.1159/000070277. [DOI] [PubMed] [Google Scholar]
- Romero FJ, Bosch-Morell F, Romero MJ, Jareno EJ, Romero B, Marin N, Roma J. Lipid peroxidation products and antioxidants in human disease. Environ Health Perspect. 1998;106(Suppl 5):1229–1234. doi: 10.1289/ehp.98106s51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado IK, Torrado AI, Santiago JM, Miranda JD. Tamoxifen and Src kinase inhibitors as neuroprotective/neuroregenerative drugs after spinal cord injury. Neural Regen Res. 2015;10(3):385–390. doi: 10.4103/1673-5374.153685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Marreilha dos Santos AP. The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology. 2012;292(2–3):90–98. doi: 10.1016/j.tox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D, Sugiyama N, Yonekubo S, Pawlak A, Murasawa H, Nakamura M, Hayashi M, Ogawa T, Moro M, Washizuka S, Amano N, Hongo K, Ohnota H. Novel oestrogen receptor beta-selective ligand reduces obesity and depressive-like behaviour in ovariectomized mice. Sci Rep. 2017;7(1):4663. doi: 10.1038/s41598-017-04946-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Honda K, Nakanishi M, Akaike A, Shimohama S. Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. FASEB J. 2000;14(9):1202–1214. doi: 10.1096/fasebj.14.9.1202. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51(6–7):333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee E, Albrecht J, Aschner M. Manganese disrupts astrocyte glutamine transporter expression and function. J Neurochem. 2009;110(3):822–830. doi: 10.1111/j.1471-4159.2009.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JB. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J Neurochem. 1994;62(1):205–216. doi: 10.1046/j.1471-4159.1994.62010205.x. [DOI] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108(21):8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, Itoh N, Sofroniew MV, Voskuhl RR. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J Neurosci. 2013;33(26):10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom JO, Theodorsson E, Theodorsson A. Order of magnitude differences between methods for maintaining physiological 17beta-oestradiol concentrations in ovariectomized rats. Scand J Clin Lab Invest. 2008;68(8):814–822. doi: 10.1080/00365510802409703. [DOI] [PubMed] [Google Scholar]
- Subramanian M, MohanKumar SM, Balasubramanian P, Northcott CA, Garver H, Fink GD, MohanKumar PS. Chronic exposure to low doses of estradiol-17ss increases blood pressure in young female rats: A possible role for central Endothelin-1. Sci Rep. 2017;7(1):139. doi: 10.1038/s41598-017-00213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Ma X, Yang H, Zhao J, Zhang J. Brain-derived neurotrophic factor prevents beta- amyloid-induced apoptosis of pheochromocytoma cells by regulating Bax/Bcl-2 expression. Neural Regen Res. 2012;7(5):347–351. doi: 10.3969/j.issn.1673-5374.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal L, Alfonso M, Campos F, Faro LR, Cervantes RC, Duran R. Effects of manganese on extracellular levels of dopamine in rat striatum: an analysis in vivo by brain microdialysis. Neurochem Res. 2005;30(9):1147–1154. doi: 10.1007/s11064-005-7775-6. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang B, Feng Y, Mo M, Du F, Li H, Yu X. 17beta-estradiol ameliorates light-induced retinal damage in Sprague-Dawley rats by reducing oxidative stress. J Mol Neurosci. 2015;55(1):141–151. doi: 10.1007/s12031-014-0384-6. [DOI] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. 2004;75(4):637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Kim DS, Lee GH, Kim KW, Kim HR, Chae HJ. Apoptosis Induced by Manganese on Neuronal SK-N-MC Cell Line: Endoplasmic Reticulum (ER) Stress and Mitochondria Dysfunction. Environ Health Toxicol. 2011;26:e2011017. doi: 10.5620/eht.2011.26.e2011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Anantharam V, Kanthasamy A. Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol Appl Pharmacol. 2011;254(2):65–71. doi: 10.1016/j.taap.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xie M, Schools GP, Feustel PF, Wang W, Lei T, Kimelberg HK, Zhou M. Tamoxifen mediated estrogen receptor activation protects against early impairment of hippocampal neuron excitability in an oxygen/glucose deprivation brain slice ischemia model. Brain Res. 2009;1247:196–211. doi: 10.1016/j.brainres.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK. Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurol. 2007;204(2):819–827. doi: 10.1016/j.expneurol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci. 2009;107(1):156–164. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]
- Zhao L, O'Neill K, Diaz Brinton R. Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res Brain Res Rev. 2005;49(3):472–493. doi: 10.1016/j.brainresrev.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278(3):403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang S, Wang B, Du F, Hu C, Li H, Feng Y, Zhu R, Mo M, Cao Y, Li A, Yu X. 17beta-Estradiol up-regulates Nrf2 via PI3K/AKT and estrogen receptor signaling pathways to suppress light-induced degeneration in rat retina. Neuroscience. 2015;304:328–339. doi: 10.1016/j.neuroscience.2015.07.057. [DOI] [PubMed] [Google Scholar]
- Zou W, Fang C, Ji X, Liang X, Liu Y, Han C, Huang L, Zhang Q, Li H, Zhang Y, Liu J, Liu J. Estrogen Receptor (ER)-alpha36 Is Involved in Estrogen-and Tamoxifen-Induced Neuroprotective Effects in Ischemic Stroke Models. PLoS One. 2015;10(10):e0140660. doi: 10.1371/journal.pone.0140660. [DOI] [PMC free article] [PubMed] [Google Scholar]