We assessed the World Health Organization algorithm for diagnosing tuberculosis in seriously ill Human Immunodeficiency Virus-infected inpatients and developed a clinical prediction rule with good diagnostic utility. The Xpert MTB/RIF assay showed high sensitivity, especially when performed on induced rather than spontaneously expectorated sputum.
Keywords: HIV, tuberculosis diagnosis, WHO algorithm, inpatients, Xpert MTB/RIF assay
Abstract
Background
The World Health Organization (WHO) algorithm for the diagnosis of tuberculosis in seriously ill human immunodeficiency virus (HIV)–infected patients lacks a firm evidence base. We aimed to develop a clinical prediction rule for the diagnosis of tuberculosis and to determine the diagnostic utility of the Xpert MTB/RIF assay in seriously ill HIV-infected patients.
Methods
We conducted a prospective study among HIV-infected inpatients with any cough duration and WHO-defined danger signs. Culture-positive tuberculosis from any site was the reference standard. A priori selected variables were assessed for univariate associations with tuberculosis. The most predictive variables were assessed in a multivariate logistic regression model and used to establish a clinical prediction rule for diagnosing tuberculosis.
Results
We enrolled 484 participants. The median age was 36 years, 65.5% were female, the median CD4 count was 89 cells/µL, and 35.3% were on antiretroviral therapy. Tuberculosis was diagnosed in 52.7% of participants. The c-statistic of our clinical prediction rule (variables: cough ≥14 days, unable to walk unaided, temperature >39°C, chest radiograph assessment, hemoglobin, and white cell count) was 0.811 (95% confidence interval, .802–.819). The classic tuberculosis symptoms (fever, night sweats, weight loss) added no discriminatory value in diagnosing tuberculosis. Xpert MTB/RIF assay sensitivity was 86.3% and specificity was 96.1%.
Conclusions
Our clinical prediction rule had good diagnostic utility for tuberculosis among seriously ill HIV-infected inpatients. Xpert MTB/RIF assay, incorporated into the updated 2016 WHO algorithm, had high sensitivity and specificity in this population. Our findings could facilitate improved diagnosis of tuberculosis among seriously ill HIV-infected inpatients in resource-constrained settings.
Tuberculosis remains a major cause of death among human immunodeficiency virus (HIV)–infected adults in resource-constrained countries in the antiretroviral therapy (ART) era [1]. Delayed or missed diagnosis contributes to tuberculosis mortality [2, 3]. World Health Organization (WHO) 2007 guidelines to diagnose smear-negative tuberculosis [4] included an algorithm for seriously ill patients with cough of 2–3 weeks and ≥1 danger sign (respiratory rate >30 breaths/minute, heart rate >120 beats/minute, temperature >39°C, and being unable to walk unaided).
A modified WHO algorithm for seriously ill patients improved clinical outcomes in 2 African cohort studies [5, 6]. However, no study has explored the ability of the 2007 WHO seriously ill algorithm to diagnose tuberculosis, which has several limitations. First, pulmonary tuberculosis commonly presents with cough duration of <14 days [7, 8]. Second, other classic tuberculosis symptoms (fever, night sweats, and weight loss), which have high negative predictive value in active case finding [9], were not included. Third, it preceded the Xpert MTB/RIF assay, which is more sensitive than smear microscopy in HIV-infected patients [10]. Finally, hemoglobin concentration and white cell count (WCC), which predict tuberculosis among HIV-infected patients [11–13], could add diagnostic value.
We conducted a prospective cohort study to attempt to improve the ability of the 2007 WHO algorithm to diagnose tuberculosis in seriously ill HIV-infected patients by evaluating any cough duration, classic tuberculosis symptoms, chest radiographic features, hemoglobin, WCC, and the Xpert MTB/RIF assay.
METHODS
Study Population
We conducted a prospective cohort study in 2 regional hospitals in Cape Town, South Africa serving high-burden HIV and tuberculosis communities, GF Jooste Hospital (November 2011–February 2013) and Khayelitsha District Hospital (March 2013–October 2014). Inclusion criteria were as follows: HIV infected, ≥18 years of age, admitted within 24 hours, coughing for any duration, and ≥1 WHO danger sign. Exclusion criteria were antituberculosis therapy that is current or completed in the previous month or defaulted within the past 6 months, exacerbation of cardiac failure or chronic obstructive pulmonary disease, and inability to produce a spontaneous or induced sputum sample. Participants were followed up at 28 and 56 days after discharge.
Data Collection
Demographic data and clinical features (at the time of admission) were recorded on a standardized case record form. Chest radiographs performed on admission were assessed by the study medical officer for features suggestive of pulmonary tuberculosis and/or bacterial pneumonia and/or Pneumocystis jirovecii pneumonia (PJP). Chest radiographs were also retrospectively reviewed by a blinded specialist radiologist, who documented the presence of specific radiographic features (enlarged hilar or mediastinal lymph nodes, pleural effusions, interstitial (“ground glass”) infiltration, nodularity, cavitation, diffuse micronodular infiltration, linear/reticulonodular infiltration, consolidation, and features of previous tuberculosis), and then classified radiographs as likely, possible, or unlikely for pulmonary tuberculosis and/or bacterial pneumonia and/or PJP. CD4 cell count was obtained on admission if none was available from the prior 6 months, and hemoglobin and WCC were done on admission. Sputum induction, using an ultrasonic nebulizer and hypertonic saline, was done on participants unable to spontaneously produce sputum. Three sputum samples from each participant were sent for Gram stain, culture, and sensitivity (1 sample); and auramine smear microscopy for acid-fast bacilli, and mycobacterial culture (BACTEC MGIT 960; Becton Dickinson, Franklin Lakes, New Jersey) (2 samples). The sputum pellet on one of the samples for mycobacterial culture was split after decontamination for Xpert MTB/RIF assay (Cepheid, Sunnyvale, California). Mycobacterial blood cultures (BacT/Alert MP; bioMérieux, Durham, North Carolina) were sent from all participants. Extrapulmonary samples (eg, pleural fluid) were sent for mycobacterial culture when appropriate.
Case Definitions and Procedures
Tuberculosis was defined as a positive culture for Mycobacterium tuberculosis from any site. The study criteria for initiating antituberculosis therapy included positive microbiological diagnosis (auramine stain and/or Xpert MTB/RIF assay and/or culture); and/or a radiological diagnosis (chest radiograph showing mediastinal and/or hilar lymph nodes or miliary infiltrates; abdominal ultrasound showing multiple enlarged lymph nodes and/or multiple splenic hypoechoic lesions and/or pericardial effusion); and/or pleural effusion/ascites showing a lymphocytic exudate; and/or no clinical improvement after 3–5 days of antibiotic therapy. Decisions to start antituberculosis therapy were made by study staff.
The diagnosis of bacterial pneumonia was made with consistent symptoms and evidence of consolidation on chest radiograph. Bacterial pneumonia was treated with a broad-spectrum β-lactam antibiotic (eg, ceftriaxone, amoxicillin-clavulanate), and a macrolide was added with CRB-65 score >2 (1 point for each of: confusion, respiratory rate ≥30/min, blood pressure systolic <90 mm Hg or diastolic ≤60 mm Hg, age ≥65 years [14, 15].
PJP was diagnosed with a cough duration ≤12 weeks, bilateral interstitial infiltration on chest radiograph, and hypoxia (defined as oxygen saturation of ≤92%) or dyspnea. PJP was treated with high-dose trimethoprim-sulfamethoxazole, and prednisone if hypoxia was present.
Statistical Analysis
A sample size of 473 was sufficient to detect an estimated 30% prevalence of culture-positive tuberculosis with 5% precision. This sample size incorporated the need for at least 10 culture-positive tuberculosis events per predictive variable for multivariate logistic regression analysis [16].
Analyses were performed using Stata version 12.1 software (StataCorp, College Station, Texas). Missing data were imputed using chained equations and 20 iterations. Baseline characteristics were described as proportions or medians. We used univariate associations to assess the ability of the following a priori selected variables to predict tuberculosis: age, sex, cough duration, individual WHO danger signs, classic tuberculosis symptoms (fever, night sweats, and weight loss), radiologist assessment of tuberculosis on chest radiograph (categorized as likely or possible), hemoglobin, and WCC [17]. A backward stepwise approach proposed by Collet [18] was used to select the most predictive variables in establishing a multivariate logistic regression model.
The model was visually assessed by a calibration plot, and by the Hosmer–Lemeshow test. An estimate of the c-statistic was used to assess discrimination (values 0.7–0.8 are deemed acceptable and 0.8–0.9 good) [19]. Internal validation used 1000 bootstrap resamples [20]. A clinical prediction rule with score chart was constructed utilizing a standard method [21]. We used the clinical prediction rule to predict the probability of having tuberculosis, and compared it with the reference standard of culture-positive tuberculosis. We calculated the diagnostic accuracy for the range of possible scores from the clinical prediction rule.
We explored associations of individual chest radiograph features recorded by the radiologist with culture-positive tuberculosis, reported as odds ratios with 95% confidence intervals (CIs). We calculated the diagnostic accuracy of sputum smear and Xpert MTB/RIF assay for diagnosing culture-positive tuberculosis.
Ethics Approval
Approval for the study was obtained from the University of Cape Town Human Research Ethics Committee. All eligible participants signed informed consent before enrollment into the study. Confused participants were enrolled and given the option to continue with participation once orientated; their data were removed from the study if consent was declined.
RESULTS
Participant Characteristics
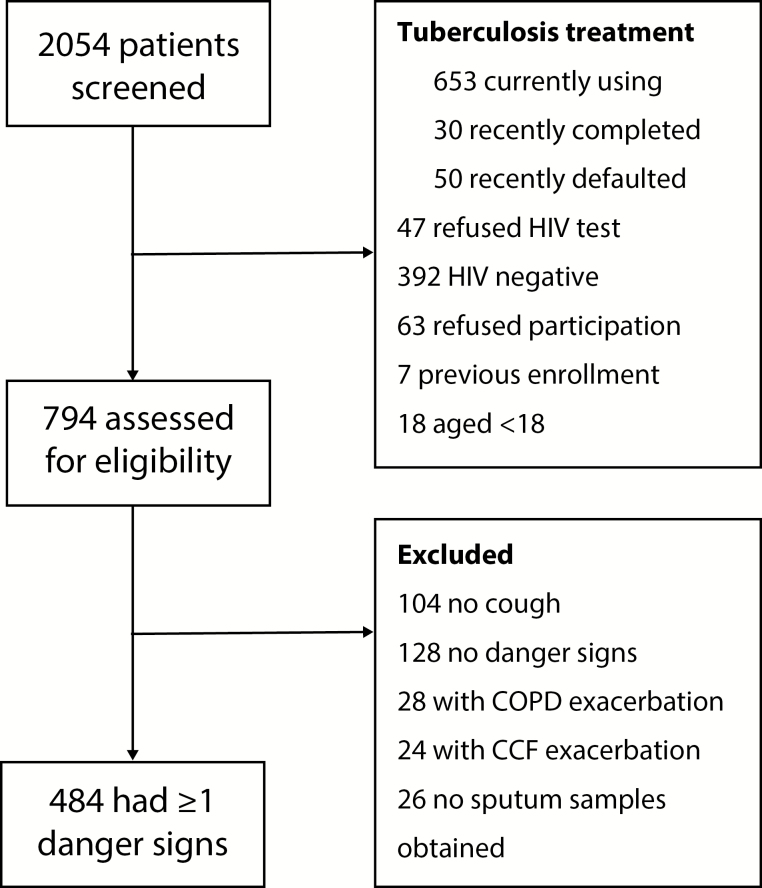
We screened 2054 patients and enrolled 484 (Figure 1). Table 1 shows participants’ baseline characteristics. All participants were commenced on antibiotic therapy at admission or at the referral site. Fifty-three percent (255/484) of participants had culture-positive tuberculosis, 50.6% (245/484) bacterial pneumonia, and 10.5% (51/484) PJP. Antituberculosis therapy was empirically started in 56 (11.6%) participants with negative sputum smears and Xpert MTB/RIF assays, of whom 22 (43.1%) had culture-positive tuberculosis. Other diagnoses (Cryptococcus neoformans, Emmonsia parva, nontuberculous mycobacteria, and malignancy) were made in 8.5% (41/484). Clinically diagnosed coinfections were common: tuberculosis with bacterial pneumonia in 25.8% (125/484) and tuberculosis with PJP in 4.3% (21/484). Different sites of culture-positive specimens (Supplementary Table 1) and pulmonary/extrapulmonary/disseminated tuberculosis are given in the Supplementary Data. The diagnostic accuracy of Xpert MTB/RIF assay and smear is shown in Table 2.
Figure 1.
Flow diagram for participant inclusion into the study. Abbreviations: CCF, congestive cardiac failure; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus.
Table 1.
Baseline Characteristics of 484 Seriously Ill Human Immunodeficiency Virus–Infected Participants Presenting With a Cough of Any Duration and ≥1 World Health Organization Danger Sign
| Characteristic | No. (%) or Median (25th–75th Percentile) |
|---|---|
| Baseline variables | |
| Age, y | 36 (30–42) |
| Sex, female | 317 (65.5) |
| Body mass indexa, kg/m2 | 20 (18–39) |
| CD4 countb, cells/µL | 89 (34–210) |
| Cough durationc, d | 14 (7–21) |
| Cough duration ≥14 d | 296 (61.2) |
| Using ART | 171 (35.3) |
| Duration on ART, y | 2.3 (0.2–5) |
| Previous tuberculosis | 236 (48.8) |
| Sputum inductiond | 312 (65.7) |
| WHO danger signs | |
| Respiratory rate >30 breaths/min | 315 (65.1) |
| Heart rate >120 beats/min | 383 (79.1) |
| Temperature >39°C | 81 (16.7) |
| Unable to walk unaided | 259 (53.5) |
| Tuberculosis symptoms | |
| Fever5 | 394 (81.6) |
| Night sweatsf | 314 (65.3) |
| Weight loss | 442 (91.3) |
| Laboratory investigations | |
| Hemoglobing g/dL | 9.5 (7.8–11.2) |
| WCCh, ×109/L | 8.6 (5.6–13.0) |
Abbreviations: ART, antiretroviral therapy; WCC, white cell count; WHO, World Health Organization.
Seventeen missing values.
One missing value.
Two missing values.
Nine missing values.
One missing value.
Three missing values.
Three missing value.
Three missing values.
Table 2.
Diagnostic Accuracy Evaluation of Sputum Auramine Smear and Xpert MTB/RIF Assay by the Reference Standard of Positive Culture From Any Site (n = 255) Among 484 Seriously Ill Human Immunodeficiency Virus–Infected Participants Presenting With a Cough of Any Duration and ≥1 World Health Organization Danger Sign
| Parameter | Xpert MTB/RIF | Auramine Smear |
|---|---|---|
| Sensitivity, % | 86.3 (81.5–90.3) | 57.0 (50.7–63.2) |
| Specificity, % | 96.1 (92.6–98.2) | 98.7 (96.2–99.7) |
| Positive predictive value, % | 96.1 (92.7–98.2) | 98.0 (94.2–99.6) |
| Negative predictive value, % | 86.2 (81.4–90.2) | 67.2 (61.9–72.2) |
| Positive likelihood ratio | 21.9 (11.5–41.6) | 43.3 (14.0–134.0) |
| Negative likelihood ratio | 0.14 (.11–.19) | 0.44 (.38–.50) |
Values in parentheses indicate the 95% confidence interval.
The yield of sputum culture was higher with sputum induction than spontaneous sputum production (56.1% [175/312] vs 45.5% [74/163]; P = .027). Similarly, the yield of smear and Xpert MTB/RIF assay was higher with sputum induction than spontaneous sputum production (32.7% [102/312] vs 30.1% [49/163], P = .553 and 51.9% [162/312] vs 41.7% [68/163], P = .035, respectively).
Seventy-two participants with negative sputum smears had features of tuberculosis on chest radiograph diagnosed by medical officers, fulfilling the criteria of the 2007 WHO algorithm for seriously ill inpatients to start empiric antituberculosis therapy with a sensitivity of 49.1% and specificity of 91.5% for the diagnosis of culture-positive tuberculosis.
Clinical Prediction Rule
Univariate associations with culture-positive tuberculosis are shown in Table 3. In multivariate logistic regression (Supplementary Table 2) the most significant predictors of culture-positive tuberculosis were being unable to walk unaided, radiologist assessment of “likely tuberculosis” on chest radiograph, and anemia. Raised WCC was a significant negative predictor of tuberculosis. The only classic tuberculosis symptom that showed a significant association with the diagnosis of tuberculosis was weight loss, but it was not significant on multivariate analysis. Cough duration ≥14 days was predictive of tuberculosis, but 28.6% (73/255) of culture-positive tuberculosis participants had cough duration <14 days.
Table 3.
Univariate Associations With Culture-Positive Tuberculosis Among 484 Seriously Ill Human Immunodeficiency Virus–Infected Participants Presenting With a Cough of Any Duration and ≥1 World Health Organization Danger Sign
| Variable | OR | (95% CI) | Wald P Value |
|---|---|---|---|
| Agea | 0.98 | (.96–1.00) | .044 |
| Sex | |||
| Female | Referent group | ||
| Male | 0.95 | (.65–1.39) | .799 |
| Cough duration ≥14 d | |||
| No | Referent group | ||
| Yes | 2.47 | (1.70–3.60) | <.001 |
| WHO danger signs | |||
| Respiratory rate >30 breaths/min | |||
| No | Referent group | ||
| Yes | 0.78 | (.54–1.14) | .207 |
| Heart rate >120 beats/min | |||
| No | Referent group | ||
| Yes | 1.53 | (.98–2.37) | .060 |
| Temperature >39°C | |||
| No | Referent group | ||
| Yes | 1.86 | (1.13–3.07) | .014 |
| Unable to walk unaided | |||
| No | Referent group | ||
| Yes | 2.77 | (1.92–4.01) | <.001 |
| Tuberculosis symptoms | |||
| Fever | |||
| No | Referent group | ||
| Yes | 1.07 | (.67–1.69) | .783 |
| Night sweats | |||
| No | Referent group | ||
| Yes | 1.18 | (.81–1.72) | .384 |
| Weight loss | |||
| No | Referent group | ||
| Yes | 3.08 | (1.54–6.17) | .002 |
| Increasing number of tuberculosis symptoms | 1.28 | (1.01–1.64) | .041 |
| Radiographic assessment of tuberculosis | |||
| Unlikely | Referent group | ||
| Possible | 2.13 | (1.00–4.54) | .052 |
| Likely | 11.01 | (4.92–24.66) | <.001 |
| Laboratory investigations | |||
| Hemoglobin, g/dLb | 0.69 | (.63–.76) | <.001 |
| WCC, ×109/Lc | 0.90 | (.87–.93) | <.001 |
Abbreviations: CI, confidence interval; OR, odds ratio; WCC, white cell count; WHO, World Health Organization.
Per 1 year increase.
Per 1 g/dL increase.
Per 1 × 109/L increase.
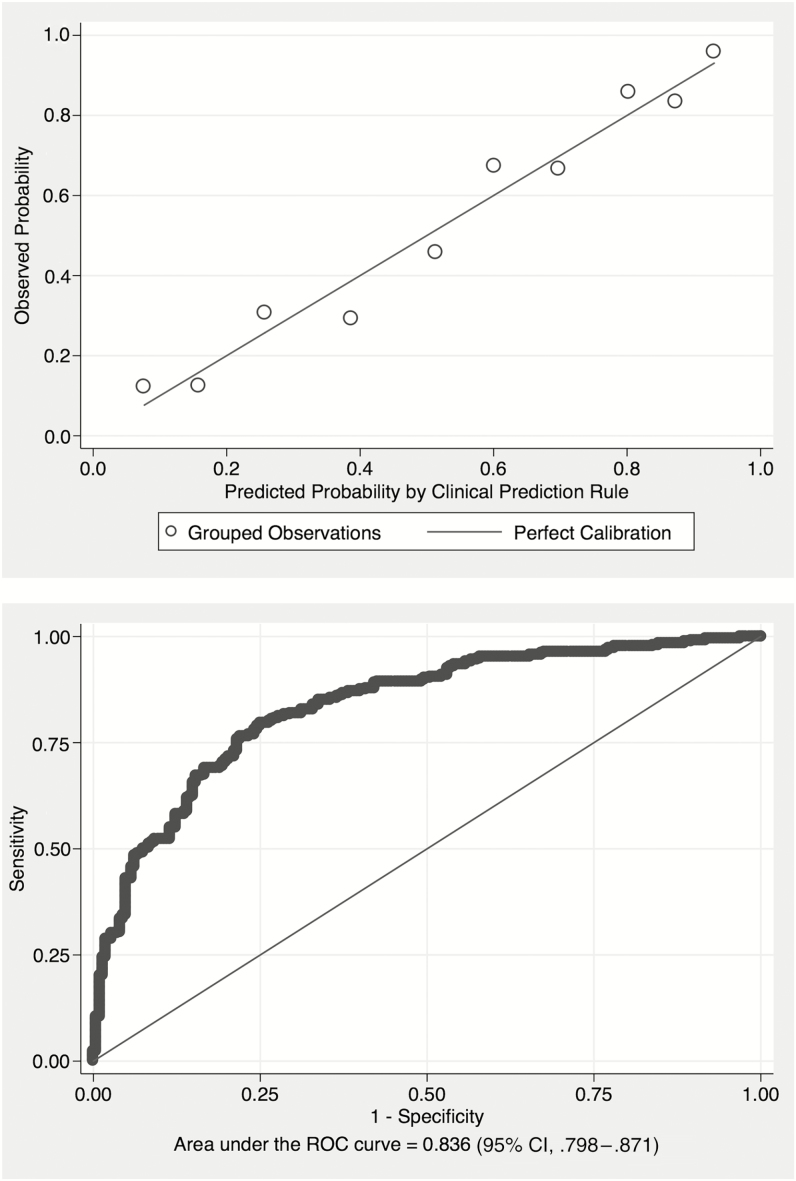
The calibration curve (Figure 2A) for the final model followed the ideal calibration line, indicating good agreement between the rate of tuberculosis estimated by the model and the tuberculosis frequency observed in the study population, confirmed by the Hosmer-Lemeshow χ2 statistic of 10.65 (P = .385). The model’s c-statistic was 0.834 (95% CI, .798–.871) (Figure 2B). The equivalents in bootstrap validation were 0.836 (95% CI, .800–.871), with an optimism estimate of 0.011 (95% CI, .010–.012), indicating good stability of the model in internal validation.
Figure 2.
Upper, Calibration plot for the assessment of variables included in a multivariate logistic regression model aimed at establishing a clinical prediction rule for the diagnosis of tuberculosis among 484 seriously ill human immunodeficiency virus (HIV)–infected participants presenting with a cough of any duration and 1 or more World Health Organization (WHO) danger sign. The line shows perfect calibration between observed and predicted tuberculosis. Lower, Discrimination of the multivariate logistic regression model aimed at establishing a clinical prediction rule for the diagnosis of tuberculosis among 484 seriously ill HIV-infected participants presenting with a cough of any duration and ≥1 WHO danger sign. Abbreviations: CI, confidence interval; ROC, receiver operating characteristic.
The clinical prediction rule with score chart was developed using the 6 selected variables (Table 4). Hemoglobin and WCC were categorized as tertiles. The diagnostic accuracy assessment based on different scores obtained from the clinical prediction rule is summarized in Table 5. The clinical prediction rule model showed a c-statistic of 0.811 (95% CI, .802–.819).
Table 4.
Clinical Prediction Rule for the Diagnosis of Culture-Positive Tuberculosis Among 484 Seriously Ill Human Immunodeficiency Virus–Infected Participants Presenting With a Cough of Any Duration and ≥1 World Health Organization Danger Sign
| Variable | Category | Points |
|---|---|---|
| Cough duration | ≥14 d | 1 |
| Temperature >39°C | Yes | 1 |
| Unable to walk unaided | Yes | 1 |
| Tuberculosis on chest radiograph | Possible | 1 |
| Likely | 5 | |
| Hemoglobin, g/dL | 3.3–8.3 | 3 |
| 8.4–10.6 | 2 | |
| White cell count, ×109/L | 1–6.5 | 1 |
| 11.2–40.4 | –2 |
Table 5.
Diagnostic Accuracy Assessment of a Clinical Prediction Rule for the Diagnosis of Culture-Positive Tuberculosis Among 484 Seriously Ill Human Immunodeficiency Virus–Infected Participants Presenting With a Cough of Any Duration and ≥1 World Health Organization Danger Sign
| Total Score | Probability of Tuberculosis | Sensitivity | Specificity | Positive Likelihood Ratio | Negative Likelihood Ratio |
|---|---|---|---|---|---|
| ≤1 | 0.1% | 100% | 0.0% | 1 | … |
| 2 | 0.2% | 95.2% | 28.8% | 1.34 | 0.17 |
| 3 | 0.5% | 92.2% | 45.4% | 1.69 | 0.17 |
| 4 | 1.1% | 87.2% | 59.3% | 2.14 | 0.23 |
| 5 | 2.5% | 78.6% | 67.7% | 2.43 | 0.31 |
| 6 | 5.6% | 66.0% | 80.3% | 3.36 | 0.42 |
| 7 | 12.2% | 50.8% | 90.5% | 5.35 | 0.54 |
| 8 | 24.4% | 39.1% | 93% | 5.58 | 0.65 |
| 9 | 43.0% | 31.2% | 96.4% | 8.67 | 0.71 |
| 10 | 63.7% | 19.5% | 97.9% | 9.13 | 0.82 |
| 11 | 80.4% | 5.9% | 99.6% | 13.44 | 0.95 |
| 12 | 90.5% | 0.2% | 100% | … | 1 |
Chest Radiograph Assessment
The radiologist assessed 416 participants’ chest radiographs: 151 (36.3%) were categorized as likely, 223 (53.6%) as possible, and 42 (10.0%) as unlikely tuberculosis. Univariate analysis of individual chest radiograph features revealed that diffuse micronodular infiltration, enlarged hilar or mediastinal lymph nodes, and nodularity >3 mm were predictors of culture-positive tuberculosis (Table 6). Bacterial pneumonia was a likely diagnosis in 9.6% (40/416) and PJP in 9.4% (39/416). Medical officers’ categorization of likely tuberculosis on chest radiograph had an odds ratio of 9.4 (95% CI, 5.7–15.6) for culture-positive tuberculosis.
Table 6.
Univariate Analysis of Individual Chest Radiograph Variables as Predictors of Culture-Positive Tuberculosis, Among the 416 Seriously Ill Human Immunodeficiency Virus–Infected Participants Presenting With a Cough of Any Duration and ≥1 World Health Organization Danger Sign and With a Specialist Radiologist Report
| Chest Radiograph Variables | no./No. (%) | Crude OR | (95% CI) | Wald P Value |
|---|---|---|---|---|
| Consolidation | 217/413 (52.5) | 0.86 | (.57–1.29) | .456 |
| Diffuse micronodular infiltration | 29/416 (6.9) | 6.45 | (2.20–18.87) | .001 |
| Linear/reticulonodular infiltration | 226/415 (54.5) | 0.93 | (.63–1.37) | .713 |
| Enlarged hilar or mediastinal lymph nodes | 210/414 (50.7) | 2.34 | (1.58–3.47) | <.001 |
| Pleural effusion | 195/414 (47.1) | 1.24 | (.84–1.82) | .284 |
| Interstitial infiltration | 259/415 (62.4) | 0.92 | (.62–1.37) | .685 |
| Nodularity (>3 mm) | 217/415 (52.3) | 2.21 | (1.49–3.28) | <.001 |
| Cavitation | 249/415 (60.0) | 1.01 | (.68–1.49) | .969 |
Abbreviations: CI, confidence interval; OR, odds ratio.
DISCUSSION
Our study is the first to evaluate the clinical, radiographic, and laboratory diagnosis of tuberculosis in a prospective cohort of inpatients fulfilling the criteria of the 2007 WHO algorithm for seriously ill HIV-infected patients. In 2016 (when enrollment into our study was completed), the WHO updated the algorithm by including classic tuberculosis symptoms and the Xpert MTB/RIF assay, and by making cough of any duration an inclusion criterion [22]. We had already incorporated all of these features into our study; therefore, we are also able to evaluate the updated WHO algorithm, but it should be noted that cough is no longer a requirement. We developed a clinical prediction rule with good diagnostic performance for tuberculosis using 6 variables, all of which are readily obtainable in most resource-constrained settings. The most significant predictors of tuberculosis were a radiologist assessment of likely tuberculosis on chest radiograph and anemia, while a raised WCC was a strong negative predictor of tuberculosis. The classic tuberculosis symptoms added no discriminatory value in diagnosing tuberculosis. Scores of 3 or 4 in our clinical prediction rule could be used to start empiric antituberculosis therapy in seriously ill patients as the sensitivity of these scores is around 90%. However, no single feature of our clinical prediction rule should be used in decisions to treat empirically for tuberculosis. The Xpert MTB/RIF assay, which has not previously been evaluated in seriously ill inpatients, had a high sensitivity of 86.3% in our participants. Implementation of our clinical prediction rule in resource-constrained settings could augment the 2016 WHO algorithm, inform the development of future algorithms, and ensure timely initiation of empiric antituberculosis treatment in seriously ill HIV-infected patients.
The 2007 WHO algorithm for seriously ill patients requires cough duration of 2–3 weeks. Although we found that cough duration of ≥14 days was predictive of tuberculosis, 28.6% of participants with confirmed tuberculosis had cough duration <14 days. Most of the data on cough duration of >14 days as a trigger for tuberculosis investigations are from studies of ambulatory patients and may not be generalizable to seriously ill inpatients. Two African studies found a high prevalence of tuberculosis among inpatients presenting acutely with pneumonia [7, 8]. These findings together with ours suggest that any cough duration was an appropriate improvement made in the 2016 WHO algorithm for the diagnosis of tuberculosis among seriously ill patients.
The classic tuberculosis symptoms fever and weight loss were sensitive, but not specific for the diagnosis of tuberculosis among HIV-infected inpatients with negative sputum smears [23]. The differential diagnosis in HIV-infected inpatients with cough and danger signs is wide, and many of these disorders and/or advanced HIV disease could reduce the diagnostic utility of the classic tuberculosis symptoms, which could explain why these were not independently associated with tuberculosis in our study. Two of the WHO danger signs, being unable to walk unaided and temperature >39°C, were significant predictors of tuberculosis in our cohort.
The WHO has recently reiterated the importance of using chest radiographic assessment to facilitate the rapid initiation of antituberculosis therapy among seriously ill HIV-infected patients [24]. Our study supports this recommendation, with a radiologist assessment of likely tuberculosis being the strongest predictor of tuberculosis in our clinical prediction rule. Other studies of hospitalized HIV-infected participants with smear-negative pulmonary tuberculosis have also reported the diagnostic value of chest radiography [17, 23, 25, 26]. The commonest individual radiographic feature associated with culture-positive tuberculosis in our study was enlarged hilar or mediastinal lymph nodes. Cavitation was not a significant clinical predictor of culture-positive tuberculosis, which might be explained by the low median CD4 count and high prevalence of previous tuberculosis. Other studies of inpatients from high-burden settings have also reported that intrathoracic lymphadenopathy was a good predictor of tuberculosis, but cavitation was not [25, 26].
Our finding that anemia was associated with tuberculosis is in keeping with other studies of inpatients and outpatients [12, 26]. A study from Cameroon found anemia and leukopenia to be independent predictors of extrapulmonary involvement in patients with pulmonary tuberculosis; many patients in our cohort had disseminated disease, which could explain why hemoglobin and WCC were good predictors of tuberculosis [13].
Sputum Xpert MTB/RIF assay performed well in our cohort, with a sensitivity of 86.3%, which is somewhat higher than the sensitivity of 79% reported in HIV-infected patients in a systematic review [10]. The high sensitivity of Xpert MTB/RIF assay we found could be explained by the high proportion of participants who had sputum induction, which increased the yield of Xpert MTB/RIF assay in our study (contrary to the findings of a study in ambulatory patients) [27]. We found a higher yield of sputum culture with sputum induction than spontaneous sputum production, which is consistent with other studies [27, 28]. In view of these findings, we call for greater access to sputum induction in resource-constrained settings.
Our study has some limitations. First, our findings may not be generalizable to patients without cough or in settings with different prevalence of tuberculosis and other pulmonary opportunistic infections. Two studies evaluated the prevalence of culture-positive tuberculosis in HIV-infected inpatients with WHO danger signs and negative sputum smears, reporting 23% in a Ugandan study [6] (51% of whom had WHO danger signs) and 23% in a study from South Africa [5], which is similar to the 22.7% culture-positive prevalence in our participants with negative sputum smears, suggesting that our findings are generalizable to sub-Saharan Africa. Tuberculosis is the leading cause of hospitalization among HIV-infected adults worldwide, with similar proportions in low- to middle-income countries, and it is thus conceivable that our findings may also be generalizable outside of Africa [29]. Second, all our participants had 1 or more WHO danger signs, which limited our ability to assess their value for the diagnosis of tuberculosis. Third, a specialist radiologist’s assessments of the chest radiographs performed well in our clinical prediction rule, but in resource-constrained settings nonspecialist doctors usually read chest radiographs and their interpretation is likely to be less accurate. Fourth, the diagnoses of bacterial pneumonia and PJP were clinical and not based on microbiological reference standards. We attempted to confirm bacterial pneumonia with blood and sputum cultures, but these were almost all negative because of antibiotic use immediately before inclusion into our study. Fifth, the reference standard for diagnosing tuberculosis was culture, which is not 100% sensitive. Finally, while bootstrap resampling has several advantages over other internal validation methods, it is not enough to demonstrate the external applicability of the derived prediction rule. Because internal validation in general is optimistic, a drop in the performance of the prediction rule could be observed when it is applied to different settings. While our measure of optimism suggests that such a drop would be marginal, external validation, ideally conducted by different investigators, is needed to confirm the performance of our prediction rule before wide uptake in routine practice. Strengths of our study are its prospective design, the use of multiple mycobacterial cultures and sputum induction to establish a reference standard for tuberculosis, and a higher than expected number of tuberculosis events, which increased our power to develop a clinical prediction rule.
In conclusion, we developed a clinical prediction rule with good performance characteristics for diagnosing tuberculosis in seriously ill HIV-infected patients and showed that the Xpert MTB/RIF assay had high sensitivity. These findings, if validated in different settings, could contribute to the development of an improved algorithm for tuberculosis in seriously ill inpatients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. Chest radiographic interpretation was done by Professor Hillel Goodman, Division of Radiology, Department of Radiation Medicine, University of Cape Town.
Author contributions. G. M., M. M., M. N., and M. X. R. designed the study. R. G., H. v. d. P., and W. S. collected the data. G. M., M. M., R. G., A. S., and A. P. K. analyzed and interpreted the data. R. G. wrote the first draft. All authors reviewed, revised, and approved the final report.
Disclaimer. The study sponsor had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Financial support. This work was supported by the National Institutes of Health (grant number R01 AI 96735-01 IRIDA).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meintjes G, Schoeman H, Morroni C et al. . Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: a cross-sectional study. BMC Infect Dis 2008; 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 2007; 369:2042–9. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO). Improving the diagnosis and treatment of smear-negative pulmonary and extra pulmonary tuberculosis among adults and adolescents, recommendations for HIV-prevalent and resource-constrained settings. WHO/HTM/TB/2007.379 Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 5. Holtz TH, Kabera G, Mthiyane T et al. . Use of a WHO-recommended algorithm to reduce mortality in seriously ill patients with HIV infection and smear-negative pulmonary tuberculosis in South Africa: an observational cohort study. Lancet Infect Dis 2011; 11:533–40. [DOI] [PubMed] [Google Scholar]
- 6. Katagira W, Walter ND, Den Boon S et al. . Empiric TB treatment of severely ill patients with HIV and presumed pulmonary TB improves survival. J Acquir Immune Defic Syndr 2016; 72:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scott JA, Hall AJ, Muyodi C et al. . Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in Kenya. Lancet 2000; 355:1225–30. [DOI] [PubMed] [Google Scholar]
- 8. Nyamande K, Lalloo UG, John M. TB presenting as community-acquired pneumonia in a setting of high TB incidence and high HIV prevalence. Int J Tuberc Lung Dis 2007; 11:1308–13. [PubMed] [Google Scholar]
- 9. Getahun H, Kittikraisak W, Heilig CM et al. . Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med 2011; 8:e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steingart KR, Schiller I, Horne DJ et al. . Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014; CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawson L, Yassin MA, Thacher TD et al. . Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand J Infect Dis 2008; 40:30–5. [DOI] [PubMed] [Google Scholar]
- 12. Kerkhoff AD, Wood R, Vogt M, Lawn SD. Predictive value of anemia for tuberculosis in HIV-infected patients in sub-Saharan Africa: an indication for routine microbiological investigation using new rapid assays. J Acquir Immune Defic Syndr 2014; 66:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yone EW, Kengne AP, Moifo B, Kuaban C. Prevalence and determinants of extrapulmonary involvement in patients with pulmonary tuberculosis in a sub-Saharan African country: a cross-sectional study. Scand J Infect Dis 2013; 45:104–11. [DOI] [PubMed] [Google Scholar]
- 14. Lim WS, van der Eerden MM, Laing R et al. . Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldman C, Brink AJ, Richards GA et al. . Management of community-acquired pneumonia in adults. S Afr Med J 2007; 97:1296–306. [PubMed] [Google Scholar]
- 16. Peduzzi P, Concato J, Kemper E et al. . A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49:1373–9. [DOI] [PubMed] [Google Scholar]
- 17. Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis 2003; 3:288–96. [DOI] [PubMed] [Google Scholar]
- 18. Collet D. Modelling binary data. 2nd ed New York: Chapman & Hall/CRC, 2003. [Google Scholar]
- 19. Hosmer D, Lemeshow S.. Applied logistic regression. New York: Wiley, 1989. [Google Scholar]
- 20. Harrell F. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: London: Springer, 2001. [Google Scholar]
- 21. Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med 2004; 23:1631–60. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach Available at: http://www.who.int/entity/hiv/pub/guidelines/keypopulations-2016/en/index.html. Accessed 8 February 2017.
- 23. Davis JL, Worodria W, Kisembo H et al. . Clinical and radiographic factors do not accurately diagnose smear-negative tuberculosis in HIV-infected inpatients in Uganda: a cross-sectional study. PLoS One 2010; 5:e9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization (WHO). Chest radiography in tuberculosis detection: summary of current WHO recommendations and guidance on programmatic approaches. Geneva, Switzerland: WHO, 2016: WHO/HTM/TB/2016.20. [Google Scholar]
- 25. Samb B, Henzel D, Daley CL et al. . Methods for diagnosing tuberculosis among in-patients in Eastern Africa whose sputum smears are negative. Int J Tuberc Lung Dis 1997; 1:25–30. [PubMed] [Google Scholar]
- 26. Le Minor O, Germani Y, Chartier L et al. . Predictors of pneumocystosis or tuberculosis in HIV-infected Asian patients with AFB smear-negative sputum pneumonia. J Acquir Immune Defic Syndr 2008; 48:620–7. [DOI] [PubMed] [Google Scholar]
- 27. Peter JG, Theron G, Pooran A et al. . Comparison of two methods for acquisition of sputum samples for diagnosis of suspected tuberculosis in smear-negative or sputum-scarce people: a randomised controlled trial. Lancet Respir Med 2013; 1:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawn SD, Kerkhoff AD, Pahlana P et al. . Diagnostic yield of tuberculosis using sputum induction in HIV-positive patients before antiretroviral therapy [short communication]. Int J Tuberc Lung Dis2012; 16:1354–7. [DOI] [PubMed] [Google Scholar]
- 29. Ford N, Matteelli A, Shubber Z et al. . TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016; 19:20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.