Abstract
As current treatment options in OA are very limited, OA patients would benefit greatly from some ability to self-manage their condition. Since diet may potentially affect OA, we reviewed the literature on the relationship between nutrition and OA risk or progression, aiming to provide guidance for clinicians. For overweight/obese patients, weight reduction, ideally incorporating exercise, is paramount. The association between metabolic syndrome, type-2 diabetes and OA risk or progression may partly explain the apparent benefit of dietary-lipid modification resulting from increased consumption of long-chain omega-3 fatty-acids from oily fish/fish oil supplements. A strong association between OA and raised serum cholesterol together with clinical effects in statin users suggests a potential benefit of reduction of cholesterol by dietary means. Patients should ensure that they meet the recommended intakes for micronutrients such as vitamin K, which has a role in bone/cartilage mineralization. Evidence for a role of vitamin D supplementation in OA is unconvincing.
Keywords: nutrition, osteoarthritis (OA), obesity, diabetes, metabolic syndrome, polyunsaturated fatty acids (PUFAs), cholesterol, anti-oxidants, vitamin D, vitamin K
Rheumatology key messages
Overweight and obese OA patients should implement a weight-loss strategy incorporating exercise tailored to mobility.
Increasing consumption of long-chain n-3 fatty-acids (oily fish/fish oil supplements) may improve pain and function in OA patients.
Reducing raised blood cholesterol and increasing intake of rich vitamin K sources may benefit OA.
Introduction
OA is the most prevalent form of arthritis [1] and the fastest growing cause of disability worldwide [2]. Globally, some 18% of women and 9.6% of men aged over 60 years have symptomatic OA, with a quarter of these individuals unable to perform routine daily activities [3]. By 2050, a projected 130 million people will suffer with OA, constituting a significant societal burden [4].
OA pathology is multifactorial, involving the remodelling of subchondral bone, synovial inflammation and loss of articular cartilage [5]. Inflammatory cytokines, notably IL-1β and TNF-α, drive catabolic pathways and perpetuate disease progression [6]. Obesity is a modifiable risk factor for OA [7] at least partly due to its associated inflammation [8]. It has recently been suggested that obesity, diabetes and the metabolic syndrome (MetS) can directly influence the development of OA [9]. A metabolic OA phenotype has been identified, which is driven by adipokines, hyperglycaemia and endocrine imbalance [1].
Current OA treatment is limited and is largely confined to symptom management [1] or total joint replacement if joint function is severely compromised [10]. Osteoarthritis Research Society International guidelines [11] recommend exercise and weight reduction in the overweight/obese, but there may be low provision of these treatments in clinical practice [2].
There is a call for a shift towards helping OA patients to self-manage their condition [11]. Since diet is a factor that may affect OA, we performed an up-to-date, literature review of the evidence for an effect of dietary factors in OA with the aim of summarizing current research findings. Guidance is targeted at clinicians, notably rheumatologists, general practitioners and dietitians and our findings form the basis of a patient information sheet (supplementary Fig. S1, available at Rheumatology online, and at https://www.bda.uk.com/foodfacts/OsteoArthritis.pdf).
Methods
For this narrative review, the PubMed database was searched for articles on the effect of OA, obesity, polyunsaturated fatty acids, cholesterol and vitamins A, C, D, E and K on risk or progression of OA. The focus was on foods or nutrients that are part of the normal diet, not on nutraceuticals, which are generally consumed in pharmacological, rather than dietary, doses. Using key search terms (Table 1), searches were conducted between October 2015 and May 2017, for papers from the past 10 years, but were extended back to 2000 for vitamins A, C, D and E and to 1995 for vitamin K owing to the paucity of information available. Human studies, including observational and cohort studies, and randomized controlled trials (RCTs) were included. Excluding duplicates, 1190 articles were retrieved.
Table 1.
Search terms used for article selection
| Nutrient | Search terms |
|---|---|
| Vitamin K | Vitamin K/phylloquinone/menaquinone AND Osteoarthritis |
| Vitamin D | Osteoarthritis AND Vitamin D |
| Vitamins A, C and E | Osteoarthritis AND: Vitamin E/tocopherols/tocotrienols |
| Osteoarthritis AND Vitamin C/ascorbic acid | |
| Osteoarthritis AND Vitamin A/carotenoids/retinol | |
| Obesity | Osteoarthritis AND Obesity AND Progression |
| Osteoarthritis AND Obesity AND Symptoms AND Review | |
| Osteoarthritis AND Obesity AND Risk AND Review | |
| Polyunsaturated fatty acids | Osteoarthritis AND PUFA/polyunsaturated fatty acids/fish oil/omega-3/omega-three/n-3 fatty acids n-6 fatty acids |
| Cholesterol | Osteoarthritis AND Cholesterol/hypercholesterolaemia |
Articles were examined and filtered by relevance. Papers were excluded if outcome measures could not be related to disease progression or symptoms, or quality was demonstrably poor. Sixty-eight articles were included in the final review. Where appropriate, reference lists were consulted for highly regarded older papers and primary evidence. Relevant conclusions or results were extracted from each article. Bradford–Hill criteria [12] and the critical appraisal skills programme (CASP) tool [13, 14] were used to appraise the evidence for each topic. With the heterogeneity of data rendering a systematic approach challenging, evidence is presented as a narrative review.
Results and discussion
Obesity
Consequences of obesity in OA
Obesity increases strain on weight-bearing joints [15] and, longitudinally, overweight and obese individuals are at considerably higher risk for knee arthroplasty [16]. An association of higher BMI with the development of hand OA [17] demonstrates an additional non-biomechanical role of obesity in OA. In the large Netherlands Epidemiology of Obesity cohort study, fat percentage was associated with hand OA: OR (95% CI): 1.34 (1.11, 1.61) in men and 1.26 (1.05, 1.51) in women [18]. Fat mass and waist-to-hip ratio were associated with hand OA, with an association with visceral fat in men [18]. Adiposity relates to risk of arthroplasty in knee and hip OA, with a relationship to central adiposity in knee OA [19].
Obesity leads to low-grade systemic inflammation and weight reduction can reduce adipose tissue and restore normal secretion patterns [20]. Adipokines could comprise a critical link between obesity and OA [21]. Leptin, an adipokine generally elevated in obesity and produced by white adipose tissue of the infra-patellar fat pad, may underlie this relationship [22]. Leptin is associated with inflammation and cartilage degradation [23] and may be involved in OA pathophysiology at a local and systemic level [24]. Figure 1 summarizes the mechanisms linking obesity to OA pathogenesis [25].
Fig. 1.
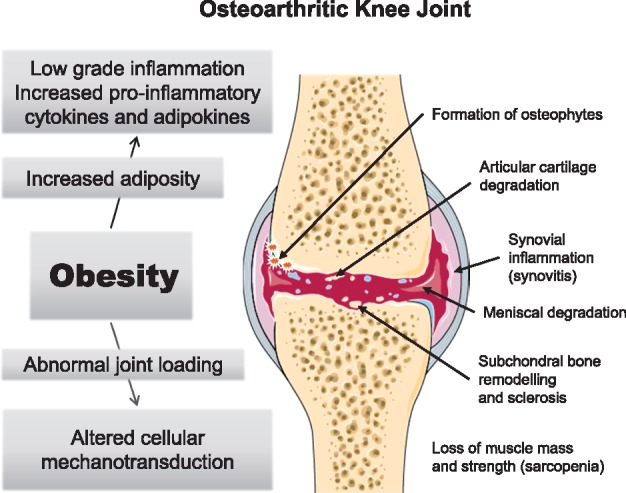
Mechanisms by which obesity leads to or exacerbates OA
Adapted by permission from Macmillan Publishers Ltd. Nat Rev Rheumatol, Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis [25]. Copyright 2013.
Relationship with MetS
Central obesity, glucose intolerance (insulin resistance), dyslipidaemia and hypertension embody the MetS [26]. OA has been suggested to be a fifth component with shared mechanisms of inflammation and oxidative stress [27]. The Research on Osteoarthritis/Osteoporosis Against Disability cohort (n = 1384) demonstrated increased radiographic incidence and progression of knee OA with the accumulation of MetS components: ⩾3 components, for incidence, OR (95% CI): 9.83 (3.57, 27.1), P < 0.001; for progression: 2.80 (1.68, 4.68), P < 0.001 [9]. A similar effect of MetS components on knee-OA risk was seen in the Melbourne Collaborative Cohort Study though no statistically significant associations were observed for hip OA, suggesting that the pathogenesis of knee and hip OA differ [28]. Prevalence of hand OA was associated with the MetS in the Netherlands Epidemiology of Obesity cohort study in 6673 participants; adjusted OR (95% CI): 1.46 (1.06, 2.02) [29].
Relationship with type-2 diabetes
Hyperglycaemia prompts local accumulation of advanced-glycation end products, impairing subchondral bone and chondrocyte function [27]. In a German cohort study, type-2 diabetes was identified as an independent risk factor for severe OA and a predictor for knee/hip replacement [30]. In a French study of patients with knee OA assessed over 3 years, type-2 diabetes was a significant predictor of joint-space-width reduction in men [31].
Clinical effects of weight reduction on OA
As outlined in Table 2 [32–35], a systematic review and meta-analysis of RCTs revealed significant improvement in physical disability in knee OA when body-weight reduction was over 5% [35]. Subsequent clinical trials demonstrated that weight reduction reduced pain and improved function in knee OA [33, 34]. In obese participants undertaking a weight-control intervention, weight reduction reduced peak knee load and pain [32], and provided symptomatic relief, irrespective of structural damage [36].
Table 2.
Findings of large trials and meta-analyses of weight reduction interventions in overweight/obese individuals with knee OA
| Study | Participants | Intervention | Mean weight reduction from baseline | Symptom outcome change from baseline |
|---|---|---|---|---|
|
|
|
|
|
| Messier et al. [34] IDEA Trialb |
|
|
|
|
|
|
|
6.1 (95% CI: 4.7, 7.6) kg; P < 0.001 |
|
The influence of weight reduction or exercise on cartilage in obese knee OA patients.
Intensive diet and exercise for arthritis. D: diet; E: exercise; ES: effect size; LED: low energy dense; RCT: randomized controlled trial; VAS: visual analogue scale; VLED: very low energy dense.
Datasets from the Osteoarthritis Initiative and the Multicentre Osteoarthritis Study demonstrate a highly significant (P < 0.003) dose–response relationship between weight change and improvement in WOMAC pain and function [37]. A reduction in body weight of 10% is associated longitudinally with increased functional capacity and reduced pain in knee OA patients [37] and is additionally beneficial for metabolic health [28].
Physical activity as an adjunct to weight reduction
Reducing adipose tissue while maintaining muscle mass is advantageous in OA, particularly for mobility [38, 39]. Physical activity generates changes in white adipose tissue, including increased mitochondrial biogenesis and an altered adipokine profile [40], and hence weight reduction programmes that combine diet and exercise have the most benefit on functional status, joint imaging and visual analogue scale pain [41]. Meta-analysis indicates that interventions combining strengthening, flexibility and aerobic exercise are most likely to improve pain and function [42]. However, caloric restriction is still integral, as highlighted in a trial in which knee compressive forces were lowest in a diet group and inflammation (IL-6) was lowest in a diet-and-exercise group, compared with exercise alone [34] (Table 2).
Effective weight management
Weight reduction and maintenance of an optimal weight is challenging, particularly when mobility is impaired [25]. Activity should be tailored to the mobility, co-morbidities and preferences of the individual [43]. Successful weight reduction interventions may incorporate dietitian input [32, 36]. In clinical practice, behaviour-change strategies, increased contact and follow-up will improve adherence to weight-management programmes [44].
Conclusions on obesity and weight reduction in OA
Weight reduction in overweight or obese OA patients reduces joint impact and injurious loading, improves inflammatory adipokine secretion patterns and positively affects metabolic-risk profile. Effective dietary interventions do not deviate greatly from general weight-management guidelines [45]. Guidance is given in Table 3 [46–56].
Table 3.
Summary of dietary interventions that may be of benefit in OA
| Intervention | Detail of recommended interventions | Points to note |
|---|---|---|
| Weight reduction in overweight or obese patients |
|
Regular clinical contact and monitoring, including dietetic input, are essential for dietary modification. Clinical input should incorporate a focus on behaviour change |
| Beneficial dietary-lipid modification in OA patients |
|
Women who are pregnant or breastfeeding should avoid fish with high levels of mercury (i.e. shark, swordfish and king mackerel) [46] and should avoid cod-liver oil due to the vitamin A content [47] |
| Dietary management of cholesterol, serum lipids and comorbidities, CVD and MetS |
|
|
| To achieve adequate levels of vitamins A, C and E |
|
US guidelines suggest an additional 30 mg/day Vitamin C for smokers |
| To increase vitamin D intake/status |
|
|
| To increase vitamin K intake |
|
The addition of a fat (such as olive oil) to a vitamin K source may increase bioavailability, as vitamin K is fat-soluble |
aNHS reference ranges/NHLBI [48] reference ranges, bSee recommendations for weight-reduction in overweight/obese OA patients, and cExcluding pregnant/lactating women. CVD: cardiovascular disease; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MetS: metabolic syndrome.
Polyunsaturated fatty acids
Dietary-lipid modification in OA
Lipids are stored in the matrix and chondrocytes of articular cartilage and may contribute towards inflammation, cartilage degradation and impaired chondrocyte structure [57]. OA joints accumulate high levels of omega-6 (n-6) fatty acids, precursors of pro-inflammatory eicosanoids [58]. In individuals with, or at high risk of, knee OA, a positive association was seen between the n-6 polyunsaturated fatty acid (PUFA) arachidonic acid (AA) and synovitis but an inverse relationship between total plasma n-3 PUFA, docosahexaenoic acid (DHA) and patellofemoral cartilage loss, as measured by MRI [59]. With diet influencing systemic lipid levels [59], it is plausible that dietary manipulation could affect articular-cartilage composition and structural damage in knee OA. A large prospective study in OA patients found that higher intakes of total and saturated fat were associated with increased knee joint space-width loss, whereas higher intakes of monounsaturated fatty acids (MUFAs) and PUFAs were associated with reduced radiographic progression [60].
Rationale for an effect of PUFAs in inflammation in OA
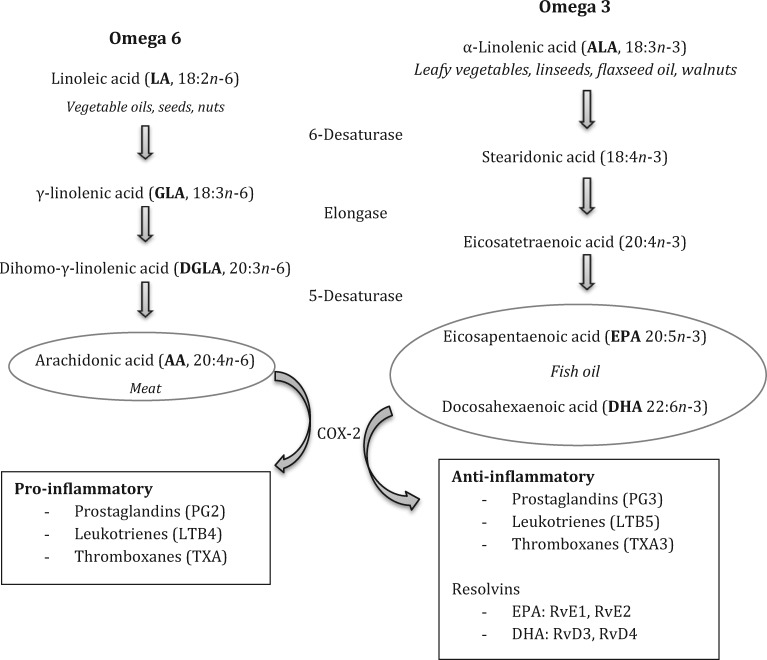
Eicosanoids are hormone-like agents that mediate and regulate inflammation. Figure 2 illustrates their formation from 20-carbon precursors [61, 62]. Eicosapentaenoic acid (EPA) and DHA create less potent inflammatory eicosanoids than those formed from the n-6 series. Indirectly, long-chain (LC) n-3 PUFAs decrease production of pro-inflammatory eicosanoids, reactive oxygen and nitrogen species and cytokines, additionally generating anti-inflammatory mediators (resolvins) [62].
Fig. 2.
Essential fatty acids: elongation and chain saturation, dietary sources and inflammatory effects
In vitro models have suggested benefits of LC n-3 PUFA supplementation for inflamed joints, with EPA proving the most effective [63, 64]. In canine OA, fish oil n-3 fatty acids improved weight bearing [65] and the arthritic condition [66].
PUFAs in the diet
The Western diet has a high ratio of n-6 to n-3 fatty acids [67], predisposing to inflammation [59]. Within the Melbourne Collaborative Cohort Study of healthy individuals, increased n-6 fatty acid consumption was associated with the development of bone-marrow lesions [68]. AA originates from meat and from the conversion of linoleic acid (LA), commonly found in vegetable oils (Fig. 2). Oils high in n-6 fatty acids include safflower oil (79%) and sunflower oil (62.2%), with low levels in olive oil, which is rich in monounsaturates [69]. The richest source of α-linolenic acid is flaxseed oil (57%) [59], but its conversion into EPA/DHA is inefficient [70]. Increasing LC n-3 PUFA status to promote an anti-inflammatory effect is best achieved with direct EPA intake alongside decreased LA intake.
EPA and DHA are found primarily in oily fish [71] (see supplementary Table S1, available at Rheumatology online). UK intakes of oily fish are below the recommended level of ‘at least one portion/week’ [72, 73] and a significant number of US adults are not meeting American Dietary Guidelines of an average consumption of 250 mg LC n-3 fatty acids per day [74]. Higher intakes of EPA/DHA, including the proposed anti-inflammatory threshold of >2.7 g/day [75], may be more easily achieved by fish oil supplementation. In a large Australian study, 32.6% of the population had recently taken fish oil or n-3 supplements, with OA patients more likely to use them [76].
EPA/DHA supplementation
The use of fish oils has shown clinical efficacy for pain reduction in RA [61, 77], but published trials of LC n-3 fatty acid supplementation in OA are limited. A systematic review and meta-analysis concluded that there was no statistically significant effect of marine oil supplements for OA pain (five trials; −0.17; 95% CI: −0.57, 0.24) [78]. However the quality of the trials included was graded as ‘very low’, and the results for the OA group were highly heterogeneous leading the authors to state that the evidence was not sufficiently robust to determine the effect of marine oil in patients with diagnoses other than RA [78]. Hill and colleagues [79] conducted a double-blind 2-year RCT in 202 knee-OA patients. There was no placebo group, attributed to efforts to maintain blinding and for ethical reasons [79]. Unexpectedly, greater benefits to WOMAC pain and function scores were found from a low-dose (0.45 g LC n-3) fish oil supplement than from a high, anti-inflammatory dose (4.5 g LC n-3) [79]. The authors concluded that there was no additional benefit of high-dose over low-dose fish oil in knee OA [79]. The low-dose supplement comparator was composed largely of Sunola oil, a monounsaturated n-9 fatty acid. Unlike olive oil, it is not rich in polyphenols with known antioxidant and potentially anti-inflammatory effects [80]. However, there may have been benefit to pain from the high oleic acid content [81], which may have effectively constituted a second active intervention [82].
Conclusions on importance of PUFAs in OA
Adding complexity to supplementation trials, the effects of fish oil can be affected by polymorphisms that alter the expression of cytokine genes [62] and it is difficult to find an appropriate placebo oil. Though further studies with appropriate controls are needed, there may be efficacy for pain reduction with a low-dose supplement equivalent to 1.5 standard capsules of 1 g fish oil/day [79]. Moreover, there is evidence for fish oil being of benefit to cardiovascular health [83], which may be relevant to this population owing to the association of OA with MetS [27]. Recommendations are summarized in Table 3.
Cholesterol
Links between elevated blood cholesterol and OA
Epidemiological studies have implicated serum cholesterol as a systemic OA risk factor [84–86]. In women from the Chingford study, knee OA was significantly associated with moderately raised serum cholesterol (OR = 2.06; 95% CI: 1.06, 3.98) [84]. In a cross-sectional study of patients with OA-related arthroplasty, hypercholesterolaemia, defined as ⩾6.2 mmol/l (239 mg/dl), was independently associated with generalized OA [85]. Hand OA has recently been associated with documented hypercholesterolaemia [86]. Longitudinal data have strengthened the evidence; in an Australian cohort of healthy women, for every 1 mmol/l increase in total cholesterol, the adjusted odds of developing a bone-marrow lesion were 1.84 (95% CI: 1.01, 3.36), P = 0.048 [87].
Cholesterol accumulation in OA
Cellular cholesterol accumulation is known to induce cytotoxicity [88] and hypercholesterolaemia can increase AA formation and production of pro-inflammatory eicosanoids [89]. Cholesterol has been found to accumulate in human OA cartilage [90]. Accumulation is typically prevented through the cholesterol efflux system [88], which may be dysregulated in OA [91]. An association has been found between OA pathogenesis and a single nucleotide polymorphism in SRBP-2, the gene for a protein involved in cholesterol homeostasis [92]. Furthermore, low-density lipoprotein (LDL)-cholesterol appears to influence OA development and progression [93]. In mice induced with OA and fed a cholesterol-rich diet, LDL accumulation led to increased synovial activation and osteophyte formation [94]. Oxidized LDL, in particular, may be involved in synovial inflammation and cartilage destruction [93].
Does reducing serum lipids reduce the burden of OA?
Reducing cholesterol accumulation with statins appears to have favourable effects in OA. A 10-year longitudinal study in a large cohort (n = 16 609) found that an increasing therapeutic statin dose vs no statin dose was incrementally associated with a decreased incidence of clinical OA [95]. In a further cohort, statin use was associated with over 50% reduction in radiographic progression of knee OA [OR (95% CI): 0.43 (0.25, 0.77)] [96]. Statins also reduce the expression of inflammatory cytokines and attenuate inflammation in OA [97]; they have been shown to exert chondroprotective effects by reducing cartilage degradation, with atorvastatin treatment inhibiting IL-1β and expression of MMP-13 in vitro [98].
Cholesterol-lowering dietary strategies applicable to OA
Cholesterol can also be lowered by dietary strategies [99]. Assuming additive effects, dietary changes could result in a 35% reduction in LDL-cholesterol, equivalent to that of a starting dose of statins [99, 100] (Fig. 3). Energy restricted weight reduction is of primary importance for reducing LDL-cholesterol in the overweight and obese [99, 101]. Saturated fat should be reduced to 11% of total energy with PUFAs and MUFAs acting as favourable substitutes [100]. Dietary cholesterol (as in eggs) is now known to exert a relatively insignificant effect on serum cholesterol compared with that of saturated fatty acids [100]. Viscous (soluble) fibre appears to lower serum cholesterol by 5–10% for a 5–10 g daily dose [102] and oat (β)-glucans have been found to be effective in a 3 g dose [103]. Meta-analysis concluded that 25 g soy protein/day contributed to total and LDL-cholesterol reduction [104]. There is strong evidence that plant stanols and sterols lower LDL-cholesterol [49]. Their richest source is fortified proprietary spreads, drinks and yogurts. A meta-analysis of 41 trials showed that intake of 2 g/day of stanols/sterols reduced LDL-cholesterol by 10% [49]. There appears to be a dose–effect relationship such that a dose of 9–10 g of plant stanols lowered LDL-cholesterol by 18% [105]. Nuts provide a source of unsaturated fats, soluble fibre and phytosterols [106]. A meta-analysis of 61 intervention trials found that tree nuts (e.g. almonds, walnuts and pistachios) significantly lowered total and LDL-cholesterol [107]. Including 30 g/day [108] of nuts in the diet could provide a simple cholesterol-lowering strategy. Dietary interventions to lower cholesterol and serum lipids and manage comorbidities are given in Table 3.
Fig. 3.
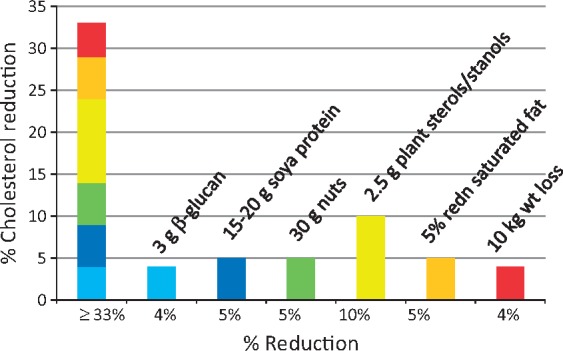
Dietary potential to lower cholesterol: different dietary strategies can add up to a total cholesterol reduction of >30%
Antioxidants and OA
A plausible rationale exists for a role of antioxidants in OA; reactive oxygen species and reactive nitrogen species may be involved in the pathophysiology of OA, and therefore suppressing these with antioxidants might delay its onset and progression [109, 110]. The antioxidant vitamins, A, C and E have received the most attention in this context with vitamin C being particularly relevant owing to its requirement for collagen formation [111].
Human studies on antioxidants and OA
Trials so far have primarily focused on the effects of vitamin E in OA [112, 113]. A systematic review of trials has investigated all three nutrients, although seven of the nine studies included were vitamin E trials [113]. Findings were conflicting, with only two short-term trials suggesting a beneficial effect of vitamin E [113]. Though vitamin C supplementation appeared to reduce OA pain, that effect has not been reproduced in other trials. A later trial of vitamin E supplementation (200 mg/day) in knee OA found a significant benefit on pain and significantly increased levels of circulating antioxidant enzymes [112]. No study to date has solely investigated vitamin A supplementation in OA, though when combined (as β-carotene) with vitamins C, E and selenium, no effect was seen [113]. Unfortunately, most of these studies were of poor quality with variations in duration, sample size, measurement outcomes, supplement dosage and form [112, 113].
Conclusions on antioxidant vitamins
At present, there are insufficient data to show benefit from antioxidant supplementation in OA. However, for general health, patients should be encouraged to eat a healthy diet that includes adequate amounts of dietary antioxidants (see recommendations in Table 3 [50–53]).
Vitamin D and OA
Although vitamin D has many biological roles, its primary function is thought to be the regulation of bone metabolism and calcium homeostasis [114]. The majority of its activity occurs via the vitamin D receptors (VDRs), a subfamily of nuclear receptors that regulate gene expression, to which it binds with high affinity [114].
Postulated role of vitamin D in OA
Acting through the VDR, vitamin D has a major role in the regulation of mineral homeostasis and bone metabolism [114]. Thus, inadequate vitamin D status is thought to impair the ability of bone to respond to the pathophysiological process of OA and influence disease progression [114, 115]. Vitamin D is also believed to have effects on inflammation and cytokine synthesis [115]. Furthermore, a number of trials have shown that vitamin D supplementation has positive effects on muscle strength [116, 117]; this may be beneficial in OA, which is often associated with marked weakness of the quadriceps muscles [118].
Human evidence on vitamin D in OA
Results from observational studies, predominantly in knee OA, though inconsistent, suggest a positive association between vitamin D deficiency, cartilage loss and OA prevalence/progression [119, 120].
Trials have had mixed findings. Three RCTs found no significant effect of vitamin D supplementation on cartilage volume or pain in knee OA [121–123], though in a post hoc analysis of one study, significant improvements in total WOMAC score and WOMAC function were found [122]. Only one RCT has had positive results, viz., a significant decrease in OA pain, and an increase in symptomatic knee function [124]. A small non-controlled intervention also found a significant increase in muscle strength following two months of vitamin D supplementation [125]. In a recent trial, people with knee OA who consistently maintained sufficient plasma vitamin D (>50 nmol/l) had significantly improved structural and functional outcomes than those consistently insufficient, suggesting a level of vitamin D status to aim for [126].
Alternative explanation for role of vitamin D in OA
There are alternative theories as to why vitamin D supplementation has not translated into positive outcomes [127, 128]. Emerging evidence suggests that low vitamin D may simply be a marker of ill-health across a number of conditions [127], for example, the inflammatory process and clinical course of OA could result in vitamin D deficiency as a consequence, rather than a cause, of disease [127]. Indeed, low serum vitamin D in inflammatory conditions may be due to the dysregulation of the VDR [128].
Conclusions on vitamin D intake/status and OA
Though it appears unlikely that vitamin D deficiency is a causal factor in OA, there appear to be benefits for muscle strength [116, 117] when patients maintain adequate vitamin D status. Vitamin D deficiency is widespread across the world [129]. Although both the Institute of Medicine [54] and the European Food Safety Authority (EFSA) [55] consider that a dietary intake that achieves a serum 25(OH)D concentration of 50 nmol/l is sufficient, other organizations prefer to define sufficiency as the higher value of 75 nmol/l [130]. Clinicians should measure vitamin D status in their OA patients and use the information in Table 3 [54, 55] to ensure that their vitamin D status reaches at least 50 nmol/l.
Vitamin K and OA
Vitamin K is a group of fat-soluble compounds, with two naturally occurring forms, vitamin K1 (phylloquinones) and vitamin K2 (menaquinones) [131]. Vitamin K1, synthesized by plants and algae, is the form most widely found in the human diet, mainly in green leafy vegetables and oils [56]. Vitamin K2 is predominantly produced by bacteria [30].
Postulated role of vitamin K in OA
Aside from its role in the complement cascade [131], vitamin K is involved in bone and cartilage mineralization [132, 133]; it is a cofactor for the enzyme γ-glutamyl carboxylase, which is responsible for the γ-carboxylation and functionality of vitamin-K-dependent (VKD) proteins [132, 133]. VKD proteins found in bone and cartilage include matrix Gla protein, periostin, gla-rich protein, gas 6 and osteocalcin. Inadequate vitamin K intake may lead to decreased carboxylation of these VKD proteins, affecting functional status [132] and resulting in abnormalities that parallel those seen in OA [133].
Human evidence on vitamin K in OA
Studies addressing the effects of vitamin K on OA in vivo are very limited and most have investigated only phylloquinone. Cross-sectional studies have shown a positive association between concentrations of plasma phylloquinone <1.0 nM and the development of OA [134] and an inverse association between knee OA and plasma phylloquinone [135]. Furthermore, phylloquinone intake has been inversely associated with individual and multiple inflammatory markers [136]. In longitudinal studies, vitamin K-deficient subjects were found to be more likely to have articular cartilage and meniscus damage, often developing OA in one or both knees [137, 138].
Despite these promising results, the only randomized, controlled trial of vitamin K failed to find a significant beneficial effect of phylloquinone supplementation against hand-OA progression [139]. However, only a subsection of the group was vitamin K insufficient at baseline and, in that subset, those who attained sufficient concentrations at follow-up had 47% less joint space narrowing (P = 0.02).
Conclusions on vitamin K and OA
As the majority of epidemiological evidence thus far is observational, causality cannot be demonstrated; however, the essential role of vitamin K in bone and cartilage health is incontrovertible. Not all populations reach the recommended vitamin K intake. Furthermore, there is no gold-standard biomarker [140]. While studies from Germany, America, the Netherlands, Japan and parts of Northern China have shown adequate intake of vitamin K according to guidelines [141–145], 59% of UK adults >65 years had an intake below recommendations, probably because of inadequate consumption of cooked green vegetables [146]. Guidance to increase vitamin K intake is given in Table 3 [56].
Conclusions
Despite the limitations of evidence that is largely based on observational studies, most of which are on knee OA, this review can offer some guidance to clinicians. With excess adiposity appearing to underlie the metabolic factors now recognized as being integral to OA, particularly of the hand and knee [28, 29], dietary modification to achieve weight reduction where appropriate, together with increased physical activity, are the strongest evidence-based recommendations. Though the evidence for benefit of dietary-lipid modification (increased LC n-3 PUFA/decreased LC n-6 PUFA) and lowering of serum cholesterol on OA is currently somewhat sparse, the recommendations proposed will at least benefit metabolic health. While vitamin/micronutrient data are limited, there is a plausible role for these nutrients in preventing/slowing OA, though at what intake levels remains to be seen. The accompanying patient-information sheet (supplementary Fig. 1, available at Rheumatology online; https://www.bda.uk.com/foodfacts/OsteoArthritis.pdf) summarizes potentially beneficial recommendations and is designed for healthcare professionals to distribute to their patients.
An ageing population and the current obesity epidemic [147] predict an increased global burden of OA. Given the current paucity of treatment options, any risk-free means of reducing progression or relieving debilitating symptoms in such a large patient group should be given a try. While we await well-designed, larger scale RCTs in OA patients of clearly defined phenotype, preferably relatively early in the disease process, our findings could be used to support the development of guidelines using the Delphi process of expert consensus to make recommendations.
Supplementary Material
Acknowledgements
A.M. has received funding from the following sources: The European Commission Framework 7 programme (EU FP7; HEALTH.2012.2.4.5-2, project number 305815; Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases). The Innovative Medicines Initiative Joint Undertaking under grant agreement No. 115770, resources of which are composed of financial contribution from the European Union's Seventh Framework programme (FP7/2007-2013) and EFPIA companies' in-kind contribution. A.M. also wishes to acknowledge funding from the European Commission through a Marie Curie Intra-European Fellowship for Career Development grant (project number 625746; acronym: CHONDRION; FP7-PEOPLE-2013-IEF) and support from the European Social Fund according to the activity ‘Improvement of researchers’ qualification by implementing world-class R&D projects' of Measure No. 09.3.3-LMT-K-712 (grant application code: 09.3.3-LMT-K-712-01-0157, agreement No. DOTSUT-215).
Contributions: S.T. and H.B. researched the topic and drafted the manuscript with the help of M.P.R. All authors contributed to discussion of the content and editing or reviewing the manuscript before submission.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Bijlsma JW, Berenbaum F, Lafeber FP.. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- 2. Conaghan PG, Porcheret M, Kingsbury SR. et al. Impact and therapy of osteoarthritis: the Arthritis Care OA Nation 2012 survey. Clin Rheumatol 2015;3:1581. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Chronic rheumatic conditions. 2016. http://www.who.int/chp/topics/rheumatic/en/ (26 December 2016, date last accessed).
- 4. World Health Organisation. Priority diseases and reason for inclusion. 2013. http://www.who.int/medicines/areas/priority_medicines/Ch6_12Osteo.pdf (26 December 2016, date last accessed).
- 5. Goldring S. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther Adv Musculoskelet Dis 2012;4:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abramson SB, Attur M.. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther 2009;11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF.. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med 1988;109:18–24. [DOI] [PubMed] [Google Scholar]
- 8. Edd SN, Giori NJ, Andriacchi TP.. The role of inflammation in the initiation of osteoarthritis after meniscal damage. J Biomech 2015;48:1420–6. [DOI] [PubMed] [Google Scholar]
- 9. Yoshimura N, Muraki S, Oka H. et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217–26. [DOI] [PubMed] [Google Scholar]
- 10. Woolf AD, Pfleger B.. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 11. McAlindon TE, Bannuru RR, Sullivan MC. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis and Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- 12. Austin BH. The environment and disease: association or causation? Bull World Health Organ 2005;83:796–8. [PMC free article] [PubMed] [Google Scholar]
- 13. Critical Appraisal Skills Programme (CASP). CASP Randomised Controlled Trial Checklist. 2013. http://www.casp-uk.net/checklists (21 July 2016, date last accessed).
- 14. Critical Appraisal Skills Programme (CASP). CASP Cohort Study Checklist. 2013. http://www.casp-uk.net/checklists (21 July 2016, date last accessed).
- 15. Messier SP, Pater M, Beavers DP. et al. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthritis Cartilage 2014;22:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leyland KM, Judge A, Javaid MK. et al. Obesity and the relative risk of knee replacement surgery in patients with knee osteoarthritis: a prospective cohort study. Arthritis Rheumatol 2016;68:817–25. [DOI] [PubMed] [Google Scholar]
- 17. Yusuf E, Nelissen RG, Ioan-Facsinay A. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010;69:761–5. [DOI] [PubMed] [Google Scholar]
- 18. Visser AW, Ioan-Facsinay A, de Mutsert R. et al. Adiposity and hand osteoarthritis: the Netherlands Epidemiology of Obesity study. Arthritis Res Ther 2014;16:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Simpson JA, Wluka AE. et al. Relationship between body adiposity measures and risk of primary knee and hip replacement for osteoarthritis: a prospective cohort study. Arthritis Res Ther 2009;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc 2005;64:163–9. [DOI] [PubMed] [Google Scholar]
- 21. Gkretsi V, Simopoulou T, Tsezou A.. Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Prog Lipid Res 2011;50:133–40. [DOI] [PubMed] [Google Scholar]
- 22. Fowler‐Brown A, Kim DH, Shi L. et al. The mediating effect of leptin on the relationship between body weight and knee osteoarthritis in older adults. Arthritis Rheumatol 2015;67:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hui W, Litherland GJ, Elias MS. et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis 2012;71:455–62. [DOI] [PubMed] [Google Scholar]
- 24. Scotece M, Mobasheri A.. Leptin in osteoarthritis: focus on articular cartilage and chondrocytes. Life Sci 2015;140:75–8. [DOI] [PubMed] [Google Scholar]
- 25. Wluka AE, Lombard CB, Cicuttini FM.. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol 2013;9:225–35. [DOI] [PubMed] [Google Scholar]
- 26. Eckel RH, Grundy SM, Zimmet PZ.. The metabolic syndrome. The Lancet 2005;365:1415–28. [DOI] [PubMed] [Google Scholar]
- 27. Zhuo Q, Yang W, Chen J, Wang Y.. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 2012;8:729–37. [DOI] [PubMed] [Google Scholar]
- 28. Monira Hussain S, Wang Y, Cicuttini FM. et al. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin Arthritis Rheum 2014;43:429–36. [DOI] [PubMed] [Google Scholar]
- 29. Visser AW, de Mutsert R, le Cessie S. et al. ; NEO Study Group. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis 2015;74:1842–7. [DOI] [PubMed] [Google Scholar]
- 30. Schett G, Kleyer A, Perricone C. et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eymard F, Parsons C, Edwards MH. et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage 2015;23:851–9. [DOI] [PubMed] [Google Scholar]
- 32. Aaboe J, Bliddal H, Messier SP, Alkjær T, Henriksen M.. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage 2011;19:822–8. [DOI] [PubMed] [Google Scholar]
- 33. Riecke BF, Christensen R, Christensen P. et al. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage 2010;18:746–54. [DOI] [PubMed] [Google Scholar]
- 34. Messier SP, Carr JJ, Williamson JD. et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcome among overweight and obese adults with knee osteoarthritis (Report). JAMA 2013;310:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen R, Bartels EM, Astrup A, Bliddal H.. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2007;66:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gudbergsen H, Boesen M, Lohmander LS. et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthritis Cartilage 2012;20:495–502. [DOI] [PubMed] [Google Scholar]
- 37. Riddle DL, Stratford PW.. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: a cohort study. Arthritis Care Res 2013;65:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rayman MP, Pattison DJ.. Dietary manipulation in musculoskeletal conditions. Best Pract Res Clin Rheumatol 2008;22:535–61. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Wluka AE, English DR. et al. Body composition and knee cartilage properties in healthy, community-based adults. Ann Rheum Dis 2007;66:1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stanford K, Middelbeek R, Goodyear L.. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 2015;64:2361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brosseau L, Wells GA, Tugwell P. et al. Ottawa Panel evidence-based clinical practice guidelines for the management of osteoarthritis in adults who are obese or overweight. Phys Ther 2011;91:843–61. [DOI] [PubMed] [Google Scholar]
- 42. Uthman OA, van der Windt DA, Jordan JL. et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ 2013;347:f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bliddal H, Leeds AR, Christensen R.. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons – a scoping review. Obes Rev 2014;15:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Messier SP. Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheum Dis Clin North Am 2008;34:713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bliddal H, Christensen R.. The management of osteoarthritis in the obese patient: practical considerations and guidelines for therapy. Obes Rev 2006;7:323–31. [DOI] [PubMed] [Google Scholar]
- 46. Wenstrom KD. The FDA’s new advice on fish: it’s complicated. Obstet Gynecol 2014;211:475–8. [DOI] [PubMed] [Google Scholar]
- 47. Expert Group on Vitamins and Minerals. Safe Upper Levels for Vitamins and Minerals. London: Food Standards Agency, 2003. https://cot.food.gov.uk/sites/default/files/vitmin2003.pdf (20 February 2017, date last accessed).
- 48. National Heart, Lung, and Blood Institute. High Blood Cholesterol: What You Need To Know. Bethesda: National Institutes of Health, 2016. https://www.nhlbi.nih.gov/health/resources/heart/heart-cholesterol-hbc-what-html (28 December 2016, date last accessed).
- 49. Katan MB, Grundy SM, Jones P. et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 2003;78:965–78. [DOI] [PubMed] [Google Scholar]
- 50. European Food Safety Authority (EFSA). Scientific opinion on dietary reference values for vitamin A. Panel on dietetic products, nutrition and allergies. EFSA J 2015;13:4028. [Google Scholar]
- 51. Institute of Medicine. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium and the Carotenoids. Washington: The National Academies Press, 2000. http://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids (28 December 2016, date last accessed). [Google Scholar]
- 52. European Food Safety Authority (EFSA). Scientific opinion on dietary reference values for vitamin C. Panel on dietetic products, nutrition and allergies. EFSA J 2013;11:3418. [Google Scholar]
- 53. European Food Safety Authority (EFSA). Scientific opinion on dietary reference values for vitamin E as α-tocopherol panel on dietetic products, nutrition and allergies. EFSA J 2015;13:4149. [Google Scholar]
- 54. Institute of Medicine. DRI Dietary Reference Intakes Calcium and Vitamin D. Washington: The National Academies Press, 2010. http://www.nap.edu/read/13050/chapter/1 (22 March 2016, date last accessed).
- 55. European Food Safety Authority (EFSA). Scientific opinion on dietary reference values for vitamin D. Panel on dietetic products, nutrition and allergies. EFSA J 2016;14:4547. [Google Scholar]
- 56. Bolton-Smith C, Price RJ, Fenton ST, Harrington DJ, Shearer MJ.. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br J Nutr 2000;83:389–99. [PubMed] [Google Scholar]
- 57. Masuko K, Murata M, Suematsu N. et al. A metabolic aspect of osteoarthritis: lipid as a possible contributor to the pathogenesis of cartilage degradation. Clin Exp Rheumatol 2009;27:347–53. [PubMed] [Google Scholar]
- 58. Plumb MS, Aspden RM.. High levels of fat and (n-6) fatty acids in cancellous bone in osteoarthritis. Lipids Health Dis 2004;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baker KR, Matthan NR, Lichtenstein AH. et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage 2012;20:382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lu B, Driban J, Xu C. et al. Dietary fat and progression of knee osteoarthritis dietary fat intake and radiographic progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res 2017;69:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rayman M, Callaghan A.. Nutrition and Arthritis. Oxford, UK: Blackwell Publishing, 2006. [Google Scholar]
- 62. Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006;83(6 Suppl):1505S–19S. [DOI] [PubMed] [Google Scholar]
- 63. Zainal Z, Longman AJ, Hurst S. et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage 2009;17:896–905. [DOI] [PubMed] [Google Scholar]
- 64. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL.. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids 2010;82:315–8. [DOI] [PubMed] [Google Scholar]
- 65. Roush JK, Cross AR, Renberg WC. et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc 2010;236:67–73. [DOI] [PubMed] [Google Scholar]
- 66. Roush JK, Dodd CE, Fritsch DA. et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc 2010;236:59–66. [DOI] [PubMed] [Google Scholar]
- 67. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 2008;233:674–88. [DOI] [PubMed] [Google Scholar]
- 68. Wang Y, Wluka AE, Hodge AM. et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage 2008;16:579–83. [DOI] [PubMed] [Google Scholar]
- 69. Orsavova J, Misurcova L, Ambrozova JV, Vicha R, Mlcek J.. Fatty acid composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci 2015;16:12871–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Davis BC, Kris-Etherton P.. Achieving optimal essential fatty acid status in vegetarians: current knowledge and practical implications. Am J Clin Nutr 2003;78:640S. [DOI] [PubMed] [Google Scholar]
- 71. Holland B. Fish and Fish Products: Third Supplement to the Fifth edition of McCance and Widdowson's The Composition of Foods. Cambridge: Royal Society of Chemistry/London: Ministry of Agriculture, Fisheries and Food, 1993. [Google Scholar]
- 72. Department of Health. National Diet and Nutrition Survey. Headline results from Years 1 and 2 (combined) of the Rolling Programme (2008/2009–2009/10). 2011. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216484/dh_128550.pdf. (28 March 2016, date last accessed).
- 73. Scientific Advisory Committee on Nutrition. Advice on Fish Consumption: Benefits and Risks. 2004. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/338801/SACN_Advice_on_Fish_Consumption.pdf (1 February 2017, date last accessed).
- 74. Papanikolaou Y, Brooks J, Reider C, Fulgoni V.. US adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J 2014;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cleland LG, James MJ, Proudman SM.. Fish oil: what the prescriber needs to know. Arthritis Res Ther 2006;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adams J, Sibbritt D, Lui CW, Broom A, Wardle J.. Ω-3 fatty acid supplement use in the 45 and Up Study Cohort. BMJ Open 2013;3:e002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. James MJ, Cleland LG.. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum 1997;27:85–97. [DOI] [PubMed] [Google Scholar]
- 78. Senftleber NK, Nielsen SM, Andersen JR. et al. Marine oil supplements for arthritis pain: a systematic review and meta-analysis of randomized trials. Nutrients 2017;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hill CL, March LM, Aitken D. et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis 2016;75:23–9. [DOI] [PubMed] [Google Scholar]
- 80. Martin-Pelaez S, Covas MI, Fito M, Kusar A, Pravst I.. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Mol Nutr Food Res 2013;57:760–71. [DOI] [PubMed] [Google Scholar]
- 81. Felson DT, Bischoff-Ferrari HA.. Dietary fatty acids for the treatment of OA, including fish oil. Ann Rheum Dis 2016;75:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kremer JM. Fish oil for OA? Don't give up yet. Ann Rheum Dis 2016;75:e42. [DOI] [PubMed] [Google Scholar]
- 83. Marchioli R, Barzi F, Bomba E. et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897–903. [DOI] [PubMed] [Google Scholar]
- 84. Hart DJ, Doyle DV, Spector TD.. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol 1995;22:1118–23. [PubMed] [Google Scholar]
- 85. Sturmer T, Sun Y, Sauerland S. et al. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J Rheumatol 1998;25:1827–32. [PubMed] [Google Scholar]
- 86. Addimanda O, Mancarella L, Dolzani P. et al. Clinical associations in patients with hand osteoarthritis. Scand J Rheumatol 2012;41:310–3. [DOI] [PubMed] [Google Scholar]
- 87. Davies-Tuck M, Hanna F, Davis SR. et al. Total cholesterol and triglycerides are associated with the development of new bone marrow lesions in asymptomatic middle-aged women – a prospective cohort study. Arthritis Res Ther 2009;11:R181–R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest 2002;110:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Prasad K, Lee P.. Suppression of oxidative stress as a mechanism of reduction of hypercholesterolemic atherosclerosis by aspirin. J Cardiovasc Pharmacol Ther 2003;8:61–9. [DOI] [PubMed] [Google Scholar]
- 90. Cillero-Pastor B, Eijkel G, Kiss A, Blanco FJ, Heeren RM.. Time-of-flight secondary ion mass spectrometry-based molecular distribution distinguishing healthy and osteoarthritic human cartilage. Anal Chem 2012;84:8909–16. [DOI] [PubMed] [Google Scholar]
- 91. Tsezou A, Iliopoulos D, Malizos KN, Simopoulou T.. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J Orthop Res 2010;28:1033–9. [DOI] [PubMed] [Google Scholar]
- 92. Kostopoulou F, Gkretsi V, Malizos KN. et al. Central role of SREBP-2 in the pathogenesis of osteoarthritis. PLoS One 2012;7:e35753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Munter W, van der Kraan PM, van den Berg WB, van Lent PL.. High systemic levels of low-density lipoprotein cholesterol: fuel to the flames in inflammatory osteoarthritis? Rheumatology 2016;55:16–24. [DOI] [PubMed] [Google Scholar]
- 94. de Munter W, Blom AB, Helsen MM. et al. Cholesterol accumulation caused by low density lipoprotein receptor deficiency or a cholesterol-rich diet results in ectopic bone formation during experimental osteoarthritis. Arthritis Res Ther 2013;15:R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kadam UT, Blagojevic M, Belcher J.. Statin use and clinical osteoarthritis in the general population: a longitudinal study. J Gen Intern Med 2013;28:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Clockaerts S, Van Osch GJ, Bastiaansen-Jenniskens YM. et al. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann Rheum Dis 2012;71:642–7. [DOI] [PubMed] [Google Scholar]
- 97. Baker JF, Walsh P, Mulhall KJ.. Statins: a potential role in the management of osteoarthritis? Joint Bone Spine 2011;78:31–4. [DOI] [PubMed] [Google Scholar]
- 98. Simopoulou T, Malizos KN, Poultsides L, Tsezou A.. Protective effect of atorvastatin in cultured osteoarthritic chondrocytes. J Orthop Res 2010;28:110–5. [DOI] [PubMed] [Google Scholar]
- 99. Jenkins DJ, Kendall CW, Vuksan V.. Viscous fibers, health claims, and strategies to reduce cardiovascular disease risk. Am J Clin Nutr 2000;71:401. [DOI] [PubMed] [Google Scholar]
- 100. Griffin BA. Nonpharmacological approaches for reducing serum low-density lipoprotein cholesterol. Curr Opin Cardiol 2014;29:360–5. [DOI] [PubMed] [Google Scholar]
- 101. Dow CA, Thomson CA, Flatt SW. et al. Predictors of improvement in cardiometabolic risk factors with weight loss in women. J Am Heart Assoc 2013;2:e000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jenkins DJ, Kendall CW, Axelsen M, Augustin LS, Vuksan V.. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lipidol 2000;11:49. [DOI] [PubMed] [Google Scholar]
- 103. Harland JI. Food combinations for cholesterol lowering. Nutr Res Rev 2012;25:249–66. [DOI] [PubMed] [Google Scholar]
- 104. Harland JI, Haffner TA.. Systematic review, meta-analysis and regression of randomised controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis 2008;200:13–27. [DOI] [PubMed] [Google Scholar]
- 105. Laitinen K, Gylling H.. Dose-dependent LDL-cholesterol lowering effect by plant stanol ester consumption: clinical evidence. Lipids Health Dis 2012;11:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kris-Etherton PM, Hu FB, Ros E, Sabate J.. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 2008;138:1746S–51S. [DOI] [PubMed] [Google Scholar]
- 107. Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D.. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr 2015;102:1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the Substantiation of Health Claims Related to Walnuts and Maintenance of Normal Blood LDL-Cholesterol Concentrations (ID 1156, 1158) and Improvement of Endothelium-dependent Vasodilation (ID 1155, 1157) Pursuant to Article 13(1) of Regulation (EC) No 1924/20061. 2011. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2011.2074/pdf (29 December 2016, date last accessed).
- 109. Grover AK, Samson SE.. Benefits of antioxidant supplements for knee osteoarthritis: rationale and reality. Nutr J 2016;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Henrotin Y, Kurz B.. Antioxidant to treat osteoarthritis: dream or reality? Curr Drug Targets 2007;8:347–57. [DOI] [PubMed] [Google Scholar]
- 111. Li Y, Schellhorn HP.. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007;137:2171–84. [DOI] [PubMed] [Google Scholar]
- 112. Bhattacharya I, Saxena R, Gupta V.. Efficacy of vitamin E in knee osteoarthritis management of North Indian geriatric population. Ther Adv Musculoskelet Dis 2012;4:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Canter PH, Wider B, Ernst E.. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology 2007;46:1223–33. [DOI] [PubMed] [Google Scholar]
- 114. Mabey T, Honsawek S.. Role of vitamin D in osteoarthritis: molecular, cellular, and clinical perspectives. Int J Endocrinol 2015;2015:383918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shen M, Luo Y, Niu Y. et al. 1,25(OH)2D deficiency induces temporomandibular joint osteoarthritis via secretion of senescence-associated inflammatory cytokines. Bone 2013;55:400–9. [DOI] [PubMed] [Google Scholar]
- 116. Beaudart C, Buckinx F, Rabenda V. et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2014;99:4336–45. [DOI] [PubMed] [Google Scholar]
- 117. Tomlinson PB, Joseph C, Angioi M.. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J Sci Med Sport 2015;18:575–80. [DOI] [PubMed] [Google Scholar]
- 118. Hall MC, Mockett SP, Doherty M.. Relative impact of radiographic osteoarthritis and pain on quadriceps strength, proprioception, static postural sway and lower limb function. Ann Rheum Dis 2006;65:865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cao Y, Winzenberg T, Nguo K. et al. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: a systematic review. Rheumatology 2013;52:1323–34. [DOI] [PubMed] [Google Scholar]
- 120. Bergink AP, Zillikens MC, Van Leeuwen JP. et al. 25-Hydroxyvitamin D and osteoarthritis: a meta-analysis including new data. Semin Arthritis Rheum 2016;45:539–46. [DOI] [PubMed] [Google Scholar]
- 121. McAlindon T, LaValley M, Schneider E. et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA 2013;309:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jin X, Jones G, Cicuttini F. et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA 2016;315:1005–13. [DOI] [PubMed] [Google Scholar]
- 123. Arden NK, Cro S, Sheard S. et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthritis Cartilage 2016;24:1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sanghi D, Mishra A, Sharma AC. et al. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin Orthop Relat Res 2013;471:3556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Heidari B, Javadian Y, Babaei M, Yousef-Ghahari B.. Restorative effect of vitamin D deficiency on knee pain and quadriceps muscle strength in knee osteoarthritis. Acta Medica Iranica 2015;53:466–47. [PubMed] [Google Scholar]
- 126. Zheng S, Jin X, Cicuttini F. et al. Maintaining vitamin D sufficiency is associated with improved structural and symptomatic outcomes in knee osteoarthritis. Am J Med 2017;130:1211–8. [DOI] [PubMed] [Google Scholar]
- 127. Autier P, Boniol M, Pizot C, Mullie P.. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2014;2:76–89. [DOI] [PubMed] [Google Scholar]
- 128. Waterhouse JC, Perez TH, Albert PJ.. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann N Y Acad Sci 2009;1173:757–65. [DOI] [PubMed] [Google Scholar]
- 129. Mithal A, Wahl DA, Bonjour JP. et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–20. [DOI] [PubMed] [Google Scholar]
- 130. Holick MF, Binkley NC, Bischoff-Ferrari HA. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 131. Shearer MJ, Newman P.. Metabolism and cell biology of vitamin K. Thromb Haemost 2008;100:530–47. [PubMed] [Google Scholar]
- 132. Beckner KL. Vitamin K dependent carboxylation. Vitam Horm 2008;78:131–56. [DOI] [PubMed] [Google Scholar]
- 133. Munroe P, Olgunturk R, Fryns J. et al. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet 1999;21:142–4. [DOI] [PubMed] [Google Scholar]
- 134. Neogi T, Booth SL, Zhang YQ. et al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum 2006;54:1255–61. [DOI] [PubMed] [Google Scholar]
- 135. Oka H, Akune T, Muraki S. et al. Association of low dietary vitamin K intake with radiographic knee osteoarthritis in the Japanese elderly population: dietary survey in a population-based cohort of the ROAD study. J Orthop Sci 2009;14:687–92. [DOI] [PubMed] [Google Scholar]
- 136. Shea MK, Booth SL, Massaro JM. et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol 2008;167:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shea MK, Kritchevsky SB, Hsu FC. et al. The association between vitamin K status and knee osteoarthritis features in older adults: the Health, Aging and Body Composition Study. Osteoarthritis Cartilage 2015;23:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Misra D, Booth SL, Tolstykh I. et al. Vitamin K deficiency is associated with incident knee osteoarthritis. Am J Med 2013;126:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Neogi T, Felson DT, Sarno R, Booth SL.. Vitamin K and hand osteoarthritis: results from a randomised controlled trial. Ann Rhuem Dis 2008;67:1570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Shea MK, Booth SL.. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients 2016;8:E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mensink GB, Beitz R.. Food and nutrient intake in East and West Germany, 8 years after the reunification—The German Nutrition Survey 1998. Eur J Clin Nutr 2004;58:1000–10. [DOI] [PubMed] [Google Scholar]
- 142. United States Department of Agriculture, Agricultural Research Service. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2009-2010. 2012. www.ars.usda.gov/ba/bhnrc/fsrg (1 February 2017, date last accessed).
- 143. Schurgers L, Geleijnse J, Grobbee D. et al. Nutritional intake of vitamins K1 (phylloquinone) and K2 (menaquinone) in the Netherlands. J Nutr Environ Med 1999;9:115–22. [Google Scholar]
- 144. Kimura N, Fukuwatari T, Sasaki R, Hayakawa F, Shibata K.. Vitamin intake in Japanese women college students. J Nutr Sci Vitaminol 2003;49:149–55. [DOI] [PubMed] [Google Scholar]
- 145. Yan L, Zhou B, Greenberg D. et al. Vitamin K status of older individuals in northern China is superior to that of older individuals in the UK. Br J Nutr 2004;92:939–45. [DOI] [PubMed] [Google Scholar]
- 146. Thane CW, Paul A, Bates CJ. et al. Intake and sources of phylloquinone (vitamin K1): variation with socio-demographic and lifestyle factors in a national sample of British elderly people. Br J Nutr 2002;87:605–13. [DOI] [PubMed] [Google Scholar]
- 147. World Health Organization. Obesity and Overweight. 2017. http://www.who.int/mediacentre/factsheets/fs311/en/ (4 February 2017, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.