This article reports a randomized, placebo‐controlled, double‐blind trial that evaluated the efficacy and safety of prophylactic antiemetic treatment with prochlorperazine for the prevention of opioidinduced nausea and vomiting in patients with cancer.
Keywords: Opioid‐induced nausea and vomiting, Prophylaxis, Antiemetics, Cancer pain, Oxycodone, Prochlorperazine
Abstract
Background.
Although opioid‐induced nausea and vomiting (OINV) often result in analgesic undertreatment in patients with cancer, no randomized controlled trials have evaluated the efficacy of prophylactic antiemetics for preventing OINV. We conducted this randomized, placebo‐controlled, double‐blind trial to evaluate the efficacy and safety of prophylactic treatment with prochlorperazine for preventing OINV.
Materials and Methods.
Cancer patients who started to receive oral oxycodone were randomly assigned in a 1:1 ratio to receive either prochlorperazine 5 mg or placebo prophylactically, given three times daily for 5 days. The primary endpoint was the proportion of patients who had a complete response (CR) during the 120 hours of oxycodone treatment. CR was defined as no emetic episode and no use of rescue medication for nausea and vomiting during 5 days. Key secondary endpoints were the proportion of patients with emetic episodes, proportion of patients with moderate or severe nausea, quality of life, and proportion of treatment withdrawal.
Results.
From November 2013 through February 2016, a total of 120 patients were assigned to receive prochlorperazine (n = 60) or placebo (n = 60). There was no significant difference in CR rates (69.5% vs. 63.3%; p = .47) or any secondary endpoint between the groups. Patients who received prochlorperazine were more likely to experience severe somnolence (p = .048).
Conclusion.
Routine use of prochlorperazine as a prophylactic antiemetic at the initiation of treatment with opioids is not recommended. Further research is needed to evaluate whether other antiemetics would be effective in preventing OINV in specific patient populations.
Implications for Practice.
Prophylactic prochlorperazine seems to be ineffective in preventing opioid‐induced nausea and vomiting (OINV) and may cause adverse events such as somnolence. Routine use of prophylactic prochlorperazine at the initiation of treatment with opioids is not recommended. Further research is needed to evaluate whether other antiemetics would be effective in preventing OINV in specific patient populations.
Introduction
Pain is one of the most common symptoms in patients with cancer [1], [2], [3]. About 38% to 80% of patients with advanced cancer experience moderate to severe pain [1], [2], [3], and opioids are generally effective for alleviating cancer pain [4], [5], [6]. However, the use of opioids often causes nausea and vomiting, especially during the early period of treatment. Opioid‐induced nausea and vomiting (OINV) occur in up to 60% of patients who start to receive opioids [7], [8], [9], [10], [11].
Although many patients eventually develop tolerance to this side effect, nausea and vomiting during the early phase of treatment often lead patients to discontinue their opioid therapy, resulting in analgesic undertreatment [9], [10], [11]. Thus, appropriate management of OINV is expected to improve patient compliance, enhance analgesic efficacy, reduce opioid‐induced complications, and improve the overall quality of life (QOL) [9], [10], [11].
The efficacy of prophylactic antiemetics in patients with cancer has been tested in various clinical settings, including chemotherapy, radiation therapy, surgical procedures, and parenteral morphine administration in an emergency setting [12], [13], [14], [15]. However, the efficacy of prophylactic antiemetics for the prevention of OINV in patients with cancer pain remains to be fully elucidated [16], [17], [18], [19], [20], [21]. To date, several preliminary observational studies have investigated the efficacy of antiemetic prophylaxis against OINV among patients with cancer pain [16], [17], [18], [19], [20]. However, to our knowledge, no randomized controlled trial has evaluated the efficacy of prophylactic treatment with antiemetics for the prevention of OINV [22].
In particular, a placebo controlled trial is lacking, and the use of placebo as a commentator is of importance. We thus conducted this randomized, placebo‐controlled, double‐blind trial to evaluate the efficacy and safety of prophylactic antiemetic treatment with prochlorperazine for the prevention of OINV in patients with cancer.
Subjects, Materials, and Methods
Study Design and Participants
This study was a single‐center, double‐blind, 1:1 placebo‐controlled, randomized trial designed to evaluate the efficacy of oral prochlorperazine for the prevention of nausea and vomiting in patients receiving oral oxycodone for cancer pain. The study was approved by the Ethics Committee at Nagoya University Hospital and was conducted according to the Declaration of Helsinki. All patients provided written informed consent for participation in this study prior to enrollment. The study is registered at University Hospital Medical Information Network (UMIN000012502).
Patients with cancer of any primary site who required oral oxycodone were enrolled in this study. The eligibility criteria included an age of ≥20 years, a life expectancy of 1 month or longer, an Eastern Cooperative Oncology Group (ECOG) performance status [23] of 0 to 3, aspartate transaminase and alanine transaminase levels ≤5× the upper limit of the normal range in the institution (ULN), a total bilirubin level of ≤3× ULN, and a creatinine clearance (Cockcroft‐Gault) of ≥30 mL/min. Patients were excluded if they were already receiving any strong opioids (i.e., morphine, oxycodone, fentanyl, or methadone); had vomiting, retching, or nausea of any causes; had received radiotherapy to the head, abdomen, or pelvis within 6 days before and during the study period; had received new drugs with emetic or antiemetic action within 48 hours before study initiation; or had a history of hypersensitivity or any contraindication to prochlorperazine. We also excluded patients who had received new chemotherapeutic agents for at least 1 week or longer before treatment with the study drug and during the study period. Patients who had received palonosetron or fosaprepitant within the last 120 hours, aprepitant within the last 72 hours, and steroids or 5‐HT3 antagonists within the last 48 hours as a part of chemotherapy‐induced nausea and vomiting prophylaxis were also excluded.
Although we had acknowledged that a history of exposure to weak opioids could confound the results, we did not exclude patients who had used weak opioids (i.e., codeine and tramadol) in order to maximize patient enrollment and to more closely reflect the real‐world situation, in which patients often will have received weak opioids before receiving oxycodone.
Randomization and Masking
Patients who satisfied all study entry criteria were randomly assigned (1:1) to the prochlorperazine group or the placebo group by a web‐based randomization system using a stratified randomized model. Randomization was stratified according to sex, age (≤55, 56–69, ≥70 years), ECOG performance status (PS: 0, 1 vs. 2, 3), and pain score (numerical rating scale [NRS]: ≥7 vs. <7). A double‐blind technique was used with a placebo identical in appearance to prochlorperazine tablets, which were encapsulated. The encapsulated prochlorperazine and placebo capsules were manufactured by an unblinded pharmacist who was not involved in providing direct care to the study patients.
Procedures
All patients received oral sustained‐release oxycodone (5 mg or 10 mg) twice daily for cancer pain (10 mg/day or 20 mg/day). Patients were randomly assigned to receive either prochlorperazine 5 mg or matched placebo orally three times daily for 5 days (15 mg/day). The initial dose of prochlorperazine or placebo was administered 30 to 60 minutes before the administration of the initial dose of oxycodone on day 1. The rationale for choosing prochlorperazine as an antiemetic for this study included the following: (a) there was no empirical evidence one antiemetic could be superior to others for the management of OINV [22], (b) some international clinical guidelines and reviews list prochlorperazine as one of the first‐line medications for OINV [6], [7], and (c) prochlorperazine is the most common antiemetic used in this setting in Japan [16], [17], [18], [19], [20], [21].
The use of immediate‐release oxycodone as rescue medication, as well as increasing the dose of sustained‐release oxycodone, was allowed at any time depending on the pain intensity. For patients who developed nausea and vomiting, metoclopramide, haloperidol, domperidone, or chlorpheniramine maleate was used for rescue treatment according to the preference of the treating physician.
Patients completed a daily diary during the study period of 5 days every 24 hours, which included information on the presence or absence of emetic episodes (if present, the times when episodes occurred), the use of rescue antiemetics (if required, the time when used), oxycodone dosage, the intensity of worst nausea according to an 11‐point Likert‐type NRS from 0 (none) to 10 (worst), and adverse effects. The NRS of nausea was formatted according to the Japanese version of the Edmonton Symptom Assessment Schedule [24], [25]. Compliance of outpatients with diary completion was checked via telephone contact. The QOL data were recorded by the patients before the study and on day 7 after the study period using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C15‐PAL questionnaire [26], [27].
Outcome Measures
Owing to the lack of established outcome measures in this setting, we decided to adopt commonly used outcome measures for chemotherapy‐induced nausea and vomiting [28], [29]. The primary endpoint of this study was the proportion of patients who had a complete response (CR) during the 120 hours of treatment with oxycodone [28], [29]. CR was defined as no emetic episode and no use of rescue medication for nausea and vomiting. An emetic episode was defined as one episode of vomiting or retching. Distinct emetic episodes were defined as episodes that were separated by the absence of emesis or retching for 5 minutes or longer [28], [29].
Furthermore, we investigated multiple secondary endpoints as follows: the proportion of patients who had at least one emetic episode during the study period; the proportion of patients who required rescue antiemetics during the study period; the cumulative number of emetic episodes in patients who had at least one emetic episode during the study period; the proportions of patients with nausea, moderate nausea (≥4), and severe nausea (≥7) [30]; QOL as assessed by the EORTC QLQ‐C15‐PAL questionnaire; the proportion of patients in whom oxycodone treatment was withdrawn for any reason; and the times to the first emetic episode and the first use of rescue antiemetics for nausea and vomiting. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0) [31].
EORTC QLQ‐C15‐PAL is a QOL questionnaire designed for use in patients receiving palliative care [26], [27]. This questionnaire includes two functional subscales (emotional and physical), seven symptom subscales (e.g., nausea/vomiting, pain), and an overall QOL question. Each subscale is converted to a score of 0–100. High scores on a functional subscale correspond to better functioning, while high scores on a symptom subscale correlate with increased symptom burden.
Statistical Analysis
Previous observational studies estimated that nausea, vomiting, or both developed in 8% to 36% of the patients who received opioids with prophylactic antiemetics [16], [17], [20], as compared with 26% to 67% of untreated patients or the study group as a whole [4], [7], [8], [9], [10], [16], [17]. Therefore, we assumed a CR rate of 80% in the prochlorperazine group and 55% in the placebo group and calculated that at least 108 patients would have to be accrued for the study to have an 80% power to detect significant differences between the groups in the primary endpoint at a two‐sided significance level of 5%, using the χ2 test. To allow for an estimated withdrawal or dropout rate of 10%, a target sample size of 120 patients (60 per arm) was selected.
Analyses of the efficacy results were performed on an intention‐to‐treat basis. Safety was evaluated in patients who received at least one dose of the study drug. No interim analyses were performed. The proportion of patients with a CR was calculated for each group and compared using the χ2 test. As secondary endpoints, the proportions of patients with emetic episodes, patients requiring rescue antiemetics, patients with nausea (any, moderate, severe), and patients in whom treatment was withdrawn were calculated for each group and compared using the χ2 test. The number of emetic episodes was compared between the groups using the Mann‐Whitney U test. Time‐to‐event endpoints (i.e., first emetic episode and first use of rescue antiemetics) were analyzed using Kaplan‐Meier methods and were tested using the log‐rank test. An analysis of covariance model was used to analyze data from the EORTC QLQ‐C15‐PAL questionnaire, including treatment group and baseline QOL as covariates.
Subgroup analyses with respect to the primary endpoint were performed and included gender, primary tumor site, opioid‐naïve status, and initial oxycodone dose as factors. Adverse events were analyzed with the use of the Cochran‐Armitage trend test. All tests were two‐sided, and p values of less than .05 were considered to indicate statistical significance. Statistical analyses were performed using JMP 11 software (SAS Institute, Inc., Cary, NC, www.sas.com).
Results
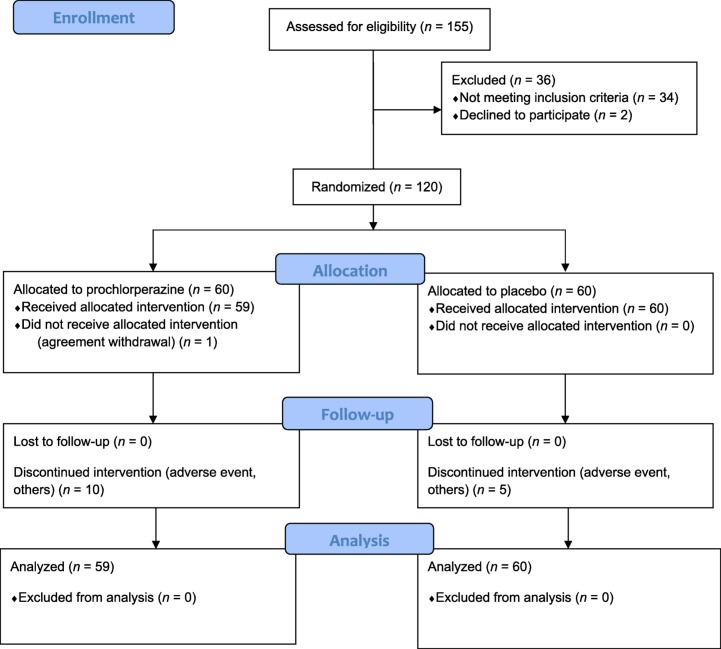
From November 2013 through February 2016, a total of 156 patients were screened, and 120 were randomly assigned, in a 1:1 ratio, to receive prochlorperazine (n = 60) or placebo (n = 60; Fig. 1). One patient in the prochlorperazine group withdrew consent after the collection of baseline data and was excluded from the efficacy analysis. Baseline patient characteristics were balanced between the groups (Table 1). The median age was 70.0 years, 63% of the patients were men, and the most common site of cancer was the lung (35%) followed by the breast (10%). The majority of patients were opioid naïve (72%).
Figure 1.
CONSORT 2010 Flow Diagram. Screening, randomization, and follow‐up.
Table 1. Patient characteristics.
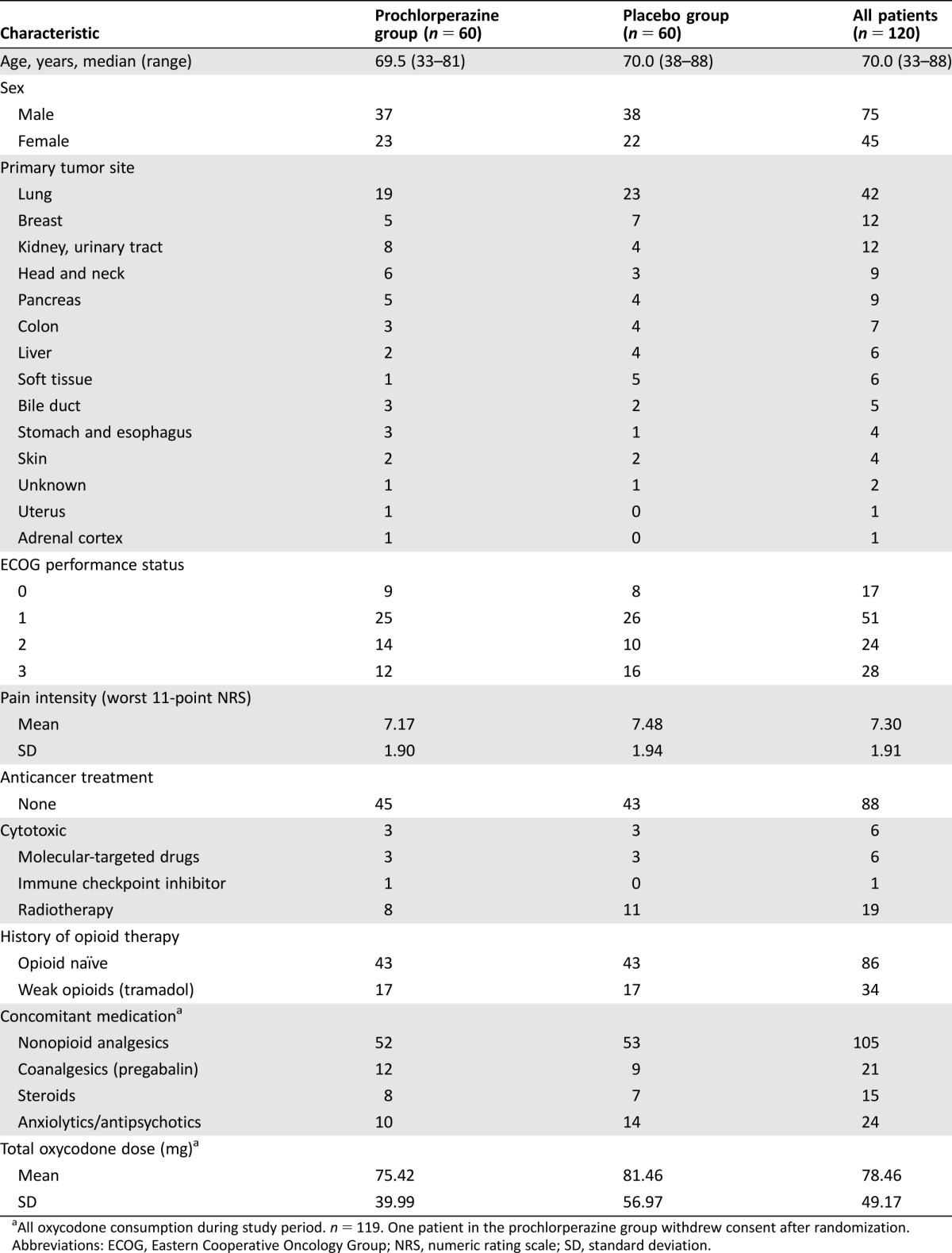
All oxycodone consumption during study period. n = 119. One patient in the prochlorperazine group withdrew consent after randomization.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NRS, numeric rating scale; SD, standard deviation.
Efficacy
There was no significant difference in the CR rate between the prochlorperazine group and the placebo group (69.5% vs. 63.3%, p = .47; Table 2). There was also no significant difference in any secondary endpoints between the groups (Table 2). Prochlorperazine had no impact on the median time to the first emetic episode or the first use of rescue antiemetic medication as compared with placebo (21.8 hours vs. 19.5 hours, p = .82; 21.3 hours vs. 22.3 hours, p = .73; Fig. 2). Ten patients in the prochlorperazine group discontinued the study because of severe vomiting (n = 5), delirium (n = 2), poor pain control (n = 2), or drug‐induced hepatic dysfunction (n = 1). In the placebo group, five patients discontinued the study because of severe vomiting (n = 3), poor pain control (n = 1), or off‐protocol drug use (n = 1).
Table 2. Primary and secondary endpoints.
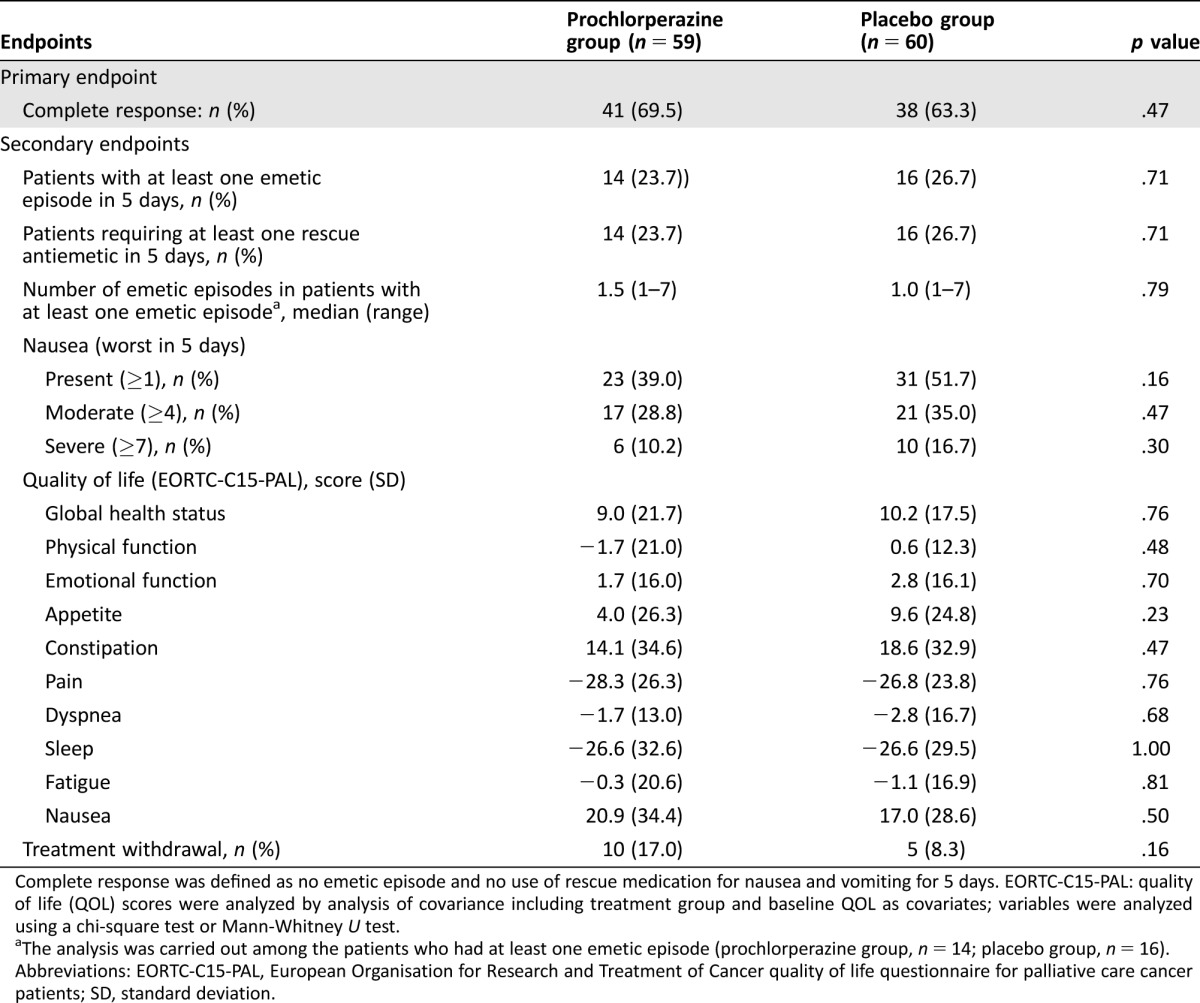
Complete response was defined as no emetic episode and no use of rescue medication for nausea and vomiting for 5 days. EORTC‐C15‐PAL: quality of life (QOL) scores were analyzed by analysis of covariance including treatment group and baseline QOL as covariates; variables were analyzed using a chi‐square test or Mann‐Whitney U test.
The analysis was carried out among the patients who had at least one emetic episode (prochlorperazine group, n = 14; placebo group, n = 16).
Abbreviations: EORTC‐C15‐PAL, European Organisation for Research and Treatment of Cancer quality of life questionnaire for palliative care cancer patients; SD, standard deviation.
Figure 2.
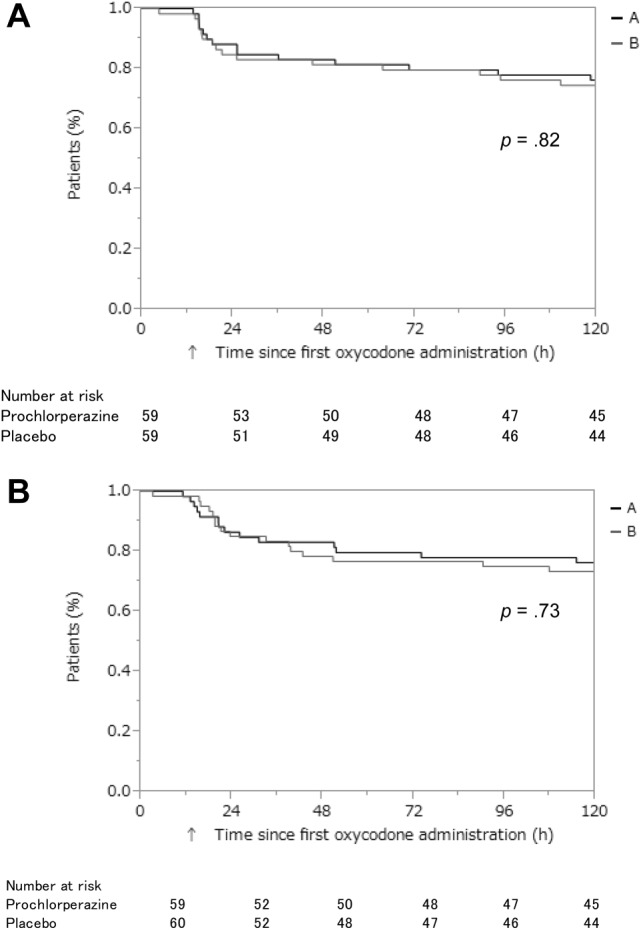
Time to first emetic episode and first rescue antiemetic medication. Time‐to‐event endpoints were analyzed using Kaplan‐Meier methods and were tested using the log‐rank test. (A): Time to first emetic episode. (B): Time to first rescue antiemetic medication.
Abbreviations: A, Prochlorperazine group; B, Placebo group; h, hours.
On subgroup analyses, the CR rate did not differ significantly between the prochlorperazine group and placebo group in any subgroups investigated (Table 3). The CR rates in patients with cancer of the digestive system (stomach, esophagus, colon, liver, bile duct, and pancreas) were lower than the CR rate in patients with lung cancer. Additional subgroup analysis by the stratification factor in forest plots revealed no statistically significant interaction of CR (Fig. 3).
Table 3. Subgroup analyses.
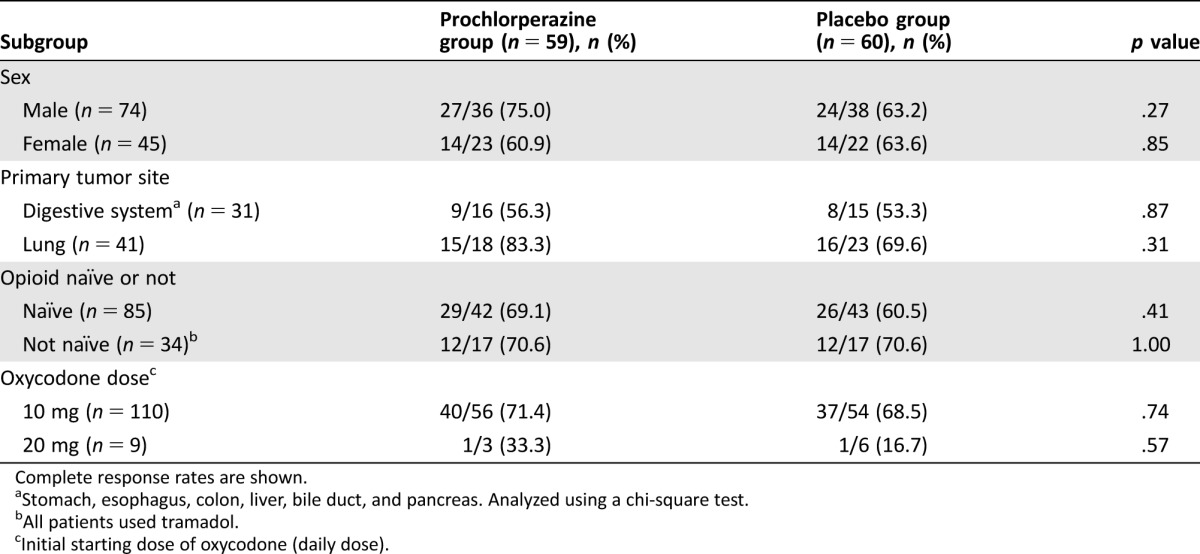
Complete response rates are shown.
Stomach, esophagus, colon, liver, bile duct, and pancreas. Analyzed using a chi‐square test.
All patients used tramadol.
Initial starting dose of oxycodone (daily dose).
Figure 3.
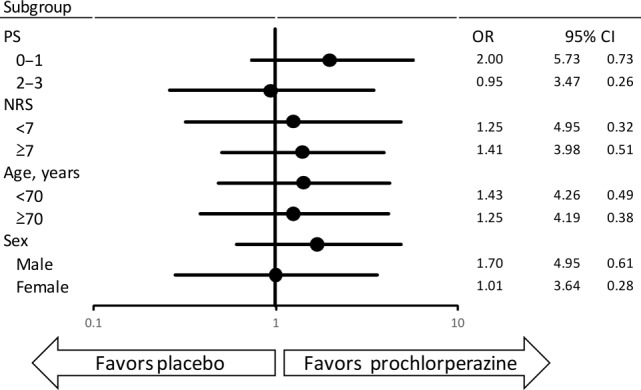
Forest plots of complete response
Abbreviations: CI, confidence interval; NRS, numeric rating scale; OR, odds ratio; PS, performance status.
Adverse Events
Adverse events were reported in 58 (98.3%) patients in the prochlorperazine group and 57 (95%) patients in the placebo group. The most common adverse events were somnolence (72.3%), constipation (65.5%), appetite loss (44.5%), nausea (44.5%), and vomiting (24.4%). More patients in the prochlorperazine group experienced somnolence (p = .048) and delirium (p = .09; Table 4).
Table 4. Adverse events.
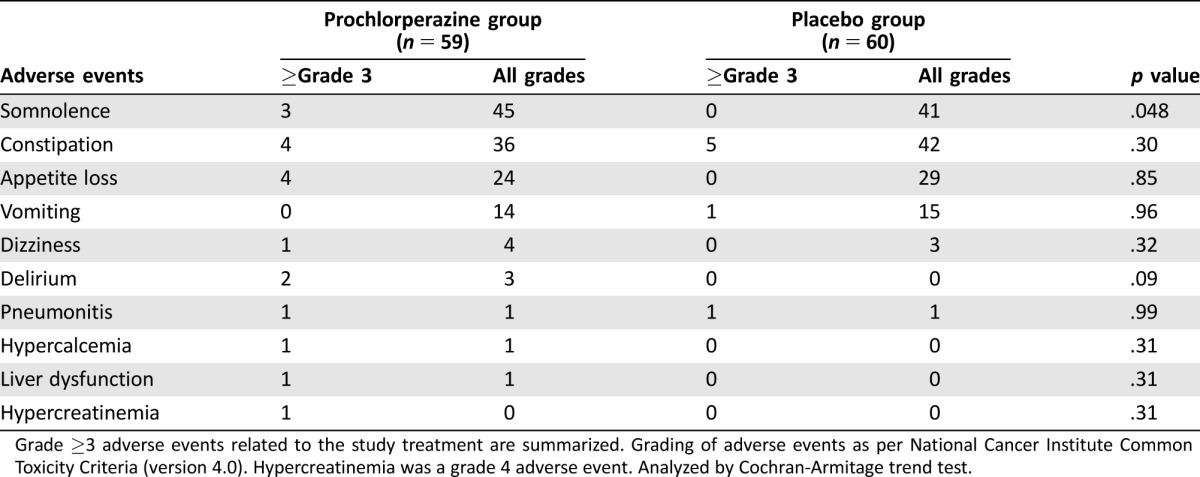
Grade ≥3 adverse events related to the study treatment are summarized. Grading of adverse events as per National Cancer Institute Common Toxicity Criteria (version 4.0). Hypercreatinemia was a grade 4 adverse event. Analyzed by Cochran‐Armitage trend test.
Other adverse events occurred with similar frequencies in both groups. Grade 4 hypercreatinemia occurred in one patient in the prochlorperazine group but was considered unrelated to the study drug.
Discussion
To the best of our knowledge, this is the first double‐blind, randomized, controlled trial to evaluate the efficacy of prophylactic antiemetic treatment against OINV in patients with cancer. The efficacy of prophylactic antiemetic treatment was investigated only in observational studies, with conflicting results. A previous single‐center study showed that interventions to promote the use of prophylactic medication might be effective in reducing the risk of opioid‐induced nausea/vomiting [16]. On the other hand, a multicenter retrospective study of 619 patients, a single‐center retrospective study of 280 patients, and another retrospective study in 416 patients reported that incidence of nausea or vomiting was similar regardless of prophylactic treatment with dopamine blockers [17], [18], [19]. In this study, there was no significant difference in the CR rates between the prochlorperazine group and placebo group. Other secondary endpoints (i.e., the number of patients who had emetic episodes, number of the patients who required antiemetics, intensity of nausea as evaluated with an NRS, median time to first emetic episode/use of rescue antiemetic medications, and quality of life subscales) did not differ significantly between the intervention group and control group. On the other hand, patients who received prochlorperazine were more likely to have severe somnolence and delirium. Therefore, we conclude that the routine use of prophylactic prochlorperazine at the initiation of treatment with opioids is not beneficial and rather might be detrimental to patients.
Our results demonstrated evidence that prochlorperazine is ineffective to prevent OINV, but it is still unclear whether the use of other antiemetics, such as olanzapine, would have resulted in beneficial outcomes, and this remains a topic for future research. Of note, we found a higher incidence of nausea and vomiting in patients with cancer of the digestive system; that is, about 50% of patients had emetic episodes after initial administration of oral oxycodone. Future research thus could focus on this high‐risk subgroup of patients.
Determining which outcome measure is most appropriate as a primary endpoint is challenging in many areas of palliative‐care research. There are no established outcomes that can be used in clinical trials assessing the efficacy of a medication to prevent OINV. In this study, we measured a variety of outcomes as secondary endpoints, and the results have the same directions. The set of outcomes adopted in this study, such as the CR rate, number of emetic episodes/rescue use of antiemetics, intensity of nausea, and the time to the first emetic episode/rescue use of antiemetics, provide interpretable findings and can be used in further studies.
Our study had several limitations. First, the study population was heterogeneous in terms of primary tumor sites and exposure to weak opioids. Although the majority of our patients had lung or breast cancer and subgroup analyses demonstrated no statistically significant difference in primary endpoints, there is a possibility that different outcomes might have been obtained in patients with homogeneous primary tumors arising in other sites. We included patients who had received weak opioids to increase feasibility, and about one third of our study patients had previously received tramadol. While subgroup analyses did not detect any significant difference in CR rates, there is also a possibility that trial on purely opioid‐naïve patients might obtain different results. Second, this was a single‐center study performed in Japanese patients, which might limit the generalizability of the findings to other populations. Third, we used oxycodone at a starting dose of 10 mg/day in most patients in this study because these dose levels are most commonly used and are approved by the Ministry of Health, Labor, and Welfare in Japan. Although the doses might be considered low in other parts of the world [4], titration of oxycodone was performed simultaneously in this study and we believe it is unlikely that the use of a higher starting dose of oxycodone would have led to different results. Fourth, we adopted CR rates as the primary endpoint of this study. The use of other measures might have led to different results, although we believe this is unlikely because all secondary outcomes had the same direction. Finally, the lack of a significant difference in emetic episodes between men and women may be related to the small number of subjects.
Conclusion
Prophylactic prochlorperazine seems to be ineffective in preventing OINV and might rather cause adverse events such as somnolence. Routine use of prophylactic prochlorperazine at the initiation of treatment with opioids is not recommended. Further research is needed to evaluate whether other antiemetics would be effective in preventing OINV in specific patient populations.
Acknowledgments
This study was supported by grants from Research Advancement on Palliative Medicine, Japanese Society for Palliative Medicine (2013). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Our study was guaranteed by monitoring and inspection, performed with the support of an external contract research organization (EPS, Tokyo, Japan). We thank the patients, their families, the investigators, and the teams who participated in this trial, and Noriko Kiriyama, Akane Kurachi, Hatue Kume, and Ai Maeda for manufacturing the study drugs.
Author Contributions
Conception/design: Hiroaki Tsukuura, Masayuki Miyazaki, Mihoko Sugishita, Yuichi Ando
Provision of study material or patients: Hiroaki Tsukuura, Masayuki Miyazaki, Mihoko Sugishita, Hiroshi Kato, Yoko Kubo, Masahiko Ando, Masashi Kondo, Kiyofumi Yamada, Yoshinori Hasegawa, Yuichi Ando
Collection and/or assembly of data: Hiroaki Tsukuura, Masayuki Miyazaki, Mihoko Sugishita, Hiroshi Kato, Yuka Murasaki
Data analysis and interpretation: Hiroaki Tsukuura, Masayuki Miyazaki, Tatsuya Morita, Mihoko Sugishita, Hiroshi Kato, Yuka Murasaki, Bishal Gyawali, Yoko Kubo, Masahiko Ando, Masashi Kondo, Kiyofumi Yamada, Yoshinori Hasegawa, Yuichi Ando
Manuscript writing: Hiroaki Tsukuura, Masayuki Miyazaki, Tatsuya Morita, Mihoko Sugishita, Hiroshi Kato, Yuka Murasaki, Bishal Gyawali, Yoko Kubo, Masahiko Ando, Masashi Kondo, Kiyofumi Yamada, Yoshinori Hasegawa, Yuichi Ando
Final approval of manuscript: Hiroaki Tsukuura, Masayuki Miyazaki, Tatsuya Morita, Mihoko Sugishita, Hiroshi Kato, Yuka Murasaki, Bishal Gyawali, Yoko Kubo, Masahiko Ando, Masashi Kondo, Kiyofumi Yamada, Yoshinori Hasegawa, Yuichi Ando
Disclosures
Yuichi Ando: Shionogi (H); Masashi Kondo: Eli Lilly & Co, Chugai Pharma, Pfizer, and Boehringer Ingelheim, Novartis, Ono Pharmaceutical (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. van den Beuken‐van Everdingen MH, de Rijke JM, Kessels AG et al. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol 2007;18:1437–1449. [DOI] [PubMed] [Google Scholar]
- 2. Breivik H, Cherny N, Collett B et al. Cancer‐related pain: A pan‐European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009;20:1420–1433. [DOI] [PubMed] [Google Scholar]
- 3. van den Beuken‐van Everdingen MH, Hochstenbach LM, Joosten EA et al. Update on prevalence of pain in patients with cancer: Systematic review and meta‐analysis. J Pain Symptom Manage 2016;51:1070–1090. [DOI] [PubMed] [Google Scholar]
- 4. Caraceni A, Hanks G, Kaasa S et al. Use of opioid analgesics in the treatment of cancer pain: Evidence‐based recommendation from the EAPC. Lancet Oncol 2012;13:e58–e68. [DOI] [PubMed] [Google Scholar]
- 5. Ripamonti CI, Santini D, Maranzano E et al. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23:139–154. [DOI] [PubMed] [Google Scholar]
- 6. Swarm R, Abernethy AP, Anghelescu DL et al. Adult cancer pain. J Natl Compr Canc Netw 2013;11:992–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNicol E, Horowicz‐Mehler N, Fisk RA et al. Management of opioid side effects in cancer‐related and chronic noncancer pain: A systematic review. J Pain 2003;4:231–256. [DOI] [PubMed] [Google Scholar]
- 8. Hanks GW, Justins DM. Cancer pain: Management. Lancet 1992;339:1031–1036. [DOI] [PubMed] [Google Scholar]
- 9. Laugsand EA, Kaasa S, Klepstad P. Management of opioid‐induced nausea and vomiting in cancer patients: Systematic review and evidence‐based recommendations. Palliat Med 2011;25:442–53. [DOI] [PubMed] [Google Scholar]
- 10. Nicholson B. Responsible prescribing of opioids for the management of chronic pain. Drugs 2003;63:17–32. [DOI] [PubMed] [Google Scholar]
- 11. Cherny N, Ripamonti C, Pereira J et al. Strategies to manage the adverse effects of oral morphine: An evidence‐based report. J Clin Oncol 2001;19:2542–2554. [DOI] [PubMed] [Google Scholar]
- 12. Basch E, Prestrud AA, Hesketh PJ et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute of Canada Clinical Trials Group (SC19), Wong RK, Paul N et al. 5‐hydroxytryptamine‐3 receptor antagonist with or without short‐course dexamethasone in the prophylaxis of radiation induced emesis: A placebo‐controlled randomized trial of the National Cancer Institute of Canada Clinical Trials Group (SC19). J Clin Oncol 2006;24:3458–3464. [DOI] [PubMed] [Google Scholar]
- 14. Schaub I, Lysakowski C, Elia N et al. Low‐dose droperidol (≤1 mg or ≤15 μg kg‐1) for the prevention of postoperative nausea and vomiting in adults: Quantitative systematic review of randomized controlled trials. Eur J Anaesthesiol 2012;29:286–294. [DOI] [PubMed] [Google Scholar]
- 15. Simpson PM, Bendall JC, Middleton PM. Review article: Prophylactic metoclopramide for patients receiving intravenous morphine in the emergency setting: A systematic review and meta‐analysis of randomized controlled trials. Emerg Med Australas 2011;23:452–457. [DOI] [PubMed] [Google Scholar]
- 16. Ishihara M, Ishihara H, Okayasu S et al. Pharmaceutical interventions facilitate premedication and prevent opioid‐induced constipation and emesis in cancer patients. Support Care Cancer 2010;18:1531–1538. [DOI] [PubMed] [Google Scholar]
- 17. Ishihara M, Ikesue H, Matsunaga H et al. A multi‐institutional study analyzing effect of prophylactic medication for prevention of opioid‐induced gastrointestinal dysfunction. Clin J Pain 2012;28:373–381. [DOI] [PubMed] [Google Scholar]
- 18. Kanbayashi Y, Hosokawa T. Predictive factors for nausea or vomiting in patients with cancer who receive oral oxycodone for the first time: Is prophylactic medication for prevention of opioid‐induced nausea or vomiting necessary? J Palliat Med 2014;17:683–687. [DOI] [PubMed] [Google Scholar]
- 19. Okamoto Y, Tsuneto S, Tsugane M et al. A retrospective chart review of opioid‐induced nausea and somnolence on commencement for cancer pain treatment. J Opioid Manag 2010;6:431–434. [DOI] [PubMed] [Google Scholar]
- 20. Tashiro M, Naito T, Ohnishi K et al. Impact of genetic and non‐genetic factors on clinical responses to prochlorperazine in oxycodone‐treated cancer patients. Clin Chim Acta 2014;429:175–180. [DOI] [PubMed] [Google Scholar]
- 21. Tsukuura H, Ando Y, Gyawali B et al. Prophylactic use of antiemetics for prevention of opioid‐induced nausea and vomiting: A questionnaire survey among Japanese physicians. J Palliat Med 2015;18:977–980. [DOI] [PubMed] [Google Scholar]
- 22. Walsh D, Davis M, Ripamonti C et al. 2016 Updated MASCC/ESMO consensus recommendations: Management of nausea and vomiting in advanced cancer. Support Care Cancer 2017;25:333–340. [DOI] [PubMed] [Google Scholar]
- 23. Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- 24. Bruera E, Kuehn N, Miller MJ et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 25. Yokomichi N, Morita T, Nitto A et al. Validation of the Japanese version of the Edmonton Symptom Assessment System‐Revised. J Pain Symptom Manage 2015;50:718–723. [DOI] [PubMed] [Google Scholar]
- 26. Groenvold M, Petersen MA, Aaronson NK et al. The development of the EORTC QLQ‐C‐15‐PAL: A shortened questionnaire for cancer patients in palliative care. Eur J Cancer 2006;42:55–64. [DOI] [PubMed] [Google Scholar]
- 27. Miyashita M, Wada M, Morita T et al. Independent validation of the Japanese version of the EORTC QLQ‐C‐15‐PAL for patients with advanced cancer. J Pain Symptom Manage 2015;49:953–959. [DOI] [PubMed] [Google Scholar]
- 28. Maemondo M, Masuda N, Sekine I et al. A phase II study of palonosetron combined with dexamethasone to prevent nausea and vomiting induced by highly emetogenic chemotherapy. Ann Oncol 2009;20:1860–1866. [DOI] [PubMed] [Google Scholar]
- 29. Saito H, Yoshizawa H, Yoshimori K et al. Efficacy and safety of single‐dose fosaprepitant in the prevention of chemotherapy‐induced nausea and vomiting in patients receiving high‐dose cisplatin: A multicentre, randomized, double‐blind, placebo‐controlled phase 3 trial. Ann Oncol 2013;24:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamaguchi T, Morita T, Nitto A et al. Establishing cutoff points for defining symptom severity using the Edmonton Symptom Assessment System‐Revised Japanese Version. J Pain Symptom Manage 2016;51:292–297. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Rockville, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009.