This article reviews the importance of rituximab in the current treatment of chronic lymphocytic leukemia (CLL) and the scientific basis for its future role in combination with CLL‐directed therapies. New and emerging agents in the treatment of CLL are examined, including rituximab biosimilars that are currently in clinical development.
Keywords: Access to health care, Biosimilars, Chronic lymphocytic leukemia, Rituximab
Abstract
Chronic lymphocytic leukemia (CLL) is managed with observation for asymptomatic or clinically silent disease; pharmacologic intervention is generally required for symptomatic patients with clinically significant adenopathy or cytopenia. In the front‐line treatment of CLL, the current standard‐of‐care includes chemotherapy in combination with an anti‐CD20 monoclonal antibody (e.g., rituximab, ofatumumab, or obinutuzumab) or ibrutinib as single agent. Despite the evolving treatment paradigm toward targeted therapy, it is likely that rituximab (plus chemotherapy), with or without targeted agents, will retain a significant role in CLL treatment. However, patents for many biologics, including rituximab, have expired or will expire in the near future. Furthermore, access to rituximab has remained challenging, particularly in countries with restricted resources. Together, these concerns have prompted the development of safe and effective rituximab biosimilars. The term “biosimilar” refers to a biologic that is highly similar to an approved reference (originator) product, notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences in purity, potency, or safety. Biosimilars are developed to treat the same condition(s) using the same treatment regimens as an approved reference biologic and have the potential to increase access to more affordable treatments. We review the importance of rituximab in the current treatment of CLL, the scientific basis of its future role in combination with chemotherapy, and the role of new and emerging agents in the treatment of CLL, which could potentially be used in combination with rituximab biosimilars. We also discuss rituximab biosimilars currently in development.
Implications for Practice.
Front‐line treatments for chronic lymphocytic leukemia (CLL) include chemotherapy in combination with an anti‐CD20 monoclonal antibody (e.g., rituximab, ofatumumab, or obinutuzumab) or ibrutinib as single agent. Despite the evolving treatment paradigm, it is likely rituximab (plus chemotherapy) and targeted agents undergoing clinical evaluation will retain a significant role in CLL treatment. However, patents for many biologics, including rituximab, have expired or will expire in the near future and, in many regions, access to rituximab remains challenging. Together, these concerns have prompted the development of safe and effective rituximab biosimilars, with the potential to increase access to more affordable treatments.
Introduction
Biologic medicines (or biopharmaceuticals) are therapeutic agents produced by a living organism or its products [1], [2]. Rituximab (Rituxan [Genentech, San Francisco, CA] in the U.S., MabThera [Roche, Basel, Switzerland] in Europe) is a chimeric anti‐CD20 monoclonal antibody [3], [4]. The mechanism of action of rituximab likely involves antibody‐dependent cellular cytotoxicity and direct signaling [5]. Rituximab is approved for the treatment of patients with rheumatoid arthritis (RA), granulomatosis with polyangiitis, and microscopic polyangiitis, as well as non‐Hodgkin's lymphomas. In combination with chemotherapy (fludarabine or bendamustine), rituximab is approved in chronic lymphocytic leukemia (CLL) [3], [4]. Although novel targeted small‐molecule therapies are emerging for the treatment of CLL, anti‐CD20 monoclonal antibodies such as rituximab are likely to continue to be prescribed with chemotherapy regimens and potentially in combination with new targeted agents.
Patents for many widely used biologics, including rituximab, have expired or will expire in the near future. Moreover, access to rituximab has been shown to be limited by factors such as availability, reimbursement, and insurance coverage [6]. Consequently, several manufacturers are developing high‐quality, safe, and effective biosimilars [7]. The U.S. Food and Drug Administration (FDA) guidance document defines a biosimilar as “highly similar to an originator [reference] biologic and for which there are no clinically meaningful differences between the two products in terms of safety, purity, and potency” [8]. The primary amino acid sequence of a biosimilar and the reference biologic must be identical, and the two must share the same route of administration, strength, and dosage form [8]. Biosimilars have the potential to mitigate the problems of access to rituximab, increase global opportunities for access to biologic medicines, and offer savings across health‐care systems.
Biosimilars have the potential to mitigate the problems of access to rituximab, increase global opportunities for access to biologic medicines, and offer savings across health‐care systems.
The purpose of this article is to review the importance of rituximab in the current treatment of CLL and the scientific basis of its role in combination with CLL‐directed therapies in the future. We examine the role of new and emerging agents in the treatment of CLL, which could potentially be used in combination with rituximab biosimilars. We also discuss rituximab biosimilars currently in clinical development.
The Current Standard‐of‐Care Treatment in CLL
Chemotherapy in Combination with Anti‐CD20 Monoclonal Antibodies
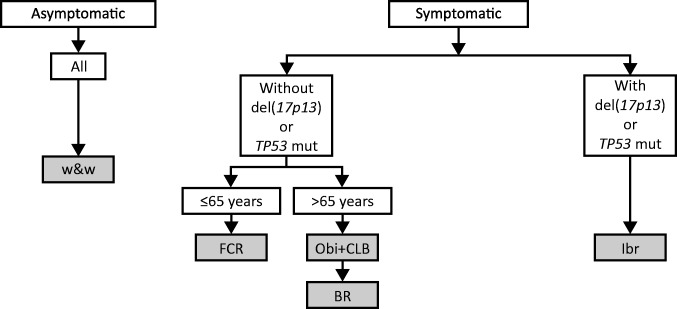
CLL is currently managed by observation for asymptomatic disease (based on the International Workshop on Chronic Lymphocytic Leukemia criteria) [9], [10]. Therapeutic intervention is generally deemed necessary for symptomatic patients with clinically significant adenopathy or cytopenia (Fig. 1) [9], [10], [11]. In the front‐line treatment of CLL, the current standard of care includes chemotherapy (fludarabine plus cyclophosphamide [FC], bendamustine, or chlorambucil [CLB]) in combination with an anti‐CD20 monoclonal antibody (e.g., rituximab, ofatumumab, or obinutuzumab) or ibrutinib as single agent [12], [13], [14]. The chemoimmunotherapy regimens are selected on the basis of patient fitness and are suitable for those without the high‐risk markers of 17p deletion or TP53 mutations, who typically have a poor prognosis and are largely resistant to chemoimmunotherapy regimens [15], [16], [17]. Ibrutinib is appropriate in the front‐line setting but of particular importance among patients with 17p deletion or TP53 mutation.
Figure 1.
First‐line regimens in the treatment of chronic lymphocytic leukemia [65].
Abbreviations: BR, bendamustine, rituximab; CLB, chlorambucil; FCR, fludarabine, cyclophosphamide, and rituximab; Ibr, ibrutinib; mut, mutations; Obi, obinutuzumab; w&w, watch and wait.
The approval of combination fludarabine, cyclophosphamide, and rituximab (FCR) as first‐line therapy was based on a randomized, phase III study in treatment‐naïve, physically fit patients with CD20‐positive CLL. Treatment with FCR resulted in improved overall survival (OS) rates versus FC alone (87% vs. 83%, respectively; p < .01). After 3 years, the progression‐free survival (PFS) rate was 65% with FCR versus 45% with FC (p < .0001). FCR was generally well tolerated in a fit, young population, although neutropenia and leukocytopenia were more frequent with FCR versus FC [12].
The absence of immunoglobulin heavy chain variable region (IGHV) gene mutations is a major adverse predictive factor in CLL [18]. Results of long‐term follow‐up in patients who participated in phase II studies of FCR as initial therapy [13], [19] showed that, after a median follow‐up of 12.8 years, the PFS rate was 54% with mutated IGHV and 9% with unmutated IGHV [20]. These findings are supported by two additional studies, including one randomized trial, that confirmed long‐term PFS in patients with CLL with mutated IGHV, with an apparent plateau observed on the PFS curve following FCR treatment [20], [21]. Together, these studies support the use of FCR in patients with mutated IGHV and highlight the importance of this regimen as first‐line therapy, even in the era of targeted therapies.
Some patients with CLL may not be eligible for FCR treatment because of older age, decreased physical fitness, impaired renal function, or a history of severe infections. These patients may require an alternative treatment regimen with a more favorable tolerability profile. Results from a randomized, phase III, noninferiority trial (German CLL Study Group CLL10) showed that FCR resulted in superior median PFS versus bendamustine plus rituximab (BR; 55.2 vs. 41.7 months, respectively) for the entire cohort of patients with low comorbidity scores (≤6) and intact renal function (glomerular filtration rate ≥70). There was no significant difference in 5‐year OS between the two treatment arms. However, severe neutropenia and infections were more frequently observed in patients treated with FCR versus BR (84% vs. 59% of patients, respectively), albeit without planned use of myeloid growth factors. This study also reported more frequent infections and more pronounced serious infectious complications in patients aged >65 years treated with FCR versus BR, even though they met the fitness eligibility criteria [22]. In the MabThera added to Bendamustine or Chlorambucil in Patients with Chronic Lymphocytic Leukemia study, patients ineligible for fludarabine treatment because of age or comorbidities were randomized to either BR or rituximab/CLB. This study demonstrated superior median PFS with BR versus rituximab/CLB (39.6 vs. 29.9 months, respectively; p = .003), with no notable differences in safety findings [23]. Based on the results of these studies, BR is a suitable treatment regimen for fit, elderly (aged >65 years) patients with CLL and for those ineligible for fludarabine treatment because of comorbidities or age.
Obinutuzumab, a humanized anti‐CD20 monoclonal antibody, was approved by the FDA for use in combination with CLB in previously untreated CLL, based on a pivotal, randomized, phase III trial. Treatment with obinutuzumab/CLB resulted in improved PFS versus patients treated with rituximab/CLB (29.2 vs. 15.4 months, respectively; p < .001) [24], [25]; however, a greater frequency of severe infusion‐related reactions was noted (21% vs. 4% of patients, respectively). Similarly, the incidence of grade 3 or 4 neutropenia was higher in patients treated with obinutuzumab/CLB versus rituximab/CLB (34% vs. 25% of patients, respectively) [24]. Based on this study, obinutuzumab/CLB is an alternative first‐line regimen for less fit or frail, elderly patients, although the choice of treatment is influenced by local practice. These findings also support the use of rituximab/CLB, as rituximab also improved PFS and was somewhat better tolerated than obinutuzumab.
Novel Approved Therapeutic Agents
Ibrutinib, a covalently binding inhibitor of Bruton's tyrosine kinase, is approved in first‐line and relapsed or refractory CLL in the U.S. and Europe. Ibrutinib is a small‐molecule drug, administered orally. Front‐line approval was based on the results of a randomized, phase III study of ibrutinib versus CLB in previously untreated elderly patients with CLL and without 17p deletion. At a median follow‐up of 18.4 months, the risk of progression or death was 84% lower with ibrutinib versus CLB (p < .001). In addition, ibrutinib significantly prolonged OS compared with CLB treatment (estimated 2‐year OS rate: 98% vs. 85%, respectively). Ibrutinib also resulted in higher overall response rate (ORR) versus CLB (86% vs. 35%; p < .001). Diarrhea, fatigue, cough, and nausea were the most frequently reported adverse events [14].
In a multicenter, randomized, open‐label, phase III study of ibrutinib versus ofatumumab in patients with relapsed or refractory CLL, ibrutinib resulted in significant improvements in OS and PFS rates versus ofatumumab, with the 3‐year PFS rate with ibrutinib estimated at 69% [14], [26]. The ORR was also significantly higher with ibrutinib versus ofatumumab (42.6% vs. 4.1%, respectively; p < .001) [27]. In a randomized, phase III study, ibrutinib/BR resulted in a PFS rate of 79% versus 24% with placebo/BR at a median follow‐up of 18 months [28].
Idelalisib is an oral phosphoinositide 3‐kinase‐delta inhibitor approved for the treatment of patients with relapsed or refractory CLL in combination with rituximab. Approval was based on results from a multicenter, randomized, double‐blind, phase III study of idelalisib/rituximab versus placebo/rituximab in patients with relapsed or refractory cancer considered unfit for chemotherapy‐based regimens, approximately half of whom had 17p deletion [29]. The median PFS was 19.4 months with idelalisib/rituximab [30]. Improvements in OS rate (92% vs. 80%; p = .02) and ORR (81% vs. 13% of patients; p < .001) were also observed with idelalisib/rituximab versus placebo/rituximab [29]. However, the use of idelalisib is not recommended in the first‐line setting because of safety issues, including infection risk, hepatotoxicity, severe diarrhea, and pneumonitis [31], [32].
Overexpression of BCL2 has been identified in CLL and regulates tumor cell survival. Venetoclax is an oral, specific inhibitor of BCL2 [33]. Venetoclax was recently approved for treatment in patients with relapsed CLL with 17p deletion, based on a phase II, single‐arm, multicenter study of venetoclax monotherapy. At a median follow‐up of 12.1 months, overall response was achieved in 79% of patients. The most frequent adverse events were neutropenia, infection, anemia, and thrombocytopenia [34].
The Role of Rituximab and Novel Agents in an Evolving Treatment Paradigm
Given the large body of supportive data from randomized trials and extensive clinical experience, it is likely that rituximab (in combination with chemotherapy) will continue to retain a significant role in the management of CLL. Although obinutuzumab/CLB does not result in prolonged OS (vs. rituximab/CLB), a randomized clinical study demonstrated improved PFS with obinutuzumab/CLB versus rituximab/CLB. In this study, obinutuzumab/CLB was associated with more frequent and severe infusion‐related reactions and neutropenia compared with rituximab/CLB [24]. This finding, together with similar toxicity reported in phase I studies of obinutuzumab with chemoimmunotherapy [35], makes it uncertain whether obinutuzumab will replace rituximab in combination with myelosuppressive chemoimmunotherapies such as FC or bendamustine. However, studies of obinutuzumab plus targeted therapies are ongoing (ClinicalTrials.gov identifiers: NCT01671904, NCT02445131, NCT02320383, NCT02950051).
Ibrutinib was approved for the treatment of relapsed/refractory CLL in 2014, and although its activity is profound, the long‐term effects are not fully understood, and data on mechanisms of resistance, as well as robust clinical data on how to salvage those who relapse, are limited [36]. Studies have shown ibrutinib plus an anti‐CD20 monoclonal antibody results in abrogation of lymphocytosis [37], [38], [39]. However, at present, there are no data from randomized clinical trials to support the combination of ibrutinib/rituximab. The evaluation of ibrutinib/rituximab versus FCR in fit patients with untreated CLL is ongoing in two phase III, randomized, open‐label, parallel‐group studies: one in the U.S. (NCT02048813) and one in the U.K. (CRUK/12037). BR versus ibrutinib (alone or with rituximab) is currently under investigation in a phase III, randomized, open‐label, crossover study in treatment‐naïve patients (NCT01886872). Rituximab is undergoing evaluation in combination with venetoclax versus BR in the relapsed/refractory setting in a phase III, randomized, open‐label, parallel‐group registration study (NCT02005471). As treatment recommendations evolve, findings from these studies may influence the role of rituximab in combination with targeted therapies.
The long‐term remission observed with FCR in some patients, such as those with mutated IGHV, and the short 6‐month duration of FCR treatment will likely result in an ongoing future role of rituximab in this regimen in CLL. The FCR regimen currently remains the treatment regimen of choice for the first‐line treatment in fit, young patients with CLL, whereas treatment with BR or obinutuzumab/CLB remain reasonable treatment choices for fit or less fit, older patients with CLL and in select patients with favorable molecular features.
Key Challenges for the Future: Access to Rituximab and Novel Agents
Rituximab in combination with FC was the first novel therapy for CLL to confer a benefit in OS. Despite this, patient access to rituximab varies globally and is limited in some countries [6]. Several factors, including acquisition cost of rituximab, insurance coverage, reimbursement, and out‐of‐pocket expenses, have the potential to restrict patient access to rituximab [6]. As with rituximab, newer agents such as ibrutinib, idelalisib, and venetoclax are likely to present similar or greater challenges to patients and physicians (e.g., increased out‐of‐pocket and direct acquisition costs as well as societal costs) that may give rise to delayed initiation of appropriate care and reduced continuity and compliance, with a potential negative impact on patient benefit [40].
A survey of 450 oncologists and hematologists from the U.S., Mexico, Turkey, Russia, and Brazil indicated that access to rituximab is limited in these countries [6]. Despite clear guidelines recommending its use in the treatment of CLL, the survey revealed many physicians chose not to treat, were unable to treat, or modified treatment duration with rituximab. Furthermore, the survey reported as few as one third of physicians in Brazil considered rituximab an easily accessible treatment option. In Mexico and Russia, 25% and 19% of physicians, respectively, reported challenges in accessibility of rituximab. In addition, the results indicated that physicians would increase their use of rituximab in clinical practice if a more affordable version were available without compromising efficacy, safety, or patient care [6]. In addition to national variations, regional disparity can affect access to rituximab, primarily because of differences in the requirements for inclusion in local formularies [41].
Biologics such as rituximab are likely to be susceptible to drug shortages. Shortages in the availability of drugs can result in delays in treatment or the use of alternative, potentially less‐effective therapeutic interventions, leading to suboptimal patient care [42].
The Rationale for Developing Rituximab Biosimilars
The patent for rituximab in Europe (MabThera) expired in 2013 and its counterpart in the U.S. (Rituxan) is due to expire in 2018 [43]. In addition, access‐related barriers and the potential of drug shortages (described above) have driven efforts to develop well‐characterized, safe, and effective biosimilars to rituximab, which may reduce the global impact of limited access [42].
Biosimilars provide additional therapeutic options and have the potential to increase access to biologics, thereby fostering greater use and appropriate duration of treatment—primarily as a result of lower costs that would reduce expense for patients and health‐care systems. European data for 2016, for example, showed increases in the use of several biologics, including erythropoietins (66%), granulocyte‐colony stimulating factors (122%), human growth hormone (41%), and antitumor necrosis factor therapies (19%), compared with the year before the availability of corresponding biosimilar versions of these agents [44]. These increases were based on the estimated number of treatment days (used by the report's authors as a measure of access to treatment) for biosimilar and referenced originator products combined, across >20 European Economic Area countries [44]. In terms of anticancer biologics, the World Health Organization (WHO) has recently announced a pilot project in which it will invite manufacturers to submit applications for prequalification of biosimilars of rituximab and trastuzumab, with the aim of improving the availability of these treatments in low‐ and middle‐income countries [45]. Many such countries rely on the WHO's list of prequalified products to guide national procurement decisions [45]. Even in the European Union, a region where resources may be less restricted, budget impact analyses suggest that the potential savings generated with the availability of rituximab biosimilars could be used to treat additional patients with rituximab, thus widening access to therapy [46], [47]. Furthermore, in Brazil, it is expected that the approval of a biosimilar version of trastuzumab will reduce the disparity in access between public and private health‐care systems [48].
Development and Approval of Biosimilars: Key Concepts
The Goal of Similarity Assessment
As biologics are primarily produced from living bacterial, animal, yeast, or plant cells, biologic molecules are not, and cannot be, identical to each other [1]. Because of the size and complexity of biologics, the manufacturing process is challenging for several reasons, including differences in expression systems and host cell lines and, therefore, must be carefully controlled [1]. The quality of biologics is influenced by several factors, such as variations in bioreactor control parameters, pH, and temperature. Manufacturers of biologics may introduce planned changes to the manufacturing processes. As such, biologics are required to undergo assessments to determine the “comparability” of the product, before and after manufacturing changes [49]. A comparability exercise involves the detailed characterization of several representative lots of the biologic, before and after changes to the manufacturing process [49]. The manufacturer of the biologic must conduct a characterization study to assess the risk of potential impact on the clinical characteristics of the biologic, and the need for preclinical or clinical data is assessed on a case‐by‐case basis [49].
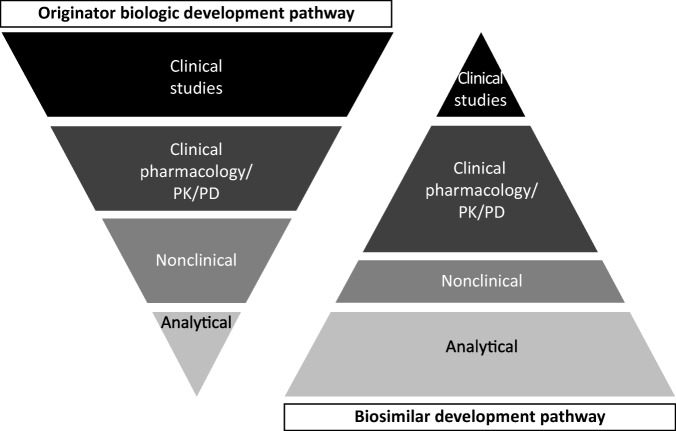
Comparability is different than a similarity assessment. Similarity assessments involve the demonstration of similar structure, function, quality, purity, biologic activity, and safety to the reference biologic, as outlined by the regulatory guidance documents [8], [50]. Indeed, the aim of a similarity assessment is not to re‐establish de novo efficacy and safety characteristics, as these studies have already been conducted to support the new drug application of the reference biologic (Fig. 2).
Figure 2.
Development pathways for originator biologics and biosimilars. Adapted from Kozlowski et al., 2012 [66].
Abbreviations: PD, pharmacodynamics; PK, pharmacokinetics.
All potential biosimilars must undergo a comprehensive regulatory review and approval process, and global regulatory guidelines from the European Medicines Agency (EMA) and FDA outline the requirements for demonstrating similarity to the reference biologic [8], [50]. Although there are slight differences in the exact regulatory definitions, the core principles are aligned to define a biosimilar as a “biological medicinal product that contains a version of the active substance of an already authorized originator biologic” [50]. Ultimately, the biosimilar developer must provide robust scientific evidence that any differences between the proposed biosimilar and the reference biologic are not clinically meaningful, based on the “totality of evidence” [8]. This is a concept whereby a regulatory authority will consider the entire data package submitted in the application from a biosimilar developer, including structural and functional characterization, nonclinical evaluation, human pharmacokinetic (PK) and pharmacodynamic (PD) data, clinical immunogenicity data, and comparative clinical efficacy and safety data [8].
Biosimilars must have the identical primary amino acid sequence and the same route of administration, strength, and dosage form as the reference biologic, all of which are achieved through a process of reverse engineering [8], [50]. The biosimilar manufacturer must conduct comprehensive and robust comparative structural and functional characterization using state‐of‐the‐art technology. Post‐translational modifications such as glycosylation are assessed using a variety of orthogonal techniques. The glycosylation profile has the potential to influence the biological activity and PK of the potential biosimilar. Sensitive assays are therefore used to identify changes in structure and function between the potential biosimilar and the reference biologic [51].
Manufacturing of Biosimilars: A Stepwise Approach
Because developers of biosimilars do not have access to the proprietary data and manufacturing processes pertaining to the reference biologic, they must conduct an extensive analysis of the reference biologic. The host cell line is selected and the amino acid sequence of the reference biologic is confirmed. The optimal host cell line is identified and the ideal clone is selected based on the desired attributes of the protein in an iterative manner [52]. The potential biosimilar must undergo purification using chromatographic and filtration techniques. Multiple lots of the reference biologic are characterized to optimize the manufacturing process and to identify differences between the proposed biosimilar and the reference biologic, particularly those that may impact the mechanism of action, such as amino acid sequence, aggregate levels, activity, and charge heterogeneity [53].
Analytical, Structural, and Functional Characterization of Biosimilars
Assessment of the primary amino acid sequence, post‐translational modifications, Fc glycosylation (which may alter the PK properties), biochemical and biophysical characteristics, and biologic activities are conducted to establish analytical similarity [53]. A rigorous assessment of the structural and functional similarity of the potential biosimilar and the reference biologic is conducted using state‐of‐the‐art techniques, physicochemical methods, and functional assays [8], [50]. Key functional assessments are also conducted using assays that best correlate with the presumptive mode of action [54].
Comparative Clinical Studies and Immunogenicity: Requirements for the Approval of Biosimilars
Demonstrating clinical similarity of a proposed biosimilar to the reference biologic involves comparative PK and PD, immunogenicity, efficacy, and safety studies, generally using an equivalence design [8]. For the originator biologic and potential biosimilar to be considered comparable, clinical endpoints for the two treatments must fall within a specific range, typically using two‐sided confidence intervals (90% or 95%) for the difference between treatments. The equivalence margins used for the primary efficacy endpoint in a comparative clinical study must be scientifically justified [55]. A confirmatory comparative clinical study is usually conducted in one therapeutic indication to show that there are no clinically meaningful differences in PK, PD, efficacy, or safety, including immunogenicity, between the potential biosimilar and the reference biologic. It is important that comparative clinical studies be conducted in a sensitive and homogeneous patient population so that any differences between the originator biologic and the potential biosimilar can be easily identified [50].
Biologic products, including biosimilars, have the potential to provoke an immunogenic response, which may alter the PK, efficacy, or safety properties [56]. Concomitant immunosuppressive agents and chemotherapy can influence the immunogenic response of all biologics. In addition, structural differences, formation of aggregates, and altered glycosylation patterns may trigger an immunogenic response [57]. As such, it is important that the formation of antidrug antibodies in patients treated with all biologics, including biosimilars, is closely monitored throughout development and during postmarketing surveillance.
Rituximab Biosimilars in Development: Current Status
Truxima (CT‐P10; a biosimilar version of rituximab) has been approved for the treatment of CLL, non‐Hodgkin's lymphoma, and RA in South Korea [58] and in Europe [59]. Another biosimilar version of rituximab, GP2013, has recently been approved in Europe for all indications of the reference medicine, MabThera [60]. Several biosimilars of rituximab are currently in clinical development (Table 1). Randomized, double‐blind, phase III comparative studies of ABP798, a potential biosimilar to rituximab, versus rituximab, are ongoing in patients with CD20‐positive Β‐cell non‐Hodgkin's lymphoma (NCT02747043) and in patients with RA (NCT02792699). Another potential rituximab biosimilar, Mabion SA, is in clinical development to evaluate comparative efficacy and safety in patients with CD20‐positive DLBCL (NCT02617485) and RA (NCT02468791). The efficacy and safety of another potential biosimilar, BI‐695500, has been studied in patients with low tumor burden FL (NCT01950273). Nonclinical studies of PF‐05280586, another proposed biosimilar to rituximab, reported similar physicochemical and in vitro functional properties to rituximab [61]. A PK study showed similarity among PF‐05280586 and rituximab sourced from both Europe and the U.S. in patients with active RA [62]. A randomized clinical trial comparing efficacy, safety, PK, and immunogenicity of PF‐05280586 in treatment‐naïve patients with CD20‐positive, low tumor burden follicular lymphoma is ongoing (NCT02213263).
Table 1. Rituximab biosimilars: Current status.
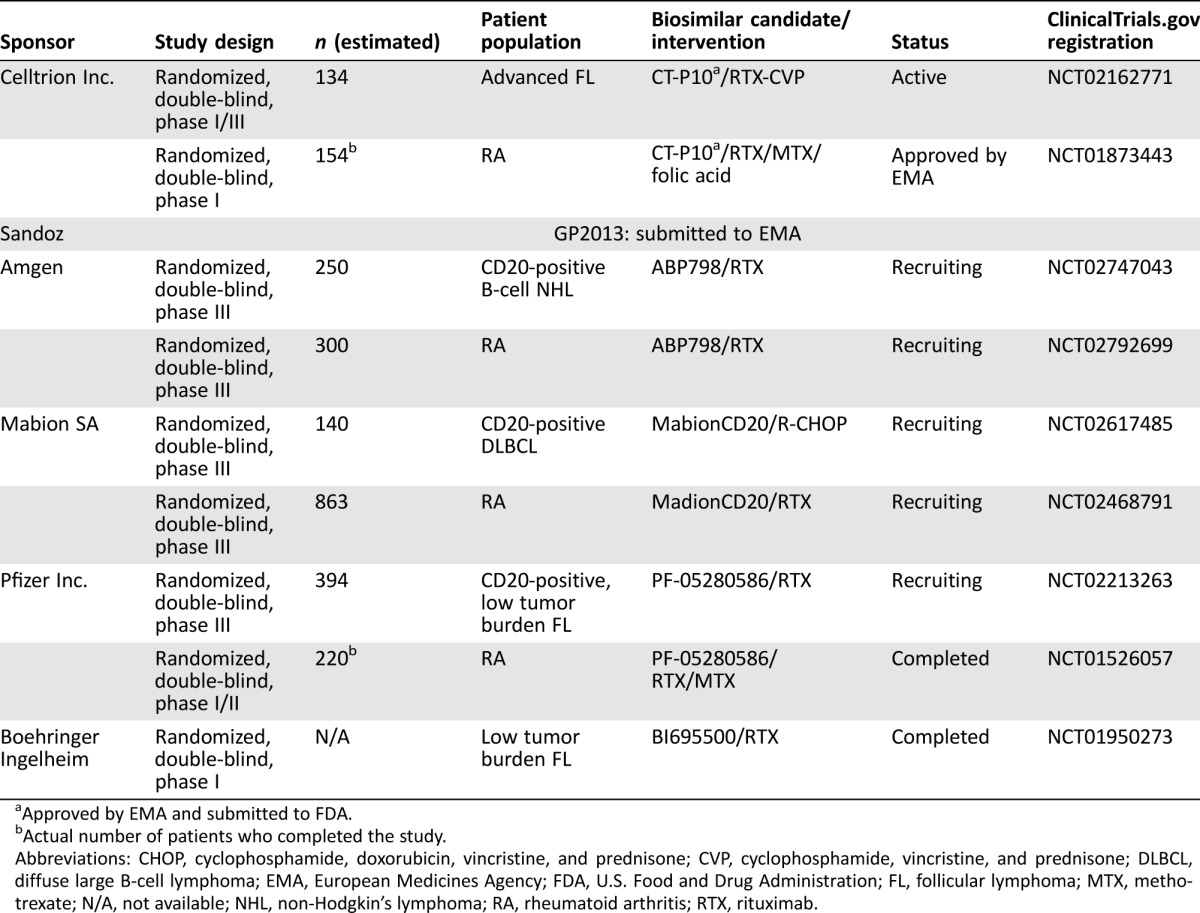
Approved by EMA and submitted to FDA.
Actual number of patients who completed the study.
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; DLBCL, diffuse large B‐cell lymphoma; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; FL, follicular lymphoma; MTX, methotrexate; N/A, not available; NHL, non‐Hodgkin's lymphoma; RA, rheumatoid arthritis; RTX, rituximab.
Considerations for the Use of Biosimilars in Clinical Practice
Several factors, such as prior experience of physicians and their understanding of biosimilars, affordability, access to drugs, and potential substitution for rituximab in curative settings, have the potential to influence the use of biosimilars in clinical practice. In contrast to treatments used in supportive care, it is likely physicians will require evidence of clinical activity of these treatments for cancer indications. For example, physicians may require assurance from PK data of rituximab and its proposed biosimilars in lymphoma and/or CLL, as there is uncertainty over extrapolation from rheumatic indications for use in cancer, in which tumor burden affects PK. Moreover, it is likely physicians will require separate confirmation of biosimilar activity in CLL, given the lower CD20 expression in this therapeutic indication compared with other lymphomas. From a clinical perspective, physicians should have an understanding of the concepts of biosimilarity and the rigorous approval processes outlined by the FDA and EMA [8], [50], which provide the necessary assurance that a biosimilar is highly similar to a reference biologic and with no clinically meaningful differences.
The potential savings and cost efficiencies in health care‐ and insurance‐related issues may also influence the uptake of biosimilars in clinical practice. Indeed, physicians and patients are likely to expect cost savings with the use of biosimilars. For example, in Europe, biosimilars are approximately 30% lower in cost, compared with the relevant originator biologic [63]. Moreover, biosimilars have the potential to offer supply chain benefits, including reduced administrative burden, as well as “wrap‐around services” such as specialist pharmacy services, patient access support mechanisms, and assistance with reimbursement administration [64]. The overall potential benefits of biosimilars will be understood with their use in clinical practice over the coming years.
Biosimilars have the potential to offer supply chain benefits, including reduced administrative burden, as well as “wrap‐around services” such as specialist pharmacy services, patient access support mechanisms, and assistance with reimbursement administration.
Extrapolation Across Indications: The Scientific Rationale
Extrapolation is an important scientific and regulatory principle that refers to the approval of a biosimilar for use in an indication held by the reference biologic and is not directly studied in a comparative clinical trial of the biosimilar and reference product. The concept of extrapolation of data from one indication to another is supported by regulatory guidelines of the FDA and EMA [8], [50]. However, the decision to extrapolate data from one indication to another is made on a case‐by‐case basis and must be supported by strong scientific justification and the totality of evidence. Although extrapolation across indications may reduce the need for repetition of clinical trials, additional studies may be required in certain cases, particularly for agents used in combination with potentially curative chemotherapy.
Conclusion
Biologics are a key component in the management of CLL. Despite the evolving treatment paradigm, it is likely that rituximab (in combination with chemotherapy) will retain a significant role in CLL. To date, rituximab has been the preferred anti‐CD20 antibody in development with targeted agents, although studies are also evaluating the role of obinutuzumab. The limited access to preferred anti‐CD20 monoclonal antibodies has prompted the need to develop safe and effective biosimilars, which have the potential to increase access to important medicines and may result in savings across health‐care systems. Further research is warranted to determine the impact of the utility of biosimilars and their implications for access to biologic medicines in global health‐care settings.
Acknowledgments
Medical writing support was provided by Neel Misra, M.Sc., of Engage Scientific Solutions, and was funded by Pfizer. This review is sponsored by Pfizer Inc.
Footnotes
For Further Reading: Hun Ju Lee, Jorge E. Romaguera, Lei Feng et al. Phase II Study of Bortezomib in Combination with Cyclophosphamide and Rituximab for Relapsed or Refractory Mantle Cell Lymphoma. The Oncologist 2017;22:549–553.
Implications for Practice: The combination of bortezomib with cyclophosphamide and rituximab represents an additional effective novel salvage regimen for mantle cell lymphoma. This combination adds to the growing list of treatment options available for patients with mantle cell lymphoma.
Author Contributions
Manuscript writing: Jennifer R. Brown, Florence Cymbalista, Jeff Sharman, Ira Jacobs, Pilar Nava‐Parada, Anthony Mato
Final approval of manuscript: Jennifer R. Brown, Florence Cymbalista, Jeff Sharman, Ira Jacobs, Pilar Nava‐Parada, Anthony Mato
Disclosures
Jennifer R. Brown: Pfizer, Roche/Genentech, Novartis, Janssen, Pharmacyclics, AstraZeneca, Gilead, AbbVie, Infinity (C/A); Florence Cymbalista: Roche (H); Jeff Sharman: Pfizer, Genentech; Ira Jacobs: Pfizer (E, OI); Pilar Nava‐Parada: Pfizer (E, OI); Anthony Mato: TG Therapeutics, AbbVie, Acerta Regeneron, DTRM, Gilead, Pharmacyclics (RF); Regeneron, TG Therapeutics (C/A).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Morrow T, Felcone LH. Defining the difference: What makes biologics unique. Biotechnol Healthc 2004;1:24–29. [PMC free article] [PubMed] [Google Scholar]
- 2. Schellekens H. Follow‐on biologics: Challenges of the “next generation.” Nephrol Dial Transplant 2005;20(suppl 4):iv31–iv36. [DOI] [PubMed] [Google Scholar]
- 3.Genentech, Inc. Rituxan (rituximab) injection prescribing information . 1997; revised August 2014. Available at https://www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed February 10, 2017.
- 4.Roche Products Limited . MabThera 100mg concentrate for solution for infusion. 2014; updated June 8, 2016. Available at http://www.medicines.org.uk/emc/medicine/2570/SPC/Mabthera+100mg+and+500mg+concentrate+for+solution+for+infusion. Accessed February 10, 2017.
- 5. Weiner GJ. Rituximab: Mechanism of action. Semin Hematol 2010;47:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baer WH II, Maini A, Jacobs I. Barriers to the access and use of rituximab in patients with non‐Hodgkin's lymphoma and chronic lymphocytic leukemia: A physician survey. Pharmaceuticals (Basel) 2014;7:530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rugo HS, Linton KM, Cervi P et al. A clinician's guide to biosimilars in oncology. Cancer Treat Rev 2016;46:73–79. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration , U.S. Department of Health and Human Services. Scientific considerations in demonstrating biosimilarity to a reference product: Guidance for industry. April 2015. Available at https://www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed February 10, 2017.
- 9. Hallek M, Cheson BD, Catovsky D et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood 2008;111:5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eichhorst B, Dreyling M, Robak T et al. Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2011;22(suppl 6):vi50–vi54. [DOI] [PubMed] [Google Scholar]
- 11. Stilgenbauer S, Furman RR, Zent CS. Management of chronic lymphocytic leukemia In: Dizon DS, ed. American Society of Clinical Oncology 2015 Educational Book. Vol 35. Alexandria, VA: American Society of Clinical Oncology, 2015:164–175. [DOI] [PubMed] [Google Scholar]
- 12. Hallek M, Fischer K, Fingerle‐Rowson G et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open‐label, phase 3 trial. Lancet 2010;376:1164–1174. [DOI] [PubMed] [Google Scholar]
- 13. Tam CS, O'Brien S, Wierda W et al. Long‐term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008;112:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burger JA, Tedeschi A, Barr PM et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Döhner H, Stilgenbauer S, Benner A et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000;343:1910–1916. [DOI] [PubMed] [Google Scholar]
- 16. Rosenwald A, Chuang EY, Davis RE et al. Fludarabine treatment of patients with chronic lymphocytic leukemia induces a p53‐dependent gene expression response. Blood 2004;104:1428–1434. [DOI] [PubMed] [Google Scholar]
- 17. Malcikova J, Smardova J, Rocnova L et al. Monoallelic and biallelic inactivation of TP53 gene in chronic lymphocytic leukemia: Selection, impact on survival, and response to DNA damage. Blood 2009;114:5307–5314. [DOI] [PubMed] [Google Scholar]
- 18. Rossi D, Terzi‐di‐Bergamo L, De Paoli L et al. Molecular prediction of durable remission after first‐line fludarabine‐cyclophosphamide‐rituximab in chronic lymphocytic leukemia. Blood 2015;126:1921–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keating MJ, O'Brien S, Albitar M et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005;23:4079–4088. [DOI] [PubMed] [Google Scholar]
- 20. Thompson PA, Tam CS, O'Brien SM et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long‐term disease‐free survival in IGHV‐mutated chronic lymphocytic leukemia. Blood 2016;127:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischer K, Bahlo J, Fink AM et al. Long‐term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial. Blood 2016;127:208–215. [DOI] [PubMed] [Google Scholar]
- 22. Eichhorst B, Fink AM, Bahlo J et al. First‐line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open‐label, randomised, phase 3, non‐inferiority trial. Lancet Oncol 2016;17:928–942. [DOI] [PubMed] [Google Scholar]
- 23. Leblond V, Laribi K, Ilhan O et al. Rituximab in combination with bendamustine or chlorambucil for treating patients with chronic lymphocytic leukemia: Interim results of a phase IIIb study (MaBLe). Blood 2012;120:2744a. [Google Scholar]
- 24. Goede V, Fischer K, Busch R et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101–1110. [DOI] [PubMed] [Google Scholar]
- 25. Goede V, Fischer K, Engelke A et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: Updated results of the CLL11 study. Leukemia 2015;29:1602–1604. [DOI] [PubMed] [Google Scholar]
- 26. Byrd JC, Furman RR, Coutre SE et al. Three‐year follow‐up of treatment‐naïve and previously treated patients with CLL and SLL receiving single‐agent ibrutinib. Blood 2015;125:2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byrd JC, Brown JR, O'Brien S et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chanan‐Khan A, Cramer P, Demirkan F et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): A randomised, double‐blind, phase 3 study. Lancet Oncol 2016;17:200–211. [DOI] [PubMed] [Google Scholar]
- 29. Furman RR, Sharman JP, Coutre SE et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014;370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharman JP, Coutre SE, Furman RR et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): Efficacy analysis in patient subpopulations with Del(17p) and other adverse prognostic factors. Blood 2014;124:330. [Google Scholar]
- 31. Lampson BL, Kasar SN, Matos TR et al. Idelalisib given front‐line for treatment of chronic lymphocytic leukemia causes frequent immune‐mediated hepatotoxicity. Blood 2016;128:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Brien SM, Lamanna N, Kipps TJ et al. A phase 2 study of idelalisib plus rituximab in treatment‐naïve older patients with chronic lymphocytic leukemia. Blood 2015;126:2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AbbVie Inc . Venclexta (venetoclax) injection prescribing information. April 2016. Available at http://www.rxabbvie.com/pdf/venclexta.pdf. Accessed October 21, 2017.
- 34. Stilgenbauer S, Eichhorst B, Schetelig J et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open‐label, phase 2 study. Lancet Oncol 2016;17:768–778. [DOI] [PubMed] [Google Scholar]
- 35. Brown JR, O'Brien S, Kingsley CD et al. Obinutuzumab plus fludarabine/cyclophosphamide or bendamustine in the initial therapy of CLL patients: The phase 1b GALTON trial. Blood 2015;125:2779–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woyach JA, Furman RR, Liu TM et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014;370:2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bennett CL, Berger JR, Sartor O et al. Progressive multi‐focal leucoencephalopathy among ibrutinib‐treated persons with chronic lymphocytic leukaemia. Br J Haematol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38. Jaglowski SM, Jones JA, Nagar V et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: A phase 1b/2 study. Blood 2015;126:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang ML, Lee H, Chuang H et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: A single‐centre, open‐label, phase 2 trial. Lancet Oncol 2016;17:48–56. [DOI] [PubMed] [Google Scholar]
- 40. Shanafelt TD, Borah BJ, Finnes HD et al. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract 2015;11:252–258. [DOI] [PubMed] [Google Scholar]
- 41. Gori S, Di Maio M, Pinto C et al. Differences in the availability of new anti‐cancer drugs for Italian patients treated in different regions. Results of analysis conducted by the Italian Society of Medical Oncology (AIOM). Tumori 2010;96:1010–1015. [PubMed] [Google Scholar]
- 42. Li E, Subramanian J, Anderson S et al. Development of biosimilars in an era of oncologic drug shortages. Drug Des Devel Ther 2015;9:3247–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Philippidis A. Biosimilars: 11 drugs to watch. Genetic Engineering & Biotechnology News, May 19, 2014. Available at http://www.genengnews.com/insight-and-intelligence/biosimilars-11-drugs-to-watch/77900135. Accessed February 10, 2017.
- 44.QuintilesIMS. The impact of biosimilar competition in Europe. May 2017. Available at https://ec.europa.eu/docsroom/documents/23102/attachments/1/translations/en/renditions/native. Accessed October 4, 2017.
- 45.World Health Organization . WHO to begin pilot prequalification of biosimilars for cancer treatment. May 4, 2017. Available at http://www.who.int/mediacentre/news/releases/2017/pilot-prequalification-biosimilars/en/. Accessed October 4, 2017.
- 46. Gulácsi L, Brodszky V, Baji P et al. The rituximab biosimilar CT‐P10 in rheumatology and cancer: A budget impact analysis in 28 European countries. Adv Ther 2017;34:1128–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rencz F, Gulácsi L, Baji P et al. Budget impact analysis of biosimilar rituximab (CT‐P10) for the treatment of chronic lymphocytic leukaemia in the 28 EU member states. Abstract presented at: 22nd Congress of the European Hematology Association; June 24, 2017; Madrid, Spain; P730.
- 48. Debiasi M, Pimentel FF, Pereira PJS et al. Biosimilars in Brazil: The beginning of an era of broader access. J Cancer Ther 2017;8:814–826. [Google Scholar]
- 49.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . ICH harmonised tripartite guideline: Comparability of biotechnological/biological products subject to changes in their manufacturing process. Q5E. November 18, 2004. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5E/Step4/Q5E_Guideline.pdf. Accessed February 10, 2017.
- 50. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology‐derived proteins as active substance: Non‐clinical and clinical issues. December 18, 2014. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf. Accessed February 10, 2017.
- 51. Shang TQ, Saati A, Toler KN et al. Development and application of a robust N‐glycan profiling method for heightened characterization of monoclonal antibodies and related glycoproteins. J Pharm Sci 2014;103:1967–1978. [DOI] [PubMed] [Google Scholar]
- 52. Lee JF, Litten JB, Grampp G. Comparability and biosimilarity: Considerations for the healthcare provider. Curr Med Res Opin 2012;28:1053–1058. [DOI] [PubMed] [Google Scholar]
- 53. Ryan AM. Frontiers in nonclinical drug development: Biosimilars. Vet Pathol 2015;52:419–426. [DOI] [PubMed] [Google Scholar]
- 54. Konishi E, Kitai Y, Kondo T. Utilization of complement‐dependent cytotoxicity to measure low levels of antibodies: Application to nonstructural protein 1 in a model of Japanese encephalitis virus. Clin Vaccine Immunol 2008;15:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chow SC, Song F, Bai H. Sample size requirement in analytical studies for similarity assessment. J Biopharm Stat 2017;27:233–238. [DOI] [PubMed] [Google Scholar]
- 56. Bendtzen K. Anti‐TNF‐alpha biotherapies: Perspectives for evidence‐based personalized medicine. Immunotherapy 2012;4:1167–1179. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization , Expert Committee on Biological Standardization. Guidelines on evaluation of similar biotherapeutic products (SBPs). 2009. Available at http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed February 14, 2017.
- 58.Generics and Biosimilars Initiative. Biosimilar rituximab approved in South Korea. February 2, 2016. Available at http://gabionline.net/Biosimilars/News/Biosimilar-rituximab-approved-in-South-Korea. Accessed December 5, 2016.
- 59.Celltrion Healthcare . Truxima, the first biosimilar mAb in oncology, granted EU marketing authorization [press release]. FirstWord Pharma, 2017. Available at https://m.firstwordpharma.com/truxima-first-biosimilar-mab-oncology-granted-eu-marketing-authorisation. Accessed March 7, 2017.
- 60.Novartis. Sandoz receives approval in Europe for Rixathon (biosimilar rituximab) to treat blood cancers and immunological diseases [press release]. June 19, 2017. Available at https://www.novartis.com/news/media-releases/sandoz-receives-approval-europe-rixathonr-biosimilar-rituximab-treat-blood. Accessed September 21, 2017.
- 61. Ryan AM, Sokolowski SA, Ng CK et al. Comparative nonclinical assessments of the proposed biosimilar PF‐05280586 and rituximab (MabThera). Toxicol Pathol 2014;42:1069–1081. [DOI] [PubMed] [Google Scholar]
- 62. Cohen S, Emery P, Greenwald M et al. A phase I pharmacokinetics trial comparing PF‐05280586 (a potential biosimilar) and rituximab in patients with active rheumatoid arthritis. Br J Clin Pharmacol 2016;82:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits 2013;6:469–478. [PMC free article] [PubMed] [Google Scholar]
- 64. Simoens S, Jacobs I, Popovian R et al. Assessing the value of biosimilars: A review of the role of budget impact analysis. Pharmacoeconomics 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Comprehensive Cancer Network . NCCN guidelines: Chronic lymphocytic leukemia/small lymphocytic leukemia. Version 1.2018. 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Accessed September 27, 2017.
- 66. Kozlowski S. Biosimilar biological products: Overview of approval pathway under the Biologics Price Competition and Innovation Act of 2009. 2012. Available at https://c.ymcdn.com/sites/casss.site-ym.com/resource/resmgr/WCBP_Speaker_Slides/2013_WCBP_Kozlowski_Steven.pdf