Abstract
Apathy is one of the most common and pervasive of the behavioral and psychological symptoms in dementia (BPSD). Apathy has profound consequences for morbidity and mortality and for caregiver burden. Current treatment of apathy has been hindered because of poor understanding of the mechanisms underlying this heterogeneous syndrome. Research has demonstrated that apathy is associated with the disruption of the frontal-striatal system in individuals with neurodegenerative disease. As with other BPSD, these neural mechanisms alone do not completely account for the syndrome—individual, caregiver and environmental factors also contribute to apathy. In this paper, we modify a current conceptual model of the factors contributing to BPSD to examine determinants of apathy. This integrative model provides a more complete and theoretically informed understanding of apathy, allowing for greater insight into potential targets for research, intervention and improved care. We end by proposing an agenda for moving the science of BPSD in general, and apathy in particular, forward.
Keywords: apathy, goal-directed behavior, behavioral and psychological symptoms of dementia
INTRODUCTION
Behavioral and psychological symptoms in dementia (BPSD) include changes in behavior, perceptions, thought content and mood disturbances such as apathy and agitation.1 They are among the most troubling symptoms accompanying neurodegenerative disease and contribute to many negative outcomes.2, 3 Significant challenges in the management of BPSD include heterogeneity of presentation, complexity of underlying neurocognitive dysfunction and the variety of precipitating individual, caregiver or environmental determinants.
In this paper, we discuss apathy as a prototype BPSD. We chose this focus for several reasons. First, apathy is one of the most prevalent and persistent BPSD across all neurodegenerative diseases.4–6 Second, as with many BPSD, apathy is a conceptually heterogenous syndrome with varied presentations, leading to the need to avoid “one size fits all” approaches to management. Third, as compared to other BPSD, there is a larger body of literature on potential causative mechanisms, indicating that the syndrome is explained in part by neuroanatomical dysfunction.7, 8 As with other BPSD, however, neural mechanisms alone do not completely account for the syndrome—determinants of apathy may also include individual, caregiver and environmental factors.1, 9 In this paper, we modify a current conceptual model1 of the factors precipitating BPSD to examine mechanisms associated with apathy. Using the latest findings related to the neurocognitive dysfunction underlying apathy, we extend the model specifically for this particular syndrome. This integrative model provides a more complete and theoretically informed understanding of apathy, allowing for greater insight into potential targets for research, intervention and improved care. Development and testing of similar models for other BPSD is recommended. We end by proposing an agenda for moving the science of BPSD in general, and apathy in particular, forward.
DEFINITION OF APATHY
The word apathy derives from the Greek word pathos or passion. While describing a state of indifference or inertia,10 over time, the concept of apathy has undergone changes in meaning, but remains vaguely defined and broadly applied.11 In 1990, Marin defined apathy as a state of motivational impairment,12 suggesting that apathy is a syndrome resulting from psychiatric, neurologic or medical disorders. While this definition represented an advance, lack of motivation is difficult to quantify and it is not the only cause of apathetic behavior.
In 2006, Levy and DuBois proposed to define apathy as “the quantitative reduction of self-generated voluntary and purposeful behaviors13.” Consistent with a model of apathy associated with a deficit in one of the three determinants of goal-directed behavior, Levy and DuBois proposed three underlying mechanisms responsible for apathy including: 1) diminished emotional-affective processing (i.e., motivation), 2) impaired cognitive processing of plans of action (i.e., planning) and 3) difficulty in initiating behavior (i.e., initiation). In this definition, apathy can occur when any one of these processes is disrupted. From this perspective, it is possible to observe and measure the various forms of apathy.7
A consensus on the diagnostic criteria for apathy in neurodegenerative conditions has been published by an international task force10 and may resolve some of the discrepancies in identifying apathy. In these criteria, apathy is described as a syndrome with cognitive, affective and behavioral dimensions. To meet criteria for apathy, the patient must: 1) display the core feature of diminished motivation with 2) reduction in two of the three following domains: a) goal-directed self-initiated or environment-stimulated behavior, b) goal-directed cognitive behavior and c) emotional response. Clinical evaluations of patients with apathy are challenging because of the variability in each individual’s goals, interests, and emotional displays. Diagnostic criteria such as those proposed by the international task force10 are necessary to operationalize this heterogeneous syndrome, both for reliable diagnosis and for distinguishing from other syndromes such as depression. Yet, there is still a need for the classification of apathy based on the underlying neural mechanisms that are foundational to the development and testing of more precise targeted treatments for apathy.
PREVALENCE
Apathy is a common behavior in neurodegenerative disorders such as Alzheimer’s disease (AD), frontotemporal degeneration (FTD), Lewy Body Disease (LBD) and Parkinson’s disease (PD). In AD, the prevalence rate has been estimated at between 51-80%.14–16 Abnormal social behavior is a hallmark symptom of FTD, and apathy is the most prevalent behavioral disorder, occurring in 90.5% of mild stage patients and 100% of moderate and severe stage patients.17 The frequency of apathy in PD and PD spectrum disorders like LBD may also be substantial, although estimates of prevalence vary more widely than in AD, ranging from 12% to 70%.18–21
Apathy is also one of the most persistent BPSD. Data from a population-based longitudinal study found that apathy was among the most stable of symptoms, having a 62% probability of continuing to be exhibited after one year.4 In this study, apathy also had a strong association with disability, poor health and high mortality.
OUTCOMES
Apathy has profound consequences. Accumulating evidence suggests that apathy is associated with a variety of undesirable outcomes, such as poor insight, poor cognitive performance, lower functional autonomy, and even increased mortality.22–25 Apathy has also been identified as an independent risk factor for the development of cognitive impairment in older adults with normal cognition26, 27 and for conversion to dementia in individuals with mild cognitive impairment (MCI).28, 29 These findings suggest that apathy contributes to global decline in cognition and every-day function and, thus, support the need to identify these at-risk patients.
PERSPECTIVES OF FAMILY AND PROFESSIONAL CAREGIVERS
People with neurodegenerative disease tend to be unconcerned about their apathetic behavior; it is quite distressing, however, for their family caregivers.30 Emotional blunting and lack of response associated with apathy reduce the relational exchange with the caregiver and patient. Indeed, caregivers often misinterpret apathy as oppositional or volitional behavior.31 Caregivers report a loss of connection to their spouse with apathy that may be related to impaired emotional responsiveness seen in the syndrome.32 Notably, in a study of family caregivers, spousal apathy had the greatest impact on deterioration of the marital relationship.33
In contrast, formal caregivers may not see apathy as a significant problem. A recent study of nursing staff in general hospitals reported a high frequency of BPSD among patients with dementia, but they did not endorse apathy/indifference as a distressing symptom.34 Similar findings have been reported in long-term care settings.35 Nursing home staff view withdrawal as common among residents but rarely was it deemed distressing to staff. Interestingly, staff distress was also not associated with dependency in activities of daily living, a core feature of apathy. Perhaps in the resource-stressed nursing home environment, “doing for” a resident is perceived as more expedient than encouraging self-care. Lack of motivation was also not endorsed as a challenging behavior by staff in Australian nursing homes,36 a finding similar to that reported earlier by Brodaty, Draper, and Low.37 In the latter study, many staff, like family caregivers, viewed symptoms as deliberate; but unlike family caregivers, formal caregivers did not report high levels of associated distress.
OVERLAP WITH DEPRESSION
Depression and apathy are distinct syndromes that are often confused. Symptoms common to both apathy and depression include anhedonia, hypersomnia and fatigue.31, 38 Starkstein and colleagues examined the differentiation of apathy and depression in AD patients using factor analysis of the Hamilton Depression Scale. They found that psychomotor retardation, agitation, and poor appetite were construed as an apathy factor. Symptoms such as sad mood, guilt, suicidal ideation, anxiety and insomnia loaded as a sadness factor, suggesting these were more commonly found in people with depression.39 Other symptoms such as self-criticism and negative thoughts about the future are common in people with depression, but absent in individuals with apathy who tend to show a lack of concern.40 This is consistent with similar findings that suggest apathy is a discrete syndrome separate from depression.31 Because apathy is so common in dementia, efforts to distinguish this syndrome from depression are imperative for guiding treatment decisions.
MEASUREMENT
Several apathy-assessment tools exist for the cognitively impaired population. Traditional instruments to assess for apathy in neurodegenerative disease include rating scales which commonly rely on proxy report (for review, see Radakovic et al., 201641). Thus, apathy is most often assessed in the context of the caregivers’ perspective and may, therefore, be subject to caregiver confounds such as burden and strain that may impact the evaluation.42, 43
Since apathy is associated with a reduction in motor behavior, others have proposed the use of objective measurements such as ambulatory actigraphy and computer-based measurements of apathy.7, 43–45 Continued work in this area is important for the development of an empirically-based, objective approach that elucidates mechanisms contributing to apathy. Lastly, utilization of instruments that include subscales to measure domains of apathy would increase the targeted treatment of apathy.41, 46
CONCEPTUAL FRAMEWORK FOR EXAMINING APATHY
In this paper, we propose an adaptation of the Kales et al., BPSD conceptual model1 to better understand apathy (see Figure 1). Factors identified in the original conceptual model are those that may either directly cause (neurodegeneration) or indirectly trigger BPSD. The original conceptual model describes how interactions between the person with dementia, caregiver and environment can trigger BPSD in the context of underlying neurodegeneration.9, 47, 48 As this is a conceptual model, the factors listed include both those with a significant evidence base as well as those that are hypothesized to be important from pratice-based experience. The model is highly useful as it details the etiologic complexity of BPSD needed for a thorough clinical assessment, and why it is likely that no single pharmacologic or non-pharmacologic approach can be used as a “magic bullet” for treatment. The model also serves as a basis for researchers to consider in studying the impact of potential etiologic causes and triggers of BPSD; these studies can ultimately lead to better, more tailored interventions than those which we have currently. Because BPSD are heterogeneous in their phenotypes (e.g. depression, psychosis, agitation, etc), have differential evidence bases and may have different underlying etiologies (e.g. different brain regions involved, etc), we believe that there is further utility to adapting the model for specific BPSD like apathy.
Figure 1.
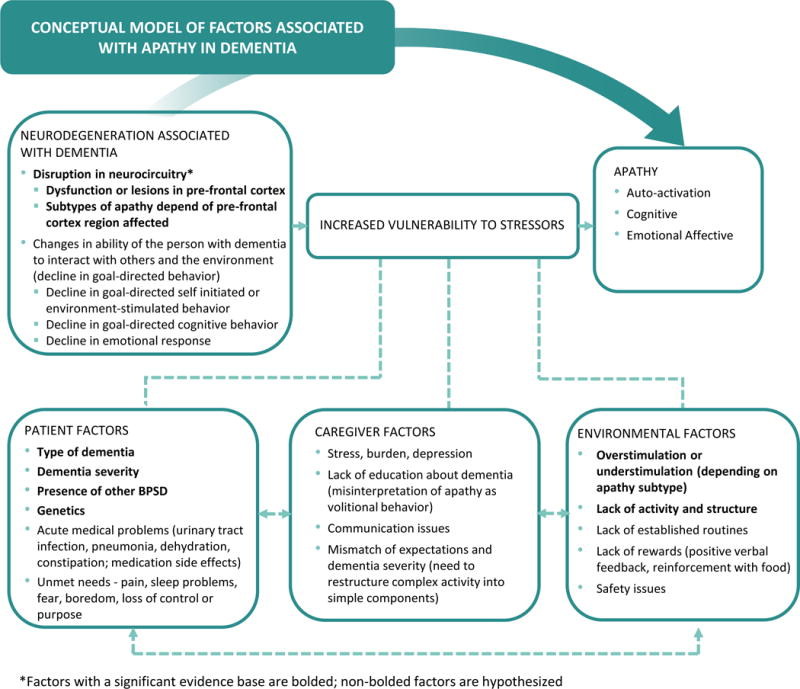
Conceptual Framework for Examining Apathy
In the specific case of apathy, incorporating the advances made in conceptualizing impairments in goal-directed behavior–initiation, planning and motivation–with their associated neuroanatomic underpinnings presents an opportunity to further improve the utility of the model for research. Thus, we have elaborated on the model to include underlying neurocognitive dysfunction thought to contribute to apathy as well as how apathy subtypes may contribute to symptom heterogeneity (see Figure 1). Ideally, this can advance the field in three ways. First, it allows researchers and clinicians the opportunity to consider apathy as arising either directly from disruptions in neurocircuitry or, alternatively, indirectly when such disruptions in neurocircuitry lower the threshold for (increase vulnerability to) specific patient, caregiver and environmental stressors. Second, it suggests distinct pathways for intervention. Third, it can point the way toward additional iterations of the model for other BPSD such as depression or psychosis, with specific attention to the neural and non-neural mechanisms pertinent to those syndromes.
In terms of neurocircuitry disruption, according to the model proposed by Levy and DuBmois (2006), apathy is the result of dysfunction in the frontal cortex or structures in the basal ganglia.13 Three goal-directed behavior processes map onto three distinct brain regions that work together in a large-scale neural network associated with apathy. In particular, three functional neuroanatomic loops underlie goal-directed behavior in the frontal area (anterior cingulate circuit, dorsolateral prefrontal circuit, orbitofrontal circuit) and appear to capture information from internal and external environments needed for enacting goal-directed behavior and performing possible actions.13 Because each circuit is functionally separate in supporting individual goal-directed behavior components, it may be plausible to distinguish different apathetic profiles or subtypes based on underlying neurocognitive dysfunction.13, 49, 50
Although the underpinnings of apathy are neurobiological in nature, it is noteworthy that patient, caregiver and environmental factors may exacerbate or trigger apathy symptoms. A granular understanding of symptom subtype and determinants are critical for effective care strategies that are person- and caregiver-centered.49
A recent scoping review focusing on BPSD followed the Kales et al. conceptual model of BPSD and used the categories of personal, caregiver and environmental determinants as a guide for searching the literature for high quality/low bias studies addressing causes or determinants of behavioral symptoms. High quality was defined using Gough’s Weight of Evidence Framework51 and low bias by the Cochrane Collaboration bias tool.52 This review found sixteen high quality/low bias studies addressing the causes or determinants of apathy.9 The operational definition of apathy varied by study. The most common instrument used to measure it was the apathy subscale of the Neuropsychiatric Inventory.53 Informant report was used most often to rate apathy, which is not surprising given that reduced insight often co-occurs with apathy.54, 55
Patient Factors
While apathy is prevalent across dementia types, there is also some limited and inconsistent data on rates by type. One study found that apathy is more common in behavioral variant FTD than AD.56 Another study found that apathy is more common in early-onset AD than late-onset AD.57 A third study among patients with AD and vascular dementia (VaD) found that apathy is more common in VaD, but the results were not statistically significant.58 In another study, apathy was most frequent in Dementia with Lewy Bodies (DLB), but again the results were not statistically significant.59
The review found strong evidence for apathy being related to the severity of cognitive impairment in dementia. Apathy was associated with both more severe cognitive impairment on Mini-Mental State Exam60, 61 and dementia severity on the Clinical Dementia Rating Scale (CDR).62 A prior study examining specific cognitive deficits in persons with AD found that apathy was associated with a greater severity of frontal lobe-related cognitive deficits.63
Several other patient-level determinants have also been implicated, including the presence of other BPSD.61 Additionally, in AD, baseline apathy and antidepressant use are associated with increasing apathy over time.64
Biologic factors appear to be most strongly associated with apathy. A number of studies have shown that neuroanatomical changes in grey matter and white matter are associated with apathy.61, 64 Apathy also appears to be associated with genetic factors including APOE e4 in AD57, 65 and c9ORF72 in FTD patients.66 Other biological factors (such as cerebral spinal fluid biomarkers in AD) do not appear to be associated with apathy.64 Finally, among patient determinants, gender does not appear to be related to apathy.60
Caregiver Factors
In the prior scoping review9, no high-quality evidence for any caregiver determinant was found. In observational studies, however, it has long been noted that social interaction (or lack thereof) can impact apathy. Other than during personal care, nursing home residents spend much of their time “doing nothing,” and negative affect as well as apathy have been observed during these unoccupied times.67, 68, 69, 70 Additionally we know that structured interactions that involve caregivers, such as recreational activities (see discussion on environmental determinants), can reduce apathy and improve affect.71
More high-quality research is needed, however, on the impact of caregiver factors, such as comuunication patterns, on exacerbation of apathy. For example, caregivers often may misinterpret apathy as oppositional or volitional behavior.32 In turn, this may lead to negative interactions in the dyad. Further, in long-term care settings,34, 35 staff may not see apathy as problematic, potentially leading to negative outcomes and the exacerbation of apathy given the lack of any intervention.
Environmental Factors
The prior scoping review found three high quality studies that evaluated environmental factors. In the first, AD patients participating in activities tailored to personality and physical ability72 showed decreased apathy. Another study of AD patients participating in cognitive stimulation also showed positive effects on apathy.73 A third study examining therapeutic conversation, also demonstrated decreases in apathy in AD patients.74 Prior work in BPSD suggests that individualizing activities provides an advantage over one-size-fits-all interventions for engaging nursing home residents with dementia. For people with apathy, activities that individuals find personally interesting supply additional intrinsic motivation.75 Since the patient environment, compared to neurobiological deficits, is relatively more modifiable, such studies are extremely important.
To summarize, a recent rigorously conducted scoping review found that most prior studies of determinants have focused on patient-related causes of apathy, particularly biologic factors. The review found strong evidence for the association of apathy with neurodegeneration. It is important to note, however, that the bulk of studies previously conducted and considered for the review were in the area of person-related factors, with no high-quality caregiver studies found and only three high-quality environmental studies found. Clearly, additional work is needed relative to the caregiver and environmental factors suggested by our adapted conceptual model (see Figure 1), particularly given their relatively greater modifiability as compared to most person-level factors such as neurodegeneration.
INTERVENTIONS FOR APATHY
Pharmacotherapy, neuromodulation and non-pharmacological approaches are among the interventions currently used for treating apathy. The evidence to support these interventions is modest and there have been no widely accepted guidelines developed for the management of apathy. Notably, treatment trial failures may relate to the commonly used simplified definition of apathy used in many trials—e.g., a lack of motivation. Given that neuroanatomical evidence supports a multicomponent approach to apathy, and that mechanisms underlying apathy are qualitatively different, different subtypes may require different interventions.49 Again, this is where our adapted model will be useful for future trials.
Pharmacotherapy
Apathy is associated with neuropathological and neurochemical alterations to frontosubcortical circuits.76 There are a number of neurotransmitters, receptors and second messengers involved in the disruption of these circuits that form the basis for pharmacotherapy. The evidence for use of pharmacologic interventions in apathy has been systematically reviewed in several papers46, 76–78 and indicates modest efficacy. Few studies have been conducted, most are retrospective and many do not have apathy as a primary outcome. Overall, cholinesterase inhibitors have the best evidence for symptomatic improvement and there is some evidence for use of memantine. One clinical trial found no evidence for modafinil in reducing apathy or improving caregiver burden.79 While the evidence for most stimulants is limited, studies of the safety and efficacy of methylphenidate (MPH) are more encouraging, and support findings that apathy may represent dopaminergic dysfunction. For example, in a recent study of community-dwelling male veterans with mild AD, individuals receiving MPH showed improvement in apathy scores over a 12 week period.80 In order to clarify the clinical efficacy of MPH, additional longitudinal studies such as The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2)81 are underway to assess change in apathy and cognition in individuals with dementia. Finally, there is evidence that antidepressants and antiepileptics do not improve apathy and may actually be harmful.
Placebo-controlled trials with apathy as the primary target are now underway which will provide much needed additional data. Because apathy has different components (behavioral, cognitive and affective), each with different underlying mechanisms, future investigations should examine separately the pharmacological effects on these aspects.
Neuromodulation
Neuromodulation approaches for treatment of apathy include repetitive transcranial stimulation (rTMS) and transcranial direct current stimulation (tDCS). Both approaches are non-invasive and deliver magnetic fields across the skull resulting in activation or inhibition of the underlying neuronal circuits involved with the generation of voluntary actions. rTMS has been efficacious for the treatment of depression in cognitively intact patients, but there is no strong evidence to support its efficacy for apathy or depression in people with dementia59 In a recent randomized clinical trial tDCS had no effect on apathy in people with moderate AD.82
Non-pharmacological Approaches
Several systematic reviews provide evidence for the efficacy of tailored activities (based on the individual past history, preferences and retained functional abilities).78, 83, 84 These methodologically heterogeneous interventions include music therapy, tailored activities, cognitive stimulation, multi-sensory behavioral therapy, art therapy and therapeutic conversation. Theoretically, tailored activities supply intrinsic motivation, a central feature of apathy, by capturing interest and providing reward. Challenges to the use of these interventions is that they can be complex and time-consuming, contributing to issues around reproducibility and sustainability.
There are limited data on the sustained effects of non-pharmacological interventions for apathy. Kolanowski and colleagues,85 however, found positive effects of individualized activities that extended one week post-intervention. Another trial of an individualized functional training program significantly reduced apathy one month post-intervention, but at 4 months apathy levels increased.86 Given that apathy often worsens with dementia progression, non-pharmacological treatment of apathy will likely require re-assessment and continuous programing.
Staff education (a month-long educational program using non-pharmacological approaches) was investigated in one study. While nursing home residents’ emotional blunting was improved, their level of interest was not.87 The investigators noted that lack of staff access to information regarding resident preferences was a major barrier to implementing non-pharmacological interventions for apathy. Poor communication around resident preferences has been identified as a barrier to person-centered care, in general, by other investigators.88, 89
Similar to pharmacologic studies, more research is needed that rigorously uses apathy diagnostic criteria and considers apathy subtypes to improve precision and effect sizes. Again, our adapted model is well suited for this. For example, multisensory stimulation may be helpful in patients with initiation difficulty, but worsen apathy in those with planning difficulties (by increasing distractibility). Needed are studies that 1) determine optimal dosage and duration of intervention and 2) test strategies to improve implementation and dissemination of evidence-based approaches. Finally, because non-pharmacological interventions have long been recommended as the first line of treatment for apathy, an up-dated review of guidelines90 is needed, given our current understanding of the determinants.
CONCLUSION
Here we suggest that apathy is a multi-component phenomenon, emerging when there is dysfunction in any component of goal-directed behavior. This adds to a conceptual model of BPSD by Kales and colleagues that describes how interactions between the person with dementia, caregiver and environment potentially trigger BPSD in the context of underlying neurodegeneration. Thus, it is likely that the pathophysiology of apathy is not a single mechanism, but rather multifaceted. Furthermore, it may be possible to identify selective impairments in goal-directed behavior which may contribute to different clinical phenotypes or subtypes of apathy.49 Understanding mechanisms underlying apathy such as neural mechanisms of goal-directed behavior in addition to factors such as those proposed by Kales and colleagues provide a necessary step forward in a proactive, targeted treatment of apathy.
IMPLICATIONS FOR OTHER BPSD
The focus here is on apathy in neurodegenerative disease, but the recommendations for advancing knowledge of this particular behavior has implications for other BPSD. BPSD is an umbrella term for a variety and range of specific symptoms such as aggression, wandering, and depression. Because BPSD are often primarily measured in the aggregate, that is, the number of symptoms displayed, this has diluted the ability to detect important associations with other variables and the effect of interventions on specific symptoms. There is a need for theoretically informed measures that provide greater precision in defining and measuring individual symptoms and syndromes.
Individual symptoms vary over time and by type of dementia. Future studies that include well-characterized samples that meet criteria for specific types of neurodegeneration and the incorporation of advanced neuroimaging techniques and other biomarkers of neurodegenerative disease will help elucidate brain mechanisms that underlie specific symptoms.91
There are many factors besides neurodegenerative disease that precipitate BPSD, including environmental context and the dyadic relationship with the caregiver. Strong conceptual frameworks that include these factors are needed to guide future research studies. Additional iteration of our apathy model for depressive or psychotic symptoms, with specific attention to the neural and non-neural mechanisms pertinent to those symptoms, would be most helpful.
TABLE 1.
RECOMMENDATIONS FOR FUTURE RESEARCH TO ADVANCE KNOWLEDGE ABOUT APATHY
| General Recommendation | Evidence/Rationale for Recommendation |
|---|---|
| Prospective clinical trials are needed with apathy as a primary outcome together with important secondary outcomes, such as function. | With few exceptions, apathy has been investigated as a secondary outcome in retrospective studies |
| Novel technology-approaches including activity-monitoring devices and eye-trackers are necessary for more objective measurement of apathy. | Apathy is often measured subjectively by the individual, caregiver or provider. |
| Use of a uniform operational definition of apathy10 and a standard measure specific to the definition would enhance precision and facilitate comparison across studies. | Apathy has been described and measured inconsistently in the literature. |
| Recruitment of well-characterized samples that meet criteria for specific types of neurodegenerative disease. | The pathophysiology of apathy may not be the same across the neurodegenerative disease spectrum. |
| Continued study of the neurobiological basis of the different apathy components using neuroimaging techniques. | Without greater neurobiological specificity, it will be difficult to understand the neuroanatomical associations with specific apathy symptoms. Greater specificity of apathy subtypes will also help investigators to more precisely identify treatment targets and to determine who is likely to respond to specific treatments. |
| Longitudinal studies of apathy are needed to allow for sufficient time to observe potential treatment effects. | Intervention trials need to be of sufficient duration to detect clinically relevant effects in the treatment arm and to observe the likelihood of worsening apathy in the control arm. In addition, given apathy’s association with conversion to MCI and AD, intervention studies should examine whether efficacious treatments delay this conversion. |
| Investigators should consider stabilization of apathy severity an important outcome of intervention in addition to delay in emergence or reduction of apathy. | Apathy worsens as dementia progresses and the type and severity of dementia likely influences response to pharmacotherapy. |
| Studies that combine biological and psychosocial approaches are needed to more successfully treat apathy. | There is a general lack of high quality research to support the use of non-pharmacological approaches. |
| Strong conceptual frameworks that go beyond condition-specific indicators of treatment success and include person-centered goals are needed to guide future studies of apathy. | Few, if any, intervention studies include outcomes that reflect goals and preferences meaningful to people with apathy and/or their caregivers. The lived experience of neurodegenerative disease can provide important ecological insight into meaningful and achievable outcomes, such as the ability to maintain social and physical activity. |
Acknowledgments
Funding Sources: Dr. Massimo is supported by the National Institute on Aging (NIA) under award K99AG056054. Dr. Kolanowski is supported by National Institute of Nursing (NINR) under award R01NR015982.
Footnotes
Conflicts of Interest: The authors have no relevant conflicts of interest to report. All authors contributed to this paper.
References
- 1.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry. 2015;23:130–140. doi: 10.1016/j.jagp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann N, Lanctot KL, Sambrook R, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry. 2006;21:972–976. doi: 10.1002/gps.1594. [DOI] [PubMed] [Google Scholar]
- 4.van der Linde RM, Matthews FE, Dening T, Brayne C. Patterns and persistence of behavioural and psychological symptoms in those with cognitive impairment: the importance of apathy. Int J Geriatr Psychiatry. 2017;32:306–315. doi: 10.1002/gps.4464. [DOI] [PubMed] [Google Scholar]
- 5.Aalten P, de Vugt ME, Jaspers N, Jolles J, Verhey FR. The course of neuropsychiatric symptoms in dementia. Part I: findings from the two-year longitudinal Maasbed study. Int J Geriatr Psychiatry. 2005;20:523–530. doi: 10.1002/gps.1316. [DOI] [PubMed] [Google Scholar]
- 6.Fauth EB, Gibbons A. Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. Int J Geriatr Psychiatry. 2014;29:263–271. doi: 10.1002/gps.4002. [DOI] [PubMed] [Google Scholar]
- 7.Massimo L, Powers JP, Evans LK, et al. Apathy in Frontotemporal Degeneration: Neuroanatomical Evidence of Impaired Goal-directed Behavior. Front Hum Neurosci. 2015;9:611. doi: 10.3389/fnhum.2015.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huey ED, Lee S, Cheran G, Grafman J, Devanand DP, Alzheimer’s Disease Neuroimaging I Brain Regions Involved in Arousal and Reward Processing are Associated with Apathy in Alzheimer’s Disease and Frontotemporal Dementia. J Alzheimers Dis. 2017;55:551–558. doi: 10.3233/JAD-160107. [DOI] [PubMed] [Google Scholar]
- 9.Kolanowski A, Boltz M, Galik E, et al. Determinants of behavioral and psychological symptoms of dementia: A scoping review of the evidence. Nurs Outlook. 2017 doi: 10.1016/j.outlook.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24:98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Chase TN. Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotox Res. 2011;19:266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- 12.Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- 13.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 14.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46:210–215. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 15.van Reekum R, Stuss DT, Ostrander L. Apathy: why care? J Neuropsychiatry Clin Neurosci. 2005;17:7–19. doi: 10.1176/jnp.17.1.7. [DOI] [PubMed] [Google Scholar]
- 16.Di Iulio F, Palmer K, Blundo C, et al. Occurrence of neuropsychiatric symptoms and psychiatric disorders in mild Alzheimer’s disease and mild cognitive impairment subtypes. Int Psychogeriatr. 2010;22:629–640. doi: 10.1017/S1041610210000281. [DOI] [PubMed] [Google Scholar]
- 17.Diehl-Schmid J, Pohl C, Perneczky R, Forstl H, Kurz A. Behavioral disturbances in the course of frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22:352–357. doi: 10.1159/000095625. [DOI] [PubMed] [Google Scholar]
- 18.Aarsland D, Bronnick K, Alves G, et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80:928–930. doi: 10.1136/jnnp.2008.166959. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen KF, Alves G, Aarsland D, Larsen JP. Occurrence and risk factors for apathy in Parkinson disease: a 4-year prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2009;80:1279–1282. doi: 10.1136/jnnp.2008.170043. [DOI] [PubMed] [Google Scholar]
- 20.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 21.Peavy GM, Salmon DP, Edland SD, et al. Neuropsychiatric features of frontal lobe dysfunction in autopsy-confirmed patients with lewy bodies and “pure” Alzheimer disease. Am J Geriatr Psychiatry. 2013;21:509–519. doi: 10.1016/j.jagp.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benoit M, Andrieu S, Lechowski L, Gillette-Guyonnet S, Robert PH, Vellas B. Apathy and depression in Alzheimer’s disease are associated with functional deficit and psychotropic prescription. Int J Geriatr Psychiatry. 2008;23:409–414. doi: 10.1002/gps.1895. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield LC, Cimino CR, Oelke LE, Hauser RA, Sanchez-Ramos J. The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology. 2010;24:721–730. doi: 10.1037/a0019650. [DOI] [PubMed] [Google Scholar]
- 24.Ishii S, Weintraub N, Mervis JR. Apathy: a common psychiatric syndrome in the elderly. J Am Med Dir Assoc. 2009;10:381–393. doi: 10.1016/j.jamda.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Nijsten JMH, Leontjevas R, Pat-El R, Smalbrugge M, Koopmans R, Gerritsen DL. Apathy: Risk Factor for Mortality in Nursing Home Patients. J Am Geriatr Soc. 2017;65:2182–2189. doi: 10.1111/jgs.15007. [DOI] [PubMed] [Google Scholar]
- 26.Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195–202. doi: 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vicini Chilovi B, Conti M, Zanetti M, Mazzu I, Rozzini L, Padovani A. Differential impact of apathy and depression in the development of dementia in mild cognitive impairment patients. Dement Geriatr Cogn Disord. 2009;27:390–398. doi: 10.1159/000210045. [DOI] [PubMed] [Google Scholar]
- 29.Delrieu J, Desmidt T, Camus V, et al. Apathy as a feature of prodromal Alzheimer’s disease: an FDG-PET ADNI study. Int J Geriatr Psychiatry. 2015;30:470–477. doi: 10.1002/gps.4161. [DOI] [PubMed] [Google Scholar]
- 30.Massimo L, Powers C, Moore P, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dementia & Geriatric Cognitive Disorders. 2009;27:96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer’s disease. J Am Geriatr Soc. 2001;49:1700–1707. doi: 10.1046/j.1532-5415.2001.49282.x. [DOI] [PubMed] [Google Scholar]
- 32.Massimo L, Evans LK, Benner P. Caring for loved ones with frontotemporal degeneration: the lived experiences of spouses. Geriatr Nurs. 2013;34:302–306. doi: 10.1016/j.gerinurse.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vugt MERS, Aalten P, Tibben A, van Swieten JC, Verhey FR. Impact of behavioural problems on spousal caregivers: a comparison between Alzheimer’s disease and frontotemporal dementia. Dementia and Geriatric Cogntive Disorders. 2006;22:35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- 34.Hessler JB, Schaufele M, Hendlmeier I, et al. Behavioural and psychological symptoms in general hospital patients with dementia, distress for nursing staff and complications in care: results of the General Hospital Study. Epidemiol Psychiatr Sci. 2017:1–10. doi: 10.1017/S2045796016001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everitt DE, Fields DR, Soumerai SS, Avorn J. Resident behavior and staff distress in the nursing home. J Am Geriatr Soc. 1991;39:792–798. doi: 10.1111/j.1532-5415.1991.tb02702.x. [DOI] [PubMed] [Google Scholar]
- 36.Koder D, Hunt GE, Davison T. Staff’s views on managing symptoms of dementia in nursing home residents. Nurs Older People. 2014;26:31–36. doi: 10.7748/nop.26.10.31.e638. [DOI] [PubMed] [Google Scholar]
- 37.Brodaty H, Draper B, Low LF. Nursing home staff attitudes towards residents with dementia: strain and satisfaction with work. J Adv Nurs. 2003;44:583–590. doi: 10.1046/j.0309-2402.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- 38.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 39.Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry. 2005;76:1070–1074. doi: 10.1136/jnnp.2004.052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marin RS. Apathy and related disorders of diminished motivation. In: Dickstein LJ, R MB, Oldham JM, editors. Review of Psychiatry. Washington, DC: American Psychiatric Press; 1996. pp. 205–242. [Google Scholar]
- 41.Radakovic R, Stephenson L, Colville S, Swingler R, Chandran S, Abrahams S. Multidimensional apathy in ALS: validation of the Dimensional Apathy Scale. J Neurol Neurosurg Psychiatry. 2016;87:663–669. doi: 10.1136/jnnp-2015-310772. [DOI] [PubMed] [Google Scholar]
- 42.Boyer F, Novella JL, Morrone I, Jolly D, Blanchard F. Agreement between dementia patient report and proxy reports using the Nottingham Health Profile. Int J Geriatr Psychiatry. 2004;19:1026–1034. doi: 10.1002/gps.1191. [DOI] [PubMed] [Google Scholar]
- 43.Valembois L, Oasi C, Pariel S, Jarzebowski W, Lafuente-Lafuente C, Belmin J. Wrist Actigraphy: A Simple Way to Record Motor Activity in Elderly Patients with Dementia and Apathy or Aberrant Motor Behavior. The journal of nutrition, health & aging. 2015;19:759–764. doi: 10.1007/s12603-015-0530-z. [DOI] [PubMed] [Google Scholar]
- 44.Konig A, Aalten P, Verhey F, et al. A review of current information and communication technologies: can they be used to assess apathy? Int J Geriatr Psychiatry. 2014;29:345–358. doi: 10.1002/gps.4017. [DOI] [PubMed] [Google Scholar]
- 45.Cummings J, Friedman JH, Garibaldi G, et al. Apathy in Neurodegenerative Diseases: Recommendations on the Design of Clinical Trials. Journal of geriatric psychiatry and neurology. 2015;28:159–173. doi: 10.1177/0891988715573534. [DOI] [PubMed] [Google Scholar]
- 46.Lanctot KL, Aguera-Ortiz L, Brodaty H, et al. Apathy associated with neurocognitive disorders: Recent progress and future directions. Alzheimers Dement. 2017;13:84–100. doi: 10.1016/j.jalz.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Jao YL, Algase DL, Specht JK, Williams K. The Association Between Characteristics of Care Environments and Apathy in Residents With Dementia in Long-term Care Facilities. Gerontologist. 2015;55(Suppl 1):S27–39. doi: 10.1093/geront/gnu166. [DOI] [PubMed] [Google Scholar]
- 48.Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Environmental correlates of neuropsychiatric symptoms in nursing home patients with dementia. Int J Geriatr Psychiatry. 2010;25:14–22. doi: 10.1002/gps.2292. [DOI] [PubMed] [Google Scholar]
- 49.Massimo L, Evans LK. Differentiating subtypes of apathy to improve person-centered care in frontotemporal degeneration. J Gerontol Nurs. 2014;40:58–65. doi: 10.3928/00989134-20140827-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quaranta D, Marra C, Rossi C, Gainotti G, Masullo C. Different apathy profile in behavioral variant of frontotemporal dementia and Alzheimer’s disease: a preliminary investigation. Curr Gerontol Geriatr Res. 2012;2012:719250. doi: 10.1155/2012/719250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gough D. Weight of evidence: a framework for the appraisal of the quality and relevance of evidence. Research Papers in Education. 2007;22:213–228. [Google Scholar]
- 52.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 53.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 54.Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol. 2012;25:127–136. doi: 10.3233/BEN-2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horning SM, Melrose R, Sultzer D. Insight in Alzheimer’s disease and its relation to psychiatric and behavioral disturbances. Int J Geriatr Psychiatry. 2014;29:77–84. doi: 10.1002/gps.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leger GC, Banks SJ. Neuropsychiatric symptom profile differs based on pathology in patients with clinically diagnosed behavioral variant frontotemporal dementia. Dement Geriatr Cogn Disord. 2014;37:104–112. doi: 10.1159/000354368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park HK, Choi SH, Park SA, et al. Cognitive profiles and neuropsychiatric symptoms in Korean early-onset Alzheimer’s disease patients: a CREDOS study. J Alzheimers Dis. 2015;44:661–673. doi: 10.3233/JAD-141011. [DOI] [PubMed] [Google Scholar]
- 58.Saz P, Lopez-Anton R, Dewey ME, et al. Prevalence and implications of psychopathological non-cognitive symptoms in dementia. Acta Psychiatr Scand. 2009;119:107–116. doi: 10.1111/j.1600-0447.2008.01280.x. [DOI] [PubMed] [Google Scholar]
- 59.Johnson DK, Watts AS, Chapin BA, Anderson R, Burns JM. Neuropsychiatric profiles in dementia. Alzheimer Dis Assoc Disord. 2011;25:326–332. doi: 10.1097/WAD.0b013e31820d89b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Proitsi P, Hamilton G, Tsolaki M, et al. A Multiple Indicators Multiple Causes (MIMIC) model of Behavioural and Psychological Symptoms in Dementia (BPSD) Neurobiol Aging. 2011;32:434–442. doi: 10.1016/j.neurobiolaging.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Reyes S, Viswanathan A, Godin O, et al. Apathy: a major symptom in CADASIL. Neurology. 2009;72:905–910. doi: 10.1212/01.wnl.0000344166.03470.f8. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez-Martinez M, Molano A, Castro J, Zarranz JJ. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease, and its relationship with cognitive impairment. Current Alzheimer research. 2010;7:517–526. doi: 10.2174/156720510792231748. [DOI] [PubMed] [Google Scholar]
- 63.Kuzis G, Sabe L, Tiberti C, Dorrego F, Starkstein SE. Neuropsychological correlates of apathy and depression in patients with dementia. Neurology. 1999;52:1403–1407. doi: 10.1212/wnl.52.7.1403. [DOI] [PubMed] [Google Scholar]
- 64.Donovan NJ, Wadsworth LP, Lorius N, et al. Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry. 2014;22:1168–1179. doi: 10.1016/j.jagp.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Prete M, Spaccavento S, Craca A, Fiore P, Angelelli P. Neuropsychiatric symptoms and the APOE genotype in Alzheimer’s disease. Neurol Sci. 2009;30:367–373. doi: 10.1007/s10072-009-0116-9. [DOI] [PubMed] [Google Scholar]
- 66.Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jao YL, Loken E, MacAndrew M, Van Haitsma K, Kolanowski A. Association between social interaction and affect in nursing home residents with dementia. Aging Ment Health. 2017:1–6. doi: 10.1080/13607863.2017.1304526. [DOI] [PubMed] [Google Scholar]
- 68.Ballard C, Fossey J, Chithramohan R, et al. Quality of care in private sector and NHS facilities for people with dementia: cross sectional survey. BMJ. 2001;323:426–427. doi: 10.1136/bmj.323.7310.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schnelle JF, MacRae PG, Giacobassi K, MacRae HS, Simmons SF, Ouslander JG. Exercise with physically restrained nursing home residents: maximizing benefits of restraint reduction. J Am Geriatr Soc. 1996;44:507–512. doi: 10.1111/j.1532-5415.1996.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 70.Perrin T. Occupational need in severe dementia: a descriptive study. J Adv Nurs. 1997;25:934–941. doi: 10.1046/j.1365-2648.1997.1997025934.x. [DOI] [PubMed] [Google Scholar]
- 71.Schreiner AS, Yamamoto E, Shiotani H. Positive affect among nursing home residents with Alzheimer’s dementia: the effect of recreational activity. Aging Ment Health. 2005;9:129–134. doi: 10.1080/13607860412331336841. [DOI] [PubMed] [Google Scholar]
- 72.Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT., Jr A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. J Am Geriatr Soc. 2011;59:1032–1041. doi: 10.1111/j.1532-5415.2011.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niu YX, Tan JP, Guan JQ, Zhang ZQ, Wang LN. Cognitive stimulation therapy in the treatment of neuropsychiatric symptoms in Alzheimer’s disease: a randomized controlled trial. Clin Rehabil. 2010;24:1102–1111. doi: 10.1177/0269215510376004. [DOI] [PubMed] [Google Scholar]
- 74.Tappen RM, Williams CL. Therapeutic conversation to improve mood in nursing home residents with Alzheimer’s disease. Res Gerontol Nurs. 2009;2:267–275. doi: 10.3928/19404921-20090428-02. [DOI] [PubMed] [Google Scholar]
- 75.Choi J, Medalia A. Factors associated with a positive response to cognitive remediation in a community psychiatric sample. Psychiatr Serv. 2005;56:602–604. doi: 10.1176/appi.ps.56.5.602. [DOI] [PubMed] [Google Scholar]
- 76.Harrison F, Aerts L, Brodaty H. Apathy in Dementia: Systematic Review of Recent Evidence on Pharmacological Treatments. Current psychiatry reports. 2016;18:103. doi: 10.1007/s11920-016-0737-7. [DOI] [PubMed] [Google Scholar]
- 77.Berman K, Brodaty H, Withall A, Seeher K. Pharmacologic treatment of apathy in dementia. Am J Geriatr Psychiatry. 2012;20:104–122. doi: 10.1097/JGP.0b013e31822001a6. [DOI] [PubMed] [Google Scholar]
- 78.Theleritis C, Siarkos K, Katirtzoglou E, Politis A. Pharmacological and Nonpharmacological Treatment for Apathy in Alzheimer Disease: A systematic review across modalities. J Geriatr Psychiatry Neurol. 2017;30:26–49. doi: 10.1177/0891988716678684. [DOI] [PubMed] [Google Scholar]
- 79.Frakey LL, Salloway S, Buelow M, Malloy P. A randomized, double-blind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer’s disease. The Journal of clinical psychiatry. 2012;73:796–801. doi: 10.4088/JCP.10m06708. [DOI] [PubMed] [Google Scholar]
- 80.Padala PR, Padala KP, Lensing SY, et al. Methylphenidate for Apathy in Community-Dwelling Older Veterans With Mild Alzheimer’s Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17030316. appiajp201717030316. [DOI] [PubMed] [Google Scholar]
- 81.Scherer RW, Drye L, Mintzer J, et al. The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial. Trials. 2018;19:46. doi: 10.1186/s13063-017-2406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suemoto CK, Apolinario D, Nakamura-Palacios EM, et al. Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer’s disease: a randomized, double-blind, sham-controlled trial. Brain Stimul. 2014;7:308–313. doi: 10.1016/j.brs.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Brodaty H, Burns K. Nonpharmacological management of apathy in dementia: a systematic review. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2012;20:549–564. doi: 10.1097/JGP.0b013e31822be242. [DOI] [PubMed] [Google Scholar]
- 84.Goris ED, Ansel KN, Schutte DL. Quantitative systematic review of the effects of non-pharmacological interventions on reducing apathy in persons with dementia. J Adv Nurs. 2016;72:2612–2628. doi: 10.1111/jan.13026. [DOI] [PubMed] [Google Scholar]
- 85.Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT., Jr A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. J Am Geriatr Soc. 2011;59:1032–1041. doi: 10.1111/j.1532-5415.2011.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lam LC, Lui VW, Luk DN, et al. Effectiveness of an individualized functional training program on affective disturbances and functional skills in mild and moderate dementia–a randomized control trial. Int J Geriatr Psychiatry. 2010;25:133–141. doi: 10.1002/gps.2309. [DOI] [PubMed] [Google Scholar]
- 87.Leone E, Deudon A, Bauchet M, et al. Management of apathy in nursing homes using a teaching program for care staff: the STIM-EHPAD study. Int J Geriatr Psychiatry. 2013;28:383–392. doi: 10.1002/gps.3836. [DOI] [PubMed] [Google Scholar]
- 88.Bangerter LR, Abbott K, Heid AR, Klumpp RE, Van Haitsma K. Health Care Preferences Among Nursing Home Residents: Perceived Barriers and Situational Dependencies to Person-Centered Care. Journal of gerontological nursing. 2016;42:11–16. doi: 10.3928/00989134-20151218-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolanowski A, Van Haitsma K, Penrod J, Hill N, Yevchak A. “Wish we would have known that!” Communication Breakdown Impedes Person-Centered Care. Gerontologist. 2015;55(Suppl 1):S50–60. doi: 10.1093/geront/gnv014. [DOI] [PubMed] [Google Scholar]
- 90.American Geriatrics S, American Association for Geriatric P. The American Geriatrics Society and American Association for Geriatric Psychiatry recommendations for policies in support of quality mental health care in U.S. nursing homes. J Am Geriatr Soc. 2003;51:1299–1304. doi: 10.1046/j.1532-5415.2003.51416.x. [DOI] [PubMed] [Google Scholar]
- 91.Rosenberg PB. New Insights Into Brain Mechanisms Underlying Neuropsychiatric Symptoms in Alzheimer Disease. Am J Geriatr Psychiatry. 2017;25:580–581. doi: 10.1016/j.jagp.2017.02.023. [DOI] [PubMed] [Google Scholar]


