Abstract
We developed methods for conditionally reprogramming (CR) primary human bronchial epithelial cells (HBECs) to extend their functional lifespan and permit their differentiation into both upper and lower airway lung epithelium. We also developed a bioreactor to support vascular perfusion and rhythmic breathing of decellularized mouse lungs reconstituted with CR HBECs isolated from patients with and without cystic fibrosis (CF). While conditionally reprogrammed cells only differentiate into an upper airway epithelium after 35 days at the air–liquid interface, in reconstituted lungs these cells differentiate into upper airway bronchial epithelium and lower airway alveolar structures after 12 days. Rapid scale-up and the ability to obtain clonal derivatives of primary patient-derived HBECs without the need for genetic manipulation may permit rapid reconstitution of the lung epithelium; facilitating the study of lung disease in tissue-engineered models.
Keywords: : multipotent, decellularized lung, ROCK inhibitor, cystic fibrosis, tissue engineering
Introduction
Engineering lungs by seeding native decellularized scaffolds with stem cells is a potential method for addressing the severe shortage of available transplants for organ failure and for studying disease ex vivo.1 Decellularized matrices can provide cells with extracellular matrix (ECM) proteins,2 mechanical queues,3 and ultrastructural architecture,4 all of which influence their differentiation. Others have reported engineering lungs using decellularized lung scaffolds from rats or larger mammals seeded with embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs).5,6 However, ESCs and iPSCs can be difficult to culture and do not always form pure populations of differentiated cells after processing. This raises safety concerns and makes it unlikely that these cells could be used to engineer transplantable tissue.7,8
HBECs are a heterogeneous population of cells containing multipotent basal cells that can be isolated from patients with minimally invasive endobronchial biopsy9 or bronchial or nasal brushings.10 HBEC basal stem-like cells are multipotent and capable of forming airway epithelium, but using currently established culturing methods these cells rapidly lose the ability to differentiate in vitro and have not been demonstrated to differentiate into lower airway cells in in vitro or ex vivo conditions tested to date.11 If these challenges could be overcome, multipotent HBECs could be used to rapidly engineer transplantable lung tissue derived from a patient's own cells, abrogating the need for lifelong immunosuppression using donor lung transplants.
Primary conditionally reprogrammed (CR) HBECs cultured in the presence of an irradiated fibroblast feeder layer and ROCK (Rho-associated coiled-coil-containing protein kinase) inhibitor have a significantly extended lifespan and retain the ability to differentiate in response to culture at an air–liquid interface (ALI).12 However, while CR HBECs have recently been used for reconstituting a tracheal matrix, they have not been shown to be capable of differentiating into lower airway cells or reconstituting lung tissue.13
The only primary bronchial basal cells known to differentiate into both upper and lower airway cells in a reconstituted lung are distal KRT5+ TP63+ bronchial basal cells; but these cells only survive a few passages and are isolated from cells of the distal airway.14 As primary HBECs are often used to screen for cystic fibrosis (CF) drugs in vitro,15 lungs seeded with CR HBECs isolated from a CF patient could represent a novel model for studying this disease ex vivo. If CR HBECs differentiate into upper and lower airway cells in response to culture in a lung, it would mean that a patient's airway epithelium could be reconstructed from their own cells isolated using a minimally invasive procedure.
In this study, we have created an ex vivo reconstituted lung system using CR HBECs seeded into a decellularized mouse lung using a bioreactor with simulated breathing and vascular perfusion. We document methods for decellularizing the murine lung while accessing the vascular and tracheal compartments. Using the bioreactor system, normal human CR HBECs (WT CR HBECs) and CR HBECs isolated from a patient with cystic fibrosis (CF CR HBECs) were seeded into decellularized murine lung matrices and maintained for up to 2 weeks. These multipotent lung-derived cells rapidly reconstitute the upper and lower airway niches and differentiate into a variety of cell types, including type I and II pneumocytes. As far as we could determine, HBECs have not been previously demonstrated to differentiate into lower airway pneumocytes. Tissue engineering of lungs using a primary adult stem-like cell population with an extended lifespan would permit iterative generation of tissue-engineered constructs with the same population of nongenetically manipulated multipotent cells. This would immediately facilitate generation of ex vivo lungs for the study of disease and ultimately transplantation.
Methods
Culture of conditionally reprogrammed HBECs
Primary normal HBECs were a generous gift of the UNC (University of North Carolina Marsico Lung Institute, The CF Center Tissue Procurement and Cell Culture Core). Primary CF HBECs were harvested and cultured from CF lung explant tissue under the UT Southwestern IRB-approved protocol No. CR00013395/STU052011020. These cells were cultured in 50/50 Bronchial Epithelial Growth Medium (BEGM) (Lonza) plus DMEM high glucose media (Thermo Fisher) supplemented with the full BEGM BulletKit +5% FBS +10 μM Rock Inhibitor (RI) Y-27632 (Enzo Life Sciences). These cells were maintained in a humidified 37°C incubator at physiologic oxygen in chambers which have been previously described.16 Approximately 500K of these cells were seeded in coculture with 500K of freshly irradiated (30 Gy) J2 3T3's in Corning 10-cm2 tissue culture dishes during passaging.
Before passage, transfer into ALI culture, or lung reconstitution, IR 3T3 J2 fibroblasts were separated from CR HBECs. In brief, when the HBEC/J2 3T3 coculture is confluent, the dishes are washed once with 10 mL of Solution A (Hepes 30 mM (pH 7.4), glucose 4 mM, KCl 3 mM, NaCl 122 nM, Na2HPO4 1 mM, and phenol red 0.5 mM) and are then washed with 3 mL of 0.02% EDTA in PBS for 5 min at 37°C to remove the fibroblasts from the culture. After 5 min, the dishes are lightly agitated to dislodge the fibroblasts. The cultures are then washed with solution A to remove residual fibroblasts. Finally, HBECs are trypsinized with 2 mL of 0.05% Trypsin 0.02% EDTA at 37°C for 10 min to detach the HBECs. The dissociated HBECs are mixed with trypsin neutralization solution before pelleting for other uses such as new 2D or ALI culture or perfused into decellularized lungs. For ALI culture and culture of reconstituted lungs after 3 days of culture with RI, we used modified differentiation ALI BEGM prepared as described previously.17
Murine lung decellularization
To harvest the lungs, we injected mice with 100 mg/kg:10 mg/kg of a ketamine/xylazine solution and a 250 U/kg heparin solution (H3393; Sigma) intraperitoneally before surgery. After surgically removing the heart–lung block, the trachea was inflated with 5 mL of a 50 U/mL PBS heparin solution in 1 mL intervals, with the lung being allowed to deflate between each interval. The right ventricle of the heart was then pierced with a 201/2-gauge needle and 3 mL of a 50 U/mL heparin solution was slowly perfused through the pulmonary circulation system. The needle was then replaced with a 201/2-gauge blunt-end needle and cannulated in place; and then 2 mL of the heparin solution was perfused through the lung. The trachea was then cannulated using a 201/2-gauge blunt needle.
The lung was loaded into a bioreactor containing 200 mL of a CHAPs solution (8 mM CHAPs, 1 M NaCl, 25 mM EDTA in PBS) and was placed in a 37°C incubator. One microliter of the CHAPs solution was infused into the tracheal compartment every half hour, and CHAPs was perfused through the lung at 1 mL/min. After 3 h of CHAPs decellularization, the lung was placed into a new jar containing 200 mL of a DNAse solution (30 μg/mL Benzoase (Sigma), 1.3 mM MgSO4, and 2 mM CaCl2 in ddH2O). One microliter of fresh DNAse solution was infused into the tracheal compartment every half hour, and DNAse was perfused through the lung at 1 mL/min for 1 h. The lung was then transferred into 200 mL of a PBS + 5X antibiotic–antimycotic solution (PBSAA) (Gemini Bio-Products) 1 mL of the PBSAA solution was infused into the tracheal compartment, and PBSAA was perfused through the lung at 1 mL/min overnight at 37°C.
After this decellularization, the lungs were then transferred to storage at 4°C until they were ready for use. One hour before use, the lung was inflated with 1 mL of a 1 mg/mL solution of fibronectin in PBS while suspended in PBS in the bioreactor. The lung was incubated in the bioreactor in a 37°C incubator for 1 h before initiating the recellularization protocol.
Murine lung picogreen DNA quantitation
DNA Quantitation of native and decellularized lungs was performed using a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Eugene, OR) following the manufacturer's protocol. Briefly, after papain digestion, lung tissue isolates were mixed with the PicoGreen reagent. They were then measured through spectrophotometry against a standard curve. The final measurements were normalized to wet tissue weight. The difference between native and decellularized lung DNA content was determined by a two-tailed Student's t-test.
Murine lung recellularization and culture
We transferred a decellularized murine lung into a bioreactor with 100 mL of BEGM. The lung was infused with 1 mL of BEGM in the tracheal compartment, and was vascularly perfused with BEGM at 1 mL/min for at least 1 h. The lung was then transferred into a jar containing 150 mL of BEGM. It was then inflated with an infusion of 1 mL of BEGM, and then 10 million PD30 CR HBECs, which have been passed through a 70 μm mesh nylon filter (Fisher Scientific) suspended in 2 mL of BEGM, were slowly infused into the murine lung immediately after the lung was inflated with 1 mL of BEGM. This infusion method produced an even distribution of cells across 90% of the lung tissue, although there were some isolated acellular patches. The bioreactor containing the lung was then transferred to a 37°C incubator for 16 h of overnight static culture to allow the cells to attach.
The next morning, the lung began vascular perfusion at 1 mL/min. Four hours later, we initiated simulated liquid breathing by attaching the tracheal chamber with 50 mL of BEGM. Negative pressure-induced breathing was performed by withdrawing 7 mL of air at 1 mL/min from the main tube and then reinfusing that same air over 7 min using an automated syringe pump (New Era Pump Systems, Inc.). For the next 2 days, the lungs would be gassed every day by infusing the primary chamber with 120 mL of air.
After 3 days of culture, the lung was transferred to new jars containing 150 mL of ALI BEGM for the main chamber and 50 mL of ALI BEGM for the tracheal chamber. The rate of liquid breathing and vascular perfusion was kept constant, and the bioreactors were manually gassed every 24 h. Media were changed every 3 days until a total of 12 days of culture had passed. The lung was then taken from the bioreactor; the two right lobes were removed and flash frozen in liquid nitrogen for protein isolation. The remaining lobe was fixed in 10% neutral buffered formalin (NBF).
ALI culture
ALI culture was performed on human placental collagen IV-coated 24-mm Transwell™ permeable (0.4 μm pore) polyester membrane supports (Costar, Ref 3450) as described previously.17 Briefly, 400K CR HBECs were seeded onto transwells with 1 mL of BEGM above the membrane and 2 mL of BEGM below the membrane. The CR HBECs became confluent after 2–3 days; after which point the BEGM was replaced with 2 mL of ALI BEGM. This medium was changed every 2–3 days.
Hematoxylin and eosin and immunofluorescence staining
We performed Hematoxylin and Eosin (H&E) and Immunofluorescence (IF) staining on slides as described previously.18 In brief; the right lobe of the lung was inflated with 10% NBF and then stored overnight in 10% NBF with gentle rocking. The lung lobes were then paraffin embedded, and slides were made with 5 μm thick slices of the tissue. The slices were rehydrated, and underwent H&E or IF staining.
Before IF staining, antigen unmasking was performed by boiling for 10 min in a pH 6.0 10 mM sodium citrate buffer. Slides were blocked and incubated overnight with the primary antibody at 4°C. After washing with PBS, slides were incubated with secondary fluorescent antibody for 1 h at room temperature, followed by a second PBS wash and counterstaining with DAPI. For IF staining we used the following antibodies, Fibronectin: ab2413 (Abcam) (1:200), Elastin: ab21610 (Abcam) (1:200), Collagen IV: ab6586 (Abcam) (1:200), Laminin: ab30320 (1:200), CCSP: AB40B73 (Abcam) (1:1000), AQP5: EPR 3747 (Abcam) (1:1000), CK14: PA5-29608 (Thermo Fisher Scientific) (1:500), Pro-SPC: AB40879 (Abcam) (1:500), Muc5B sc-20119 (Santa Cruz Biotechnology) (1:500), and acetylated alpha tubulin T6793 (Sigma-Aldrich) (1:500).
Protein isolation and western blotting
Protein was isolated using lysis buffer (1% Triton-X100, 1 mM EDTA, 12 mM NaCl in TRIS buffer at 7.4 pH with freshly added protease inhibitor [Roche Product No. 05892791001]). Flash-frozen lung lobes were pulverized with a mortar and pestle. The pulverized lung lobe was suspended in 100 μL of lysis buffer and was drawn through a 201/2-gauge needle five times. The suspension was centrifuged at 21,000 RCF and 4°C for 15 min. The supernatant was transferred to a new tube and stored at −80°C. Protein was isolated from ALI cultures by first washing the membrane with sterile PBS two times. One hundred microliters of lysis buffer was added to the well and the nylon membrane was scrapped with the back of a sterile pipette tip. The suspension was collected and the nylon membrane was washed with another 50 μL of lysis buffer. The protein suspension was stored at −80°C.
For western blotting, we loaded 25 μg of the protein lysate for 2D culture and ALI culture and 35 μg of protein for native, decellularized, and reconstituted lungs onto a Mini-PROTEAN TGX Precast Gel (Bio-Rad Cat. 456-1086) and ran the gel at 100 V for 30 min. After transferring the protein to a PFA membrane using a Trans-Blot Turbo Transfer Pack (Bio-Rad Cat. 170-4157), we used blocking buffer (5% dry milk in PBST) for 1 h and performed primary and secondary immunostaining as described previously.19 For western blotting, we used the following primary antibodies; CCSP: AB40873 (Abcam) (1:1000), AQP5: EPR3747 (Abcam) (1:1000), CK14: PA5-29608 (Thermo Fisher Scientific) (1:500), Pro-SPC: AB40879 (Abcam) (1:500), and Beta Actin (ab8227) (Abcam) 1:1000.
Biomechanical testing
We assayed the tensile strength and Young's modulus of native and decellularized lungs using an Instron 5848 with a 10-newton load cell. We took a strip of the mouse lung of ∼9–12 mm in length and 3–6 mm in width and placed them into the load cell after sandwiching each end of the lung strip in sand paper. The sample dimensions were recorded using an area micrometer (with a resolution of 1 μm) by lightly compressing the sample into a rectangular cross-section. The samples were preloaded to reach the toe region and preconditioned with six cycles of 10% cyclic engineering strain (grip to grip) at 1 s per cycle, and then were loaded until failure.
For each lung slice strain was measured as ɛ = dL/Lo, where ɛ = strain, dL = change in length, and Lo = Initial length. Stress was measured as σ = Fn/A, where σ = stress, Fn = applied force, and A = Area. The Young's modulus was measured as the linear regression of stress divided by strain during deformation. The tensile stiffness was measured as a linear regression of k = F/δ during deformation, where k = tensile stiffness, F = applied force, and δ = displacement. The statistical significance of differences between native and decellularized matrix bulk–Young's modulus and stiffness was determined by a two-tailed Student's t-test.
Results
Mouse lung decellularization preserves ECM proteins and ultrastructure
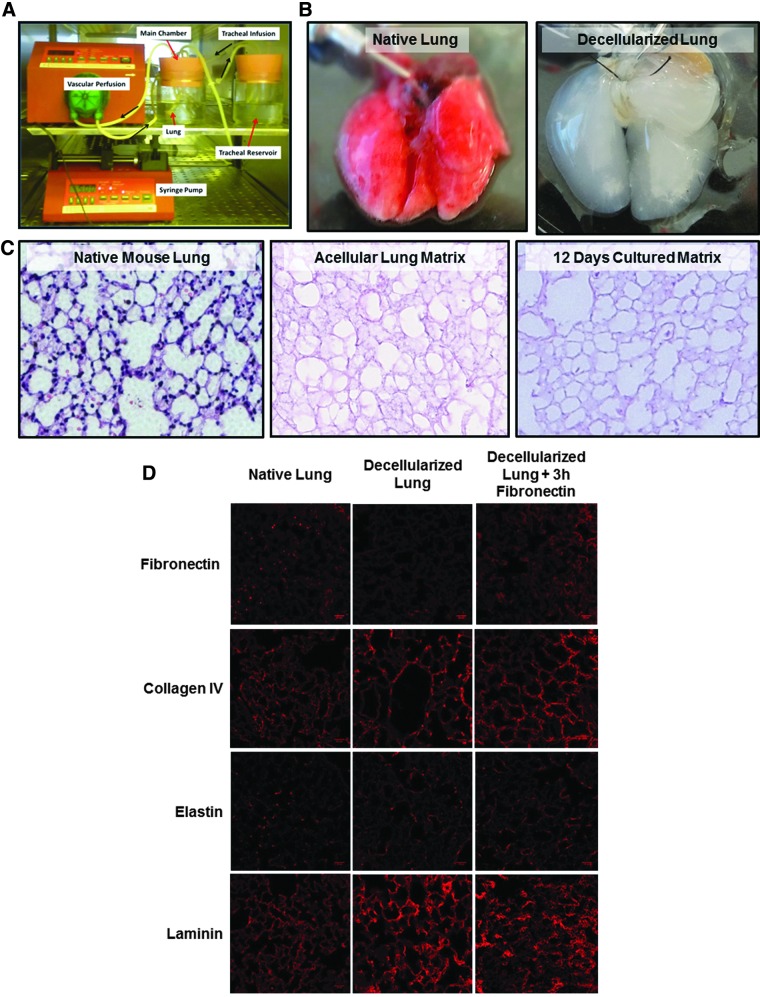
A bioreactor system for decellularization of murine lungs and extended culture of introduced human lung epithelial cells with vascular perfusion and simulated breathing was developed (Fig. 1A). The native murine lung (Fig. 1B, left) was loaded into the bioreactor through cannulation of the trachea and right ventricle with blunted needles. In this bioreactor system, simulated breathing was performed through a syringe pump withdrawing air from the pressurized main bioreactor chamber, lowering the pressure and drawing liquid or air from the tracheal chamber into the lung through a breathing loop originally developed by Petersen et al.,20 whereas vascular perfusion was performed by circulation of culture medium into the cannulated right ventricle by a peristaltic pump (Fig. 1A).
FIG. 1.
Mouse lung decellularization. (A) The bioreactor system for decellularization of murine lungs and culture of recellularized lungs with vascular perfusion. The vascular perfusion is effected by a peristaltic pump, whereas liquid breathing is performed by a programmable syringe pump withdrawing air from the main culture chamber, creating negative pressure which induces the lung to expand. (B) Comparison of native lung and the postdecellularization ghost lung. (C) H + E staining comparing a native murine lung to a decellularized matrix and a decellularized matrix cultured for 12 days. (D) IF staining comparing the presence of ECM proteins fibronectin, collagen IV, elastin, and laminin before and after decellularization in a murine lung. ECM, extracellular matrix; IF, immunofluorescence; H+E, Hematoxylin and Eosin.
After decellularizing the lung in this bioreactor system for 3 h by vascular perfusion with a CHAPs solution followed by a 1 h DNAse wash, the resulting tissue takes on a ghost-like or translucent appearance as seen by others21 (Fig. 1B, right). A comparison of H&E staining of native (Fig. 1C, left) versus decellularized mouse lungs (Fig. 1C, middle) demonstrates that while the cells have been removed from the lung matrix, the ECM morphological structures do not change with no evidence of murine cellular regrowth even after the decellularized lung has been cultured for 12 days in the bioreactor system (n = 3) (Fig. 1C, right).
Decellularization of the murine lung with this protocol leaves less than 50 ng of DNA/mg of lung tissue after 12 days in culture conditions in a bioreactor, conforming with generally accepted tolerances for decellularization of tissue for tissue engineering applications.22 In optimizing this protocol, we found that decellularization for 2 h was not sufficient to fully decellularize the lung matrix, whereas decellularization for 5 h with the CHAPs solution had no additional effect on the amount of residual DNA present in the matrix (n = 4) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
Detergent-mediated decellularization can remove or damage lung ECM proteins, which are essential for the mechanical function of the lung and epithelial cell attachment and spreading.23–25 Analysis of ECM proteins in decellularized lungs made by IF staining (n = 2) shows some retention of elastin, collagen IV, and laminin and loss of fibronectin over the course of decellularization (Fig. 1D). Other mouse lung decellularization protocols using CHAPs have shown retention of collagen IV and laminin after CHAPs-mediated decellularization.26
While excess fibronectin production is a significant component of interstitial lung fibrosis, fibronectin has been shown to inhibit HBEC apoptosis in vitro and promote cell attachment to culture surfaces, indicating that supplementing the lung with some fibronectin may improve attachment and survival of HBECs to the lung matrix.27 For loss of fibronectin, we supplemented the lung with fibronectin for 1–3 h before reconstituting the lung, resulting in restoration of near-native amounts of fibronectin (Fig. 1D, right). These results indicate that the decellularization protocol produces a mouse lung matrix with mostly preserved architecture and ECM components with no intact murine cells remaining viable to repopulate the lung over 12 days.
Conditionally reprogrammed HBECs rapidly reconstitute mouse lungs
Investigations of lung diseases, such as CF, are commonly performed with primary human bronchial epithelial cells (HBECs) cultured at the ALI by culture on a microporous membrane (Transwell).28 As far as could be determined, there have been no studies comparing the dynamics of murine lungs reconstituted with HBECs to HBECs cultured at the ALI. In addition, we sought to determine if CR HBECs would respond to the decellularized murine lung niche by producing both upper and lower airway cells. We utilized two human cell strains isolated from primary tracheal/bronchial tissue, one derived from a patient free of lung disease, and the other from a patient with CF caused by a homozygous Δ-F508 mutation in the CFTR gene. We first determined that both populations can divide past 50 population doublings in vitro using the CR conditions, but senesce earlier using standard lung epithelial cell culture conditions (Supplementary Fig. S2).
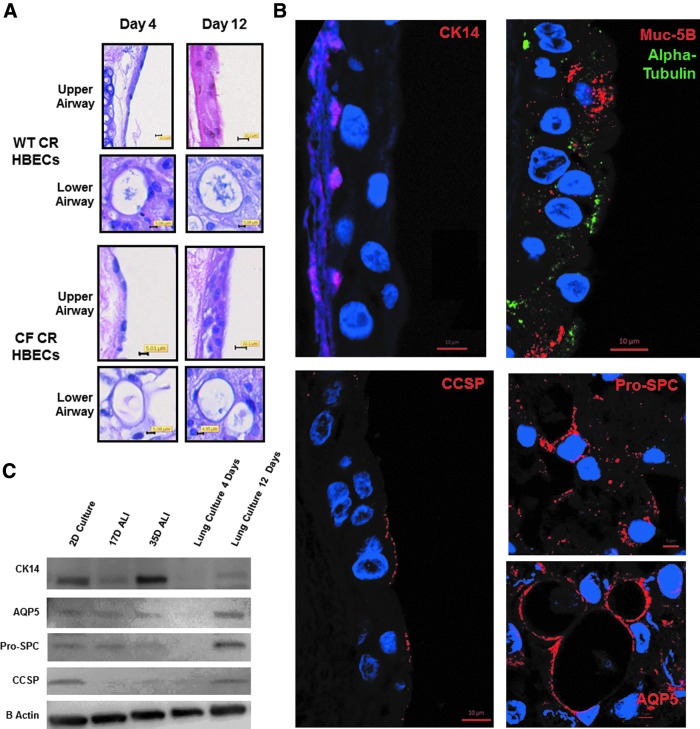
Reconstitution of decellularized mouse lungs was next performed with 10 million WT CR HBECs or CF CR HBECs which had undergone 30 population doublings (PDs) since their isolation. Over the course of culture in the bioreactor the lungs were fed with BEGM. After 3 days in culture in the bioreactor, lungs were switched to ALI BEGM supplemented with additional CaCl2 and retinoic acid. After 4 days of culture in the bioreactor, lungs seeded with CF or wild-type (WT) CR HBECs showed only partial differentiation into lung epithelium (Fig. 2A representative of three separate experiments (n = 3). However, after 12 days of culture in a bioreactor, H&E staining of recellularized mouse lungs seeded with WT CR HBECs (n = 3) and CF CR HBECs (n = 3) morphologically showed the formation of alveolar type cells in the lower airway niche and ciliated pseudostratified epithelium in the upper airway compartment that contained basal and goblet-appearing cells (Fig. 2A).
FIG. 2.
Differentiation of CR HBECs in reconstituted lung culture. (A) H + E staining of reconstituted lungs seeded with WT CR HBECs (top four images) and CF CR HBECs (bottom four images) for 4 days or 12 days. (B) IF Staining of basal cells (CK14), mucin-producing goblet cells (Muc-5B), ciliated cells (Alpha Tubulin), and Clara cells (CCSP) in the upper airways and with alveoli type I cells (AQP5), and alveoli type II cells (Pro-SPC) in the lower airways in a WT CR HBEC reconstituted lung cultured for 12 days in a bioreactor. (C) Western blot comparing AQP5, CK14, Pro-SPC, and CCSP protein in WT CR HBECs cultured in 2-D, at ALI for 12 days and 35 days, and in reconstituted lung culture for 4 days and 12 days. CCSP, Clara cell secretory protein; CR, conditional reprogrammed; HBEC, human bronchial epithelial cell; WT, wild type.
In optimizing the seeding protocol, we found that removing conditional reprogramming conditions from CR HBECs 7 days before seeding them into lungs produced an exaggerated columnar upper airway epithelium with no basal cells and no lower airway epithelium; suggesting that the reprogrammed state of CR HBECs is essential to their capacity to differentiate into lower airway cells (n = 2) (Supplementary Fig. S3). Seeding the murine lung with either 1 or 4 million CR HBECs and culturing them for 12 days produced reconstituted lungs, which did not display well-differentiated epithelium, underscoring the necessity of the proper ratio of CR HBECs to lung matrix surface area for proper differentiation (n = 2) (Supplementary Fig. S4). From a morphological standpoint, this indicates that CR HBECs respond to signals in the reconstituted murine lung system by forming both upper and lower airway niche-appropriate structures.
IF staining was then used to evaluate the epithelial cell lineages that CR HBECs differentiate into after culture in a reconstituted lung for 12 days. Murine lungs recellularized with WT CR HBECs were observed to contain cytokeratin 14 (CK14)-positive basal cells, α tubulin-positive cilia buds, Muc 5B-positive goblet cells, and Clara cell secretory protein (CCSP)-positive club cells in the upper airway (n = 3) (Fig. 2B). WT CR HBECs in the lower airways stained for aquaporin 5 (AQP5) and pro-surfactant protein C (Pro-SPC), indicating their differentiation into alveolar type I and II cells, respectively (n = 3) (Fig. 2B).
To further compare WT CR HBECs cultured in 2D, ALI, and in reconstituted lungs, western blotting for AQP5, CK14, Pro-SPC, and CCSP was performed. CK14, an epithelial basal stem cell marker, is high in 2D culture and ALI culture. This is to be expected as CK14-positive basal cells are highly represented in pseudostratified epithelia. AQP-5 and Pro-SPC, markers of alveolar type I and II cells, respectively, are more abundant in reconstituted lungs compared with ALI or 2D cultures, which do not have peripheral lung differentiated markers. CCSP, a marker of terminal bronchioles, is found in 12-day reconstituted lung cultures, but not in 35-day ALI cultures (n = 3) (Fig. 2C). These results can be interpreted to indicate that CR HBECs are multipotent and have the capacity to differentiate into both upper and lower airway cells when presented with the appropriate niches.
Reconstitution of decellularized lung matrices with conditionally reprogrammed HBECs restores mechanical integrity lost during decellularization
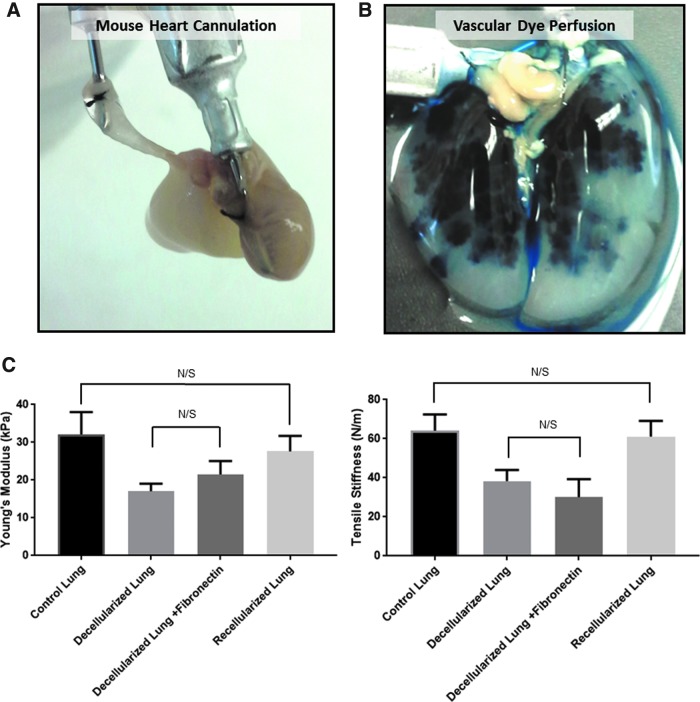
We next tested the preservation of vascular access to the lung and biomechanical properties of the matrix after decellularization and recellularization to determine the integrity of the mouse lung matrix for tissue engineering applications. In this bioreactor system, a blunted 201/2-gauge needle was secured into the right ventricle to gain access to the mouse lung vasculature (Fig. 3A). The lung was then perfused with Evan's Blue Dye through the right ventricle to evaluate access to the vascular compartment after decellularization. The Evan's Blue Dye perfused through the right ventricle permeated the lung's vascular compartment without visible leakage, demonstrating that the decellularized vascular compartment could hold imposed hydraulic pressure over time (Fig. 3B).
FIG. 3.
Preservation of vascular access after decellularization and restoration of lung mechanics after recellularization. (A) Access to the vascular system of the heart–lung block is attained by securing a blunt-end needle to the right ventricle. (B) A decellularized murine lung infused with a 15% Evan's blue dye solution in PBS. (C) Quantitation of the Young's modulus, a measure of elasticity, and tensile stiffness of native lung (n = 10), decellularized lung (n = 11), decellularized lung after fibronectin coating (n = 3), and lung tissue reconstituted with CF CR HBECs (n = 3) analyzed by uniaxial stress/strain testing. N/S, not significant.
We next evaluated lung elasticity (Young's modulus) and tensile stiffness of native lung (n = 10), mouse lungs after decellularization (n = 11), decellularized lungs coated with fibronectin (n = 3), and lungs reconstituted with 10 million CF CR HBECs cultured in our bioreactor system (n = 3) by measuring the load/stress of strips of the lung matrix after uniaxial deformation/strain as previously described.29 While the lung remained intact and held tensile stress (up to 16 kPa) after decellularization, the Young's modulus and tensile stiffness were reduced by 48% and 54%, respectively, after decellularization (Fig. 3C) that could be due to loss of ECM components or lack of a cellular component.
While addition of fibronectin did not significantly change the tensile stiffness or elasticity of the decellularized lungs, reconstitution with CR HBECs plus fibronectin restored the tensile strength and elasticity of the lung to near-native levels with no statistically significant difference to native lung tissue. In summary, the described mouse lung decellularization protocol and bioreactor system accesses both the tracheal and vascular compartments while preserving some of their mechanical integrity. Importantly, most of the mechanical integrity can be restored by reconstitution with CR HBECs.
Multipotency and clonogenicity of conditionally reprogrammed HBECs
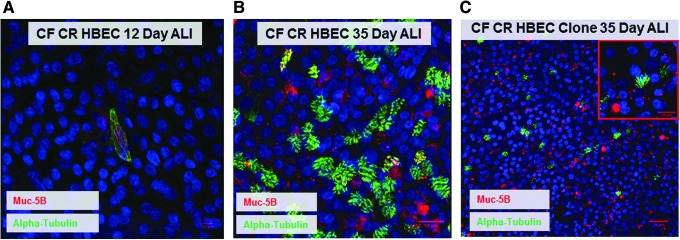
If CF CR HBECs are able to support clonal selection in vitro while maintaining their capacity for differentiation, these cells may permit modeling of gene therapy or genetic manipulation in tissue-engineered systems. At ALI, CR HBECs fail to undergo terminal differentiation into a pseudostratified epithelium in 12 days; indicating that these cells remain in an undifferentiated state (Fig. 4A). After culture at ALI for 35 days, CF CR HBECs form a pseudostratified epithelium displaying both ciliated cells and mucus-producing goblet cells as detected by IF staining for acetylated alpha tubulin and Muc 5B, respectively (Fig. 4B).
FIG. 4.
Differentiation and clonogenicity of CR HBECs at ALI. (A) IF staining of CR HBECs cultured at ALI in a transwell for 12 days showing a lack of differentiation. (B) IF staining for ciliated cells (alpha-tubulin) and goblet cells (Mub5-B) in CR HBECs cultured at ALI in a Transwell for 35 days. (C) IF staining of a CF CR HBEC clone cultured at ALI for 35 days. ALI, air–liquid interface.
We next isolated individual cultured CF CR HBEC clones and cultured them at an ALI for 35 days. These clones produced a comparable pseudostratified epithelium displaying both ciliated and goblet cells (Fig. 4C) demonstrating that single CR HBECs are multipotent. To our knowledge, this is the first successful differentiation of CR HBEC clones at the ALI. These results indicate that CF CR HBECs can tolerate clonal selection while maintaining their ability to differentiate. This makes them a valuable cellular resource for studying genetic correction approaches of diseases in tissue-engineered models.
Discussion
In this investigation, we have used a HBEC conditional reprogramming protocol and mouse lung bioreactor system to recapitulate the upper and lower airway epithelium in a decellularized lung within only 12 days. Overcoming the small size and fragility of the mouse heart–lung block by cannulating the right ventricle to access the pulmonary vasculature, the present studies demonstrate the feasibility of using lung matrices derived from the large array of murine disease models of lung disease.
Conditionally reprogrammed HBECs may address an unmet need in tissue engineering for both disease modeling and transplantation; a source of primary patient-derived tissue that can be rapidly expanded from a biopsy into sufficient number of cells to create engineered tissue without losing the ability to differentiate.30 Estimates have suggested that ∼45 billion human epithelial cells would be required to seed a human scale decellularized lung matrix.31 Some techniques can generate human-derived lung progenitors from iPSCs in 12–15 days, after which point they would need to be expanded to about 45 billion cells.32
It may take considerably less time and resources to generate enough primary cells to perform human scale lung tissue engineering using CR HBECs. For example, a typical nasal brushing isolates ∼1.4 × 106 primary HBECs.10 CR HBECs in culture have a population doubling rate of 3 days. While scaling up to 45 billion cells, primary CR HBECs isolated from a typical nasal brushing will have undergone ∼14 population doublings over the course of 45 days, at which age the cells would still be able to differentiate into lung epithelium.
Therapeutic applications of lungs engineered with CR HBECs may be limited by the need for HBECs isolated from a patient. If the patient suffers from a genetic disease such as CF, lungs tissue generated from their cells would yield another diseased lung. Gene therapy-mediated correction of this defect in isolated CR HBECs may overcome this limitation, particularly as CR HBECs can support clonal culture. However, it remains to be seen if CR HBECs can undergo CRISPR-mediated gene editing while preserving their capacity to differentiate. Additionally, extremely unhealthy lung tissue may not yield cells sufficient for scale-up. For these patients, iPSCs generated from their skin, which did not undergo such stress, may be a more optimal route; particularly considering nonintegrative iPSC generation methods which may significantly improve the safety profile of these cells.33
Cells used in this study came from two donors, one with no lung disease and one with CF. In vitro these cell populations did not behave differently, although the WT CR HBECs did have a longer lifespan. The CF patient was a delta-F508 homozygous knockout, and the defective CFTR protein was preserved over the course of culture as shown by Ussing chamber analysis (data not shown). As we could not detect differences in the ability of WT and CF HBECs to reconstitute lung tissue, this may permit the study of subtle differences between CF and WT-engineered lungs, such as the isolation and characterization of their protein excretions, as has been done in ALI culture models.34
To the best of our understanding, this is the first study to demonstrate the ability of HBECs to differentiate into lower airway type I and II cells; a trait previously only associated with lung stem cells isolated from the distal bronchoalveolar junction.35 The scalability of the methods described in this report allows for iterative optimization experiments to improve lung reconstitution protocols without having to constantly rederive new primary cells. Achieving an even seeding density and reconstitution of the vascular tree across a decellularized lung is one of the major barriers facing translation of reconstituted lungs to clinical applications.36,37
This experimental paradigm allowed us to optimize the described seeding protocol by varying the seeding density, optimizing timing for removing reprogramming conditions, and dispersing the HBECs into a single cell suspension before application to the lungs all with the same patient-derived cell populations. In our current experiments, seeding10 million single cell suspension HBECs with no recovery time from conditional reprogramming conditions was optimal for generating even coverage of the reconstituted lung. With these conditions, we achieved about 90% cell coverage with appropriate differentiation of CR HBECs in the various compartments of the lung.
The broad array of genetically engineered mouse models and tools available in mice afford much more flexibility and utility compared with rat lungs, which have comparatively fewer genetic tools and disease models. Previous studies have demonstrated that matrices derived from decellularized lungs isolated from patients with chronic obstructive pulmonary fibrosis or idiopathic pulmonary fibrosis (IPF) have altered mechanical properties and that normal lung cells do not survive on these diseased matrices.38,39
While other murine lung bioreactor systems have been developed,40 they do not support continuous vascular perfusion of media or cells in the bioreactor, which may prevent recapitulation of a vascular endothelium, simulation of vascular delivery of compounds to the tissue-engineered construct, or the collection of circulating tumor cells seen in 4D rat lung perfusable tumor models.41,42 Utilizing mouse models of lung diseases such as IPF may allow investigators to examine the role of fibrotic or damaged matrices on cell behavior in the context of simulated breathing and vascular perfusion. These studies will be particularly important for developing gene and cell therapy applications in a controlled ex vivo environment. This experimental system provides a format for elucidating the causes of lung disease, and separating lung epithelial cell autonomous effects from those of the ECM.
One of the consistent challenges facing the study of CF in vivo is that mouse models of the disease made by knockout of CFTR do not spontaneously develop lung disease, as they have an alternate chloride channel that is not present in human lung epithelial cells.43,44 This CR HBEC culture method may permit rapid simulation of genetic correction of CFTR in patients; which has only been done thus far using cell lines in organoid culture systems.45 Tissue-engineered lungs made from HBECs isolated from patients with CF should allow for simulating the behavior of CF HBECs in a lung context without chronic inflammation or infection, which are common confounding factors in the study of CF in animal models.46
The only cure for most chronic lung diseases is transplantation, but there is a significant shortage of such donor lungs.47 Reconstituted lungs show promise for transplantation, but using previously described methods and cell sources the lungs fail shortly after transplantation.1 A scalable lung culture system allows for the optimization of lung tissue engineering methods in the context of tissue in part by increasing the number of experiments that can be done with a single primary cell lineage. This may in turn permit optimization of lung tissue engineering and the generation of reconstituted lungs for transplantation.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute (Lung SPORE P50CA70907 to JWS), a pilot grant from the Cystic Fibrosis Foundation (to JWS), a Cystic Fibrosis Foundation Postdoctoral Award (PETERS15FO), and NCI-designated Comprehensive Cancer Center Support Grant (5P30 CA142543). This work was also funded by the HHMI Med into Grad Scholars Program, The TL1 Scholars Program (TL1TR001104), and a National Cancer Institute T32 training grant (CA124334) to JP-H and RL. This work was performed in laboratories constructed with support from the National Institute of Health grant C06 RR30414. Immunofluorescence microscopy was supported by NH S10 RR029731-01 to Kate Luby-Phelps.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gilpin S.E., et al. Bioengineering lungs for transplantation. Thorac Surg Clin 26, 163, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Shojaie S., et al. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: requirement of matrix-bound HS proteoglycans. Stem Cell Rep 4, 419, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh D., et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong F., et al. Interplay of cell shape and division orientation promotes robust morphogenesis of developing epithelia. Cell 159, 415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaedi M., et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 123, 4950, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song J.J., et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg 92, 998; discussion 1005–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Peterson S.E., and Loring J.F. Genomic instability in pluripotent stem cells: implications for clinical applications. J Biol Chem 289, 4578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S.M., and Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 13, 497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott M.J., et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380, 994, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Souza N., Avila P.C., and Widdicombe J.H. Polarized cultures of human airway epithelium from nasal scrapings and bronchial brushings. In Vitro Cell Dev Biol Anim 39, 266, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Fulcher M.L., et al. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107, 183, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Suprynowicz F.A., et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci U S A 109, 20035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler C.R., et al. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 194, 156, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilpin S.E., et al. Regenerative potential of human airway stem cells in lung epithelial engineering. Biomaterials 108, 111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentzsch M., et al. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 56, 568, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright W.E., and Shay J.W. Inexpensive low-oxygen incubators. Nat Protoc 1, 2088, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., et al. Dexamethasone-mediated repression of MUC5AC gene expression in human lung epithelial cells. Am J Respir Cell Mol Biol 34, 338, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaisani A., et al. Branching morphogenesis of immortalized human bronchial epithelial cells in three-dimensional culture. Differentiation 87, 119, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado O., et al. Multipotent capacity of immortalized human bronchial epithelial cells. PLoS One 6, e22023, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen T.H., et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ott H.C., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Crapo P.M., Gilbert T.W., and Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown B.N., and Badylak S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res 163, 268, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faulk D.M., et al. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater 10, 183, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly G.C., and Engler A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 43, 55, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wallis J.M., et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods 18, 420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han S.W., and Roman J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: pro-oncogenic effects mediated by PI3-kinase and NF-kappa B. Oncogene 25, 4341, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Wu R., Zhao Y.H., and Chang M.M. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J 10, 2398–2403, 1997 [DOI] [PubMed] [Google Scholar]
- 29.O'Neill J.D., et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg 96, 1046 discussion 1055–1056, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez J.J., et al. Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng Part A 20, 1735, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calle E.A., et al. Strategies for whole lung tissue engineering. IEEE Trans Biomed Eng 61, 1482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilpin S.E., et al. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann Thorac Surg 98, 1721 discussion 1729, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren L., et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters-Hall J.R., et al. Quantitative proteomics reveals an altered cystic fibrosis in vitro bronchial epithelial secretome. Am J Respir Cell Mol Biol 53, 22, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajstura J., et al. Evidence for human lung stem cells. N Engl J Med 364, 1795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Stabler C.T., et al. Revascularization of decellularized lung scaffolds: principles and progress. Am J Physiol Lung Cell Mol Physiol 309, L1273, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarritt M.E., Pashos N.C., and Bunnell B.A. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol 3, 43, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner D.E., et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35, 3281, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Booth A.J., et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186, 866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godin L.M., et al. Decreased laminin expression by human lung epithelial cells and fibroblasts cultured in acellular lung scaffolds from aged mice. PLoS One 11, e0150966, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vishnoi M., et al. Circulating tumor cells from a 4-dimensional lung cancer model are resistant to cisplatin. J Thorac Cardiovasc Surg 148, 1056; discussion 1063–1064, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Mishra D.K., et al. Gene expression profile of A549 cells from tissue of 4D model predicts poor prognosis in lung cancer patients. Int J Cancer 134, 789, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kent G., et al. Phenotypic abnormalities in long-term surviving cystic fibrosis mice. Pediatr Res 40, 233, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Clarke L.L., et al. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci U S A 91, 479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwank G., et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Yan Z., et al. Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Hum Gene Ther Clin Dev 26, 38, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valapour M., et al. OPTN/SRTR 2013 annual data report: lung. Am J Transplant 15 Suppl 2, 1, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.