Abstract
Background
Next-generation sequencing is revealing genomic heterogeneity in localized prostate cancer (CaP). Incomplete sampling of CaP multiclonality has limited the implications for molecular subtyping, stratification, and systemic treatment.
Objective
To determine the impact of genomic and transcriptomic diversity within and among intraprostatic CaP foci on CaP molecular taxonomy, predictors of progression, and actionable therapeutic targets.
Design, setting, and participants
Four consecutive patients with clinically localized National Comprehensive Cancer Network intermediate- or high-risk CaP who did not receive neoadjuvant therapy underwent radical prostatectomy at Roswell Park Cancer Institute in June–July 2014. Presurgical information on CaP content and a customized tissue procurement procedure were used to isolate nonmicroscopic and noncontiguous CaP foci in radical prostatectomy specimens. Three cores were obtained from the index lesion and one core from smaller lesions. RNA and DNA were extracted simultaneously from 26 cores with ≥90% CaP content and analyzed using whole-exome sequencing, single-nucleotide polymorphism arrays, and RNA sequencing.
Outcome measurements and statistical analysis
Somatic mutations, copy number alternations, gene expression, gene fusions, and phylogeny were defined. The impact of genomic alterations on CaP molecular classification, gene sets measured in Oncotype DX, Prolaris, and Decipher assays, and androgen receptor activity among CaP cores was determined.
Results and limitations
There was considerable variability in genomic alterations among CaP cores, and between RNA- and DNA-based platforms. Heterogeneity was found in molecular grouping of individual CaP foci and the activity of gene sets underlying the assays for risk stratification and androgen receptor activity, and was validated in independent genomic data sets. Determination of the implications for clinical decision-making requires follow-up studies.
Conclusions
Genomic make-up varies widely among CaP foci, so care should be taken when making treatment decisions based on a single biopsy or index lesions.
Patient summary
We examined the molecular composition of individual cancers in a patient’s prostate. We found a lot of genetic diversity among these cancers, and concluded that information from a single cancer biopsy is not sufficient to guide treatment decisions.
Keywords: Prostatic adenocarcinoma, Androgen deprivation therapy, Castration, Radiation therapy, Somatic alterations, Active surveillance, Indolent, Personalized medicine, Actionable target
1. Introduction
The majority of radical prostatectomy (RP) specimens harbor multiple topographically separate prostate cancer (CaP) foci [1,2]. It has long been recognized that these intraprostatic CaPs vary in biological aggressiveness and progress at different rates [1,3–7]. Recent application of next-generation (NextGen) sequencing has identified significant diversity in the genomic make-up of prostate-confined CaPs [8], both among index CaP lesions in general [9–13] and among index lesions with the same Gleason score (Gleason 7) [14], and in some cases among CaP foci from different regions of the same RP specimen [14,15].
Most of these NextGen results have been obtained using whole-genome sequencing, whole-exome sequencing, or copy number analyses. The extent to which heterogeneity in somatic alterations at the DNA level is reflected in, or overlaps with, differential gene expression or the presence of gene fusions at the RNA level remains uncertain. To the best of our knowledge, no study has systematically assessed the extent to which such variability applies to the individual CaPs in an RP specimen. These are important considerations because urologists increasingly rely on molecular classifications and prediction based on gene expression profiles for CaP risk stratification and clinical decision-making [16], and the relevant information is derived from single CaP core biopsies or index lesions.
This study was conducted to systematically define the intratumoral and intertumoral genomic heterogeneity in all nonmicroscopic and noncontiguous intraprostatic CaP foci from patients at higher risk of metastatic progression. Genomic heterogeneity within and among CaP lesions was assessed for its impact on classification of CaP foci according to recently proposed molecular taxonomy [13], on expression profiles of gene sets that underlie the Oncotype DX (http://oncotypedx.com), Prolaris (http://prolaris.myriad.com), and Decipher (http://deciphertest.com) prognosticators [16], and on androgen receptor (AR) activity, which is targeted by androgen deprivation therapy and radiation therapy for recurrent CaP after RP [17–20].
2. Patients and methods
2.1. Patient selection
Four consecutive patients (CAP-001, -002, -003, and -004) who presented with National Comprehensive Cancer Network intermediate-risk (n = 1) or high-risk (n = 3) CaP [17] and underwent RP at Roswell Park Cancer Institute (RPCI) in June–July 2014 were selected for the study. None of the patients had received neoadjuvant therapy. The study received institutional review board approval before initiation. Patient and CaP characteristics are shown in Table 1.
Table 1.
Clinical characteristics for four patients who underwent radical prostatectomy for intermediate- or high-risk prostate cancer
| Variable | CAP-001 | CAP-002 | CAP-003 | CAP-004 |
|---|---|---|---|---|
| Age at surgery (yr) | 64 | 61 | 72 | 60 |
| Pre-RP PSA (ng/ml) | 2.3 | 3.6 | 4.23 | 8.33 |
| Clinical primary GG | 5 | 3 | 4 | 4 |
| Clinical secondary GG | 4 | 4 | 3 | 5 |
| Clinical T stage | 2a | 1c | 3a | 2a |
| Clinical N stage | 0 | 0 | 0 | 0 |
| Clinical M stage | 0 | 0 | 0 | 0 |
| Pathologic primary GG | 4 | 3 | 4 | 4 |
| Pathologic secondary GG | 5 | 4 | 3 | 3 |
| Capsular invasion | Yes | Yes | Yes | Yes |
| Extent of capsular invasion | Diffuse | Focal | Diffuse/multifocal | Focal |
| Perineural invasion | Yes | Yes | Yes | Yes |
| Seminal vesicle invasion | Yes | No | No | No |
| Bladder neck invasion | Yes | No | Yes | No |
| Margin involvement | Diffuse | Negative | Focal | Negative |
| Lymph node metastasis | No | No | No | No |
| RP specimen prostate cancer (%) | 20 | 15 | 35 | 15 |
| Pathologic T stage | 3b | 3a | 3a | 3a |
| Pathologic N stage | 0 | 0 | 0 | 0 |
| Pathologic M stage | 0 | 0 | 0 | 0 |
| Persistent disease | Yes | No | No | Unknown |
| Biochemical recurrence | No | No | No | Unknown |
| Biochemical failure | Yes | No | No | Unknown |
| RP failure | Yes | No | No | Unknown |
| Metastatic disease | No | No | No | No |
| Post-RP radiation | Yes | No | No | No |
| Time to radiation (mo) | 4.64 | – | – | – |
| Follow-up (mo) | 7.5 | 26.4 | 19.2 | 0.3 |
| Cancer status at last follow-up | Evidence of disease | No evidence | No evidence | Unknown |
| Patient status at last contact | Alive | Alive | Alive | Alive |
RP = radical prostatectomy; PSA = prostate-specific antigen; GG = Gleason grade; persistent disease = PSA levels did not fall to undetectable levels after RP; biochemical recurrence = biochemical recurrence according to National Comprehensive Cancer Network guideline definition; biochemical failure = biochemical recurrence or biochemical persistence according to National Comprehensive Cancer Network guideline definitions.
2.2. Tissue procurement
Nonmicroscopic, noncontiguous CaP foci tissues from RP specimens were obtained using a central quadrant procurement method developed at RPCI [21] with modifications as detailed in the Supplementary information. For each patient, the number and location of CaP foci with ≥90 neoplastic nuclei that were obtained are described in Supplementary Table 1. A blood sample was obtained before operation for isolation of germline DNA.
2.3. NextGen sequencing studies
RNA and DNA were isolated using an AllPrep DNA/RNA/protein kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Detailed descriptions of whole-exome sequencing (WES), copy number alteration (CNA), and RNA sequencing (RNA-Seq) assays, analyses, validation, and bioinformatics subanalyses are included in the Supplementary information.
3. Results
All nonmicroscopic and noncontiguous CaP foci within the RP specimens from four CaP patients (CAP-001 to CAP-004) were procured. Three independent tissue cores (denoted a–c) were obtained from the index lesion (Td1), and one core was obtained from each of the other lesions (Td2–5) (Supplementary Fig. 1, Supplementary Table 1) to define genomic heterogeneity within the index CaP lesion (intratumoral heterogeneity) and between CaP lesions (intertumoral heterogeneity). RNA and DNA were extracted simultaneously for each tissue core (n = 26).
Each DNA sample was analyzed for somatic alterations using a SureSelect XT WES assay (Agilent Technologies, Santa Clara, CA, USA). The median sequencing depth ranged from 88× to 127×. All samples had at least 90% of targeted regions covered with 30× or greater coverage (Supplementary Table 2). The number of somatic CaP mutations varied from 26 to 120 per patient, which is consistent with previous reports [10,11,13]. Customized HaloPlex targeted sequencing of the same DNA samples validated 90.1% of mutations identified in the WES discovery phase (Fig. 1, Supplementary Table 3). The variant allele frequency for mutations measured using WES and customized HaloPlex targeted sequencing were highly consistent (Supplementary Fig. 3). Some mutations have been reported in CaP (eg, PTEN, AKT1, MLL2) [11,13,22,23], whereas others affect known cancer census genes (eg, JAK1) [24], or have not yet been reported at high frequency in organ-confined CaP but have been implicated in other human malignancies (eg, GNAO1) [25]. Of the 210 somatic mutations that were validated, 141 are predicted to cause amino acid changes, including missense and nonsense single-nucleotide variations (SNVs), splice SNVs, and indels (Supplementary Table 3). Of these 141, 133 mutations affect genes known to be subject to somatic mutations in clinical CaP (0.3–13.1% of cases, Supplementary Table 4). The significantly higher frequency (p < 0.001) of mutations affecting the genes encoding CELSR3, CSMD1, FGD5, MINK1, MLL2, SCN5A, USH2A, and ZFHX3 in CaP that has failed androgen deprivation therapy in comparison to treatment-naïve CaP suggests that these may be potential driver mutations.
Fig. 1.
Intratumoral and intertumoral heterogeneity in somatic alterations. For each patient, a heatmap was generated that lists genes affected by somatic alterations (top panel), genes affected by copy number alterations (CNAs; middle panel), and genes involved in gene fusions (bottom panel). Note the absence of focal CNAs in CAP-004. From left to right: the status of the mutation across the cores of the index lesion (Td1) and the other lesions (Td2–5).
Significant intratumoral and intertumoral heterogeneity was observed at the level of individual somatic mutations (Fig. 1). Each alteration was found to occur in one patient only and was detected in one to all of that patient’s CaP foci. Tumor heterogeneity was characterized using the presence or absence of shared and private somatic alterations. Among the three tissue cores from the index lesion for a representative case (CAP-003), Td1a and Td1c shared a very similar mutation profile that was markedly different from that of Td1b, which suggests the presence of intratumoral heterogeneity. At the intertumoral level, high levels of similarity were found between Td1a and Td1c of the index lesion and the nonindex lesion Td4, while another core of the index lesion, Td1b, was more similar to lesion Td3. The mutation profiles of Td2 and Td5 were distinct from that of the other foci. For example, a PTEN Q248* nonsense mutation was not detected in Td2 and Td5, but was found in other foci. The results indicate both intertumoral and intratumoral heterogeneity in somatic alterations.
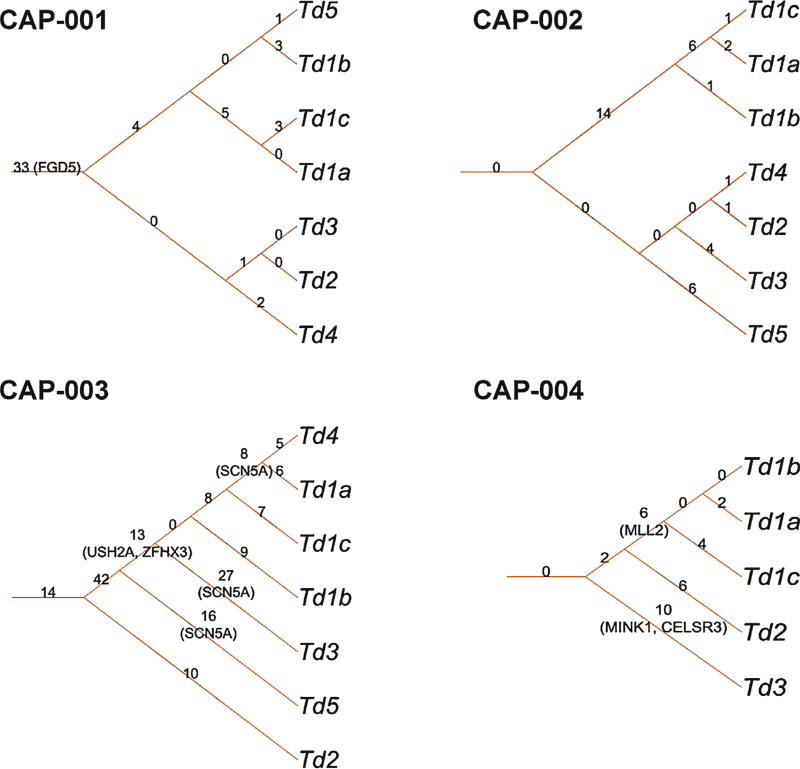
For each patient, a phylogenetic tree was generated based on somatic mutations. Clonal evolution of CaP foci can be inferred from these trees, and different levels of intratumoral and intertumoral heterogeneity were observed (Fig. 2). CAP-001 and CAP-003 showed greater intratumoral heterogeneity, with foci from the same index lesion clustered with another lesion (Td5 and Td4, respectively) compared to CAP-002 and CAP-004. The phylogenetic tree modeling also showed that different foci obtain additional private somatic mutations during clonal evolution.
Fig. 2.
Prostate cancer (CaP) genomic heterogeneity evidenced in phylogenetic trees based on somatic mutations. For each patient, a phylogenetic tree was generated according to the somatic mutation status of his CaP foci. The root edge label shows the number of mutations shared by all intraprostatic CaP foci. The internal edge label shows the number of mutations shared only by foci of the subtree. The leaf edge shows the number of private mutations present only in the leaf focus. The genes shown in parentheses (FGD5, SCN5A, USH2A, ZFHX3, MLL2, MINK1, CELSR3) are associated with putative driver somatic mutations (Supplementary Table 4).
CaP DNA was analyzed for CNAs using OmniExpress assays (Illumina, San Diego, CA, USA), except for two tissue cores (Supplementary Table 5) for which insufficient DNA remained. Few CNAs were identified per core, and the vast majority were deletions (Supplementary Fig. 4). Specific Reference Sequence (http://www.ncbi.nlm.nih.gov/refseq/) database genes targeted by focal CNAs are listed in Figure 1, and include previously reported alterations in localized CaP (eg, PTEN, ZFHX3, ETV1) [13]. These alterations were verified in gene expression data (eg, PTEN) and gene fusion data (eg, ETV1) from RNA-Seq experiments on the same tissue cores. Both intratumoral and intertumoral CNA heterogeneity was found (Fig. 1).
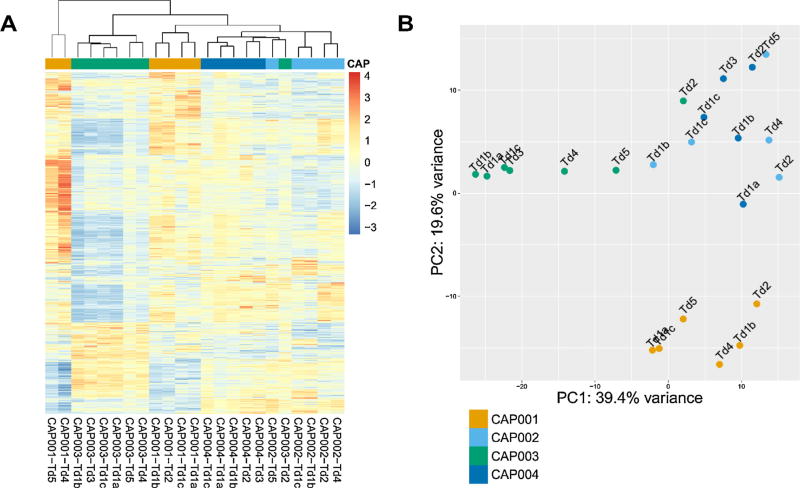
Sufficient RNA was isolated to yield high-quality TruSeq (Illumina) RNA-Seq data for 23 CaP foci (Supplementary Table 5). An average of 115 million reads per sample and an average mapping rate of 93% were achieved (Supplementary Table 6). When gene expression patterns were evaluated via unsupervised clustering using significantly differentially expressed genes (Fig. 3A), CaP cores from the same patient or the same index lesion did not always group together. Principal component analysis of gene expression profiles confirmed this heterogeneity (Fig. 3B). Pairwise comparison of differentially expressed genes (at least twofold difference and p < 0.05) in CaP foci from each patient confirmed both intratumoral and intertumoral heterogeneity in gene expression patterns (Supplementary Fig. 5). The number and range of differentially expressed genes within and among CaP foci of all four patients are shown in Supplementary Tables 7–10. For each patient, intertumoral heterogeneity was greater than the diversity in gene expression patterns within different regions of the index lesion.
Fig. 3.
Intratumoral and intertumoral heterogeneity in gene expression data derived from RNA sequencing. (A) Unsupervised clustering of CaP foci using significantly differentially expressed genes (DEGs). (B) Principal component analysis (PCA) with the first component plotted on the x-axis and the second component on the y-axis.
RNA-Seq data were also mined using two independent prediction algorithms (Cicero and DeFuse) to identify gene fusions. Well-known CaP gene fusions such as TMPRSS2-ERG and SLC45A3-ETV1, as well as novel gene rearrangements (eg, DHCR24-MFSD4), were present in one or more (but not all) of each patient’s cores. Rearrangements that involved the same genes but differed in breakpoint location were found (eg, TMPRSS2-ERG). As predictions in this manner are prone to generate false positives, efforts were focused on those fusions returned by both programs (Fig. 1). Predicted gene rearrangements were validated using endpoint polymerase chain reaction (PCR) on cDNA generated from CaP-derived RNA and capillary sequencing of PCR products. The presence of rearrangements and breakpoint locations were validated for seven of eight predicted gene fusions (eg, DHCR24-MFSD4; Supplementary Table 11). The exception was a predicted KLK2-KLK3 fusion, for which RNA-Seq data mapped to sequences with high homology in the KLK2 and KLK3 gene sequences. In general, fusions predicted by both algorithms were validated, those not predicted by either were not, and some were verified after prediction by one program (eg, LRIG1-CDC7 in CAP-003; Supplementary Fig. 6). The majority of DNA-derived genomic heterogeneity was conserved at the RNA level, but additional variability in gene expression and gene fusions was identified when analyzing RNA.
Molecular pathologic information derived from a single prostate biopsy or index lesion is increasingly used to guide CaP treatment decisions [16,26]. The impact of genomic heterogeneity among CaPs, and between DNA- and RNA-based assays, on assigning a patient’s individual CaP foci according to a recently proposed molecular taxonomy [13] that is based on gene fusion status (ERG, ETV1/4, FLI1) or somatic mutations (SPOP, FOXA1, IDH1) was determined. CAP-002 and CAP-004 CaPs harbored TMPRSSR-ERG fusions (Fig. 4A), which is consistent with the high prevalence of this gene rearrangement [26]. However, only two of five foci from each patient contained these rearrangements. Similarly, not all foci from patient CAP-003 harbored ETV1 fusions. The vast majority of foci could not be classified as belonging to any of the seven subgroups in this taxonomic system. Data from four independent studies that analyzed 163 independent CaP foci from 60 RP specimens [14,15,27,28] validated these conclusions (Supplementary Tables 12–15, Fig. 4B). Of 60 patients studied, 17 had CaP foci that fell into different The Cancer Genome Atlas (TCGA) classes. For two patients, the same CaP focus was classified according to two TCGA classifiers (both ERG and ETV1); and CaP cores from 24 patients could not be classified according to any of the seven TCGA classifiers. Analysis of SPINK1 mRNA levels, recently proposed as an alternative molecular subtyping method for localized CaP [29], revealed similar heterogeneity among CaP foci. Differential SPINK1 expression (twofold or greater) was found between Td1a and Td5, Td1b and Td5, and Td2 and Td5 in CAP-003 (Supplementary Tables 7–10).
Fig. 4.
Molecular taxonomy classification of individual prostate cancer (CaP) foci. (A) The discovery cohort. Each CaP focus was classified into seven groups that were the basis for a recently proposed molecular taxonomy for localized CaP. Classification was performed according to the presence of gene fusions involving ERG, ETV1, ETV4, or FLI1, or somatic mutations affecting SPOP, FOXA1, or IDH. ERG, ETV1, ETV4, and FLI1 gene fusions were predicted by both DeFuse and Cicero algorithms. Yes = CaP focus was classified into a molecular taxonomy group; No = CaP focus could not be classified; black cells = sample was not assayed; yellow highlights = predicted by DeFuse only; blue highlights = predicted by Cicero only. (B) Validation using publicly available data sets. Independent CaP foci from multifocal CaP radical prostatectomy specimens procured from 60 patients in four independent studies [14,15,27,28] were evaluated for the presence of seven The Cancer Genome Atlas (TCGA) classifiers as above. WGS = whole-genome sequencing; WES = whole-exome sequencing; FISH = fluorescent in situ hybridization. Evaluable = the TCGA classifiers can be evaluated in the data set used to profile the CaP focus; N/A = classifier not present; concordant foci = CaP foci classified according to the same TCGA classifier; discordant foci = CaP foci classified according to different TCGA classifier; classified 2× = same CaP focus classified according to two TCGA classifiers; not classified = CaP foci that could not be not classified according to any of the seven TCGA classifiers.
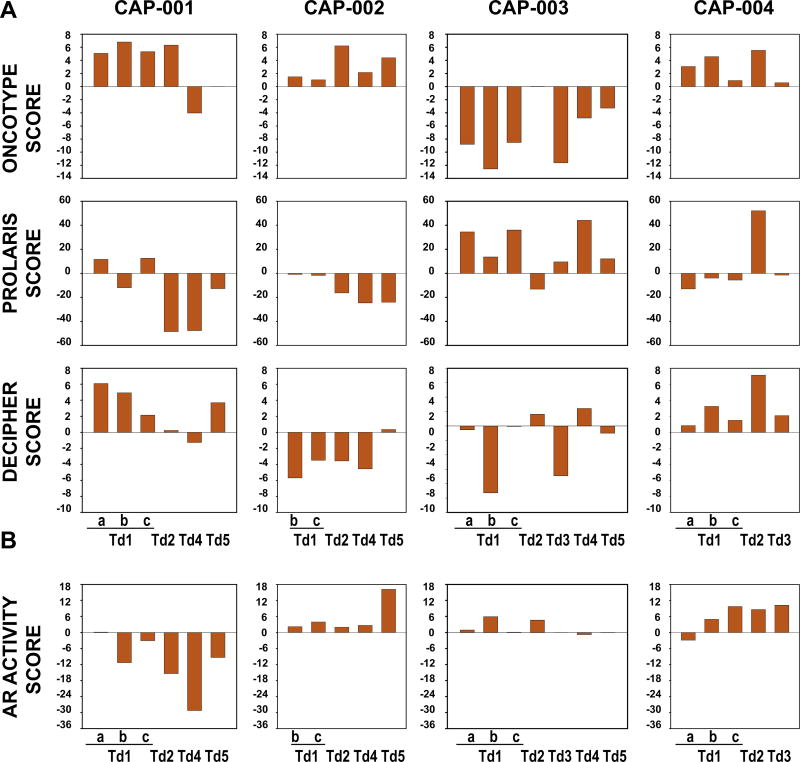
These findings indicate that sampling different regions of the same RP specimen can have a major impact on CaP (mis)classification. These results prompted us to quantitate, for each CaP focus, the expression of gene signatures that are the foundation of increasingly popular genomic prognostic tests used to stratify indolent versus aggressive CaP. Scores were generated that summarize the composite expression of: (1) 12 cancer genes that the Oncotype DX test uses to predict adverse pathology at RP and recurrent CaP after RP; (2) 31 cell-cycle progression genes that the Prolaris test uses to stratify patients according to CaP aggressiveness; and (3) the 22 genes that the Decipher assay uses to estimate the probability of metastatic disease [16] (Supplementary Table 16). Two or more cores from each patient had similar scores for each prognosticator, but considerable intratumoral and intertumoral diversity in score range and directionality was observed. Differences were most pronounced for the gene profiles of the Prolaris test, and somewhat less pronounced for the gene signature underlying the Oncotype DX assay (Fig. 5A). No correlation was observed between the scores for the assays, between scores and CaP core location (anterior vs posterior prostate, left vs right, among levels etc.), or between the scores and the Gleason score for a CaP focus (Supplementary Table 17). While some scores showed a consistent pattern with phylogenetic trees (eg, Prolaris in CAP-002 and AR in CAP-004), such consistency was not clear or generalizable overall (Supplementary Fig. 7). Similar variability was found for scores derived from matched preoperative CaP biopsies and CaP cores from the same prostate area for an independent cohort of 24 patients [30] (Supplementary Fig. 8A).
Fig. 5.
Intraprostatic CaP heterogeneity in performance of genomic prognosticator and AR activity scores. (A) For each CaP focus from which RNA sequencing data were generated, the gene expression signatures measured in the Oncotype DX, Prolaris, and Decipher assays were quantified as corresponding scores. (B) Using gene expression profiles from the same CaP foci, expression of 20 AR-dependent genes was quantified as an AR activity score. The gene expression signatures used to generate these scores are listed in Supplementary Table 16. AR = androgen receptor.
Androgen deprivation and/or radiation therapy are the default treatments for CaP recurrence after RP [17]. These treatments affect AR activity [18–20,31] but lead to variable responses in individual patients. As three of the four patients studied here were at high risk of recurrence, an assessment of AR activity was performed for each CaP focus by measuring the expression of a select set of AR target genes [13,32] (Supplementary Table 16). We found remarkable diversity in AR activity scores both within and among CaPs from CAP-001 to CAP-004. AR activity did not correlate with any other scores or with the prostate region from which the cores were obtained (Fig. 5B) and did not map consistently to phylogenetic trees, but did correlate with Gleason score (Supplementary Fig. 7, Supplementary Table 17). These conclusions also held true for comparison of 24 pairs of matching biopsies and prostatectomy cores (Supplementary Fig. 8B).
4. Discussion
The ultimate goal of genomic classifiers and prognosticators to predict the aggressiveness of clinically localized, untreated CaP is to individualize risk stratification and optimize treatment plans [16,26]. The clinical utility and cost-effectiveness of genomic assays to inform on diverse clinical CaP behavior depend on their ability to measure in a representative manner the CaP genomic composition for an individual patient. Capturing the multifocal nature of CaP is critical, since a patient’s index lesion or a preoperative biopsy is not necessarily the lesion that leads to lethal CaP [33]. Our findings of considerable intratumoral and intertumoral CaP heterogeneity provide insight into the limitations of the classification tools available and opportunities to improve on their performance. The majority of foci characterized (104/189, 55%) did not fit any of seven molecular taxonomy categories derived from index lesions from 333 primary CaPs [13]. These observations are in accordance with previous reports that TMPRSS2-ERG fusion status, one of the classifiers used, differs among intraprostatic CaP foci. Inconsistent reports of association between TMPRSS2-ERG status and postoperative risk of CaP progression [26] may be because of inadequate tissue sampling. Similar problems may be encountered when recent TCGA-derived classifications [13] are developed further to guide treatment decisions regarding active surveillance and (multi-)treatment modalities. The presence of the same somatic mutation in index lesions and smaller CaP foci within the same prostate brings into focus the potential relevance of monoclonal or polyclonal origin of multifocal CaP [8,14,15]. Our tissue procurement method reduces the likelihood of monoclonal origin because of field cancerization or seeding to spatially contiguous prostate regions. Low correlation between the location of mutations and intraprostatic blood or lymph flow makes monoclonal intraprostatic reseeding less plausible. These patients presented with localized CaP without any evidence of metastases, so reseeding of oligometastatic CaP to the prostate also seems unlikely. Polyclonal CaP origin in which the same genes are affected by the same somatic alteration in different CaP foci may be linked to patient-specific germline, microenvironment, or lifestyle factors.
This study quantitated, for the first time in the same tissue core, the expression levels of genes that inform the Decipher, Prolaris, and Oncotype DX prognosticator scores [16], as well as AR activity [13,32]. These analyses revealed considerable variability within and among CaP foci. Although for some of the 28 patients studied here at least two CaP foci had comparable values for one or more scores, in general the correlation of those values between different intraprostatic CaPs was low. Sampling issues may explain previous reports of similarity (Oncotype DX (GPS)34) and dissimilarity (8 of 24 incongruent Decipher risk categories between matched biopsies and cores obtained from same prostate area [30]) in score values derived from two CaPs from an RP specimen [30,34]. Care is required when interpreting data from our analyses since we did not utilize the commercial Decipher, Prolaris, and Oncotype DX tests, but quantitated expression of the genes measured in these tests on another gene expression platform, RNA-Seq instead of oligoarray (Decipher) or real-time reverse transcriptase–PCR (Oncotype DX), which could have affected the scores. Analyses of gene profiles herein may also be a factor, since algorithms that are used in commercial assays were not available. The identity of three transcripts (Supplementary Table 16) from the commercial Decipher assay is proprietary information. The specific AR-dependent gene set studied may have influenced the results; we quantitated 20 AR-dependent genes previously characterized in clinically localized and metastatic CaP [13,32]. Additional confounders included the focus on different CaP risk categories among studies [16] and the isolation of nucleic acids from frozen versus formalin-fixed, paraffin-embedded tissues. These factors could contribute to our finding that CaP location did not correlate with molecular taxonomy, Decipher score, or AR activity, in contrast to another report [35].
Notwithstanding these limitations, the values and ranges for the scores shown in Figure 5 and Supplementary Figure 8 indicate that is reasonable to assume that for a nontrivial proportion of patients, the specific CaP that is assayed can alter their risk assessment/prognosis. For example, a 20% change in Oncotype DX score (20 on a scale from 0 to 100) is sufficient to alter prediction of stage and grade [34]. Direct comparison between cutoffs used in the commercial assays and the values of scores obtained from our quantitation method proved difficult because of the proprietary nature of commercial quantitation methods, the unavailability of three Decipher score transcripts, and difficulty in assessing how the tissues analyzed in this study compared to those used to generate the commercial assays.
The use of genomic classifiers to personalize treatment plans shows promise but is in its infancy [16,26]; additional validation and optimization are required to ensure optimal clinical decision-making. Our data indicate that taking into account the range rather than the absolute value of scores, the average score from two or more intraprostatic CaP foci, cooperativity between scores from different assays on the same tissues, and inclusion of DNA-based (eg, CNA [9]) data may improve the performance of current and future prognosticators. Such fine-tuning will require systematic analyses of representative CaP foci, both retrospectively and prospectively, from larger multi-institutional cohorts of patients for whom clinical follow-up and responses to postoperative treatment are available. Such information will be critical in the development of prognosticators that provide information that is accurate enough to allow clinicians to recommend treatment only when necessary and maximize therapeutic benefit when treatment is given.
Supplementary Material
Acknowledgments
The authors thank Dr. Cassandra Talerico for review of the manuscript and acknowledge that the next-generation sequencing was carried out by the Genomics Shared Resource at Roswell Park Cancer Institute, which is supported by NCI grant P30CA016056.
Funding/Support and role of the sponsor: This work was supported by an award from the Roswell Park Alliance Foundation and NIH/NCI grant CA166440 (to H.V.H.). The funding agencies did not influence the design or conduct of the study.
Footnotes
Author contributions: Hannelore V. Heemers and Song Liu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heemers, Mohler, Azabdaftari, Liu, Wei, Wang, Morrison.
Acquisition of data: Wei, Wang, Azabdaftari, Mohler, Liu, Heemers.
Analysis and interpretation of data: Wei, Wang, Lampert, Hu, Gomez, Murakam, Liu, Heemers.
Drafting of the manuscript: Heemers, Liu.
Critical revision of the manuscript for important intellectual content: Wei, Wang, Lampert, Schlanger, DePriest, Hu, Gomez, Murakam, Glenn, Conroy, Azabdaftari, Mohler, Morrison, Liu, Heemers.
Statistical analysis: Wei, Wang, Hu, Gomez, Murakam, Liu.
Obtaining funding: Mohler, Heemers.
Administrative, technical, or material support: Lampert, DePriest, Schlanger, Glenn, Conroy.
Supervision: Heemers, Liu, Mohler.
Other: None.
Financial disclosures: Hannelore V. Heemers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.07.008.
References
- 1.Aihara M, Wheeler TM, Ohori M, Scardino PT. Heterogeneity of prostate cancer in radical prostatectomy specimens. Urology. 1994;43:60–6. doi: 10.1016/s0090-4295(94)80264-5. [DOI] [PubMed] [Google Scholar]
- 2.Murphy GP, Busch C, Abrahamsson PA, et al. Histopathology of localized prostate cancer. Scand J Urol Nephrol Suppl; Consensus Conference on Diagnosis and Prognostic Parameters in Localized Prostate Cancer; Stockholm, Sweden. May 12–13, 1993; 1994. pp. 7–42. [PubMed] [Google Scholar]
- 3.Miller GJ, Cygan JM. Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol. 1994;152:1709–13. doi: 10.1016/s0022-5347(17)32368-6. [DOI] [PubMed] [Google Scholar]
- 4.Ruijter ET, van de Kaa CA, Schalken JA, Debruyne FM, Ruiter DJ. Histological grade heterogeneity in multifocal prostate cancer. Biological and clinical implications. J Pathol. 1996;180:295–9. doi: 10.1002/(SICI)1096-9896(199611)180:3<295::AID-PATH663>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Song SY, Pretlow TG, et al. Evidence of independent origin of multiple tumors from patients with prostate cancer. J Natl Cancer Inst. 1998;90:233–7. doi: 10.1093/jnci/90.3.233. [DOI] [PubMed] [Google Scholar]
- 6.Macintosh CA, Stower M, Reid N, Maitland NJ. Precise microdissection of human prostate cancers reveals genotypic heterogeneity. Cancer Res. 1998;58:23–8. [PubMed] [Google Scholar]
- 7.Cheng L, Bostwick DG, Li G, et al. Allelic imbalance in the clonal evolution of prostate carcinoma. Cancer. 1999;85:2017–22. doi: 10.1002/(sici)1097-0142(19990501)85:9<2017::aid-cncr20>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Gerlinger M, Catto JW, Orntoft TF, Real FX, Zwarthoff EC, Swanton C. Intratumour heterogeneity in urologic cancers: from molecular evidence to clinical implications. Eur Urol. 2015;67:729–37. doi: 10.1016/j.eururo.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–9. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–77. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–25. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutros PC, Fraser M, Harding NJ, et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat Genet. 2015;47:736–45. doi: 10.1038/ng.3315. [DOI] [PubMed] [Google Scholar]
- 15.Cooper CS, Eeles R, Wedge DC, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet. 2015;47:367–72. doi: 10.1038/ng.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falzarano SM, Ferro M, Bollito E, Klein EA, Carrieri G, Magi-Galluzzi C. Novel biomarkers and genomic tests in prostate cancer: a critical analysis. Minerva Urol Nefrol. 2015;67:211–31. [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Clinical practice guide-lines in oncology. Prostate cancer guidelines. www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 18.Heemers HV. Targeting androgen receptor action for prostate cancer treatment: does the post-receptor level provide novel opportunities? Int J Biol Sci. 2014;10:576–87. doi: 10.7150/ijbs.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin JF, Schiewer MJ, Dean JL, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–71. doi: 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–53. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison C, Cheney R, Johnson CS, Smith G, Mohler JL. Central quadrant procurement of radical prostatectomy specimens. Prostate. 2009;69:770–3. doi: 10.1002/pros.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Futreal PA, Coin L, Marshall M, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–83. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kan Z, Jaiswal BS, Stinson J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–73. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 26.Bostrom PJ, Bjartell AS, Catto JW, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68:1033–44. doi: 10.1016/j.eururo.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg J, Klevebring D, Liu W, et al. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. Eur Urol. 2013;63:347–53. doi: 10.1016/j.eururo.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 28.Svensson MA, LaFargue CJ, MacDonald TY, et al. Testing mutual exclusivity of ETS rearranged prostate cancer. Lab Invest. 2011;91:404–12. doi: 10.1038/labinvest.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomlins SA, Alshalalfa M, Davicioni E, et al. Characterization of 1577 primary prostate cancers reveals novel biological and clinicopathologic insights into molecular subtypes. Eur Urol. 2015;68:555–67. doi: 10.1016/j.eururo.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knudsen BS, Kim HL, Erho N, et al. Application of a clinical whole-transcriptome assay for staging and prognosis of prostate cancer diagnosed in needle core biopsy specimens. J Mol Diagn. 2016;18:395–406. doi: 10.1016/j.jmoldx.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spratt DE, Evans MJ, Davis BJ, et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 2015;75:4688–96. doi: 10.1158/0008-5472.CAN-15-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, Coleman I, Morrissey C, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22:369–78. doi: 10.1038/nm.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–22. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;68:550–60. doi: 10.1016/j.eururo.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Faisal FA, Sundi D, Tosoian JJ, et al. Racial variations in prostate cancer molecular subtypes and androgen receptor signaling reflect anatomic tumor location. Eur Urol. 2016;70:14–7. doi: 10.1016/j.eururo.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.