Abstract
Psoriatic arthritis (PsA) is an inflammatory rheumatic disorder that occurs in patients with psoriasis and predominantly affects musculoskeletal structures, skin and nails. The etiology of PsA is not well understood but evidence supports interplay of genetic, immunologic and environmental factors which promote pathological bone remodeling and joint damage in PsA. Localized and systemic bone loss due to increased activity of osteoclasts is well established in PsA based on animal models and translational studies. In contrast, the mechanisms responsible for pathological bone remodeling in PsA remain enigmatic although new candidate molecules and pathways have been identified. Recent reports have revealed novel findings related to bone erosion and pathologic bone formation in PsA. Many associated risk factors and contributing molecular mechanisms have also been identified. In this review, we discuss new developments in the field, point out unresolved questions regarding the pathogenetic origins of the wide array of bone phenotypes in PsA and discuss new directions for investigation.
Keywords: axial spondyloarthritis, bone remodeling, osteoblast, osteoclast-, psoriasis, psoriatic arthritis
Introduction
Psoriatic arthritis (PsA) is a chronic systemic inflammatory disease associated with psoriasis and nail dystrophy that affects peripheral joints, soft tissues and the axial skeleton. In most cases, patients first develop psoriatic lesions and subsequently up to a third of patients develop arthritis. Given that more than 7 million people manifest psoriasis (Ps) [1], from 1.5 to 2 million individuals in the US may eventually develop PsA. PsA is a heterogeneous disease and shares many clinical features such as sacroiliitis, spondylitis, enthesitis, and uveitis with the larger group of seronegative spondyloarthropathies (SpA) [2]. Inflammation and pathological bone remodeling in the joints are key features of PsA which may result in chronic pain, decreased quality of life and functional impairment.
Unfortunately, diagnosis of PsA is often delayed and patients may show irreversible joint damage at presentation [3]. Pathological bone remodeling in PsA arises from a dramatic alteration in the tightly regulated bone homeostatic pathways that maintain normal bone integrity and the pathologic events are promoted by release of an array of cytokines and other factors in inflamed synovial tissues. Recent research has uncovered pivotal molecules and cellular interactions that underlie altered pathological bone remodeling. In particular, certain clinical features combined with genetic and environmental risk factors increase the risk for joint damage and altered bone remodeling in PsA. Although newer therapies have considerably reduced the progression of bone damage, remission is uncommon and many patients progress to disability at some level. In this review, we discuss the mechanistic insights into the pathways that alter tightly regulated bone homeostasis in psoriatic disease and review the impact of recent therapeutic advances on progressive bone damage. Furthermore, we also highlight current knowledge gaps and point out areas where further studies are required to improve treatment outcomes in PsA.
Clinical features of bone involvement in psoriatic disease
One of the most striking features of PsA is the wide array of bone phenotypes associated with this disease. For the most part, presentation is asymmetric in the appendicular skeleton with a propensity for ray distribution involving some digits with high severity and completely sparing others. Joint deformities arise from tendon involvement, bone erosion or joint space narrowing and aberrant bone formation presenting as peripheral joint fusion or ankylosis. In the axial skeleton patients may develop syndesmophytes or large paramarginal syndesmophytes and changes in the sacroiliac joints [4]. Enthesitis is a common feature with calcification or new bone formation (enthesophytes) noted at insertion sites [5]. Lastly, dactylitis, characterized by diffuse swelling of a toe or finger may be accompanied by bone erosion or pathologic new bone formation [6]. Altered new bone formation is not just limited to PsA. In recent study, high-resolution peripheral quantitative CT (HR-pQCT) scans of the metacarpophalangeal joints revealed, significantly higher prevalence of bone formation at entheseal sites of psoriasis patients who presented no signs of joint manifestations as compared to the healthy controls [7]. This recent finding suggests that subclinical bone formation may be linked to psoriasis even in the absence of clinical evidence of joint involvement.
The bone phenotypes in PsA and rheumatoid arthritis are quite distinct although overlapping features can be observed in individual patients. RA tends to involve the metacarpal phalangeal (MCP), proximal interphalangeal (PIP) and metacarpal phalangeal (MTP) joints in a symmetric manner with erosions beginning at the site of capsular insertion on the bone[8].The pathologic process is dominated by pathologic bone resorption with inadequate bone repair resulting in both local and systemic bone loss[9]. Axial involvement is uncommon and generally limited to the C1 and C2 vertebral structures. In contrast, PsA is more likely to be asymmetric involving any of the peripheral joints including distal interphalangeal joints which develop characteristic large eccentric erosions coupled frequently with new bone formation manifested as paramarginal and vertical syndesmophytes in the axial skeleton and enthesophytes, periostitis and bony ankylosis in the peripheral joints [10]. Dactylitis and enthesitis are also common clinical features observed in psoriatic but not typically rheumatoid arthritis. Thus, RA is largely a resorptive disorder of peripheral joints whereas PsA affects both axial and peripheral joints with a mixed erosive-proliferative phenotype. In contrast to RA and PsA, the bone changes in ankylosing spondylitis (AS) are largely confined to the axial skeleton and take the form of vertical syndesmophytes. Patients most often demonstrate bilateral sacroillitis and bony involvement of peripheral joints is uncommon. Pathologic bone erosion in the spine is observed but the predominant pathology is pathologic bone formation.
Pathological aspects of PsA
Uncoupling of OB and OC interactions and pathological bone remodeling in PsA
The integrity of bone is maintained by the dynamic interaction of matrix forming cells, osteoblasts (OB) and bone resorbing cells, osteoclasts (OCs). OBs originate from the mesenchymal stem cells (MSCs) in the presence of key osteogenic factors while OCs arise from circulating osteoclast precursor cells (OCPs) of myeloid origin. In normal bone homeostasis, OB and OC coordinate activities to maintain the structural and functional components of bone through a continuous process of removal and replacement of matrix. Most importantly, OB and OC communicate through an array of secreted molecular factors that regulate cell differentiation, maturation and function via activation of biological receptors by their respective ligands. RANK and RANKL serve as a prime example of a receptor-ligand interaction that profoundly modulates bone remodeling [11]. In the case of inflammatory arthritis, the coordinated activities of OC and OB are disrupted resulting in altered bone remodeling and structural deformities [12]. In PsA, an array of inflammatory cytokines, growth factors and altered response to biomechanical forces converge resulting in altered bone remodeling and pathologic subtypes observed in PsA [12]. The pivotal molecules that alter the differentiation, maturation and function of the OB and OC cells in the context of PsA are discussed in the following section.
A. Pathologic bone resorption in psoriatic arthritis
Contrasts in pathological bone remodeling in PsA and RA
Significant bone resorption and cartilage damage in inflamed joints is a prominent clinical feature of PsA [2]. Localized bone destruction is also seen in other inflammatory arthropathies such as rheumatoid arthritis (RA). Although similar patterns of erosion are present in some patients with RA and PsA, significant differences exist between these disorders in regards to articular and periarticular inflammation.
Most importantly, pathologic bone resorption takes place in both diseases. As a result, radiographic manifestations of early disease in PsA and RA may be difficult to distinguish until more distinctive features appear in more established disease. Radiographic and ultrasound features of bone erosion and joint space narrowing may overlap with those of RA and inflammatory osteoarthritis, particularly early in disease [13]. Distinguishing radiographic features of PsA appear in the more advanced disease and become evident in the peripheral synovial joints as well as in the fibro-cartilaginous joints, sacroiliac region and axial spine, and the entheses [14,15].
Significant differences in the skeletal pathologies of PsA and RA have been reported [16]. One of the key differences between PsA and RA bone loss is the lack of osteoblastic response to pathologic resorption present in RA, while in PsA new bone formation may be exuberant. A recent study reported changes in hand bone mineral density (BMD) using digital X-ray radiogrammetry (DXR-BMD) in early PsA and contrasted them with RA patients. Periarticular bone loss in the hand progressed over time in RA whereas periarticular bone density in PsA patients remained unchanged [17]. These findings suggest that different pathological mechanisms are involved in PsA and RA. Significant differences also exist in terms of the anatomical localization of inflammatory lesions and in periarticular bone changes [17,18].
Systemic bone loss in psoriasis and PsA
In RA, both localized and systemic bone loss takes place, manifesting as focal erosions, joint space narrowing and decrease bone mineral density in the spine and hip [16]. While localized bone loss is a well-established as a key feature of PsA bone damage, studies also revealed lower bone mineral density and higher risk of bone fracture in Ps and PsA patients compared to healthy controls [19]. PsA patients have higher body mass index (BMI) than RA patients and controls and when this variable was entered into the model, bone density was similarly decreased in both RA and PsA [20]. It is important to mention that there are some inconsistencies related to these overall findings, for example, no association between psoriasis and risk of fracture was observed in the HUNT study which utilized the hospital-driven fracture data from Nord-Trondelag County (1995–2013) linking to psoriasis severity, BMD measurement and other related data [21]. Chandran et al. conducted a systemic review where they found 13 of 21 published reports indicated increased bone loss in PsA patients [22] while the remainder did not reveal this association. Nonetheless, one large cohort study of patients with psoriasis and PsA revealed elevated risk for fracture in Ps and PsA patients [19] further providing evidence of systemic bone loss in Ps and PsA patients.
Tumor Necrosis Factor (TNF) and Receptor activator of nuclear factor κB ligand (RANKL) dependent and independent pathways of bone resorption
RANKL
The cytokine RANKL is regarded as the critical factor that promotes differentiation of multinuclear bone resorbing osteoclast cells (OCs) from mononuclear progenitor cells. Exposure to M-CSF increases expression of the transmembrane protein receptor activator of nuclear factor-κB (RANK) on the osteoclast progenitor cells (OCPs) which interact with its ligand, RANKL expressed by osteoblasts, activated T lymphocytes and synovial fibroblastoid cells. RANK-RANKL interactions initiate a cascade of signaling events that involve activation of TRAF6, NFκB and other signaling molecules. A second signal provided by activation of triggering receptor expressed on myeloid cells 2 (TREM2) and osteoclast-associated receptor (OSCAR) converge on NFATc1 which translocates to the nucleus and transcribes multiple key osteoclastic genes such as Dendritic Cell-Specific Transmembrane Protein (DC-STAMP), Osteoclast-Stimulatory Transmembrane (OC-STAMP), v-ATPase V0 subunit d2 (ATPV0D2), cathepsin K (CTSK) which promote cell fusion and the development of the secretory molecules and the ruffled membrane required for bone resorption. Importantly, RANKL is expressed by Th17 and not Th1 cells. The notion that Th17 cells are primary cells responsible for bone resorption, however, was recently challenged in animal studies where cell specific deletion of RANKL indicated that RANKL expression was limited to synovial fibroblastoid cells and B cells. These findings suggest that these two cell lineages and not Th17 cells are the prime cell types promoting pathologic bone resorption [23]. Taken together, both in vitro and animal studies demonstrate that RANKL, in the presence of M-CSF, are major factors involved in osteoclastogenesis. Past studies demonstrated elevated frequency of RANK and RANKL expressing cells in the synovium of PsA patients compared to osteoarthritis and RANKL expression was dramatically upregulated in the synovial lining layer [24]. Moreover, serum RANKL levels in PsA patients were significantly higher compared to patients with plaque psoriasis or healthy individuals [25].These data provide additional support for a central role of RANKL in promoting osteoclastogenesis and bone loss in PsA.
TNF-α
RANKL is in the TNF superfamily of molecules so the observation that TNF can also induce osteoclastogenesis in the presence or absence of RANKL underscores parallel effects on bone. Indeed in the presence of permissive doses of RANKL, TNF-α substantially upregulates in vitro osteoclastogenesis [26]. TNF-α, in the absence of RANKL, can also induce osteoclastogenesis from OCPs in vitro [27]. Indeed, prolonged exposure to TNF-α induced increased expression of NFATc1 and sustained calcium oscillation in human macrophages and promoted osteoclastogenesis [28]., Zhao et al., demonstrated that myeloid-specific deletion of the transcription factor recombinant recognition sequence binding protein at the Jκ site (RBP-J) in mice reduced TNF-α induced OC differentiation by suppressing NFATc1 and attenuating AP-1 activation [27]. Overexpression of human TNF in the TNF-tg mice leads to increase in the number of circulating CD11b+ OCPs and increased bone resorption [29]. Analysis of serum, synovial fluid and synovial tissue isolated from PsA patients demonstrated elevated levels of TNF and soluble TNF-R55 [30]. Moreover, phase III clinical trials of anti-TNF agents in PsA demonstrated inhibition of radiographic progression (Table 1). Lastly, reduced radiographic progression and alterations in osteitis after anti-TNF therapy correlated with marked reduction in the number of circulating OCPs [31]. Collectively, these studies demonstrate the TNF-α can amplify osteoclastogenesis in the presence of RANKL but that it also has direct effects on OC formation when RANKL is not present.
Table 1.
Similarities and differences between PsA and AS bone pathologies
| Characteristics | PsA | AS | References |
|---|---|---|---|
|
| |||
| Definitive radiographic sacroiliitis | − | + | [143] |
|
| |||
| Sacroiliitis distribution | Bilateral | Unilateral or bilateral | [144] |
|
| |||
| Grade 4 sacroiliitis | −/+ | +/++ | [145] |
| Not corroborated by subsequent studies | |||
|
| |||
| Spondylitis | + | ++ | [47] |
|
| |||
| Distribution of syndesmophytes | Asymmetrically distributed | Symmetrically distributed | [144] |
| Progress randomly along the spine | [146] | ||
|
| |||
| Bridging Syndesmophytes | −/+ | −/+/++ | [47] |
|
| |||
| SIJ ankylosis | + | ++ | [47] |
|
| |||
| Radiographic severity | + | ++ | [143] |
'−' indicates absence,'+' and '++' indicate presence of specific features
IL-17
IL-17 secretion by Th17 has been shown to be significantly elevated in both PsA and psoriasis compared to healthy controls [32]. The members of IL-17 family include at least six members in humans and mice, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. Among these, the role of IL-17A in arthritis and bone remodeling has been well studied. In vitro studies revealed that IL-17A can increase osteoclastogenesis in the presence or absence of OBs through direct and indirect effects. When OB and OCPs are cultured together, IL-17A can increase osteoclastogenesis by upregulating RANKL expression on OB cells [33]. It has been also shown that IL-17A increases secretion of inflammatory mediators such as IL-1β, TNF-α and PGE2 by OBs [34] which further potentiate osteoclastogenesis. Similarly, IL-17A can promote osteoclastogenesis from CD11b+ human OCPs even in the absence of OB or RANKL stimulation, and this effect is partially dependent on TNF-α given that osteoclastogenesis was blocked by infliximab in vitro. [35]. These findings, however, stand in contrast to findings reported with the RAW264.7 cell line where high doses of IL-17A suppressed RANKL-induced osteoclastogenesis and release of OC-related effector molecules such as cathepsin K and MMPs in mature OCs [36]. Nonetheless, recent studies demonstrated that treatment of PsA patients with mAbs targeting IL-17 lessened joint inflammation and reduced X-ray progression in PsA patients [37].
IL-6
Elevated expression of IL-6 has been noted in synovial tissue of the PsA patients and IL-6 levels correlate with the number of affected joints and elevation of the C-reactive protein (CRP) [38,39]. The mechanism by which IL-6 contributes to joint damage and pathological bone remodeling in PsA patients is still not well understood. Past in vitro studies showed, IL-6 can support OC formation from human CD14+ osteoclast progenitor cells by a RANKL-independent mechanism [40]. Despite the therapeutic potential of IL-6 from a mechanistic perspective a clinical trial of the anti-IL-6R mAb, tocilizumab, failed to show a significant treatment response in PsA [41]. In contrast, recent clinical trial data showed that blockade of IL-6 with the mAb clazakizumab ameliorated musculoskeletal manifestations in PsA patients [42]. Studies in animal models showed that exposure of mouse OCPs to IL-6 and TNF-α, increased formation of multinuclear functional OCs that efficiently erode mineralized tissue [43]. Using mice with germline or conditional deletion of RANK in myeloid cells, O'Brien et al. further demonstrated that combined exposure to IL-6 and TNF-α induce OC differentiation that is independent of RANKL [44]. Further studies are now needed to confirm these in vitro findings in human cells and to examine if IL-6 is a reasonable target in PsA.
B. Pathological bone formation in PsA patients
Some characteristic basic distinctive anatomical features in PsA
A characteristic feature that differentiates PsA from other erosive inflammatory arthritis such as RA is the exuberant pathological bone formation. Importantly, such development of bony nodules can be seen even at sites distant from bone resorption [2]. The new bone formation can present in the axial skeleton as marginal or paramarginal syndesmophytes or in the peripheral joints as enthesophytes, periosteal bone formation (‘whiskering on plain X-ray’) or joint ankylosis [15,45]. Enthesitis, inflammation of sites where ligaments, tendons and joint capsules attached to bone, is often accompanied by heterotopic cartilage and bone formation in the enthesis [15,45]. Another fascinating but poorly understood feature present in the PsA is the ''ray distribution" of involvement in which one digit may demonstrate bony fusion while adjacent digits exhibit no abnormality or bone erosion [46].
Pathological bone formation and joint damage in PsA and ankylosing spondylitis (AS), similarities and differences
Pathological bone formation is shared between PsA and AS presenting as syndesmophytes, sacroiliitis, and sacroiliac joint (SIJ) ankylosis [47–49]. Despite the apparent similarities, significant differences in these bone phenotypes are observed in these two diseases in terms of the extent and distribution of sacroiliitis and syndesmophytes (outlined in Table 1). Sacroiliac joint involvement in PsA is more likely to be asymmetric. The syndesmophytes in PsA are asymmetrically distributed and progress randomly along the spine whereas in AS, syndesmophytes are symmetrically distributed. Similarly, the bridging syndesmophytes are much more common in AS and seen less frequently in PsA patients. These divergent morphological features provide preliminary support for separate disease pathways in these disorders.
BMP and WNT signaling in the context of pathological bone formation in PsA patients
A key aspect of PsA is the pathologic bone deposition which results from aberrant proliferation, differentiation and activity of bone forming osteoblasts (OB) in which multiple signaling pathways are involved. Members of the bone morphogenetic protein (BMP)-bone morphogenetic protein receptor (BMPR) pathway as well as WNT signaling pathway and molecules that inhibit WNT signaling, such as DKK1, and SOST are especially relevant for PsA and related spondyloarthritic diseases such as AS.
Relevance of BMP signaling
BMPs are members of the TGF-β superfamily and are well established as key regulators of bone formation. The BMP molecules increase the activity of key osteogenic transcription factors, RUNX2 and osterix, in mesenchymal stem cells and thereby increase the rate of osteoblast differentiation. Similarly, BMPs increase mineral deposition by the maturing osteoblast cells through enhanced expression of alkaline phosphatases (ALP), type I collagen, osteocalcin and bone sialoprotein in the OB cells [50,51]. Previous animal studies indicated that altered BMP signaling was involved in joint ankyloses [52]. Moreover, elevated expression of BMP and phosphorylated SMAD1/5 were detected in the ankylosing enthesis in a murine model with joint pathology similar to that observed PsA [52]. Moreover, overexpression of noggin, a non-specific antagonist of BMP decreased the pathological severity in this murine model with ankylosing enthesitis [52] clearly establishing a key role of BMP signaling in ankylosis. Of particular relevance is a recent study in which serum levels of BMP-7, were significantly elevated in PsA patients compared to healthy controls and BMP-7 levels correlated with the Glasgow Ultrasound Enthesitis Scoring System (GUESS) - a measure of enthesitis [53]. These findings are in agreement with the findings of Lories et al who also reported positive significant correlation between serum BMP-7 levels and lower limb enthesitis in male DBA/1 mice [54] and provide support for a prominent contribution from BMP-7 in altered bone remodeling and enthesitis in PsA. The specificity of this finding to PsA was bought into question, however by Yuan el al. who reported that BMP-7 is also elevated in RA and AS patients and that BMP-7 levels correlated with serum levels of RUNX2 [51].
Relevance of WNT signaling
The Wnt family consists of number of small secreted glycoproteins that regulate a range of cellular activities and developmental processes. Prior in vivo studies clearly established the critical role of Wnt/β-catenin signaling (canonical WNT pathway) in regulating OB differentiation from mesenchymal stem cells [55]. Regulation of Wnt/β-catenin signaling is driven by the production of two antagonists of WNT signaling, Dickkopf-related protein 1(DKK-1) and Sclerostin (SOST and DKK-1 functions as a master regulator of joint remodeling [56]. Specifically, DKK1 elevation in RA patients correlates with increased bone erosion and lower BMD [57]. In AS patients, a correlation between decreased serum levels of functional DKK-1 (as compared to healthy controls) and syndesmophyte development was reported [58]. Another group reported that serum levels of DKK-1 in a cohort of female PsA patients were significantly lower than in healthy controls and RA patients [59] while increased serum levels of DKK1 were noted in PsA patients [60]. These differences arise from divergent methods for DKK-1 quantification, different characteristics of the patients population or differences in the vitamin D serum levels Interestingly, another study, Daoussis et al, noted elevated levels of total serum DKK1 in AS but it was dysfunctional in its biologic effects [61].
It is important to note that administration of anti-DKK1 antibody to transgenic TNF-α overexpressing (TNF-Tg) mice was associated with osteophyte formation in inflamed joints but not in the joints of untreated mice [56]. As TNF-Tg mice typically do not develop osteophytes, the above mentioned findings indicate DKK-1 can inhibit local bone formation in some conditions. The findings from these animal studies align with recent reports that revealed decreased DKK1 levels in PsA patients. This finding is of particular interest given that low DKK1 levels may contribute to the increased osteophyte formation reported in psoriasis and PsA [56].
TNF α, IL-17 and related cytokines in pathologic bone formation
The cytokines TNF-α and IL-17 exhibit pro-inflammatory and bone resorbing functions and have been demonstrated to promote pathologic bone resorption as described above. Under specific in vitro conditions and in certain animal models, however, the inflammatory cytokines including TNF-α, IL-17 as well as IL-23 and IL-22 can increase osteogenic differentiation. While the contribution of TNF-α and IL-17 to in vitro osteoclastogenesis is well-established, the overall impact of these cytokines on pathologic bone formation is not well understood. Previous reports demonstrated that TNF-α blocks OB differentiation by inhibiting insulin-like growth factor-1 (IGF-1) [62], Runx2[63] and osterix (Osx also known as SP7)[64] whereby increased apoptosis [65] or effects on other pathways [66] may also be important. On the other hand, several studies with murine and human MSCs and related cell lines found that TNF-α induces osteogenic differentiation [67–70]. In particular, low dose TNF-α promotes OB differentiation in vitro [71,72]. Osteogenesis was dependent on increased NF-κB signaling in the MSCs [67]. Furthermore, local in vivo administration of exogenous rhTNF-α accelerated fracture healing in mice [71]
The role of IL-17 in bone formation is also very dependent on the experimental condition and animal model. Indeed, two in vitro studies reported that IL-17 inhibits OB differentiation [73,74], while several others found that IL-17 can increase OB differentiation [75–80]. Importantly, in the K/BxN serum transfer arthritis model, it was reported that IL-17 deficiency promotes periosteal bone formation [81] indicating IL-17 blockade can increase pathologic bone formation, a major problem in SpA. Uluçkan et al. crossed K14-IL17Aind (mice that conditionally overexpressing IL-17A together with EGFP) with JunBΔep (mice with JunB deletion in epidermal cells) and manifest chronic IL-17A–mediated skin inflammation. In this model, IL-17A decreased bone formation by inhibiting WNT signaling in OBs resulting in increased bone loss without a decline in OC activity. [82]. In a study with IL-17A−/− mice, Ono et al., demonstrated that IL-17A produced by γδ T cells increased proliferation and osteoblastic differentiation of mesenchymal progenitor cells and promoted bone formation facilitating bone fracture healing [83]. Together, these data indicate that IL-17 as well as TNF-α can inhibit or promote bone formation in a context dependent manner. Further studies will elucidate which of these findings translate most appropriately to psoriatic disease.
In the passively induced collagen-induced arthritis (CIA) murine model, in vivo expression of IL-23 was associated with enthesitis via activation of entheseal residing IL-23R expressing CD3+CD4−CD8− T cells which produced IL-17, TNF-α and IL-22. These mice demonstrated entheseal and periosteal bone formation and pathologic bone resorption [84]. Recent in vitro studies demonstrated that IL-22 can enhance osteogenic differentiation in human MSCs while a combination of TNF and IFN-ɣ with IL-22 suppress it [85]. Overall, the systemic effects of these cytokines in the context of pathologic bone formation require detailed analysis in PsA patients. Ongoing and future longitudinal imaging studies on PsA patients treated with therapeutic agents with a combined Omics analysis may provide insights into the local tissue and bone factors that underlie aberrant bone remodeling in PsA.
Genetic and environmental factors that can influence bone pathologies in PsA patients
1) Genetic factors and bone sub-phenotypes in PsA
It is well established that several genomic loci are associated with PsA [86]. In early reports, HLA-B*27 was noted to be associated with increased risk of axial disease in PsA patients and this association was confirmed in other cohorts [87]. More recent studies revealed that significant genotypic heterogeneity exists in PsA patients and certain specific haplotypes (HLA-B*08, HLA-B*27, HLA-B*38, HLA-B*39 and HLA-C*06:02) are present at higher frequencies than controls or psoriasis patients [88]. It was also noted that some extended haplotypes were associated with specific sub-phenotypes. Peripheral synovitis was associated with HLA-B*08:01 whereas asymmetric sacroiliitis showed association with the HLA-B*08:01-C*07:01 haplotype and its constituent alleles [88]. Interestingly, comparison of the genetic risk loci present in Ps and PsA revealed that specific risk allele, HLA-C*06:02, previously thought to be a risk factor for development of arthritis in Ps patients, was not associated with PsA but rather the amino acid position 97 of HLA-B differentiated PsA from psoriasis[89]. This impressive finding, coupled with the same association reported in ankylosing spondylitis, may provide novel insights into immunogenetic factors that contribute to the development of arthritis and altered bone phenotypes in spondyloarthritis.
2) Potential links between psoriatic plaques and joint inflammation
On average, Ps precedes the onset of PsA by 8–10 years [90]. Despite this observation, mechanisms that directly link psoriatic skin and joint inflammation have not been forthcoming. In one model, psoriatic inflammation acts as a trigger for arthritis through the systemic circulation of skin-derived inflammatory cytokines which act upon target cell populations in the entheses, soft tissues of the digits and the joints [2]. In another model, loss of suppression mediated by T cells or other cell lineages permit activation of pathways that lead to bone resorption and formation that is modulated by genetic, epigenetic and environmental factors.
In vitro studies and experiments in animal models, however, may provide insights into a skin-joint axis in PsA. A recent study revealed that exposure to TNF-α, IL-17, OPN and IL-33 can induce pro-osteoclastogenic factors such as RANKL from the skin which can further promote human monocytes to differentiate into OCs [91]. In one preclinical study, K5.Stat3 mice which express activation-prone Stat3 and have psoriasiform lesions were crossed with CF759 mice which demonstrate persistent Stat3 activation due to impairment in SOCS3-dependent negative regulation. The offspring of these mice manifested severe psoriasiform lesions and arthritis within 3 weeks of age whereas the K5.Stat3 or CF759 mice developed skin inflammation and joint damage at a later age and the penetrance was lower for the skin and joint phenotypes [92]. These results indicate that psoriatic inflammation may temporally relate to joint inflammation although a causal pathway has not been established.
As noted above, a recent report detailed interactions between cutaneous cytokines and osteoclastogenesis but another recently published study revealed how skin inflammation can impact OB differentiation. Uluçkan et al., demonstrated that skin-epithelial-specific expression of IL-17A in mice leads to bone loss [82]. Furthermore, under inflammatory conditions, skin-resident γδ T cells, innate lymphoid cells (ILCs) and keratinocytes; express IL-17A, which act systemically to inhibit OB and osteocyte function through inhibition of Wnt signaling. They further showed that inhibition of Wnt signaling and inhibition of IL-17A signaling can rescue such bone loss [82]. Taken together, these findings imply the existence of a network with the potential to link psoriasis with synovial inflammation and in some cases joint damage in PsA patients via mechanisms that promote altered OC and OB activity.
3) Arthritis triggered by trauma in the setting of psoriasis-clues to pathogenesis or coincidental events?
The Koebner phenomenon, first recognized in 1876, is the formation of psoriasiform lesions in uninvolved skin of psoriasis patients following trauma [93]. Similarly, it was also noted joint trauma can trigger inflammatory joint inflammation and less commonly, the full spectrum of PsA, termed deep Koebner phenomenon [94,95]. Together these clinical findings may reflect interactions triggered by biomechanical stress, trauma and microtrauma which lead to sustained musculoskeletal inflammation. The reports of deep Koebner phenomenon, however, have been confined to case reports or small case series. Thus, the publication of a prospective, longitudinal cohort study that revealed physical trauma to be independently associated with increased risk of PsA with the highest risk associated with bone and joint trauma adds additional credibility to this putative pathway [96]. Interestingly, of great relevance, was the analysis in an RA cohort where the risk of arthritis was not increased following trauma [96]. These findings suggest that trauma may increase the risk for PsA in some individuals by mechanisms that are not evident at this time.
4) Microbial dysbiosis and gut inflammation likely regulate bone remodeling in PsA
Gut microbiota are well known to play crucial role in regulating overall immune response in mice [97]. Dysbiosis has been associated with gut and joint inflammation [98,99]. The finding that HLA-B27-transgenic rats raised in germ free environments, do not develop peripheral joint disease or gut inflammation but once reconstituted with Bacteroides, disease manifestations arise, kindled interest in this relationship over 20 years ago [100]. Importantly, recent microbiome studies demonstrate that the gut microbiome in many patients with inflammatory arthritis differs from healthy individuals [101,102]. Intestinal dysbiosis has also been noted in AS patients [103]. In another report, Scher et al., found microbial dysbiosis in patients with PsA and reported lower abundancies of specific bacterial genera including Akkermansia and Ruminococcus in PsA patients [104]. Interestingly, they also found that the local gut immune response in PsA is characterized by a decrease RANKL and increase fecal sIgA levels relative to healthy controls. The implication and clinical relevance of these findings await further study, particularly to determine if the dysbiosis precedes or follows the onset of arthritis and joint damage.
Recently, work from Ciccia et al showed that subclinical gut inflammation in PsA patients is characterized by high levels of tissue inflammation, Paneth cell hyperplasia and Th17 and Th22, but not a Th1 driven immune response [105]. Interestingly, their work further demonstrated that another inflammatory cytokine, IL-9 was specifically overexpressed in the gut of PsA patients (especially in patients with inflammatory gut lesions) but not in the gut of patients with AS or Crohn's disease (CD) patients, or controls. Moreover, when stimulated in vitro with IL-9, isolated epithelial cells from PsA gut tissues showed increased IL-23p19 [105] mRNA levels. They did not examine joint phenotypes, however, it was reported that IL-9 can induce differentiation of Th17 [106]. Elevated levels of IL-9 were identified in PsA synovial tissues [105]. It is known that IL-9 can act as a survival factor for the pathologic T cells localized in the synovium of inflammatory arthritis [107]. Thus, these findings indicate a potential relationship between microbial dysbiosis and altered production of IL-9, IL-23 and IL-17 with the potential to circulate to joint tissues and promote arthritis.
5) Obesity and metabolic dysregulation
Population based studies revealed that obesity is associated with increased risk for Ps and PsA [108,109]. Obesity was also shown to be associated with lower probability of achieving sustained minimal disease activity (MDA) state in PsA patients [110,111]. Moreover, successful weight loss was more strongly associated with joint response (as assessed by MDA) in obese PsA patients treated with TNF-α blockers when compared to PsA patients who did not lose weight. The state of obesity is associated with low grade chronic inflammation in the adipose tissue and other organs resulting into a pro-inflammatory environment [112]. The ranges of pro-inflammatory cytokines generated in fat tissues are now well established [112]. Adipocytes secrete a wide variety of mediators, including TNF-α and IL-6 [113] which are known to increase osteoclastogenesis. Therefore, obesity may serve as a risk factor for increased pathological bone remodeling in PsA by increasing an array of proinflammatory cytokines. Obesity can also result in many other alterations (increased adipokine levels and decreased vitamin D) with the potential to modulate skeletal homeostasis in PsA patients. Interestingly, high levels of the adipokine leptin was reported in obese PsA patients compared to the obese Ps patients [114]. Similarly, low levels of vitamin D have been noted in PsA patients [115]. Interestingly, past studies indicated that in RA, obesity is associated with increased Disease Activity Score 28 (DAS28) but lower radiographic joint damage [116–118] and baseline circulating serum adipokine levels predict radiographic protection [119–121]. Whether such relationships between joint damage, obesity and adipokine levels holds true for PsA remains to be determined.
6) Smoking and exposure to pollutants
Diverse environmental factors impact development of inflammatory joint diseases, including PsA. Exposure to cigarette smoke is one of such major environmental risk factors associated with increased risk for PsA [122,123]. Smoking is also associated with decreased therapeutic responses to anti-TNF-α agents [124]. The mechanisms linking cigarette smoke to the development of PsA and lower response to anti-TNF-α agents are not well understood. It has been reported that smoking increases TNF-α [125]. As TNF-α is a potent inducer of OCs that contribute in the pathogenesis and bone erosion in PsA [24,126], it is conceivable that smoking worsens bone health in PsA patients by elevating TNF-α levels and promoting osteoclastogenesis and elevated bone resorption in PsA.
Therapeutic benefits and inhibition of structural damage in PsA
Over the past 15 years, the treatment of PsA has progressed at breakneck speed. Prior to 2003, only DMARDs were available to treat this disorder and currently 7 biologic agents and apremilast are FDA approved in the US. Several agents that inhibit a diverse array of mechanisms (IL-17A and F, JAK1, 2, 3 and IL-23) are in clinical trials (some are outlined in table 2). A major consideration when considering a treatment for psoriatic joint disease is whether a patient has baseline joint damage because evidence indicates these patients are more likely to show radiographic progression [127]. In table 2, the effect of agents approved for PsA or in clinical trials on bone damage is outlined. It must be emphasized that to date, no agents have been demonstrated to block new bone formation although the effect of early anti-inflammatory therapy of joint disease on development of syndesmophytes, enthesophytes or bony ankyloses is thought to be quite plausible.
Interestingly, despite the widespread use of conventional DMARDs in PsA, no formal studies have been performed to examine if these agents inhibit or slow radiographic progression. Likewise, apremilast has not been analyzed for its effect on radiographic progression. All five available anti-TNF agents do inhibit X-ray progression [128–135]. In a combined analysis of the PSUMMIT 1 and 2 trials, ustekinumab significantly inhibited X-ray progression in the anti-TNF naive patients [136] but progression was not significantly decreased when the PSUMMIT 2 trial data was analyzed alone presumably because patients previously exposed to anti-TNF agents were included [137]. Another consideration in this study was the smaller sample size. Both secukinumab and ixekizumab inhibited radiographic progression [138–140]. The ability of guselkumab, bizekizumab and tofacitinib to block X-ray progression awaits further study. It is also important to point out that the extent of baseline structural damage in studies carried out over the last several years is less than was observed in the anti-TNF trials. The lower degree of baseline damage may explain why the magnitude of the change in radiographic scores following treatment is not as large in the more recent phase III trials. Two recent studies demonstrated the efficacy and safety of tofacitinib in PsA for patients who were inadequate responders to DMARDs (OPAL Broaden) or anti-TNF agents (OPAL Beyond) [141,142]. In the OPAL Broaden study, radiographs were assessed at baseline and 12 months using the van der Heijde–modified total Sharp score although the patients on placebo were only assessed through month 3 [141]. At month 12, the minimal mean changes from baseline in the modified total Sharp score were observed across all the trial groups, including the groups of patients who switched from placebo to tofacitinib at month 3. A total of 91 to 98% of patients across all trial groups met the radiographic criteria for non-progression [141].
Unresolved questions in psoriatic arthritis
Recent preclinical and clinical investigations have revealed new disease pathways and shed light on the underlying molecular mechanisms related to pathological joint damage and altered bone remodeling in PsA. However, many critical questions remain (Table 3). One of the most perplexing aspects of this disease is the wide array of bone phenotypes and the asymmetric distribution which in the feet and hands often takes on a ray distribution [2]. Another enigmatic finding is the association of nail disease and distal interphalangeal joint (DIP) involvement. Is this truly an example of enthesitis with adjacent bone involvement and if so, where does the process begin, in the nail or the bone? One of the major unanswered questions is what are the factors responsible for new bone formation in the axial and appendicular skeleton? Lastly, is there a link between the bone phenotypes and dysbiosis- a skin to joint or a gut to joint axis in PsA?
Table 3.
Unresolved questions regarding bone pathologies in psoriatic arthritis
|
Conclusion
In spite of the recent advancements and scientific discoveries, PsA still remains a disease of unknown etiology that affects millions of individuals worldwide causing chronic pain, discomfort, suffering and disability. While research has revealed much about the molecular and cellular events that promote altered bone phenotypes in PsA, many questions still remain to be answered. We have now expanded understanding of the key events that promote pathologic bone resorption but our understanding regarding pathological bone formation is quite limited. Recent studies have revealed association between specific gene variants but we lack insight into how these variants promote or enhance disease phenotypes. Moreover, the contribution of other key factors such as skin and gut inflammation, mechanical stress, microbial dysbiosis, obesity and smoking, to potentiate the progression of the pathological bone remodeling and impact treatment outcomes in PsA patients is not well understood. Based on the rapid pace of scientific discoveries, answers to these questions will be forthcoming in the near future. Advances in imaging, Omics technology and analytic tools that permit integrated analysis and precision science will provide new insights that will dramatically improve our knowledge regarding these unresolved questions.
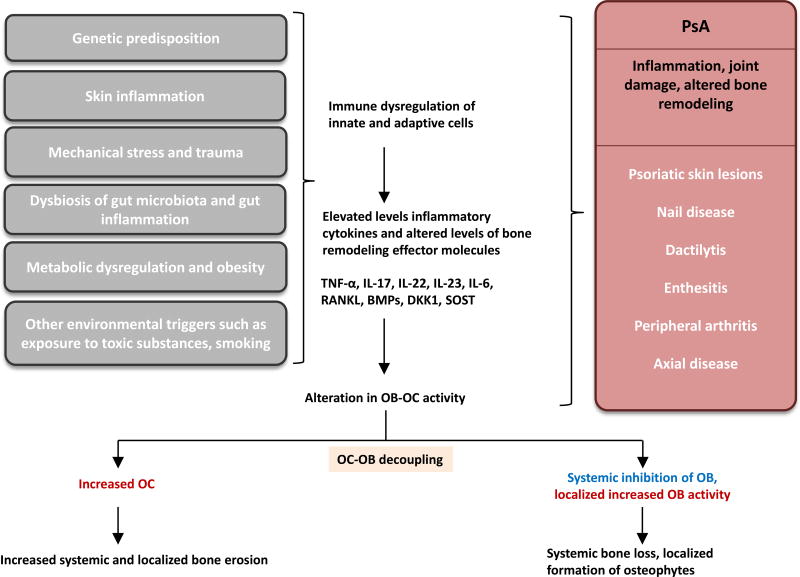
Figure 1. Schematic representation of bone remodeling in PsA.
Genetic predisposition, skin inflammation, mechanical stress, dysbiosis in gut microbiota, gut inflammation can lead to immune dysregulation causing elevation of various inflammatory cytokines and alteration in key signaling molecules that regulate inflammation and the activity OC and OB cells. These resulting systemic and localized alterations manifest as clinical symptoms of psoriatic diseases. Metabolic dysregulation, obesity and environmental triggers can further compound such alterations. Such alterations results into decoupling of OB-OC interactions ultimately lead to increased bone erosion as well as aberrant formation in PsA patients.
Table 2.
Approved and promising therapeutic agents targeting key pathways to treat PsA
| Therapeutic Agent | Target | Inhibition of radiographic progression |
References |
|---|---|---|---|
|
| |||
| DMARDS (methotrexate, azulfidine, leflunomide) | Several targets | No supporting evidence | [129] |
|
| |||
| Apremilast | PDE4 inhibitor | Not known | No studies on radiographic progression |
|
| |||
| Infliximab, | TNF inhibition | ++ | [128,129] |
| Etanercept, | [130,131] | ||
| Adalimumab, | [132,133] | ||
| Golimumab, | [134]. | ||
| Certolizumab | [135] | ||
|
| |||
| Ustekinmab | Anti p40 blocks IL-12/23 | ++* | [137,147] |
|
| |||
| Secukinumab, | IL-17A inhibition | ++ | [138,139] |
| Ixekizumab | [140] | ||
|
| |||
| Guselkumab | IL-23 inhibition | Not known | No studies on radiographic progression |
|
| |||
| Brodalumab | Anti-IL 17RA receptor antagonist | Not known | No radiographic studies |
|
| |||
| Bimekizumab | Dual IL-17A and IL-17F inhibitor | Not known | No radiographic studies |
|
| |||
| Tofacitinib | JAK 1–3 inhibition | Not clear | [141,142] |
'++' indicate inhibition of radiographic progression,
PSUMMIT 2 trial did not show significant inhibition of radiographic progression in patients previously exposed to anti-TNF-α agents
Acknowledgments
None
Financial support and sponsorship
Ananta Paine’s research works are supported by Health Sciences Research Using High Performance Computational Resources (HSCCI) Pilot Grant, Lung Biology Strategic Plan High Risk Project Pilot Research Award, and Department of Medicine Research Pilot Projects Award from University of Rochester. Christopher Ritchlin’s research works are supported by funds from the National Institutes of Health (NIH) AR0169000, P30AR069655 and 5UL1TR000042-09 grants.
Footnotes
Conflicts of interest
Christopher Ritchlin has research grants from Amgen, Abbvie, and UCB. He is also a consultant for Amgen, Abbvie, Boehringer Ingelheim, Celgene, Janssen, and Novartis. Ananta Paine has no conflicts of interest.
Contributor Information
Ananta Paine, Allergy, Immunology & Rheumatology Division, University of Rochester Medical Center, Rochester, New York 14623.
Christopher Ritchlin, Allergy, Immunology & Rheumatology Division, University of Rochester Medical Center, Rochester, New York 14623.
References
- 1.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. 2017;376:2095–2096. doi: 10.1056/NEJMc1704342. [DOI] [PubMed] [Google Scholar]
- 3.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045–1050. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]
- 4.Paparo F, Revelli M, Semprini A, Camellino D, Garlaschi A, Cimmino MA, Rollandi GA, Leone A. Seronegative spondyloarthropathies: what radiologists should know. Radiol Med. 2014;119:156–163. doi: 10.1007/s11547-013-0316-5. [DOI] [PubMed] [Google Scholar]
- 5.Narvaez J, Narvaez JA, de Albert M, Gomez-Vaquero C, Nolla JM. Can magnetic resonance imaging of the hand and wrist differentiate between rheumatoid arthritis and psoriatic arthritis in the early stages of the disease? Semin Arthritis Rheum. 2012;42:234–245. doi: 10.1016/j.semarthrit.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Brockbank JE, Stein M, Schentag CT, Gladman DD. Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheum Dis. 2005;64:188–190. doi: 10.1136/ard.2003.018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon D, Faustini F, Kleyer A, Haschka J, Englbrecht M, Kraus S, Hueber AJ, Kocijan R, Sticherling M, Schett G, et al. Analysis of periarticular bone changes in patients with cutaneous psoriasis without associated psoriatic arthritis. Ann Rheum Dis. 2016;75:660–666. doi: 10.1136/annrheumdis-2014-206347. [DOI] [PubMed] [Google Scholar]
- 8.Llopis E, Kroon HM, Acosta J, Bloem JL. Conventional Radiology in Rheumatoid Arthritis. Radiol Clin North Am. 2017;55:917–941. doi: 10.1016/j.rcl.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Stavre Z, Upchurch K, Kay J, Gravallese EM. Differential Effects of Inflammation on Bone and Response to Biologics in Rheumatoid Arthritis and Spondyloarthritis. Curr Rheumatol Rep. 2016;18:72. doi: 10.1007/s11926-016-0620-x. [DOI] [PubMed] [Google Scholar]
- 10.Siannis F, Farewell VT, Cook RJ, Schentag CT, Gladman DD. Clinical and radiological damage in psoriatic arthritis. Ann Rheum Dis. 2006;65:478–481. doi: 10.1136/ard.2005.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paine A, Ritchlin C. Bone remodeling in psoriasis and psoriatic arthritis: an update. Curr Opin Rheumatol. 2016;28:66–75. doi: 10.1097/BOR.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 13.Sudol-Szopinska I, Matuszewska G, Kwiatkowska B, Pracon G. Diagnostic imaging of psoriatic arthritis. Part I: etiopathogenesis, classifications and radiographic features. J Ultrason. 2016;16:65–77. doi: 10.15557/JoU.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright V. Seronegative polyarthritis: a unified concept. Arthritis Rheum. 1978;21:619–633. doi: 10.1002/art.1780210603. [DOI] [PubMed] [Google Scholar]
- 15.McGonagle D. Imaging the joint and enthesis: insights into pathogenesis of psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii58–60. doi: 10.1136/ard.2004.034264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldring SR. Differential mechanisms of de-regulated bone formation in rheumatoid arthritis and spondyloarthritis. Rheumatology (Oxford) 2016;55:ii56–ii60. doi: 10.1093/rheumatology/kew345. [DOI] [PubMed] [Google Scholar]
- 17.Szentpetery A, Heffernan E, Haroon M, Kilbane M, Gallagher P, McKenna MJ, FitzGerald O. Striking difference of periarticular bone density change in early psoriatic arthritis and rheumatoid arthritis following anti-rheumatic treatment as measured by digital X-ray radiogrammetry. Rheumatology (Oxford) 2016;55:891–896. doi: 10.1093/rheumatology/kev443. [DOI] [PubMed] [Google Scholar]
- 18.Finzel S, Englbrecht M, Engelke K, Stach C, Schett G. A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann Rheum Dis. 2011;70:122–127. doi: 10.1136/ard.2010.132423. [DOI] [PubMed] [Google Scholar]
- 19.Ogdie A, Harter L, Shin D, Baker J, Takeshita J, Choi HK, Love TJ, Gelfand JM. The risk of fracture among patients with psoriatic arthritis and psoriasis: a population-based study. Ann Rheum Dis. 2017;76:882–885. doi: 10.1136/annrheumdis-2016-210441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy SM, Anandarajah AP, Fisher MC, Mease PJ, Greenberg JD, Kremer JM, Reed G, Chen R, Messing S, Kaukeinen K, et al. Comparative analysis of disease activity measures, use of biologic agents, body mass index, radiographic features, and bone density in psoriatic arthritis and rheumatoid arthritis patients followed in a large U.S. disease registry. J Rheumatol. 2010;37:2566–2572. doi: 10.3899/jrheum.100483. [DOI] [PubMed] [Google Scholar]
- 21.Modalsli EH, Asvold BO, Romundstad PR, Langhammer A, Hoff M, Forsmo S, Naldi L, Saunes M. Psoriasis, fracture risk and bone mineral density: the HUNT Study, Norway. Br J Dermatol. 2017;176:1162–1169. doi: 10.1111/bjd.15123. [DOI] [PubMed] [Google Scholar]
- 22.Chandran S, Aldei A, Johnson SR, Cheung AM, Salonen D, Gladman DD. Prevalence and risk factors of low bone mineral density in psoriatic arthritis: A systematic review. Semin Arthritis Rheum. 2016;46:174–182. doi: 10.1016/j.semarthrit.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, Nakashima T, Takayanagi H. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis. 2016;75:1187–1195. doi: 10.1136/annrheumdis-2014-207137. [DOI] [PubMed] [Google Scholar]
- 24.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–831. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin TE, ElFar NN, Ghaly NR, Hekal MM, Hassan AM, Elsaadany HM. Serum level of receptor activator of nuclear factor kappa-B ligand in patients with psoriasis. Int J Dermatol. 2016;55:e227–233. doi: 10.1111/ijd.13159. [DOI] [PubMed] [Google Scholar]
- 26.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao B, Grimes SN, Li S, Hu X, Ivashkiv LB. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J Exp Med. 2012;209:319–334. doi: 10.1084/jem.20111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarilina A, Xu K, Chen J, Ivashkiv LB. TNF activates calcium-nuclear factor of activated T cells (NFAT)c1 signaling pathways in human macrophages. Proc Natl Acad Sci U S A. 2011;108:1573–1578. doi: 10.1073/pnas.1010030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Z, Li P, Zhang Q, Schwarz EM, Keng P, Arbini A, Boyce BF, Xing L. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem. 2006;281:11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 30.Partsch G, Wagner E, Leeb BF, Dunky A, Steiner G, Smolen JS. Upregulation of cytokine receptors sTNF-R55, sTNF-R75, and sIL-2R in psoriatic arthritis synovial fluid. J Rheumatol. 1998;25:105–110. [PubMed] [Google Scholar]
- 31.Anandarajah AP, Schwarz EM, Totterman S, Monu J, Feng CY, Shao T, Haas-Smith SA, Ritchlin CT. The effect of etanercept on osteoclast precursor frequency and enhancing bone marrow oedema in patients with psoriatic arthritis. Ann Rheum Dis. 2008;67:296–301. doi: 10.1136/ard.2007.076091. [DOI] [PubMed] [Google Scholar]
- 32.Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, Smith M, Thomas R, Gaston H. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther. 2013;15:R136. doi: 10.1186/ar4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng L, Wang W, Ni J, Mao X, Song D, Liu T, Wei J, Zhou H. Role of autophagy in tumor necrosis factor-alpha-induced apoptosis of osteoblast cells. J Investig Med. 2017;65:1014–1020. doi: 10.1136/jim-2017-000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yago T, Nanke Y, Ichikawa N, Kobashigawa T, Mogi M, Kamatani N, Kotake S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: a novel mechanism of osteoclastogenesis by IL-17. J Cell Biochem. 2009;108:947–955. doi: 10.1002/jcb.22326. [DOI] [PubMed] [Google Scholar]
- 36.Kitami S, Tanaka H, Kawato T, Tanabe N, Katono-Tani T, Zhang F, Suzuki N, Yonehara Y, Maeno M. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie. 2010;92:398–404. doi: 10.1016/j.biochi.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 37.van der Heijde D, Landewe RB, Mease PJ, McInnes IB, Conaghan PG, Pricop L, Ligozio G, Richards HB, Mpofu S. Brief Report: Secukinumab Provides Significant and Sustained Inhibition of Joint Structural Damage in a Phase III Study of Active Psoriatic Arthritis. Arthritis Rheumatol. 2016;68:1914–1921. doi: 10.1002/art.39685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Kuijk AW, Reinders-Blankert P, Smeets TJ, Dijkmans BA, Tak PP. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. 2006;65:1551–1557. doi: 10.1136/ard.2005.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celis R, Planell N, Fernandez-Sueiro JL, Sanmarti R, Ramirez J, Gonzalez-Alvaro I, Pablos JL, Canete JD. Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features. Arthritis Res Ther. 2012;14:R93. doi: 10.1186/ar3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 41.Ogata A, Kumanogoh A, Tanaka T. Pathological role of interleukin-6 in psoriatic arthritis. Arthritis. 2012;2012:713618. doi: 10.1155/2012/713618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mease PJ, Gottlieb AB, Berman A, Drescher E, Xing J, Wong R, Banerjee S. The Efficacy and Safety of Clazakizumab, an Anti-Interleukin-6 Monoclonal Antibody, in a Phase IIb Study of Adults With Active Psoriatic Arthritis. Arthritis Rheumatol. 2016;68:2163–2173. doi: 10.1002/art.39700. [DOI] [PubMed] [Google Scholar]
- 43.Yokota K, Sato K, Miyazaki T, Kitaura H, Kayama H, Miyoshi F, Araki Y, Akiyama Y, Takeda K, Mimura T. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014;66:121–129. doi: 10.1002/art.38218. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, Aliprantis AO, Charles JF. RANK-Independent Osteoclast Formation and Bone Erosion in Inflammatory Arthritis. Arthritis Rheumatol. 2016;68:2889–2900. doi: 10.1002/art.39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polachek A, Li S, Chandran V, Gladman DD. Clinical Enthesitis in a Prospective Longitudinal Psoriatic Arthritis Cohort: Incidence, Prevalence, Characteristics, and Outcome. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.23174. [DOI] [PubMed] [Google Scholar]
- 46.Gladman DD. Clinical, radiological, and functional assessment in psoriatic arthritis: is it different from other inflammatory joint diseases? Ann Rheum Dis. 2006;65(Suppl 3):iii22–24. doi: 10.1136/ard.2006.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G, Jobling A, Shaddick G, Bi J, Winchester R, et al. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis. 2017;76:701–707. doi: 10.1136/annrheumdis-2016-209853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ory PA, Gladman DD, Mease PJ. Psoriatic arthritis and imaging. Ann Rheum Dis. 2005;64(Suppl 2):ii55–57. doi: 10.1136/ard.2004.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schett G, Coates LC, Ash ZR, Finzel S, Conaghan PG. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res Ther. 2011;13(Suppl 1):S4. doi: 10.1186/1478-6354-13-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan TL, Chen J, Tong YL, Zhang Y, Liu YY, Wei JC, Liu Y, Zhao Y, Herrmann M. Serum Heme Oxygenase-1 and BMP-7 Are Potential Biomarkers for Bone Metabolism in Patients with Rheumatoid Arthritis and Ankylosing Spondylitis. Biomed Res Int. 2016;2016:7870925. doi: 10.1155/2016/7870925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–1579. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abd-Elsalam N, Kamel N, Zamzam M, El-Hilaly R, Sobhib M, Sabryc M. The relation between serum bone morphogenetic protein-7 and severity of enthesitis in psoriatic arthritis. Egyptian Rheumatology and Rehabilitation. 2013;40:129–133. [Google Scholar]
- 54.Lories RJ, Matthys P, de Vlam K, Derese I, Luyten FP. Ankylosing enthesitis, dactylitis, and onychoperiostitis in male DBA/1 mice: a model of psoriatic arthritis. Ann Rheum Dis. 2004;63:595–598. doi: 10.1136/ard.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 57.Rossini M, Viapiana O, Adami S, Fracassi E, Idolazzi L, Dartizio C, Povino MR, Orsolini G, Gatti D. In patients with rheumatoid arthritis, Dickkopf-1 serum levels are correlated with parathyroid hormone, bone erosions and bone mineral density. Clin Exp Rheumatol. 2015;33:77–83. [PubMed] [Google Scholar]
- 58.Heiland GR, Appel H, Poddubnyy D, Zwerina J, Hueber A, Haibel H, Baraliakos X, Listing J, Rudwaleit M, Schett G, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:572–574. doi: 10.1136/annrheumdis-2011-200216. [DOI] [PubMed] [Google Scholar]
- 59.Fassio A, Idolazzi L, Viapiana O, Benini C, Vantaggiato E, Bertoldo F, Rossini M, Gatti D. In psoriatic arthritis Dkk-1 and PTH are lower than in rheumatoid arthritis and healthy controls. Clin Rheumatol. 2017 doi: 10.1007/s10067-017-3734-2. [DOI] [PubMed] [Google Scholar]
- 60.Dalbeth N, Pool B, Smith T, Callon KE, Lobo M, Taylor WJ, Jones PB, Cornish J, McQueen FM. Circulating mediators of bone remodeling in psoriatic arthritis: implications for disordered osteoclastogenesis and bone erosion. Arthritis Res Ther. 2010;12:R164. doi: 10.1186/ar3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, Yiannopoulos G, Andonopoulos AP. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010;62:150–158. doi: 10.1002/art.27231. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 64.Lu X, Gilbert L, He X, Rubin J, Nanes MS. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NF kappa B pathways. J Biol Chem. 2006;281:6297–6306. doi: 10.1074/jbc.M507804200. [DOI] [PubMed] [Google Scholar]
- 65.Ghali O, Chauveau C, Hardouin P, Broux O, Devedjian JC. TNF-alpha's effects on proliferation and apoptosis in human mesenchymal stem cells depend on RUNX2 expression. J Bone Miner Res. 2010;25:1616–1626. doi: 10.1002/jbmr.52. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. Am J Physiol Endocrinol Metab. 2005;288:E1011–1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- 67.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45:367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 68.Lu Z, Wang G, Dunstan CR, Zreiqat H. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem Cells Dev. 2012;21:2420–2429. doi: 10.1089/scd.2011.0589. [DOI] [PubMed] [Google Scholar]
- 69.Marupanthorn K, Tantrawatpan C, Tantikanlayaporn D, Kheolamai P, Manochantr S. The Effects of TNF-alpha on Osteogenic Differentiation of Umbilical Cord Derived Mesenchymal Stem Cells. J Med Assoc Thai. 2015;98(Suppl 3):S34–40. [PubMed] [Google Scholar]
- 70.Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, Kim CD, Jung JS. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168–177. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 71.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J, Yang P. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44:420–427. doi: 10.1111/j.1365-2184.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z, Jia Y, Du F, Chen M, Dong X, Chen Y, Huang W. IL-17A Inhibits Osteogenic Differentiation of Bone Mesenchymal Stem Cells via Wnt Signaling Pathway. Med Sci Monit. 2017;23:4095–4101. doi: 10.12659/MSM.903027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang JR, Pang DD, Tong Q, Liu X, Su DF, Dai SM. Different Modulatory Effects of IL-17, IL-22, and IL-23 on Osteoblast Differentiation. Mediators Inflamm. 2017;2017:5950395. doi: 10.1155/2017/5950395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osta B, Lavocat F, Eljaafari A, Miossec P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Front Immunol. 2014;5:425. doi: 10.3389/fimmu.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Croes M, Oner FC, van Neerven D, Sabir E, Kruyt MC, Blokhuis TJ, Dhert WJ, Alblas J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–270. doi: 10.1016/j.bone.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, Lee Y, Kim HH. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 78.Huang W, La Russa V, Alzoubi A, Schwarzenberger P. Interleukin-17A: a T-cell-derived growth factor for murine and human mesenchymal stem cells. Stem Cells. 2006;24:1512–1518. doi: 10.1634/stemcells.2005-0156. [DOI] [PubMed] [Google Scholar]
- 79.Kocic J, Santibanez JF, Krstic A, Mojsilovic S, Dordevic IO, Trivanovic D, Ilic V, Bugarski D. Interleukin 17 inhibits myogenic and promotes osteogenic differentiation of C2C12 myoblasts by activating ERK1,2. Biochim Biophys Acta. 2012;1823:838–849. doi: 10.1016/j.bbamcr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Nam D, Mau E, Wang Y, Wright D, Silkstone D, Whetstone H, Whyne C, Alman B. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS One. 2012;7:e40044. doi: 10.1371/journal.pone.0040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw AT, Maeda Y, Gravallese EM. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res Ther. 2016;18:104. doi: 10.1186/s13075-016-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uluckan O, Jimenez M, Karbach S, Jeschke A, Grana O, Keller J, Busse B, Croxford AL, Finzel S, Koenders M, et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci Transl Med. 2016;8:330ra337. doi: 10.1126/scitranslmed.aad8996. [DOI] [PubMed] [Google Scholar]
- 83.Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, Takayanagi H. IL-17-producing gammadelta T cells enhance bone regeneration. Nat Commun. 2016;7:10928. doi: 10.1038/ncomms10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 85.El-Zayadi AA, Jones EA, Churchman SM, Baboolal TG, Cuthbert RJ, El-Jawhari JJ, Badawy AM, Alase AA, El-Sherbiny YM, McGonagle D. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford) 2017;56:488–493. doi: 10.1093/rheumatology/kew384. [DOI] [PubMed] [Google Scholar]
- 86.O'Rielly DD, Rahman P. Genetic, Epigenetic and Pharmacogenetic Aspects of Psoriasis and Psoriatic Arthritis. Rheum Dis Clin North Am. 2015;41:623–642. doi: 10.1016/j.rdc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 88.FitzGerald O, Haroon M, Giles JT, Winchester R. Concepts of pathogenesis in psoriatic arthritis: genotype determines clinical phenotype. Arthritis Res Ther. 2015;17:115. doi: 10.1186/s13075-015-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bowes J, Ashcroft J, Dand N, Jalali-Najafabadi F, Bellou E, Ho P, Marzo-Ortega H, Helliwell PS, Feletar M, Ryan AW, et al. Cross-phenotype association mapping of the MHC identifies genetic variants that differentiate psoriatic arthritis from psoriasis. Ann Rheum Dis. 2017;76:1774–1779. doi: 10.1136/annrheumdis-2017-211414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogdie A, Weiss P. The Epidemiology of Psoriatic Arthritis. Rheum Dis Clin North Am. 2015;41:545–568. doi: 10.1016/j.rdc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raimondo A, Lembo S, Di Caprio R, Donnarumma G, Monfrecola G, Balato N, Ayala F, Balato A. Psoriatic cutaneous inflammation promotes human monocyte differentiation into active osteoclasts, facilitating bone damage. Eur J Immunol. 2017;47:1062–1074. doi: 10.1002/eji.201646774. [DOI] [PubMed] [Google Scholar]
- 92.Yamamoto M, Nakajima K, Takaishi M, Kitaba S, Magata Y, Kataoka S, Sano S. Psoriatic inflammation facilitates the onset of arthritis in a mouse model. J Invest Dermatol. 2015;135:445–453. doi: 10.1038/jid.2014.426. [DOI] [PubMed] [Google Scholar]
- 93.Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231–236. doi: 10.1016/j.clindermatol.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Punzi L, Pianon M, Bertazzolo N, Fagiolo U, Rizzi E, Rossini P, Todesco S. Clinical, laboratory and immunogenetic aspects of post-traumatic psoriatic arthritis: a study of 25 patients. Clin Exp Rheumatol. 1998;16:277–281. [PubMed] [Google Scholar]
- 95.McGonagle D, Ash Z, Dickie L, McDermott M, Aydin SZ. The early phase of psoriatic arthritis. Ann Rheum Dis. 2011;70(Suppl 1):i71–76. doi: 10.1136/ard.2010.144097. [DOI] [PubMed] [Google Scholar]
- 96.Thorarensen SM, Lu N, Ogdie A, Gelfand JM, Choi HK, Love TJ. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Ann Rheum Dis. 2017;76:521–525. doi: 10.1136/annrheumdis-2016-209334. [DOI] [PubMed] [Google Scholar]
- 97.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horta-Baas G, Romero-Figueroa MDS, Montiel-Jarquin AJ, Pizano-Zarate ML, Garcia-Mena J, Ramirez-Duran N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J Immunol Res. 2017;2017:4835189. doi: 10.1155/2017/4835189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diamanti AP, Manuela Rosado M, Lagana B, D'Amelio R. Microbiota and chronic inflammatory arthritis: an interwoven link. J Transl Med. 2016;14:233. doi: 10.1186/s12967-016-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manasson J, Scher JU. Spondyloarthritis and the microbiome: new insights from an ancient hypothesis. Curr Rheumatol Rep. 2015;17:10. doi: 10.1007/s11926-014-0487-7. [DOI] [PubMed] [Google Scholar]
- 102.Scher JU, Littman DR, Abramson SB. Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol. 2016;68:35–45. doi: 10.1002/art.39259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Costello ME, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, Marshall M, Kenna TJ, Triolo G, Brown MA. Brief Report: Intestinal Dysbiosis in Ankylosing Spondylitis. Arthritis Rheumatol. 2015;67:686–691. doi: 10.1002/art.38967. [DOI] [PubMed] [Google Scholar]
- 104.Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, Marmon S, Neimann A, Brusca S, Patel T, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ciccia F, Guggino G, Ferrante A, Raimondo S, Bignone R, Rodolico V, Peralta S, Van Tok M, Cannizzaro A, Schinocca C, et al. Interleukin-9 Overexpression and Th9 Polarization Characterize the Inflamed Gut, the Synovial Tissue, and the Peripheral Blood of Patients With Psoriatic Arthritis. Arthritis Rheumatol. 2016;68:1922–1931. doi: 10.1002/art.39649. [DOI] [PubMed] [Google Scholar]
- 106.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kundu-Raychaudhuri S, Abria C, Raychaudhuri SP. IL-9, a local growth factor for synovial T cells in inflammatory arthritis. Cytokine. 2016;79:45–51. doi: 10.1016/j.cyto.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 108.Love TJ, Zhu Y, Zhang Y, Wall-Burns L, Ogdie A, Gelfand JM, Choi HK. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71:1273–1277. doi: 10.1136/annrheumdis-2012-201299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gladman DD, Callen JP. Early-onset obesity and risk for psoriatic arthritis. JAMA. 2010;304:787–788. doi: 10.1001/jama.2010.1162. [DOI] [PubMed] [Google Scholar]
- 110.di Minno MN, Peluso R, Iervolino S, Lupoli R, Russolillo A, Scarpa R, di Minno G. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res (Hoboken) 2013;65:141–147. doi: 10.1002/acr.21711. [DOI] [PubMed] [Google Scholar]
- 111.Eder L, Thavaneswaran A, Chandran V, Cook RJ, Gladman DD. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis. 2015;74:813–817. doi: 10.1136/annrheumdis-2013-204448. [DOI] [PubMed] [Google Scholar]
- 112.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 113.Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 114.Eder L, Jayakar J, Pollock R, Pellett F, Thavaneswaran A, Chandran V, Rosen CF, Gladman DD. Serum adipokines in patients with psoriatic arthritis and psoriasis alone and their correlation with disease activity. Ann Rheum Dis. 2013;72:1956–1961. doi: 10.1136/annrheumdis-2012-202325. [DOI] [PubMed] [Google Scholar]
- 115.Urruticoechea-Arana A, Martin-Martinez MA, Castaneda S, Piedra CA, Gonzalez-Juanatey C, Llorca J, Diaz-Gonzalez F, Gonzalez-Gay MA Group CPC. Vitamin D deficiency in chronic inflammatory rheumatic diseases: results of the cardiovascular in rheumatology [CARMA] study. Arthritis Res Ther. 2015;17:211. doi: 10.1186/s13075-015-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67:769–774. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 117.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56:3575–3582. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 118.Vidal C, Barnetche T, Morel J, Combe B, Daien C. Association of Body Mass Index Categories with Disease Activity and Radiographic Joint Damage in Rheumatoid Arthritis: A Systematic Review and Metaanalysis. J Rheumatol. 2015;42:2261–2269. doi: 10.3899/jrheum.150224. [DOI] [PubMed] [Google Scholar]
- 119.Klein-Wieringa IR, van der Linden MP, Knevel R, Kwekkeboom JC, van Beelen E, Huizinga TW, van der Helm-van Mil A, Kloppenburg M, Toes RE, Ioan-Facsinay A. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2011;63:2567–2574. doi: 10.1002/art.30449. [DOI] [PubMed] [Google Scholar]
- 120.Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1562–1568. doi: 10.1136/ard.2011.150813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ebina K, Fukuhara A, Ando W, Hirao M, Koga T, Oshima K, Matsuda M, Maeda K, Nakamura T, Ochi T, et al. Serum adiponectin concentrations correlate with severity of rheumatoid arthritis evaluated by extent of joint destruction. Clin Rheumatol. 2009;28:445–451. doi: 10.1007/s10067-008-1074-y. [DOI] [PubMed] [Google Scholar]
- 122.Li W, Han J, Qureshi AA. Smoking and risk of incident psoriatic arthritis in US women. Ann Rheum Dis. 2012;71:804–808. doi: 10.1136/annrheumdis-2011-200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tillett W, Jadon D, Shaddick G, Cavill C, Korendowych E, de Vries CS, McHugh N. Smoking and delay to diagnosis are associated with poorer functional outcome in psoriatic arthritis. Ann Rheum Dis. 2013;72:1358–1361. doi: 10.1136/annrheumdis-2012-202608. [DOI] [PubMed] [Google Scholar]
- 124.Hojgaard P, Glintborg B, Hetland ML, Hansen TH, Lage-Hansen PR, Petersen MH, Holland-Fischer M, Nilsson C, Loft AG, Andersen BN, et al. Association between tobacco smoking and response to tumour necrosis factor alpha inhibitor treatment in psoriatic arthritis: results from the DANBIO registry. Ann Rheum Dis. 2015;74:2130–2136. doi: 10.1136/annrheumdis-2014-205389. [DOI] [PubMed] [Google Scholar]
- 125.Petrescu F, Voican SC, Silosi I. Tumor necrosis factor-alpha serum levels in healthy smokers and nonsmokers. Int J Chron Obstruct Pulmon Dis. 2010;5:217–222. doi: 10.2147/copd.s8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110:1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bond SJ, Farewell VT, Schentag CT, Gladman DD. Predictors for radiological damage in psoriatic arthritis: results from a single centre. Ann Rheum Dis. 2007;66:370–376. doi: 10.1136/ard.2006.056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kavanaugh A, Krueger GG, Beutler A, Guzzo C, Zhou B, Dooley LT, Mease PJ, Gladman DD, de Vlam K, Geusens PP, et al. Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis. 2007;66:498–505. doi: 10.1136/ard.2006.058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van der Heijde D, Kavanaugh A, Gladman DD, Antoni C, Krueger GG, Guzzo C, Zhou B, Dooley LT, de Vlam K, Geusens P, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: Results from the induction and maintenance psoriatic arthritis clinical trial 2. Arthritis Rheum. 2007;56:2698–2707. doi: 10.1002/art.22805. [DOI] [PubMed] [Google Scholar]
- 130.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Tsuji W. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–2272. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 131.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Dunn M, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol. 2006;33:712–721. [PubMed] [Google Scholar]
- 132.Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–3289. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 133.Gladman DD, Mease PJ, Cifaldi MA, Perdok RJ, Sasso E, Medich J. Adalimumab improves joint-related and skin-related functional impairment in patients with psoriatic arthritis: patient-reported outcomes of the Adalimumab Effectiveness in Psoriatic Arthritis Trial. Ann Rheum Dis. 2007;66:163–168. doi: 10.1136/ard.2006.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, Hsia EC. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: Results through week 24 of the GO-VIBRANT study. Arthritis Rheumatol. 2017 doi: 10.1002/art.40226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann Rheum Dis. 2014;73:48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kavanaugh A, Ritchlin C, Rahman P, Puig L, Gottlieb AB, Li S, Wang Y, Noonan L, Brodmerkel C, Song M, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000–1006. doi: 10.1136/annrheumdis-2013-204741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, Wang Y, Shen YK, Doyle MK, Mendelsohn AM, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–999. doi: 10.1136/annrheumdis-2013-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewe R, Conaghan PG, Gottlieb AB, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 139.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewe R, Nash P, Pricop L, Yuan J, et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med. 2015;373:1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 140.Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, Lin CY, Braun DK, Lee CH, Gladman DD, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, Cieslak D, Graham D, Wang C, Menon S, et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. N Engl J Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 142.Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, Kudlacz E, Wang C, Menon S, Hendrikx T, et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N Engl J Med. 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 143.Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. N Engl J Med. 2016;375:1303. doi: 10.1056/NEJMc1609622. [DOI] [PubMed] [Google Scholar]
- 144.McEwen C, DiTata D, Lingg C, Porini A, Good A, Rankin T. Ankylosing spondylitis and spondylitis accompanying ulcerative colitis, regional enteritis, psoriasis and Reiter's disease. A comparative study. Arthritis Rheum. 1971;14:291–318. doi: 10.1002/art.1780140302. [DOI] [PubMed] [Google Scholar]
- 145.Gladman DD, Brubacher B, Buskila D, Langevitz P, Farewell VT. Differences in the expression of spondyloarthropathy: a comparison between ankylosing spondylitis and psoriatic arthritis. Clin Invest Med. 1993;16:1–7. [PubMed] [Google Scholar]
- 146.Helliwell PS, Hickling P, Wright V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis. 1998;57:135–140. doi: 10.1136/ard.57.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]