Abstract
As antibodies continue to gain predominance in drug discovery and development pipelines, efforts to control and optimize their activity in vivo have matured to incorporate sophisticated abilities to manipulate engagement of specific Fc binding partners. Such efforts to promote diverse functional outcomes include modulating IgG-Fc affinity for FcγRs to alternatively potentiate or reduce effector functions, such as antibody-dependent cellular cytotoxicity and phagocytosis. While a number of natural and engineered Fc features capable of eliciting variable effector functions have been demonstrated in vitro and in vivo, elucidation of these important functional relationships has taken significant effort through use of diverse genetic, cellular and enzymatic techniques. As an orthogonal approach, we demonstrate use of FcγR as chromatographic affinity ligands to enrich and therefore simultaneously identify favored binding species from a complex mixture of serum-derived pooled polycloncal human IgG, a load material that contains the natural repertoire of Fc variants and post-translational modifications. The FcγR-enriched IgG was characterized for subclass and glycoform composition and the impact of this bioseparation step on antibody activity was measured in cell-based effector function assays including Natural Killer cell activation and monocyte phagocytosis. This work demonstrates a tractable means to rapidly distinguish complex functional relationships between two or more interacting biological agents by leveraging affinity chromatography followed by secondary analysis with high-resolution biophysical and functional assays and emphasizes a platform capable of surveying diverse natural post-translational modifications that may not be easily produced with high purity or easily accessible with recombinant expression techniques.
Keywords: IgG, antibody, post-translational modification, glycosylation, Fc Receptor
Introduction
Antibodies are a growing therapeutic class addressing a number of diseases including autoimmunity, cancer and pathogenic infection. While antigen binding is important for IgG efficacy, in vivo studies have also exposed the role of IgG-Fc engaging FcγR on immune effector cells to provide therapeutic benefit for a number of diseases (Ferrant et al. 2004, Hessell et al. 2007, Hessell et al. 2009, Abboud et al. 2010, Pal et al. 2013, DiLillo et al. 2014). Notably, additional in vivo studies support a direct link between enhanced IgG-FcγR affinity and improved therapeutic efficacy (Cartron et al. 2002, Olinger et al. 2012, Bournazos et al. 2014, Varshney et al. 2014) revealing an opportunity to develop enhanced therapeutics through a comprehensive understanding of tunable IgG Fc features that modulate the IgG-FcγR interaction.
In humans, FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa and FcγRIIIb are differentially expressed on immune cells including macrophages, neutrophils, and natural killer (NK) cells, which engage in variable effector functions such as antibody dependent cell-mediated cytotoxicity (ADCC), phagocytosis (ADCP) and virus inhibition (ADCVI) (recently reviewed(Boesch et al. 2015)). FcγRI, FcγRIIa (allotypes H/R131) and FcγRIIIa (allotypes V/F158) are activating receptors while FcγRIIb is an inhibitory receptor. FcγRIIc is an activating receptor with the extracellular domain identical to FcγRIIb and an intracellular domain identical to FcγRIIa. FcγRIIIb (allotypes NA1/NA2/SH), in contrast to the other FcγR, lacks an intracellular domain and can exhibit either activating or inhibitory behavior. Functional relationships between receptor engagement and cellular activity are sophisticated given FcγR are structurally diverse, have variable preferences for IgG subclasses and their expression profiles are cell type dependent. Additionally, the ratio of IgG crosslinking with activating versus inhibitory receptors on a single cell can potentiate the cellular activity and actuated effector function (Boruchov et al. 2005).
The specificity of IgG subclasses for each FcγR has been previously investigated using effector cells isolated from human blood (Vance et al. 1993), FcγR-transfected cell lines (Bruhns et al. 2009) and by surface plasmon resonance using recombinant FcγR extracellular domains against serum derived (Bruhns et al. 2009) or recombinant (Warncke et al. 2012) IgG subclasses. These studies have revealed that IgGs differentially bind FcγR. For instance, IgG2 lacks affinity for FcγRI and demonstrates weaker binding towards the FcγRIIa R131 versus H131 allotype (Warncke et al. 2012) while IgG1 and IgG3 exhibit preferential binding to FcγRIIIa (Vance et al. 1993). Beyond subclass, engineered cell lines have been used to probe glycoform specificity for FcγR where IgG-Fc glycans that lack Fucose (Shields et al. 2002, Shibata-Koyama et al. 2009) or contain a bisecting GlcNac (Umana et al. 1999), which exhibit improved binding towards FcγRIII, independent of subclass (Niwa et al. 2005). Antibodies with variable fucose and bisected glycan content have also been produced using cell culture additives (Kanda et al. 2007) or enzymatic techniques (Hodoniczky et al. 2005, Wei et al. 2008, Zou et al. 2011, Lin et al. 2015). Importantly, the differential binding of IgG subclasses and glycoforms to FcγR has shown a direct link between Fc features and biological activity in vitro; high affinity IgGs more effectively actuate ADCC (Umana et al. 1999, Shields et al. 2002, Niwa et al. 2005) and phagocytosis (Shibata-Koyama et al. 2009, Goh et al. 2011). Similar observations have been made from associations with outcomes in vaccination, post-exposure treatment and disease pathogenesis studies in vivo (Olinger et al. 2012, Ackerman et al. 2013, Rombouts et al. 2015).
As an alternative to genetic or cellular techniques to examine IgG-FcγR functional relationships, proteins with specificity towards IgG-Fc or Fc glycans have been immobilized on chromatography media and used as affinity ligands to enrich high affinity species, sometimes in combination with in vitro enzymatic techniques. Fc receptors such as protein A have been employed as affinity ligands to differentially separate IgG subclasses from serum derived IgG (Hjelm et al. 1972, Duhamel et al. 1979, Martin 1982); however, protein A does not have selectivity for IgG Fc glycans (Nose and Wigzell 1983). To separate IgGs by Fc glycoforms, researchers have employed carbohydrate-binding lectins to enrich IgG Fcs that are sialylated (Kaneko et al. 2006), fucosylated (Shinkawa et al. 2003, Tojo et al. 2009) or mannosylated (Tojo et al. 2009). More recently, human FcγRIII and FcγRIIa-based affinity chromatography has demonstrated the ability to separate nonfucosylated or galactosylated IgG1 (Bolton et al. 2013, Dashivets et al. 2015, Thomann et al. 2015).
To expand on these previous studies employing genetic, cellular, enzymatic or purification strategies to control or enrich IgG-Fc features, we investigate the ability of all known human FcγR and their respective allotypes to simultaneously isolate high affinity subclasses and glycoforms from multi-donor pooled human serum IgG, a polyclonal starting material representative of the natural human Fc repertoire. The enriched material was further characterized for subclass and glycoform composition. To compare experimental results with expectations from binding models, we also utilized the Langmuir competitive binding model (Weber and DiGiano 1996, Mahamadi and Nharingo 2010) to calculate chromatographic enrichment factors and compare to our results as well as predict serum IgG subclass occupancy of FcγR on effector cells in vivo. Functional relationships of the load and FcγR-enriched IgG were examined by evaluating NK cell activation and monocyte-mediated phagocytosis. These tests identified FcγRIII as the most effective separation ligand for enhancing these effector functions, an activity associated with its ability to enrich IgG3 and nonfucosylated/bisected IgG-Fc glycoforms.
Materials and Methods
Protein expression and purification
Human FcγR expression vectors were prepared as previously described (Boesch et al. 2014). HEK-293F cells were transfected with FcγR vectors using 25 kD branched PEI (Polyscience) and cultured for 7 days in Freestyle serum free media (Invitrogen) at 37°C with 8% CO2. Cells were separated by centrifugation and the supernatant filtered (Steritop Express, Millipore). The cell free broth was purified over a nickel charged Sepharose 4 Fast Flow column (GE) using an AKTA FPLC. The eluates were filtered (Steriflip, EMD-Millipore), concentrated with a 10 kD membrane (Amicon Ultra-15, EMD-Millipore) and aggregates were removed with a Superdex 75 (GE) size exclusion chromatography (SEC) column.
The VRC01 IgG1 wild type protein, the genetically aglycosylated N297Q mutant, the oligomannose/nonfucosylated Fc glycovariant, and subclass-switched variants were produced as previously described (Boesch et al. 2014, Brown et al. 2017). Protein A was used to purify IgG1, IgG2 and IgG4 and protein G for IgG3 using an AKTA FPLC (GE). The eluates were titrated with 1 M Tris pH 8.5 to neutralize the pH. The neutralized peaks were filtered (Steriflip, EMD-Millipore), concentrated with a 30 kD membrane (Amicon Ultra-15, EMD-Millipore) and aggregates were removed with a Sephacryl S-200/HR (GE) SEC column.
The pPPI4 vector encoding JR-FL SOSIP gp140 and the pcDNA3.1-Furin vector were kind gifts of Dr. Rogier Sanders (Binley et al. 2000, Sanders et al. 2000). The gp140 and furin vectors were co-transfected into HEK293F as described above. The soluble gp140 was purified over a custom affinity 5 ml NHS-activated Sepharose 4 Fast Flow chromatography column (GE) produced under the manufacturers recommendation by coupling 20 mg wild type VRC01 IgG1 to the resin for use as a functional gp140 affinity ligand. The VRC01 column was loaded with cell free gp140 broth at neutral pH using an AKTA FPLC (GE). gp140 was eluted with 100 mM glycine pH 2.7 and neutralized with 1 M Tris pH 8.5. Column flow through was reloaded until no peak was visible by A280. The neutralized eluates were filtered (Steriflip, EMD-Millipore), concentrated with a 30 kD membrane (Amicon Ultra-15, EMD-Millipore) and aggregates were removed with a Sephacryl S-200/HR (GE) SEC column. After use the VRC01 affinity column was stored in PBS with 20% ethanol at 4°C for up to 12 months without loss of activity.
Fc receptor affinity chromatography
1 or 5 ml NHS activated Sepharose 4 Fast Flow prepacked columns (GE) were produced under the manufacturers recommendations by coupling 1.75-4 mg of FcγR in PBS per ml of NHS resin. Each FcγR column was equilibrated with PBS. IgG from pooled human serum (Athens Research) was diluted to 1-2 mg/ml with PBS. 20 mg of IgG was loaded onto each FcγR column at a rate of approximately 1 ml/min. A wash with PBS was used to remove any weakly binding IgG. Tightly bound IgG was eluted from the columns using 100 mM glycine pH 3.0. The elution pools were neutralized with 1M MES at pH 8.5. The peaks were filtered (Steriflip, EMD-Millipore), concentrated with a 10 kD membrane (Amicon Ultra-15, EMD-Millipore) and buffer exchanged by concentration/dilution by five 15 ml washes using PBS. The aggregation state of FcγR load and eluates was analyzed using an SRT-150 analytical SEC column (Sepax Technologies) on an HPLC system (Agilent Technologies) under the manufacturer’s recommended conditions. The FcγR I column lost binding activity after 1 cycle and was not used in further analysis. The other FcγR columns did not lose activity after 1 or more cycles. After use, FcγR columns were stored in PBS with 20% ethanol at 4 °C for up to 12 months without loss of activity.
IgG subclass characterization
A quantitative multiplexed bead-based assay was used to measure the concentration and proportion of each subclass in the FcγR column loads and eluates using an adaptation of a method described previously (Brown et al. 2012). Briefly, subclass specific capture antibodies (Southern Biotech: anti-IgG JDC-10, anti-IgG2 31-7-4, anti-IgG4 HP6025; Invitrogen: anti-IgG1 A10630, anti-IgG3 053600) were coupled to magnetic fluorescent beads (Luminex). A master mix of subclass specific beads was prepared by combining individual bead sets to achieve a final count of 500 microspheres of each specificity in each well of a 384-well plate (Greiner). Sample was first added to the wells followed by the bead master mix for a total of 50 μl in PBS. The plate was covered, submerged in a sonicator for 15 seconds and then incubated on a XYZ-plane plate shaker at room temperature for 2 hr. The plate was washed five times with 60 μl of PBS 0.05% tween-20 (PBS-T) (Thermo) using a plate washer (Biotek). Subclass specific anti-human IgG PE-conjugated detection antibody (Southern Biotech: anti-IgG 9040-09, anti-IgG1 9052-09, anti-IgG2 9070-09, anti-IgG3 9210-09, anti-IgG4 9200-09) was diluted to 0.65 μg/ml and 40 μl was added to each well. The plate was covered, sonicated and incubated for 30 min at room temperature on a XYZ-plane plate shaker. The beads were washed fives times with PBS-T as before, and after the washes, the microspheres were resuspended in 35 μl of Luminex Sheath Fluid. The plate was covered, sonicated and placed in a Bio-plex array reader (FlexMap 3D, Bio-Plex Manager 5.0, Bio-Rad). The Median Fluorescence Intensity (MFI) of PE signal was determined for each bead set in each well. Background signal, defined as the average MFI observed for each microsphere set when incubated with detection reagent(s) in the absence of test antibody, was subtracted from the MFI of each sample. Individual IgG subclasses (Athens Research) were serially diluted to generate standard curves for calculating the subclass concentration from the original undiluted sample. Individual composition percentages were calculated by dividing an individual subclass concentration by the sum of all four subclass concentrations measured in a unique sample.
IgG-Fc glycan characterization
The Fc specific glycan composition was characterized using a method previously described (Mahan et al. 2015). Briefly, purified human IgG was digested into Fc and F(ab′)2 fragments using IdeS, (FabRICATOR, Genovis). Twenty micrograms of IgG at 1 mg/ml in PBS, was digested with enzyme under manufacturers recommendations. To separate the digested fragments, protein G magnetic beads (EMD-Millipore) were used to bind and enrich the Fc portions. F(ab′)2 and Fab fragments, remained in the supernatant, along with IdeS while the bead-bound fraction contained Fc and any incompletely digested IgG. Glycans were released from IgG-bound beads using enzymatic digestion with PNGaseF using the manufacturers instructions (New England Biolabs). Ice-cold ethanol was added to each to precipitate protein and separate released glycans. Plates were incubated in ethanol at −20 °C and precipitated proteins, and beads and were pelleted by centrifugation. Glycan containing supernatants were transferred and dried completely in a centrivap (Labconco) and stored at −20 °C until labeling. Thoroughly dried glycans were labeled by reductive amination using APTS (Life Technologies). Unreacted dye was removed using fresh P2 size-exclusion columns (Biorad) releasing labeled glycan in the flow-through. Glycans were stored at 4 °C until analysis on a DNA sequencer. Each sample was diluted in a 96-well PCR plate and loaded onto a 3130XL ABI DNA sequencer and run as previously described(Laroy et al. 2006). Converted files were analyzed using MATLAB (The MathWorks, Inc.) to align peaks and calculate the area under the curve for each peak using a custom script. Calibration of the assay for proper peak identification was previously performed using glycan standards (Prozyme) and controls with known compositions were run alongside unknown samples to ensure assay consistency.
Natural Killer cell degranulation
The NK cell activity of the IgG samples was assessed using a plate-based assay by an adaptation of previously described assays (Alter et al. 2004, Al-Hubeshy et al. 2011) where NK cell degranulation is measured by CD107a surface expression, a marker correlated with cytokine release and cytotoxic activity (Alter et al. 2004) and ADCC (Fischer et al. 2006, Chung et al. 2009). The NK-92 human NK cell line (NantKwest, formerly Conkwest) was cultured in tissue culture flasks at 37 °C 5% CO2, splitting the cells 1:5 every 3-4 days and maintaining the cells at 600,000 cell/ml or less using RPMI 1610 with L-glutamine (Corning) 2.5% horse serum (ATCC) 12.5% fetal bovine serum (Biowest) 10 IU/ml IL-2 (AIDS Reagent Program) 1 mM sodium pyruvate (Corning) + 1x MEM nonessential amino acids (Corning) as culturing media. Sterile 96-well polystyrene plates (Costar) were coated overnight at 4 °C with FcγR column load and eluates starting at 3 μg/ml, or 5 μg/ml SOSIP gp140 in PBS. After coating, plates were washed 5 times with 250 ml of PBS in an automatic plate washer (Biotek), blocked with 250 μl of 1% BSA in PBS for 30 min at room temperature and washed another 5 times. 100 μl of cell culture media was added to FcγR column load/eluate plates and 100 μl of 3-0.01 μg/ml of VRC01 subclasses in cell culture media was added to the SOSIP gp140 coated plates. 100,000 NK-92 cells in 100 μl of cell culture media were added to each well. On each plate, four coated/blocked wells containing 2.5 μg/ml PMA (Sigma-Aldrich) and 0.5 μg/ml ionomycin (Sigma-Aldrich) were used as positive controls and four coated/blocked wells containing no test IgG were used as negative controls. Additionally, 5 μl of Alexafluor-647 anti-human CD107a detection reagent (Southern Biotech) was added to each well. After incubation of the plates for 1 hr at 37 °C 5% CO2, Brefeldin A (final concentration 10 μg/ml, Sigma-Aldrich) and mononensin (Golgistop, final concentration 6 μg/ml, Sigma-Aldrich) was added to each well. After incubation, cells were washed with cold PBS, centrifuged and resuspended in 100 μl of cold PBS, supplemented with 100 μl of Fix & Perm Medium A (Life Technologies), and incubated for 15 min. 50 μl of PBS with 5% FBS was added to each well to quench the reaction and cells were centrifuged, washed again with 200 μl of PBS with 5% FBS, and resuspended in 200 μl of PBSF (PBS, 0.1% BSA). Data was acquired on a MACSQuant flow cytometer, and analyzed using Flowjo V10. Data was analyzed in GraphPad Prism.
The extent of IgG loading to plates or VRC01 subclass binding to the SOSIP gp140 plates was measured by performing an identical dilution in a 96-well plate as performed for the degranulation assay. VRC01 subclasses were incubated on the SOSIP gp140 coated plates for 1 hr at room temperature. After IgG loading, the plates were washed with PBS 0.1% BSA, incubated with 0.65 μg/ml anti-human IgG H+L HRP (Thermo) in PBS 0.1% BSA for 30 minutes, washed again, incubated with 150 μl of APTS (Thermo) for 30 minutes and quenched with 100 μl of 1% SDS. Data was acquired at 405 nm with a UV-vis plate reader (SpectraMax, Molecular Diagnostics), and analyzed using Flowjo V10 and GraphPad Prism.
Antibody-dependent cellular phagocytosis (ADCP)
The phagocytic activity of VRC01 IgG subclasses was assessed by adaption of previously described phagocytosis assay (Ackerman et al. 2011, McAndrew et al. 2011). Briefly, FluoSpheres® Carboxylate-Modified Microspheres, 1.0 μm, yellow-green fluorescent (Life Technologies) were conjugated by NHS/EDC (Thermo) activation with anti-human Fab (Jackson ImmunoResearch) for evaluating the FcγR column load/eluates or SOSIP pg140 for evaluating the VRC01 subclasses. The beads were diluted to 800,000 beads per 50 μl in RPMI complete media (10% FBS supplemented with Penstrep). The THP-1 human monocyte cells (gift from Dr. Brent Berwin) were diluted to 20,000 cells per 150 μl RPMI. The assay was run in a 96-well tissue culture plate by pipetting 50 μl of beads, then 150 μl of cells and lastly 50 μl of either RPMI alone or antibody diluted into RPMI at 5x the final target concentration. The cells were incubated at 37°C at 5% CO2 for 4 hours. All centrifugation steps were performed at 1300 RPM for 8 min at 4°C. After incubation, cells were fixed as described for the degranulation assay. Data was acquired on a MACSQuant flow cytometer, and analyzed using Flowjo V10 and GraphPad Prism. A background-subtracted phagocytosis score was calculated by multiplying the percentage of bead positive cells by their MFI, followed by subtraction of the phagocytosis score observed in the absence of antibody.
Consistent IgG loading to the anti-human Fab and SOSIP gp140 beads was confirmed by performing an identical dilution in a 96-well plate as performed for the phagocytosis assay with exception of the VRC01 IgG2, 3 and 4. Beads were incubated with IgG for 1 hr at room temperature, washed with PBS 0.1% BSA, incubated with 0.65 μg/ml anti-human IgG H+L Alexafluor647 (Thermo) in PBS 0.1% BSA for 30 minutes and washed again prior to reading. Data was acquired with a MACSQuant flow cytometer, and analyzed using Flowjo V10 and GraphPad Prism.
Results and Discussion
Characteristics of FcγR-chromatography columns
With exception of FcγRI, all of the FcγR could be repeatedly cycled with minimal change in the IgG capacity. The IgG capacity per ml of resin is displayed in Table 1. Additionally, the percent utilization was calculated by dividing the total number of moles/ml of IgG bound versus the theoretical moles/ml that should bind based on a 1:1 IgG-FcγR stoichiometry and accounting for the molecular weights of IgG (~150 kDa) and the FcγR. The percent utilization number represents the percentage of FcγR bound to the chromatography media that are still active for IgG binding after coupling through free amine groups on solvent exposed lysines of the FcγR. It is notable that the percent utilization is relatively low which may be partially related to the high density of solvent exposed lysines in the FcγR region that contacts IgG-Fc (Ferrara et al. 2011, Ramsland et al. 2011, Mimoto et al. 2013, Lu et al. 2015). Instead of coupling to the chromatography media via free amines, it is possible that utilization would improve if C-terminus site-specific conjugation techniques were employed (Bellucci et al. 2013, Thomann et al. 2015).
Table 1.
FcγR variants and chromatography characteristics.
| Receptor | Approximate MW [Da] | Density [mg/ml] | Capacityavg [mg/ml] | Utilization (%) |
|---|---|---|---|---|
|
| ||||
| FcγRI | 75000 | 1.75 | 0.05 | 1.3 |
|
| ||||
| FcγRIIA H131 | 32000 | 4 | 0.75 | 4.0 |
| FcγRIIA R131 | 32000 | 4 | 0.08 | 0.4 |
|
| ||||
| FcγRIIB/C | 32000 | 4 | 0.72 | 3.8 |
|
| ||||
| FcγRIIIA V158 | 45000 | 4 | 1.06 | 8.0 |
| FcγRIIA F158 | 45000 | 4 | 0.67 | 5.0 |
|
| ||||
| FcγRIIIB NA1 | 45000 | 4 | 0.27 | 2.0 |
| FcγRIIIB NA2 | 45000 | 3.2 | 0.41 | 3.8 |
| FcγRIIIB SH | 45000 | 4 | 0.42 | 3.1 |
Biophysical characterization of IgG enriched by FcγR chromatography
IgG subclass
Based on both FcγR affinity and concentration in solution, each IgG subclass is expected to differentially compete for available immobilized receptor on the chromatographic media based on its affinity relative to the other subclasses, thus generating a proportion of each subclass on the solid surface that may differ from its original proportion in solution. To assess this competitive behavior, the load and FcγR-enriched IgG from the chromatography runs were analyzed for the proportion of each IgG subclass in the mixture. Subclass prevalences in the load material (Figure 1A) were consistent with previous studies (Teschner et al. 2007, van der Poel et al. 2011). Both FcγRIIa allotypes H131 and R131 peaks possess a higher proportion of IgG2, displaying a 2-2.5-fold enrichment factor (Figure 1B). This result may not be surprising for the H131 allotype given IgG2 has previously displayed the highest affinity relative to the other subclasses (Warncke et al. 2012). However, the enrichment of IgG2 by the R131 FcγRIIa allotype was surprising given it has the lowest affinity relative to the other subclasses. One possible explanation is preferential enrichment of high affinity glycoforms such as agalactosylated species which are more prevalent in the IgG2 subclass (Selman et al. 2012) or selective enrichment of covalently dimeric IgG2 that can comprise up to 0.4% of serum IgG2 (Yang et al. 2014). FcγRIIa H131 also enriched IgG3 two-fold, however the R131 allotype did not. FcγRIIb unexpectedly enriched IgG3 five-fold, though its affinity is similar or only slightly higher than IgG1 though 10-fold higher than IgG2 (Bruhns et al. 2009, Warncke et al. 2012). FcγRIIIa/b receptors depleted IgG2 and IgG4 and enriched IgG3 3-5-fold relative to the load, consistent with IgG3 possessing the highest affinity amongst all subclasses for FcγRIII receptors (Bruhns et al. 2009).
Figure 1. IgG Subclass distribution and enrichment factors of the load and FcγR-enriched samples.
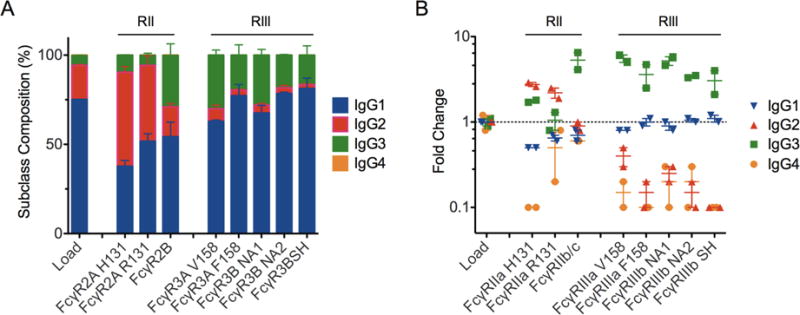
A. Prevalence of each subclass in load and FcγR-enriched samples. Error bars represent the standard error observed between duplicates. B. Fold change of subclass prevalence relative to load sample proportion in load and FcγR-enriched samples. Points represent purification duplicates and bars indicate the mean. A dotted line is plotted at 1 to indicate the baseline prevalence.
Given the levels of high molecular weight (HMW) species in an IVIG product can directly impact its effect in vivo (Teeling et al. 2001), we evaluated the enrichment IgG-aggregates such as dimers or higher order oligomers using analytical size exclusion chromatography. While the load contained 5% HMW species, the FcγRIII receptors did not alter the level of HMW in the elution peaks (samples contained 4.7-5.1% HMW). In contrast FcγRIIa H131, FcγRIIa R131 and FcγRIIb/c contained 7%, 7.5% and 13% HMW species, respectively. Preferential enrichment of HMW species may be expected for the low affinity FcγRII receptors which have previously exhibited a 200-fold increase in affinity towards dimeric IgG versus monomer in contrast to the high affinity FcγRI receptor with 2-3-fold enhanced affinity for dimeric IgG (Luo et al. 2009). However, enrichment of HMW by the low affinity FcγRIIIb receptor, which has previously shown an 800-fold increase in affinity for dimeric IgG relative to monomeric IgG (Luo et al. 2009), was not observed. Instead, the lack of HMW enrichment with FcγRIII columns may be due to IgG aggregates being outcompeted by high affinity monomeric nonfucosylated and bisected IgG glycoforms.
IgG-Fc glycans
The combined effects of IgG subclass and glycosylation can dramatically tune the affinity for FcγRs (Niwa et al. 2005). To explore the glycan enrichment capabilities of immobilized FcγR-chromatography media, the elution peaks were analyzed for Fc-only glycans (Mahan et al. 2014). The proportion of Fc-glycan species and the IgG glycoform enrichment level varied between immobilized receptors (Figure 2). Agalactosylated species (G0) were enriched 8-14-fold from the load material for all of the FcγRs consistent with agalactosylated antibodies being pro-inflammatory (Winkler et al. 2013) as well as previous findings for FcγRIIIa (Ackerman et al. 2013, Bolton et al. 2013) although there have been conflicting results in literature (Houde et al. 2010, Dashivets et al. 2015, Thomann et al. 2015). FcγRIIa H131 and R131 enriched in G0 species the most which may be partially related to the enrichment of IgG2 given this subclass has the highest proportion of G0 species in serum derived IgG (Wuhrer et al. 2007, Selman et al. 2012). However, enrichment of G0 was also observed for FcγRIIb/c, which did not enrich in IgG2. The proportional increase of G0 species was mainly driven by the enrichment of G0F glycans for FcγRIIa/b-chromatrography eluates, which translates to an apparent depletion of nonfucosylated species as shown in Figure 2B. Interestingly, G0FB glycans were undetectable in the FcγRIIa/b peaks in contrast to the load and FcγRIII eluates and significantly depleted of G1. In contrast, FcγRIII-chromatography enriched nonfucosylated IgG glycoforms approximately 1.5-2-fold, consistent with our previous study of nonfucosylated monoclonal IgG1(Bolton et al. 2013) and other more recent studies (Dashivets et al. 2015, Thomann et al. 2015). We also observed 2-fold enrichment of bisected IgG glycoforms. There is an apparent enrichment of sialylated IgG (G2S1, Figure 2B) in the FcγRIII peaks, however this may be more related to those sialylated species also being nonfucosylated (Figure 2B).
Figure 2. Glycan composition of elution peaks from FcγR affinity chromatography columns.
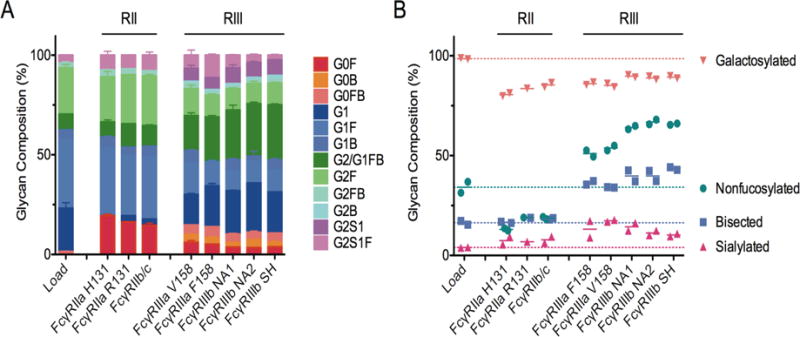
A. Prevalence of individual IgG-Fc glycoforms in the load and FcγR-enriched samples. Error bars represent the standard error observed between duplicates. B. Prevalence of summary glycotypes including total galactosylated, nonfucosylated, bisected and sialylated IgG-Fc glycans. Points represent purification duplicates and bars indicate the mean. A dotted line is plotted at the average for each summary glycoform to indicate the baseline prevalence.
Modeling of IgG subclass occupancy of FcγR
In humans, blood contains approximately 10 g/L of IgG. Given the high concentration of IgG in serum and tissues due to extravasation, most FcγR on effector cells are already occupied with polyclonal serum IgG as previously estimated using the one-component Langmuir isotherm (van der Poel et al. 2011). While this model is useful for calculating a single IgG subclass occupying surface FcγR, it does not account for competition between all four IgG subclasses for individual FcγR based on their differential binding affinities. In contrast, the Langmuir competitive model (LCM) (Weber and DiGiano 1996, Mahamadi and Nharingo 2010) can account for n competing species, in this case, the four IgG subclasses competing for one type of surface FcγR. Using Equation 1 and previously published IgG subclass affinities for FcγR (Bruhns et al. 2009, Warncke et al. 2012), the percentage of FcγR occupied by each subclass from serum IgG was estimated as shown in Figure 3A-B. This estimation indicates that generally, 90% or more of FcγR on the surface of effector cells are pre-loaded with serum IgG. This result is consistent with the previous analysis (van der Poel et al. 2011) but with further clarification of the proportion of each IgG subclasses on surface FcγR as compared to solution conditions. For instance, IgG2 comprises approximately 20% of human serum IgG, however, per the LCM, this subclass occupies only 3% or less of surface FcγR. In contrast, IgG3 is 5% in serum, but can occupy 20-30% of FcγRIII receptors according to the binding affinities reported in Bruhns, et al. Relative to IgG1, the higher affinity of IgG2 for FcγRIIa, but lower affinity of IgG3 for FcγRIIIa are responsible for the differences observed in predicted subclass occupancy based on data from Warncke, et al. as compared to that from Bruhns, et al. Qualitatively, neither model is perfect, but aspects of both are reflected in the experimental data; the results of enrichment experiments (Figure 1A) conducted here are consistent with enrichment of IgG2 as modeled from affinities reported in Warncke, et al., and IgG3 as modeled from data in Bruhns, et al.
Figure 3. Comparison of model and experiment.
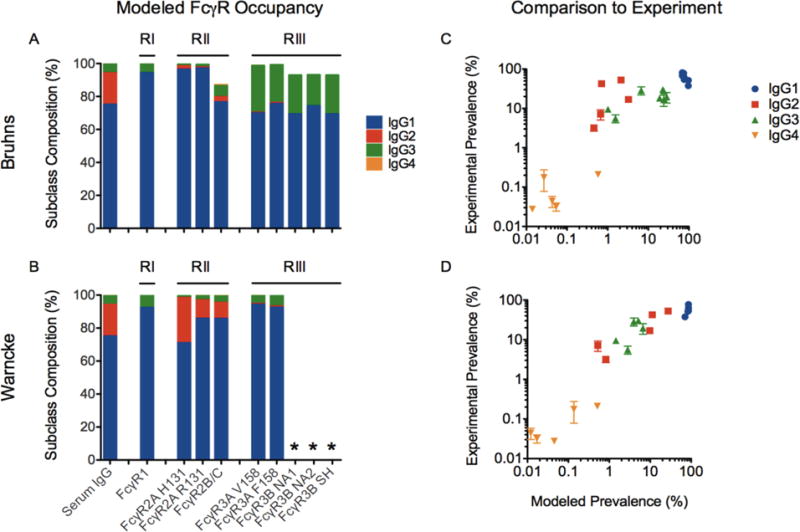
A-B. Estimation of FcγR occupancy by IgG subclasses based on the Langmuir competitive model (LCM) using affinity data from Bruhns, et al (A) and Warncke, et al (B). Asterisks indicate receptors that were not tested. C-D. Comparison of predicted enrichment of IgG subclasses versus experimentally observed values for FcγRIIa, FcγRIIb, and FcγRIIIa variants using affinity data from Bruhns, et al (C) and Warncke, et al (D). Error bars represent the standard error of experimental duplicates.
In addition to estimating the pre-loading of surface FcγR with serum IgG subclasses, the LCM was also used to predict the percentage of each subclass in the FcγR-chromatography eluates using the experimentally measured IgG subclass concentrations and the previously measured affinities (Bruhns et al. 2009, Warncke et al. 2012) (Figure 3C-D). The predicted percentages are in reasonable agreement with the measured eluate samples, suggesting that the effective affinities of the subclasses in the preparation of polyclonal serum IgG assayed here recapitulated some but not all aspects of the antibody samples evaluated in binding affinity studies. Specifically, none of the reference studies account for the preferential binding of different IgG-Fc glycoforms with FcγR which presented differences in enrichment as described earlier and in previous studies using FcγR-chromatography (Bolton et al. 2013, Dashivets et al. 2015, Thomann et al. 2015). Not accounting for glycoform preferences in the model may explain some deviation from unity (y = x). The LCM could be easily extended to capture a finer level of granularity if the equilibrium dissociation constants (KDs) were known for the relevant glycoforms of each IgG subclass. The refinement of IgG-Fc competition for FcγR by both subclass and glycoform preferences could provide further insight into how these Fc features translate into functional differences in vitro and in vivo.
| Equation 1 |
Effector function characterization of FcγR-enriched IgG
NK cell activation
The NK cell activation activity of the FcγR-enriched samples, monoclonal VRC01 IgG subclasses and VRC01 IgG1 glycovariants was assessed by measurement of CD107a surface expression on a human NK cell line expressing FcγRIIIa V158. To evaluate the polyclonal FcγR-enriched samples, 96-well plates were directly coated with a dilution series of the IgG. Given the extent of coating was somewhat variable between FcγR-enriched samples (data not shown), the x-axis for the degranulation results was normalized by A405 nm signal from an identically prepared plate. As shown in Figure 4A, FcγRIII- enrichment improved the NK cell activation potency by nearly 1-log relative to the load material, consistent with the high affinity of FcγRIIIa for IgG3 (Bruhns et al. 2009) as well as its preference for nonfucosylated (Shields et al. 2002, Niwa et al. 2005, Shibata-Koyama et al. 2009) and bisected (Umana et al. 1999, Hodoniczky et al. 2005) IgG-Fc glycans, all of which were enriched over the FcγRIII columns. In addition, the monoclonal subclass results confirm that IgG1 and IgG3 produce the strongest potentiation of NK cell activity and that nonfucosylated IgG1 dramatically improves this activity (Figure 4B). The FcγRIIb/c elution peak also displayed improved NK cell activation, which may be due to its enrichment of IgG3, and unexpectedly the FcγRIIa R131 elution also improved NK cell activity, however these results may be confounded by the enrichment of high molecular weight species.
Figure 4. NK cell degranulation activity of control and enriched antibody pools.
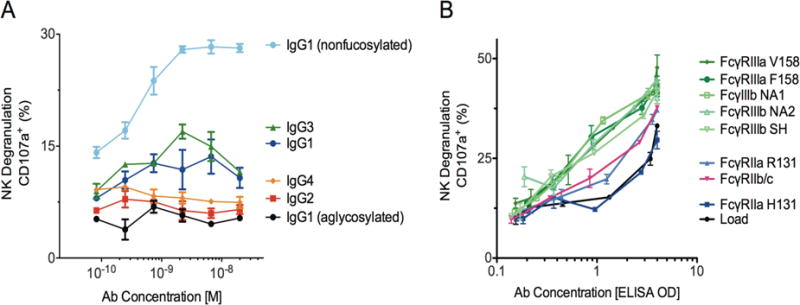
A. NK degranulation activity of monoclonal VRC01 IgG subclasses and glycovariants using SOSIP gp140 coated plates. B. NK degranulation activity of polyclonal IgG load and FcγR-enriched samples using plates directly coated with IgG. Error bars represent standard error of duplicates.
Antibody-dependent cellular phagocytosis (ADCP)
The phagocytic activity of the subclass controls and FcγR-enriched samples was assessed using a human THP-1 monocyte cell line that expresses FcγRI, FcγRIIa, FcγRIIb and FcγRIIIa. The IgG subclass rank order of IgG3, IgG1, IgG4 followed by IgG2 having the weakest phagocytic activity is consistent with previous studies using monocytes (Rozsnyay et al. 1989, Goh et al. 2011). The lack of potentiation using a nonfucosylated IgG is consistent with previous findings where the preferential blocking of FcγRIIIa did not modulate THP-1 monocyte phagocytosis (Ackerman et al. 2011, Moldt et al. 2011) but conflicts with studies using freshly isolated monocytes (Richards et al. 2008, Herter et al. 2014). This discrepancy in FcγRIIIa-mediation between cell types may be partially explained by the dramatically lower expression of FcγRIIIa relative to FcγRI/II on THP-1 cells versus freshly isolated cells (Richards et al. 2008, Ackerman et al. 2011, Herter et al. 2014). Similar to the NK cell activity results, FcγRIII-enriched samples exhibited the greatest potentiation of phagocytic activity (Figure 5B). In contrast to the NK cell results, this enhanced effector function is likely driven by the enrichment of IgG3 and not the enrichment of nonfucosylated species as demonstrated with the monoclonal IgG subclasses and glycovariants (Figure 5A).
Figure 5. Phagocytic activity of control and enriched antibody pools.
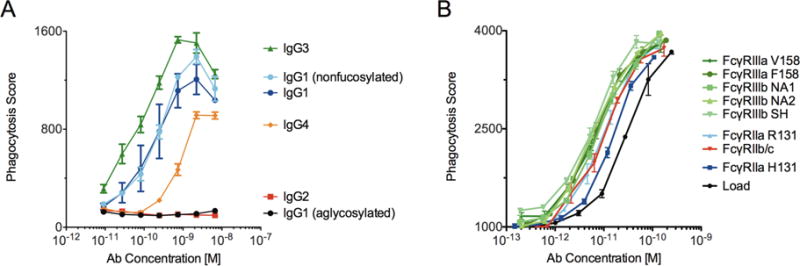
A. Phagocytic activity of monoclonal VRC01 IgG subclasses and glycovariants. B. Phagocytic activity of polyclonal IgG load and FcγR-enriched samples. Error bars represent standard error of duplicates.
The FcγRII-enriched samples did show an improvement in phagocytic activity over the load material, however these results are confounded in that the samples contained more aggregate than the load. That aside, FcγRIIb elution enriched IgG3 which has high affinity towards FcγRIIa (Bruhns et al. 2009) and the FcγRII H131 and R131 eluates were enriched in IgG2 which preferentially binds FcγRIIa, the receptor previously shown to strongly contribute towards monocyte phagocytosis (Richards et al. 2008, Ackerman et al. 2011, Moldt et al. 2011). That being said, the monoclonal IgG2 mediated phagocytosis similar to the aglycosylated variant displaying a complete lack of activity. Given the inability of IgG2 to bind FcγR1, this attenuation may be expected given FcγRI engagement has been previously shown to partially contribute towards IgG phagocytic activity (Goh et al. 2011) therefore, the enhanced phagocytic potential of the enriched IgG is more likely explained by the enrichment of agalactosylated species which according to the results described earlier, preferentially bind FcγRIIa.
Conclusion
We explored the isolation of high affinity IgG subclasses and glycoforms from a complex human-derived mixture using FcγR affinity ligands. When analyzing the FcγR-enriched samples using biophysical and effector function assays, we observed functional relationships similar to those previously demonstrated using genetic, cellular, enzymatic and purification techniques. For instance, the FcγRIII columns enriched IgG3 and nonfucosylated/bisected IgG-Fc glycoforms that translated to the greatest enhancement of NK cell and monocyte activity. Additionally, there were unexpected observations such as both FcγRIIa allotypes (H/R131) enriching IgG2 and G0 species and these eluates displayed greater phagocytic activity relative to the load material despite monoclonal IgG2 have zero phagocytic capacity indicating the enrichment of G0 may be functionally relevant. While nonfucosylated IgG greatly improved NK cell activity, and has previously been shown to improve monocyte/macrophage phagocytosis with freshly isolated cells (Richards et al. 2008, Herter et al. 2014), we did not observe an enhancement with THP-1 cells likely due to the low expression of FcγRIIIa on this cell line (Richards et al. 2008, Ackerman et al. 2011, Herter et al. 2014). Additionally, to our knowledge this is the first the demonstration of using FcγRIII-chromatography to enrich bisected IgG-Fc glycoforms.
These observations highlight the possibility of gaining new insights by analyzing human-derived biological materials given these most closely represent their natural state in vivo. It also points out limitations in the secondary analysis used in this study given there are additional Fc features or structural relationships established or posited to affect FcγR engagement that could be explored further such as the composition of IgG high molecular weight species (Teeling et al. 2001), composition of IgG disulfide bond variants (Liu and May 2012), the composition of GM allotypes (de Lange 1989, Kumpel et al. 1989, Pandey 2013, Pandey 2014), individual subclass glycoform specificity (Niwa et al. 2005), and the glycosylation of FcγRs (Edberg and Kimberly 1997, Drescher et al. 2003, Shibata-Koyama et al. 2009, Ferrara et al. 2011).
Beyond the use of FcγR-chromatography as an analytical tool, there is potential for its use in manufacturing new compositions of intravenous immunoglobulin (IVIG) therapeutics, which are currently used for their anti-inflammatory and immunomodulatory properties (Hartung et al. 2009). FcγR-based separations could be performed in conjunction with glycan remodeling techniques to improve yield by varying the proportion of bisected, galactosylated and sialylated IgG glycans (Hodoniczky et al. 2005, Wei et al. 2008, Tojo et al. 2009, Zou et al. 2011, Bolton et al. 2013, Dashivets et al. 2015, Lin et al. 2015, Thomann et al. 2015) as desired (defucoyslation of the IgG-Fc attached glycan is inefficient due to steric hindrance (Yazawa et al. 1986, Yamane-Ohnuki and Satoh 2009)).
In general, evaluations of any two binding partners could be performed using affinity chromatography to interrogate primary and higher order protein structures as well post-translational modifications such as glycosylation. For instance other Fc receptors may be of interest as affinity ligands to evaluate immunoglobulin preferences such as the neonatal Fc receptor (FcRn) (Schlothauer et al. 2013, Schoch et al. 2015), FcR-Like receptors (FcRLs) (Wilson et al. 2010, Wilson et al. 2012), FcαR, FcεR, FcμR, Fcα/μR, IgD-R, glycan binding lectins (Dong et al. 1999, Arnold et al. 2006, Anthony et al. 2008, Keeble et al. 2008, Karsten et al. 2012, Boesch et al. 2014), bacterial surface proteins (Forsgren and Sjoquist 1966, Bjorck and Kronvall 1984, Bjorck 1988), phage-encoded proteins (Muller et al. 2013) and viral capsid proteins (Para et al. 1980, Dubin et al. 1990, Litwin et al. 1992, Namboodiri et al. 2007, Corrales-Aguilar et al. 2014). Conversely, Fc receptor preferences for non-immunoglobulins such as pentraxins (Lu et al. 2012) or pathogen-secreted antagonists(Stemerding et al. 2013) may also be of interest. More broadly, therapeutically relevant ligand/receptor pairs such as erythropoietin (EPO)-EPOR (Tsuda et al. 1990), other glycosylated proteins/peptides (reviewed in (Sola and Griebenow 2010)), or de novo discovery of new binding pairs from complex mixtures such as bacterial cultures (Stemerding et al. 2013), human tissue samples (Liu et al. 2006) or from large-scale proteomic screens leveraging the cloning and expression of open reading frames (Phizicky et al. 2003).
Analogous to co-immunoprecipitation-coupled mass spectroscopy to identify unique binding partners (Free et al. 2009), we explored use of affinity chromatography to enrich high affinity primary and higher order protein structures from a natural complex mixture of potential binders followed by the characterization of the load and eluates. To exemplify this generalizable technique we identified IgG subclass and glycoform structural specificity for FcγR and their functional implications. An advantage of affinity chromatography as part of this workflow is its scalable productivity enabling high-resolution secondary analysis of flow through and eluates using biophysical and functional characterization in in vitro assays. Probing the recognition properties of binding partners whether engineered or naturally derived represents an important methodology to enhance our understanding of their structural specificity and potential functional role in activity in vivo.
Acknowledgments
AWB is a co-inventor on intellectual property regarding the use of regarding use of human FcγR3A for purification of IgG subtypes, owns stock in, and serves as CEO of Zepteon, Inc., which markets this resin. These studies were supported in part by the Bill and Melinda Gates Foundation (OPP1032817, OPP1146996, and OPP1114729) and the National Institutes of Health (R01AI102691, R01AI131975 and P01AI120756).
References
- Abboud N, Chow SK, Saylor C, Janda A, Ravetch JV, Scharff MD, Casadevall A. A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. The Journal of experimental medicine. 2010;207(11):2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. The Journal of clinical investigation. 2013;123(5):2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366(1-2):8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. Journal of immunological methods. 2011;366(1-2):8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hubeshy ZB, Coleman A, Nelson M, Goodier MR. A rapid method for assessment of natural killer cell function after multiple receptor crosslinking. Journal of immunological methods. 2011;366(1-2):52–59. doi: 10.1016/j.jim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. Journal of immunological methods. 2004;294(1-2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JN, Dwek RA, Rudd PM, Sim RB. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunology letters. 2006;106(2):103–110. doi: 10.1016/j.imlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bellucci JJ, Amiram M, Bhattacharyya J, McCafferty D, Chilkoti A. Three-in-one chromatography-free purification, tag removal, and site-specific modification of recombinant fusion proteins using sortase A and elastin-like polypeptides. Angewandte Chemie. 2013;52(13):3703–3708. doi: 10.1002/anie.201208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. Journal of virology. 2000;74(2):627–643. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorck L. Protein L. A novel bacterial cell wall protein with affinity for Ig L chains. Journal of immunology. 1988;140(4):1194–1197. [PubMed] [Google Scholar]
- Bjorck L, Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. Journal of immunology. 1984;133(2):969–974. [PubMed] [Google Scholar]
- Boesch AW, Alter G, Ackerman ME. Prospects for engineering HIV-specific antibodies for enhanced effector function and half-life. Current opinion in HIV and AIDS. 2015;10(3):160–169. doi: 10.1097/COH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch AW, Brown EP, Cheng HD, Ofori MO, Normandin E, Nigrovic PA, Alter G, Ackerman ME. Highly parallel characterization of IgG Fc binding interactions. mAbs. 2014;6(4):915–927. doi: 10.4161/mabs.28808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton GR, Ackerman ME, Boesch AW. Separation of nonfucosylated antibodies with immobilized FcgammaRIII receptors. Biotechnology progress. 2013;29(3):825–828. doi: 10.1002/btpr.1717. [DOI] [PubMed] [Google Scholar]
- Bolton GR, Ackerman ME, Boesch AW. Separation of nonfucosylated antibodies with immobilized FcgammaRIII receptors. Biotechnology progress. 2013 doi: 10.1002/btpr.1717. [DOI] [PubMed] [Google Scholar]
- Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. The Journal of clinical investigation. 2005;115(10):2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournazos S, Chow SK, Abboud N, Casadevall A, Ravetch JV. Human IgG Fc domain engineering enhances antitoxin neutralizing antibody activity. The Journal of clinical investigation. 2014;124(2):725–729. doi: 10.1172/JCI72676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EP, Dowell KG, Boesch AW, Normandin E, Mahan AE, Chu T, Barouch DH, Bailey-Kellogg C, Alter G, Ackerman ME. Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles. J Immunol Methods. 2017;443:33–44. doi: 10.1016/j.jim.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EP, Licht AF, Dugast AS, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. Journal of immunological methods. 2012;386(1-2):117–123. doi: 10.1016/j.jim.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. Journal of immunology. 2009;182(2):1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- Corrales-Aguilar E, Hoffmann K, Hengel H. CMV-encoded Fcgamma receptors: modulators at the interface of innate and adaptive immunity. Seminars in immunopathology. 2014;36(6):627–640. doi: 10.1007/s00281-014-0448-2. [DOI] [PubMed] [Google Scholar]
- Dashivets T, Thomann M, Rueger P, Knaupp A, Buchner J, Schlothauer T. Multi-Angle Effector Function Analysis of Human Monoclonal IgG Glycovariants. PloS one. 2015;10(12):e0143520. doi: 10.1371/journal.pone.0143520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange GG. Polymorphisms of human immunoglobulins: Gm, Am, Em and Km allotypes. Experimental and clinical immunogenetics. 1989;6(1):7–17. [PubMed] [Google Scholar]
- DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nature medicine. 2014;20(2):143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Storkus WJ, Salter RD. Binding and uptake of agalactosyl IgG by mannose receptor on macrophages and dendritic cells. Journal of immunology. 1999;163(10):5427–5434. [PubMed] [Google Scholar]
- Drescher B, Witte T, Schmidt RE. Glycosylation of FcgammaRIII in N163 as mechanism of regulating receptor affinity. Immunology. 2003;110(3):335–340. doi: 10.1046/j.1365-2567.2003.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin G, Frank I, Friedman HM. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. Journal of virology. 1990;64(6):2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel RC, Schur PH, Brendel K, Meezan E. pH gradient elution of human IgG1, IgG2 and IgG4 from protein A-sepharose. Journal of immunological methods. 1979;31(3-4):211–217. doi: 10.1016/0022-1759(79)90133-9. [DOI] [PubMed] [Google Scholar]
- Edberg JC, Kimberly RP. Cell type-specific glycoforms of Fc gamma RIIIa (CD16): differential ligand binding. Journal of immunology. 1997;159(8):3849–3857. [PubMed] [Google Scholar]
- Ferrant JL, Benjamin CD, Cutler AH, Kalled SL, Hsu YM, Garber EA, Hess DM, Shapiro RI, Kenyon NS, Harlan DM, Kirk AD, Burkly LC, Taylor FR. The contribution of Fc effector mechanisms in the efficacy of anti-CD154 immunotherapy depends on the nature of the immune challenge. International immunology. 2004;16(11):1583–1594. doi: 10.1093/intimm/dxh162. [DOI] [PubMed] [Google Scholar]
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umana P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Penack O, Gentilini C, Nogai A, Muessig A, Thiel E, Uharek L. The anti-lymphoma effect of antibody-mediated immunotherapy is based on an increased degranulation of peripheral blood natural killer (NK) cells. Experimental hematology. 2006;34(6):753–759. doi: 10.1016/j.exphem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Forsgren A, Sjoquist J. “Protein A” from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. Journal of immunology. 1966;97(6):822–827. [PubMed] [Google Scholar]
- Free RB, Hazelwood LA, Sibley DR. Identifying novel protein-protein interactions using co-immunoprecipitation and mass spectroscopy. Current protocols in neuroscience/editorial board, Jacqueline N Crawley … [et al] 2009 doi: 10.1002/0471142301.ns0528s46. Chapter 5: Unit 5.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh YS, Grant AJ, Restif O, McKinley TJ, Armour KL, Clark MR, Mastroeni P. Human IgG isotypes and activating Fcgamma receptors in the interaction of Salmonella enterica serovar Typhimurium with phagocytic cells. Immunology. 2011;133(1):74–83. doi: 10.1111/j.1365-2567.2011.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung HP, Mouthon L, Ahmed R, Jordan S, Laupland KB, Jolles S. Clinical applications of intravenous immunoglobulins (IVIg)–beyond immunodeficiencies and neurology. Clinical and experimental immunology. 2009;158(Suppl 1):23–33. doi: 10.1111/j.1365-2249.2009.04024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. Journal of immunology. 2014;192(5):2252–2260. doi: 10.4049/jimmunol.1301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nature medicine. 2009;15(8):951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H, Hjelm K, Sjoquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Letters. 1972;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnology progress. 2005;21(6):1644–1652. doi: 10.1021/bp050228w. [DOI] [PubMed] [Google Scholar]
- Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Molecular & cellular proteomics : MCP. 2010;9(8):1716–1728. doi: 10.1074/mcp.M900540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, Shitara K, Satoh M. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17(1):104–118. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Kohl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M, Kohl J. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nature medicine. 2012;18(9):1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(16):6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpel BM, Wiener E, Urbaniak SJ, Bradley BA. Human monoclonal anti-D antibodies. II. The relationship between IgG subclass, Gm allotype and Fc mediated function. British journal of haematology. 1989;71(3):415–420. doi: 10.1111/j.1365-2141.1989.tb04300.x. [DOI] [PubMed] [Google Scholar]
- Laroy W, Contreras R, Callewaert N. Glycome mapping on DNA sequencing equipment. Nature protocols. 2006;1(1):397–405. doi: 10.1038/nprot.2006.60. [DOI] [PubMed] [Google Scholar]
- Lin CW, Tsai MH, Li ST, Tsai TI, Chu KC, Liu YC, Lai MY, Wu CY, Tseng YC, Shivatare SS, Wang CH, Chao P, Wang SY, Shih HW, Zeng YF, You TH, Liao JY, Tu YC, Lin YS, Chuang HY, Chen CL, Tsai CS, Huang CC, Lin NH, Ma C, Wong CH. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(34):10611–10616. doi: 10.1073/pnas.1513456112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin V, Jackson W, Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. Journal of virology. 1992;66(6):3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. mAbs. 2012;4(1):17–23. doi: 10.4161/mabs.4.1.18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Qian WJ, Mottaz HM, Gritsenko MA, Norbeck AD, Moore RJ, Purvine SO, Camp DG, 2nd, Smith RD. Evaluation of multiprotein immunoaffinity subtraction for plasma proteomics and candidate biomarker discovery using mass spectrometry. Molecular & cellular proteomics : MCP. 2006;5(11):2167–2174. doi: 10.1074/mcp.T600039-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Chu J, Zou Z, Hamacher NB, Rixon MW, Sun PD. Structure of FcgammaRI in complex with Fc reveals the importance of glycan recognition for high-affinity IgG binding. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(3):833–838. doi: 10.1073/pnas.1418812112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Marjon KD, Mold C, Du Clos TW, Sun PD. Pentraxins and Fc receptors. Immunological reviews. 2012;250(1):230–238. doi: 10.1111/j.1600-065X.2012.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Lu Z, Raso SW, Entrican C, Tangarone B. Dimers and multimers of monoclonal IgG1 exhibit higher in vitro binding affinities to Fcgamma receptors. mAbs. 2009;1(5):491–504. doi: 10.4161/mabs.1.5.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamadi C, Nharingo T. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresource technology. 2010;101(3):859–864. doi: 10.1016/j.biortech.2009.08.097. [DOI] [PubMed] [Google Scholar]
- Mahan AE, Tedesco J, Dionne K, Baruah K, Cheng HD, De Jager PL, Barouch DH, Suscovich T, Ackerman M, Cripsin M, Alter G. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. Journal of immunological methods. 2014 doi: 10.1016/j.jim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AE, Tedesco J, Dionne K, Baruah K, Cheng HD, De Jager PL, Barouch DH, Suscovich T, Ackerman M, Crispin M, Alter G. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. Journal of immunological methods. 2015;417:34–44. doi: 10.1016/j.jim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LN. Chromatographic fractionation of rhesus monkey (Macaca mulatta) IgG subclasses using deae cellulose and protein A-sepharose. Journal of immunological methods. 1982;50(3):319–329. doi: 10.1016/0022-1759(82)90170-3. [DOI] [PubMed] [Google Scholar]
- McAndrew EG, Dugast AS, Licht AF, Eusebio JR, Alter G, Ackerman ME. Determining the phagocytic activity of clinical antibody samples. J Vis Exp. 2011;(57):e3588. doi: 10.3791/3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimoto F, Katada H, Kadono S, Igawa T, Kuramochi T, Muraoka M, Wada Y, Haraya K, Miyazaki T, Hattori K. Engineered antibody Fc variant with selectively enhanced FcgammaRIIb binding over both FcgammaRIIa(R131) and FcgammaRIIa(H131) Protein engineering, design & selection : PEDS. 2013;26(10):589–598. doi: 10.1093/protein/gzt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. Journal of virology. 2011;85(20):10572–10581. doi: 10.1128/JVI.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MG, Ing JY, Cheng MK, Flitter BA, Moe GR. Identification of a phage-encoded Ig-binding protein from invasive Neisseria meningitidis. Journal of immunology. 2013;191(6):3287–3296. doi: 10.4049/jimmunol.1301153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namboodiri AM, Budkowska A, Nietert PJ, Pandey JP. Fc gamma receptor-like hepatitis C virus core protein binds differentially to IgG of discordant Fc (GM) genotypes. Molecular immunology. 2007;44(15):3805–3808. doi: 10.1016/j.molimm.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. Journal of immunological methods. 2005;306(1-2):151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinger GG, Jr, Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly M, Whaley KJ, Lear CM, Biggins JE, Scully C, Hensley L, Zeitlin L. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(44):18030–18035. doi: 10.1073/pnas.1213709109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, Smit JM, Fremont DH, Pierson TC, Heise MT, Diamond MS. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS pathogens. 2013;9(4):e1003312. doi: 10.1371/journal.ppat.1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey JP. Immunoglobulin GM allotypes as effect modifiers of cytomegalovirus-spurred neuroblastoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(11):1927–1930. doi: 10.1158/1055-9965.EPI-13-0612. [DOI] [PubMed] [Google Scholar]
- Pandey JP. Immunoglobulin GM Genes, Cytomegalovirus Immunoevasion, and the Risk of Glioma, Neuroblastoma, and Breast Cancer. Frontiers in oncology. 2014;4:236. doi: 10.3389/fonc.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para MF, Baucke RB, Spear PG. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. Journal of virology. 1980;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky E, Bastiaens PI, Zhu H, Snyder M, Fields S. Protein analysis on a proteomic scale. Nature. 2003;422(6928):208–215. doi: 10.1038/nature01512. [DOI] [PubMed] [Google Scholar]
- Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, Powell MS, Tan PS, Cendron AC, Wines BD, Scott AM, Hogarth PM. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. Journal of immunology. 2011;187(6):3208–3217. doi: 10.4049/jimmunol.1101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Molecular cancer therapeutics. 2008;7(8):2517–2527. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, Huizinga TW, Wuhrer M, van Schaardenburg D, Toes RE, Scherer HU. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Annals of the rheumatic diseases. 2015;74(1):234–241. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- Rozsnyay Z, Sarmay G, Walker M, Maslanka K, Valasek Z, Jefferis R, Gergely J. Distinctive role of IgG1 and IgG3 isotypes in Fc gamma R-mediated functions. Immunology. 1989;66(4):491–498. [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Schiffner L, Master A, Kajumo F, Guo Y, Dragic T, Moore JP, Binley JM. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. Journal of virology. 2000;74(11):5091–5100. doi: 10.1128/jvi.74.11.5091-5100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlothauer T, Rueger P, Stracke JO, Hertenberger H, Fingas F, Kling L, Emrich T, Drabner G, Seeber S, Auer J, Koch S, Papadimitriou A. Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. mAbs. 2013;5(4):576–586. doi: 10.4161/mabs.24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch A, Kettenberger H, Mundigl O, Winter G, Engert J, Heinrich J, Emrich T. Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(19):5997–6002. doi: 10.1073/pnas.1408766112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman MH, Derks RJ, Bondt A, Palmblad M, Schoenmaker B, Koeleman CA, van de Geijn FE, Dolhain RJ, Deelder AM, Wuhrer M. Fc specific IgG glycosylation profiling by robust nano-reverse phase HPLC-MS using a sheath-flow ESI sprayer interface. Journal of proteomics. 2012;75(4):1318–1329. doi: 10.1016/j.jprot.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K, Satoh M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Experimental hematology. 2009;37(3):309–321. doi: 10.1016/j.exphem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Shibata-Koyama M, Iida S, Okazaki A, Mori K, Kitajima-Miyama K, Saitou S, Kakita S, Kanda Y, Shitara K, Kato K, Satoh M. The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: an inhibitory element for high Fc gamma RIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 2009;19(2):126–134. doi: 10.1093/glycob/cwn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. The Journal of biological chemistry. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. The Journal of biological chemistry. 2003;278(5):3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- Sola RJ, Griebenow K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2010;24(1):9–21. doi: 10.2165/11530550-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerding AM, Kohl J, Pandey MK, Kuipers A, Leusen JH, Boross P, Nederend M, Vidarsson G, Weersink AY, van de Winkel JG, van Kessel KP, van Strijp JA. Staphylococcus aureus formyl peptide receptor-like 1 inhibitor (FLIPr) and its homologue FLIPr-like are potent FcgammaR antagonists that inhibit IgG-mediated effector functions. Journal of immunology. 2013;191(1):353–362. doi: 10.4049/jimmunol.1203243. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Jansen-Hendriks T, Kuijpers TW, de Haas M, van de Winkel JG, Hack CE, Bleeker WK. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimers: studies in experimental immune thrombocytopenia. Blood. 2001;98(4):1095–1099. doi: 10.1182/blood.v98.4.1095. [DOI] [PubMed] [Google Scholar]
- Teschner W, Butterweck HA, Auer W, Muchitsch EM, Weber A, Liu SL, Wah PS, Schwarz HP. A new liquid, intravenous immunoglobulin product (IGIV 10%) highly purified by a state-of-the-art process. Vox sanguinis. 2007;92(1):42–55. doi: 10.1111/j.1423-0410.2006.00846.x. [DOI] [PubMed] [Google Scholar]
- Thomann M, Schlothauer T, Dashivets T, Malik S, Avenal C, Bulau P, Ruger P, Reusch D. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PloS one. 2015;10(8):e0134949. doi: 10.1371/journal.pone.0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo S, Okazaki A, Wakitani M, Shinkawa T, Uchida K, Suzawa T. A chromatographic approach for elevating the antibody-dependent cellular cytotoxicity of antibody composites. Biological & pharmaceutical bulletin. 2009;32(9):1604–1608. doi: 10.1248/bpb.32.1604. [DOI] [PubMed] [Google Scholar]
- Tsuda E, Kawanishi G, Ueda M, Masuda S, Sasaki R. The role of carbohydrate in recombinant human erythropoietin. European journal of biochemistry/FEBS. 1990;188(2):405–411. doi: 10.1111/j.1432-1033.1990.tb15417.x. [DOI] [PubMed] [Google Scholar]
- Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nature biotechnology. 1999;17(2):176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- van der Poel CE, Spaapen RM, van de Winkel JG, Leusen JH. Functional characteristics of the high affinity IgG receptor, FcgammaRI. Journal of immunology. 2011;186(5):2699–2704. doi: 10.4049/jimmunol.1003526. [DOI] [PubMed] [Google Scholar]
- Vance BA, Huizinga TW, Wardwell K, Guyre PM. Binding of monomeric human IgG defines an expression polymorphism of Fc gamma RIII on large granular lymphocyte/natural killer cells. Journal of immunology. 1993;151(11):6429–6439. [PubMed] [Google Scholar]
- Varshney AK, Wang X, Aguilar JL, Scharff MD, Fries BC. Isotype switching increases efficacy of antibody protection against staphylococcal enterotoxin B-induced lethal shock and Staphylococcus aureus sepsis in mice. mBio. 2014;5(3):e01007–01014. doi: 10.1128/mBio.01007-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. Journal of immunology. 2012;188(9):4405–4411. doi: 10.4049/jimmunol.1200090. [DOI] [PubMed] [Google Scholar]
- Weber WJ, DiGiano FA. Process dynamics in environmental systems. New York: Wiley; 1996. [Google Scholar]
- Wei Y, Li C, Huang W, Li B, Strome S, Wang LX. Glycoengineering of human IgG1-Fc through combined yeast expression and in vitro chemoenzymatic glycosylation. Biochemistry. 2008;47(39):10294–10304. doi: 10.1021/bi800874y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TJ, Fuchs A, Colonna M. Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. Journal of immunology. 2012;188(10):4741–4745. doi: 10.4049/jimmunol.1102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TJ, Gilfillan S, Colonna M. Fc receptor-like A associates with intracellular IgG and IgM but is dispensable for antigen-specific immune responses. Journal of immunology. 2010;185(5):2960–2967. doi: 10.4049/jimmunol.1001428. [DOI] [PubMed] [Google Scholar]
- Winkler A, Berger M, Ehlers M. Anti-rhesus D prophylaxis in pregnant women is based on sialylated IgG antibodies. F1000Research. 2013;2:169. doi: 10.12688/f1000research.2-169.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Stam JC, van de Geijn FE, Koeleman CA, Verrips CT, Dolhain RJ, Hokke CH, Deelder AM. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7(22):4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. mAbs. 2009;1(3):230–236. doi: 10.4161/mabs.1.3.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Goetze AM, Flynn GC. Assessment of naturally occurring covalent and total dimer levels in human IgG1 and IgG2. Molecular immunology. 2014;58(1):108–115. doi: 10.1016/j.molimm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Madiyalakan R, Chawda RP, Matta KL. alpha-L-fucosidase from aspergillus niger: demonstration of a novel alpha-L-(1----6)-fucosidase acting on glycopeptides. Biochemical and biophysical research communications. 1986;136(2):563–569. doi: 10.1016/0006-291x(86)90477-8. [DOI] [PubMed] [Google Scholar]
- Zou G, Ochiai H, Huang W, Yang Q, Li C, Wang LX. Chemoenzymatic synthesis and Fcgamma receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcgammaIIIa receptor. Journal of the American Chemical Society. 2011;133(46):18975–18991. doi: 10.1021/ja208390n. [DOI] [PMC free article] [PubMed] [Google Scholar]


