Abstract
Esophageal adenocarcinoma (EAC) is characterized by resistance to chemotherapy and poor outcome. Although Cisplatin (CDDP) has been used as a first-line therapy in patients with EAC, resistance remains a major clinical problem. The AXL receptor tyrosine kinase, originally isolated as a transforming gene from leukemia, is overexpressed in several solid tumors. Herein, we assessed AXL protein expression in human EACs and examined its role in CDDP resistance in human EAC cells. AXL overexpression was detected in >50% of tumors examined. Elevating AXL in non-overexpressing cells doubled the sensitivity to CDDP cytotoxicity and increased cell survival 3-fold, whereas attenuating AXL in ovexpressing cells reduced survival 2-fold. The effects of AXL modulation on cell survival associated with changes in cellular and molecular markers of apoptosis. Mechanistic investigations revealed that AXL blocked CDDP-induced activation of endogenous p73β (TP73), reducing its protein half-life, and inhibited CDDP-induced levels of p-c-ABL(Y412) and p-p73β(Y99). These changes were associated with a disruption of c-ABL/p73β protein interactions due to association with c-ABL in the cytoplasm, thereby blocking nuclear accumulation of c-ABL and phosphorylation of p73β in response to DNA damage. Together, our results establish that AXL promotes CDDP resistance in esophageal adenocarconima and argue that therapeutic targeting of AXL may sensitize these cancers to DNA damaging drugs.
Keywords: AXL, cisplatin, CDDP, c-ABL, p73
Introduction
The incidence of esophageal adenocarcinoma (EAC) has sharply increased by 6-fold in the United States in the last few decades (1, 2). One of the major risk factors for developing EAC is gastroesophageal reflux disease (GERD), which may lead to Barrett's esophagus, a known pre-malignant lesion that transforms into invasive cancer by progressing through intermediate stages of low-grade and high-grade dysplasia (reviewed by (3)). Unfortunately, the majority of patients with EAC are mostly diagnosed at an advanced stage of the disease, which may reflect their poor prognosis (4). Generally, the treatment of advanced EAC involves neo-adjuvant chemotherapy and radiation followed by esophagectomy (5).
The cancer chemotherapeutic agent cisplatin (CDDP, cis-diamminedichloroplatinum) exerts its cytotoxic effect by inducing DNA damage and causing apoptosis through activation of the tumor-suppressor p53 protein (6). Although deficient p53 expression renders cancer cells less responsive to cisplatin-based chemotherapy, drug resistance is not complete. In fact, the p73 protein, a member of p53 family, can also induce apoptosis in response to genotoxic stress (7) and cisplatin (6). A previous study showed that cisplatin increased p73 protein levels through activation of the non-receptor tyrosine kinase c-ABL. Yuan et al, (8) demonstrated that c-ABL binds and phosphorylates the p73 protein on a tyrosine residue (Y99) in response to DNA damage, and disruption of the c-ABL/p73 interaction blocked apoptosis. Cisplatin alone or in combination with other drugs has been used in the treatment of patients with advanced esophageal cancer, but cisplatin resistance remains a serious clinical challenge (9, 10). In addition to aberrant p53 expression, negative regulation of p73 could potentially play a significant role in promoting cisplatin resistance. This is of major importance given the fact that the majority of EACs are deficient or mutant in p53 (11, 12).
The AXL receptor tyrosine kinase is a member of the TAM sub-family that also includes Tyro3 and Mer, and was originally isolated as a transforming gene from human leukemia cells (13, 14). Elevated AXL expression and interaction with its ligand Gas6 (growth arrest-specific 6) have been associated with cell survival, proliferation, and migration in solid tumors (15-17). These effects are mediated through activation of the MAPK/ERK and PI3K/AKT pathways (reviewed by Linger et al.) (18). A recent study identified AXL activation as a novel mechanism of acquired resistance to EGFR inhibitors in non-small cell lung cancer (19, 20). Overexpression of AXL has been reported in various neoplasms, including melanomas (21), lung (22), and breast cancers (23). A previous study indicated that AXL was increasingly up-regulated during a multistep esophageal carcinogenesis and as an adverse prognostic marker in EAC (24).
The primary aim of this report was to study the role of AXL in mediating CDDP resistance in EAC, and identify the molecular mechanism that regulates this effect. We have uncovered that the AXL protein is frequently overexpressed in human EAC primary tumors and cell lines. We also demonstrate that AXL suppresses c-ABL/p73 signaling in response to CDDP, thereby blocking apoptosis and promoting drug resistance in EAC. These findings provide evidence that AXL/c-ABL/p73 axis might be exploited as a therapeutic target to sensitize tumors to DNA-damaging drugs in EAC.
Materials and Methods
Cell lines and reagents
The human esophageal adenocarcinoma cancer cell lines OE33, OE19, SK-GT-4, and FLO-1 were kindly provided by Dr. David Beer (University of Michigan, Ann Arbor, MI). JH-EsoAd1 cell line was a kind gift from Dr. James Richard Eshleman (Johns Hopkins, Baltimore, MD). HEK-293 cells were purchased from ATCC (Manassas, VA). These cells were maintained in F12 (HAM) medium (GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen Life Technologies, Carlsbad, CA) and 1% penicillin/streptomycin (GIBCO). The immortalized cell lines originated from normal esophageal squamous epithelia, HEEC, was purchased from ScienCell Research Laboratories (Carlsbad, CA); and EPC2, was kindly provided by Dr. Hiroshi Nakagawa (University of Pennsylvania, Philadelphia, PA). HEEC cells were cultured in EpiCM-2 medium (ScienCell) supplemented with 5% FBS. EPC2 cells were grown in keratinocyte SFM medium supplemented with 40 mg/ml bovine pituitary extract and 1 ng/ml epidermal growth factor (Invitrogen). Cisplatin (CDDP, cis-diamminedichloroplatinum) (APP Pharmaceutical, LLC., Schaumburg, IL) stock solution (3.3 mmol/L) prepared in sterile water was provided by TVC Outpatient Pharmacy, Vanderbilt University Medical Center, Nashville, TN. Cycloheximide was purchased from Sigma-Aldrich. AXL, PUMA, c-ABL, p-c-ABL(Y412), cleaved caspase-3 and -9, cleaved PARP, and β-actin antibodies were obtained from Cell Signaling Technology (Danvers, MA). p-AXL(Y779) and HDM2 antibodies were purchased from R&D Systems (Minneapolis, MN) and Calbiochem (Billerica, MA), respectively. p73 antibody was obtained from Bethyl Laboratories (Montgomery, TX). Lamin B antibody was purchased Santa Cruz Biotechnology (Santa Cruz, CA).
AXL expression and plasmids
The constructs of pcDNA4/AXL-myc-His and pcDNA4 (a gift from Dr. Rosa Marina Melillo, University of Naples, Italy) (25) were utilized to generate stable expression cells. Briefly, OE33 cells were transfected using Iipofectamine 2000 (Invitrogen). Stably transfected OE33 cells expressing AXL or vector control (empty pcDNA4) were selected with 100 μg/ml zeocin (Invitrogen) following standard protocols (26).
The AXL coding sequence from pcDNA3.1/AXL plasmid was sub-cloned into the adenoviral shuttle vector (pACCMV). The recombinant adenovirus-expressing AXL was generated by co-transfecting HEK-293 cells with the shuttle and backbone adenoviral (pJM17) plasmids using the Calcium Phosphate Transfection kit (Applied Biological Materials Inc., Richmond, BC). The pcDNA3/FLAG-p73β and pcDNA3/GFP-c-Abl IV (mouse type IV) plasmids were kindly provided by Dr. Alex Zaika (Vanderbilt University Medical Center, Nashville, TN). The pcDNA3-lacZ plasmid was a gift from Dr. Michael K. Cooper (Vanderbilt University, Nashville, TN).
Small hairpin RNA
Lentivirus particles expressing control shRNA or a cocktail of five different clones of AXL shRNA were produced and validated by Sigma-Aldrich. FLO-1 cells that express high levels of endogenous AXL were transduced with lentivirus particles and selected with 1 μg/ml puromycin for 10 days.
Immunoblot analysis
Cells were lysed in RIPA buffer (50 mmol/L Tris-HCl buffer, pH 7.4, 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) supplemented with 1× Halt protease inhibitor cocktail and 1× Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL). Proteins were separated and Western blot analysis was carried out as described previously (27).
Immunoprecipitation
Cells were lysed in RIPA buffer supplemented with 1% Halt protease and phosphatase inhibitors (Pierce). The protein concentration was determined by the Bio-Rad Protein Assay. Immunoprecipitations of equal total protein amounts (200 μg) were performed at room temperature for 1h by using a primary antibody previously bound to 50 μl Dynabeads Protein G (Invitrogen). The beads were washed three times with ice-cold PBS. The beads in each tube were heated to 100°C for 5 min in 30 μl of 2× sample buffer. The proteins were then eluted by magnet and subjected to immunoblot analysis.
Immunohistochemistry
Tissue microarrays (TMA) containing 27 de-identified archival cases of esophageal adenocarcinomas, including 7 esophageal normal epithelial tissue samples, were kindly provided by Dr. Wael El-Rifai (Vanderbilt University Medical Center, Nashville, TN). 5 μm of TMA sections were used for IHC staining of AXL receptor tyrosine kinase with polyclonal goat AXL antibody (1:200) (AF154; R&D Systems). The intensity and frequency of staining were graded as described previously (28).
Cell viability assay
Cells (5×103 per well) were seeded in triplicate onto a 96-well plate. The next day, cells were treated with vehicle or various concentrations of CDDP for 48h. Cell viability was determined using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI) following the supplier's instructions.
Clonogenic survival assay
Cells were plated in triplicate at low density (2×103 per well) in 6-well plates. The next day, cells were treated with vehicle or CDDP for 48h. Culture media were replaced and cells were grown for two weeks. Cell colonies were then fixed with 2% paraformaldehyde and stained with 0.05% crystal violet. Cell colonies were semi-quantitatively analyzed by densitometry using ImageJ software (NIH Image).
Apoptosis analysis
Cells (105 per well) were plated in triplicate in 6-well plates and treated with vehicle or CDDP for 48h. Cells were then harvested and stained with Annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (PI) (R&D Systems). The samples were subjected to fluorescence-activated cell sorting (FACS) analysis by a flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Apoptotic cell death was determined by counting the cells that stained positive for Annexin-V FITC and negative for PI.
Cycloheximide (CHX)-based 73β protein stability assay
OE33 cells were transiently co-transfected with pcDNA3/p73β-Flag in combination with pcDNA4/AXL-myc-His or pcDNA4 vector control using lipofectamine 2000 (Invitrogen). Next day, cells were treated with 80 μg/ml of CHX and harvested at different time points. Proteins were analyzed by Western blotting to evaluate p73β protein stability. Protein bands intensities were semi-quantitatively analyzed by densitometry using ImageJ software (NIH Image). The p73β protein bands intensities for each time point were normalized to their corresponding β-actin. The protein degradation curves were generated by plotting relative band intensities as a function of the time-period of CHX treatment. Linear regression was applied and the protein half-life (t1/2) of p73β, which is expressed as the time for degradation of 50% of the protein, was calculated from the fitted line equation (29).
Luciferase assay
To investigate the transcriptional activity of p73, we used the PG13-Luc vector that contains 13-tandem repeats of the p53 consensus DNA binding site. In cells harboring non-functional p53, activated endogenous p73 protein binds to the p53 binding site, thereby transcription is induced and the reporter gene is expressed (30). OE33 cells stably expressing AXL or pcDNA4 empty vector, and OE19 cells infected with recombinant adenovirus expressing AXL or vector control were seeded in triplicate in 24-well plates (25×103 per well). The next day, cells were transiently co-transfected with 200 ng of the PG13-Luc and 100 ng of pcDNA3-lacZ plasmid, under the control of CMV, using Fugene 6 (Promega) according to the manufacturer's instructions. The next day, cells were treated with CDDP (10 μmol/L) for 48h. The luciferase activity was measured using the luciferase reporter assay kit (Promega) according to the manufacturer's instructions. The β-galactosidase activity was determined by incubation of cell lysates with the enzyme substrate ONP-β-D-galactopyranoside, and measuring light absorbance at 410 nm. Firefly luciferase activities were normalized to β-galactosidase levels.
Statistical analysis
The results were expressed as the mean with ± SD. The statistical significance of the studies was determined by either the parametric unpaired Student's t test or two-way ANOVA followed by Bonferroni post hoc test. Differences with ≤0.05 are considered significant. The difference in AXL protein expression between normal and EAC tissue samples was assessed by Fisher's exact test.
Results
Frequent overexpression of AXL in human esophageal adenocarcinoma
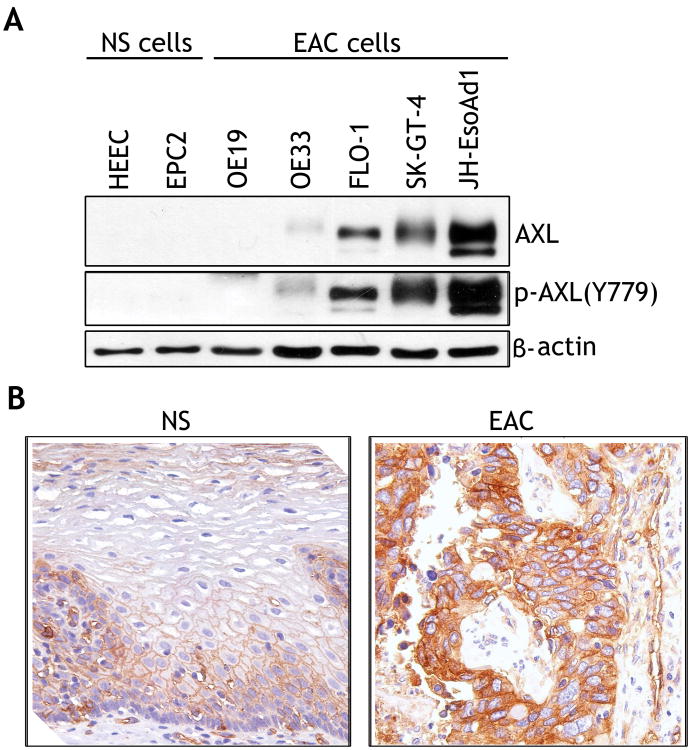
The Western blot analysis data indicated increased levels of AXL and p-AXL(Y779) proteins in 3/5 EAC cell lines, and 2/2 normal esophageal squamous cell lines were negative for AXL expression (Figure 1A). We evaluated AXL protein expression in tissue microarrays containing 27 EAC and 7 esophageal normal squamous tissue samples by immunohistochemical staining with anti-AXL specific antibody. The IHC data showed that AXL expression was relatively low in normal tissue samples, as depicted by weak membrane staining predominantly in the lower third of the mucosa (Figure 1B, left panel). In contrast, in some EAC tissue samples, AXL was highly expressed as indicated by strong cytoplasmic and membrane staining (Figure 1B, right panel). A comprehensive analysis of the IHC data, whereby IHC index cutoff of 3 was used to indicate overexpression, revealed that AXL was overexpressed in 51.8% of EAC tumor samples (14/27) (Table 1). None of the tested normal tissue samples exhibited AXL overexpression (IHC index ≤2) (Table 1). The difference in AXL protein expression between normal tissue samples and EAC was statistically significant (p= 0.0068).
Figure 1. AXL is overexpressed in esophageal adenocarcinomas.
A) Protein extracts from normal esophageal squamous epithelial cell lines (NS) and esophageal adenocarcinoma cell lines (EAC) were subjected to Western blot analysis of AXL and p-AXL(Y779) proteins. B) A representative AXL immunohistochemical staining (IHC) of normal esophageal squamous epithelial tissue sample (NS) (left panel), showing a weak membrane staining (brown color) predominantly in the lower third of the mucosa. A representative AXL IHC staining of a moderately differentiated esophageal adenocarcinoma tissue sample (EAC) (right panel) indicating a strong cytoplasmic and membrane staining.
Table 1. AXL receptor tyrosine kinase is frequently overexpressed in esophageal adenocarcinomas.
| IHC Index Score | ||||
|---|---|---|---|---|
|
|
||||
| 0 - 1 | 2 | 3 | * p value | |
| NS | 2 | 5 | 0 | 0.0068 |
| EAC | 8 (29.6%) | 5 (18.5%) | 14 (51.8%) | |
Abbreviations: IHC, immunohistochemistry; NS, normal esophageal squamous epithelial tissue; EAC, esophageal adenocarcinoma tissue
The Fisher's exact test to assess the difference in AXL index score between normal and tumor tissue samples
AXL enhances cell survival in esophageal adenocarcinoma
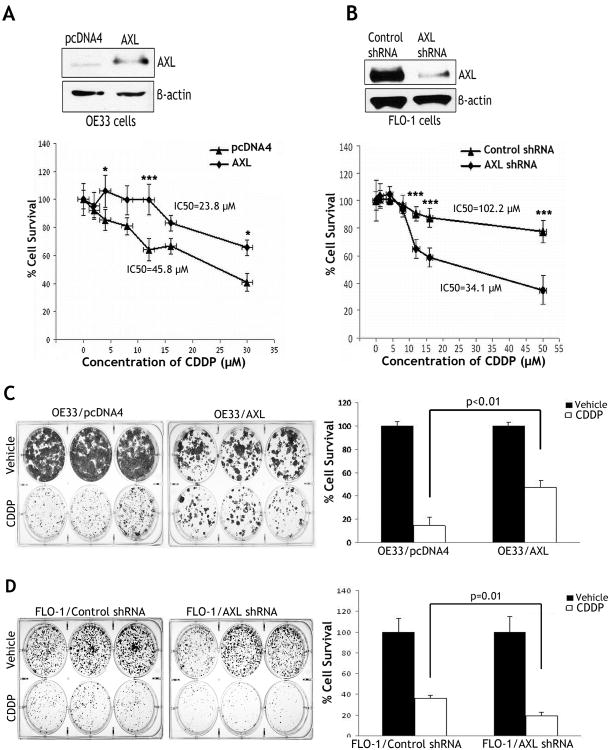
The cell viability assay data indicated that reconstitution of AXL expression in OE33 cells significantly increased cell survival relative to control cells in response to 48h treatment with various concentrations of CDDP (p<0.05) (Figure 2A). In fact, the CDDP IC50 was 45.8 μmol/L in AXL-expressing cells as opposed to 23.8 μmol/L in control cells (Figure 2A). To further confirm the role of AXL in regulating cell survival, we subjected FLO-1 cells stably expressing AXL shRNA or control shRNA to the CellTiter cell viability assay after treatment with various concentrations of CDDP for 48h. The results clearly indicated that knockdown of endogenous AXL significantly sensitized cells to CDDP (p<0.001) (Figure 2B). In fact, the CDDP IC50 was 102.2 μmol/L in FLO-1/control shRNA cells, and 34.1 μmol/L in FLO-1/AXL shRNA cells (Figure 2B).
Figure 2. AXL promotes survival of esophageal adenocarcinoma cells.
A) Cell viability of OE33 cells stably expressing AXL or empty vector in response to CDDP was assessed by CellTiter-Glo Luminescent Cell Viability Assay. Western blot analysis of AXL in OE33/AXL and OE33/pcDNA4 stable cells is shown (upper panel). Cell survival of AXL-expressing cells was significantly higher than control cells in response to CDDP (lower panel). B) Cell viability of FLO-1 cells stably expressing AXL shRNA or control shRNA in response to CDDP was determined as in panel A. Immunoblot of AXL is shown (upper panel). Knockdown of AXL in FLO-1 cells significantly decreased cell viability in response to CDDP (lower panel). C) OE33 cells stably expressing AXL or pcDNA4 were subjected to clonogenic survival assay after treatment with vehicle or CDDP (2.5 μmol/L) for 48h. Quantitative data (right panel) showed significantly higher cell survival in AXL-expressing cells than control cells (p<0.01). D) FLO-1 cells stably expressing AXL shRNA or control shRNA were treated with CDDP (5 μmol/L) for 48h, and subjected to clonogenic survival assay. Quantitative data (right panel) indicated that knockdown of endogenous AXL significantly decreased cell survival relative to control in response to CDDP (p=0.01). Results are representative of at least three experiments and shown as the mean ± SD. *p<0.05, ***p<0.001.
To confirm the short-term survival assay data, we subjected OE33 cells stably expressing AXL or empty vector, and FLO-1 cells stably expressing AXL shRNA or control shRNA to long-term clonogenic survival assay. The data indicated that the reconstitution of AXL expression in OE33 cells enhanced cell survival by 3-fold relative to control (p<0.01) in response to CDDP (Figure 2C). Conversely, knockdown of endogenous AXL in FLO-1 cells decreased cell survival by approximately 2-fold relative to control (p=0.01) in response to CDDP (Figure 2D).
AXL suppresses DNA damage-induced apoptosis and activation of caspases
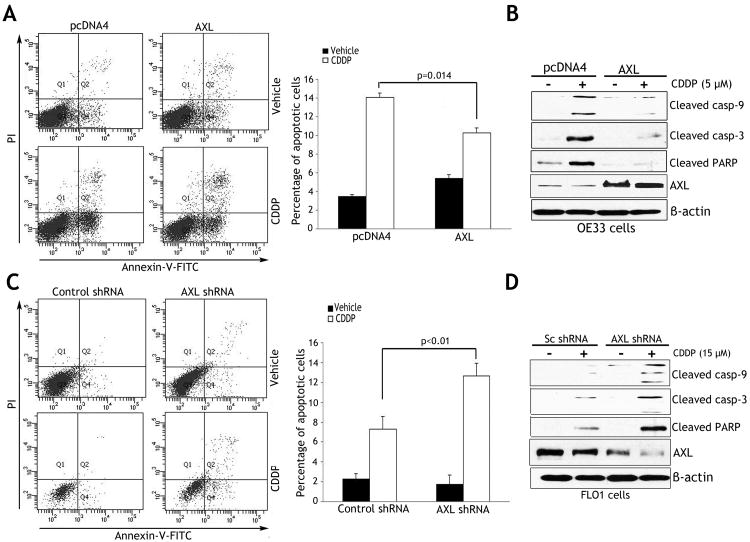
The Annexin V/PI staining and FACS analysis data indicated that the reconstitution of AXL expression in OE33 cells inhibited early apoptosis events by 25% relative to control in response to CDDP (p=0.014) (Figure 3A). In line with this result, Western blot analysis indicated significantly higher protein levels of cleaved forms of caspase-9 and -3, and PARP in control cells than AXL-expressing cells after treatment with CDDP (Figure 3B). In contrast, knockdown of endogenous AXL in FLO-1 cells increased early apoptosis by 73.5% relative to control (p<0.01) in response to CDDP (Figure 3C). Accordingly, Western blot analysis results showed that knockdown of endogenous AXL significantly increased protein levels of cleaved forms of caspase-9 and -3, and PARP relative to control in response to CDDP (Figure 3D).
Figure 3. AXL expression inhibits CDDP-induced apoptosis.
A) Apoptosis in OE33 cells stably expressing AXL or empty vector after treatment with vehicle or CDDP (10 μmol/L) for 48h, was determined by Annexin-V/propidium iodide (PI) staining and FACS analysis. Quantitative data (right panel) showed significantly less apoptosis in AXL-expressing cells than control cells (p=0.014) in response to CDDP. B) Western blot analysis of cleaved caspase-3 and -9, cleaved PARP, and AXL proteins in OE33 cells after treatment with vehicle or CDDP as described in panel A. C) Apoptosis in FLO-1 cells stably expressing AXL shRNA or control shRNA after treatment with vehicle or CDDP (15 μmol/L) for 48h was evaluated as in panel A. Quantitative data (right panel) indicated that knocking down endogenous AXL induced significantly more apoptosis than control cells (p<0.01) in response to CDDP. D) Western blot analysis of cleaved caspase-3 and -9, cleaved PARP, and AXL proteins in FLO-1 cells after treatment with vehicle or CDDP as described in panel C. Results are representative of at least three experiments and shown as the mean ± SD.
AXL blocks DNA damage-induced activation and nuclear accumulation of p73 and decreases p73 protein stability
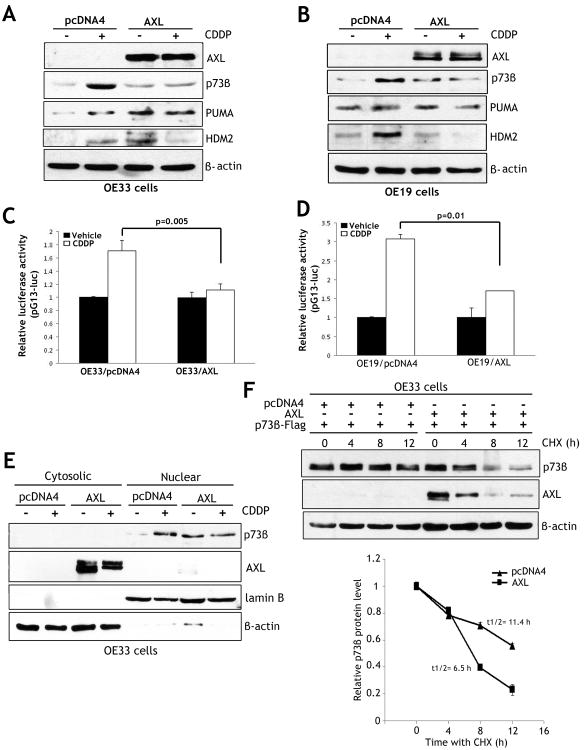
To examine if AXL expression has an effect on DNA damage-induced activation of p73, we utilized OE33 and OE19 cell models. Western blot analysis data indicated that endogenous p73β protein level was significantly higher in control cells than AXL-expressing cells in response to CDDP (Figures 4A&4B). Accordingly, protein expression of p73 downstream transcriptional targets PUMA and HDM2 was significantly induced by CDDP in control cells but not in AXL-expressing cells (Figures 4A&4B). Consistent with these data, qRT-PCR analysis showed that relative mRNA expression of PUMA and HDM2 was 3.5-fold and 2-fold higher in control cells than AXL-expressing cells, respectively, in response to CDDP (Supplemental Figure 1). To confirm the role of AXL in regulating p73 transcriptional activity, we performed the luciferase reporter assay using pG13-luc plasmid. In OE33 cells, luciferase activity was 54.7% higher in control cells than AXL-expressing cells (p=0.005) after treatment with CDDP (Figure 4C). Similarly, in OE19 cells, the luciferase activity was 81.1% higher in control cells than AXL-expressing cells (p=0.01) in response to CDDP (Figure 4D). Western blot analysis of cytosolic and nuclear protein fractions unequivocally confirmed CDDP-induced accumulation of p73β protein in the nucleus in OE33 control cells (Figure 4E). Conversely, CDDP treatment had no effect on p73β level or localization in AXL-expressing OE33 cells (Figure 4E).
Figure 4. AXL blocks CDDP-induced activation and nuclear accumulation of p73 and decreases its protein stability.
A-B) Western blot analysis of AXL, p73β, PUMA, and HDM2 proteins in OE33 cells stably expressing AXL or pcDNA4, and OE19 cells infected with control adenovirus (10 MOI) or AXL adenovirus (10 MOI). All cells were treated with vehicle or CDDP (10 μmol/L) for 24h. The treatment with CDDP increased levels of p73β, PUMA, and HDM2 proteins, and these effects were abrogated by AXL. C-D) The pG13 luciferase activity was 54.5% higher in OE33/pcDNA4 control cells than OE33/AXL cells (p=0.005), and 81.1% higher in OE19/pcDNA4 control cells than OE19/AXL cells (p=0.01) in response to CDDP. E) Protein accumulation and localization of endogenous p73β in OE33/AXL and OE33/pcDNA4 stable cells was evaluated by Western blot analysis of cytosolic and nuclear protein fractions after treatment with vehicle or CDDP (10 μmol/L) for 48h. AXL expression blocked CDDP-induced nuclear accumulation of p73β. F) Protein stability of exogenous p73β transiently expressed in OE33/AXL or OE33/pcDNA4 stable cells was assessed by Western blot analysis after treatment with 80 μg/ml CHX for the indicated times. The protein degradation results indicate that AXL reduced the protein half-life of p73β from 11.4h to 6.5h relative to control (lower panel).
Based on our finding that AXL suppressed CDDP-induced activation of p73β, we hypothesized that AXL could potentially regulate p73β protein stability. To test this hypothesis, we assessed the protein stability of exogenous p73β transiently expressed in OE33/AXL or OE33/pcDNA4 stable cells by Western blot analysis after treatment with CHX. Indeed, the protein degradation data indicated that AXL significantly reduced the protein half-life of p73β from 11.4h to 6.5h relative to control (Figure 4F).
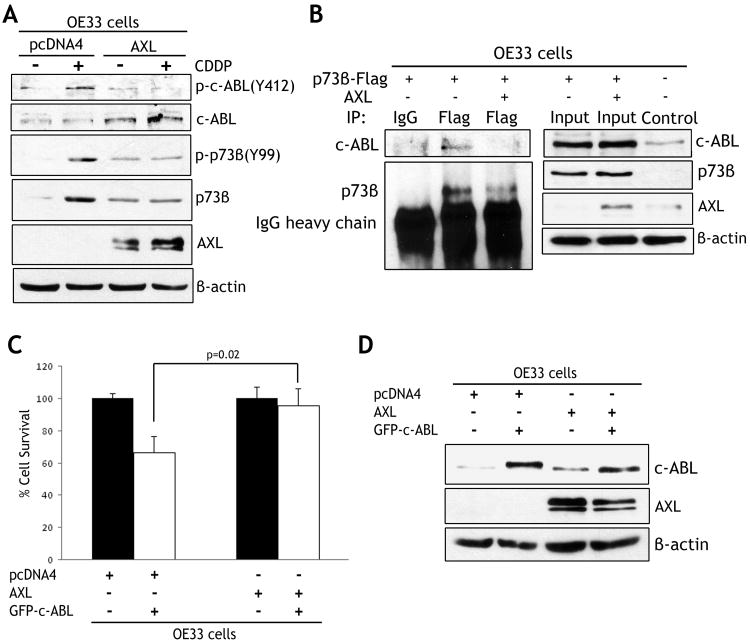
AXL attenuates DNA damage-induced phosphorylation of c-ABL and disrupts c-ABL/p73 protein association
As the non-receptor tyrosine kinase c-ABL is the primary regulator of p73 in response to DNA damage, we postulated that AXL could negatively regulate c-ABL, thereby inhibiting p73 activation. Western blot analysis data indicated that CDDP treatment significantly increased p-c-ABL(Y412) and p-p73β(Y99) in OE33/pcDNA4 cells. Conversely, AXL expression substantially attenuated CDDP-induced phosphorylation of c-ABL and p73β proteins in OE33/AXL cells (Figure 5A). Interestingly, the basal levels of c-ABL and p73β proteins were higher in AXL-expressing cells than control cells (Figure 5A). We next examined if AXL interferes with c-ABL binding to p73β in OE33 cells. Immunoprecipitation and Western blot data indicated that endogenous c-ABL bound to exogenous p73β protein as expected. However, the reconstitution of AXL expression significantly disrupted the c-ABL/p73β protein complex (Figure 5B). To confirm that AXL blocked CDDP-induced apoptosis through regulation of c-ABL, we verified whether AXL could directly suppress c-ABL-induced cell death. The cell viability assay data showed that transient expression of c-ABL in OE33 cells induced approximately 40% less cell survival relative to control cells (p=0.02) (Figure 5C). On the other hand, expression of AXL in combination with c-ABL completely restored survival to the level of control cells (Figure 5C). Western blot analysis data confirmed protein expression of c-ABL alone or in combination with AXL in OE33 cells (Figure 5D).
Figure 5. AXL blocks binding of c-ABL to p73β protein and attenuates CDDP-induced phosphorylation of c-ABL.
A) OE33 cells stably expressing AXL or pcDNA4 were treated with vehicle or CDDP (10 μmol/L) for 48h. The Western blot data indicate that CDDP treatment significantly increased p-c-ABL(Y412), p-p73β(Y99), and p73β protein levels in control cells. In contrast, AXL expression abrogated these effects in response to CDDP. B) Western blot analysis of immunoprecipitated exogenous p73β protein with M2-flag antibody in OE33 cells transfected with p73β-Flag alone or in combination with AXL. Exogenous p73β interacted with endogenous c-ABL protein, and the p73β/c-ABL protein complex was disrupted by AXL. C) Cell viability of OE33 cells transfected with pcDNA4 alone, AXL alone or in combination with GFP-c-ABL was assessed 48h post-transfection. AXL expression blocked c-ABL-induced cell death. D) Western blot analysis of c-ABL and AXL proteins in OE33 cells transiently transfected as in panel C.
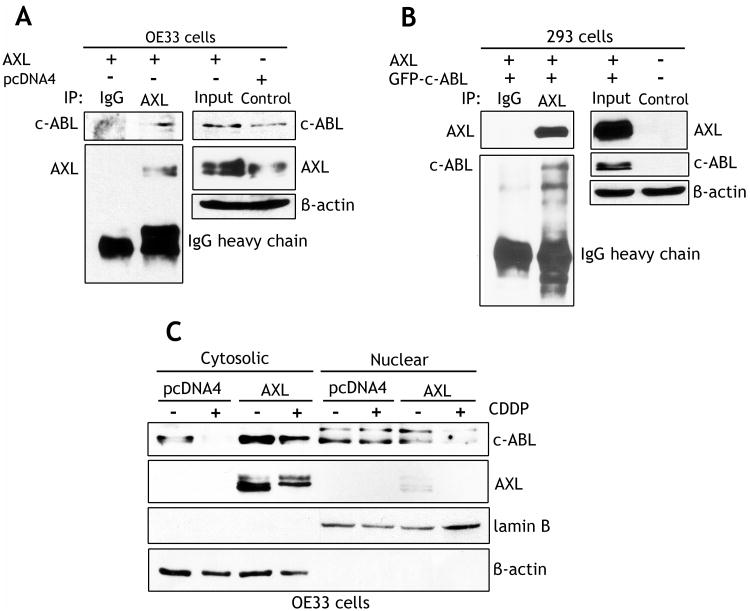
AXL associates with c-ABL protein and prevents DNA damage-induced accumulation of c-ABL in the nucleus
Based on our results showing that AXL interfered with c-ABL binding to p73, and blocked phosphorylation of c-ABL, we hypothesized that AXL could interact with c-ABL forming a protein complex that prevents accumulation of c-ABL in the nucleus and its interaction with p73 in response to DNA damage. To test this hypothesis, we performed immunoprecipitation with the AXL antibody followed by Western blot analysis of AXL and c-ABL proteins in OE33 and HEK-293 cells. The data demonstrated protein association of exogenous AXL with endogenous c-ABL in OE33 cells (Figure 6A), or with exogenous c-ABL in HEK-293 cells (Figure 6B). We next treated OE33 cells stably expressing AXL or pcDNA4 with vehicle or CDDP (10 μmol/L) for 48h, and subjected the cytosolic and nuclear protein fractions to Western blot analysis of c-ABL and AXL proteins. The results clearly indicated that cytosolic c-ABL significantly decreased in response to CDDP in control cells, but AXL expression counteracted this effect (Figure 6C). Overall, the protein level of cytosolic c-ABL was significantly higher in AXL-expressing cells than control cells. Conversely, the nuclear c-ABL protein expression level was higher in control cells than AXL-expressing cells (Figure 6C). In addition, the data showed that AXL protein expression was limited to the cytosolic fraction (Figure 6C). Taken together, these results strongly suggest that AXL sequesters c-ABL in the cytosol and prevents targeting of c-ABL to the nucleus in response to DNA damage.
Figure 6. AXL interacts with c-ABL protein and attenuates CDDP-induced accumulation of c-ABL in the nucleus.
A) Western blot analysis of immunoprecipitated proteins with AXL antibody in OE33 cells stably expressing pcDNA4 or AXL. The data demonstrate protein association of AXL with endogenous c-ABL. B) Western blot analysis of immunoprecipitated proteins with AXL antibody in HEK-293 cells transiently co-transfected with AXL and GFP-c-ABL. The results indicate protein interaction between AXL and exogenous c-ABL. C) Western blot analysis of cytosolic and nuclear fractions of OE33 cells stably expressing pcDNA4 or AXL and treated with vehicle or CDDP (10 μmol/L) for 48h. The data show accumulation of c-ABL in the nucleus in control cells whereas c-ABL was mainly sequestered in the cytosol and undetected in the nucleus in AXL-expressing cells in response to CDDP.
Discussion
Although the DNA damaging agent CDDP alone or in combination with other drugs has been used as a first-line therapy in patients with advanced esophageal cancer, resistance to CDDP, unfortunately, remains a major clinical problem (9, 10). Identification of the mechanisms that control resistance to CDDP is essential to predicting response to therapy, and to developing new drugs that can overcome resistance. Based on the fact that AXL was implicated in promoting cell survival and proliferation through activation of downstream growth and survival pathways in various cancers (15, 18), we evaluated AXL protein expression and examined its role in CDDP resistance in EAC.
In this study, we demonstrated frequent overexpression of the AXL protein in EAC primary tumors (51.8%) and showed that AXL was exclusively expressed in EAC cell lines, but not in normal esophageal squamous cell lines. Our results clearly indicated that reconstitution of AXL expression enhanced survival and attenuated apoptosis after treatment with CDDP. Conversely, knockdown of endogenous AXL sensitized cells to CDDP. Our findings strongly indicate that AXL promotes resistance to CDDP in EAC cells.
CDDP induces DNA damage causing apoptosis through activation of the tumor suppressor p53 protein (6). However, CDDP can also induce apoptosis in p53-deficient cells through activation of the p73 protein, a member of the p53 family (6). This is particularly of major importance, given the majority of EACs are deficient or mutant in p53 (11). To identify the mechanism by which AXL mediates resistance to CDDP, we hypothesized that AXL could block activation of p73 in response to CDDP in EAC cells. Indeed, our data showed that the reconstitution of AXL expression significantly attenuated CDDP-induced transcriptional activation of endogenous p73β as confirmed by decreased levels of p73β, PUMA, and HDM2 proteins, and PG13-luc luciferase activity. Interestingly, AXL expression increased the basal endogenous p73β protein level, albeit blocking CDDP-induced activation of p73β. This suggests that the reconstitution of AXL oncoprotein expression in our p53-deficient EAC cells could decrease growth rate or induce some apoptosis through activation of p73. Consistent with this observation, our results indicated significantly less colony formation (Figure 2C) and more apoptosis (Figure 3A) in vehicle-treated AXL-expressing cells than control cells. A previous report showed that endogenous p73α and p73β proteins were up-regulated in p53-deficient cancer cells in response to oncogenes (30). Additional studies will be required to fully determine the mechanism by which AXL regulates endogenous p73 basal expression independent of stress signals. As the protein stability of p73 was shown to be enhanced by CDDP (6), we postulated that AXL could destabilize p73 protein, thereby blocking its activation in response to DNA damage. Indeed, we demonstrated that AXL expression significantly decreased the somewhat stable exogenous p73β protein half-life from 11.4 h to 6.5 h.
Several studies indicated that the functional non-receptor tyrosine kinase, c-ABL, is required for CDDP-induced up-regulation of p73 protein stability, as activated c-ABL binds through its SH3 domain to the carboxy-terminal homo-oligomerization domain of p73 and phosphorylates it on a tyrosine residue at position 99 in response to DNA damage (6, 8, 31). We confirmed that AXL blocked CDDP-induced phosphorylation of c-ABL and p73β proteins, and demonstrated that AXL expression disrupted c-ABL/p73β protein association. Accordingly, our data showed that AXL significantly attenuated c-ABL-induced cell death, strongly suggesting that AXL regulation of p73 is mediated by c-ABL. c-ABL localization is controlled by three nuclear localization signals (NLS) and one nuclear export signal (NES) that are responsible for shuttling of c-ABL between the cytoplasm and nucleus (32). It has been shown that nuclear c-ABL relays pro-apoptotic signals from ATM to p53 and p73 in response to DNA damage (8, 33). Another study indicated that c-ABL increases p73 protein levels in the nucleus in a kinase-dependent manner. Phosphorylation of p73 (Y99) promotes tight interaction with the SH2 domain of c-ABL that may enhance p73 protein stability (34). Based on these studies and our findings, we postulated that AXL interacts with cytosolic c-ABL, thereby blocking nuclear accumulation of c-ABL in the apoptotic response to DNA damage. Our results clearly demonstrated protein association between AXL and either exogenous or endogenous c-ABL, although we did not confirm direct binding of AXL to c-ABL. Furthermore, we showed that AXL blocked nuclear accumulation of c-ABL protein in response to DNA damage, hence disrupting the apoptotic signaling cascade. In a similar study, Raina et al (35) indicated that the MUC1 transmembrane glycoprotein sequesters c-ABL in the cytoplasm and thereby attenuates apoptosis in response to anticancer agents mediated DNA damage. Interestingly, our data showed that AXL dramatically increased the basal protein expression level of c-ABL in the cytosol independent of DNA damage response. Further studies will be necessary to elucidate the mechanism by which AXL regulates cytosolic c-ABL expression, and investigate its functional implication.
In conclusion, our results indicate that frequent overexpression of AXL in EAC underlies a CDDP resistance phenotype. We demonstrate that AXL expression inhibits c-ABL/p73 signaling in response to DNA damage, hence blocking apoptosis and mediating drug resistance in EAC. Therefore, our findings provide evidence that AXL/c-ABL/p73 axis could be exploited as a therapeutic target to sensitize tumors to DNA-damaging drugs in EAC.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by grants from Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103) and the Vanderbilt Digestive Disease Research Center (DK058404). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of Vanderbilt University.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Zhang HY, Spechler SJ, Souza RF. Esophageal adenocarcinoma arising in Barrett esophagus. Cancer Lett. 2009;275:170–7. doi: 10.1016/j.canlet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Kountourakis P, Ajani JA, Davila M, Lee JH, Bhutani MS, Izzo JG. Barrett's Esophagus: A Review of Biology and Therapeutic Approaches. Gastrointest Cancer Res. 2012;5:49–57. [PMC free article] [PubMed] [Google Scholar]
- 4.Tomizawa Y, Wang KK. Screening, surveillance, and prevention for esophageal cancer. Gastroenterol Clin North Am. 2009;38:59–73. viii. doi: 10.1016/j.gtc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku GY, Ilson DH. Preoperative therapy for esophageal cancer. Gastroenterol Clin North Am. 2009;38:135–52. ix. doi: 10.1016/j.gtc.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 7.Lin KW, Nam SY, Toh WH, Dulloo I, Sabapathy K. Multiple stress signals induce p73beta accumulation. Neoplasia. 2004;6:546–57. doi: 10.1593/neo.04205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan ZM, Shioya H, Ishiko T, et al. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–7. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 9.Grunberger B, Raderer M, Schmidinger M, Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27:2705–14. [PubMed] [Google Scholar]
- 10.Gravalos C, Gomez-Martin C, Rivera F, et al. Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol. 2011;13:179–84. doi: 10.1007/s12094-011-0637-6. [DOI] [PubMed] [Google Scholar]
- 11.Schneider PM, Stoeltzing O, Roth JA, et al. P53 mutational status improves estimation of prognosis in patients with curatively resected adenocarcinoma in Barrett's esophagus. Clin Cancer Res. 2000;6:3153–8. [PubMed] [Google Scholar]
- 12.Hamelin R, Flejou JF, Muzeau F, et al. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett's esophagus. Gastroenterology. 1994;107:1012–8. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 13.O'Bryan JP, Frye RA, Cogswell PC, et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–31. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neubauer A, O'Bryan JP, Fiebeler A, Schmidt C, Huhn D, Liu ET. Axl, a novel receptor tyrosine kinase isolated from chronic myelogenous leukemia. Semin Hematol. 1993;30:34. [PubMed] [Google Scholar]
- 15.Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–53. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204:36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- 18.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postel-Vinay S, Ashworth A. AXL and acquired resistance to EGFR inhibitors. Nat Genet. 2012;44:835–6. doi: 10.1038/ng.2362. [DOI] [PubMed] [Google Scholar]
- 21.van Ginkel PR, Gee RL, Shearer RL, et al. Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer Res. 2004;64:128–34. doi: 10.1158/0008-5472.can-03-0245. [DOI] [PubMed] [Google Scholar]
- 22.Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001;37:2264–74. doi: 10.1016/s0959-8049(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 23.Berclaz G, Altermatt HJ, Rohrbach V, Kieffer I, Dreher E, Andres AC. Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Ann Oncol. 2001;12:819–24. doi: 10.1023/a:1011126330233. [DOI] [PubMed] [Google Scholar]
- 24.Hector A, Montgomery EA, Karikari C, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10:1009–18. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avilla E, Guarino V, Visciano C, et al. Activation of TYRO3/AXL tyrosine kinase receptors in thyroid cancer. Cancer Res. 2011;71:1792–804. doi: 10.1158/0008-5472.CAN-10-2186. [DOI] [PubMed] [Google Scholar]
- 26.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005;65:6583–92. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 27.Belkhiri A, Dar AA, Zaika A, Kelley M, El-Rifai W. t-Darpp promotes cancer cell survival by up-regulation of Bcl2 through Akt-dependent mechanism. Cancer Res. 2008;68:395–403. doi: 10.1158/0008-5472.CAN-07-1580. [DOI] [PubMed] [Google Scholar]
- 28.Vangamudi B, Peng DF, Cai Q, El-Rifai W, Zheng W, Belkhiri A. t-DARPP regulates phosphatidylinositol-3-kinase-dependent cell growth in breast cancer. Mol Cancer. 2010;9:240. doi: 10.1186/1476-4598-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Trausch-Azar JS, Ciechanover A, Schwartz AL. Ubiquitin-proteasome-mediated degradation, intracellular localization, and protein synthesis of MyoD and Id1 during muscle differentiation. J Biol Chem. 2005;280:26448–56. doi: 10.1074/jbc.M500373200. [DOI] [PubMed] [Google Scholar]
- 30.Zaika A, Irwin M, Sansome C, Moll UM. Oncogenes induce and activate endogenous p73 protein. J Biol Chem. 2001;276:11310–6. doi: 10.1074/jbc.M005737200. [DOI] [PubMed] [Google Scholar]
- 31.Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature. 1999;399:809–13. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 32.Taagepera S, McDonald D, Loeb JE, et al. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci U S A. 1998;95:7457–62. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Zeng L, Wang J, et al. A positive role for c-Abl in Atm and Atr activation in DNA damage response. Cell Death Differ. 2011;18:5–15. doi: 10.1038/cdd.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai KK, Yuan ZM. c-Abl stabilizes p73 by a phosphorylation-augmented interaction. Cancer Res. 2003;63:3418–24. [PubMed] [Google Scholar]
- 35.Raina D, Ahmad R, Kumar S, et al. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. Embo J. 2006;25:3774–83. doi: 10.1038/sj.emboj.7601263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Rifai W, Moskaluk CA, Abdrabbo MK, et al. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.