Summary
In a multisite birth-cohort study, Giardia spp were detected by enzyme immunoassay at least once in two-thirds of the children. Early persistent infection with Giardia, independent of diarrhea, was associated with deficits in both weight and length at 2 years of age.
Keywords: children, Giardia, growth, intestinal permeability, risk factors.
Abstract
Background.
Giardia are among the most common enteropathogens detected in children in low-resource settings. We describe here the epidemiology of infection with Giardia in the first 2 years of life in the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED), a multisite birth-cohort study.
Methods.
From 2089 children, 34916 stool samples collected during monthly surveillance and episodes of diarrhea were tested for Giardia using an enzyme immunoassay. We quantified the risk of Giardia detection, identified risk factors, and assessed the associations with micronutrients, markers of gut inflammation and permeability, diarrhea, and growth using multivariable linear regression.
Results.
The incidence of at least 1 Giardia detection varied according to site (range, 37.7%–96.4%) and was higher in the second year of life. Exclusive breastfeeding (HR for first Giardia detection in a monthly surveillance stool sample, 0.46 [95% confidence interval (CI), 0.28–0.75]), higher socioeconomic status (HR, 0.74 [95% CI, 0.56–0.97]), and recent metronidazole treatment (risk ratio for any surveillance stool detection, 0.69 [95% CI, 0.56–0.84]) were protective. Persistence of Giardia (consecutive detections) in the first 6 months of life was associated with reduced subsequent diarrheal rates in Naushahro Feroze, Pakistan but not at any other site. Giardia detection was also associated with an increased lactulose/mannitol ratio. Persistence of Giardia before 6 months of age was associated with a −0.29 (95% CI, −0.53 to −0.05) deficit in weight-for-age z score and −0.29 (95% CI, −0.64 to 0.07) deficit in length-for-age z score at 2 years.
Conclusions.
Infection with Giardia occurred across epidemiological contexts, and repeated detections in 40% of the children suggest that persistent infections were common. Early persistent infection with Giardia, independent of diarrhea, might contribute to intestinal permeability and stunted growth.
INTRODUCTION
Giardia lamblia, also known as Giardia duodenalis and Giardia intestinalis, is the most common etiology of intestinal parasitic infection in the first 2 years of life in low-resource settings. Although Giardia is a recognized pathogen of waterborne diarrhea outbreaks [1] and a common cause of diarrhea among travelers [2–4] and after recreational water exposure [5], the impact of endemic pediatric giardiasis is less clear. Two large studies of global etiologies of endemic pediatric diarrhea, the Global Enterics Multicenter Study (GEMS) [6] and the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) [7], found Giardia significantly more often in nondiarrheal than diarrheal stools. Similarly, Giardia was not associated with acute diarrhea in a meta-analysis of 12 acute pediatric diarrhea studies [4], and Giardia had a protective effect against acute diarrhea in 2 longitudinal studies [8, 9].
Evidence for an association between Giardia infection and child growth outcomes has been mixed [10–15]. Giardia infection is associated with disrupted villus architecture [16], an elevated lactulose/mannitol ratio (a marker of intestinal permeability) [17, 18], and zinc and vitamin A deficiencies [19–21], which suggests gut dysfunction and inadequate nutrient uptake. These associations, however, have been inconsistent and limited in ascribing directionality, because vitamin A deficiency, for example, can increase susceptibility to Giardia infection [22].
The multisite MAL-ED birth-cohort study [23] provides high-resolution prospective data to clarify early-life Giardia epidemiology in high-prevalence settings. Longitudinal analysis specifically enables assessment of the temporality between detection of Giardia, diarrhea, micronutrient status, markers of intestinal permeability and inflammation, and the estimation of longer-term effects on growth. Here, we describe the determinants, burden, and impact of Giardia infection in the first 2 years of life in 8 low-resource sites.
METHODS
The MAL-ED study design and methods have been described [23]. In brief, the study was conducted between November 2009 and February 2014 at sites in Dhaka, Bangladesh (BGD), Fortaleza, Brazil (BRF), Vellore, India (INV), Bhaktapur, Nepal (NEB), Naushahro Feroze, Pakistan (PKN), Loreto, Peru (PEL), Venda, South Africa (SAV), and Haydom, Tanzania (TZH). Children were followed from birth (<17 days of age) via twice-weekly home visits for illness surveillance, medicines, and breastfeeding practices and monthly for anthropometry until they reached 2 years of age [24]. Nondiarrheal surveillance stool samples were collected and tested for 40 enteropathogens [25] monthly in the first year (0–12 months) of life and quarterly in the second year (12–24 months) of life. Stool samples were collected and tested also during every diarrhea episode reported during the twice-weekly surveillance visits. Diarrhea was defined as maternal report of 3 or more loose stools in 24 hours or 1 stool with visible blood [24]. Weight-for-age (WAZ) and length-for-age (LAZ) z scores were calculated using the 2006 World Health Organization child growth standards [26]. Sociodemographic information was assessed biannually and summarized using the Water, Assets, Maternal Education, Income (WAMI) score, which is based on monthly household income, maternal education, wealth measured by 8 assets, and access to improved water and sanitation [27], as defined by World Health Organization guidelines [28]. Plasma zinc and retinol concentrations were assessed at 7, 15, and 24 months of age [29]. Urinary lactulose/mannitol excretion ratios, measured at 3, 6, 9, and 15 months of age, were converted into sample-based z scores (LMZs) using the BRF cohort as the internal reference population [30]. All sites received ethical approval from their respective governmental, local institutional, and collaborating institutional ethical review boards. Informed written consent was obtained from the parent or guardian of each child.
Data and Definitions
We included in the analysis all monthly surveillance and diarrheal stool samples that were tested for Giardia by enzyme immunoassay (EIA) (TechLab, Blacksburg, VA), the majority of which were also tested by wet-prep microscopy. The laboratory methods for detecting other enteropathogens and gut biomarkers, including α-1-antitrypsin (ALA), myeloperoxidase (MPO), neopterin (NEO), and α-1-acid glycoprotein (AGP), a marker of systemic inflammation, have been described [25, 29, 31].
Definitions of incident Giardia-related diarrhea were defined with increasing specificity for diarrhea of true Giardia etiology as follows: (1) Giardia-positive diarrhea, Giardia was detected in a diarrheal stool sample; (2) new Giardia-positive diarrhea, Giardia was detected in a diarrheal stool sample, and the most recent previous stool sample tested negative for Giardia or was taken more than 2 months earlier; (3) Giardia-positive diarrhea-associated pathogens–negative diarrhea, Giardia was detected in a diarrheal stool sample, but no diarrhea-associated pathogens that were previously identified in MAL-ED were detected (13 of 40 pathogens tested, ie, norovirus GII, rotavirus, astrovirus, adenovirus, Campylobacter, Cryptosporidium, heat-stable enterotoxin-producing enterotoxigenic Escherichia coli, typical enteropathogenic E coli, heat-labile enterotoxin-producing enterotoxigenic E coli, Shigella, enteroinvasive E coli, Entamoeba histolytica, and Salmonella [7]); and (4) Giardia-positive-only diarrhea, Giardia was detected in a diarrheal stool sample, and no other enteropathogens among all 40 tested were detected [25]. Persistence of Giardia detection was defined as 2 consecutive stool samples that tested positive for Giardia (2 consecutive months in the first year of life or 2 consecutive quarters in the second year). Prolonged persistence was defined as 3 consecutive stool samples that tested positive for Giardia.
Data Analysis
Risk factors for the first detection of Giardia in surveillance stool samples were identified using pooled logistic regression to estimate hazard ratios (HRs) and adjusting for site and a restricted quadratic spline [32] for age. Variables in the multivariable model were included on the basis of statistical significance, model fit by the quasi-likelihood information criterion, covariance between factors, and variability of factors within sites for site-specific models. Comparing by the Akaike information criterion (AIC) to models with linear week of the year, seasonality was assessed by modeling Giardia detection with linear, quadratic, and cubic terms for the week of the year (w), and the terms sin(2πw/52), cos(2πw/52), sin(4πw/52), and cos(4πw/52). We used Poisson regression to evaluate associations between zinc and vitamin A status with Giardia detection in surveillance stool samples and adjusted for previous Giardia detection and potential confounders included in the multivariable risk factor model. We estimated the effect of Giardia detection on subsequent diarrheal rates using pooled logistic regression with general estimating equations (GEEs) and robust variance to account for correlation between outcomes within children and adjusted for the same confounders and illness symptoms during the exposure periods. We estimated the effect of Giardia in all stools on gut biomarker concentrations using multivariable linear regression with GEEs and adjusted for stool consistency and presence of the 2 other pathogens of highest prevalence, enteroaggregative E coli (EAEC) and Campylobacter. Last, we estimated the effect of Giardia detection in surveillance stools on WAZ and LAZ attainment at 2 years of age using multivariable linear regression with GEEs. Confounders, listed in the table footnotes, included baseline sociodemographic characteristics associated with Giardia detection identified above and EAEC and Campylobacter stool positivity. Data from SAV were excluded from zinc-related analyses and data from PKN were excluded from length-related analyses because of measurement quality concerns at those sites. For analyses limited to surveillance stool samples, results (not shown) were consistent when we repeated analyses with diarrheal stool samples.
RESULTS
Diagnostics
Of 34916 stool samples (27092 surveillance and 7824 diarrheal) tested for Giardia by an EIA, 33796 (96.8%) were also tested for Giardia by wet-prep microscopy. Compared to EIA, the sensitivity of microscopy was 46.2%, and its specificity was 99.3%. Giardia positivity by microscopy was 21% less likely if the stool was watery or liquid than if it was soft or formed (risk ratio [RR], 0.79 [95% confidence interval (CI), 0.68–0.90]), but we found no association between stool consistency and EIA results (RR, 0.94 [95% CI, 0.86–1.03]).
Incidence and Persistence
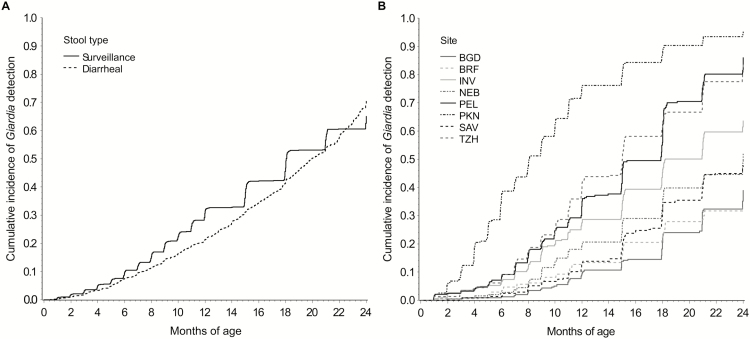
Among 2089 children with at least 1 tested stool, the overall Giardia prevalence according to the EIA in stool samples was 14.7% (n = 5135). Giardia was detected at least once in two-thirds (n = 1178) of the 1741 children followed to 2 years of age (range, 37.7% [BRF] to 96.4% [PKN]). The overall median times to Giardia detection, which varied according to site, were 18.0 and 20.0 months for surveillance and diarrheal stool samples, respectively (Figure 1).
Figure 1.
Cumulative incidence of Giardia detection in surveillance and diarrheal stool samples across all sites (A) and in surveillance stool samples within each site (B) among 2089 children in the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) cohort with at least 1 stool tested for Giardia.
The incidence of Giardia-positive diarrhea was 40.4 cases per 100 person-years. However, measures of Giardia-related diarrhea incidence decreased by approximately 60%, 75%, and more than 80% when we required the previous stool sample to have tested negative for Giardia, the current stool sample to have no detection of diarrhea-associated pathogens, and the current stool sample to have no detection of any other pathogens, respectively (Table 1).
Table 1.
Prevalence of Giardia Detection in Surveillance Stools and Incidence of Giardia-Related Diarrhea According to Site Among 2089 Children in the MAL-ED Birth Cohort
| Site | Prevalence (%) of Giardia Detection in Monthly Surveillance Stools | Incidencea of Giardia- Positive Diarrhea | Incidencea of New Giardia- Positive Diarrheab | Incidencea of Giardia-Positive Diarrhea- Associated Pathogens–Negative Diarrheac | Incidencea of Giardia-Positive- Only Diarrhead |
|---|---|---|---|---|---|
| BGD | 4.2 | 22.8 (18.7–27.7) | 12.3 (9.4–16.1) | 1.8 (0.9–3.6) | 1.4 (0.6–3.0) |
| BRF | 7.3 | 4.6 (2.9–7.4) | 1.8 (0.9–3.8) | 1.5 (0.7–3.4) | 1.0 (0.4–2.8) |
| INV | 13.1 | 21.4 (17.6–26.1) | 8.1 (5.9–11.2) | 6.6 (4.6–9.4) | 4.4 (2.8–6.8) |
| NEB | 9.2 | 18.2 (14.6–22.6) | 7.5 (5.4–10.5) | 6.6 (4.6–9.5) | 5.1 (3.4–7.7) |
| PEL | 16.4 | 115.8 (106.3–126.2) | 40.4 (34.9–46.7) | 28.7 (24.1–34.0) | 18.7 (15.1–23.2) |
| PKN | 35.2 | 126.2 (116.3–136.9) | 42.5 (36.9–48.9) | 28.3 (23.8–33.6) | 15.7 (12.4–19.7) |
| SAV | 6.4 | 4.1 (2.6–6.3) | 2.4 (1.4–4.3) | 1.0 (0.4–2.4) | 0.61 (0.20–1.9) |
| TZH | 16.2 | 7.0 (5.0–9.9) | 3.7 (2.3–6.0) | 0.44 (0.11–1.8) | 0.22 (0.03–1.6) |
| All | 13.6 | 40.4 (38.4–42.5) | 15.0 (13.8–16.3) | 9.5 (8.5–10.5) | 5.9 (5.2–6.8) |
Abbreviations: BGD, Dhaka, Bangladesh; BRF, Fortaleza, Brazil; INV, Vellore, India; NEB, Bhaktapur, Nepal; PEL, Loreto, Peru; PKN, Naushahro Feroze, Pakistan; SAV, Venda, South Africa; TZH, Haydom, Tanzania.
aRate per 100 person-years.
bNew Giardia-positive diarrhea was defined if Giardia was detected in the diarrheal stool and the most recent previous stool tested negative for Giardia or was taken more than 2 months earlier (n = 539 of 1452 [37.1%] of all Giardia-positive diarrheal stools).
c Giardia-positive diarrhea-associated pathogens–negative diarrhea was defined if Giardia was detected in the diarrheal stool and no diarrhea-associated pathogens were detected (n = 341 of 1291 [26.4%] of all completely tested Giardia-positive diarrheal stools). Diarrhea-associated pathogens (norovirus GII, rotavirus, astrovirus, adenovirus, Campylobacter, Cryptosporidium, heat-stable enterotoxin-producing enterotoxigenic E coli, typical enteropathogenic E coli, heat-labile enterotoxin-producing enterotoxigenic E coli, Shigella, enteroinvasive E coli, E. histolytica, and Salmonella) were associated with diarrhea in the first or second year of life [7].
d Giardia-positive-only diarrhea was defined if Giardia was detected in the diarrheal stool and no other pathogens were detected (n = 214 of 1291 [16.6%] of all completely tested Giardia-positive diarrheal stool samples).
The overall prevalence of Giardia detected in surveillance stool samples was 13.6% (Table 1). However, the prevalence decreased by more than half (6.0%) when we required the previous surveillance stool to have tested negative for Giardia. Repeated Giardia detections in surveillance stool samples occurred in 838 (40.1%) children (Supplementary Figure 1). The prevalence of persistence was less than 5% before 6 months of age in all except the PKN site but increased to 31.8% overall in the second year of life.
Risk Factors
Giardia detection increased with age over the first 2 years of life; a 1-month increase in age was associated with an 11% increase in the risk of Giardia detection in surveillance stool samples (RR, 1.11 [95% CI, 1.10–1.12]). The percentage of days in the previous month that the child was exclusively breastfed was a strongly protective factor against first Giardia detection (Table 2). Socioeconomic factors, including increased socioeconomic score (Water, Assets, Maternal Education, Income score [27]), household income, and older maternal age, were also protective. Metronidazole exposure in the previous 15 days was associated with a 31% relative decrease (95% CI, 16–44) in Giardia detection in surveillance stool samples, but we found no association with exposure more than 15 days earlier or with exposure to any other antibiotics.
Table 2.
Risk Factors for First Giardia Detection in Monthly Surveillance Stool Samples Among 2088 Children in the MAL-ED Cohort With at Least 1 Surveillance Stool
| Risk Factor | Semi-Univariable (HR [95% CI])a | Multivariable (HR [95% CI])b |
|---|---|---|
| Child characteristics | ||
| Female (vs male) | 1.02 (0.89–1.16) | |
| Enrollment weight (per 1 z score) | 1.05 (0.98–1.12) | |
| Exclusively breastfed since birth | 0.67 (0.37–1.20) | |
| Percent days exclusively breastfed in last month (100% vs 0%) | 0.43 (0.27–0.70) | 0.46 (0.28–0.75) |
| WAZ (per 1 z score)c | 0.94 (0.88–1.00) | 0.97 (0.91–1.03) |
| LAZ (per 1 z score)c,d | 0.96 (0.89–1.03) | |
| WLZ (per 1 z score)c,d | 0.94 (0.88–1.00) | |
| Metronidazole used in previous 15 days | 0.69 (0.47–1.01) | 0.71 (0.48–1.04) |
| Metronidazole used in previous 16–30 days | 1.02 (0.73–1.42) | |
| Sociodemographic | ||
| Socioeconomic score (WAMI [27], per 0.5 unit) | 0.54 (0.42–0.69) | 0.74 (0.56–0.97) |
| Household income at or above site-specific median income | 0.79 (0.69–0.91) | |
| Maternal age (per 5 y) | 0.83 (0.75–0.93) | 0.86 (0.77–0.96) |
| Maternal education (≥6 y completed) | 0.74 (0.64–0.86) | |
| Mother married | 1.24 (0.95–1.60) | |
| Child has siblings | 1.36 (1.18–1.57) | 1.26 (1.09–1.46) |
| Mean no. of people per room in the household (per 1 unit) | 1.09 (1.04–1.16) | |
| Water | ||
| Improved (vs unimproved) drinking water [28] | 0.95 (0.72–1.25) | |
| Time to access water (>10 min) | 1.18 (0.97–1.44) | |
| Treated (vs untreated) water | 0.69 (0.54–0.88) | 0.78 (0.61–1.00) |
| Sanitation and hygiene | ||
| Improved (vs unimproved) sanitation [28] | 0.89 (0.71–1.10) | |
| Always washed hands after child defecated | 0.79 (0.71–0.89) | 0.80 (0.68–0.95) |
| Always washed hands before preparing food | 0.85 (0.77–0.95) | |
| Always washed hands after using toilet | 0.82 (0.73–0.92) | |
| Used toilet paper | 0.86 (0.76–0.98) | 0.95 (0.83–1.08) |
| Shared toilet facility | 1.10 (0.89–1.35) | |
| Environmental | ||
| Dirt floor | 1.34 (1.10–1.62) | 1.13 (0.92–1.38) |
| Household owned cows | 1.04 (0.80–1.35) | |
| Household owned chickens | 1.38 (1.12–1.70) | 1.35 (1.10–1.66) |
Abbreviations: CI, confidence interval; HR, hazard ratio; LAZ, length-for-age z score; WAMI, Water, Assets, Maternal Education, Income; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
aAdjusted for site and age only (using restricted quadratic splines).
bAdjusted for site, age, and all other variables with estimates in this column (significant or strong association with Giardia detection in semi-univariable analysis, and not collinear with other variables).
cAt most recent measurement before stool collection.
dExcluding PKN.
Multiple hygiene and environmental risk factors were associated with Giardia detection. Hand-washing, treatment of drinking water, and increased water access were protective, whereas the presence of siblings was a strong risk factor. Associations with having a dirt floor and owning chickens indicate the importance of environmental exposure to Giardia (Table 2). The distribution of environmental factors differed according to site, and although risk factor trends were generally consistent, there were site-to-site variations in the magnitude and even the direction of associations in some cases (Supplementary Figure 2).
Giardia was positively correlated with Campylobacter detection (Pearson correlation coefficient [PCC], 0.15; P < .0001) but not with the detection of EAEC (PCC, −0.02; P = .0001) or viruses (PCC, −0.00; P = .7) in all stool samples, which suggests that the routes of transmission and/or age-susceptibility patterns are similar to those of Campylobacter.
Seasonality
We found a significant increase in first Giardia detections in surveillance stool samples in July through September and a smaller peak in March/April in the south Asian sites (BGD, INV, NEB, and PKN) (Supplementary Figure 3). Giardia seasonality was variable at the other sites, with peaks in December/January in the BRF and PEL sites and a small peak in March/April in the TZH and SAV sites. In contrast, we found no evidence of seasonality when we included all Giardia detections. No association between site-specific mean temperature or rainfall and Giardia positivity was found. The seasonality of Giardia detection in diarrheal stool samples matched the seasonality of all-cause diarrhea, which suggests that many Giardia-positive diarrheal episodes were caused by other pathogens (not shown).
Zinc, Vitamin A, and Giardia
Higher plasma zinc and retinol concentrations at 7 months of age were associated with decreased subsequent Giardia detection (Supplementary Table 1). A combined 1 standard deviation greater zinc and retinol concentration was associated with an adjusted 22% (95% CI, 2%–37%) lower Giardia-detection rate in surveillance stool samples from 8 to 24 months of age. A higher retinol concentration at 15 months of age was also associated with an approximate 10% decrease in the subsequent Giardia-detection rate. There were no associations between zinc status and Giardia detection at 15 months of age and no associations at either time period between zinc or vitamin A status and incidence of Giardia-positive diarrhea.
Giardia detection in surveillance stool samples in the period between vitamin A status measurements at 7 and 15 months was associated with an adjusted −1.58 mg/dL (95% CI, −2.82 to −0.34 mg/dL) change in retinol concentration over that time period. There were no associations between Giardia and change in zinc concentration.
Giardia and Risk of Acute Diarrhea
Giardia detection in surveillance stool samples was not associated with short-term diarrheal risk (adjusted RR for diarrhea in the following 30 days, 1.07 [95% CI, 0.96–1.19]). In addition, the apparent negative association between Giardia detection and diarrhea previously reported (RR adjusted for age and site, 0.90 [95% CI, 0.86–0.95]) [7] might be explained by treatment of 37% of all diarrhea episodes with metronidazole, such that Giardia might have been cleared before the diarrheal stool was collected. When we adjusted for recent metronidazole exposure, the association between Giardia detection and diarrhea moved toward the null (RR, 0.95 [95% CI, 0.90–1.00]).
Giardia detection was not associated with subsequent diarrheal rates in any except the PKN site (Supplementary Table 2). Giardia persistence before 6 months of age was common at the PKN site (17.4%) and was associated with an adjusted 28% relative decrease (95% CI, 11–41]) in diarrheal rates from 6 to 24 months of age. Giardia persistence in the first year of life at the PKN site (47.7%) was also associated with an adjusted decrease in subsequent diarrheal rates (adjusted incidence rate ratio, 0.81 [95% CI, 0.64–1.03]). The protective effect was driven largely by protection against subsequent diarrhea in which enteropathogenic bacteria were detected.
Associations With Gut-Function Biomarkers
The presence of Giardia in stool samples was associated with an elevated marker of increased intestinal permeability, LMZ, across sites (Table 3), as indicated by an average increase in lactulose (z-score difference, 0.12 [95% CI, −0.00 to 0.24]) and a decrease in mannitol (z-score difference, −0.15 [95% CI, −0.26 to −0.04]). We found no significant differences in the associations across ages, although the magnitude was greatest in the second year of life (adjusted LMZ difference at 15 months, 0.25 [95% CI, 0.10–0.40]). Among markers of inflammation, Giardia was also associated with a decrease in NEO concentration (Table 3) but not consistently with MPO, ALA, or AGP.
Table 3.
Associations Between Giardia Detection and Markers of Inflammation and Gut Permeability in All Stool Samples Among 2076 Children in the MAL-ED Cohort With at Least 1 Biomarker Measurement
| Site | LMZ Differencea (95% CI) (n = 4203) |
ALA Differencea (95% CI) (n = 24769) | MPO Differencea (95% CI) (n = 24769) | NEO Differencea (95% CI) (n = 24769) |
AGP Differencea (95% CI) (n = 3550) |
|---|---|---|---|---|---|
| BGD | 0.29 (0.00 to 0.59) | 0.1 (−0.06 to 0.27) | −0.22 (−0.43 to −0.02) | −0.07 (−0.35 to 0.21) | −0.44 (−12.01 to 11.14) |
| BRF | 0.03 (−0.28 to 0.35) | 0.00 (−0.22 to 0.23) | −0.18 (−0.46 to 0.09) | −0.20 (−0.42 to 0.02) | 3.53 (−10.99 to 18.05) |
| INV | 0.25 (0.04 to 0.46) | −0.08 (−0.2 to 0.03) | 0.00 (−0.12 to 0.13) | −0.12 (−0.23 to −0.01) | −1.05 (−8.66 to 6.57) |
| NEB | 0.18 (−0.15 to 0.51) | 0.11 (−0.03 to 0.24) | 0.01 (−0.14 to 0.16) | −0.10 (−0.21 to 0.01) | −1.05 (−11.62 to 9.51) |
| PEL | 0.23 (0.07 to 0.39) | −0.02 (−0.16 to 0.11) | −0.12 (−0.25 to 0.02) | −0.19 (−0.30 to −0.08) | −5.59 (−15.91 to 4.74) |
| PKN | 0.19 (0.01 to 0.38) | 0.05 (−0.06 to 0.16) | 0.00 (−0.11 to 0.10) | 0.00 (−0.09 to 0.10) | 7.39 (0.35 to 14.43) |
| SAV | 0.17 (−0.27 to 0.61) | −0.01 (−0.19 to 0.18) | 0.07 (−0.11 to 0.25) | −0.20 (−0.38 to −0.01) | 4.45 (−11.05 to 19.94) |
| TZH | 0.01 (−0.42 to 0.44) | 0.01 (−0.13 to 0.15) | −0.06 (−0.19 to 0.08) | −0.23 (−0.39 to −0.07) | 5.20 (−7.40 to 17.80) |
| All | 0.22 (0.12 to 0.32) | −0.04 (−0.09 to 0.01) | −0.09 (−0.14 to −0.03) | −0.11 (−0.16 to −0.06) | 1.83 (−1.82 to 5.47) |
Abbreviations: AGP, α-1-acid glycoprotein (mg/dL); BGD, Dhaka, Bangladesh; BRF, Fortaleza, Brazil; CI, confidence interval; INV, Vellore, India; LMZ, urinary lactulose/mannitol excretion ratio z score (BRF cohort was the internal reference population); ALA, α-1-antitrypsin (log[mg/g]); MPO, myeloperoxidase (log[ng/mL]); NEB, Bhaktapur, Nepal; NEO: neopterin (log[nmol/L]); PEL, Loreto, Peru; PKN, Naushahro Feroze, Pakistan; SAV, Venda, South Africa; TZH, Haydom, Tanzania.
aAdjusted for site, age, stool consistency, sex, WAMI (Water, Assets, Maternal Education, Income) score, mother’s age, presence of siblings, water treatment, routine hand-washing after child defecation, use of toilet paper, dirt floor, ownership of chickens, percent exclusive breastfeeding, and presence of enteroaggregative E coli and/or Campylobacter in stool sample.
Effects on Growth
Giardia detection was associated with reduced weight and length attainment at 2 years of age (Table 4). Compared with those with low Giardia exposure (the 10th percentile of Giardia positivity in surveillance stool samples over the first 2 years of life), children with high exposure (the 90th percentile of Giardia positivity) had an adjusted −0.12 LAZ decrement (95% CI, −0.25 to 0.01) and −0.11 WAZ decrement (95% CI, −0.23 to −0.00) at 2 years of age. Giardia persistence in the first 6 months of life was statistically significantly associated with more than double that decrement in both weight and length at 24 months of age (Table 4).
Table 4.
Effects of Giardia Detection in Monthly Surveillance Stool Samples on Weight and Length Attainment at 2 Years of Age Among 1727 Children in the MAL-ED Cohort With Anthropometric Measurements at 2 Years
| Exposure | n (N = 1727) | WAZ Differencea (95% CI) | LAZ Differencea,b (95% CI) |
|---|---|---|---|
| Giardia persistence in first 6 months | 62 | −0.29 (−0.53 to −0.05) | −0.29 (−0.64 to 0.07) |
| Any Giardia detection in first 6 months | 173 | −0.01 (−0.16 to 0.14) | 0.08 (−0.12 to 0.28) |
| Giardia persistence in first year | 290 | −0.04 (−0.16 to 0.09) | −0.01 (−0.15 to 0.13) |
| Any Giardia detection in first year | 549 | −0.07 (−0.18 to 0.03) | −0.07 (−0.18 to 0.03) |
| Giardia persistence in second year | 549 | −0.05 (−0.15 to 0.05) | −0.09 (−0.19 to 0.01) |
| Any Giardia detection in second year | 976 | −0.01 (−0.11 to 0.08) | −0.05 (−0.14 to 0.05) |
| Any Giardia persistence | 663 | −0.05 (−0.15 to 0.04) | −0.07 (−0.17 to 0.03) |
| Any Giardia detection | 1101 | −0.02 (−0.12 to 0.08) | −0.04 (−0.13 to 0.06) |
| Any Giardia-related diarrheac | 557 | 0.07 (−0.04 to 0.19) | −0.03 (−0.15 to 0.10) |
| Percent positive surveillance stool samples (per 38% increased) | −0.11 (−0.23 to −0.00) | −0.12 (−0.25 to 0.01) |
Abbreviations: CI, confidence interval; LAZ, length-for-age z score; WAZ, weight-for-age z score.
aAdjusted for site, anthropometric measurement at enrollment, sex, WAMI (Water, Assets, Maternal Education, Income) score, mother’s age, presence of siblings, water treatment, routine hand-washing after child defecation, use of toilet paper, dirt floor, ownership of chickens, age at stopping exclusive breastfeeding, and percentage of surveillance stool samples that tested positive for Campylobacter and enteroaggregative E coli in the first 2 years of life.
bAll LAZ estimates excluded PKN (n = 1478).
cAlso adjusted for Giardia persistence in surveillance stool samples to assess independent effect of diarrhea with Giardia detection.
dThe difference between the 10th and 90th percentile of cumulative percent positivity across all sites was 38%, which represents a contrast between high and low Giardia exposure in the context of the MAL-ED cohort.
DISCUSSION
The prospective MAL-ED birth-cohort study provided a unique opportunity to investigate the complex relationships between Giardia exposure, micronutrient status, intestinal permeability, diarrhea, and growth. Giardia detection was common at all sites and increased in frequency through the second year of life. Major reductions in Giardia diarrhea incidence when more specific definitions were used suggest that true Giardia-caused cases of diarrhea are difficult to identify in settings of high endemicity. In the absence of molecular typing, evidence of visual clustering of Giardia detections within children and seasonal patterns limited to first detections suggest that repeated detections can often represent persistent infections. We identified both hygiene and environmental risk factors for Giardia infection and confirmed that the fecal-oral and waterborne routes both might be important modes of transmission. Host factors, including zinc and vitamin A deficiencies, might also contribute to Giardia susceptibility, because children with higher micronutrient concentrations had less subsequent Giardia detection. This relationship might be bidirectional, because Giardia detection from 7 to 15 months of age was also associated with a decrease in retinol concentration during that period.
In contrast to data from previous reports [8, 9], our longitudinal data did not suggest that Giardia infection was protective against diarrhea across the sites. The protective effect of Giardia on subsequent acute diarrheal risk was limited to early exposures at the PKN site, which might be explained by the uniquely high detection rate in the first year of life at this site, environment-specific unidentified biological susceptibility factors, Giardia strain variability, and coinfection factors. Because the diagnostic EIA was insensitive to stool consistency, dilution of Giardia in diarrheal stools does not explain previously reported inverse associations with diarrhea [4, 7]. In contrast, clearance of Giardia in diarrheal stools by metronidazole, the most common antibiotic used for diarrhea treatment in MAL-ED (17% of all episodes were treated with metronidazole [33]), might contribute to this inverse association.
Even in the absence of diarrheal symptoms, Giardia infection, especially early persistent infection, was associated with reduced weight and height attainment at 2 years. This finding was reported recently from an independent cohort study in Bangladesh [34]. This early impact might be the result of a critical period of susceptibility in which the infant gut and intestinal microbiota are developing [35, 36]. The increased lactulose/mannitol excretion ratio associated with Giardia suggests a mechanism through increased intestinal permeability and malabsorption, components of environmental enteropathy [18]. Giardia was not associated with increased markers of intestinal inflammation (ALA, MPO, or NEO), which suggests that Giardia might disrupt epithelial cells through pathways different from those of chronic immune activation typical of environmental enteropathy. Given the divergence between diarrhea and growth outcomes associated with Giardia infection, diarrhea-independent mechanisms are likely responsible for the growth impact such that physiologic insult occurs without necessary manifestation of diarrhea.
We found that the subset of children with early and persistent Giardia infection experienced a significant disease burden caused by Giardia. However, no association was observed between Giardia detection and growth in the comprehensive risk factor models from MAL-ED that compared high and low levels of Giardia exposures both defined by no detections in the first 6 months (MAL-ED Network Investigators, unpublished data). Although other determinants might influence growth at later ages more strongly, targeting Giardia early in life might confer substantial benefit.
This study was limited by fewer surveillance stool samples tested in the second year of life when Giardia was most common. In addition, although more sensitive than microscopy, the EIA has a lower sensitivity than nucleic acid–based detection methods [37, 38]. We also did not use Assemblage typing, which might have helped distinguish persistent infection from reinfection and explain variability in clinical outcomes [39, 40]. Last, antihelminthic medications that are active against Giardia (eg, albendazole or mebendazole) were not recorded, although they might have been used at the individual level and potentially through mass treatment campaigns. Despite these limitations, the breadth of data available, including markers of gut inflammation and permeability, enabled robust analysis of the effects and potential mechanisms of Giardia infection.
The MAL-ED study enabled a comprehensive description of Giardia epidemiology in children early in life across 8 diverse sites. In general, interventions to limit exposure might reduce Giardia burden better than treatment, because metronidazole only transiently reduced detection. Interventions to reduce early exposure (eg, through sustained exclusive breastfeeding) might have the greatest effect on long-term outcomes, given increased the susceptibility of children to the effects of Giardia during their first few months of life.
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online.
Supplementary Material
Notes
We thank the investigators and staff of the MAL-ED Network for their important contributions and the caregivers and children who participated in the study.
Financial support. MAL-ED is carried out as a collaborative project supported by the Bill and Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the National Institutes of Health Fogarty International Center (grant D43-TW009359 to E.T.R.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hanevik K, Hausken T, Morken MH, et al. Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J Infect 2007; 55:524–30. [DOI] [PubMed] [Google Scholar]
- 2. Bartelt LA, Sartor RB. Advances in understanding Giardia: determinants and mechanisms of chronic sequelae. F1000Prime Rep 2015; 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freedman DO, Weld LH, Kozarsky PE, et al. ; GeoSentinel Surveillance Network Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med 2006; 354:119–30. [DOI] [PubMed] [Google Scholar]
- 4. Muhsen K, Levine MM. A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clin Infect Dis 2012; 55:S271–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stuart JM, Orr HJ, Warburton FG, et al. Risk factors for sporadic giardiasis: a case-control study in southwestern England. Emerg Infect Dis 2003; 9:229–33. [DOI] [PubMed] [Google Scholar]
- 6. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 7. Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veenemans J, Mank T, Ottenhof M, et al. Protection against diarrhea associated with Giardia intestinalis is lost with multi-nutrient supplementation: a study in Tanzanian children. PLoS Negl Trop Dis 2011; 5:e1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muhsen K, Cohen D, Levine MM. Can Giardia lamblia infection lower the risk of acute diarrhea among preschool children? J Trop Pediatr 2014; 60:99–103. [DOI] [PubMed] [Google Scholar]
- 10. Prado MS, Cairncross S, Strina A, et al. Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology 2005; 131:51–6. [DOI] [PubMed] [Google Scholar]
- 11. Boeke CE, Mora-Plazas M, Forero Y, Villamor E. Intestinal protozoan infections in relation to nutritional status and gastrointestinal morbidity in Colombian school children. J Trop Pediatr 2010; 56:299–306. [DOI] [PubMed] [Google Scholar]
- 12. Sackey ME, Weigel MM, Armijos RX. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J Trop Pediatr 2003; 49:17–23. [DOI] [PubMed] [Google Scholar]
- 13. Ajjampur SS, Koshy B, Venkataramani M, et al. Effect of cryptosporidial and giardial diarrhoea on social maturity, intelligence and physical growth in children in a semi-urban slum in south India. Ann Trop Paediatr 2011; 31:205–12. [DOI] [PubMed] [Google Scholar]
- 14. Hollm-Delgado MG, Gilman RH, Bern C, et al. Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. Am J Epidemiol 2008; 168:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centeno-Lima S, Rosado-Marques V, Ferreira F, et al. Giardia duodenalis and chronic malnutrition in children under five from a rural area of Guinea-Bissau. Acta Med Port 2013; 26:721–4. [PubMed] [Google Scholar]
- 16. Hjelt K, Paerregaard A, Krasilnikoff PA. Giardiasis causing chronic diarrhoea in suburban Copenhagen: incidence, physical growth, clinical symptoms and small intestinal abnormality. Acta Paediatr 1992; 81:881–6. [DOI] [PubMed] [Google Scholar]
- 17. Goto R, Panter-Brick C, Northrop-Clewes CA, et al. Poor intestinal permeability in mildly stunted Nepali children: associations with weaning practices and Giardia lamblia infection. Br J Nutr 2002; 88:141–9. [DOI] [PubMed] [Google Scholar]
- 18. Denno DM, VanBuskirk K, Nelson ZC, et al. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis 2014; 59:S213–9. [DOI] [PubMed] [Google Scholar]
- 19. Astiazaran-Garcia H, Lopez-Teros V, Valencia ME, et al. Giardia lamblia infection and its implications for vitamin A liver stores in school children. Ann Nutr Metab 2010; 57:228–33. [DOI] [PubMed] [Google Scholar]
- 20. Ertan P, Yereli K, Kurt O, et al. Serological levels of zinc, copper and iron elements among Giardia lamblia infected children in Turkey. Pediatr Int 2002; 44:286–8. [DOI] [PubMed] [Google Scholar]
- 21. Abou-Shady O, El Raziky MS, Zaki MM, Mohamed RK. Impact of Giardia lamblia on growth, serum levels of zinc, copper, and iron in Egyptian children. Biol Trace Elem Res 2011; 140:1–6. [DOI] [PubMed] [Google Scholar]
- 22. Quihui-Cota L, Astiazarán-García H, Valencia ME, et al. Impact of Giardia intestinalis on vitamin A status in schoolchildren from northwest Mexico. Int J Vitam Nutr Res 2008; 78:51–6. [DOI] [PubMed] [Google Scholar]
- 23. MAL-ED Network Investigators. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 2014; 59:S193–206. [DOI] [PubMed] [Google Scholar]
- 24. Richard SA, Barrett LJ, Guerrant RL, et al. ; MAL-ED Network Investigators Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin Infect Dis 2014; 59:S220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Houpt E, Gratz J, Kosek M, et al. Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis 2014; 59:S225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age, Methods and Development 2006. Available at: http://www.who.int/childgrowth/standards/Technical_report.pdf?ua=1 Accessed May 9, 2016.
- 27. Psaki SR, Seidman JC, Miller M, et al. Measuring socioeconomic status in multicountry studies: results from the eight-country MAL-ED study. Popul Health Metr 2014; 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization and United Nations Children’s Fund Joint Monitoring Programme for Water Supply and Sanitation (JMP). Progress on Drinking Water and Sanitation: Special Focus on Sanitation. UNICEF, New York and WHO, Geneva, 2008 Available at: http://www.wssinfo.org/fileadmin/user_upload/resources/1251794333-JMP_08_en.pdf Accessed February 5, 2016.
- 29. Caulfield LE, Bose A, Chandyo RK, et al. Infant feeding practices, dietary adequacy, and micronutrient status measures in the MAL-ED study. Clin Infect Dis 2014; 59:S248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kosek MN, Lee GO, Guerrant RL, et al. The MAL-ED study: Age and sex normalization of intestinal permeability for the assessment of enteropathy in infancy. J Pediatric Gastroenterol Nutrition 2016; in press. [DOI] [PubMed] [Google Scholar]
- 31. Kosek M, Guerrant RL, Kang G, et al. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Dis 2014; 59:S239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Howe CJ, Cole SR, Westreich DJ, et al. Splines for trend analysis and continuous confounder control. Epidemiology 2011; 22:874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donowitz JR, Alam M, Kabir M, et al. A prospective longitudinal cohort to investigate the effects of early life giardiasis on growth and all cause diarrhea. Clin Infect Dis 2016; 63:792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murgas Torrazza R, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol 2011; 31:S29–34. [DOI] [PubMed] [Google Scholar]
- 36. Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics 2012; 129:950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calderaro A, Gorrini C, Montecchini S, et al. Evaluation of a real-time polymerase chain reaction assay for the laboratory diagnosis of giardiasis. Diagn Microbiol Infect Dis 2010; 66:261–7. [DOI] [PubMed] [Google Scholar]
- 38. Schuurman T, Lankamp P, van Belkum A, et al. Comparison of microscopy, real-time PCR and a rapid immunoassay for the detection of Giardia lamblia in human stool specimens. Clin Microbiol Infect 2007; 13:1186–91. [DOI] [PubMed] [Google Scholar]
- 39. Kohli A, Bushen OY, Pinkerton RC, et al. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans R Soc Trop Med Hyg 2008; 102:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laishram S, Kang G, Ajjampur SSR. Giardiasis: a review on assemblage distribution and epidemiology in India. Indian J Gastroenterol 2012; 31:3–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.