Abstract
Introduction
The Mediterranean and Dietary Approaches to Stop Hypertension diets have been associated with lower dementia risk. We evaluated dietary inflammatory potential in relation to mild cognitive impairment (MCI)/dementia risk.
Methods
Baseline food frequency questionnaires from n = 7085 women (aged 65–79 years) were used to calculate Dietary Inflammatory Index (DII) scores that were categorized into four groups. Cognitive function was evaluated annually, and MCI and all-cause dementia cases were adjudicated centrally. Mixed effect models evaluated cognitive decline on over time; Cox models evaluated the risk of MCI or dementia across DII groups.
Results
Over an average of 9.7 years, there were 1081 incident cases of cognitive impairment. Higher DII scores were associated with greater cognitive decline and earlier onset of cognitive impairment. Adjusted hazard ratios (HRs) comparing lower (anti-inflammatory; group 1 referent) DII scores to the higher scores were group 2-HR: 1.01 (0.86–1.20); group 3-HR: 0.99 (0.82–1.18); and group 4-HR: 1.27 (1.06–1.52).
Conclusions
Diets with the highest pro-inflammatory potential were associated with higher risk of MCI or dementia.
Keywords: Diet, Cognitive decline, Mild cognitive impairment, Dementia, Inflammation, Survival analysis
1. Introduction
As the U.S. population ages, cognitive decline and dementia have become large and costly public health problems. Although some lifestyle interventions may help to prevent vascular cognitive impairment, currently, there are no interventions to stop or slow the progression of Alzheimer’s disease (AD). In the absence of any effective primary or secondary prevention strategies, the field has focused, in recent years, on primordial [1] prevention, that is, targeting modifiable risk factors to prevent or delay the onset of symptoms. These modifiable factors include dietary habits and comorbid chronic conditions that are influenced by diet such as type 2 diabetes and hypertension.
Diet has a strong influence on health, and the benefits of a healthy diet could potentially yield an estimated $114.5 billion in medical savings [2] (not including the potentially beneficial effects of diet on cognition). The Mediterranean and the Dietary Approaches to Stop Hypertension (DASH) diets have been associated with better cognition and a lower incidence of dementia [3–6]. The Mediterranean diet emphasizes the intake of fruits, vegetables, whole grains, low-fat dairy products, olive oil, fish, poultry, and wine with meals. Similarly, the DASH diet emphasizes the intake of fruits, vegetables, and low-fat dairy intake, as well as legumes, nuts, and emphasizes lower consumption of animal protein, sweets, and sodium. Both diets have been associated with lower plasma levels of inflammatory markers [7].
Inflammation has been implicated in both AD and vascular cognitive impairment [8,9]; therefore, it is important to study whether inflammation associated with diet affects cognitive function and impairment. It is possible that this modifiable risk factor, either on its own or when paired with other risk factors (e.g., hypertension or type 2 diabetes), accelerates an individual’s cognitive decline and increases the risk of dementia. Conversely, a diet with greater anti-inflammatory properties may lower the risk. The Dietary Inflammatory Index (DII) was developed to characterize an individual’s overall diet based on its inflammatory potential [10]. Higher DII scores, indicating greater inflammatory potential, have been associated with inflammatory biomarker levels (interleukin-6 [IL-6], high sensitivity C-reactive protein [hs-CRP], and tumor necrosis factor α [TNF-α2]) in the Women’s Health Initiative (WHI) [11]. The objective of the present study is to evaluate the associations, over time, between dietary inflammatory potential and global cognition and incident mild cognitive impairment (MCI) or probable dementia in a cohort of older women. We hypothesize that women reporting diets with higher pro-inflammatory potential will perform worse on cognitive tests over time and will have a higher incidence of MCI or dementia than women consuming diets with lower inflammatory potential.
2. Methods
2.1. Study population
The Women’s Health Initiative Memory Study (WHIMS) participants were recruited from 39 sites of the larger WHI study in 1996 to 1999. The WHIMS consisted of parallel randomized clinical trials for the evaluation of the effects of estrogen alone (E-Alone) or in combination with progestin (E+P) on incident MCI or probable dementia. The study design has been reported previously [12–16]. The WHI E+P trial ended in 2002 [12,14], and the E-Alone trial ended in 2004 [13,17,18]. Participants continued post-trial cognitive assessments, returning for annual clinic visits to evaluate cognitive function until 2007/2008 when the study transitioned to the WHIMS–Epidemiology of Cognitive Health Outcomes. The WHIMS–Epidemiology of Cognitive Health Outcomes is ongoing, and annual cognitive assessments are now conducted by telephone. WHIMS participants completed the WHI Food Frequency Questionnaire (WHI-FFQ [16,19,20]; based on the Block FFQ [21,22]) at baseline.
Participants were eligible for inclusion in the present analysis if they completed the WHI-FFQ at baseline; did not have cognitive impairment at the baseline assessment of cognitive function; and completed more than one follow-up cognitive examinations over the course of the study. Participants missing important covariates such as age, race, or education levels were excluded.
2.2. The Dietary Inflammatory Index
Dietary patterns, or indices that take into account multiple dietary factors, can provide a more comprehensive assessment of diet and may be more predictive of disease processes and outcomes than single nutrients or foods. Researchers at the University of South Carolina Cancer Prevention and Control Program created the DII based on evidence suggesting that dietary factors influence inflammation [10]. Rather than being a specific food-based index, the DII represents a mixture of eight pro-inflammatory nutrients, 19 anti-inflammatory nutrients, 10 whole foods and spices, caffeine, flavan-3-ol, flavones, flavonols, flavanones, anthocyanidins, and isoflavones. For the purposes of this analysis, 32 food parameters were available from the WHI-FFQ. A detailed description of the DII method [10] and its construct validation [23] have been published elsewhere. Briefly, following a review of the literature, 45 food items and nutrients (Table 1) were found to be associated with six well-known inflammatory biomarkers (IL-1β, IL-4, IL-6, IL-10, TNF-α, and CRP) [10]. The strength of the associations between the 45 dietary factors and these inflammatory biomarkers reported in the literature were then scored: +1, 0, or −1 for a positive, null, or inverse association, respectively. Scores were weighted by the type of study design supporting the associations. Weighted scores were tallied to obtain component-specific inflammatory effect scores. Dietary intake data were standardized to a representative global diet database that was constructed based on 11 data sets representing diverse populations around the world. The standardized dietary intake estimates were converted to centered percentiles for each DII component, to improve interpretability; centered percentiles were then multiplied by the corresponding DII component-specific inflammatory effect scores and summed to obtain the overall DII score for each individual. The DII score characterizes an individual’s diet on a continuum from maximally anti-inflammatory to maximally pro-inflammatory, with a higher DII score indicating a more pro-inflammatory diet and a lower DII score indicating a more anti-inflammatory diet. The construct validity of the DII has been demonstrated in four studies [11,23–25].
Table 1.
DII food parameters
| • Alcohol | • Riboflavin |
| • Vitamin B12 | • Saffron* |
| • Vitamin B6 | • Saturated fat |
| • β-Carotene | • Selenium |
| • Caffeine | • Thiamin |
| • Carbohydrate | • Trans fat |
| • Cholesterol | • Turmeric* |
| • Energy kcal | • Vitamin A |
| • Eugenol* | • Vitamin C |
| • Total fat | • Vitamin D |
| • Fiber | • Vitamin E |
| • Folic acid | • Zinc |
| • Garlic* | • Green/black tea |
| • Ginger* | • Flavan-3-ol* |
| • Iron | • Flavones* |
| • Magnesium | • Flavonols |
| • MUFA | • Flavanones* |
| • Niacin | • Anthocyanidins* |
| • Omega 3 | • Isoflavones |
| • Omega 6 | • Pepper* |
| • Onion | • Thyme or oregano* |
| • Protein | • Rosemary* |
| • PUFA |
Abbreviations: DII, Dietary Inflammatory Index; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; WHI-FFQ, Women’s Health Initiative–Food Frequency Questionnaire.
Items not collected in WHI-FFQ and thus not used in computation of DII.
2.3. Cognitive function and impairment outcomes
The outcomes of interest were cognitive decline over time (i.e., over the first 10 years of observation) and incident cognitive impairment defined as either MCI or probable dementia (for the entire 20 years of observation). In the first phase of WHIMS (years 1–10), women received in-person examinations, as described previously [15]. Briefly, a modified version of the Mini–Mental State Examination (Modified Mini–Mental State Examination [3MS]) [26] was administered annually to all participants. Women scoring below preset cut points based on education were referred for further evaluation including a clinical evaluation by a board-certified physician and additional neuropsychological testing including portions of the Consortium to Establish a Registry for Alzheimer’s Disease battery [27], the Mini– Mental State Examination [28], Trail Making Test parts A and B [29], a structured psychiatric interview (PRIME-MD) [30], the 15-item Geriatric Depression Scale-short form [31,32], and the acquired cognitive and behavioral changes interview [15] completed by a knowledgeable friend or family member. All data were centrally adjudicated by a panel of expert clinicians who classified each participant as normal, MCI [33], or probable dementia [34].
In the extension phase of WHIMS, the study transitioned to a telephone follow-up interview and cognitive assessment with the modified Telephone Interview for Cognitive Status (TICS-m) [35], the Oral Trail Making Test parts A and B [36], East Boston Memory Test [37], Digit Span [38], and Verbal Fluency-Animals [39]. Functional status was assessed with the Dementia Questionnaire (DQ) [40]. The DQ was administered to a proxy informant when TICS-m scores fell below 31 points. All data were then used to adjudicate MCI, probable dementia, or no impairment as described previously.
2.4. Covariates
Covariates, which were measured or self-reported at enrollment in the WHI, included age, education, total energy intake (derived from the FFQ), and body mass index (BMI; kg/m2). Physical activity levels were determined from a detailed series of questions on the frequency and duration of mild, moderate, and strenuous exercise [41]. A weekly expenditure score in metabolic equivalents was then determined using a standardized classification of energy expenditures from physical activity [42]. History of non-steroidal anti-inflammatory drug (NSAID) use was determined by direct examination of pill bottles at baseline [19] and defined as usage at least twice per week for the last 2 weeks [43]. We accounted for treatment assignment (E-Alone or E1P) and recruitment region. We adjusted for APOE ε4 carriage and neighborhood socioeconomic status (NSES) in the subsets of participants for whom these data were available. Other covariates included race/ethnicity and baseline self-reported diabetes, high cholesterol, hypertension, and cigarette smoking (current, past, and never).
2.5. Analytic approach
Participants were classified into four groups based on baseline DII scores (< −2; −2 to 0; 0 to 2; and >2). This categorical classification was chosen to allow for replication in other studies. Comparisons between categorical variables by DII groups were evaluated using χ2 statistics, and analyses of variance were used to evaluate continuous variables. For the assessment of cognitive change, 3MS scores obtained from the first 10 years of observation were compared between DII groups using mixed models adjusted for baseline age, age [2], education, race, treatment arm, diabetes, hypertension, NSAID use, BMI, geographical region, energy intake, high cholesterol treated with medication, hours of exercise/week, and smoking history. We considered only the first 10 years of observation to evaluate cognitive trajectories because this period is more proximal to the dietary assessment and because the cognitive assessment was later modified (telephone administration) for the remainder of the follow-up period. APOE ε4 carrier status was an added covariate in a subset of 5614 women for whom genetic data were available.
For the comparison of cognitive impairment, Kaplan-Meier plots [44] were produced, depicting unadjusted survival to incident cognitive impairment (including both MCI and probable dementia) over the course of up to 20 years of observation. Outcomes of cognitive impairment by DII group were analyzed using Cox proportional hazard models [45] adjusted for baseline measures of hormone therapy assignment, race, baseline age, age2, diabetes, education, hypertension, NSAID use, BMI, geographic region, energy intake, physical activity, high cholesterol requiring pills, and smoking. We tested for trend with ordinal models, and DII scores also were modeled as a continuous variable and adjusted for all the same covariates as the categorical models.
Several sensitivity analyses were conducted: APOE ε4 carrier status was added as a covariate in analyses of the subset of women with available APOE data. Similarly, we added an indicator for NSES as relationships between NSES and health behaviors and cognitive function has been demonstrated [46,47]. We also ran an analysis in which we adjusted for incident cardiovascular disease (CVD) (defined as coronary heart disease, myocardial infarction, or stroke); and another in which we implemented a 3-year lag time for onset of MCI or dementia to evaluate whether women with low 3MS scores at baseline were already progressing to the onset of MCI or probable dementia at the start of the study. All statistical analyses were conducted using SAS, v9.4 (SAS Institute, Cary, NC, USA), and all tests were deemed statistically significant at a 0.05 level of significance.
3. Results
A total of 7479 WHIMS women provided WHI-FFQ responses at baseline when their average dietary intake over the prior 3 months was reported. Nine women were excluded because of compromised cognitive function at baseline (either MCI or probable dementia); 69 were excluded because of extreme values for BMI (<15 kg/m2 and >50 kg/m2); 292 were excluded because of extreme values for energy intake (<500 and >5000 kcal/day); 3 were missing race, and 21 were missing education levels. Our final sample consisted of 7085 women who were assessed with the WHI-FFQ at baseline and were followed over time to assess cognitive function (Fig. 1). DII scores were derived using WHI-FFQ data. Participants were divided into four groups based on DII scores <−2 (group 1; n = 2694); −2 to 0 (group 2; n = 1655); 0 to 2 (group 3; n = 1269); and >2 (group 4; n = 1467). Table 2 provides a description of the demographic characteristics of the sample by the DII group. The group with the lowest DII score (most anti-inflammatory dietary potential) was older, had the highest proportion of White participants, the highest level of education and exercise, the lowest BMI, and the highest energy intake. There were no differences between groups in prevalence of self-reported hypertension or diabetes. The group with the lowest DII scores also had the lowest proportion of current smokers, reported more frequent alcohol intake, more of them used NSAIDs, and they had the highest NSES levels. Among the subsample with measures of C-reactive protein (n = 5833), the group with the lowest DII scores also had the lowest level of inflammation.
Fig. 1.
Sample Selection. Abbreviations: BMI, body mass index; FFQ, Food Frequency Questionnaire.
Table 2.
Demographic characteristics of 7085 WHIMS participants
| DII categories
|
||||||
|---|---|---|---|---|---|---|
| All N = 7085 |
< −2 N = 2694 |
−2 to 0 N = 1655 |
0 to 2 N = 1269 |
>2 N = 1467 |
||
| Variable | Mean ± SD or N (%) |
Mean ± SD or N (%) |
Mean ± SD or N (%) |
Mean ± SD or N (%) |
Mean ± SD or N (%) |
P > F |
| DII score | −0.6 ± 2.6 | −3.2 ± 0.9 | −1.1 ± 0.6 | 1.0 ± 0.6 | 3.3 ± 0.8 | <.0001 |
| Age (years) | 71.0 ± 3.9 | 71.1 ± 3.9 | 71.2 ± 3.9 | 70.8 ± 3.8 | 70.7 ± 3.8 | .0005 |
| Race | ||||||
| White | 6211 (87.7) | 2443 (90.7) | 1459 (88.2) | 1090 (85.9) | 1219 (83.1) | <.0001 |
| African American | 471 (6.6) | 116 (4.3) | 110 (6.6) | 105 (8.3) | 140 (9.5) | <.0001 |
| Max education HS | 2073 (29.3) | 641 (23.8) | 478 (28.9) | 397 (31.3) | 557 (38.0) | <.0001 |
| BMI (kg/m2) | 28.4 ± 5.4 | 28.1 ± 5.3 | 28.4 ± 5.5 | 28.9 ± 5.5 | 28.7 ± 5.5 | <.0001 |
| Exercise (MET-hours/week) | 11.3 ± 13.3 | 13.2 ± 14.2 | 11.4 ± 13.5 | 10.2 ± 11.7 | 8.8 ± 11.9 | <.0001 |
| 3MS | 95.3 ± 4.2 | 95.7 ± 3.9 | 95.3 ± 4.1 | 95.3 ± 4.2 | 94.5 ± 4.7 | <.0001 |
| HT arm = EPLC | 1384 (19.5) | 503 (18.7) | 319 (19.3) | 254 (20.0) | 308 (21.0) | <.0001 |
| HT arm = ERT | 1375 (19.4) | 450 (16.7) | 331 (20.0) | 266 (21.0) | 328 (22.4) | <.0001 |
| HT arm = PERT | 2124 (30.0) | 836 (31.0) | 500 (30.2) | 373 (29.4) | 415 (28.3) | <.0001 |
| HT arm = PPLC | 2202 (31.1) | 905 (33.6) | 505 (30.5) | 376 (29.6) | 416 (28.4) | <.0001 |
| Dietary energy (kcals) | 1599 ± 628 | 1805 ± 664 | 1634 ± 50 | 1579 ± 515 | 1201 ± 93 | <.0001 |
| Hypertension | 2744 (39.1) | 1013 (38.1) | 654 (39.9) | 522 (41.6) | 555 (38.2) | .15 |
| Diabetes | 587 (8.3) | 209 (7.8) | 138 (8.4) | 113 (8.9) | 127 (8.7) | .59 |
| High Chol + pills | 1260 (18.0) | 519 (19.5) | 265 (16.3) | 236 (18.8) | 240 (16.6) | .02 |
| NSAID use | 1808 (25.5) | 721 (26.8) | 475 (28.7) | 275 (21.7) | 337 (23.0) | <.0001 |
| 1+ Alcoholic drinks/week | 2504 (35.6) | 1098 (41.0) | 548 (33.3) | 416 (33.2) | 442 (30.5) | <.0001 |
| Current smoker | 493 (7.1) | 134 (5.1) | 124 (7.6) | 96 (7.7) | 139 (9.6) | <.0001 |
| Past smoker | 2804 (40.1) | 1149 (43.4) | 635 (38.7) | 474 (38.0) | 546 (37.6) | <.0001 |
| Region NE | 1910 (27.0) | 739 (27.4) | 465 (28.1) | 317 (25.0) | 389 (26.5) | .09 |
| Region South | 1484 (20.9) | 541 (20.1) | 351 (21.2) | 265 (20.9) | 327 (22.3) | .09 |
| Region MidW | 1718 (24.2) | 623 (23.1) | 400 (24.2) | 345 (27.2) | 350 (23.9) | .09 |
| Region West | 1973 (27.8) | 791 (29.4) | 439 (26.5) | 342 (27.0) | 401 (27.3) | .09 |
| CRP* | 2.70 (2.17) | 2.58 (2.10) | 2.70 (2.17) | 2.78 (2.25) | 2.87 (2.23) | .001 |
| NSES Index | 75.2 (8.2) | 75.9 (7.7) | 75.1 (8.5) | 75.0 (8.1) | 74.0 (8.7) | <.0001 |
Abbreviations: 3MS, Modified Mini–Mental State Examination; BMI, body mass index; CRP, C-reactive protein; DII, Dietary Inflammatory Index; EPLC, estrogen placebo arm; ERT, estrogen only; High Chol + pills, high cholesterol requiring pills; HS, high school; HT, hormone therapy; MET, metabolic equivalent; MidW, midwest; NE, northeast; NSAID, non-steroidal anti-inflammatory drug; NSES, neighborhood socioeconomic status; PERT, estrogen + progestin; PPLC, progestin placebo arm; SD, standard deviation; WHIMS, Women’s Health Initiative Memory Study.
CRP is only available on a subset of 5833 participants.
3.1. Cognitive performance over time
In Table 3, least square mean results from mixed effect models for cognitive performance over the first 10 years of the study are presented. The group with the lowest DII scores performed the best overall compared with the other groups. The group with the highest DII scores performed the worst, on average, scoring more than a half point lower on the 3MS compared to the group with the lowest DII scores in the fully adjusted model. Between-group comparisons were significant (P < .05), with the exception of comparisons between groups 1 and 3 and between groups 2 and 4. When APOE ε4 carrier status was included as a covariate for the subsample with available data, the results were not substantially changed in direction or magnitude (Supplementary Table 3a).
Table 3.
Mixed-model least square means of 3MS scores of 7085 WHIMS participants
| Model | Group 1 DII < −2 |
Group 2 DII −2 to 0 |
Group 3 DII 0 to 2 |
Group 4 DII ≥2 |
|---|---|---|---|---|
| Model 1 | 96.14 (95.98–96.30) | 95.55 (95.34–95.75) | 95.85 (95.62–96.08) | 95.07 (94.85–95.28) |
| Model 2 | 93.91 (93.71–94.11) | 93.55 (93.33–93.77) | 93.87 (93.63–94.10) | 93.32 (93.10–93.54) |
| Model 3 | 93.51 (93.27–93.76) | 93.14 (92.87–93.41) | 93.51 (93.23–93.80) | 92.92 (92.64–93.19) |
Abbreviation: BMI, body mass index.
NOTE. Model 1: unadjusted. Model 2: adjusted for age, education, race, and hormone therapy treatment. Model 3: adjusted for model 2 items plus diabetes, hypertension, high cholesterol requiring pills, NSAID use, BMI, physical activity, energy intake, geographic region, and smoking. Between-group comparisons are significant (P < .05) with the exception of comparisons between groups 1 and 3 and between groups 2 and 4.
3.2. Cognitive impairment survival analyses
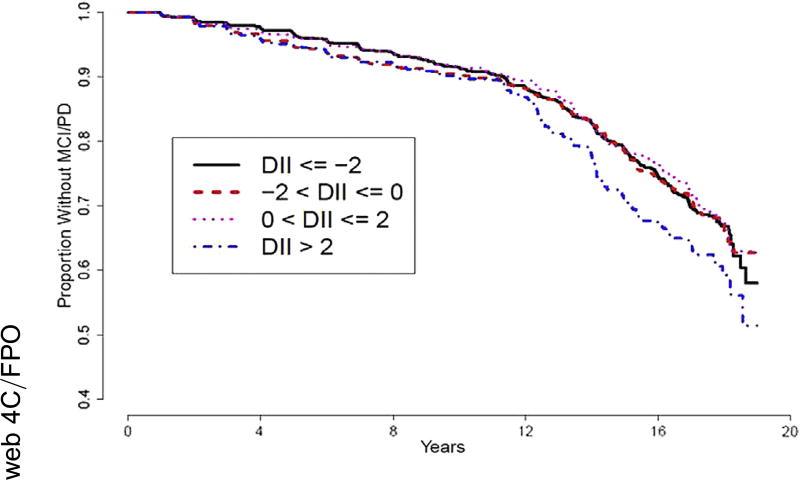
Kaplan-Meier curves for survival to onset of MCI or probable dementia are shown in Fig. 2. The group with the highest DII scores (most pro-inflammatory dietary inflammatory potential) had the shortest average impairment-free survival. The curve representing the group with the lowest DII scores overlaps the two other groups.
Fig. 2.
Kaplan-Meier curves of survival to MCI or dementia onset over 20 years stratified by DII group. Abbreviations: DII, Dietary Inflammatory Index; MCI, mild cognitive impairment; PD, Probable Dementia.
Hazard ratios from Cox regression models are shown in Table 4. The group with the lowest DII scores was the reference group. Model 1 was unadjusted; model 2 was adjusted for age, race, education, and hormone therapy treatment arm; and model 3 was additionally adjusted for diabetes, hypertension, high cholesterol requiring medication, NSAID use, BMI, physical activity level, energy intake, region of the country, and smoking status. In all models, the group with higher inflammatory potential had a significantly increased risk for MCI or probable dementia compared to the group with the lowest inflammatory potential. The P-values for ordinal trends in each model were significant (Table 4; P <.05). Consistent with the Kaplan-Meier curves, groups 2 and 3 did not differ from the referent, group 1.
Table 4.
Hazard ratios comparing the risk of MCI or probable dementia by groups of DII scores
| Hazard ratios (95% CIs)
|
|||||
|---|---|---|---|---|---|
| Model | Group 1, reference | Group 2 versus Group 1 | Group 3 versus Group 1 | Group 4 versus Group 1 | Test for trend P value |
| Model 1 | 1.00 | 1.06 (0.90–1.24) | 0.97 (0.82–1.16) | 1.30 (1.10–1.52) | .01 |
| Model 2 | 1.00 | 0.99 (0.84–1.16) | 0.95 (0.80–1.13) | 1.25 (1.06–1.47) | .01 |
| Model 3 | 1.00 | 1.01 (0.86–1.20) | 0.99 (0.82–1.18) | 1.27 (1.06–1.52) | .03 |
Abbreviations: BMI, body mass index; CI, confidence interval; DII, Dietary Inflammatory Index; MCI, mild cognitive impairment.
NOTE. Model 1: unadjusted. Model 2: adjusted for age, education, race, hormone therapy treatment. Model 3: adjusted for model 2 items plus diabetes, hypertension, high cholesterol requiring pills, NSAID use, BMI, physical activity, energy intake, geographic region, and smoking.
Sensitivity analyses within a subsample of participants with APOE genotype data showed attenuated effects for groups 2 and 3; however, adjusted comparisons (equivalent to Table 4 models 2 and 3) between groups 4 and 1 remained statistically significant. Overall tests for ordinal trend for models 1 to 3 were no longer significant. However, it should be noted that most participants with APOE genotypes were White, so this smaller subsample of the cohort is biased (Supplementary Table 4a). A second sensitivity analysis included an indicator for NSES in the subset of participants for whom these data were available and results were relatively unchanged from those shown in Table 4 (Supplementary Table 4b; P-value for ordinal trend = .07 in fully adjusted model). To test the possibility that the effect of diet was due to modification of CVD risk, we fitted a model that was adjusted for incident CVD and found very modest changes in results (Supplementary Table 4c; P-value for ordinal trend = .03 in fully adjusted model). Finally, to evaluate the possibility that women with lower 3MS scores at baseline may have been at higher risk for MCI or probable dementia at the beginning of the study, we tested a model lagged for MCI or probable dementia onset by three years; there was similarly little change in our estimates (Supplementary Table 4d; P-value for ordinal trend = .03 in fully adjusted model).
4. Discussion
In this study, self-reported diets higher in pro-inflammatory properties, were associated with worse cognitive function over time and an increased risk of incident MCI and probable dementia. While we predicted that a diet with higher inflammatory potential would be associated with a steeper decline in cognitive function over time and a higher risk for incident dementia, we did not see the anticipated dose-response effect across groups defined by DII levels. It may be that there is a threshold beyond which a diet higher or lower in inflammatory properties does not offer added cognitive risk or protection.
Although we found a significant difference in cognitive performance between the highest and lowest DII groups, the differences in scores do not appear to be clinically meaningful. However, considering the cumulative effect over time, there is potential for a delay in the onset of MCI or dementia, which is meaningful on an individual level. Nonetheless, these findings are not readily applicable in clinical settings as the DII does not represent a specific diet. Rather, it is a tool that is designed to evaluate and describe the inflammatory potential of diet. Further replication in other cohorts is needed to confirm these findings. As results from other studies confirm these, then it might be reasonable to design diets to lower DII scores to reduce the risk of MCI or dementia.
Our findings are in line with other studies that have found associations between dietary patterns, inflammation, and cognitive function [48] or cognitive decline [49]. In a sample of 3080 participants from the Supplémentation en Vitamines et Minérauz Antioxydants Study, higher DII scores at midlife were associated with poorer cognitive function ~13 years later [48]. In the Whitehall II study, reduced rank regression was used to identify an inflammatory dietary pattern associated with IL-6 levels based on a set of predefined food groups [49]. The pattern was characterized by higher intake of red meat, processed meat, peas, legumes, and fried food and lower intake of whole grains. An association between their inflammatory diet pattern and faster declines in global cognition over time was found.
Adherence to other diets such as the Mediterranean and DASH diets has been associated with reduced cognitive decline and lower incidence of dementia in some studies [3–6]. Both diets are high in antioxidants, and their recommended dietary intakes appear to be aligned with lower inflammatory potential. However, not all studies have replicated these findings [50–52]. Inconsistencies could be due to differences in study populations, methodologies, or dietary scoring methods [53,54]. The most recent null finding came from another analysis from the WHIMS, using essentially the same sample and FFQ data as those used in the present study [55]. The study found no association between cognitive decline and healthy dietary patterns including the alternate Mediterranean dietary score, the Healthy Eating Index 2010 [56,57], the Alternate Healthy Eating Index 2010 [58,59], and the DASH diet [60,61]. The primary difference between these dietary patterns and the DII is the strong emphasis of the DII on the pro- and anti-inflammatory potential of the dietary constituents (i.e., food parameters).
The mechanisms by which healthy diets influence cognition may be indirect, via reduction in risk for CVD and better metabolic control, conditions that are associated with chronic inflammation. Reductions in dietary inflammatory potential also may directly reduce the chronic inflammation associated with neurodegenerative diseases such as AD [62] In the WHI cohort, higher DII scores have been correlated with higher concentrations of inflammatory markers including IL-6, TNF-α, and hs-CRP [11]. In the Seasonal Variation of Blood Cholesterol Study [23], higher DII scores derived from both 24-hour dietary and 7-day dietary recalls also were associated with higher levels of hs-CRP. Finally, the DII includes saturated and trans fats, components of the Western diet with inflammatory potential, which have been associated with poor cognitive function in other studies [63,64].
4.1. Limitations
Although WHIMS has up to ~20 years of follow-up observation for survival analyses, the evaluation of cognitive decline over time is limited to the first ~10 years in this report. This is due to a transition from face-to-face cognitive evaluations using the 3MS, to a validated telephone cognitive assessment format using the TICS-m. We chose to evaluate cognitive decline over the first 10 years as this is more proximal to the FFQ evaluation. On a national level, diets have changed over the last 20 years, and there may be fewer trans fats in today’s diet than there were when the WHIMS women were measured, limiting generalizability to today’s diets. FFQs have known measurement error, including underreporting, which may lead to attenuated associations between the DII and outcomes. The FFQ in our study was evaluated only at baseline, and diet can change over time and due to health conditions or other motivation. The seasonality of FFQ data collection was not taken into account in the derivation of the DII. This could have influenced the nutrients consumed by season and by region of the country in which participants reside. We did, however, control for region in our analysis. In a subsample from the larger WHI cohort, a modest decrease in DII scores was observed over time [65]. It is likely that some of the individuals included in the group with the diet that had the highest anti-inflammatory potential were following a healthy diet due to illness. A number of food parameters were not measured by the WHI-FFQ. Ginger, turmeric, garlic, oregano, pepper, rosemary, eugenol, saffron, flavan-3-ol, flavones, flavonols, flavanones, and anthocyanidins were not included in the computation of DII scores for participants. Because all of these dietary factors are anti-inflammatory, the DII scores in this study likely underestimate the anti-inflammatory properties of participants’ diets [11]. Furthermore, the absence of these components should have minimal impact in general as they represent nutrients that are typically consumed in small quantities. There were significant differences between groups on quite a few variables, that is, age, education, race, BMI, energy intake, exercise, NSAID use, alcohol, smoking, and NSES. Many of these variables are associated with each other as well as with cognition. Although we adjusted for these conditions, it is likely that there was some residual confounding. As this is an observational study, we can only adjust for potential sources of confounding that were collected. We have demonstrated an association between DII scores and these outcomes. However, a strong causal inference cannot be made from a single study. Additionally, because we do not have information about women’s dietary patterns before baseline, we cannot know if dietary choices were made before or after the beginning of cognitive decline for some women. We attempted to address this by evaluating associations lagged by 3 years, and our results were essentially unchanged.
5. Conclusions
A diet with pro-inflammatory properties is associated with worse cognitive function in later life and with higher risk of MCI or probable dementia. Further replication of our work is needed to confirm these findings, as our results show a nonlinear association, suggesting the possible existence of a threshold.
It is unlikely that long-term dietary approaches could be successfully implemented in a clinical trial setting. Yet, the results of this observational legacy study can be informative. Studies such as this help to generate hypotheses by providing information about potential interventions and, with further study, may form the basis for developing new biomarkers of disease. Animal models could be used to study the effects of foods on inflammation in the brain and on AD pathology. This may be an appropriate way to address the issues of residual confounding and reverse causality that are likely to be found in any study of human diets. The present study highlights the importance of observational studies and the foundations they provide for development of more focused research questions [66]. What are the mechanisms for the effects of diet on cognition? Can better measures of diet be developed, that is, using new technologies? Are there biological measures of diet or indicators of dietary intake that can be derived from the microbiome, proteome, or metabolome that can be used to probe these associations further? Clearly, a number of open questions requiring further research remain.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic Review: We searched the literature for studies on diet and cognition with a focus on dietary inflammation. Most studies on diet and cognition have been focused on dietary approaches such as the Mediterranean diet, the DASH diet, or the MIND diet. The Dietary Inflammatory Index (DII) is a relatively new measure, and therefore it has only been studied for an association with cognition in one other study to date.
Interpretation: Few studies have focused on the pro-or anti-inflammatory potential of diets. Unlike the Mediterranean or DASH diets, the DII is an assessment of the inflammatory potential of a diet rather than a prescription of foods that can be followed. The DII, with its association with inflammatory bio-markers, cognitive decline, and risk of MCI or probable dementia, together with results of prior studies on diet suggests this may be a potent route for primordial prevention interventions.
Future directions: This work should be replicated, preferably in studies that have data supporting AD clinical diagnoses and pathology. Correlations between β-amyloid deposition and tau would further enhance our understanding of the association between diets with pro-inflammatory potential and cognitive decline associated with AD.
Acknowledgments
Funding: The Women’s Health Initiative (WHI) was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN 268201600001C, HHSN268201600002C, HHSN26820 1600003C, and HHSN268201600004C. The Women’s Health Initiative Memory Study (WHIMS) was funded in part by Wyeth Pharmaceuticals, St. Davids, PA. The WHIMS Extension was funded by the National Institute on Aging contract HHSN26820044221C, and WHIMS-ECHO was funded by the National Institute on Aging through contracts HHSN271201100004C, HHSN26820044221C, and HHSN 271201100004C.
J.R.H. and N.S. were supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant number R44DK103377. F.K.T. was supported by the National Cancer Institute grant number K99CA207736.
ClinicalTrials.gov identifiers: NCT00017953 (WHIMS-Y) and NCT01124773 (WHIMS/WHIMS-ECHO).
The authors wish to acknowledge the contributions of the WHI investigators: Program office (National Heart, Lung, and Blood Institute, Bethesda, MD, USA): Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA, USA): Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA: J.E.M.; MedStar Health Research Institute/Howard University, Washington, DC, USA: Barbara V. Howard; Stanford Prevention Research Center, Stanford, CA, USA: Marcia L. Stefanick; The Ohio State University, Columbus, OH, USA: Rebecca Jackson; University of Arizona, Tucson/Phoenix, AZ, USA: Cynthia A. Thomson; University at Buffalo, Buffalo, NY, USA: Jean Wactawski-Wende; University of Florida, Gainesville/Jacksonville, FL, USA: Marian Limacher; University of Iowa, Iowa City/Davenport, IA, USA: Jennifer Robinson; University of Pittsburgh, Pittsburgh, PA, USA: Lewis Kuller; Wake Forest University School of Medicine, Winston-Salem, NC, USA: Sally Shumaker; University of Nevada, Reno, NV, USA: Robert Brunner; University of Minnesota, Minneapolis, MN,USA: Karen L. Margolis. WHIMS (Wake Forest University School of Medicine, Winston-Salem, NC, USA): Mark Espeland.
For a list of all the investigators who have contributed to WHI science, please visit https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jalz.2017.04.004.
Disclosure: J.R.H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the Dietary Inflammatory Index from the University of South Carolina to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. The subject matter of this article will have no direct bearing on the work of CHI nor has any CHI-related activity exerted any influence on this project. N.S. is an employee of CHI.
References
- 1.Last JM, Abramson JH, Greiedman GD, Porta M, Spas-off RA, Thuriaux M, editors. A dictionary of epidemiology. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 2.Anekwe TD, Rahkovsky I. Economic costs and benefits of healthy eating. Curr Obes Rep. 2013;2:225–34. [Google Scholar]
- 3.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–30. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 4.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–17. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–21. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on memory, health and aging. Am J Clin Nutr. 2013;98:1263–71. doi: 10.3945/ajcn.112.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. 2015;6:738–47. doi: 10.3945/an.115.009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham C, Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimers Res Ther. 2015;7:33. doi: 10.1186/s13195-015-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, et al. Construct validation of the dietary inflammatory index among post-menopausal women. Ann Epidemiol. 2015;25:398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 13.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 14.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 15.Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–21. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 16.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 18.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 19.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 20.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 23.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutr. 2014;17:1825–33. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivappa N, Hebert JR, Marcos A, Diaz LE, Gomez S, Nova E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. 2016 doi: 10.1002/mnfr.201600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113:665–71. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 27.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 30.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care: The prime-md 1000 study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 31.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4:173–8. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 32.Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS) Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 33.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–42. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–7. [Google Scholar]
- 36.Ricker JH, Axelrod BN. Analysis of an oral paradigm for the Trail Making Test. Assessment. 1994;1:47–52. doi: 10.1177/1073191194001001007. [DOI] [PubMed] [Google Scholar]
- 37.Gfeller JD, Horn GJ. The East Boston Memory Test: a clinical screening measure for memory impairment in the elderly. J Clin Psychol. 1996;52:191–6. doi: 10.1002/(SICI)1097-4679(199603)52:2<191::AID-JCLP10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. Wechsler Memory Scale, Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 39.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 40.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. Avalidation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–6. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 41.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 42.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 43.Bavry AA, Thomas F, Allison M, Johnson KC, Howard BV, Hlatky M, et al. Nonsteroidal anti-inflammatory drugs and cardiovascular outcomes in women: results from the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes. 2014;7:603–10. doi: 10.1161/CIRCOUTCOMES.113.000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 45.Cox DR. Regression models and life tables. J R Stat Soc Ser B (Methodological) 1972;34:187–220. [Google Scholar]
- 46.Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349–70. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shih RA, Ghosh-Dastidar B, Margolis KL, Slaughter ME, Jewell A, Bird CE, et al. Neighborhood socioeconomic status and cognitive function in women. Am J Public Health. 2011;101:1721–8. doi: 10.2105/AJPH.2011.300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kesse-Guyot E, Assmann KE, Andreeva VA, Touvier M, Neufcourt L, Shivappa N, et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.-MAX Study. Eur J Nutr. 2016 doi: 10.1007/s00394-016-1211-3. [DOI] [PubMed] [Google Scholar]
- 49.Ozawa M, Shipley M, Kivimaki M, Singh-Manoux A, Brunner EJ. Dietary pattern, inflammation and cognitive decline: the Whitehall II prospective cohort study. Clin Nutr. 2017;36:506–12. doi: 10.1016/j.clnu.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cherbuin N, Anstey KJ. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH Through Life Study. Am J Geriatr Psychiatry. 2012;20:635–9. doi: 10.1097/JGP.0b013e31823032a9. [DOI] [PubMed] [Google Scholar]
- 51.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–48. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, et al. Mediterranean diet and cognitive function in older age. Epidemiology. 2013;24:490–9. doi: 10.1097/EDE.0b013e318294a065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367:31–7. doi: 10.1111/nyas.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beto JA, Champagne CM, Dennett CC, Harris JE. The challenge of connecting dietary changes to improved disease outcomes: the balance between positive, neutral, and negative publication results. J Acad Nutr Diet. 2016;116:917–20. doi: 10.1016/j.jand.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, et al. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women’s Health Initiative Memory Study. J Acad Nutr Diet. 2016;116:921–930. doi: 10.1016/j.jand.2015.12.017. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–80. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–8. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 58.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9:152–7. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 60.The DASH diet. Dietary Approaches to Stop Hypertension. Lippincotts Prim Care Pract. 1998;2:536–8. [PubMed] [Google Scholar]
- 61.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 62.Gardener SL, Rainey-Smith SR, Martins RN. Diet and inflammation in Alzheimer’s disease and related chronic diseases: a review. J Alzheimers Dis. 2015;50:301–34. doi: 10.3233/JAD-150765. [DOI] [PubMed] [Google Scholar]
- 63.Gardener SL, Rainey-Smith SR, Barnes MB, Sohrabi HR, Weinborn M, Lim YY, et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol Psychiatry. 2015;20:860–6. doi: 10.1038/mp.2014.79. [DOI] [PubMed] [Google Scholar]
- 64.Shakersain B, Santoni G, Larsson SC, Faxen-Irving G, Fastbom J, Fratiglioni L, et al. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement. 2016;12:100–9. doi: 10.1016/j.jalz.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Tylavsky FA, et al. Longitudinal changes in the dietary inflammatory index: an assessment of the inflammatory potential of diet over time in postmenopausal women. Eur J Clin Nutr. 2016;70:1374–80. doi: 10.1038/ejcn.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.OECD. Emerging Trends in Biomedicine and Health Technology Innovation: Addressing the Global Challenge of Alzheimer’s. Vol OECD Science, Technology and Industry Policy Papers, No. 6. Paris: OECD Publishing; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.