Abstract
Understanding the neurobiology of social bonding in non-human primates is a critical step in understanding the evolution of monogamy, as well as understanding the neural substrates for emotion and behavior. Coppery titi monkeys (Callicebus cupreus) form strong pair bonds, characterized by selective preference for their pair mate, mate-guarding, physiological and behavioral agitation upon separation, and social buffering. Mate-guarding, or the “maintenance” phase of pair bonding, is relatively under-studied in primates. In the current study, we used functional imaging to examine how male titi monkeys viewing their pair mate in close proximity to a stranger male would change regional cerebral glucose metabolism. We predicted that this situation would challenge the pair bond and induce “jealousy” in the males. Animals were injected with [18F]-fluorodeoxyglucose (FDG), returned to their cage for 30 min of conscious uptake, placed under anesthesia, and then scanned for 1 hour on a microPET P4 scanner. During the FDG uptake, males (n=8) had a view of either their female pair mate next to a stranger male (“jealousy” condition) or a stranger female next to a stranger male (control condition). Blood and cerebrospinal fluid samples were collected and assayed for testosterone, cortisol, oxytocin, and vasopressin. Positron emission tomography (PET) was co-registered with structural magnetic resonance imaging (MRI), and region of interest analysis was carried out. Bayesian multivariate multilevel analyses found that the right lateral septum (Pr(b>0)=93%), left posterior cingulate cortex (Pr(b>0)=99%), and left anterior cingulate (Pr(b>0)=96%) showed higher FDG uptake in the jealousy condition compared to the control condition, while the right medial amygdala (Pr(b>0)=85%) showed lower FDG uptake. Plasma testosterone and cortisol concentrations were higher during the jealousy condition. During the jealousy condition, duration of time spent looking across at the pair mate next to a stranger male was associated with higher plasma cortisol concentrations. The lateral septum has been shown to be involved in mate-guarding and mating-induced aggression in monogamous rodents, while the cingulate cortex has been linked to territoriality. These neural and physiological changes may underpin the emotion of jealousy, which can act in a monogamous species to preserve the long-term integrity of the pair.
Keywords: monogamy, mate-guarding, mating-induced aggression, testosterone, lateral septum, cingulate cortex, vasopressin, cortisol
INTRODUCTION
Shakespeare’s “green-eyed monster” has been written about for centuries (Shakespeare, 1988), but the scientific study of jealousy is relatively young. Jealousy is an aspect of romantic relationships that works to maintain the relationship, but which can develop into intimate partner violence when unrestrained (Buss, 2002; Neal and Edwards, 2015). Jealousy may have given a fitness advantage to humans in our ancestral environment; current evidence shows that culture also plays a role (Harris, 2003). The emotion of jealousy is a form of social rejection that occurs when another individual (partner, parent, etc.) appears to devalue a relationship because of an outside third party (Leary, 2015). Because jealousy is an emotion that often occurs in the context of reproductive relationships, it is relevant to our understanding of the evolution and neurobiology of pair bonds.
The neural basis of jealousy in humans is not well understood in part because eliciting jealousy requires complex social interactions which may be difficult to create in a laboratory setting (Harmon-Jones et al., 2009). However, a large body of studies has suggested that social rejection of various types is mediated by the anterior cingulate cortex (Eisenberger, 2015). A recent functional magnetic resonance imaging (fMRI) study asked participants to imagine that an early stage romantic partner “did not prefer” them over a romantic rival. This jealousy condition provoked activation in the dorsal and ventral striatum (dopaminergic areas), as well as the cingulate cortex (Sun et al., 2016). Dopamine agonist therapy is also associated with delusional jealousy in Parkinson’s patients (Poletti et al., 2012).
Investigating the neurobiology of jealousy in non-human primates that form pair bonds is an important step in understanding the evolution of monogamy. Male sexual “jealousy” was studied in the context of rhesus monkey consortships using positron emission tomography (PET) imaging (Rilling et al., 2004). Rhesus monkeys are not socially monogamous, but do form short-term consortships in which a male guards an estrus female (Manson, 1997; Palombit, 2014). When rhesus males viewed their consort next to a stranger male, they had increased [18F]-fluorodeoxyglucose (FDG) uptake in areas including the right amygdala and right superior temporal sulcus; plasma testosterone concentrations also increased (Rilling et al., 2004). Theoretically, a threat to a long-term reproductive and affiliative relationship might be even more salient than a threat to a short-term sexual consortship, because it is an attachment bond and there are more resources to lose (Ellis and Weinstein, 1986). Unlike rhesus monkeys, titi monkeys are socially monogamous and form long-term pair bonds, and thus might even have stronger “jealousy” reactions than rhesus monkeys (which only form consortships).
Social monogamy is displayed by a small minority of mammals, usually estimated at 3–5% of mammalian species (Kleiman, 1977; Lukas and Clutton-Brock, 2013; Diaz-Munoz and Bales, 2016; Tecot et al., 2016). In socially monogamous animals, the development of an adult attachment relationship or “pair bond” is associated with the onset of mate-guarding in both males and females (Mason, 1966; Winslow et al., 1993; McGuire and Getz, 1998; Fernandez-Duque et al., 2000; Bowler et al., 2002; Getz et al., 2003; Fisher-Phelps et al., 2016; Tabbaa et al., 2016). This behavior helps maintain the relationship through aggression towards both same- and opposite-sex individuals. The pair bond is a construct encompassing a preference for the familiar partner, distress upon separation, and the ability of the pair mate to buffer stress (Mason and Mendoza, 1998). As such, it is very similar to a human romantic relationship (Hazan and Shaver, 1987; Sbarra and Hazan, 2008) and the pair mates might be expected to feel jealousy if a third party threatened that relationship. While the neurobiology of pair bonding has been best studied in a rodent model, the socially monogamous prairie vole (Microtus ochrogaster), the many potential differences between rodent and primate nervous systems make a primate model for pair bonding desirable as well (Phillips et al., 2014; Bales et al., 2017).
Titi monkeys (genus Callicebus) are small, arboreal primates which display social monogamy (including “jealousy” behavior) both in the field (Mason, 1966; Spence-Aizenberg et al., 2016; Van Belle et al., 2016) and in the laboratory (Mason, 1974; Mendoza and Mason, 1997; Carp et al., 2016). For example, in the wild, a male was observed placing himself in between his female pair mate and intruding male, and physically restraining his female pair mate to keep her from moving towards an “intruder” male (Mason, 1966). Wild titi monkeys of both sexes respond to conspecific playbacks by duetting and approaching the speaker, which may function as both territorial and mate defense (Caselli et al., 2015). Both males and females show strong arousal reactions towards outsiders, including tail-lashing and arched-back displays, and restraint of the pair mate to keep her/him away from the stranger, although males have stronger reactions than females (Cubiciotti and Mason, 1978; Fernandez-Duque et al., 2000). This jealousy reaction can be duplicated in a laboratory context either with live intruders (Fernandez-Duque et al., 2000) or by introduction of a mirror in which the pair sees their own reflections (Fisher-Phelps et al., 2016). Titi monkeys provide an ideal non-human primate to examine a challenge to the pair bond that could elicit a “jealousy” response. Ellis and Weinstein (Ellis and Weinstein, 1986) proposed that three conditions are necessary for eliciting jealousy: (1) an attachment relationship between two individuals, (2) valued resources that are part of the attachment bond, and (3) intrusion by a third individual that is perceived by one partner as wanting to become a receiver of resources. Titi monkeys fit these criteria since the (1) adult male and female form an attachment relationship with each other (unlike most other monkeys), and (2) titi monkeys naturally respond to “intruders” in the wild and captivity.
In addition to the potential neural changes associated with jealousy, we were also interested in the potential hormonal changes. In the rhesus monkey study, males who viewed their consort next to a stranger male had an increase in plasma testosterone concentrations (Rilling et al., 2004). While testosterone is the hormone most often associated with male jealousy or mate-guarding (Wingfield et al., 1990; Gray et al., 2017), there is also evidence for the role of vasopressin in aggression from both animals (Winslow et al., 1993; Ferris and Delville, 1994; Stribley and Carter, 1999; Gobrogge and Wang, 2016; Simmons et al., 2017) and humans (Marshall, 2013). Vasopressin and oxytocin are also involved in the neurobiology of pair bond formation (Numan and Young, 2016). A role for cortisol in jealousy is also plausible based on its responses to challenging social situations (Breuner and Hahn, 2003; Casto and Edwards, 2016; Beehner and Bergman, 2017; Mendoza, 2017).
In the current study, we examined potential changes in the neural and hormonal substrates in response to a challenge to the pair bond of male titi monkeys, using the previously mentioned rodent, rhesus monkey, and human studies as our guides for the outcome measures. We exposed our subjects to two conditions in which they viewed either (1) their female pair mate next to a stranger male (jealousy condition) or (2) a stranger female next to a stranger male (control condition). We expected to see increased [18F]-fluorodeoxyglucose (FDG) uptake in the lateral septum; this could be due to up-regulation of dopamine D1 receptors as has been observed in monogamous prairie voles who mate-guard (Aragona et al., 2006; Resendez et al., 2016) and titi monkeys who were recently paired (Hostetler et al., 2017). We also examined other areas implicated in jealousy in rodents (i.e. posterior cingulate cortex, medial amygdala, anterior hypothalamus), rhesus monkeys (i.e. insular cortex, superior temporal sulcus (Rilling et al., 2004)), or humans (i.e. anterior cingulate, nucleus accumbens, caudate, putamen, ventral pallidum (Sun et al., 2016)). While we do not specifically know the distribution of androgen receptors in titi monkeys, we did have a strong a priori prediction of increased plasma testosterone concentrations, because of testosterone’s association with mating-related aggression and competition (Gray et al., 2017; Wingfield, 2017). Similarly, we predicted increases in plasma hormone concentrations of cortisol, oxytocin, and vasopressin due to their association with social challenge (Mendoza, 2017).
METHODS
All experimental procedures were approved by the Animal Care and Use Committee of the University of California, Davis, and complied with National Institutes of Health ethical guidelines as set forth in the Guide for Lab Animal Care.
Subjects
Subjects were eight captive-born adult male titi monkeys (Callicebus cupreus) housed at the California National Primate Research Center (CNPRC) in Davis, CA. Subjects were a mean age of 7.7 years old (median 7.0, range 4.0 – 12.8), and were living with their female pair mates for a mean of 2.5 years (median 1.7, range 0.7 – 9.9). All subjects were parents of offspring living in the cage. Animals were fed twice daily (0830 and 1330 h) a diet consisting of New World monkey chow, rice cereal, banana, apples, raisins, and baby carrots and water was available ad libitum. Further details of husbandry and training are available elsewhere (Tardif et al., 2006).
Experimental design and PET scanning with FDG
Functional imaging was used to examine how males viewing their pair mate in close proximity to a stranger male would differ in their regional cerebral glucose metabolism compared to viewing a stranger male next to a stranger female in adjacent cages. Subjects, pair mates and young offspring (less than one year old) were relocated to a metabolism room 48 hours prior to their positron emission tomography (PET) scan. As in our previous PET studies (Bales et al., 2007; Hinde et al., 2016; Maninger et al., 2017), animals were relocated prior to the scan in order to reduce the possible effect of novel housing on brain metabolism. Animals were fasted 6–12 h prior to the scan, with water available throughout the pre-scan period. On the day of the scan, all of the animals were caught and removed from the cage. The subject was manually restrained while he received a bolus injection of [18F]-fluorodeoxyglucose (FDG, PETNET Solutions, Sacramento, CA, up to 2 mCi/kg IV, administered in a volume of <2 ml) into the saphenous vein.
Following the FDG injection, the male was put back in his cage alone (since his pair mate and offspring were removed) for the 30 minute conscious uptake period, where he had visual access to another cage that housed two animals separated by a wire mesh. In the jealousy condition, the two animals in the viewing cage were the subject’s female pair mate and a stranger male (Figure 1). The stranger was a male who was unfamiliar to the subjects. Viewing a stranger male adjacent to his female pair mate was designed to challenge the pair bond and induce “jealousy” in the male subjects. In the control condition, there was a stranger female and a stranger male monkey (note that this was a different animal from the jealousy condition) in the viewing cage. Because titi monkeys are territorial animals when paired and can show aggression to opposite-sex strangers (Fernandez-Duque et al., 2013), the male and female in the viewing cage were separated by a wire mesh in order to prevent any physical aggression (and potential wounding) between the unfamiliar animals. This was important because there were no humans in the room during the FDG uptake period to stop any fights. During the control condition, the female pair mate was moved out of the testing room. The offspring were moved out of the testing room for both the control and jealousy conditions. A camera was placed at the side of the subject’s cage and the male was filmed during the uptake period for 30 minutes, while all of the humans left the room. Each of the eight males experienced both the jealousy and control conditions on separate days; there was a mean of 5.2 weeks (range 3 – 6.3) between testing days. The order of conditions was counter-balanced, such that four males experienced the jealousy condition before the control condition and four males experienced the control condition before the jealousy condition.
Figure 1. Research Design.

Subject males (n = 8) each underwent two conditions: a jealousy condition and a control condition. In the jealousy condition, they viewed their female pair mate next to a stranger male. In the control condition, males viewed two stranger animals, a stranger male and stranger female.
After the FDG uptake period, subjects were anesthetized with ketamine (25 mg/kg IM) and administered medetomidine (0.05 mg/kg IM). After the subject was sedated, a 1 ml blood sample was collected from the femoral vein and put into a heparin-containing tube, and a sample of cerebrospinal fluid (CSF) was collected and put on ice. In order to ensure that hormonal outcomes were not influenced by the considerable disturbances involved prior to collection of blood samples, care was taken to ensure that the time of day was comparable for a given subject tested in each condition. Testing started between 0800–0839 h for the first subject, and the second subject was tested approximately 1.5–2 hours later. Cortisol concentrations for males tested in the first (earlier) group averaged 94.1 ± 5.8 μg/dl, while males tested in the later group averaged 55.7 ± 7.2 μg/dl. While time of day was correlated with cortisol concentrations (r = −0.575), this effect of circadian rhythm was accounted for in the design of the study by carrying out both of an individual’s scans (control and jealousy) in the same time grouping (early or late). For example, both of 32878’s scans were carried out in the early group, and both of 31716’s scans were carried out in the late group. We also measured the duration of time between capture of the subject following FDG uptake until bloodand CSF sample collection (i.e. “disturbance time”). Disturbance time for blood samples was a mean of 5.22 minutes (median 4.80, range 1.72–16.15) after capture and sedation (raw data in Appendix 1). This disturbance time was not statistically correlated with plasma cortisol concentrations (r = 0.102). CSF samples were collected a mean of 9.50 minutes (median 9.88, range 5.75–13.13) after capture and sedation (raw data in Appendix 1; no statistical analysis on CSF data was carried out).
Following collection of blood and CSF samples, an endotracheal tube was placed and a catheter was placed in the saphenous vein in order to administer IV fluids (lactated ringers solution, 10 ml/kg/hr). Atipamazole was used to reverse medetomidine, and anesthesia was maintained with isoflurane (1–2%), while the male was positioned on the scanner bed feet first and the brain of the animal was positioned in the center of the scanner. PET imaging was performed on a microPET P4 scanner (Siemens Preclinical Solutions, Knoxville, TN). Image acquisition began a mean of 69.49 (SD ± 7.52) minutes post-FDG administration, and static PET scans were acquired for 60 minutes. Anesthesia was maintained throughout the scan. Animals were housed in metabolism cages for 24 h after scanning, at which time radiation was decayed to background levels and animals were returned to their home cages.
MRI Scanning
Structural magnetic resonance imaging (MRI) scans were conducted in a GE Signa LX 9.1 scanner (General Electric Corporation, Milwaukee, WI) with a 1.5 T field strength and a 3″ surface coil. Each male was fasted 8–12 h before the procedure. At the start of the procedure, the male was sedated with ketamine (10 mg/kg IM) and medazolam (0.1 mg/kg IM), and an endotracheal tube was placed. A catheter was also placed in the saphenous vein in order to administer fluids as necessary. Anesthesia was maintained with isoflurane (1–2%) while the male was positioned in the MRI scanner. Each scan lasted approximately 20 min and consisted of a 3D SPGR pulse sequence in a coronal plane. Images of the entire brain were collected using the following parameters: echo time TE=7.9 ms, repetition time TR=22.0 ms, flip angle=30.0°, field of view=8 cm, number of excitations=3, matrix=256×256, and slice thickness=1 mm. As a precautionary measure, the male’s EtCO2, oxygen saturation, heart rate and blood pressure were monitored throughout.
PET and MRI Coregistration, Quantification of FDG Uptake
We determined which regions of interest (ROIs) to quantify based on three groups of studies: rodent studies of aggression (lateral septum, medial amygdala, posterior cingulate cortex, and anterior hypothalamus), the Rilling rhesus monkey study of consortship (superior temporal cortex, insular cortex), and human studies of social pain and jealousy (anterior cingulate cortex, nucleus accumbens, ventral pallidum, caudate, and putamen) (Figure 2).
Figure 2.
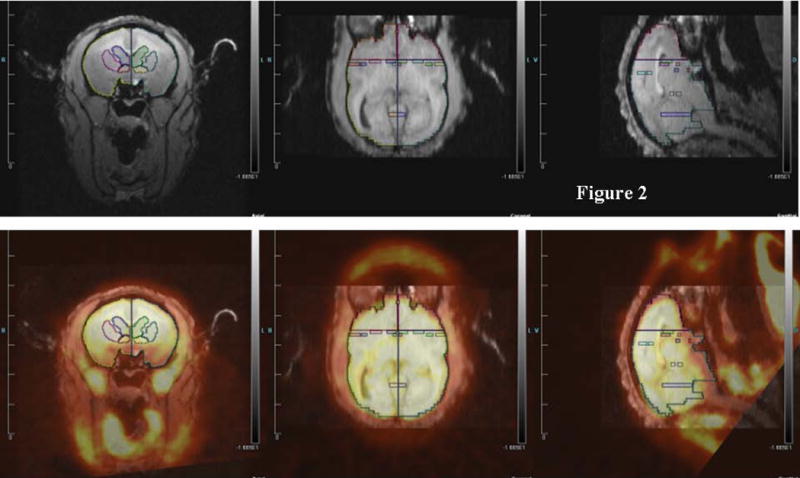
Top: MRI image with regions of interest. Bottom: MRI image co-registered with PET for titi monkey in jealousy condition.
ROI structures were individually drawn on each subject’s MRI image, for both left and right hemispheres, using landmarks as a guide, in Siemen’s Inveon Research Workplace software (IRW, Siemens Healthcare, USA). ROIs were drawn prior to co-registrations with the PET image, so they were drawn blind with regard to PET image/FDG uptake and to experimental condition. The same ROIs were used for both the jealousy and control conditions. Static PET images were reconstructed with a 3DRP reconstruction protocol. MRI images were co-registered with PET scan images using the automatic rigid registration algorithm in IRW and checked visually for registration accuracy. Mean activity for the PET images were determined in IRW by applying ROIs defined on the MRI images to the PET images. Data are presented in proportions of whole brain activity, which was calculated by dividing the mean activity in the ROI (in units of microcuries per cubic centimeter) by mean activity of whole brain ROI.
Behavioral Coding
Males were filmed during the 30 min FDG uptake period. After all of the PET scans were completed, the videos were scored by a trained coder (T.S.) who was blind to experimental condition and validated against previous scoring done in the laboratory. Videos were scored on Behavior Tracker 1.5 (behaviortracker.com) for duration of the behaviors in the ethogram (see Table 1). Behaviors included lip smacking (an affiliative behavior), tail lashing (an arousal behavior), arching (an arousal behavior), as well as looking across at the stimulus cage, locomotion, chewing, drinking, and “off camera.” Data analyses were performed on the total duration of each behavior (i.e. the absolute length of time the behavior was performed).
Table 1.
Ethogram.
| Behavior | Definition |
|---|---|
| Look Across | Male’s eyes gaze in the direction of the stimulus cage. |
| Lip smack | Male makes rapid lip movement accompanied by smacking sound. |
| Arch | Male raises dorsal surface of his back. May be accompanied by piloerection. (This behavior along with tail lash are “arousal behavior”) |
| Tail lash | Male whips his tail back and forth laterally. (This behavior along with arch are “arousal behavior”) |
| Locomotion | Male moves at least one body length. |
| Chew/pick bandage | Male manipulates bandage (which covers his injection site) with his mouth or hands. |
| Drink | Male drinks water. Begins when mouth touches lixit and ends when drinking terminates. |
| Off camera | Male is out of view of the camera. |
All behaviors were analyzed as total durations
Blood Sampling and Hormone Analysis
Blood and CSF samples were collected after animals were sedated for the PET scan following the FDG uptake period, and placed on ice. Blood samples in heparin-containing tubes were centrifuged at 3000 RPM for 15 minutes at 4° C. Plasma was aliquoted, and plasma and CSF samples were stored at −70° C until assay. CSF samples were assayed for oxytocin (OT) and vasopressin (AVP). Plasma samples were assayed for testosterone, cortisol, OT, and AVP. While the veterinarians collected as many CSF samples as possible, often they were unable to get a sample due to the small size of the animals (male subjects weighed a mean of 1.3 kg, median 1.2, range 1.1 – 1.6). Therefore, we present CSF values in Appendix 1, but did not have an adequate sample size to analyze them statistically.
AVP and OT concentrations were estimated in duplicate using commercial enzyme immunoassay kits (Enzo Life Sciences, Farmingdale, NY) previously validated for titi monkeys. Assay sensitivity was 2.34 pg/ml for AVP and 15.55 pg/ml for OT. Intra- and inter-assay coefficients of variation (CV) were 3.36% and 14.34% respectively for AVP, and 10.62% and 12.78%, respectively for OT. Plasma cortisol and testosterone concentrations were estimated in duplicate using commercial radioimmunoassay kits (Siemens Healthcare, Malvern, PA). Prior to cortisol assay, plasma samples were diluted 1:4 in PBS gel buffer. Cortisol assay procedures were modified with the addition of 0.5 and 2.35 μg/dl concentrations of standards along with the provided range of 1.0 to 49 μg/dl. Assay sensitivity was 0.261 μg/dl. Intra- and inter-assay CV were 3.20% and 6.26%, respectively. Prior to testosterone assay, plasma samples were diluted 1:2 in PBS gel buffer. Testosterone assay procedures were modified with the addition of 57 and 197.5 ng/dl concentrations of standards along with the provided range of 24 to 1667 ng/dl. Testosterone assay sensitivity was 4.58 ng/dl. All samples were run in the same assay and intra-assay CV was 1.02%.
Data Analysis
All models were fitted in a fully Bayesian multivariate multilevel framework for several reasons. First, due to our small sample size and large number of outcomes, multivariate models could not be estimated with least squares or maximum likelihood methods. Bayesian multivariate methods allowed for estimation of hypothesized regions of interest that included numerous correlated outcomes in one model. In addition, Bayesian multilevel methods fully account for uncertainty across levels of hierarchically structured data (McElreath, 2015), which was important due to our within-subjects design. Third, Bayesian methods allow for incorporating prior information into the model which improves precision of the parameter estimates (Gelman et al., 2008; Kruschke and Vanpaemel, 2015)(see Supplementary Material for model details). Finally, parameter estimates have probabilistic interpretations, which allows for estimating the probability of a positive or negative experimental effect (Zucker et al., 1997; Lee, 2011).
In total, we fit five multivariate multilevel models. The first three models were based on hypothesized brain regions previously implicated as modulating jealousy-like behavior in different species: (1) mate-guarding in rodents; (2) jealousy or social pain in humans; and (3) bilateral regions from the rhesus monkey study. The next two multivariate multilevel models assessed hormonal and behavioral differences. The final models were exploratory, in that outcomes were determined from our results. These final models were not multilevel, but multivariate examining correlations between look duration and hormones as well as FDG uptake in ROIs (for the jealousy condition only). For these models, we standardized the predictors and response variables so that the estimates were on r scale (correlation coefficient). All model based estimates are provided in Tables (2–6), whereas the raw means and standard deviations are provided with the model checks (Appendix 2).
Variance was partitioned into two components (Gelman and Hill, 2007): 1) the variance between subjects (i.e., varying intercepts); and 2) the residual variance . As a measure of residual variance explained by subject, we computed intra-class correlation coefficients (ICC) (Quene and Van Den Bergh, 2004). Each multivariate model included one fixed effect, which provided a contrast from the control group. The parameter estimates were summarized with 95% credibility intervals (CrI) that, by definition, have a 95% probability of containing the true parameter (Morey et al., 2015). Bayesian methods provide probabilistic estimates that allow for explicit statements about likely values for the true treatment effect. We thus computed posterior probabilities of a positive or negative effect (Pr(b > 0) and Pr(b < 0)) (Gelman, 2013; Greenland and Poole, 2013). This is not possible with classical methods, since parameters are assumed to be fixed point estimates (probability distributions cannot exist). Posterior probabilities > 80% are reported in the results section, but all estimates are provided in tables. The 80% figure was chosen merely as a convenient figure in order to simplify the reporting of the results. Finally, an effect size parameter (δT) was obtained from dividing the estimate (b) by the square root of the variance components summed (Hedges, 2007). Interpretation of (δT) follows Cohen’s d (Cohen, 2009) (small = 0.2, medium = 0.5, large = 0.8).
The jealousy condition for one male was not usable for technical reasons, thus the final sample size was 8 control scans and 7 jealousy scans, for 8 total subjects. Due to the complexity of the multivariate multilevel models, we examined fit with posterior predictive checks and posterior predictive p-values (Gelman et al., 1996; de la Horra and Rodriguea-Bernal, 1999). Here, the fitted model was used to simulate data, from which a properly specified model will provide replications that look like the observed data and non-extreme p-values (0.95 > p-value > 0.05). For most models, the posterior predictive p-values indicated that model fit was adequate (Appendix 2).
All computation was done in R (Team, 2016).The package brms (Buerkner, 2015), a front end to the probabilistic programming language Stan (Stan_Development_Team, 2015), was used to fit all regression models (all R script and data are available upon request).
A Note on Interpretation of Results
The use of Bayesian statistics remains relatively uncommon. This may be seen as a limitation when comparing our results to the extant literature. Indeed, our use of Bayesian methods has different goals than typically pursued: we did not focus on rejecting a null hypothesis. Instead, our analysis sought to quantify the most probable values for the “true” effect of jealousy on regional cerebral glucose metabolism, hormones and behavior in titi monkeys. This is not possible with classical methods (e.g., ANOVA) in which evidential quantities (e.g., p-values) are in reference to counterfactual sampling procedures. When inferring from our results, the posterior probabilities can be directly interpreted as probabilities (how probability is used in everyday language). The present approach does not include thresholds (i.e., cut-offs, but of course a probability of 99% provides stronger evidence than 85%, assuming equal prior odds). A meaningful probability can be determined in light of theory, past research, the quality of this study (including limitations), and the reported results.
RESULTS
FDG Uptake
Our first multivariate multilevel model simultaneously estimated areas implicated by rodent studies as modulating mate-guarding behavior: the lateral septum (LS), anterior hypothalamus (AH), posterior cingulate cortex (PCC) and medial amygdala (MeA). The probability of a positive effect of jealousy condition on FDG uptake in the right LS was 93% (b = 0.04, CrI = [−0.02 – 0.10], δT = 0.55; Figure 3A) and, similarly, 99% in the left PCC (b = 0.04, CrI = [0.01 – 0.07], δT = 1.02; Figure 3B), while there was some evidence for reduced uptake in the right MeA (Pr(b < 0) = 85%, b = −0.03, CrI = [−0.10 – 0.03], δT = − 0.40; Figure 3A). The posterior probabilities for the other comparisons were below 80% and are reported in Table 2. Notably, the amount of variation explained by subject across outcomes ranged from 9% (left AH: ICC = 0.09, CrI = [0.00 – 0.44]) to 42% (right LS: ICC = 0.42, CrI = [0.004 – 0.87]).
Figure 3.
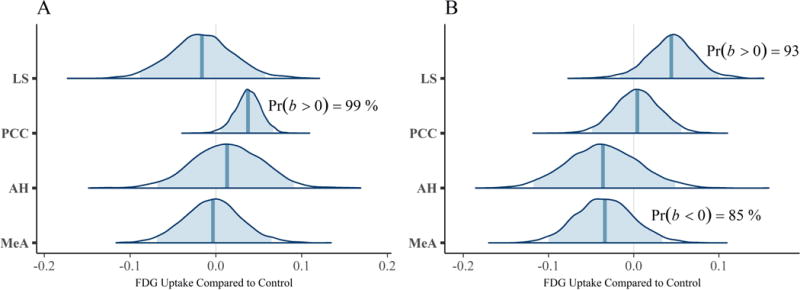
Posterior distributions from the multivariate multilevel mate guarding model. The density plots are the parameter estimates (contrasts from the control group). The shaded blue regions are the 95% credible regions, while the dark blue lines are the point estimates. Panel A: left ROIs. Panel B: right ROIs.
Table 2.
Multivariate multilevel model estimates for regions of interest implicated by rodent studies as modulating mate-guarding behavior.
| ROI | b | Post. SD | 95-% CrI | δT | Pr(b > 0) | Pr(b < 0) | |
|---|---|---|---|---|---|---|---|
| LS-L | Intercept | 0.96 | 0.03 | 0.87, 1.02 | – | – | – |
| Jealousy | −0.02 | 0.04 | −0.08, 0.06 | −0.18 | 31 % | 69 % | |
| LS-R | Intercept | 0.91 | 0.03 | 0.85, 0.97 | – | – | – |
| Jealousy | 0.04 | 0.03 | −0.02, 0.10 | 0.55 | 93 % | 7 % | |
| AH-L | Intercept | 0.67 | 0.03 | 0.61, 0.74 | – | – | – |
| Jealousy | 0.01 | 0.04 | −0.07, 0.10 | 0.14 | 63 % | 37 % | |
| AH-R | Intercept | 0.69 | 0.03 | 0.62, 0.76 | – | – | – |
| Jealousy | −0.04 | 0.04 | −0.12, 0.05 | −0.35 | 20 % | 80 % | |
| PCC-L | Intercept | 1.07 | 0.01 | 1.01, 1.09 | – | – | – |
| Jealousy | 0.04 | 0.02 | 0.01, 0.07 | 1.02 | 98 % | 2 % | |
| PCC-R | Intercept | 1.09 | 0.02 | 1.05, 1.14 | – | – | – |
| Jealousy | 0.01 | 0.03 | −0.05, 0.06 | 0.05 | 55 % | 45 % | |
| MeA-L | Intercept | 0.70 | 0.03 | 0.64, 0.75 | – | – | – |
| Jealousy | −0.01 | 0.03 | −0.07, 0.06 | −0.04 | 44 % | 56 % | |
| MeA-R | Intercept | 0.70 | 0.03 | 0.64, 0.76 | – | – | – |
| Jealousy | −0.03 | 0.03 | −0.10, 0.03 | −0.40 | 16 % | 84 % |
Note. Intercept is the model estimate for the control condition. Jealousy is the difference from the control condition—the jealousy effect. Post. SD is the posterior standard deviation of the estimate. These estimates were all obtained from the same model. Note: interpretation of (δT) follows Cohen’s d (small = 0.2, medium = 0.5, large = 0.8).
Our second multivariate multilevel model examined several cortical areas that were shown to be associated with jealousy or social pain in human studies (anterior cingulate cortex (AC), caudate (Ca), putamen (P), nucleus accumbens (NAcc), and ventral pallidum (VP)). The probability of a positive effect of jealousy condition on FDG uptake in the left AC was 96% (b= 0.05, CrI = [− 0.01 – 0.10], δT = 0.79)), while there was some evidence for reduced uptake in the right Ca (Pr(b > 0) = 90%, b= 0.04, CrI = [−0.03 – 0.10], δT = 0.41), right VP (Pr(b > 0) = 86%, b= 0.05, CrI = [−0.03 –0.13], δT = 0.38), and the left VP (Pr(b> 0) = 88%, b= 0.04, CrI = [−0.03 – 0.13], δT = 0.38). According to the posterior predictive checks, model fit was adequate. The other comparisons are provided in Table 3. The amount of residual variation explained by subject across outcomes ranged from 13% (left VP: ICC = 0.13, CrI = [0.00 – 0.56]) to 53% (right Ca: ICC = 0.53, CrI = [0.02 – 0.89]).
Table 3.
Multivariate multilevel model estimates for regions of interest shown to be associated with jealousy and social pain in humans.
| ROI | b | Post. SD | 95-% CrI | δT | Pr(b > 0) | Pr(b < 0) | |
|---|---|---|---|---|---|---|---|
| AC-L | Intercept | 1.06 | 0.02 | 1.04, 1.10 | – | – | – |
| Jealousy | 0.05 | 0.03 | −0.01, 0.10 | 0.79 | 96 % | 4 % | |
| AC-R | Intercept | 1.08 | 0.02 | 1.04, 1.12 | – | – | – |
| Jealousy | 0.01 | 0.02 | −0.04, 0.05 | 0.09 | 60 % | 40 % | |
| Ca-L | Intercept | 1.13 | 0.03 | 1.07, 1.18 | – | – | – |
| Jealousy | −0.01 | 0.03 | −0.08, 0.06 | −0.14 | 36 % | 64 % | |
| Ca-R | Intercept | 1.06 | 0.03 | 0.99, 1.13 | – | – | – |
| Jealousy | 0.04 | 0.03 | −0.03, 0.09 | 0.41 | 90 % | 10 % | |
| P-L | Intercept | 1.18 | 0.02 | 1.14, 1.23 | – | – | – |
| Jealousy | 0.02 | 0.02 | −0.03, 0.06 | 0.26 | 79 % | 21 % | |
| P-R | Intercept | 1.15 | 0.04 | 1.06, 1.24 | – | – | – |
| Jealousy | −0.02 | 0.05 | −0.12, 0.08 | −0.19 | 31 % | 69 % | |
| NAcc-L | Intercept | 0.96 | 0.05 | 0.86, 1.06 | – | – | – |
| Jealousy | 0.02 | 0.06 | −0.10, 0.12 | 0.27 | 76 % | 24 % | |
| NAcc-R | Intercept | 0.89 | 0.03 | 0.83, 0.96 | – | – | – |
| Jealousy | 0.02 | 0.04 | −0.05, 0.09 | 0.11 | 62 % | 38 % | |
| VP-L | Intercept | 0.83 | 0.04 | 0.76, 0.90 | – | – | – |
| Jealousy | 0.05 | 0.04 | −0.04, 0.13 | 0.38 | 88 % | 12 % | |
| VP-R | Intercept | 0.79 | 0.03 | 0.72, 0.85 | – | – | – |
| Jealousy | 0.03 | 0.03 | −0.03, 0.10 | 0.45 | 86 % | 14 % |
Note. Intercept is the model estimate for the control condition. Jealousy is the difference from the control condition—the jealousy effect. Post. SD is the posterior standard deviation of the estimate. These estimates were all obtained from the same model Note: interpretation of (δT) follows Cohen’s d (small = 0.2, medium = 0.5, large = 0.8).
The third multivariate multilevel model estimated bilateral ROIs from the rhesus monkey study (insular cortex (IC) and superior temporal sulcus (ST)). This model produced negligible probabilities for an effect of jealousy, and residual variation attributed to subjects was minimal (all instances < 5%). Importantly, posterior predictive check indicated a misfit between the observed and the model implied standard deviations (Appendix 2; Table 4). Assuming equal variances was problematic, but unfortunately a heteroskedastic model could not be fit (due to an already complex model).
Table 4.
Multivariate multilevel model estimates for regions from the rhesus monkey study.
| ROI | b | Post. SD | 95-% CrI | δT | Pr(b > 0) | Pr(b < 0) | |
|---|---|---|---|---|---|---|---|
| IC-L | Intercept | 1.09 | 0.03 | 1.03, 1.14 | – | – | – |
| Jealousy | −0.01 | 0.03 | −0.07, 0.04 | −0.16 | 27 % | 73 % | |
| IC-R | Intercept | 1.07 | 0.03 | 1.02, 1.13 | – | – | – |
| Jealousy | 0.01 | 0.03 | −0.05, 0.06 | 0.09 | 63 % | 37 % | |
| ST-L | Intercept | 1.01 | 0.05 | 0.91, 1.12 | – | – | – |
| Jealousy | 0.01 | 0.04 | −0.07, 0.09 | 0.07 | 62 % | 38 % | |
| ST-R | Intercept | 1.04 | 0.11 | 0.84, 1.25 | – | – | – |
| Jealousy | 0.02 | 0.07 | −0.11, 0.15 | 0.06 | 62 % | 38 % |
Note. Intercept is the model estimate for the control condition. Jealousy is the difference from the control condition—the jealousy effect. Post. SD is the posterior standard deviation of the estimate. These estimates were all obtained from the same model. Note: interpretation of (δT) follows Cohen’s d (small = 0.2, medium = 0.5, large = 0.8).
Hormones
Our multivariate multilevel model estimated plasma hormone concentrations of oxytocin (OT), vasopressin (AVP), cortisol, and testosterone in the jealousy condition compared to the control condition. There was a positive effect (Pr(b> 0) = 93%) of jealousy condition on plasma testosterone (b = 190.17, CrI = [− 85.38 – 440.42], δT = 0.48) as well as similar evidence (Pr(b > 0) = 92%) for a positive effect on plasma cortisol (b = 10.13, CrI = [−4.83 – 24.48], δT = 0.40). Posterior predictive checks indicated that the model adequately described the observed data. The amount of residual variation explained by subjects ranged from 17% (AVP: ICC = 0.17, CrI = [0.00 – 0.67]) to 63% (cortisol: ICC = 0.62, CrI = [0.03 – 0.92]). Plasma OT and AVP concentrations had lower probabilities (65% and 74%, respectively) for differences between conditions (Table 5; also presented are model estimates and confidence intervals).
Table 5.
Multivariate multilevel model estimates for plasma hormone concentrations.
| Hormone | b | Post. SD | 95−% CrI | δT | Pr(b > 0) | Pr(b < 0) | |
|---|---|---|---|---|---|---|---|
| OT | Intercept | 509.65 | 67.16 | 378.36, 647.11 | – | – | – |
| Jealousy | 23.01 | 68.26 | −120.78, 155.07 | 0.13 | 65 % | 35 % | |
| AVP | Intercept | 263.17 | 25.60 | 212.61, 316.62 | – | – | – |
| Jealousy | 17.72 | 29.51 | −42.55, 75.83 | 0.25 | 74 % | 26 % | |
| Cortisol | Intercept | 75.67 | 9.32 | 56.76, 93.98 | – | – | – |
| Jealousy | 10.13 | 7.35 | −4.83, 24.48 | 0.41 | 92 % | 8 % | |
| Testosterone | Intercept | 410.88 | 160.66 | 129.45, 723.02 | – | – | – |
| Jealousy | 190.17 | 129.97 | −85.38, 440.42 | 0.48 | 93 % | 7 % |
Note. Intercept is the model estimate for the control condition. Jealousy is the difference from the control condition—the jealousy effect. Post. SD is the posterior standard deviation of the estimate. These estimates were all obtained from the same model. Note: interpretation of (δT) follows Cohen’s d (small = 0.2, medium = 0.5, large = 0.8).
Behavior
The multivariate multilevel model estimated differences in total durations of behavior between the control and jealousy conditions (for ethogram see Table 1). Due to excessive zeroes, drinking and time off camera were not analyzed. There was some evidence for a positive effect (Pr(b > 0) = 90%) of jealousy condition on lip smacking duration (b = 7.60, CrI = [−4.19 – 19.75], δT = 0.55). The residual variance explained by subject ranged from 14% (chewing: ICC = 0.14, CrI = [0 – 0.59]) to 99% (tail lashing: ICC = 0.99, CrI = [0.97 – 1.0]). All estimates are reported in Table 6.
Table 6.
Multivariate multilevel model estimates for behaviors (duration)
| Behavior | b | Post. SD | 95-% CrI | δT | Pr(b > 0) | Pr(b < 0) | |
|---|---|---|---|---|---|---|---|
| Tail lash | Intercept | 1.41 | 1.81 | −2.20, 4.90 | – | – | – |
| Jealousy | 0.14 | 0.18 | −0.23, 0.51 | 0.03 | 75 % | 15 % | |
| Arch | Intercept | 15.45 | 8.07 | −0.58, 31.15 | – | – | – |
| Jealousy | −10.51 | 9.88 | −29.60, 9.75 | −0.49 | 13 % | 87 % | |
| Look across | Intercept | 145.72 | 45.57 | 55.98, 239.23 | – | – | – |
| Jealousy | 6.28 | 54.11 | −101.40, 116.81 | 0.05 | 55 % | 45 % | |
| Lip smack | Intercept | 3.01 | 5.38 | −7.66, 13.50 | – | – | – |
| Jealousy | 7.60 | 5.90 | −4.19, 19.75 | 0.55 | 90 % | 10 % | |
| Locomotion | Intercept | 261.85 | 87.39 | 78.18, 437.65 | – | – | – |
| Jealousy | −14.51 | 104.07 | −222.03, 195.26 | −0.06 | 44 % | 56 % | |
| Chew | Intercept | 123.73 | 42.85 | 40.55, 280.42 | – | – | – |
| Jealousy | −19.81 | 59.22 | −139.99, 99.02 | −0.17 | 37 % | 63 % |
Note. Intercept is the model estimate for the control condition. Jealousy is the difference from the control condition—the jealousy effect. Post. SD is the posterior standard deviation of the estimate. These estimates were all obtained from the same model. Note: interpretation of (δT) follows Cohen’s d (small = 0.2, medium = 0.5, large = 0.8).
Correlations: Jealousy condition
Durations of behaviors were standardized prior to analysis, resulting in correlations (r). Look duration via a single-level multivariate model was positively correlated with cortisol (b = 0.63, CrI = [−0.07 – 0.98], Pr(b > 0) = 97%). The correlation with testosterone was also positive, but the interval was very wide (b = 0.31, CrI = [−0.67 – 0.94], Pr(b > 0) = 78%). Using a single-level multivariate model to investigate associations between behavior and brain region of interest, we found that look across duration had a probability of a positive correlation on FDG uptake in the right LS of 92% (b = 0.53, CrI = [−0.32 – 0.97]; Figure 4A). There were substantial negative correlations with look duration in the left PCC (b= −0.46, CrI = [−0.97 – 0.35], Pr(b < 0) = 90%; Figure 4B) and the right MeA (b= −0.74, CrI = [−0.99 – −0.22], Pr(b < 0) = 99%; Figure 4C).
Figure 4.

Correlations between look duration and ROIs: a) right lateral septum, b) left posterior cingulate cortex, and c) right medial amygdala. Points are the observed data. The blue shaded areas are the fitted 95% credible regions for the correlations.
The probability of a negative correlation between plasma testosterone and FDG uptake in the right MeA was 96% (b = −0.57, CrI = [−0.97 – 0.07]), 48% for the right LS (b = −0.03, CrI = [−0.84 – .80]), and 79% for the left PCC (b = −0.57, CrI = [−0.93, 0.60]). There were negative correlations between cortisol concentrations and FDG uptake in the left PCC (b = −0.19, CrI = [−0.91, 0.70], Pr(b < 0) = 72%), and right MeA (b = −0.54, CrI = [−0.97, 0.22], Pr(b < 0) = 93%). There was a positive correlation between cortisol concentrations and FDG uptake in the right LS (b = 0.31, CrI = [−0.66, 0.94], Pr(b > 0) = 81 %), but the interval was very wide.
DISCUSSION
After seeing his female pair mate next to a stranger male, male titi monkeys showed increased FDG uptake in the right lateral septum (LS), left posterior cingulate cortex (PCC) and left anterior cingulate (AC), and decreased uptake in the right medial amygdala (MeA) compared to the control condition. Our subjects also had higher plasma testosterone and cortisol concentrations and spent more time lip smacking in the jealousy condition compared to the control condition. In the jealousy condition, the amount of time looking at the pair mate next to a stranger male was associated with higher plasma cortisol concentrations. These neural and physiological changes may underpin the emotion of jealousy, which can act in a monogamous species to preserve the long-term integrity of the pair.
We now have multiple lines of evidence suggesting that the lateral septum plays a role in both pair bond formation and pair bond maintenance in titi monkeys. The lateral septum is innervated by vasopressin fibers in many mammalian species, including a number of primate species (Ragen and Bales, 2013). In titi monkeys it contains oxytocin receptors but not vasopressin receptors (Freeman et al., 2014), suggesting that any actions of vasopressin in that area are mediated through oxytocin receptors (Barberis and Tribollet, 1996). In addition, it receives dopaminergic input from the ventral tegmental area (Sheehan et al., 2004). In our initial cross-sectional study comparing pair bonded males to males that were housed alone, FDG uptake in the lateral septum was statistically different between the two groups, with a difference of 9% (Bales et al., 2007). Dopamine D1 receptor binding in the lateral septum of male titi monkeys is also statistically significantly up-regulated 4–9 weeks following pair bonding (Hostetler et al., 2017). In socially monogamous prairie voles, up-regulation of D1 receptors is associated with the onset of mate-guarding, although in that case the sensitive neural area is the nucleus accumbens (Aragona et al., 2006). The lateral septum also plays an important role in social memory (Everts and Koolhaas, 1999) and in the preference formation aspects of pair bonding (Liu et al., 2001). The lateral septum also modulates stress in many species, via an oxytocinergic mechanism (Singewald et al., 2011; Guzman et al., 2013), and stress can modulate the process of social bonding (DeVries et al., 1996). In this study, the medium-large effect size that we found suggests not just a long-term change in dopamine neurochemistry (Hostetler et al., 2017), but also a strong involvement in acute responses to a threat to the pair bond.
In the present study we also found higher FDG uptake in the left posterior cingulate cortex in the jealousy condition, as well as evidence for a positive effect in the left anterior cingulate. In semi-free-ranging prairie voles, higher vasopressin receptor binding in the posterior cingulate was associated with higher fidelity to the partner (Ophir et al., 2008), which theoretically could be related to a stronger pair bond or more time spent in proximity mate-guarding. The fact that we also found evidence for higher FDG uptake in the anterior portion of the cingulate (and that the effect sizes for both posterior and anterior were large and remarkably similar in magnitude) suggests that our “jealousy” condition affects the left cingulate cortex as a whole, and is not just confined to the posterior cingulate. There are well-studied associations between anterior cingulate cortex and socially painful situations (Eisenberger, 2015), which fits with the view of jealousy as social rejection.
Our study found lateralized effects of the jealousy condition on regional cerebral glucose metabolism, such that male titi monkeys showed increased FDG uptake in the right lateral septum (LS), left posterior cingulate cortex (PCC) and left anterior cingulate (AC), and decreased FDG uptake in the right medial amygdala (MeA) in the jealousy condition compared to the control. Lateralized effects have also been found in human studies of jealousy or other forms of social exclusion. In a human study on jealousy using electroencephalogram (EEG) to measure electrical activity of the brain, jealousy evoked by a computerized ball-tossing game was associated with greater relative left frontal activation (Harmon-Jones et al., 2009). In that study, the authors concluded that their left frontal activation finding was consistent with jealousy being associated with approach motivation. This finding was interpreted within long-standing research in human emotion that greater left-sided brain activity is associated with approach behavior and predominantly positive affect, while relative greater right-sided activity is associated with avoidance behavior and negative emotions (Davidson and Fox, 1982). Like our male titi monkeys, Takahashi et al (2006) found men who read about infidelity showed functional magnetic resonance imaging (fMRI) changes in the amygdala and the cingulate cortex (Takahashi et al., 2006). Takahashi et al (2006) found that men who read statements about sexual infidelity had increased fMRI activation in the right amygdala (as well as other areas), while men who read statements about emotional infidelity had greater fMRI activation in the left and right cingulate cortex (as well as other areas). Unlike Takahashi and colleagues, who found right and left activation of the cingulate cortex with jealousy, Sun and colleagues (Sun et al., 2016) found that the left posterior cingulate gyrus fMRI activation was associated with romantic jealousy and the left anterior cingulate gyrus was associated with romantic happiness. Using EEG, fMRI and regional cerebral glucose metabolism PET/MRI methods allow us to visualize what areas of the brain are associated with behavior and social scenarios, but they do not allow us to know what types of receptors are being activated or what neurotransmitters are changing in the brain. Future research on jealousy using PET with specific radiotracers could allow us to measure changes in neurotransmitter availability and potentially in release in this model (Hostetler et al., 2017). While it is commonly assumed that lateralization is a human trait, brain (and behavior) asymmetries are not the exception but the norm, and can be found in all taxa of the animal kingdom (Gunturkun and Ocklenburg, 2017).
We found lower probabilities that our experimental condition affected plasma hormone concentrations of OT (65%) or AVP (74%), and a small effect size for AVP. Some human studies have found relationships between elevated plasma oxytocin levels and socially painful situations such as troubled romantic relationships (Taylor et al., 2010) or other types of relationship distress (Taylor et al., 2006), mainly in women. In contrast to women, higher levels of plasma vasopressin, but not oxytocin, were associated with relationship problems in men (Taylor et al., 2010). Although we did not find large differences in our peripheral measure of plasma OT and AVP peptide hormones, this does not mean that central nervous system changes in OT and AVP did not occur. Plasma and other peripheral measures of these peptide hormones are considered imperfect reflections of central nervous system levels (Freeman et al., 2016). An additional explanation for why we did not find a larger effect for plasma OT or AVP is the timing of when we collected blood samples from our subjects. While sampling blood following the 30 minute FDG uptake period was reasonable timing to see effects of steroids such as testosterone and cortisol (Mendoza, 2017), it would almost certainly be past the peak timing to see effects of a behavioral stimulus on plasma oxytocin (Kenkel et al., 2012). Lastly, these blood samples also were taken after animals were sedated, so they do not represent “baseline” blood samples.
As predicted, there were positive associations between the jealousy condition and plasma steroid hormone concentrations of testosterone and cortisol. Testosterone concentrations were measurably higher in the jealousy condition, with a small to medium effect size. This increase is not surprising given testosterone’s association with mating-related aggression (Wingfield et al., 1990; Wingfield, 2017). The “challenge hypothesis” predicts that androgens should respond acutely to social challenges, and then return to baseline in order to avoid adverse effects of steroids (Wingfield et al., 1990). This has been generally supported in the literature, including that on non-human primates (Bales et al., 2006) and humans (Archer, 2006). Cortisol was marginally higher during the jealousy condition (92% probability of a true effect), and it was significantly correlated with the time that the subject spent gazing at his pair mate and the stranger male, suggesting that this stimulus does constitute a social stressor (Mendoza, 2017). The increased time spent lip smacking during the jealousy condition compared to the control condition was possibly affiliative behavior directed towards his pair mate, an attempt to get her attention, or a form of self-soothing behavior. We did not tape the stimulus pair, so we do not know what specific behavior our subject was viewing. This is a limitation of the current study which should be corrected in future studies.
We also cannot say definitively that the subjects in our experiment experienced the emotion of “jealousy”. Similarly, with humans we would need verbal confirmation that participants experienced this emotion. In particular, since the pair mate was separated from the stranger by a barrier, the stimulus may have been less potent for the subject than if the pair mate and stranger had full access to each other. The higher testosterone concentrations experienced by our male subjects when viewing their pair mate next to a stranger, as well as the positive correlations between duration of time spent looking across at them and both cortisol concentrations and FDG uptake in the lateral septum, do suggest that this situation may have been viewed as a challenge to the pair bond or sexual relationship. However, it is worth noting that the emotion we attribute to the subjects was not shown unambiguously through behavior.
A neural model of pair bonding in titi monkeys is beginning to coalesce, and the available evidence suggests both similarities and differences to the current, rodent-based model (Gobrogge and Wang, 2015; Numan and Young, 2016). When forming pair bonds, both prairie voles and titi monkeys recruit neural areas rich with oxytocin and/or vasopressin receptors and involved in social memory (such as the lateral septum), and dopaminergic areas involved in reward (such as the nucleus accumbens) (Bales et al., 2017). The involvement of these two systems suggests that the initial pair bond formation, and subsequent mating, serve both as learning and as positive reinforcing stimuli, involving the neural systems involved in other motivated behaviors (Tops et al., 2014). The maintenance phase of pair bonding is thought to be based on negative reinforcement; i.e. avoidance of aversive stimuli such as separation (Resendez et al., 2016), and to involve the opioid and dopamine systems as well. The lateral septum in titi monkey brain contains oxytocin receptors (Freeman et al., 2014), dopamine D1 receptors (Hostetler et al., 2017), dopamine D2 receptors [Bales, unpublished data], and both μ and ĸ opioid receptors (Ragen et al., 2015). Thus, the lateral septum appears to be a hot-spot for both the formation and the maintenance of pair bonding in male titi monkeys. The neural substrates of primate pair bonding thus appear to involve the same principles and neurochemistry, but differing neural areas, as rodent pair bonding. Based on current mammalian phylogenies, it is likely that monogamy evolved multiple times (Lukas and Clutton-Brock, 2013), and it is therefore not surprising for the details of neurobiological mechanism to differ. Convergent evolution on nonapeptide mechanisms, however, seems likely given the outcomes of this and other studies.
Previous findings, as well as the present study, have suggested an important role for the lateral septum. Future research might focus on this area and particularly on interactions between the oxytocin, dopamine, and opioid systems, in order to continue dissecting the underpinnings of pair bonding in primates. Special attention will need to be paid to other potential differences from rodents, such as the longer time that it takes for primates to form a pair bond (Rothwell, unpublished data). Studying these neural substrates of social bonds may give us important clues with which to approach health and welfare problems such as addiction (Tops et al., 2014), autism (Anagnostou et al., 2014), and partner violence (Marshall, 2013). Finally, they may help inform us as to the evolutionary origin and maintenance of monogamy as a social system.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (grants HD053555 and P51OD011107), and the Good Nature Institute. The authors report no conflicts of interest. KLB, SPM, and WAM designed the study and obtained the funding. The animal experiments were carried out by NM and TS. SRC and DJR oversaw the methodology and analysis of the imaging data. DRW performed the statistical analysis. NM and KLB wrote the paper and all authors edited the final version.
We also gratefully acknowledge the following for research assistance: Luana Griffin, Rebecca Larke, Carlos Almeida, Ben Ragen, and Bales lab undergraduate volunteers; Jaleh Janatpour and Kevin Theis for animal care; Dr. Angela Colagross-Schouten, Dr. Kari Christe, Dr. Laura Summers, and the veterinary staff for care and assistance with the PET scans; Vanessa Bakula, Sarah Grisso, Deborah Kent, and Research Services at CNPRC; Michelle Connell and Jennifer Fung at the Center for Molecular and Genomic Imaging; and Richard Larson and the Center for Imaging Sciences at UC Davis.
References Cited
- Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Research. 2014 doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Bales KL, Arias del Razo R, Conklin QA, Hartman S, Mayer HS, Rogers FD, et al. Titi monkeys as a novel non-human primate model for the neurobiology of pair bonding. Yale Journal of Biology and Medicine. 2017 [PMC free article] [PubMed] [Google Scholar]
- Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia) Hormones and Behavior. 2006;49:88–95. doi: 10.1016/j.yhbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Research. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Critical Reviews in Neurobiology. 1996;10(1):119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ. The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Hormones and Behavior. 2017;91:68–83. doi: 10.1016/j.yhbeh.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiology & Behavior. 2002;76(4–5):559–566. doi: 10.1016/s0031-9384(02)00755-2. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Hahn TP. Integrating stress physiology, environmental change, and behavior in free-living sparrows. Hormones and Behavior. 2003;43:115–123. doi: 10.1016/s0018-506x(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Buerkner PC. brms: Bayesian regression models using Stan. R Package Version 0.6.0 2015 [Google Scholar]
- Buss DM. Human mate guarding. Neuro endocrinology letters. 2002;S4:23–29. [PubMed] [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, Bales KL. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus) American Journal of Primatology. 2016;78:326–339. doi: 10.1002/ajp.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli CB, Mennill DJ, Gestich CC, Setz EZ, Bicca-Marques JC. Playback responses of socially monogamous black-fronted monkeys to simulated solitary and paired intruders. American Journal of Primatology. 2015;77:1135–1142. doi: 10.1002/ajp.22447. [DOI] [PubMed] [Google Scholar]
- Casto KV, Edwards DA. Testosterone, cortisol, and human competition. Hormones and Behavior. 2016;82:21–37. doi: 10.1016/j.yhbeh.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. New York: Taylor & Francis; 2009. [Google Scholar]
- Cubiciotti DDI, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behavioral Ecology and Sociobiology. 1978;3:311–322. [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in humans infants. Science. 1982;218:1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- de la Horra J, Rodriguea-Bernal M. The posterior predictive p-value for the problem of goodness of fit. Test. 1999;8:117–128. [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences. 1996;93(21):11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Munoz SL, Bales KL. “Monogamy” in primates: variability, trends, and synthesis. American Journal of Primatology. 2016;78:283–287. doi: 10.1002/ajp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. Social pain and the brain: controversies, questions, and where to go from here. Annual Review of Psychology. 2015;66:601–629. doi: 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- Ellis C, Weinstein E. Jealousy and the social psychology of emotional experience. Journal of Social and Personal Relationships. 1986;3:337–357. [Google Scholar]
- Everts HGJ, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behavioural Brain Research. 1999;99(1):7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Di Fiore A, De Luna AG. Pair-mate relationships and parenting in equatorial saki monkeys (Pithecia aequatorialis) and red titi monkeys (Callicebus discolor) of Ecuador. In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA, editors. Evolutionary Biology and Conservation of Titis, Sakis, and Uakaris. Cambridge: Cambridge University Press; 2013. pp. 295–302. [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mason WA. Effects of pair-bond and social context on male-female interactions in captive titi monkeys (Callicebus moloch, Primates: Cebidae) Ethology. 2000;106:1067–1082. [Google Scholar]
- Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology. 1994;19(5–7):593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Fisher-Phelps ML, Mendoza SP, Serna S, Griffin LL, Schaefer TJ, Jarcho MR, et al. Laboratory simulations of mate-guarding as a component of the pair-bond in male titi monkeys, Callicebus cupreus. American Journal of Primatology. 2016;78:573–582. doi: 10.1002/ajp.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GG, et al. lasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology. 2016;66:185–194. doi: 10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, et al. euroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A. Commentary: P values and statistical practice. Epidemiology. 2013;24:69–72. doi: 10.1097/EDE.0b013e31827886f7. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Gelman A, Jakulin A, Pittau MG, Su YS. A weakly informative default prior distribution for logistic and other regression models. The Annals of Applied Statistics. 2008;2 [Google Scholar]
- Gelman A, Meng XL, Stern H. Posterior predictive assessment of model fitness via realized discrepancies. Statistica Sinica. 1996;6:733–807. [Google Scholar]
- Getz LL, McGuire B, Carter CS. Social behavior, reproduction and demography of the prairie vole, Microtus ochrogaster. Ethology Ecology & Evolution. 2003;15:105–118. [Google Scholar]
- Gobrogge K, Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: Lessons from voles. Hormones and Behavior. 2015;76:91–105. doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Wang ZX. The ties that bond: neurochemistry of attachment in voles. Current Opinion in Neurobiology. 2016;38:80–88. doi: 10.1016/j.conb.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PB, McHale TS, Carre JM. A review of human male field studies of hormones and behavioral reproductive effort. Hormones and Behavior. 2017;91:52–67. doi: 10.1016/j.yhbeh.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Greenland S, Poole C. Living with p-values. Epidemiology. 2013;24:62–68. doi: 10.1097/EDE.0b013e3182785741. [DOI] [PubMed] [Google Scholar]
- Gunturkun O, Ocklenburg S. Ontogenesis of lateralization. Neuron. 2017;94:249–263. doi: 10.1016/j.neuron.2017.02.045. [DOI] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, et al. Fear-enhancing effects of septal oxytocin receptors. Nature Neuroscience. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Peterson CK, Harris CR. Jealousy: novel methods and neural correlates. Emotion. 2009;9:113–117. doi: 10.1037/a0014117. [DOI] [PubMed] [Google Scholar]
- Harris CR. A review of sex differences in sexual jealousy, including self-report data, psychophysiological responses, interpersonal violence, and morbid jealousy. Personality and Social Psychology Review. 2003;7:102–128. doi: 10.1207/S15327957PSPR0702_102-128. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver P. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Effect sizes in cluster-randomized designs. Journal of Educational and Behavioral Sciences. 2007;32:341–370. [Google Scholar]
- Hinde K, Muth C, Maninger N, Ragen BJ, Larke RH, Jarcho MR, et al. Challenges to the pair bond: Neural and hormonal effects of separation and reunion in a monogamous primate. Frontiers in Behavioral Neuroscience. 2016;10:221. doi: 10.3389/fnbeh.2016.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Hinde K, Maninger N, Mendoza SP, Mason WA, Rowland DJ, et al. Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus) American Journal of Primatology. 2017;79:1–9. doi: 10.1002/ajp.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. Journal of Neuroendocrinology. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kruschke JK, Vanpaemel W. Bayesian estimation in hierarchical models. In: Busemeyer J, Townsend J, Wang ZJ, Eidels A, editors. The Oxford Handbook of Computational and Mathematical Psychology. Oxford: Oxford University Press; 2015. pp. 279–299. [Google Scholar]
- Leary MR. Emotional responses to interpersonal rejection. Dialogues in Clinical Neuroscience. 2015;17:435–441. doi: 10.31887/DCNS.2015.17.4/mleary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ. Demystify statistical significance - time to move on from the p value to Bayesian analysis. Journal of the National Cancer Institute. 2011;103:2–3. doi: 10.1093/jnci/djq493. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang ZX. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2001;115(4):910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341:526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- Maninger N, Hinde K, Mendoza SP, Mason WA, Larke RH, Ragen BJ, et al. Pair bond formation leads to a sustained increase in global cerebral glucose metabolism in monogamous male titi monkeys (Callicebus cupreus) Neuroscience. 2017;348:302–312. doi: 10.1016/j.neuroscience.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JH. Primate consortships: a critical review. Current Anthropology. 1997;38:353–374. [Google Scholar]
- Marshall AD. Posttraumatic stress disorder and partner-specific social cognition: a pilot study of sex differences in the impact of arginine vasopressin. Biological Psychology. 2013;93:296–303. doi: 10.1016/j.biopsycho.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA. Social organization of the South American monkey, Callicebus moloch: a preliminary report. Tulane Studies in Zoology. 1966;13:23–28. [Google Scholar]
- Mason WA. Comparative studies of Callicebus and Saimiri: Behaviour of male-female pairs. Folia Primatologica. 1974;22:1–8. doi: 10.1159/000155614. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: Parents, offspring and mates. Psychoneuroendocrinology. 1998;23(8):765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- McElreath R. rethinking: Statistical Rethinking book package. 2015 Retrieved from https://github.com/rmcelreath/rethinking)
- McGuire B, Getz LL. The nature and frequency of social interactions among free-living prairie voles (Microtus ochrogaster) Behavioral Ecology and Sociobiology. 1998;43:271–279. [Google Scholar]
- Mendoza SP. Hormones, Brain, and behavior. 3rd 2017. Social stress: concepts, assumptions, and animal models. [Google Scholar]
- Mendoza SP, Mason WA. Attachment relationships in New World primates. Annals of the New York Academy of Sciences. 1997;807:203–209. doi: 10.1111/j.1749-6632.1997.tb51921.x. [DOI] [PubMed] [Google Scholar]
- Morey RD, Hoekstra R, Rouder JN, Lee MD, Wagenmakers EJ. The fallacy of placing confidence in confidence intervals. Psychonomic Bulletin & Review. 2015;23:103–123. doi: 10.3758/s13423-015-0947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AM, Edwards KM. Perpetrators’ and victims’ attributions for IPV: a critical review of the literature. Trauma, Violence & Abuse. 2015 doi: 10.1177/1524838015603551. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proceedings of the National Academy of Sciences. 2008;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombit RA. Sexual conflict in nonhuman primates. Advances in the Study of Behavior. 2014;46:191–280. [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, et al. Why primate models matter. American Journal of Primatology. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Perugi G, Logi C, Romano A, Del Dotto P, Ceravolo R, et al. Dopamine agonists and delusional jealousy in Parkinson’s disease: a cross-sectional prevalence study. Movement Disorders. 2012;27:1679–1682. doi: 10.1002/mds.25129. [DOI] [PubMed] [Google Scholar]
- Quene H, Van Den Bergh H. On multi-level modeling of data from repeated measures designs: a tutorial. Speech Communication. 2004;43:103–121. [Google Scholar]
- Ragen BJ, Bales KL. Oxytocin and vasopressin in non-human primates. In: Choleris E, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of behavior. Cambridge University Press; 2013. pp. 288–306. [Google Scholar]
- Ragen BJ, Freeman SM, Laredo SA, Mendoza SP, Bales KL. μ and ĸ opioid receptor distribution in the monogamous titi monkey (Callicebus cupreus): implications for social behavior and endocrine functioning. Neuroscience. 2015;290:421–434. doi: 10.1016/j.neuroscience.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, et al. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife. 2016;5:e15325. doi: 10.7554/eLife.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Winslow JT, Kilts CD. The neural correlates of mate competition in dominant male rhesus macaques. Biological Psychiatry. 2004;56:364–375. doi: 10.1016/j.biopsych.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: an integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Personality and Social Psychology Review. 2008;12:141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- Shakespeare W. Four Tragedie: Hamlet, Othello, King Lear, Macbeth. Bantam Books; 1988. [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Research. Brain Research Reviews. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Simmons TC, Balland JF, Dhauna J, Yang SY, Traina JL, Vazquez J, et al. Early intranasal vasopressin administration impairs partner preference in adult male prairie voles (Microtus ochrogaster) Frontiers in Endocrinology. 2017;8:145. doi: 10.3389/fendo.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald GM, Rjabokon A, Singewald N, Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology. 2011;36:793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence-Aizenberg A, Di Fiore A, Fernandez-Duque E. Social monogamy, male-female relationships, and biparental care in wild titi monkeys (Callicebus discolor) Primates. 2016;57:103–112. doi: 10.1007/s10329-015-0489-8. [DOI] [PubMed] [Google Scholar]
- Stan_Development_Team. Stan Modeling Language User’s Guide and Reference Manual 2015 [Google Scholar]
- Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proceedings of the National Academy of Sciences. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yu H, Chen J, Liang J, Lu L, Zhou X, et al. Neural substrates and behavioral profiles of romantic jealousy and its temporal dynamics. Scientific Reports. 2016;6:27469. doi: 10.1038/srep27469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbaa M, Paedae B, Liu Y, Wang Z. Neuropeptide regulation of social attachment: the prairie vole model. Comprehensive Physiology. 2016;7:81–104. doi: 10.1002/cphy.c150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Matsuura M, Yahata N, Koeda M, Suhara T, Okuba Y. Men and women show distinct brain activations during imagery of sexual and emotional infidelity. NeuroImage. 2006;32:1299–1307. doi: 10.1016/j.neuroimage.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Tardif S, Bales K, Williams L, Moeller E, Abbott D, Schultz-Darken N, et al. Preparing New World monkeys for laboratory research. I.L.A.R. Journal. 2006;47:307–315. doi: 10.1093/ilar.47.4.307. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenal axis activity in older women. Psychosomatic Medicine. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Team, R.C. R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- Tecot SR, Singletary B, Eadie E. Why “monogamy” isn’t good enough. American ournal of Primatology. 2016;78:340–354. doi: 10.1002/ajp.22412. [DOI] [PubMed] [Google Scholar]
- Tops M, Koole SL, IJzerman H, Buisman-Pijlman FTA. Why social attachment and oxytocin protect against addiction and stress: insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology, Biochemistry, and Behavior. 2014;119:39–48. doi: 10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Van Belle S, Fernandez-Duque E, Di Fiore A. Demography and life history of wild red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequoatorialis) in Amazonian Ecuador: A 12-year study. American Journal of Primatology. 2016;78:204–215. doi: 10.1002/ajp.22493. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. The challenge hypothesis: Where it began and relevance to humans. Hormones and Behavior. 2017;92:9–12. doi: 10.1016/j.yhbeh.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Duffy AM, Jr, Ball GF. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist. 1990;136:829–846. [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Zucker DR, Schmid CH, McIntosh MW, D’Agostino RB, Selker HP, Lau J. Combining single patient (N-of-1) trials to estimate population treatment effects and to evaluate individual patient responses to treatment. Journal of Clinical Epidemiology. 1997;50:401–410. doi: 10.1016/s0895-4356(96)00429-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


