Abstract
Background
Cancer screening rates are lowest in those without insurance or a regular provider. Since 2008, the Colorectal Cancer Prevention Network (CCPN) has provided open-access colonoscopy to uninsured South Carolinians through established, statewide partnerships and patient navigation. Herein, we describe the structure, implementation, and clinical outcomes of this program.
Methods
The CCPN provides access to colonoscopy screening at no cost to uninsured, asymptomatic patients aged 50–64 (African Americans age 45–64 are eligible) who live at or below 150% of the poverty line and seek medical care in free medical clinics, federally qualified health centers, or hospital-based indigent practices in SC. Screening is done by board-certified gastroenterologists. Descriptive statistics and regression are used to describe the population screened, and to assess compliance rates and colonoscopy quality metrics.
Results
From >4,000 patients referred to the program, 1,854 were deemed eligible, 1,144 attended an in-person navigation visit, and 909 completed a colonoscopy. Nearly 90% of participants exhibited good-to-excellent bowel preparation. An overall cecal intubation rate of 99% was measured. The polyp detection rate (PDR) and adenoma detection rate (ADR) were 63% and 36%, respectively, with male gender and urban residence positively associated with polyp/adenoma detection. Over 13% of participants had an advanced polyp, and 1% had a cancer diagnosis or surgical intervention.
Conclusions
The CCPN program is characterized by strong collaboration with clinicians statewide, low no-show rates, and high colonoscopy quality. Future work will assess the effectiveness of the navigation approach, and will explore the mechanisms driving higher polyp/adenoma detection in urban participants.
Keywords: Colonoscopy, Colorectal Neoplasms, Early Detection of Cancer, Patient Navigation, South Carolina
Introduction
Colorectal cancer (CRC) is the fourth most common form of cancer, and the second leading cause of cancer death in the United States (U.S.). It is most prevalent in those over 50 years of age.1 South Carolina experiences approximately 2,300 incident cases of CRC each year and over 800 deaths. Racial disparities in CRC are profound both across the nation and in South Carolina.2–5 Incidence rates for African Americans (AA) are higher in the U.S. (46.7 per 100,000) and South Carolina (44.3 per 100,000) compared to whites (38.9 per 100,000 nationally, 36.9 per 100,000 in SC); mortalities among AA are also higher than for other races.6 These disparities may be due to lower socioeconomic status and a relative lack of insurance among AA populations.7, 8, 9 Timely screening allows identification and removal of pre-cancerous colorectal lesions before they progress to cancer, and is associated with reduced disease incidence and mortality. 10, 11
In the general U.S. population, about 65% of average-risk adults follow U.S. Preventive Services Task Force guidelines for CRC screening.12 Yet, screening rates are particularly low in medically underserved populations,12, 13 resulting in elevated numbers of late-stage cancers, exacerbated health care costs, and increased deaths.14–16 Poverty, poor access to care, and lack of insurance coverage contribute to reduced engagement in screening or diagnostic testing within these populations.17, 18 Community-based health systems, such as federally-qualified health centers (FQHCs) and free medical clinics (FMCs), provide primary care services to a large proportion of underinsured and uninsured individuals. In South Carolina, 90.8% of FQHC patients live at or below 200% of the federal poverty line, 29.2% are uninsured, and 32.2% have undergone CRC screening.19 The majority of FQHC patients (62.3% nationally and 64.9% in SC in 2016) represent racial and/or ethnic minorities.20 The interplay between race/ethnicity, socioeconomic vulnerability, and inadequate insurance coverage has been shown to affect observed disparities in CRC burden21–25, making FQHCs and FMCs uniquely positioned to benefit from programs aimed at increasing CRC screening rates.
In recent years, efforts have focused on improving CRC screening awareness and participation through evidence-based practices, such as reminder letters, patient navigation, elimination of financial barriers, among others.26–29 Navigation in the clinical and community setting has been successful in increasing cancer screening participation, and has great potential in reducing cancer disparities.30–36
In the current study, we describe the programmatic structure, implementation, and outcomes of an open-access colonoscopy screening program for uninsured patients in South Carolina. Patient navigation and organized linkages to board-certified gastroenterologists are central features of the program, which has the over-arching goal of increased participation in high-quality CRC screening among the uninsured.
Methods
Programmatic Structure of the Colon Cancer Screening Program
In 2008, to address disparities in care for uninsured individuals in South Carolina, the Center for Colon Cancer Research at the University of South Carolina established the Colorectal Cancer Prevention Network (CCPN), which provides CRC education, awareness, and high-quality screening services statewide. Comprehensive patient navigation and organized linkages among referring clinics, board-certified gastroenterologists, pathologists, and cancer treatment specialists are utilized in the program.
Eligibility
The CCPN provides colonoscopy screening at no cost to individuals age 50–64 (African Americans are eligible at age 45) who are under the care of safety-net practices, live at or below 150% of the federal poverty line, and are uninsured. Patient eligibility is determined through chart reviews and phone interviews managed by patient navigators, who are employed by the CCPN but work remotely within the communities they serve. Exclusion criteria for the program include: a prior colonoscopy within the past 10 years, a history of colorectal neoplasia, recent onset of symptoms associated with CRC (i.e., significant rectal bleeding, iron deficiency anemia, unintended weight loss greater than 10%, diarrhea or constipation, abdominal distention, pain or cramps, nausea or vomiting), a diagnosis of an inherited CRC disorder, a known history of inflammatory bowel disease, or a personal history of cancer other than a non-melanoma skin cancer. Although very rare, participating gastroenterologists may also decline to provide services based on additional medical factors (e.g., allowable BMI range) that vary across practices.
Clinical partnerships
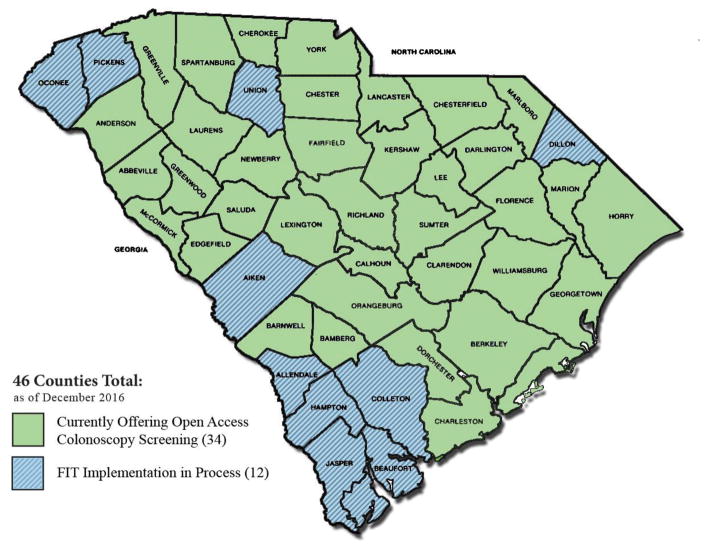
The CCPN recruits patients from 36 counties throughout South Carolina (see Figure 1). A network of health system partners, including 112 FMCs, FQHCs, and hospital-based indigent practices, provide patient referrals to CCPN navigators, who assess medical eligibility, and as appropriate, coordinate navigation appointments, educate patients on CRC and screening, conduct reminder calls, schedule colonoscopies, and provide follow-up information as needed. Procedures are carried out by 86 board-certified gastroenterologists representing 19 endoscopy facilities and 5 hospital-based practices, all of whom donate their time to the CCPN. In addition, 12 pathology organizations and 13 anesthesiology practices provide services to the program.
Figure 1.
Map of SC Counties Served by the CCPN Screening Program
Implementation of the Program
Patient navigation
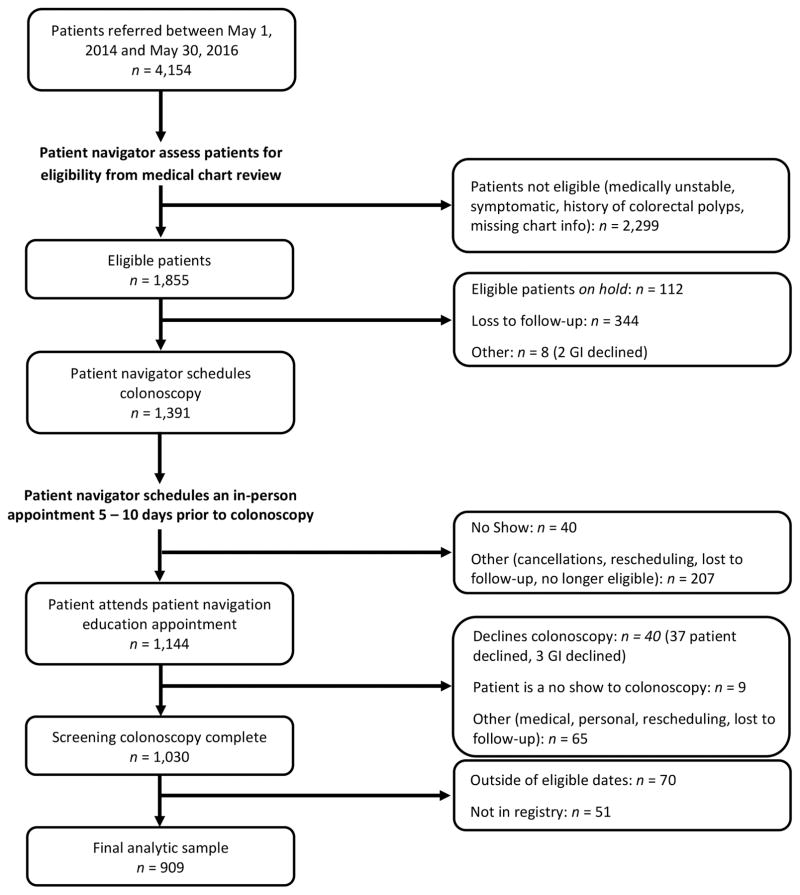
A comprehensive patient navigation approach is used to recruit, educate, and screen patients that are referred to the program (see Figure 2). Once a clinic makes a referral, a navigator conducts a medical chart review to ensure the patient’s eligibility. Upon being declared tentatively eligible, the patient is contacted and interviewed via telephone, a step that is particularly important since a complete health history is not always available in the medical records. Following confirmation of eligibility, the navigator sends patient files to an endoscopy center and receives the colonoscopy appointment once approved. An in-person navigation visit is scheduled 5–10 days prior to the procedure, during which time the patient is educated on CRC and the importance of screening, procedure details and instructions are given, and the patient has an opportunity ask questions (see Appendix for select navigation materials used). After the in-person visit, the navigator follows up with the patient as needed the day before and the day of the procedure. The navigator is available to the patient via telephone at any time 24 hours a day 7 days a week, which helps reduce no-shows and any other barriers that arise (e.g., assistance getting patient a car ride to colonoscopy through church-based, neighbors, or other community organizations).
Figure 2.
Flow Diagram for Referral, Patient Navigation and Open Access Colonoscopy for CCPN Screening Program
Data collection and analysis
During each step of the patient navigation process, data are collected in real time, managed by CCPN navigators, and housed in the Data Coordinating Center (DCC). The DCC is supported by a web-based, HIPAA-compliant database by Dacima Software, Inc. 37 In addition to patient contact information and eligibility criteria, the database captures over 1,000 variables, including details about health behaviors, personal/family medical history, endoscopy details, pathology findings, and other specialized care reports.
Outcomes assessment
Analysis of program outcomes were conducted for patients screened between May 2014 and May 2016. The following quality metrics and clinical outcomes were evaluated: colonic preparation quality (% excellent/good preparation), cecal intubation rate (% of patients where cecum was reached during screening), polyp detection rate (PDR, i.e., % of colonoscopies where one or more polyps was removed during the procedure), and adenoma detection rate (ADR, i.e., % of colonoscopies where one or more adenomas was identified via pathological examination). Patients were further classified based upon the most advanced polyp biopsied during colonoscopy or procedure outcome, as follows: (1) cancer, carcinoid, or lesion requiring surgical intervention; (2) advanced polyp(s), classified as any polyp ≥ 1 cm or greater in diameter, including hyperplastic, traditional serrated or sessile serrated, and any polyp with villous components and/or high-grade dysplasia38–41 ; (3) non-advanced adenoma(s) <1 cm in diameter; (4) hyperplastic polyp(s) <1 cm in diameter; (5) biopsied polyp(s) with non-significant pathology; or (6) no biopsies performed. Patients who had a colonoscopy with polypectomy, but no associated pathology report, were excluded from this analysis.
Descriptive statistical analyses were carried out for the quality metrics and clinical outcomes overall, and by patient characteristics (gender, age, race/ethnicity, urban/rural location based on 2010 ZIP Rural-Urban Commuting Area/RUCA codes, language spoken, and educational attainment). Multilevel logistic regression (level 1: patients, level 2: physicians), with a physician-level random intercept, was performed for PDR and ADR as a function of patient characteristics, with significance defined at the alpha=0.05 level.
Results
Among 1,855 patients referred to the program and deemed eligible, 1,391 were scheduled for colonoscopy, 1,144 attended an in-person navigation visit, and 1,030 completed the procedures. Of the completed procedures, 909 patients with complete records (colonoscopy and pathology) were included in the analytic sample (see Figure 2). No-shows for the scheduled patient navigation visit and for the colonoscopy were exceptionally low (40/1,391 = 2.9% and 9/1,144 = 0.8%, respectively). The colonoscopy participation rate, which is defined as the proportion of eligible, navigated individuals who completed their scheduled colonoscopy, was 90% (1,030/1,144). The majority of patients screened were female (62%), non-Hispanic black (53%), and high school graduates or less (68%). Less than 3% of patients spoke a language other than English. Mirroring the SC population, 32% of the patients screened resided in a rural area (Table 1).
Table 1.
CCCR Screening Program Participant Characteristics and Screening Metrics, May 2014 – May 2016 (N=909)
| Characteristics | Excellent/Good Preparation | Cecum Intubation Rate | Polyp Detection Rate (PDR) | Adenoma Detection Rate (ADR)a | |
|---|---|---|---|---|---|
| N (%) | n (%) | n (%) | n (%) | n (%) | |
| Total | 909 | 812 (89.32) | 905 (99.556) | 570 (62.71) | 291 (35.62) |
|
| |||||
| Gender | p = 0.2997 | p = 0.985 | p = 0.0453 | p = 0.0123 | |
|
| |||||
| Female | 564 (62.05) | 509 (90.25) | 561 (99.47) | 339 (60.11) | 167 (32.36) |
| Male | 345 (37.95) | 303 (87.83) | 344 (99.71) | 231 (66.96) | 124 (41.33) |
|
| |||||
| Ageb | p = 0.0330 | p = 0.1473 | p = 0.3231 | p = 0.0899 | |
|
| |||||
| 45–49 | 47 (5.17) | 43 (91.49) | 46 (97.87) | 29 (61.70) | 9 (22.50) |
| 50–54 | 405 (44.55) | 358 (88.40) | 405 (100) | 241 (59.51) | 123 (33.70) |
| 55–59 | 288 (31.68) | 268 (93.06) | 286 (99.31) | 189 (65.62) | 105 (40.70) |
| 60–64 | 169 (18.59) | 143 (84.62) | 168 (99.41) | 111 (65.68) | 54 (35.29) |
|
| |||||
| Race/ethnicity | p = 0.6211 | p = 0.1707 | p = 0.2763 | p = 0.0924 | |
|
| |||||
| Non-Hispanic White | 335 (36.89) | 297 (88.66) | 335 (100) | 221 (65.97) | 120 (39.47) |
| Non-Hispanic Black | 483 (53.19) | 431 (89.23) | 479 (99.17) | 292 (60.46) | 139 (32.25) |
| Other | 90 (9.91) | 83 (92.22) | 90 (100) | 56 (62.22) | 32 (40.00) |
|
| |||||
| Location | p = 0.4883 | p = 1 | p = 0.0043 | p = 0. 0031 | |
|
| |||||
| Urban | 614 (67.55) | 552 (89.90) | 611 (99.66) | 405 (65.96) | 218 (39.14) |
| Rural | 295 (32.45) | 260 (88.14) | 294 (99.51) | 165 (55.93) | 73 (28.19) |
|
| |||||
| Language | p = 0.3821 | p = 1 | p = 0.5633 | p = 0.6839 | |
|
| |||||
| English | 882 (97.03) | 786 (89.12) | 878 (99.55) | 555 (62.93) | 281 (35.48) |
| Non-English | 27 (2.97) | 26 (96.30) | 27 (100) | 15 (55.56) | 10 (41.67) |
|
| |||||
| Education | p = 0.6103 | p = 0.4610 | p = 0.7124 | p = 0.6287 | |
|
| |||||
| Less than HS | 249 (27.42) | 216 (86.75) | 249 (100) | 161 (64.66) | 83 (37.05) |
| HS Diploma | 366 (40.31) | 333 (90.98) | 363 (99.18) | 227 (62.02) | 108 (33.44) |
| Some College or Associate’s | 230 (25.33) | 205 (89.13) | 229 (99.57) | 146 (63.48) | 80 (38.46) |
| Bachelor’s or Higher | 63 (6.94) | 57 (90.48) | 63 (100) | 36 (57.14) | 20 (33.33) |
P-values were calculated from a Chi-square or Fisher’s exact test (for small cell sizes); bolded values are statistically significant at alpha level 0.05.
Adenoma detection rate based on 816 patients for which both colonoscopy and pathology data (if applicable) are available.
Age at inclusion; the age group of 45–49 includes African Americans only.
Overall, 89% of screened patients were rated as having good-to-excellent bowel preparations. In addition, cecal intubation rates were >99%. No significant differences were noted in bowel preparation or cecal intubation rate by race/ethnicity, gender, education, language or rurality (Table 1). Significant differences in bowel preparation were noted for age (p=0.033), with persons aged 60–64 having the lowest prevalence of good-to-excellent bowel preparation (85%).
PDR and ADR were 63% and 36%, respectively. Both PDR and ADR were higher in males than in females (p=0.045 and p=0.012; see Table 1). Persons residing in rural areas were significantly less likely to have a polyp (p=0.0043) or adenoma (p=0.0031; Table 1). No significant differences in detection rates were noted across race/ethnicity, language spoken, or education.
Among all completed colonoscopies, 36% had no biopsy performed. Among the lesions sent for pathological assessment (n=486), 6.6% were histologically normal, 41.6% were hyperplastic polyps, 28.0% were non-advanced adenomas, and 22.0% were advanced adenomas/polyps (Table 2). About 1% of patients were diagnosed with CRC or a carcinoid tumor, and were referred for surgical resection. Males were more likely than females to have advanced polyps (p=0.029) and cancer/carcinoid tumors (p=0.015).
Table 2.
Screening and Pathology Findings among CCCR Screening Program Participants, May 2014-May 2016 (N=816)
| Characteristic n (%) | No Biopsies Performed | Biopsy with NSP* | Hyperplastic Polyps < 1cm | Adenoma < 1cm | Advanced Polypsa | Surgical intervention, Cancer, or Carcinoidb |
|---|---|---|---|---|---|---|
| Total | 330 (36.30) | 32 (3.92) | 202 (24.72) | 136 (16.65) | 107 (13.11) | 9 (1.10) |
|
| ||||||
| Gender | p = 0.0252 | p = 0.6362 | p = 1 | p = 1 | p = 0.0288 | p = 0.0146 |
|
| ||||||
| Female | 221 (39.18) | 22 (4.26) | 128 (24.81) | 86 (16.67) | 57 (11.05) | 2 (0.39) |
| Male | 109 (31.59) | 10 (3.33) | 74 (24.58) | 50 (16.61) | 50 (16.67) | 7 (2.33) |
|
| ||||||
| Race/ethnicity | p = 0.2902 | p = 0.2130 | p = 0.5830 | p = 0.4855 | p = 0.1352 | p = 0.2177 |
|
| ||||||
| Non-Hispanic White | 111 (33.13) | 8 (2.63) | 78 (25.57) | 54 (17.70) | 47 (15.46) | 6 (1.97) |
| Non-Hispanic Black | 186 (38.51) | 22 (5.10) | 107 (24.83) | 66 (15.31) | 47 (10.90) | 3 (0.70) |
| Other | 33 (36.67) | 2 (2.50) | 16 (20.00) | 16 (20.00) | 13 (16.25) | 0 |
P-values were calculated from a Chi-square or Fisher’s exact test (for small cell sizes); bolded values are statistically significant at alpha level 0.05.
Advanced polyps are defined as any polyp 1 cm or greater, including hyperplastic, any traditional serrated or sessile serrated adenoma/polyp, any polyp w/villous components, and/or high-grade dysplasia
Includes patients with surgical intervention, cancer or carcinoid diagnosis. P-values were calculated from a Chi-square or Fisher’s exact test (for small cell sizes)
NSP = No significant pathology.
In a multilevel, adjusted logistic regression model, male gender (OR = 1.54, 95% CI: 1.13, 2.11) and rural residence (OR = 0.67, 95% CI: 0.45, 0.99) were significantly associated with the presence of adenomas, with rates in males and urban residents being higher than in females and rural residents, respectively (Table 3). Male gender was also significantly associated with polyp detection, although to a lesser extent, in both unadjusted and adjusted models. After controlling for physician variability and other individual-level covariates, rural residence was no longer associated with polyp detection (OR = 0.70, 95% CI: 0.48, 1.02). No differences were noted based on age, race/ethnicity, language spoken, or education in adjusted models.
Table 3.
Factors Associated with Polyp and Adenoma Detection at Baseline Screening among CCCR Screening Program Participants, May 2014-May 2016
| Polyp Detection | Adenoma Detectiona | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Unadjusted | Multivariable* | Unadjusted | Multivariable* | ||||||
| Characteristic | N (%) | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
|
| |||||||||
| Gender | |||||||||
|
| |||||||||
| Female | 564 (62.05) | REF | NA | REF | NA | REF | NA | REF | NA |
| Male | 345 (37.95) | 1.34 | (1.02, 1.78) | 1.45 | (1.07, 1.97) | 1.47 | (1.10, 1.98) | 1.54 | (1.13, 2.11) |
|
| |||||||||
| Ageb | |||||||||
|
| |||||||||
| 45–49 | 47 (5.17) | 0.84 | (0.43, 1.64) | 0.91 | (0.44, 1.91) | 0.53 | (0.24, 1.20) | 0.56 | (0.24, 1.33) |
| 50–54 | 405 (44.55) | 0.77 | (0.53, 1.12) | 0.73 | (0.49, 1.09) | 0.93 | (0.63, 1.38) | 0.87 | (0.57, 1.32) |
| 55–59 | 288 (31.68) | 1.00 | (0.67, 1.49) | 1.01 | (0.66 1.55) | 1.26 | (0.83, 1.90) | 1.18 | (0.76, 1.83) |
| 60–64 | 169 (18.59) | REF | NA | REF | NA | REF | NA | REF | NA |
|
| |||||||||
| Race/ethnicity | |||||||||
|
| |||||||||
| Non-Hispanic White | 335 (36.89) | REF | NA | REF | NA | REF | NA | REF | NA |
| Non-Hispanic Black | 483 (53.19) | 0.79 | (0.59, 1.05) | 0.75 | (0.55, 1.04) | 0.73 | (0.54, 0.99) | 0.76 | (0.54, 1.06) |
| Other | 90 (9.91) | 0.85 | (0.52, 1.38) | 1.00 | (0.55, 1.80) | 1.02 | (0.62, 1.69) | 1.03 | (0.57, 1.88) |
|
| |||||||||
| Location | |||||||||
|
| |||||||||
| Urban | 614 (67.55) | REF | NA | REF | NA | REF | NA | REF | NA |
| Rural | 295 (32.45) | 0.65 | (0.49, 0.87) | 0.70 | (0.48, 1.02) | 0.61 | (0.44, 0.84) | 0.67 | (0.45, 0.99) |
|
| |||||||||
| Language | |||||||||
|
| |||||||||
| English | 882 (97.03) | REF | NA | REF | NA | REF | NA | REF | NA |
| Non-English | 27 (2.97) | 0.74 | (0.34, 1.59) | 0.65 | (0.25, 1.71) | 1.30 | (0.57, 2.96) | 1.13 | (0.42, 3.08) |
|
| |||||||||
| Education | |||||||||
|
| |||||||||
| < HS | 249 (27.42) | REF | NA | REF | NA | REF | NA | REF | NA |
| HS Diploma | 366 (40.31) | 0.89 | (0.64, 1.25) | 0.84 | (0.59, 1.21) | 0.85 | (0.60, 1.22) | 0.86 | (0.59, 1.25) |
| Some College or Associate’s | 230 (25.33) | 0.95 | (0.65, 1.38) | 0.93 | (0.63, 1.39) | 1.06 | (0.72, 1.57) | 1.11 | (0.73, 1.68) |
| Bachelor’s or Higher | 63 (6.94) | 0.73 | (0.42,1.28) | 0.59 | (0.32, 1.08) | 0.85 | (0.47, 1.55) | 0.72 | (0.38, 1.36) |
Excludes those that did not have a pathology report (n = 93)
Age at inclusion; the age group of 45–49 includes African Americans only
Multivariable model includes a physician-level random intercept.
Discussion
Provision of high-quality CRC screening that includes comprehensive patient navigation to uninsured and medically underserved South Carolinians ensures that barriers to participation are addressed, that patients are knowledgeable and prepared for their procedures, and that linkages to follow-up treatment are made available. The quality of the CCPN program is evidenced by low no-show rates, favorable colonoscopy quality metrics, strong collaborations with referring clinics and board-certified gastroenterologists, and prevention of CRC through identification and removal of pre-cancerous polyps. Its statewide reach is also a major strength, as the program works with over 110 health clinics in 36 counties to obtain patient referrals. Others have reported increased CRC screening rates in uninsured and underserved populations in similar programs using patient navigation.33, 42–47 In a study of primarily uninsured patients from Suffolk County, NY, Lane et al.46 found that providing 800 FQHC patients direct access to colonoscopy resulted in a 37% PDR, a 26% ADR, a 3% no-show rate, a 95–99% cecal intubation rate, and a 92% good-to-excellent bowel preparation rate. Wolf et al. 44 implemented a statewide colonoscopy screening program including 13,000 uninsured Colorado residents, and reported a 93% adequate bowel preparation rate, a 97% cecal intubation rate, and a 28% ADR.
Several features of the current study are noteworthy. The PDR and ADR for the CCPN program (63% and 36%, respectively) are among the highest reported to date, which may be due to the high-quality bowel preparation and cecal intubation rates we observed, exclusive engagement of board-certified gastroenterologists, and study location (e.g., high obesity rate in SC). The observation of higher ADR and PDR in men compared to women is consistent with prior reports;48, 49 however, the finding of a lower ADR among rural relative to urban residents was rather unexpected. This may be due to any of a number of factors, including but not limited to differing characteristics of rural vs. urban patients. Ad-hoc analyses to determine whether the higher ADR in rural areas might be attributed to patient demographic differences revealed no statistically significant differences between the demographics of rural vs. urban patients in our program (Supplementary Table 1). Further studies will be required to understand the contribution of other patient and provider-level factors on ADR variability.
Studies of racial/ethnic differences in polyp/adenoma detection among uninsured patients have shown mixed results. In a study of uninsured individuals visiting community health clinics in NYC, Collazo et al.47 found a significant difference in polyp detection across race/ethnicity, but did not find statistically significant differences in polyp type, location or advanced pathology across groups. Similarly, Lane et al.46 did not find statistically significant differences in risk of having an adenoma between African American and White uninsured patients from Suffolk County, NY. Our study found no significant differences in either PDR or ADR across racial/ethnic groups, although further studies are needed to determine whether racial/ethnic disparities vary across geographic regions with differing population demographics, lifestyle behaviors, and healthcare infrastructure and insurance systems.
The finding that persons aged 60–64 in our program were least likely to have good-to-excellent bowel preparation aligns with a recent meta-analysis showing a pooled odds ratio of 1.14 for age, indicating higher odds of poor bowel preparation with increasing age.50 Similar, Gandhi et al.50 reported greater likelihood of poor bowel preparation in persons with diabetes, hypertension, stoke or dementia, all of which are more common in older populations. The lower PDR/ADR observed among 60–64 year olds may be partially due to this lower rate of good-to-excellent bowel preparation, we well as the exclusion of 60–64 year olds from the program who had a colonoscopy <10 years ago or a polyp history.
There are several limitations to the current study. By offering CRC screening only to uninsured patients, the CCPN program lacks a comparison group to a general, insured population. Additionally, only 3% of our patients were non-English speakers, limiting our generalizability to programs in regions with many non-English speaking patients. It is noteworthy, however, that bowel preparation quality and overall clinical outcomes did not differ by language spoken. Furthermore, the CCPN was unable to eliminate all selection bias, as only patients using FMCs or FQHCs as a regular source of care were referred to the program. Finally, the navigation approach utilized in the present study requires both in-person and over-the-phone patient eligibility assessment and education, and requires significant manpower and resources. Replication of the program may not be trivial. A study to examine which elements of patient navigation are most cost effective and result in the best clinical outcomes would be critical as an important next step. Future research should also examine the patient characteristics associated with non-compliance and loss to follow-up prior to colonoscopy. Finally, a study to investigate physician-level outcomes as a function of patient case-mix, practice selection criteria, and other performance factors should be performed. Despite these caveats, the current analysis provides insights into the structure, operation, and outcomes of a statewide CRC screening program that will be useful in future efforts aimed at increasing screening rates, particularly within uninsured and medically underserved communities that are vulnerable to high CRC burden.
Supplementary Material
Acknowledgments
Funding: Funding for this program leverages public and private funding including The Duke Endowment, Blue Cross Blue Shield of SC Foundation, the state of South Carolina, the National Institutes of Health (4P30GM103336), the University of South Carolina, in-kind contributions from board-certified gastroenterologists, anesthetists, pharmacists, pathologists, individual donations, and other sources. Dr. Eberth is supported in part by a grant from the American Cancer Society (MRSG-15-148-01-CPHPS), and MJ is supported by grant T32-FM081740 from the NIH-National Institute of General Medical Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Footnotes
Conflicts of interests: None to disclose.
Author contributions: JME, AT, FGB, and RC conceptualized the study goals. Methodology was designed by JME and EP, with feedback from all co-authors. Software coding and formal analysis was performed by MJJ and BQ. Collection of data required for the investigation was conducted by RC, AT and DL, with assistance from CCPN Program navigators. Data visualizations were created by JME, MJJ and BQ. Project supervision was provided by JME, FGB, and EP, and administration by JME and FGB. JME prepared the original manuscript draft, with input from all co-authors. All authors provided critical feedback and editing for the final manuscript. Funding acquisition for the CCPN Program was done by FGB, AT and RC.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial Disparities in Stage-Specific Colorectal Cancer Mortality: 1960–2005. Am J Public Health. 2010;100:1912–1916. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauh J, Brawley OW, Berger M. Racial Disparities in Colorectal Cancer. Curr Probl Cancer. 2007;31:123–133. doi: 10.1016/j.currproblcancer.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Hébert JR, Daguise VG, Hurley DM, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115:2539–2552. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace K, Hill EG, Lewin DN, et al. Racial disparities in advanced-stage colorectal cancer survival. Cancer Causes Control. 2013;24:463–471. doi: 10.1007/s10552-012-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. State Cancer Profiles. 2016. [Google Scholar]
- 7.On Views of Race and Inequality, Blacks and Whites are Worlds Apart. Washington, D.C: Pew Research Center; 2016. Demographic trends and economic well-being. [Google Scholar]
- 8.Moonsinghe RCM, Truman BI. Health insurance coverage - United States, 2008 and 2010. MMWR Suppl. 2013;62:61–64. [PubMed] [Google Scholar]
- 9.Niu X, Pawlish KS, Roche LM. Cancer Survival Disparities by Race/Ethnicity and Socioeconomic Status in New Jersey. J Health Care Poor Underserved. 2010;21:144–160. doi: 10.1353/hpu.0.0263. [DOI] [PubMed] [Google Scholar]
- 10.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. New Eng J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. New Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 12.Vital signs: colorectal cancer screening test use--United States, 2012. MMWR. 2013;62:881. [PMC free article] [PubMed] [Google Scholar]
- 13.Baker DW, Liss DT, Alperovitz-Bichell K, et al. Colorectal Cancer Screening Rates at Community Health Centers that Use Electronic Health Records: A Cross Sectional Study. J Health Care Poor Underserved. 2015;26:377–390. doi: 10.1353/hpu.2015.0030. [DOI] [PubMed] [Google Scholar]
- 14.Ho C, Kornfield R, Vittinghoff E, Inadomi J, Yee H, Somsouk M. Late presentation of colorectal cancer in a vulnerable population. Am J Gastroenterol. 2013;108:466–470. doi: 10.1038/ajg.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mobley LR, Kuo T-M, Watson L, Brown GG. Geographic Disparities in Late-Stage Cancer Diagnosis: Multilevel Factors and Spatial Interactions. Health & place. 2012;18:978–990. doi: 10.1016/j.healthplace.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley CJ, Given CW, Roberts C. Late stage cancers in a Medicaid-insured population. Med Care. 2003;41:722–728. doi: 10.1097/01.MLR.0000065126.73750.D1. [DOI] [PubMed] [Google Scholar]
- 17.Thomson MD, Siminoff LA. Finding medical care for colorectal cancer symptoms: experiences among those facing financial barriers. Health Educ Behav. 2015;42:46–54. doi: 10.1177/1090198114557123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojinnaka C, Vuong A, Helduser J, et al. Determinants of variations in self-reported barriers to colonoscopy among uninsured patients in a primary care setting. J Community Health. 2015;40:260–270. doi: 10.1007/s10900-014-9925-8. [DOI] [PubMed] [Google Scholar]
- 19.Health Resources and Services Administration. [accessed January 25, 2017];Health Center Data: Health Center Program Grantee Data. 2015 Available from URL: https://bphc.hrsa.gov/uds/datacenter.aspx.
- 20.Health Resources and Services Administration. [accessed September 12, 2017];Health Center Data. 2016 Available from URL: https://bphc.hrsa.gov/datareporting/index.html.
- 21.Tawk R, Abner A, Ashford A, Brown CP. Differences in Colorectal Cancer Outcomes by Race and Insurance. Int J Environ Res Public Health. 2015;13 doi: 10.3390/ijerph13010048. ijerph13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfson JA, Sun CL, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: Results from a population-based study. Cancer. 2015;121:3885–3893. doi: 10.1002/cncr.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorey KM, Haji-Jama S, Bartfay E, Luginaah IN, Wright FC, Kanjeekal SM. Lack of access to chemotherapy for colon cancer: multiplicative disadvantage of being extremely poor, inadequately insured and African American. BMC Health Serv Res. 2014;14:133. doi: 10.1186/1472-6963-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitzkorski JR, Willis AI, Nick D, Zhu F, Farma JM, Sigurdson ER. Association of race and socioeconomic status and outcomes of patients with rectal cancer. Ann Surg Oncol. 2013;20:1142–1147. doi: 10.1245/s10434-012-2837-x. [DOI] [PubMed] [Google Scholar]
- 25.Miranda PY, Johnson-Jennings M, Tarraf W, Gonzalez P, Vega WA, Gonzalez HM. Using colorectal trends in the U.S. to identify unmet primary care needs of vulnerable populations. Prev Med. 2012;55:131–136. doi: 10.1016/j.ypmed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality. Toolkit for the System Approach to Tracking and Increasing Screenng for Public Health Improvement of Colorectal Cancer Intervention. 2010. [Google Scholar]
- 27.National Colorectal Cancer Roundtable. Tools & Resources - How to Increase Colorectal Cancer Screening Rates in Practice: A Primary Care Clinician’s Evidence-Based Toolbox and Guide. [Google Scholar]
- 28.Centers for Disease Control and Prevention. [accessed January 25, 2017];Screen for Life: National Colorectal Cancer Action Campaign. Available from URL: https://www.cdc.gov/cancer/colorectal/sfl/index.htm.
- 29.Steinwachs D, Allen JD, Barlow WE, et al. NIH state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. NIH Consens State Sci Statements. 2010;27:1–31. [PubMed] [Google Scholar]
- 30.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171:906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 31.Hendren S, Griggs J, Epstein R, et al. Study protocol: a randomized controlled trial of patient navigation-activation to reduce cancer health disparities. BMC Cancer. 2010;10:551. doi: 10.1186/1471-2407-10-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jean-Pierre P, Hendren S, Fiscella K, et al. Understanding the processes of patient navigation to reduce disparities in cancer care: perspectives of trained navigators from the field. J Cancer Educ. 2010;26:111–120. doi: 10.1007/s13187-010-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Percac-Lima S, Grant R, Green A, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Intern Med. 2009;24:211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelton RC, Thompson HS, Jandorf L, et al. Training experiences of lay and professional patient navigators for colorectal cancer screening. J Cancer Educ. 2011;26:277–284. doi: 10.1007/s13187-010-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battaglia TA, Burhansstipanov L, Murrell SS, Dwyer AJ, Caron SE. Assessing the impact of patient navigation: prevention and early detection metrics. Cancer. 2011;117:3553–3564. doi: 10.1002/cncr.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61:237–249. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dacima Software, Inc. [accessed January 25, 2017]; Available from URL: http://www.dacimasoftware.com/
- 38.MIT, JCA Serrated polyps: clinical implications and future directions. Curr Gastroenterol Rep. 2013;15:342. doi: 10.1007/s11894-013-0342-4. [DOI] [PubMed] [Google Scholar]
- 39.Short M, Layton M, Teer B, Domagalski J. Colorectal Cancer Screening and Surveillance. Am Fam Physician. 2015;91:93–100. [PubMed] [Google Scholar]
- 40.Obuch J, Pigott C, Ahnen D. Sessile Serrated Polyps: Detection, Eradication and Prevention of the Evil Twin. Curr Treat Options Gastroenterol. 2015;13:156–170. doi: 10.1007/s11938-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Choi Y, Kwon H, et al. Simple colonoscopy reporting system checking the detection rate of colon polyps. World J Gastroenterol. 2015;21:9380–9386. doi: 10.3748/wjg.v21.i31.9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naylor K, Fritz C, Polite B, Kim K. Evaluating screening colonoscopy quality in an uninsured urban population following patient navigation. Prev Med Rep. 2017;5:194–199. doi: 10.1016/j.pmedr.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shokar NK, Byrd T, Salaiz R, et al. Against colorectal cancer in our neighborhoods (ACCION): A comprehensive community-wide colorectal cancer screening intervention for the uninsured in a predominantly Hispanic community. Prev Med. 2016;91:273–280. doi: 10.1016/j.ypmed.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 44.Wolf HJ, Dwyer A, Ahnen DJ, et al. Colon cancer screening for Colorado’s underserved: a community clinic/academic partnership. Am J Prev Med. 2015;48:264–270. doi: 10.1016/j.amepre.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Honeycutt S, Green R, Ballard D, et al. Evaluation of a patient navigation program to promote colorectal cancer screening in rural Georgia, USA. Cancer. 2013;119:3059–3066. doi: 10.1002/cncr.28033. [DOI] [PubMed] [Google Scholar]
- 46.Lane DS, Messina CR, Cavanagh MF, Anderson JC. Delivering colonoscopy screening for low-income populations in Suffolk County: strategies, outcomes, and benchmarks. Cancer. 2013;119(Suppl 15):2842–2848. doi: 10.1002/cncr.28160. [DOI] [PubMed] [Google Scholar]
- 47.Collazo T, Jandorf L, Thelemaque L, Lee K, Itzkowitz S. Screening colonoscopy among uninsured and underinsured urban minorities. Gut Liver. 2015;9:502–508. doi: 10.5009/gnl14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gohel TD, Burke CA, Lankaala P, et al. Polypectomy rate: a surrogate for adenoma detection rate varies by colon segment, gender, and endoscopist. Clin Gastroenterol Hepatol. 2014;12:1137–1142. doi: 10.1016/j.cgh.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 49.Sanaka MR, Gohel T, Podugu A, et al. Adenoma and sessile serrated polyp detection rates: variation by patient sex and colonic segment but not specialty of the endoscopist. Dis Colon Rectum. 2014;57:1113–1119. doi: 10.1097/DCR.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 50.Gandhi K, Tofani C, Sokach C, Patel D, Kastenberg D, Daskalakis C. Patient characteristics associated with quality of colonoscopy preparation: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2017.08.016. Published ahead of print on August 18, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.