Abstract
Vaccinia virus (VACV) A14 is a major envelope protein and a dominant antibody target in the smallpox vaccine. However, the role of anti-A14 antibodies in immunity against orthopoxviruses is unclear. Here, we characterized 22 A14 monoclonal antibodies (mAb) from two mice immunized with VACV. Epitope mapping showed that 21 mAbs targeted the C-terminal hydrophilic region, while one mAb recognized the middle region predicted to be across the viral envelope from the C-terminus. However, none of the mAbs bound to virions in studies with electron microscopy. Interestingly, some mAbs showed low VACV neutralization activities in the presence of complement and provided protection to SCID mice challenged with VACV ACAM2000. Our data showed that, although A14 is an immunodominant antigen in smallpox vaccine, its B cell epitopes are either enclosed within the virions or are inaccessible on virion surface. Anti-A14 antibodies, however, could contribute to protection against VACV through a complement-dependent pathway.
Keywords: smallpox, vaccinia, antibody, A14, immunization, epitope, neutralization
Graphical abstract
Introduction
Smallpox was once a deadly disease afflicting millions of people before being eradicated through strategies that included immunization with live vaccinia virus (VACV), an orthopoxvirus closely related to variola virus (Moss, 2007). The cession of routine smallpox vaccination following the eradication led to a population that is largely immune naïve to orthopoxviruses, some of which still cause zoonotic infections in humans (Shchelkunov, 2013). Monkeypox virus (Parker et al., 2007), previously found only in Africa, caused a brief outbreak in the U.S. in 2003 (Reed et al., 2004). Cowpox virus and vaccinia virus have been reported to cause infection of domesticated animals and their human handlers in Europe, South America and the Indian subcontinent (Essbauer et al., 2010; Megid et al., 2012; Singh et al., 2012; Trindade et al., 2007).
Despite the success of VACV as the smallpox vaccine, the immunological basis of smallpox vaccine has only been studied in recent years with modern biology. In animal models and human vaccinees, neutralizing antibodies have been shown to play an essential role in protection against orthopoxvirus infection (Belyakov et al., 2003; Hopkins and Lane, 2004). VACV produces two antigenically different forms of virions (Condit et al., 2006; Moss, 2007; Smith et al., 2002), and antibodies against both virion forms are required for optimal protection against orthopoxviruses (Lustig et al., 2005). The intracellular mature virions (MVs) stay within the cells until cell lysis, while the extracellular enveloped viruses (EVs) exit the cells via exocytosis (Smith et al., 2002). MVs have an envelope embedded with more than 20 viral proteins, while EVs have an additional envelope with at least six viral proteins. VACV B5 is the major target of neutralizing antibodies against EV (Bell et al., 2004; Benhnia et al., 2009; Putz et al., 2006), as depletion of anti-B5 antibodies from sera of vaccinated individuals greatly reduced neutralization of EVs (Bell et al., 2004; Putz et al., 2006). In contrast, no single protein has been found to be the dominant MV-neutralizing target. Neutralizing antibody levels in at least subsets of vaccinated individuals correlate with human IgG responses to several MV proteins, including H3, A27, D8, L1 and A14 (Benhnia et al., 2008). H3 (Davies et al., 2005), A27 (Kaever et al., 2016; Rodriguez et al., 1985), D8 (Hsiao et al., 1999) and L1 (Ichihashi and Oie, 1996; Wolffe et al., 1995) are known to be the targets of MV-neutralizing antibodies, but whether A14 is a neutralizing target is unknown. Depletion of individual or a combination of the major MV-neutralizing antibodies from sera of the vaccinees did not significantly reduce neutralization of MV (Aldaz-Carroll et al., 2005; Benhnia et al., 2008; He et al., 2007), indicating that additional candidates such as A14 warrant further testing.
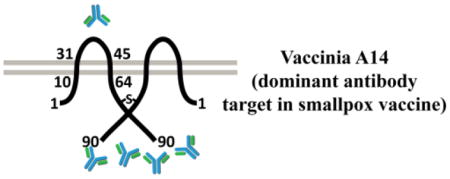
A14 is a major MV envelope protein and plays an essential role in viral assembly (Rodriguez et al., 1998; Salmons et al., 1997). It is a small protein of only 90 amino acids (aa) and predicted to have two transmembrane domains (residues 13-31 and 45-64) that are separated by a 13-aa hydrophilic loop (residues 32-44) (Mercer and Traktman, 2003) (Fig. 1C). The orientation of A14 protein in respect to MV envelope is unclear. The formation of an intermolecular disulfide bond involving a cysteine near the C-terminus suggested that the C-terminus was internal to the virion envelope (Mercer and Traktman, 2003). However, an opposite orientation of A14 was suggested by a more recent model of virion assembly (Maruri-Avidal et al., 2013; Weisberg et al., 2017), which involves the budding of ER membranes into ER lumen.
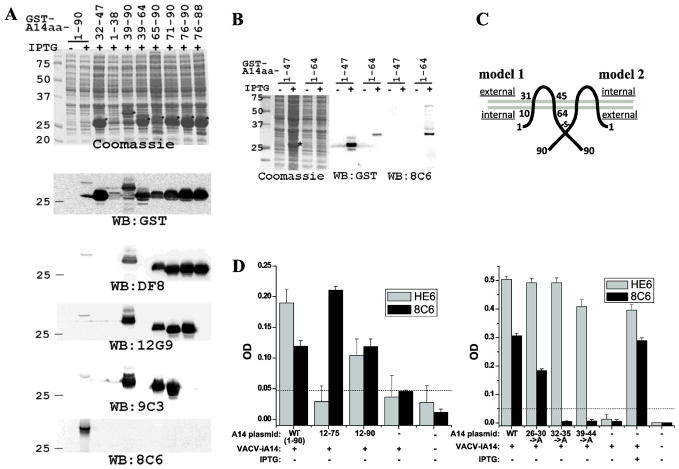
Figure 1. Mapping the epitopes of A14 mAbs. A and B).
Mapping the epitopes by Western blot of GST-A14 proteins. E. coli strains were either not induced (−) or induced with IPTG (+) to express GST fusion protein with the indicated A14 fragments. Proteins from the whole cell lysates were resolved by SDS-PAGE and analyzed by either Coomassie staining or by Western blot with the indicated antibodies. Prominent protein bands that are only present in induced samples are marked with *. Only Western blots with representative antibodies are shown. C). Predicted topology of A14 on MV with two possible orientations. The two grey lines represent the viral envelope, and the dark lines represent an A14 dimer. The amino acid residue numbers are indicated. The “-S-” denotes the disulfide bond via Cys71. The internal and external side of the virion are indicated in the two models. D). Further define the 8C6 epitope by ELISA of A14 mutants. 293T cells were transfected with plasmids encoding A14 alleles under the control of a VACV promoter and subsequently infected with an IPTG-inducible A14 mutant VACV (VACV-iA14) either in the absence of IPTG. The cell lysates were used to coat a microtiter plate, and ELISA was performed with either 8C6 or HE6. The A14 plasmids are named after the A14 residues expressed (12-75, 12-90) or A14 residues substituted with alanines (26–30->A, 32–35->A, 39–44->A).
In this study, we isolated 22 anti-A14 monoclonal antibodies (mAb) from two mice that had been infected with VACV. We characterized this large panel of mAbs in terms of their epitope, antibody sequence, in vitro MV neutralization and in vivo protection efficacy.
Material and Methods
Hybridoma generation and characterization
The A14 antibodies were developed in two batches. The generation of the first batch of A14 antibodies has been described (Meng et al., 2011). The second batch of A14 antibodies were generated similarly. Briefly, a BALB/c mouse was infected intranasally with 5 x 103 plaque-forming-unit (PFU) of VACV WR. Seven weeks after the infection, the mouse was injected intravenously with 7 x 107 PFU of UV-inactivated WR virus. Three days afterwards, the spleen of the mouse was harvested for hybridoma generation. The hybridomas secreting anti-VACV antibodies were identified with an immunofluorescence assay of VACV-infected cells as described (Meng et al., 2011). Those that are positive for anti-A14 were identified based on both immunoprecipitation and immunofluorescence results as described (Meng et al., 2011). Anti-H3 mAb #41 (McCausland et al., 2010), anti-L1 mAb M12B9 (Kaever et al., 2014), and anti-A10 mAb BG3 (Meng et al., 2011) have been described.
Antibody production and purification
Hybridoma cells were cultured with hybridoma serum-free medium (Invitrogen) supplemented with OPI Media Supplement (sigma-aldrich). Antibodies were purified from the conditioned culture media with a HiTrap Protein G-Sepharose column (GE Healthcare Life Sciences). The antibodies are in PBS after going through a PD-10 desalting column (GE Healthcare Life Sciences).
Epitope mapping with Western blot
The plasmids for expressing the fusion of Glutathione S-transferase (GST) and A14 were constructed by PCR-amplifying the viral gene from VACV WR DNA and cloning the PCR fragment into pGEX6P-1 (GE Healthcare Life Sciences). The expression of the fusion protein in E. coli BL21 strain was induced with isopropyl-beta-D-thiogalactoside (IPTG; Invitrogen). The bacteria were lysed via sonication in SDS-PAGE sample buffer, and the clarified cell lysates were directly used in Western blot analysis.
Epitope mapping with ELISA of infected cells
Plasmids containing mutant alleles of the A14 gene under the control of a VACV promoter were described previously (Mercer and Traktman, 2003). The plasmids were transfected into 293T cells with Lipofectamine 2000 (Invitrogen). 6 hours later, the cells were infected with iA14/GFP (Wu et al., 2012), an ITPG-inducible A14 mutant VACV, at an MOI of 5 in the presence or absence of IPTG. Cells were harvested at 24 hpi, and the cell lysates were used to coat 96-well microtiter plates. ELISA were performed with 1 μg/ml anti-A14 antibodies as described previously (Kaever et al., 2014).
Antibody sequencing
Antibody sequencing was performed as described previously (Kaever et al., 2014). Briefly, total RNA from hybridoma cells was isolated, and cDNA was amplified using the OneStep RT-PCR kit (Qiagen). The reverse transcription PCR was performed using primers 5′MsVHE and 3′Cy1 (for isotype IgG1 antibodies), 3′Cy2c outer (for isotype IgG2a antibodies) or 3′Cy2b outer (for isotype IgG2b antibody) for the heavy chains, and primers 5′mVkappa and 3′mCκ for the kappa light chains (Tiller et al., 2009). PCR products were gel-purified and subsequently sequenced. Sequences include V-D-J regions for heavy chains and V-J regions for light chains. Finally, antibody germ lines were determined using IMGT’s V-Quest service (Brochet et al., 2008). The sequences of the antibodies have been submitted to GenBank (Access numbers from KP300823 to KP300866).
Immunogold EM
Sucrose-gradient purified virions were adsorbed to 200 nm carbon-coated nickel grids (EM Sciences) and blocked with 5% BSA. Grids were then incubated with various antibodies at 2 μg/ml for 1 h. After washing 3x with 0.1M Tris-Cl (pH 7.4), grids were incubated with secondary gold-conjugated antibodies (12 nm anti-mouse, JacksonImmuno) for 1 h. Grids were washed 3x with 0.1M Tris-Cl and then fixed with 4% paraformaldehyde/2% glutaraldehyde. Grids were stained with uranyl-acetate and visualized with a JEOL 1230 transmission electron microscope at the UTHSCSA Electron Microscopy lab.
MV neutralization assay
WR viruses used in neutralization and in vivo protection assay were purified successively through a sucrose cushion and a sucrose gradient step, according to the standard protocol (Earl et al., 1998). The viruses were mixed with PBS or purified antibodies for 1 h at 4°C (in a total volume of 1 ml). When complement is used in neutralization, baby rabbit complement (Cedarlane Laboratories) was added to the mixtures at a final concentration of 2%. For each neutralization condition, three independent mixtures were set up and inoculated onto monolayers of BS-C-1 cells in 6-well plate. After 1 h incubation at 37°C, the inoculum was replaced with 2 ml DMEM supplemented with 1% FBS and 0.5% (w/v) methylcellulose (Sigma-aldrich). The plates were incubated for 2 days at 37°C before being processed for plaque counting.
In vivo protection
Groups of six SCID mice were given half intraperitoneal (i.p.), half retro-orbital injection of a total of 100 μg antibodies or PBS in equivalent volume. One day later, the mice were retro-orbitally injected with 1 x 105 PFU of VACV ACAM2000. Mice were weighed in increments of 3 days to assess disease progression. The clinical score, a composite score of the pox lesion abundance on the four paws plus the tail, was evaluated as described previously (McCausland et al., 2010).
Results
Generation of anti-A14 mAbs
Previously, when we screened a large panel of B cell hybridomas derived from a mouse immunized with VACV, we found 17 out of 66 anti-VACV mAbs were against A14 (Meng et al., 2011). In a similar screen for additional anti-VACV mAbs, we identified another 7 anti-A14 mAbs. Notably, we used live VACV for immunization and screened hybridomas with immunofluorescence of infected cells, which did not favor any VACV antigen, so the spectrum of the monoclonal antibodies generated from both screens matched roughly the profile of polyclonal antibody responses in humans and animals immunized with the smallpox vaccine (Davies et al., 2007). As part of our efforts to map B cell epitopes in smallpox vaccine, we characterized all the A14 mAbs from the two screens except for one that failed the purification.
Mapping the epitopes of anti-A14 mAbs
All the A14 mAbs recognized the native A14 protein expressed by VACV, as they were positive against VACV-infected cells in immunofluorescence assay and specifically precipitated A14 from VACV-infected cells ((Meng et al., 2011) and data not shown). They were also found to recognize the recombinant, GST-A14 fusion protein in Western blot (Fig. 1A). We thus tried to map the epitopes of the A14 mAbs by performing Western blot on GST fusion proteins containing different A14 fragments. The results with all the A14 mAbs are summarized in Table 1, and representative results with selected antibodies were shown in Fig. 1. 21 out of the 22 mAbs recognized the 20-aa C-terminus (residues 71-90). Among them, 9C6 from the second screen did not recognize any smaller A14 protein fragment, while four mAbs from the second screen (3G6, 4C5, 12G9 and 12B4) recognized a 15-aa fragment (residues 76-90), and all 16 mAbs from the first screen (HE6, AD10, BF8, DF8, GH3, FE11, LE11, JG8, KD8, CE11, TH6, JE1, AD5, BB5, LB8, and BF9) recognized an even smaller, 13-aa fragment (residues 76-88). 8C6 from the second screen was the only mAb that did not recognize the C-terminus. Instead, it recognized residues 1-64 in Western blot (Fig. 1B). 8C6 did not recognize any smaller fragment within the first 64 residues, suggesting that its epitope is conformational.
Table 1.
Summary of Epitope Mapping by Western blot of GST-A14 fusion proteins
| A14 protein fragment | minimal linear epitope | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 1-90 | 39-90 | 65-90 | 71-90 | 76-90 | 76-88 | 1-64 | 1-38 | 1-47 | 39-64 | 32-47 | 32-64 | ||
| AD5 | + | + | + | + | + | + | − | − | − | − | − | − | |
| AD10 | + | + | + | + | + | + | − | − | − | − | − | − | |
| BF8 | + | + | + | + | + | + | − | − | − | − | − | − | |
| FE11 | + | + | + | + | + | + | − | − | − | − | − | − | |
| GH3 | + | + | + | + | + | + | − | − | − | − | − | − | 76-88 |
| JG8 | + | + | + | + | + | + | − | − | − | − | − | − | |
| JE1 | + | + | + | + | + | + | − | − | − | − | − | − | |
| LB8 | + | + | + | + | + | + | − | − | − | − | − | − | |
| CE11 | + | + | + | + | + | + | − | − | − | − | − | − | |
| DF8 | + | + | + | + | + | + | − | − | − | − | − | − | |
| KD8 | + | + | + | + | + | + | − | − | − | − | − | − | |
| LE11 | + | + | + | + | + | + | − | − | − | − | − | − | |
| TH6 | + | + | + | + | + | + | − | − | − | − | − | − | |
| BF9 | + | + | + | + | + | + | − | − | − | − | − | − | |
| HE6 | + | + | + | + | + | + | − | − | − | − | − | − | |
| BB5 | + | + | + | + | + | + | − | − | − | − | − | − | |
| 3G6 | + | + | + | + | + | − | − | − | − | − | − | − | |
| 4C5 | + | + | + | + | + | − | − | − | − | − | − | − | 76-90 |
| 12G9 | + | + | + | + | + | − | − | − | − | − | − | − | |
| 12B4 | + | + | + | + | + | − | − | − | − | − | − | − | |
| 9C3 | + | + | + | + | − | − | − | − | − | − | − | − | 71-90 |
| 8C6 | + | − | − | − | − | − | + | − | − | − | − | − | 1-64 |
To further define the epitope of 8C6, we expressed A14, wild type or mutant, in mammalian cells in the context of VACV infection, and tested 8C6 binding of infected cells by ELISA and immunofluorescence. Plasmids encoding different A14 alleles, regulated by a VACV promoter, were transfected into cells, which were subsequently infected with an A14 inducible VACV mutant in the absence of the inducer. The cells were then processed for immunofluorescence or ELISA with the A14 mAbs. Since the results from immunofluorescence and ELISA are similar, only the relatively quantitative ELISA results were shown in Fig. 1D. 8C6 was positive for A14 of residues 12-90, and the signal was very similar to that for the full-length A14 (Fig. 1D, left), indicating that the N-terminal 11 residues were not part of 8C6 epitope. Alanine substitution of residues 26-30 reduced but did not abolish binding with 8C6. In contrast, alanine substitution of residues 32-35 or 39-44 abolished binding with 8C6 (Fig. 1D, right). These A14 mutants were expressed at similar level in the cells as they were equally recognized by HE6, whose epitope lies outside the mutated regions. Altogether, the ELISA results and the Western blot results indicated that the epitope for 8C6 is present in residues 12-64 and that the hydrophilic loop (residues 32-44) between the two transmembrane domains is part of the epitope.
The dominant A14 epitopes at the C-terminus are recognized by multiple antibody groups
It is interesting that all but one of the 22 mAbs recognized the C terminus (residues 71-90). To find out whether these antibodies are closely related, we determined the sequences of the A14 mAbs (Table II and III). Comparison of the antibody sequences showed that the 16 mAbs that recognized the minimal linear epitope of 76-88 could be divided into 4 groups based on their heavy chain (HC) sequence (groups A, B, C and D). Two of the four groups (A&B) shared a very similar light chain (LC, group a). Antibodies within the same group have either identical sequence or a small number of substitutions in the CDR regions, indicating that they are derived from the same B cell lineage. Antibodies from different groups had divergent sequences, especially in their HC CDR3. The 4 mAbs that recognized the minimal linear epitope of 76-90 belongs to 2 groups with different HC (group E, F) and LC (group e, d). Among them, 12B4 has a LC that is similar to that of BB5 (LC group d). 9C3, the mAb that recognizes the minimal linear epitope of 71-90, belongs to a unique group. 8C6, the only mAb that recognizes an epitope outside residues 71-90, has a unique HC, but has a LC that is similar to that of group e. Altogether, the data showed that a diverse group of antibodies recognized epitopes present in the C-terminal 20-aa (residue 71-90).
Table 2.
Summary of antibody sequencing
| HC | LC | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CDR1 | CDR2 | CDR3 | Group | CDR1 | CDR2 | CDR3 | Group | |
| AD5 | GYSFTNYY | IDPFNGNT | VPHFDV | ODIGSF | ATS | LQYASSPYT | ||
| AD10 | GYSFTNYY | IDPFNGNT | VPHFDV | ODIGSF | NTT | QQHDSSPYT | ||
| BF8 | GYSFTNYY | IDPFNGNT | VPHFDV | ODIGSF | ATS | LQYASSPYT | ||
| FE11 | GYSFTNYY | IDPFNGNT | VPHFDV | A | ODIGSF | ATS | LQYASSPYT | |
| GH3 | GYSFTNYY | IDPFNGNT | VPHFDV | ODIGSF | ATS | LQYASSPYT | ||
| JG8 | GYSFTNYY | IDPFNGGT | VPHFHV | ODIGYN | ATS | LQYASSPYT | ||
| JE1 | GYSFPTYY | IDPFNGGT | VPHFHV | ODIGSS | ATS | LQYATSPYT | a | |
| LB8 | GYSFPTYY | IDPFNGGT | VPHFHV | ODIGGH | ATS | LQYATSPYT | ||
| CE11 | GYTFTSYW | IYPGSGIT | TGYFDV | ODIGNN | ATS | LQYATSPYT | ||
| DF8 | GYTFTSYW | IYPGSGTT | TGYFDV | ODIGNN | ATS | LQYATSPYT | ||
| KD8 | GYTFTSYW | IYPGSGIT | TGYFDV | B | ODIGNS | ATS | LQYASSPYT | |
| LE11 | GYTFTSYW | IYPGSGTT | TGYFDV | ODIGTS | ATS | LQYASSPYT | ||
| TH6 | GYTFTSYW | IYPGSGTT | TGYFDV | ODIGNN | ATS | LQYATSPYT | ||
| BF9 | GYTFTSYY | IYPGDGYT | VRQLGAH | C | PSLLAGDGKTY | LVS | WQGTHFPRT | c |
| HE6 | GYTFTSYY | IYPGDGVT | VRQLGAH | PSLLAGDGKTY | LVS | WQGTHFPRT | ||
| BB5 | GYSFTSNY | IFPGSGKT | ARMAYYGRDYDAMDY | D | QSVLYSSNQKNY | WSS | HQYLSSWT | d |
| 3G6 | GFTFNIYA | IRNKTTNYAT | VRRRSIMIAGFAY | STVRY | DTS | QQWSSYPLT | ||
| 4C5 | GFTFNIYA | IRNKTTNYAT | VRRRSIMIAGFAY | E | SSVRY | DTS | QQWSSYPLT | e |
| 12G9 | GFTFNTYA | IRTKSNNYAT | VRRRSIMIAGFAY | SSIRY | DTS | QQWTSYPLT | ||
| 12B4 | GYTFTDYN | VNPKFDTT | ARMRRGAFYFDY | F | QSVLYSSDQKNY | WAS | HQYLSSFT | d |
| 9C3 | GYTFTSYI | VDPYNDGI | ARDGYALDY | G | KSVSTSGYSY | LAS | QHSRDLPPT | g |
| 8C6 | GYSFTGYN | IDPFFGGT | ARQGGYFLAMDY | H | SSVSY | DTS | QQWSSFPPT | e |
Residues that are varied between members of the same group are underlined.
Table 3.
Summary of antibody characterization
| isotype | Minimal linear epitope | Ab sequences | Protection against VACVb | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HC | LC | |||||
| Mouse #1 | AD5 | 2b | ||||
| AD10 | 3 | |||||
| BF8 | 1 | − | ||||
| FE11 | 2b | A | ||||
| GH3 | 3 | |||||
| JG8 | 3 | |||||
| JE1 | 3 | a | ||||
| LB8 | 3 | 76-88 | ||||
| CE11 | 3 | |||||
| DF8 | 3 | |||||
| KD8 | 3 | B | ||||
| LE11 | 3 | |||||
| TH6 | 3 | |||||
| BF9 | 2b | C | c | |||
| HE6 | 2b | ++ | ||||
| BB5 | 3 | D | d | |||
| 3G6 | 2b | |||||
| 4C5 | 3 | 76-90 | E | e | ||
| Mouse #2 | 12G9 | 3 | ||||
| 12B4 | 3 | F | d | |||
| 9C3 | 2a | 71-90 | G | g | ++ | |
| 8C6 | 2a | ~12-64 | H | e | + | |
++, similar to positive control (anti-H3 #41); −, similar to negative control (anti-A10, BG3); +, intermediate between positive and negative
A14 epitopes of the mAbs are inaccessible on MV
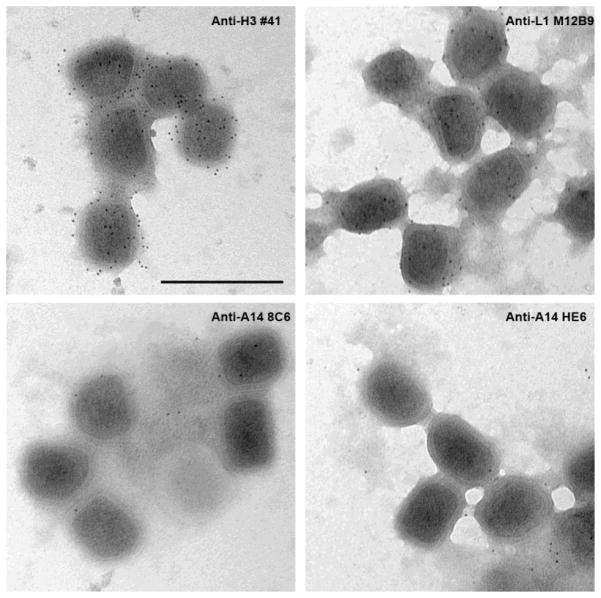
As antibodies neutralize virus predominantly by binding epitopes exposed on virion surface, we then tested whether any of the A14 epitopes are exposed on MV by performing electron microscopy analysis of purified MV following antibody staining. Antibodies against MV envelope protein H3 (mAb #41) (McCausland et al., 2010) and L1 (M12B9) (Kaever et al., 2014) were used as the positive control, while antibody against the internal core protein A10 (BG3) (Meng et al., 2011) was used as the negative control. There was no staining of MV by anti-A10 (data not shown), but both anti-H3 and anti-L1 consistently stained MV envelopes (Fig. 2). There were generally more gold particles from staining with the anti-H3 than with the anti-L1 antibodies, consistent with H3 being a major virion membrane protein. Surprisingly, although 8C6 and HE6 presumably target the opposite sides of the viral envelope, neither antibodies stained MV envelope significantly, indicating that their epitopes are either internal or inaccessible on virion surfaces. The latter possibility may due to the presence of other viral proteins on virion envelope that interfere with antibody bindings to the epitopes.
Figure 2. Immunogold staining of purified VACV MV with anti-VACV antibodies.
Sucrose-gradient purified virions were incubated with the indicated antibodies at 2 μg/ml followed by incubation with secondary gold-conjugated antibodies. The virions were stained with uranyl-acetate and visualized with an electron microscope.
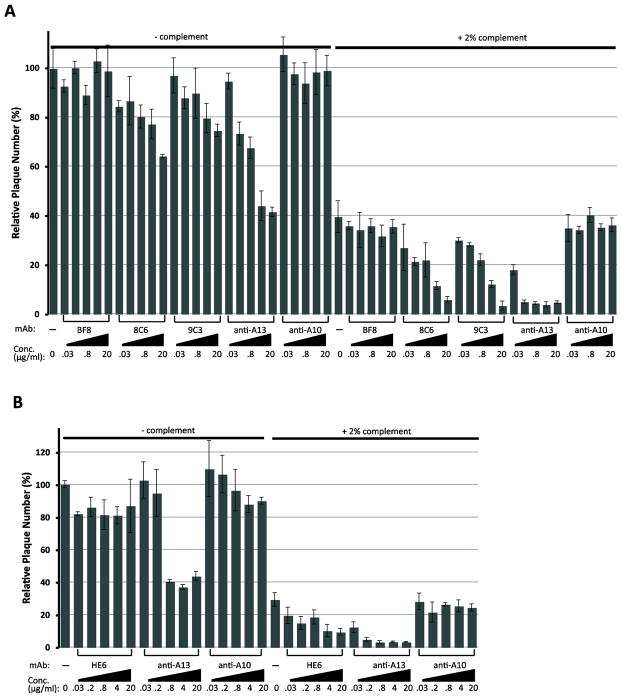
Anti-A14 mAbs showed low MV neutralization activities in the presence of complement
To test whether anti-A14 could neutralize MV, we performed plaque reduction assay with representative A14 mAbs (Fig. 3). A MV-neutralizing mAb (anti-A13 11F7) and a non-neutralizing mAb (anti-A10 BG3) were included as the positive and negative control, respectively. BF8 (against 76-88, IgG1) did not reduce plaque number either in the absence or presence of 2% rabbit complement, similar to the negative control BG3 (Fig. 3). Only at high antibody concentrations (20 μg/ml), 8C6 (against 12-64, IgG2a) and 9C3 (against 71-90, IgG2) reduced plaque number by ~40% and 20%, respectively (Fig. 3A). Their neutralization efficiency is low compared to the positive control anti-A13 mAb, which could neutralize 60% of MV at a lower antibody concentration (5 μg/ml). Complement (2%) alone reduced the plaque number by ~60%, and the addition of 8C6 or 9C3 further reduced the plaque number by another 30–40%. Again, this compared unfavorably with the anti-A13 mAb, which achieved the same neutralizing effect at a much lower antibody concentration. In a separate experiment (Fig. 3B), HE6 (against 76-88, IgG2b) also showed a small neutralization effect in the presence of 2% complement. Altogether, these data showed that high concentrations of A14 mAbs of the complement fixing isotypes had a small neutralization effect in the presence of complement.
Figure 3. Neutralization of VACV MV by A14 mAbs.
Sucrose-gradient purified VACV mature virions were incubated with the indicated concentration of purified antibodies in the presence or absence of rabbit complement for 1 hr at 4°C. The mixture was then added to monolayers of BS-C-1 cells, and the inoculum was removed after one hour. The number of plaques that appeared after 2 days was enumerated. The number of plaques obtained under the indicated condition as the percentage to the number of plaques from untreated inoculums is shown. The average and standard deviation are from three independent inoculums.
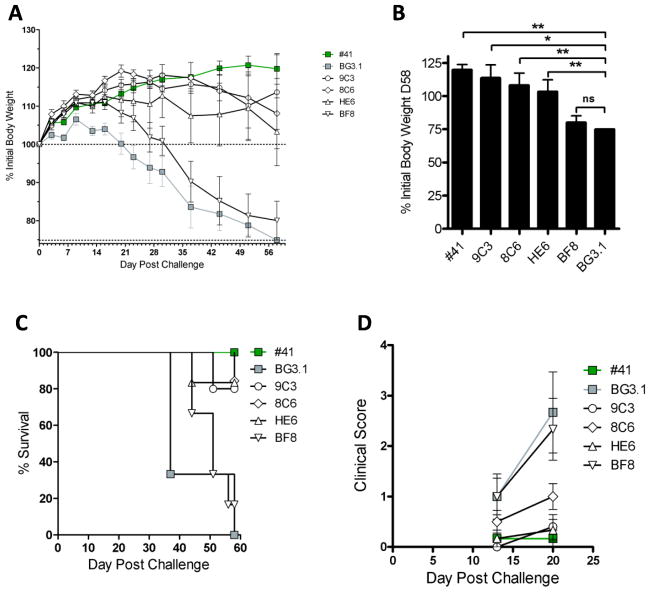
Anti-A14 mAbs showed some protection against in vivo vaccinia virus challenge
To see whether the small neutralization effects of the antibodies could translate into in vivo protection, we tested representative A14 mAbs for their ability to protect SCID mice against the challenge of a lethal dose (105 PFU) of VACV ACAM2000. Groups of six SCID mice were given by the intraperitoneal (i.p.) and retro-orbital route purified antibodies and challenged subsequently with 105 PFU ACAM2000 retro-orbitally. Anti-A10 mAb BG3 was used as a negative control, and anti-H3 #41 was used as a positive control. As expected, anti-H3 #41 provided substantial protection against disease, as the treated mice did not suffer weight loss, having 100% survival, and fewer clinical lesions. In this model, 9C3 and HE6 each provided protection against weight loss (p=0.0150 and p=0.0096 at day 58, respectively) (Fig. 4A&B), death (p=0.0016 and p=0.0005 respectively) (Figure 4C), and pox lesion at day 20 (p=0.0181 and p=0.0099 respectively) (Figure 4D). 8C6 had a more modest efficacy (p=0.0096 weight loss, p=0.0005 death and p=0.0704 pox). BF8 had no significant efficacy. The result was largely consistent with in intro neutralization result with the exception that the efficacy of 8C6 in in vivo was worse than what we had predicted from in vitro neutralization result.
Figure 4. Protection of SCID mice from intravenous VACV challenge by anti-A14 mAbs.
Groups of six SCID mice were treated with either 400 μL PBS or 400 μL of the indicated antibodies at a concentration of 0.25 mg/mL (=100 μg) on day −1. PBS/antibodies were injected at 200 μL intraperitoneal and 200 μL retro-orbitally. Then, mice were challenged with 1 x 105 PFU (200 μL) of VACV ACAM2000 by the retro-orbital route on day 0. (A) Body weights. (B) Body weight loss at day 58. (C) Survival. (D) Clinical scores over time.
Discussion
A14 is one of the dominant antibody targets in smallpox vaccine (Davies et al., 2007), but no B cell epitope has previously been identified in A14. We showed in this study that at least two regions of A14 are targets of antibody responses, with the C-terminus being the dominant one. A14 is a small protein predicted to have two transmembrane domains, leaving three hydrophilic regions as potentially accessibly to antibodies. They are the 12-aa N-terminus (residues 1-12), the 26-aa C-terminus (residues 65-90) and the 13-aa middle region (residues 32-44). Relative to this small number of potential antibody binding sites, we have isolated a large number of mAbs that presumably cover the majority of the potential epitopes. While the process of mAb generation could be stochastic, an overwhelming number of antibodies from two separate screens all target the C-terminus, indicating that the C-terminus is the dominant target for antibody responses. The 13-aa middle region is targeted by one antibody out of the 22 mAbs, indicating that it is also an antibody target but a relatively minor one at least in mice.
Although A14 is a dominant antibody target, whether it could serve as a neutralizing target was previously unknown. A14 is a major virion membrane protein, representing the fourth most abundant MV protein by molar concentration (8% of MV) (Chung et al., 2006). However, we showed in this study that A14 is an inherently poor neutralizing target due to a lack of antibody binding sites in A14 on virion surface. The presence of two transmembrane domains in A14 dictates that either the middle region (residues 32-44) or the N- and C-termini are protruded out of MV. However, EM studies showed that both the antibodies against the middle region (8C6) and the C-terminus did not bind to MV. Besides the C-terminus and the middle region, another possible antibody target on A14 is the N-terminal 12-aa. Since the N-terminus is predicted to be on the same side of virion membrane as the C-terminus, antibodies against the N-terminal 12-aa should behave similarly as antibodies against the C-terminal 20-aa in terms of their virion binding and neutralizing abilities. Altogether, our studies suggest that all possible antibody targets on A14 are either enclosed within virions or largely inaccessible for antibody binding on virion surface, suggesting that A14 is an inherently poor target for neutralizing antibodies.
All our anti-A14 mAbs demonstrated no or very poor MV-neutralizing activities in the absence of complement, consistent with the lack of antibody binding sites in A14 on virion surface. In the presence of complement, both 8C6 and antibodies against the C-terminus showed some MV-neutralizing activities at high antibody concentrations. It is possible that complement may breach the integrity of the virions and make the epitopes more accessible for antibody binding. An antibody of the complement-fixing isotype is required for the neutralization, as BF8 (IgG1) did not neutralize MV in the presence of complement. This is consistent with the findings that the majority of MV-neutralizing antibodies require complement for efficient neutralization. In our previous studies of protective effects of various MV-neutralizing antibodies, we have used both intravenous challenge of SCID mice and intranasal challenge of immunocompetent mice and found the former challenge model to be much more MV dependent (Kaever et al., 2014; McCausland et al., 2010; Xu et al., 2011). While the most potent MV-neutralizing antibodies, anti-L1, could only protect against death against intranasal challenge, they could protect against weight loss, pox lesion as well as death in the SCID mouse model (Kaever et al., 2014). Therefore, we chose the SCID mouse challenge as the more sensitive model for assessing the protective effect of anti-A14 antibodies. In this model, anti-A14 antibodies of the complement-fixing isotype protected against death, weight loss and pox lesion caused by ACAM2000. The protective effect largely correlates with the complement-mediated neutralization of MV. We do not think NK cell-mediated antibody-dependent cellular cytotoxicity played a role in the protection, as VACV MV membrane proteins such as A14 are rarely found on the surface of infected cells. 8C6, which showed a slightly better in vitro neutralization than 9C3, provided a poorer protection than 9C3. This may due to a difference in pharmacokinetics of the antibodies in vivo.
Highlights.
Vaccinia virus A14 is a dominant antibody target in the smallpox vaccine, but whether it is a neutralizing target is unknown.
22 anti-A14 monoclonal antibodies from vaccinia-immunized mice were characterized as to their epitopes and neutralization abilities.
A14 epitopes are either enclosed within the virions or are inaccessible on virion surface.
Anti-A14 antibodies could contribute to protection against infection through a complement-dependent pathway.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200900048C and grant AI079217. We thank Christopher Lao for his initial contribution in in vivo protection studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Pannell LK, Lebowitz J, Fogg C, White CL, Moss B, Cohen GH, Eisenberg RJ. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005;341:59–71. doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Bell E, Shamim M, Whitbeck JC, Sfyroera G, Lambris JD, Isaacs SN. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325:425–431. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. PNAS. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia MR, McCausland MM, Moyron J, Laudenslager J, Granger S, Rickert S, Koriazova L, Kubo R, Kato S, Crotty S. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J Virol. 2009;83:1201–1215. doi: 10.1128/JVI.01797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia MR, McCausland MM, Su HP, Singh K, Hoffmann J, Davies DH, Felgner PL, Head S, Sette A, Garboczi DN, Crotty S. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J Virol. 2008;82:3751–3768. doi: 10.1128/JVI.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, Chen CH, Ho MY, Huang CY, Liao CL, Chang W. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J Virol. 2006;80:2127–2140. doi: 10.1128/JVI.80.5.2127-2140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79:11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DH, Molina DM, Wrammert J, Miller J, Hirst S, Mu Y, Pablo J, Unal B, Nakajima-Sasaki R, Liang X, Crotty S, Karem KL, Damon IK, Ahmed R, Villarreal L, Felgner PL. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678–1686. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of Recombinant Vaccinia Viruses. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. John Wiley & Sons, Inc; 1998. pp. 16.17.11–16.17.19. [Google Scholar]
- Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010;140:229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Manischewitz J, Meseda CA, Merchlinsky M, Vassell RA, Sirota L, Berkower I, Golding H, Weiss CD. Antibodies to the A27 protein of vaccinia virus neutralize and protect against infection but represent a minor component of Dryvax vaccine--induced immunity. J Infect Dis. 2007;196:1026–1032. doi: 10.1086/520936. [DOI] [PubMed] [Google Scholar]
- Hopkins RJ, Lane JM. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39:819–826. doi: 10.1086/422999. [DOI] [PubMed] [Google Scholar]
- Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J Virol. 1999;73:8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y, Oie M. Neutralizing epitope on penetration protein of vaccinia virus. Virology. 1996;220:491–494. doi: 10.1006/viro.1996.0337. [DOI] [PubMed] [Google Scholar]
- Kaever T, Matho MH, Meng X, Crickard L, Schlossman A, Xiang Y, Crotty S, Peters B, Zajonc DM. Linear Epitopes in Vaccinia Virus A27 Are Targets of Protective Antibodies Induced by Vaccination against Smallpox. J Virol. 2016;90:4334–4345. doi: 10.1128/JVI.02878-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaever T, Meng X, Matho MH, Schlossman A, Li S, Sela-Culang I, Ofran Y, Buller M, Crump RW, Parker S, Frazier A, Crotty S, Zajonc DM, Peters B, Xiang Y. Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in l1 protein. Journal of virology. 2014;88:11339–11355. doi: 10.1128/JVI.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol. 2005;79:13454–13462. doi: 10.1128/JVI.79.21.13454-13462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruri-Avidal L, Weisberg AS, Moss B. Direct formation of vaccinia virus membranes from the endoplasmic reticulum in the absence of the newly characterized L2-interacting protein A30.5. Journal of virology. 2013;87:12313–12326. doi: 10.1128/JVI.02137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCausland MM, Benhnia MR, Crickard L, Laudenslager J, Granger SW, Tahara T, Kubo R, Koriazova L, Kato S, Crotty S. Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model. Antivir Ther. 2010;15:661–675. doi: 10.3851/IMP1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megid J, Borges IA, Abrahao JS, Trindade GS, Appolinario CM, Ribeiro MG, Allendorf SD, Antunes JM, Silva-Fernandes AT, Kroon EG. Vaccinia virus zoonotic infection, Sao Paulo State, Brazil. Emerging infectious diseases. 2012;18:189–191. doi: 10.3201/eid1801.110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhong Y, Embry A, Yan B, Lu S, Zhong G, Xiang Y. Generation and characterization of a large panel of murine monoclonal antibodies against vaccinia virus. Virology. 2011;409:271–279. doi: 10.1016/j.virol.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Traktman P. Investigation of structural and functional motifs within the vaccinia virus A14 phosphoprotein, an essential component of the virion membrane. J Virol. 2003;77:8857–8871. doi: 10.1128/JVI.77.16.8857-8871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2905–2946. [Google Scholar]
- Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- Putz MM, Midgley CM, Law M, Smith GL. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nat Med. 2006;12:1310–1315. doi: 10.1038/nm1457. [DOI] [PubMed] [Google Scholar]
- Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, Swain GR, Olson VA, Sargent EK, Kehl SC, Frace MA, Kline R, Foldy SL, Davis JP, Damon IK. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Rodriguez JF, Janeczko R, Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985;56:482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JR, Risco C, Carrascosa JL, Esteban M, Rodriguez D. Vaccinia virus 15-kilodalton (A14L) protein is essential for assembly and attachment of viral crescents to virosomes. J Virol. 1998;72:1287–1296. doi: 10.1128/jvi.72.2.1287-1296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons T, Kuhn A, Wylie F, Schleich S, Rodriguez JR, Rodriguez D, Esteban M, Griffiths G, Locker JK. Vaccinia virus membrane proteins p8 and p16 are cotranslationally inserted into the rough endoplasmic reticulum and retained in the intermediate compartment. J Virol. 1997;71:7404–7420. doi: 10.1128/jvi.71.10.7404-7420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchelkunov SN. An increasing danger of zoonotic orthopoxvirus infections. PLoS pathogens. 2013;9:e1003756. doi: 10.1371/journal.ppat.1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Balamurugan V, Bhanuprakash V, Venkatesan G, Hosamani M. Emergence and reemergence of vaccinia-like viruses: global scenario and perspectives. Indian J Virol. 2012;23:1–11. doi: 10.1007/s13337-012-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Tiller T, Busse CE, Wardemann H. Cloning and expression of murine Ig genes from single B cells. Journal of immunological methods. 2009;350:183–193. doi: 10.1016/j.jim.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Trindade GS, Emerson GL, Carroll DS, Kroon EG, Damon IK. Brazilian vaccinia viruses and their origins. Emerging infectious diseases. 2007;13:965–972. doi: 10.3201/eid1307.061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg AS, Maruri-Avidal L, Bisht H, Hansen BT, Schwartz CL, Fischer ER, Meng X, Xiang Y, Moss B. Enigmatic origin of the poxvirus membrane from the endoplasmic reticulum shown by 3D imaging of vaccinia virus assembly mutants. Proc Natl Acad Sci U S A. 2017;114:E11001–E11009. doi: 10.1073/pnas.1716255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe EJ, Vijaya S, Moss B. A Myristylated Membrane Protein Encoded by the Vaccinia Virus L1R Open Reading Frame Is the Target of Potent Neutralizing Monoclonal Antibodies. Virology. 1995;211:53–63. doi: 10.1006/viro.1995.1378. [DOI] [PubMed] [Google Scholar]
- Wu X, Meng X, Yan B, Rose L, Deng J, Xiang Y. Vaccinia virus virion membrane biogenesis protein A11 associates with viral membranes in a manner that requires the expression of another membrane biogenesis protein, A6. Journal of virology. 2012;86:11276–11286. doi: 10.1128/JVI.01502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Meng X, Yan B, Crotty S, Deng J, Xiang Y. An epitope conserved in orthopoxvirus A13 envelope protein is the target of neutralizing and protective antibodies. Virology. 2011;418:67–73. doi: 10.1016/j.virol.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]