Abstract
Objective
Previous studies have shown that both pre- and post-natal adversities, the latter including exposures to stress during childhood and adolescence, explain variations in structural properties of white matter (WM) in the brain. While previous studies have examined effects of independent stress exposures within one developmental period, such as childhood, we examine effects of stress across development using data from a prospective longitudinal study. More specifically, we ask how stressful events during prenatal development, childhood, and adolescence relate to variation in WM properties in early adulthood in men recruited from a birth cohort.
Method
Using data from 393 mother-son pairs from a community-based birth cohort from England (Avon Longitudinal Study of Parents and Children), we examined how stressful life events relate to variation in different structural properties of WM in the corpus callosum and across the whole brain in early adulthood in men aged 18–21 years. We distinguish between stress occurring during three developmental periods: a) prenatal maternal stress, b) postnatal stress within the first four years of life, c) stress during adolescence (age 12–16 years). To obtain a comprehensive quantification of variation in WM, we assess structural properties of WM using four different measures, namely fractional anisotropy (FA), mean diffusivity (MD), magnetization transfer ratio (MTR) and myelin water fraction (MWF).
Results
The developmental model shows that prenatal stress is associated with lower MTR and MWF in the genu and/or splenium of the corpus callosum, and with lower MTR in global (lobar) WM. Stress during early childhood is associated with higher MTR in the splenium, and stress during adolescence is associated with higher MTR in the genu and lower MD in the splenium. We see no associations between postnatal stress and variation in global (lobar) WM.
Conclusions
The current study found evidence for independent effects of stress on WM properties during distinct neurodevelopmental periods. We speculate that these independent effects are due to differences in the developmental processes unfolding at different developmental time points. We suggest that associations between prenatal stress and WM properties may relate to abnormalities in neurogenesis, affecting the number and density of axons, while postnatal stress may interfere with processes related to myelination or radial growth of axons. Potential consequences of prenatal glucocorticoid exposure should be considered in obstetric care.
Suggested keywords: Adversity, stress, white matter, DTI, MTR, MWF
Previous neuroimaging studies have documented differences in structural properties of white matter (WM) related to adverse experiences such as of prenatal stress (Sarkar et al., 2014), stressful life events during childhood (Paul et al., 2008), childhood neglect (J L Hanson et al., 2013), maltreatment (Choi, Jeong, Polcari, Rohan, & Teicher, 2012; W. L. Huang, Harper, Evans, Newnham, & Dunlop, 2001; Jackowski et al., 2008), institutionalization (Bick et al., 2015; Eluvathingal et al., 2006) and exposure to inter-parental verbal aggression during late childhood and early adolescence (Choi, Jeong, Rohan, Polcari, & Teicher, 2009; Teicher, Samson, Sheu, Polcari, & McGreenery, 2010). White matter accounts for approximately 38% of the total brain volume and is composed mostly of myelinated axons (Leonard et al., 2008; Paus, Pesaresi, & French, 2014). Axons constitute the “highway” for information transfer within the brain via electrochemical signaling and transport of cellular components between the neuronal cell body and synapses (Paus et al., 2014). Myelination begins during late stages of fetal development and continues well into early adulthood (Dean et al., 2014; Lebel et al., 2012). Structural properties of WM are influenced by various neurobiological features including the number of axons, their diameter, and the thickness of the myelin sheath (Paus, 2010). These features are shaped by different neurodevelopmental processes that are guided by a combination of genetic influences, as well as spontaneous electrochemical activity of neurons, which, in turn, may be impacted by experiences (Ben-Ari & Spitzer, 2010). Different structural properties of WM can be assessed using different neuroimaging techniques. T1-weighted images allow one to measure global or regional volumes of WM. Diffusion tensor imaging (DTI) (Tournier, Mori, & Leemans, 2011), quantitative T1 & T2 (qT1, qT2) imaging (Deoni, 2010), magnetization transfer ratio (MTR) and myelin water fraction (MWF) assess different structural properties of WM globally or locally within specific fiber tracts. DTI measures the diffusion of water and is believed to reflect the microstructural organization of WM, while MTR and MWF are believed to be more sensitive to the content of macromolecule-bound protons in lipid membranes, cholesterol, and the myelin sheath (Deoni, 2011; MacKay et al., 2006; Paus, 2010). Studies of early brain development show that these different WM metrics provide distinct and complementary information about structural properties of WM (Deoni et al., 2011; Deoni, Dean, O’Muircheartaigh, Dirks, & Jerskey, 2012).
While previous studies suggest that stressful experiences can have a negative impact on structural properties of WM, little is know about the importance of the timing of these effects. White matter development unfolds over different stages during pre- and post-natal periods. Distinguishing between differential effects of stress during different developmental periods is important because different neurodevelopmental processes may be affected depending on the timing of an exposure. The aim of this study was to examine whether stressful events during distinct developmental periods relate to variation in WM properties when modeled within the same developmental model. To do this, we examine how stressful experiences during three key developmental periods, namely prenatal development, early childhood, and adolescence, relate prospectively to WM outcomes in the corpus callosum, as well as across global (lobar) WM. We use longitudinal data from a large prospective birth cohort of men from the general population in England who have been exposed to various levels of stressful life events during their development. The use of such a sample recruited from the general population may be particularly informative for interventions given the high prevalence of stressful life events even within the general population and the strong association between early adverse experiences and a range of poor physical, cognitive and mental health outcomes (Berens, Jensen, & Nelson, 2017; Felitti et al., 1998; Jensen, Berens, & Nelson, 2017). We focused on WM in the corpus callosum because this is the WM tract that has been most consistently found to be associated with early adverse experiences including early life stress (Paul et al., 2008; Seckfort et al., 2008) as well as more severe stressors such as early institutionalization (Bick et al., 2015) and maltreatment in subjects with or without post-traumatic stress disorder (PTSD) (H. Huang, Gundapuneedi, & Rao, 2012; Jackowski et al., 2008; Teicher et al., 2004). Similar to previous studies, we investigated structural variation within the genu and splenium, i.e. the most anterior and posterior parts of the corpus callosum. The genu contains inter-hemispheric fibers connecting mainly frontal cortical regions while the splenium contains inter-hemispheric connections connecting mostly posterior cortical regions (Hofer, Merboldt, Tammer, & Frahm, 2008; Pandya, Karol, & Heilbronn, 1971). Previous studies have observed differential developmental trajectories of WM in the genu and splenium (Deoni et al., 2012). Such difference may suggest that different substructures of the corpus callosum are differentially affected by experiences due to differences in their composition and/or developmental timing. To obtain a comprehensive quantification of variations in WM, we used four different imaging-based measures, namely FA, MD, MTR and MWF. Based on previous literature, we hypothesized that both pre- and postnatal stressful events would relate to low FA, MTR, and MWF and high MD in the splenium and genu splenium of the corpus callosum (Daniels, Lamke, Gaebler, Walter, & Scheel, 2013). We expected that these effects would be observed in the corpus callosum, but not in global (lobar) WM properties. Given high rate of neurodevelopmental change during fetal development, we predicted that prenatal stress would have the most profound effects on WM, followed by early postnatal stress.
2. METHODS AND MATERIALS
2.1 Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC: http://www.alspac.bris.ac.uk) is a birth cohort designed to investigate the influence of various factors on health trajectories. All pregnant women residing in the former Avon Health Authority in South-West England, who had an estimated date of delivery between 1 April 1991 and 31 December 1992, were invited to participate in the study. This resulted in a cohort of 14,541 pregnancies (Boyd et al., 2013; Fraser et al., 2012). Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees.
2.1.1 Subsample with brain MRI data
Brain imaging was carried out in a subsample of 507 young men when they were 18 to 21.5 years old (see Table 1 for sample characteristics). Only men were scanned owing to the focus of the NIH grant funding this work. Participants were selected based on their domicile being within a 3-hour journey (1-way) from the scanning site and availability of three or more longitudinal samples of blood used for measuring levels of sex hormones during puberty (Khairullah et al., 2014). We included the first 507 men who met these criteria and accepted the invitation to take part in the MRI substudy. Of these, 456 males completed all scanning using the same protocol (structural MRI, DTI, MTR and MWF). We excluded participants who failed to pass quality control of the image-analysis pipeline. Quality control consisted of visual inspection of the raw images for movement or image artifacts and inspection of registration quality (see “Quality control” for more details). Participants were also excluded if they were extreme outliers (defined as ±3 SD) on any of the WM measures. A total of 393 mother-son pairs were included in the model predicting variation in WM in the genu and splenium; a subsample of 377 was included in the analyses predicting variation across all global (lobar) WM.
Table 1.
| VARIABLE NAME | Summary statistics | |
|---|---|---|
| BACKGROUND INFORMATION AND COVARIATES | Mean [SD] or % | |
| Mean Age [years] | 19.63 [1.84] | |
| Mean IQ [assessed at age 15.5 years] | 96.33 [12.42] | |
| Ethnicity [% white] | 98% | |
| Maternal educational level [% O level or less, A-levels, degree] | 44%, 32%, 24% | |
| Breastfeeding [breastfed for > 6 months] | 45% | |
| Birth complications index [Any] | 20% | |
| PREVALENCE OF STRESSFUL LIFE EVENTS | Mean | SD |
| Prenatal events | 2.57 | 2.14 |
| At 8 months | 3.42 | 2.39 |
| At 21 months | 4.02 | 2.68 |
| At 33 months | 4.49 | 2.7 |
| At 47 months | 3.95 | 2.71 |
| Adolescence (12–16 years) | 1.52 | 1.45 |
SD = Standard deviation.
2.2 Measures
2.2.1 Prenatal stressful events (mother)
At 18 weeks of gestation, mothers completed a questionnaire examining the presence of 35 stressful life events including death of a close family member, family instability, and domestic violence since the beginning of the pregnancy (see appendix). This questionnaire was used to create a cumulative prenatal-stress index.
2.2.2. Early stressful life events
Stressful events during early childhood were assessed repeatedly via maternal reports when their sons were between 0 and 4 years old using the questionnaire mentioned above. A latent factor was created based on the cumulative indices to assesse “continuity of stressful events” between age 8, 21, 33, and 47 months. Confirmatory Factor Analyses indicated good fit (χ2(19) = 30.046, P< 0.051).
2.2.3. Adolescent stressful life events
Stressful events between age 12 and 16 were assessed using a self-reported questionnaire at age 16. This questionnaire was adapted slightly to be more relevant to this age group (see appendix).
2.2.4. Control variables
Analyses predicting variation in WM controlled for obstetric complications, duration of breastfeeding, maternal education, and the participant’s age at the time of the scan as all of these factors may impact WM development.
2.2.5. MRI acquisition
MR images, including MT, DTI, mcDESPOT, and T1-weighted sequences were acquired on a GE 3T magnet HDx system (General Electric, Waukesha, USA) using an 8-channel receiver-only head coil (See Supplemental Methods for details).
2.2.6. Fiber tractography
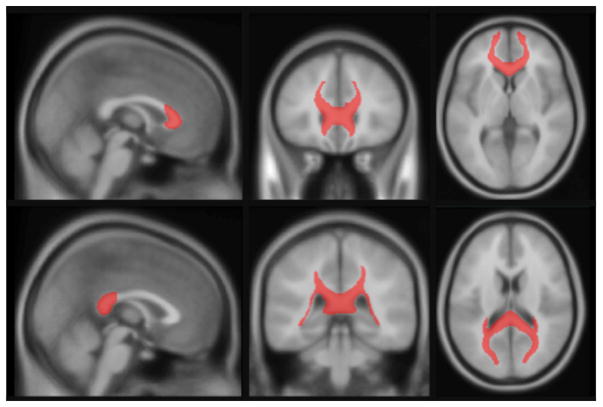
Tractography was conducted in ExploreDTI (v4.8.3) (Leemans & Jones, 2009) for MATLAB (R2010a, v7.10.0.499). We applied the following corrections to the diffusion weighted MRI: head motion, eddy current distortions, and EPI deformations. Diffusion tensors were estimated with the REKINDLE approach [kappa = 6; (Tax, Otte, Viergever, Dijkhuizen, & Leemans, 2015)] using an iteratively reweighted linear least squares approach (Veraart, Sijbers, Sunaert, Leemans, & Jeurissen, 2013). Next, we constructed probabilistic maps of the genu and splenium of the corpus callosum (see supporting information). These maps were thresholded at 50%, binarized and used as masks (see figure 1) to calculate mean values of FA, MD, MTR and MWF for each tract in each individual by projecting the probabilistic maps back to the native space of each individual to avoid interpolation artifacts (i.e., the transformation matrix consisted of the concatenation of nonlinear transformations from atlas space to native T1W image and then from T1W to FA/MD/MTR/MWFspace).
Figure 1. A view of the final binarized mask of the genu (top) and splenium (bottom) in ICBM-152.
Image of the mask used to extract FA, MD, MTR and MWF values in the genu and splenium of the corpus callosum.
2.2.7. Global (lobar) WM
The analysis of the T1W images was consistent with previous studies in our laboratory (Pangelinan et al., 2016). After correction for intensity inhomogeneities and slice-wise artifacts, images underwent linear and non-linear registration using the ANTs algorithm (Collins, Holmes, Peters, & Evans, 1995; Grabner et al., 2006) to an adolescent template of 808 T1W images on which the tissue classification priors were predefined (Tohka, Zijdenbos, & Evans, 2004). WM was classified and segmented into the eight cerebral lobes, namely the left and right occipital, parietal, temporal and frontal lobes. The lobar WM masks were projected back to the T1W native space of each individual. To obtain a global (lobe-based) WM estimate for each measure, we projected the lobar WM masks in T1W space back to the native space of the FA/MD/MTR/MWF images (i.e., the transformation matrix consisted of the concatenation of nonlinear transformations from atlas space to native T1W and then from T1W to FA/MD/MTR/MWF space).
2.2.8. Quality control
The quality of the registration (i.e., back-projection of the probabilistic maps of the genu and splenium and global (lobar) WM masks) to the native space for each modality was visually inspected for each participant.
2.2.9. Estimation of WM measures
All images were co-registered linearly to the T1W image to correct for head motion. Non-brain tissue was removed using a mask computed with the BET algorithm (Smith, 2002). Registration and brain masking were performed with FSL (http://www.fmrib.ox.ac.uk/fsl/). Images were then corrected for B1 inhomogeneities and off-resonance artifacts, using maps generated from the IR-SPGR and phase-cycling SSFP acquisitions, respectively. MTR was computed as the percent signal change between the MT off and MT on image sequences. The MTR signal was averaged across all voxels for the genu, splenium, and global (lobar) WM. MWF was extracted from the mcDESPOT sequence (Deoni, Rutt, Arun, Pierpaoli, & Jones, 2008) and averaged across all voxels for the genu, splenium, and global (lobar) WM. The same approach was applied to the FA and MD parametric maps derived from DTI images using ExporeDTI, as described above.
2.2.10. Statistical Analysis
We first ran bivariate correlations to explore the associations between study variables. A significant correlation was determined using a statistical threshold of p=<0.05. Prospective associations between: 1) prenatal, 2) childhood, and 3) adolescent stressful events, and variation in WM structure (FA, MD, MTR and MWF) in young adulthood were examined in two multivariate latent path models computed in Mplus version 7 (Muthén & Muthén, 2012). The first model examined variation in WM properties in the genu and splenium, while the second model examined variations in global (lobar) WM. Mplus uses full information maximum likelihood estimation, which allows for inclusion of respondents with missing data on the independent variables (Asparouhov & Muthén, 2010). Model fit was assessed using various assessments, namely the chi-square test with a non-significant p-value (p>0.05) indicating good model fit; the comparative fit index (CFI) and the Tucker-Lewis index (TLI) with acceptable fit defined as ≥ 0.9; and the root mean square error of approximation (RMSEA) with acceptable fit defined as ≤ 0.08) (Hooper, Mullen, Coughlan, & Mullen, 2008). Individual paths in the multivariate model were evaluated using a statistical threshold of p<0.05. The multivariate path model accounts for covariance among variables within and across time, as well as potential confounding data structures (e.g. breastfeeding, birth complications, maternal education and age at scan) resulting in conservative estimates of effects. Moreover, the use of overall fit indices provides an extra test of the appropriateness of the model above and beyond individual paths. More specifically, the fit indices allow for examination of the goodness of fit of the overall model (i.e., the hypothesized covariance of variables) to the data (i.e. the observed covariance of the data). We did not correct for multiple comparisons due to the conservative nature of the multivariate model, and because corrections, such as Bonferroni correction, assume independence of the individual tests, which is not the case in the currently model which estimates multiple developmental pathways between correlated stress exposures and correlated WM outcomes.
3. RESULTS
The bivariate correlations among the study variables are provided in Table 2. Stressful events occurring during prenatal development, early childhood, or adolescence were moderately correlated suggesting some degree of continuity in stress exposures over developmental time. The different WM measures were also moderately correlated suggesting that FA, MD, MTR and MWF measure related, but also distinct properties of WM. As expected FA, MTR and MWF were positively correlated, while MD was negatively correlated with the other metrics. Prenatal stress was negatively correlated with MTR in the genu and splenium, MWF in the splenium, and FA in the genu of the corpus callosum. We did not see any significant correlations between stress during childhood and WM in the corpus callosum, and only one positive correlation between stress during adolescence and variation in MTR in the genu of the corpus callosum. Among the covariates we saw that birth complications were related to MWF in the genu and splenium. Age at scan was correlated with MTR and MD in the splenium. Correlations between stressful events and global (lobar) WM can be found in the supporting information.
Table 2.
Bivariate correlations among main study variables
| VARIABLE NAME | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stressful experiences | |||||||||||
| 1. Prenatal stress | |||||||||||
| 2. Childhood stress | 0.537** | ||||||||||
| 3. Adolescent stress | 0.151** | 0.282** | |||||||||
| White matter properties | |||||||||||
| 4. MWF Genu | −0.089° | −0.014 | 0.089° | ||||||||
| 5. MWF Splenium | −0.139** | 0.000 | 0.077 | 0.700** | |||||||
| 6. MTR Genu | −0.176** | −0.069 | 0.106* | 0.110* | 0.171** | ||||||
| 7. MTR Splenium | −0.132** | 0.056 | 0.055 | 0.166** | 0.320** | 0.671** | |||||
| 8. FA Genu | −0.105* | −0.041 | 0.058 | 0.253** | 0.217** | 0.076 | 0.099* | ||||
| 9. FA Splenium | −0.052 | −0.017 | −0.014 | 0.208** | 0.406** | 0.090° | 0.258** | 0.536** | |||
| 10. MD Genu | 0.097° | 0.016 | −0.101* | −0.350** | −0.305** | −0.155** | −0.227** | −0.612** | −0.387** | ||
| 11. MD Splenium | 0.065 | 0.038 | −0.091° | −0.180** | −0.431** | −0.090° | −0.266** | −0.493** | −0.574** | 0.556** | |
| Control variables | |||||||||||
| 12. Breast feeding | −0.062 | −0.033 | −0.009 | 0.008 | 0.022 | −0.030 | −0.015 | 0.075 | −0.037 | −0.077 | −0.053 |
| 13. Birth complications | 0.039 | 0.021 | 0.006 | 0.110* | 0.111* | −0.058 | 0.012 | 0.028 | 0.083 | −0.033 | −0.085° |
| 14. Maternal education | −0.080 | −0.043 | −0.012 | 0.097° | 0.067 | 0.015 | 0.014 | 0.041 | 0.055 | −0.061 | −0.073 |
| 15. Age at scan | 0.000 | 0.000 | 0.000 | 0.028 | −0.032 | 0.064 | 0.108* | −0.031 | −0.014 | 0.027 | 0.113* |
MWF = Myelin Water Fraction; MTR = Magnetic Transfer Ratio; FA = Fractional Anisotropy; MD = Mean diffusivity
P <0.01
P < 0.05
P <0.1
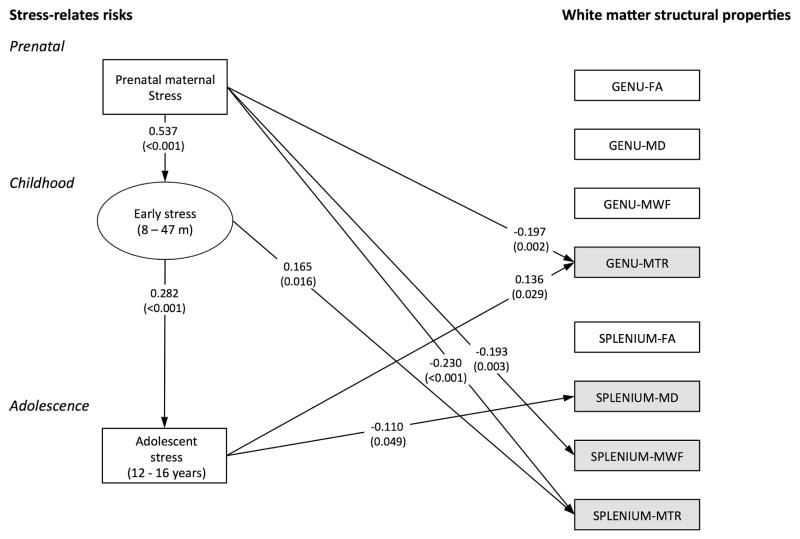
The multivariate path model predicting continuity in stress, and independent associations between stressful events at each of the three time points and variation in WM in the genu and splenium (Figure 2) showed good fit to the data (χ2(62) = 78.518, P=0.077; CFI = 0.991; TLI = 0.979; RMSEA = 0.026, 90% CI 0.000–0.042). The model illustrated high continuity in stressful events, namely that higher levels of prenatal stress predicted high levels of early childhood stress, which in turn predicted high levels of stress during adolescence. Prenatal stress, childhood stress, and stress during adolescence each related independently to variations in one or more WM outcomes. Prenatal stress predicted lower MWF and MTR in the genu and splenium. Childhood stress predicted higher MTR in the splenium. Stress during adolescence predicted higher MTR in the genu, and lower MD in the splenium.
Figure 2. splenium of the corpus callosum by stressful life event.
The multivariate path model showing associations between stress-related risks (left side) and the WM outcomes in the corpus callosum (right side). Illustration includes only significant association between the main variables of interest. A rectangular box indicates that a variable was based on a single assessment; an oval box indicates that a latent factor was creased based on numerous assessments. All estimates relating to one of the WM outcomes controlled for birth complications and duration of breastfeeding, above and beyond the stress at each time point. The model included all correlations among the four WM modalities (FA, MD, MTR and MWF) for the genu and splenium respectively.
The multivariate model predicting variation in global WM also showed good fit to the data (χ2(50) = 50.591, P=0.450 CFI = 0.999; TLI = 0.999; RMSEA = 0.005, 90% CI 0.000–0.033). This model, however, resulted in only one association between stress and WM, namely a negative association between prenatal stress and global (lobar) MTR (β= −0.195, p = 0.002).
4. DISCUSSION
The developmental model considering concurrently stressful experiences across three time points illustrates strong prospective relationships between stress exposures during different developmental periods, reflecting high levels of continuity in stress. This suggests that children whose mothers were stressed during pregnancy were more likely to be born into families exposed to higher levels of stress throughout their post-natal development.
Stress occurring during different developmental periods related independently to variation in WM properties in the genu and/or splenium of the corpus callosum: (1) prenatal stress was associated with lower MTR in the genu and splenium and lower MWF in the splenium; (2) early childhood stress was associated with higher MTR in the splenium; and (3) stress during adolescence was associated with higher MTR in the genu and lower MD in the splenium. Prenatal stress, but not postnatal stress, was also associated with lower MTR values in global (lobar) WM, suggesting that this association between prenatal stress and WM properties may not be specific to the corpus callosum but reflect a more global impact of prenatal stress on MTR across the whole brain. There were no associations between childhood or adolescent stress and global WM, suggesting that the association between stress in childhood and adolescence, and variability in WM in the corpus callosum were not accounted for by global impacts on WM.
We highlight two main findings: (1) Prenatal stress was consistently associated with lower MTR and MWF in the genu and/or splenium, and with lower global (lobar) MTR; (2) within the same developmental model, childhood stress and stress during adolescence was associated with higher MTR in the splenium or genu, and associated with lower MD in the splenium.
The finding relating prenatal stress to lower MTR/MWF in the genu and splenium are consistent with previous findings linking early adversity to abnormalities in WM properties in the corpus callosum. Most previous studies have used DTI to examine variation in FA and MD and observed low FA in the corpus callosum in relation to a wide range of early adversities including stressful life events (Paul et al., 2008; Seckfort et al., 2008), institutionalization (Bick et al., 2015), and exposure to maltreatment and verbal abuse (H. Huang et al., 2012; Jackowski et al., 2008; Teicher et al., 2004). Fractional anisotropy is often interpreted such that higher values reflect normative developmental trajectories. MTR and MWF tend to correlate with FA, but are considered more sensitive to myelin content. The interpretations of these WM metrics are, however, based on simplifications since in vivo neuroimaging provides only indirect measures of structural variation in WM properties, and effects are averaged over thousands of axons contained in a scanned WM unit (~6 mm3) (Bartzokis et al., 2012; Deoni et al., 2012). In contrast to previous studies we did not see any associations between stressful events and variation in FA, and we saw only one association between stress during adolescence and MD within the multivariate path model. Still, the finding of lower MTR and MWF in individuals exposed to higher levels of prenatal stress is in line with our hypothesis that stress would relate to lower MTR, MWF and FA, and higher MD. We hypothesized that this effect would be limited to the corpus callosum, but found that prenatal stress also predicted lower MTR globally across the eight hemispheric lobes and, as such, was not limited to a single fiber tract.
The finding of positive associations between postnatal stress and MTR in the genu and splenium and a negative association between adolescent stress and MD in the splenium was contrary to our hypothesis. This hypothesis was based on previous studies relating childhood stressors to lower FA (Daniels et al., 2013), which would normally correspond with lower MTR and higher MD. A key challenge in comparing current findings with the previous literature, however, is that many studies have focused on more severe stressors (e.g. trauma and maltreatment) and have recruited children or adults with PTSD, which may have impacted findings. Indeed, PTSD has been associated with low FA in the corpus callosum (Daniels et al., 2013). Interestingly, some studies have reported higher WM volumes in the cingulate and prefrontal cortex of physically abused children in whom WM volumes were positively correlated with self-reported stressors (Jamie L Hanson et al., 2010), and in adults with PTSD after acute trauma (Abe et al., 2006). Moreover, a study in mice found that early weaning (compared with later weaning) was associated with accelerated formation of myelin, increased density of myelinated axons, and smaller axon diameters in the amygdala (Ono et al., 2008). These latter findings suggest that stress could potentially lead to increases in myelin (which may be one of the drivers of increases in WM volumes).
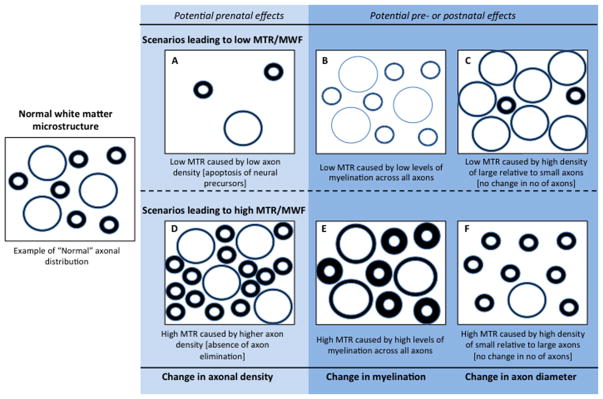
Here, we discuss three scenarios that may relate variation in MTR, MWF, FA, and MD to specific WM properties: (1) variation in the number/density of axons; (2) variation in the thickness of the myelin sheath around the axons; (3) variation in the radial size (diameter) of the axons (see Fig. 3). The model is organized such that the three scenarios in the top part of the model would lead to low MTR/MWF/FA and high MD (scenarios A, B, and C), while the three scenarios in the lower part of the model would lead to high MTR/MWF/FA and low MD (scenarios D, E and F). While adjudication between the scenarios presented in Figure 3 would require histological (i.e., post mortem) data, animal studies and in vitro studies of cortical culture provide some neurobiological evidence in support of the suggested scenarios.
Figure 3.
Illustration of suggested potential drivers of changes in white matter properties associated with prenatal, childhood and adolescent stress. Scenarios on the light shaded background represent effects that may relate to prenatal or early postnatal experiences only i.e. variation in the number/density of axons. Scenarios on the darker shaded background represent effects that can be either pre- or postnatal, i.e., variation in the thickness of the myelin sheath and variation in the radial size (diameter) of the axons. MTR = Magnetization Transfer Ratio; MWF = Myelin Water Fraction.
The first scenario (Fig. 3, A and D), namely variation in the number of neurons/axons, is mostly relevant to prenatal or early postnatal experiences where the density of neurons is determine by neurodevelopmental processes related to neurogenesis and apoptosis. Both neurogenesis and apoptosis are shaped by both genetic influences and experience (neuronal activity). Prenatal maternal stress in particular, but also early postnatal stress has been associated with reduced neurogenesis and enhanced apoptosis in the hippocampus in rodents (Lemaire, Koehl, Le Moal, & Abrous, 2000; Lemaire, Lamarque, Le Moal, Piazza, & Abrous, 2006; Mirescu, Peters, & Gould, 2004; Van den Hove et al., 2006) and non-human primates (Coe et al., 2003; Uno et al., 1990). Most studies have focused on the hippocampus yet some studies have found evidence of functional cellular and structural changes in the cerebral cortex following psychosocial stress during early postnatal development which, in theory, could affect the development of WM tracts including the corpus callosum (Koo et al., 2003; Van den Hove et al., 2006). In contract with stress, environmental enrichment, such as training and stimulation, has been associated with increased neurogenesis in the hippocampus (Nilsson, Perfilieva, Johansson, Orwar, & Eriksson, 1999). The extent to which neurogenesis occurs in regions other than the hippocampus during postnatal development is unclear.
The second scenario (Fig. 3. B and E) considers abnormal myelination of axons as the driver of variation in WM. Similar to neurogenesis and apoptosis, myelination is guided by genetic influences and neuronal activity (Gibson et al., 2014). Most commonly, stress has been related to reduced myelination (as indicated by lower MTR/MWF/FA) whereas experiences typically associated with increased myelination include environmental enrichment (Gibson et al., 2014). Still, some studies suggest that stress can lead to increase in myelination and thus higher MTR/MWF/FA. One study in rodents, for instance, found that early weaning (which is considered a source of stress) was associated with markers of increased myelination (Ono et al., 2008) and some studies in humans have related stressful experiences to greater WM volumes that may reflect an expansion of WM (Abe et al., 2006; Jamie L Hanson et al., 2010). A possible explanation for increased myelination may be that moderate levels of stress, as opposed to severe levels of stress, may lead to increased myelination as an adaptation to a stressful environment. Regarding the molecular mechanisms underlying increases or decreases in myelination studies in rodents, primates, and sheep have found that prenatal exposure to glucocorticoids delays oligodendrocyte and astrocyte maturation (Antonow-Schlorke, Schwab, Li, & Nathanielsz, 2003). Both oligodendrocytes and astrocytes are essential to myelin biosynthetic pathways and long-term maintenance of myelin. One study also found that prenatal administration of betamethasone, a synthetic glucocorticoid, was associated with thinning of the myelin sheath in the corpus callosum in sheep (W. L. Huang et al., 2001).
Scenarios C and F invoke variation in the proportion of large-to-small axons caused by variation in the radial growth of axons. This relative proportion can affect WM properties because the thickness of the myelin sheath varies depending on the size of the axon. This relationship is expressed by the g-ratio, the ratio between the axon and the fiber diameter. Large axons have relatively thin myelin sheath compared with small axons (Paus et al., 2014), low MTR and MWF may therefore reflect high density of large axons, whereas high MTR and MWF may reflect high density of small axons with relatively thicker myelin sheaths. Similar to neurogenesis and myelination, high density of large-relative- to-small axons (scenario C), is likely explained by increased neuronal and axonal growth due to neuronal activity associated with positive stimulation. Moderate level of stress could therefore theoretically have a positive impact on radial axonal growth viewed here as an adaptation to a stressful environment. This is unlikely, however, in response to excess stress. Attenuated radial growth of the axon, resulting in more small relative to large axons (Scenario F), could be caused by suboptimal levels of stimulation, or by stress-induced disturbances in the transport of biological molecules and organelles (Paus, 2010; Paus et al., 2014). Indeed, animal studies have found that prenatal stress is associated with lower expression of microtubules (an important component of the axonal cytoskeleton important for axonal transport) in the hippocampus in rodents (Bianchi, Heidbreder, & Crespi, 2003; Liu & Brady, 2002) and non-human primates (Uno et al., 1990). Moreover, studies in sheep have found that prenatal exposure to synthetic glucocorticoids is associated with reduced diameter of myelinated axons in the corpus callosum (Antonow-Schlorke et al., 2003; W. L. Huang et al., 2001).
Importantly, the three scenarios outlined above are not mutually exclusive but may co-occur. The pervasive effect of prenatal stress observed in the current study likely reflects a combination of different processes. Indeed, myelination and radial growth of the axon likely inter-relate because the thickness of the myelin sheath varies depending on the size of the axon as explained above.
The study had a few limitations. First, the study was limited to men and findings may not generalize to women. Previous studies in both children and adolescents provide evidence suggesting that WM development follows sex-specific patterns that may impact how stress affects normative developmental processes (Deoni et al., 2012; Perrin et al., 2009). Moreover, various studies have shown that sex moderates the effects of pre- and early postnatal adversity on physical health and behavioral outcomes, such as cognition, with converging evidence suggesting that male fetuses and infants are more vulnerable to early biological and psychosocial adversity compared with females (DiPietro & Voegtline, 2017). Future research will need to examine if the results presented here also apply to females. Another limitation is that we focused on the corpus callosum as a region of interest. The corpus callosum was chosen based on previous studies of the effects of stress on WM in which lower FA in the corpus callosum has been the most consistent finding (Daniels et al. 2013). Given that our model is complex, we opted against increasing this complexity further by adding more WM tracts. Finally, we note that focusing on the corpus callosum takes advantage of its uniform geometry (i.e., mostly parallel fibre orientation) and histological knowledge from animal and postmortem human studies (Björnholm et al., 2017). The latter helps guide the interpretation of potential cellular features underlying observed variation in MTR, MWF, FA and MD discussed in Figure 3. We also ran the model with global (lobar) volume as the outcome to test whether effects in the corpus callosum were driven by global effects, yet we cannot rule out the possibility that other tracts may also be impacted by early life stress.
5. CONCLUSIONS
The main findings of the current study were the independent associations between WM properties, prenatal stress, and stress occurring during childhood or adolescence. We proposed three developmental processes that may drive observed variations in WM: (1) abnormal neurogenesis/apoptosis; (2) abnormal myelination; (3) abnormal radial growth of axons. During prenatal development, we found that increased levels of (maternal) stress were associated with low MTR and MWF. This finding may reflect any of three proposed scenarios explaining increased MTR/MFW in conceptual model. Because myelination of axons occurs late in prenatal and mostly during postnatal development, we suggest that the effect of prenatal stress may most likely reflect scenario A (low axon density) or a combination of multiple scenarios. Postnatal stress, whether occurring during childhood or adolescence, was associated with higher MTR and stress during adolescence was associated with lower MD. This finding is unlikely to be explained by scenario D (less apoptosis), which is most relevant to prenatal development. Increased MTR in relation to postnatal stress may therefore reflect scenarios E (more myelination) or F (less radial growth). Future longitudinal studies should provide multimodal characterization of WM both before and after adverse events to capture the dynamics of these relationships. Moreover, animal models may help characterize neurodevelopmental changes associated with stress during different developmental periods. The pervasive effect of prenatal stress is important from a clinical perspective because it highlight potential long-term consequences of maternal exposure to stress or synthetic cortisol. Synthetic cortisol is sometimes used as part of antenatal care if a mother is at risk of going into labor preterm to speed up lung maturation in the fetus. Maternal cortisol exposure has previously been associated with altered neuroendocrinological functioning and poorer cognitive outcomes in exposed children (Davis & Sandman, 2010; Welberg & Seckl, 2001) and our findings support the view that fetal and perinatal exposure to stress hormones may have implications for WM development, which in turn may impact behavioral and health outcomes including cognition. Screening for, and intervention, against maternal stress during pregnancy should be considered part of obstetric care, and the potential neurodevelopmental consequence of cortisol exposure should be studied more intensely as children exposed to prenatal cortisol, whether natural or synthetic, may benefit from early intervention to support neural development.
Supplementary Material
Table S1. Correlations between stress and global WM volumes
Acknowledgments
We are extremely grateful to the families who took part in this study and the ALSPAC team, including midwifes, interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We also acknowledge The UK Medical Research Council, the Wellcome Trust (Grant ref: 092731), and the University of Bristol for providing support for The Avon Longitudinal Study of Parents and Children.
Financial disclosure
The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. This paper was supported by grants from the National Institutes of Health (R01MH085772 to T Paus; R01HD068437 to ED Barker) and Canadian Institutes of Health Research (MOP125892 to T Paus). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research of A.L. is supported by VIDI Grant 639.072.411 from the Netherlands Organisation for Scientific Research (NWO). None of the authors report any conflicts of interest.
Footnotes
Disclosure: None of the authors report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, … Ohtomo K. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Research: Neuroimaging. 2006;146(3):231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. The Journal of Physiology. 2003;547(1):117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T, Muthén B. Weighted least squares estimation with missing data 2010 [Google Scholar]
- Bartzokis G, Lu PH, Heydari P, Couvrette A, Lee GJ, Kalashyan G, … Altshuler LL. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biological Psychiatry. 2012;72(12):1026–1034. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Spitzer NC. Phenotypic checkpoints regulate neuronal development. Trends in Neurosciences. 2010;33(11):485–492. doi: 10.1016/j.tins.2010.08.005. Retrieved from . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens AE, Jensen SKG, Nelson CA. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Medicine. 2017;15(1):135. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Heidbreder C, Crespi F. Cytoskeletal changes in the hippocampus following restraint stress: role of serotonin and microtubules. Synapse. 2003;49(3):188–194. doi: 10.1002/syn.10230. [DOI] [PubMed] [Google Scholar]
- Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, Nelson CA. Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatrics. 2015;169(3):211–219. doi: 10.1001/jamapediatrics.2014.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnholm L, Nikkinen J, Kiviniemi V, Nordström T, Niemelä S, Drakesmith M. NeuroImage Structural properties of the human corpus callosum : Multimodal assessment and sex differences. 2017;152(December 2016):108–118. doi: 10.1016/j.neuroimage.2017.02.056. [DOI] [PubMed] [Google Scholar]
- Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, … Davey Smith G. Cohort Profile: the “children of the 90s”--the index offspring of the Avon Longitudinal Study of Parents and Children. International Journal of Epidemiology. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage. 2012;59(2):1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65(3):227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biological Psychiatry. 2003;54(10):1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3(3):190–208. [Google Scholar]
- Daniels JK, Lamke J, Gaebler M, Walter H, Scheel M. White matter integrity and its relationship to PTSD and childhood trauma—A systematic review and meta-analysis. Depression and Anxiety. 2013;30(3):207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81(1):131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Walker L, … Deoni S. Modeling healthy male white matter and myelin development: 3 through 60months of age. NeuroImage. 2014;84:742–752. doi: 10.1016/j.neuroimage.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL. Quantitative relaxometry of the brain. Topics in Magnetic Resonance Imaging: TMRI. 2010;21(2):101–113. doi: 10.1097/RMR.0b013e31821e56d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL. Magnetic resonance relaxation and quantitative measurement in the brain. In: Modo M, Bulte JWM, editors. Magnetic Resonance Neuroimaging. Vol. 711. Springer; 2011. pp. 65–108. [DOI] [PubMed] [Google Scholar]
- Deoni SCL, Dean DC, O’Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63(3):1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, … Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31(2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Voegtline KM. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. doi: 10.1016/j.neuroscience.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, … Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, … Lawlor DA. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2012;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, … Zuchero JB. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. International Conference on Medical Image Computing and Computer-Assisted Intervention; Berlin Heidelberg: Springer; 2006. pp. 58–66. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev. 2013;84(5):1566–1578. doi: 10.1111/cdev.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. The Journal of Neuroscience. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Merboldt KD, Tammer R, Frahm J. Rhesus monkey and human share a similar topography of the corpus callosum as revealed by diffusion tensor MRI in vivo. Cereb Cortex. 2008;18(5):1079–1084. doi: 10.1093/cercor/bhm141. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Mullen J, Coughlan J, Mullen MR. Structural Equation Modelling: Guidelines for Determining Model Fit Structural Equation Modelling: Guidelines for Determining Model Fit. The Electronic Journal of Business Research Methods. 2008;6(1):53–60. Retrieved from www.ejbrm.com. [Google Scholar]
- Huang H, Gundapuneedi T, Rao U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology. 2012;37(12):2693–2701. doi: 10.1038/npp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays astrocyte and capillary tight junction maturation in fetal sheep. International Journal of Developmental Neuroscience. 2001;19(5):487–493. doi: 10.1016/s0736-574801)00035-1. [DOI] [PubMed] [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, … Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Research. 2008;162(3):256–61. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SKG, Berens AE, Nelson CA. Effects of poverty on interacting biological systems underlying child development. The Lancet Child & Adolescent Health. 2017 doi: 10.1016/S2352-4642(17)30024-X. [DOI] [PubMed] [Google Scholar]
- Khairullah A, Klein L Cousino, Ingle SM, May MT, Whetzel CA, Susman EJ, Paus T. Testosterone trajectories and reference ranges in a large longitudinal sample of male adolescents. PloS One. 2014;9(9):e108838. doi: 10.1371/journal.pone.0108838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Park CH, Choi SH, Kim NJ, Kim HS, Choe JC, Suh YH. The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. The FASEB Journal. 2003;17(11):1556–1558. doi: 10.1096/fj.02-1032fje. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal Stimulation of the Pups Counteracts Prenatal Stress-Induced Deficits in Hippocampal Neurogenesis. Biological Psychiatry. 2006;59(9):786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, Chiarello C. Size matters: cerebral volume influences sex differences in neuroanatomy. Cerebral Cortex. 2008;18(12):2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HW, Brady ST. Journal of Neurochemistry. Vol. 81. England: Blackwell Publishing; 2002. Neuronal cold-stable tubulin induced by chronic glucocorticoid stress. In; p. 64. [Google Scholar]
- MacKay A, Laule C, Vavasour I, Bjarnason T, Kolind S, Madler B. Insights into brain microstructure from the T2 distribution. Magnetic Resonance Imaging. 2006;24(4):515–525. doi: 10.1016/j.mri.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience. 2004;7(8):841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. The Comprehensive Modelling Program for Applied Researchers: User’s Guide. 2012;7 [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. Journal of Neurobiology. 1999;39(4):569–78. doi: 10.1002/(SICI)1097-4695(19990615)39:4<569::AID-NEU10>3.0.CO;2-F. [pii] [DOI] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156(4):1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Karol EA, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Research. 1971;32(1):31–43. doi: 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- Pangelinan MM, Leonard G, Perron M, Pike GB, Richer L, Veillette S, … Paus T. Puberty and testosterone shape the corticospinal tract during male adolescence. Brain Structure and Function. 2016;221(2):1083–1094. doi: 10.1007/s00429-014-0956-9. [DOI] [PubMed] [Google Scholar]
- Paul R, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, … Cohen RA. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatric Disease and Treatment. 2008;4(1):193–201. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain and Cognition. 2010;72(1):26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T, Pesaresi M, French L. White matter as a transport system. Neuroscience. 2014;276:117–125. doi: 10.1016/j.neuroscience.2014.01.055. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, … Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45(4):1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Dell’Acqua F, O’Connor TG, Catani M, Deeley Q, … Murphy DG. Prenatal stress and limbic-prefrontal white matter microstructure in children aged 6–9 years: a preliminary diffusion tensor imaging study. World J Biol Psychiatry. 2014;15(4):346–352. doi: 10.3109/15622975.2014.903336. [DOI] [PubMed] [Google Scholar]
- Seckfort DL, Paul R, Grieve SM, Vandenberg B, Bryant RA, Williams LM, … Gordon E. Early Life Stress on Brain Structure and Function Across the Lifespan: A Preliminary Study. Brain Imaging and Behavior. 2008;2(1):49–58. doi: 10.1007/s11682-007-9015-y. [DOI] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax CMW, Otte WM, Viergever MA, Dijkhuizen RM, Leemans A. REKINDLE: Robust extraction of kurtosis INDices with linear estimation. Magnetic Resonance in Medicine. 2015;73(2):794–808. doi: 10.1002/mrm.25165. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/mrm.25165/full. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56(2):80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Sheu YS, Polcari A, McGreenery CE. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. American Journal of Psychiatry. 2010;167:1464–1471. doi: 10.1176/appi.ajp.2010.10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tournier J, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magnetic Resonance in Medicine. 2011;65(6):1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Developmental Brain Research. 1990;53(2):157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Van den Hove DLA, Steinbusch HWM, Scheepens A, Van de Berg WDJ, Kooiman LAM, Boosten BJG, … Blanco CE. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137(1):145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. Neuroimage. 2013;81:335–346. doi: 10.1016/j.neuroimage.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology. 2001;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlations between stress and global WM volumes