Abstract
Androgen administration protects against musculoskeletal deficits in models of sex-steroid deficiency and injury/disuse. It remains unknown, however, whether testosterone prevents bone loss accompanying spinal cord injury (SCI), a condition that results in a near universal occurrence of osteoporosis. Our primary purpose was to determine whether testosterone-enanthate (TE) attenuates hindlimb bone loss in a rodent moderate/severe contusion SCI model. Forty (n=10/group), 14 week old male Sprague-Dawley rats were randomized to receive: (1) Sham surgery (T9 laminectomy), (2) moderate/severe (250 kdyne) SCI, (3) SCI+Low-dose TE (2.0 mg/week), or (4) SCI+High-dose TE (7.0 mg/week). Twenty-one days post-injury, SCI animals exhibited a 77–85% reduction in hindlimb cancellous bone volume at the distal femur (measured via μCT) and proximal tibia (measured via histomorphometry), characterized by a >70% reduction in trabecular number, 13–27% reduction in trabecular thickness, and increased trabecular separation. A 57% reduction in cancellous volumetric bone mineral density (vBMD) at the distal femur and a 20% reduction in vBMD at the femoral neck were also observed. TE dose dependently prevented hindlimb bone loss after SCI, with high-dose TE fully preserving cancellous bone structural characteristics and vBMD at all skeletal sites examined. Animals receiving SCI also exhibited a 35% reduction in hindlimb weight bearing (triceps surae) muscle mass and a 22% reduction in sublesional non-weight bearing (levator ani/bulbocavernosus [LABC]) muscle mass, and reduced prostate mass. Both TE doses fully preserved LABC mass, while only high-dose TE ameliorated hindlimb muscle losses. TE also dose dependently increased prostate mass. Our findings provide the first evidence indicating that high-dose TE fully prevents hindlimb cancellous bone loss and concomitantly ameliorates muscle loss after SCI, while low-dose TE produces much less profound musculoskeletal benefit. Testosterone-induced prostate enlargement, however, represents a potential barrier to the clinical implementation of high-dose TE as a means of preserving musculoskeletal tissue after SCI.
Key words: : androgen, microCT, osteoporosis, sex steroids, skeletal
Introduction
Approximately 270,000 persons currently have a spinal cord injury (SCI) in the United States, with an estimated 12,000 new SCIs occurring each year.1 Persons experience a myriad of functional and physiological deficits after SCI, including reductions in lower extremity bone mineral density (BMD) and skeletal muscle mass.2 Osteoporosis in the lower extremities develops in most persons with a functionally complete SCI; the condition is characterized by extensive cancellous bone loss within the first 2–3 years after injury and cortical bone loss continuing for 10 or more years.3 In this regard, bone loss ranges from 50–60% at cancellous bone sites and 25–35% at cortical bone sites4 within several years of injury, underlying the 100-fold greater bone fracture risk in those with SCI compared with healthy age-matched persons.5 Similarly, progressive skeletal muscle loss occurs rapidly after SCI, resulting in an approximate 18–46% reduction in muscle cross-sectional area (CSA) within several months of injury.6,7 Importantly, the extensive bone loss, high fracture risk, and severe muscle loss within this population represent detrimental outcomes that impede strategies to maintain musculoskeletal and metabolic health after SCI.
Neurological impairment and a complete reduction in lower extremity load bearing are the primary factors underlying the musculoskeletal deficits after SCI.2 Rodent studies demonstrate that androgen administration ameliorates the rapid hindlimb muscle losses that occur within several weeks of spinal cord transection8,9 and contusion SCI,10 and a single (open label) clinical trial has reported that testosterone treatment improves lower extremity lean mass in men with motor complete (AIS A-C) SCI.11 Administration of testosterone (and other androgens) also prevents musculoskeletal deficits occurring within several days to several weeks after nerve injury,12 disuse,13 or gonadectomy.14–16
The bone loss resulting from SCI, however, is much more severe and characteristically different than that resulting from sex-steroid deficiency17 or from other disuse/injury models.18,19 In particular, severe cancellous bone deficits are present within 21 days of SCI in several rodent models.20,21 In this regard, we are unaware of any study that has examined whether testosterone administration prevents the SCI-induced bone loss that occurs rapidly after injury.
The primary purposes of this study were to (1) develop a male rodent contusion SCI model of acute bone loss and (2) determine whether testosterone administration prevents bone loss in this model. Secondary purposes were to examine the effects of testosterone administration on other androgen-sensitive tissues, including hindlimb weight bearing and sublesional non-weight bearing skeletal muscle and the prostate, as well as to determine whether elevated systemic estradiol (that occurs after aromatization of testosterone) is needed for the musculoskeletal benefit of administered testosterone. We hypothesized that testosterone administration would prevent bone loss and ameliorate muscle loss in a dose-dependent manner and that high-dose, but not low-dose, testosterone would elevate prostate mass.
Methods
Animal care
Barrier-raised and specific pathogen-free male Sprague-Dawley rats aged 14 weeks were obtained from Charles River Laboratories (Wilmington, MA). Rodent models are commonly used to assess the effectiveness of therapeutic interventions after SCI22 because rats closely reproduce the musculoskeletal responses that occur after traumatic SCI in humans.23 Animals were individually housed in a temperature- and light-controlled room on a 12-h light, 12-h dark cycle. Rats were fed commercially available Harlan rodent chow containing 3.1 kcal/g, distributed as 58% carbohydrate, 24% protein, and 18% fat (2018 Teklad Global 18% Protein Rodent Diet, Harlan Laboratories Inc., Indianapolis, IN) and tap water ad libitum. Macronutrient breakdown of rodent chow was verified by the manufacturer. All experimental procedures conformed to the Institute for Laboratory Animal Research Guide to the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee at the Gainesville VA Medical Center.
Experimental design
Rats (n=10/group) were blocked according to initial body weight and stratified into the following groups: (1) Sham surgery (T9 laminectomy)+vehicle (SHAM), (2) T9 laminectomy+moderate/severe (250 kdyne) contusion SCI+vehicle (SCI), (3) SCI+low-dose testosterone-enanthate (SCI+LowTE), and (4) SCI+high-dose TE groups (SCI+HighTE). Animals received TE (low-dose=2.0 mg/week in 0.1 mL sesame oil or high-dose=7.0 mg/week in 0.1 mL sesame oil) or vehicle (0.1 mL/week, sesame oil) treatments via intramuscular injection at time of surgery and at weekly intervals thereafter. Blood was sampled via the tail tip amputation method before initial drug administration and at 7 day intervals thereafter. Animals were assessed for open field locomotion by two blinded observers using the Basso-Beattie-Bresnahan (BBB) locomotor rating scale at weekly intervals24 and were injected subcutaneously with declomycin and calcein (all chemicals obtained from Sigma-Aldrich, St. Louis, MO, unless noted) at a dose of 15 mg/kg body weight 10 and 3 days before sacrifice, respectively, for fluorochrome labeling of bone surfaces.
Rats were sacrificed 21 days after surgery, via an intraperitoneal injection of 120 mg/kg pentobarbital. Blood was acquired by intracardiac puncture, and the left and right femurs and tibiae, soleus, and gastrocnemius/plantaris (comprising the triceps surae muscle complex that experiences exceptional disuse atrophy for upward of 12 weeks after midthoracic contusion SCI),25 spinal column, levator ani/bulbocavernosus (LABC) muscle complex (a sublesional non-weight bearing highly androgen sensitive skeletal muscle),26 prostate, and heart were excised and weighed.
Blood samples were centrifuged at 3000g, and serum aliquots were separated and stored at −80°C until analyzed. The femora were wrapped in saline-soaked gauze to prevent dehydration and stored at −20°C for future microcomputed tomography (μCT) analysis and bone mechanical strength testing. Tibia were fixed in formalin for 48 h and then stored at 4°C in 70% ethanol before histomorphometric analysis. A section of the spinal cord (including the lesion site) was fixed in paraformaldehyde (PFA) and stored at 4°C for 7 days before histomorphometric analysis to confirm the severity of injury. The remaining soft tissue, vertebrae, and spinal cord dura were then removed and the cord was trimmed to a section including segments extending 2 mm rostral and 2 mm caudal to the lesion site, and placed in fresh PFA for 14 days to ensure fixation and then transferred to 70% ethanol for histologic assessment. The remaining tissues were snap frozen in liquid nitrogen and stored at −80°C until further analysis.
Surgery and post-surgical care
Animals were kept on a circulating water heated pad to maintain body temperature and remained under isoflurane anesthesia during all surgical procedures. The spinal cord was exposed by laminectomy under sterile conditions and a 250 kdyne force was applied to the T9 segment of the spinal cord by the IH Impactor, which induces a moderate/severe mid-thoracic contusion SCI. Confirmation of T9 laminectomy/injury was verified at time of surgery using anatomical landmarks and during spinal column dissection after sacrifice. Animals were given subcutaneous injections of buprenorphine (0.05 mg/kg) and ketoprofen (5.0 mg/kg) to reduce pain and inflammation for 36 h after SCI, and ampicillin was administered for 5 days after surgery. Postoperative care of the animals included daily examination for signs of distress, weight loss, dehydration, fecal clearance, and bladder dysfunction, and animals were monitored for the possibility of urinary tract infection (none occurred). Manual expression of bladders was performed at least twice daily until spontaneous voiding returned. Ringer solution was provided subcutaneously after surgery to promote rehydration. A nutritional supplement (Jell-O® cube with added protein and fat) and sliced apples were provided to expedite recovery and assist in maintenance of body weight.
Drug administration
Testosterone-enanthate (TE, Savient Pharmaceutical, East Brunswick, NJ), a slowly released testosterone ester that elevates serum testosterone concentrations for 1 week,14 was dissolved in vehicle (sesame oil) and injected intramuscularly into alternating quadriceps musculature once every 7 days, under isoflurane anesthesia. The high-dose of TE (7.0 mg/week in 0.1 mL sesame oil) was chosen because it consistently maintains serum testosterone concentrations in the supraphyiologic range and because it has previously been shown to prevent cancellous bone loss and to produce potent myotrophic effects in male rodents after orchiectomy.14–16 The low-dose of TE (2.0 mg/week in 0.1 mL sesame oil) was chosen because it is reported to produce physiologic serum testosterone concentrations within several days of injection27 and to restore sex-organ weight and normal sexual and nonsexual behavior in sex-hormone deficient rats.28 In addition, the use of low-dose TE allows us to clarify whether maintenance of supraphysiologic testosterone within the circulation is necessary for musculoskeletal effects after SCI.
Bone histomorphometry and structural analyses
We evaluated cancellous bone characteristics in proximal tibial metaphysis and cortical bone characteristics in the tibial diaphysis using standard histologic techniques, as described previously in detail.29 All bone histologic measurements and nomenclature prescribe to the recommendations of the American Society of Bone and Mineral Research (ASBMR)30 and are further defined in Supplementary Table 1 (see online supplementary material at ftpliebert.com).
Briefly, the right tibia was excised from each animal at sacrifice and cut in half, cross-sectionally, with a hand-held rotary saw (Dremel Moto Tool, Racine, WI). The proximal and distal tibiae were placed in 10% phosphate-buffered formalin for 48 h for tissue fixation, dehydrated in ethanol, and embedded undecalcified in methyl methacrylate. The proximal tibiae were sectioned longitudinally at 4 μm and 8 μm thicknesses with a Leica/Jung 2065 microtome. The 4 μm bone sections were stained by the Von Kossa method with a tetrachrome counterstain (Polysciences Inc., Warrington, PA) for the assessment of cancellous bone structure, and the 8 μm bone sections of the proximal tibial metaphysis remained unstained to measure fluorochrome-based indices of bone formation. The tibial diaphyses 1–2 mm proximal to the tibiofibular junction were sawed in 200 μm-thick cross sections with an Isomet low-speed saw (Buehler, Lake Bluff, IL) and subsequently ground to a thickness of 50 μm between roughened glass plates before the histomorphometric evaluation of cortical bone structure and fluorochrome-based indices of bone formation.
Cancellous and cortical bone structural variables were measured in metaphyseal and diaphyseal sections, respectively, with the Osteomeasure System (Osteometrics, Atlanta, GA). The sample area within the proximal tibial metaphysis began 1 mm distal to the growth plate to exclude the primary spongiosa and cancellous bone tissue within 0.25 mm of the endocortical surfaces. The following bone variables were measured or calculated, according to previously described methods31: cancellous bone volume (as a percentage of bone tissue area, Cn.BV/TV %), trabecular number (Tb.N, #/mm), trabecular width (Tb.Wi, μm), trabecular separation (Tb.Sp, μm) measured at the proximal tibia metaphysis and cortical tissue area (Ct.T.Ar, mm2), cortical bone area (Ct.B.Ar, mm2), marrow area (Ma.Ar, mm2), and cortical width (distance between the periosteal and endocortical surfaces, Ct.Wi, μm) measured at the tibial diaphysis.
Fluorochrome-based indices of cancellous (proximal tibial metaphysis) and cortical (tibial diaphysis) bone formation were measured under ultraviolet illumination. The percentages of cancellous (osteoblast [Ob.S/BS, %], osteoclast [Oc.S/BS, %], and osteoid [OS/BS, %] as percentages of total cancellous perimeter), periosteal (as percentage of total periosteal perimeter, Ps.MS/BS, %), and endocortical (as percentage of total endocortical perimeter, Ec.MS/BS, %) bone surfaces with double fluorochrome labels were measured with the Osteomeasure System. Mineralizing surface (MS/BS), an index of active bone formation, was calculated as the percentage of cancellous bone surface with a double fluorochrome label. Mineral apposition rate (MAR), an index of osteoblast activity, was calculated by dividing the interlabel distance by the time interval between administration of fluorochrome labels (i.e., 7 days). Bone formation rate (BFR/BS) was calculated by multiplying MS/BS by MAR.
μCT and mechanical evaluation of bone
The right femoral metaphysis, diaphysis, and femoral neck were scanned by μCT with a Bruker Skyscan 1172 (Kontich, Belgium) in accordance with recommendations of the ASBMR32 and are further defined in Supplementary Table 1 (see online supplementary material at ftp.liebertpub.com). The acquisition settings were as follows: 80 kVP and current 120 μA, and a 0.5 mm thick aluminum filter was used for beam hardening reduction. Bones were scanned with a large camera resolution (1 k) at a voxel size of 19.2 μm, with a rotation step of 0.5 degrees, and tomographic rotation of 180 degrees.
The regions of interest (ROI) at the femoral metaphysis began 1.5 mm proximal to the growth plate and encompassed a total of 4 mm. The ROI at the diaphysis encompassed a 2 mm region beginning at 55% of the femur length to avoid the third trochanter that is present in rat femora. The ROI at the femoral neck encompassed 0.35 mm surrounding the smallest diameter of the neck.
Cross-sectional images were reconstructed using a filtered back-projection algorithm (NRecon, Kontich, Belgium). Two dimensional and three-dimensional morphometric measurements were calculated using CTan (version #1.13.1.1) software (Bruker Skyscan, Kontich, Belgium) and include cancellous measurement at the distal femur: BV/TV, Tb.N, Tb.Sp, trabecular thickness (Tb.Th), structural model index (SMI), and cortical measurements at the femoral midshaft: total cross-sectional bone (plus medullary) area (Tt.Ar), cortical bone area (Ct.Ar), medullary area (Ma.Ar), cortical area fraction (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th). In addition, medullary volumetric (v)BMD (cancellous bone only) was evaluated using the previously defined ROI within the distal femur, total/integral vBMD (cortical plus cancellous bone) and medullary vBMD were assessed using the previously defined femoral neck ROI, and cortical volumetric tissue mineral density (vTMD) was assessed using the previously defined femoral diaphysis ROI. Densities were determined after calibration with hydroxyapatite phantoms.
Subsequent to μCT, the femora underwent mechanical testing. The femora were thawed to room temperature and remained wrapped in saline soaked gauze except during measurements. The femoral neck was subjected to a compression and bending test using a Lloyd material testing machine (LR5K, J.J. Lloyd Instruments, Southhampton, UK), as previously described.15 Briefly, a pre-load (10N/0.1 mm/sec) was applied on the femoral head using a flat steel fixture. The bending load was applied at 1.0 mm/sec until failure of the specimen. The breaking load of the femoral neck was determined from the load-deformation curve.
Spinal cord histology
The spinal cord was embedded in paraffin and sectioned with a microtome (10 μm thickness). The sections were stained with hematoxylin and eosin, and light microscopic images were obtained using a Zeiss Axio Imager Z2 microscope (Carl Zeiss, Göttingen, Germany) at 2.5X magnification to qualitatively verify injury consistency among groups receiving SCI.
Serum measurements
Serum measurements were performed in duplicate on a single plate. Testosterone was determined by enzyme immunoassay (EIA) that requires 10 μL serum, has a sensitivity of 0.02 ng/mL, and an intra-assay coefficient of variation (CV) <9%, and estradiol was determined by ultra-sensitive enzyme-linked immunosorbent assay that requires 100 μL serum, has a sensitivity of 3 pg/mL, and an intra-assay CV <7.9% (ALPCO Diagnostics, Salem, NH). Osteocalcin (a circulating marker of whole-body bone formation) was determined by an EIA that requires 20 μL serum, has a sensitivity of 50 ng/mL, and an intra-assay CV <5.0%, and C-telopeptide (a circulating marker of whole-body bone resorption) was determined using an EIA that requires 20 μL serum and has a sensitivity of 2.0 ng/mL and an intra-assay CV below 9.2% (Immunodiagnostic systems, Fountain Hills, AZ).
Statistical analysis
Results are reported as means±standard deviation, and an α level of p<0.05 was defined as the threshold of significance. Mixed-Model Repeated Measures analyses of variance (ANOVAs) were used to analyze variables that were assessed at multiple time points. One-way ANOVAs were used to analyze normally distributed data that were assessed at only a single time point. Tukey posthoc tests were performed for multiple comparisons among groups when appropriate. The non-parametric Kruskal-Wallis and Mann-Whitney tests were performed when data were not normally distributed. Linear dependence between the serum sex-steroid hormones and specific bone/muscle structural variables were evaluated with Pearson correlations. Hormone values that were below the lowest detectable standard are reported as such and were assigned a value equal to the sensitivity of each individual assay for the purposes of statistical analysis. All statistical analyses were performed with the SPSS v15.0.0 statistical software package (IBM, Chicago, IL).
Results
Injury severity and locomotor function
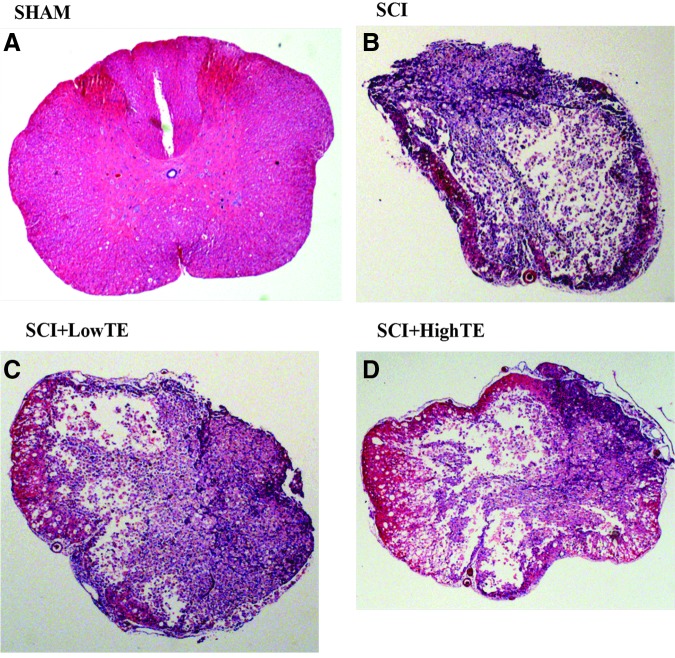
The injury force and velocity for the moderate/severe contusion SCI were 282±50 kdyne and 121±3 mm/sec in SCI animals, 282±42 kdyne and 122±4 mm/sec in SCI+LowTE animals, and 278±47 kdyne and 123±4 mm/sec in SCI+HighTE animals, respectively. No differences in injury force or in velocity were present among groups. Light microscopic studies of the spinal cord indicated that the injuries were symmetrical in most cases with complete destruction of central gray matter leaving only a thin rim of white matter mostly at the ventral and ventrolateral regions. There was no viable residual tissue sparing in dorsal regions. The central gray areas were filled with tissue debris. Under higher magnification, the spared white matter presented numerous vacuoles, from loss of tissue. Overall, histological assessment of the spinal cords indicated that a moderate to severe injury intensity was consistently present at the epicenter in the majority of the specimens receiving SCI (Fig. 1).
FIG. 1.
Histologic images of the spinal cord (hematoxylin and eosin stain, 2.5X magnification) from animals subjected to Sham surgery (T9 laminectomy) or moderate/severe (250 kdyne) spinal cord injury (SCI) alone or in combination with a low- (SCI+LowTE) or high-dose (SCI+HighTE) of testosterone-enanthate (TE). Note reduced mass of pink-stained white matter indicative of moderate/severe SCI (B, C, D) versus A (Sham surgery) indicating a qualitatively consistent injury intensity in all groups receiving SCI. Color image is available online at www.liebertpub.com/neu
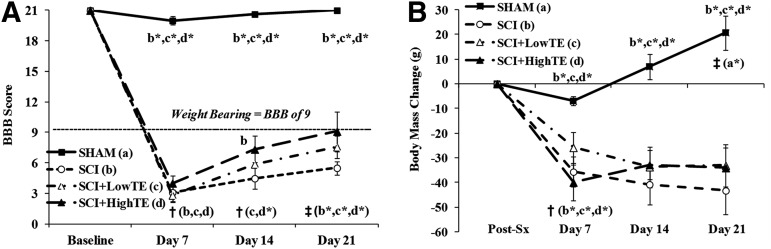
At Day 7, hindlimb locomotor function (BBB score) was reduced to a similar magnitude in all animals receiving SCI (Fig. 2A, p<0.001), and values remained lower than baseline and lower than SHAMs throughout the 21 day experiment (Fig. 2A, p<0.001). From Day 7 to Day 21, locomotor function progressively improved in all animals receiving SCI, with some differences present between groups. Specifically, locomotor function was improved at Day 14 (vs. Day 7) in SCI+LowTE (p<0.05) and in SCI+HighTE animals (p<0.01), while no significant improvement in locomotor function was present in SCI animals at this time point. At Day 21, locomotor function further improved such that BBB score was higher than Day 7 in all groups (p<0.01). In addition, locomotor function in SCI+HighTE animals was higher at Day 14 (but not Day 21) when compared with SCI animals (p<0.05).
FIG. 2.
Basso-Beattie-Bresnahan (BBB) scores (A) and body mass change (B) from animals subjected to Sham surgery (T9 laminectomy) or moderate/severe (250 kdyne) spinal cord injury (SCI) alone or in combination with a low- (SCI+LowTE) or high-dose (SCI+HighTE) of testosterone-enanthate (TE). Values are means±standard error of n=8–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or *p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE). †indicates within-groups difference compared with previous time point and ‡ indicates within-group difference compared with Day 7 for respectively labeled groups.
Body mass
At Day 7, the body mass of all groups receiving SCI was reduced 6–9% (p<0.01), while body mass of SHAM animals was not different than post-surgical weight (Fig. 2B). Subsequently, body mass of SHAM animals increased until sacrifice, while body mass in all groups receiving SCI remained mostly stable. When compared with SHAMs, body mass of all groups receiving SCI was 6–8% lower at Day 7 (p<0.05), 9–11% lower at Day 14 (p<0.01), and 12–13% lower at sacrifice (p<0.001), with no body mass differences present among SCI animals with or without TE.
Serum sex-steroid concentrations and circulating markers of bone turnover
Baseline serum sex steroids, osteocalcin, and C-telopeptide concentrations were within normal physiologic ranges and similar between groups (data not shown). At post-surgery Day 7, testosterone concentrations in SCI animals were 42% below SHAMs (non-significant, Table 1) and concentrations remained 45% below SHAMs at sacrifice (p=0.063, trend). In TE treated animals, testosterone concentrations peak within 1 day of injection and gradually decline over the following 7 days.14 Peak testosterone concentrations in SCI+LowTE and SCI+HighTE animals were ∼100% (p=0.005) and five-fold (p<0.001) above SHAM concentrations, respectively, while nadir concentrations were within the normal physiologic range and similar to SHAMs after LowTE administration and 70% higher than SHAMs after HighTE administration (p<0.05).
Table 1.
Serum Sex-Steroid Concentrations and Systemic Markers of Bone Formation and Resorption after Sham Surgery (T9 Laminectomy) or Moderate/Severe (250 kdyne) Spinal Cord Injury Alone or in Combination with a Low or High Dose of Testosterone-Enanthate
| SHAM (a) | SCI (b) | SCI+LowTE (c) | SCI+HighTE (d) | |
|---|---|---|---|---|
| Day 7 (nadir) testosterone, ng/mL | 3.1±1.9d | 1.8±0.9d* | 2.3±0.5d* | 4.9±0.7a,b*,c* |
| Day 21 (peak) testosterone, ng/mL | 4.5±2.6c*,d* | 2.5±1.4c*,d* | 9.4±2.6a*,b*,d* | 24.6±3.9a*,b*,d* |
| Estradiol, pg/mL | 6.1±2.5 | 5.7±2.3 | 5.2±1.5 | 8.2±4.4 |
| Osteocalcin, ng/mL | 407±73c,d* | 379±149d | 285±94a | 231±47a*,b |
| C-telopeptide, ng/mL | 57±17 | 58±17 | 53±11 | 43±14 |
Values are means±standard deviation of n=8–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or *p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE). Testosterone/vehicle was administered once weekly via intramuscular injection. Peak and nadir testosterone was evaluated 1 day and 7 days after testosterone/vehicle administration, respectively. All other values were acquired at sacrifice.
SCI, spinal cord injury; SCI+LowTE, spinal cord injury plus low-dose testosterone-enanthate; SCI+HighTE, spinal cord injury plus high-dose testosterone-enanthate.
Testosterone produced a dose-dependent reduction in osteocalcin with SCI+LowTE being 30% lower than SHAM (p=0.038, Table 1) and SCI+HighTE being 29% lower than SCI (p=0.015) and 43% lower than SHAM (p<0.001). No differences were present among groups for serum C-telopeptide. At sacrifice, no differences in serum estradiol were present among groups. In groups receiving SCI, serum testosterone and estradiol were positively correlated (r=0.418, p<0.05).
Cancellous bone histomorphometric analyses
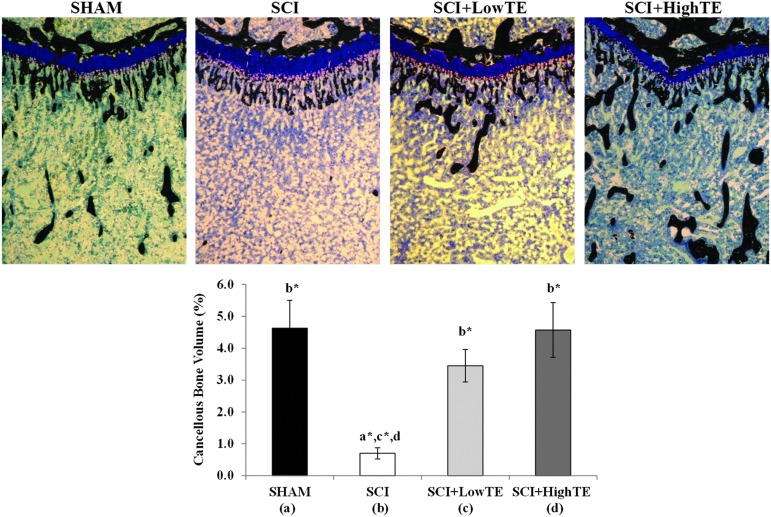
Histomorphometric analysis of the proximal tibia indicated that cancellous bone volume was 83% lower in SCI animals compared with SHAMs (p=0.002, Table 2, Fig. 3). This difference was characterized by a 75% reduction in trabecular number (p=0.004), 27% reduction in trabecular width (p<0.05), and >three-fold increase in trabecular separation (p=0.004). Conversely, testosterone administration resulted in dose-dependent prevention of cancellous bone loss, with cancellous bone volume being four-fold to five-fold higher in LowTE and HighTE animals compared with SCI (p<0.001). Ultimately, all cancellous morphometric variables in the proximal tibia of SCI+High TE animals were maintained at the level of SHAMs. Serum testosterone was positively correlated with several tibial morphometric outcomes (Supplementary Table 2; see online supplementary material at ftp.liebertpub.com), while no correlations were observed between estradiol and any bone variable.
Table 2.
Cancellous Histomorphometric Characteristics at the Proximal Tibial Metaphysis after Sham Surgery (T9 Laminectomy) or Moderate/Severe (250 Kdyne) Spinal Cord Injury Alone or in Combination with a Low or High Dose of Testosterone-Enanthate
| SHAM (a) | SCI (b) | SCI+LowTE (c) | SCI+HighTE (d) | |
|---|---|---|---|---|
| Cn.BV/TV, % | 4.6±2.6b* | 0.8±0.5a*,c*,d* | 3.5±1.4b* | 4.6±2.6b* |
| Tb.N, #/mm | 3.7±1.9b* | 0.9±0.6a*,c*,d* | 3.3±1.3b* | 4.1±2.1b* |
| Tb.Wi, μm | 14.6±3.2b | 10.7±3.0a,d | 12.5±1.2 | 13.0±1.4b |
| Tb.Sp, μm | 463±566b* | 1520±911a*,c*,d* | 384±300b* | 322±224b* |
| Oc.S/BS, % | 4.2±1.5 | 6.3±3.6d | 4.2±1.1 | 3.2±0.7b |
| Ob.S/BS, % | 19.2±4.6 | 16.0±8.4 | 14.5±4.3 | 14.1±6.6 |
| OS/BS, % | 16.1±3.6 | 13.5±7.0 | 12.2±4.4 | 11.8±5.3 |
| MS/BS, % | 13.5±4.2b*,c* | 2.6±1.6a*,c*,d | 5.7±2.3a*,b* | 8.9±6.5b |
| MAR, μm/day | 1.2±0.5b | 0.6±0.4a,d | 0.8±0.3 | 1.2±0.4b |
| BFR/BS (dL), μm3/μm2/day | 16.6±7.8b*,c* | 1.2±0.9a*,c*,d* | 4.9±3.3a*,b* | 11.4±11.7b* |
Values are means±standard deviation of n=8–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or * p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE). SCI, spinal cord injury; SCI+LowTE, spinal cord injury plus low-dose testosterone-enanthate; SCI+HighTE, spinal cord injury plus high-dose testosterone-enanthate. Cn.BV/TV, cancellous bone volume; Tb.N, trabecular number, Tb.Wi, trabecular width; Tb.Sp, trabecular separation; Oc.S/BS, osteoclast surface; Ob.S/BS, osteoblast surface; OS/BS, osteoid surface; MS/BS, mineralizing surface; MAR, mineral apposition rate, BFR/BS, bone formation rate/bone surface.
FIG. 3.
Histologic images of the proximal tibial metaphysis (von Kossa/tetrachrome stain, 40X) from animals subjected to Sham surgery (T9 laminectomy) or moderate/severe (250 kdyne) spinal cord injury (SCI) alone or in combination with a low- (SCI+LowTE) or high-dose (SCI+HighTE) of testosterone-enanthate (TE). Note reduced mass of black-stained cancellous bone (B) indicative of cancellous osteopenia in SCI animals; high-dose TE (D) fully prevented cancellous bone loss. Values are means±standard error of n=8–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or *p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE). Color image is available online at www.liebertpub.com/neu
Cancellous BFR/BS was 93% lower (p=0.001) in the proximal tibia of SCI animals compared with SHAMs (Table 2). Similarly, cancellous MS/BS was 80% lower (p<0.001) and MAR was 50% lower (p<0.05) in SCI animals compared with SHAMs. Conversely, TE partially prevented the SCI-induced reduction in cancellous BFR/BS at this bone site with LowTE and HighTE being>four-fold (p=0.005) and>nine-fold higher (p=0.001) than SCI, respectively. TE also protected against the SCI-induced reductions in cancellous MS/BS with LowTE preventing ∼30% of the decline (p=0.003) and HighTE preventing ∼58% of the decline (p<0.05). HighTE also produced a strong trend toward preventing the decline in MAR resulting from SCI (p=0.052). Ultimately, cancellous BFR/BS, MS/BS, and MAR in SCI+HighTE animals were not different than SHAMs. No changes in osteoblast or osteoid surfaces were present among groups. Osteoclast surface was 49% lower in SCI+HighTE animals compared with SCI animals (p<0.05). No other differences were present among groups.
Cortical bone histomorphometric analyses
Histomorphometric analyses indicated no differences in cortical bone structural characteristics were present among groups at the tibial diaphyses (Table 3). Periosteal MS/BS and BFR/BS were 74% (p=0.001) and 76% lower (p=0.004) in SCI animals compared with SHAMs, respectively. Because of lack of double fluorochrome labels, periosteal and endocortical MAR were not measurable and endocortical MS/BS and BFR/BS were undetectable in animals receiving SCI. TE administration did not prevent reductions in periosteal or endocortical MS/BS or BFR/BS at either dose. Values for all experimental groups were below that of SHAMs (p<0.001), with no differences among groups.
Table 3.
Cortical Histomorphometric Characteristics at the Tibial Diaphysis after Sham Surgery (T9 Laminectomy) or Moderate/Severe (250 Kdyne) Spinal Cord Injury Alone or in Combination with Low- or High-Dose Testosterone-Enanthate
| SHAM (a) | SCI (b) | SCI+LowTE (c) | SCI+HighTE (d) | |
|---|---|---|---|---|
| Ct.T.Ar, mm2 | 5.7±0.5 | 5.5±0.2 | 5.5±0.4 | 5.2±0.4 |
| Ct.B.Ar, mm2 | 4.7±0.5 | 4.5±0.2 | 4.5±0.4 | 4.3±0.4 |
| Ma.Ar, mm2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.2 | 0.9±0.1 |
| Ct.Wi, μm | 840±61 | 809±64 | 812±54 | 777±65 |
| Ps.MS/BS, % | 47±22b*,c*,d* | 12±8a* | 11±7a* | 10±4a* |
| Ps.MAR, μm/day | 1.4±0.6 | 1.0±0.3 | 1.1±0.4 | 1.1±0.5 |
| Ps.BFR/BS, μm3/μm2/day | 72±62b*,c*,d* | 17±11a* | 15±10a* | 16±13a* |
| Ec.MS/BS, % | 5.5±6.6b,c,d | 0.0±0.0a | 0.0±0.0a | 0.0±0.0a |
| Ec.MAR, μm/day | 0.5±0.5b,c,d | 0.0±0.0a | 0.0±0.0a | 0.0±0.0a |
| Ec.BFR/BS, μm3/μm2/day | 8.9±11.8b,c,d | 0.0±0.0a | 0.0±0.0a | 0.0±0.0a |
Values are means±standard deviation of n=7–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or *p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE). SCI, spinal cord injury; SCI+LowTE, spinal cord injury plus low-dose testosterone-enanthate; SCI+HighTE, spinal cord injury plus high-dose testosterone-enanthate. Ct.T.Ar, cortical tissue area; Ct.B.Ar, cortical bone area; Ma.Ar, marrow area; Ct.Wi, cortical width; Ps.MS/BS, periosteal mineralizing surface; Ps.MAR, periosteal mineral apposition rate; Ps.BFR/BS, periosteal bone formation rate; Ec.MS/BS, endocortical mineralizing surface; Ec.MAR, endocortical mineral apposition rate; Ec.BFR/BS, endocortical bone formation rate.
μCT and mechanical analyses of bone
Similar to histomorphometric analysis, μCT analysis of the distal femur indicated that cancellous BV/TV was 77% lower in SCI animals compared with SHAMs (p<0.001, Table 4). This difference was characterized by a 73% reduction in Tb.N (p<0.001), 13% reduction in Tb.Th (p=0.004), and 48% increase in Tb.Sp (p=0.021). TE dose dependently prevented bone loss with cancellous BV/TV being nearly three-fold (p=0.011) and 3.5-fold (p=0.001) higher in LowTE and HighTE animals, respectively. Structural model index (SMI) was also increased by SCI (p<0.001) at this bone site, and HighTE prevented this increase (p=0.002), while LowTE did not. Ultimately, all cancellous bone variables in the distal femur of SCI+HighTE animals were maintained at the level of SHAMs.
Table 4.
μCT Analysis of Three-Dimensional Cancellous Structural Characteristics at the Distal Femur after Sham Surgery (T9 Laminectomy) or Moderate/Severe (250 Kdyne) Spinal Cord Injury Alone or in Combination with Low- or High-Dose Testosterone-Enanthate
| SHAM (a) | SCI (b) | SCI+LowTE (c) | SCI+HighTE (d) | |
|---|---|---|---|---|
| BV/TV, % | 10.7±3.5b* | 2.5±1.1a*,c,d* | 7.3±2.1b | 8.6±3.4b* |
| Tb.N, #/mm | 0.91±0.27b* | 0.24±0.11a*,c*,d* | 0.65±0.17b* | 0.74±0.23b* |
| Tb.Th, mm | 0.116±0.006b* | 0.101±0.004a*,d | 0.111±0.006a* | 0.112±0.011b |
| Tb.Sp, mm | 0.86±0.26b | 1.27±0.38a,c*,d* | 0.69±0.12b* | 0.74±0.18b* |
| SMI | 2.31±0.17b*,c* | 2.90±0.20a*,d* | 2.67±0.19a* | 2.54±0.17b* |
Values are means±standard deviation of n=7–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or *p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE). SCI, spinal cord injury; SCI+LowTE, spinal cord injury plus low-dose testosterone-enanthate; SCI+HighTE, spinal cord injury plus high-dose testosterone-enanthate. BV/TV, bone volume fraction, Tb.Th, trabecular thickness; Tb.N, trabecular number, Tb.Sp, trabecular separation; SMI=structure model index.
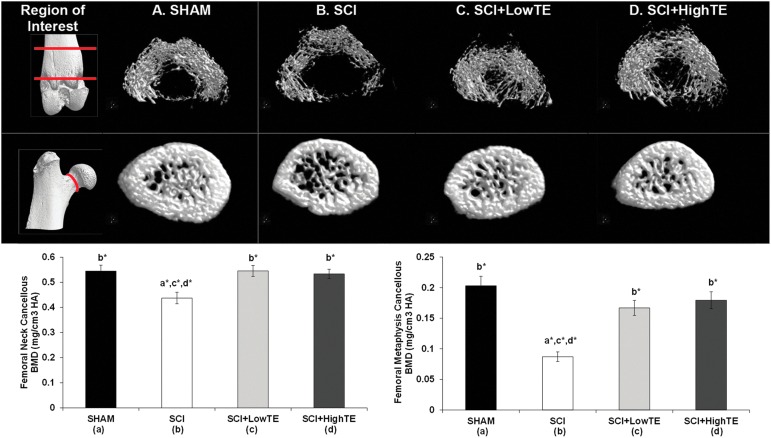
Cancellous (medullary) vBMD was 20% lower at the femoral neck (p=0.007) and 57% lower at the distal femoral metaphysis (p<0.001) of SCI animals compared with SHAMs (Fig. 4). TE (regardless of dose) prevented these reductions, with cancellous vBMD values being 92–106% higher at the distal femur (p<0.001) and 22–25% higher at the femoral neck (p=0.008) compared with SCI animals and not different than SHAMs. No differences were present among groups for cortical bone structural characteristics or cortical vTMD at the femoral diaphyses or for total/integral vBMD at the femoral neck (data not shown). The maximal (breaking) load of the femoral neck was 90.3±14.6 N (SHAMs), 77.9±23.6 N (SCI), 82.6±18.9 N (SCI+LowTE), and 89.7±10.3 N (SCI+HighTE), with no significant differences among groups. Serum testosterone was positively correlated with several femoral cancellous morphometric outcomes (Supplementary Table 2; see online supplementary material at ftp.liebertpub.com), while no associations were observed between estradiol and any femoral bone variable.
FIG. 4.
μCT images of cancellous bone at the distal femoral metaphysis and femoral neck of animals subjected to Sham surgery (T9 laminectomy) or moderate/severe (250 kdyne) spinal cord injury (SCI) alone or in combination with a low- (SCI+LowTE) or high-dose (SCI+HighTE) of testosterone-enanthate (TE). Values are means±standard error of n=8–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or *p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE). BMD, bone mineral density. Color image is available online at www.liebertpub.com/neu
Androgen sensitive peripheral tissue mass
LABC muscle mass was 22% lower in SCI animals compared with SHAMs (p=0.002, Table 5). Similarly, soleus, gastrocnemius/plantaris, and triceps surae muscle mass was 32–40% lower in SCI compared with SHAMs (p<0.001). Testosterone completely prevented the reduction in LABC mass, with SCI+LowTE being 30% higher (p=0.002) and SCI+HighTE being 44% higher (p<0.001) than SCI animals and equal to that of SHAMs. High-dose TE also ameliorated hindlimb muscle loss, with soleus, gastrocnemius/plantaris, and combined triceps surae muscle mass being 33% (p<0.05), 19% (trend, p=0.060), and 20% (p<0.05) higher than SCI animals, respectively; although, values remained 20–22% below SHAMs (p<0.05). In contrast, low-dose TE did not prevent SCI-induced reductions in hindlimb muscle mass.
Table 5.
Androgen Sensitive Peripheral Tissue Mass after Sham Surgery (T9 Laminectomy) or Moderate/Severe (250 Kdyne) Spinal Cord Injury Alone or in Combination with Low- or High-Dose Testosterone-Enanthate
| SHAM (a) | SCI (b) | SCI+LowTE (c) | SCI+HighTE (d) | |
|---|---|---|---|---|
| LABC, g | 1.38±0.21b* | 1.07±0.15a*,c*,d* | 1.39±0.14b* | 1.54±0.13b* |
| Soleus, g | 0.22±0.04b*,c*,d | 0.13±0.02a*,d | 0.15±0.03a* | 0.17±0.04a,b |
| Gastrocnemius/plantaris, g | 2.95±0.23b*,c*,d* | 2.00±0.43a* | 2.24±0.27a* | 2.37±0.22a* |
| Triceps surae, g | 3.22±0.21b*,c*,d* | 2.12±0.45a*,d | 2.40±0.30a* | 2.54±0.25a*b |
| Prostate, g | 0.52±0.11b,c,d* | 0.40±0.15a,c*,d* | 0.84±0.34a,b* | 0.96±0.37a,b* |
| Heart, g | 1.24±0.04 | 1.11±0.13 | 1.13±0.14 | 1.14±0.10 |
| Retroperitoneal fat, g | 2.12±0.52 | 1.59±0.43 | 2.03±0.78 | 1.51±0.31 |
Values are means±standard deviation of n=7–9/group. Letters a–d indicate differences from respectively labeled groups at p<0.05 or *p<0.01 (a=vs. SHAM, b=vs. SCI, c=vs. SCI+LowTE, d=vs. SCI+HighTE).
SCI, spinal cord injury; SCI+LowTE, spinal cord injury plus low-dose testosterone-enanthate; SCI+HighTE, spinal cord injury plus high-dose testosterone-enanthate; LABC, levator ani/bulbocavernosus.
Prostate mass was also reduced 24% in SCI animals compared with SHAMs (p<0.05, Table 5). Conversely, TE administration produced dose-dependent prostate enlargement, with SCI+LowTE being 61% higher (p<0.05) and 110% higher (p=0.004) and SCI+HighTE being 82% higher (p<0.01) and 140% higher (p=0.001) than SHAMs and SCI animals, respectively. No differences in heart mass or retroperitoneal fat mass were present among groups. LABC mass and prostate mass were positively correlated with serum testosterone concentration (Supplementaary Table 2) but not serum estradiol. No significant associations were present between testosterone or estradiol and hindlimb musculature.
Discussion
Our results provide the first evidence demonstrating that TE produces a dose-dependent protection against SCI-induced cancellous bone loss in the hindlimbs of rodents. In addition, our results confirm previous reports indicating that testosterone ameliorates the loss of hindlimb musculature after SCI8–10 and expands on these findings by demonstrating TE fully prevents muscle loss in the highly androgen sensitive LABC muscle. Specifically, we observed that a moderate/severe contusion SCI produced dramatic cancellous bone loss in both the proximal tibia and distal femur that was characterized by concomitant reductions in trabecular number and trabecular width, and increased trabecular separation occurring within 21 days of injury. Low-dose TE partially prevented these reductions and also prevented LABC muscle loss, but did not improve hindlimb muscle mass. Alternatively, high-dose TE fully prevented hindlimb cancellous bone loss, increased LABC muscle mass, and ameliorated hindlimb muscle loss. Prostate mass was also reduced after SCI, while TE produced dose-dependent prostate enlargement.
Bone loss and bone turnover after SCI
Severe lower extremity bone loss occurs clinically after SCI and is thought to result from increased bone resorption and reduced bone formation.20,33,34 This bone loss is characterized by large reductions in cancellous bone mass within the first few years after SCI with cortical bone loss persisting for >10 years.3 Similarly, the severe cancellous bone loss that we and others20,21,35 observed after SCI appears to have resulted from a combination of increased bone resorption, as indicated by an increased osteoclast surface, and reduced bone formation, as indicated by reduced cancellous MAR and BFR/BS. Interestingly, no changes in circulating osteocalcin or C-telopeptide (markers of whole body bone formation and resorption, respectively) were observed after SCI, which is somewhat inconsistent with previous reports indicating increased circulating markers of bone resorption occur in male and female rodents after spinal cord transection.20,34
These apparent inconsistencies likely result from bone loss and (elevated) bone turnover remaining isolated to bone below the lesion level after SCI (as has been reported in humans)36 and/or because our model differed in sex (male vs. female), age at time of injury (14 weeks vs. 6–9 weeks), and mode of injury (moderate-severe contusion SCI vs. spinal cord transection) in comparison with previous studies.20,34 We also observed that animals subjected to SCI exhibited deleterious microstructural alterations in the shape (architecture) of the remaining trabeculae (reported as SMI) indicative of reduced plate-like architecture or increased rod-like trabecular architecture18 and that high-dose TE prevented these changes. SMI is highly predictive of the maximal strength of cancellous bone, with the change from plate-like to rod-like structures reducing bone strength.37 Despite this, we observed only minimal alterations in femoral neck strength, most likely because cortical bone deficits were not observed within the short time frame of this study. In this regard, femoral neck strength is dependent on both the quantity and quality of cortical and cancellous bone.
Rodent SCI models
Several rodent models of SCI-induced bone loss exist in the literature.20,21,35 Of these, spinal cord transection induces the most severe bone loss,20 which exceeds that resulting from ovariectomy,17 sciatic neuroectomy,19 or hindlimb immobilization.18 Within 3 weeks of spinal cord transection, large cancellous bone deficits are observable, and these deficits are slightly worsened over the subsequent 6 months, ultimately reaching 70–80% cancellous bone loss that is characterized by a 60–80% loss of trabecular number/thickness and>four-fold increase in trabecular separation.20
Despite this severe bone loss, the spinal cord transection model does not mimic the histopathologic features of the most common type of SCI in humans, which primarily occur via a blow to the spinal cord followed by a period of spinal compression. Alternatively, the rodent mid-thoracic contusion SCI model is commonly used to assess therapeutic interventions focused on musculoskeletal tissue changes after SCI25 because this model closely reproduces the histopathological features observed after traumatic SCI in humans.23,24 In this regard, severe contusion SCI produces progressive hindlimb bone loss in female rodents, reaching a level of ∼67% cancellous bone loss and ∼20% cortical bone loss 8 weeks after injury.21 Interestingly, within 21 days of SCI we observed a magnitude of hindlimb cancellous bone loss that exceeded that of other severe contusion SCI models21 and was similar to that of the long-term (>6 month) transection SCI model.20
Our SCI model differed from previous rodent contusion and transection SCI bone loss models in that we used male rats (previous studies used females) that were of a significantly older age at time of injury (14 weeks in our study vs. 6–9 weeks in previous studies).20,21 We chose to use slightly older male rats because >80% of the clinical SCI population are adult men, and the rapid bone formation rates occurring in younger rats (at time of injury)38 may not be characteristic of bone formation rates in adults and because variations in bone mineral characteristics exist between males and females that result largely from differences in the endogenous sex-steroid milieu.39
Testosterone deficiency and musculoskeletal deficits after SCI
Androgens influence the development and maintenance of musculoskeletal tissue in males, as demonstrated by the ability of various aromatizable and non-aromatizable androgens to prevent musculoskeletal deficits resulting from gonadectomy.14–16 Approximately 40–80% of men with SCI exhibit low testosterone, with a higher incidence and a greater severity of hypogonadism occurring closer to time of injury,40 which coincides with the time frame in which men experience the greatest musculoskeletal deficits after SCI.2 We observed that testosterone concentrations were reduced by >40% in SCI animals beginning at 7 days post-SCI and persisting until sacrifice, when compared with SHAMs, suggesting that testosterone deficits may have influenced the SCI-induced reductions in musculoskeletal tissue that we observed.
The pathophysiology of SCI-induced bone and muscle loss is multifactorial, however, being primarily influenced by disuse and the severity of the neurologic insult.2 Despite this, our results indicate that TE dose dependently prevents sublesional musculoskeletal deficits after SCI, with high-dose TE fully preserving hindlimb cancellous bone characteristics. Interestingly, our results corroborate recent findings indicating that nandrolone (a synthetic androgen) treatment produces skeletal benefit subsequent to spinal cord transection.41 The magnitude of skeletal protection induced by either low- or high-dose TE far exceeded that of nandrolone, most likely because TE administration was initiated immediately post-SCI in our study, while Sun and associates,41 did not begin treatment until 28 days after SCI.
Interestingly, our results appear to be androgen-mediated, given that (1) estradiol was unchanged even by supraphysiologic TE administration and (2) testosterone concentrations were associated with several bone/muscle structural outcomes, while estradiol was not associated with any musculoskeletal outcome. Conversely, TE did not restore periosteal and endocortical bone formation after SCI, which is in direct contrast to the known role of testosterone in promoting radial expansion of cortical bone in uninjured animals42 and the ability of testosterone to prevent reductions in periosteal bone formation resulting from orchiectomy.43
The implications of these findings are that testosterone exerts divergent effects on cancellous and cortical bone after SCI, perhaps because androgens and mechanical loading provide a complementary influence on the maintenance of bone mass, as has been demonstrated in the hindlimb suspended androgen receptor knockout.44 Clearly, longer duration studies that pair testosterone treatment with mechanical loading are needed to directly address this intriguing possibility and to determine whether the beneficial effects of TE on musculoskeletal tissue persist beyond the initial time frame of this study.
Muscle preservation after SCI
Testosterone is known to produce dose-dependent myotrophic effects in non-neurologically impaired men, with higher doses producing more profound effects.45 Interestingly, both TE doses that we administered fully prevented LABC muscle loss after SCI, while only high-dose TE ameliorated hindlimb muscle loss. In this regard, the myotrophic effects of testosterone are primarily mediated by androgen receptor (AR) expression in muscle fibers.26,46 Importantly, the rodent LABC muscle highly expresses AR positive myonuclei and is highly sensitive to androgen status, while certain hindlimb muscles have much lower AR expressions,46 perhaps underlying the reduced androgen-mediated muscle preservation in the hindlimb musculature of rats in comparison with that of the LABC. In contrast, rodent hindlimb musculature responds more robustly to mechanical load and in a more classical manner to androgen status because of relatively lower AR expressions,46 with only continuously sustained supraphysiologic testosterone producing myotrophic adaptations. In support of this contention, both low- and high-dose TE produced peak testosterone concentrations in the supraphysiologic range, while only high-dose TE sustained testosterone concentrations above the normal physiologic range throughout the intervention and ameliorated muscle loss.
We also report that TE produced small dose-dependent improvements in hindlimb locomotor function (BBB), similar to results that have been reported by others.47 We are confident that these improvements were androgen-mediated and not simply an artifact resulting from a slightly different injury severity between groups because (1) the force and velocity of the injury impact were identical between groups, (2) BBB score was identical between groups at post-injury Day 7, and (3) qualitative histological examination of the spinal cord indicated that rodents received a moderate-severe injury that was consistent across all groups. This slight androgen-mediated improvement in hindlimb locomotor function raises the possibility that enhanced mechanical loading may have influenced the musculoskeletal improvements discussed above. We find this possibility unlikely, given that hindlimb weight support and hindlimb stepping ability were not restored until 21 days after injury in animals receiving SCI+HighTE and that quadrupedal ambulation- and/or exercise-induced improvements in cancellous BMD are not observable (subsequent to disuse) within this time frame in other rodent disuse models.48 Regardless, future research that quantitatively evaluates spared white matter after TE administration of SCI animals would assist in verifying the potential neuroprotective effects of testosterone and whether these changes influence musculoskeletal preservation.
Mechanisms of testosterone action
Despite the musculoskeletal benefits associated with TE administration, several known androgenic side effects exist with testosterone treatment, of which benign prostatic hyperplasia is a clinical concern. We observed a reduction in prostate mass in rodents receiving SCI, which is similar to that which occurs in men after SCI49 and may be related to the high prevalence of hypogonadism within this population.40 In an attempt to limit androgen-induced prostate enlargement, we administered TE in a low-dose (that has previously been reported to restore sex-organ weight and normal sexual and nonsexual behavior in chemically castrated rats).28 Both low- and high-dose TE, however, resulted in peak testosterone concentrations that were above SHAM values and produced prostate enlargement, demonstrating one barrier to the clinical implementation of high-dose testosterone as a means of preventing SCI-induced musculoskeletal deficits.
In this regard, the 5α-reduction of testosterone to dihydrotestosterone (DHT) is a direct stimulus for androgen-mediated prostate enlargement.46 As evidence, ORX rats receiving supraphysiologic testosterone experience robust prostate enlargement, while those receiving testosterone plus MK-434 (a rodent type I/II 5α-reductase inhibitor),50 trenbolone (a non-5α reducible synthetic testosterone analogue),15,16 or any of a variety of selective androgen receptor modulators (SARMs)26,46 maintain prostate mass at or below that of intact animals and experience musculoskeletal improvements. Importantly, the 5α-reduction of testosterone to DHT is not required for the myotrophic46 or bone protective effects of androgens.15,16,50 As such, future research evaluating the musculoskeletal effects of non-5α-reducible androgens, SARMs, or high-dose testosterone in combination with pharmacologic 5α-reductase inhibitors after SCI appears warranted and may provide a means of acquiring androgen-mediated bone and muscle maintenance in a safer manner.
Conclusion
We present the first-ever evidence demonstrating that testosterone dose dependently prevents bone loss after SCI, with high-dose TE providing full protection against the loss of cancellous bone. In addition, high-dose TE completely prevented the loss of non-weight bearing sublesional muscle (LABC) and ameliorated hindlimb muscle loss. Despite these benefits, TE produced prostate enlargement and was unable to prevent reductions in cortical bone formation. These latter findings illustrate potential barriers to clinical implementation of high-dose TE administration as a means of restoring musculoskeletal tissue after SCI. Future research examining the interactions of mechanical loading and testosterone administration on hindlimb musculoskeletal tissue after SCI may assist in providing a mechanistic understanding of the potential divergent role of testosterone in cancellous and cortical bone after SCI and may improve the therapeutic potential of testosterone treatment within this population.
Supplementary Material
Acknowledgments
This work was funded by a Department of Veterans' Affairs RR&D CDA-2 (#B7733-2) to JFY.
We thank Raj Manoharan (Micro Photonics, Inc.) for his excellent technical assistance with μCT analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Devivo M.J. (2012). Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372 [DOI] [PubMed] [Google Scholar]
- 2.Giangregorio L., and McCartney N. (2006). Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J. Spinal Cord Med. 29, 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zehnder Y., Luthi M., Michel D., Knecht H., Perrelet R., Neto I., Kraenzlin M., Zach G., and Lippuner K. (2004). Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos. Int. 15, 180–189 [DOI] [PubMed] [Google Scholar]
- 4.Eser P., Frotzler A., Zehnder Y., Wick L., Knecht H., Denoth J., and Schiessl H. (2004). Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34, 869–880 [DOI] [PubMed] [Google Scholar]
- 5.Frisbie J.H. (1997). Fractures after myelopathy: the risk quantified. J. Spinal Cord Med. 20, 66–69 [DOI] [PubMed] [Google Scholar]
- 6.Castro M.J., Apple D.F., Jr., Hillegass E.A., and Dudley G.A. (1999). Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. Occup. Physiol. 80, 373–378 [DOI] [PubMed] [Google Scholar]
- 7.Spungen A.M., Wang J., Pierson R.N., Jr., and Bauman W.A. (2000). Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J. Appl. Physiol. 88, 1310–1315 [DOI] [PubMed] [Google Scholar]
- 8.Gregory C.M., Vandenborne K., Huang H.F., Ottenweller J.E., and Dudley G.A. (2003). Effects of testosterone replacement therapy on skeletal muscle after spinal cord injury. Spinal Cord 41, 23–28 [DOI] [PubMed] [Google Scholar]
- 9.Wu Y., Zhao J., Zhao W., Pan J., Bauman W.A., and Cardozo C.P. (2012). Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J. Neurotrauma 29, 1663–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byers J.S., Huguenard A.L., Kuruppu D., Liu N.K., Xu X.M., and Sengelaub D.R. (2012). Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J. Comp. Neurol. 520, 2683–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauman W.A., Cirnigliaro C.M., La Fountaine M.F., Jensen A.M., Wecht J.M., Kirshblum S.C., and Spungen A.M. (2011). A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm. Metab. Res. 43, 574–579 [DOI] [PubMed] [Google Scholar]
- 12.Cardozo C.P., Qin W., Peng Y., Liu X., Wu Y., Pan J., Bauman W.A., Zaidi M., and Sun L. (2010). Nandrolone slows hindlimb bone loss in a rat model of bone loss due to denervation. Ann. N. Y. Acad. Sci. 1192, 303–306 [DOI] [PubMed] [Google Scholar]
- 13.Wimalawansa S.M., Chapa M.T., Wei J.N., Westlund K.N., Quast M.J., and Wimalawansa S.J. (1999). Reversal of weightlessness-induced musculoskeletal losses with androgens: quantification by MRI. J. Appl. Physiol. 86, 1841–1846 [DOI] [PubMed] [Google Scholar]
- 14.Yarrow J.F., Conover C.F., Purandare A.V., Bhakta A.M., Zheng N., Conrad B., Altman M.K., Franz S.E., Wronski T.J., and Borst S.E. (2008). Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am. J. Physiol. Endocrinol. Metab. 295, E1213–E1222 [DOI] [PubMed] [Google Scholar]
- 15.McCoy S.C., Yarrow J.F., Conover C.F., Borsa P.A., Tillman M.D., Conrad B.P., Pingel J.E., Wronski T.J., Johnson S.E., Kristinsson H.G., Ye F., and Borst S.E. (2012). 17beta-Hydroxyestra-4,9,11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone 51, 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarrow J.F., Conover C.F., McCoy S.C., Lipinska J.A., Santillana C.A., Hance J.M., Cannady D.F., VanPelt T.D., Sanchez J., Conrad B.P., Pingel J.E., Wronski T.J., and Borst S.E. (2011). 17beta-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am. J. Physiol. 300, E650–E660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang S.D., Shen C., Jiang L.S., and Dai L.Y. (2007). Differences of bone mass and bone structure in osteopenic rat models caused by spinal cord injury and ovariectomy. Osteoporos. Int. 18, 743–750 [DOI] [PubMed] [Google Scholar]
- 18.Liu D., Zhao C.Q., Li H., Jiang S.D., Jiang L.S., and Dai L.Y. (2008). Effects of spinal cord injury and hindlimb immobilization on sublesional and supralesional bones in young growing rats. Bone 43, 119–125 [DOI] [PubMed] [Google Scholar]
- 19.Jiang S.D., Jiang L.S., and Dai L.Y. (2006). Spinal cord injury causes more damage to bone mass, bone structure, biomechanical properties and bone metabolism than sciatic neurectomy in young rats. Osteoporos. Int. 17, 1552–1561 [DOI] [PubMed] [Google Scholar]
- 20.Jiang S.D., Jiang L.S., and Dai L.Y. (2007). Changes in bone mass, bone structure, bone biomechanical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif. Tissue Int. 80, 167–175 [DOI] [PubMed] [Google Scholar]
- 21.Voor M.J., Brown E.H., Xu Q., Waddell S.W., Burden R.L., Jr., Burke D.A., and Magnuson D.S. (2012). Bone loss following spinal cord injury in a rat model. J. Neurotrauma 29, 1676–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erbayraktar Z., Gokmen N., Yilmaz O., and Erbayraktar S. (2013). Experimental traumatic spinal cord injury. Methods Mol. Biol. 982, 103–112 [DOI] [PubMed] [Google Scholar]
- 23.Gregory C.M., Vandenborne K., Castro M.J., and Dudley G.A. (2003). Human and rat skeletal muscle adaptations to spinal cord injury. Can. J. Appl. Physiol. 28, 491–500 [DOI] [PubMed] [Google Scholar]
- 24.Basso D.M., Beattie M.S., and Bresnahan J.C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 [DOI] [PubMed] [Google Scholar]
- 25.Liu M., Bose P., Walter G.A., Thompson F.J., and Vandenborne K. (2008). A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal Cord 46, 488–493 [DOI] [PubMed] [Google Scholar]
- 26.Yarrow J.F., McCoy S.C., and Borst S.E. (2010). Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids 75, 377–389 [DOI] [PubMed] [Google Scholar]
- 27.Klapcinska B., Jagsz S., Sadowska-Krepa E., Gorski J., Kempa K., and Langfort J. (2008). Effects of castration and testosterone replacement on the antioxidant defense system in rat left ventricle. J. Physiol. Sci. 58, 173–177 [DOI] [PubMed] [Google Scholar]
- 28.Fielder T.J., Peacock N.R., McGivern R.F., Swerdloff R.S., and Bhasin S. (1989). Testosterone dose-dependency of sexual and nonsexual behaviors in the gonadotropin-releasing hormone antagonist-treated male rat. J. Androl. 10, 167–173 [DOI] [PubMed] [Google Scholar]
- 29.Iwaniec U.T., Wronski T.J., and Turner R.T. (2008). Histological analysis of bone. Methods Mol. Biol. 447, 325–341 [DOI] [PubMed] [Google Scholar]
- 30.Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R., and Parfitt A.M. (2013). Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recker R.R., Kimmel D.B., Dempster D., Weinstein R.S., Wronski T.J., and Burr D.B. (2011). Issues in modern bone histomorphometry. Bone 49, 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., and Muller R. (2010). Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 33.Roberts D., Lee W., Cuneo R.C., Wittmann J., Ward G., Flatman R., McWhinney B., and Hickman P.E. (1998). Longitudinal study of bone turnover after acute spinal cord injury. J. Clin. Endocrinol. Metab. 83, 415–422 [DOI] [PubMed] [Google Scholar]
- 34.Qin W., Sun L., Cao J., Peng Y., Collier L., Wu Y., Creasey G., Li J., Qin Y., Jarvis J., Bauman W.A., Zaidi M., and Cardozo C. (2013). The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J. Biol. Chem. 288, 13511–13521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morse L., Teng Y.D., Pham L., Newton K., Yu D., Liao W.L., Kohler T., Muller R., Graves D., Stashenko P., and Battaglino R. (2008). Spinal cord injury causes rapid osteoclastic resorption and growth plate abnormalities in growing rats (SCI-induced bone loss in growing rats). Osteoporos. Int. 19, 645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dauty M., Perrouin Verbe B., Maugars Y., Dubois C., and Mathe J.F. (2000). Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27, 305–309 [DOI] [PubMed] [Google Scholar]
- 37.Mittra E., Rubin C., and Qin Y.X. (2005). Interrelationship of trabecular mechanical and microstructural properties in sheep trabecular bone. J. Biomech. 38, 1229–1237 [DOI] [PubMed] [Google Scholar]
- 38.Horton J.A., Bariteau J.T., Loomis R.M., Strauss J.A., and Damron T.A. (2008). Ontogeny of skeletal maturation in the juvenile rat. Anat. Rec. (Hoboken) 291, 283–292 [DOI] [PubMed] [Google Scholar]
- 39.Compston J.E. (2001). Sex steroids and bone. Physiol. Rev. 81, 419–447 [DOI] [PubMed] [Google Scholar]
- 40.Clark M.J., Schopp L.H., Mazurek M.O., Zaniletti I., Lammy A.B., Martin T.A., Thomas F.P., and Acuff M.E. (2008). Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am. J. Phys. Med. Rehabil. 87, 758–767 [DOI] [PubMed] [Google Scholar]
- 41.Sun L., Pan J., Peng Y., Wu Y., Li J., Liu X., Qin Y., Bauman W.A., Cardozo C., Zaidi M., and Qin W. (2013). Anabolic steroids reduce spinal cord injury-related bone loss in rats associated with increased Wnt signaling. J. Spinal Cord Med. 36, 616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderschueren D., Vandenput L., Boonen S., Lindberg M.K., Bouillon R., and Ohlsson C. (2004). Androgens and bone. Endocr. Rev. 25, 389–425 [DOI] [PubMed] [Google Scholar]
- 43.Turner R.T., Wakley G.K., and Hannon K.S. (1990). Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J. Orthop. Res. 8, 612–617 [DOI] [PubMed] [Google Scholar]
- 44.Saita Y., Nakamura T., Mizoguchi F., Nakashima K., Hemmi H., Hayata T., Ezura Y., Kurosawa H., Kato S., and Noda M. (2009). Combinatory effects of androgen receptor deficiency and hind limb unloading on bone. Horm. Metab. Res. 41, 822–828 [DOI] [PubMed] [Google Scholar]
- 45.Bhasin S., Woodhouse L., Casaburi R., Singh A.B., Mac R.P., Lee M., Yarasheski K.E., Sinha-Hikim I., Dzekov C., Dzekov J., Magliano L., and Storer T.W. (2005). Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J. Clin. Endocrinol. Metab. 90, 678–688 [DOI] [PubMed] [Google Scholar]
- 46.Yarrow J.F., McCoy S.C., and Borst S.E. (2012). Intracrine and myotrophic roles of 5alpha-reductase and androgens: a review. Med. Sci. Sports Exerc. 44, 818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeman R.J., Bauman W.A., Wen X., Ouyang N., Etlinger J.D., and Cardozo C.P. (2009). Improved functional recovery with oxandrolone after spinal cord injury in rats. Neuroreport 20, 864–868 [DOI] [PubMed] [Google Scholar]
- 48.Yarrow J.F., McCoy S.C., Ferreira J.A., Pingel J.E., Conrad B.P., Wronski T.J., Williams A.A., Borst S.E., and Brown M. (2012). A rehabilitation exercise program induces severe bone mineral deficits in estrogen-deficient rats after extended disuse. Menopause 19, 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pannek J., Bartel P., Gocking K., and Frotzler A. (2013). Prostate volume in male patients with spinal cord injury: a question of nerves? BJU Int. 112, 495–500 [DOI] [PubMed] [Google Scholar]
- 50.Borst S.E., Conover C.F., Carter C.S., Gregory C.M., Marzetti E., Leeuwenburgh C., Vandenborne K., and Wronski T.J. (2007). Anabolic effects of testosterone are preserved during inhibition of 5alpha-reductase. Am. J. Physiol. Endocrinol. Metab. 293, E507–E514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.