Abstract
OBJECTIVE
To examine open-flame and/or high-temperature cooking (grilling/barbecuing, broiling, or roasting) and doneness preferences (rare, medium, or well done) for red meat, chicken, and fish in relation to type 2 diabetes (T2D) risk among U.S. adults who consumed animal flesh regularly (≥2 servings/week).
RESEARCH DESIGN AND METHODS
The prospective studies included 52,752 women from the Nurses’ Health Study (NHS) (followed during 1996–2012), 60,809 women from NHS II (followed during 2001–2013), and 24,679 men from the Health Professionals Follow-Up Study (HPFS) (followed during 1996–2012) who were free of diabetes, cardiovascular disease, and cancer at baseline. Incident cases of T2D were confirmed by validated supplementary questionnaires.
RESULTS
We documented 7,895 incident cases of T2D during 1.74 million person-years of follow-up. After multivariate adjustments including baseline BMI and total consumption of red meat, chicken, and fish, higher frequency of open-flame and/or high-temperature cooking was independently associated with an elevated T2D risk. When comparing open-flame and/or high-temperature cooking >15 times/month with <4 times/month, the pooled hazard ratio (HR) (95% CI) of T2D was 1.28 (1.18, 1.39; Ptrend <0.001). When comparing the extreme quartiles of doneness-weighted frequency of high-temperature cooking, the pooled HR (95% CI) of T2D was 1.20 (1.12, 1.28; Ptrend <0.001). These associations remained significant when red meat and chicken were examined separately. In addition, estimated intake of heterocyclic aromatic amines was also associated with an increased T2D risk.
CONCLUSIONS
Independent of consumption amount, open-flame and/or high-temperature cooking for both red meat and chicken is associated with an increased T2D risk among adults who consume animal flesh regularly.
Introduction
The role of diet as one of the modifiable factors precipitating diabetes incidence has been well established (1,2). Based on current evidence gleaned from observational studies and clinical trials, the U.S. Dietary Guidelines recommend eating an overall healthful diet for the prevention of major chronic diseases (3). Regarding protein intake, the guidelines recommend seafood, poultry, and lean meats, among other sources of protein, as components of a healthful diet (3).
These sources of protein, however, may exert differing health effects on type 2 diabetes (T2D) risk, as suggested by evidence from human observational studies (4–7). For example, red meat consumption, especially processed red meat, has been consistently associated with an increased risk of T2D (4,8). For fish intake, although two meta-analyses of prospective studies consistently demonstrated no overall relationship with T2D risk, opposing associations have been observed when separating the studies based on geographical region: a positive association in the U.S. and Europe but an inverse association in Asia and Australia (7,9). For chicken or poultry intake and T2D risk, previous studies have also yielded mixed results (5,6,10). In a previous study, we found that red meat cooking methods were associated with risk of developing T2D in women (11). Further investigations are needed to substantiate the association between cooking methods of other kinds of meats and diabetes risk.
Moreover, accumulating evidence has suggested that cooking meats at high temperature can produce several hazardous chemicals, including heterocyclic aromatic amines (HAAs), polycyclic aromatic hydrocarbons (PAHs), and advanced glycation end products (AGEs) (12–14), which are known carcinogens or can impact inflammation and insulin sensitivity (15–19). Cooking temperature, duration, and doneness level can significantly affect the levels of these chemicals in cooked meats (12–14). To date, no study has comprehensively examined meat cooking methods (such as grilling/barbecuing, broiling, or roasting), doneness level (rare, medium, or well done), and dietary HAA intake in relation to T2D risk.
To fill these knowledge gaps, we prospectively investigated associations of open-flame and/or high-temperature cooking methods for different types of meats, doneness preferences, and estimated HAA intake with the risk of developing T2D in three large prospective cohort studies of U.S. men and women.
Research Design and Methods
Study Population
We used data from three prospective cohort studies: the Nurses’ Health Study (NHS), NHS II, and the Health Professionals Follow-Up Study (HPFS). The NHS included 121,700 U.S. female nurses aged 30 to 55 years enrolled in 1976 from 11 states. The NHS II, established in 1989, included 116,671 younger female registered nurses aged 25 to 42 years from 14 states. The HPFS, established in 1986, included 51,529 U.S. men aged 40 to 75 years from 50 states. More details on the three cohorts and data collection can be found elsewhere (20,21). At baseline and every 2 years thereafter, participants of the three cohorts updated information on lifestyle factors, medical history, and newly diagnosed diseases through self-administered questionnaires, with a cumulative response rate over 90%. A semiquantitative food frequency questionnaire (FFQ) was administered in 1980 in NHS, 1991 in NHS II, and 1986 in HPFS, and diet information was updated every 2–4 years in these cohorts using the FFQs (22). Reasonable reproducibility and validity of the questionnaires have been detailed elsewhere (23,24).
In the current analysis, the study baseline was 1996 for NHS and HPFS and 2001 for NHS II, when detailed information on different cooking methods for different types of meats was collected. Participants were excluded if they reported a diagnosis of diabetes, cardiovascular disease, or cancer at baseline (n = 14,323 in NHS, n = 12,177 in NHS II, and n = 7,140 in HPFS); if they reported implausible daily caloric intake (<500 or >3,500 kcal/day for women, <800 or >4,200 kcal/day for men); or if they had missing information on all cooking methods for red meat, chicken, and fish (n = 4,129 in NHS, n = 9,806 in NHS II, and n = 4,450 in HPFS). In addition, we restricted the analysis to the participants (81.1% of NHS, 88.8% of NHS II, and 86.5% of HPFS) who consumed red meat, poultry, or fish regularly (≥2 servings/week). For the participants who reported the frequency of cooking methods for meats but did not respond about the doneness level (<3%), missing values were imputed with the mode. After exclusions, 52,752 women in NHS, 60,809 women in NHS II, and 24,679 men in HPFS were included in the final analysis with 12–16 years of follow-up (the end of follow-up was 2012 for NHS and HPFS and 2013 for NHS II). In a sensitivity analysis, we excluded the participants with missing data on meat cooking method and doneness level. The study protocol was approved by the institutional review boards at the Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital. The return of the questionnaires was considered implied consent.
Assessment of Diet and Cooking Methods for Meats
The intake of foods and nutrients was calculated and updated based on the validated FFQ every 4 years from baseline to the end of follow-up (22). The 2010 Alternative Healthy Eating Index (AHEI) was calculated (22). All nutrients were adjusted for total energy intake using the residual method (25).
In the 1996 (NHS and HPFS) and 2001 (NHS II) questionnaires, participants were asked the frequency of cooking chicken by pan-frying, broiling, and grilling/barbecuing, the frequency of cooking fish by broiling, and the frequency of cooking hamburger, beef, or steak by pan-frying, roasting, and grilling/barbecuing—with seven prespecified response categories (never, less than 1 time/month, 1 time/month, 2–3 times/month, 1 time/week, 2–3 times/week, and 4+ times/week). Moreover, participants were also asked about the doneness level (lightly browned, medium browned, well browned, and blackened/charred) for each individual cooking method for different types of meats. In HPFS, the information on the frequency of each cooking method and the doneness level for each meat was asked again in the 2004 questionnaire. A previous study demonstrated reasonable validity of our questionnaire assessments of HAA intake when compared with a more detailed questionnaire (e.g., the deattenuated correlation coefficients were 0.60 for 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline [MeIQx] and 0.36 for 2-amino-1-methyl-6-phenylimiazol[4,5-b]pyridine [PhIP]) (26). In addition, in HPFS, cooking method frequencies assessed in 1996 and 2004 were moderately correlated (Pearson correlation coefficients ranged from 0.33 to 0.40). Although the validity of the cooking questionnaire assessments was not directly assessed, moderate correlations were observed between the frequency of each cooking method and the amount of consumption of each animal food assessed in the 1994 FFQ in NHS and HPFS and the 1999 FFQ in NHS II (Spearman correlation coefficients range 0.3–0.5, all P < 0.001).
In addition to the individual cooking methods for red meat, chicken, and fish, we also summed the frequency of grilling/barbecuing, broiling, and roasting to reflect the overall frequency of open-flame and/or high-temperature cooking methods for red meat (hamburger, beef, and steak) (11) and chicken. Because the questionnaire only inquired about broiling fish, for which the frequency was quite low among our participants (e.g., only 4.3% of participants broiled fish ≥2 times/week), we did not examine fish broiling as a separate method in the main analysis. When we estimated the frequency of open-flame and/or high-temperature cooking methods for total meats (red meat, chicken, and fish), we included fish broiling frequency. Pan-frying was not considered as a potentially risky cooking method because this method was not associated with higher T2D risk in our previous study in NHS (which used a different questionnaire for red meat cooking methods that was only administered in 1986) (11). The overall frequency of open-flame and/or high-temperature cooking methods was categorized into five groups in the three cohorts (based on the quintiles of cooking frequencies with minor adjustments to have common absolute cut points across cohorts): for total meats (red meat, chicken, and fish), <4, 4–7, 8–11, 12–15, and >15 times/month; for red meat, <1, 1, 2–3, 4–5, and >5 times/month; and for chicken, <2, 2–4, 5–7, 8–10, and >10 times/month. In a sensitivity analysis, the cooking frequency was categorized into simple quintiles in each cohort.
Regarding doneness level, because there were few participant responses in the blackened/charred category (e.g., 0.2% for broiling chicken and 0.9% for barbecuing steak in NHS), we combined well browned and blackened/charred into one category. Meat doneness levels were assigned values of 1, 2, or 3 for lightly browned, medium browned, and well browned or blackened/charred, respectively. A doneness-weighted frequency of high-temperature cooking was generated by multiplying the assigned value (1, 2, or 3) for each doneness level for open-flame and/or high-temperature cooking methods (i.e., broiling, barbecuing/grilling, and roasting) by the frequency of each cooking method and then summing.
Assessment of HAA Intake
In the current study, the estimated dietary HAA intake was derived by multiplying the frequency of cooking meats with a prespecified portion size with HAA levels (ng/g meat) according to specific cooking methods and doneness levels and then summing. In this calculation, we used the CHARRED database (dceg.cancer.gov/tools/design/charred), an online database containing HAA levels measured in meat samples cooked using different methods by various doneness levels (26,27). More details are described in Supplementary Data Appendix 1.
Ascertainment of T2D
A validated supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy was mailed to participants who reported having diabetes in the biennial questionnaires. The validity of self-reported T2D diagnosis has been documented previously (28,29). More details are described in Supplementary Data Appendix 1.
Assessment of Covariates
In the biennial follow-up questionnaires, information was updated on demographic, socioeconomic, and lifestyle factors, including cigarette smoking, alcohol consumption, physical activity, marital status, menopausal status (women only), use of postmenopausal hormones (women only), and multivitamin use. BMI was calculated as self-reported weight in kilograms divided by the square of height in meters (kg/m2). Physical activity was estimated as METs per week based on the average hours spent on various activities, weighted by the intensity level.
Statistical Analysis
Pearson correlation coefficients (rs) were calculated to evaluate correlations between the frequencies of cooking methods. Person-years were calculated from the return of the baseline questionnaire to the date of T2D diagnosis, death, or loss to follow-up or to the end of follow-up (30 June 2012 for NHS, 30 June 2013 for NHS II, and 31 January 2012 for HPFS), whichever came first. Cox proportional hazards models were applied to calculate hazard ratios (HRs) and 95% CIs for the associations of meat intake, frequency of individual cooking methods for red meat, chicken, or fish, frequency of open-flame and/or high-temperature cooking, doneness-weighted frequency of high-temperature/open-flame cooking, and dietary intake of HAAs with the risk of T2D. To minimize sample size reduction due to missing covariates (<2%), indicator variables were created for missing categorical variables. In a sensitivity analysis, we restricted our analyses to the participants without missing data for covariates. In the multivariate model, in addition to age and calendar year, we further adjusted for ethnicity, marital status (married, not married, or missing), smoking status (never smoker, past smoker, current smoker [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol consumption (g/day: 0, 0.1–4.9, 5.0–14.9, or ≥15.0 in women; 0, 0.1–4.9, 5.0–29.9, or ≥30.0 in men; or missing), physical activity (METs/week: 0–2.9, 3–8.9, 9–17.9, 18–26.9, ≥27.0, or missing), family history of diabetes (yes or no), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal [never, former, or current hormone use], or missing), total energy intake (kcal/day), and dietary quality as measured by the AHEI. To control for potential confounding by meat consumption, total intake of chicken, fish, and red meat were further adjusted, and analyses were also stratified by intake of total meats, red meat, and chicken (in tertiles). Moreover, baseline BMI was further controlled. In the multivariate model, the baseline exposures and covariates were used in NHS and NHS II, while the time-varying exposures and covariates were used in HPFS in which cooking method information was updated once during follow-up. In a sensitivity analysis in HPFS, we only used baseline exposures and covariates in the multivariate model. In addition, the linear trend was tested by assigning a median value to each category as a continuous variable. A joint analysis was conducted to examine the potential interaction between frequency of open-flame and/or high-temperature cooking and meat doneness preference score (cooking frequency was not taken into account) in relation to T2D risk. In the current study, the proportional hazards assumption was tested by using a likelihood ratio test comparing models with and without multiplicative interaction terms between exposure and calendar year, and the proportional hazards assumption was not violated in any analysis.
Linear regression models were applied to examine the associations of frequency of high-temperature cooking methods for total meats with 4-year weight change among the participants who were younger than 60 years at baseline, because differential body composition changes at older ages might influence the associations of interest. In addition, the association of frequency of open-flame and/or high-temperature cooking methods with the risk of obesity was also examined, with the exclusion of participants who were obese (BMI ≥30 kg/m2) at baseline. In the multivariate model, the same covariates mentioned above were included.
In sensitivity analyses, instead of adjusting for diet quality (as indicated by the AHEI), individual dietary factors were controlled for in the multivariate model. Considering that cooking methods may differ across regions of the country, geographic location (north, middle, south, or unknown) was further adjusted for in the multivariate model. The association of high-temperature cooking frequency with T2D risk was also examined, stratifying by baseline BMI. To minimize the potential confounding by total energy intake, in a sensitivity analysis we repeated the analyses using the energy-adjusted residues of all food intakes and cooking methods calculated using linear regression (25). To reduce the possibility that participants with high risk of T2D or prediabetes may change their cooking practice, we excluded participants who reported incident T2D diagnosed in the first 4-year follow-up. In addition, we excluded the participants (<3%) who reported information on cooking frequency but were missing information on meat doneness level. In another sensitivity analysis, participants with low meat consumption (<2 servings/week) were also included.
Stratified analyses were conducted by age (<60 years, ≥60 years), BMI (<30 kg/m2, ≥30 kg/m2), physical activity (< median level, ≥ median level), and current smoking status (yes, no) to determine potential effect modification by these factors. The P values for the product terms between median frequency of high-temperature cooking methods and stratification variables were used to estimate the significance of interactions.
The Cochran Q statistic and the I2 statistic were used to examine the heterogeneity of associations among the cohorts. All analyses were conducted separately in each cohort and the results were then pooled using a fixed-effects model or a random-effects model if there was heterogeneity. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, NC). Two-sided P < 0.05 was considered statistically significant.
Results
The age-adjusted distribution of baseline characteristics of the study populations are shown in Table 1. Participants who reported higher frequency of open-flame and/or high-temperature cooking of total meats tended to be younger (primarily in NHS and HPFS), have a higher BMI and physical activity level, and have a higher consumption of alcohol, total energy, total red meat, chicken, fish, vegetables, fruits, soda, and protein. The rs values between frequencies of different cooking methods at baseline are shown in Supplementary Table 1. For each animal food (chicken and beef/steak), there were modest positive correlations between frequency of broiling, barbecuing, and roasting (rs range 0.10–0.40).
Table 1.
Age-adjusted baseline characteristics according to frequency of open-flame and/or high-temperature cooking of total meats among participants who consumed red meat, chicken, or fish regularly (≥2 servings/week)a
| Frequency of open-flame and/or high-temperature cooking of total meats (red meat, chicken, and fish)b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| NHS | NHS II |
HPFS |
|||||||
| <4 times/month | 8–11 times/month | >15 times/month | <4 times/month | 8–11 times/month | >15 times/month | <4 times/month | 8–11 times/month | >15 times/month | |
| Number of participantsc | 12,496 | 11,521 | 6,677 | 9,108 | 14,832 | 10,714 | 3,401 | 6,418 | 4,680 |
| Age, years | 62.7 ± 7.1 | 61.1 ± 6.9 | 60.7 ± 6.9 | 46.3 ± 4.7 | 46.0 ± 4.7 | 46.6 ± 4.5 | 64.3 ± 9.4 | 61.9 ± 9.1 | 61.2 ± 8.8 |
| BMI, kg/m2 | 25.8 ± 5.0 | 26.4 ± 5.0 | 26.7 ± 5.2 | 25.7 ± 5.8 | 26.4 ± 5.8 | 27.0 ± 6.2 | 25.5 ± 3.4 | 25.9 ± 3.4 | 26.0 ± 3.5 |
| Waist circumference, cm | 85.6 ± 5.3 | 86.1 ± 5.3 | 86.5 ± 5.5 | 85.7 ± 5.6 | 86.8 ± 5.6 | 87.7 ± 5.8 | 94.2 ± 7.4 | 94.7 ± 7.9 | 94.4 ± 8.3 |
| Physical activity, METs/week | 17.1 ± 22.2 | 18.2 ± 20.8 | 21.6 ± 24.5 | 18.5 ± 23.6 | 20.4 ± 23.9 | 26.1 ± 32.0 | 33.7 ± 40.8 | 36.9 ± 39.8 | 41.1 ± 44.7 |
| Alcohol intake, g/day | 4.4 ± 8.7 | 5.7 ± 9.3 | 6.0 ± 9.3 | 3.1 ± 6.7 | 4.5 ± 7.5 | 4.8 ± 7.8 | 9.4 ± 13.8 | 11.8 ± 14.8 | 12.8 ± 15.2 |
| Current smoking | 12 | 12 | 11 | 8 | 9 | 8 | 5 | 6 | 4 |
| White race | 98 | 98 | 97 | 95 | 97 | 96 | 95 | 96 | 96 |
| Family history of diabetes | 25 | 24 | 26 | 14 | 15 | 16 | 20 | 20 | 23 |
| Multivitamin use | 52 | 53 | 54 | 58 | 58 | 60 | 54 | 52 | 55 |
| Any use of postmenopausal hormone | 59 | 62 | 62 | 23 | 25 | 26 | NA | NA | NA |
| Married | 74 | 82 | 82 | 82 | 88 | 87 | 86 | 91 | 91 |
| Dietary intake | |||||||||
| Total energy, kcal/day | 1,671 ± 433 | 1,791 ± 440 | 1,863 ± 459 | 1,700 ± 485 | 1,839 ± 497 | 1,932 ± 517 | 1,869 ± 498 | 2,019 ± 531 | 2,129 ± 553 |
| Total red meat, servings/day | 1.00 ± 0.50 | 1.13 ± 0.50 | 1.11 ± 0.53 | 0.65 ± 0.50 | 0.80 ± 0.52 | 0.82 ± 0.62 | 0.83 ± 0.69 | 1.09 ± 0.77 | 1.07 ± 0.83 |
| Chicken, servings/day | 0.33 ± 0.24 | 0.41 ± 0.25 | 0.52 ± 0.30 | 0.41 ± 0.33 | 0.52 ± 0.35 | 0.69 ± 0.47 | 0.35 ± 0.28 | 0.42 ± 0.27 | 0.55 ± 0.33 |
| Fish, servings/day | 0.20 ± 0.19 | 0.27 ± 0.21 | 0.35 ± 0.27 | 0.18 ± 0.20 | 0.24 ± 0.21 | 0.31 ± 0.29 | 0.22 ± 0.21 | 0.28 ± 0.24 | 0.39 ± 0.32 |
| Total vegetables, servings/day | 2.9 ± 1.4 | 3.4 ± 1.4 | 3.9 ± 1.6 | 2.8 ± 1.8 | 3.3 ± 1.8 | 4.0 ± 2.3 | 2.9 ± 1.5 | 3.2 ± 1.4 | 3.7 ± 1.7 |
| Total fruits, servings/day | 2.2 ± 1.3 | 2.4 ± 1.2 | 2.7 ± 1.3 | 1.1 ± 0.8 | 1.2 ± 0.8 | 1.4 ± 1.0 | 2.3 ± 1.4 | 2.4 ± 1.3 | 2.7 ± 1.5 |
| Total dairy products, servings/day | 2.1 ± 1.1 | 2.1 ± 1.1 | 2.2 ± 1.1 | 2.2 ± 1.5 | 2.3 ± 1.5 | 2.4 ± 1.6 | 1.9 ± 1.2 | 1.9 ± 1.2 | 1.9 ± 1.2 |
| Soda, servings/day | 0.7 ± 0.9 | 0.8 ± 0.9 | 0.9 ± 0.9 | 1.2 ± 1.4 | 1.3 ± 1.4 | 1.4 ± 1.5 | 0.7 ± 0.8 | 0.8 ± 0.9 | 0.9 ± 1.0 |
| Whole grains, g/day | 19.8 ± 13.5 | 18.1 ± 11.3 | 18.9 ± 11.7 | 24.4 ± 15.5 | 22.2 ± 13.2 | 22.8 ± 13.4 | 28.1 ± 18.6 | 24.9 ± 15.9 | 25.7 ± 16.1 |
| Sodium, g/day | 2.1 ± 0.4 | 2.1 ± 0.4 | 2.1 ± 0.4 | 2.1 ± 0.3 | 2.1 ± 0.3 | 2.1 ± 0.3 | 2.5 ± 0.5 | 2.5 ± 0.5 | 2.4 ± 0.5 |
| Carbohydrate, % energy | 51.6 ± 7.0 | 50.1 ± 6.5 | 50.0 ± 6.7 | 52.1 ± 7.0 | 50.4 ± 6.6 | 50.0 ± 6.7 | 50.6 ± 7.7 | 48.3 ± 6.9 | 48.4 ± 7.2 |
| Protein, % energy | 18.0 ± 2.7 | 18.7 ± 2.5 | 19.8 ± 2.8 | 18.1 ± 3.0 | 18.8 ± 2.8 | 19.7 ± 2.9 | 17.3 ± 2.6 | 18.0 ± 2.5 | 18.9 ± 2.6 |
| Trans fatty acids, % energy | 1.5 ± 0.5 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.6 ± 0.6 | 1.6 ± 0.5 | 1.5 ± 0.5 | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.5 |
| P/S ratio | 0.56 ± 0.16 | 0.56 ± 0.14 | 0.58 ± 0.14 | 0.53 ± 0.15 | 0.53 ± 0.13 | 0.54 ± 0.14 | 0.59 ± 0.17 | 0.57 ± 0.15 | 0.60 ± 0.17 |
| AHEId | 46.6 ± 9.4 | 47.4 ± 8.6 | 50.0 ± 8.7 | 48.9 ± 10.3 | 49.6 ± 9.9 | 51.9 ± 10.0 | 47.3 ± 10.0 | 46.8 ± 9.5 | 49.0 ± 9.5 |
Data are mean ± SD or percentage. NA, not applicable; P/S ratio, polyunsaturated fatty acids–to–saturated fatty acids ratio.
aOpen-flame and/or high-temperature cooking of meats included broiling, barbecuing, or roasting of chicken, fish, or red meat.
bNot all the frequency categories (<4, 4–7, 8–11, 12–15, >15 times/month) are shown in the table because of limited space.
cThe total participants were 52,752 women in the NHS, 60,809 women in the NHS II, and 24,679 men in the HPFS.
dAlcohol consumption was not included in the AHEI score.
Frequency of Individual Cooking Methods for Red Meat, Chicken, and Fish and T2D Risk
During 1.74 million person-years of follow-up, we documented 7,895 incident cases of T2D. Supplementary Table 2 shows the associations of frequency of individual cooking methods for red meat, chicken, and fish with risk of T2D. After multivariate adjustment including total intake of chicken, fish, and red meat, higher frequency of broiling and barbecuing chicken and higher frequency of roasting beef and grilling/barbecuing steak were each associated with an increased T2D risk. In contrast, the frequency of pan-frying chicken, pan-frying steak or hamburger, or broiling fish was not significantly associated with T2D risk (Supplementary Table 2).
Frequency of High-Temperature Cooking and T2D Risk
Associations between frequency of open-flame and/or high-temperature cooking methods for meats and risk of T2D are shown in Table 2. After multivariate adjustment of covariates including baseline BMI and total intake of chicken, fish, and red meat, a higher frequency of high-temperature/open-flame cooking was associated with an increased T2D risk. When comparing open-flame and/or high-temperature cooking >15 times/month with <4 times/month, the pooled HR (95% CI) was 1.28 (1.18, 1.39; Ptrend <0.001; Pheterogeneity = 0.06) (model 4, Table 2). The results remained significant when red meat and chicken were analyzed separately: the pooled HR (95% CI) of T2D was 1.42 (1.29, 1.55; Ptrend <0.001) for red meat and 1.15 (1.07, 1.25; Ptrend = 0.002) for chicken. The associations did not materially change with further adjustment of baseline waist circumference (instead of baseline BMI). Similar results were observed when the cooking frequency was categorized into quintiles in each cohort. Supplementary Fig. 1 shows the associations between frequency of open-flame and/or high-temperature cooking and T2D risk according to baseline BMI categories.
Table 2.
HR (95% CI) of T2D according to frequency of open-flame and/or high-temperature cooking of meats among participants who consumed red meat, chicken, or fish regularly (≥2 servings/week)
| Frequency of open-flame and/or high-temperature cookinga |
||||||
|---|---|---|---|---|---|---|
| NHS | ||||||
| Total meatsb | <4 times/month | 4–7 times/month | 8–11 times/month | 12–15 times/month | >15 times/month | Ptrend |
| Servings/day | 1.5 ± 0.6 | 1.7 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.6 | 2.0 ± 0.6 | |
| Cases/person-years | 817/172,353 | 1,181/217,250 | 955/160,731 | 537/89,476 | 579/92,656 | |
| Model 1 | 1.00 | 1.15 (1.05, 1.25) | 1.24 (1.13, 1.37) | 1.26 (1.13, 1.40) | 1.31 (1.18, 1.46) | <0.001 |
| Model 2 | 1.00 | 1.21 (1.10, 1.32) | 1.38 (1.25, 1.52) | 1.42 (1.27, 1.58) | 1.53 (1.37, 1.71) | <0.001 |
| Model 3 | 1.00 | 1.18 (1.08, 1.29) | 1.32 (1.20, 1.45) | 1.34 (1.20, 1.50) | 1.42 (1.27, 1.59) | <0.001 |
| Model4 | 1.00 | 1.14 (1.04, 1.25) | 1.23 (1.12, 1.36) | 1.24 (1.11, 1.39) | 1.26 (1.12, 1.41) | <0.001 |
| Red meat | <1 times/month | 1 time/month | 2–3 times/month | 4–5 times/month | >5 times/month | Ptrend |
| Cases/person-years | 513/122,551 | 710/144,224 | 1,241/230,370 | 1,121/172,994 | 484/63,328 | |
| Model 1 | 1.00 | 1.18 (1.05, 1.32) | 1.28 (1.16, 1.42) | 1.55 (1.39, 1.72) | 1.81 (1.60, 2.05) | <0.001 |
| Model 2 | 1.00 | 1.19 (1.06, 1.34) | 1.27 (1.15, 1.42) | 1.54 (1.38, 1.71) | 1.77 (1.56, 2.02) | <0.001 |
| Model 3 | 1.00 | 1.17 (1.04, 1.31) | 1.23 (1.11, 1.37) | 1.46 (1.31, 1.63) | 1.66 (1.45, 1.90) | <0.001 |
| Model4 | 1.00 | 1.12 (1.00, 1.25) | 1.14 (1.02, 1.27) | 1.32 (1.18, 1.48) | 1.40 (1.23, 1.60) | <0.001 |
| Chicken | <2 times/month | 2–4 times/month | 5–7 times/month | 8–10 times/month | >10 times/month | Ptrend |
| Cases/person-years | 1,521/293,004 | 1,160/206,932 | 710/118,115 | 243/44,715 | 435/69,700 | |
| Model 1 | 1.00 | 1.08 (1.00, 1.16) | 1.15 (1.05, 1.25) | 1.04 (0.91, 1.19) | 1.20 (1.08, 1.33) | 0.001 |
| Model 2 | 1.00 | 1.15 (1.06, 1.24) | 1.26 (1.15, 1.38) | 1.17 (1.02, 1.34) | 1.34 (1.20, 1.50) | <0.001 |
| Model 3 | 1.00 | 1.14 (1.05, 1.23) | 1.23 (1.12, 1.35) | 1.14 (1.00, 1.31) | 1.28 (1.14, 1.43) | <0.001 |
| Model 4 |
1.00 |
1.09 (1.01, 1.18) |
1.14 (1.04, 1.25) |
1.07 (0.93, 1.22) |
1.16 (1.04, 1.30) |
0.008 |
| NHS II | ||||||
|---|---|---|---|---|---|---|
| Total meats | <4 times/month | 4–7 times/month | 8–11 times/month | 12–15 times/month | >15 times/month | Ptrend |
| Servings/day | 1.3 ± 0.7 | 1.4 ± 0.7 | 1.5 ± 0.7 | 1.6 ± 0.7 | 1.8 ± 0.9 | |
| Cases/person-years | 271/104,111 | 635/194,217 | 561/168,696 | 354/103,040 | 530/121,317 | |
| Model 1 | 1.00 | 1.29 (1.12, 1.49) | 1.30 (1.12, 1.50) | 1.33 (1.14, 1.56) | 1.64 (1.42, 1.90) | <0.001 |
| Model 2 | 1.00 | 1.41 (1.22, 1.62) | 1.52 (1.31, 1.76) | 1.58 (1.35, 1.85) | 2.02 (1.74, 2.34) | <0.001 |
| Model 3 | 1.00 | 1.38 (1.19, 1.59) | 1.48 (1.28, 1.71) | 1.53 (1.30, 1.80) | 1.92 (1.65, 2.23) | <0.001 |
| Model 4 | 1.00 | 1.27 (1.10, 1.47) | 1.33 (1.14, 1.54) | 1.26 (1.07, 1.48) | 1.46 (1.25, 1.70) | <0.001 |
| Red meat | <1 times/month | 1 time/month | 2–3 times/month | 4–5 times/month | >5 times/month | Ptrend |
| Cases/person-years | 217/96,864 | 299/114,986 | 731/220,014 | 718/186,001 | 386/73,515 | |
| Model 1 | 1.00 | 1.18 (0.99, 1.41) | 1.53 (1.32, 1.78) | 1.78 (1.53, 2.07) | 2.34 (1.98, 2.76) | <0.001 |
| Model 2 | 1.00 | 1.16 (0.97, 1.38) | 1.49 (1.28, 1.74) | 1.74 (1.49, 2.04) | 2.21 (1.86, 2.62) | <0.001 |
| Model 3 | 1.00 | 1.14 (0.96, 1.36) | 1.45 (1.25, 1.70) | 1.68 (1.43, 1.96) | 2.07 (1.74, 2.46) | <0.001 |
| Model 4 | 1.00 | 1.05 (0.88, 1.26) | 1.26 (1.08, 1.47) | 1.38 (1.18, 1.62) | 1.49 (1.26, 1.78) | <0.001 |
| Chicken | <2 times/month | 2–4 times/month | 5–7 times/month | 8–10 times/month | >10 times/month | Ptrend |
| Cases/person-years | 523/164,322 | 682/209,414 | 517/149,811 | 201/62,694 | 428/105,139 | |
| Model 1 | 1.00 | 1.06 (0.94, 1.19) | 1.14 (1.01, 1.28) | 1.04 (0.88, 1.22) | 1.29 (1.14, 1.47) | <0.001 |
| Model 2 | 1.00 | 1.18 (1.05, 1.33) | 1.32 (1.17, 1.50) | 1.23 (1.05, 1.45) | 1.52 (1.33, 1.73) | <0.001 |
| Model 3 | 1.00 | 1.18 (1.05, 1.32) | 1.32 (1.16, 1.49) | 1.23 (1.04, 1.45) | 1.50 (1.31, 1.71) | <0.001 |
| Model 4 |
1.00 |
1.11 (0.99, 1.25) |
1.18 (1.04, 1.33) |
1.01 (0.86, 1.19) |
1.24 (1.08, 1.41) |
0.01 |
| HPFS | ||||||
|---|---|---|---|---|---|---|
| Total meats | <4 times/month | 4–7 times/month | 8–11 times/month | 12–15 times/month | >15 times/month | Ptrend |
| Servings/day | 1.5 ± 0.8 | 1.6 ± 0.8 | 1.8 ± 0.8 | 1.8 ± 0.8 | 2.0 ± 0.9 | |
| Cases/person-years | 193/46,089 | 364/75,005 | 400/79,757 | 226/49,533 | 299/67,095 | |
| Model 1 | 1.00 | 1.15 (0.96, 1.37) | 1.20 (1.01, 1.42) | 1.11 (0.91, 1.34) | 1.10 (0.92, 1.32) | 0.83 |
| Model 2 | 1.00 | 1.18 (0.99, 1.41) | 1.29 (1.09, 1.54) | 1.23 (1.01, 1.50) | 1.23 (1.02, 1.48) | 0.11 |
| Model 3 | 1.00 | 1.16 (0.97, 1.38) | 1.24 (1.04, 1.48) | 1.18 (0.97, 1.43) | 1.15 (0.95, 1.39) | 0.42 |
| Model 4 | 1.00 | 1.17 (0.98, 1.40) | 1.23 (1.03, 1.47) | 1.13 (0.93, 1.38) | 1.12 (0.92, 1.35) | 0.79 |
| Red meat | <1 times/month | 1 time/month | 2–3 times/month | 4–5 times/month | >5 times/month | Ptrend |
| Cases/person-years | 175/51,231 | 184/43,392 | 363/85,710 | 486/87,242 | 274/49,905 | |
| Model 1 | 1.00 | 1.20 (0.97, 1.48) | 1.22 (1.02, 1.46) | 1.62 (1.36, 1.93) | 1.62 (1.34, 1.96) | <0.001 |
| Model 2 | 1.00 | 1.20 (0.98, 1.48) | 1.22 (1.01, 1.46) | 1.62 (1.36, 1.94) | 1.61 (1.32, 1.97) | <0.001 |
| Model 3 | 1.00 | 1.19 (0.96, 1.46) | 1.17 (0.98, 1.41) | 1.52 (1.27, 1.83) | 1.45 (1.18, 1.78) | <0.001 |
| Model 4 | 1.00 | 1.20 (0.97, 1.48) | 1.15 (0.96, 1.39) | 1.45 (1.21, 1.75) | 1.35 (1.10, 1.66) | 0.002 |
| Chicken | <2 times/month | 2–4 times/month | 5–7 times/month | 8–10 times/month | >10 times/month | Ptrend |
| Cases/person-years | 503/103,127 | 454/97,576 | 282/58,245 | 84/23,232 | 159/35,299 | |
| Model 1 | 1.00 | 0.96 (0.84, 1.09) | 1.00 (0.87, 1.16) | 0.78 (0.62, 0.98) | 0.95 (0.79, 1.13) | 0.35 |
| Model 2 | 1.00 | 1.01 (0.89, 1.15) | 1.09 (0.94, 1.27) | 0.85 (0.68, 1.08) | 1.03 (0.86, 1.24) | 0.91 |
| Model 3 | 1.00 | 1.00 (0.88, 1.14) | 1.07 (0.92, 1.24) | 0.85 (0.67, 1.07) | 1.01 (0.84, 1.22) | 0.90 |
| Model 4 |
1.00 |
0.99 (0.87, 1.13) |
1.07 (0.92, 1.25) |
0.84 (0.66, 1.06) |
0.98 (0.66, 1.06) |
0.68 |
| Pooled | ||||||
|---|---|---|---|---|---|---|
| Total meats | <4 times/month | 4–7 times/month | 8–11 times/month | 12–15 times/month | >15 times/month | Ptrend |
| Model 3 | 1.00 | 1.22 (1.14, 1.31) | 1.34 (1.25, 1.44) | 1.36 (1.25, 1.48) | 1.49 (1.37, 1.62) | <0.001 |
| Pheterogeneity | 0.16 | 0.27 | 0.12 | 0.001 | 0.001 | |
| Model 4 | 1.00 | 1.18 (1.10, 1.26) | 1.26 (1.17, 1.35) | 1.23 (1.13, 1.33) | 1.28 (1.18, 1.39) | <0.001 |
| Pheterogeneity | 0.44 | 0.71 | 0.69 | 0.09 | 0.06 | |
| Red meat | <1 times/month | 1 time/month | 2–3 times/month | 4–5 times/month | >5 times/month | Ptrend |
| Model 3 | 1.00 | 1.17 (1.07, 1.27) | 1.28 (1.18, 1.38) | 1.53 (1.41, 1.66) | 1.72 (1.57, 1.89) | <0.001 |
| Pheterogeneity | 0.96 | 0.14 | 0.38 | 0.03 | 0.03 | |
| Model 4 | 1.00 | 1.11 (1.02, 1.22) | 1.17 (1.08, 1.27) | 1.36 (1.26, 1.48) | 1.42 (1.29, 1.55) | <0.001 |
| Pheterogeneity | 0.65 | 0.56 | 0.67 | 0.73 | 0.73 | |
| Chicken | <2 times/month | 2–4 times/month | 5–7 times/month | 8–10 times/month | >10 times/month | Ptrend |
| Model 3 | 1.00 | 1.12 (1.06, 1.18) | 1.22 (1.14, 1.30) | 1.13 (1.01, 1.23) | 1.30 (1.20, 1.40) | <0.001 |
| Pheterogeneity | 0.15 | 0.11 | 0.03 | 0.01 | 0.01 | |
| Model 4 | 1.00 | 1.08 (1.02, 1.14) | 1.14 (1.07, 1.22) | 1.00 (0.91, 1.11) | 1.15 (1.07, 1.25) | 0.002 |
| Pheterogeneity | 0.36 | 0.64 | 0.23 | 0.13 | 0.16 | |
Data for servings/day are mean ± SEM. Model 1 was adjusted for age. Model 2 was further adjusted for ethnicity, smoking status (never smoker, past smoker, current smoker [1–14, 15–24, or ≥ 25 cigarettes/day], or missing), alcohol intake (g/day: 0, 0.1–4.9, 5.0–14.9, or ≥15.0 in women; 0, 0.1–4.9, 5.0–29.9, or ≥30.0 in men; or missing), family history of diabetes (yes or no), marital status (married, not married, or missing), menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, former, or current hormone use], or missing) (for women), physical activity (METs/week: 0–2.9, 3–8.9, 9–17.9, 18–26.9, ≥ 27.0, or missing), total energy intake (kcal/day), and AHEI without alcohol intake. Model 3 was further adjusted for total intake of chicken, fish, and red meat. Model 4 was further adjusted for baseline BMI.
aOpen-flame and/or high-temperature cooking consisted of the frequency of broiling, barbecuing, or roasting of chicken, fish, or red meat.
bTotal meats included red meat, chicken, and fish.
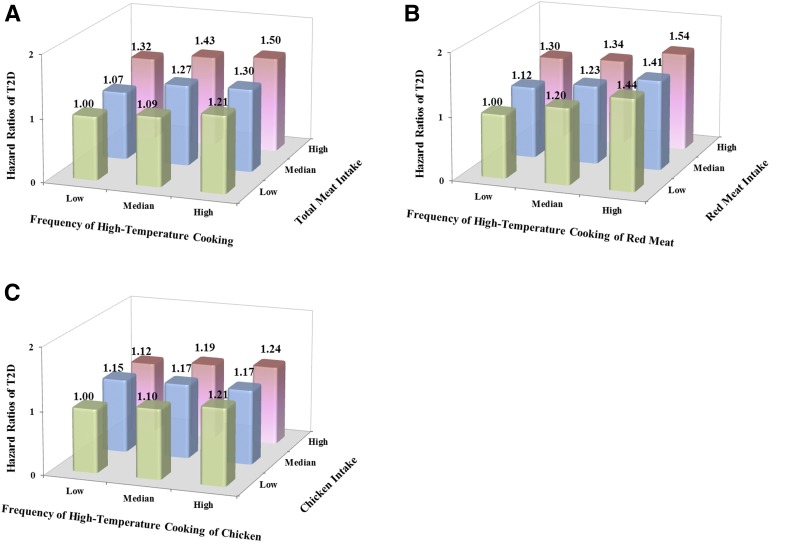
Figure 1 shows the joint associations between frequency of open-flame and/or high-temperature cooking and meat intake and T2D risk. Compared with participants who were in the lowest tertiles of both open-flame and/or high-temperature cooking frequency and meat intake, participants in the highest tertiles had a pooled HR (95% CI) of T2D of 1.50 (1.37, 1.64) for total meats, 1.54 (1.41, 1.68) for red meat, and 1.24 (1.14, 1.35) for chicken. No interaction was detected between open-flame and/or high-temperature cooking and intake of total meats, red meat, and chicken.
Figure 1.
Joint analysis of open-flame and/or high-temperature cooking frequency and meat intake (red meat, chicken, and fish) in relation to T2D risk among participants who consumed red meat, chicken, or fish regularly (≥2 servings/week). A: Total meat (red meat, chicken, and fish) intake. B: Red meat intake. C: Chicken intake. HRs were adjusted for age, ethnicity (Caucasian, African American, Hispanic, or Asian), smoking status (never smoker, past smoker, current smoker [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake (g/day: 0, 0.1–4.9, 5.0–14.9, or ≥15.0 in women; 0, 0.1–4.9, 5.0–29.9, or ≥30.0 in men; or missing), family history of diabetes (yes or no), marital status (married, not married, or missing), menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, former, or current hormone use], or missing) (women only), physical activity (METs/week: 0–2.9, 3–8.9, 9–17.9, 18–26.9, ≥27.0, or missing), total energy intake (kcal/day), AHEI without alcohol intake, and baseline BMI. For panels B and C, red meat, chicken, and fish intake were mutually adjusted.
Doneness-Weighted Frequency of High-Temperature Cooking and T2D Risk
The associations between doneness-weighted frequency of open-flame and/or high-temperature cooking and risk of T2D are shown in Table 3. After multivariate adjustment including baseline BMI and total intake of chicken, fish, and red meat, a higher doneness-weighted frequency of high-temperature cooking was associated with an increased T2D risk. Comparing extreme quartile frequencies, the pooled HR (95% CI) of T2D was 1.20 (1.12, 1.28; Ptrend <0.001; Pheterogeneity = 0.05). The results remained significant when red meat and chicken were analyzed separately: comparing extreme quartiles, the pooled HR (95% CI) of T2D was 1.28 (1.19, 1.37; Ptrend <0.001) for red meat and 1.10 (1.03, 1.17; Ptrend = 0.03) for chicken (model 4, Table 3). Supplementary Fig. 2 shows the joint associations of open-flame and/or high-temperature cooking frequency and doneness preference score in relation to T2D risk.
Table 3.
HR (95% CI) of T2D according to doneness-weighted frequency of open-flame and/or high-temperature cooking among participants who consumed red meat, chicken, or fish regularly (≥2 servings/week)
| Quartile of doneness-weighted frequency of high-temperature cooking | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Ptrend | |
| NHS | |||||
| Total meats | |||||
| Servings/day | 0.97 ± 0.5 | 1.08 ± 0.5 | 1.14 ± 0.5 | 1.15 ± 0.5 | |
| Cases/person-years | 868/186,189 | 923/172,446 | 1,090/188,224 | 1,188/185,607 | |
| Model 1 | 1.00 | 1.15 (1.05, 1.26) | 1.24 (1.13, 1.35) | 1.36 (1.25, 1.49) | <0.001 |
| Model 2 | 1.00 | 1.18 (1.07, 1.29) | 1.31 (1.20, 1.44) | 1.47 (1.34, 1.60) | <0.001 |
| Model 3 | 1.00 | 1.15 (1.05, 1.26) | 1.26 (1.15, 1.37) | 1.37 (1.26, 1.51) | <0.001 |
| Model 4 | 1.00 | 1.11 (1.01, 1.22) | 1.18 (1.08, 1.29) | 1.23 (1.12, 1.35) | <0.001 |
| Red meat | |||||
| Cases/person-years | 675/155,302 | 1,122/226,516 | 1,015/169,240 | 1,257/181,408 | |
| Model 1 | 1.00 | 1.14 (1.03, 1.25) | 1.37 (1.24, 1.51) | 1.58 (1.44, 1.73) | <0.001 |
| Model 2 | 1.00 | 1.13 (1.03, 1.25) | 1.31 (1.19, 1.45) | 1.48 (1.35, 1.63) | <0.001 |
| Model 3 | 1.00 | 1.11 (1.00, 1.22) | 1.26 (1.14, 1.40) | 1.40 (1.27, 1.55) | <0.001 |
| Model 4 | 1.00 | 1.06 (0.96, 1.16) | 1.18 (1.07, 1.30) | 1.23 (1.11, 1.36) | <0.001 |
| Chicken | |||||
| Cases/person-years | 891/173,123 | 1,177/212,384 | 969/174,797 | 1,032/172,162 | |
| Model 1 | 1.00 | 1.07 (0.98, 1.17) | 1.08 (0.98, 1.18) | 1.15 (1.05, 1.26) | <0.001 |
| Model 2 | 1.00 | 1.13 (1.04, 1.23) | 1.18 (1.07, 1.29) | 1.29 (1.18, 1.41) | <0.001 |
| Model 3 | 1.00 | 1.12 (1.03, 1.22) | 1.15 (1.05, 1.26) | 1.24 (1.13, 1.36) | <0.001 |
| Model 4 | 1.00 | 1.08 (0.99, 1.19) | 1.09 (1.00, 1.19) | 1.13 (1.03, 1.24) | 0.03 |
| NHS II | |||||
|---|---|---|---|---|---|
| Total meats | |||||
| Servings/day | 0.65 ± 0.5 | 0.75 ± 0.5 | 0.80 ± 0.5 | 0.83 ± 0.6 | |
| Cases/person-years | 494/178,588 | 538/166,740 | 597/174,365 | 722/171,687 | |
| Model 1 | 1.00 | 1.19 (1.05, 1.34) | 1.25 (1.11, 1.41) | 1.51 (1.35, 1.69) | <0.001 |
| Model 2 | 1.00 | 1.23 (1.09, 1.39) | 1.33 (1.18, 1.50) | 1.63 (1.45, 1.83) | <0.001 |
| Model 3 | 1.00 | 1.21 (1.07, 1.36) | 1.29 (1.14, 1.45) | 1.56 (1.39, 1.76) | <0.001 |
| Model 4 | 1.00 | 1.16 (1.03, 1.32) | 1.18 (1.05, 1.33) | 1.29 (1.14, 1.45) | <0.001 |
| Red meat | |||||
| Cases/person-years | 442/187,773 | 522/161,280 | 630/179,307 | 757/163,021 | |
| Model 1 | 1.00 | 1.42 (1.25, 1.61) | 1.53 (1.36, 1.73) | 2.00 (1.78, 2.25) | <0.001 |
| Model 2 | 1.00 | 1.31 (1.15, 1.49) | 1.41 (1.25, 1.60) | 1.73 (1.53, 1.95) | <0.001 |
| Model 3 | 1.00 | 1.29 (1.13, 1.46) | 1.37 (1.21, 1.55) | 1.65 (1.46, 1.87) | <0.001 |
| Model 4 | 1.00 | 1.19 (1.05, 1.36) | 1.26 (1.12, 1.43) | 1.38 (1.22, 1.56) | <0.001 |
| Chicken | |||||
| Cases/person-years | 536/168,973 | 568/169,190 | 600/180,114 | 647/173,105 | |
| Model 1 | 1.00 | 1.09 (0.97, 1.23) | 1.09 (0.97, 1.23) | 1.21 (1.08, 1.35) | <0.001 |
| Model 2 | 1.00 | 1.18 (1.05, 1.33) | 1.23 (1.09, 1.38) | 1.38 (1.22, 1.55) | <0.001 |
| Model 3 | 1.00 | 1.18 (1.05, 1.33) | 1.21 (1.07, 1.36) | 1.35 (1.20, 1.52) | <0.001 |
| Model 4 | 1.00 | 1.12 (0.99, 1.26) | 1.11 (0.99, 1.25) | 1.13 (1.01, 1.28) | 0.10 |
| HPFS | |||||
|---|---|---|---|---|---|
| Total meats | |||||
| Servings/day | 0.84 ± 0.7 | 0.99 ± 0.7 | 1.07 ± 0.8 | 1.10 ± 0.8 | |
| Cases/person-years | 365/82,105 | 346/76,854 | 391/79,395 | 380/79,126 | |
| Model 1 | 1.00 | 1.02 (0.88, 1.19) | 1.12 (0.97, 1.29) | 1.10 (0.95, 1.27) | 0.13 |
| Model 2 | 1.00 | 1.03 (0.89, 1.20) | 1.18 (1.02, 1.36) | 1.15 (1.00, 1.34) | 0.03 |
| Model 3 | 1.00 | 1.00 (0.86, 1.16) | 1.13 (0.97, 1.30) | 1.08 (0.93, 1.25) | 0.22 |
| Model 4 | 1.00 | 0.98 (0.84, 1.13) | 1.06 (0.91, 1.23) | 1.01 (0.86, 1.17) | 0.77 |
| Red meat | |||||
| Cases/person-years | 320/90,284 | 291/63,713 | 442/85,041 | 429/78,440 | |
| Model 1 | 1.00 | 1.30 (1.10, 1.52) | 1.46 (1.26, 1.69) | 1.54 (1.33, 1.78) | <0.001 |
| Model 2 | 1.00 | 1.29 (1.10, 1.51) | 1.41 (1.21, 1.63) | 1.48 (1.27, 1.73) | <0.001 |
| Model 3 | 1.00 | 1.26 (1.07, 1.48) | 1.33 (1.14, 1.55) | 1.35 (1.15, 1.58) | 0.002 |
| Model 4 | 1.00 | 1.22 (1.04, 1.43) | 1.26 (1.08, 1.46) | 1.22 (1.04, 1.43) | 0.06 |
| Chicken | |||||
| Cases/person-years | 402/83,872 | 342/70,026 | 396/85,174 | 342/78,406 | |
| Model 1 | 1.00 | 1.03 (0.89, 1.19) | 1.00 (0.87, 1.15) | 0.95 (0.82, 1.09) | 0.34 |
| Model 2 | 1.00 | 1.09 (0.94, 1.26) | 1.07 (0.93, 1.24) | 1.02 (0.88, 1.19) | 0.97 |
| Model 3 | 1.00 | 1.08 (0.93, 1.25) | 1.05 (0.91, 1.21) | 1.00 (0.86, 1.17) | 0.73 |
| Model 4 | 1.00 | 1.03 (0.89, 1.19) | 1.03 (0.89, 1.19) | 0.97 (0.83, 1.13) | 0.55 |
| Pooled | |||||
|---|---|---|---|---|---|
| Total meats | |||||
| Model 3 | 1.00 | 1.13 (1.06, 1.21) | 1.24 (1.16, 1.32) | 1.37 (1.28, 1.46) | <0.001 |
| Pheterogeneity | 0.15 | 0.34 | 0.01 | 0.01 | |
| Model 4 | 1.00 | 1.10 (1.03, 1.17) | 1.15 (1.08, 1.23) | 1.20 (1.12, 1.28) | <0.001 |
| Pheterogeneity | 0.19 | 0.44 | 0.03 | 0.05 | |
| Red meat | |||||
| Model 3 | 1.00 | 1.19 (1.11, 1.27) | 1.31 (1.22, 1.40) | 1.47 (1.37, 1.58) | <0.001 |
| Pheterogeneity | 0.13 | 0.57 | 0.06 | 0.02 | |
| Model 4 | 1.12 (1.05, 1.21) | 1.22 (1.14, 1.31) | 1.28 (1.19, 1.37) | <0.001 | |
| Pheterogeneity | 1.00 | 0.18 | 0.64 | 0.30 | 0.17 |
| Chicken | |||||
| Model 2 | 1.00 | 1.13 (1.06, 1.20) | 1.15 (1.07, 1.22) | 1.22 (1.15, 1.31) | <0.001 |
| Pheterogeneity | 0.65 | 0.32 | 0.01 | 0.01 | |
| Model 4 | 1.00 | 1.08 (1.02, 1.15) | 1.09 (1.01, 1.15) | 1.10 (1.03, 1.17) | 0.03 |
| Pheterogeneity | 0.69 | 0.71 | 0.20 | 0.18 | |
Data for servings/day are mean ± SEM. Model 1 was adjusted for age. Model 2 was further adjusted for ethnicity, smoking status (never smoker, past smoker, current smoker [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake (g/day: 0, 0.1–4.9, 5.0–14.9, or >15.0 in women; 0, 0.1–4.9, 5.0–29.9, or ≥30.0 in men; or missing), family history of diabetes (yes or no), marital status (married, not married, or missing), menopause status and postmenopausal hormones use (premenopause, postmenopause [never, former, or current hormone use], or missing) (for women), physical activity (METs/week: 0–2.9, 3–8.9, 9–17.9, 18–26.9, ≥27.0, or missing), total energy intake (kcal/day), and AHEI without alcohol intake. Model 3 was further adjusted for total intake of chicken, fish, and red meat. Model 4 was further adjusted for baseline BMI. Q, quartile.
After multivariate adjustment, higher estimated intake of HAAs was significantly associated with an increased risk of T2D (Supplementary Table 3). Comparing extreme quintiles, the pooled HR (95% CI) of T2D was 1.47 (1.20, 1.81; Ptrend <0.001). Higher frequency of open-flame and/or high-temperature cooking methods for total meats was associated with a greater weight gain during the first 4-year follow-up (Supplementary Table 4). Positive associations were also observed for the risk of developing obesity. Comparing extreme cooking frequency categories, the pooled HR (95% CI) of obesity was 1.59 (1.50, 1.69; Ptrend <0.001). Regarding open-flame and/or high-temperature cooking and T2D risk, the associations were markedly attenuated but remained significant with further adjustment for HAA intake and BMI change during follow-up (Supplementary Fig. 3). More details are described in Supplementary Data Appendix 1 (30).
Sensitivity Analyses
These observations were similar by different follow-up duration or after further adjustment for geographic location (north, middle, south, or unknown) and individual dietary factors (instead of AHEI) in the multivariate models (data not shown). When the participants with low meat consumption (<2 servings/week) were included in the analyses, the results did not materially change. For open-flame and/or high-temperature cooking, when analyses were stratified by age (<60 years, ≥60 years), BMI (<30 kg/m2, ≥30 kg/m2), physical activity (< median level, ≥ median level), and current smoking status (yes, no), the associations persisted in the strata of these variables (Supplementary Table 5) and no significant interactions were observed. For doneness-weighted frequency of high-temperature cooking and HAA intake, the results also persisted in the stratified analyses (data not shown). Similar results were observed when we excluded the participants with missing data for covariates or when only baseline exposures and covariates were used in the multivariate model in HPFS. The results did not change materially when energy-adjusted residuals of all food intake and cooking methods were used in the analyses. In other sensitivity analyses, when excluding the participants with incident T2D diagnosed in the first 4-year follow-up or participants who had information on cooking frequency but were missing information on doneness level, the results were similar.
Conclusions
In the three large prospective cohort studies among U.S. men and women who consumed red meat, chicken, or fish regularly, a higher frequency of open-flame and/or high-temperature cooking for both red meat and chicken was independently associated with an increased T2D risk during 12–16 years of follow-up. In addition, higher estimated intake of HAAs was also linked with an increased risk of T2D. These associations were independent of baseline BMI and total consumption of chicken, fish, and red meat. Moreover, a higher frequency of open-flame and/or high-temperature cooking was associated with greater weight gain and higher obesity risk.
Comparison With Other Studies
Different types of meat consumption have been differentially associated with diabetes risk (4–7). Red meats, particularly processed red meats, were consistently associated with an increased risk of developing T2D in prospective cohort studies (4,8). Findings regarding fish and chicken intake were less consistent. Two meta-analyses demonstrated a positive association of fish intake with T2D risk in U.S. and European populations but an inverse association in Asian and Australian populations (7,9). A recent large prospective study among Swedish men (n = 35,583) found that higher fried fish consumption was associated with an increased risk of T2D, although no overall association was demonstrated for total fish intake (31). For chicken or poultry, some studies reported null or inverse associations with T2D risk (5,10), whereas an increased risk was demonstrated in the European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct study (6) and the Singapore Chinese Health Study (32). In the current study, we found a positive association between red meat intake and T2D risk after further adjusting for cooking methods. In addition, chicken and fish intake was also associated with a modest increased risk of T2D in our three cohorts. These findings were largely consistent with those in earlier analyses of data in our cohorts (4). The reasons for inconsistent findings regarding chicken and fish intake and T2D risk are unknown, although potential differences in population characteristics and environmental contamination (such as methylmercury or polychlorinated biphenyls in fish) may play a role. Furthermore, methods preferred for cooking meats may differ among different study populations. For example, in China, stewing, braising, steaming, and stir-frying are commonly used to cook meats (33), while grilling/barbecuing, broiling, roasting, and pan-frying are more widely practiced in Western countries (34). In our previous study in the NHS cohort (with cooking information for red meat only), we found that a higher frequency of broiling, barbecuing, and roasting red meat, but not stewing/boiling or pan-frying, was independently associated with an increased T2D risk in women (11). In the current study with detailed cooking information for red meat, chicken, and fish, we not only replicated a positive association between open-flame and/or high-temperature cooking methods for red meat and T2D risk in both men and women but also found that open-flame and/or high-temperature cooking methods for chicken also independently increased T2D risk. In the current study, we did not observe a significant association between the frequency of broiling fish and T2D risk, although this fish cooking method was not common among our participants. For example, only 4.3% participants broiled fish ≥2 times/week, and few participants (<1%) cooked fish until well browned and/or blackened/charred. Therefore, our analysis may not have an adequate statistical power to detect a weak-to-modest association for broiling fish.
In addition to open-flame and/or high-temperature cooking methods, the degree of meat doneness might also modify the associations between meat intake and T2D risk. Some previous studies suggested that the meat doneness level, especially very well done, could increase the risk of certain cancers (35,36), although other studies reported null associations (37). In the current study, we also demonstrated that a higher doneness-weighted frequency of high-temperature/open-flame cooking for both red meat and chicken was significantly associated with an increased risk of T2D. Furthermore, the current study found that open-flame and/or high-temperature cooking methods were positively associated with weight gain and obesity risk and that HAA intake and changes in BMI during follow-up might partially explain the positive associations between high-temperature cooking and T2D risk. Overall, these results suggested that, independent of the amount of meat consumption, open-flame and/or high-temperature cooking methods for both red meat and chicken were associated with an increased T2D risk.
Potential Biological Mechanisms
Although the exact mechanisms underlying the observed associations remain unknown, some studies have suggested that certain hazardous chemicals including HAAs, PAHs, nitrosamines, and AGEs, which are produced during high-temperature cooking of meats, might be involved in the development of diabetes (14,16,19). A recent in vitro study by Rogers et al. (16) demonstrated that low-dose HAA exposure could induce gene expression changes in JAK/STAT and MAPK pathways linked with inflammation and diabetes. Evidence from in vitro and in vivo studies suggested that PAHs might induce proinflammatory cytokine production, interfere with insulin secretion, and consequently increase the risk of developing diabetes (38). Using data from the National Health and Nutrition Examination Survey (NHANES), several cross-sectional studies also found that urinary PAH biomarkers were associated with inflammation and an increased prevalence of diabetes (19,39). In addition, another study in rat pups showed that nitrosamine exposure caused lipid peroxidation, elevated expression of proinflammatory cytokine, and promoted insulin resistance (40). For AGEs, previous studies have demonstrated a strong link with inflammation, oxidative stress, and insulin resistance in animals and humans (14). Nevertheless, more investigations are warranted to establish the underlying mechanisms.
Strengths and Limitations
To our knowledge, this is the first study to examine open-flame and/or high-temperature cooking frequency and meat doneness level in relation to T2D risk among men and women who consumed red meat, chicken, or fish regularly. This is also the first study linking higher estimated dietary HAA intake with an increased T2D risk. In addition, the strengths of this study also include the large sample size, long follow-up period, a detailed questionnaire on different cooking methods and doneness levels for different types of meats, and careful adjustments for a multitude of potential risk factors.
Several limitations should be acknowledged as well. First, the study participants were all health professionals, and most of them were Caucasians. Although the relative homogeneity could alleviate confounding by socioeconomic status, it also limits the generalizability of the findings. Second, although information on high-temperature cooking methods for red meat, chicken, or fish was collected twice in HPFS, we only collected this information once in NHS and NHS II, which might not represent long-term cooking practices. Considering that the correlations between the 1996 and 2004 assessments of cooking methods in HPFS were moderate (rs range 0.30–0.44), which might be due to changes in cooking behaviors over time or measurement errors in self-reported data, more prospective studies, particularly with repeated measurements of cooking methods and doneness levels, are warranted to confirm our findings. Third, although measurement errors in self-reported assessments of diet are inevitable, our validation studies demonstrated reasonable validity of the FFQ: for example, the rs between the FFQ and multiple dietary records ranged from 0.38 to 0.70 for various red meat intakes (23,24). Moreover, such measurement errors are likely to be nondifferential in this prospective study but may result in residual confounding by dietary factors. Fourth, the current study did not have data on PAHs and nitrosamines, and the validity of the cooking questionnaire was not directly assessed. In addition, the cooking questionnaire did not include all cooking methods (such as boiling/stewing and stir-frying) for other types of meats (such as pork, lamb, and fish [only the frequency of broiling fish was inquired about in the current study]), which warrant more investigations in future studies. Fifth, we simply added the frequency of each cooking method or doneness level to reflect overall meat-cooking preferences, assuming an additive association across different cooking methods for different meats with equal health effects. In addition, adiposity might not be perfectly controlled in our study because BMI is not a direct measure of adiposity, although similar results were observed when waist circumference was adjusted for in the models. Sixth, some of the associations, especially those for chicken cooking methods, did not achieve statistical significance among men. However, we did not observe clear evidence of heterogeneity in associations across the three cohorts. Meanwhile, the associations for red meat cooking frequencies and HAA intake were highly consistent between men and women. Future studies are needed to explore potential sex differences in these associations. Finally, with the observed associations markedly attenuated after further controlling for BMI, it was unknown whether this observation reflected true mediation effects or residual confounding by other factors related to weight gain.
Implications of Findings
Our study provides novel evidence that open-flame and/or high-temperature cooking may independently contribute to the development of T2D beyond the risk of high meat intake. These findings imply that avoiding the use of open-flame and/or high-temperature cooking methods, including grilling/barbecuing, broiling, and roasting, may help reduce T2D risk among individuals who consume red meat, chicken, or fish regularly. Regarding potential strategies for diabetes prevention, this study also provides further evidence in support of the reduction of meat intake, especially red meat consumption.
Conclusion
The current study suggests that, independent of the consumption amount, open-flame and/or high-temperature cooking for both red meat and chicken is associated with an increased risk of T2D. More prospective studies are warranted to confirm these findings.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants for their dedication and contribution to the research.
Funding. This study was supported by National Heart, Lung, and Blood Institute grants CA186107, CA176726, CA167552, DK082486, and DK058845. Q.S. was supported by National Institutes of Health grants HL035464, ES022981, and ES021372.
Duality of Interest. D.M.E. has served as a consultant and scientific advisory committee member for The Culinary Institute of America; has performed consulting services with regard to nutrition science and its application to novel web-based educational products for Nutrition Development Group, LLC; has performed scientific consulting with regard to the design of clinical studies to test herbal products for Infinitus (China) Company, Ltd.; has served as a nutrition advisory board member for the Barilla Center for Food & Nutrition (Italy); and has performed consulting services in an effort to promote health and wellness educational programs in Japan for Campus for Health (Japan). In each instance, he received a consulting fee but does not own equity or stock. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.L. and Q.S. participated in the study concept and design and statistical analysis and interpretation. G.L. drafted the manuscript. All authors participated in critical revision and approved the final version of the manuscript. G.L. and Q.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Heart Association EPI|Lifestyle 2017 Scientific Sessions, Portland, OR, 7–10 March 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1992/-/DC1.
References
- 1.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34:1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietary Guidelines Advisory Committee Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC, U.S. Department of Agriculture and U.S. Department of Health and Human Services, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbrecher A, Erber E, Grandinetti A, Kolonel LN, Maskarinec G. Meat consumption and risk of type 2 diabetes: the Multiethnic Cohort. Public Health Nutr 2011;14:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendinelli B, Palli D, Masala G, et al.; InterAct Consortium . Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia 2013;56:47–59 [DOI] [PubMed] [Google Scholar]
- 7.Wallin A, Di Giuseppe D, Orsini N, Patel PS, Forouhi NG, Wolk A. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes: systematic review and meta-analysis of prospective studies. Diabetes Care 2012;35:918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence. Curr Atheroscler Rep 2012;14:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xun P, He K. Fish consumption and incidence of diabetes: meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care 2012;35:930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Woudenbergh GJ, Kuijsten A, Tigcheler B, et al. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam Study. Diabetes Care 2012;35:1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G, Zong G, Hu FB, Willett WC, Eisenberg DM, Sun Q. Cooking methods for red meats and risk of type 2 diabetes: a prospective study of U.S. women. Diabetes Care 2017;40:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knize MG, Salmon CP, Pais P, Felton JS. Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv Exp Med Biol 1999;459:179–193 [DOI] [PubMed] [Google Scholar]
- 13.Knize MG, Dolbeare FA, Carroll KL, Moore DH 2nd, Felton JS. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem Toxicol 1994;32:595–603 [DOI] [PubMed] [Google Scholar]
- 14.Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 2010;110:911–916.e912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha R, Rothman N. Role of well-done, grilled red meat, heterocyclic amines (HCAs) in the etiology of human cancer. Cancer Lett 1999;143:189–194 [DOI] [PubMed] [Google Scholar]
- 16.Rogers LJ, Basnakian AG, Orloff MS, et al. 2-Amino-1-methyl-6-phenylimidazo(4,5-b) pyridine (PhIP) induces gene expression changes in JAK/STAT and MAPK pathways related to inflammation, diabetes and cancer. Nutr Metab (Lond) 2016;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uribarri J, Cai W, Ramdas M, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care 2011;34:1610–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alshaarawy O, Elbaz HA, Andrew ME. The association of urinary polycyclic aromatic hydrocarbon biomarkers and cardiovascular disease in the US population. Environ Int 2016;89-90:174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alshaarawy O, Zhu M, Ducatman AM, Conway B, Andrew ME. Urinary polycyclic aromatic hydrocarbon biomarkers and diabetes mellitus. Occup Environ Med 2014;71:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62 [DOI] [PubMed] [Google Scholar]
- 21.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–468 [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136 [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 Suppl.):1220S–1228S; discussion 1229S–1231S [DOI] [PubMed]
- 26.Byrne C, Sinha R, Platz EA, et al. Predictors of dietary heterocyclic amine intake in three prospective cohorts. Cancer Epidemiol Biomarkers Prev 1998;7:523–529 [PubMed] [Google Scholar]
- 27.Sinha R, Cross A, Curtin J, et al. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res 2005;49:648–655 [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 29.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 30.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515–1527 [DOI] [PubMed] [Google Scholar]
- 31.Wallin A, Di Giuseppe D, Orsini N, Åkesson A, Forouhi NG, Wolk A. Fish consumption and frying of fish in relation to type 2 diabetes incidence: a prospective cohort study of Swedish men. Eur J Nutr 2017;56:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talaei M, Wang YL, Yuan JM, Pan A, Koh WP. Meat, dietary heme iron, and risk of type 2 diabetes mellitus: the Singapore Chinese Health Study. Am J Epidemiol 2017;186:824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam KC, Jo C, Lee M. Meat products and consumption culture in the East. Meat Sci 2010;86:95–102 [DOI] [PubMed] [Google Scholar]
- 34.Swatland HJ. Meat products and consumption culture in the West. Meat Sci 2010;86:80–85 [DOI] [PubMed] [Google Scholar]
- 35.Sinha R, Chow WH, Kulldorff M, et al. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res 1999;59:4320–4324 [PubMed] [Google Scholar]
- 36.Tasevska N, Sinha R, Kipnis V, et al. A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am J Clin Nutr 2009;89:1884–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabat GC, Cross AJ, Park Y, et al. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int J Cancer 2009;124:2430–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalil A, Villard PH, Dao MA, et al. Polycyclic aromatic hydrocarbons potentiate high-fat diet effects on intestinal inflammation. Toxicol Lett 2010;196:161–167 [DOI] [PubMed] [Google Scholar]
- 39.Alshaarawy O, Zhu M, Ducatman A, Conway B, Andrew ME. Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation. A positive association that is more evident in men. Environ Res 2013;126:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer’s disease. J Alzheimers Dis 2009;17:827–844 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.