Abstract
The comparative analysis of complex behavioral phenotypes is valuable as a reductionist tool for both drug discovery and defining chemical bioactivity. Flavonoids are a diverse class of chemicals that elicit robust neuroactive and hormonal actions, though bioactivity information is limited for many, particularly for neurobehavioral endpoints. Here, we used a zebrafish larval chemomotor response (LCR) bioassay to comparatively evaluate a suite of 24 flavonoids, and in addition a panel of 30 model neuroactive compounds representing diverse modes of action (e.g. caffeine, chlorpyrifos, methamphetamine, nicotine, picrotoxin). Naïve larval zebrafish were exposed to concentration ranges of each compound at 120 hours post-fertilization (hpf) and locomotor activity measured for 5 hours. The model neuroactive compounds were largely behaviorally bioactive (20 of 30) with most effects phenotypic of their known modes of action. Flavonoids rapidly and broadly elicited hyperactive locomotor effects (22 of 24). Multidimensional analyses compared responses over time and identified three distinct bioactive groups of flavonoids based on efficacy and potency. Using GABAergics to modulate hyperactive responses, two flavonoids, (S)-equol and kaempferol were tested for GABAA receptor antagonism, as well as a known GABAA receptor antagonist, picrotoxin. Pharmacological intervention with positive allosteric modulators of the GABAA receptor, alfaxalone and chlormethiazole, ameliorated the hyperactive response to picrotoxin, but not for (S)-equol or kaempferol. Taken together, these studies demonstrate that flavonoids are differentially bioactive and that the chemobehavioral effects likely do not involve a GABAA receptor mediated mode of action. Overall, the integrative zebrafish platform provides a useful framework for comparatively evaluating high-content chemobehavioral data for sets of structurally- and mechanistically-related flavonoids and neuroactive compounds.
Keywords: behavior, flavonoids, GABA receptor, locomotion, neurotoxicity, zebrafish
1. Introduction
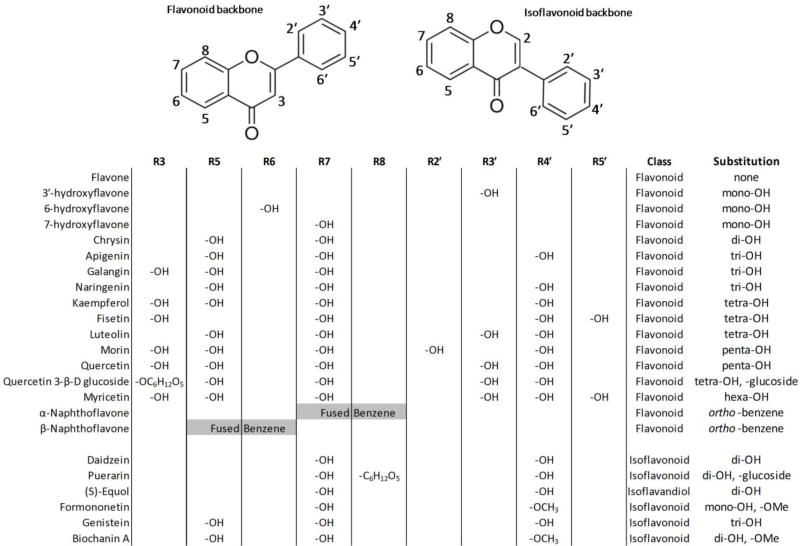
Flavonoids are a large class of structurally diverse compounds that exhibit highly bioactive and structure-dependent properties (Fig. 1). A number of naturally occurring food sources contain flavonoids (i.e. fruits, vegetables), though relatively high levels are found in processed soy-based products and over-the-counter dietary supplements. As a result, flavonoids are commonly studied for their widely varied and potentially beneficial nutraceutical properties, including anti-oxidant, anti-cancer, anti-inflammatory, and hormonal bioactivities (reviewed by: Nijveldt et al., 2001; Cornwell et al., 2004; Kumar and Pandey, 2013). Due to the ubiquity of these compounds in the diets of certain populations, flavonoids are frequently detected in biological fluids, such as urine (Valentin-Blasini et al., 2003; Valentin-Blasini et al., 2005) and blood plasma (Peeters et al., 2007). Several bioactive flavonoids are also detected in amniotic fluid and umbilical cord blood (Adlercreutz et al., 1999; Foster et al., 2002; Todaka et al., 2005; Mustafa et al., 2007), as well as breast milk (Choi et al., 2002; Song et al., 2013). Relatively high levels are also detected in soy-based baby formulas (Setchell et al., 1997; Setchell et al., 1998; Choi et al., 2002; Song et al., 2013), thus raising concerns regarding adverse developmental effects from the potent neuroactive and hormonal bioactive properties, though potential consequences of such exposures remain unclear. Taken together, there is a need to comparatively evaluate and comprehensively define the multifaceted bioactive effects of flavonoids.
Fig. 1.
Backbones and substituent groups for all tested flavonoids and isoflavonoids. Also tested but not shown is resveratrol, a flavonoid-like stilbenoid.
In higher vertebrates, diverse types of neurobehavioral effects have been reported for select flavonoids, including modulation of anxiety (Lund and Lephart, 2001), depression (Wang et al., 2010), fear (Garey et al., 2001), learning (Kohara et al., 2014), and seizure-like activity (Medina et al., 1990; Avallone et al., 2000). For some flavonoids (e.g. phytoestrogens), the behavioral effects in adults may in part be the result of hormonal action affecting endocrine pathways throughout the HPG axis and effects on reproductive activity (Lephart et al., 2002; Ball et al., 2010). However, many of the widely varying and complex behavioral effects of flavonoids can be attributed to interactions with a variety of important neuroreceptors throughout the nervous system. Neurotransmitter signaling and neuroreceptor function can be directly modulated by flavonoids in vitro, including acetylcholinesterase (reviewed by: Uriarte-Pueyo and I. Calvo, 2011), adenosine receptors (Moro et al., 1998), glycine receptors (Huang and Dillon, 2000), opioid receptors (Katavic et al., 2007), serotonin and dopamine receptors (Katavic et al., 2007), and nicotinic acetylcholine receptors (Lee et al., 2011). Perhaps most well understood are the mixed interactions of several flavonoids with GABAA receptors in vitro, particularly as antagonists (reviewed by: Wang et al., 2010; Hanrahan et al., 2011). Previously, we demonstrated that developmental exposure to several flavonoids elicits seizure-like spasms and hyperactive behaviors (spastic pectoral fin movement and body twitching), though the etiology of this neurodevelopmental effect is unclear (Bugel et al., 2016). For the vast majority of flavonoids, in vivo bioactivity information for integrated apical endpoints, such as behavior, is limited.
The zebrafish has many advantages that provide a useful framework to comparatively investigate chemical bioactivity in vivo (reviewed by: Bugel et al., 2014; Caro et al., 2016). The zebrafish genome also shares 70% homology with the human genome, which is valuable for translational medicine, drug discovery, and large-scale chemical screens (Howe et al., 2013). The development of zebrafish-based chemobehavioral assays have shown potential for improving our understanding of how chemicals affect neurobehavior in vivo, and has shown considerable promise for translating discoveries to clinical applications (reviewed by: Kokel and Peterson, 2008; Bruni et al., 2014). Low-complexity chemobehavioral bioassays in zebrafish are also highly amenable to high-throughput interrogation of large sets of compounds (Kokel et al., 2010). These reductionist approaches have led to powerful applications in drug discovery for profiling previously unknown compounds for neuroactive modes of action that could lead to either undesirable toxic side effects or potentially beneficial applications as anti-psychotic (Ellis and Soanes, 2012; Bruni et al., 2016) and anti-seizure treatments (Baraban et al., 2005; Ellis et al., 2012). Overall, the zebrafish provides a powerful platform that permits the comparative evaluation of chemobehavior for structurally-related and mechanistically-related compounds.
Here, we present a comparative evaluation of locomotor behavioral responses for a diverse group of neuroactive compounds in zebrafish using a low-complexity larval chemomotor response (LCR) bioassay. We first tested 30 model neuroactive chemicals as proof-of-principle to validate the capacity of the bioassay to detect behavioral responses for known neuroactive substances. This panel included 14 drugs and toxicants representing a variety of known modes of action, and 16 GABAergic drugs (Table 1). Second, we evaluated the chemobehavioral responses of 24 flavonoid and flavonoid-like chemicals to determine chemical relationships (Fig. 1, Table 1). Finally, we used two GABAA receptor specific drugs (alfaxalone and chlormethiazole) to test the involvement of GABAA receptors as a potential mode of action for stimulatory compounds found to elicit hyperactivity, specifically picrotoxin, (S)-equol, kaempferol.
Table 1.
List of 30 model neuroactive chemicals tested with known modes of action and concentrations evaluated. The primary mode of action is provided for each compound, though several elicit effects involving mixed modes of action (e.g. ethanol).
| Chemical | Concentrations (µM) |
Purity | CAS # | Primary MoA |
|---|---|---|---|---|
| Neuroactive drugs and toxicants (14 total) | ||||
| Bumetanide | 1, 10, 100, 1000 | 98%1 | 28395-03-1 | Na-K-Cl (NKCC1) co-transporter inhibitor |
| Caffeine | 1, 10, 100, 1000 | 99%1 | 58-08-2 | Adenosine receptor antagonist |
| Chlorpyrifos | 1, 10, 100, ‡ | 98%1 | 2921-88-2 | Acetylcholinesterase inhibitor |
| Dopamine | 1, 10, 100, 1000 | 98%1 | 62-31-7 | Prototypical dopamine receptor agonist |
| Epinephrine | 1, 10, 100, 1000 | 99%1 | 51-43-4 | Adrenergic receptor agonist |
| Fluoxetine | 1, 10, ‡ | 98%1 | 56296-78-7 | Selective serotonin reuptake inhibitor |
| Imidacloprid | 1, 10, 100, 1000 | 99%1 | 138261-41-3 | Nicotinic acetylcholine receptor agonist |
| S-(+)-Ketamine | 1, 10, 100, 1000 | 98%1 | 33795-24-3 | NDMA receptor antagonist |
| Mecamylamine | 1, 10, 100, † | 98%1 | 60-40-2 | Nicotinic acetylcholine receptor antagonist |
| (+)-Methamphetamine | 1, 10, 100, 1000 | 98%1 | 51-57-0 | Dopaminergic/norepinephrinergic/serotoner gic |
| Methyllycaconitine | 1, 10, 100, † | 96%1 | 21019-30-7 | Nicotinic acetylcholine receptor antagonist |
| (−)-Nicotine | 1, 10, 100, ‡ | 99%1 | 54-11-5 | Prototypical nicotinic acetylcholine receptor agonist |
| N-methyl-D-aspartic acid (NMDA) | 1, 10, 100, 1000 | 98%1 | 6384-92-5 | Prototypical NMDA receptor agonist |
| Tricaine mesylate (MS-222) | 1, 10, 100, ‡ | 95%4 | 886-86-2 | Na+channel blocker |
| GABA agonists and positive modulators (10 total) | ||||
| Alfaxalone (5α-pregnan-3α-ol-11,20-dione) | 1, 10, † | 98%1 | 23930-19-0 | GABAA receptor (+)-allosteric modulator |
| (±)-Baclofen | 1, 10, 100, 1000 | 98%1 | 1134-47-0 | GABAB receptor agonist |
| Carbamazepine | 1, 10, 100, 1000 | 98%1 | 298-46-4 | GABAA receptor agonist |
| CGP 13501 | 1, 10, 100, 1000 | 98%3 | 56189-68-5 | GABAB receptor positive allosteric modulator |
| Chlormethiazole | 1, 10, 100, ‡ | 98%1 | 6001-74-7 | GABAA receptor positive allosteric modulator |
| Ethanol | 1, 10, 100, 1000 | 99%1 | 64-17-5 | GABAA receptor positive allosteric modulator |
| gamma-Aminobutyric Acid (GABA) | 1, 10, 100, 1000 | 99%1 | 56-12-2 | Prototypical GABA receptor agonist |
| Muscimol | 1, 10, 100, ‡ | 98%1 | 2763-96-4 | GABAA receptor agonist |
| SKF 97541 (CGP 35024) | 1, 10, 100, 1000 | 98%3 | 127729-35-5 | GABAB receptor agonist |
| Valproic Acid | 1, 10, 100, 1000 | 98%1 | 99-66-1 | Increases levels of GABA and Na+ channel blocker |
| GABA antagonists and negative modulators (6 total) | ||||
| Bicuculline | 1, 10, 100, ‡ | 97%1 | 485-49-4 | GABAA receptor antagonist |
| Dehydroisoandrosterone 3-sulfate | 1, 10, 100, 1000 | 98%1 | 78590-17-7 | GABAA receptor negative allosteric modulator |
| Pentylenetetrazol | 1, 10, 100, 1000 | 99%1 | 54-95-5 | GABAA receptor blocker and proconvulsant |
| Picrotoxin | 1, 10, 100, ‡ | 98%1 | 124-87-8 | GABAA receptor antagonist |
| Pregnenolone sulfate | 1, 10, 100, 1000 | 98%1 | 1247-64-9 | GABAA receptor negative allosteric modulator |
| SCH 50911 | 1, 10, 100, 1000 | 98%3 | 160415-07-6 | GABAB receptor antagonist |
Purity and sources are indicated:
Sigma Aldrich (St. Louis, MO),
Cayman Chemical Company (Ann Arbor, MI),
Argent (Redmond, WA),
Tocris Bioscience (Minneapolis, MN). Solubility issues that prohibited testing of higher concentrations are indicated.
Solubility issues that prohibited testing of higher concentrations are indicated.
Acute lethality associated with higher concentrations are also indicated. All compounds were prepared using DMSO as a carrier solvent.
2. Materials and methods
2.1. Chemicals
The structural backbones and substituents for the flavonoids and flavonoid-like chemicals tested are shown in Fig. 1. A list of all model neuroactive chemicals and flavonoids tested are provided in Tables 1 and 2, respectively, with the concentrations tested and sources for each compound. All chemical stocks were prepared in DMSO as a carrier solvent. Final concentrations of DMSO in control and treatment groups were either 0.1% for concentration-response studies, or 1% for pharmacological intervention studies. These exposure concentrations of DMSO are lower than maximum tolerated concentrations reported for developmental exposures, and were necessary to achieve target concentrations while minimizing precipitation in stock solutions (Maes et al., 2012). Dimethyl sulfoxide (≥99.9%, DMSO, CAS: 67-68-5) was obtained from Avantor Performance Materials (Center Valley, PA).
Table 2.
List of 24 flavonoids and flavonoid-like compounds tested and concentrations evaluated.
| Chemical | Concentrations (µM) | Purity | CAS # |
|---|---|---|---|
| Flavonoids (24 total) | |||
| 3'-Hydroxyflavone | 1, 5, 10, 25, 50 | 98%1 | 70460-18-3 |
| 6-Hydroxyflavone | 1, 5, 10, 25, 50 | 98%1 | 6665-83-4 |
| 7-Hydroxyflavone | 1, 5, 10, 25, 50 | 98%1 | 6665-86-7 |
| α-Naphthoflavone | 1, 5, 10, 25, 50 | 98%1 | 604-59-1 |
| β-Naphthoflavone | 1, 5, 10, 25, 50 | 98%1 | 6051-87-2 |
| Apigenin | 1, 5, 10, 25, 50 | 98%2 | 520-36-5 |
| Biochanin A | 1, 5, 10, 25, 50 | 99%1 | 491-80-5 |
| Chrysin | 1, 5, 10, 25, 50 | 97%1 | 480-40-0 |
| Daidzein | 1, 5, 10, 25, 50 | 95%2 | 486-66-8 |
| (S)-Equol | 1, 5, 10, 25, 50 | 98%2 | 531-95-3 |
| Fisetin | 1, 5, 10, 25, 50 | 98%1 | 345909-34-4 |
| Flavone | 1, 5, 10, 25, 50 | 99%1 | 525-82-6 |
| Formononetin | 1, 5, 10, 25, 50 | 98%1 | 485-72-3 |
| Galangin | 1, 5, 10, 25, 50 | 95%1 | 548-83-4 |
| Genistein | 1, 5, 10, 25, 50 | 98%2 | 446-72-0 |
| Kaempferol | 1, 5, 10, 25, 50 | 98%2 | 520-18-3 |
| Luteolin | 1, 5, 10, 25, 50 | 98%2 | 491-70-3 |
| Morin | 1, 5, 10, 25, 50 | 95%2 | 654055-01-3 |
| Myricetin | 1, 5, 10, 25, 50 | 98%2 | 529-44-2 |
| Naringenin | 1, 5, 10, 25, 50 | 98%1 | 67604-48-2 |
| Puerarin | 1, 5, 10, 25, 50 | 98%1 | 3681-99-0 |
| Quercetin | 1, 5, 10, 25, 50 | 95%2 | 6151-25-3 |
| Quercetin 3-β-D glucoside | 1, 5, 10, 25, 50 | 90%1 | 482-35-9 |
| Resveratrol | 1, 5, 10, 25, 50 | 99%1 | 501-36-0 |
Purity and sources are indicated:
Sigma Aldrich (St. Louis, MO),
Cayman Chemical Company (Ann Arbor, MI),
Argent (Redmond, WA),
Tocris Bioscience (Minneapolis, MN). All compounds were prepared using DMSO as a carrier solvent.
2.2. Zebrafish husbandry
Wild-type 5D zebrafish (Danio rerio) were maintained on a 14:10 light/dark cycle at the Sinnhuber Aquatic Research Laboratory at Oregon State University (Corvallis, OR), in accordance with protocols approved by the Institutional Animal Care and Use Committee. A genetically heterogeneous tropical 5D (T5D) strain was used for all studies (Balik-Meisner et al., 2018). Embryos were collected from group spawns and enzymatically dechorionated at 4–5 hours post-fertilization (hpf) in embryo medium with 0.1 mg/mL Pronase (Roche, Indianapolis, IN) following previously described protocols (Mandrell et al., 2012). Dechorionated embryos were individually transferred into clear BD 353075 Falcon 96-well plates (Corning, Corning, NY) using robotic automation (Mandrell et al., 2012). Each well contained 100 µL embryo medium (15 mM NaCl, 0.5 mM KCl, 1 mM CaCl2, 0.15 mM KH2PO4, 0.05 mM Na2HPO4, 1 mM MgSO4, 0.05 mM NaHCO3, pH 7.3). Plates were sealed with parafilm and incubated at 28.5 °C on a 14:10 light/dark cycle until 120 hpf.
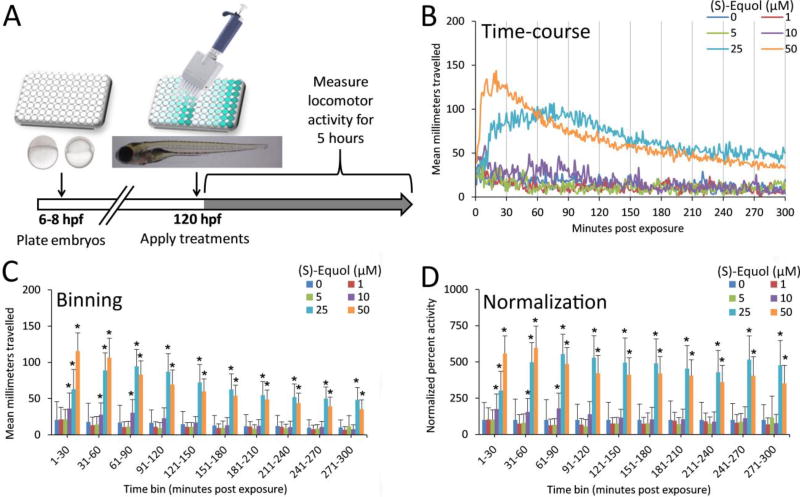
2.3. Chemical exposure and larval chemomotor response (LCR) behavioral analysis
The larval chemomotor response bioassay exposure paradigm is shown in Fig 2A. At 120 hpf, 100 µL of 2X treatment prepared in embryo medium were added to achieve the desired concentrations of each test chemical with a final concentration of either 0.1% or 1% DMSO in all control and treatment groups. Following addition of treatment and onset of exposure, plates were placed on an illuminated ViewPoint Zebrabox system and allowed to rest for 10 minutes to settle following handling of the plate and application of treatments. Locomotor activity was then recorded for 5 hours using ZebraLab video tracking software (ViewPoint Life Sciences, Lyon, France). All studies were performed using full illumination to maintain stable and low levels of locomotor activity observed under normal control conditions. Locomotor activity was measured for each minute, providing a time-course concentration-response (Fig. 2B). Activity data was then binned into 30 min segments (Fig. 2C), and normalized to the respective control group within each time-bin (Fig. 2D).
Fig. 2.
Exposure paradigm and data analysis pipeline for the larval chemomotor response assay. (A) Naïve larval zebrafish were cultured from 6–120 hpf, then exposed to test chemicals at 120 hpf to evaluate acute locomotor behavioral effects of various flavonoids and neuropharmacologicals across a concentration range. (B) Example of a 5-hour time-course of locomotor activity following exposure to (S)-equol across a broad concentration range (1–50 µM) in 0.1% DMSO. (C) Locomotor activity was binned into 30 min time-bins across the 5-hour exposure. (D) Within each 30 min time-bin, locomotor activity was normalized to the relative control (percent activity). Heat maps of locomotor activity were generated using binned and normalized data to comparatively evaluate locomotor responses of chemicals sets. *Significance determined using two-way repeated measures ANOVA with Tukey’s post-hoc, p ≤ 0.05, N = 16 animals per concentration. Data transformed using Box-Cox power transformation for normality prior to analysis. Data are reported as mean ± SD for normalized activity relative to the control within each time group. Summary data and statistics (time-course, binned, and normalized plots, 95% CI, N-values and p-values) for all tested flavonoids and neuropharmacologicals are provided in Supplementary Materials.
For the neuroactive drugs and GABA receptor agonists/antagonists tested, a broad range of concentrations was evaluated (0, 1, 10, 100 and 1000 µM). However, solubility limits prohibited testing higher concentrations and are indicated in Table 1. The three general groups of neuroactive compounds tested included a group of 14 model drugs and toxicants with varied modes of action (e.g. caffeine, chlorpyrifos, nicotine), a group of 10 GABA receptor agonists and positive allosteric modulators (e.g. alfaxalone, baclofen, carbamazepine), and a group of six GABA receptor antagonists and negative allosteric modulators (e.g. bicuculline, picrotoxin, SCH 50911). For flavonoids, a standard concentration range (0, 1, 5, 10, 25, 50 µM) was tested (Table 2). These nominal concentrations of flavonoids account for solubility limits (typically 50 to 100 µM), and are relatable to low-micromolar levels measured in infant blood plasma and soy based baby formulas as well as concentrations previously used to define developmental bioactivity and toxicity using the zebrafish embryo-larval toxicity bioassay (Cassidy et al., 1994; Setchell et al., 1997; Bugel et al., 2016).
For pharmacological intervention studies requiring co-treatment of the test compounds (picrotoxin, (S)-equol, kaempferol) with each GABAA receptor specific drug (alfaxalone and chlormethiazole), compounds were added simultaneously to determine whether each GABAergic drug ameliorated the hyperactive locomotor responses typically observed with the test compound alone. The three test compounds were: picrotoxin, a model GABAA receptor antagonist and chloride channel blocker, and two highly efficacious flavonoids, (S)-equol and kaempferol. Pharmacologicals alfaxalone and chlormethiazole are GABAA receptor specific positive allosteric modulators, and were used in these studies at concentrations that did not elicit major effects on locomotor activity throughout the assay time-course. Concentrations used were 1 µM for alfaxalone and 100 µM for chlormethiazole. For reference, reported in vitro GABAA receptor IC50 values for alfaxalone and chlormethiazole are 8.4 and 140 µM, respectively (Cross et al., 1989; Horne et al., 1992). Picrotoxin (100 µM), (S)-equol (25 µM) and kaempferol (25 µM) elicited robust responses throughout the assay and were used for intervention studies.
For all chemicals and studies, N=16, 24 or 32 animals per treatment group, and exact replicate values are provided in Supplementary Materials. However, prior to chemical exposures at 120 hpf, untreated plates were evaluated for wells containing malformed or dead animals, which were either excluded from the study or replaced by viable normal animals. Upon completion of the assay, treatment groups were evaluated for acute lethality during the 5-hour exposure, which were excluded from analysis and indicated in Table 1 and Supplemental Materials.
2.4. Data and statistical analyses
Statistical tests were performed using SigmaPlot™ (v. 13.0), XLSTAT (2016), and R (v. 3.2.2). A p-value ≤ 0.05 was universally regarded as significantly different for all studies. A Box-Cox power transformation function (MASS R-package) was used to optimally transform binned activity data for normality prior to statistical analysis. Significance was determined using a two-way repeated measures ANOVA (Tukey’s post-hoc) to evaluate the effect of chemical treatment on binned locomotor activity across the 5-hour exposure period. Morpheus (Broad Institute) was used to generate heat maps of significant findings exceeding a 1.5 fold change cutoff threshold, and for agglomerative hierarchical clustering analysis (HCA). HCA for the activity heat map was performed using the Euclidean metric with complete linkage. Principle component analysis (PCA) was performed using a Pearson (n) metric. A bootstrapped k-means clustering algorithm was applied to the PCA to determine cluster centers (Trace(W) criterion, 1000 iterations, 0.00001 convergence, 100 repetitions with random initial partition). For each chemical, all summary data and statistics are provided in Supplementary Materials, including time-course, binned and normalized concentration response curves, mean ± SD for normalized activity with 95% confidence intervals, N-values and p-values.
3. Results
3.1. Model validation and evaluation of model neuroactive drugs
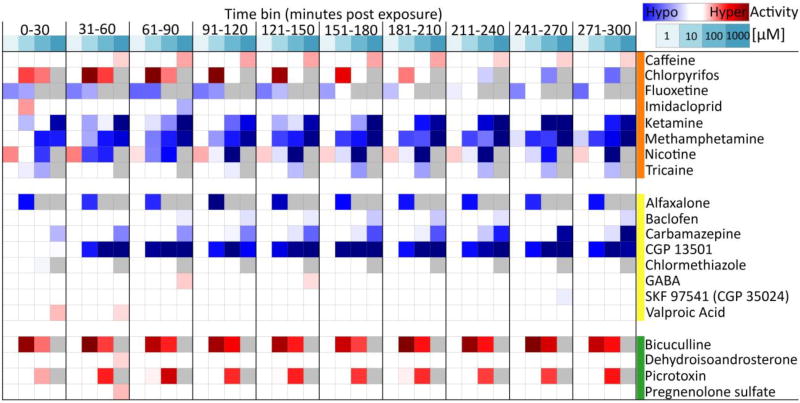
To validate the LCR bioassay and test the robustness and types of responses, a diverse panel of 30 known model neuroactive compounds was evaluated, including 14 drugs and toxicants representing a variety of modes of action, and 16 GABAergic drugs (Table 1, Fig. 3). A complete summary of the results for each individual model neuroactive compound tested are provided (Supplementary Materials).
Fig. 3.
A concentration-response heat map for comparatively evaluating acute locomotor behavioral responses to various neuroactive drugs and toxicants (orange group), GABA receptor agonists and positive allosteric modulators (yellow group), and GABA receptor antagonists and negative allosteric modulators (green group). All 30 chemicals in Table 1 were tested, and those that did not elicit any significant effects on locomotor activity exceeding the 1.5 fold change cutoff threshold are not shown (bumetanide, dopamine, epinephrine, ethanol, mecamylamine, methyllycaconitine, NMDA, pentylenetetrazol, muscimol, SCH 50911). A standard concentration range of 1, 10, 100, and 1000 µM was evaluated, with solubility and lethality issues permitting (see Table 1 and Supplemental Materials). Concentrations unable to be tested are shaded gray. Locomotor activity was binned into 30 min blocks and normalized to the respective time-bin control group (0.1% DMSO). Shaded cells indicate the normalized mean percent activity of animals relative to controls only when significantly different from control groups and exceeding a 1.5 fold change cutoff threshold. Significance determined using two-way repeated measures ANOVA with Tukey’s post-hoc, p ≤ 0.05, N = 16–32 animals per treatment group. Summary statistics for all 30 tested compounds are provided in Supplementary Materials.
Of the 14 widely used model neuroactive drugs and toxicants tested with varying and known modes of action, eight elicited significant changes on locomotor activity relative to controls (Fig. 3). Three stimulants elicited stable hyperactive effects throughout the LCR assay time-course: caffeine (1000 µM), chlorpyrifos (10 and 100 µM), and nicotine (1 µM). However, chlorpyrifos and nicotine elicited bimodal concentration-dependent effects also. With prolonged exposure in the higher concentration groups, nicotine also elicited hypoactive effects at 10 and 100 µM, and chlorpyrifos elicited hypoactive effects at 100 µM. Imidacloprid elicited a minor transient hyperactive effect at 10 µM from 0–30 min post exposure, but not at other concentrations or time-bins. A number of compounds elicited consistent hypoactive effects throughout the assay, including fluoxetine, ketamine, methamphetamine, and tricaine. Six model compounds did not elicit changes in locomotor activity from 1–1000 µM, including bumetanide, dopamine, epinephrine, mecamylamine, methyllycaconitine, NMDA.
Of the 10 GABA receptor agonists and positive allosteric modulators tested, eight elicited significant changes in locomotor activity relative to controls (Fig. 3). Four elicited robust and sustained hypoactive locomotor behavioral effects throughout the assay time-course (alfaxalone, baclofen, carbamazepine, CGP 13501). Several others elicited spurious effects that were generally not robust or sustained responses at the concentrations tested. Chlormethiazole and SKF 97541 (CGP 35024) elicited minor transient hypoactive effects at 100 and 1000 µM, respectively, though only at a single time-bin (0–30 and 241–270 min post exposure, respectively). Two GABA receptor agonists and positive allosteric modulators caused spurious and minor hyperactive effects (GABA and valproic acid) that were generally sustained. Ethanol and muscimol were the only compounds of the ten tested GABA receptor agonists and positive allosteric modulators to not elicit any significant changes in locomotor activity (Supplemental Materials).
Of the six GABA receptor antagonists and negative allosteric modulators tested, four significantly altered locomotor activity relative to controls (Fig. 3). Two elicited robust hyperactive locomotor effects (bicuculline, picrotoxin) that were sustained responses across the duration of the assay, though the response to bicuculline was greater at the intermediate concentration (10 µM) compared to 100 µM. Two elicited transient hyperactive effects that were not sustained across the assay time-course (dehydroisoandrosterone and pregnenolone sulfate), and were only observed in the 31–60 min post exposure time-bin. Pentylenetetrazol and SCH 50911 were the only compounds to not elicit changes in locomotor activity in the group of tested GABA receptor antagonists and negative allosteric modulators.
3.2. Comparative evaluation of neurobehavioral effects of flavonoids and flavonoid-like compounds
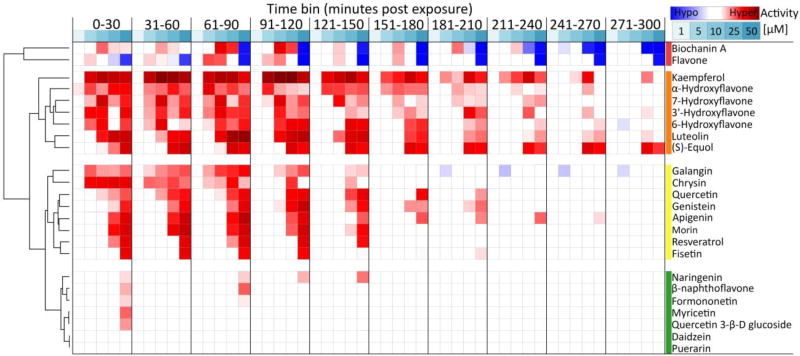
The LCR bioassay was used to comparatively evaluate the acute and rapid acting neurobehavioral effects for 24 flavonoids using two multidimensional approaches to compare and classify responses of flavonoids (Table 2, Figs. 4 and 5). A complete summary of the results for each individual flavonoid and flavonoid-like compound tested are provided (Supplementary Materials).
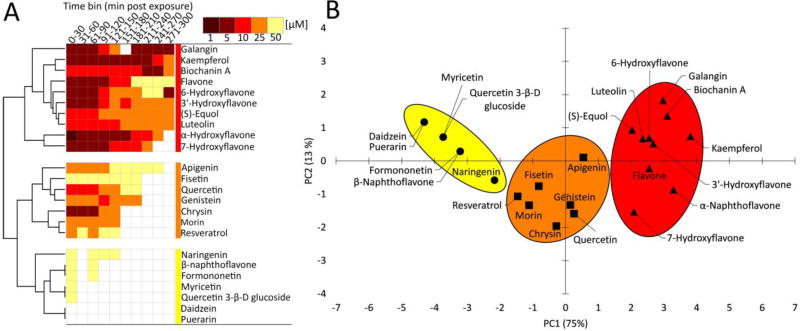
Fig. 4.
A concentration-response heat map for comparatively evaluating acute locomotor behavioral responses to flavonoids and flavonoid-like compounds. All 24 chemicals in Table 2 were tested and a standard concentration range of 1, 5, 10, 25, and 50 µM was evaluated. Locomotor activity was binned into 30 min blocks and normalized to the respective time-bin control group (0.1% DMSO). Shaded cells indicate the normalized mean percent activity of animals relative to controls only when significantly different from control groups and exceeding a 1.5 fold change cutoff threshold. Significance determined using two-way repeated measures ANOVA with Tukey’s post-hoc, p ≤ 0.05, N = 16 animals per treatment group. A Euclidian distance metric with complete linkage was used for hierarchical clustering analysis (HCA). Summary statistics for all 24 flavonoids tested are provided in Supplementary Materials.
Fig. 5.
Comparative evaluation of lowest effect levels (LEL) for flavonoid effects on locomotor activity across all time-bins using (A) hierarchical clustering analysis (HCA) and (B) principle component analysis. For HCA, a Euclidian distance metric with complete linkage was used. For PCA, a Pearson (n) metric with bootstrapped k-means clustering algorithm was used.
Hierarchical clustering analysis (HCA) was used to comparatively evaluate locomotor activity responses across concentrations and time for all flavonoids as an integrated analysis of potency and efficacy (Fig. 4). Of 24 flavonoids evaluated, 22 elicited significant locomotor responses within the tested concentration range (1–50 µM) and 5-hour assay duration. Puerarin and daidzein were the only flavonoids that did not significantly alter locomotor activity. All other flavonoids elicited differential effects with regards to concentration, the robustness of responses, and the time-course kinetics of responses. HCA was used to integrate each of these variables and comparatively evaluate the concentration-dependent responses across the 5-hour assay. This classified flavonoids into four general groups based on response similarity (Fig. 4). The first and second groups elicited effects at generally lower concentrations, and with persistent effects throughout the 5-hour assay duration. The first group contained two flavonoids (biochanin A and flavone) that elicited hyperactive effects at lower concentrations, and hypoactive responses at higher concentrations. The second group contained seven flavonoids that elicited long lasting hyperactive effects at low to mid micromolar concentrations (kaempferol, α-naphthoflavone, 7-hydroxyflavone, 3′-hydroxyflavone, 6-hydroxyflavone, luteolin, (S)-equol). The third group included eight flavonoids that generally elicited hyperactivity at mid to high micromolar concentrations and were relatively short-lived, lasting 91–180 minutes post exposure (galangin, chrysin, quercetin, genistein, apigenin, morin, resveratrol, fisetin). The fourth and least bioactive group contained the remaining seven tested flavonoids, which spuriously elicited minor hyperactive effects only at high micromolar concentrations (naringenin, β-naphthoflavone, formononetin, myricetin, quercetin 3- β-D glucoside), or no effects at all (daidzein, puerarin).
To further classify flavonoid neurobehavioral bioactivity, HCA and principle component analysis (PCA) were used as dimensional reduction tools to comparatively evaluate lowest effect levels (LELs) as a surrogate measure of potency (Fig. 5). Comparative LEL analysis resulted in three major potency groups (low, intermediate and high) of flavonoids that were identical between HCA and PCA methods. The high potency group included 10 flavonoids, which elicited relatively sustained long term effects at low micromolar concentrations (5–10 µM), included: galangin, kaempferol, biochanin A, flavone, 6-hydroxyflavone, 3'-hydroxyflavone, (S)-equol, luteolin, α-naphthoflavone, and kaempferol. The intermediate potency group contained seven flavonoids with effects in this group occurring at mid micromolar concentrations (25–50 µM) for an intermediate length of time, which included apigenin, fisetin, quercetin, genistein, chrysin, morin, and resveratrol, The low potency group included seven flavonoids that elicited either highly transient effects at 50 µM, naringenin, β-naphthoflavone, formononetin, myricetin, quercetin 3-β-D glucoside, or no effects within any time-bin, daidzein and puerarin.
3.3. Investigation of the role of the GABA receptors as a mediator of flavonoid hyperactivity using pharmacological intervention
Pharmacological intervention with GABAA receptor specific drugs (alfaxalone and chlormethiazole) was used to test whether the hyperactive locomotor activity effect of flavonoids involved a GABAA receptor dependent mode of action (Fig. 6). A complete summary of results for each co-treatment pharmacological intervention study is provided (Supplementary Materials).
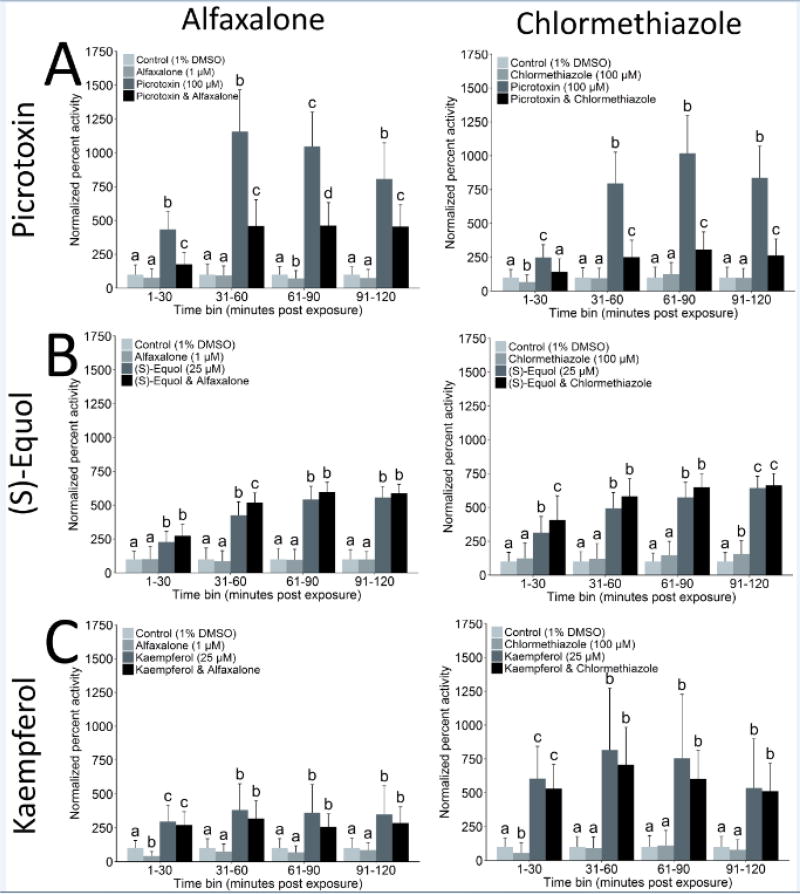
Fig 6.
GABAA receptor specific pharmacological intervention of hyperactive behavioral effects for (A) picrotoxin as a model GABAA receptor antagonist, and two efficacious flavonoids: (B) (S)-equol and (C) kaempferol. Locomotor activity was measured for 5 hours immediately following application of treatments. Concentrations tested for picrotoxin, (S)-equol, and kaempferol were previously demonstrated to elicit a robust hyperactive response, and were 100, 25, and 25 µM, respectively. Two GABAA receptor specific positive allosteric modulators tested were alfaxalone (1 µM) and chlormethiazole (100 µM), and concentrations were previously determined as no effect levels. Data are reported as mean ± SD for normalized activity relative to the control (1% DMSO) within each time group. Significance determined using two-way repeated measures ANOVA with Tukey’s post-hoc, p ≤ 0.05, N = 32 animals per treatment group. Significance is indicated using compact letter designations, and bars not labelled with the same letter within each time-bin were significantly different. The first four time-bins are shown (2-hours post-exposure), though each study was conducted for 5 hours and are included in Supplementary Materials with summary statistics for all intervention studies.
Overall, the hyperactive locomotor activity induced by picrotoxin was broadly inhibited by co-treatment with alfaxalone and with chlormethiazole (Fig. 6A, Supplementary Materials). The GABAA receptor drugs effectively inhibited the hyperactivity of a known GABAA receptor antagonist validating the co-exposure paradigm for pharmacological intervention. Although each pharmacological reduced the induction of the hyperactive locomotor response by picrotoxin across the full 5-hour assay, co-treatment did not completely block the induction of hyperactivity. Alfaxalone co-treatment significantly reduced the hyperactive response of picrotoxin in the 1–210 min post exposure time-bins, whereas there were no significant differences between the picrotoxin and co-treatment groups in the 211–300 min post exposure time-bins (Supplementary Materials). Similarly, chlormethiazole did not completely inhibit the hyperactive responses to picrotoxin, though co-treatment resulted in a long-term repression of hyperactivity (Supplementary Materials).
In contrast, hyperactive responses to (S)-equol and kaempferol were not broadly inhibited by the GABAA receptor specific positive allosteric modulators. Alfaxalone did have a spurious and minor synergistic effect on (S)-equol hyperactivity in single time-bins (31–60 min post exposure), though this was not observed in other time-bins or with kaempferol. Similarly, chlormethiazole had inconsistent minor synergistic increases of hyperactive responses for a single time-bin for (S)-equol (1–30 min post exposure), and two time-bins for kaempferol (211–240 and 271–300 min post exposure).
4. Discussion
In the present studies, we used a larval chemomotor response bioassay as a multidimensional platform to comparatively evaluate structurally-related flavonoids and other mechanistically-diverse neuroactive compounds in vivo. Several neuroactive flavonoids modulate the activity of important receptors throughout the nervous system, and thus, potentially alter behavior. However, behavioral data for the vast majority of flavonoids is either limited or unavailable. It was therefore the objective of these studies to provide high-content locomotor behavioral data with the LCR bioassay for flavonoid chemical classification and investigate a potential mode of action (i.e. GABAA receptor activity). Our findings suggested that larval zebrafish behavior is highly responsive to a multitude of well-known neuroactive compounds (20 of 30 tested) with varying known modes of action (Fig. 3). Notably, the majority of GABAergic drugs tested were behaviorally bioactive (12 of 16). Likewise, the suite of flavonoids tested broadly elicited hyperactive locomotor activity (22 of 24 tested). However, flavonoids were differentially bioactive with distinct and complex chemical-specific differences in responses (Figs. 4 and 5), which was not dependent on GABAA receptor specific activity for two model flavonoids (Fig. 6). Overall, these studies demonstrate the potential for large-scale chemobehavioral approaches with larval zebrafish for classifying bioactivity and for elucidating neurobehavioral modes of action in vivo.
Larval zebrafish behavioral assays are increasingly being used to identify drug target pathways and discover novel neuropharmacological action (reviewed by: McCarroll et al., 2016). To this end, we interrogated 30 neuroactive compounds using the LCR bioassay, including 14 drugs representing diverse modes of action, 10 GABA agonists and positive allosteric modulators, and six GABA antagonists and negative allosteric modulators. This panel of model drugs elicited three general types of responses, which were (1) characteristic for the known mode of action (e.g. caffeine, picrotoxin), (2) bimodal and concentration-dependent (e.g. chlorpyrifos, nicotine), or (3) atypical for the known mode of action (e.g. GABA, valproic acid). Seven stimulants elicited characteristic hyperactivity, including caffeine, chlorpyrifos, bicuculline, dehydroisoandrosterone 3-sulfate, nicotine, picrotoxin, and pregnenolone sulfate. Eight compounds with depressant, anxiolytic, or sedative modes of action elicited characteristic hypoactive effects, including fluoxetine, ketamine, tricaine (MS-222), alfaxalone, baclofen, carbamazepine, CGP 13501, and SKF 97541 (CGP 35024). However, responses to several chemicals were not necessarily consistent throughout the time-course. For example, while picrotoxin and bicuculline elicited characteristic hyperactivity, responses attenuated over time. For these two compounds these time-dependent effects may be due to desensitization of GABAA receptors by prolonged ligand binding and activation (Chang et al., 2002). Similarly, three compounds (chlorpyrifos, imidacloprid, and nicotine) elicited concentration-dependent bimodal responses that were characteristically hyperactive at low concentrations and hypoactive at high concentrations. These responses were likely due to acute stimulant intoxication followed by overdose-associated effects (e.g. prolonged muscle contraction), typical for acetylcholinesterase inhibition in neuromuscular junctions. Additionally, acetylcholinesterase inhibition by chlorpyrifos is dependent on the conversion to the active oxygenated metabolites chlorpyrifos-oxon and paraoxon, which suggested that rapid metabolism may influence behavioral time-course responses (Kousba et al., 2004). In contrast, three compounds elicited atypical responses for their known action (GABA, valproic acid, and methamphetamine). Hypoactive effects were anticipated for GABA, an inhibitory neurotransmitter, and valproic acid, a CNS depressant, yet both compounds modestly increased locomotion. In higher vertebrates, low doses of GABA similarly elicits modest increases in locomotion whereas high doses elicit the hypoactive effects expected for neuroinhibition (Jones et al., 1981). Contrastingly, amphetamine is a potent CNS stimulant and elicits bimodal hyperactive locomotor responses that attenuate with increased dosage (Yates et al., 2007). In our study, robust hypoactive effects were observed for methamphetamine. Related dose-dependent bimodal behavioral effects have also been reported for cannabinoids (Childs et al., 2017), opioids (Kuzmin et al., 2000), and nicotine (File et al., 1998). While many of the neuroactive drugs affected locomotor activity, eleven compounds were ineffective with the chemobehavior assay (1–1000 µM), including bumetanide, dopamine, epinephrine, ethanol, mecamylamine, methyllycaconitine, NMDA, pentylenetetrazole, chlormethiazole, muscimol, and SCH 50911. For these, false negative behavioral effects are likely the result of the experimental conditions, and results may differ with improved test conditions designed to target specific chemicals. Hyperactive responses to the broad CNS stimulant pentylenetetrazole have been reported for larval zebrafish, though typically with short exposures to 10 mM or higher (Berghmans et al., 2007; Afrikanova et al., 2013). Lack of behavioral responses for some of the other ineffective neuroactive compounds was likely due to similarly not achieving sufficient physiologically relevant doses. Additionally, behavioral effects for some compounds may have been overlooked because of the broad test concentration range. For example, no behavioral effects were observed for muscimol, a potent GABAA agonist, whereas high concentrations were acutely lethal. Taken together, concentration-dependent bimodal and atypical behavioral effects are likely to be common for neuroactive drugs with diverse types of targets. These proof-of-principle studies help to establish potential limitations of the model and emphasize the importance of well-defined concentration ranges and time-courses. However, our studies used primarily positive control compounds and did not include negative control compounds to determine the potential for false positives. Other studies have demonstrated false positive and false negative behavioral outcomes occur with larval zebrafish behavioral assays, and that secondary assays could be used to validate results (Afrikanova et al., 2013). Overall, the concomitant evaluation of well-known drugs aids with the interpretation of different types of behavioral responses and helps to contextualize responses to compounds with unknown modes of action (e.g. flavonoids).
The present study provides the largest comparative evaluation of acute flavonoid behavioral responses to date, and is comprised of 24 flavonoids and flavonoid-like compounds. The structural diversity of flavonoids has spurred many comparative and structure-activity relationship studies aimed at the varied bioactive effects on specific endpoints. Large-scale comparative studies have included developmental toxicity (Bugel et al., 2016), GABAA receptor binding (Huang et al., 2001; Duchowicz et al., 2008), anti-oxidant activity (Rasulev et al., 2005), aryl hydrocarbon receptor activation (Denison and Nagy, 2003), cytochrome p450 inhibition (Iori et al., 2005; Roy and Roy, 2008), and anti-cancer activity (Zhang et al., 2005). To our knowledge, the direct and rapid acting effects on locomotor activity for many flavonoids has not been comparatively evaluated for a large and broad set of flavonoids until now. Overall, flavonoids broadly elicited hyperactive locomotor behavioral responses, though with varied chemical-, concentration-, and time-dependent effects. To integrate comprehensive datasets for flavonoid bioactivity in larval zebrafish, we used similar multidimensional analysis methods with the same set of flavonoids and concentrations used previously to evaluate developmental toxicity (Bugel et al., 2016). HCA and PCA were used as data reduction tools to integrate response profiles across time and concentration, and ultimately rank acute behavioral bioactivity for all flavonoids. The basis for this approach is that locomotor activity is an apical outcome resulting from the perturbation of single or multiple putative target receptor(s) in varied cell- and tissue-types throughout the central and peripheral nervous system. As a result, response profiles thus serve as a surrogate integrated measurement of chemical toxicokinetics, efficacy, and potency. Using this approach, we identified several major groups of bioactive flavonoids, which did not generally cluster according to chemical subclasses (i.e. flavonoids vs. isoflavonoids).
Overall, the studies presented here demonstrated that minor structural alterations can dramatically change behavioral response profiles, and ultimately, chemical classification. Specifically, this set of flavonoids illustrates the potential effects of structural components including hydroxyl, O-methyl, and glucoside substituents on biological activity (Fig. 1). Structure-activity relationships in flavonoids are essential because complex metabolism in vivo commonly results in the addition or removal of substituent groups, including metabolism by the gut microbiome (e.g. hydrolysis of flavonoid glycosides), phase II conjugation (e.g. UGT, SULT, COMT), and to a lesser extent phase I hydroxylation by cytochrome P450 enzymes (reviewed by: Chen et al., 2014). There was no clear and direct relationship between sequential hydroxylation state and bioactivity when comparing the parent flavone to the mono-, di-, tri-, tetra-, penta-, and hexa-hydroxyflavone derivatives (7-hydroxyflavone, chrysin, naringenin, luteolin/kaempferol, quercetin, myricetin, respectively). This subset represents structurally related flavonoids with a step-wise addition of hydroxyl substituents from the parent flavone to the hexa-hydroxyflavone derivative. Of these, high bioactivity was observed for the parent flavone and mono-hydroxyflavone, and activity decreased for the di- and tri-hydroxflavones, chrysin and naringenin. However, the tetra-hydroxyflavone isomers, luteolin and kaempferol, had elevated bioactivity relative to the di- and tri-hydroxyflavones and grouped with the parent flavone and mono-hydroxyflavone. Relative bioactivity again decreased with further hydroxylation to the penta- and hexa-hydroxyflavone congeners, quercetin and myricetin. This suggested that general hydroxylation state alone does not determine bioactivity, and that responses are congener specific. Furthermore, O-methylation and hydroxylation greatly increased activity of isoflavonoids. When comparing the inactive isoflavonoid daidzein to formononetin, substitution of the R4′ hydroxyl group on daidzein for an O-methyl group (formononetin) modestly increased activity. Similarly, substitution of the R4′ hydroxyl group on genistein for an O-methyl group (biochanin A) greatly increased activity. Hydroxylation at the R5 position of genistein and biochanin A greatly increased activity in both cases relative to their R5 unhydroxylated derivatives, daidzein and formononetin. Taken together, these suggest that hydroxylation and O-methylation of isoflavonoids together may generally increase activity for specific congeners. Furthermore, hydrolysis of glucoside congeners, quercetin 3-β-D and puerarin, generally increased activity. Quercetin 3-β-D was a relatively inactive flavonoid with a minor and transient behavioral effect, whereas the parent compound quercetin elicited much greater hyperactivity. Intestinal microflora plays an important role in hydrolyzing glucoside substituents on puerarin to produce daidzein, which is further metabolized to the (S)-equol specific enantiomer (Kim et al., 1998; Setchell et al., 2005). In the present study, with acute non-dietary exposures, (S)-equol was a highly bioactive isoflavandiol whereas daidzein and puerarin were completely inactive, which may support a microbiome role in activating some isoflavonoids. Overall, these studies emphasize the need for large sets of flavonoids to elucidate important structural components that affect bioactivity, efficacy and potency. Small changes in substituent functional groups could significantly alter pharmacodynamics, and future studies should include additional flavonoids or endpoints to further elucidate structure-activity relationships. Additionally,
For the majority of behavioral studies conducted with flavonoids, anxiolytic properties are typically the most commonly reported. However, it is difficult to relate anxiolytic properties with the direct and rapid acting locomotor effects reported in the present study. Locomotor activity is low-complexity and interpretation is thereby limited, and different types of behavioral effects are likely divergent modes of action. While the anxiolytic effects of flavonoids are believed to be GABAA receptor mediated, hypoactive locomotor effects including sedation and myorelaxation are sometimes associated with high doses and it is unclear whether they are due to a GABA receptor interaction (Zanoli et al., 2000; Fernandez et al., 2009; de Carvalho et al., 2011). In contrast, a number of studies have reported hyperactive locomotor activity observed concomitantly with anxiolytic effects for several flavonoids (Ognibene et al., 2008; Zeng et al., 2010). Previously, we demonstrated that developmental exposure to flavonoids can elicit seizure-like spasms and hyperactive behaviors (spastic pectoral fin movement and body twitching), which was observed for seven flavonoids: 3′- hydroxyflavone, 6- hydroxyflavone, and 7- hydroxyflavone, (S)-equol, kaempferol, β-naphthoflavone, and biochanin A. (Bugel et al., 2016). Seizurelike activity is phenotypic of abnormal or excessive neurostimulation, which implicated a role for neuroreceptor modulation in mediating these adverse neurodevelopmental effects, and the locomotor effects were hypothesized to be mediated by a GABA receptor dependent mode of action. While the anxiolytic effects of flavonoids are largely believed to be GABA receptor mediated, there is compelling evidence that GABA receptor interactions are not due to classical benzodiazepine binding site modulation (Goutman et al., 2003; Campbell et al., 2004). Furthermore, behavioral effects of flavonoids are complex and are likely flavonoid-, dose-, assay- and life-stage dependent. To evaluate potential anxiolytic properties of flavonoids in future studies, it may be useful to use a more complex larval scototaxis behavioral assay, such as the locomotor photoresponse (LPR) assay as a surrogate measure of anxiety-related responses.
Although flavonoids interact with diverse types of neuroreceptors and targets, the antagonism and resulting modulation of GABAA receptors through interactions with the benzodiazepine binding site in vitro is perhaps the most widely reported neuroactive property. Drugs that antagonize inhibitory GABA receptors in the central nervous system commonly elicit phenotypic stimulant-like effects followed by convulsions and tonic-clonic seizures (reviewed by: Treiman, 2001). In the present study, we report a potentially related bioactive property of flavonoids that elicited an acute and rapid-acting hyperactive locomotor response broadly observed across this chemical class. We therefore hypothesized that these hyperactive locomotor behavioral effects were due in part to the known GABAA receptor modulation by flavonoids. One objective was to test whether GABAA receptor activity antagonism mediated the hyperactive locomotor responses to two select flavonoids with robust stimulant-like effects, (S)-equol and kaempferol. Picrotoxin was also evaluated concomitantly as a well-known GABAA receptor non-competitive antagonist and chloride channel blocker. In the present studies, the hyperactive response to picrotoxin was ameliorated by both GABAA receptor specific drugs, alfaxalone and chlormethiazole, though the hyperactive effects of (S)-equol and kaempferol were not reduced by either drug. It is important to note that the GABAA receptor specific drugs elicited a modest increase of the hyperactive responses to (S)-equol and kaempferol in several time-bins, but not consistently across the time-course (Supplementary Materials). Additionally, the lowest effect levels of (S)-equol and kaempferol that elicited hyperactive effects (10 and 5 µM, respectively) in the present study are also much lower than in vitro binding affinities for the GABAA receptor benzodiazepine site (Ki = 80–93 µM), though other flavonoids have much higher affinities (Paladini et al., 1999). Taken together, GABAA receptor antagonism is unlikely to be responsible for the hyperactive locomotor effects of (S)-equol and kaempferol, whereas the pharmacological intervention study demonstrated a clear GABAA receptor mediated mode of action for picrotoxin. Thus, considering the promiscuity of flavonoids with various neuroreceptors, the hyperactive locomotor effects may result from a cumulative effect on multiple pharmacological targets in several different cell- or tissue-types. However, it is inefficient to further interrogate individual putative target neuroreceptors without compelling evidence. To more rapidly and comprehensively explore the involvement of various neuroreceptors that may mediate the chemobehavioral effects of flavonoids, a global non-biased approach could prove useful by utilizing a broad library of small molecules with known pharmacological modes of action to modulate signaling pathways for all major target classes (e.g. LOPAC®1280). Our results suggest that LCR bioassay experimental parameters (e.g. bin-size, assay duration) can be optimized to reduce assay length and improve throughput capacity for such future applications. The amenability and potential of large-scale high-throughput larval zebrafish locomotor-based behavioral assays for drug discovery is clear (Kokel et al., 2010). Studies have already shown promise for identifying compounds with anti-seizure activity, and mode of action discovery is a logical extension of this application (Berghmans et al., 2007; Winter et al., 2008). The present study serves to further demonstrate the amenability of the larval chemomotor response bioassay for mode of action discovery for neuroactive compounds in zebrafish, and to this end, the rapid-throughput interrogation of potential target neuroreceptors.
Conclusions
In summary, our studies used the zebrafish LCR bioassay to comparatively evaluate 24 structurally-related flavonoids, and 30 pharmacologically-diverse neuroactive compounds. Of the 24 flavonoids tested, 22 bioactive flavonoids modulated behavior and elicited hyperactive locomotor activity, which indicates that vertebrate behavior is broadly sensitive to flavonoids. However, the profiles of the behavioral effects were flavonoid-specific, and multidimensional analysis determined several differentially active groups based on potency, efficacy, and time-course kinetics of the responses. Pharmacological intervention studies suggested that the hyperactivity elicited by two flavonoids, (S)-equol and kaempferol, did not involve a GABAA receptor mediated mode of action, whereas there was a clear GABAA receptor mediated mode of action for the behavioral effects of picrotoxin. Given the scarcity of bioactivity data for the broad class of flavonoids, and the potential for both health benefits and health concerns surrounding this chemical class, further studies are warranted to elucidate modes of action and potential neuroreceptors responsible for neuroactive properties. Overall, these studies emphasize the utility of a multidimensional zebrafish platform for comparatively examining behavioral effects for large sets of related compounds.
Supplementary Material
Highlights.
A larval locomotor assay provides an integrative platform for comparative analysis
Neuroactive drugs elicit varied types of locomotor responses
Larval zebrafish locomotion is highly responsive to GABAergic drugs
Flavonoids broadly and rapidly elicit hyperactive locomotor responses
GABAA receptor positive allosteric modulators do not block flavonoid hyperactivity
Acknowledgments
We would also like to thank the staff at the Oregon State University Sinnhuber Aquatic Research Laboratory for animal and husbandry support.
Funding information:
This work was supported by the following U.S. National Institute of Environmental Health Sciences grants: K99 ES025280, P30 ES000210, P42 ES016465, and T32 ES007060. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- GABA
gamma-aminobutyric acid
- HCA
hierarchical clustering analysis
- hpf
h post-fertilization
- LCR
larval chemomotor response
- LEL
lowest effect level
- PCA
principle component analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Materials. Summary data and statistics for all tested compounds and chemical inhibition studies, including time-course, binned and normalized concentration response curves, mean ± SD for normalized activity with 95% confidence intervals, lethality, N-values, and p-values (two-way repeated measures ANOVA with Tukey’s post-hoc, p ≤ 0.05).
The authors declare they have no potential conflicts of interest.
Authors’ contributions:
SMB: Designed and performed experiments, analyzed data, and prepared manuscript
RLT: Supervised overall performance of project and prepared manuscript
References
- Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am. J. Obstet. Gynecol. 1999;180:737–743. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- Afrikanova T, Serruys AS, Buenafe OE, Clinckers R, Smolders I, de Witte PA, Crawford AD, Esguerra CV. Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs. PloS one. 2013;8:e54166. doi: 10.1371/journal.pone.0054166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000;59:1387–1394. doi: 10.1016/s0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Balik-Meisner M, Truong L, Scholl EH, Tanguay RL, Reif DM. Population genetic diversity in zebrafish lines. Mamm. Genome. 2018 doi: 10.1007/s00335-018-9735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball ER, Caniglia MK, Wilcox JL, Overton KA, Burr MJ, Wolfe BD, Sanders BJ, Wisniewski AB, Wrenn CC. Effects of genistein in the maternal diet on reproductive development and spatial learning in male rats. Horm. Behav. 2010;57:313–322. doi: 10.1016/j.yhbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–768. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy research. 2007;75:18–28. doi: 10.1016/j.eplepsyres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Bruni G, Lakhani P, Kokel D. Discovering novel neuroactive drugs through high-throughput behavior-based chemical screening in the zebrafish. Frontiers in pharmacology. 2014;5:153. doi: 10.3389/fphar.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni G, Rennekamp AJ, Velenich A, McCarroll M, Gendelev L, Fertsch E, Taylor J, Lakhani P, Lensen D, Evron T, Lorello PJ, Huang XP, Kolczewski S, Carey G, Caldarone BJ, Prinssen E, Roth BL, Keiser MJ, Peterson RT, Kokel D. Zebrafish behavioral profiling identifies multitarget antipsychotic-like compounds. Nat. Chem. Biol. 2016;12:559–566. doi: 10.1038/nchembio.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel SM, Bonventre JA, Tanguay RL. Comparative developmental toxicity of flavonoids using an integrative zebrafish system. Toxicol. Sci. 2016;154:55–68. doi: 10.1093/toxsci/kfw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel SM, Tanguay RL, Planchart A. Zebrafish: A marvel of high-throughput biology for 21st century toxicology. Current environmental health reports. 2014;1:341–352. doi: 10.1007/s40572-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Chebib M, Johnston GA. The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA(A) receptors. Biochem. Pharmacol. 2004;68:1631–1638. doi: 10.1016/j.bcp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Caro M, Iturria I, Martinez-Santos M, Pardo MA, Rainieri S, Tueros I, Navarro V. Zebrafish dives into food research: effectiveness assessment of bioactive compounds. Food & function. 2016;7:2615–2623. doi: 10.1039/c6fo00046k. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am. J. Clin. Nutr. 1994;60:333–340. doi: 10.1093/ajcn/60.3.333. [DOI] [PubMed] [Google Scholar]
- Chang Y, Ghansah E, Chen Y, Ye J, Weiss DS. Desensitization mechanism of GABA receptors revealed by single oocyte binding and receptor function. J. Neurosci. 2002;22:7982–7990. doi: 10.1523/JNEUROSCI.22-18-07982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zheng S, Li L, Jiang H. Metabolism of flavonoids in human: a comprehensive review. Curr. Drug Metab. 2014;15:48–61. doi: 10.2174/138920021501140218125020. [DOI] [PubMed] [Google Scholar]
- Childs E, Lutz JA, de Wit H. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug Alcohol Depend. 2017;177:136–144. doi: 10.1016/j.drugalcdep.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MH, Kim KR, Hong JK, Park SJ, Chung BC. Determination of non-steroidal estrogens in breast milk, plasma, urine and hair by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:2221–2228. doi: 10.1002/rcm.845. [DOI] [PubMed] [Google Scholar]
- Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Stirling JM, Robinson TN, Bowen DM, Francis PT, Green AR. The modulation by chlormethiazole of the GABAA-receptor complex in rat brain. Br. J. Pharmacol. 1989;98:284–290. doi: 10.1111/j.1476-5381.1989.tb16893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho RS, Duarte FS, de Lima TC. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011;221:75–82. doi: 10.1016/j.bbr.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Duchowicz PR, Vitale MG, Castro EA, Autino JC, Romanelli GP, Bennardi DO. QSAR modeling of the interaction of flavonoids with GABA(A) receptor. Eur. J. Med. Chem. 2008;43:1593–1602. doi: 10.1016/j.ejmech.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Ellis LD, Seibert J, Soanes KH. Distinct models of induced hyperactivity in zebrafish larvae. Brain Res. 2012;1449:46–59. doi: 10.1016/j.brainres.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Ellis LD, Soanes KH. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav. Brain Res. 2012;233:450–457. doi: 10.1016/j.bbr.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Nguyen M, Yow TT, Chu C, Johnston GA, Hanrahan JR, Chebib M. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem. Res. 2009;34:1867–1875. doi: 10.1007/s11064-009-9969-9. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal A-M. Bimodal modulation by nicotine of anxiety in the social interaction test: Role of the dorsal hippocampus. Behav. Neurosci. 1998;112:1423–1429. doi: 10.1037/0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- Foster WG, Chan S, Platt L, Hughes CL., Jr Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol. Lett. 2002;129:199–205. doi: 10.1016/s0378-4274(02)00018-8. [DOI] [PubMed] [Google Scholar]
- Garey J, Morgan MA, Frohlich J, McEwen BS, Pfaff DW. Effects of the phytoestrogen coumestrol on locomotor and fear-related behaviors in female mice. Horm. Behav. 2001;40:65–76. doi: 10.1006/hbeh.2001.1660. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Waxemberg MD, Doñate-Oliver F, Pomata PE, Calvo DJ. Flavonoid modulation of ionic currents mediated by GABAA and GABAC receptors. Eur. J. Pharmacol. 2003;461:79–87. doi: 10.1016/s0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]
- Hanrahan JR, Chebib M, Johnston GA. Flavonoid modulation of GABA(A) receptors. Br. J. Pharmacol. 2011;163:234–245. doi: 10.1111/j.1476-5381.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne AL, Hadingham KL, Macaulay AJ, Whiting P, Kemp JA. The pharmacology of recombinant GABAA receptors containing bovine α1, β1, γ2L sub-units stably transfected into mouse fibroblast L-cells. Br. J. Pharmacol. 1992;107:732–737. doi: 10.1111/j.1476-5381.1992.tb14515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Direct inhibition of glycine receptors by genistein, a tyrosine kinase inhibitor. Neuropharmacology. 2000;39:2195–2204. doi: 10.1016/s0028-3908(00)00046-0. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu T, Gu J, Luo X, Ji R, Cao Y, Xue H, Wong JT-F, Wong BL, Pei G, Jiang H, Chen K. 3D–QSAR Model of Flavonoids Binding at Benzodiazepine Site in GABAA Receptors. J. Med. Chem. 2001;44:1883–1891. doi: 10.1021/jm000557p. [DOI] [PubMed] [Google Scholar]
- Iori F, da Fonseca R, Ramos MJ, Menziani MC. Theoretical quantitative structure-activity relationships of flavone ligands interacting with cytochrome P450 1A1 and 1A2 isozymes. Bioorg. Med. Chem. 2005;13:4366–4374. doi: 10.1016/j.bmc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ, Wu M. Injections of dopaminergic, cholinergic, serotoninergic and gabaergic drugs into the nucleus accumbens: effects on locomotor activity in the rat. Neuropharmacology. 1981;20:29–37. doi: 10.1016/0028-3908(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Katavic PL, Lamb K, Navarro H, Prisinzano TE. Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships. J. Nat. Prod. 2007;70:1278–1282. doi: 10.1021/np070194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Yu KU, Bae EA, Han MJ. Metabolism of puerarin and daidzin by human intestinal bacteria and their relation to in vitro cytotoxicity. Biol. Pharm. Bull. 1998;21:628–630. doi: 10.1248/bpb.21.628. [DOI] [PubMed] [Google Scholar]
- Kohara Y, Kuwahara R, Kawaguchi S, Jojima T, Yamashita K. Perinatal exposure to genistein, a soy phytoestrogen, improves spatial learning and memory but impairs passive avoidance learning and memory in offspring. Physiol. Behav. 2014;130:40–46. doi: 10.1016/j.physbeh.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, Healey D, Kim S, Werdich AA, Haggarty SJ, Macrae CA, Shoichet B, Peterson RT. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010;6:231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D, Peterson RT. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Briefings in functional genomics & proteomics. 2008;7:483–490. doi: 10.1093/bfgp/eln040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousba AA, Sultatos LG, Poet TS, Timchalk C. Comparison of chlorpyrifos-oxon and paraoxon acetylcholinesterase inhibition dynamics: potential role of a peripheral binding site. Toxicol. Sci. 2004;80:239–248. doi: 10.1093/toxsci/kfh163. [DOI] [PubMed] [Google Scholar]
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. The Scientific World Journal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J. Pharmacol. Exp. Ther. 2000;295:1031–1042. [PubMed] [Google Scholar]
- Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Lee SM, Paik HD, Kim HC, Nah SY. Effects of quercetin on alpha9alpha10 nicotinic acetylcholine receptor-mediated ion currents. Eur. J. Pharmacol. 2011;650:79–85. doi: 10.1016/j.ejphar.2010.09.079. [DOI] [PubMed] [Google Scholar]
- Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol. Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/s0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- Maes J, Verlooy L, Buenafe OE, de Witte PA, Esguerra CV, Crawford AD. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PloS one. 2012;7:e43850. doi: 10.1371/journal.pone.0043850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, Simonich MT, Tanguay RL. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. Journal of laboratory automation. 2012;17:66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll MN, Gendelev L, Keiser MJ, Kokel D. Leveraging large-scale behavioral profiling in zebrafish to explore neuroactive polypharmacology. ACS chemical biology. 2016;11:842–849. doi: 10.1021/acschembio.5b00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JH, Paladini AC, Wolfman C, Levi de Stein M, Calvo D, Diaz LE, Pena C. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 1990;40:2227–2231. doi: 10.1016/0006-2952(90)90716-x. [DOI] [PubMed] [Google Scholar]
- Moro S, van Rhee AM, Sanders LH, Jacobson KA. Flavonoid derivatives as adenosine receptor antagonists: a comparison of the hypothetical receptor binding site based on a comparative molecular field analysis model. J. Med. Chem. 1998;41:46–52. doi: 10.1021/jm970446z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AM, Malintan NT, Seelan S, Zhan Z, Mohamed Z, Hassan J, Pendek R, Hussain R, Ito N. Phytoestrogens levels determination in the cord blood from Malaysia rural and urban populations. Toxicol. Appl. Pharmacol. 2007;222:25–32. doi: 10.1016/j.taap.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Bovicelli P, Adriani W, Saso L, Laviola G. Behavioral effects of 6-bromoflavanone and 5-methoxy-6,8-dibromoflavanone as anxiolytic compounds. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:128–134. doi: 10.1016/j.pnpbp.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Paladini AC, Marder M, Viola H, Wolfman C, Wasowski C, Medina JH. Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J. Pharm. Pharmacol. 1999;51:519–526. doi: 10.1211/0022357991772790. [DOI] [PubMed] [Google Scholar]
- Peeters PH, Slimani N, van der Schouw YT, Grace PB, Navarro C, Tjonneland A, Olsen A, Clavel-Chapelon F, Touillaud M, Boutron-Ruault MC, Jenab M, Kaaks R, Linseisen J, Trichopoulou A, Trichopoulos D, Dilis V, Boeing H, Weikert C, Overvad K, Pala V, Palli D, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita HB, van Gils CH, Skeie G, Jakszyn P, Hallmans G, Berglund G, Key TJ, Travis R, Riboli E, Bingham SA. Variations in plasma phytoestrogen concentrations in European adults. J. Nutr. 2007;137:1294–1300. doi: 10.1093/jn/137.5.1294. [DOI] [PubMed] [Google Scholar]
- Rasulev BF, Abdullaev ND, Syrov VN, Leszczynski J. A quantitative structure-activity relationship (QSAR) study of the antioxidant activity of flavonoids. Qsar & Combinatorial Science. 2005;24:1056–1065. doi: 10.1002/qsar.200430013. [DOI] [Google Scholar]
- Roy K, Roy PP. Comparative QSAR studies of CYP1A2 inhibitor flavonoids using 2D and 3D descriptors. Chemical biology & drug design. 2008;72:370–382. doi: 10.1111/j.1747-0285.2008.00717.x. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am. J. Clin. Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- Song BJ, Jouni ZE, Ferruzzi MG. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition. 2013;29:195–202. doi: 10.1016/j.nut.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, Osada H, Ikezuki Y, Tsutsumi O, Iguchi T, Mori C. Fetal exposure to phytoestrogens - the difference in phytoestrogen status between mother and fetus. Environ. Res. 2005;99:195–203. doi: 10.1016/j.envres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Uriarte-Pueyo II, Calvo M. Flavonoids as Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2011;18:5289–5302. doi: 10.2174/092986711798184325. [DOI] [PubMed] [Google Scholar]
- Valentin-Blasini L, Blount BC, Caudill SP, Needham LL. Urinary and serum concentrations of seven phytoestrogens in a human reference population subset. J. Expo. Anal. Environ. Epidemiol. 2003;13:276–282. doi: 10.1038/sj.jea.7500278. [DOI] [PubMed] [Google Scholar]
- Valentin-Blasini L, Sadowski MA, Walden D, Caltabiano L, Needham LL, Barr DB. Urinary phytoestrogen concentrations in the U.S. population (1999-2000) J. Expo. Anal. Environ. Epidemiol. 2005;15:509–523. doi: 10.1038/sj.jea.7500429. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang L, Hua L, Xing D, Du L. Effect of flavonoids in scutellariae radix on depression-like behavior and Brain Rewards: Possible in dopamine system. Tsinghua Science and Technology. 2010;15:460–466. doi: 10.1016/s1007-0214(10)70088-2. [DOI] [Google Scholar]
- Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin JP, Hutchinson TH. Validation of a larval zebrafish locomotor assay for assessing the seizure liability of early-stage development drugs. J. Pharmacol. Toxicol. Methods. 2008;57:176–187. doi: 10.1016/j.vascn.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci. Lett. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli P, Avallone R, Baraldi M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia. 2000;71(Suppl 1):S117–123. doi: 10.1016/s0367-326x(00)00186-6. [DOI] [PubMed] [Google Scholar]
- Zeng S, Tai F, Zhai P, Yuan A, Jia R, Zhang X. Effect of daidzein on anxiety, social behavior and spatial learning in male Balb/c J mice. Pharmacol. Biochem. Behav. 2010;96:16–23. doi: 10.1016/j.pbb.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang X, Coburn RA, Morris ME. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem. Pharmacol. 2005;70:627–639. doi: 10.1016/j.bcp.2005.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.