Summary
Endophentoypes, quantifiable traits lying on the causal chain between a clinical phenotype and etiology, can be used to accelerate genomic discovery in Obsessive-Compulsive Disorder (OCD). Here we identify the neuroanatomic changes that are shared by 22 OCD adult and adolescent patients and 25 of their unaffected siblings who are at genetic risk for the disorder. Comparisons were made against 47 age and sex matched healthy controls. We defined the surface morphology of the striatum, globus pallidus and thalamus, and thickness of the cerebral cortex. Patients with OCD show significant surface expansion compared to healthy controls, following adjustment for multiple comparisons, in interconnected regions of the caudate, thalamus and right orbitofrontal cortex. Their unaffected siblings show similar, significant expansion, most marked in the ventromedial caudate bilaterally, the right pulvinar thalamic nucleus and the right orbitofrontal cortex. These regions define a network that has been consistently implicated in OCD. Additionally both patients with OCD and unaffected siblings showed similar increased thickness of the right precuneus which receives rich input from the thalamic pulvinar nuclei and the left medial temporal cortex. Anatomic change within the orbitofrontostriatal and posterior brain circuitry thus emerges as a promising endophenotype for OCD.
Keywords: Obsessive compulsive disorder, basal ganglia, Endophenotypes, genetics, Striatum, Thalamus, Orbitofrontal cortex, Neuroanatomy
Introduction
Obsessive-compulsive disorder (OCD) is characterized by the presence of unwanted, repetitive and intrusive thoughts and unnecessary, repetitive behaviors and affects around 2.3% of individuals.1 OCD is highly heritable with between 27-47% of variance of the phenotype attributable to heritable factors.2-5 Meta-analysis of genetic association studies has implicated serotonin system polymorphisms in the disorder (5-HTTLPR and HTR2A), although the increased risk conferred by each genetic variant is small, with odds ratios hovering around 1.2.6 A common variant near the BTBD3 gene which codes for a transcription factor attained genome wide significance in trio but not case-control analyses.7 Notwithstanding this progress, there is a need for approaches that might accelerate genomic progress. One strategy is to define endophenotypes, such as brain-based changes which are found both in those with the disorder and in their unaffected relatives.8 Endophenotypes are held to lie closer to the etiology of a disorder than more distal clinical phenotypes and are thus ideal for exploring genetic factors driving OCD. This approach has proved powerful in delineating anomalous brain activity associated with a genetic liability to OCD and here we extend the approach to brain structure.9-13
OCD has been consistently linked with anomalies of the circuitry interconnecting the prefrontal cortex, the striatum, globus pallidus and thalamus.14-17 These regions form information processing ‘loops’ which are pivotal in much of human cognition. The circuit most frequently implicated in pathogenesis of OCD links the lateral orbitofrontal cortex to ventral and medial regions of the caudate nucleus. This caudate region then projects to the internal globus pallidus and then to the ventral anterior and mediodorsal thalamic nuclei. The thalamus projects back to predominately lateral orbitofrontal cortical regions, completing the loop. Other fronto-striato-thalamic loops are implicated in OCD, and the various loops are richly interconnected.14, 18
Recent models of OCD implicate disruption not only of fronto-striatal-thalamic circuitry but also networks incorporating interactions between parietal and thalamic regions. Parietal dysfunction, particularly of the supramarginal and angular gyri is implicated by the deficits seen both in patients with OCD and their unaffected relatives in motor inhibition, planning and attentional control.13, 19-22 These parietal regions are richly interconnected with the thalamus, receiving dense input from the pulvinar nuclei.23, 24 The pulvinar nuclei also have reciprocal connections with the precuneus, a key hub in the default mode network. This network partly subserves introspective cognitive processes and has been found to have anomalous activity in OCD.25-28 Recent models have also incorporated limbic regions which support psychological processes disrupted in OCD.14, 29 These include the parahippocampal cortex implicated in implicit learning, and the amygdala, implicated in abnormal fear conditioning. In summary, while models of OCD focus on anomalies within orbitofronto-striatal circuitry, recent conceptualizations include disruption within large-scale networks incorporating parietal and limbic regions.
Neuroanatomic change in subcortical structures has been reported in OCD, predominately increased volume of the striatum and thalamus.30, 31 While informative, such studies usually examine change at the level of an entire structure and thus cannot define the subregions which might be most compromised in the disorder. Recent advances in neuroimaging analytic techniques allow the definition of surface morphology at the level of millimeters in the striatum, globus pallidus and thalamus. Two such surface mapping studies found expansion in the anterior-superior caudate.32, 33 The sole study to examine the thalamus reported expansion of the surface of the right anterolateral thalamus and loss of typical asymmetry in the pulvinar thalamic nuclei.34 To date, no study has examined surface morphology in unaffected relatives.
Neuroanatomic change has also been found in the cerebral cortex in OCD. A meta-analysis of ten voxel- based morphometric studies, which define change in grey matter density, found increase in the lateral orbitofrontal cortex in OCD.35 Several recent studies have measured the thickness of the cortex at thousands of points across the cerebral cortex allowing the definition of structural change with exquisite resolution. While findings are mixed, there are reports of increased thickness of the orbitofrontal gyrus in adults with OCD.36-38 A theme emerges of congruent increase in the dimensions of richly interconnected regions of the lateral orbitofrontal cortex, striatum and thalamus in OCD.
Here, we map differences in the morphology of the striatum, globus pallidus and thalamus and cerebral cortex in OCD. We aimed to replicate and extend the finding of expansion of the surfaces of the striatum and thalamus in patients with the disorder. We hypothesized that there would increase in cortical dimensions in regions that are richly interconnected with the striatum, specifically increased thickness of the orbitofrontal cortex. Additionally we determined whether these morphological changes are also present in the unaffected siblings of those with the disorder, and might thus serve as endophenotypes for future genomic studies.
Methods
Participants
Twenty two individuals with OCD and 25 of their unaffected siblings were contrasted against 47 age and sex matched healthy controls. Participants with OCD and their siblings were recruited from both general adult psychiatry and specialist OCD clinics. All participants with OCD were in outpatient treatment. Control subjects were recruited by advertisement and drawn from the same geographic region. The primary inclusion criterion for patients was the presence of DSM-IVR defined OCD. The primary psychiatric exclusion criteria were substance dependence and psychotic disorders. Patients with other comorbid diagnoses (which were predominately depression, anxiety disorders and tics) were not excluded provided that OCD was the primary diagnosis and main problem for which treatment was sought.
Subjects were evaluated for OCD and other psychiatric disorders using the Structured Clinical Interview for DSM-IV and OCD symptom severity was measured using the Yale-Brown Obsessive-Compulsive Scale (YBOCS). Assessment of adolescent and adult ADHD was obtained through clinical interviews using the clinician administered ADHD-RS-IV, providing examples and prompts appropriate for the adolescents and adults. 39 For those under 18 years of age, parents were also interviewed using the Parent Diagnostic Interview for Children and Adolescents.40 Unaffected siblings did not meet DSM-IVR criteria for OCD. Other psychiatric disorders were not exclusionary for siblings except for psychosis and substance dependence. Control subjects were free of all axis 1 psychiatric diagnoses and had no first-degree relatives with OCD. The general exclusion criteria were a full-scale IQ of less than 80, head injury resulting in loss of consciousness and presence of a neurological disorder, with the exception of tics. The study was approved by the IRB of the NIMH and all individuals gave written informed consent.
Neuroimaging
All participants had neuroanatomic magnetic resonance imaging on a 1.5-T General Electric (Milwaukee, Wisconsin) Signa scanner. T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained using three- dimensional spoiled gradient recalled echo in the steady state. Imaging parameters were echo time of 5 msec, repetition time of 24 msec, flip angle of 45°, acquisition matrix of 256 xh192, number of excitations = 1, and 24 cm field of view.
The striatum (caudate and putamen), globus pallidus and thalamus were automatically identified using a recently developed segmentation method known as the MAGeT Brain algorithm.41 To summarize the technique, single atlases for the striatum, the globus pallidus and the thalamus that were previously defined using a three-dimensional reconstruction of serial histological data42 were warped to an MRI-based template. The atlases were then customized to a subset of the dataset (30 randomly selected subjects) using a nonlinear transformation estimated in a region-of-interest defined around the subcortical structures.41-43 This set of subjects now acts as a set of templates and all other subjects are now warped to these templates. This provides thirty candidate segmentations for each subject’s striatum, globus pallidus and thalamus. The final segmentation is decided upon using a voxel-wise majority vote, that is, the label occurring most frequently at a specific location is retained.44 Volumes of each structure were determined from these segmentations. These methods are reliable in comparisons against ‘gold standard’ manual definitions of the striatum and thalamus (Kappa = 0.86).41 The templates used are shown in Supplemental Figure 1.
To determine shape, first surface-based representations of the basal ganglia and thalamus defined on the input atlas were estimated using the marching cubes algorithm 45 and morphologically smoothed using the AMIRA software package (Visage Imaging; San Diego, CA, USA). The resulting surfaces have about 6,300 vertices per striatum, 1,300 per globus pallidus and 3,100 per thalamus. The nonlinear portions of the 30 transformations that map each subject to the input template were concatenated and averaged across the template library in order to limit the effects of noise and error and to increase precision and accuracy.46 These surface based representations were warped to fit each template, and as in the case of the segmentation, each these surfaces was warped to match each subject. This yields thirty possible surface representations per subject that are then merged by creating a new surface representation of the striatum, pallidum or thalamus by estimating the median coordinate representation at each location. At this point a third of the surface of each triangle is assigned to each vertex within the triangle. The surface area value stored at each vertex is the sum of all such assignments from all connected triangles.47 Finally, surface area values were blurred with a surfaced-base diffusion smoothing kernel (5mm for the striatum and thalamus and 3mm pallidum).
The Constrained Laplacian Anatomic Segmentation using Proximity surface extraction procedure was used to determine cerebral cortical thickness at over 40,000 vertices in each hemisphere. First, surface meshes representing white and gray matter interfaces were generated.48 The root mean square thickness between corresponding nodes on the surface meshes was calculated in native space. A 30-mm surface blurring algorithm, which preserves cortical topologic features, was used to reduce noise in thickness measurements.49 Thickness measurements were aligned using surface registration to maximize thickness value correspondence between participants in terms of gyral patterning.50
Analyses
We first tested the hypothesis of increased subcortical and cortical dimensions in the OCD group compared to controls using t tests. Adjustment for multiple comparisons was made using the false discovery rate procedure (setting q<0.05). Results of these vertex level analyses were projected onto subcortical and cortical templates. We calculated the total surface area and mean cortical thickness values for each group within these regions, and converted these values to Z scores to facilitate comparison (one Z value for the subcortical regions, and one Z value for the cortical regions). We then tested the hypothesis that the unaffected siblings would also show significant expansion in these regions compared to controls, in keeping with a potential endophenotype. A repeated measures ANOVA was used, with group as the between subjects factor and the Z scores for the cortex and subcortex as a within subjects factor. Again, results at the vertex level for the contrast of unaffected siblings against controls were projected onto brain templates.
Results
Clinical
The groups did not differ in age, sex distribution nor IQ- see Table 1. YBOCS scores were significantly higher in the affected individuals compared to their unaffected siblings. Eleven of the patients with OCD were treated with SSRIs, clomipramine or venlafaxine at the time of the study and one patient was also taking a low-dose antipsychotic. All but one sibling and all healthy controls were unmedicated. While three of the OCD subjects endorsed hoarding as a symptom, they also endorsed other obsessions and compulsions.
Table 1.
Demographic and clinical characteristics of the OCD group, their unaffected siblings and healthy controls.
| OCD patients | Unaffected siblings | Healthy controls | Test of significance | |
|---|---|---|---|---|
| Number | 22 | 25 | 47 | |
| Sex | Male 14 | Male 14 | Male 28 | X2=0.33, p=0.85 |
| Female 8 | Female 11 | Female 18 | ||
| Age at assessment, years(mean, SD) | 26 (SD 13) | 24 (SD 13) | 23 (SD 9) | F(2,91)=0.53, p=0.52 |
| IQ | 110 (17) | 113 (12) | t(34)=0.74, p=0.46 | |
| Y-BOCS- means (SD) | 22 (9) | 2 (3) | NA | t=10.2, p<0.0001 |
| Current symptoms: N(%) of patients | ||||
| Taboo | 15 (68) | NA | NA | |
| Symmetry | 10 (45) | |||
| Hoarding | 3 (14) | |||
| Contamination | 14 (64) | |||
| Checking | 8 (36) | |||
| Lifetime symptoms | ||||
| Taboo | 19 (86) | NA | NA | |
| Symmetry | 15 (68) | |||
| Hoarding | 7 (31) | |||
| Contamination | 18 (82) | |||
| Checking | 14(64) | |||
| Medication | ||||
| SSRI/clomipramine/Venlafaxine | 11 | 1 | 0 | |
| Current comorbidity | ||||
| None | 16 | 22 | NA | |
| Major depression | 2 | 0 | NA | |
| Generalized Anxiety disorder | 1 | 2 | NA | |
| Dysthymia | 2 | 1 | NA | |
| Tics | 3 | 0 | NA | |
Neuroimaging
There was a significant increase of surface area in the OCD group relative to controls, following adjustment for multiple comparisons bilaterally in the tail and head of caudate and the thalamus in the region of the anterior and pulvinar nuclei-. There was a trend to expansion in the globus pallidus, pars interna which did not survive adjustment for multiple comparisons- see Supplemental Figure 2. At the level of the cerebral cortex, there was a significant increase in thickness, surviving adjustment for multiple comparisons in the right lateral orbitofrontal cortex, the left medial temporal cortex and the right posterior cingulate cortex. We then tested the hypothesis that the unaffected siblings would also show increase in surface area and cortical thickness within these regions. This was confirmed as the unaffected siblings had increased subcortical and cortical dimensions compared to controls (p=0.018), similar to the increased dimensions shown by the patients compared to controls (p<0.0001). The overall significant increase in unaffected siblings compared to controls was driven at a subcortical level by expansion of surface area in ventromedial region of the caudate bialterally, extending along its tail and in the region of the right pulvinar thalamic nuclei.- Figures 1 and 2. In these regions, there was increased surface area compared to controls for both patients with OCD (p<0.0001) and the unaffected siblings (p=0.01). The patients also had a significant increase surface area expansions compared to their siblings (p=0.03) and the values for these regions are shown in Figure 4.
Figure 1.
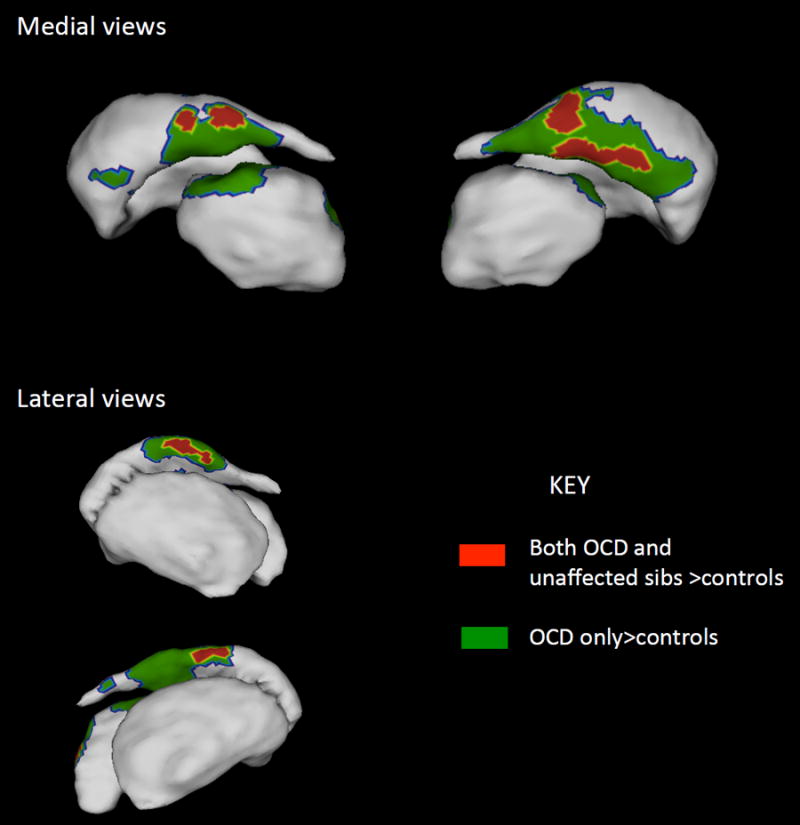
Regions where both the OCD paDents and unaffected siblings showed significant increase in striatal and thalamic surface area compared to controls are shown in red. These regions are candidate endophenotypes. Regions where only the OCD group showed significant increase compared to controls are shown in green. More detailed views of the thalamus are given in Figure 2 and results for the globus pallidus are given in Supplemental Figure 2.
Figure 2.
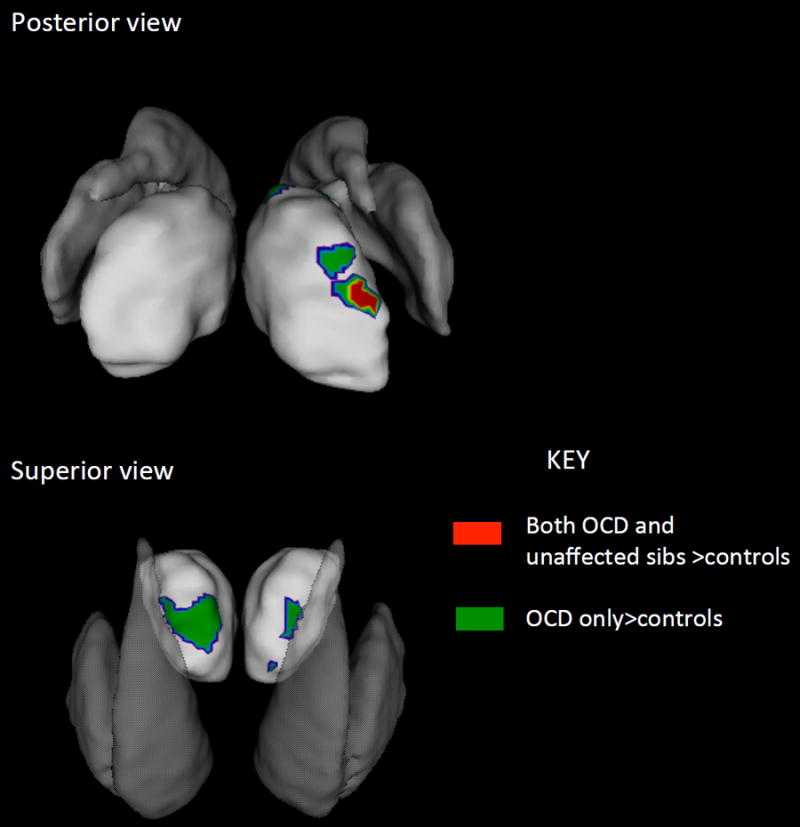
Detailed views of the thalamic regions where both the OCD patients and unaffected siblings showed significant increase in surface area compared to controls are shown in red. Regions where only the OCD group showed significant increase compared to controls are shown in green. The striatum are overlaid in shadow to help with orientation.
Figure 4.
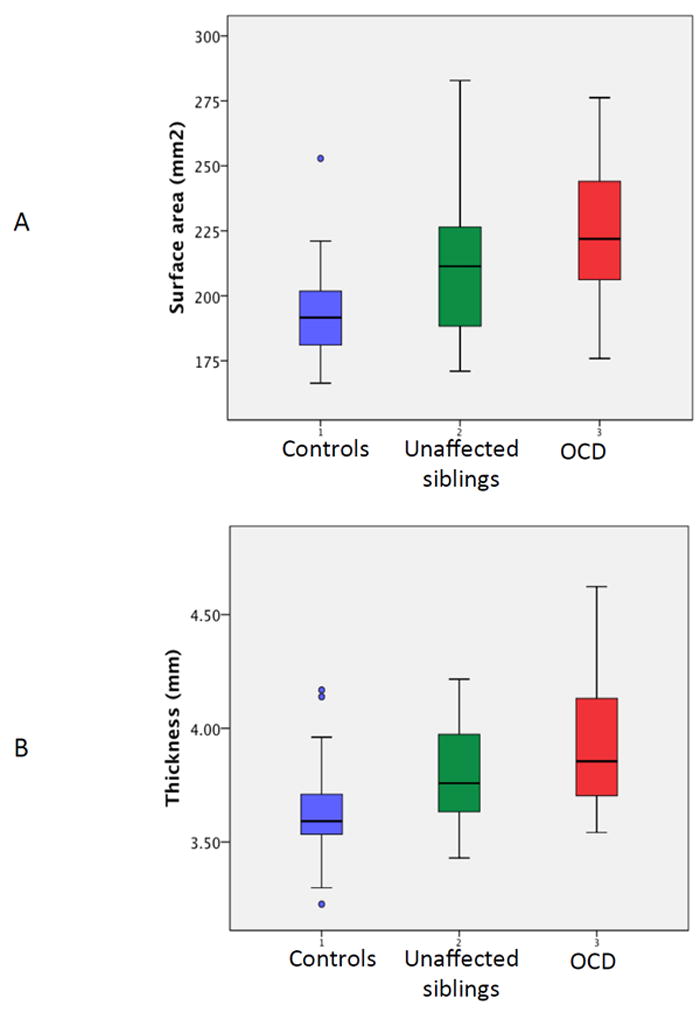
Plots showing the minimum, maximum, median and quartile values for (A) the total surface area of regions where both the OCD patients and unaffected siblings had significant expansion, shown in Figures 1 and 2; and (B) the average mean thickness of the cortex for the regions shown in Figure 3.
At the cortical level, the unaffected siblings showed increase compared to controls in thickness of the right lateral prefrontal cortex, the left medial temporal cortex and the right precuneus- Figure 3. In these regions, there was an increase in cortical thickness in both the patients (p<0.00001) and unaffected siblings (p=0.003). The patients and unaffected siblings did not differ significantly in thickness (p=0.11).
Figure 3.
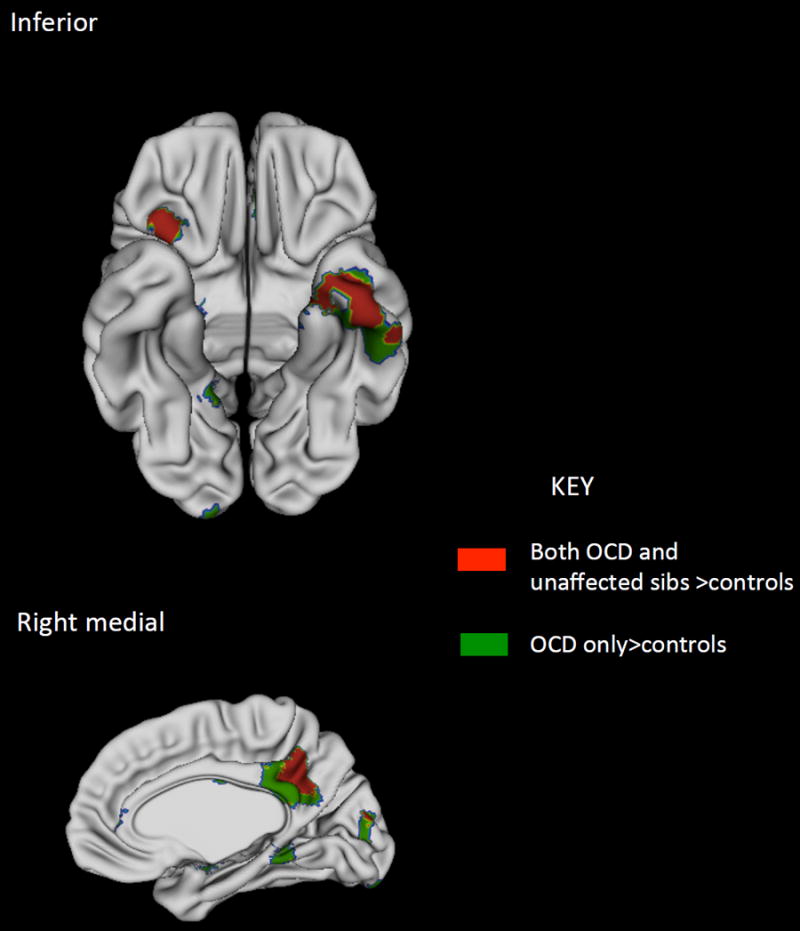
Cortical regions where both the OCD patients and unaffected siblings showed significant increase in surface area compared to controls are shown in red. Regions where only the OCD group showed significant increase compared to controls are shown in green.
There were no group differences in cerebral grey matter volumes (all p>0.1; see Supplemental Table 1). The thalamus was larger bilaterally in OCD patients relative to controls (right thalamus: OCD patients mean 6157, (SD 680)mm3 , controls 5813 (524) mm3, p=0.03; left thalamus, OCD patients, mean 6218 (699) mm3, controls 5861 (497) mm3, p=0.02). There were no other significant volumetric group differences.
There was no significant interaction between diagnostic group and age in the determination of surface areas, suggesting that this effect held across the age range studied. The pattern of results did not differ significantly between medicated and unmedicated OCD patients, nor between OCD patients with and without comorbid disorders- see Supplemental Table 2.
Discussion
Two novel findings emerge from this study, which is the first to define the changes in subcortical surface morphology and cortical thickness shared by patients with OCD and their unaffected siblings. First, we find an increase in subcortical and cortical dimensions in OCD that localized to interconnected regions of the orbitofrontal cortex, caudate and thalamus. Second, unaffected siblings also show significant increases within a restricted portion of these regions. At the subcortical level, unaffected siblings showed surface area expansion in the ventromedial caudate and the right pulvinar thalamic nuclei. At a cerebral cortical level, unaffected siblings showed increased thickness of the right lateral orbitofrontal cortex, left medial temporal cortex and right precuneus.
These differences in the unaffected siblings were not detected using conventional volumetric region of interest approach and the findings thus illustrate the power of detailed spatial morphological mapping. The striatal findings both confirm and extend previous reports of surface expansion in OCD, and the increased thickness of the orbitofrontal is consonant with the increase in grey matter density reported in OCD.35
The regions showing endophenotypic properties are consonant with models of OCD. 14, 15, 51 The ventromedial caudate receives input from not only the orbitofrontal cortex but also the limbic regions, including medial temporal regions and the amygdala.29 The ventromedial caudate has direct projections to the internal globus pallidus, a region where we find a trend to surface area expansion in OCD patients. Dysfunction within this orbitofrontostriatal circuitry in OCD has been demonstrated in meta-analyses of both task-based and resting state functional imaging studies.14 This dysfunction extends to those at genetic risk for the disorder. Chamberlain and colleagues found decreased activation of the orbitofrontal cortex during reversal learning in both those with OCD and their unaffected relatives.9 Motor inhibition deficits in unaffected relatives have been linked with an increase in striatal grey matter and interconnected prefrontal cortical regions.13 Another relevant study compared brain activity during a planning task in monozygotic twins concordantly high on obsessive-compulsive symptoms against monozygotic twins concordantly low in symptoms.10 This comparison, which defines brain activation differences linked to genetic factors, found differential activity in the left caudate and globus pallidus and interconnected inferior prefrontal regions. A further contrast within monozygotic twin pairs who are discordant for obsessive-compulsive symptoms, parsed out brain activation differences that were likely to reflect environmental factors. Notably, no differences in basal ganglia, thalamic or orbitofrontal activation were found between these monozygotic twins who differed in their level of obsessive-compulsive symptomatology.
The current study converges with the only previous examination of thalamic surfaces in OCD to find anomalies in the region of the anterior and pulvinar nuclei.34 The pulvinar nuclei are richly interconnected with the precuneus, another region we find to have increased dimensions in unaffected siblings.25 The precuneus is a key component of the default mode network, which partly subserves introspective cognitive processes.28, 52, 53 Abnormal activity within this network in OCD has been proposed as a contributor to the excessive focus on internally generated thoughts and feeling found in the disorder.26, 27 The pulvinar nuclei also project to the angular and supramarginal gyri. These parietal regions support planning and response inhibition, and deficits in these cognitive domains extend to both patients with OCD and their unaffected relatives.13, 19-22,54 The finding of increased thickness of left medial temporal regions in both patients with OCD and their unaffected siblings is in keeping models which integrate limbic regions into circuitry for OCD.14, 29 These models have been largely driven by both by findings of anomalies of amygdala-based fear learning and extinction in OCD and the demonstration of dysfunction within the pararhippocampal cortex during implicit learning.29, 55, 56 While there are some reports of increased grey matter density in medial temporal regions, results are mixed.36-38 Finding increased dimensions of the pulvinar nuclei, precuneus and limbic regions in unaffected siblings suggests that disruption of large scale brain systems beyond frontostriatal networks may reflect genetic vulnerability to OCD.14, 29
The neuroanatomic anomalies found in unaffected siblings are both more regionally confined and less severe than those seen in those with OCD. This is compatible with a cycle in which a genetically vulnerable neural substrate leads to symptoms, which in turn exacerbate the underlying neural deficits.
Orbitofrontal-striato-thalamic expansion is an attractive endophenotype for OCD. Endophenotypes should ideally be more heritable than the associated clinical disorder or phenotype. Twin studies suggest that orbitofrontal and striatal dimensions are under tight genetic control, with heritability estimates of around 0.9 for the striatum and 0.7 for the orbitofrontal cortex, well above the estimated heritability of OCD.57-59 There are candidate genes which might contribute to the orbitofrontostriatal morphological changes we report. For example, mice exhibit obsessive-compulsive like behaviors when the SAP90/PSD95-associated protein (SAPAP-3) gene is knocked-out, and this post-synaptic scaffolding protein is richly expressed in the striatum.60 Behavioral and associated neurophysiological anomalies are rescued among SAPA-3 knock-out mice by serotonin re-uptake inhibitors, induced striatal expression of SAPAP-3 and optogenetic stimulation of orbitofrontal-striatal circuitry.61 There is preliminary evidence that polymorphisms within the gene are associated with pathological grooming, although not OCD per se. 62 Additionally, polymorphisms of the dopamine transporter gene, which has rich striatal expression, show non-significant trends in meta-analysis of association studies of OCD.6, 63
Our surface area findings may also have some diagnostic specificity for OCD. For example, pulvinar surface contraction characterizes Attention-Deficit/Hyperactivity Disorder which contrasts with the expansion we find in OCD and unaffected siblings.64 Additionally, meta-analyses find that anxiety disorders other than OCD and Attention-Deficit Hyperactivity Disorder are associated with decreased striatal dimensions, unlike the striatal expansions we and others find in OCD.31, 65
There are limitations to the study. First, many of the OCD subjects were on medication at the time of the study. However, the presence of similar surface area expansion in unaffected siblings who were unmedicated argues strongly that the findings in the OCD group cannot be attributed to medication. The pattern of findings was the same for OCD patients either on or off medication. Second, while we controlled for multiple comparisons, these findings require replication.
We thus identify altered morphology within orbitofrontal-striato-thalamic and posterior brain circuits as candidate endophenotypes for OCD.
Supplementary Material
Acknowledgments
The study was funded by the Intramural Programs of the National Human Genome Research Institute and the National Institute of Mental Health
Footnotes
Supplementary information is available at Molecular Psychiatry’s website.
All authors declare no conflict of interest.
References
- 1.Ruscio A, Stein D, Chiu W, Kessler R. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Molecular psychiatry. 2008;15(1):53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: a review. Twin Research and Human Genetics. 2005;8(05):450–458. doi: 10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- 3.Jonnal AH, Gardner CO, Prescott CA, Kendler KS. Obsessive and compulsive symptoms in a general population sample of female twins. American journal of medical genetics. 2000;96(6):791–796. doi: 10.1002/1096-8628(20001204)96:6<791::aid-ajmg19>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Pauls DL. The genetics of obsessive-compulsive disorder: a review. Dialogues in clinical neuroscience. 2010;12(2):149. doi: 10.31887/DCNS.2010.12.2/dpauls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mataix-Cols D, Boman M, Monzani B, Ruck C, Serlachius E, Langstrom N, et al. Population-Based, Multigenerational Family Clustering Study of Obsessive-Compulsive Disorder. JAMA psychiatry (Chicago, III) 2013:1–9. doi: 10.1001/jamapsychiatry.2013.3. [DOI] [PubMed] [Google Scholar]
- 6.Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.76. [DOI] [PubMed] [Google Scholar]
- 7.Stewart SE, Yu D, Scharf JM, Neale BM, Fagerness JA, Mathews CA, et al. Genome-wide association study of obsessive-compulsive disorder. Mol Psychiatry. 2013;18(7):843–843. doi: 10.1038/mp.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321(5887):421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 10.den Braber A, van ’t Ent D, Cath DC, Wagner J, Boomsma DI, de Geus EJ. Brain activation during cognitive planning in twins discordant or concordant for obsessive-compulsive symptoms. Brain. 2010;133(10):3123–3140. doi: 10.1093/brain/awq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, et al. Presupplementary motor area hyperactivity during response inhibition: A candidate endophenotype of Obsessive-Compulsive Disorder. American journal of psychiatry. 2012;169(10):1100–1108. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- 12.Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. American Journal of Psychiatry. 2011;168(3):317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- 13.Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen C-H, Del Campo N, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130(12):3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 14.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience & Biobehavioral Reviews. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. British Journal of Psychiatry. 1998 [PubMed] [Google Scholar]
- 16.Graybiel AM, Rauch SL. Toward a neurobiology review of obsessive-compulsive disorder. Neuron. 2000;28:343. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 17.Mataix-Cols D, van den Heuvel OA. Common and distinct neural correlates of obsessive-compulsive and related disorders. The Psychiatric clinics of North America. 2006;29(2):391. doi: 10.1016/j.psc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Delorme R, Gousse V, Roy I, Trandafir A, Mathieu F, Mouren-Simeoni M-C, et al. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. European Psychiatry. 2007;22(1):32–38. doi: 10.1016/j.eurpsy.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanath B, Janardhan Reddy Y, Kumar KJ, Kandavel T, Chandrashekar C. Cognitive endophenotypes in OCD: a study of unaffected siblings of probands with familial OCD. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(4):610–615. doi: 10.1016/j.pnpbp.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. The American journal of psychiatry. 2007;164(2):335. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennertz L, Rampacher F, Vogeley A, Schulze-Rauschenbach S, Pukrop R, Ruhrmann S, et al. Antisaccade performance in patients with obsessive Aicompulsive disorder and unaffected relatives: further evidence for impaired response inhibition as a candidate endophenotype. European archives of psychiatry and clinical neuroscience. 2012;262(7):625–634. doi: 10.1007/s00406-012-0311-1. [DOI] [PubMed] [Google Scholar]
- 23.Grieve KL. The primate pulvinar nuclei: vision and action. Trends Neurosci. 2000;23(1):35. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 24.Snow JC, Allen HA, Rafal RD, Humphreys GW, Desimone R. Impaired Attentional Selection following Lesions to Human Pulvinar: Evidence for Homology between Human and Monkey. Proc Natl Acad Sci U S A. 2009;106(10):4054–4059. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 26.Jang JH, Kim J-H, Jung WH, Choi J-S, Jung MH, Lee J-M, et al. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neuroscience letters. 2010;474(3):158–162. doi: 10.1016/j.neulet.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PloS one. 2012;7(5):e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, et al. Altered Corticostriatal Functional Connectivity in Obsessive-compulsive DisorderAltered Corticostriatal Functional Connectivity in OCD. Archives of general psychiatry. 2009;66(11):1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 29.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends in cognitive sciences. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotge J-Y, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, et al. Meta-Analysis of Brain Volume Changes in Obsessive-Compulsive Disorder. Biological Psychiatry. 2009;65(1):75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Archives of General Psychiatry. 2010;67(7):701. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 32.Zarei M, Mataix-Cols D, Heyman I, Hough M, Doherty J, Burge L, et al. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biological psychiatry. 2011;70(11):1083–1090. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Choi J-S, Kim SH, Yoo SY, Kang D-H, Kim C-W, Lee J-M, et al. Shape deformity of the corpus striatum in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 2007;155(3):257–264. doi: 10.1016/j.pscychresns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Kang D-H, Kim SH, Kim C-W, Choi J-S, Jang JH, Jung MH, et al. Thalamus surface shape deformity in obsessive-compulsive disorder and schizophrenia. Neuroreport. 2008;19(6):609–613. doi: 10.1097/WNR.0b013e3282fa6db9. [DOI] [PubMed] [Google Scholar]
- 35.Rotge J-Y, Langbour N, Guehl D, Bioulac B, Jaafari N, Allard M, et al. Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2009;35(3):686–691. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan VM, Narr KL, Phillips OR, Thompson PM, Toga AW, Szeszko PR. Greater regional cortical gray matter thickness in obsessive-compulsive disorder. Neuroreport. 2008;19(15):1551. doi: 10.1097/WNR.0b013e3283112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallucca E, MacMaster FP, Haddad J, Easter P, Dick R, May G, et al. Distinguishing between major depressive disorder and obsessive-compulsive disorder in children by measuring regional cortical thickness. Archives of general psychiatry. 2011;68(5):527. doi: 10.1001/archgenpsychiatry.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin YÄ, Yoo SY, Lee JK, Ha TH, Lee KJ, Lee JM, et al. Cortical thinning in obsessive compulsive disorder. Human brain mapping. 2007;28(11):1128–1135. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DuPaul GJ, Power JD, Anastopouls AA, Reid R. ADHD Rating Scale-IV: Checklists, Norms and Clinical Interpretation. The Guilford Press; New York: 1998. [Google Scholar]
- 40.Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39(1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarty M, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, et al. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage. 2006;30(2):359–376. doi: 10.1016/j.neuroimage.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 43.Chakravarty MM, Sadikot AF, Germann J, Bertrand G, Collins DL. Towards a validation of atlas warping techniques. Med Image Anal. 2008;12(6):713–726. doi: 10.1016/j.media.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.04.193. [DOI] [PubMed] [Google Scholar]
- 45.Proceedings of the ACM Siggraph Computer Graphics. ACM; 1987. Marching cubes: A high resolution 3D surface construction algorithm. [Google Scholar]
- 46.Borghammer P, Ostergaard K, Cumming P, Gjedde A, Rodell A, Hall N, et al. A deformation-based morphometry study of patients with early-stage Parkinson’s disease. European Journal of Neurology. 2010;17(2):314–320. doi: 10.1111/j.1468-1331.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- 47.Lyttelton OC, Karama S, Ad-Dab’bagh Y, Zatorre RJ, Carbonell F, Worsley K, et al. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 2009;46(4):895–903. doi: 10.1016/j.neuroimage.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 48.Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27(1):210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 49.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24(1):163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 50.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34(4):1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 51.Rapoport JL. Childhood obsessive-compulsive disorder. The Clinical Guide to Child Psychiatry. 1985:208. [Google Scholar]
- 52.Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- 53.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biological psychiatry. 2010;67(12):1178–1184. doi: 10.1016/j.biopsych.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Rauch SL, Savage CR, Alpert NM, Dougherty D, Kendrick A, Curran T, et al. Probing striatal function in obsessive-compulsive disorder: a PET study of implicit sequence learning. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(4):568–573. doi: 10.1176/jnp.9.4.568. [DOI] [PubMed] [Google Scholar]
- 56.Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive Aicompulsive disorder. Biological psychiatry. 2007;61(3):330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 57.Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Pol H, Hilleke E. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 2007;28(6):464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein JL, Hibar DP, Madsen SK, Khamis M, McMahon KL, de Zubicaray GI, et al. Discovery and replication of dopamine-related gene effects on caudate volume in young and elderly populations (N=1198) using genome-wide search. Mol Psychiatry. 2011;16(9):927–937. doi: 10.1038/mp.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human brain mapping. 2009;30(1):163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding J-D, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burguilere E, Monteiro P, Feng G, Graybiel AM. Optogenetic Stimulation of Lateral Orbitofronto-Striatal Pathway Suppresses Compulsive Behaviors. Science. 2013;340(6137):1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bienvenu O, Wang Y, Shugart Y, Welch J, Grados M, Fyer A, et al. Sapap3 and pathological grooming in humans: results from the OCD collaborative genetics study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150(5):710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, et al. The dopamine transporter: immunochemical characterization and localization in brain. The Journal of neuroscience. 1995;15(3):1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov I. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. The American journal of psychiatry. 2010;167(4):397. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry. 2011;168(11):1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


