Abstract
The importance of subzero temperature interactions with elevated CO2 on plant carbon metabolism has received rather little attention, despite their likely role in influencing future vegetation productivity and dynamics. Here we focused on the critical issues of CO2-enrichment effects on leaf-freezing temperatures, subsequent membrane damage, and recovery of the photosynthetic system. We show that growth in elevated CO2 (70 Pa) results in a substantial and significant (P < 0.01) increase (up to 4°C) in the ice nucleation temperature of leaves of Maidenhair tree (Ginkgo biloba), which was observed consistently throughout the 1999 growing season relative to their ambient CO2 (35 Pa) counterparts. We suggest that increased sensitivity of leaves to ice damage after growth in elevated CO2 provides an explanation for increased photoinhibition observed in the field early and late in the growing season when low nighttime temperatures are experienced. This new mechanism is proposed in addition to the earlier postulated explanation for this phenomenon involving a reduction in the rate of triose-P utilization owing to a decrease in the rate of carbohydrate export from the leaf.
The continued rise in the partial pressure of atmospheric CO2 is the most certain feature of future global environmental change (IPCC, 1995). Many studies have identified the direct and indirect action of a CO2-enriched atmosphere on plant carbon metabolism (for review, see Drake et al., 1997), but rather less attention has been paid to its capacity to influence interactions between plants and low, chilling, or freezing temperatures. Such interactions may be important for understanding future changes in vegetation dynamics since low temperatures regulate plant distribution limits through their action on a variety of different temperature-sensitive life cycle processes (Woodward, 1987). Of those studies investigating these phenomena, contrasting effects have been observed. Leaves of the evergreen, Eucalyptus pauciflora, showed higher ice nucleation temperatures (Lutze et al., 1998) in response to CO2 enrichment, leading to more severe frost damage, whereas needles of Scots pine (Pinus sylvestris; Repo et al., 1996) and buds of the deciduous birch, Betula allaghanensis, (Wayne et al., 1998) had increased frost resistance under high CO2. Spruce (Picea abies) needles showed no changes in frost hardiness, however (Wiemken et al., 1996). Although somewhat inconclusive, these studies provide some evidence for the capacity for elevated CO2 to influence the freezing temperatures or frost damage of plant tissues.
An additional and equally important aspect of the interaction between plants, elevated CO2, and low temperature is the expectation that a CO2-enriched atmosphere will influence rates of leaf photosynthetic carbon gain and the recovery of the photosynthetic apparatus after exposure to chilling and freezing temperatures. The expectation is 2-fold. First, plants exposed to these low temperature events typically suffer membrane disruption and desiccation when ice crystal formation occurs (Long et al., 1994), leading to a corresponding reduction in photochemical production of NADPH and ATP. Countering this loss is the potential for elevated CO2 to increase the proportion of these products utilized in photosynthetic carbon fixation rather than photorespiration (Osborne et al., 1997). Second, in cold temperate climate zones terrestrial plant leaves are frequently exposed to high irradiance under low, chilling, or freezing temperatures with associated increased photoinhibition, (Long et al., 1994). New experimental evidence indicates that under such conditions, but with CO2 enrichment, photoinhibition can increase, as indicated by loss in the efficiency of photosystem II and CO2 fixation (Hymus et al., 1999; Roden et al., 1999).
Based on these earlier CO2-enrichment field-studies we hypothesize that plant growth with elevated CO2 raises the freezing point of leaves compared with leaves from ambient CO2-grown plants. We suggest that this effect renders their photosynthetic apparatus more sensitive to damage by cold temperatures, as evidenced by increased photoinhibition. Here these hypotheses were tested by focusing on the deciduous Maidenhair tree (Ginkgo biloba), saplings of which have been exposed to an increased partial pressure of atmospheric CO2 for the past five years. Maidenhair tree is a so called “living fossil” (Beerling et al., 1998; Beerling, 1999), representing one of the most ancient seed plants. It attained a circumpolar distribution in the Northern hemisphere and extended into several regions of the Southern hemisphere between the late Mesozoic and early Tertiary, but with a rapid decline from the Miocene to the Quaternary (Rothwell and Holt, 1997). It is interesting that over much of the Mesozoic and Tertiary, the partial pressure of atmospheric CO2 is predicted by geochemical models to have been 2 to 5 times the pre-industrial value (30 Pa; Berner, 1997). From a paleobiology perspective therefore Maidenhair tree is a taxon that has a long history of exposure to elevated CO2.
We first measured the effect long-term exposure to elevated CO2 on the temperatures at which leaves of Maidenhair tree freeze, and the potential for leaf cellular membrane damage, following the application of a suite of artificial early autumnal frosting events with a varying, but controlled, degree of severity. Measurements of leaf-freezing temperatures were made throughout the growing season to assess whether natural physiological cold hardening influenced the action of CO2. Leaf-gas exchange responses and the recovery of the photosynthetic apparatus from ambient and elevated CO2-grown plants were measured following the application of the frosting treatment (2 h) and 3 weeks later to monitor post-treatment recovery. The response of photoinhibition was interpreted through concurrent measurements of modulated chlorophyll fluorescence to detect changes in the partitioning of absorbed energy between non-photochemical and photochemical processes in the thylakoid membrane. These measurements were made on leaves after the application of frosting events of different severities to allow us to test the hypothesis that increased photoinhibition in leaves, observed previously in the field with CO2 enrichment (Hymus et al., 1999; Roden et al., 1999), is directly related to physical damage of the cellular proteins and membranes by ice crystal formation rather than being solely linked to the inability of plants to export carbohydrates out of leaves, reductions in linear electron transport, and triose-P utilization (TPU) limitation (Hymus et al., 1999).
RESULTS
Ice Nucleation Temperatures
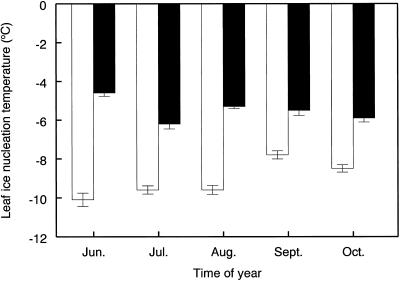
Leaf-ice nucleation temperatures were consistently and significantly (P < 0.01) higher throughout the growing season (+3.6°C averaged across all sampling dates) in plants grown with CO2 enrichment compared with the ambient CO2 plants (Fig. 1). The largest difference detected was +5.5°C during midsummer, thereafter this increase in ice nucleation temperature declined to about 2.5°C by mid-October when the leaves began to senesce (as indicated by yellowing). The reduction in treatment differences as the season progressed points to a possible influence of cold hardening reducing the effect of CO2 on ice nucleation temperatures since they occurred in parallel to a small decrease in the temperature at which the leaves of the elevated CO2 plants froze (from −4.5°C to −6°C) and a small increase for those leaves from ambient CO2 plants (from −10°C to −8°C; Fig. 1).
Figure 1.
Seasonal changes in ice nucleation temperatures of leaves of Maidenhair tree after long-term (5 years) growth at ambient (□) and elevated (▪) CO2 partial pressures. Values are means of four leaves, one per plant at each CO2 partial pressure ± se. All measurements were made mid-month. All differences between means at ambient and elevated CO2 partial pressures were significant (P < 0.001, two sample t tests).
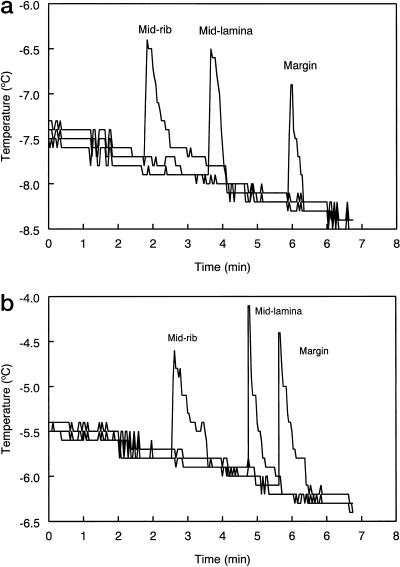
Exotherm freezing traces taken from the thermocouples placed at three positions across the leaf surface recorded the propagation of the freezing wave through the tissues (Fig. 2). Measurements made on similar-sized leaves from ambient (Fig. 2a) and elevated (Fig. 2b) CO2-grown plants showed that freezing was initiated close to the midrib and then moved rapidly toward the edge of the leaf (Fig. 2). These results are in agreement with data obtained from infrared video thermography images capturing the time-course of freezing in whole leaves (Wisniewski et al., 1997; Lutze et al., 1998). There was also a CO2 effect on the rate at which the freezing wave was propagated through the leaf. The duration of time from the detection of initial freezing in the mid-rib to the freezing of the leaf margin was 3.3 min for ambient CO2-grown leaves and 2.4 min for elevated CO2-grown leaves (means of two leaves per treatment).
Figure 2.
Temperature cooling traces of individual Maidenhair tree leaves measured from thermocouples located at three positions on the abaxial leaf surface for plants grown under ambient (a) and elevated (b) CO2 conditions. Leaves from both ambient and elevated CO2 treatments used were of similar size.
Membrane Damage
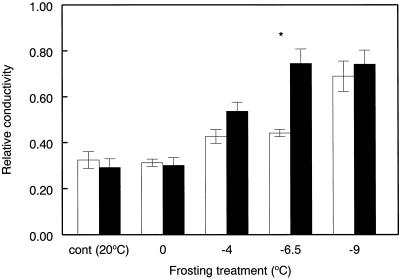
Electrolyte leakage rates provided no evidence of chilling damage to leaf cellular membranes when leaves were cooled to 0°C in either elevated or ambient CO2-grown plants (Fig. 3). At freezing temperatures of between −4 and −6.5°C, membrane damage significantly (P < 0.05) increased, and Rc (relative conductivity) rose from 0.3 to 0.7 in leaves of plants grown with CO2 enrichment, a response that was absent from ambient CO2-grown plants between these temperatures (Fig. 3). The leaves from plants grown at ambient CO2 did, however, show a marked increase in membrane damage when freezing temperatures fell to between −6.5 and −9°C.
Figure 3.
Electrolyte leakage rates of cellular membranes, expressed as Rc, of leaves of Maidenhair tree in response to the frosting treatments after long-term (5 years) growth at ambient (□) and elevated (▪) CO2 partial pressures. Values are means of four leaves, one per plant at each CO2 partial pressure ± se. All measurements were made mid-month. No significant differences between CO2 partial pressures for each CO2 partial pressure, except at −6.5°C (P < 0.05, two sample t tests).
These differences were directly correlated with the temperature at which ice nucleation occurred. Leaves from elevated CO2 plants froze at −6.0°C (Fig. 1) and showed severe cellular membrane damage when temperatures fell below this threshold (Fig. 3). Leaves from plants grown at ambient CO2 froze at the lower temperature of −8.5°C, and consequently, is was not until temperatures decreased below this value that significant membrane damage was detected (Fig. 3). The correspondence between leaf-ice nucleation temperatures and cellular membrane damage indicates that the measured ice nucleation temperatures were those critical for the development of damaging ice crystal formation.
Photosynthesis
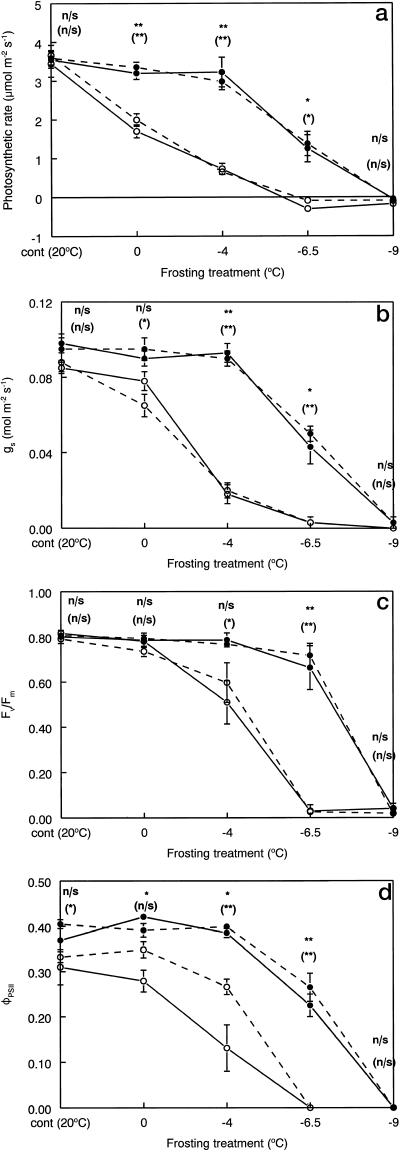
Leaf-gas exchange and chlorophyll fluorescence exhibited marked differences between plants grown at ambient and elevated CO2 partial pressures both 2 h and 3 weeks after the application of the frosting treatments (Fig. 4). Ambient CO2-grown plants showed little or no inhibition of photosynthesis until cooling temperature pretreatments were below −4°C, whereas elevated CO2-grown plants exhibited reductions with chilling below 0°C and more pronounced reductions at all freezing temperatures below that (Fig. 4a). Furthermore, the inhibitory effect of the frosting treatments on the net photosynthetic rates of leaves showed no recovery in either group of plants 3 weeks after treatment. When leaves were cooled to below the ice nucleation temperatures (−8.5°C and −6.0°C for ambient and elevated CO2 plants, respectively), photosynthesis was completely inhibited (Fig. 4a), indicating substantial tissue damage.
Figure 4.
Effect of frosting treatment temperatures on leaf net photosynthetic rates (a), stomatal conductance (gs; b), variable to maximum chlorophyll fluorescence ratios (Fv/Fm; c),and quantum efficiency of photosystem II (ΦPSII; d) for attached leaves of Maidenhair tree grown under long-term ambient (●) and elevated (○) CO2 partial pressures. Measurements were made 2 h (continuous line) and 3 weeks (broken line) after frosting treatment for dark adapted plants. Values are means of four leaves, one per plant at each CO2 partial pressure ± se. All measurements were made dates. Significant differences between ambient and elevated CO2 leaves were tested for 2 h (uppermost result) and 3 weeks (value in parentheses) after the frosting treatment. n/s, No significant difference; *, P < 0.05; **, P < 0.01.
Leaf stomatal conductance of plants from elevated CO2 was marginally lower than those from ambient CO2 (Fig. 4b), as reported previously for Maidenhair tree (Beerling et al., 1998). Stomatal conductance responses to the frost treatments paralleled those of photosynthetic rate for both the ambient and elevated CO2 plants, with no sign of recovery after 3 weeks (Fig. 4b). The tracking of leaf photosynthetic rates by stomatal conductance indicates that stomatal function was retained even when leaf photosynthesis was completely inhibited.
Chlorophyll fluorescence parameters showed significant CO2 effects between the two groups of plants, but these were dependent upon temperature (Fig. 4). After the 0°C treatment, no changes in Fv/Fm were observed in the leaves from plants grown with elevated CO2. Reduced rates of electron transport due to damage to PSII were therefore not in response to the decreased net photosynthetic rate of these plants at this temperature compared with the ambient CO2-grown plants (Fig. 4a). When leaf temperatures were cooled to −4°C, Fv/Fm was reduced by about 30% for elevated CO2 plants, with no major effects on plants from ambient CO2 (Fig. 4c). This shift is indicative of a reduced maximum photochemical efficiency of PSII and would be consistent with freeze-induced damage of thylakoid membranes in response to chilling and freezing injury. The Fv/Fm of leaves of ambient CO2-grown plants showed a conserved response across all freezing temperatures until these fell below ice nucleation values (Fig. 4c). Post-treatment monitoring revealed no appreciable recovery of Fv/Fm in either group of plants after 3 weeks (Fig. 4c).
Plants grown with elevated CO2 had a significantly reduced relative quantum efficiency of PSII photochemistry (ΦPSII) after all frost treatments (Fig. 4d). In contrast to the other gas exchange measurements, however (Fig. 4, a–c), ΦPSII showed some ability to recover from frost treatments providing they were above the measured ice nucleation temperatures, with recovery almost complete at −4°C for ambient and 0°C for elevated CO2 plants.
DISCUSSION
Our results show that leaves of the deciduous woody living fossil Maidenhair tree become more susceptible to freezing at higher temperatures when grown with CO2 enrichment. Such a response is consistent with the findings of Lutze et al. (1998) for the evergreen frost-hardy eucalypt E. pauciflora. Moreover, when leaves were cooled to below leaf-ice nucleation thresholds, photochemical and cellular membrane damage ensued resulting in the complete inhibition of photosynthetic CO2 fixation. Recovery from such damage was negligible over a 3-week period, suggesting that early season freezing injury could persist into the growing season, limiting photosynthetic carbon gain, especially for plants in a high CO2 environment.
The mechanism by which elevated CO2 alters leaf-freezing temperatures remains uncertain. Enhanced freezing tolerance at the current partial pressure of CO2 has been correlated with changes in solute accumulation (e.g. soluble sugars; Steponkus, 1984), cold-induced gene expression (Thomashow, 1998), and antifreeze proteins (Jiang et al., 1999). Therefore, for CO2 enrichment to increase the susceptibility of leaves to freezing, it must reduce the expression of these features of the plants' physiology. Little is known of this possibility at present, but the recognition that abscisic acid can influence the freezing tolerance through changes in the accumulation of soluble proteins and soluble sugars (Mantyla et al., 1995; Bravo et al., 1998) may offer a possible signal pathway susceptible to the action of CO2 worthy of further investigation. A further possibility is that leaves developing in a CO2-enriched atmosphere support greater populations of ice nucleating bacteria, making them more susceptible to freezing (Lutze et al., 1998).
There have been few field studies reporting leaf photosynthetic measurements on plants grown at elevated CO2 and experiencing early or late season subzero temperatures. Reports on leaf-gas exchange on the evergreen species, Pinus taeda (Hymus et al., 1999), E. pauciflora (Roden et al., 1999), and seedlings of the deciduous tree, Fagus sylvatica (Leverenz et al., 1999) all show an increase in photoinhibition with CO2 enrichment at these times, as characterized by a decrease in the Fv/Fm ratio. One hypothesis to explain this response (Hymus et al., 1999) is that photosynthesis becomes progressively limited by the rate of TPU owing to decreased rates of carbohydrate transport out of the leaf or reduced carbohydrate requirement. The latter may be compounded by an accumulation of storage reserves in sink tissues during the previous growing season (for evergreen trees) when photosynthesis is stimulated by elevated CO2. Limitation of photosynthesis by the rate of TPU could prevent an increase in the rate of the carboxylation reaction of Rubisco (vc) at elevated CO2, but would depress the rate of the oxygenation reaction (vo; Leegood et al., 1985; Sharkey, 1985). This would then reduce the requirement for NADPH produced by the electron transport chain, and could lead to an increase in damage as evidenced by an increase in energy dissipation via non-photochemical mechanisms and photoinhibition.
However, for this proposed mechanism to explain our observations in Maidenhair tree an increase in leaf carbohydrate concentration is required. We have not measured this directly, but earlier CO2-enrichment experiments with Maidenhair tree indicate its leaves typically have high non-structural carbohydrate and starch contents when grown at ambient CO2 and show only small increases (10%–11%) with CO2 enrichment (600 μL L−1; Korner et al., 1995). Therefore, some other mechanism(s) may be operating to increase the sensitivity of photoinhibition in elevated CO2 to low temperatures in addition to TPU limitation. Our data clearly show that for the deciduous tree, Maidenhair tree, loss of photosynthetic function can occur due to a frosting event alone (i.e. in the absence of light) when leaf temperatures approach the ice nucleation threshold. Given large differences (up to 4°C) in ice nucleation temperatures between ambient and elevated CO2-grown plants throughout the growing season (Fig. 1), we suggest that early spring and late autumnal nighttime frosts could induce substantial differences in the extent of low temperature damage to plants grown at ambient and elevated CO2, both leading to subsequent loss of photosynthetic efficiency and photoinhibitory damage. This could provide an additional explanation for the apparent increased photoinhibition reported for early and late in the growing season of elevated CO2 field-grown plants (Hymus et al., 1999; Leverenz et al., 1999; Roden et al., 1999). Further experiments are currently in progress which aim to resolve the relative importance of the two possible mechanisms (ice damage versus TPU limitation) using parallel measurements of ice nucleation temperatures, membrane damage, leaf carbohydrates, photosynthesis, and temperature.
Our data and the findings of others have considerable implications for the climatic and palaeoecological interpretation of high palaeo-latitude fossilized plant remains dating to the Mesozoic and early Tertiary, when the CO2 partial pressure is thought to have been several times greater than the present value (Berner, 1997). Coldest monthly mean continental temperatures estimated from the climatic limits of nearest living relatives in a contemporary climate and CO2 are, for example, likely to be too cool by at least several degrees. There is also the possibility that the productivity of vegetation was curtailed by photoinhibition during polar summers in the high latitudes where there was a warm climate in conjunction with continuous 24-h daylight and a high CO2 environment. Further work is required to bridge plant physiological studies with those of palaeobiology if the phenomenon of polar fossil forests growing up to 85 °S and 80 °N (Chaloner and Creber 1989; Francis, 1990; Taylor et al., 1992) are to be properly understood (Beerling, 1998, 1999).
MATERIALS AND METHODS
Plant Material and Environmental Conditions
Five-year-old Maidenhair tree (Gingko biloba L.) saplings of uniform size were grown in four heated greenhouses in Sheffield, UK (Beerling et al., 1998), under either ambient (36–40 Pa) or elevated CO2 (56–60 Pa) environments between 1995 and 1997 and subsequently at 70 to 74 Pa CO2 between 1997 and 1999. Greenhouse temperatures were controlled to track ambient air temperature, except during the winter months when saplings were leafless, and a minimum temperature of 3°C was set to provide frost protection. Daytime photon flux density during the summer months generally varied between 100 and 1,500 μmol m−2 s−1 and was typically between 350 and 450 μmol m−2 s−1. Relative humidity in each greenhouse was maintained above a minimum of 75%. All measurements were made between mid-June and mid-October 1999, before the onset of leaf senescence that preceded leaf fall, which in 1999 occurred during mid-November. No difference in leaf fall date was observed in the two treatments.
Determination of Ice Nucleation Temperatures
Detached leaves (one per plant, four plants per CO2 treatment) of Maidenhair tree were secured onto a peltier cooling plate (ST3353–05, Marlow Industries, Dallas; see www.marlow.com for details), abaxial surface uppermost. Two thermocouples (76 μm in diameter) were placed on each leaf midway between the mid-rib and leaf margin and secured with plastic insulation tape. Two leaves were accommodated on the plate beneath a perspex cover. The plate was cooled at a controlled rate 6°C h−1 using a programmable power supply (se5010, Marlow Industries) and the temperature of the leaves logged at 2-s intervals using a datalogger (Squirrel 1000 series, Grant Instruments, Cambridge, UK). Leaf-freezing temperatures were determined by observing exotherms (Wisniewski et al., 1997), characterized by an almost instantaneous increase in leaf temperature of between 2°C to 3°C (due to ice crystal formation) and subsequent cooling back to the controlled temperature curve within about 1 min. This method allowed determination of the ice nucleation temperatures to within 0.1°C. The cooling treatment was imposed over the range 10°C to −12°C for each run.
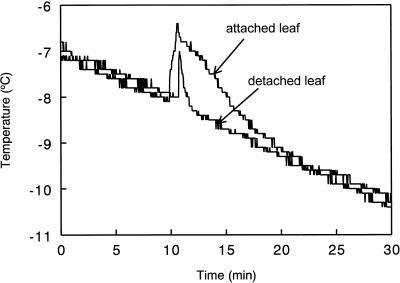
Pilot studies were conducted to determine if the rate of cooling or if the effect of detaching leaves influenced ice nucleation temperatures determined using this system. Cooling the leaves at either 3°C h−1 or 6°C h−1 had minimal effects on ice nucleation temperatures determined in this way (values within 0.2°C of each other). The effect of detaching leaves on ice nucleation temperatures was assessed with replicate measurements made on four leaves from ambient CO2-grown plants. No marked differences in ice nucleation temperatures were detected between attached (−7.9°C ± 0.04°C, mean ±se) and detached (−8.1°C ± 0.05°C) leaves (Fig. 5), although differences were evident in the respective characteristic exotherm traces. Traces from attached leaves showed a 0.5°C to 1°C higher sharp rise in temperature and the nucleation spike was broader, lasting for about 10 min compared with about 1 min for detached leaves. We attributed this slower decline in the exotherm of attached leaves to the continued supply of water from the xylem through the petiole into the leaf. For convenience all leaves were detached and cooled at a standard rate (6°C h−1) with the thermocouples placed at standard positions.
Figure 5.
Temperature cooling traces showing exotherms for attached and detached leaves of Maidenhair tree grown at ambient CO2. Note that despite differences in the shape of the curves, exotherms of the attached and detached leaves occur at very similar temperatures.
Controlled Frosting Treatments
Controlled frosting treatments were applied to individual attached leaves of Maidenhair tree in early October. One leaf from each of four plants grown at each CO2 partial pressure (one from each of the four replicate ambient and elevated CO2 environments) were selected for each frosting treatment. Freezing and chilling injury of leaves in light or darkness has different effects on the photosynthetic system (Flexas et al., 1999). Since in nature plants are typically exposed to early autumnal frosts in the dark, our plants were dark conditioned for 60 min prior to the frosting treatment. Frosting treatments were applied by placing leaves on a peltier-cooled freezing plate at an initial temperature of 10°C and cooled at a rate of 6°C h−1 to one of four target temperatures (0°C, −4°C, −6.5°C, and −9°C). In all cases leaves were held for 1 h at the target temperature before being warmed back to 10°C at 6°C h−1. These treatments effectively simulated temperatures experienced during early autumnal frosting events, after the plants had begun the process of physiological cold hardening.
Assessment of Frost Damage
Cellular (membrane) damage to leaves of Maidenhair tree after the frosting treatments were assessed objectively by measurement of electrolyte leakage from tissues using a conductivity meter (PCM1, Jenway Products, Leeds, UK; Caporn et al., 1994). The treated leaf tissue was placed in de-ionized water and regular measurements of conductivity were made over a period of 25 h. Changes in conductivity over a given time interval (Ct) were compared with measurements obtained after autoclaving the sample at 105°C for 4 min to provide an estimate of maximum electrolyte leakage (Cm), normalized for leaves of different sizes. The degree of frost damage to the leaves was assessed using the term of Rc (Rc = Ct/Cm).
Photosynthetic Measurements
Short-term changes in the operation of the photosynthetic apparatus were monitored by measuring photosynthesis in situ on replicate (n = 4) leaves of plants 2 h and 3 weeks after the application of the frosting treatments using an open gas exchange system incorporating an infrared gas analyzer with a Parkinson leaf chamber (model LCA3, Analytical Development Company, Hoddesdon, UK). All gas exchange measurements (photosynthesis and stomatal conductance) were made at a CO2 partial pressure of 35 Pa, 20°C, a photon flux density of 450 μmol m−2 s−1 (selected to represent a typical value experienced during growth), and a mean leaf-to-air vapor pressure deficit of 0.94 kPa.
Chlorophyll Fluorescence
Chlorophyll a fluorescence was measured simultaneously with gas-exchange using a modified gas exchange chamber to allow attachment of a fiber optic connected to a modulated fluorescence system (PAM 101 fluorimeter, H. Walz, Effeltrich, Germany). Plants were dark-adapted for at least 60 min prior to measurements to ensure full oxidation of PSII and relaxation of chlorophyll fluorescence quenching processes. An initial measurement of Fo and Fm was taken to allow calculation of the Fv/Fm ratio, a general indicator of photoinhibition of PSII, and hence photosynthetic efficiency brought about by an inability to dissipate excess light energy (Krause and Weis, 1984, 1991; Owens, 1991). Subsequently the ΦPSII was determined under actinic illumination as described by Genty et al. (1989). Steady-state rates of photosynthesis were indicated by constant rates of leaf-CO2 exchange and yield of chlorophyll fluorescence.
ACKNOWLEDGMENTS
We thank Dr. C.P. Osborne and Prof. R.C. Leegood for helpful comments on the manuscript.
Footnotes
D.J.B. gratefully acknowledges funding of this work through awards from the Natural Environment Research Council of the United Kingdom (GR8/4223 and GR3/11900) and a Royal Society University Research Fellowship.
LITERATURE CITED
- Beerling DJ. The future as the key to the past for palaeobotany? Trends Ecol Evol. 1998;13:311–316. doi: 10.1016/s0169-5347(98)01334-2. [DOI] [PubMed] [Google Scholar]
- Beerling DJ. Atmospheric carbon dioxide, past climates and the plant fossil record. Bot J Scotl. 1999;51:49–68. [Google Scholar]
- Beerling DJ, McElwain JC, Osborne CP. Stomatal responses of the ‘living fossil’ Ginkgo biloba L. to changes in atmospheric CO2 concentrations. J Exp Bot. 1998;49:1603–1607. [Google Scholar]
- Berner RA. The rise of land plants and their effect on weathering and atmospheric CO2. Science. 1997;276:544–546. [Google Scholar]
- Bravo LA, Zuniga GE, Alberdi M, Corcuera LJ. The role of ABA in freezing tolerance and cold acclimation in barley. Physiol Plant. 1998;103:17–23. [Google Scholar]
- Caporn SJM, Risager M, Lee JA. Effect of nitrogen supply on frost hardiness in Calluna vulgaris (L.) Hull. New Phytol. 1994;128:461–468. doi: 10.1111/j.1469-8137.1994.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Chaloner WG, Creber GT. The phenomenon of forest growth in Antarctica: a review. In: Crame AJ, editor. Origins and Evolution of the Antarctic Biota. Geological Society of London Special Publication No. 47, London; 1989. pp. 85–88. [Google Scholar]
- Drake BG, Gonzàlez-Meler MA, Long SP. More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- Flexas J, Badger M, Chow WS, Medrano H, Osmond CB. Analysis of the relative increase in photosynthetic O2 uptake when photosynthesis in grapevine leaves is inhibited following night temperatures and/or water stress. Plant Physiol. 1999;121:675–684. doi: 10.1104/pp.121.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis JE. Polar fossil forests. Geol Today. 1990;6:92–95. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Hymus GJ, Ellsworth DS, Baker NR, Long SP. Does free-air carbon dioxide enrichment affect photochemical energy use by evergreen trees in different seasons? A chlorophyll fluorescence study of mature loblolly pine. Plant Physiol. 1999;120:1183–1191. doi: 10.1104/pp.120.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change. The Science of Climate Change. In: Houghton JT, Meira Filho LG, Callander BA, Harris N, Kattenberg A, Moss RH, editors. Climate Change 1995. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- Korner C, Pelaez-Riedl S, Van Bel AJE. CO2 responsiveness in plants: a possible link to phloem loading. Plant Cell Environ. 1995;20:867–880. [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence as a tool in plant physiology. Photosynth Res. 1984;5:139–157. doi: 10.1007/BF00028527. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Jiang Y, Jia SR, Fei YB, Tan KH. Antifreeze proteins and their role in plant anti-freeze physiology. Acta Bot Sin. 1999;41:677–685. [Google Scholar]
- Leegood LC, Walker DA, Foyer CH. Regulation of the Calvin cycle. In: Barber R, Baker NR, editors. Topics in Photosynthesis 6. Amsterdam: Elsevier; 1985. pp. 189–258. [Google Scholar]
- Leverenz JW, Bruhn D, Saxe H. Responses of two provenances of Fagus sylvatica seedlings to a combination of four temperatures and two CO2 treatments during the first growing season: gas exchange of leaves and roots. New Phytol. 1999;144:437–454. doi: 10.1046/j.1469-8137.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- Long SP, Humphries S, Falkowski PG. Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:633–662. [Google Scholar]
- Lutze JL, Roden JS, Holly CJ, Wolfe J, Egerton JJG, Ball MC. Elevated atmospheric [CO2] promotes frost damage in evergreen tree seedlings. Plant Cell Environ. 1998;21:631–635. [Google Scholar]
- Mantyla E, Lang V, Palva ET. Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins. Plant Physiol. 1995;107:141–148. doi: 10.1104/pp.107.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Drake BG, LaRoche J, Long SP. Does long-term elevation of CO2 concentration increase photosynthesis in forest floor vegetation? Plant Physiol. 1997;114:337–344. doi: 10.1104/pp.114.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens TG. Fluorescence and energy transduction. In: Demers S, editor. Individual Cell and Particle Analysis in Oceanography. NATO/ASI Workshop. Berlin: Springer-Verlag; 1991. pp. 187–203. [Google Scholar]
- Repo T, Hanninen H, Kellomaki S. The effects of long-term elevated of air temperature and CO2 on the frost hardiness of Scots pine. Plant Cell Environ. 1996;19:209–216. [Google Scholar]
- Roden JS, Egerton JJG, Ball MC. Effect of elevated [CO2] on photosynthesis and growth of snow gum (Eucalyptus pauciflora) seedlings during winter and spring. Aust J Plant Physiol. 1999;26:37–46. [Google Scholar]
- Rothwell GW, Holt B. Fossils and phenology in the evolution of Ginkgo biloba. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Trémouillaux-Guîller J, Tobe H, editors. Gingko biloba: A Global Treasure: From Biology to Medicine. Tokyo: Springer-Verlag; 1997. pp. 223–230. [Google Scholar]
- Sharkey TD. O2-insensitive photosynthesis in C3 plants: its occurrence and a possible explanation. Plant Physiol. 1985;78:71–75. doi: 10.1104/pp.78.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus PL. Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol. 1984;35:543–584. [Google Scholar]
- Taylor EL, Taylor TN, Cúneo NR. The present is not the key to the past: a polar forest from the Permian of Antarctica. Science. 1992;257:1675–1677. doi: 10.1126/science.257.5077.1675. [DOI] [PubMed] [Google Scholar]
- Thomashow MF. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998;118:1–7. doi: 10.1104/pp.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne PM, Reekie EG, Bazzaz FA. Elevated CO2 ameliorates birch response to high temperature and frost stress: implications for modelling climate-induced geographic range shifts. Oecologia. 1998;114:335–342. doi: 10.1007/s004420050455. [DOI] [PubMed] [Google Scholar]
- Wiemken V, Kossatz L, Ineichen K. Frost hardiness of Norway spruce grown under elevated atmospheric CO2 and increased nitrogen fertilizing. J Plant Physiol. 1996;149:433–438. [Google Scholar]
- Wisniewski M, Londow SE, Ashworth EN. Observations of ice nucleation and propagation in plants using infrared video thermography. Plant Physiol. 1997;113:327–334. doi: 10.1104/pp.113.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward FI. Climate and Plant Distribution. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]