Abstract
Luteinizing hormone (LH) acts on the granulosa cells that surround the oocyte in mammalian preovulatory follicles to cause meiotic resumption and ovulation. Both of these responses are mediated primarily by an increase in cyclic adenosine monophosphate (cAMP) in the granulosa cells, and the activity of cAMP phosphodiesterases (PDEs), including PDE4, contributes to preventing premature responses. However, two other cAMP-specific PDEs, PDE7 and PDE8, are also expressed at high levels in the granulosa cells, raising the question of whether these PDEs also contribute to preventing uncontrolled activation of meiotic resumption and ovulation. With the use of selective inhibitors, we show that inhibition of PDE7 or PDE8 alone has no effect on the cAMP content of follicles, and inhibition of PDE4 alone has only a small and variable effect. In contrast, a mixture of the three inhibitors elevates cAMP to a level comparable with that seen with LH. Correspondingly, inhibition of PDE7 or PDE8 alone has no effect on meiotic resumption or ovulation, and inhibition of PDE4 alone has only a partial and slow effect. However, the fraction of oocytes resuming meiosis and undergoing ovulation is increased when PDE4, PDE7, and PDE8 are simultaneously inhibited. PDE4, PDE7, and PDE8 also function together to suppress the premature synthesis of progesterone and progesterone receptors, which are required for ovulation. Our results indicate that three cAMP PDEs act in concert to suppress premature responses in preovulatory follicles.
Multiple cAMP phosphodiesterases function together to maintain the mouse ovarian follicle in an arrested state before stimulation by luteinizing hormone.
In cells in which physiological responses are elicited by hormones that elevate cyclic adenosine monophosphate (cAMP), it is critical that cAMP stays low before hormonal stimulation. To ensure that cAMP-dependent processes do not occur prematurely, cAMP phosphodiesterases (PDEs) are continuously active in many tissues (see “Discussion”), including granulosa cells of the ovarian follicle (1–3). The mammalian ovarian follicle is a spherical complex of thousands of granulosa cells surrounding an oocyte in the center. The oocyte’s meiotic cell cycle is paused at prophase, and cyclic nucleotide levels must be precisely controlled to maintain the arrest until, with each reproductive cycle, a subset of follicles responds to luteinizing hormone (LH) (4–6). LH acts on its receptors in the granulosa cells to activate the G-protein, Gs, thus activating adenylyl cyclase and elevating cAMP, and the cAMP rise in the granulosa cells is a primary stimulus for reinitiation of meiosis in the oocyte and for ovulation (5, 6). LH signaling triggers other responses as well, including activation of the glycine transporter, GLYT1, in the oocyte, allowing cell volume regulation (7), and the activation of genes leading to granulosa cell luteinization (8). All of these responses occur following a preovulatory increase in LH, during which serum levels of LH stay elevated for a period of several hours (9). Although the LH receptor activates other G-proteins in addition to Gs (5), prevention of untimely cAMP elevation is essential for preventing premature responses in the follicle.
Previous studies have indicated that activity of PDE4 contributes to preventing meiosis and ovulation from occurring prematurely. PDE4 inhibitors (PDE4i) promote resumption of meiosis in isolated follicles (2, 3), and injection of some (but not other) PDE4 inhibitors into rats causes progesterone production and ovulation (10). However, PDE4 accounts for only 20% to 30% of the total cAMP PDE activity in mouse preovulatory ovaries (11, 12) and for only 15% to 20% of the cAMP PDE activity in bovine and rat mural granulosa cells (13, 14).
In preovulatory mural granulosa cells of mice, rats, and humans, levels of mRNA encoding the cAMP-specific PDEs PDE7A, PDE7B, PDE8A, and PDE8B are much higher than those encoding the four isoforms of PDE4 (14–16). Furthermore, PDE8 accounts for ∼40% of the cAMP PDE activity in bovine and porcine mural granulosa cells (13, 17). Studies of a T cell line have indicated that inhibition of PDE7 and PDE8 results in phosphorylation of a set of proteins distinct from those that are phosphorylated when PDE4 is inhibited (18). These findings, as well as extensive evidence that PDE4, PDE7, and PDE8 often act together in other cells to control cAMP-dependent processes (see “Discussion”), led us to investigate the function of these three PDEs in mouse ovarian follicles. Here, we use a new PDE7 inhibitor (PDE7i), alone or in combination with PDE4i and PDE8 inhibitors (PDE8i), to test the effects of inhibiting these PDEs on cAMP levels, meiotic resumption, ovulation, and production of progesterone and its receptors.
Materials and Methods
PDE inhibitors
The PDE4i rolipram was obtained from Tocris Bioscience (Bristol, United Kingdom). PDE7i and PDE8i (also called PF-04957325) were obtained from Pfizer Worldwide Research and Development (Groton, CT). The chemical structure of PDE7i is shown in Supplemental Fig. 1. For comparison, this figure also shows the previously published structures of rolipram (19) and PF-04957325 (20). PF-04957325 is now available from MedChemExpress (Monmouth Junction, NJ). Stock solutions were made in dimethyl sulfoxide (DMSO) and stored at −80°C. Final DMSO concentrations after dilution were as follows: 1 μM PDE7i, 0.003% DMSO; 1 μM PDE8i, 0.002% DMSO; 5 μM rolipram, 0.005% DMSO; 20 μM rolipram, 0.02% DMSO; 5 μM rolipram + 1 μM PDE7i + 1 μM, 0.01% DMSO. By itself, 0.1% DMSO did not cause meiotic resumption or ovulation in cultured ovarian follicles and did not affect the time course of meiotic resumption in response to LH (Supplemental Fig. 2). As the highest concentration of DMSO that was used in the solutions of PDE inhibitors was less than this (0.02%), DMSO was not included in controls without PDE inhibitors.
Rolipram has a half-maximal inhibitory concentration (IC50) for PDE4 of 70 to 140 nM, measured with 1 μM cAMP substrate, whereas IC50 values for all other cyclic nucleotide PDEs are >100 μM (21–24). PDE7i and PDE8i are also highly selective. Dissociation constant of the enzyme inhibitor complex (Ki) values for PDE7i and PDE8i were determined as previously described (25) for a member of each cyclic nucleotide PDE family. These data, shown in Tables 1 and 2, were supplied by Steve Jenkinson (Pfizer Worldwide Research and Development).
Table 1.
Selectivity Data for PDE7i and PDE8i
|
|
|
PDE7i
|
PDE8i
|
|---|---|---|---|
| PDE | Substrate | Ki, nM | Ki, nM |
| PDE1B1 | cAMP | 7200 | >9900 |
| PDE2A1 | cGMP | >9900 | >9900 |
| PDE3A1 | cAMP | >9800 | >9800 |
| PDE4D3 | cAMP | 3800 | 3000 |
| PDE5A1 | cGMP | >9600 | >9600 |
| PDE6 | cGMP | >9900 | >9900 |
| PDE7B | cAMP | 7.8 | 2500 |
| PDE8B | cAMP | >9500 | 0.1 |
| PDE9A1 | cGMP | >9600 | >9600 |
| PDE10A1 | cAMP | >9300 | >9300 |
| PDE11A4 | cGMP | >9200 | >9200 |
Assays were performed using recombinant human PDE proteins, except for PDE6, which was extracted from bovine retina. Mean values for the dissociation constant of the enzyme inhibitor complex (Ki) for PDE7i and for PDE8i, determined for the indicated PDEs and substrates.
Abbreviations: cGMP, cyclic guanosine monophosphate; n, number of assays.
Table 2.
PDE7i and PDE8i
| PDE | pKi, Means ± SEM | Ki, nM | n |
|---|---|---|---|
| PDE7i | |||
| PDE7B | 8.11 ± 0.05 | 7.8 | 6 |
| PDE1B1 | 5.14 ± 0.16 | 7200 | 4 |
| PDE4D3 | 5.42 ± 0.24 | 3800 | 4 |
| PDE8i | |||
| PDE8B | 8.74 ± 0.06 | 0.1 | 3 |
| PDE7B | 5.60 ± 0.14 | 2500 | 4 |
| PDE4D3 | 5.52 ± 0.05 | 3000 | 4 |
Assays were performed using recombinant human PDE proteins. Means ± standard error of the mean (SEM) values for the negative of the base 10 logarithm of Ki (pKi), testing PDE7i with the indicated PDEs. Mean IC50 values for PDE8i inhibition of PDE8A and PDE8B were previously reported to be 0.7 and <0.3 nM, respectively (20) (substrate concentration not specified) and 3.1 and 0.4 nM, respectively (26) (12 to 14 nM cAMP substrate).
Abbreviation: n, number of assays.
In brief, PDE activity was measured using a scintillation proximity assay (SPA) that measures the inhibition by the indicated compounds of the activity of the human recombinant PDEs listed in Tables 1 and 2 (except for PDE6, which was extracted from bovine retina), as determined in vitro using lysates or FLAG-tag-purified enzymes. The assays were performed in a 384-well format. These assays were used to measure the hydrolysis of [3H]cyclic guanosine monophosphate (cGMP) to the 5′ nucleotide [3H]guanosine monophosphate (GMP) or of [3H]cAMP to the 5′ nucleotide [3H]adenosine monophosphate (AMP), as indicated in Tables 1 and 2. The enzyme stocks were thawed slowly and diluted in assay buffer containing 50 mM Tris HCl buffer (pH 7.5 at room temperature) and 1.3 mM MgCl2. Additionally, the PDE1 assay buffers contained 2.8 mM CaCl2 and the activator calmodulin at a final assay concentration of 100 U/mL. The enzyme concentration for each isoform was determined by enzyme titration experiments to achieve 20% to 30% substrate turnover at 30 minutes, with the exception of the PDE6 reactions, which were incubated for 60 minutes. The final substrate concentration used was at sub–Michaelis-Menten constant (Km) levels (20 nM for [3H]cAMP and 50 nM for [3H]cGMP) so that IC50 values would approximate the Ki values. In all cases, these reactions were well within the linear portion of the assay.
The reaction was stopped by the addition of PDE SPA beads (PerkinElmer, Waltham, MA) at a final assay concentration of 0.2 mg/well. The PDE9 assay required the extra addition of a potent PDE9i, PF-00509783, at a high concentration (10 μM) before the addition of beads to stop the reaction completely. The products, [3H]GMP and [3H]AMP, bound preferentially to yttrium silicate SPA beads and were detected by scintillation counting in a Microbeta Trilux Counter (PerkinElmer). The inhibition of enzyme activity was calculated relative to the activity of uninhibited controls (DMSO) and fully inhibited (high concentration of known inhibitor). To determine IC50 values, the compounds were tested in duplicate using a half-log dilution scheme with the top concentration of 10 μM. The corresponding IC50 values of the compounds for the inhibition of PDE activities were determined from the concentration-effect curves by using a four-parameter logistic fit. Apparent Ki values were estimated by using the Cheng-Prusoff equation: −Ki = (IC50)/(1 + [S]/Km), where S is the substrate, and Km is the substrate concentration at which one-half of the enzyme’s active sites are occupied by substrate.
The mean Ki for PDE7i inhibition of PDE7B is 7.8 nM compared with ≥3.8 μM for all other cyclic nucleotide PDE families (Table 1). The Ki for PDE7i inhibition of PDE7A was not determined. The mean Ki for PDE8i inhibition of PDE8B is 0.1 nM compared with ≥2.5 μM for all other cyclic nucleotide PDE families (Table 1). These measurements are close to previously published IC50 values for this inhibitor (20, 26, 27). These previous studies reported IC50 values for this inhibitor that are approximately two to eight times higher for PDE8A than for PDE8B but still much lower than for all other cyclic nucleotide PDE families (see Table 2 legend).
Mice and follicle culture
Prepubertal B6SJLF1 mice (23 to 25 days old), from The Jackson Laboratory (Bar Harbor, ME), were used for all experiments. Procedures were approved by the Animal Care Committee of the University of Connecticut Health Center.
Ovarian follicles, 320 to 400 μm in diameter, were isolated using fine forceps. Before use, the follicles were cultured for 24 to 30 hours on Millicell organotypic membranes (Merck Millipore, Cork, Ireland; PICMORG50) (28, 29). Bovine serum albumin (3 mg/mL) was included in the culture medium in place of serum. Follicle-stimulating hormone (FSH; 1 nM; 30 ng/mL) was included in the medium to stimulate follicle growth and expression of LH receptors. The use of FSH at 1 nM instead of 0.3 nM (10 ng/mL), which we have used previously, was an improvement; with 1 nM FSH, 100% of follicle-enclosed oocytes underwent nuclear envelope breakdown (NEBD) by 6 hours after LH exposure, whereas with 0.3 nM FSH, the percentage was typically ∼80% (30, 31). LH was used at 0.1, 1, 10, or 300 nM (0.3 ng/mL to 10 μg/mL), as indicated. Highly purified ovine FSH (AFP7558C) and ovine LH (ovine LH-26) were obtained from A. F. Parlow (National Hormone and Peptide Program, Torrance, CA).
Observations of follicles on Millicell membranes were made using an upright microscope with a 20×/0.4 numerical aperture long-working distance objective. LH or PDE inhibitors were applied to the medium in the dish holding the Millicell membrane, and oocytes within follicles were observed for the presence or absence of a nuclear envelope and nucleolus at 1-hour intervals. Photographs of follicles were taken on the Millicell membranes. Ovulation was scored at 24 hours.
cAMP measurement
Follicles were sonicated in 0.1 M HCl, and cAMP was measured with a direct cAMP enzyme-linked immunosorbent assay kit from Enzo (Farmingdale, NY; ADI-900-066). This kit is highly selective for cAMP and shows only 0.33% cross-reactivity with AMP, 0.12% with adenosine triphosphate, and <0.001% with cGMP, GMP, guanosine triphosphate, cyclic uridine monophosphate, and cytidine triphosphate (manufacturer’s product information sheet). Interassay precision was determined by comparing the standard curves obtained in 16 separate assays using cAMP solutions prepared from a stock solution provided with the kit. The coefficient of variation (standard deviation/mean ×100) at the midpoint concentration was 14.5%. Intra-assay precision was determined by the assaying of 14 replicates of the midpoint concentration in a single assay; the coefficient of variation was 1.2%.
Progesterone measurement
Progesterone in the culture media was measured using an enzyme-linked immunosorbent assay kit from Cayman Chemical (Ann Arbor, MI; 582601). This kit is selective for progesterone but shows a small amount of cross-reactivity with pregnenolone (14%), 17β-estradiol (7.2%), 5β-pregnan-3α-ol-20-one (6.7%), and 17α-hydroxyprogesterone (3.6%) (manufacturer’s product information sheet). Interassay precision was determined by comparing the standard curves obtained in 16 separate assays, using progesterone solutions prepared from a stock solution provided with the kit. The coefficient of variation at the midpoint concentration was 24.3%. Intra-assay precision was determined by assaying seven replicates of the midpoint concentration in a single assay; the coefficient of variation was 6.2%.
Western blotting
Samples for Western blotting were prepared by sonication of follicles in Laemmli sample buffer containing 75 mM dithiothreitol, 10 mM NaF, 1 mM Na3VO4, 1 mM Pefabloc (Sigma-Aldrich, St. Louis, MO), and Complete Mini EDTA-free protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). The progesterone receptor (PGR) antibody was obtained from Abcam (Cambridge, MA; ab16661, rabbit monoclonal, clone SP2; Table 3). This antibody recognizes both PGR-A and PGR-B isoforms (32). The tissue-culture supernatant was used at a dilution of 1:500. The PGR antibody was detected using a fluorescent secondary antibody (IRDye 800CW, 926-32211; Li-cor Biosciences, Lincoln, NE). The binding of the secondary antibody was quantified using the Odyssey infrared imaging system (Li-cor Biosciences). Variability in the amount of total protein loaded in each lane was <5%, as confirmed by densitometry of staining with Swift Membrane Stain (G-Biosciences, St. Louis, MO).
Table 3.
Antibody Descriptions
| Protein Target | Antigen | Name of Antibody | Manufacturer, Catalog Number | Species, Monoclonal/ Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| PGR, PGR-A and PGR-B isoforms | Amino acids 412 to 526 of human PGR | Clone SP2 | Abcam, ab16661 | Rabbit, monoclonal | 1:500 | AB_443421 |
| Rabbit IgG | Rabbit IgG (heavy and light chains) | Goat anti-rabbit IgG, IRDye 800CW-conjugated antibody | Li-cor Biosciences, 926-32211 | Goat, polyclonal | 1:15000 | AB_621843 |
Abbreviations: IgG, immunoglobulin G; RRID, Research Resource Identifier.
Measurement of cAMP PDE activity in follicle lysates
Methods were the same as previously described (16), except that [3H]cAMP was used instead of [3H]cGMP.
Statistics
Data were analyzed by one-way analysis of variance or unpaired t test, followed by the Holm-Sidak correction for multiple comparisons, as appropriate, using Prism software (GraphPad, La Jolla, CA). Percent data were transformed by arc sine square root transformation before analysis. All values indicate mean ± standard error of the mean (SEM). Numbers in parentheses indicate the numbers of independent experiments, and asterisks and different letters indicate significant differences (P < 0.05). Each experiment included 8 to 16 follicles.
Results
PDE4, PDE7, and PDE8 act together to suppress premature cAMP elevation in preovulatory follicles
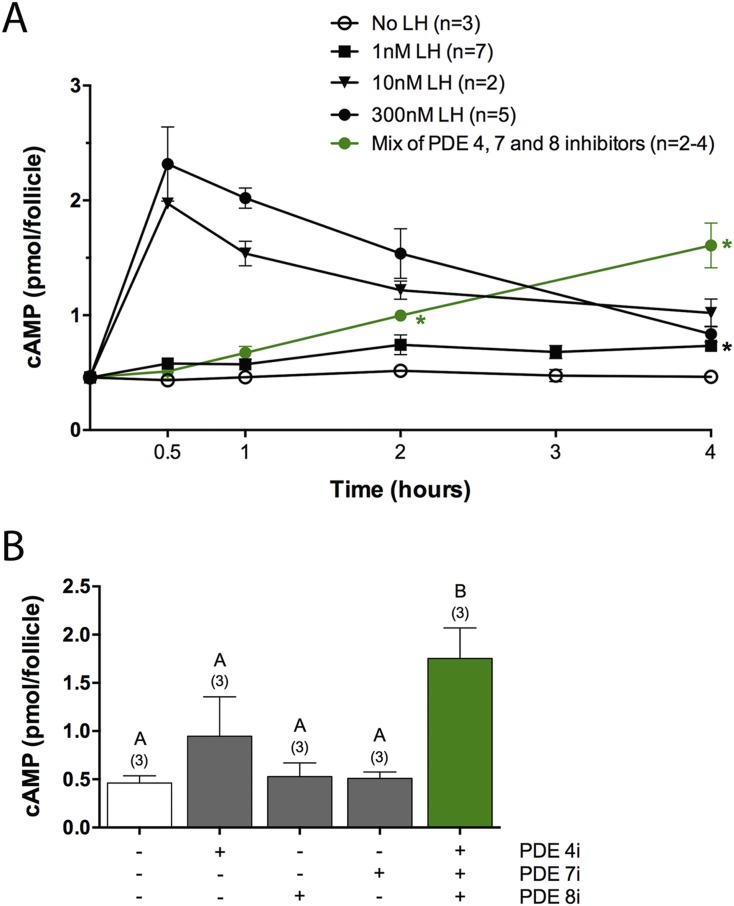
The LH receptor stimulates cAMP formation by activating the G-protein, Gs, in granulosa cells, and the resulting elevation of cAMP is a major component of the signal that causes meiotic resumption from prophase arrest (5, 6). As background for investigation of the role of PDEs in suppression of premature cAMP elevation, we first characterized the LH-induced cAMP increase under our experimental conditions by measuring the cAMP content of mouse preovulatory follicles at various times after application of various concentrations of LH (Fig. 1A). Concentrations of LH that were sufficient to stimulate meiotic resumption maximally in our culture system (≥10 nM; see Fig. 2B) increased the cAMP content by approximately fivefold at 30 minutes. The cAMP content then declined gradually but was still above baseline at 4 hours (Fig. 1A). These cAMP levels are similar to those determined in a previous study in which the LH receptor of mouse ovarian follicles was stimulated with human chorionic gonadotropin (33).
Figure 1.
PDE4, PDE7, and PDE8 act together to suppress premature cAMP elevation in preovulatory follicles. (A) Time course of cAMP elevation after exposure of follicles to various concentrations of LH or to a mixture of inhibitors of three cAMP PDEs: PDE4i (rolipram, 5 μM), PDE7i (1 μM), and PDE8i (PF04957325, 1 μM). The LH data were obtained from the indicated number of independent experiments, each including all time points. The PDE inhibitor data include two to four measurements for each time point. t Tests were performed to test the statistical significance of the difference between the measurements following no LH and 1 nM LH treatments; the black asterisk indicates a significant difference (P < 0.05 after correction for multiple comparisons). t Tests were also used to test the difference between measurements following no LH and PDE inhibitor treatments; the green asterisks indicate significant differences. (B) cAMP content of follicles after a 4-hour exposure to each of the inhibitors listed in (A) or to a mixture of the three inhibitors. Numbers in parentheses indicate the number of independent experiments. Different letters indicate significant differences (P < 0.05).
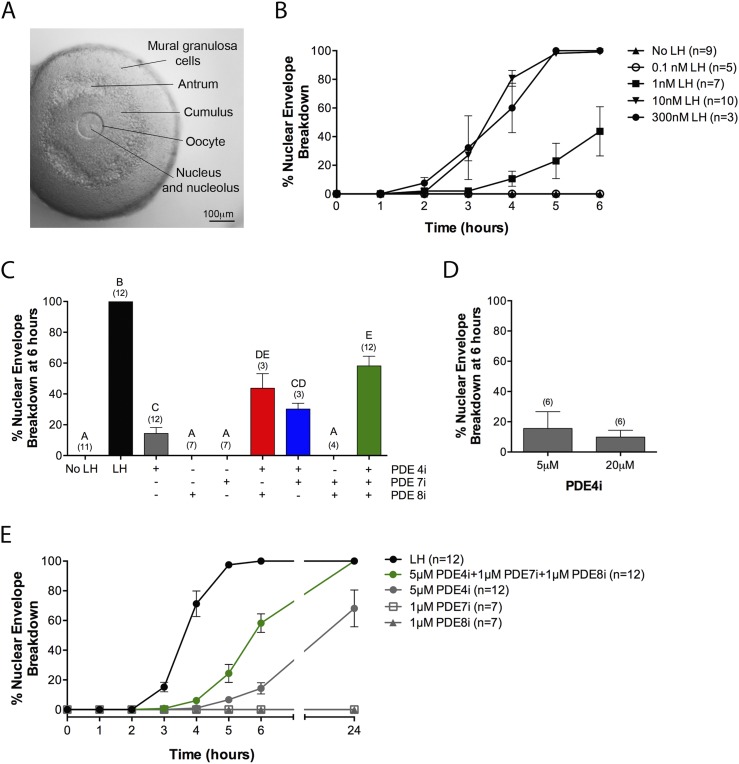
Figure 2.
PDE4, PDE7, and PDE8 act together to suppress premature NEBD in follicle-enclosed oocytes. (A) A follicle before exposure to LH or a PDE inhibitor; an intact nucleus is visible in the oocyte. (B) Time course of NEBD in response to various concentrations of LH. (C) Percent NEBD at 6 hours after treatment of follicles with a saturating concentration of LH (300 nM), individual PDE inhibitors, pairs of inhibitors, or all three inhibitors together (5 μM rolipram, 1 μM PDE7i, 1 μM PDE8i). (D) Similar percent NEBD in response to 5 and 20 μM rolipram. (E) Time course of NEBD in response to LH (300 nM), individual PDE inhibitors [concentrations as in (C)], or a mixture of the three inhibitors. Numbers in parentheses indicate the number of independent experiments. Different letters indicate significant differences (P < 0.05).
A smaller and slower elevation of cAMP occurred with 1 nM LH (Fig. 1A), corresponding to a slower resumption of meiosis (Fig. 2B). At 0.5 to 3 hours after 1 nM LH addition, the cAMP content of the follicles was not statistically different from that of follicles that were incubated without LH (Fig. 1A). However, measurements at 4 hours after addition of 1 nM LH showed a significant increase in cAMP (Fig. 1A). One nanomolar is similar to the peak serum concentration of LH during the preovulatory surge in mice (9, 34), although the concentration dependence of meiotic resumption in response to purified ovine LH applied to follicles in vitro may not be identical to that when the native hormone is delivered to follicles in vivo.
To investigate which cAMP PDEs contribute to suppression of premature elevation of cAMP before LH exposure, we measured the cAMP content of follicles after exposure to selective inhibitors of PDE4 (rolipram, 5 μM), PDE7 (1 μM), or PDE8 (1 μM), alone or in combination. The inhibitor concentrations were chosen based on the in vitro selectivity data described in Tables 1 and 2. Because inhibitor concentrations in the cytoplasm may be lower than those in the medium, as a result of limited permeability of the plasma membrane, we used the highest concentrations at which the inhibitors showed selectivity among different PDEs. At 5 μM, rolipram inhibition of PDE7 and PDE8 is insignificant, as the IC50 for PDE7 and PDE8 is >100 μM (21–24). At 1 μM, there could be minor inhibition by the PDE7i of PDE4 and PDE8 and minor inhibition by PDE8i of PDE4 and PDE7, based on measurements of the effects of these inhibitors on activity of recombinant proteins in vitro (Tables 1 and 2). Higher concentrations of the PDE7i and PDE8i were not tested, because of the possibility of crossreactivity with other PDEs, and these inhibitors were used at 1 μM for all subsequent experiments.
Measurements made at 30 or 60 minutes after application of a mixture of the three inhibitors showed little or no increase in cAMP content (Fig. 1A), consistent with the relatively slow action of these inhibitors on biological responses, as described later. However, when measurements were made at 2 or 4 hours, the three inhibitors together increased the cAMP content by two- to fourfold (Fig. 1A and 1B).
A 4-hour treatment with rolipram alone caused a small increase in cAMP content in two of three experiments, but the mean value for the three experiments was not statistically different from the cAMP content with no treatment. A 4-hour treatment with either the PDE7i or the PDE8i had no detectable effect (Fig. 1B). A large increase in cAMP content was seen, however, in response to a 4-hour treatment with a mixture of the three inhibitors (Fig. 1B), indicating that these three PDEs act together to maintain cAMP at a low level before LH exposure. This synergistic action of the three cAMP inhibitors, when applied together, was also seen in various biological responses, as described later.
PDE4, PDE7, and PDE8 act together to suppress premature meiotic resumption in follicle-enclosed oocytes
To monitor the time course of meiotic resumption, follicles were cultured on the surface of Millicell membranes, such that they were slightly flattened, allowing observation of the oocyte and its nucleus within the intact follicle (Fig. 2A) (28). The first obvious sign of meiotic resumption is NEBD, marking the transition from prophase to metaphase. Under the culture conditions used here, 100% of oocytes underwent NEBD by 6 hours after exposure to 10 or 300 nM LH (Fig. 2B). LH (1 nM) also caused NEBD in some follicles, but the response was delayed (Fig. 2B), consistent with the lower amplitude and slower time course of cAMP elevation in response to LH (Fig. 1A).
With the use of this culture system, we investigated the effect of PDE4i, PDE7i, and PDE8i on maintenance of meiotic arrest. At 6 hours, ∼15% of the follicles treated with 5 μM of the PDE4i rolipram had undergone NEBD (Fig. 2C). A higher concentration of rolipram (20 μM) did not increase the fraction of oocytes resuming meiosis (Fig. 2D), consistent with a previous report (2). Therefore, we used 5 μM rolipram for all subsequent experiments, except as noted.
None of the follicles treated with 1 μM PDE7i or PDE8i had undergone NEBD at 6 hours (Fig. 2C). However, when follicles were treated with a mixture of 5 μM rolipram and 1 μM each PDE7i and PDE8i, ∼60% of the follicles had undergone NEBD at 6 hours (Fig. 2C). These results indicate that PDE4, PDE7, and PDE8 act together to suppress spontaneous NEBD. Mixtures of PDE4i and PDE7i or of PDE4i and PDE8i also showed a synergistic stimulation of NEBD (Fig. 2C). Based on their effects on recombinant proteins in vitro (Tables 1 and 2), 1 μM PDE8i or PDE7i could cause a slight inhibition of PDE4 if this concentration was reached in the cytoplasm. However, this is unlikely to account for the synergistic effect of the combination of 1 μM PDE7i or PDE8i with 5 μM rolipram, as no increase in NEBD was seen with 20 μM vs 5 μM rolipram (Fig. 2D).
The time course of NEBD, in response to the mixture of PDE inhibitors, showed an ∼2-hour delay compared with the response to a saturating level of LH (300 nM; Fig. 2E). Correspondingly, the cAMP content of follicles was not significantly elevated from baseline until 2 hours after application of the mixture of PDE inhibitors (Fig. 1A). Coincubation with 0.1 or 1 nM LH did not increase the rate of NEBD in response to the mixture of cAMP PDE inhibitors (Supplemental Fig. 3).
When follicles were observed at 24 hours after treatment with rolipram alone, ∼70% had undergone NEBD (Fig. 2E). This finding is consistent with previous studies in which follicles were exposed to rolipram for 24 hours (2, 3) [David Calebiro, personal communication regarding the timing of the experiments reported in Lyga et al. (3)]. Even at 24 hours, no NEBD was seen in follicles treated with 1 μM PDE7i or PDE8i, alone or in combination (Fig. 2E). However, when these inhibitors were combined with rolipram, the percentage of NEBD at 24 hours increased to 100% vs ∼70% with rolipram alone (Fig. 2E).
PDE4, PDE7, and PDE8 act together to suppress premature ovulation
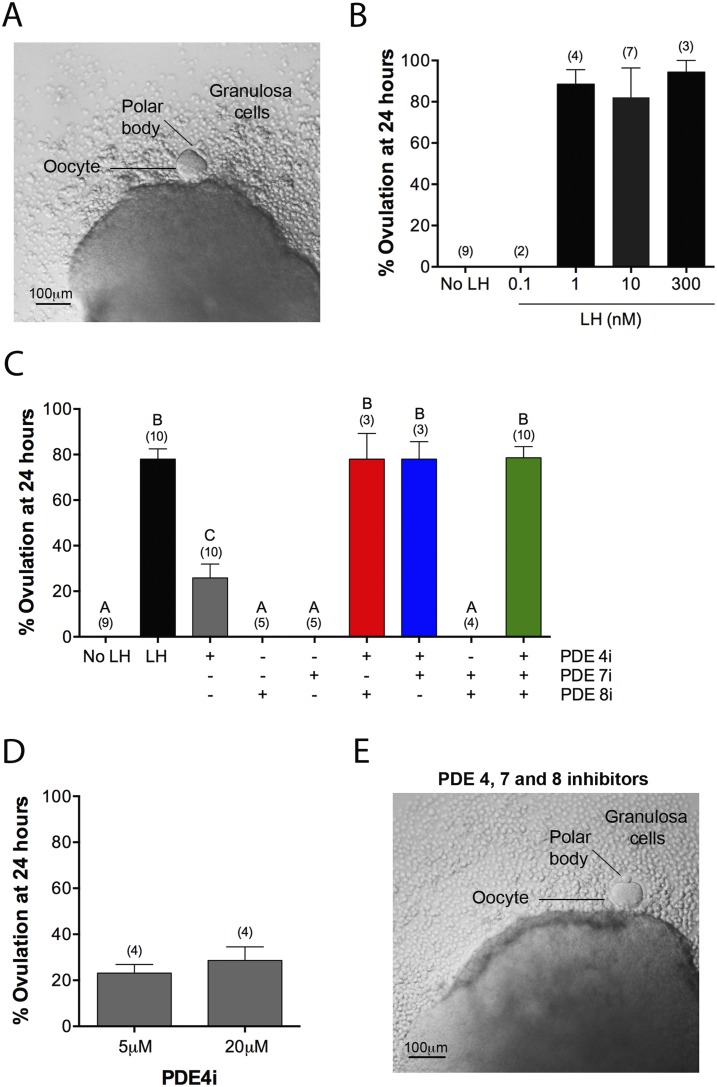
LH causes ovulation, at least in part, by signaling through Gs to elevate cAMP (5, 33), although signaling through Gq/11 to elevate inositol trisphosphate also contributes (5, 35). At 24 hours after applying LH to isolated follicles in culture, the oocyte has been released, and often, a polar body can be seen (Fig. 3A). Some of the granulosa cells are also released. Approximately 80% of follicles treated with 1 to 300 nM LH ovulated (Fig. 3B). To investigate which cAMP PDEs contribute to suppression of premature ovulation, we investigated the effects of cAMP PDE inhibitors on this process.
Figure 3.
PDE4, PDE7, and PDE8 act together to suppress premature ovulation. (A) A follicle at 24 hours after exposure to LH (300 nM). Ovulation has occurred, as indicated, by extrusion of the oocyte and some granulosa cells. (B) Percent ovulation at 24 hours after exposure to various concentrations of LH. (C) Percent ovulation at 24 hours after treatment of follicles with LH (300 nM), individual PDE inhibitors, pairs of inhibitors, or all three inhibitors together (5 μM rolipram, 1 μM PDE7i, 1 μM PDE8i). (D) Similar percent ovulation in response to 5 and 20 μM rolipram. (E) A follicle at 24 hours after treatment with a mixture of PDE4i, PDE7i, and PDE8i [concentrations as in (C)], showing ovulation, as seen with LH. Numbers in parentheses indicate the number of independent experiments. Different letters indicate significant differences (P < 0.05).
Inhibition of PDE4 alone, using 5 or 20 μM rolipram, caused ovulation in ∼20% of follicles (Fig. 3C and 3D). PDE7i and PDE8i, used at 1 μM, alone or in combination, did not cause ovulation (Fig. 3C). However, when PDE7i or PDE8i or both were applied together with rolipram, ∼80% of follicles ovulated, as seen with LH (Fig. 3C and 3E). Thus, as with the stimulation of cAMP elevation and meiotic resumption, PDE4, PDE7, and PDE8 act together to suppress premature ovulation.
PDE4, PDE7, and PDE8 all contribute to suppression of premature production of progesterone and PGRs
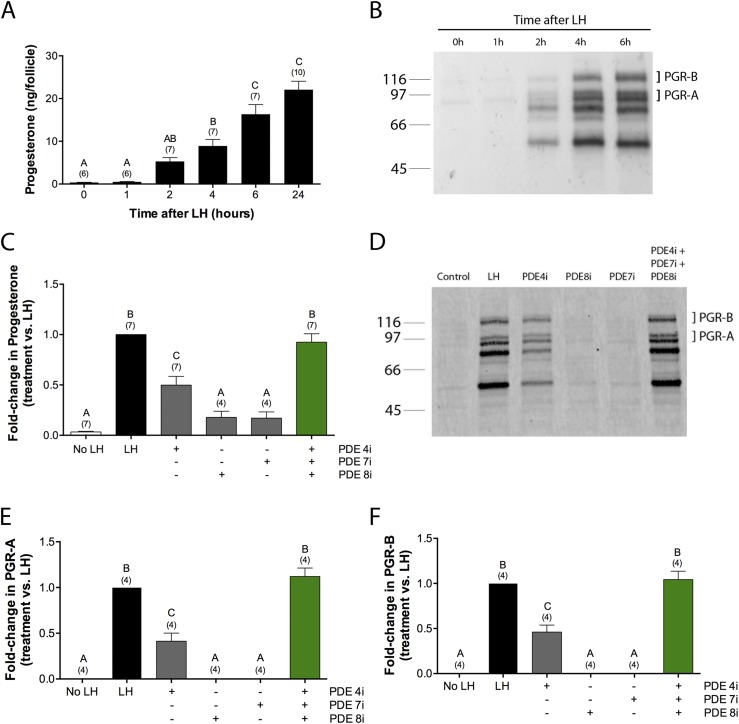
An aspect of the LH-induced signaling network that is essential for ovulation, although not for meiotic progression, is the synthesis by the granulosa cells of progesterone and its receptors PGR-A and PGR-B (36–40). Synthesis of progesterone and its receptors started between 1 and 2 hours after exposure to LH (300 nM; Fig. 4A and 4B), consistent with previous studies (32, 38, 41–44).
Figure 4.
PDE4, PDE7, and PDE8 all contribute to suppression of spontaneous production of progesterone and PGRs. (A and B) Time course of synthesis of (A) progesterone and (B) its receptors, PGR-A and PGR-B, in response to LH (300 nM). (C–F) Stimulation of synthesis of (C) progesterone and (D–F) PGRs by incubation of follicles for 6 hours in the presence of LH (300 nM) or PDE4i (rolipram, 5 μM), PDE7i (1 μM), and/or PDE8i (1 μM). (B and D) Molecular weight in kDa is shown at left. The doublet near the 97 kDa marker corresponds to the PGR-A isoform, and the doublet just above the 116 kDa marker corresponds to the PGR-B isoform; the doublets are thought to result from phosphorylation (32). The lower bands, in the range of ∼50 to 70 kDa, are unidentified but have been seen in a previous study as well (32). Numbers in parentheses indicate the number of independent experiments. Different letters indicate significant differences (P < 0.05).
To determine whether premature progesterone production by the follicle is suppressed by constitutive cAMP PDE activity, we measured progesterone released into the medium at 6 hours after application of PDE inhibitors. As for the previous analyses, rolipram was used at a concentration of 5 μM, and PDE7i and PDE8i were both used at 1 μM. Application of rolipram alone resulted in a progesterone level of ∼50% of that seen with LH (Fig. 4C). Application of either PDE7i or PDE8i alone each resulted in a progesterone level of ∼20% of that seen with LH (Fig. 4C). A mixture of the three inhibitors resulted in a progesterone level that was the same as that seen with LH (Fig. 4C). The effect of the three inhibitors on progesterone production was additive but not synergistic.
To investigate if inhibition of PDE4, PDE7, or PDE8 results in synthesis of PGRs, follicles were treated with inhibitors of each of these PDEs, alone or in combination (Fig. 4D–4F). Treatment for 6 hours with rolipram resulted in a level of PGR protein that was ∼40% of that seen with LH, whereas neither PDE7i nor PDE8i resulted in detectable PGR protein expression. However, treatment with a mixture of the three inhibitors reproduced the rise in PGR protein seen with LH, indicating that these cAMP PDEs act together to suppress PGR synthesis.
LH signaling does not cause a rapid decrease in cAMP PDE activity
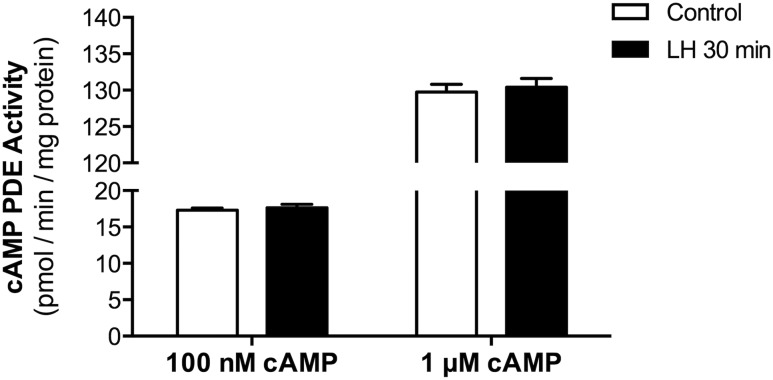
Although LH signaling elevates cAMP by stimulating Gs and increasing the activity of adenylyl cyclase (5), this signaling could be amplified by an LH-induced decrease in cAMP PDE activity. To investigate this possibility, we measured cAMP PDE activity in lysates of follicles that had been treated with or without LH (300 nM). Because the peak cAMP increase after LH exposure occurs at 30 minutes (Fig. 1A), samples were prepared at this time point. Activity was assayed with two different cAMP substrate concentrations: 0.1 or 1 μM. No difference in activity was seen as a result of the LH treatment, indicating that at least on the time scale investigated, LH signaling does not decrease cAMP PDE activity, as detected in cell lysates (Fig. 5). By 2 hours after LH receptor stimulation, the amounts of PDE4D protein and PDE4 activity actually increase (12). These results support the conclusion that although cAMP PDEs contribute to suppression of spontaneous NEBD and ovulation before the preovulatory increase in LH, the initial rise in cAMP after LH exposure is not amplified by a decrease in cAMP PDE activity.
Figure 5.
LH signaling does not cause a rapid decrease in cAMP PDE activity. cAMP PDE activity was measured in lysates of follicles, with or without a 30-minute exposure to LH (300 nM). Activity was assayed with two different cAMP substrate concentrations: 0.1 or 1 μM. Bars show the means ± SEM for two independent experiments.
Discussion
Our findings indicate that three families of cAMP PDEs—PDE4, PDE7, and PDE8—act together in mouse ovarian follicles to suppress premature cAMP elevation, meiotic resumption, and ovulation before the preovulatory increase in LH. The observation that exposure to PDE7i and PDE8i has no effect in the absence of PDE4 inhibition indicates that the amount of PDE4 is sufficient to prevent premature resumption of meiosis under these experimental conditions. PDE4 inhibition alone, however, produces a submaximal response that is potentiated by concurrent inhibition of PDE7 and PDE8. These observations indicate a “redundancy” of PDEs regulating meiotic arrest and ovulation, with PDE4 by itself being sufficient, but with PDE7 and PDE8 serving as “back-up” regulators.
However, this description of the roles of PDE7 and PDE8 as back-up regulators applies only to the specific biological responses that we investigated. cAMP is a pleiotropic second messenger, and there may be unique roles for PDE7 and PDE8 vs PDE4, in particular, cAMP-mediated pathways in ovarian follicles. Consistent with this concept, our observations at 24 hours after inhibition of PDE4 activity indicate a more pronounced effect on ovulation than on NEBD.
Studies of other cells also indicate differential effects of inhibition of PDE4, PDE7, and PDE8. Phosphoproteomic analysis of a T cell line has shown that PDE4 inhibition (when combined with PDE3 inhibition) results in phosphorylation of a set of proteins that is distinct from the set of proteins that are phosphorylated as a result of inhibition of PDE7 and PDE8 (when combined with PDE1 inhibition) (18). Correspondingly, the biological processes regulated by these different PDE inhibitor combinations differ (18). Likewise, individual PDEs have been demonstrated to have distinct functional roles in cardiac myocytes, where the raising of intracellular cAMP content through PDE2 inhibition has antihypertrophic effects in neonatal rat ventricular myocytes, whereas inhibition of PDE3 generates prohypertrophic signals (45). Correspondingly, the subcellular localization of PDE2 differs from that of PDE3 (45). Spatially distinct cAMP PDE signaling domains may be present in ovarian follicles as well, where they may contribute to the divergent signaling pathways that control different responses to LH.
Cooperative function of cAMP-hydrolyzing PDEs has also been found in Leydig cells of the testis, which produce testosterone in response to LH. Inhibition of either of two different cAMP-specific PDEs (PDE4 and PDE8) can induce steroid production in these cells, and inhibition of both of these enzymes increases steroid production more than the sum of either alone (27, 46). However, whereas PDE4 has the primary role and PDE8 a supporting role in regulation of meiotic arrest in granulosa cells, the situation is reversed is in Leydig cells, with PDE8 having the primary role and PDE4 a supporting role.
Multiple cAMP PDEs act together to suppress cAMP-dependent responses in other cells as well. These include cells of the adrenal cortex (47, 48), smooth muscle (49, 50), adipose tissue (51), and leukocytic cell lines (52, 53). The presence of more than one type of PDE protein present to suppress premature cAMP elevation could help to ensure that conditions that might reduce the activity of any individual PDE would not disrupt essential cellular processes. Other factors, such as cAMP buffering by high concentrations of the regulatory subunits of protein kinase A, may also contribute to preventing premature, cAMP-dependent responses (54).
Supplementary Material
Acknowledgments
We acknowledge the generous contributions of Anne W. Schmidt, Tom Chappie, Patrick Verhoest, Steve Jenkinson, and Christopher J. Schmidt (Pfizer Worldwide Research and Development), who supplied PDE7i and PDE8i, as well as data on their selectivity. We also thank Joe Beavo, Dan Bernard, Stefan Brocke, Paul Epstein, and Frank Menniti for helpful discussions and Judith Krall for technical assistance.
Financial Support: This work was supported by a grant (R37HD014939) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to L.A.J).
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- AMP
adenosine monophosphate
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- DMSO
dimethyl sulfoxide
- FSH
follicle-stimulating hormone
- GMP
guanosine monophosphate
- IC50
half-maximal inhibitory concentration
- Ki
dissociation constant of the enzyme inhibitor complex
- Km
Michaelis-Menten constant
- LH
luteinizing hormone
- NEBD
nuclear envelope breakdown
- PDE
phosphodiesterase
- PDE4i
phosphodiesterase 4 inhibitor
- PDE7i
phosphodiesterase 7i inhibitor
- PDE8i
phosphodiesterase 8 inhibitor
- PGR
progesterone receptor
- SEM
standard error of the mean
- SPA
scintillation proximity assay
References
- 1. Conti M, Kasson BG, Hsueh AJ. Hormonal regulation of 3′,5′-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology. 1984;114(6):2361–2368. [DOI] [PubMed] [Google Scholar]
- 2. Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1996;178(2):393–402. [DOI] [PubMed] [Google Scholar]
- 3. Lyga S, Volpe S, Werthmann RC, Götz K, Sungkaworn T, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized LH receptors in ovarian follicles. Endocrinology. 2016;157(4):1613–1621. [DOI] [PubMed] [Google Scholar]
- 4. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330(6002):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunzicker-Dunn M, Mayo K. Gonadotrophin signaling in the ovary In: Plant TM and Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. 4th ed.San Diego: Academic Press; 2015:895–945. [Google Scholar]
- 6. Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu Rev Physiol. 2017;79(1):237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richard S, Baltz JM. Preovulatory suppression of mouse oocyte cell volume-regulatory mechanisms is via signalling that is distinct from meiotic arrest. Sci Rep. 2017;7(1):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117–149. [DOI] [PubMed] [Google Scholar]
- 9. Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, Campbell RE, Herbison AE. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794–4802. [DOI] [PubMed] [Google Scholar]
- 10. McKenna SD, Pietropaolo M, Tos EG, Clark A, Fischer D, Kagan D, Bao B, Chedrese PJ, Palmer S. Pharmacological inhibition of phosphodiesterase 4 triggers ovulation in follicle-stimulating hormone-primed rats. Endocrinology. 2005;146(1):208–214. [DOI] [PubMed] [Google Scholar]
- 11. Jin SL, Richard FJ, Kuo WP, D’Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci USA. 1999;96(21):11998–12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JY, Richard F, Chun SY, Park JH, Law E, Horner K, Jin SL, Conti M. Phosphodiesterase regulation is critical for the differentiation and pattern of gene expression in granulosa cells of the ovarian follicle. Mol Endocrinol. 2003;17(6):1117–1130. [DOI] [PubMed] [Google Scholar]
- 13. Sasseville M, Albuz FK, Côté N, Guillemette C, Gilchrist RB, Richard FJ. Characterization of novel phosphodiesterases in the bovine ovarian follicle. Biol Reprod. 2009;81(2):415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen TS, Stahlhut M, Andersen CY. Phosphodiesterases in the rat ovary: effect of cAMP in primordial follicles. Reproduction. 2015;150(1):11–20. [DOI] [PubMed] [Google Scholar]
- 15. Petersen TS, Kristensen SG, Jeppesen JV, Grøndahl ML, Wissing ML, Macklon KT, Andersen CY. Distribution and function of 3′,5′-cyclic-AMP phosphodiesterases in the human ovary. Mol Cell Endocrinol. 2015;403:10–20. [DOI] [PubMed] [Google Scholar]
- 16. Egbert JR, Uliasz TF, Shuhaibar LC, Geerts A, Wunder F, Kleiman RJ, Humphrey JM, Lampe PD, Artemyev NO, Rybalkin SD, Beavo JA, Movsesian MA, Jaffe LA. Luteinizing hormone causes phosphorylation and activation of the cGMP phosphodiesterase PDE5 in rat ovarian follicles, contributing, together with PDE1 activity, to the resumption of meiosis. Biol Reprod. 2016;94(5):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergeron A, Guillemette C, Sirard MA, Richard FJ. Active 3′-5′ cyclic nucleotide phosphodiesterases are present in detergent-resistant membranes of mural granulosa cells. Reprod Fertil Dev. 2017;29(4):778–790. [DOI] [PubMed] [Google Scholar]
- 18. Beltejar MG, Lau HT, Golkowski MG, Ong SE, Beavo JA. Analyses of PDE-regulated phosphoproteomes reveal unique and specific cAMP-signaling modules in T cells. Proc Natl Acad Sci USA. 2017;114(30):E6240–E6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwabe U, Miyake M, Ohga Y, Daly JW. 4-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone (ZK 62711): a potent inhibitor of adenosine cyclic 3′,5′-monophosphate phosphodiesterases in homogenates and tissue slices from rat brain. Mol Pharmacol. 1976;12(6):900–910. [PubMed] [Google Scholar]
- 20. Vang AG, Ben-Sasson SZ, Dong H, Kream B, DeNinno MP, Claffey MM, Housley W, Clark RB, Epstein PM, Brocke S. PDE8 regulates rapid Teff cell adhesion and proliferation independent of ICER. PLoS One. 2010;5(8):e12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soderling SH, Bayuga SJ, Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci USA. 1998;95(15):8991–8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sasaki T, Kotera J, Yuasa K, Omori K. Identification of human PDE7B, a cAMP-specific phosphodiesterase. Biochem Biophys Res Commun. 2000;271(3):575–583. [DOI] [PubMed] [Google Scholar]
- 23. Gamanuma M, Yuasa K, Sasaki T, Sakurai N, Kotera J, Omori K. Comparison of enzymatic characterization and gene organization of cyclic nucleotide phosphodiesterase 8 family in humans. Cell Signal. 2003;15(6):565–574. [DOI] [PubMed] [Google Scholar]
- 24. Bian H, Zhang J, Wu P, Varty LA, Jia Y, Mayhood T, Hey JA, Wang P. Differential type 4 cAMP-specific phosphodiesterase (PDE4) expression and functional sensitivity to PDE4 inhibitors among rats, monkeys and humans. Biochem Pharmacol. 2004;68(11):2229–2236. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, Hoffman WE, Lebel LA, McCarthy SA, Nelson FR, Proulx-LaFrance C, Majchrzak MJ, Ramirez AD, Schmidt K, Seymour PA, Siuciak JA, Tingley FD III, Williams RD, Verhoest PR, Menniti FS. Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther. 2008;325(2):681–690. [DOI] [PubMed] [Google Scholar]
- 26. Tsai LC, Beavo JA. Regulation of adrenal steroidogenesis by the high-affinity phosphodiesterase 8 family. Horm Metab Res. 2012;44(10):790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimizu-Albergine M, Tsai LC, Patrucco E, Beavo JA. cAMP-specific phosphodiesterases 8A and 8B, essential regulators of Leydig cell steroidogenesis. Mol Pharmacol. 2012;81(4):556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135(19):3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140(5):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shuhaibar LC, Egbert JR, Norris RP, Lampe PD, Nikolaev VO, Thunemann M, Wen L, Feil R, Jaffe LA. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc Natl Acad Sci USA. 2015;112(17):5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shuhaibar LC, Egbert JR, Edmund AB, Uliasz TF, Dickey DM, Yee SP, Potter LR, Jaffe LA. Dephosphorylation of juxtamembrane serines and threonines of the NPR2 guanylyl cyclase is required for rapid resumption of oocyte meiosis in response to luteinizing hormone. Dev Biol. 2016;409(1):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191(3):525–535. [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez KF, Couse JF, Jayes FL, Hamilton KJ, Burns KA, Taniguchi F, Korach KS. Insufficient luteinizing hormone-induced intracellular signaling disrupts ovulation in preovulatory follicles lacking estrogen receptor-β. Endocrinology. 2010;151(6):2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jayes FL, Burns KA, Rodriguez KF, Kissling GE, Korach KS. The naturally occurring luteinizing hormone surge is diminished in mice lacking estrogen receptor Beta in the ovary. Biol Reprod. 2014;90(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breen SM, Andric N, Ping T, Xie F, Offermans S, Gossen JA, Ascoli M. Ovulation involves the luteinizing hormone-dependent activation of G(q/11) in granulosa cells. Mol Endocrinol. 2013;27(9):1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsafriri A, Popliker M, Nahum R, Beyth Y. Effects of ketoconazole on ovulatory changes in the rat: implications on the role of a meiosis-activating sterol. Mol Hum Reprod. 1998;4(5):483–489. [DOI] [PubMed] [Google Scholar]
- 37. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 38. Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97(9):4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robker RL, Akison LK, Russell DL. Control of oocyte release by progesterone receptor-regulated gene expression. Nucl Recept Signal. 2009;7:e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim J, Bagchi IC, Bagchi MK. Control of ovulation in mice by progesterone receptor-regulated gene networks. Mol Hum Reprod. 2009;15(12):821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsafriri A, Lieberman ME, Barnea A, Bauminger S, Lindner HR. Induction by luteinizing hormone of ovum maturation and of steroidogenesis in isolated Graafian follicles of the rat: role of RNA and protein synthesis. Endocrinology. 1973;93(6):1378–1386. [DOI] [PubMed] [Google Scholar]
- 42. Park OK, Mayo KE. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol. 1991;5(7):967–978. [DOI] [PubMed] [Google Scholar]
- 43. Carbajal L, Biswas A, Niswander LM, Prizant H, Hammes SR. GPCR/EGFR cross talk is conserved in gonadal and adrenal steroidogenesis but is uniquely regulated by matrix metalloproteinases 2 and 9 in the ovary. Mol Endocrinol. 2011;25(6):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci USA. 2011;108(4):1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zoccarato A, Surdo NC, Aronsen JM, Fields LA, Mancuso L, Dodoni G, Stangherlin A, Livie C, Jiang H, Sin YY, Gesellchen F, Terrin A, Baillie GS, Nicklin SA, Graham D, Szabo-Fresnais N, Krall J, Vandeput F, Movsesian M, Furlan L, Corsetti V, Hamilton G, Lefkimmiatis K, Sjaastad I, Zaccolo M. Cardiac hypertrophy is inhibited by a local pool of cAMP regulated by phosphodiesterase 2. Circ Res. 2015;117(8):707–719. [DOI] [PubMed] [Google Scholar]
- 46. Shimizu-Albergine M, Van Yserloo B, Golkowski MG, Ong SE, Beavo JA, Bornfeldt KE. SCAP/SREBP pathway is required for the full steroidogenic response to cyclic AMP. Proc Natl Acad Sci USA. 2016;113(38):E5685–E5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsai LC, Shimizu-Albergine M, Beavo JA. The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland. Mol Pharmacol. 2011;79(4):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsai LC, Beavo JA. The roles of cyclic nucleotide phosphodiesterases (PDEs) in steroidogenesis. Curr Opin Pharmacol. 2011;11(6):670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Challiss RA, Adams D, Mistry R, Nicholson CD. Modulation of spasmogen-stimulated Ins(1,4,5)P3 generation and functional responses by selective inhibitors of types 3 and 4 phosphodiesterase in airways smooth muscle. Br J Pharmacol. 1998;124(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palmer D, Tsoi K, Maurice DH. Synergistic inhibition of vascular smooth muscle cell migration by phosphodiesterase 3 and phosphodiesterase 4 inhibitors. Circ Res. 1998;82(8):852–861. [DOI] [PubMed] [Google Scholar]
- 51. Kraynik SM, Miyaoka RS, Beavo JA. PDE3 and PDE4 isozyme-selective inhibitors are both required for synergistic activation of brown adipose tissue. Mol Pharmacol. 2013;83(6):1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alaamery MA, Wyman AR, Ivey FD, Allain C, Demirbas D, Wang L, Ceyhan O, Hoffman CS. New classes of PDE7 inhibitors identified by a fission yeast-based HTS. J Biomol Screen. 2010;15(4):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dong H, Zitt C, Auriga C, Hatzelmann A, Epstein PM. Inhibition of PDE3, PDE4 and PDE7 potentiates glucocorticoid-induced apoptosis and overcomes glucocorticoid resistance in CEM T leukemic cells. Biochem Pharmacol. 2010;79(3):321–329. [DOI] [PubMed] [Google Scholar]
- 54. Carr DW, Cutler RE Jr, Cottom JE, Salvador LM, Fraser ID, Scott JD, Hunzicker-Dunn M. Identification of cAMP-dependent protein kinase holoenzymes in preantral- and preovulatory-follicle-enriched ovaries, and their association with A-kinase-anchoring proteins. Biochem J. 1999;344(2):613–623. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.