Abstract
Pectin methyl esterase (PME) (EC 3.1.1.11) catalyzes the hydrolysis of methylester groups of cell wall pectins. We investigated the role of this enzyme in dormancy termination and germination of yellow cedar (Chamaecyparis nootkatensis [D. Don] Spach) seeds. PME activity was not detected in dormant seeds of yellow cedar but was induced and gradually increased during moist chilling; high activity coincided with dormancy breakage and germination. PME activity was positively correlated to the degree of dormancy breakage of yellow cedar seeds. The enzyme produced in different seed parts and in seeds at different times during moist chilling, germination, and early post-germinative growth consisted of two isoforms, both basic with isoelectric points of 8.7 and 8.9 and the same molecular mass of 62 kD. The pH optimum for the enzyme was between 7.4 and 8.4. In intact yellow cedar seeds, activities of the two basic isoforms of PME that were induced in embryos and in megagametophytes following dormancy breakage were significantly suppressed by abscisic acid. Gibberellic acid had a stimulatory effect on the activities of these isoforms in embryos and megagametophytes of intact seeds at the germinative stage. We hypothesize that PME plays a role in weakening of the megagametophyte, allowing radicle emergence and the completion of germination.
Following dispersal from the parent tree, seeds of yellow cedar (Chamaecyparis nootkatensis [D. Don] Spach) are dormant and require several months (up to 1 year in wild stands) to undergo moist chilling before germinating (Pawuk, 1993). Maintenance of dormancy is not a consequence of embryo immaturity at the time of seed dispersal (Xia and Kermode, 1999). Yellow cedar embryos germinate when they are excised from mature dormant seeds and placed in water, indicating that the seed tissues enclosing the embryo (the testa, remnants of the nucellus, and the megagametophyte) prevent radicle emergence. The megagametophyte plays a primary role in inhibiting embryo germination (Ren and Kermode, 1999). Abscisic acid (ABA) is involved to some extent in the dormancy mechanism of yellow cedar seeds. Fluridone (an inhibitor of carotenoid biosynthesis that diminishes endogenous ABA), when used with gibberellic acid (GA3), is effective in relieving the dormancy of whole seeds of yellow cedar in the complete absence of moist chilling. Furthermore, upon dormancy termination, the embryo exhibits a reduced sensitivity to ABA and an enhanced capacity to metabolize ABA (Schmitz et al., 2000; N. Schmitz, S. Abrams, and A. Kermode, unpublished data). The dormancy mechanism of yellow cedar is complex and is not exclusively imposed by the megagametophyte (although this is the primary mode of dormancy regulation) (Ren and Kermode, 1999). Furthermore, in addition to chemical inhibition (mediated by ABA), the megagametophyte also acts as a mechanical barrier to prevent radicle protrusion, a factor that may also involve regulation by ABA and other hormones such as gibberellins (through regulation of cell wall rigidity). For example, the micropylar megagametophyte decreases in mechanical strength following a dormancy-breaking treatment, and during germination, the cells of the megagametophyte in the area immediately surrounding the radicle exhibit a loss of their internal structure, which would represent significant weakening to allow radicle emergence (Ren and Kermode, 1999). Concurrently, the embryo exhibits increased turgor and a reduced sensitivity to low osmotic potentials.
In a dormancy mechanism involving mechanical restraint, weakening of the cell walls of the megagametophyte, especially at the micropylar region is proposed to be a prerequisite for germination; this is thought to be achieved by the induction of cell wall hydrolases (for review, see Bewley and Black, 1994; Bewley, 1997). Thus, radicle protrusion may depend upon concomitant weakening of cell walls of the surrounding tissues, thereby decreasing the force required by the radicle to penetrate them. In seeds of white spruce (Picea glauca), weakening of the micropylar end of the megagametophyte and nucellus precedes radicle protrusion, and this weakening is associated with endo-β-mannanase activity (Downie et al., 1997).
Primary cell walls of plants are thought to be comprised of three structurally independent but interacting parts: a framework constructed of cellulose microfibrils and hemicelluloses (mainly xyloglucans), a matrix made of pectins, and structural glycoproteins such as extensin (Carpita and Gibeaut, 1993). The endosperm cell walls of certain seeds (e.g. tomato and fenugreek) contain relatively large amounts of galactomannans (Groot et al., 1988), which are a carbohydrate reserve (Reid, 1985). Pectins are major components of the primary cell wall and are especially abundant in the middle lamella. These polysaccharides are a heterogeneous and complex group. Smooth regions, comprised of linear polymers of up to 100 residues of d-GalUA residues (α-1,4-linked), are interrupted at regular intervals by so-called “hairy regions,” in which multiple side chains of neutral sugars are attached (Carpita and Gibeaut, 1993; Thibault et al., 1993). The GalUA residues in the smooth regions can be methyl esterified to a varying degree and in a non-random fashion with blocks of polygalacturonans being completely methyl esterified (De Vries et al., 1986). The density of pectin methyl-esterified galacturonan residues can determine the character of the cell wall, including wall porosity; it may also provide charged surfaces that modulate wall pH and ion balance, limit access to cell wall hydrolytic enzymes, and serve as recognition molecules that signal appropriate developmental responses to symbiotic organisms, pathogens, and insects (Moustacas et al., 1986; Carpita and Gibeaut, 1993).
Pectin methyl esterase (PME) (EC 3.1.1.11) catalyzes the hydrolysis of methylester groups of cell wall pectins. It has been found in all plant tissues and in some of plant cell wall-degrading microorganisms or insects (Campbell and Shea, 1990; Christgau et al., 1996) and has been implicated in a number of processes including cell growth (Moustacas et al., 1991), fruit ripening (Gaffe et al., 1994; Tieman and Handa, 1994; Steele et al., 1997), abscission and senescence (Liners and van Cutsem, 1992), pathogenesis (Collmer and Keen, 1986; Baayen et al., 1997), and cambial cell differentiation (Guglielmino et al., 1997).
Although PME activities increase rapidly in some seeds following germination (Nighojkar et al., 1994; Alexandre et al., 1997), the involvement of PME in the termination of seed dormancy has not yet been investigated. In the present study, we investigate the potential role of PME in dormancy termination and the germination of yellow cedar seeds and characterize the enzyme biochemically.
RESULTS
PME Isoforms in Yellow Cedar Seeds
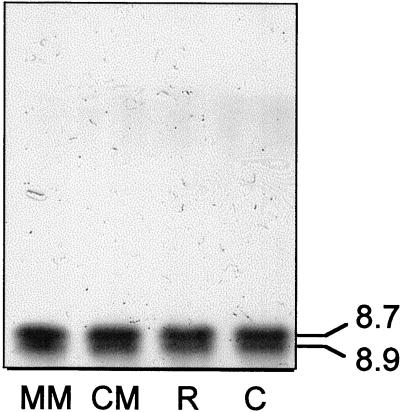
PME activity is detected in germinating yellow cedar seeds, and we sought to characterize the enzyme as a prelude to examining its role in dormancy termination. Crude extracts were generated from different parts of germinated seeds and subjected to isoelectric focusing (IEF) followed by ruthenium red staining. Two isoforms of PME were detected, both had pIs of 8.7 and 8.9, indicative of basic proteins (Fig. 1).
Figure 1.
IEF gel showing PME by ruthenium red staining. Seed parts were extracted after their excision from germinated seeds having radicle lengths of 15 mm. Equal amounts of protein (15 μg) in crude extracts from different seed parts were loaded in each lane. MM, Micropylar end of the megagametophyte; CM, chalazal end of the megagametophyte; R, radicle; C, cotyledons.
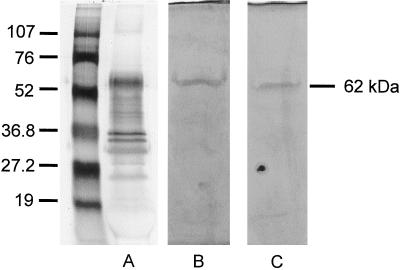
PME, partially purified by a cellulose anion-exchange column, was fractionated by SDS-PAGE and subjected to silver staining (Fig. 2A) or activity staining (Fig. 2, B and C). A single active band was detected under reducing or non-reducing conditions (Fig. 2, B and C); thus, PME of yellow cedar seeds is comprised of two proteins with the same molecular mass. In the silver-stained gel, a protein corresponding to the same molecular mass (62 kD) was abundant.
Figure 2.
SDS-PAGE under reducing (A and C) and non-reducing (B) conditions showing PME by silver staining (A) or active staining (B and C). Partially purified PME protein (2 μg) was loaded in A; equal activities of PME (40 nkat) were loaded in B and C.
Thermal Stability and the Effects of pH and Cations on PME Activity
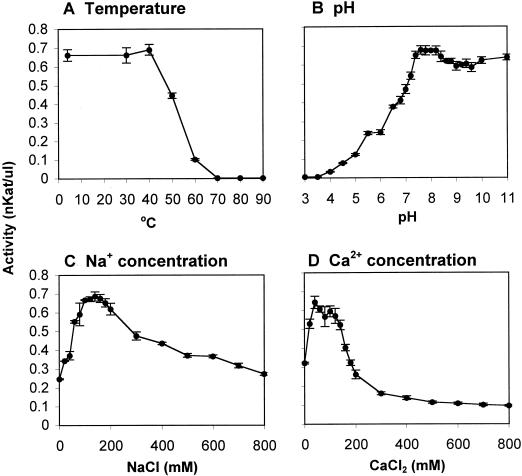
PME was relatively stable over time at 4°C, maintaining most of its activity over a 30-d period; only 33% or 16% of its original activity was maintained when the enzyme was stored at room temperature or at 37°C, respectively (data not shown). A 5-min incubation of PME extracts at temperatures below 40°C had little effect on PME activity, but higher temperatures rapidly abolished activity (Fig. 3A). The apparent pH optimum for the enzyme was between 7.4 and 8.4, but PME retained most of its activity over a wide pH range (7.4–11) (Fig. 3B). Both Na+ and Ca2+ stimulated PME activity when present at low concentrations (60–200 mm NaCl or 20–140 mm CaCl2), but Ca2+ became inhibitory at higher concentrations (Fig. 3, C and D).
Figure 3.
Effects of temperature, pH, and cations on PME activity. Data are the average of three replicates ± se. A, Thermostability of PME. Partially purified PME extracts were maintained for 5 min at the temperatures indicated and then assayed for activity. B, Effects of pH on PME activity. The activities of partially purified PME extracts were assayed in gels having different pH values. C and D, Effects of Na+ (C) or Ca2+ (D) on PME activity. The activities of the partially purified PME extracts were determined using gels containing different concentrations of NaCl (C) or CaCl2 (D).
Pattern of Increase of PME Activities before, during, and after Dormancy Termination of Yellow Cedar Seeds
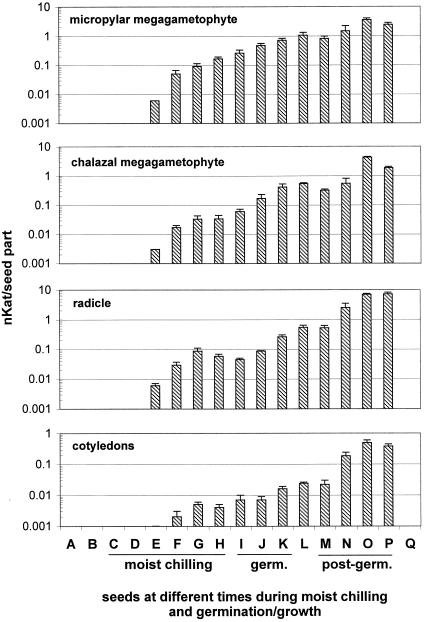
No PME activity was detectable in dormant seeds of yellow cedar (i.e. those subjected to a 3-d soak or to a control treatment in which seeds were maintained in warm [25°C] moist conditions for 90 d) (Fig. 4). PME activities became detectable after 15 d of moist chilling and increased gradually thereafter during moist chilling and during and following germination. Thus, high PME activity coincides with dormancy breakage of yellow cedar seeds; in the absence of an effective dormancy-breaking treatment, no enzyme activity is produced. PME activities were consistently higher in the radicle than in the cotyledons throughout moist chilling and germination. Prior to the completion of germination, PME activities were higher in the micropylar megagametophyte than in the chalazal megagametophyte (Fig. 4).
Figure 4.
Changes in PME activities in different seed parts of yellow cedar at different times prior to, and following, dormancy breakage. The PME assay was conducted by the gel diffusion method outlined in Downie et al. (1998). Data are based on three replicates of 10 seed parts each (±se). The different seed parts were isolated from mature seeds as follows: A, No treatment (mature dry); B, 3-d soak; C and D, 3-d soak and warm, moist conditions for 15 (C) or 30 d (D). E through H, Three-day soak and 30 d in warm, moist conditions followed by moist chilling for 15 (E), 30 (F), 45 (G), or 60 d (H). I through K, Full dormancy-breaking treatment (3-d soak, 30-d warm, moist conditions, and 60-d moist chilling) followed by germination conditions for 1 (I), 2 (J), or 3 d (K). L, After expansion of the megagametophyte. M through P, After the completion of germination, in which radicle lengths were 1 (M), 5 (N), 10 (O), and 15 mm (P). Q, A control treatment that consisted of a 3-d soak and a 90-d period in warm, moist conditions.
Correlation of PME Activities and the Capacity for Germination
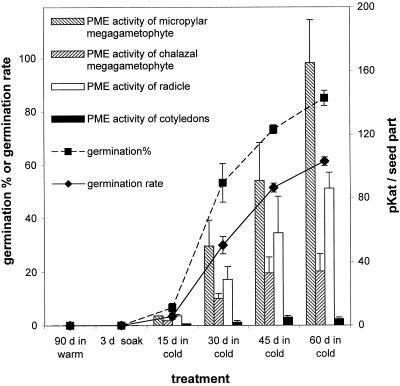
Moist chilling is essential not only for dormancy breakage (and therefore, optimal germination) but also for the enhancement of post-germinative growth. The longer the period of moist chilling that seeds were subjected to (following the prior 30-d treatment in warm, moist conditions), the greater their capacity for completing germination (Fig. 5, compare germination percentages and rates after 15, 30, 45, and 60 d of moist chilling). Moreover, the capacity for germination was well correlated with a capacity to produce PME activities (particularly in the micropylar megagametophyte and in the radicle) (Fig. 5). For example, after 60 d of moist chilling, which elicited 85% germination, PME activities increased to 165 pkat in the micropylar region of the megagametophyte and to 86 pkat in the radicle. Treatments that were not effective in breaking dormancy (i.e. mature seeds subjected to a 3-d soak or to a control treatment in which seeds were maintained in warm [25°C], moist conditions for 90 d) did not lead to any induction of PME activities (Fig. 5). Therefore, a strong and positive correlation between dormancy-breakage and PME activity exists in yellow cedar seeds.
Figure 5.
A comparison of PME activities and germination capacity of yellow cedar seeds. Mature seeds were subjected to a 3-d soak (only) or to a 3-d soak and 30 d of warm, moist conditions followed by different periods of moist chilling (15, 30, 45, or 60 d, the latter representing the full dormancy-breaking treatment). As a control, seeds were subjected to a 3-d soak followed by 90 d of warm, moist conditions. At the times indicated, one set of seeds was assayed for PME activity, and the remainder of the seeds were placed in germination conditions for 30 d to monitor the germination capacity (rate and percentage of germination). Data are based on three replicates of 10 seed parts for PME activities or on three replicates of 20 seeds each for germination measures (±se). Germination rate indicates the speed of germination and was calculated according to the formula noted in “Materials and Methods.”
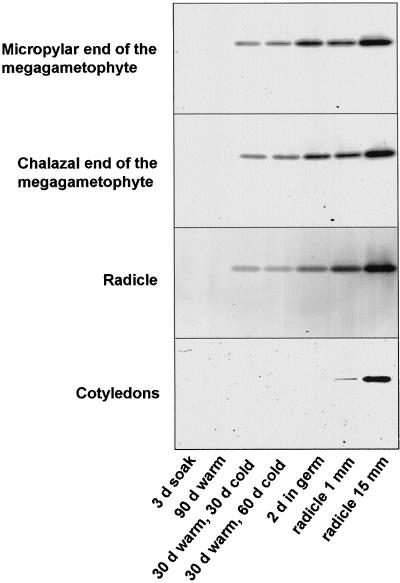
To further examine increases in PME activity at different stages and in different seed parts, crude extracts were subjected to acidic continuous native protein-gel electrophoresis (Fig. 6). In dormant seeds, no active PME bands were detected. During moist chilling (after a previous 30-d period in warm, moist conditions), PME activity was induced (which appeared as a single band on the gel) in the megagametophyte and radicle. Following transfer of seeds to germination conditions, the activity associated with this single band increased during germination and post-germinative growth. In cotyledons, PME activity was detected only after the completion of germination (Fig. 6).
Figure 6.
Native acidic continuous PAGE showing PME by ruthenium red staining in different seed parts excised from seeds at different times during a dormancy-breaking treatment and during germination/growth. Equal amounts of protein (50 μg) in crude extracts from different seed parts were loaded in each lane. d in cold, Length of time of moist chilling following a previous 30-d warm, moist treatment; d in germ, days in germination conditions; subsequent time points (radicle 1 and 15 mm) are times following the completion of germination.
Regulation of PME Isoforms by ABA and GA3
In a dormancy mechanism involving the megagametophyte as a mechanical barrier, it is possible that regulation of cell wall rigidity and the induction of cell wall hydrolases that weaken the megagametophyte are controlled by ABA and other hormones such as gibberellins. Following dormancy breakage, ABA (which could have an inhibitory effect on cell wall hydrolase production) may decline, whereas gibberellins may be produced, allowing cell wall hydrolase induction, megagametophyte weakening, and radicle protrusion.
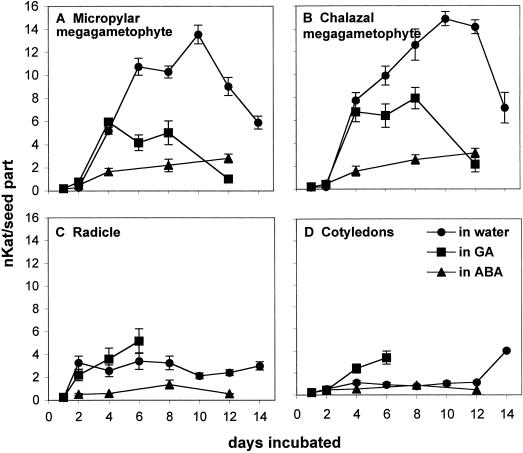
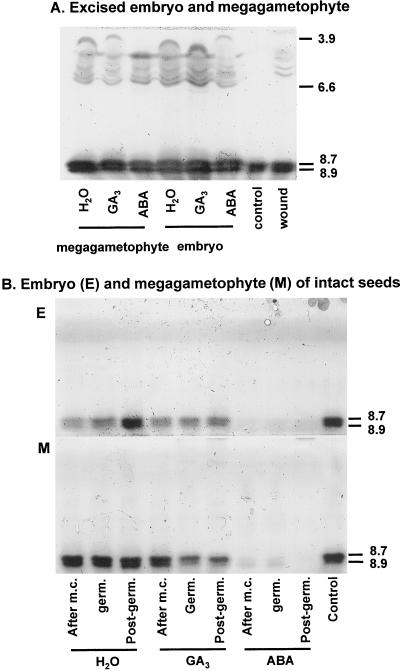
When embryos are excised from mature dormant seeds, they exhibit 100% germination. Whereas the megagametophyte inhibits completion of germination of the embryo in the intact dormant seed, it is not clear what influence the embryo has over the megagametophyte in terms of inhibiting germinative/post-germinative events, including hydrolase production. To examine hormonal regulation of PME activities, megagametophytes and embryos were excised from mature dormant seeds (subjected to only a 3-d soak) and then placed on water, 0.1 mm ABA, or 0.5 mm GA3. When megagametophytes and embryos were excised from the dormant seed and placed on water, PME activities were induced to high levels, particularly in the megagametophyte (Fig. 7). IEF gels (Fig. 8A) revealed an induction of several new acidic isoforms of PME (with pIs between 3.9 and 6.6), in addition to the two basic isoforms (pIs 8.7–8.9) associated with germination and post-germinative growth (Fig. 1). The acidic isoforms were induced primarily as a result of wounding; similar isoforms were induced when embryos and megagametophytes were kept within the intact seeds but pierced with forceps (Fig. 8A, “wound”). PME activities were detected in isolated megagametophytes and embryos treated with ABA, although the plant growth regulator had a distinct inhibitory effect (Fig. 7). GA3 appeared to have some effect on the total activities within the megametophyte and embryo (Fig. 7), being inhibitory in the megagametophyte and promotive in the radicle, but this was less evident on the IEF gels (Fig. 8A; compare GA3 versus water).
Figure 7.
Total PME activities in the micropylar megagametophyte (A), chalazal megagametophyte (B), radicle (C), and cotyledons (D), following incubation of isolated megagametophytes and embryos in water, ABA, or GA3. Megagametophytes and embryos were excised from mature dormant seeds (subjected to only a 3-d soak) and then placed on water, 0.1 mm ABA, or 0.5 mm GA3 for 2 weeks. PME activities were monitored by activity assays every 2 d, and the incubation solutions (water, ABA, and GA3) were changed every 3 d.
Figure 8.
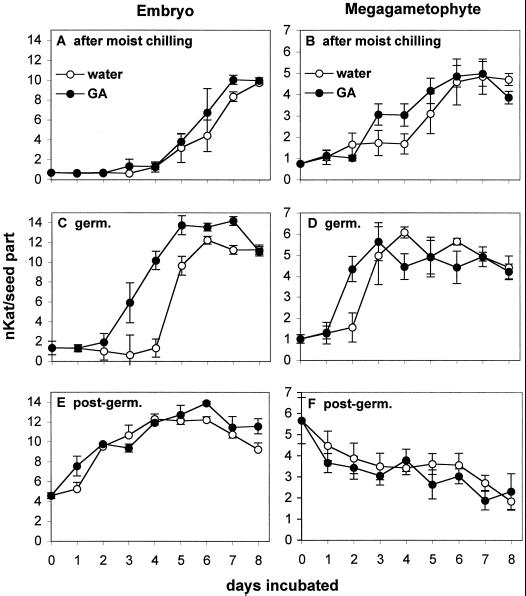
IEF gels showing the effects of GA3 and ABA on PME isoform activities in isolated embryos and megagametophytes excised from dormant seeds (A) and in embryos and megagametophytes (B) of intact seeds following a full dormancy-breaking treatment and during the germinative and post-germinative stages. A, Megagametophytes and embryos were excised from mature dormant seeds (subjected to only a 3-d soak) and then placed on water, 0.1 mm ABA, or 0.5 mm GA3 for 6 d. To test whether the acidic PME isoforms in the embryos and megagametophytes excised from dormant seeds were due to wounding during their excision, the seed parts were kept within the intact seed and wounded by piercing them with forceps (wound). The control is the partially purified PME from intact germinated seeds. B, Intact yellow cedar seeds were subjected to a full dormancy-breaking treatment (after m.c., moist chilling) or were subjected to the dormancy-breaking treatment and then transferred to germination conditions for 4 d (germ.) or until the seeds had achieved radicle lengths of 1 mm (post-germ.). Following moist chilling or during the germinative and post-germinative phases, seeds were incubated in water, 0.1 mm ABA, or 0.5 mm GA3 for 6 d and the PME isoform activities determined in the embryo and megagametophyte of the intact seeds.
Since the above experiment did not address the role of PME in dormancy breakage and germination and the potential hormonal regulation of hydrolases specific to these processes, intact yellow cedar seeds were subjected to the full dormancy-breaking treatment consisting of a 3-d soak, 30 d of warm, moist conditions and 60 d of moist chilling. Following this, one set of seeds was placed on water, 0.1 mm ABA, or 0.5 mm GA3 (Figs. 8B and 9, A and B). The remaining seeds were transferred to germination conditions for 4 d (germinative stage) or until seeds had completed germination and had radicle lengths of 1 mm (post-germinative stage) and then incubated on water, 0.1 mm ABA, or 0.5 mm GA3 (Figs. 8B and 9, C–F). In intact yellow cedar seeds that had been subjected to the full dormancy-breaking treatment or were at the germinative or post-germinative stages, ABA led to a significant suppression of PME activities within the embryo and megagametophyte (data not shown). IEF gels also revealed that the activities of the two basic isoforms of PME induced during and following dormancy breakage were greatly suppressed by ABA (Fig. 8B). GA3 had a stimulatory effect on the total PME activities of embryos at the germinative phase (especially between d 2 and 5 of incubation) and in megagametophytes after moist chilling and at the germinative phase (Fig. 9C, embryo, and B and D, megagametophyte). This was less obvious on the IEF gels (Fig. 8B), but in this case the incubation time was for 6 d (after the time when GA3 exhibited a promotive effect on PME in Fig. 9). No acidic isoforms of PME were induced in intact seeds (Fig. 8B).
Figure 9.
Time course of PME activities in the embryo and megagametophyte following incubation of intact seeds at different stages in water or GA3. Intact yellow cedar seeds were subjected to a full dormancy-breaking treatment (A and B, after moist chilling), or were subjected to the dormancy-breaking treatment and then transferred to germination conditions for 4 d (C and D, germ.) or until the seeds had germinated and had achieved radicle lengths of 1 mm (E and F, post-germ.). Following moist chilling or during the germinative and post-germinative stages, seeds were incubated in water or 0.5 mm GA3 and the total PME activities determined in the embryo and megagametophyte of the intact seeds by activity assays over an 8-d period. The incubation solutions (water and GA3) were changed every 3 d.
DISCUSSION
We investigated the potential role of PME in dormancy termination and the germination of yellow cedar seeds and characterized the enzyme biochemically. The two isoforms of PME in yellow cedar seeds have the same molecular mass of 62 kD and different pIs of 8.7 and 8.9, similar to the pIs of other PME isoforms characterized so far (Bordenave and Goldberg, 1994; Alonso et al., 1997). The enzyme from yellow cedar showed considerable stability when stored at 4°C, although it is not as thermally stable as the PMEs found in some fruits (Versteeg et al., 1980; Seymour et al., 1991; Laratta et al., 1995). Yellow cedar PME was active over a wide pH range (between 7.4–11.0), somewhat similar to that of other PMEs in which the optimum pH range is 7 to 9 (Rexova-Benkova and Markovic, 1976). Most isoforms of PMEs (particularly those that bind tenaciously to the cell wall) are strongly activated by the presence of salts (cations) in the reaction mixture (Bordenave and Goldberg, 1994; Nighojkar et al., 1994). Although the mechanism is not fully understood, the cations are thought to primarily interact with the substrate rather than with the enzyme (Nari et al., 1991). Activities of yellow cedar PME were also stimulated by low concentrations of Na+ or Ca2+ ions.
In a dormancy mechanism involving mechanical restraint, weakening of the cell walls of the megagametophyte especially at the micropylar region is proposed to be a prerequisite for germination. This process, mediated by cell wall hydrolases, would decrease the force required by the radicle to penetrate them. Hydrolytic enzymes implicated in dormancy termination include endo-β-mannanase (Downie et al., 1997) and oxalate oxidase (Grzelczak et al., 1985). In tomato seeds, chemical weakening of the surrounding endosperm is caused by enzymes produced under the influence of the embryo: dormant seeds are unable to produce the cell wall degrading enzymes. In these seeds, the endosperm cell walls contain relatively large amounts of galactomannans (Groot et al., 1988), which are a carbohydrate reserve (Reid, 1985). Three enzymes contribute to the hydrolysis of the galactomannans: α-galacto-sidase, a mannohydrolase, and endo-β-mannanase. More recent studies (focusing on endo-β-mannanase) have revealed that only the isoforms of the enzyme that are produced during germination have a potential role in weakening the endosperm (Nonogaki and Morohashi, 1996; Toorop et al., 1996; Voigt and Bewley, 1996). Endo-β-mannanase is also produced in the endosperm of lettuce seeds when the seeds are released from dormancy by GA or red light (Halmer et al., 1976) and seeds of Datura ferox produce endo-β-mannanase and β-mannosidase in the micropylar region of the endosperm after red-light stimulation, several hours before the radicle protrudes through it (Sanchez and de Miguel, 1997). In seeds of white spruce, weakening of the micropylar end of the megagametophyte and nucellus precedes radicle protrusion, and this weakening is associated with endo-β-mannanase activity (Downie et al., 1997).
Induction of PME in developing and germinated seeds has been examined (Bordenave and Goldberg, 1994; Nighojkar et al., 1994; Ebbelaar et al., 1996; Downie et al., 1998); however, studies to date have not addressed the role of this enzyme in dormancy breakage. In a previous report (Ren and Kermode, 1999), we detected a predominance of pectins in the cell walls of tissues surrounding the yellow cedar embryo. In the present study, the pattern of increase in PME activities in yellow cedar seeds coincided with the process of dormancy breakage, and the amount of enzyme activity produced was strongly correlated with the ability of seeds to germinate. Furthermore, in the absence of effective dormancy-breaking treatments (i.e. when mature seeds were subjected to a 3-d soak or to a control treatment comprised of a 90-d period in warm, moist conditions), no enzyme activity was induced. ABA caused significant suppression of the PME isoforms associated with dormancy breakage and germination/growth in embryos and megagametophytes of intact seeds. In isolated megagametophytes, and even in isolated embryos capable of completing germination, this inhibitory effect of ABA was somewhat reduced (Fig. 8A). GA3 had a promotive effect on PME activities in the embryo and megagametophyte at the germinative stage. However, the role of endogenous gibberellins in dormancy breakage of yellow cedar seeds is by no means clear, and preliminary analyses indicate that endogenous GAs are extremely low during germination (D. Stewart, N. Schmitz, R. Pharis, and A. Kermode, unpublished data).
The precise role of PME in yellow cedar seed germination is unknown. De-esterification of cell wall pectins, mediated by PMEs, has been shown to alter the characteristics of cell walls and hence mediate various physiological and/or biochemical processes in plant tissues. The proposed mechanisms of PME action in these processes can be summarized as follows: (a) creating an acidic environment within the cell wall as a result of de-esterification of pectins and thus promoting cell wall extension or growth (Moustacas et al., 1991); (b) facilitating hydrolysis of polygalacturonic chains by pectinases, a process thought to promote fruit ripening (Huber and O'Donoghue, 1993; Gaffe et al., 1994; Tieman and Handa, 1994; Steele et al., 1997) and seed germination (Sitrit et al., 1999); and (c) promoting formation of Ca2+ cross-linkages (through demethylation of pectins) that ultimately change the state of the pectin matrix by generating free carboxyl groups that are able to bind Ca2+ (Fry, 1986).
These changes are believed to increase the firmness of fresh vegetables (e.g. potato tubers, green bean pods, and pepper fruits) (Bartolome and Hoff, 1972; Ebbelaar et al., 1996; Sethu et al., 1996). It is possible that PME in the megagametophyte of yellow cedar de-esterifies pectins in cell walls such that the pectin chains are rendered more susceptible to the action of polygalacturonases, softening the cells walls of the megagametophyte, and thus promoting radicle protrusion. In a previous report (Ren and Kermode, 1999), dormancy breakage of yellow cedar seeds was correlated not only with a weakening of the micropylar megagametophyte, but also with an increased growth potential of the embryo; whether PME of the embryo is involved in this latter process (e.g. by promoting the formation of Ca2+ cross-linkages in cell walls) remains to be determined. Both mechanisms (i.e. megagametophyte weakening and increased growth potential of the embryo) could contribute to dormancy breakage of yellow cedar seeds (Ren and Kermode, 1999).
The same basic PME isoforms were detected in the different parts of yellow cedar seeds during dormancy breakage even though the cell wall changes mediated by PME in the embryo and megametophyte may be quite different (e.g. increased growth potential of the embryo and weakening of the megagametophyte). This indicates that the reaction pathways of the cell wall pectins after their de-esterification by PME are likely not determined by PME itself but rather by the micro-environments that surround the cell wall pectins (Carpita and Gibeaut, 1993). In some plants only one PME isoform is present (Nighojkar et al., 1994); however, in most plant tissues, a number of PME isoforms have been isolated (Gaffe et al., 1992; Lim and Chung, 1993; Bordenave and Goldberg, 1994). Four isoforms are detected in hypocotyls of mung bean; the acidic isoforms are either free in the intercellular fluid or weakly bound to cell walls, whereas the basic isoforms are tightly bound to cell walls. Binding of the enzyme to the cell wall is thought to modulate its activity both temporally and spatially (Bordenave and Goldberg, 1994).
In summary the present study demonstrates a strong positive correlation between PME activity and dormancy breakage of yellow cedar seeds. Further studies are necessary to reveal its precise mechanism of action in dormancy termination and the completion of germination.
MATERIALS AND METHODS
Seed Materials and Warm/Cold Treatments of Mature Seeds to Break Dormancy
Mature seeds of yellow cedar (Chamaecyparis nootkatensis) seed lot 30156 (previously collected from natural stands by MacMillan Bloedel and obtained from the Tree Seed Centre (Surrey, BC, Canada) were used. This seed lot was used exclusively because of its high viability. A 90-d warm/cold, moist treatment is effective in breaking dormancy of yellow cedar seeds (Ren and Kermode, 1999). Seeds were subjected to a 72-h running water treatment at 23°C followed by surface sterilization in a 1% (w/v) sodium hypochlorite solution for 10 min and four rinses with sterile distilled water. Seeds were then kept hydrated in near darkness at 26°C for 30 d (warm, moist treatment) and then transferred to 4°C for 60 d (moist chilling). To maintain high-moisture conditions throughout the warm and cold treatments, seeds were placed between two layers of moistened number 1 filter paper (Whatman, Clifton, NJ) on a mesh tray in a seed box (Hoffman Manufacturing, Albany, OR) with sterile water in the bottom of the seed box to maintain 100% relative humidity.
Seed Germination
After the 90-d dormancy-breaking treatment, seeds were placed in germination conditions (30°C d, 20°C nights with an 8-h photoperiod; light intensity at 100 μmol m−2 s−1, photosynthetically active radiation 400–700 nm) after transferring them to Petri dishes (100 × 15 mm) containing Whatman number 1 filter paper moistened with 3 mL of sterilized water. Percent germination (i.e. the number of seeds exhibiting radicle emergence) was monitored daily. The germination percentage and germination rate are used to determine germination capacity. Germination rate indicates the speed of germination. The formula used was:
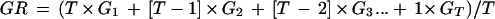 |
where T is the duration of the germination test in days (T = 30 d) and G1, G2, … , GT are the percentages of seeds germinated on d 1, 2, … , T (Xu, 1990).
Extraction of PME
A variety of methods have been used to extract PMEs from plant tissues, depending upon the nature of the plant tissue or the developmental stage. Some methods use high-salt buffers to extract PME from the residue or pellet after an initial extraction with distilled water (Baldwin and Pressey, 1988; Nighojkar et al., 1994; Alonso et al., 1997). PMEs can be tightly bound to cell walls; thus, others have used the strategy of isolating cell walls first (by the use of a Suc gradient series, from 0.4–1.0 m) followed by the extraction of the PMEs from the cell wall debris using a high-salt buffer (Goldberg, 1977; Bordenave and Goldberg, 1994). However, not all isoforms are tightly associated with the cell wall, rather some are in the intercellular fluid (Bordenave and Goldberg, 1994). Low-salt buffers (0.1–0.2 m NaCl) (Sethu et al., 1996) and high-salt buffers (1 m NaCl) (Ebbelaar et al., 1996; Downie et al., 1998) have been used, as well as 80% (v/v) ethanol to precipitate and concentrate the PME extract (Cruickshank and Wade, 1980). Although PME of yellow cedar seeds was extracted using water, a low-salt buffer or 0.4 m Suc, the most efficient buffer was a high-salt buffer. Unless otherwise stated, PME was extracted by grinding the seed parts at 4°C in 0.1 m citrate to 0.2 m Na2HPO4 buffer containing 1.0 m NaCl, pH 5.0. The homogenized slurry was centrifuged for 10 min at 14,000g in a microfuge (Eppendorf Scientific, Westbury, NY) at 4°C, and the supernatant was collected and stored at −20°C.
PME Activity Assay
The PME activity was quantified by the gel diffusion assay as described in Downie et al. (1998) with some modifications. A gel mold was made by placing a support-gel bond (245 × 125 mm, Amersham-Pharmacia Biotech, Piscataway, NJ) with its hydrophobic side facing down on a glass plate. The gel bond was then covered with another U-frame glass plate and the cassette was clamped together. A 50-mL mixture containing 6.25 mL of 0.1 m citric acid, 12.5 mL of 0.2 m Na2HPO4, 0.1% (w/v) of 90% esterified pectin, and 1% (w/v) agarose, pH 6.3, was boiled to dissolve the agarose. Following cooling to 60°C, the gel was cast into the gel mold using a syringe and then polymerized at room temperature for 1 h (Collmer et al., 1988). Two-millimeter-diameter wells were made in the 0.5-mm-thick gel with a cork-borer, and the excised gel was removed with a pipette connected to a vacuum. PME samples of 2 μL were loaded into each well, and the gel was sealed in a container and incubated at 37°C overnight (16 h). Gels were stained with 0.02% (w/v) ruthenium red for 1 h and destained with water, and the diameters of the red-stained areas were measured with a calipers to determine the amount of activity that was calculated according to a standard curve made from commercial PME (Fluka Chemika and Biochemika, Ronkonkoma, NY) under the same conditions.
Acidic Continuous Native PAGE
PME bands were isolated by acidic continuous native PAGE according to Hames and Rickwood (1981) with some modifications. Gels were comprised of 10% (w/v) acrylamide, 24 mm KOH, 0.86% (v/v) glacial acetic acid, 0.075% (w/v) ammonium persulphate, and 0.5% (v/v) TEMED (N,N,N′,N′-tetramethylethylenediamine), pH 4.3. PME samples were mixed with an equal amount of sample buffer (24 mm KOH, 0.86% [v/v] glacial acetic acid, 10% [w/v] glycerol, and 0.5 μL of methyl green dye, pH 4.3), loaded into wells, and electrophoresed at 4°C at 100 V of constant voltage with the polarity reversed; the running buffer was comprised of 24 mm KOH and 0.86% (v/v) glacial acetic acid, pH 4.3. After electrophoresis, gels were equilibrated for 5 min in 0.1 m citrate to 0.2 m Na2HPO4 buffer, pH 6.3, and then incubated for 90 min in the same buffer plus 0.5% (w/v) 90% esterified pectin as substrate at 37°C. Following a brief rinse with water, gels were stained with 0.02% (w/v) ruthenium red and destained with water.
IEF-Gel Electrophoresis
IEF-gel electrophoresis was performed using a Mini IEF Cell according to the manufacturer's instructions (model 111, Bio-Rad Laboratories, Richmond, CA). PME samples were loaded onto gels comprised of 5% (w/v) acylamide, 5% (w/v) glycerol, 2% (v/v) ampholyte (pH 3–10), 0.015% (w/v) ammonium persulfate, 0.0005% (w/v) riboflavin, and 0.06% (v/v) TEMED and focused at 100 V for 15 min, 200 V for 15 min, and 450 V for 60 min. Gels were then equilibrated for 5 min in 0.1 m citrate to 0.2 m Na2HPO4 buffer, pH 6.3, and incubated for 30 min in the same buffer plus 0.5% (w/v) 90% esterified pectin as substrate at 37°C. Following a brief rinse with water, gels were stained with 0.02% (w/v) ruthenium red and destained with water.
Partial Purification of PME from Yellow Cedar Seeds
PME was extracted from germinated seeds by grinding them in a mortar and pestle in 20 mm Tris [tris(hydroxy-methyl)aminomethane]-HCl buffer containing 1 m NaCl, pH 7.5. The slurry was centrifuged (4°C) at 7,000g for 30 min. The supernatant was collected, concentrated by polyethylene glycol 8,000 for 4 h and dialyzed against 10 mm Tris-HCl buffer, pH 7.5, overnight. This desalted PME crude extract was partially purified by anion chromatography with a DEAE-cellulose anion column. PME did not bind to the column and was eluted; the eluant was concentrated by polyethylene glycol 8,000 and dialyzed against 10 mm Tris buffer, pH 7.5. This partially purified PME was used for the enzyme characterization studies.
SDS-PAGE
Extracts were fractionated by SDS-PAGE on 10% (w/v) gels according to the method of Laemmli (1970) using a minigel system (Bio-Rad Laboratories, Richmond, CA). Protein samples were mixed with SDS sample buffer (65 mm Tris-HCl, pH 6.8, 2% [w/v] SDS, 10% [w/v] glycerol, and 0.01% [w/v] bromphenol blue), with or without 2% (v/v) β-mercaptoethanol, incubated overnight at 4°C, and loaded onto gels on the basis of equal protein. Following electrophoresis, gels were either stained by silver staining or subjected to PME activity staining as outlined below.
Activity Staining of PME following SDS-PAGE
After SDS-PAGE, bands with PME activity were detected by activity staining (Hou and Lin, 1998). The gels were immersed for 10 min with agitation in 25% (v/v) isopropanol in 10 mm Tris buffer (pH 7.9) (with two changes) to wash out the SDS and then washed three times in 10 mm Tris buffer for 15 min each. For activity staining, gels were incubated in the dark at 37°C for 15 to 20 min in freshly prepared substrate-dye solution and then destained with 10% (v/v) acetic acid. The substrate-dye solution consisted of 40 mg of β-naphthyl acetate in 16 mL of N,N-dimethylformamide that was brought to 160 mL with 144 mL of 10 mm Tris buffer (pH 7.9) in which 80 mg of tetrazotized o-dianisidine was dissolved.
Effect of pH and Different Concentrations of Cations on PME Activity
Gels used for the activity assays contained 0.1% (w/v) of 90% esterified pectin, 1% (w/v) agarose, and 0.05% (w/v) Na3N. After polymerization, the gel was cut into small pieces and each piece was soaked in a solution of different pH for 2 h before samples were loaded onto gels later subjected to activity assays. The pH of the gel soaking solutions were adjusted by mixing different volumes of 0.05 m citrate and 0.1 m Na2HPO4 (pH 3.0–7.5) or by using 50 mm Tris buffers of pH 6.8 to 11.0. Each soaking solution also contained 0.1% (w/v) of 90% esterified pectin. The use of two different buffers to assess the effects of pH on PME activity (from pH 3.0–7.5 and from pH 6.8–11.0) was valid since PME activity was not significantly altered by the different buffers in the range of pH overlap (6.8–7.5). To determine the effects of different concentrations of cations on PME activity, the gel pieces were soaked in solutions containing different concentrations of NaCl or CaCl2, 50 mm Tris-base, pH 7.5, and 0.1% (w/v) of 90% esterified pectin.
Effects of ABA and GA3 on Activities of PME Isoforms
PME was assessed by both activity assays and IEF gels subjected to ruthenium red staining (as described above). Two different experiments were conducted. In the first, megagametophytes and embryos were excised from dormant seeds and then incubated in water, 0.1 mm ABA or 0.5 mm GA3. PME was monitored every 2 d over a 2-week period during which the incubation solution was changed every 3 d. In the second experiment, intact yellow cedar seeds were subjected to a full dormancy-breaking treatment consisting of a 3-d soak, 30 d of warm, moist conditions, and 60 d of moist chilling. Following this, one set of seeds was placed on water, 0.1 mm ABA or 0.5 mm GA3. The remaining seeds were transferred to germination conditions for 4 d (germinative stage) or until seeds had germinated and had radicle lengths of 1 mm (post-germinative stage) and then incubated in water, 0.1 mm ABA, or 0.5 mm GA3. As before, the incubation solutions were changed every 3 d. In this experiment, PME was monitored at d 6 (Fig. 8B) or daily over the 8-d study period (Fig. 9).
ACKNOWLEDGMENTS
We are grateful to Stan Wheat and Mike Gerhard (MacMillan Bloedel Reforestation Centre, Nanaimo, BC, Canada), John Russell (British Columbia Forest Service, Lake Cowichan, BC, Canada), and Dave Kolotelo (British Columbia Ministry of Forests, Tree Seed Centre, Surrey, BC, Canada) for their help in obtaining mature seed of yellow cedar.
Footnotes
This work was supported by the Forest Renewal B.C. (grant no. HQ96232–RE to A.R.K.).
LITERATURE CITED
- Alexandre F, Morvan O, Gaffe J, Mareck A, Jauneau A, Dauchel H, Balange AP, Morvan C. Pectin methylesterase pattern in flax seedlings during their development. Plant Physiol Biochem. 1997;35:427–436. [Google Scholar]
- Alonso J, Howell N, Canet W. Purification and characterization of two pectinmethylesterases from persimmon (Diospyros kaki) J Sci Food Agric. 1997;75:352–358. [Google Scholar]
- Baayen RP, Schoffelmeer EAM, Toet S, Elgersma DM. Fungal polygalacturonase activity reflects susceptibility of carnation cultivars to fusarium wilt. Eur J Plant Pathol. 1997;103:15–23. [Google Scholar]
- Baldwin EA, Pressey R. Tomato polygalacturonase elicits ethylene production in tomato fruit. J Am Soc Hortic Sci. 1988;113:92–95. [Google Scholar]
- Bartolome LG, Hoff JE. Firming of potatoes: biochemical effects of preheating. J Agric Food Chem. 1972;20:266–270. [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds. Physiology of Development and Germination. Ed 2. New York: Plenum Press; 1994. [Google Scholar]
- Bordenave M, Goldberg R. Immobilized and free apoplastic pectinmethylesterases in mung bean hypocotyl. Plant Physiol. 1994;106:1151–1156. doi: 10.1104/pp.106.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, Shea PJ. A simple staining technique for assessing feeding damage by Leptoglossus occidentalis Heidemann on cones. Can Entomol. 1990;122:963–968. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Christgau S, Kofod LV, Halkier T, Andersen LN, Hockauf M, Dorreich K, Dalboge H, Kauppinen S. Pectin methyl esterase from Aspergillus aculeatus: expression cloning in yeast and characterization of the recombinant enzyme. Biochem J. 1996;319:705–712. doi: 10.1042/bj3190705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer A, Keen T. The role of pectic enzymes in plant pathogenesis. Annu Rev Phytopathol. 1986;24:383–409. [Google Scholar]
- Collmer A, Ried JL, Mount M. Assay methods for pectic enzymes. Methods Enzymol. 1988;161:329–335. [Google Scholar]
- Cruickshank RH, Wade GC. Detection of pectic enzymes in pectin-acrylamide gels. Anal Biochem. 1980;107:177–181. doi: 10.1016/0003-2697(80)90508-4. [DOI] [PubMed] [Google Scholar]
- De Vries JA, Hansen M, Soderberg J, Glahn PE, Pecersen JK. Distribution of methoxyl groups in pectins. Carbohydr Polym. 1986;6:165–176. [Google Scholar]
- Downie B, Dirk LMA, Hadfield K, Wilkins TA, Bennett AB, Bradford KJ. A gel diffusion assay for quantification of pectin methylesterase activity. Anal Biochem. 1998;264:149–157. doi: 10.1006/abio.1998.2847. [DOI] [PubMed] [Google Scholar]
- Downie B, Hilhorst HWM, Bewley JD. Endo-β-mannanase activity during dormancy alleviation and germination of white spruce (Picea glauca) seeds. Physiol Plant. 1997;101:405–415. [Google Scholar]
- Ebbelaar MEM, Tucker GA, Laats MM, van Dijk C, Stolle-Smits T, Recourt K. Characterization of pectinases and pectin methylesterase cDNAs in pods of green beans (Phaseolus vulgaris L.) Plant Mol Biol. 1996;31:1141–1151. doi: 10.1007/BF00040831. [DOI] [PubMed] [Google Scholar]
- Fry SC. Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol. 1986;37:165–186. [Google Scholar]
- Gaffe J, Morvan C, Jauneau A, Demarty M. Partial purification of flax cell wall pectin methylesterase. Phytochemistry. 1992;31:761–765. [Google Scholar]
- Gaffe J, Tieman DM, Handa AK. Pectin methylesterase isoforms in tomato (Lycopersicon esculentum) tissues: effects of expression of pectin methylesterase antisense gene. Plant Physiol. 1994;105:199–203. doi: 10.1104/pp.105.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. On possible connections between auxin induced growth and cell wall glucanase activities. Plant Sci Lett. 1977;8:233–242. [Google Scholar]
- Groot SPC, Kieliszewska-Rokicka B, Vermeer E, Karssen CM. Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta. 1988;174:500–504. doi: 10.1007/BF00634479. [DOI] [PubMed] [Google Scholar]
- Grzelczak ZF, Rahman S, Kennedy TD, Lane BG. Germin: compartmentation of the protein, its translable mRNA, and its biosynthesis among roots, stems, and leaves of wheat seedlings. Can J Biochem Cell Biol. 1985;63:1003–1013. [Google Scholar]
- Guglielmino N, Liberman M, Catesson AM, Mareck A, Prat R, Mutaftschiev S, Goldberg R. Pectin methylesterases from poplar cambium and inner bark: localization, properties, and seasonal changes. Planta. 1997;202:70–75. doi: 10.1007/s004250050104. [DOI] [PubMed] [Google Scholar]
- Halmer P, Bewley JD, Thorpe TA. An enzyme to degrade lettuce endosperm cell walls: appearance of a mannanase following phytochrome- and gibberellin-induced germination. Planta. 1976;130:189–196. doi: 10.1007/BF00384419. [DOI] [PubMed] [Google Scholar]
- Hames BD, Rickwood D. Gel Electrophoresis of Proteins: A Practical Approach. Oxford: IRL Press; 1981. pp. 1–42. [Google Scholar]
- Hou WC, Lin YH. Activity staining of pectinesterase on polyacrylamide gels after acidic or sodium dodecyl sulfate electrophoresis. Electrophoresis. 1998;19:692–694. doi: 10.1002/elps.1150190515. [DOI] [PubMed] [Google Scholar]
- Huber DL, O'Donoghue EM. Polyuronides in avocado (Persea americana) and tomato (Lycopersicon esculentum) fruits exhibit markedly different patterns of molecular weight downshifts during ripening. Plant Physiol. 1993;102:473–480. doi: 10.1104/pp.102.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laratta B, Loiudice R, Giovane A, Quagliuolo L, Servilllo L, Castaldo D. Thermostability of three pectinesterase isoenzymes in tomato fruit. Food Chem. 1995;52:415–418. [Google Scholar]
- Lim YM, Chung MCM. Isolation and characterization of pectin methylesterase from papaya. Arch Biochem Biophys. 1993;307:15–20. doi: 10.1006/abbi.1993.1553. [DOI] [PubMed] [Google Scholar]
- Liners F, van Cutsem P. Distribution of pectic polysaccharides throughout walls of suspension-cultured carrot cells. Protoplasma. 1992;170:10–21. [Google Scholar]
- Moustacas AM, Nari J, Borell M, Noat G, Ricard J. Pectin methylesterase: metal ions and plant cell wall extension. The role of metal ions in plant cell wall extension. J Biochem. 1991;279:351–354. doi: 10.1042/bj2790351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustacas AM, Nari J, Diamantidis G, Noat G, Crasnier M, Borel M, Ricard J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells: II. The role of pectin methyl esterase in the modulation of electrostatic effects in soybean cell walls. Eur J Biochem. 1986;155:191–197. doi: 10.1111/j.1432-1033.1986.tb09476.x. [DOI] [PubMed] [Google Scholar]
- Nari J, Noat G, Ricard J. Pectin methylesterase, metal ions and plant cell-wall extension: hydrolysis of pectin by plant cell-wall pectin methylesterase. Biochem J. 1991;279:343–350. doi: 10.1042/bj2790343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighojkar A, Srivastava S, Kumar A. Pectin methylesterase from germinating Vigna sinensis seeds. Plant Sci. 1994;103:115–120. [Google Scholar]
- Nonogaki H, Morohashi Y. An endo-β-mannanase develops exclusively in the micropylar endosperm of tomato seed prior to germination. Plant Physiol. 1996;110:555–559. doi: 10.1104/pp.110.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawuk WH. Germination of Alaska cedar seed. Tree Planters' Notes. 1993;44:21–24. [Google Scholar]
- Reid JSG. Cell wall storage carbohydrate in seeds: biochemistry of the seed “gums and hemicelluloses.”. Adv Bot Res. 1985;11:125–155. [Google Scholar]
- Ren C, Kermode AR. Analyses to determine the role of the megagametophyte and other seed tissues in dormancy maintenance of yellow cedar (Chamaecyparis nootkatensis) seeds: morphological, cellular and physiological changes following moist chilling and during germination. J Exp Bot. 1999;50:1403–1419. [Google Scholar]
- Rexova-Benkova L, Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:23–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- Sanchez RA, de Miguel L. Phytochrome promotion of mannan-degrading enzymes in the micropylar endosperm of Datura ferox seeds requires the presence of the embryo and gibberellin synthesis. Seed Sci Res. 1997;7:27–33. [Google Scholar]
- Schmitz N, Abrams SR, Kermode AR. Changes in abscisic acid content and embryo sensitivity to (+)-abscisic acid during the termination of dormancy of yellow cedar seeds. J Exp Bot. 2000;51:1159–1162. doi: 10.1093/jexbot/51.347.1159. [DOI] [PubMed] [Google Scholar]
- Sethu KMP, Prabha TN, Tharanathan RN. Post-harvest biochemical changes associated with the softening phenomenon in capsicum annuum fruits. Phytochemistry. 1996;42:961–966. [Google Scholar]
- Seymour TA, Preston JF, Wicker L, Lindsay JA, Wei CH, Marshall MR. Stability of pectinesterases of marsh white grapefruit pulp. J Agric Food Chem. 1991;39:1075–1079. [Google Scholar]
- Sitrit Y, Hadfield KA, Bennett AB, Bradford KJ, Downie AB. Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol. 1999;121:419–428. doi: 10.1104/pp.121.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele NM, McCann MC, Roberts K. Pectin modification in cell walls of ripening tomatoes occurs in distinct domains. Plant Physiol. 1997;114:373–381. doi: 10.1104/pp.114.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault JF, Renard C, Axelos MAV, Roger P, Crepeau MJ. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr Res. 1993;238:271–286. [Google Scholar]
- Tieman DM, Handa AK. Reduction in pectin methylesterase activity modifies tissue integrity and action levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol. 1994;106:429–436. doi: 10.1104/pp.106.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toorop PE, Bewley JD, Hilhorst HWM. Endo-β-mannanase isoforms are present in the endosperm and embryo of tomato seeds, but are not essentially linked to the completion of germination. Planta. 1996;200:153–158. [Google Scholar]
- Versteeg C, Rambouts FM, Spaansen CH, Pilnik W. Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. J Food Sci. 1980;45:969–972. [Google Scholar]
- Voigt B, Bewley JD. Developing tomato seeds when removed from the fruit produce multiple forms of germinative and post-germinative endo-β-mannanase: responses to desiccation, abscisic acid and osmoticum. Planta. 1996;200:71–77. [Google Scholar]
- Xia JH, Kermode AR. Analyses to determine the role of embryo immaturity in dormancy maintenance of yellow-cedar (Chamaecyparis nootkatensis) seeds: synthesis and accumulation of storage proteins and proteins implicated in desiccation tolerance. J Exp Bot. 1999;50:107–118. [Google Scholar]
- Xu N. The regulatory effects of surrounding tissues on embryo development and germination in alfalfa (Medicago sativa L.). MSc thesis. Ontario, Canada: University of Guelph; 1990. [Google Scholar]