Abstract
The inducible expression of polyphenol oxidase (PPO), a presumed antiherbivore enzyme, was examined in hybrid poplar (Populus trichocarpa × Populus deltoides). Following mechanical wounding simulating insect damage, PPO activity increased dramatically in wounded and unwounded leaves on wounded plants beginning at 24 and 48 h, respectively. A hybrid poplar PPO cDNA was isolated and its nucleotide sequence determined. On northern blots, PPO transcripts were detected within 8 h of wounding, and reached peak levels at 16 and 24 h in wounded and unwounded leaves, respectively. Methyl jasmonate spray and feeding by forest tent caterpillar also induced PPO expression. The induction of PPO was strongest in the youngest four leaves, which were generally avoided by caterpillars in free feeding experiments. This wound- and herbivore-induced expression of PPO in hybrid poplar supports the defensive role of this protein against insect pests.
The genus Populus, which includes both the poplars and aspens, has become a model organism for tree molecular biology, forest biotechnology, and most recently, tree genomics (Sterky et al., 1998). The attributes making Populus spp. so useful for research are their rapid growth, ease of vegetative propagation, a relatively small genome, and tractability to Agrobacterium-mediated transformation. These features also underlie the significant economic importance of Populus spp. worldwide; for example, artificial hybrids of poplar grown in large-scale plantations are becoming increasingly important for pulp production in North America. Such plantation forestry may eventually be expanded to include transgenic poplars. Genetically modified poplar with improved processing characteristics, in particular, reduced lignin content, has been a major thrust in forest biotechnology research (Boudet, 1998). However, faster growth and enhanced pest and pathogen resistance are traits that may also be improved in transgenic trees (Tzfira et al., 1998).
Poplars are known to be hosts for many herbivorous insects and pathogens (Whitman et al., 1996), and hybrid poplar (Populus trichocarpa × Populus deltoides) plantations consisting of trees of similar genotype and age are particularly susceptible to outbreaks of insect pests. Pest resistance in poplar is very variable among genotypes (Robison and Raffa, 1994; Havill and Raffa, 1999); however, the mechanisms underlying such differences still need to be elucidated. Greater knowledge of how poplars defend themselves against insect herbivores will provide novel genes for genetic engineering of resistance, as well as markers for selection of superior genotypes. It will also provide a basis for understanding defense mechanisms of woody plants in general. How defense in long-lived plants such as trees compares with that in short-lived annuals and crops plants is one of the outstanding questions in the field of plant-insect and plant-pathogen interactions.
In a survey of several interspecific poplar hybrids for host suitability for lepidopteran defoliators, Robison and Raffa (1994, 1997) found no correlation of resistance with moisture, fiber, or nitrogen content. However, preliminary evidence suggested that differences in insect performance are due to varying levels of defensive chemicals. Many previous studies on defense mechanisms in poplar and other trees have focused on secondary plant metabolites (Mattson et al., 1988; Tallamy and Raupp, 1991). Phenolic compounds such as tannins are often correlated with resistance to herbivores, and in some trees their accumulation is induced by previous herbivory (for review, see Constabel, 1999). Populus as a genus is rich in a variety of phenolic compounds; in trembling aspen (Populus tremuloides) leaves, for example, the phenolic glycosides, salicortin and tremuloidin, can constitute up to 4% of leaf dry weight. These have been shown to contribute to defense against forest tent caterpillar (FTC) and gypsy moth (Lindroth and Hwang, 1996).
In addition to toxic or deterrent secondary plant metabolites, a variety of protein-based antiherbivore defenses have been described in herbaceous plants (Ryan, 1990; Duffey and Felton, 1991). Prominent examples of antiherbivore proteins are protease inhibitors, lectins, and oxidative enzymes. Defensive proteins are often antinutritive rather than directly toxic. Furthermore, in many species they are inducible and accumulate only following insect attack (for review, see Constabel, 1999). Few studies of antiherbivore proteins or their corresponding genes have been conducted in forest trees; however, early pioneering work by Gordon and coworkers described wound-induced genes in a P. trichocarpa × P. deltoides (TD) poplar hybrid (Parsons et al., 1989). Several cDNAs were isolated and these were shown to encode a Kunitz-type trypsin inhibitor, chitinase, and β-glucanase, as well as a storage protein-like gene (Bradshaw et al., 1991; Davis et al., 1993). Protease inhibitors are effective defenses that prevent and reduce herbivore damage (Hilder et al., 1987; Johnson et al., 1989), and chitinase and β-glucanase have been implicated in pathogen defenses (Buell, 1999). It is significant that wounding induces expression of these genes in the damaged as well as unwounded (systemically wounded) leaves; their wound inducibility suggests that the corresponding defense proteins play an important role in the poplar defense. Furthermore, in other poplar hybrids prior leaf damage or feeding was shown to reduce subsequent damage by pests (Robison and Raffa, 1997; Havill and Raffa, 1999), underscoring the biological importance of inducible defense mechanisms in poplar.
We recently observed that wounding of TD hybrid poplar leaves causes a strong induction of polyphenol oxidase (PPO) activity (Constabel and Ryan, 1998). PPO is an enzyme catalyzing the oxidation of o-diphenolic compounds to o-quinones (diphenolase; EC 1.10.3.2), as well as the hydroxylation of monophenols to o-diphenols (monophenolase; EC 1.14.18.1; Steffens et al., 1994). The enzyme is responsible for the typical browning of plant extracts and damaged tissues caused by the spontaneous polymerization and crosslinking of the o-quinones. Fruit commonly contains large amounts of PPO (Macheix et al., 1990); the involvement of PPO in enzyme-mediated browning during food processing has motivated many studies in PPOs from food plants, and PPO cDNAs have been cloned from potato, apple, grape, and sugarcane (for review, see Constabel et al., 1996). However, the physiological function of PPO in fruit and other organs in healthy plants is still uncertain (Steffens et al., 1994).
By contrast, a role of foliar PPO in the defense against leaf-eating insects has been proposed and documented (Felton et al., 1989; Duffey and Felton, 1991). During chewing and feeding, the mixing of PPO and phenolic substrates generates the o-quinones; these highly reactive compounds are then able to covalently modify free amino and sulfhydryl groups in dietary proteins within the mouth and gut of the insect. The resulting phenolic adducts prevent efficient assimilation of the alkylated amino acids, and thus reduce the nutritive value of protein (Felton et al., 1992). This antinutritive effect of PPO has been well documented for defense in tomato, where PPO can be induced to high levels (Felton et al., 1989). PPO induction occurs coordinately with several other known defense proteins in tomato and is signaled by the wound hormone systemin, further supporting the importance of PPO in defense in this plant (Constabel et al., 1995; Bergey et al., 1996).
Our observation that wounding induces PPO in hybrid poplar leaves indicates that this enzyme may also be important for defense in poplar. To further address this question we have investigated hybrid poplar PPO at the molecular level. Here we describe the cDNA cloning of hybrid poplar PPO, and characterize PPO gene expression and induction by wounding and insect herbivory. Our results support the hypothesis that PPO has a defensive role in hybrid poplar.
RESULTS
Optimization of PPO Assay and Wound Induction of PPO Activity
To optimize mechanical wound treatments in hybrid poplar, we first tested different methods of wounding leaves for their effects on PPO induction. Leaves were damaged by slicing with a razor blade, removing tissue from leaf margins with a hole punch, abrading the leaf surface with sandpaper, or crushing the leaf blade margins with pliers. All these treatments resulted in elevated PPO activity after 3 d; however, crushing leaves with pliers and removal of leaf material using a hole punch resulted in the strongest PPO induction (data not shown). Crushing leaf tissue with pliers was the most practical and easiest to standardize. This technique was therefore used for all subsequent experiments.
We optimized the assay for PPO in leaf extracts by testing several commercially available phenolic PPO substrates. A wounded leaf extract with high PPO activity was used in these assays, and PPO activity was measured with an oxygen electrode to assay consumption of oxygen. This assay allowed for the direct comparisons of reaction rates regardless of the absorptivity of the individual products formed. The relative rates of oxidation of the various substrates in decreasing order was 4-methyl catechol > catechol > dihydroxyphenylalanine (DOPA) > chlorogenic acid. Assays carried out using DOPA as the substrate were the most linear and sufficiently sensitive; this substrate was therefore used for subsequent experiments.
To confirm that the DOPA oxidation observed was due to a PPO rather than other oxidative enzymes, we tested several inhibitors and activators of PPO activity (Table I). As expected, kojic acid and tropolone inhibited the oxidation of DOPA, consistent with the properties of phenolases in general (Chen et al., 1991; Valero et al., 1991). Furthermore, ferulic acid inhibited, whereas SDS activated, the oxidation of DOPA in poplar extracts, both characteristic for PPO (o-diphenol oxidase) activity (Walker and McCallion, 1980). By contrast, laccase (p-diphenolase) is affected by neither SDS nor ferulic acid (Walker and McCallion, 1980). Cetyltrimethylammonium bromide, previously reported to be an inhibitor of PPO (Walker and McCallion, 1980), had no inhibitory effect on hybrid poplar PPO activity (Table I). Overall, these data indicate that the DOPA-oxidizing activity in hybrid poplar leaf extracts was indeed due to a typical PPO.
Table I.
Effects of PPO inhibitors and activators on DOPA- oxidizing activity in extracts prepared from wounded hybrid poplar leaves
| Chemical Compound | Concentration | Effect |
|---|---|---|
| Ferulic acid | 1 mm | Inhibits |
| Tropolone | 0.025 mm | Inhibits |
| Kojic acid | 1 mm | Inhibits |
| SDS | 0.15% | Activates |
| CTAB | 10 mm | No effect |
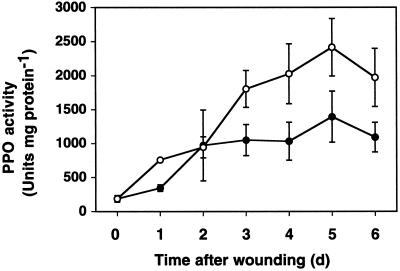
To investigate the kinetics of PPO induction in hybrid poplar following wounding, we performed time course experiments. Three-month-old saplings were wounded three times on the margins of six fully expanded leaves, and both the wounded and unwounded leaves (systemically wounded leaves) were collected at 1-d intervals and frozen for analysis. PPO activity increased detectably at 1 d post-wounding in the wounded leaf, and by 2 d post-wounding in the systemically wounded leaves (Fig. 1). The increase in PPO activity in wounded and systemically wounded leaves continued until 5 d post-wounding and then began to decline. This peak at 5 d was observed in several independent experiments and may represent the return of the leaf to its prior unwounded state. In systemically wounded leaves, PPO activity generally increased in parallel to the induction observed in wounded leaves, but the increases seen were consistently smaller. These experiments clearly demonstrated that hybrid poplar responds to mechanical damage with a rapid systemic defense response resulting in elevated PPO activity.
Figure 1.
Wound induction of PPO in hybrid poplar leaves. Young hybrid poplar saplings were wounded as described in “Material and Methods,” and wounded leaves (○) and unwounded leaves on wounded plants (●) were harvested at the indicated times for PPO assays. Each point represents the means (±se), determined independently from samples of three wounded plants.
Isolation and Analysis of Hybrid Poplar PPO cDNA
To further characterize PPO expression at the molecular level, we isolated a hybrid poplar cDNA. To obtain a probe for PPO, reverse transcriptase-PCR experiments were carried out using hybrid poplar mRNA as a template and degenerate primers designed from conserved regions within the copper-binding sites of known plant PPO sequences (van Gelder et al., 1997). A fragment of the expected size was amplified and its identity confirmed by nucleotide sequencing. A cDNA library constructed from mRNA isolated from systemically wounded hybrid poplar leaves was subsequently screened with the PPO cDNA fragment. Approximately 0.15% of the 5 × 105 plaques screened hybridized with the probe, suggesting that PPO cDNAs were relatively abundant in the cDNA library. A cDNA 1,912 bp in length was isolated and its nucleotide sequence determined on both strands. Since the original cDNA clone extended only a few basepairs upstream of the predicted start codon, 5′ RACE was carried out to extend this sequence. The presence of several in-frame stop codons indicated that this ATG was in fact the translation start site. This was also confirmed with database comparisons (see below).
Conceptual translation of the cDNA predicted a protein of 563 amino acids with a molecular mass of 64 kD (Fig. 2). BLAST sequence comparisons with the GenBank sequence databases confirmed the cDNA as encoding hybrid poplar PPO, which was named PtdPPO. PtdPPO had the highest BLAST similarity scores with PPO cDNAs isolated from apple (Boss et al., 1995; Haruta et al., 1998) and grape (Dry and Robinson, 1994), with protein sequence identities of 59% and 55%, respectively. The predicted hybrid poplar PPO protein included an N-terminal transit peptide of 67 amino acid residues, which targets the protein for import into the chloroplast and thylakoid lumen (Fig. 2). All plant PPOs sequenced to date have been found to contain such thylakoid targeting peptides, consistent with the observed plastidic localization for PPO protein and PPO activity (Steffens et al., 1994). The predicted PtdPPO transit peptide was calculated to be somewhat shorter in length than the corresponding regions from other PPO genes. However, it contains the typical features of thylakoid targeting sequences: an N-terminal hydroxy amino acid-rich region required for import into the stroma, followed by a hydrophobic core domain ending in the Ala-X-Ala consensus recognized by the thylakoid processing peptidase (Steffens et al., 1994; Robinson and Mant, 1997). Other features of PtdPPO include two conserved copper-binding domains, each with three highly conserved His residues that complex the catalytically active Cu2+ (Steffens et al., 1994).
Figure 2.
Nucleotide and predicted protein sequences of hybrid poplar PtdPPO cDNA. The thylakoid transit peptide is double underlined, and the copper-binding domains are single underlined. Within the copper-binding domains, the primers used to amplify the PPO fragment employed as a probe are double underlined, and conserved His residues are marked with an asterisk. The PtdPPO cDNA has been deposited in GenBank (accession no. AF 263611).
Using the PtdPPO cDNA as a probe, we performed Southern analyses on hybrid poplar genomic DNA to estimate the size of the PPO gene family in the H11-11 TD hybrid. We also analyzed DNA from a closely related TD hybrid 53-246, as well as its female parent P. trichocarpa 93-268. In both TD poplar hybrids, the PPO probe hybridized with three to six bands, depending on the restriction enzyme used (Fig. 3). In P. trichocarpa 93-968 only two to three hybridizing bands were observed. These represented a subset of the bands visualized in hybrid 53-246. The most probable interpretation is that in both hybrids the banding pattern is due to the presence of two alleles at two polymorphic PPO loci. From these experiments we conclude that both TD hybrids likely contain two PPO genes.
Figure 3.
Southern analysis of PPO in poplar. The entire PtdPPO cDNA was used as a probe. H11-11 is the TD hybrid used in this study, whereas 53-246 is a related TD hybrid; 93-968 is the P. trichocarpa parent of 53-246. E, EcoRI; H, HindIII; X, XbaI.
Induction of PPO mRNA in Response to Wounding and Methyl Jasmonate (MeJa) Treatment
The availability of the PtdPPO cDNA probe allowed us to use northern analyses to determine if the observed PPO induction observed using enzyme assays was due to increased PtdPPO gene expression or enzyme activation. Plants were wounded as previously described, and both the wounded and systemically wounded leaves were harvested at various times for RNA extraction. PPO transcripts, approximately 2.1 kb in size, accumulated to detectable levels within 8 h of the treatment in the wounded leaves, and within 16 h in systemically wounded upper leaves (Fig. 4). In wounded leaves, peak accumulation was observed at 16 h, and at in systemically wounded leaves at 24 h. For most time points, PPO mRNA was more abundant in the wounded leaves. Overall, the pattern observed on northern analyses was consistent with the kinetics of PPO activity following wounding (Fig. 1) For comparison, the same blot was reprobed with a trembling aspen cDNA encoding a Kunitz-type proteinase inhibitor (M. Haruta, M.E. Christopher, J.J. Patton, I.T. Major, and C.P. Constabel, unpublished data; Bradshaw et al., 1989). This mRNA showed a pattern of wound induction similar to what was observed with PPO (data not shown), and indicated that other defense genes were also induced by our treatments.
Figure 4.
Northern analysis of PPO mRNA accumulation following wounding. Wounded and unwounded (systemic) leaves on wounded plants were harvested at the indicated times for RNA extraction. A, Top panel, X-ray film image of blots hybridized with PPO probe; bottom panel, the ethidium bromide-stained gel as a loading control. B, Graphical representation of PPO transcript intensity as determined from phosphor imager. □, Wounded leaves; ●, unwounded leaves.
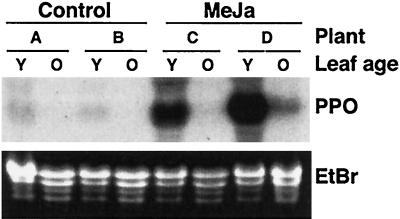
The similar wound induction of both PPO and trypsin inhibitor mRNAs following wounding suggests that these defense responses are coordinately controlled. In other plants the coordinate systemic induction of diverse defense proteins including PPO and protease inhibitors is known to involve jasmonate signaling intermediates (Bergey et al., 1996). We therefore tested MeJa for its effect on PPO expression in hybrid poplar. Saplings were sprayed with either MeJa in 0.1% (v/v) Triton X-100, or mock-sprayed with Triton X-100 alone. Leaves were sampled after 24 h for RNA extraction and northern analysis from both younger (leaves 5–8) and older (leaves 15–20) parts of the plant. PPO mRNA was strongly induced in the younger leaves of MeJa-treated plants, but only slightly induced in older leaves (Fig. 5). This differential responsiveness was also seen in wounding experiments (see below). A small induction was sometimes observed in the control leaves, most likely due to the Triton X-100. Overall, MeJa clearly has the potential to induce PPO expression in hybrid poplar leaves (Fig. 5). However, in direct comparisons of wounding and MeJa treatment, we observed that wounding was generally a stronger inducer of PPO mRNA than MeJa (data not shown).
Figure 5.
Northern analysis of PPO mRNA induction by MeJa. Saplings were treated with MeJa or mock-sprayed, and analyzed after 24 h. A, Top panel, x-ray film image of blots hybridized with PPO probe; bottom panel, the ethidium bromide-stained gel as a loading control. A through D refer to individual plants tested. Y, Young leaves; O, old leaves.
Localization of PPO Induction by Herbivory and Wounding
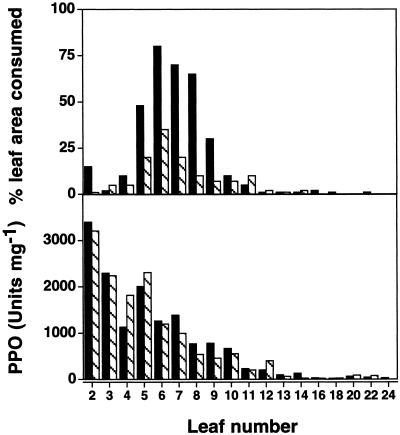
We studied the effects of actual herbivory on PPO activity by subjecting hybrid poplar saplings to feeding by FTC. The preferred host plant of these oligophagous Lepidopteran defoliators in western Canada is trembling aspen, but preliminary experiments indicated that TD hybrid poplars are also a suitable food plant for these herbivores. Herbivore damage was induced throughout entire hybrid poplar saplings by free-roaming FTC, as described under “Materials and Methods.” Allowing the insects free range of movement represented a realistic situation. However, it was difficult to control the extent of the damage, and in different plants and experiments varying amounts of leaf material was consumed (Fig. 6). Therefore, results were not pooled and representative data from only one experiment are shown. Despite differences between individual plants in the total amount of leaf material consumed, in both cases the larvae strongly preferred to feed on leaves 5 to 9 (Fig. 6). This preference was unexpected, but highly reproducible, and was observed in several other feeding trials. Since the position of each leaf along the stem of the plant is proportional to its age, the FTC's feeding preference must reflect a preferred leaf age.
Figure 6.
FTC feeding preference and induction of PPO activity in hybrid poplar saplings. Top panel, Percentage of material consumed from individual leaves after 6 d of FTC feeding. Bottom panel, PPO activity in remaining leaf material. Hatched and black bars represent individual plants.
PPO activity was very strongly induced after 6 d of insect feeding, to levels comparable with those induced by wounding (Fig. 6, compare with Fig. 1). Control plants in cages without FTCs had low PPO activity, typically less than 100 units mg−1 protein (data not shown). It is surprising that the induction of PPO activity within the damaged plant did not exactly mirror the pattern of tissue damage. Although the leaves with the greatest damage (leaves 5–9) showed a very strong induction of PPO, even higher levels of PPO activity were measured in leaves 1 to 4. These very young leaves sustained little direct herbivory, but were presumably induced by the systemic wound signal to strongly express PPO. In a similar manner, low levels of herbivore damage were observed on older leaves (10 and above); these leaves, however, contained only relatively low levels of PPO (Fig. 6).
The high degree of PPO induction in the youngest leaves despite low levels of direct herbivore damage could be due to a greater responsiveness of younger leaves to damage, to the systemic signal moving primarily upwards, or to a combination of both factors. To test these possibilities, we compared the inducibility of PPO in leaves of different ages along the axis of a 3-month-old plant. Leaves from a range of positions were mechanically wounded as before, and 24 h later the wounded leaves were harvested for northern-blot analysis (Fig. 7). Control samples were representative leaves from the same plant (leaf positions 2, 9, and 16) taken immediately prior to wounding. Leaves 3 to 5 (the youngest leaves tested) clearly responded to damage with the greatest induction of PPO mRNA, showing at least 5-fold higher PPO transcript levels compared with leaves 8 and above. This result indicated a much greater responsiveness to tissue damage in leaves 3 to 5 compared with other leaves, and could explain the very high PPO activity despite low levels in FTC damage (compare Fig. 6). Older leaves (leaf positions 10–20) showed a lower PPO mRNA inducibility (Fig. 7). Therefore the pattern of PPO induction caused by herbivory in Figure 6 could be explained by this differential responsiveness to wound induction. However, a predominantly upwards movement of the systemic wound signal may also be involved (see “Discussion”).
Figure 7.
Northern analysis of PPO wound induction in leaves of different ages. A range of leaves of a young poplar sapling were mechanically wounded as described in “Materials and Methods,” and analyzed after 24 h. Leaves 2, 9, and 16 were used as controls, and harvested immediately prior to the wounding treatment. A, Top panel, X-ray film image of blots hybridized with PPO probe. Control and treatment panels were from the same blot; however, the control panel was exposed for 68 h to visualize very low PPO mRNA levels, whereas the wound treatment panel was exposed for 6.5 h. Bottom panel, The ethidium bromide-stained gel as a loading control. B, Graphical representation of PPO transcript intensity as determined from phosphor imager.
DISCUSSION
Resistance of trees to insect pests is of major importance to forest health, yet our understanding of the molecular mechanisms underlying this resistance is only rudimentary. In this report we describe the cloning of a cDNA encoding a potentially antiherbivore protein, PPO, from hybrid poplar. We show that wounding and herbivory by leaf-eating insects resulted in a plant-wide induction of PPO activity, that this increase was preceded by elevated levels of PPO transcripts, and that the signaling compound MeJa was an effective inducer of this response. We also investigated developmental aspects of PPO induction in hybrid poplar. Our results support the hypothesis that in hybrid poplar, PPO is an important component of the defense of poplar against leaf-eating insects.
We isolated a fragment of PtdPPO using PCR and primers complementary to the highly conserved copper-binding domains of other plant PPOs, and this fragment was subsequently used as a probe to isolate a full-length hybrid poplar PPO cDNA clone. The molecular mass of the mature peptide was predicted to be 56.6 kD, comparable with the 54 to 68 kD of PPOs from other plant species (van Gelder et al., 1997). Database sequence comparisons confirmed that the identity of the cDNA as a PPO. It is interesting that the most similar sequences in GenBank were PPO cDNA sequences from apple (59%) and grape (55%), two other woody plants. The copper-binding domains (Cu-A and Cu-B) of PtdPPO showed much higher similarity (84% and 76%) with their respective domains in the apple cDNAs than did the cDNAs overall, consistent with the essential role of copper in catalysis. By contrast, the transit peptides that direct PPO to the thylakoid lumen are very poorly conserved among plant PPOs (van Gelder et al., 1997).
However, the predicted PtdPPO transit peptide does contain the typical hydroxy amino acid-rich N-terminal region, followed by a hydrophobic domain and the Ala-X-Ala processing site (Robinson and Mant, 1997). Recent insight into import of thylakoid lumen proteins has delineated two independent pathways, a prokaryotic type Sec system that requires ATP, and a ΔpH-dependent system (Robinson and Mant, 1997). The twin Arg motif at position 44 just preceding the hydrophobic domain and a basic residue at position 63 of PtdPPO are essential for protein import via the ΔpH-dependent import system (Chaddock et al., 1995; Robinson and Mant, 1997). Hybrid poplar and other plant PPOs thus belong to those lumenal proteins that are imported into the thylakoids via the ΔpH-dependant pathway. This could be demonstrated experimentally with isolated chloroplasts, where disruption of the pH gradient by photosynthetic electron transport inhibitors blocks the import of tomato PPO into thylakoids (Sommer et al., 1994).
Southern analysis suggested that hybrid poplar most likely contains two PPO genes, with polymorphic alleles at each locus (Fig. 3). Although three to six bands are visible on Southern blots of poplar hybrid H11-11, comparisons of the banding pattern in the related TD hybrid (53-246) with its female parent (93-968) suggested that the more complex banding pattern of the hybrids must be due to the detection of both parental alleles. Likewise, in the closely related trembling aspen, we have detected only two PPO genes (M. Haruta, J.A. Pedersen, and C.P. Constabel, unpublished data). In tomato and potato, PPO genes have been studied in great detail, and gene families with seven and six members, respectively, were identified (Hunt et al., 1993; Thygesen et al., 1995). Detailed analysis of expression of all seven tomato PPO genes indicated that only one gene family member is wound inducible, whereas the others are developmentally regulated (Thipyapong and Steffens, 1997; Thipyapong et al., 1997). For apple, Boss et al. (1995) suggest that there are likely to be at least four PPO genes, and in Phytolacca sp. at least two genes are known to be present (Joy et al., 1995). Since PPO in hybrid poplar is expressed as a function of wounding as well as development (J. Wang and C.P. Constable, unpublished data), it will be interesting to determine if there are distinct developmentally and stress-regulated hybrid poplar PPO genes.
We used northern analyses to demonstrate that the local and systemic induction of PPO activity was due to an increased abundance of PPO transcript accumulation (Fig. 2). Therefore, transcriptional activation of PPO genes and de novo enzyme synthesis, rather than enzyme activation, is the most likely mechanism underlying the wound-induced increase in PPO activity (Fig. 1). This is an important result because many plant PPOs are latent and require chemical activation to be fully active (Jimenez and Garcia-Carmona, 1996, and refs. therein). Wounding or pathogen attack typically result in a modified local chemical environment due to the release of vacuolar and cellular constituents, and thus the potential activation of PPO has made some previous studies of PPO induction difficult to interpret (for discussion, see Steffens et al., 1994). Defense protein induction via transcriptional activation and de novo enzyme synthesis is indicative of an active response to tissue damage and is observed for many other insect defense proteins (Bergey et al., 1996), supporting the hypothesis that in hybrid poplar PPO is important for pest defense.
Our results also demonstrate that herbivory by FTC is a very effective inducer of PPO activity and mRNA. Due to the unpredictable feeding behavior of live insects it was difficult to directly compare mechanical wounding with actual herbivory. However, in some experiments herbivory was a stronger stimulus than mechanical wounding (not shown). This could be an indication that FTC feeding not only releases endogenous defense signals, but that herbivore-specific signals such as described in other plant-herbivore interactions may be involved (Alborn et al., 1997). Overall, the induction of expression of PPO by wounding and caterpillar feeding supports a defensive role of PPO in hybrid poplar.
Several previous studies have demonstrated the wound inducibility of PPO gene expression in leaves of tomato, potato, and apple, as well as in apple fruit (Boss et al., 1995; Thipyapong et al., 1995; Constabel et al., 1996). Other species such as apricot failed to show wound-induced accumulation of PPO mRNA (Chevalier et al., 1999). Such species-specific differences are consistent with previous work demonstrating a clear wound induction of PPO activity in only a subset of crop plants tested (Constabel and Ryan, 1998). This suggests that although PPO may be ubiquitous in the plant kingdom, it has evolved into a defensive role in only a few species. It should be noted, however, that many studies have focused on PPO expression in relation to tissue browning and food processing, and that the effects of wounding were not specifically investigated.
In hybrid poplar, PPO induction also shows a developmentally regulated component, since younger leaves accumulated higher levels of PPO mRNA and PPO activity in response to localized damage than older leaves (Figs. 6 and 7). Likewise, PPO-induction by MeJa was also much stronger in younger than older leaves (Fig. 5). This type of pattern is also observed for other wound-induced PPOs, since in potato and tomato PPO mRNA accumulation was induced by wounding only in the youngest leaves (Hunt et al., 1993; Thipyapong et al., 1995; Thipyapong and Steffens, 1997). Preferential PPO induction in young leaves may be an adaptation against herbivory, since young leaves are often preferred by herbivorous insects. For example, survival of young FTC is enhanced if they have access to the very youngest leaves of trembling aspen during bud break rather than slightly older foliage (Parry et al., 1998). A strong inducible defense in these young leaves may thus help protect the tree. Furthermore, the youngest leaves may also be the major target of the systemic signal that induces PPO and other defense proteins. Previous work monitoring trypsin inhibitor gene expression in poplar indicated that wound-induced systemic gene induction was coordinate with assimilate movement from source to sink tissues (Davis et al., 1991). Since the youngest leaves are very strong sinks, they should be more rapidly induced by the systemically mobile wound signal than older leaves.
In inducing damage on whole poplar saplings with FTC we observed a strong preference of these herbivores for leaves 5 to 9 (Fig. 6). This result was unexpected, although a similar preference (leaves 2–8) had been described previously on different poplar hybrids (Robison and Raffa, 1997). For TD hybrid H11-11 grown in our environmental chambers, the leaves older than position 9 become noticeably tougher compared with younger leaves (C.P. Constable, unpublished data), which could explain why the larvae avoided leaves 9 and older. In this context, the induction of Phe ammonia lyase and O-methyl transferase mRNA by wounding of hybrid poplar is significant (Koch et al., 1998), since it suggests there is deposition of lignin or other phenolics in the cell wall. However, leaves 1 to 4 were also avoided; we speculate that this was due to the strong induction of PPO (and other defense proteins) in these leaves (see Fig. 7). The preference of leaves 5 to 9 by FTC larvae may thus represent a compromise between leaf toughness and the induced biochemical defenses. More detailed experiments will have to be performed to test this hypothesis.
The ability of MeJa to induce PPO (Fig. 5) provides additional support for the proposed defensive role of PPO against herbivores. Jasmonates and related octadecanoid metabolites are known inducers of defense responses against pests and pathogens in many species (Weiler, 1997). In tomato, MeJa and the octadecanoid defense-signaling pathway induce PPO in addition to a battery of antiherbivore proteins, including Ser, Cys, and aspartic proteinase inhibitors, lipoxygenase, carboxypeptidase, as well as proteins that help regulate the defense response itself (Bergey et al., 1996). Disruption of the octadecanoid pathway in mutants or via chemical inhibitors leads to an increased susceptibility to insect herbivores, clearly demonstrating that jasmonate-mediated responses are essential for defense (Doares et al., 1995; Howe et al., 1996). Induction of hybrid poplar PPO by MeJa indicates that the octadecanoid defense-signaling pathway mediates herbivore defenses in poplars, which further supports the idea that PPO is an antiherbivore defense in poplar. In addition, Kunitz-type protease inhibitor mRNA, previously shown to be wound induced in this poplar hybrid (Bradshaw et al., 1989), is MeJa-inducible as well (M.E. Christopher and C.P. Constabel, unpublished data). Wounded TD poplar hybrids also systemically accumulate mRNA encoding chitinases, β-glucanases, and a bark storage protein-like polypeptide (Bradshaw et al., 1991; Davis et al., 1993). In a Populus maximozii × P. trichocarpa hybrid, wounding was shown to induce Phe ammonia lyase and O-methyltransferase enzymes involved in lignification (Koch et al., 1998). It thus appears that in poplar, much like tomato, jasmonates and the octadecanoid pathway regulates a battery of defense proteins.
In summary we have cloned and characterized the expression of a wound-induced hybrid poplar PPO. We suggest that this protein plays an important role in the defense of hybrid poplar against folivore insects for the following reasons: (a) PPO induction by wounding and herbivory is systemic, which suggests that it functions in reducing additional herbivore damage rather than in wound repair; (b) the induction is mediated by increased mRNA accumulation, which is typical of a variety of induced defenses proteins; and (c) poplar PPO is induced by MeJa, which is known to regulate the herbivore defense in other plant species. Experiments to overexpress as well as suppress PPO in transgenic poplar are under way, which will allow us to test the defensive role of PtdPPO directly.
MATERIALS AND METHODS
Plant Material
Poplar hybrid H11-11 (Populus trichocarpa × Populus deltoides [TD]), originating from the University of Washington/Washington State University Poplar Research Program, were obtained from G. Radamaker (Washington State University). The 53-246 TD hybrid and 98-968 P. trichocarpa parent were obtained from Dr. C. Douglas (University of British Columbia). Plants were propagated from hardwood or greenwood cuttings in peat (Terra-Lite Redi-Earth, WR Grace, Ajax, ON, Canada) in 15-cm-diameter pots. All plants were maintained in the University of Alberta Biotron's environmental chambers under 16-h days at 18°C and 75% relative humidity. Light intensity was 300 μEm2/s at pot height, composed of approximately 20% incandescent (2,700-W) and 80% cool-white (11,880-W) lights. Plants were watered daily with a solution containing 1 g L−1 20–20-20 Plant-Prod fertilizer (Plant Products, Brampton, ON). All side shoots were pruned as they developed so that each plant consisted of a single main stem, no less than 2 weeks prior to wounding.
Wounding, MeJa, and Herbivore Treatments
Plants were approximately 10- to 12-weeks-old when used for experiments. The most apical unfolded, but not yet expanded, leaf was designated as leaf position one, and the other leaves were numbered sequentially down the stem. For wounding experiments, leaves were mechanically wounded by crushing leaf blades at the margins with pliers. Each leaf was wounded at least twice, 1.5 h apart. For time course experiments, six fully expanded leaves were wounded. Tissue was frozen in liquid nitrogen and stored at −80°C until analyzed.
MeJa (Bedoukian Research, Danbury, CT) was diluted 1:10 with 95% (v/v) ethanol, and then rediluted 1:500 with water:0.1% (v/v) Triton X-100. Leaves were sprayed to the point of runoff. Controls were mock-sprayed with the same solution without MeJa. Herbivore treatments using FTC were carried out in 75-cm-high insect cages within the environmental chambers. Three-month-old plants, consisting of one main stem and approximately 20 to 25 leaves, were maintained in large insect cages and 15 FTC larvae (fourth instar, obtained from Dr. A. Keddie, University of Alberta) were placed at random on each plant. The larvae, being highly mobile, moved over the entire plant and fed at will for the duration of the experiment. The amount of leaf material consumed was monitored daily. After 6 d the experiment was terminated, the proportion of leaf material eaten was visually estimated, and the remaining leaf tissue was sampled for PPO analysis. Similar trees were placed in a cage without larvae for controls.
PPO Enzyme Assays
Frozen leaves were extracted into extraction buffer (100 mm NaPO4, pH 7.0, 0.1% [v/v] Triton X-100, and 5% [w/v] polyvinylpyrrolidone), clarified by centrifugation, and the supernatant was immediately assayed for PPO activity. For routine assays, the conversion of DOPA to dopaquinone was measured spectrophotometrically as described (Sherman et al., 1991). The rates of oxidation of DOPA and that of other substrates was compared by measuring oxygen consumption with a Clark-type oxygen electrode (Yellow Springs Instrument Co., Yellow Springs, OH). Protein concentrations were determined by the method of Bradford (1976) using bovine serum albumin as a standard. Specific activity was calculated using an extinction coefficient of e = 3,600 m−1 cm−1 for dopaquinone (Burton and Kirchmann, 1997).
cDNA Library Construction and PPO Cloning
Total RNA was isolated from hybrid poplar leaves (systemically wounded for 28 h) as described (Wingate et al., 1989). Poly(A+) RNA was isolated using magnetic beads (PolyATract mRNA isolation kit, Promega, Madison, WI) and used to construct a cDNA library (Lambda Zap II cDNA Synthesis Kit, Stratagene, La Jolla, CA) using the manufacturer's protocol. Approximately 5 × 105 plaques were screened using a fragment of the PPO cDNA previously amplified by PCR with PPO-specific degenerate oligonucleotide primers (5′-TTC/TGCIC/TTICCNTAC/ TTGGAAC/TTGG-3′; 5′-CCACATICG/TG/ATCIACG/ ATTNGCG/ATGG/ATG-3′) and cloned into T-tailed pBluescript vectors (Marchuk et al., 1991). The clone with the longest cDNA insert was identified by PCR and its sequence determined.
Sequence Analysis and 5′ RACE
DNA sequencing was carried out on both strands using a fluorescently labeled dideoxyterminator sequencing kit (Thermosequenase, Amersham, Buckinghamshire, UK) and analyzed using an ABI Prism 377 DNA Sequencer (Applied Biosystems, Foster City, CA). RACE (5′ RACE) to confirm the 5′ end of the poplar cDNA was performed using the 5′ RACE System (Gibco/BRL, Gaithersburg, MD). Amplified products were cloned into pBluescript-derived T- vectors, and the clones with the largest inserts were sequenced. Sequence analyses were carried out with the Wisconsin Package Version 9.1 (Genetics Computer Group, Madison, WI).
RNA and DNA Hybridization Analysis
Twenty micrograms of total RNA per lane was loaded onto 1.2% (w/v) agarose-formaldehyde gels in MOPS [3-(N-morpholino)-propanesulfonic acid] buffer (pH 7) and transferred by capillary blotting onto Zeta-Probe membranes (Bio-Rad, Hercules, CA). The RNA was cross-linked to membranes using a GS Gene Linker UV chamber (Bio-Rad) prior to prehybridization for 2 h at 42°C in 5× sodium chloride/sodium phosphate/EDTA (SSPE), 50% (v/v) formamide, 5× Denhardt's solution, 1% (w/v) SDS, 10% (w/v) dextran sulfate, and 100 mg mL−1 denatured salmon sperm DNA. DNA probes were obtained by random priming of the entire PtdPPO coding sequence (T7 Quickprime kit, Pharmacia Biotech, Piscataway, NJ) and hybridization carried out for 16 to 18 h. The membranes were washed twice with 5× SSPE and 1% (w/v) SDS for 15 min at room temperature, twice with 1× SSPE/1% (w/v) SDS at 65°C for 30 min, and once with 0.1× SSPE/1% (w/v) SDS at 65°C for 30 min. The blots were exposed to x-ray film as well as on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) for quantification of signal intensities (ImageQuant software, Molecular Dynamics). Ethidium bromide staining of RNA was used to verify equal loading of lanes.
For Southern analyses, genomic DNA was isolated from young leaves as described (Doyle and Doyle, 1990), with the extraction buffer modified to contain 4% (w/v) cetyltrimethylammonium bromide and 1% (w/v) polyvinylpolypyrrolidone. Fifteen micrograms of DNA was digested with EcoRI, HindIII, and XbaI (Gibco/BRL), electrophoresed through a 0.8% (w/v) agarose gel, and blotted onto Zeta-Probe membranes (Bio-Rad) using standard protocols (Sambrook et al., 1989). Hybridization and analysis was carried out as described above.
ACKNOWLEDGMENTS
The authors thank Sean Gregg for help with PPO assays and insect experiments, Andrew Keddie for supplying the forest tent caterpillar larvae, and Gary Radamaker and Carl Douglas for providing poplar cuttings.
Footnotes
This work was funded by a research grant from the Natural Sciences and Engineering Research Council of Canada (to C.P.C.).
LITERATURE CITED
- Alborn H, Turlings T, Jones T, Stenhagen G, Loughrin J, Tumlinson J. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- Bergey D, Howe G, Ryan CA. Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss P, Gardner R, Janssen B, Ross G. An apple polyphenol oxidase cDNA is up-regulated in wounded tissues. Plant Mol Biol. 1995;27:429–433. doi: 10.1007/BF00020197. [DOI] [PubMed] [Google Scholar]
- Boudet A-M. A new view of lignification. Trends Plant Sci. 1998;3:67–71. [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem. 1976;71:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Jr, Hollick JB, Parsons TJ, Clarke HRG, Gordon MP. Systemically wound-responsive genes in poplar trees encode proteins similar to sweet potato sporamins and legume Kunitz trypsin inhibitors. Plant Mol Biol. 1989;14:51–59. doi: 10.1007/BF00015654. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Parsons TJ, Gordon MP. Wound-responsive gene expression in poplars. For Ecol Manag. 1991;43:211–224. [Google Scholar]
- Buell R. Genes involved in plant-pathogen interactions. In: Agrawaal AA, Tuzun S, Bent E, editors. Induced Plant Defenses Against Herbivores and Pathogens. APS Press, St. Paul. 1999. pp. 73–93. [Google Scholar]
- Burton SG, Kirchmann S. Optimised detergent-based method for extraction of a chloroplast membrane-bound enzyme: polyphenol oxidase from tea (Camellia sinensis) Biotechnol Tech. 1997;11:645–648. [Google Scholar]
- Chaddock AM, Mant A, Karnauchov I, Brink S, Hermann RG, Klösgen RB, Robinson C. A new type of signal peptide: central role of a twin arginine motif in transfer signals for the ΔpH-dependent thylakoidal protein translocase. EMBO J. 1995;14:2715–2722. doi: 10.1002/j.1460-2075.1995.tb07272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Wei C-I, Marshall M. Inhibition mechanism of kojic acid on polyphenol oxidase. J Agric Food Chem. 1991;39:1897–1901. [Google Scholar]
- Chevalier T, de Rigal D, Mbéguié-A-Mbéguié D, Gauillard F, Richard-Forget F, Fils-Lycaon BR. Molecular cloning and characterization of apricot fruit polyphenol oxidase. Plant Physiol. 1999;119:1261–1269. doi: 10.1104/pp.119.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constabel CP. A survey of herbivore-inducible defensive proteins and phytochemicals. In: Agrawaal AA, Tuzun S, Bent E, editors. Induced Plant Defenses Against Herbivores and Pathogens. APS Press, St. Paul. 1999. pp. 137–166. [Google Scholar]
- Constabel CP, Bergey D, Ryan CA. Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc Natl Acad Sci USA. 1995;92:407–411. doi: 10.1073/pnas.92.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constabel CP, Bergey DR, Ryan CA. Polyphenol oxidase as a component of the inducible defense response in tomato against herbivores. In: Romeo JT, Saunders JA, Barbosa P, editors. Phytochemical Diversity and Redundancy in Ecological Interactions. New York: Plenum Press; 1996. pp. 231–252. [Google Scholar]
- Constabel CP, Ryan CA. A survey of wound- and methyl jasmonate-induced leaf polyphenol oxidase in crop plants. Phytochemistry. 1998;47:507–511. [Google Scholar]
- Davis J, Egelkrout E, Coleman G, Chen T, Haisig B, Riemenschneider D, Gordon M. A family of wound-induced genes in Populus shares common features with genes encoding vegetative storage proteins. Plant Mol Biol. 1993;23:135–143. doi: 10.1007/BF00021426. [DOI] [PubMed] [Google Scholar]
- Davis J, Gordon M, Smit B. Assimilate movement dictates remote sites of wound-induced gene expression in poplar leaves. Proc Natl Acad Sci USA. 1991;88:2393–2396. doi: 10.1073/pnas.88.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares S, Syrovets T, Weiler E, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. BRL Focus. 1990;12:13–15. [Google Scholar]
- Dry I, Robinson S. Molecular cloning and characterization of grape berry polyphenol oxidase. Plant Mol Biol. 1994;26:495–502. doi: 10.1007/BF00039560. [DOI] [PubMed] [Google Scholar]
- Duffey SS, Felton GW. Enzymatic antinutritive defenses of the tomato plant against insects. In: Hedin PA, editor. Naturally Occurring Pest Bioregulators. Washington, DC: ACS Press; 1991. pp. 167–197. [Google Scholar]
- Felton GW, Donato KK, Broadway RM, Duffey SS. Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol. 1992;38:277–285. [Google Scholar]
- Felton GW, Donato KK, Del Vecchio RJ, Duffey SS. Activation of foliar oxidases by insect feeding reduces nutritive quality of dietary protein of foliage for noctuid herbivores. J Chem Ecol. 1989;15:2667–2693. doi: 10.1007/BF01014725. [DOI] [PubMed] [Google Scholar]
- Haruta M, Murata M, Hiraide A, Kadokura H, Yamasaki M, Sakuta M, Shimizu S, Homma S. Cloning genomic DNA encoding apple polyphenol oxidase and comparison of the gene product in Escherichia coli and in apple. Biosci Biotechnol Biochem. 1998;62:358–362. doi: 10.1271/bbb.62.358. [DOI] [PubMed] [Google Scholar]
- Havill NP, Raffa KF. Effects of elicitation treatment and genotypic variation on induced resistance in Populus: impacts on gypsy moth (Lepidoptera: Lymantriidae) development and feeding behavior. Oecologia. 1999;120:295–303. doi: 10.1007/s004420050861. [DOI] [PubMed] [Google Scholar]
- Hilder V, Gatehouse A, Sheerman S, Barker R, Boulter D. A novel mechanism of insect resistance engineered into tobacco. Nature. 1987;330:160–163. [Google Scholar]
- Howe G, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M, Eannetta N, Yu H, Newman S, Steffens J. cDNA cloning and expression of potato polyphenol oxidase. Plant Mol Biol. 1993;21:59–68. doi: 10.1007/BF00039618. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Garcia-Carmona F. The effect of sodium dodecyl sulfate on polyphenol oxidase. Phytochemistry. 1996;42:1503–1509. [Google Scholar]
- Johnson R, Narvaez J, An G, Ryan CA. Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci USA. 1989;86:9871–9875. doi: 10.1073/pnas.86.24.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy IVR, Sugiyama M, Fukuda H, Komamine A. Cloning and characterization of polyphenol oxidase cDNAs of Phytolacca americana. Plant Physiol. 1995;197:1083–1089. doi: 10.1104/pp.107.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JR, Scherzer AJ, Eshita SM, Davis KR. Ozone sensitivity in hybrid poplar is correlated with a lack of defense-gene activation. Plant Physiol. 1998;118:1243–1252. doi: 10.1104/pp.118.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroth RL, Hwang S-Y. Diversity, redundancy, and multiplicity in chemical defense systems of aspen. In: Romeo JT, Saunders JA, Barbosa P, editors. Phytochemical Diversity and Redundancy in Ecological Interactions. New York: Plenum Press; 1996. pp. 25–56. [Google Scholar]
- Macheix J-J, Fleuriet A, Billot J. Fruit Phenolics. Boca Raton, FL: CRC Press; 1990. p. 378. [Google Scholar]
- Marchuk D, Drumm M, Saulino A, Collins FS. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991;19:1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WJ, Levieux J, Bernard-Dagan C. Mechanisms of woody plant defenses against insects. New York: Springer-Verlag; 1988. pp. 157–169. [Google Scholar]
- Parry D, Spence JR, Volney WJA. Budbreak phenology and natural enemies mediate survival of first-instar forest tent caterpillar (Lepidoptera: Lasiocampidae) Environ Entomol. 1998;27:1368–1374. [Google Scholar]
- Parsons TJ, Bradshaw HD, Jr, Gordon MP. Systemic accumulation of specific mRNAs in response to wounding in poplar trees. Proc Natl Acad Sci USA. 1989;86:7895–7899. doi: 10.1073/pnas.86.20.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Mant A. Targeting of proteins into and across the thylakoid membrane. Trends Plant Sci. 1997;2:431–437. [Google Scholar]
- Robison DJ, Raffa KF. Characterization of hybrid poplar clones for resistance to the forest tent caterpillar. For Sci. 1994;40:686–714. [Google Scholar]
- Robison DJ, Raffa KF. Effects of constitutive and inducible traits of hybrid poplars on forest tent caterpillar feeding and population ecology. For Sci. 1997;43:252–267. [Google Scholar]
- Ryan CA. Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopathol. 1990;28:425–449. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sherman T, Vaughn K, Duke S. A limited survey of the phylogenetic distribution of polyphenol oxidase. Phytochemistry. 1991;30:2499–2506. [Google Scholar]
- Sommer A, Ne'eman E, Steffens JC, Mayer AM, Harel E. Import, targeting, and processing of a plant polyphenol oxidase. Plant Physiol. 1994;105:1301–1311. doi: 10.1104/pp.105.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens J, Harel E, Hunt M. Polyphenol oxidase. In: Ellis BE, Kuroki GW, Stafford HA, editors. Genetic Engineering of Plant Secondary Metabolism. New York: Plenum Press; 1994. pp. 276–304. [Google Scholar]
- Sterky F, Regan S, Karlsson J, Hertzberg M, Rohde A, Holmberg A, Amini B, Bhalerao R, Larsson M, Villarroel R. Gene discovery in the wood-forming tissues of poplar: analysis of 5,692 expressed sequence tags. Proc Natl Acad Sci USA. 1998;95:13330–13335. doi: 10.1073/pnas.95.22.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallamy DW, Raupp MJ. Phytochemical Induction by Herbivores. New York: John Wiley; 1991. [Google Scholar]
- Thipyapong P, Hunt M, Steffens J. Systemic wound induction of potato (Solanum tuberosum) polyphenol oxidase. Phytochemistry. 1995;40:673–676. [Google Scholar]
- Thipyapong P, Joel D, Steffens J. Differential expression and turnover of the tomato polyphenol oxidase gene family during vegetative and reproductive development. Plant Physiol. 1997;113:707–718. doi: 10.1104/pp.113.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thipyapong P, Steffens J. Tomato polyphenol oxidase: differential response of the polyphenol oxidase F promoter to injuries and wound signals. Plant Physiol. 1997;1152:409–418. doi: 10.1104/pp.115.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen P, Dry I, Robinson S. Polyphenol oxidase in potato: a multigene family that exhibits differential expression patterns. Plant Physiol. 1995;109:525–531. doi: 10.1104/pp.109.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfira T, Zuker A, Altman A. Forest-tree biotechnology: genetic transformation and its application to future forests. Trends Biotechnol. 1998;16:439–446. [Google Scholar]
- Valero E, Garcia-Moreno M, Varon R, Garcia-Carmona F. Time-dependent inhibition of grape polyphenol oxidase by tropolone. J Agric Food Chem. 1991;39:1043–1046. [Google Scholar]
- van Gelder CWG, Flurkey WH, Wichers HJ. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry. 1997;45:1309–1323. doi: 10.1016/s0031-9422(97)00186-6. [DOI] [PubMed] [Google Scholar]
- Walker JRL, McCallion RF. The selective inhibition of ortho- and para-diphenol oxidases. Phytochemistry. 1980;19:373–377. [Google Scholar]
- Weiler EW. Octadecanoid mediated signal transduction in higher plants. Naturwissenschaften. 1997;84:340–349. [Google Scholar]
- Whitman TG, Floate KD, Martinsen GD, Driebe EM, Keim P. Ecological and evolutionary implications of hybridizations: Populus-herbivore interactions. In: Stettler RF, Bradshaw Jr HD, Heilman PE, Hinckley TM, editors. Biology of Populus and Its Implications for Management and Conservation. Ottawa, Canada: NRC Research Press; 1996. pp. 247–275. [Google Scholar]
- Wingate V, Broadway R, Ryan CA. Isolation and characterization of a novel, developmentally regulated proteinase inhibitor I protein and cDNA from the fruit of a wild species of tomato. J Biol Chem. 1989;264:17734–17738. [PubMed] [Google Scholar]